Abstract
In vertebrate smooth/non-muscle myosins, phosphorylation of the regulatory light chains by a specific calmodulin-activated kinase controls both myosin head interaction with actin and assembly of the myosin into filaments. Previous studies have shown that the C-terminal domain of the regulatory light chain is crucial for the regulation of these myosin functions. To further dissect the role of this region of the light chain in myosin regulation, a series of chicken smooth muscle myosin regulatory light chain mutants has been constructed with successive C-terminal deletions. These mutants were synthesized in Escherichia coli and analysed by their ability to restore Ca2+ regulation to scallop myosin that had been stripped of its native regulatory light chains ('desensitized'). The results show that regulatory light chain mutants with deletions in the C-terminal helix in subdomain 4 were able to reform the regulatory Ca2+ binding site on the scallop myosin head, but had lost the ability to suppress scallop myosin filament assembly and interaction with actin in the absence of Ca2+. Further deletions in the C-terminal domain led to a gradual loss of ability to restore the regulatory Ca2+ binding site. Thus, the regions in the C-terminal half of the regulatory light chain responsible for myosin regulation can be identified.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
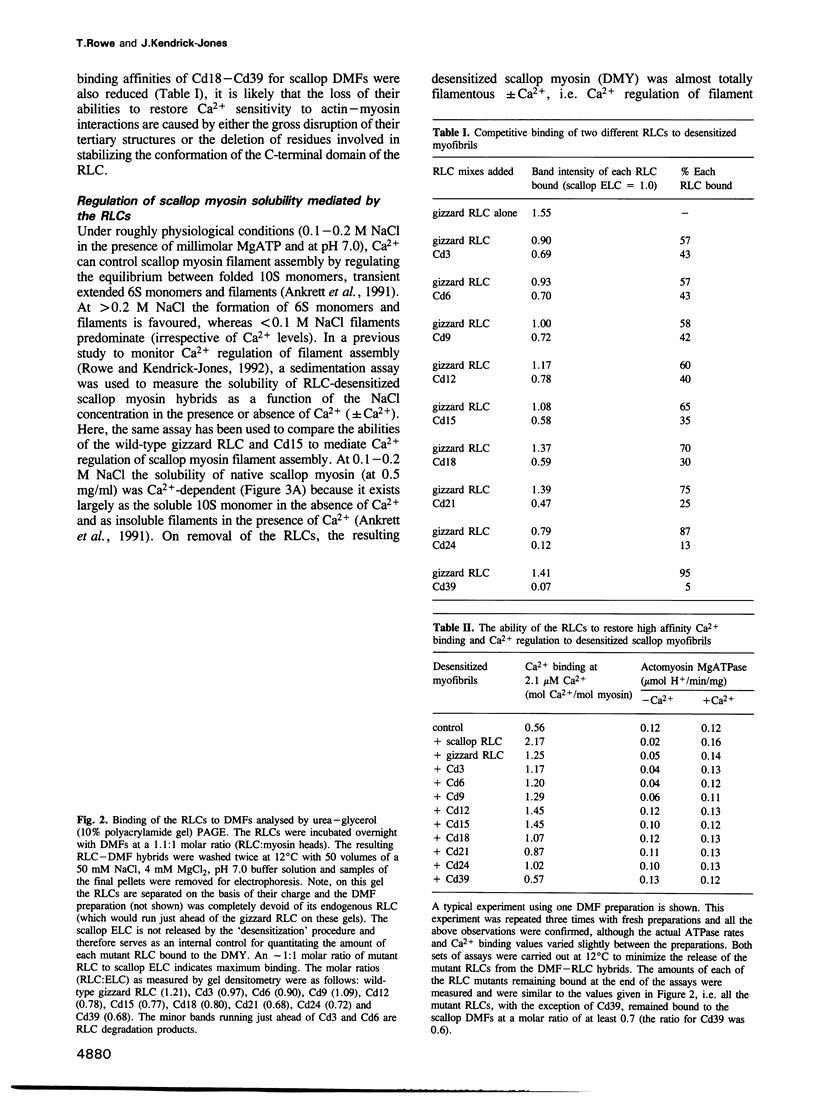
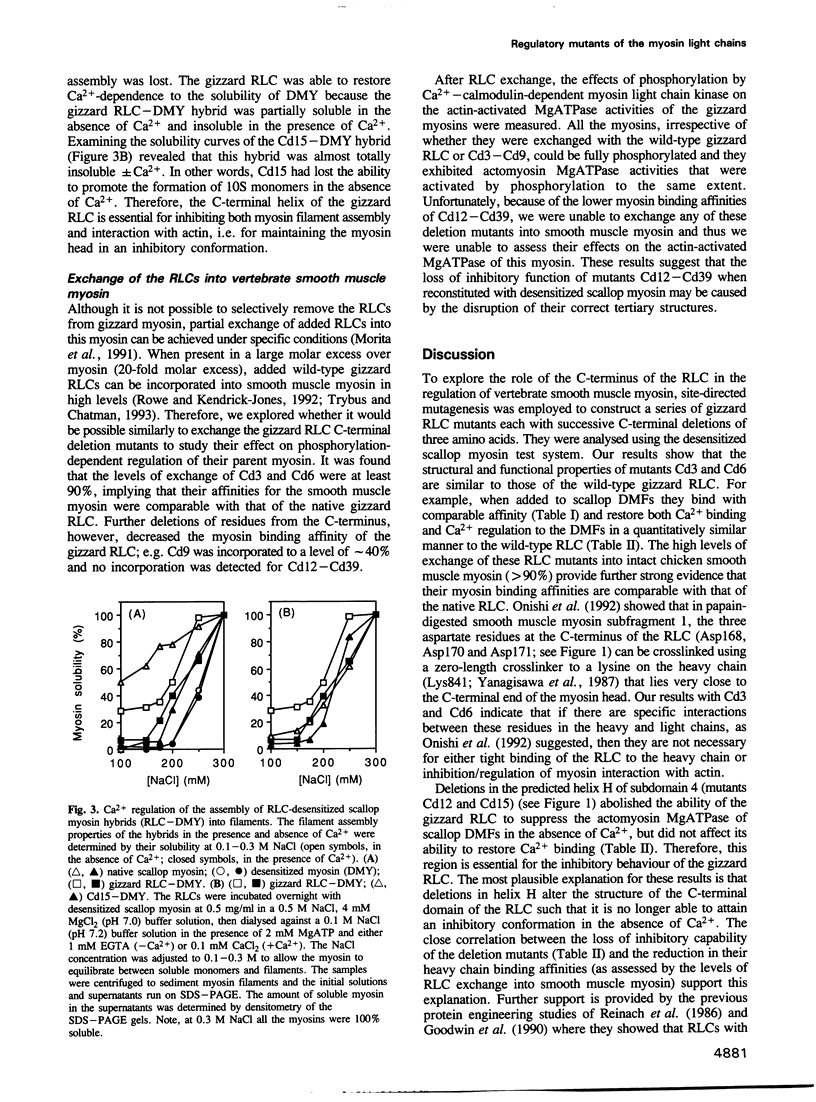
- Ankrett R. J., Rowe A. J., Cross R. A., Kendrick-Jones J., Bagshaw C. R. A folded (10 S) conformer of myosin from a striated muscle and its implications for regulation of ATPase activity. J Mol Biol. 1991 Jan 20;217(2):323–335. doi: 10.1016/0022-2836(91)90546-i. [DOI] [PubMed] [Google Scholar]
- Chantler P. D., Szent-Györgyi A. G. Regulatory light-chains and scallop myosin. Full dissociation, reversibility and co-operative effects. J Mol Biol. 1980 Apr 15;138(3):473–492. doi: 10.1016/s0022-2836(80)80013-1. [DOI] [PubMed] [Google Scholar]
- Citi S., Kendrick-Jones J. Regulation in vitro of brush border myosin by light chain phosphorylation. J Mol Biol. 1986 Apr 5;188(3):369–382. doi: 10.1016/0022-2836(86)90161-0. [DOI] [PubMed] [Google Scholar]
- Collins J. H. Homology of myosin DTNB light chain with alkali light chains, troponin C and parvalbumin. Nature. 1976 Feb 26;259(5545):699–700. doi: 10.1038/259699a0. [DOI] [PubMed] [Google Scholar]
- Craig R., Smith R., Kendrick-Jones J. Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. 1983 Mar 31-Apr 6Nature. 302(5907):436–439. doi: 10.1038/302436a0. [DOI] [PubMed] [Google Scholar]
- Cross R. A., Cross K. E., Sobieszek A. ATP-linked monomer-polymer equilibrium of smooth muscle myosin: the free folded monomer traps ADP.Pi. EMBO J. 1986 Oct;5(10):2637–2641. doi: 10.1002/j.1460-2075.1986.tb04545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R. A., Jackson A. P., Citi S., Kendrick-Jones J., Bagshaw C. R. Active site trapping of nucleotide by smooth and non-muscle myosins. J Mol Biol. 1988 Sep 5;203(1):173–181. doi: 10.1016/0022-2836(88)90100-3. [DOI] [PubMed] [Google Scholar]
- Goodwin E. B., Leinwand L. A., Szent-Györgyi A. G. Regulation of scallop myosin by mutant regulatory light chains. J Mol Biol. 1990 Nov 5;216(1):85–93. doi: 10.1016/S0022-2836(05)80062-2. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Chang X. J., Edwards K. A., Kulkarni S., Aguilera I., Kiehart D. P. The regulatory light chain of nonmuscle myosin is encoded by spaghetti-squash, a gene required for cytokinesis in Drosophila. Cell. 1991 Jun 28;65(7):1177–1189. doi: 10.1016/0092-8674(91)90013-o. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Cande W. Z., Tooth P. J., Smith R. C., Scholey J. M. Studies on the effect of phosphorylation of the 20,000 Mr light chain of vertebrate smooth muscle myosin. J Mol Biol. 1983 Mar 25;165(1):139–162. doi: 10.1016/s0022-2836(83)80247-2. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Rasera da Silva A. C., Reinach F. C., Messer N., Rowe T., McLaughlin P. Recombinant DNA approaches to study the role of the regulatory light chains (RLC) using scallop myosin as a test system. J Cell Sci Suppl. 1991;14:55–58. doi: 10.1242/jcs.1991.supplement_14.11. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Szentkiralyi E. M., Szent-Györgyi A. G. Regulatory light chains in myosins. J Mol Biol. 1976 Jul 15;104(4):747–775. doi: 10.1016/0022-2836(76)90180-7. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kwon H., Goodwin E. B., Nyitray L., Berliner E., O'Neall-Hennessey E., Melandri F. D., Szent-Györgyi A. G. Isolation of the regulatory domain of scallop myosin: role of the essential light chain in calcium binding. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4771–4775. doi: 10.1073/pnas.87.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H., Melandri F. D., Szent-Györgyi A. G. Role of gizzard myosin light chains in calcium binding. J Muscle Res Cell Motil. 1992 Jun;13(3):315–320. doi: 10.1007/BF01766459. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Messer N., Kendrick-Jones J. Chimaeric myosin regulatory light chains: sub-domain switching experiments to analyse the function of the N-terminal EF hand. J Mol Biol. 1991 Apr 20;218(4):825–835. doi: 10.1016/0022-2836(91)90270-g. [DOI] [PubMed] [Google Scholar]
- Morita J., Takashi R., Ikebe M. Exchange of the fluorescence-labeled 20,000-dalton light chain of smooth muscle myosin. Biochemistry. 1991 Oct 1;30(39):9539–9545. doi: 10.1021/bi00103a022. [DOI] [PubMed] [Google Scholar]
- Onishi H., Maita T., Matsuda G., Fujiwara K. Interaction between the heavy and the regulatory light chains in smooth muscle myosin subfragment 1. Biochemistry. 1992 Feb 4;31(4):1201–1210. doi: 10.1021/bi00119a033. [DOI] [PubMed] [Google Scholar]
- Onishi H., Wakabayashi T. Electron microscopic studies of myosin molecules from chicken gizzard muscle I: the formation of the intramolecular loop in the myosin tail. J Biochem. 1982 Sep;92(3):871–879. doi: 10.1093/oxfordjournals.jbchem.a134001. [DOI] [PubMed] [Google Scholar]
- Pearson R. B., Jakes R., John M., Kendrick-Jones J., Kemp B. E. Phosphorylation site sequence of smooth muscle myosin light chain (Mr = 20 000). FEBS Lett. 1984 Mar 12;168(1):108–112. doi: 10.1016/0014-5793(84)80216-1. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Reinach F. C., Nagai K., Kendrick-Jones J. Site-directed mutagenesis of the regulatory light-chain Ca2+/Mg2+ binding site and its role in hybrid myosins. Nature. 1986 Jul 3;322(6074):80–83. doi: 10.1038/322080a0. [DOI] [PubMed] [Google Scholar]
- Rowe T., Kendrick-Jones J. Chimeric myosin regulatory light chains identify the subdomain responsible for regulatory function. EMBO J. 1992 Dec;11(13):4715–4722. doi: 10.1002/j.1460-2075.1992.tb05576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals J. R., McDonald J. M., Bruns D., Jarett L. A sensitive and precise isotopic assay of ATPase activity. Anal Biochem. 1978 Oct 15;90(2):785–795. doi: 10.1016/0003-2697(78)90169-0. [DOI] [PubMed] [Google Scholar]
- Seidel J. C. Fragmentation of gizzard myosin by alpha-chymotrypsin and papain, the effects on ATPase activity, and the interaction with actin. J Biol Chem. 1980 May 10;255(9):4355–4361. [PubMed] [Google Scholar]
- Sellers J. R., Chantler P. D., Szent-Györgyi A. G. Hybrid formation between scallop myofibrils and foreign regulatory light-chains. J Mol Biol. 1980 Dec 15;144(3):223–245. doi: 10.1016/0022-2836(80)90088-1. [DOI] [PubMed] [Google Scholar]
- Stafford W. F., 3rd, Szentkiralyi E. M., Szent-Györgyi A. G. Regulatory properties of single-headed fragments of scallop myosin. Biochemistry. 1979 Nov 27;18(24):5273–5280. doi: 10.1021/bi00591a002. [DOI] [PubMed] [Google Scholar]
- Strynadka N. C., James M. N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A. G., Szentkiralyi E. M., Kendrick-Jonas J. The light chains of scallop myosin as regulatory subunits. J Mol Biol. 1973 Feb 25;74(2):179–203. doi: 10.1016/0022-2836(73)90106-x. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Chatman T. A. Chimeric regulatory light chains as probes of smooth muscle myosin function. J Biol Chem. 1993 Feb 25;268(6):4412–4419. [PubMed] [Google Scholar]
- Trybus K. M., Huiatt T. W., Lowey S. A bent monomeric conformation of myosin from smooth muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6151–6155. doi: 10.1073/pnas.79.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way M., Pope B., Gooch J., Hawkins M., Weeds A. G. Identification of a region in segment 1 of gelsolin critical for actin binding. EMBO J. 1990 Dec;9(12):4103–4109. doi: 10.1002/j.1460-2075.1990.tb07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Hamada Y., Katsuragawa Y., Imamura M., Mikawa T., Masaki T. Complete primary structure of vertebrate smooth muscle myosin heavy chain deduced from its complementary DNA sequence. Implications on topography and function of myosin. J Mol Biol. 1987 Nov 20;198(2):143–157. doi: 10.1016/0022-2836(87)90302-0. [DOI] [PubMed] [Google Scholar]




