Abstract
The recruitment of asymptomatic volunteers has been identified as a critical factor that is delaying development and validation of preventive therapies for Alzheimer’s disease (AD). Typical recruitment strategies involve the use of convenience samples or soliciting participation of older adults with a family history of AD from clinics and outreach efforts. However, high risk groups, such as ethnic/racial minorities, are traditionally less likely to be recruited for AD prevention studies, thus limiting the ability to generalize findings for a significant proportion of the aging population. A community-engagement approach was used to create a registry of 2,311 research-ready, healthy adult volunteers who reflect the ethnically diverse local community. Furthermore, the registry’s actual commitment to research was examined, through demonstrated participation rates in a clinical study. The approach had varying levels of success in establishing a large, diverse pool of individuals who are interested in participating in pharmacological prevention trials and meet criteria for primary prevention research trials designed to delay the onset of AD. Our efforts suggest that entry criteria for clinical trials need to be carefully considered to be inclusive of African Americans, and that sustained effort is needed to engage African Americans in pharmacological prevention approaches.
INTRODUCTION
Among the impediments for Alzheimer’s disease (AD) primary prevention trials is the difficulty of quickly identifying large numbers of healthy, yet high risk individuals who are willing to participate in treatment studies that are designed to delay the onset of AD. As stated in the National Plan to Address Alzheimer’s Disease, The United States Department of Health and Human Services (HHS) recognized that the rapidity of scientific progress in prevention research depends on a diverse pool of participants who are readily available to participate in prevention research1. The National Alzheimer’s Project Act (NAPA) signed into law in 2011, has served as a catalyst for coordinating prevention and treatment efforts, including prevention research. However, the success of prevention trials depends on effective, coordinated, funded efforts for engaging and enrolling a pool of high risk, asymptomatic older adults.
The challenge of recruiting asymptomatic volunteers has been identified as a critical, rate-limiting factor that is delaying development and validation of preventive therapies for AD 2. The goal of AD primary prevention studies is to delay the onset of AD among healthy, cognitively normal older adults. Herein lies a conundrum. Healthy, normal subjects do not necessarily seek out clinical trials for AD prevention. Without readily available registries from which to recruit, how can investigators identify large numbers of asymptomatic adults in an efficient, cost-effective manner? Typical recruitment strategies for population-based samples involve time consuming, costly methods that yield low response rates. For example, in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT), approximately 2.8 million mailings to Medicare-eligible older adults were sent in order to enroll 2,518 subjects3. Prevention studies for other diseases (such as coronary heart disease) have had a screening-to-enrollment ratio of 28:1 4, and it has been recommended that this recruitment to enrollment ratio be closer to 10:1 to meet enrollment goals for AD prevention studies3.
An alternative method of recruitment relies on convenience samples. Registries of healthy volunteers with increased risk for AD have been highlighted as an essential first step towards mobilizing primary prevention studies2. One approach is to solicit participation of older adults from registries of healthy volunteers with dominantly inherited Alzheimer’s disease. Such registries have been used to recruit for large primary prevention trials that target pre-symptomatic ethnically diverse members of large, early-onset, dominantly inherited AD kindreds, such as the Alzheimer’s Prevention Initiative, and the Dominantly Inherited Alzheimer’s Network5,6. However, efforts are also needed that develop registries of individuals at risk for the more commonly occurring sporadic form of AD. One such recruitment method involves seeking individuals with a family history of AD from clinics. However, registries that inquire about sensitive information, such as family history of sporadic or dominantly inherited AD, may deter otherwise willing volunteers. Alternative strategies are needed in order to build registries, using minimal information that can appeal to a wide representation of the aging population.
A further complication to recruitment for prevention is ensuring population representativeness. It is well known that clinical trials for AD do not typically include large numbers of African-Americans (AAs) for a variety of complex historical and societal reasons7,8. Recruitment strategy and methods for selecting inclusion/exclusion criteria are prone to create underrepresentation of ethnically diverse participants in these trials7,9. Because of a reluctance to enroll in medical experiments, ethnic minorities may be less likely to attend research centers that are involved in recruitment, and may be less likely to report a family history of AD 5,9. For studies that require a positive family history, health disparities may lead to underreporting of a family history of AD, perhaps due to premature death of a family member from other chronic health conditions, or decreased access to medical care where a diagnosis would have been made for a family member. Underrepresentation of ethnic and racial minorities in prevention trials is concerning because some studies have shown that these populations are at up to two times the risk of developing AD compared to Caucasians10. Thus, the inability to recruit and enroll ethnic/racial minority groups in clinical trials and preventive studies limits the generalizability of findings for a significant proportion of the aging population.
A successful method for enriching studies with ethnic minority participants that qualify for prevention trials involves a proactive community-engagement approach that includes active outreach, community relationship building that is characterized by reciprocity whereby participants benefit from involvement in the research, and deliberate attempts to increase access to prevention research 8,11. The use of such an approach resulted in a four fold increase in minority participation in longitudinal research at the University of California, Davis Alzheimer’s Disease Center (UCD ADC) 12. The UCD ADC previously attempted using minority-serving satellite clinics to bolster recruitment; however, this approach yielded volunteers with advanced dementia and low participation rates of minorities. The success of their new approach was due in large part to creating ties with representatives of the community and disseminating educational information in the community through presentations, health fairs, distribution of flyers, and word-of-mouth referrals. The UCD ADC community-engagement approach was also successful in yielding more participants with a broad range of cognitive function, including individuals with normal cognition. Therefore, the community-engagement approach has the potential to promote recruitment of ethnically diverse, asymptomatic volunteers that are eligible for Alzheimer’s disease prevention trials.
The Alzheimer’s Disease Prevention Registry (ADPR) of the Joseph and Kathleen Bryan Alzheimer’s Disease Research Center (Bryan ADRC) at Duke, was designed to develop a “research-ready” representative registry of normal older adult community volunteers who are willing to participate in clinical studies facilitating the development of AD prevention and treatment strategies. Herein we describe coordinated efforts to create this registry of healthy volunteers who reflect the ethnically diverse local community. Furthermore, the registry’s actual commitment to research will be examined, by examining the characteristics of individuals from the ADPR who later enrolled in clinical studies.
METHODS
ADPR recruitment strategy
Recruitment of individuals for the Alzheimer’s Disease Prevention Registry (ADPR) is an ongoing effort beginning in July 2009 and was coordinated through the Bryan ADRC Education Core and its African American Community Outreach Program (AACOP). Individuals who registered for the ADPR will be referred to as ‘registrants’. The strategy for recruiting ADPR registrants was to utilize a community-engagement approach by introducing the ADPR to individuals who attended outreach events. The Education Core provides AD educational outreach to the North Carolina region, and the Research Triangle in particular (including Durham, Chapel Hill, and Raleigh). Established in 1995, AACOP works to disseminate AD information to underserved AA communities across the state of NC. The outreach activities rely on established community partnerships and personal contacts through regularly scheduled regional and local conferences, including the former Bryan ADRC scientific conferences for researchers, patients and caregivers, as well as health fairs and events. Outreach was also facilitated by Duke University Medical Center and Bryan ADRC websites, regular newsletters, ads from Duke University and the local media, feature stories from newspapers and online newsletters, and referrals from the Center for the Study of Aging and Human Development at Duke University. Regularly scheduled events included presentations at senior health fairs, family reunions, civic and senior groups, and educational venues at medical centers, senior housing facilities, lifelong learning groups, Duke University retiree associations, and churches. Additional details about the ADPR recruitment methods are provided in the online supplement and Figure S1. A key emphasis of all outreach activities was on community ownership in medical research that advances local community interests.
Individuals recruited for the ADPR were community residents, age 55 years or older, who reported that they did not have diagnosis of dementia. Registrants provided minimal personal information, including contact information, gender, ethnicity, age category, and research interest. Registration for the ADPR was completed in person at a recruitment event, by telephone, or through a secured website. Registration in the ADPR indicated that participants were interested in being contacted for AD prevention studies that would be offered by the ADRC in the future. Participants could indicate interest in being contacted for any/all potential studies, or they could opt in or out of studies depending on their interest (e.g., imaging/biomarkers, pharmacological interventions, and/or non-pharmacological interventions). All registrants were assured that registration did not constitute consent to participate in any study. As IRB approved studies became available in the Bryan ADRC, participants in the registry were invited to enroll. The “PREPARE” study was among the first of these studies. Designed to characterize genetic and medical factors contributing to cognitive heterogeneity in normal aging, a goal of the PREPARE study was to determine the subset and characteristics of participants who would be eligible for primary prevention studies. All ADPR registrants were approached to participate. See Figure 1 for a description of participant flow. ADPR participants are referred to the PREPARE study and to a number of other prevention and longitudinal studies.
Figure 1.
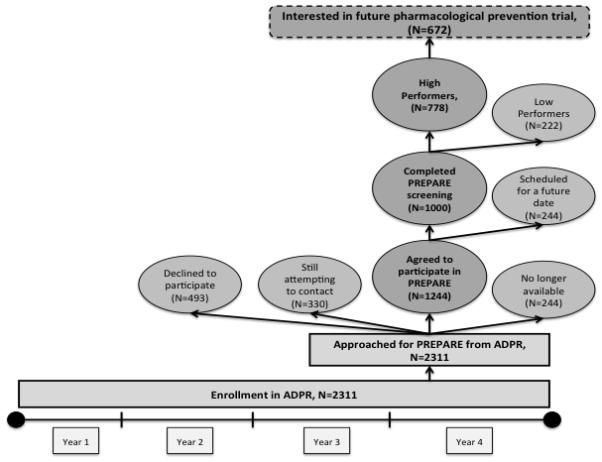
Flow of Participants and Timeline
PREPARE study recruitment and protocol
Participants in the PREPARE study were recruited from the ADPR and other subject research registries and studies at Duke. Registrants were contacted over a period of approximately 1.5 years and invited to enroll in PREPARE (see Figure 1). Some of the registrants could not be contacted, or declined to participate. The PREPARE protocol involved a one-hour assessment that included a brief medical history, cognitive testing, collection of vital signs, and a blood sample to gather genetic information. The cognitive tests included the Montreal Cognitive Assessment (MoCA) 13, the 10-item word list memory task from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) battery14, and Trail Making Test, Part B15. Participants were classified into two groups, broadly defined as High Performers who scored above specified cut points, and Low Performers who scored below the cut points. This means of classification was made to estimate the proportion of high performing individuals who are more likely to be classified as cognitively normal if given a more comprehensive assessment. The High Performers would likely be eligible for future primary prevention trials. It should be noted that a proportion of the individuals who were classified as Low Performers may actually be classified as cognitively normal if given a more comprehensive clinical evaluation. Individuals were considered to be High Performers if they met the following cognitive criteria: Obtained a passing score on all three cognitive measures; a passing score was defined as a MoCA score ≥ 25, CERAD delayed word list recall score ≥4, and Trails B completion time ≤ 180 seconds. At the conclusion of the study visit, participants were asked:
“Within a year, we will be launching a prevention trial for Alzheimer’s disease that includes a compound that is already in common use for controlling blood sugar. Would you consider participating?”
Both the ADPR registry and PREPARE study are ongoing. The ADPR registry and PREPARE study were approved by the Institutional Review Board at Duke University Medical Center. Figure 1 shows the flow of participants in the PREPARE study.
RESULTS
ADPR (registry) sample
Over the first 3 years and 3 months since the registry commenced (July 2009-October 2012), N=2311 individuals registered. The majority of registrants were female (74.0%) and White (65.9%). The second largest group was African American (30.9%), 1.6% reported other ethnicities (including American Indian, Hispanic, Asian, or other), and <2% did not report ethnicity information. See Table 1 for a comparison of demographic characteristics between AAs and Caucasians. The largest proportion of registrants was in the younger 55-64 age category (46.2%), followed by ages 65-74 (35.6%), and 75-84 (15.5%). Only 2.7% were age 85 or older. There was no significant difference in the proportion of Caucasians and AAs in each of the age categories.
Table 1.
Comparison of African American and Caucasian registrants in the ADPR registry and participants in the PREPARE study.
| ADPR registrants | PREPARE participants | |||||
|---|---|---|---|---|---|---|
| Caucasians, N=1522 (65.9%) |
AA, N=715 (30.9%) |
p value | Caucasians, N=837 (83.7%) |
AA, N=146 (14.6%) |
p value, | |
| Age, N(%) | p<.01 | p<.05 | ||||
| 55-64 | 680(44.7%) | 351(49.1%) | 316(37.8%) | 65(44.5%) | ||
| 65-74 | 522(34.3%) | 274(38.3%) | 333(39.8%) | 63(43.2%) | ||
| 75-84 | 273(17.9%) | 75(10.5%) | 176(21.0%) | 16(11.0%) | ||
| 85+ | 47(3.1%) | 15(2.1%) | 12(1.4%) | 2(1.4%) | ||
| Family history of dementia, % | - | p<.001 | ||||
| yes | Not asked | Not asked | - | 435(52.0%) | 49(33.6%) | |
| no | Not asked | Not asked | - | 373(44.6%) | 91(62.3%) | |
|
Interest in pharmacological preventive
studies, N (%) |
*p<001 | p <.01 | ||||
| Yes, interested | 1153(78.5%) | 436(61.0%) | 732(87.5%) | 114(78.1%) | ||
| No, not interested |
316(21.5%) | 279(39.0%) | 103(12.3%) | 32(21.9%) | ||
Note: Numbers may not add to 100% because only African Americans (AA) and Caucasians were compared. Pearson χ2 test was used to compare groups.
Data missing from N=58 registrants
The majority of registrants signed up for the ADPR on-site following a presentation by an ADRC staff or faculty member (22.5%), at health fairs (14.17%), or at church presentations (14.7%). The remaining registrants signed up in response to radio/newspaper/newsletter articles (9.6%), on the ADRC website (7.6%), during a research visit for other ADRC research studies (6.8%), in response to a flyer or poster (6.4 %), at Duke University Medical Center (4.7%), by word-of-mouth from a family or friend (4.5%), at former annual ADRC conferences (3.2%), at health agencies (1.8%), at community service groups (1.2%), and from other sources (2.7%). Many of the registrants who signed up via the website were introduced to the ADPR at health fairs or presentations. The majority were interested in all types of studies (70.5%). A smaller percentage (29.5%) expressed an interest in only imaging studies and/or non-pharmacological interventions. We examined ethnic group differences between AA and Caucasians in research interest; we did not evaluate group differences in the other ethnic categories because there was <2% representation in each of the other ethnic group categories. Compared to Caucasians, a greater proportion of African Americans specifically preferred non-pharmacological interventions (including neuroimaging and/or other nonpharmacological studies) over pharmacological approaches, Pearson χ2 (3, N= 2184) = 74.39, p < .001, see Table 1.
PREPARE sample
An initial letter was sent to 2311 registrants. After attempting to contact them by phone, email, or mail, a portion of the registrants could not be reached (N=330), some declined to participate when they were contacted (N=493), and others were no longer available (N=244) because they were too medically ill, deceased, had dementia, or lived too far to travel to Duke. Among the registrants who could be contacted (N=1981), 63% agreed to participate (N=1244), and a total of 1000 participants have enrolled and completed their PREPARE research study visit as of 10/03/2012.
The age of participants ranged from 55-93, with a mean age of 68.2 (7.8SD). Half of the participants reported a family history of dementia (49%, N=490). A greater proportion of participants were in younger age categories, with 38.8% in the 55-64 age range, 40.3% were age 65-74, 19.5% were age 75-84, and 1.4% age 85 and older. The majority of PREPARE participants were Caucasian (83.7%), and AAs made up the second largest racial group (14.6%), see Table 1. There were 2% or fewer of each of the following racial/ethnic groups: Hispanic, American Indian/Alaska Native, Asian, or ‘other race’. Caucasians and AAs were compared according to the following characteristics: age, family history of dementia, and interest in participating in a pharmacological prevention trial. Compared to Caucasians, AAs were younger, less likely to report a family history of dementia, and less likely to express interest in participating in an upcoming pharmacological prevention trial, see Table 1.
Among those who completed the research visit, 77.8% (N=778) were classified as High Performers. The majority of participants who completed their research visit (85.9%, N=859) stated that they would consider participating in a pharmacological prevention trial. Among those classified as High Performers, 86.3% (N=672) expressed interest in a pharmacological intervention, and would therefore be more likely to be eligible for a primary prevention trial. Characteristics of individuals who were and were not interested in a pharmacological prevention trial are shown in Table 2. Participants who were interested in a pharmacological prevention trial were more likely to be younger, Caucasian, female, and classified as a high performer, but did not differ according to family history of dementia.
Table 2.
Comparison of PREPARE participants who would vs. would not consider participation in a pharmacological prevention study.
| Yes, would consider a pharmacological prevention trial (N=859) |
No, would not consider a pharmacological prevention trial (N=141) |
p value | |
|---|---|---|---|
| Age, mean(SD) | 67.8(7.7) | 70.3(8.0) | p<.001 |
| Female (%) | 84.1% | 15.8% | p<.05 |
| Male (%) | 90.4% | 9.6% | |
| Caucasian (%) | 87.5% | 12.3% | p<.05 |
| African American (%) | 77.9% | 22.1% | |
| Positive Family History of dementia (%) |
87.8% | 12.2% | ns |
| Negative Family History of dementia (%) |
83.7% | 16.3% | |
| Cognitive status, % of High Performers |
86.3% | 13.7% | p<.05 |
| Cognitive status, % of Low Performers |
85.3% | 14.7% |
Independent samples t-test was used to compare differences in age. Pearson χ2 test was used to compare difference in gender, race, family history of dementia, and cognitive status.
DISCUSSION
Our goal was to develop a “research-ready” representative registry of normal older adult community volunteers who are willing to participate in AD prevention studies. Of particular interest, was to create a registry of volunteers who represent the diverse local community, including a large proportion of AAs. Using very little personal information, our community-engagement approach was successful in recruiting a large, ethnically/racially diverse group of over 2000 individuals in the ADPR who have expressed interest in AD prevention studies. Our success supports the literature that suggests that a community-engagement approach can successfully increase participation of diverse cohorts in AD clinical trials 8,11,12.
Actual interest in participating in clinical research was demonstrated by the introduction of the PREPARE study. Although not a clinical trial, the PREPARE study demonstrated that a high proportion of participants (63%, n=1244) were willing to participate in a research study involving detailed clinical assessment and genetic testing. Among those who enrolled in PREPARE and completed their study visit (n=1000), nearly 2/3 (n=672) were willing to consider participation in a pharmacological prevention trial and are high performers on cognitive screening tests. Most participants were recruited from areas in close proximity to Duke University Medical Center, which will further facilitate their participation in the upcoming primary prevention trial.
The recommendation of at least a 10:1 recruitment-to-enrollment ratio by Meinert and Brietner 3 appears to be consistent with our efforts summarized in the current registry and pre-screening study. Our efforts thus far have yielded a 3:1 ratio – approximately 29% of the individuals from the registry enrolled in PREPARE, were classified as high performers, and indicated interest in a pharmacological prevention trial. When we estimate the number of PREPARE participants who will eventually meet all eligibility criteria for the pharmacological prevention trial, the 10:1 ratio is an appropriate estimate for planning recruitment and enrollment targets. Our results also point to a number of factors that could improve the recruitment-to-enrollment ratio. Some of the initial registrants were contacted for the PREPARE study over a year after registering for the ADPR. Previous successful outreach efforts have been characterized by maintaining an active presence in the community; therefore, for some of the early registrants, the time lag of up to 18 months between registering for the ADPR and being contacted for the PREPARE study may have affected participation rates. Another consideration is that, although the ADPR did not ask sensitive questions about family history of AD, the PREPARE study involved collection of a blood sample for genetic testing. This may have had a mixed effect, for example, individuals with a family history of AD may be motivated to participate in a genetic study to help future generations, yet others may be deterred if they feel uncomfortable sharing genetic information in a research context. Finally, results of the current investigation indicated that additional efforts will be required to increase interest in pharmacological prevention studies among older individuals and AA participants.
The success in registering a high proportion of AAs (31%) into the ADPR did not translate to an equally high rate of enrollment into the PREPARE study (15%). Although the representation of AAs in the PREPARE study currently exceeds published rates of participation in AD clinical trials (typically 10% or less8,16), the percentage of AA participants who would eventually meet all eligibility criteria for the upcoming pharmacological prevention trial will likely be reduced. The reduction in AA participation in the PREPARE study may have been because the PREPARE study was conducted in preparation for a clinical trial. However, the PREPARE study itself did not involve participation in a pharmacological prevention trial, discussion of a future pharmacological trial was not specifically mentioned in introductory materials, and participation in the PREPARE study did not require any commitment to participate in a clinical trial. An additional possibility for the reduction in participation rates could be related to fewer AA reporting a family history of AD. For many individuals, family history of AD is a driving force for participating in AD prevention research. If there is less awareness of family history of dementia, there may be less motivation to engage in research, particularly if there is any indication that the research requires the use of medication for individuals that do not yet have any cognitive impairment. Overall, results suggest that targeted, coordinated efforts are needed in order to recruit and enroll AA participants who would consider participation in a pharmacological clinical trial.
We examined the prevalence of risk factors that have been used by other prevention studies to identify high risk individuals for Alzheimer’s prevention research. Results show that the use of family history of dementia as an indicator for risk would have resulted in lower AA eligibility for the future prevention trial. Among the AA PREPARE participants, only 31.8% reported a family history of dementia, whereas 55.8% of Caucasian participants reported a family history of dementia. These rates are inconsistent with literature that reports a 2 fold increase in risk of AD among AAs compared to Caucasians10. This may be partially related to AAs having less access to family medical history, specifically related to a dementia diagnosis. Another risk factor is age. A greater proportion of the total sample were under age 75, so if a primary prevention study were to target older ages, fewer participants in the current sample would be eligible. However, recruitment efforts are continuing, including targeted recruitment of individuals in older age groups.
Although 4 years of coordinated effort may seem like a lengthy amount of time and resources to devote to recruitment efforts, the enabling activities associated with the ADPR registry and the PREPARE study have served a number of aims. It has established a large sample of research-ready, cognitively normal participants who meet criteria for a primary prevention trial related to cognitive impairment. Therefore, as the clinical trial launches, there will be a pool of participants ready to enroll, and who are more likely to meet cognitive inclusion criteria. In August 2013, the TOMMORROW study launched, a clinical trial involving 50 global sites, designed to test the effectiveness of low dose pioglitazone in delaying the onset of Mild Cognitive Impairment due to Alzheimer’s disease 17. The ADPR registry participants are actively being contacted to participate and the registry continues to grow as the local community hears of the launching studies. In addition to the primary aim, ADPR and PREPARE participants have enrolled in other primary and secondary prevention studies being conducted at the Bryan ADRC. This approach therefore provides a means for recruiting participants for multiple primary and secondary prevention studies. If similar registries were implemented across other national Alzheimer’s Disease Centers, prevention studies could be quickly initiated, speeding the development of therapies in representative populations and avoiding the need for expensive, less efficient recruitment campaigns.
Supplementary Material
Acknowledgements
NIA grant: P30 AG028377, ADCC Genomic Medicine Approach to MCI and Dementia (Bryan ADRC); NIA grant: P30AG028377-04S1, Research Supplement to Promote Diversity in Health-Related Research (Dr. Romero); Zinfandel Pharmaceuticals, Inc.
REFERENCES
- 1.U.S. Department of Health & Human Services [Accessed November 1st, 2013];National plan to address alzheimer’s disease: 2013 update. http://aspe.hhs.gov/daltcp/napa/NatlPlan2013.shtml. Updated 2013.
- 2.Khachaturian ZS, Barnes D, Einstein R, et al. Developing a national strategy to prevent dementia: Leon thal symposium 2009. Alzheimers & Dementia. 2010;6(2):89–97. doi: 10.1016/j.jalz.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meinert CL, Breitner JCS. Chronic disease long-term drug prevention trials: Lessons from the alzheimer’s disease anti-inflammatory prevention trial (ADAPT) Alzheimers & Dementia. 2008;4(1):S7–S14. doi: 10.1016/j.jalz.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Neaton J, Grimm R, Cutler J. Recruitment of participants for the multiple risk factor intervention trial (mrfit) Control Clin Trials. 1987;8(4):S41–S53. doi: 10.1016/0197-2456(87)90006-7. [DOI] [PubMed] [Google Scholar]
- 5.Reiman EM, Langbaum JBS, Fleisher AS, et al. Alzheimer’s prevention initiative: A plan to accelerate the evaluation of presymptomatic treatments. Journal of Alzheimers Disease. 2011;26:321–329. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman RJ, Aisen PS, De Strooper B, et al. Autosomal-dominant alzheimer’s disease: A review and proposal for the prevention of alzheimer’s disease. Alzheimer’s research & therapy. 2011;3(1) doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsh K, Ballard E, Nash F, Raiford K, Harrell L. Issues affecting minority participation in research studies of alzheimer-disease. Alzheimer Dis Assoc Dis. 1994;8:38–48. [PubMed] [Google Scholar]
- 8.Olin JT, Dagerman KS, Fox LS, Bowers B, Schneider LS. Increasing ethnic minority participation in alzheimer disease research. Alzheimer Disease & Associated Disorders. 2002;16:S82–S85. doi: 10.1097/00002093-200200002-00009. [DOI] [PubMed] [Google Scholar]
- 9.McCallum JM, Arekere DM, Green BL, Katz RV, Rivers BM. Awareness and knowledge of the US public health service syphilis study at tuskegee: Implications for biomedical research. J Health Care Poor Underserved. 2006;17(4):716–733. doi: 10.1353/hpu.2006.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alzheimer’sAssociation alzheimer’s disease facts and figures. Alzheimers & Dementia. 2010;2010;6(2):158–94. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Schneider LS. Drug development, clinical trials, cultural heterogeneity in alzheimer disease - the need for pro-active recruitment. Alzheimer Disease & Associated Disorders. 2005;19(4) doi: 10.1097/01.wad.0000190808.97878.b8. [DOI] [PubMed] [Google Scholar]
- 12.Hinton L, Carter K, Reed BR, et al. Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer Disease & Associated Disorders. 2010;24(3) doi: 10.1097/WAD.0b013e3181c1ee01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasreddine ZS, Chertkow H, Phillips N, Whitehead V, Collin I, Cummings JL. The montreal cognitive assessment (MoCA): A brief cognitive screening tool for detection of mild cognitive impairment. Neurology. 2004;62(7):A132–A132. [Google Scholar]
- 14.Welsh KA, Butters N, Mohs RC, et al. The consortium to establish a registry for alzheimer’s disease (CERAD). part V. A normative study of the neuropsychological battery. Neurology. 1994;44(4):609–14. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 15.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19(5) doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 16.Tariot P, Farlow M, Grossberg G, et al. Memantine treatment in patients with moderate to severe alzheimer disease already receiving donepezil - A randomized controlled trial. JAMA-J Am Med Assoc. 2004;291(3):317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 17.Crenshaw DG, Gottschalk WK, Lutz MW, et al. Using genetics to enable studies on the prevention of alzheimer’s disease. Clinical Pharmacology & Therapeutics. 2013;93(2):177–185. doi: 10.1038/clpt.2012.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


