Abstract
Percutaneous interventions for portal hypertension have been available since the 1990s. Over time, improved technology—including covered stent grafts—and clinical understanding has expanded the available procedures for percutaneous portal decompression. While transjugular intrahepatic portosystemic shunt creation is the most commonly cited percutaneous intervention, direct intrahepatic portocaval shunt and percutaneous mesocaval shunt creation are important alternatives with specific advantages and applications. This article reviews contemporary, minimally invasive interventional approaches to percutaneous portosystemic shunt creation in terms of procedure rationale, patient selection, interventional technique, and technical outcomes.
Keywords: percutaneous, transjugular intrahepatic portosystemic shunt, direct intrahepatic portocaval shunt, mesocaval shunt, interventional radiology
Objectives: Upon completion of this article, the reader will be able to identify the different percutaneous portosystemic shunts used for decompression of the portal venous system, identify indications, contraindications, and techniques for percutaneous shunt creation, and describe the technical outcomes of percutaneous shunt treatment in the setting of portal hypertension.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Portosystemic shunts aim to mitigate the severe consequences of portal hypertension—including medically refractory variceal hemorrhage and intractable ascites—through decompression of the portal and mesenteric venous systems. While effective, traditional surgical portosystemic shunts—such as distal splenorenal (Warren), mesocaval, and portocaval shunts—are associated with nontrivial operative morbidity1 2 3 and present limited applicability in cases of advanced liver disease, portal or mesenteric venous thrombosis, and medical comorbidity.3 4 5 Recent advances in endovascular methodologies and technological improvements in interventional devices have spurred the development of minimally invasive treatment options for the relief of portal hypertension through creation of stent-lined extra-anatomic conduits. These percutaneous procedures, performed in the interventional radiology (IR) suite using real-time imaging guidance, are associated with high technical success, safety, and efficacy, and have established a growing role in the treatment of portal hypertensive disease.6 7 8 The current article aims to review contemporary percutaneous portosystemic shunt approaches—including transjugular intrahepatic portosystemic shunts (TIPSs), direct intrahepatic portocaval shunts (DIPSs), percutaneous mesocaval shunts, and other percutaneous shunts—with a focus on procedure rationale, patient selection, interventional technique, and technical outcomes.
Portal Hypertension
The liver is a high-flow, low-resistance vascular bed. Native pressure in the portal vein varies between 5 and 10 mm Hg,9 10 and the portosystemic pressure gradient (PSG), or pressure difference between portal vein and systemic circulation (often measured in a hepatic vein), is generally 3 to 6 mm Hg. Portal hypertension, defined as a PSG exceeding 6 mm Hg or an absolute portal pressure above 10 mm Hg,11 represents a common sequela of cirrhotic liver disease that can trigger decompensation and precipitate complications such as ascites and varices. Ascites occurs when sinusoidal hypertension induces systemic vasodilation, reduced splanchnic vascular resistance, and increased capillary filtration causing fluid extravasation (the “forward-flow” theory).12 Development of varices, which are prone to bleeding, occurs as pressure builds in the portal system and natural collaterals expand to shunt blood into the systemic circulation. These portal hypertensive complications occur frequently and are often lethal; chronic liver disease and cirrhosis results in 30,000 deaths per year in the United States.13 Patients with uncomplicated cirrhosis have a 50% chance of developing ascites within 10 years, after which the 2-year mortality rate is nearly 50%.14 Gastroesophageal varices are found in approximately 30 to 40% of patients with cirrhosis,15 and when varices bleed, the risk of death is over 50% within 1 year.16
Conventional Medical and Surgical Therapies
Traditional therapy for portal hypertension is directed at controlling secondary effects. Diuretic therapy and sodium restriction are used for ascites management, although 10% of patients fail to respond to a high dose of diuretics or develop a contraindication to therapy, including hyponatremia or renal failure.14 Nonselective β-blockers, somatostatin analogues, and endoscopic ligation and/or banding are used to prevent or manage esophageal varices,17 but 15% will fail therapy within 5 days.18 While medical and endoscopic management is considered first-line therapy for patients with portal hypertensive complications, refractory cases may benefit from portal system decompression with either creation of a surgical shunt, percutaneous shunt placement, or liver transplantation.6
Surgical options for portosystemic decompression include the distal splenorenal (Warren), mesocaval, and portocaval shunts.19 The distal splenorenal shunt is created via a bilateral subcostal incision through which the splenic vein is identified after superior mobilization of the stomach and take-down of the splenocolic ligament, and sewn in an end-to-side fashion to the left renal vein after dissection of this vessel within the left retroperitoneum; the procedure is completed with left gastric vein ligation.20 Surgical mesocaval shunts between the superior mesenteric vein (SMV) and inferior vena cava (IVC) are created through a transverse abdominal incision using an 8 to 12 mm interposition graft,21 while portocaval shunts may be constructed through a right subcostal incision using an interposition graft22 or anastomoses created in a side-to-side or end-to-side fashion.23
Since the 1990s, use of surgical shunts has decreased significantly due to development of alternative management options5 and difficulty in performance for patients with severe liver cirrhosis due to poor overall health, challenging anatomy including splenic enlargement, abundant varices, abdominal distension, and naturally occurring tributaries acting as portosystemic shunts.24 These factors may also contribute to a paucity of surgeons with experience and expertise in executing these complex operations and who reliably achieve outcomes similar to those obtained at centers of excellence.
Interventional Radiology Management Approaches and Rationale
Percutaneously created portosystemic shunts alleviate portal hypertension by rerouting mesenteric, splenic, and portal blood flow through low-resistance outflow pathways into the systemic circulation. These conduits convey between 80 and 100% of portal venous blood flow into the central venous system through a controlled, synthetic channel,25 relieving sinusoidal hypertension and rerouting blood flow away from physiologically occurring extrahepatic varices. In reducing the hepatic venous pressure gradient, such shunts address the fundamental pathophysiology underlying portal hypertension, rather than merely tackling the sequela thereof, and sustainably avert portal hypertensive complications when durably patent.
Transjugular Intrahepatic Portosystemic Shunt
TIPSs are artificial channels created within the hepatic parenchyma connecting the portal and hepatic venous systems, which are aimed at decompressing the mesenteric, splenic, and portal veins. First described by Rösch et al in 1969,26 TIPSs were initially employed in humans in 1987,27 but were not considered an effective treatment for portal hypertensive complications until stent technology improved shunt patency rates in the 1990s.28 29 TIPS was originally developed to treat medically refractory variceal hemorrhage, gaining favor over operative shunt creation because its minimal invasive technique was more suitable for patients with severe liver disease who could not tolerate surgery. Today, TIPS is employed as an effective treatment for many complications of portal hypertension, including variceal bleeding30 and abdominal ascites.31 Advantages of TIPS include a long track record and robust clinical experience, minimally invasive nature, and well-described technique that is familiar to most practicing IRs, enabling widespread application. Potential disadvantages encompass reduced portal venous perfusion potentially resulting in hepatic deterioration,32 increased risk of hepatic encephalopathy,32 and possibility of shunt dysfunction mandating the need for surveillance imaging.33
Direct Intrahepatic Portocaval Shunt (DIPS)
A modification of the TIPS procedure, known as DIPS, was first described in 2001,34 and serves to create an artificial communication between the IVC and portal vein through the caudate lobe. The DIPS approach has two main benefits compared with TIPS: first, venous shunting into the larger IVC avoids chronic issues of hepatic vein stenosis that may complicate TIPSs that fail to extend to the confluence of the hepatic vein and IVC; second, the shorter liver parenchymal shunt tract leads to less susceptibility for pseudointimal hyperplasia development and subsequent shunt dysfunction.35 Moreover, DIPS is an advantageous approach for portosystemic shunting when the hepatic veins are unsuitable or inaccessible, as in Budd–Chiari syndrome.36 For these reasons, DIPS has become the preferred portal hypertensive treatment in some IR practices. Drawbacks of DIPS can include technical issues in the setting of calcific IVC, potential for bleeding either due to IVC puncture or liver capsular transgression, and possible technical interference with subsequent liver transplantation due to the stent location within the main portal vein and hepatic level IVC.37
Percutaneous Mesocaval Shunts
Portosystemic shunts involving the mesenteric veins were originally a surgical technique most commonly created between the SMV and the renal vein or IVC. Although operative mesocaval shunt creation using vitallium tubes was first described in 1945,38 the first percutaneous construction of a mesocaval shunt was not reported until 1996.39 Benefits of using mesenteric vessels as conduits to the systemic vasculature rather than a patent portal vein include maintenance of portal flow to the liver and preservation of native portal venous anatomy for subsequent liver transplantation.40 On the contrary, patients with chronic portal vein occlusion who would not routinely be candidates for TIPS creation, barring application of endovascular recanalization techniques, may be eligible for mesocaval shunt creation in the setting of mesenteric vessel accessibility.39 Drawbacks of the percutaneous mesocaval shunt approach include limited past performance and narrow clinical experience, as well as a challenging and potentially precarious procedural approach requiring transmesenteric, intraperitoneal vessel puncture.
Other Percutaneous Shunts
Although not in clinical use, other percutaneous shunts have been experimentally evaluated for feasibility. The percutaneous retroperitoneal splenorenal shunt (PRESS) functions to connect the left renal vein and the proximal splenic vein through the retroperitoneal cavity via a stent-lined channel; the viability of this procedure has been tested in swine with technical success41 42 but has not been clinically translated into humans. The purported benefits of PRESS parallel those of surgical distal splenorenal shunts, namely, selective decompression of varices, preservation of portal venous blood flow, and maintenance of mesenteric venous hypertension.41
Procedure Indications and Patient Selection
Major indications for TIPS include secondary prevention of variceal bleeding, treatment of acute refractory variceal hemorrhage, and intractable ascites.6 8 43 Other promising procedure indications include treatment of hepatic hydrothorax, Budd–Chiari syndrome, hepatorenal syndrome, and hepatopulmonary syndrome.6 44 45 46 Early first-line treatment for acute hemorrhage47 48 and management of portal vein thrombosis49 represent emerging TIPS indications. Patients are typically excluded as TIPS candidates in the setting of marked hepatic insufficiency (bilirubin levels greater than 3.0 mg/dL herald increased risk50), overt hepatic encephalopathy, right-sided heart failure (right atrial pressure exceeding 20 mm Hg),51 and uncontrolled infection or sepsis. Other contraindications can include biliary obstruction, polycystic liver disease, pulmonary hypertension (mean pulmonary arterial pressure greater than 45 mm Hg),6 coagulopathy, and thrombocytopenia.
The indications for DIPS and percutaneous mesocaval shunt creation overlap those of TIPS, but are typically reserved for select cases in which TIPS is anatomically unfeasible. For example, DIPS may be applied in the setting of hepatic vein occlusion, as in Budd–Chiari syndrome. DIPS may also be useful in cases where the hepatic parenchymal tract for TIPS is inadequate due to tumor,36 cysts, or dilated bile ducts. Mesocaval shunt creation may be considered in cases of portal vein occlusion with a patent mesenteric venous system.
Preprocedure risk assessment prior to portosystemic shunt creation is critically important for proper patient selection and counseling, and in current clinical practice is principally based on the Model for End-Stage Liver Disease (MELD) score. This system may be used to stratify patients according to chronic liver disease severity and predict clinical outcomes after shunt creation,52 53 54 55 56 as MELD scores have high predictive capacity for short- and intermediate-term patient mortality.56 In the setting of TIPS creation, patients may be classified into low (MELD less than 18), intermediate (MELD 19–25), and high (MELD greater than 25) risk categories, with 3-month overall mortality approximating 15, 33, and 80%, respectively.56
Procedural Technique
General Considerations
Cross-sectional imaging with computed tomography (CT), magnetic resonance imaging, or ultrasound with color Doppler imaging should be obtained prior to portosystemic shunt creation to assess the liver and vascular anatomy for adequate preprocedure planning.57 Regarding intravenous antibiotics, there are no data supporting benefit, although routine prophylaxis is recommended prior to TIPS by some clinical practice guidelines.58 Patient sedation may be accomplished via general anesthesia, monitored anesthesia care, or intravenous moderate sedation59; however, general anesthesia has the benefit of assured patient comfort and pain relief, airway control, and hemodynamic monitoring by an anesthesiologist, allowing the IR to fully concentrate on technical aspects of the procedure.
TIPS Creation
The detailed technical approach to TIPS creation has been previously described,30 60 and a standard TIPS is depicted in Fig. 1. In the setting of conventional liver configuration and vascular anatomy, a straightforward TIPS procedure can routinely be completed in one hour, although several pathologic or anatomic scenarios—such as bland or tumor-related portal vein thrombosis or portal vein cavernous transformation—can considerably increase the difficulty of the procedure or preclude TIPS creation altogether.
Figure 1.

Representative TIPS created in a 49-year-old woman with intractable ascites. Post-TIPS venogram displays covered stent graft lined hepatic intraparenchymal shunt (arrowheads) extending from right portal vein (black arrow) to confluence of right hepatic vein and IVC (white arrow). IVC, inferior vena cava; TIPS, transjugular intrahepatic portosystemic shunt.
Several procedure steps warrant specific consideration. Transhepatic portal venous puncture is typically the crucial, rate-limiting step of the procedure, but may be facilitated through visualization of the catheterized hepatic vein—which guides direction of transhepatic needle puncture—coupled with performance of high-quality wedged portography, which provides a portal venous target. Methods to achieve retrograde portal venous filling have been previously described,60 and may employ a wedged sheath or balloon occlusion catheter with injection of iodinated contrast material or carbon dioxide. While several transjugular access sets (Rosch-Uchida, Ring, and Haskal transjugular access sets, Cook Medical, Bloomington, IN, or Hawkins transjugular access set, AngioDynamics, Queensbury, NY) are commercially available, close familiarity with the performance characteristics of one or the other of the devices will also optimize puncture proficiency.
As covered stent grafts have been shown to improve TIPS patency,61 62 63 64 the specialized expanded polytetrafluoroethylene (e-PTFE) covered Viatorr stent graft (W.L. Gore and Associates, Flagstaff, AZ) is now widely used for TIPS creation. In selecting and deploying the covered stent graft, care must be taken to choose the correct length and to cover the entire extent of the shunt to the level of the hepatic vein and IVC confluence. As the lack of distal coverage may prompt distal stenosis and TIPS dysfunction,65 additional stents should be utilized to extend the shunt if the distal (hepatic vein) portion of the covered stent graft falls short of the junction of the hepatic vein and IVC.
Current clinical guidelines endorse the preferred degree of PSG reduction to less than 12 mm Hg for patients with esophageal variceal hemorrhage (possibly less for gastric variceal bleeding) and ≤ 12 mm Hg for intractable ascites.6 While the 12 mm Hg threshold is known to significantly reduce the risk for bleeding,66 the optimal PSG threshold for treatment of ascites is not known.31 Although covered stent grafts may be initially underdilated to achieve the desired PSG levels without overreduction that may prompt liver insufficiency or hepatic encephalopathy, it is currently unknown whether such underexpanded devices spontaneously expand to nominal diameter over time based on intrinsic radial force.
The necessity of concurrent variceal embolization at the time of TIPS creation is debatable. While embolization may limit variceal filling and avoid recanalization, embolotherapy increases procedure duration, cost, and radiation exposure. Qi et al assessed the efficacy of variceal embolization performed contemporaneously with TIPS in a meta-analysis,67 and found that adjunctive embolotherapy was associated with a significantly lower rate of rebleeding. Nonetheless, in many clinical practices embolization of gastroesophageal varices is still performed solely at the discretion of the primary IR operator.
DIPS Creation
The methodological approach to DIPS creation has been reported,36 and a typical DIPS is portrayed in Fig. 2. Much of the DIPS procedure technique parallels TIPS creation, but the crux of this intervention lies in the safe transcaval puncture of the portal vein through the hepatic caudate lobe. This procedural step is widely performed using intravascular ultrasound (IVUS) guidance, with the benefits of real-time imaging including direct visualization of portal venous needle entry, decreased radiation use, and decreased procedure time.36 IVUS imaging performed from a femoral approach guides transjugular access into the portal vein while ensuring safety of the needle puncture. A modified “gun-sight” approach68 may be used as an alternative when IVUS is unavailable. For this maneuver, a snare placed into the portal vein via a percutaneous transhepatic approach or through a recanalized paraumbilical vein is used as a target for transcaval puncture. Because the retrohepatic IVC is outside the liver capsule, there is a risk for bleeding into the abdominal cavity during DIPS, and caution with needle puncture and attentiveness to stent positioning is therefore critical to prevent intraprocedural hemoperitoneum.69
Figure 2.

DIPS created in a 46-year-old man with variceal hemorrhage. Final venogram shows covered stent graft lined shunt (arrowheads) within liver caudate lobe, which spans from main portal vein (black arrow) to IVC (white arrow) (case courtesy of John D. Louie, MD, and Rajesh P. Shah, MD, Division of Interventional Radiology, Department of Radiology, Stanford University Medical Center). DIPS, direct intrahepatic portocaval shunt; IVC, inferior vena cava.
Percutaneous Mesocaval Shunt Creation
The methods for percutaneous mesocaval shunt creation are scantly described. A representative percutaneously created mesocaval shunt is illustrated in Fig. 3. The reported technique for percutaneous mesocaval shunt creation is based on a standard DIPS procedure: a transjugular access set needle is passed through the suprarenal IVC wall into the SMV using IVUS guidance.70 Once the extravascular tract is established, the procedure is completed with deployment of a covered stent graft, which is required because of risk of bleeding into peritoneum via a bare metal stent. In the absence of IVUS, simultaneous transabdominal puncture of the SMV and IVC under CT guidance has been used to facilitate percutaneous mesocaval shunt construction.39
Figure 3.
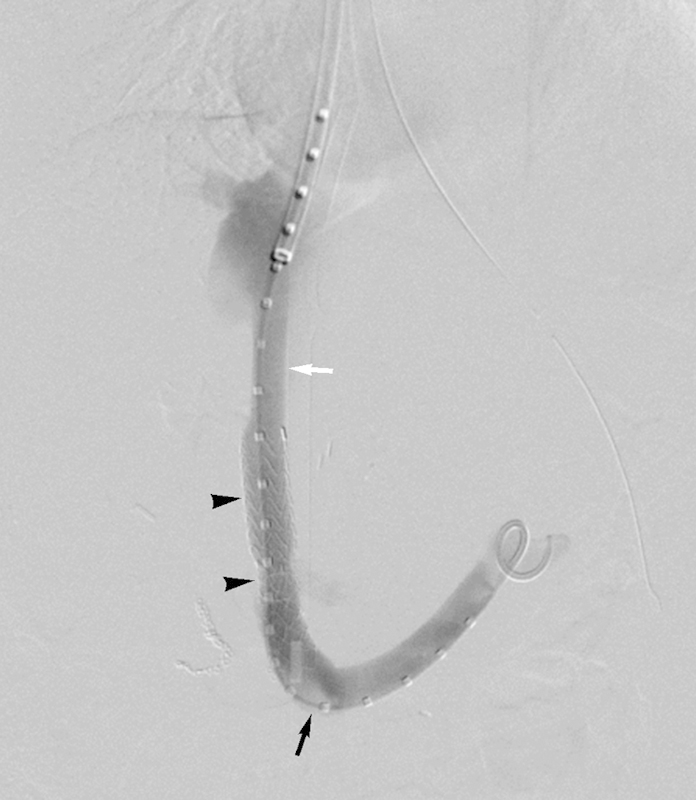
Percutaneous mesocaval shunt created in a 9-year-old girl with biliary atresia status postliver transplant complicated by portal vein thrombosis and development of varices. Completion venogram demonstrates covered stent graft lined intraperitoneal shunt (arrowheads), which crosses from confluence of SMV and splenic vein (black arrow) to IVC (white arrow) (case courtesy of John D. Louie, MD, and Rajesh P. Shah, MD, Division of Interventional Radiology, Department of Radiology, Stanford University Medical Center). IVC, inferior vena cava; SMV, superior mesenteric vein.
Procedure Technical Outcomes
TIPS
TIPS procedural success is judged based on technical and hemodynamic parameters. Technical success is defined as successful shunt creation between a hepatic vein and intrahepatic portal vein branch, while hemodynamic success indicates PSG reduction below a selected threshold, typically 12 mm Hg.71 Technical and hemodynamic success rates approximate 95% or higher in most series.72 TIPS-related major adverse events occur with a low incidence (approximating 3%).72 Complications may include: wedged venography-related liver injury; biliary puncture and fistula formation; hepatic arterial and nontarget organ puncture; shunt malposition; device migration; vessel trauma during coil embolization; post-TIPS liver ischemia, infarction, and failure; TIPS occlusion; and radiation injury.60 Finally, despite the considerable improvement in TIPS patency rates afforded by e-PTFE covered stent grafts, routine imaging surveillance is still recommended to ensure persistence of an open shunt.6
A discussion of clinical efficacy of TIPS is beyond the scope of the current review, but comparative outcomes assessment against conventional surgical shunts warrants brief mention. Surgical shunts are historically associated with higher 30-day morbidity,3 and whereas some studies indicate that TIPS and surgical (distal splenorenal) shunts are equally effective at managing variceal bleeding,73 others suggest that operative (portocaval) shunts are more effective than TIPS in controlling bleeding.74 A Cochrane Collaboration review of portosystemic shunts found that total surgical shunt, distal splenorenal shunts, and TIPS with endoscopic therapy provided similar outcomes.33 A major limitation of all of these studies is comparison to TIPS created using bare metal stents, which are outdated and associated with higher rates of shunt dysfunction.75 76
DIPS
Similar to TIPS, DIPSs are created using covered stent grafts with a high-rate technical and hemodynamic success: 100% for both in two large series, with a PSG goal of 15 mm Hg.35 69 The most commonly reported complication is bleeding in 5 to 6%, either due to extrahepatic portal vein puncture or related to the bare portion of covered stent graft being placed in the extrahepatic shunt tract.35 69 This procedure is associated with high shunt patency, with long-term primary patency approximating 75 to 100%.35 69
Percutaneous Mesocaval Shunts
Percutaneous mesocaval shunts have been infrequently discussed in the literature since the first case report in 1996. Two recent reports have documented the use of percutaneous mesocaval shunts: one report of three successful mesocaval shunts using IVUS guidance,70 and another in a pediatric patient, creating an end-to-end complete diversion of SMV flow into the IVC for more complete portal decompression.77 As the number of reported clinical cases is small, it is not possible to estimate the overall procedure technical success rate and long-term shunt patency. Similarly, the adverse event profile of percutaneous mesocaval shunt creation is unknown, although theoretical risks and complications include intraperitoneal hemorrhage, shunt migration, or inadvertent bowel or nearby organ perforation; puncture of the uncinate process of the pancreas has also been reported.70
Conclusion
Percutaneous portosystemic shunts have become an established component in the management of portal hypertension. While TIPS remains the most widely applied intervention, technical variations have emerged that aim to address methodological hurdles and expand the number of patient conditions amenable to percutaneous portal decompression. Future studies will continue to affirm the procedural and clinical benefits of percutaneous portosystemic shunts, clarify technique selection between shunting approaches, and develop new percutaneous portosystemic shunt methodologies.
Footnotes
Financial Support, Disclosures, and Conflicts of Interest None.
References
- 1.Jenkins R L, Gedaly R, Pomposelli J J, Pomfret E A, Gordon F, Lewis W D. Distal splenorenal shunt: role, indications, and utility in the era of liver transplantation. Arch Surg. 1999;134(4):416–420. doi: 10.1001/archsurg.134.4.416. [DOI] [PubMed] [Google Scholar]
- 2.Sarfeh I J, Rypins E B. Partial versus total portacaval shunt in alcoholic cirrhosis. Results of a prospective, randomized clinical trial. Ann Surg. 1994;219(4):353–361. doi: 10.1097/00000658-199404000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orozco H, Mercado M A, Granados Garcia J. et al. Selective shunts for portal hypertension: current role of a 21-year experience. Liver Transpl Surg. 1997;3(5):475–480. doi: 10.1002/lt.500030501. [DOI] [PubMed] [Google Scholar]
- 4.Hermann R E Henderson J M Vogt D P Mayes J T Geisinger M A Agnor C Fifty years of surgery for portal hypertension at the Cleveland Clinic Foundation. Lessons and prospects Ann Surg 19952215459–466., discussion 466–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson J M, Nagle A, Curtas S, Geisinger M, Barnes D. Surgical shunts and TIPS for variceal decompression in the 1990s. Surgery. 2000;128(4):540–547. doi: 10.1067/msy.2000.108209. [DOI] [PubMed] [Google Scholar]
- 6.Boyer T D Haskal Z J; American Association for the Study of Liver Diseases. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009 Hepatology 2010511306. [DOI] [PubMed] [Google Scholar]
- 7.Runyon B A; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update Hepatology 20094962087–2107. [DOI] [PubMed] [Google Scholar]
- 8.Salerno F, Cammà C, Enea M, Rössle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007;133(3):825–834. doi: 10.1053/j.gastro.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Sherlock S. Portal circulation and portal hypertension. Gut. 1978;19(1):70–83. doi: 10.1136/gut.19.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galambos J T. Portal hypertension. Semin Liver Dis. 1985;5(3):277–290. doi: 10.1055/s-2008-1040624. [DOI] [PubMed] [Google Scholar]
- 11.Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7(2):141–155. doi: 10.1586/egh.12.83. [DOI] [PubMed] [Google Scholar]
- 12.Rössle M, Gerbes A L. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut. 2010;59(7):988–1000. doi: 10.1136/gut.2009.193227. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention, National Center for Health Statistics Compressed Mortality File 1999–2010 on CDC WONDER Online Database, released January 2013 Data are compiled from Compressed Mortality File 1999–2010 Series 20 No. 2P, 2013. Available at: http://wonder.cdc.gov/cmf-icd10.html. Accessed December 9, 2013
- 14.Runyon B A; Practice Guidelines Committee, American Association for the Study of Liver Diseases (AASLD). Management of adult patients with ascites due to cirrhosis Hepatology 2004393841–856. [DOI] [PubMed] [Google Scholar]
- 15.Kovalak M, Lake J, Mattek N, Eisen G, Lieberman D, Zaman A. Endoscopic screening for varices in cirrhotic patients: data from a national endoscopic database. Gastrointest Endosc. 2007;65(1):82–88. doi: 10.1016/j.gie.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 16.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Gøtzsche P C, Hróbjartsson A. Somatostatin analogues for acute bleeding oesophageal varices. Cochrane Database Syst Rev. 2008;(3):CD000193. doi: 10.1002/14651858.CD000193.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Amico G De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators Hepatology 2003383599–612. [DOI] [PubMed] [Google Scholar]
- 19.Warren W D. Control of variceal bleeding. Reassessment of rationale. Am J Surg. 1983;145(1):8–16. doi: 10.1016/0002-9610(83)90159-9. [DOI] [PubMed] [Google Scholar]
- 20.Henderson J M. Heidelberg, Germany: Springer; 2007. Distal splenorenal shunt; pp. 653–664. [Google Scholar]
- 21.Mercado M A, Orozco H. Heidelberg, Germany: Springer; 2007. Low-diameter mesocaval shunt; pp. 665–674. [Google Scholar]
- 22.Rosemurgy A S, Korkolis D P. Heidelberg, Germany: Springer; 2007. 8MM interposition portacaval shunt; pp. 675–686. [Google Scholar]
- 23.Orloff M J, Orloff M S, Orloff S L. Heidelberg, Germany: Springer; 2007. Portacaval shunts: side-to-side and end-to-side; pp. 687–702. [Google Scholar]
- 24.Khanna R, Mishra S, Khanna S, Khanna A K. Inferior meso-caval shunt for portal hypertension with bleeding esophageal varices. Indian J Gastroenterol. 2003;22(3):108–109. [PubMed] [Google Scholar]
- 25.Walser E M, Harris V M, Harman J T, Park H M, Siddiqui A R. Quantification of intrahepatic portosystemic shunting after placement of a transjugular intrahepatic portosystemic shunt. J Vasc Interv Radiol. 1996;7(2):263–267. doi: 10.1016/s1051-0443(96)70775-3. [DOI] [PubMed] [Google Scholar]
- 26.Rösch J, Hanafee W N, Snow H. Transjugular portal venography and radiologic portacaval shunt: an experimental study. Radiology. 1969;92(5):1112–1114. doi: 10.1148/92.5.1112. [DOI] [PubMed] [Google Scholar]
- 27.Gordon J D, Colapinto R F, Abecassis M. et al. Transjugular intrahepatic portosystemic shunt: a nonoperative approach to life-threatening variceal bleeding. Can J Surg. 1987;30(1):45–49. [PubMed] [Google Scholar]
- 28.Richter G M, Noeldge G, Palmaz J C. et al. Transjugular intrahepatic portacaval stent shunt: preliminary clinical results. Radiology. 1990;174(3 Pt 2):1027–1030. doi: 10.1148/radiology.174.3.174-3-1027. [DOI] [PubMed] [Google Scholar]
- 29.Haskal Z J, Davis A, McAllister A, Furth E E. PTFE-encapsulated endovascular stent-graft for transjugular intrahepatic portosystemic shunts: experimental evaluation. Radiology. 1997;205(3):682–688. doi: 10.1148/radiology.205.3.9393521. [DOI] [PubMed] [Google Scholar]
- 30.Gaba R C, Omene B O, Podczerwinski E S. et al. TIPS for treatment of variceal hemorrhage: clinical outcomes in 128 patients at a single institution over a 12-year period. J Vasc Interv Radiol. 2012;23(2):227–235. doi: 10.1016/j.jvir.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Parvinian A, Bui J T, Knuttinen M G, Minocha J, Gaba R C. Transjugular intrahepatic portosystemic shunt for the treatment of medically refractory ascites. Diagn Interv Radiol. 2014;20(1):58–64. doi: 10.5152/dir.2013.13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freedman A M, Sanyal A J, Tisnado J. et al. Complications of transjugular intrahepatic portosystemic shunt: a comprehensive review. Radiographics. 1993;13(6):1185–1210. doi: 10.1148/radiographics.13.6.8290720. [DOI] [PubMed] [Google Scholar]
- 33.Khan S, Tudur Smith C, Williamson P, Sutton R. Portosystemic shunts versus endoscopic therapy for variceal rebleeding in patients with cirrhosis. Cochrane Database Syst Rev. 2006;(4):CD000553. doi: 10.1002/14651858.CD000553.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen B, Uchida B T, Timmermans H, Keller F S, Rosch J. Intravascular US-guided direct intrahepatic portacaval shunt with a PTFE-covered stent-graft: feasibility study in swine and initial clinical results. J Vasc Interv Radiol. 2001;12(4):475–486. doi: 10.1016/s1051-0443(07)61887-9. [DOI] [PubMed] [Google Scholar]
- 35.Petersen B, Binkert C. Intravascular ultrasound-guided direct intrahepatic portacaval shunt: midterm follow-up. J Vasc Interv Radiol. 2004;15(9):927–938. doi: 10.1097/01.RVI.0000133703.35041.42. [DOI] [PubMed] [Google Scholar]
- 36.Petersen B D, Clark T W. Direct intrahepatic portocaval shunt. Tech Vasc Interv Radiol. 2008;11(4):230–234. doi: 10.1053/j.tvir.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Hutchins R R, Patch D, Tibballs J, Burroughs A, Davidson B R. Liver transplantation complicated by embedded transjugular intrahepatic portosystemic shunt: a new method for portal anastomosis- a surgical salvage procedure. Liver Transpl. 2000;6(2):237–238. doi: 10.1002/lt.500060203. [DOI] [PubMed] [Google Scholar]
- 38.Blakemore A H, Lord J W. The Technic of Using Vitallium Tubes in Establishing Portacaval Shunts for Portal Hypertension. Ann Surg. 1945;122(4):476–489. doi: 10.1097/00000658-194510000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyman U R, Semba C P, Chang H, Hoffman C, Dake M D. Percutaneous creation of a mesocaval shunt. J Vasc Interv Radiol. 1996;7(5):769–773. doi: 10.1016/s1051-0443(96)70847-3. [DOI] [PubMed] [Google Scholar]
- 40.Scudamore C H, Erb S R, Morris C. et al. Medium aperture meso-caval shunts reliably prevent recurrent variceal hemorrhages. Am J Surg. 1996;171(5):490–494. doi: 10.1016/S0002-9610(97)89610-9. [DOI] [PubMed] [Google Scholar]
- 41.Kaminou T, Rösch J, Yamada R. et al. Percutaneous retroperitoneal splenorenal shunt: an experimental study in swine. Radiology. 1998;206(3):799–802. doi: 10.1148/radiology.206.3.9494504. [DOI] [PubMed] [Google Scholar]
- 42.Wang L G, Fan W J, Zhang L, Wang B, Tang T, Li X. [Percutaneous retroperitoneal splenorenal stent shunt: an experimental study in swine model with portal hypertension] Zhonghua Yi Xue Za Zhi. 2010;90(17):1216–1219. [PubMed] [Google Scholar]
- 43.Saab S, Nieto J M, Lewis S K, Runyon B A. TIPS versus paracentesis for cirrhotic patients with refractory ascites. Cochrane Database Syst Rev. 2006;(4):CD004889. doi: 10.1002/14651858.CD004889.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bilbao J I, Pueyo J C, Longo J M. et al. Interventional therapeutic techniques in Budd-Chiari syndrome. Cardiovasc Intervent Radiol. 1997;20(2):112–119. doi: 10.1007/s002709900117. [DOI] [PubMed] [Google Scholar]
- 45.Guevara M, Ginès P, Bandi J C. et al. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology. 1998;28(2):416–422. doi: 10.1002/hep.510280219. [DOI] [PubMed] [Google Scholar]
- 46.Spencer E B, Cohen D T, Darcy M D. Safety and efficacy of transjugular intrahepatic portosystemic shunt creation for the treatment of hepatic hydrothorax. J Vasc Interv Radiol. 2002;13(4):385–390. doi: 10.1016/s1051-0443(07)61741-2. [DOI] [PubMed] [Google Scholar]
- 47.García-Pagán J C, Caca K, Bureau C. et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Pagán J C, Di Pascoli M, Caca K. et al. Use of early-TIPS for high-risk variceal bleeding: results of a post-RCT surveillance study. J Hepatol. 2013;58(1):45–50. doi: 10.1016/j.jhep.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 49.D'Avola D, Bilbao J I, Zozaya G. et al. Efficacy of transjugular intrahepatic portosystemic shunt to prevent total portal vein thrombosis in cirrhotic patients awaiting for liver transplantation. Transplant Proc. 2012;44(9):2603–2605. doi: 10.1016/j.transproceed.2012.09.050. [DOI] [PubMed] [Google Scholar]
- 50.Rajan D K, Haskal Z J, Clark T W. Serum bilirubin and early mortality after transjugular intrahepatic portosystemic shunts: results of a multivariate analysis. J Vasc Interv Radiol. 2002;13(2 Pt 1):155–161. doi: 10.1016/s1051-0443(07)61932-0. [DOI] [PubMed] [Google Scholar]
- 51.Valji K. Philadelphia, PA: Elsevier Inc.; 2006. Hepatic, splenic, and portal vascular systems; pp. 269–319. [Google Scholar]
- 52.Kamath P S, Wiesner R H, Malinchoc M. et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 53.Salerno F, Merli M, Cazzaniga M. et al. MELD score is better than Child-Pugh score in predicting 3-month survival of patients undergoing transjugular intrahepatic portosystemic shunt. J Hepatol. 2002;36(4):494–500. doi: 10.1016/s0168-8278(01)00309-9. [DOI] [PubMed] [Google Scholar]
- 54.Schepke M, Roth F, Fimmers R. et al. Comparison of MELD, Child-Pugh, and Emory model for the prediction of survival in patients undergoing transjugular intrahepatic portosystemic shunting. Am J Gastroenterol. 2003;98(5):1167–1174. doi: 10.1111/j.1572-0241.2003.07515.x. [DOI] [PubMed] [Google Scholar]
- 55.Ferral H, Gamboa P, Postoak D W. et al. Survival after elective transjugular intrahepatic portosystemic shunt creation: prediction with model for end-stage liver disease score. Radiology. 2004;231(1):231–236. doi: 10.1148/radiol.2311030967. [DOI] [PubMed] [Google Scholar]
- 56.Gaba R C Couture P M Bui J T et al. Prognostic capability of different liver disease scoring systems for prediction of early mortality after transjugular intrahepatic portosystemic shunt creation J Vasc Interv Radiol 2012; In press [DOI] [PubMed] [Google Scholar]
- 57.Scanlon T, Ryu R K. Portal vein imaging and access for transjugular intrahepatic portosystemic shunts. Tech Vasc Interv Radiol. 2008;11(4):217–224. doi: 10.1053/j.tvir.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Venkatesan A M Kundu S Sacks D et al. Practice guidelines for adult antibiotic prophylaxis during vascular and interventional radiology procedures[corrected]J Vasc Interv Radiol 201021111611–1630., quiz 1631 [DOI] [PubMed] [Google Scholar]
- 59.DeGasperi A, Corti A, Corso R. et al. Transjugular intrahepatic portosystemic shunt (TIPS): the anesthesiological point of view after 150 procedures managed under total intravenous anesthesia. J Clin Monit Comput. 2009;23(6):341–346. doi: 10.1007/s10877-009-9167-y. [DOI] [PubMed] [Google Scholar]
- 60.Gaba R C, Khiatani V L, Knuttinen M G. et al. Comprehensive review of TIPS technical complications and how to avoid them. AJR Am J Roentgenol. 2011;196(3):675–685. doi: 10.2214/AJR.10.4819. [DOI] [PubMed] [Google Scholar]
- 61.Vignali C, Bargellini I, Grosso M. et al. TIPS with expanded polytetrafluoroethylene-covered stent: results of an Italian multicenter study. AJR Am J Roentgenol. 2005;185(2):472–480. doi: 10.2214/ajr.185.2.01850472. [DOI] [PubMed] [Google Scholar]
- 62.Maleux G, Nevens F, Wilmer A. et al. Early and long-term clinical and radiological follow-up results of expanded-polytetrafluoroethylene-covered stent-grafts for transjugular intrahepatic portosystemic shunt procedures. Eur Radiol. 2004;14(10):1842–1850. doi: 10.1007/s00330-004-2359-4. [DOI] [PubMed] [Google Scholar]
- 63.Hausegger K A, Karnel F, Georgieva B. et al. Transjugular intrahepatic portosystemic shunt creation with the Viatorr expanded polytetrafluoroethylene-covered stent-graft. J Vasc Interv Radiol. 2004;15(3):239–248. doi: 10.1097/01.rvi.0000116194.44877.c1. [DOI] [PubMed] [Google Scholar]
- 64.Jung H S, Kalva S P, Greenfield A J. et al. TIPS: comparison of shunt patency and clinical outcomes between bare stents and expanded polytetrafluoroethylene stent-grafts. J Vasc Interv Radiol. 2009;20(2):180–185. doi: 10.1016/j.jvir.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 65.Parvinian A, Omene B O, Bui J T, Knuttinen M G, Minocha J, Gaba R C. Angiographic patterns of transjugular intrahepatic portosystemic shunt dysfunction and interventional approaches to shunt revision. J Clin Imaging Sci. 2013;3:19. doi: 10.4103/2156-7514.111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casado M, Bosch J, García-Pagán J C. et al. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology. 1998;114(6):1296–1303. doi: 10.1016/s0016-5085(98)70436-6. [DOI] [PubMed] [Google Scholar]
- 67.Qi X, Liu L, Bai M. et al. Transjugular intrahepatic portosystemic shunt in combination with or without variceal embolization for the prevention of variceal rebleeding: a meta-analysis. J Gastroenterol Hepatol. 2014;29(4):688–696. doi: 10.1111/jgh.12391. [DOI] [PubMed] [Google Scholar]
- 68.Haskal Z J, Duszak R Jr, Furth E E. Transjugular intrahepatic transcaval portosystemic shunt: the gun-sight approach. J Vasc Interv Radiol. 1996;7(1):139–142. doi: 10.1016/s1051-0443(96)70750-9. [DOI] [PubMed] [Google Scholar]
- 69.Hoppe H, Wang S L, Petersen B D. Intravascular US-guided direct intrahepatic portocaval shunt with an expanded polytetrafluoroethylene-covered stent-graft. Radiology. 2008;246(1):306–314. doi: 10.1148/radiol.2461062191. [DOI] [PubMed] [Google Scholar]
- 70.Hong R, Dhanani R S, Louie J D, Sze D Y. Intravascular ultrasound-guided mesocaval shunt creation in patients with portal or mesenteric venous occlusion. J Vasc Interv Radiol. 2012;23(1):136–141. doi: 10.1016/j.jvir.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 71.Haskal Z J Rees C R Ring E J Saxon R Sacks D; Society of Interventional Radiology Technology Assessment Committee. Reporting standards for transjugular intrahepatic portosystemic shunts J Vasc Interv Radiol 2003149 Pt 2S419–S426. [DOI] [PubMed] [Google Scholar]
- 72.Haskal Z J, Martin L, Cardella J F. et al. Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2003;14(9 Pt 2):S265–S270. [PubMed] [Google Scholar]
- 73.Henderson J M, Boyer T D, Kutner M H. et al. Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: a randomized trial. Gastroenterology. 2006;130(6):1643–1651. doi: 10.1053/j.gastro.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 74.Orloff M J, Vaida F, Haynes K S, Hye R J, Isenberg J I, Jinich-Brook H. Randomized controlled trial of emergency transjugular intrahepatic portosystemic shunt versus emergency portacaval shunt treatment of acute bleeding esophageal varices in cirrhosis. J Gastrointest Surg. 2012;16(11):2094–2111. doi: 10.1007/s11605-012-2003-6. [DOI] [PubMed] [Google Scholar]
- 75.Sommer C M, Gockner T L, Stampfl U. et al. Technical and clinical outcome of transjugular intrahepatic portosystemic stent shunt: bare metal stents (BMS) versus viatorr stent-grafts (VSG) Eur J Radiol. 2012;81(9):2273–2280. doi: 10.1016/j.ejrad.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 76.Barrio J, Ripoll C, Bañares R. et al. Comparison of transjugular intrahepatic portosystemic shunt dysfunction in PTFE-covered stent-grafts versus bare stents. Eur J Radiol. 2005;55(1):120–124. doi: 10.1016/j.ejrad.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 77.Burke C, Taylor A G, Ring E J, Kerlan R K Jr. Creation of a percutaneous mesocaval shunt to control variceal bleeding in a child. Pediatr Radiol. 2013;43(9):1218–1220. doi: 10.1007/s00247-013-2643-z. [DOI] [PubMed] [Google Scholar]


