Abstract
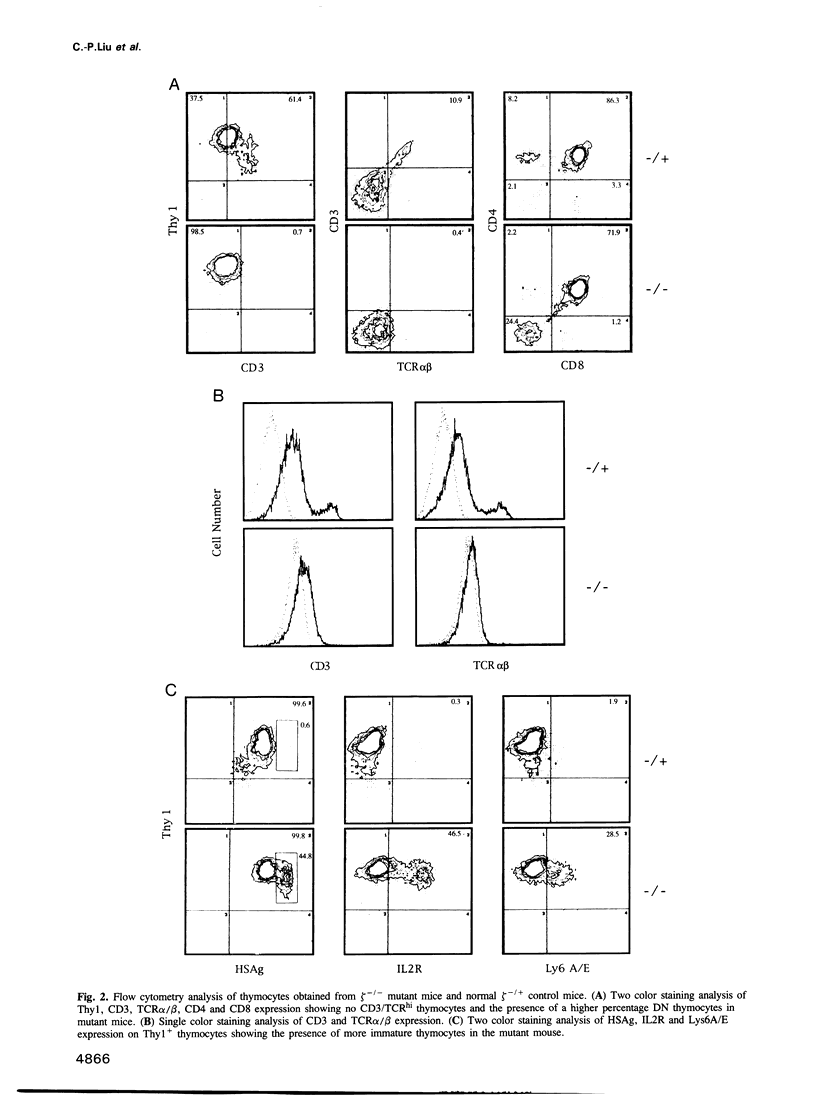
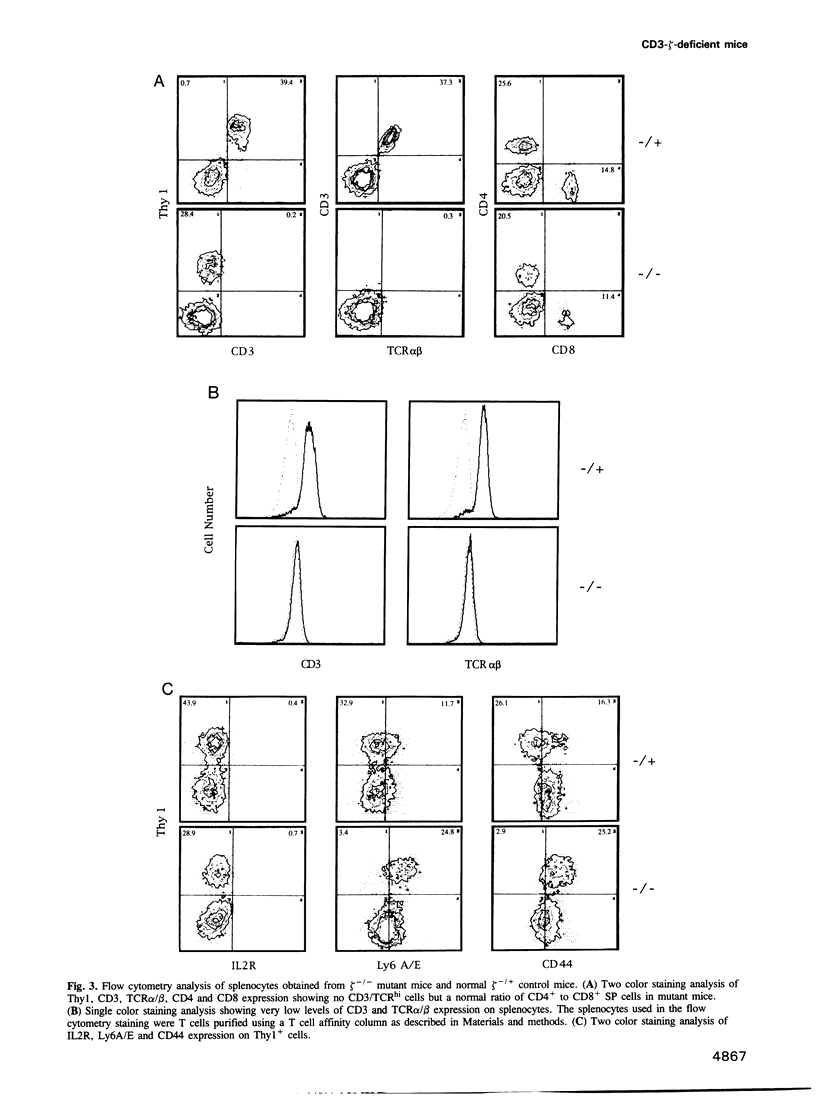
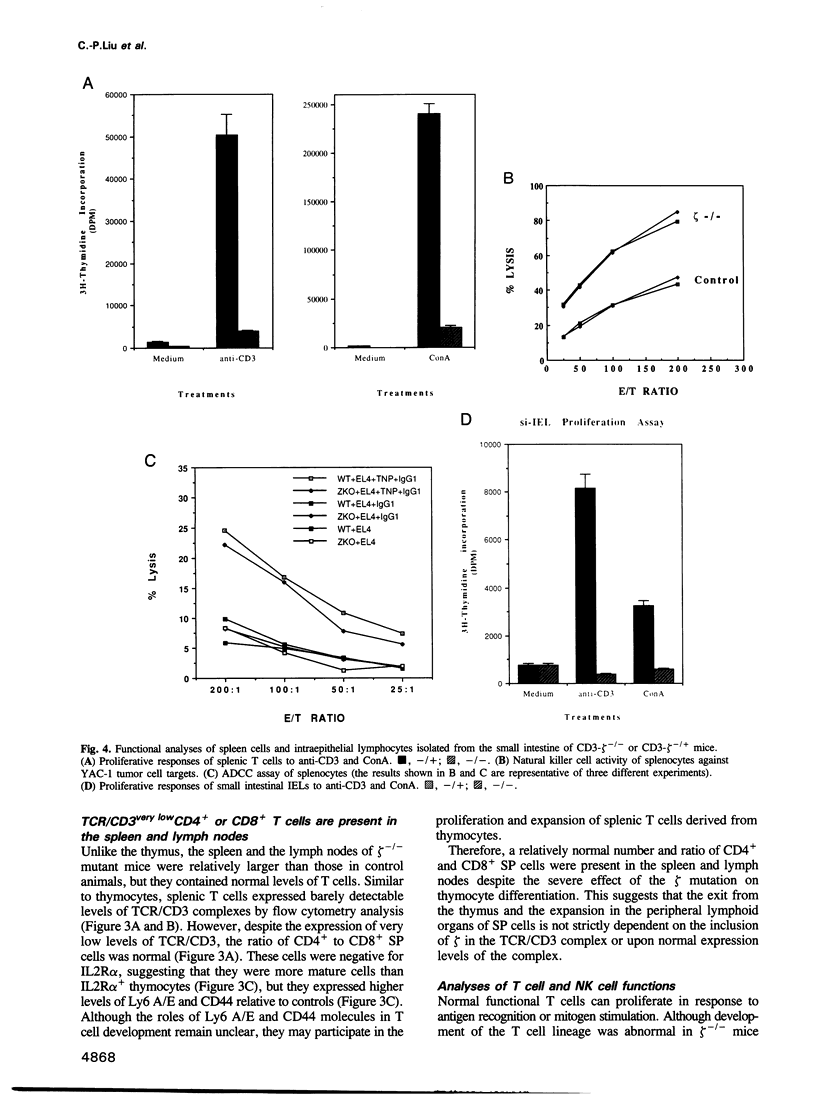
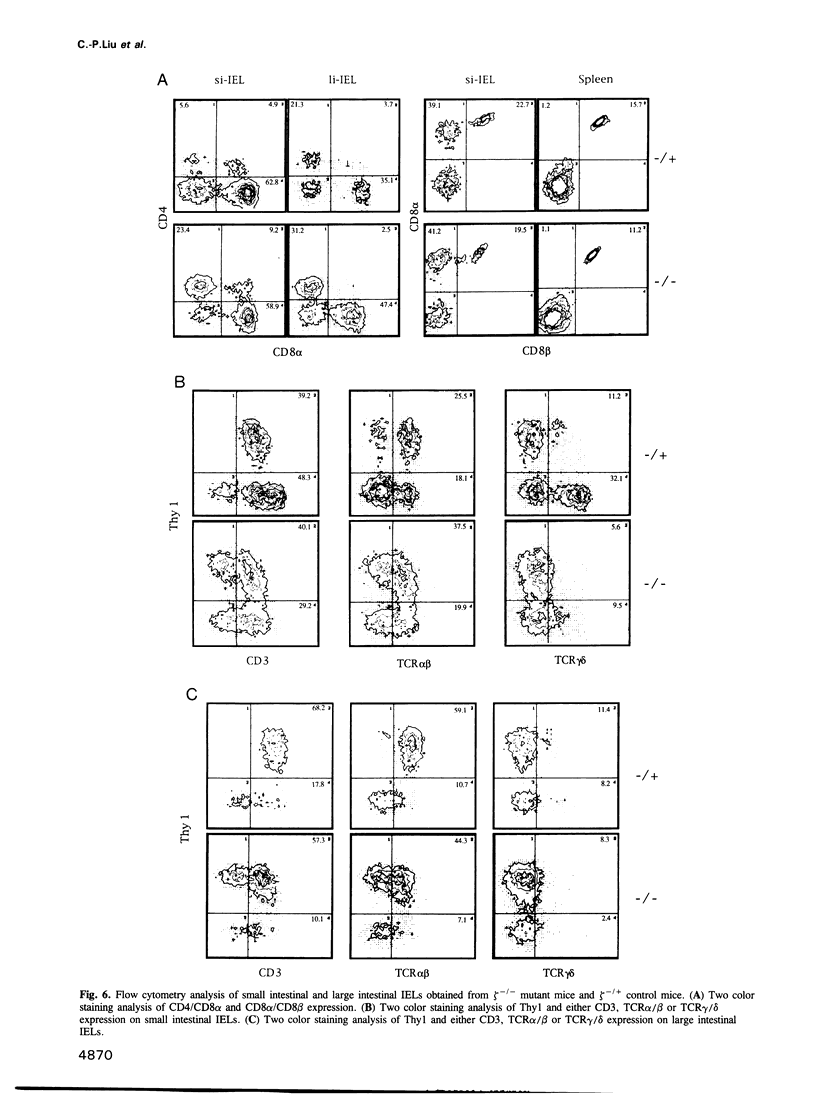
The T cell antigen receptor (TCR)-associated invariable membrane proteins (CD3-gamma, -delta, -epsilon and -zeta) are critical to the assembly and cell surface expression of the TCR/CD3 complex and to signal transduction upon engagement of TCR with antigen. Disruption of the CD3-zeta gene by homologous recombination resulted in a structurally abnormal thymus which primarily contained CD4- CD8- and TCR/CD3very lowCD4+CD8+ cells. Spleen and lymph nodes of CD3-zeta-/- mutant mice contained a normal number and ratio of CD4+ and CD8+ single positive cells that were TCR/CD3very low. These splenocytes did not respond to antibody cross-linking or mitogenic triggering. The V beta genes of CD4-CD8- and CD4+CD8+ thymocytes and splenic T cells were productively rearranged. These data demonstrated that (i) in the absence of the CD3-zeta chain, the CD4- CD8- thymocytes could differentiate to CD4+CD8+ TCR/CD3very low thymocytes, (ii) that thymic selection might have occurred, (iii) but that the transition to CD4+CD8- and CD4-CD8+ cells took place at a very low rate. Most strikingly, intraepithelial lymphocytes (IELs) isolated from the small intestine or the colon expressed normal levels of TCR/CD3 complexes on their surface which contained Fc epsilon RI gamma homodimers. In contrast to CD3-zeta containing IELs, these cells failed to proliferate after triggering with antibody cross-linking or mitogen. In comparison to thymus-derived peripheral T cells in the spleen and lymph nodes, the preferential expression of normal levels of TCR/CD3 in intestinal IELs suggested they mature via an independent extrathymic pathway.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcon B., Berkhout B., Breitmeyer J., Terhorst C. Assembly of the human T cell receptor-CD3 complex takes place in the endoplasmic reticulum and involves intermediary complexes between the CD3-gamma.delta.epsilon core and single T cell receptor alpha or beta chains. J Biol Chem. 1988 Feb 25;263(6):2953–2961. [PubMed] [Google Scholar]
- Alarcón B., Ley S. C., Sánchez-Madrid F., Blumberg R. S., Ju S. T., Fresno M., Terhorst C. The CD3-gamma and CD3-delta subunits of the T cell antigen receptor can be expressed within distinct functional TCR/CD3 complexes. EMBO J. 1991 Apr;10(4):903–912. doi: 10.1002/j.1460-2075.1991.tb08023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Caligiuri M., O'Brien C., Manley T., Ritz J., Schlossman S. F. Fc gamma receptor type III (CD16) is included in the zeta NK receptor complex expressed by human natural killer cells. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2274–2278. doi: 10.1073/pnas.87.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Caligiuri M., Ritz J., Schlossman S. F. CD3-negative natural killer cells express zeta TCR as part of a novel molecular complex. Nature. 1989 Sep 14;341(6238):159–162. doi: 10.1038/341159a0. [DOI] [PubMed] [Google Scholar]
- Ashwell J. D., Klusner R. D. Genetic and mutational analysis of the T-cell antigen receptor. Annu Rev Immunol. 1990;8:139–167. doi: 10.1146/annurev.iy.08.040190.001035. [DOI] [PubMed] [Google Scholar]
- Baniyash M., Hsu V. W., Seldin M. F., Klausner R. D. The isolation and characterization of the murine T cell antigen receptor zeta chain gene. J Biol Chem. 1989 Aug 5;264(22):13252–13257. [PubMed] [Google Scholar]
- Barrett T. A., Gajewski T. F., Danielpour D., Chang E. B., Beagley K. W., Bluestone J. A. Differential function of intestinal intraepithelial lymphocyte subsets. J Immunol. 1992 Aug 15;149(4):1124–1130. [PubMed] [Google Scholar]
- Benhamou M., Gutkind J. S., Robbins K. C., Siraganian R. P. Tyrosine phosphorylation coupled to IgE receptor-mediated signal transduction and histamine release. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5327–5330. doi: 10.1073/pnas.87.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron C. A., van den Elsen P., Tutt M. M., Medveczky P., Kumar V., Terhorst C. Murine natural killer cells stimulated in vivo do not express the T cell receptor alpha, beta, gamma, T3 delta, or T3 epsilon genes. J Immunol. 1987 Sep 1;139(5):1704–1710. [PubMed] [Google Scholar]
- Blumberg R. S., Ley S., Sancho J., Lonberg N., Lacy E., McDermott F., Schad V., Greenstein J. L., Terhorst C. Structure of the T-cell antigen receptor: evidence for two CD3 epsilon subunits in the T-cell receptor-CD3 complex. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7220–7224. doi: 10.1073/pnas.87.18.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Cosson P., Klausner R. D. Colocalized transmembrane determinants for ER degradation and subunit assembly explain the intracellular fate of TCR chains. Cell. 1990 Nov 2;63(3):503–513. doi: 10.1016/0092-8674(90)90447-m. [DOI] [PubMed] [Google Scholar]
- Bosma M. J., Carroll A. M. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- Chen J., Trounstine M., Kurahara C., Young F., Kuo C. C., Xu Y., Loring J. F., Alt F. W., Huszar D. B cell development in mice that lack one or both immunoglobulin kappa light chain genes. EMBO J. 1993 Mar;12(3):821–830. doi: 10.1002/j.1460-2075.1993.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Cosgrove D., Gray D., Dierich A., Kaufman J., Lemeur M., Benoist C., Mathis D. Mice lacking MHC class II molecules. Cell. 1991 Sep 6;66(5):1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Exley M., Terhorst C., Wileman T. Structure, assembly and intracellular transport of the T cell receptor for antigen. Semin Immunol. 1991 Sep;3(5):283–297. [PubMed] [Google Scholar]
- Frank S. J., Niklinska B. B., Orloff D. G., Merćep M., Ashwell J. D., Klausner R. D. Structural mutations of the T cell receptor zeta chain and its role in T cell activation. Science. 1990 Jul 13;249(4965):174–177. doi: 10.1126/science.2371564. [DOI] [PubMed] [Google Scholar]
- Godfrey D. I., Kennedy J., Suda T., Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993 May 15;150(10):4244–4252. [PubMed] [Google Scholar]
- Gramzinski R. A., Adams E., Gross J. A., Goodman T. G., Allison J. P., Lefrançois L. T cell receptor-triggered activation of intraepithelial lymphocytes in vitro. Int Immunol. 1993 Feb;5(2):145–153. doi: 10.1093/intimm/5.2.145. [DOI] [PubMed] [Google Scholar]
- Grusby M. J., Johnson R. S., Papaioannou V. E., Glimcher L. H. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991 Sep 20;253(5026):1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Malassis-Seris M., Briottet C., Vassalli P. Cytotoxic differentiation of mouse gut thymodependent and independent intraepithelial T lymphocytes is induced locally. Correlation between functional assays, presence of perforin and granzyme transcripts, and cytoplasmic granules. J Exp Med. 1991 Jun 1;173(6):1549–1552. doi: 10.1084/jem.173.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C., Berkhout B., Alarcon B., Sancho J., Wileman T., Terhorst C. Requirements for cell surface expression of the human TCR/CD3 complex in non-T cells. Int Immunol. 1991 Apr;3(4):359–368. doi: 10.1093/intimm/3.4.359. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Uchida N., Friedman J., Weissman I. L. Lymphocyte development from stem cells. Annu Rev Immunol. 1992;10:759–783. doi: 10.1146/annurev.iy.10.040192.003551. [DOI] [PubMed] [Google Scholar]
- Jin Y. J., Clayton L. K., Howard F. D., Koyasu S., Sieh M., Steinbrich R., Tarr G. E., Reinherz E. L. Molecular cloning of the CD3 eta subunit identifies a CD3 zeta-related product in thymus-derived cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3319–3323. doi: 10.1073/pnas.87.9.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Marrack P., Kappler J. W., Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Yu G., Phillips J. H. Analysis of Fc gamma RIII (CD16) membrane expression and association with CD3 zeta and Fc epsilon RI-gamma by site-directed mutation. J Immunol. 1991 Mar 1;146(5):1571–1576. [PubMed] [Google Scholar]
- Lanier L. L., Yu G., Phillips J. H. Co-association of CD3 zeta with a receptor (CD16) for IgG Fc on human natural killer cells. Nature. 1989 Dec 14;342(6251):803–805. doi: 10.1038/342803a0. [DOI] [PubMed] [Google Scholar]
- Lefrancois L., Goodman T. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-gamma delta+ intraepithelial lymphocytes. Science. 1989 Mar 31;243(4899):1716–1718. doi: 10.1126/science.2564701. [DOI] [PubMed] [Google Scholar]
- Lefrancois L. Phenotypic complexity of intraepithelial lymphocytes of the small intestine. J Immunol. 1991 Sep 15;147(6):1746–1751. [PubMed] [Google Scholar]
- Letourneur F., Klausner R. D. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 epsilon. Science. 1992 Jan 3;255(5040):79–82. doi: 10.1126/science.1532456. [DOI] [PubMed] [Google Scholar]
- Liu C. P., Auerbach R. In vitro development of murine T cells from prethymic and preliver embryonic yolk sac hematopoietic stem cells. Development. 1991 Dec;113(4):1315–1323. doi: 10.1242/dev.113.4.1315. [DOI] [PubMed] [Google Scholar]
- Liu C. P., Auerbach R. Ontogeny of murine T cells: thymus-regulated development of T cell receptor-bearing cells derived from embryonic yolk sac. Eur J Immunol. 1991 Aug;21(8):1849–1855. doi: 10.1002/eji.1830210811. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Lees R. K., Bron C., Sordat B., Miescher G. T cell antigen receptor expression in athymic (nu/nu) mice. Evidence for an oligoclonal beta chain repertoire. J Exp Med. 1987 Jul 1;166(1):195–209. doi: 10.1084/jem.166.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The T cell receptor. Science. 1987 Nov 20;238(4830):1073–1079. doi: 10.1126/science.3317824. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Miller J. B. Myoblast diversity in skeletal myogenesis: how much and to what end? Cell. 1992 Apr 3;69(1):1–3. doi: 10.1016/0092-8674(92)90111-o. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H., O'Shea J. J., Longo D. L., Loeffler C. M., McVicar D. W., Ochoa A. C. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992 Dec 11;258(5089):1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Clarke A. R., Rudnicki M. A., Iacomini J., Itohara S., Lafaille J. J., Wang L., Ichikawa Y., Jaenisch R., Hooper M. L. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992 Nov 19;360(6401):225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R. S., Herrup K., Tonegawa S., Papaioannou V. E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992 Mar 6;68(5):869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Mosley R. L., Klein J. R. Peripheral engraftment of fetal intestine into athymic mice sponsors T cell development: direct evidence for thymopoietic function of murine small intestine. J Exp Med. 1992 Nov 1;176(5):1365–1373. doi: 10.1084/jem.176.5.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley R. L., Whetsell M., Klein J. R. Proliferative properties of murine intestinal intraepithelial lymphocytes (IEL): IEL expressing TCR alpha beta or TCR tau delta are largely unresponsive to proliferative signals mediated via conventional stimulation of the CD3-TCR complex. Int Immunol. 1991 Jun;3(6):563–569. doi: 10.1093/intimm/3.6.563. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., MacKenzie S., Baca M. E., Felstein M. V., Parrott D. M. Functional characteristics of intraepithelial lymphocytes from mouse small intestine. II. In vivo and in vitro responses of intraepithelial lymphocytes to mitogenic and allogeneic stimuli. Immunology. 1986 Aug;58(4):627–634. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., McInnes I. B., Parrott D. M. Functional properties of intra-epithelial lymphocytes from mouse small intestine. IV. Investigation of the proliferative capacity of IEL using phorbol ester and calcium ionophore. Immunology. 1989 Mar;66(3):398–403. [PMC free article] [PubMed] [Google Scholar]
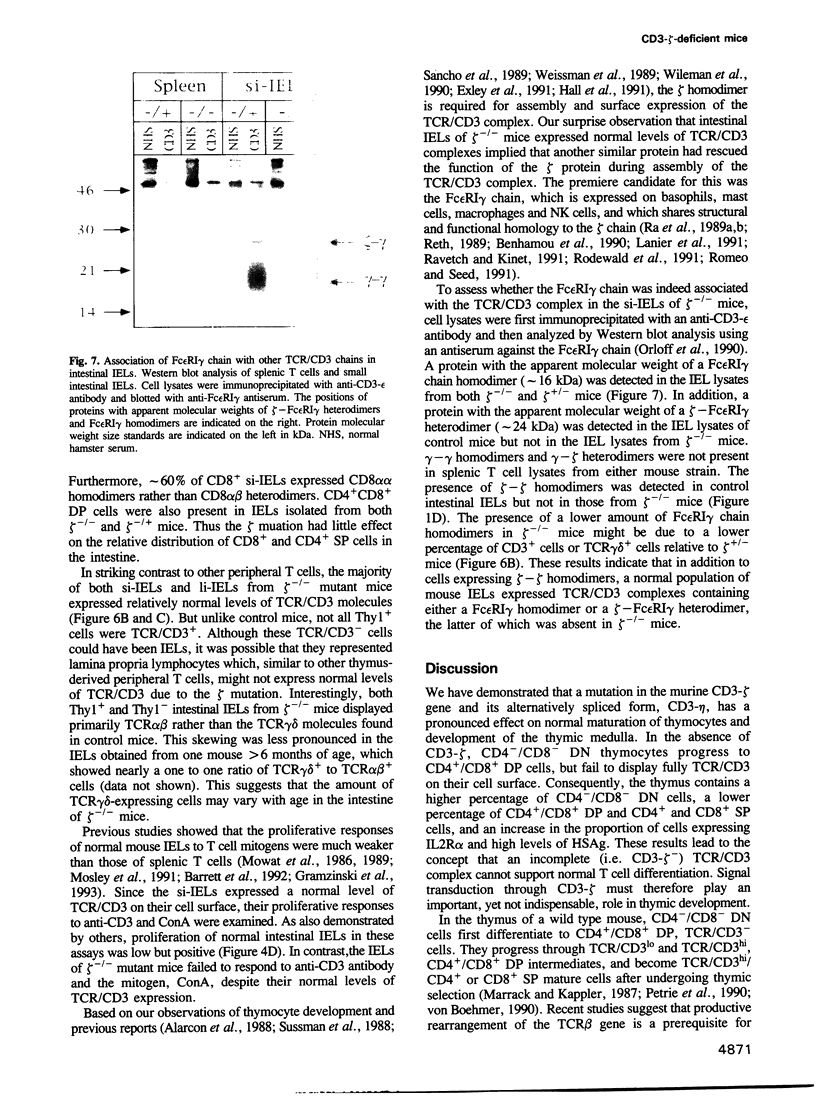
- Orloff D. G., Ra C. S., Frank S. J., Klausner R. D., Kinet J. P. Family of disulphide-linked dimers containing the zeta and eta chains of the T-cell receptor and the gamma chain of Fc receptors. Nature. 1990 Sep 13;347(6289):189–191. doi: 10.1038/347189a0. [DOI] [PubMed] [Google Scholar]
- Paolini R., Jouvin M. H., Kinet J. P. Phosphorylation and dephosphorylation of the high-affinity receptor for immunoglobulin E immediately after receptor engagement and disengagement. Nature. 1991 Oct 31;353(6347):855–858. doi: 10.1038/353855a0. [DOI] [PubMed] [Google Scholar]
- Petrie H. T., Hugo P., Scollay R., Shortman K. Lineage relationships and developmental kinetics of immature thymocytes: CD3, CD4, and CD8 acquisition in vivo and in vitro. J Exp Med. 1990 Dec 1;172(6):1583–1588. doi: 10.1084/jem.172.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott K. L., Viney J. L., Kay G., Rastan S., Gardiner E. M., Chae S., Hayday A. C., Owen M. J. Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science. 1992 Jun 5;256(5062):1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- Poussier P., Edouard P., Lee C., Binnie M., Julius M. Thymus-independent development and negative selection of T cells expressing T cell receptor alpha/beta in the intestinal epithelium: evidence for distinct circulation patterns of gut- and thymus-derived T lymphocytes. J Exp Med. 1992 Jul 1;176(1):187–199. doi: 10.1084/jem.176.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ra C., Jouvin M. H., Blank U., Kinet J. P. A macrophage Fc gamma receptor and the mast cell receptor for IgE share an identical subunit. Nature. 1989 Oct 26;341(6244):752–754. doi: 10.1038/341752a0. [DOI] [PubMed] [Google Scholar]
- Ra C., Jouvin M. H., Kinet J. P. Complete structure of the mouse mast cell receptor for IgE (Fc epsilon RI) and surface expression of chimeric receptors (rat-mouse-human) on transfected cells. J Biol Chem. 1989 Sep 15;264(26):15323–15327. [PubMed] [Google Scholar]
- Ravetch J. V., Kinet J. P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Reth M. Antigen receptor tail clue. Nature. 1989 Mar 30;338(6214):383–384. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- Rocha B., Vassalli P., Guy-Grand D. The V beta repertoire of mouse gut homodimeric alpha CD8+ intraepithelial T cell receptor alpha/beta + lymphocytes reveals a major extrathymic pathway of T cell differentiation. J Exp Med. 1991 Feb 1;173(2):483–486. doi: 10.1084/jem.173.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B., Vassalli P., Guy-Grand D. The extrathymic T-cell development pathway. Immunol Today. 1992 Nov;13(11):449–454. doi: 10.1016/0167-5699(92)90074-H. [DOI] [PubMed] [Google Scholar]
- Rodewald H. R., Arulanandam A. R., Koyasu S., Reinherz E. L. The high affinity Fc epsilon receptor gamma subunit (Fc epsilon RI gamma) facilitates T cell receptor expression and antigen/major histocompatibility complex-driven signaling in the absence of CD3 zeta and CD3 eta. J Biol Chem. 1991 Aug 25;266(24):15974–15978. [PubMed] [Google Scholar]
- Romeo C., Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991 Mar 8;64(5):1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- Sancho J., Chatila T., Wong R. C., Hall C., Blumberg R., Alarcon B., Geha R. S., Terhorst C. T-cell antigen receptor (TCR)-alpha/beta heterodimer formation is a prerequisite for association of CD3-zeta 2 into functionally competent TCR.CD3 complexes. J Biol Chem. 1989 Dec 5;264(34):20760–20769. [PubMed] [Google Scholar]
- Sancho J., Ledbetter J. A., Choi M. S., Kanner S. B., Deans J. P., Terhorst C. CD3-zeta surface expression is required for CD4-p56lck-mediated upregulation of T cell antigen receptor-CD3 signaling in T cells. J Biol Chem. 1992 Apr 15;267(11):7871–7879. [PubMed] [Google Scholar]
- Shinkai Y., Koyasu S., Nakayama K., Murphy K. M., Loh D. Y., Reinherz E. L., Alt F. W. Restoration of T cell development in RAG-2-deficient mice by functional TCR transgenes. Science. 1993 Feb 5;259(5096):822–825. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K. P., Oltz E. M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A. M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992 Mar 6;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Sussman J. J., Bonifacino J. S., Lippincott-Schwartz J., Weissman A. M., Saito T., Klausner R. D., Ashwell J. D. Failure to synthesize the T cell CD3-zeta chain: structure and function of a partial T cell receptor complex. Cell. 1988 Jan 15;52(1):85–95. doi: 10.1016/0092-8674(88)90533-8. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Viney J. L., Kilshaw P. J., MacDonald T. T. Cytotoxic alpha/beta+ and gamma/delta+ T cells in murine intestinal epithelium. Eur J Immunol. 1990 Jul;20(7):1623–1626. doi: 10.1002/eji.1830200734. [DOI] [PubMed] [Google Scholar]
- Wegener A. M., Letourneur F., Hoeveler A., Brocker T., Luton F., Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992 Jan 10;68(1):83–95. doi: 10.1016/0092-8674(92)90208-t. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Frank S. J., Orloff D. G., Merćep M., Ashwell J. D., Klausner R. D. Role of the zeta chain in the expression of the T cell antigen receptor: genetic reconstitution studies. EMBO J. 1989 Dec 1;8(12):3651–3656. doi: 10.1002/j.1460-2075.1989.tb08539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Carson G. R., Concino M., Ahmed A., Terhorst C. The gamma and epsilon subunits of the CD3 complex inhibit pre-Golgi degradation of newly synthesized T cell antigen receptors. J Cell Biol. 1990 Apr;110(4):973–986. doi: 10.1083/jcb.110.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]