Abstract
The corticobulbar projection to the hypoglossal nucleus was studied from the frontal, parietal, cingulate and insular cortices in the rhesus monkey using high-resolution anterograde tracers and stereology. The hypoglossal nucleus received bilateral input from the face/head region of the primary (M1), ventrolateral pre- (LPMCv), supplementary (M2), rostral cingulate (M3), and caudal cingulate (M4) motor cortices. Additional bilateral corticohypoglossal projections were found from the dorsolateral premotor cortex (LPMCd), ventrolateral proisocortical motor area (ProM), ventrolateral primary somatosensory cortex (S1), rostral insula and pregenual region of the anterior cingulate gyrus (areas 24/32). Dense terminal projections arose from the ventral region of M1, moderate projections from LPMCv and rostral part of M2, with considerably less hypoglossal projections arising from the other cortical regions. These findings demonstrate that extensive regions of the non-human primate cerebral cortex innervate the hypoglossal nucleus. The widespread and bilateral nature of this corticobulbar connection suggests recovery of tongue movement after cortical injury that compromises a subset of these areas, may occur from spared corticohypoglossal projection areas located on the lateral, as well as medial surfaces of both hemispheres. Since functional imaging studies have shown that homologous cortical areas are activated in humans during tongue movement tasks, these corticobulbar projections may exist in the human brain.
Keywords: Corticofugal, Tongue, Dysarthria, Dysphagia, Stroke, Brainstem, Medulla, Cranial Nerves
INTRODUCTION
Vocalization, chewing, swallowing and respiration are extremely complex motor activities requiring a significant and timely contribution of tongue movements (Miller, 2002). It has long been recognized that tongue movements are comprehensively influenced by assemblies of integrated neuronal circuits located within the lower brainstem (Holstege and Kuypers, 1977; Holstege et al., 1977, 1983; Lowe, 1981; Jean, 1984, 2001; Miller, 1999; Sawczuk and Mosier, 2001; Jürgens, 2002; Gestreau et al., 2005; Hannig and Jürgens, 2005; Lund and Kolata 2006; Yamada et al., 2005). However, very little is known about the role of the cerebral cortex in mediating tongue movements which is quite surprising, since clinical observations have long noted that oromotor deficits occur in patients sustaining localized cortical injury (e.g., Meadows, 1973; Willoughby and Anderson, 1984; Horner et al., 1988; Robbins and Levin, 1988; Martin and Sessle, 1993; Robins et al., 1993; Daniels and Foundas, 1997; Hamdy et al., 1997). Indeed, tongue weakness, dysarthria, dysphagia and aspiration are common in patients suffering the most frequently occurring stroke, middle cerebral artery (MCA) occlusion (Miller, 1999; Umapathi et al., 2000; Falsetti et al., 2009; for review see Hamdy et al., 2000; Singh and Hamdy, 2006; Michou and Hamdy 2009). Reinforcing the existence of a cortical influence on swallowing and tongue movements are seminal reports showing that surface stimulation of the lateral precentral cortex in humans evokes swallowing, tongue and mastication movements (e.g., Penfield and Boldery, 1937; Penfield and Welch, 1949; Woolsey et al., 1979) while more recent studies show similar effects following transcranial magnetic stimulation (TMS) of the precentral region (e.g., Meyer et al., 1997; Krings et al., 1997; Muellbacher et al., 1998, 1999, 2001; Rödel et al., 2003; D’Ausilio et al., 2009; Boudreau et al., 2013. Although the TMS findings have been enlightening, renewed interest in the role of the cerebral cortex in mediating oromotor movements has largely transpired from functional imaging studies showing that multiple cortical sites are active when healthy human subjects perform tongue movements in isolation, or during integrated movements such as swallowing and speaking (e.g., Birn et al., 1998; Corefield et al., 1999; Hamdy et al., 1999; Mosier et al., 1999; Zald and Pardo, 1999; Kern et al., 2001; Martin et al., 2001, 2004; Mosier and Bereznaya, 2001; Malandraki et al., 2009; Sörös et al., 2009; Grabski et al., 2012). From these observations, a common neural network of cortical areas appear to influence tongue/orofacial movement including the ventral portion of the primary motor and somatosensory cortices, insula, anterior cingulate gyrus, supplementary motor cortex, and parietal cortex (Humbert and Robbins, 2007; Miller, 2008; Michou and Hamdy, 2009).
Of these brain areas, cortex forming the ventral extension of the primary motor cortex (M1) and adjacent ventral premotor region has frequently been associated with mediation of tongue movements in non-human primates. Aspiration resection of both cortical areas have long been known to induce immediate post-surgical tongue dysfunction (e.g., Green and Walker, 1938; Luschei and Goodwin, 1975; Larson et al., 1980). Complimenting these observations are physiological studies in non-human primates demonstrating that face, tongue and mandibular movements occur following stimulation of this cortex (e.g., Horsley and Schäfer, 1888; Leyton and Sherrington, 1917; Walker and Green, 1938; Woolsey et al., 1952; Cure and Rasmussen, 1954; Luschei et al., 1971, 1974; McGuinness et al., 1980; Sessle and Wiesendanger, 1982; Huang et al., 1988; Murray and Sessle, 1992; Hatanaka et al., 2005). These physiological observations are grounded, in part, by anatomical findings in monkeys showing that the ventral region of M1projects to multiple cranial nerve motor nuclei including the hypoglossal nucleus (Kuypers, 1958a; Kuypers and Lawrence, 1967; Jürgens and Alipour, 2002), facial nucleus (Kuypers, 1958a, Lawrence and Kuypers, 1967; Jenny and Saper, 1987; Morecraft et al., 2001), and trigeminal motor nucleus (Kuypers, 1958a; Kuypers and Lawrence, 1967).
As for the other constituents of the proposed cortical tongue/swallowing network, far less is known about their role mediating tongue movements, but some observations are suggestive. Foremost are stimulation studies showing that orofacial responses occur following stimulation of the rostral region of M2 in monkey (Woolsey et al., 1952; Mitz and Wise, 1987; Luppino et al., 1991; Godschalk et al., 1995) and humans (Penfield and Welch, 1951; Talairch and Bancaud, 1966; Fried et al., 1991). In monkey, orofacial movements have also been observed following microstimulation of cortex lining the lower bank of the cingulate sulcus, possibly corresponding to the rostral part of the rostral cingulate motor cortex (Godschalk et al., 1995) and rostral part of the caudal cingulate motor cortex (Luppino et al., 1991; Godschalk, et al., 1995). Previous monkey tracer studies have been less convincing indicating that M2 (Künzle, 1978; Jürgens, 1984; Wiesendanger and Wiesendanger, 1984) and the ventrolateral premotor cortex may not project to the hypoglossal nucleus (Künzle, 1978; Simonyan and Jürgens, 2003), which in part, prompted the current investigation using high-resolution tract tracing methodology.
What has attracted our attention is that a number of homologous regions in the monkey cortex, that have been shown in human neuroimaging work to be associated with tongue movements, have been shown to project to the facial nucleus of the lower pons (Jenny and Saper, 1987; Morecraft et al., 2001; Gong et al., 2005). Based upon these findings, physiological observations (Woolsey et al., 1952, Mitz and Wise, 1987; Luppino et al., 1991; Godschalk et al., 1995) and topographical patterns of cortical interconnections with the face region of M1 (Muakkassa and Strick, 1979; Morecraft and Van Hoesen, 1992; Morecraft et al., 1996; Tokuno et al., 1997), we proposed the existence of an integrated network of face regions including the ventral part of M1and lateral premotor cortex (LPMCv), as well as the rostral part of the supplementary (M2), rostral cingulate (M3), and caudal cingulate (M4) motor cortices that may uniquely influence volitional and emotional components of facial expression (Morecraft et al., 1996; 2001; 2004; 2007a). Whether or not these cortical face regions constitute orofacial representations, or craniocervical representations, has yet to be determined.
Given that the neuroscientific corner-stones characterizing the M1 face/head region include the demonstration of physiologically evoked cranial movements and direct corticobulbar projections to multiple cranial nerve motor nuclei, it is possible that other frontal and cingulate regions shown physiologically to elicit orofacial movements, and anatomically to issue corticofacial projections, may also project to other cranial nerve motor nuclei much like ventral M1. This could reinforce perspectives on cortical face/head representation in the debate on somatotopic motor organization currently dominated by viewpoints focusing on cortical forelimb and hind limb representation. Such findings could broaden our current approach to investigating the potential role of cortical areas, in addition to ventral M1, that may contribute to neurological disorders adversely affecting cranial nerve motor function. Clinical intuition would also suggest that this information may assist in expanding our initiative of localizing cortical areas that may favorably contribute to the recovery of orofacial movements following supratentorial brain injury. Such considerations prompted the present investigation to explore the possibility that corticobulbar projections to the hypoglossal nucleus arise from regions of the monkey cerebral cortex which innervate the facial nucleus and correspond to homologous components of the human cortical swallowing network indicated in the functional imaging literature. Since the insula, frontal operculum, anterior cingulate gyrus and lateral parietal cortex have also been implicated in this network, these areas were included in our investigative analysis.
MATERIALS AND METHODS
The corticobulbar projection to the hypoglossal nucleus was studied using 39 cortical injection sites in 20 Rhesus monkeys (Macaca mulatta) (Figs. 1–3, Tables 1–3). All experimental and surgical procedures used in this study followed United States Department of Agriculture, National Institutes of Health, and Society for Neuroscience guidelines for the ethical treatment of animals and were approved by the Institutional Animal Care and Use Committee at The University of South Dakota. Details of the experimental methods used to accomplish the goals of this study are provided below.
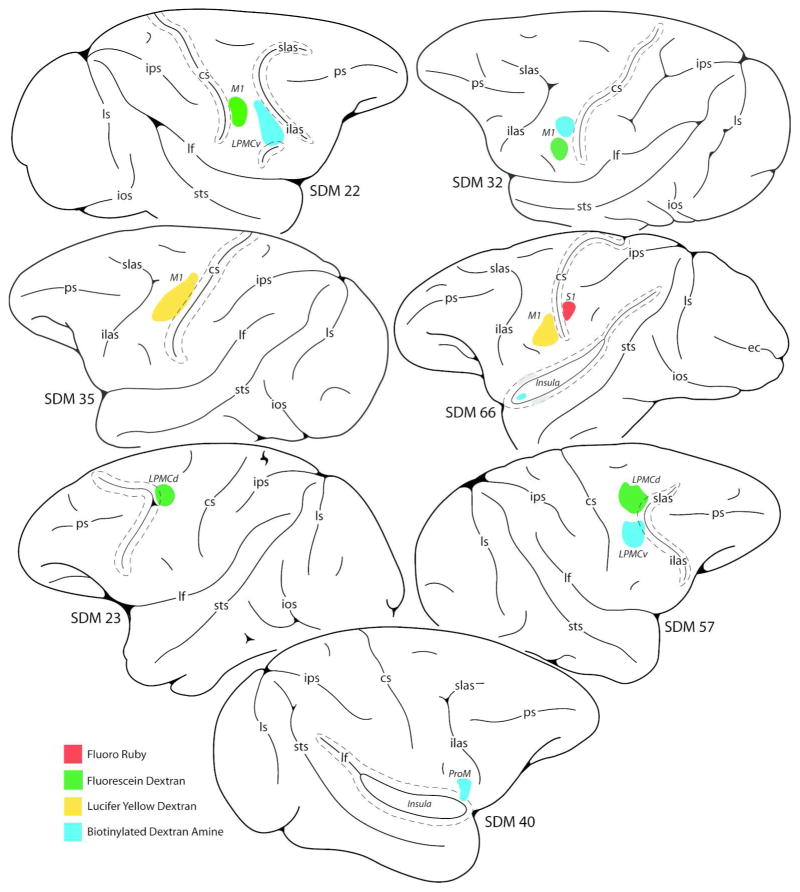
Figure 1.
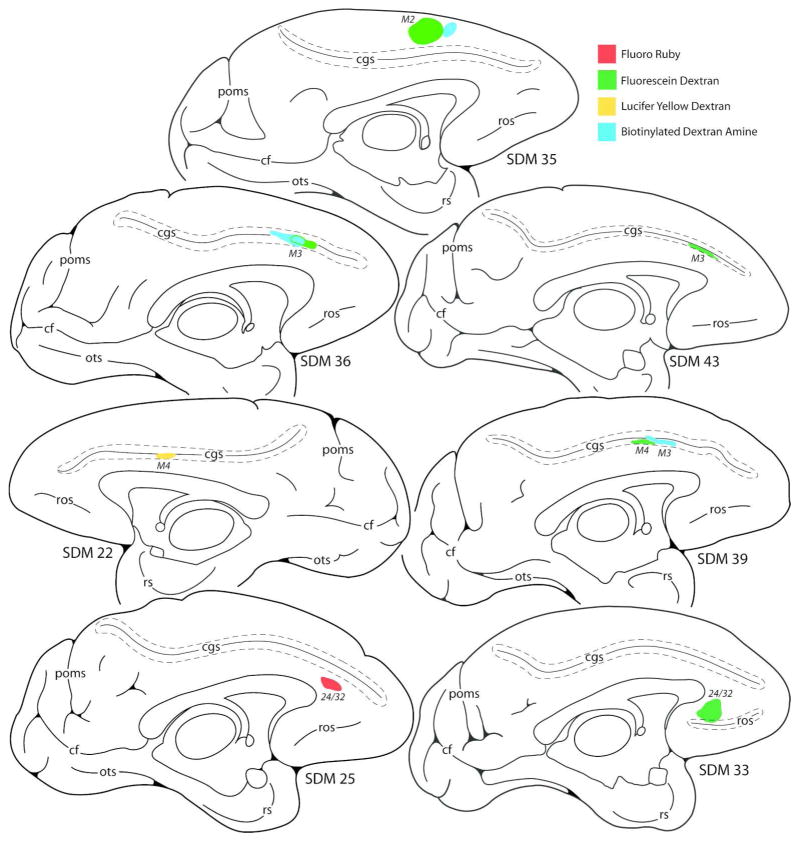
Line drawings of cortical injection sites on the lateral wall of the monkey (Macaca mulatta) cerebral hemisphere giving rise to terminal labeling in the hypoglossal nucleus of cranial nerve XII. Cortical sulci that are “opened” are indicated by the dashed lines around the solid line (representing the fundus). Abbreviations: cs, central sulcus; ec, ectocalcarine sulcus; ilas, inferior limb of the arcuate sulcus; ios, inferior occipital sulsus; ips, intraparietal sulcus; LPMCd, dorsal lateral premotor cortex; LPMCv, ventral lateral premotor cortex; lf, lateral fissure; ls, lunate sulcus; M1, primary motor cortex; ProM, proisocortical motor area (according to the terminology of Sanides, 1968, 1970); ps, principle sulcus; S1, primary somatosensory cortex; SDM, South Dakota Monkey; slas, superior limb of the arcuate sulcus; STS, superior temporal sulcus.
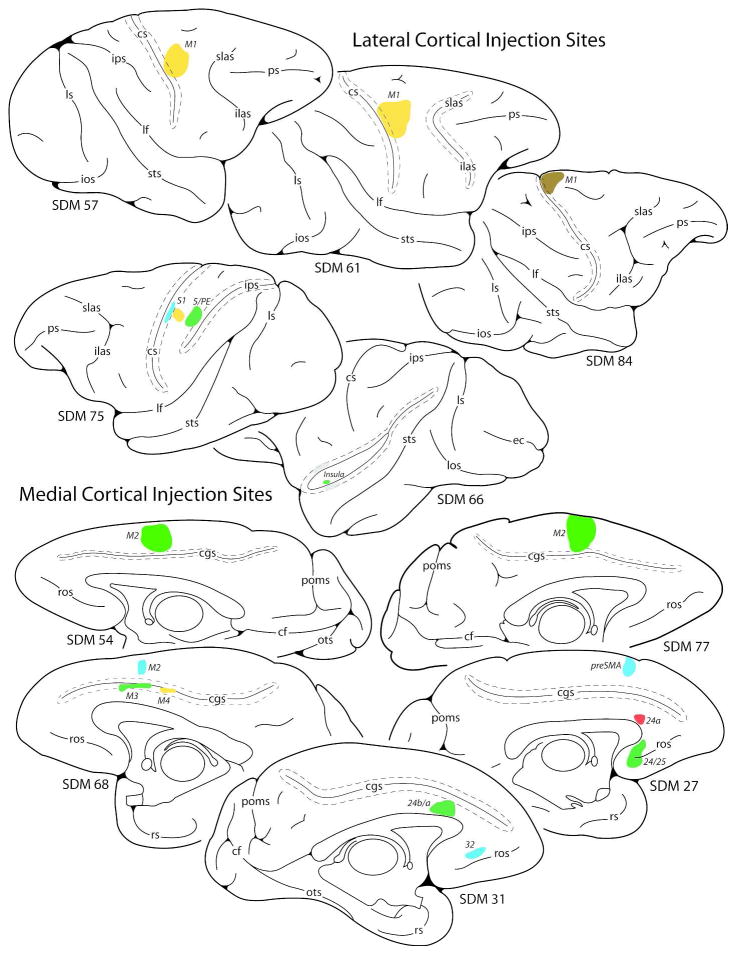
Figure 3.
Line drawings of cortical injection sites on the lateral (top) and medial (bottom) walls of the monkey (Macaca mulatta) cerebral hemisphere giving rise to little to no terminal labeling in the hypoglossal nucleus of cranial nerve XII. Cortical sulci that are “opened” are indicated by the dashed lines around the solid line (representing the funds). Abbreviations: preSMA, pre-supplementary motor area. For other abbreviations see Figs. 1 and 2.
Table 1.
Description of the Experimental Parameters of the Lateral Cortical Injection Cases Resulting in Hypoglossal Labeling
| Case | Sex | Weight (kg) | Area Injected | Tracer/Injections | Total Volume (μL) | Post-injection survival (days) |
|---|---|---|---|---|---|---|
| SDM22 | M | 6.0 | M1 Face/dorsal | FD/4 | 1.2 | 29 |
| SDM32 | M | 5.6 | M1 Face/dorsal | BDA/1 | 0.4 | 30 |
| SDM32 | M | 5.6 | M1 Face/ventral | FD/1 | 0.4 | 30 |
| SDM35 | F | 3.5 | M1 Face-dorsal/M1 Arm/LPMCv | LYD/4 | 1.6 | 29 |
| SDM66 | F | 8.1 | M1 Face/ventral | LYD/3 | 1.2 | 33 |
| SDM22 | M | 6.0 | LPMCv Face | BDA/4 | 1.2 | 29 |
| SDM57 | F | 6.0 | LPMCv Face/arm | BDA/3 | 1.2 | 32 |
| SDM57 | F | 6.0 | LPMCd Arm | FD/3 | 1.2 | 32 |
| SDM23 | F | 8.5 | LPMCd Arm | FD/3 | 1.0 | 29 |
| SDM40 | F | 4.8 | ProM | BDA/1 | 0.3 | 27 |
| SDM66 | F | 8.1 | Insula - anterior | BDA/1 | 0.4 | 33 |
| SDM66 | F | 8.1 | S1 Face (Areas1/2) | FR/3 | 1.2 | 33 |
Table 3.
Description of the Experimental Parameters of Cortical Injection Cases Resulting in Little to No Hypoglossal
| Case | Sex | Weight (kg) | Area Injected | Tracer/Injections | Total Volume (μL) | Post-injection survival (days) |
|---|---|---|---|---|---|---|
| Frontal Cases | ||||||
| SDM57 | F | 6 | M1 Arm | LYD/3 | 1.2 | 32 |
| SDM61 | F | 4.3 | M1 Arm | LYD/3 | 1.2 | 33 |
| SDM84 | M | 11.7 | M1 Leg | DA488/2 | 0.8 | 33 |
| SDM54 | M | 9.2 | M2 Arm | FD/3 | 1.2 | 33 |
| SDM77 | M | 9.6 | M2 Arm | FD/3 | 1.2 | 33 |
| SDM68 | F | 7.9 | M2 Arm | BDA/3 | 1.2 | 33 |
| SDM27 | F | 8.7 | PreSMA/Area 8Bm | BDA/2 | 0.5 | 28 |
| Cingulate Cases | ||||||
| SDM68 | F | 7.9 | M4 Arm | LYD/3 | 1.2 | 33 |
| SDM68 | F | 7.9 | M3 Arm | FD/3 | 1.2 | 33 |
| SDM31 | F | 3 | Area 24a/b | FD/1 | 0.2 | 30 |
| SDM27 | F | 8.7 | Area 24a | FR/2 | 0.4 | 28 |
| SDM31 | F | 3 | Area 24/32 | BDA/2 | 0.4 | 30 |
| SDM27 | F | 8.7 | Area 25/32 | FD/1 | 0.4 | 28 |
| Parietal Cases | ||||||
| SDM75 | M | 9.5 | S1 Arm-Areas 3/1 | BDA/3 | 1.2 | 33 |
| SDM75 | M | 9.5 | S1 Arm-Areas 1/2 | LYD/3 | 1.2 | 33 |
| SDM75 | M | 9.5 | Area 5 | FD/3 | 1.2 | 33 |
| Insula Case | ||||||
| SDM66 | F | 8.1 | Insula – Rostral/Inferior | FD/1 | 0.4 | 33 |
Neurosurgical Exposure and Cortical Tract Tracer Injection
All neurosurgical procedures were performed under sterile conditions. Preoperatively, each monkey was immobilized with atropine (0.5mg/kg) then ketamine hydrochloride (10mg/kg). The monkey was then intubated, placed on a mechanical ventilator and anesthetized with either pentobarbital (early cases), or a mixture of 1.0–1.5% isoflurane and surgical grade air/oxygen. The monkey was subsequently placed into a neurosurgical head holder and administered mannitol intravenously (1.0–1.5g/kg). For surgical exposure of the cerebral cortex, a suitable skin flap was made and turned followed by a craniotomy over the desired portion of cortex to be injected with neuronal tract tracer. The cortical surface was exposed by opening the dura by a flap incision or paired cruciate incisions. Details of the lateral and midline surgical procedures have been reported in our previous studies (Morecraft et al., 2001, 2002, McNeal et al., 2010).
In selected cases, electrophysiological microstimulation was used in combination with ketamine (10 mg/kg) and diazepam (1.0 mg/kg) to map M1, LPMC and M2 which has been described in detail in our previous reports (McNeal et al., 2010; Morecraft et al., 2007a, 2013a). Briefly, stimulation was performed using a Grass Square Pulse Stimulator system (model S28; Grass Technologies, West Warwick, RI) with an attached tungsten electrode (impedance 0.5–1.5 MΩ). The electrode was secured in an electrode micromanipulator unit (Kopf Instruments, Model 1760–61, Tujunga, CA) and advanced in the gray matter at 500 μm intervals. Evoked movements were induced using a train duration of 50 ms and pulse duration of 0.2 ms delivered at 330 Hz. Current intensity ranged between 1 and 90 μA. Each stimulation site location was recorded for data reconstruction purposes. Electrode penetration sites were intentionally minimized to avoid gray matter tissue damage while maximizing optimal tract tracer uptake and transport from corticobulbar projection neurons.
In all cases, multiple tract tracer injections were placed 3–4 mm below the cortical surface. The anterograde tracers that were injected included 10% biotinylated dextran amine (BDA) in 0.9% saline, 10% lucifer yellow dextran (LYD) in 0.9% sterile saline, 10% fluorescein dextran (FD) in sterile water, 10% fluoro ruby (FR) in sterile water and 5% DA488 in sterile water (Invitrogen/Molecular Probes Inc, Eugene, OR, USA) (Tables 1 and 2). The FD solution was composed of an equal mixture of 3,000 and 10,000 MW volumes, as was the FR solution. Our method of localization of M1, LPMC, M2, M3, and M4 using anatomical landmarks has been previously described (Morecraft et al., 2001, 2002). The injections were made using a surgical microscope and Hamilton microsyringe that was secured in a specially designed Hamilton microdrive attached to the Kopf micromanipulator unit. In all experimental cases, total injection volumes ranged from 0.2 μL to 1.6 μL (Tables 1–3). In selected cases, the retrograde tracers fast blue (FB: 4–5% in 0.1M phosphate buffer) and diamidino-yellow (DY: 4–5% in 0.1M phosphate buffer) were injected into the body of the tongue to assist in localizing the hypoglossal nucleus during the data collection and analysis process. Following the injection procedure, the surgical field was irrigated with 0.9% sterile saline then gently swabbed with cottonoids. The dura was repositioned and closed with sutures and the bone flap was replaced and anchored. The temporalis muscle was reattached and skin flap was closed using standard neurosurgical technique. The animal was monitored and Bicillin L-A was used as pre- and post-operative prophylaxis antibiotic and buprenorphine (0.01 mg/kg) was used as a post-operative analgesic.
Table 2.
Description of the Experimental Parameters of the Medial Cortical Injection Cases Resulting in Hypoglossal Labeling
| Case | Sex | Weight (kg) | Area Injected | Tracer/Injections | Total Volume (μL) | Post-injection survival (days) |
|---|---|---|---|---|---|---|
| SDM35 | F | 3.5 | M2 Arm/Face | FD/2 | 0.6 | 29 |
| SDM35 | F | 3.5 | preSMA/M2 Face | BDA/1 | 0.3 | 29 |
| SDM36 | M | 12.1 | M3 Face | FD/2 | 0.6 | 35 |
| SDM36 | M | 12.1 | M3 Face/Arm | BDA/2 | 0.6 | 35 |
| SDM43 | F | 4.8 | M3 Face | FD/3 | 1.2 | 34 |
| SDM22 | M | 6.0 | M4 Face | LYD/1 | 0.4 | 29 |
| SDM39 | F | 5.0 | M4 Face | FD/3 | 1.2 | 32 |
| SDM25 | M | 5.0 | Area 24/32 | FR/3 | 0.9 | 29 |
| SDM33 | M | 3.0 | Area 24/32 | FD/2 | 0.6 | 30 |
| SDM39 | F | 5.0 | M3 Arm/M4 Face | BDA/3 | 1.2 | 32 |
Tissue Processing
Following a survival period of 27–35 days after tract tracer injection, each monkey was deeply anesthetized with an overdose of pentobarbital (50 mg/kg or more) and perfused transcardially with 0.9% saline. Saline infusion was followed by 2 liters of 4% paraformaldehyde in 0.1M phosphate buffer at pH 7.4 (PB) to fix the tissue, then one liter each of 10% and 30% sucrose in 0.1M PB for tissue cryoprotection. The central nervous system was carefully extracted, placed in 30% sucrose in 0.1M PB and stored for 2 to 5 days at 4° C.
In all cases the cerebral cortex was frozen sectioned in the coronal plane on a sliding microtome (American Optical 860, Buffalo, NY, USA) at a thickness of 50 μm in cycles of 10, forming 10 complete series of evenly spaced tissue sections respectively. Each brainstem was blocked from the central nervous system, frozen with dry ice and cut horizontally on the sliding microtome at a thickness of 50 μm in cycles of 5, 7 or 10 forming 5, 7 or 10 complete series of evenly spaced tissue sections respectively. For both the cortex and brainstem, one series of tissue sections was mounted on subbed slides, dried and eventually stained for Nissl substance using thionin to evaluate cytoarchitectonic organization (Morecraft et al., 1992, 2004, 2012).
In all monkeys involved in this study, BDA was injected into a cortical region of interest as were other dextran tract tracers. Accordingly, one series of tissue sections through the cortex and brainstem were immunohistochemically processed for BDA alone. Then, several additional series of tissue sections were processed for double labeling of tract tracer (e.g., BDA + LYD; BDA+FR; BDA+FD) (e.g., Morecraft et al., 2007a, 2007b, 2013a) (Figs. 4, 5).
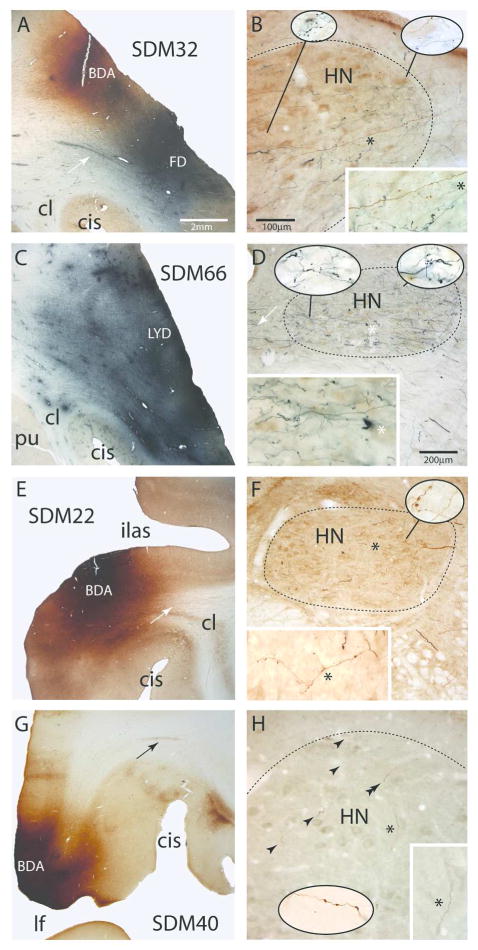
Figure 4.
Plate of photomicrographs showing representative examples of cortical injection sites on the lateral wall of the monkey (Macaca mulatta) cerebral hemisphere (left panels, A,C,E,G) giving rise to terminal labeling in the hypoglossal nucleus (HN) of cranial nerve XII (right panels, B,D,F,H). A) Coronal section showing the dorsally located BDA injection site (brown) and ventrally located FD injection site (blue) in the M1 orofacial representation in case SDM32. The white arrow denotes a well-defined bundle of FD labeled axons emerging from the cortical FD injection site. The micron bar is also applicable to panels C,E and G. B) Photomicrographic image of a horizontal section showing BDA (brown) and FD (blue) labeled fibers and terminals in the ipsilateral hypoglossal nucleus (HN) in case SDM32. The micron bar also applies to the main panel in plate H. Note the extensive terminal labeling throughout the nucleus and dense distribution of fibers coursing in the ventrolateral region toward more medially located regions of the nucleus. The asterisk in the main panel denotes the location of the higher powered inset in the bottom right hand corner of the panel. The pull-out spheres show higher power (100x) images from selected regions of the nucleus. C) Coronal section showing the LYD injection site (blue) in the ventral region of the M1 orofacial representation in case SDM66. D) Photomicrographic image of a horizontal section illustrating FD (blue) labeled fibers and terminals in the contralateral hypoglossal nucleus in case SDM66. Heavy terminal labeling is notable throughout the nucleus and the dense distribution of fibers can be seen coursing across in the lateral region of the nucleus toward more medially located targets. The white arrows show fibers that decussate across the midline at the level of the HN. The asterisk in the main panel denotes the location of the higher powered inset in the bottom right hand corner of the panel. The pull-out spheres show higher power (100x) images from selected regions of the nucleus. The micron bar applies to the main panel in plate F. E) Coronal section through part of the BDA injection site in LPMCv in case SDM22. The white arrow in the subcortical white matter identifies a coalesced bundle of BDA fibers emerging from the injection site and passing dorsal to the claustrum. F) Horizontal section through the contralateral hypoglossal nucleus showing BDA labeled fibers and terminal boutons in the HN in case SDM22. The asterisk in the main panel denotes the location of the higher powered inset in the bottom left hand corner of the panel. The image of the main axon shown in the inset has been rotated 90°. The pull-out sphere shows a higher power (100x) images from the selected region of the nucleus G) Photomicrograph of a coronal section through the BDA injection site in area ProM in case SDM40. The black arrow in the subcortical white matter identifies a bundle of BDA fibers arching over the circular sulcus (CiS). H) Horizontal section through the contralateral hypoglossal nucleus showing BDA labeled fibers and terminal boutons (black arrowheads) in the HN. The asterisk in the main panel denotes the location of the higher powered inset in the bottom right hand corner of the panel. The pull-out sphere shows a higher power (100x) image of labeled boutons in the dorsolateral region of the hypoglossal nucleus (approximating the region identified by the double arrowhead) from a section inferior to the main image. Abbreviations: CiS, circular sulcus; HN, hypoglossal nucleus; ilas, inferior limb of the arcuate sulcus.
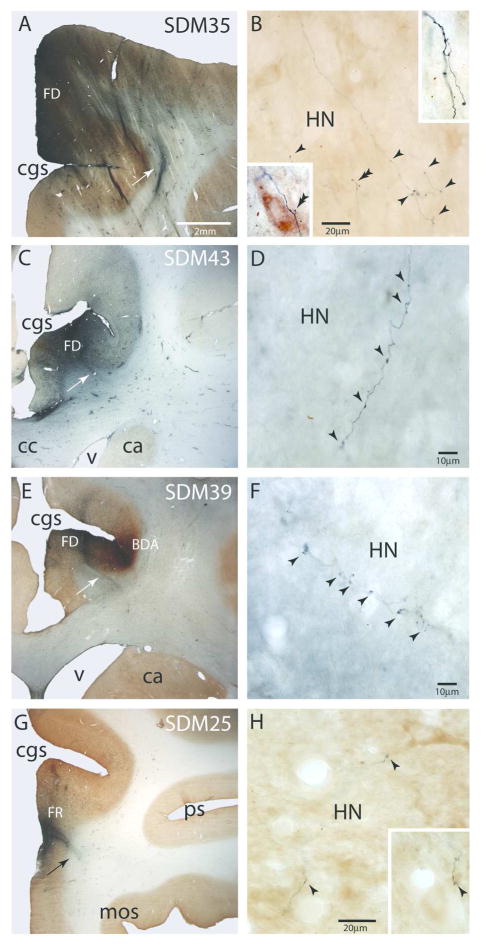
Figure 5.
Plate of photomicrographs showing representative examples of cortical injection sites on the medial wall of the monkey (Macaca mulatta) cerebral hemisphere (main panels) producing terminal labeling in the hypoglossal nucleus (HN) of cranial nerve XII (insets). A) Photomicrograph of a coronal section showing the large FD injection site (blue) in the rostral region of M2 in case SDM35. The white arrow identifies a densely labeled bundle of FD fibers that is descending from the FD injection site. The micron bar applies to all other main panels showing the respective injection sites (C, E, G). B) Horizontal section through the contralateral hypoglossal nucleus showing FD labeled fibers and terminal boutons (black arrowheads). The insets are higher power images (100x) from adjacent tissue sections showing detailed terminal fields. The double arrowhead identifies labeled boutons in close proximity to large cell bodies. C) Photomicrograph of a coronal section showing the localized FD injection site (blue) in the rostral region of M3 in case SDM43. The white arrow identifies a densely labeled bundle of FD fibers emerging from the ventral region of the FD injection site. D) Horizontal section through the ipsilateral hypoglossal nucleus showing a FD labeled fiber with terminal boutons (black arrowheads) in the dorsolateral region of the HN. E) Photomicrograph of a coronal section showing the localized FD injection site (blue) in the rostral region of M4 medial to the BDA injection site that involved primarily the caudal-most part of M3 in case SDM39. The white arrow identifies a densely labeled bundle of FD fibers emerging from the ventral region of the FD injection site. F. High power photomicrograph of a FD labeled terminals in the dorsolateral part of the ipsilateral hypoglossal nucleus. The arrowheads indicate the locations of labeled boutons. G) Low power photomicrograph of a coronal section showing the small localized FR injection site (blue) in the rostral region of cingulate area 24/32 in case SDM25. The black arrow identifies a densely labeled bundle of FD fibers emerging from the FD injection site. H) High power photomicrograph showing FD (blue) labeled fibers and terminal boutons in the ipsilateral hypoglossal nucleus (HN). The inset shows a fiber fragment with labeled boutons in an adjacent tissue section at the same magnification as the main panel. The arrow heads identify labeled boutons in close opposition to a proximal dendrite of a large neuron in the nucleus. Abbreviatons: ca, caudate nucleus; cc, corpus callosum; cgs, cingulate sulcus; cl, claustrum; HN, hypoglossal nucleus; mos, medial orbital sulcus; ps, principle sulcus; v, ventricle.
To accomplish this plan, BDA was immunohistochemically processed in a complete cortical and brainstem series using the avidin-biotin (ABC) labeling procedure in combination with the 3, 3′ diaminobenzidine tetrahydrochloride (DAB) staining method yielding a brown BDA reaction product (e.g., Fig. 4E) (Morecraft et al., 2007a, 2007b, 2013a). Once completed, the tissue sections were mounted on subbed slides (50×75×1.2 mm plain end slides, Brain Research Laboratories, Newton, MA), dried overnight and coverslipped (No. 1, 40×50mm glass coverslips, Brain Research Laboratories, Newton, MA) using Permount (Fisher Scientific, Fair Lawn, NJ).
In subsequent series of tissue sections, BDA was processed in the same manner as described above which was followed by immunohistochemical localization of a second tract tracer (e.g., LYD; Fig. 4C, D). Details of this double labeling procedure for BDA/LYD, BDA/FD and BDA/FR have been previously reported (Morecraft et al., 2007a, 2007b, 2013a). Thus, in the double labeling experiments, BDA was typically stained brown and the second dextran tracer (e.g., LYD) was processed and stained blue using the Vector SG blue substrate kit (SK-4700). In some of our early monkey experiments, BDA was colorized black using nickel enhancement and DAB and the second tracer was colorized brown using DAB alone (Morecraft et al., 2001, 2002) or vice versa. Once the double labeling procedure was completed, the tissue was rinsed and the sections were mounted on subbed glass slides and dried overnight. Then the tissue was dehydrated and coverslipped using Permount and glass coverslips.
Our method for localizing DA-488 in monkey SDM84 (Fig. 3) followed the immunohistochemical protocol described by Rosenzweig and colleagues (Rosenzweig et al., 2010). For the double labeling procedure to visualize DA488 (Invitrogen/Molecular Probes, D-22910), BDA was processed in serial cortical and brainstem tissue sections using the avidin-biotin (ABC) labeling procedure in combination with the 3, 3′ diaminobenzidine tetrahydrochloride (DAB) staining method yielding a brown reaction product. Following this procedure, the tissue was then incubated/rotated in rinse buffer (5% normal goat serum, 0.4% Triton X-100 in 0.05M tris buffered saline at pH 7.4) overnight at 4° C. The tissue was transferred to a rinse buffer solution containing rabbit antibody to DA488 (Invitrogen, A11094) at a dilution of 1:5000 for approximately 48 hours at 4° C. The tissue sections were thoroughly rinsed in 0.05M tris buffered saline (TBS) then incubated in a rinse buffer solution of biotinylated anti-rabbit IgG (H and L) (Vector Laboratories, BA-1000) at a dilution of 1:200 for 3 hours at room temperature. The sections were again thoroughly rinsed in TBS then incubated in an Avidin Biotin Complex (ABC) solution for 4 hours at room temperature. The ABC solution consisted of 5μL Triton X-100, 0.4 drop of reagent A, 0.4 drop reagent B per mL of TBS used which was prepared and gently stirred for 30 minutes prior to use. The sections were subsequently rinsed in TBS then incubated in Vector SG blue substrate staining solution (Vector Laboratories, SK-4700) until a visually acceptable reaction was evident (approximately 2–5 minutes). The Vector SG substrate solution consisted 3 drops of chromagen and 3 drops of hydrogen peroxide per 5 mL of TBS of solution. This procedure resulted in staining DA488 blue and BDA brown.
Data Analysis
Localization of the cortical injection site and the terminal boutons within the hypoglossal nucleus was accomplished using brightfield illumination on a BX-51 Olympus microscope (Leeds Precision Instruments, Minneapolis, MN). Attached to the microscope was a high resolution MAC 5000 motorized stage (Ludl Electronic Products, Hawthorne, NY, USA) which was interfaced with Neurolucida and Stereo Investigator neuroanatomical data collection software (Microbrightfield, Colchester, VT, USA, RRID:nif-0000-10294, RRID:nif-0000-00124) in a Dell computer. Cases with cortical injections of dextran tracers from our non-human primate microscopic slide collection were initially evaluated using the brightfield microscope and screened for positive and negative hypoglossal terminal labeling. Injection sites located in the frontal, cingulate, parietal and insular cortices were included in this process. In cases that were found to contain hypoglossal terminal labeling, the Neurolucida system was used to plot the locations of the injection site in the cortex and terminal-like profiles (boutons) in the hypoglossal nucleus. In brainstem sections, the locations of terminal boutons were plotted in the hypoglossal nucleus in every tissue section to obtain a general characterization of the topography and relative density of the projection using Olympus UPlanApo 20x–40x microscope objectives (Leeds Precision Instruments, Minneapolis, MN). Matching Nissl stained sections were consulted to confirm the cytoarchitectonic affiliation of each cortical injection site and determine the anatomical boundaries of the hypoglossal nucleus as determined by motor neuron soma representation. In 2 additional animals, a NeuN stained series through the hypoglossal nucleus was used to verify peripheral and rostro-caudal hypoglossal boundary characteristics. A detailed description of the NeuN immunohistochemical processing procedure used for these 2 cases is presented in the “Materials and Methods” section of our recent paper on corticospinal projections from the primary motor cortex (Morecraft et al., 2013a). In selected monkey cases, every section through the hypoglossal nucleus in the immunohistochemically processed series of brainstem sections was evaluated to estimate total terminal bouton number within the hypoglossal nucleus using stereology. Immunoreactive terminal-like varicosities (i.e., putative boutons or terminal-like profiles) were defined as small swellings along the terminal fibers that were 0.5 – 3.5 μm in diameter (Wouterlood and Groenewegen, 1985; Freese and Amaral, 2006; Morecraft et al., 2007b). In our stereological analysis, microscopic identification of all boutons was accomplished using an Olympus PlanApo 100x oil objective (Leeds Precision Instruments, Minneapolis, MN).
Stereological Analysis
Using Stereo Investigator 7 (MicroBrightField Inc. Williston VT, USA), unbiased estimates of terminal boutons were obtained in the hypoglossal nucleus using the Optical Fractionator probe. We designed and constructed the parameters of the probes according to our recently published material (McNeal et al., 2010; Morecraft et al., 2007b, 2013a). Briefly, the stereological parameters included the counting brick dimensions, tissue thickness, counting brick placement, guard zones and dissector height. The same counting frame (109.2/71.4 μm) and X/Y grid placement (125.3/241.9 μm) were applied to all case material when performing stereology. The counting target consisted of immunoreactive terminal-like varicosities that are 0.5 – 3.5 μm in diameter which were counted using unbiased counting rules.
Data Reconstruction and Presentation
Publication quality images of injection sites and labeled fibers were captured using a Spotflex 64 Mp shifting pixel camera, (Diagnostic Instruments Inc., Sterling Heights, MI, USA, version 4.6), mounted on an Olympus BX51 microscope. Photographic montages of the injection sites and labeled fibers were made using Adobe PhotoShop 7.0 (Adobe Systems Inc., San Jose, CA, USA). Brightness and contrast were adjusted in the images. Cortical reconstructions were developed as previously described using metrically calibrated images of the cortical surface (Morecraft and Van Hoesen, 1992, 1993). Publication quality illustrations were created using Slidewrite 6.0 for data graphs (Rancho Santa Fe, CA, USA) and Adobe Illustrator 10.0 for line drawings (Adobe Systems Inc., San Jose, CA, USA).
RESULTS
Thirty nine, high-resolution anterograde tract tracer injection sites, from 20 animals were used to study the potential corticofugal projection to the hypoglossal nucleus in the medulla. The findings from lateral surface injection sites that resulted in terminal hypoglossal labeling will be presented first (Fig. 1; Table 1) followed by medial surface injection sites (Fig. 2; Table 2) which produced hypoglossal terminal labeling. Then we will present findings from additional injection site cases available in our slide library which produced little to no hypoglossal labeling (Figure 3; Table 3). Collectively, this approach was designed to assist in localizing the corticohypoglossal projection by identifying both positive and negative cortical areas innervating the hypoglossal nucleus as well as areas that do so, but only very weakly. Due to the large number and variety of injection sites used for this investigation, the specific anatomical location of each cortical injection site will be described together with the reported hypoglossal findings for clarity.
Figure 2.
Line drawings of cortical injection sites on the medial wall of the monkey (Macaca mulatta) cerebral hemisphere producing terminal labeling in the hypoglossal nucleus of cranial nerve XII. Cortical sulci that are “opened” are indicated by the dashed lines around the solid line (representing the funds). Abbreviations: cf, calcarine fissure; cgs, cingulate sulcus; M2, supplementary motor cortex; M3, rostral cingulate motor cortex; M4, caudal cingulate motor cortex; ots, occipitotemporal sulcus; pos, medial parietooccipitaol sulcus; ros, rostral sulcus; rs, rhinal sulcus.
Hypoglossal Projections from the Lateral Cortical Surface
Hypoglossal Projections from M1
Terminal labeling in the hypoglossal nucleus was studied from five individual injection sites located in the ventral region of the M1 (SDM22-FD, SDM32-BDA, SDM32-FD, SDM35-LYD and SDM66-LYD) (Figs. 1, 4A–D; Tables 1, 4). All face/head representations were localized using intracortical microstimulation with the exception of SDM35-LYD which was estimated using anatomical landmarks. Based upon the results from all 5 cases, this projection was by far the largest in terms of estimated total bouton number (Table 4). This overall projection was characterized as bilateral with a contralateral predominance with the exception of SDM32-FD, which demonstrated a slight ipsilateral predominance (Fig. 6A). In all M1 cases, heavy labeling occupied extensive regions throughout the nucleus at all rostral to caudal levels (Figs. 4B, 4D, 7).
Table 4.
Hypoglossal Nucleus Bouton Estimation of the Lateral Cortical Injection Cases
| Case | Area Injected | Tracer Injected | Total Contralateral Boutons | Total Ipsilateral Boutons |
|---|---|---|---|---|
| SDM22 | M1 Face/dorsal | FD | 30,189 | 29,416 |
| SDM32 | M1 Face/dorsal | BDA | 21,238 | 16,983 |
| SDM32 | M1 Face/ventral | FD | 24,399 | 39,056 |
| SDM35 | M1 Face-dorsal/M1Arm/LPMCv | LYD | 61,911 | 52,188 |
| SDM66 | M1 Face/ventral | LYD | 120,701 | 116,987 |
| SDM22 | LPMCv Face | BDA | 11,035 | 8,657 |
| SDM57 | LPMCv Arm/Face | BDA | 1,131 | 393 |
| SDM57 | LPMCd Arm | FD | 2,458 | 1,967 |
| SDM23 | LPMCd Arm | FD | 1,052 | 382 |
| SDM40 | ProM | BDA | 2,596 | 3,685 |
| SDM66 | Insula – rostral | BDA | 231 | 306 |
| SDM66 | S1 Face | FR | 157 | 31 |
Figure 6.
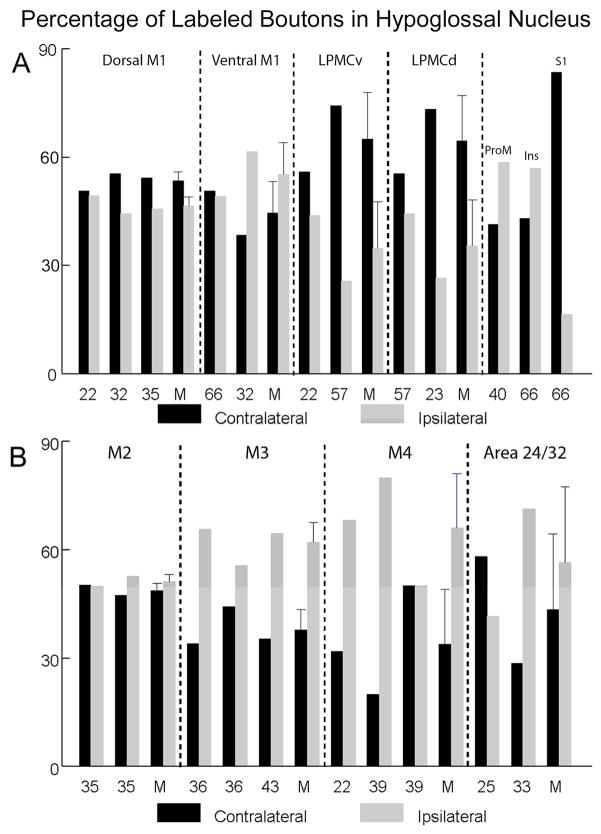
Estimated percentage of labeled boutons in the ipsilateral and contralateral hypoglossal nucleus following injections of high-resolution anterograde dextran tract tracers in lateral (A, top) and medial (B, bottom) cortical regions. Each individual SDM monkey case is identified on the abscissa as well as the mean (M) for each major cortical region (separated by the vertical dashed lines) when more than one animal case was available for study. The error bars on the mean for each major cortical projection represent standard errors when more than one case was available for study.
Figure 7.
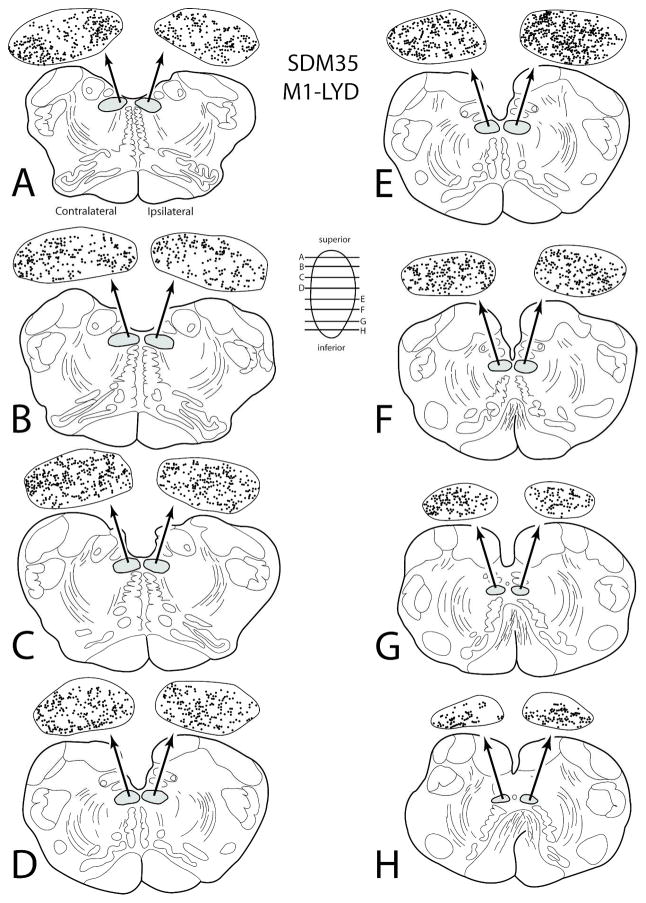
Line drawings of horizontal sections through rostral to caudal (A–H) levels of the medulla in case SDM35 showing the topographical distribution of labeled terminal boutons in the hypoglossal nucleus (see black dots on HN pullout) following an injection of LYD in the orofacial representation of M1 (Case SDM35-LYD). The presence of labeled fibers (without boutons) are not illustrated in the hypoglossal nucleus pullouts. The contralateral and ipsilateral sides indicated in panel A apply to all horizontal sections. Note the widespread and heavy bilateral terminal labeling in the HN at all horizontal levels.
The injection site in case SDM22-FD was located in the dorsal region of the M1 face representation (Fig. 1). Stereological analysis demonstrated that an estimated 30,189 labeled boutons were located in the contralateral nucleus, whereas 29,416 boutons were estimated in the ipsilateral nucleus (Table 4). The injection site in case SDM32-BDA was also located in the dorsal part of the face M1 area (Figs. 1, 4A) producing an estimated 21,238 boutons in the contralateral nucleus and 16,983 in the ipsilateral nucleus (Fig. 4B; Table 4). In the same experimental animal (SDM32) FD was injected ventral to the BDA injection site (Figs. 1, 4A) to examine potential differences in the hypoglossal projection from the dorsal versus ventral regions of M1. Our analysis demonstrated that the ventral injection site (FD) produced a greater number of hypoglossal terminals than the dorsal site (BDA) (Table 4). For example, in case SDM32-FD, 24,399 boutons were found in the contralateral nucleus and 39,056 in the ipsilateral nucleus. Thus, there was a shift in laterality showing that the dorsal region of the orofacial representation (SDM32-BDA) gave rise to a slightly more contralateral projection with the ventral region (SDM32-FD) issuing a slightly more ipsilateral projection (Fig. 6A).
In the next series of experiments, the results from 2 large injection sites placed into M1 that gave rise to a heavy corticohypoglossal projection were compared. In the first experiment, a large injection of LYD (SDM35-LYD) was placed dorsally in the M1 face area, but this injection site spread to involve the adjacent region of LPMCv (as determined cytoarchitectonically) as well as the adjacent M1 arm area (as determined by a robust corticospinal projection to cervical spinal levels) (Figs. 1,7; Tables 1, 4). In the second experiment, a large injection of LYD (SDM66-LYD) was placed into the ventral region of the face/head region of M1 (Figs. 1, 4B; Tables 1, 4). As found with case SDM32, which examined potential differences in the dorsal M1 and ventral M1 hypoglossal projection, the results from this set of experiments also demonstrated that the ventral region gives rise to a more robust corticohypoglossal projection than the dorsal region. For instance, in case SDM35-LYD (dorsal injection site), approximately 61,910 boutons were estimated in the contralateral nucleus with 52,188 in the ipsilateral nucleus. However, in case SDM66-LYD (ventral injection site), 120,701 boutons were found within the contralateral nucleus and 116,987 within the ipsilateral nucleus. Thus in terms of laterality, the dorsal injection was slightly more contralateral as demonstrated in the SDM32-BDA experiment summarized in the previous paragraph, whereas the large ventral injection site in SDM66 was bilateral compared to the ipsilateral predominance in the ventral SDM32-FD injection site (Fig. 6A).
Hypoglossal Projections from LPMCv and LPMCd
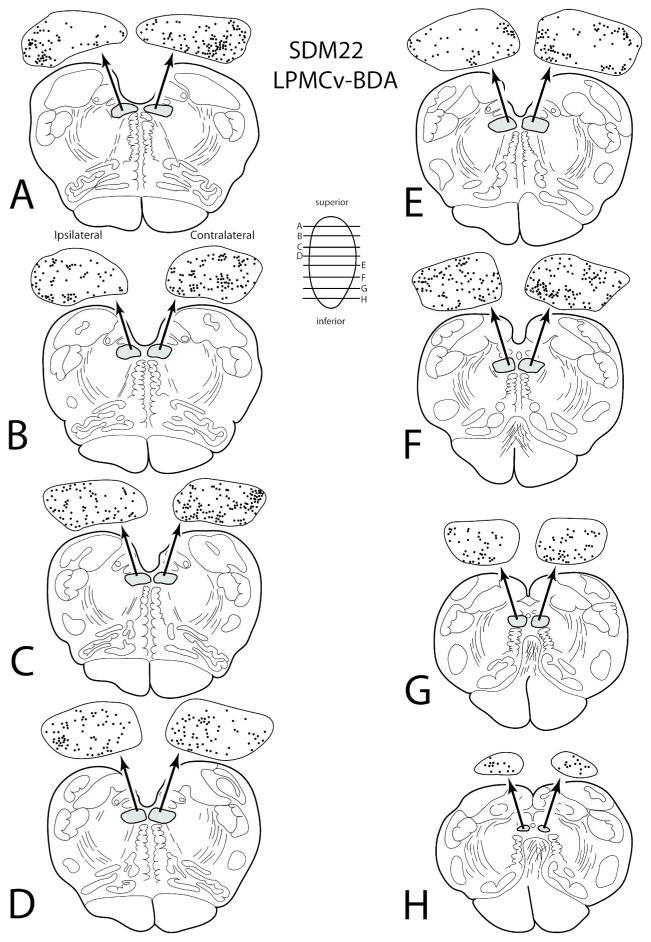
Terminal labeling was found in the hypoglossal nucleus from two LPMCv injection sites (SDM22-BDA and SDM57-BDA) (Fig. 1; Tables 1, 4). The corticohypoglossal projection in both cases was characterized as bilateral with a contralateral predominance (Fig. 6A). This projection was also considerably stronger from the ventral region of LPMCv. For example, an estimated 11,035 boutons were found in the contralateral hypoglossal nucleus and an estimated 8,657 boutons were found within the ipsilateral hypoglossal nucleus of case SDM22-BDA in which the injection site was located ventrally in LPMCv (Figs. 1, 8). In experiment SDM57-BDA which had an injection in the dorsal region of LPMCv, an estimated 1,131 boutons were found in the contralateral hypoglossal nucleus with 393 boutons in the ipsilateral nucleus. In terms of topography, labeling appeared to be diffusely distributed from rostral to caudal levels of the hypoglossal nucleus (Fig. 8).
Figure 8.
Line drawings of horizontal sections through rostral to caudal (A–H) levels of the medulla in case SDM22 showing the topographical distribution of labeled terminal boutons in the hypoglossal nucleus (see black dots on HN pullout) following an injection of BDA in the orofacial representation of LPMCv (Case SDM22-BDA). The presence of labeled fibers (without boutons) are not illustrated in the hypoglossal nucleus pullouts. The contralateral and ipsilateral sides indicated in panel A apply to all horizontal sections. Note the significant bilateral terminal labeling throughout the HN.
In addition to the predicted projection from the ventral lateral premotor cortex, an unanticipated projection to the hypoglossal nucleus was found following injections for anterograde tracer placed into the dorsal part of the lateral premotor cortex (LPMCd) (Fig. 1). Specifically, in two experiments, intracortical microstimulation was used to localize LPMCd. In case SDM 57-LYD, shoulder and elbow movements were observed and the injection was placed within these sites. In case SDM23-FD, the injection was placed in a region characterized electrophysiologically by upper extremity and tongue movements (Morecraft et al., 2001, see Fig. 6, SDM23). In both cases, the projection was characterized as bilateral with a contralateral predominance (Fig. 6A). In injection case SDM57-FD, which was located dorsal to the arcuate spur (Fig. 1), 2,458 boutons were estimated in the contralateral hypoglossal nucleus with 1,967 boutons estimated in the ipsilateral nucleus. A weaker corticohypoglossal projection was found in SDM23-FD which was located in the same general region of LPMCd but involved a smaller portion of this cortex (compare the injection sites in SDM23-FD and SDM57-FD in Fig. 1). In SDM23-FD 1,052 boutons were found contralaterally with 382 ipsilaterally.
Hypoglossal Projections from ProM
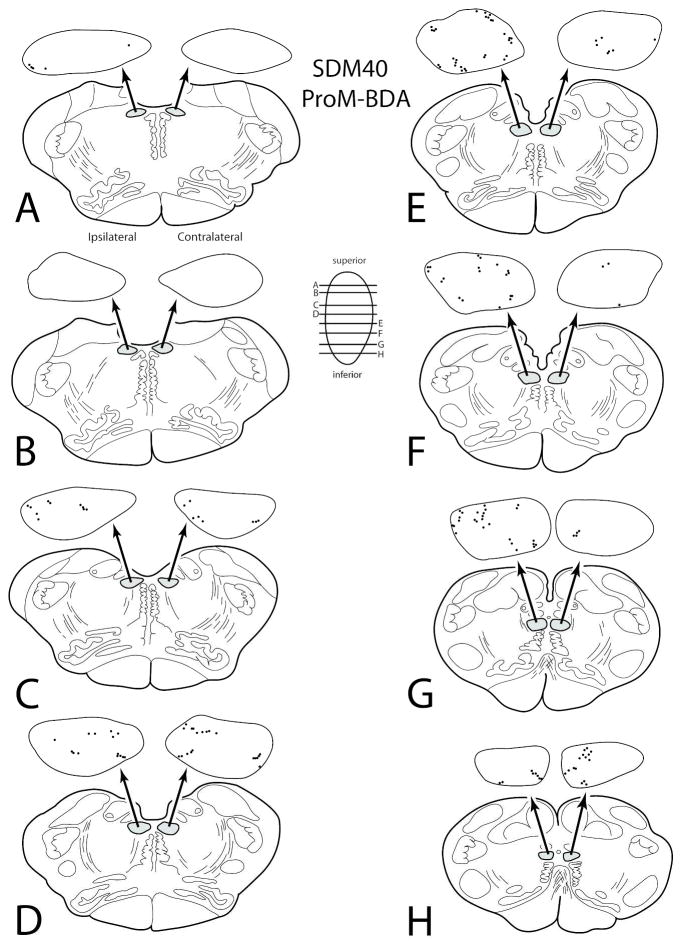
In the hypoglossal nucleus, light terminal labeling was found following a small injection of BDA into the rostral opercular portion of area ProM (SDM40-BDA) (Figs. 1, 9). This projection was characterized as bilateral with an ipsilateral predominance (Fig. 6A; Table 4). Although there was no distinguishable topography, labeling was light and diffuse with some boutons occasionally clustered near the periphery of the nucleus at several horizontal levels (Fig. 9). In this experiment, an estimated 2,596 boutons were found in the contralateral hypoglossal nucleus and an estimated 3,685 boutons were located within the ipsilateral hypoglossal nucleus.
Figure 9.
Line drawings of horizontal sections through rostral to caudal (A–H) levels of the medulla in case SDM40 showing the topographical distribution of labeled terminal boutons in the hypoglossal nucleus (see black dots on HN pullout) following an injection of BDA in the lateral opercular region corresponding to area ProM (Case SDM40-BDA). The presence of labeled fibers (without boutons) are not illustrated in the hypoglossal nucleus pullouts. The contralateral and ipsilateral sides indicated in panel A apply to all horizontal sections. Note the diffuse bilateral terminal labeling throughout the HN.
Hypoglossal Projections from the Insula
Terminal labeling in the hypoglossal nucleus was found from one small, localized injection site positioned in the anterior region of the insula (SDM66-BDA) (Fig. 1). Cytoarchitectonically, this region corresponded with area Idg, or the dysgranular part of the insula (of Mesulam and Mufson, 1982). This projection was characterized as bilateral, sparse and with a slight ipsilateral predominance (Fig. 6A; Table 4). There was no discernible topography as labeling was scattered in the medial and lateral dimension of the nucleus. Specifically, an estimated 231 boutons were found in the contralateral hypoglossal nucleus and an estimated 305 boutons were located within the ipsilateral hypoglossal nucleus.
Hypoglossal Projections from S1
Hypoglossal labeling was found from one localized injection site that was placed in the face/head representation of the primary somatosensory cortex (S1) which was identified using intracortical microstimulation (SDM66-FR) (Fig. 1). This injection site involved the gyral surface of S1 corresponding to areas 1/2 following cytoarchitectonic analysis of corresponding tissue sections containing the injection sites. The corticohypoglossal projection was characterized as bilateral with a slight contralateral predominance (Fig. 6A; Table 4). Overall, very few boutons were noted which could be related to the relatively small injection site (Fig. 1). For instance, an estimated 157 boutons were found within the contralateral hypoglossal nucleus and an estimated 31 boutons were found within the ipsilateral hypoglossal nucleus.
Hypoglossal Projections from the Medial Cortical Surface
Hypoglossal Projections from Supplementary Motor Cortex (M2)
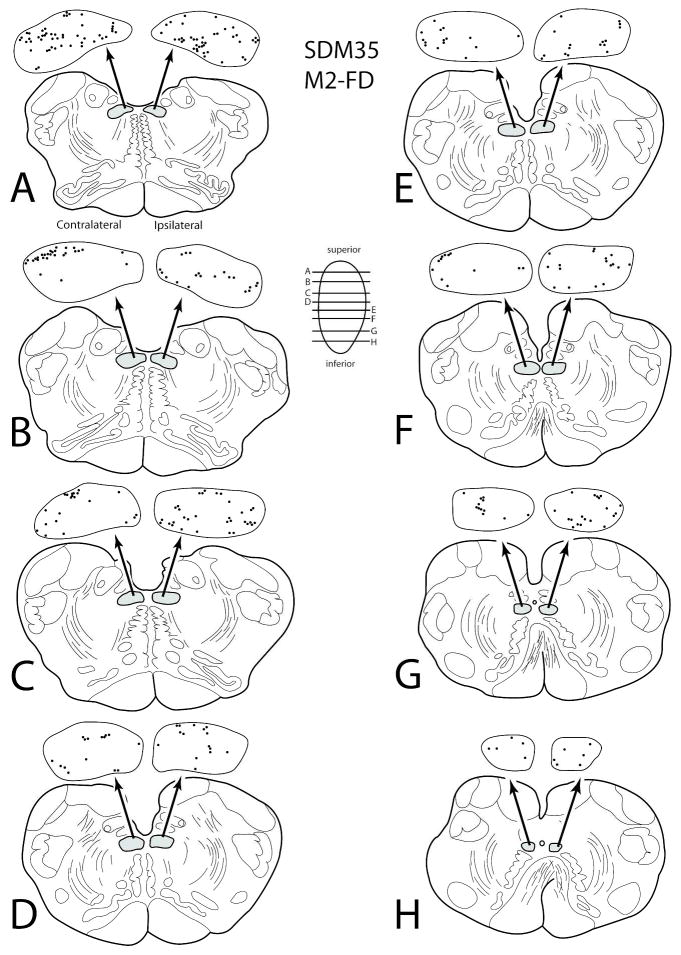
Terminal labeling was found in the hypoglossal nucleus from two injection sites placed on the medial wall of the superior frontal gyrus in one monkey (SDM35-FD and SDM35-BDA). Due to an extensive network of bridging veins arching over the interhemispheric sulcus (joining the superior sagittal sinus) found during neurosurgical exposure, and the presence of a large network of arterial vessels lining the medial cortical surface, intracortical microstimulation was not used to map this brain region. Therefore, the coronal level corresponding with the genu of the arcuate sulcus was used to estimate the center of the M2 arm representation (e.g., Macpherson et al., 1982; Tanji and Kurata, 1985; Mitz and Wise, 1987; Aizawa et al., 1991; Mushciake et al., 1991; Wang et al., 2001) and a relatively large FD injection was made 1–2 mm anterior to this plane (Figs. 2, top, 5A) which corresponds with the general location of the face representation and rostral part of the arm representation of M2 (Mitz and Wise, 1987). Immediately anterior to this injection site, a small injection of BDA was made (Fig. 2, top). Cytoarchitectonic analysis and injection site reconstruction revealed the FD injection involved the rostral half of area 6m and the BDA injection site involved the pre-SMA with potential involvement of the rostral most portion of area 6m.
In case SDM35-FD, labeled corticospinal axons and terminals in the cervical spinal cord verified the involvement of the M2 arm area. Similarly, a substantial corticohypoglossal projection from SDM35-FD verified concomitant injection site involvement of the face area of M2 (Fig. 5B (inset), 10). The M2 hypoglossal projection was bilateral and represented the most powerful hypoglossal projection found from the medial wall experiments (Figs. 6B, 10; Table 5). In terms of topography, labeling in case SDM35-FD was found scattered throughout the hypoglossal nucleus (Fig. 10). Quantitatively, an estimated 16,704 boutons were discovered within the contralateral hypoglossal nucleus and an estimated 16,602 boutons within the ipsilateral nucleus (Fig. 6; Table 5). In contrast, the projection from the preSMA/rostral M2 face region in case SDM35-BDA (Fig. 2) was characterized as being very weak, with a very slight ipsilateral predominance as an estimated 426 boutons found within the contralateral nucleus and an estimated 473 boutons were found within the ipsilateral hypoglossal nucleus (Fig. 6B; Table 5).
Figure 10.
Line drawings of horizontal sections through rostral to caudal (A–H) levels of the medulla in case SDM35 showing the topographical distribution of labeled terminal boutons in the hypoglossal nucleus (see black dots on HN pullout) following an injection of FD in the mid- to rostral region of M2 (Case SDM350-FD). The presence of labeled fibers (without boutons) are not illustrated in the hypoglossal nucleus pullouts. The contralateral and ipsilateral sides indicated in panel A apply to all horizontal sections. Note the diffuse bilateral terminal labeling throughout the HN
Table 5.
Hypoglossal Bouton Estimation of the Medial Cortical Injection Cases
| Case | Area Injected | Tracer Injected | Total Contralateral Boutons | Total Ipsilateral Boutons |
|---|---|---|---|---|
| SDM35 | M2 Arm/Face | FD | 16,704 | 16,602 |
| SDM35 | PreSMA/M2 Face | BDA | 426 | 473 |
| SDM36 | M3 Face | FD | 477 | 920 |
| SDM36 | M3 Face/Arm | BDA | 920 | 1,158 |
| SDM43 | M3 Face | FD | 1,766 | 3,230 |
| SDM22 | M4 Face | LYD | 104 | 223 |
| SDM39 | M4 Face | FD | 88 | 351 |
| SDM39 | M3 Arm/M4 Face | BDA | 44 | 44 |
| SDM25 | Area 24/32 | FR | 758 | 542 |
| SDM33 | Area 24/32 | FD | 72 | 179 |
Hypoglossal Projections from Rostral Cingulate Motor Cortex (M3)
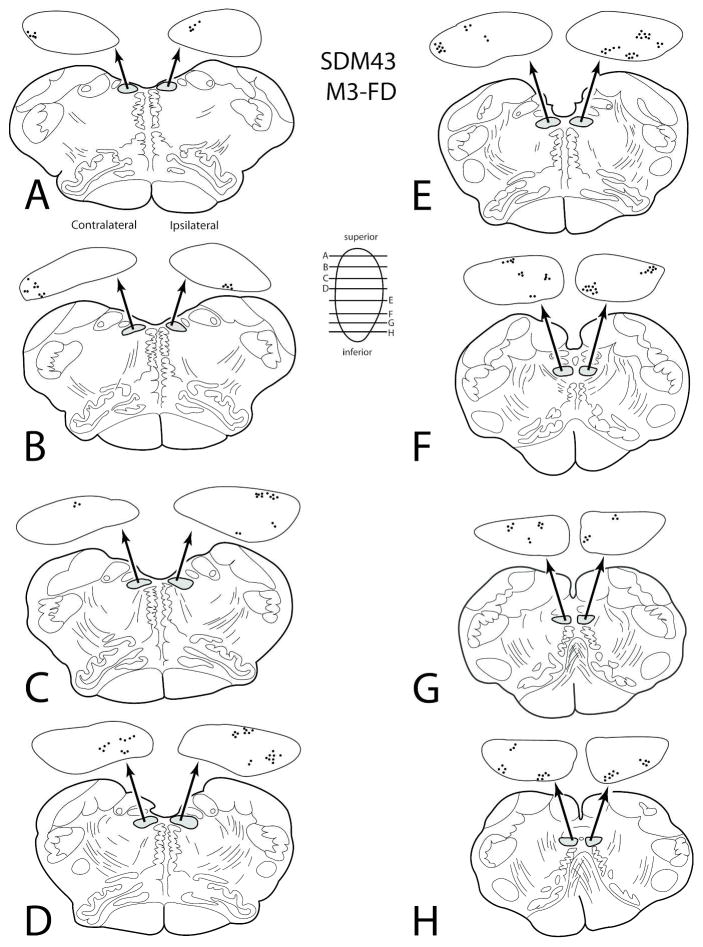
Terminal labeling was found in the hypoglossal nucleus from three injection sites located in the rostral region of M3 (SDM36-BDA; SDM36-FD and SDM43-FD) (Figs. 2, 5B). The corticohypoglossal projection was characterized as bilateral, with all three cases demonstrating an ipsilateral predominance (Fig. 6B). Overall, this projection was the second strongest of all medial wall experiments (Table 5). For example, in case SDM36-FD which was located in coronal levels over the genu of the corpus callosum (Fig. 2), an estimated 477 boutons were found within the contralateral nucleus and an estimated 920 boutons were found within the ipsilateral hypoglossal nucleus. In case SDM36-BDA in which the injection site was located caudal to the SDM36-FD injection site with some overlap (Fig. 2), 920 boutons were found within the contralateral nucleus and 1,158 boutons were estimated in the ipsilateral hypoglossal nucleus. In case SDM43-FD the injection site was located in a similar location over the genu of the corpus callosum as injection site SDM36-FD, but extended more rostral that the SDM36-BDA injection (Figs. 2, 5B). In this experiment, an estimated 1,766 boutons were found within the contralateral nucleus and an estimated 3,230 boutons were localized within the ipsilateral hypoglossal nucleus (Figs. 5B (inset), 11; Table 5).
Figure 11.
Line drawings of horizontal sections through rostral to caudal (A–H) levels of the medulla in case SDM43 showing the topographical distribution of labeled terminal boutons in the hypoglossal nucleus (see black dots on HN pullout) following an injection of FD in the rostral region of M3 (Case SDM43-FD). The presence of labeled fibers (without boutons) are not illustrated in the hypoglossal nucleus pullouts. The contralateral and ipsilateral sides indicated in panel A apply to all horizontal sections.
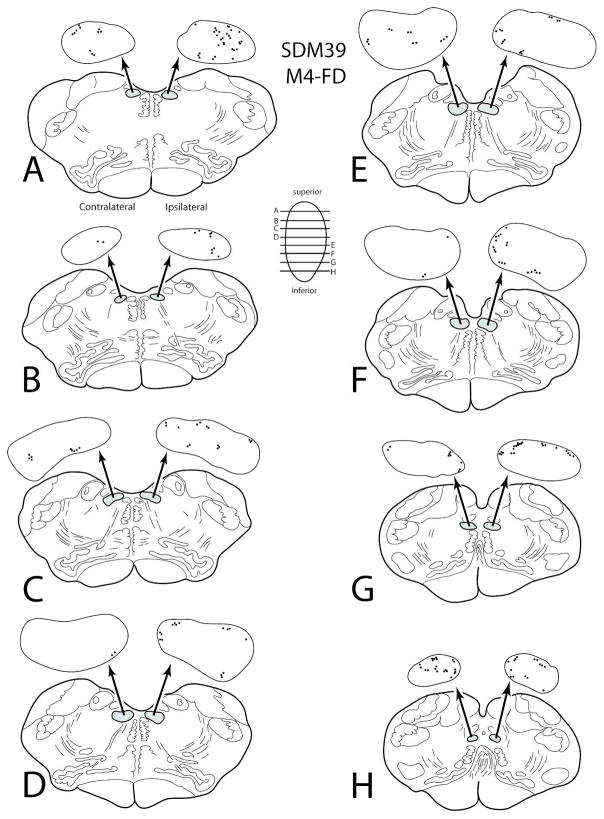
Hypoglossal Projections from Caudal Cingulate Motor Cortex (M4)
Hypoglossal labeling was found from two injection sites located in the rostral region (face/head area) of M4 (SDM22-LYD and SDM39-FD) and one injection site that involved the caudal/middle region of M3 (the arm area) with minor overlap with the rostral part of M4 (SDM39-BDA) (Figs. 2, 5C). This corticohypoglossal projection was characterized as bilateral with ipsilateral predominance in 2 of the 3 experiments and a bilateral projection in one case (Figs. 6B, 12; Table 5). Overall, labeling was very light and scattered throughout the hypoglossal nucleus (Figs. 5C (inset), 12; Table 5). In case SDM22-LYD, an estimated 104 boutons were found in the contralateral hypoglossal nucleus and 223 in the ipsilateral nucleus. The injection site in case SDM39-FD (Figs. 2, 5C) was located in a nearly identical location as in case SDM22-LYD (coronal levels containing the anterior commissure - see Fig. 2) and produced a very similarly sized projection. For example, 88 boutons were found in the contralateral hypoglossal nucleus and 351 boutons were estimated in the ipsilateral nucleus (Fig. 5C (inset, 11; Table 5). Finally, the injection site in case SDM39-BDA (Figs. 2, 5C) gave rise to very few labeled hypoglossal boutons (44 contralaterally and 44 ipsilaterally) but a substantial number of labeled axons and terminals in cervical levels of the spinal cord demonstrating primary injection site involvement of the rostrally positioned M3 arm representation.
Figure 12.
Line drawings of horizontal sections through rostral to caudal (A–H) levels of the medulla in case SDM39 showing the topographical distribution of labeled terminal boutons in the hypoglossal nucleus (see black dots on HN pullout) following an injection of FD in the rostral region of M4 (Case SDM39-FD). The presence of labeled fibers (without boutons) are not illustrated in the hypoglossal nucleus pullouts. The contralateral and ipsilateral sides indicated in panel A apply to all horizontal sections.
Hypoglossal Projections from Perigenual Cingulate Cortex
Terminal labeling in the hypoglossal nucleus was found from two injection sites located in the perigenual cingulate cortex (areas 24/32) (SDM25-FR and SDM33-FD) (Figs. 2, 5D, 13). In both cases this projection was characterized as bilateral (Fig. 6B). The injection site in case SDM25-FR was located anterior to the corpus callosum and gave rise to an estimated 758 boutons in the contralateral hypoglossal nucleus and 542 boutons in the ipsilateral nucleus (Table 5). The injection site in case SDM33-FD was located anterior to the corpus callosum genu but more ventral than the SDM25-FR injection. This injection site labeled an estimated 72 boutons in the contralateral hypoglossal nucleus and 179 in the ipsilateral hypoglossal nucleus. Thus, the more dorsally located injection site demonstrated a slight ipsilateral predominance whereas the ventral injection site in SDM25 was more contralateral (Fig. 6B).
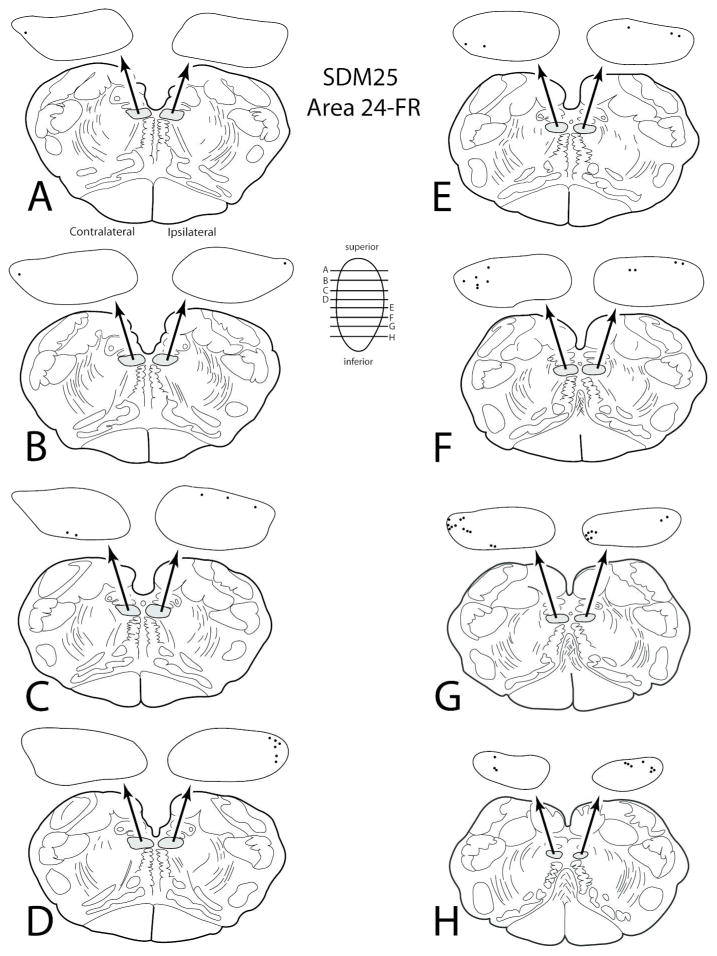
Figure 13.
Line drawings of horizontal sections through rostral to caudal (A–H) levels of the medulla in case SDM25 showing the topographical distribution of labeled terminal boutons in the hypoglossal nucleus (see black dots on HN pullout) following an injection of FR in the pregenual region of anterior cingulate gyrus (Case SDM25-FR). The presence of labeled fibers (without boutons) are not illustrated in the hypoglossal nucleus pullouts. The contralateral and ipsilateral sides indicated in panel A apply to all horizontal sections. Note the consistant labeling in the dorsolateral region of the hypoglossal nucleus.
Cortical Areas Associated with Little to No Hypoglossal Projections
Frontal Lobe Cases
Several additional frontal lobe cases with injections placed in M1, M2 and the preSMA were evaluated for potential corticohypoglossal projections (Fig. 3). In cases SDM57-LYD and SDM61-LYD, in which the injection site was located in the electrophysiologically defined arm area of M1, (see Morecraft et al., 2013a, Fig. 1) an extremely sparse number of labeled terminals were found in the hypoglossal nucleus. In contrast, both injection cases produced heavy corticospinal terminal labeling throughout cervical levels of the contralateral spinal cord (see Morecraft et al., 2013a; Table 2). In case SDM84-DA488, which had an injection of DA488 in the electrophysiologically defined hind limb region of M1 (Fig. 3), no labeled terminals were found in the hypoglossal nucleus but abundantly labeled fibers were found in the corticospinal tract of the cervical spinal cord. Much like the M1 arm cases described above, hypoglossal terminals were rarely found in the three M2 cases that had tract tracer injections placed into the physiologically defined arm representation of M2 (SDM54-FD, SDM77FD, SDM68-BDA) (Fig. 3). For example, in case SDM68-BDA 4 fibers were found and only 3 with boutons. Finally, in case SDM27-BDA, which had an injection of BDA placed in the preSMA (Fig. 3, bottom), no terminals were found in the hypoglossal nucleus. However, tracer transport to the lower medulla was evident as a few labeled fibers and terminals were located in the cell sparse region between the hypoglossal nucleus and dorsal motor nucleus of the vagus and a few BDA labeled fibers without boutons were found in the hypoglossal nucleus (possibly en passant).
Cingulate Cortex Cases
Six additional injection sites placed into the cingulate cortex were evaluated for potential corticohypoglossal projections. In case SDM68-FD the injection site was placed in the cortex lining the lower bank of the cingulate sulcus, in a location corresponding to the arm region of M3 (Fig. 3, bottom). In the same monkey, an injection of LYD (case SDM68-LYD) was also placed into cortex lining the lower bank of the cingulate sulcus but more posterior, in a region corresponding to the arm region of M4 (Fig. 3, bottom). In both cases very few labeled terminals were found in the hypoglossal nucleus which was in contrast to the extensive corticospinal labeling found at cervical levels of the spinal cord indicating that the FD injection site involved primarily the arm region of M3 and the LYD injection the arm region of M4. In case SDM31-FD the injection site was placed on the anterior gryal convexity, in a location directly over the genu of the corpus callosum (Fig. 3, bottom). Cytoarchitectonically, this injection site involved both areas 24a and 24b. No hypoglossal projection was found but effective tracer transport to the lower medullary levels was noted by the presence of labeled terminals in gray matter that was located adjacent to the hypoglossal nucleus (i.e., the intercalated nuclear region). In the same monkey an injection of BDA was placed in the ventral part of area 32 (Fig. 3, bottom), just above the rostral sulcus (case SDM31-BDA) which did not give rise to label terminals in the hypoglossal nucleus. Finally, 2 cingulate gyrus injections were evaluated for probable hypoglossal labeling in monkey SDM27 (Fig. 3, bottom). The FR injection site was located on the medial surface, directly over the anterior region of the corpus callosum genu (SDM27-FR) and within area 24a. The second, FD injection site was located below the genu involving area 24b as well as area 25. In both cases, terminal labeling in the hypoglossal nucleus did not occur but effective transport of tracer was found in the brainstem.
Parietal Lobe Cases
In one monkey, two adjacent tracer injections were placed in the electrophysiologically identified arm area of S1 (Fig. 3). Specifically, case SDM75-BDA involved cortex lining the caudal bank of the central sulcus (areas 3 and 1) and injection site case SDM75-LYD involved the adjacent parietal region located on the gyral surface (areas 1 and 2). A third injection, case SDM77-FD was made caudal to the LYD injection and involved the rostral part of area 5 (or area PE) (Fig. 3, middle). No BDA labeling was found in the hypoglossal nucleus, but several LYD and FD labeled fibers were noted of which one single FD fiber had a very small cluster of labeled boutons.
Insula Case
One very small injection was placed in the ventral region of the anterior insula, corresponding to the ventral part of the dysgranular sector of the insula (area Idg (of Mesulam and Mufson, 1982) (Fig. 3, middle). No hypoglossal labeling was detected in this experimental case.
DISCUSSION
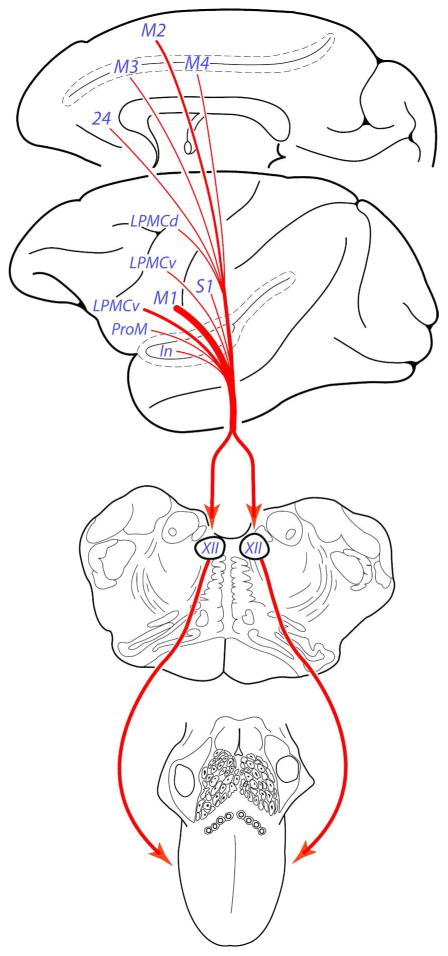
Dating back to the seminal works of Hans Kuypers on descending cortical projections to the lower brainstem (Kuypers 1956, 1958a, 1958b, 1958c), it was recognized that the hypoglossal nucleus receives a direct, and massive cortical projection from the ventral region of the precentral cortex in the human and non-human primate brain. Our findings reinforce this classical discovery in the monkey model and show for the first time that the corticohypoglossal projection is not unique to the ventral precentral area, as it arises in much lesser intensity, from many parts of the cortical mantle (Fig. 14). Although not as prominent as the M1 projection, we found a fairly robust corticohypoglossal projection from the ventral part of LPMCv and rostral part of M2. A comparatively weaker, but potentially influential projection arose from area ProM, the periarcuate genu region of LPMCv and LPMCd, rostral part of M3 and rostral part of the M4, including an unsuspected projection from the pregenual region of the anterior cingulate gyrus. A small projection was also found to the hypoglossal nucleus from the face/head area of the primary somatosensory cortex and rostral region of the insula. From the perspective of corticomotor somatotopy, the sizable hypoglossal projection from the ventral part of M1 and LPMCv, as well as notable projection from rostral parts of the medial motor cortices (M2, M3 and M4) strengthen the proposed concept of five orofacial motor representations located in the frontal, and cingulate motor cortices in the non-human primate brain (Morecraft et al., 2001, 2004). Our experimental findings additionally show that the cortical orofacial representations are positioned ventral (for lateral motor areas) and anterior (for medial motor areas) to adjacent arm representations. Finally, our experiments indicate that cortex thought to predominately regulate arm movements in the dorsolateral premotor region (LPMCd), may to some degree, influence hypoglossal motor mechanisms.
Figure 14.
Summary diagram illustrating the main findings of the present study. We localized bilateral projections to the hypoglossal nucleus from 11 different regions of the cortical mantle involving frontal, cingulate, parietal, and insular territories of the telencephalon. Given the highly diverse, and specialized functions regulated by these cortical regions, our findings suggest that hypoglossal mechanisms are likely to be influenced by motor and sensory related parts of the cerebral cortex, as well as multimodal association and limbic related regions of the cortex. The bilateral nature and widespread origin of these projections suggests cortical input to the non-human primate hypoglossal nucleus is highly protected, and the redundancy of this connection may underlie favorable recovery of tongue movement following localized supratentorial brain injury.
Corticohypoglossal Projections from the Primary Motor Cortex (M1)
The corticohypoglossal projection from the ventral region of M1 was by far, the most intense corticofugal projection found in our study (Figs. 4B, 4D, 7; Table 4). The finding of a powerful and bilateral projection from the ventral region of M1 in the monkey is consistent with previous studies (Kuypers, 1958a; Jürgens and Alipour, 2002). In terms of laterality, we found a slightly predominant contralateral projection in 4 of the 5 experiments conducted (Fig. 6A; Table 4) which is in agreement with Kuypers observations in monkey and chimpanzee (Kuypers, 1958a). These observations correlate with human TMS studies showing that the corticohypoglossal projection from M1 is bilateral (Urban et al., 1994, 1996; Meyer et al., 1997; Ghezzi and Baldini, 1998; Muellbacher et al., 1998, 1999, 2001; Rödel et al., 2003; Bawa et al., 2004) with more contralateral influence in most individuals, characterized physiologically by lower motor thresholds and larger mean evoked motor potentials (Ghezzi and Baldini, 1988; Muellbacher et al., 1998, 1999, 2001; Rödel et al., 2003; Bawa et al., 2004). Our findings of a slight contralateral predominance in most experiments may correlate with recent findings that a small angle of tongue deviation occurs in the majority of healthy individuals (Wei et al., 2012), although hemispheric dominance, handedness and direction of tongue deviation in healthy controls were not considered.
Our series of neurological experiments were in part, designed to gain insight into possible connectional differences in the projection from dorsal versus ventral regions of the M1 orofacial representation. We found the corticohypoglossal projection is much stronger from the ventral part of the M1 orofacial representation than dorsal part. For example, in case SDM 32, an equal amount of a compatible tract tracer was injected in a single location in the dorsal (SDM32-BDA) and ventral sectors (SDM32-FD) of M1 (Figs. 1, 4A,B). The results demonstrated a greater number of estimated boutons from the ventral site, both on the ipsilateral and contralateral side of termination (Table 4). Likewise, large injection sites of LYD were made dorsally in case SDM35, and ventrally in case SDM66, with the latter being slightly less in tracer volume (Fig. 1; Table 1). Both injection sites gave rise to the highest number of estimated boutons found in all cases with the ventral injection site (SDM66-LYD) giving rise to double the number of ipsilateral and contralateral boutons compared to the dorsal injection site (SDM35-LYD), despite more tracer injected into SDM35. This finding would correlate with reports in monkeys showing that electrophysiologically evoked facial movements occur more commonly in the dorsal M1 orofacial region, with tongue responses occurring more frequently ventrally (McGuinness et al., 1980; Huang et al., 1988; Murray and Sessle, 1992; Hatanaka et al., 2005). This finding closely corresponds to human TMS work showing overlap of M1 face and tongue movements with the primary lingual area located laterally (Rödel et al., 2003).
Another interesting feature of the M1 projection is the relatively heavy labeling found in many regions of the nucleus (i.e., medial, lateral, dorsal and ventral) (Figs. 4B, 4D, 7) which is in contrast to the ventral M1 projection to the facial nucleus, which is topographically specific. For instance, in the monkey the M1 projection dominantly innervates the ventrolateral region of the contralateral facial nucleus (Kuypers, 1958a; Jenny and Saper, 1987; Morecraft et al., 2001) which contains motor neurons supplying the lower facial muscles (e.g., Jenny and Saper, 1987; Morecraft et al., 2001). Thus, injury to ventral M1 in non-human primates produces lower facial paralysis, with sparing of the upper facial muscles (Green and Walker, 1938; Symon et al., 1975; Larson et al., 1980), a condition that characteristically occurs following unilateral MCA infarction in stroke patients (Afifi and Bergman, 1980; Brodal, 1981; Adams et al., 1997). Given this example, the widespread nature of M1 labeled terminals occurring within the hypoglossal nucleus (Figs. 4B, 4D, 7) does not readily foster predictions regarding which tongue muscles may be more adversely affected following ventral M1 damage. However, Sokoloff and Deacon (1992) have shown in Macaca fascicularis that the hypoglossal nucleus is musculotopically organized. Thus, future studies should be pursued to determine if terminal bouton density varies within these musculotopic subsectors and relates to preferential M1 innervation of some intrinsic and extrinsic tongue muscles.
In our efforts to further localize the corticohypoglossal projection from M1, we examined potential hypoglossal nucleus labeling from injection sites located in the arm and leg representations of M1 (Fig. 3). We were surprised to find evidence of very few labeled corticohypoglossal terminals following injections into the electrophysiologically defined arm area of M1. A finding which is in extreme contrast to the estimated 218,847 corticospinal terminal boutons located in the cervical enlargement (C5-T1) estimated for the SDM61 injection site (Morecraft et al., 2013a -SDM61-LYD, Table 2). The virtual absence of a projection to the hypoglossal nucleus from the arm area of M1 in our material correlates well with Kuypers (1958a) and Leichnitz (1986) observations who found no evidence of a corticohypoglossal projection from the arm region using older tract tracing methodology. Similarly, in one M1 hind limb injection case (Fig. 3, top), we were unable to find terminal labeling in the hypoglossal nucleus in sound agreement with Kuypers (1958a). Overall, these findings provide strong connectional support for the general view of a somatotopically organized M1 simiusculus in monkey (Woolsey et al., 1952) and by association, an ordered homunculus in the human primary motor cortex (Penfield and Boldery 1937; Penfield and Welch, 1951; Woolsey, et al., 1979). However, our findings of an extremely sparse corticohypoglossal projection from the arm/hand region of M1 may have scientific value in predicting that a few labeled cells may occur in the arm/hand representation area of M1 following injections of retrograde transneuronal viral tracer into the non-human primate tongue, or following injection of retrograde tract tracer into the hypoglossal nucleus.
Corticohypoglossal Projections from the Lateral Premotor Cortex and ProM
We localized a significant corticohypoglossal projection from the ventral region of the lateral premotor cortex (area 6Vb) which accounted for the second strongest corticobulbar projection in our investigation (Table 4). Several previous studies have examined the possibility of a corticohypoglossal projection from the ventrolateral premotor region in the monkey (Kuypers, 1958a, Künzle, 1978; Simonyan and Jürgens, 2003; Borra et al., 2010). Among these, Kuypers (1958a) report was the only investigation to indicate that cortex corresponding to ventral area 6 (area FBA and area FCBm) may project to the hypoglossal nucleus as he inconclusively stated that this cortical region “does not contribute substantially to the projections” innervating the hypoglossal nucleus when compared to the ventral area 4 (M1). With respect to the report of Simonyan and Jürgens, their BDA injection sites were placed into an electrophysiologically defined laryngeal region of area 6Vb (Simonyan and Jürgens, 2003, see their Fig. 1). As a result, they found heavy terminal labeling in the reticular formation of the lower medulla indicating an adequate post-injection survival interval for BDA transport to the lower brainstem, but found no terminal labeling in the adjacent hypoglossal nucleus. Several explanations may account for this incongruity. First, the injection site in the Simonyan and Jürgens work was very close in proximity to the area 47L cytoarchitectonic border of the prefrontal cortex, thus being located slightly anterior and inferior to our ventral LPMCv injection site (SDM22-BDA) (see their Fig. 1 compared to our Fig. 1). Likewise, our ProM injection site (SDM40-BDA) was located on the lateral opercular convexity of the frontal cortex (Figs. 1, 4G), slightly more ventral than the 3 injection sites analyzed by Simonyan and Jürgens. In Künzle’s comprehensive study (1978), the lack of terminal hypoglossal labeling from his ventrolateral premotor injection cases may be due to technical/methodological limitations. For instance, Künzle used the autoradiographic tract tracing procedure which is less sensitive than the dextran tract tracing method and the short post-injection survival period (2–5 days) may not have been long enough for sufficient long distance axonal transport of the injected amino acids. Lastly, Borra and colleagues (2010) investigated brainstem projections from the dorsal part of area 6Va (area F5 according to their nomenclature) and did not report a hypoglossal projection but injection site location may account for this discrepancy. Our injection site (SDM57-BDA, Fig. 1) was located on the gyral convexity of dorsal area 6Va (Fig. 1), whereas the 3 injection cases studied by Borra and co-workers were located in cortex lining the posterior bank of the arcuate sulcus, directly below the arcuate spur. From this, it can be deduced that the corticohypoglossal projection arises from cortex on the gyral surface of dorsal area 6Va and not from cortex lining the adjacent arcuate sulcus region. Our observation is reinforced by intracortical microstimulation work demonstrating that orofacial movements are evoked from this cortical location (Gentilucci et al., 1988; Godschalk et al., 1995; Graziano et al., 2002). The finding of corticohypoglossal projection neurons in this area is also supported by the observation that the dorsal region of area 6Va may be involved with tongue deviation and protrusion that accompanies the action of bringing a grasped hand to the mouth (Graziano et al., 2002). However, it is likely that the long duration stimulus trains used to evoke these movements would evoke temporal summation in multiple frontal cortical regions that receive cortical projections from LPMCv and also innervate the hypoglossal nucleus, including ventral M1.
We also encountered an unanticipated corticohypoglossal projection from lateral premotor cortex located just dorsal to the genu/spur region of the arcuate sulcus (SDM23-FD and SDM57-FD; Fig. 1; Table 4). Traditionally, this frontal region is recognized for its involvement in the motor preparation and execution of voluntary upper extremity movements (e.g., Davare et al., 2006; Hoshi and Tanji, 2007). Interestingly this cortex has been shown to issue corticofugal projections to the spinal cord (Martino and Strick, 1987; Dum and Strick, 1991; He et al., 1993) as well as the facial nucleus (see Morecraft et al., 2001, SDM23-FD, Fig 12). Taken jointly, these structural observations show that the dorsal periarcuate region contains either a mixture of corticospinal and corticobulbar projection neurons, neurons that give rise to collateral projections to multiple motor targets in the brainstem and spinal cord, or a combination of these potential neuronal phenotypes. Understanding the functional significance of these orofacial projection neurons in this physiologically characterized arm-related cortex in the non-human primate would appear to be of considerable interest as they may be spared following ventrolateral brain injury and play a role in the recovery of tongue and facial movements.
Corticohypoglossal Projections from the Supplementary Motor Cortex
In the early 1950’s, a somatotopically organized supplementary motor cortex, anchored by a face/head representation rostrally, was described in the human (Penfield and Welch, 1951) and monkey (Woolsey et al., 1952) brain. Yet the available neuroanatomical literature concerned with studying the brainstem projections from the supplementary motor cortex did not reveal a corticohypoglossal projection (DeVito and Smith, 1959; Künzle, 1978; Jürgens, 1984; Wiesendanger and Wiesendanger, 1984) which is in contrast to the bilateral projection found in our study (Figs., 5B, 10; Table 5). This difference is likely due to the less sensitive tract tracing methods available at the time these previous investigations were carried out. In terms of pinpointing the origin of this projection, our comparative analysis of corticofugal projections from multiple injection sites allowed us to localize the corticohypoglossal projection to the rostral (face/head) region of M2. For example, the injection site in the pre-SMA (SDM27-BDA), did not give rise to hypoglossal afferents and the injection site in the physiologically defined arm region of M2 gave rise to very few hypoglossal projections and numerous corticospinal labeled terminals (e.g., see McNeal et al., 2010, Table 2, SDM54, SDM77). In contrast, a considerable portion of the SDM35-FD injection site was located anterior to the coronal plane through the anterior commissure (Fig. 2), and gave rise to numerous hypoglossal labeled terminals as well as corticospinal labeling, indicating involvement of both the rostral part of the M2 arm representation and its adjacent orofacial representation. Furthermore, the injection in case SDM35-BDA was positioned immediately anterior to the SDM35-FD injection (Fig. 2) and produced very few hypoglossal labeled terminals (Table 5). Collectively, this set of experiments provide strong support for a somatotopically organized supplementary motor cortex with face/head representation being positioned rostral to the arm representation (Woolsey et al., 1952; Mitz and Wise, 1987; Godschalk et al., 1995; Luppino et al., 1991).
Corticohypoglossal Projections from the Cingulate Cortex
This is the first study to investigate the corticohypoglossal projection from the anterior cingulate cortex. We found 3 distinct cingulate regions giving rise to a corticohypoglossal projection including the rostral part of M3, rostral part of M4 and the pregenual region (area 24/32) of the gyral component of this cortex. Although these are considerably weaker than the ventral frontal lobe projections, their functional contribution to tongue movement may be related to motivational and other higher-order behaviors. Particularly when considering the widespread and diverse cortical inputs to these cingulate regions (e.g., Vogt and Pandya, 1987; Carmichael and Price, 1994, 1995; Barbas and Blatt, 1995; Morecraft and Van Hoesen, 1998; Barbas et al., 1999; Morecraft et al., 2004; 2012) versus the highly localized cortical inputs converging on the ventrolateral precentral and premotor regions (e.g., Matelli et al., 1986; Morecraft and Van Hoesen, 1993; Tokuno et al., 1997). Indeed, the functions mediated by the anterior cingulate include the integration of negative affect, pain and cognitive control (Shackman et al., 2011), set-shifting (Bissonette et al., 2013), conflict monitoring and decision making (Carter and van Veen, 2007; Botvinick, 2013) as well as self-regulation (Posner et al., 2007). Anatomically, we have shown that cortex lining the lower bank of the cingulate sulcus, corresponding to the orofacial region of M3 and M4 receive an extensive set of higher-order cortical inputs including multimodal association and limbic cortical afferents from prefrontal, orbitofrontal and medial temporal sources (Morecraft et al., 2004 (see case 6 for M4), 2012 (see cases 5 and 6 for M3)) which link this cortex to such complex associative cingulate functions. Similarly, the pregenual region of the anterior cingulate gyrus receives strong medial temporal lobe inputs that are coupled to higher-order memory functions (Barbas and Blatt, 1995, Carmichael and Price, 1995, 1996; Barbas et al., 1999; Morecraft et al., 2012). Potentially adding to this rich complexity of cortical behavioral control is the fact that both M3 and the pregenual cingulate region receive extensive amygdala inputs (Van Hoesen, 1981; Amaral and Price, 1984; Barbas and De Olomos, 1990; Carmichael and Price, 1995; Morecraft et al 2007a) implying a particularly strong emotionally related influence on these cingulate corticohypoglossal projection areas.
Corticohypoglossal Projections from the Parietal Cortex and Insula
In one experimental case we found a light corticohypoglossal projection from the ventral region of the gyral portion of the somatosensory cortex (areas 1 and 2) (Table 4). The significance of this weak projection is unclear, but it is interesting to note that the paucity of a strong projection to this brainstem “motor” nucleus parallels, in principle, the paucity of direct corticospinal projections to motor neurons in lamina IX of the cervical spinal cord following injections of tract tracer into the gyral part of the arm region of S1(areas 1 and 2) and upper part of cortex lining the posterior bank of the central sulcus (areas 3b and 1) (Coulter and Jones 1977). However, our injection site in the face/head region of S1 in case SDM66-FR did not involve the cortex lining the depths of the central sulcus which could potentially increase the likelihood of detecting a more significant corticohypoglossal projections from S1 than found in the current study because the anterior bank of the central sulcus harbors the majority of descending monosynaptic projections to spinal motor neurons compared to the gyral portion of the frontal motor cortex (e.g., Cheney et al., 1991; Rathelot and Strick, 2006, 2009). Indeed, future experiments are required to more thoroughly address this issue with injections of dextran tracers placed into cortex forming the posterior bank of the central sulcus (areas 3 and 1). It is possible that S1 (and M1) projections to the hypoglossal nucleus mediate hypoglossal interneurons and first-order axons from tongue muscle afferents that project to the hypoglossal nucleus for sensory influences on control of tongue motion.
To our knowledge, a projection from the insula to the hypoglossal nucleus has not been reported in the monkey model. In our experiment, the projection was found to be light (Table 1, case SDM66-BDA). However, this must be interpreted cautiously as the BDA injection site itself was very small and the resultant labeling may not be fully representative of the entire insular-hypoglossal projection. Yet our finding does demonstrate that the non-human primate insula has a direct projection to the hypoglossal nucleus and that further study is justified to reinvestigate this issue with larger injection sites of high-resolution anterograde tracers. Functionally, it has been shown with magnetoencephalography (MEG) that cingulate and insular activation begin 2000 ms prior to the initiation of swallowing and continue throughout swallowing, perhaps being essential for the initiation of swallowing (Watanabe et al., 2004). This is supported by the finding that dysphasia occurs in patients with an isolated stroke confined to the anterior insula, but not posterior insula (Daniels and Foundas, 1997).
Somatotopy
One goal of the current study was to investigate the possibility that regions of the monkey frontal and cingulate cortex issuing corticobulbar projections to the facial motor nucleus of cranial nerve VII (Morecraft et al., 2001), also give rise to corticobulbar projections to the hypoglossal motor nucleus of cranial nerve XII. Our incentive to investigate this originated in part, from the idea that demonstrating common cortical regions projecting to multiple cranial nerve motor nuclei could reinforce perspectives on cortical face/head representation in the debate on somatotopic corticomotor organization which is dominated by investigations and viewpoints focusing on forelimb and hind limb representation (e.g., Murray and Coulter, 1981; Hutchins et al., 1988; Luppino et al., 1994, He et al., 1993, 1995). We found that the same general regions of the frontal lobe and cingulate cortex known to project to the facial nucleus also issued projections to the hypoglossal nucleus, thus underscoring their recognition of cortical orofacial representations. Furthermore, as discussed earlier, these regions are located ventral to the motor arm representations on the lateral surface (i.e., M1 and LPMCv), and anterior to arm representations on the medial cortical surface (i.e., M2, M3 and M4). However, future studies are needed to examine whether a general topography for these corticobulbar projections exists within each orofacial representation, and whether there are projections to the trigeminal motor nucleus of cranial nerve V.
Clinical Considerations of Potential Widespread Corticohypoglossal Projections
Although the most common form of stroke involves the middle cerebral artery (MCA), which supplies the orofacial representation of M1, the occurrence of clinically detectable tongue paresis following peri-Rolandic injury in humans is rare (e.g., Urban et al., 1996, 1997; Muellbacher et al., 1998). However, this clinical outcome has been described following small, localized unilateral M1 injury (e.g., Willoughby and Anderson, 1984; Ghika, et al., 1989; Dureiu and Leys, 1994; Urban et al., 1996, 1997; Muellbacher et al., 1998) and following transient ischemic attacks (Wei et al. 2012). Experimentally, it has been shown in stroke patients with contralesional lingual paresis that TMS stimulation of the lesioned hemisphere did not evoke lingual movement in the 5 patients studied, but stimulation of the non-lesioned hemisphere did evoke bilateral genioglossus muscle activity and tongue movement despite the clinical presence of paresis (Muellbacher et al., 1998). In a follow-up study, after complete recovery of tongue movement, stimulation of the lesioned hemisphere again did not produce lingual movements in 4 of the 5 patients (Muellbacher et al., 1999). However, TMS of the non-lesioned hemisphere elicited symmetric lingual movement demonstrating a potential role for uncrossed corticobulbar projections from the intact hemisphere in the recovery process. Our findings are consistent with this evidence as we found very prominent ipsilateral terminal labeling from M1 in all of our experimental cases (Table 4). Our work also suggests that spared cortical areas on the dorsolateral surface (LPMCd,) as well as medial surface (M2, M3, M4 and the anterior cingulate gyrus) may support tongue motor recovery following unilateral cortical stroke, which needs to be assessed in future patient studies. Indeed, if present in the human brain, our findings of medial wall corticohypoglossal projections may be of particular importance for favorable recovery of tongue movement control in patients with a history of MCA strokes that initially affect one hemisphere, followed by a second stroke affecting the other hemisphere (e.g., Umapathi et al., 2000), or in patients sustaining bilateral opercular MCA occlusion (e.g., Ferrari et al., 1979; Grattan-Smith et al., 1989; Nisipeanu et al., 1997; Suresh and Deepa, 2004)
Conversely, damage to the medial wall of the hemisphere from anterior cerebral artery (ACA) stroke is a rare clinical phenomenon (e.g., Bogousslavsky and Regli, 1990; Kazui et al, 1993; Kumral et al., 2002; Carrera et al., 2007, for review see Brust, 1992) as are alterations in clinically detectable simple voluntary tongue movements after such injury (Critchley, 1930). However, some observations indicate that ACA stroke results in higher-order oromotor complications including speech disturbances, deficits in articulation (dysarthria) and swallowing difficulties (dysphagia) (Critchley, 1930; Bogousslavsky and Regli, 1990; Chamorro et al., 1997). Thus, it is possible that the hypoglossal projections from the medial wall, if present in the human brain, may subserve a complex or supportive role in articulation and swallowing compared to a more movement specific-related role for the lateral corticohypoglossal projections.
Study Limitations and Future Research
The present study attempted to estimate the number of terminal-like immuoreactive particles (boutons) within the anatomically defined boundary of the hypoglossal nucleus. It is likely that many of these terminals interact with cell bodies and dendrites of hypoglossal motor neurons that are confined to this anatomical region but some caution should be exercised in this interpretation. Specifically, we do not know from the present work if these axon projections terminate on hypoglossal motor neuron somas or their dendritic processes, or hypoglossal interneurons (e.g., Cooper, 1981; Sokoloff and Deacon, 1992). Furthermore, it is possible that corticohypoglossal terminals form synaptic contacts with dendrites located within the anatomical confines of the hypoglossal nucleus which arise from cell bodies residing in the opposite hypoglossal nucleus (Cooper, 1981) or adjacent reticular formation. Additionally, we do not know from our material if these terminals are excitatory or inhibitory. Indeed, future studies are required to address these intriguing synaptic possibilities.
As pointed out for the M1 projection, it will be of significant interest to determine if the projections arising from each cortical area preferentially terminate in distinct musculotopic subsectors of the hypoglossal nucleus based upon the monkey musculotopic template mapped out by Sokoloff and Deacon (1992). Indeed, in contrast to the heavy and widespread projection from M1 as discussed previously that included peripheral and central targets of the nucleus, many of the non-M1 projections tended to innervate the peripheral regions of the nucleus which has been shown by Sokoloff and Deacon to harbor motor neurons innervating the intrinsic tongue muscles.
As we have stressed, it is not likely that any of the non-M1 projections will rival the anatomical density and physiological influence of the M1 hypoglossal projection. However, it is important to note that the terminal density of many of the non-M1 hypoglossal projections may be underestimated in our study, including the insular, cingulate motor area, and pregenual cingulate projections, since these values were derived from experimental cases that had very small injection sites (Figs. 1, 2, 4G, 5C,E,G; Tables 1, 2). The small, localized nature of these injection sites were intended to avoid tracer spread into adjacent cortical architectonic areas and nearby subcortical gray matter structures. Indeed, the core of nearly all of our cingulate motor area injection sites did not involve the cortex lining the upper and lower parts of the cingulate sulcus fundus, which if involved, would have likely increased the total number of estimated terminal boutons for these pathways. Future studies with large cingulate and insular injection sites could appropriately address this issue. However, a distinct advantage of these small injection sites lies in the fact that they were highly effective in pinpointing the specific cytoarchitectonic origin of these non-frontal corticohypoglossal projections.
Finally it is important to point out that there are many indirect pathways of cortical origin that are likely to have significant influence tongue movements including corticostriate, corticopontine and corticoreticular projections for example. In fact, the corticoreticular projection from the ventral region of M1 and adjacent ventrolateral premotor cortex in the monkey has been shown to be quite extensive (Kuypers 1958a). Other related work has shown that premotor neurons in the pontine and medullary parvocellular reticular formation project directly to the hypoglossal nucleus (Holstege and Kuypers, 1977; Holstege et al., 1977, 1983). Specifically, the lateral propriobulbar projection system is derived primarily from the lateral part of the lateral tegmental field and innervates the ipsilateral hypoglossal nucleus whereas the medial propriobulbar projection system originates from the medial part of the lateral tegmentum and tends to project bilaterally to the hypoglossal nucleus (Holstege and Kuypers, 1977; Holstege et al., 1977). In addition to contributing to this multisynaptic projection system, it is possible that direct cortical projections to the lower bulbar reticular formation may terminate on dendrites originating from cell bodies residing in the hypoglossal nucleus that extend out into the nearby reticular formation (Cooper, 1981). Thus, future studies are required to investigate the anatomical characteristics of the indirect, corticoreticular projection from the non-M1 regions found to directly innervate the hypoglossal nucleus. The possibility that these descending corticoreticular projections may be quite strong compared to their corticohypoglossal counterparts could significantly add to our current level of understanding of cortical contributions to hypoglossal motor mechanisms.
Summary and Conclusions
Although there is renewed interest in understanding the role of the cerebral cortex in mediating swallowing and tongue movement (Humbert and Robbins, 2007; Michou and Hamdy 2009), a literature review clearly shows scholarly interest in this area of motor control has been limited, particularly when comparing the number of tongue related studies to the overwhelming number of studies investigating cortical influence on upper extremity movement. One possible reason is that following unilateral cortical injury, adequate levels of tongue motor recovery occur rapidly and important residual, subclinical motor deficits are not apparent using standard bedside neurological examination (Urban et al., 1996, 1997). Indeed, the widespread nature of bilateral projections found in our study (Fig. 14) indicate that cortical influence on the non-human primate hypoglossal nucleus is highly protected. However, the common and persistent occurrence of complex swallowing and orofacial deficits that accompany cortical injury indicate that cortical contributions to hypoglossal control mechanisms may be more significant than currently recognized, involving both direct corticohypoglossal projections as identified in the present study, as well as the indirect cortical projection systems. Thus, our findings provide a strong template for exploring the possible existence of these non-M1 corticohypoglossal projections in the human brain, investigating potential roles for multiple cortical areas in neurological disorders adversely affecting oral-related motor function, and expanding our initiative to localize cortical areas that may favorably contribute to the recovery of tongue movements following cortical stroke, traumatic brain injury and neurosurgical resection.
Acknowledgments
GRANT SUPPORT: Supported by National Institutes of Health grants NS 046367 and NS 33003 and The Benign Essential Blepharospasm Research Foundation.
The authors would like to Konstantin Avramov, Ann Marie Cross, Katherine Degan, Zeljko Dvanajscak, Matthew Gedney, Alexia Gillian, Gina Ann Good Crow, James Herrick, Chris Jensen, Jeffery Kennedy, Jennifer Louie, David McNeal, Jonovan Ottenbacher and Clinton Schroeder for surgical assistance and contributions to the tissue processing procedures that were critical for developing some of the case material used for this study.
LIST OF ABBREVIATIONS
- BDA
biotinylated dextran amine
- cf
calcarine sulcus
- cgs
cingulate sulcus
- cis
circular sulcus
- Cs
central sulcus
- ec
ectocalcarine sulcus
- FD
fluorescein dextran
- FR
fluro ruby
- HN
hypoglossal nucleus
- lf
lateral fissure
- ilas
inferior limb of the arcuate sulcus
- ios
inferior occipital sulcus
- In
insula
- ips
intraparietal sulcus
- LPMC
lateral premotor cortex
- LPMCd
dorsal lateral premotor cortex
- LPMCv
ventral lateral premotor cortex
- ls
lunate sulcus
- LYD
lucifer yellow dextran
- M1
primary motor cortex
- M2
supplementary motor cortex
- M3
rostral cingulate motor cortex
- M4
caudal cingulate motor cortex
- mos
medial orbital sulcus
- ots
occipitotemporal sulcus
- poms
medial parieto-occipital sulcus
- preSMA
pre-supplementary motor cortex
- ps
principle sulcus
- ros
rostral sulcus
- rs
rhinal sulcus
- S1
primary somatosensory cortex
- slas
superior limb of the arcuate sulcus
- sts
superior temporal sulcus
- v
ventricle
Footnotes
Conflict of interest statement.
All authors do not have any conflict of interest that could inappropriately influence, or be perceived to influence, this work.
Role of authors. All authors listed on the paper contributed significantly to the elaboration of the paper and/or to the research that led to preparation of the manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: RJM. Development of data: RJM, KSS-M, JG, KMS-C. Acquisition of data: KMS-C, JG, RJM; Interpretation of data: RJM, KMS-C, WGD, KSS-M, JG. Drafting of the manuscript: RJM, KSS-M, WGD, KMS-C, JG. Obtained funding: RJM. Study supervision: RJM.
References
- Adams RD, Victor M, Ropper AH. Principles of neurology. 6. New York: McGraw-Hill; 1997. [Google Scholar]
- Afifi AK, Bergman RA. Basic neuroscience. Baltimore-Munich: Urbran and Schwarzenberg; 1980. [Google Scholar]
- Aizawa H, Inase M, Mushiake H, Shima K, Tanji J. Reorganization of activity in the supplementary motor area associated with motor learning and functional recovery. Exp Brain Res. 1991;84:668–671. doi: 10.1007/BF00230980. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol. 1990;300:549–571. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol. 1999;410:343–367. doi: 10.1002/(sici)1096-9861(19990802)410:3<343::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Bawa P, Hamm JD, Dhillon P, Gross PA. Bilateral responses of upper limb muscles to transcranial magnetic stimulation in human subjects. Exp Brain Res. 2004;158:385–390. doi: 10.1007/s00221-004-2031-x. [DOI] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Jesmanowicz A, Shaker R. Magnetic field changes in the human brain due to swallowing or speaking. Magn Reson Med. 1998;40:55–60. doi: 10.1002/mrm.1910400108. [DOI] [PubMed] [Google Scholar]
- Bogousslavsky J, Regli F. Anterior cerebral artery territory infarction in the Lausanne stroke registry: clinical and etiologic patterns. Arch Neurol. 1990;47:144–150. doi: 10.1001/archneur.1990.00530020040012. [DOI] [PubMed] [Google Scholar]
- Boudreau SA, Lontis ER, Caltenco H, Svensson P, Sessle BJ, Andreasen Struijk LN, Arendt-Nielsen L. Features of cortical neuroplasticity associated with multidirectional novel motor skill training: a TMS mapping study. Exp Brain Res. 2013;225:513–526. doi: 10.1007/s00221-012-3391-2. [DOI] [PubMed] [Google Scholar]
- Borra E, Belmalih A, Gerbella M, Rozzi S, Luppino G. Projections of the hand field of the macaque ventral premotor area F5 to the brainstem and spinal cord. J Comp Neurol. 2010;518:2570–2591. doi: 10.1002/cne.22353. [DOI] [PubMed] [Google Scholar]
- Brodal A. Neurological anatomy: in relation to clinical medicine. 3. Oxford: Oxford University Press; 1981. [Google Scholar]
- Brust JCM. Anterior cerebral artery disease. In: Barnett HJM, Stein BM, Mohr JP, Yatsim FM, editors. Stroke: pathophysiology, diagnosis, management. New York: Churchill-Livingstone; 1992. pp. 337–360. [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbitofrontal and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Carrera E, Maeder-Ingvar M, Rossetti AO, Devuyst G, Bogousslavsky Trends in risk factors, patterns and causes in hospitalized strokes over 25 years: the Lausanne stroke registry. Cerebrovas Dis. 2007;24:97–103. doi: 10.1159/000103123. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Marshall RS, Valls-Solé J, Tolosa E, Mohr JP. Motor behavior in stroke patients with isolated medial frontal ischemic infarction. Stroke. 1997;28:1755–1760. doi: 10.1161/01.str.28.9.1755. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE, Mewes K. Neural mechanisms underlying corticospinal and rubrospinal control of limb movements. Prog Brain Res. 1991;87:213–252. doi: 10.1016/s0079-6123(08)63054-x. [DOI] [PubMed] [Google Scholar]
- Cooper MH. The hypoglossal nucleus of the primate: a Golgi study. Neurosci Lett. 1981;21:249–254. doi: 10.1016/0304-3940(81)90212-3. [DOI] [PubMed] [Google Scholar]
- Corfield DR, Murphy K, Josephs O, Fink GR, Frackowiak RS, Guz A, Adams L, Turner R. Cortical and subcortical control of tongue movement in humans: a functional neuroimaging study using fMRI. J Appl Physiol. 1999;86:1468–1477. doi: 10.1152/jappl.1999.86.5.1468. [DOI] [PubMed] [Google Scholar]
- Coulter JD, Jones EG. Differential distribution of corticospinal projections from individual cytoarchitectonic fields in the monkey. Brain Res. 1977;129:335–340. doi: 10.1016/0006-8993(77)90012-9. [DOI] [PubMed] [Google Scholar]
- Critchley M. The anterior cerebral artery and its syndromes. Brain. 1930;53:120–165. doi: 10.1177/003591573002300524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cure C, Rasmussen T. Effects of altering the parameters of electrical stimulating currents upon motor responses from the precentral gyrus of Macaca mulatta. Brain. 1954;77:18–33. doi: 10.1093/brain/77.1.18. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Foundas AL. The role of the insular cortex in dysphagia. Dysphagia. 1997;12:146–156. doi: 10.1007/PL00009529. [DOI] [PubMed] [Google Scholar]
- D’Ausilio A, Pulvermüller F, Salmas P, Bufalari I, Begliomini C, Fadiga L. The motor somatotopy of speech. Curr Biol. 2009;19:381–385. doi: 10.1016/j.cub.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Davare M, Andres M, Cosnard G, Thonnard J-L, Olivier E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci. 2006;26:2260–2268. doi: 10.1523/JNEUROSCI.3386-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito JL, Smith OA. Projections from the mesial frontal cortex (supplementary motor area) to the cerebral hemispheres and brain stem of the Macaca mulatta. J Comp Neurol. 1959;111:261–277. doi: 10.1002/cne.901110204. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16:6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieu I, Leys D. Lingual motility in unilateral hemispheric vascular complications. Study of the cortico-hypoglossal afferences. Rev Neurol (Paris) 1994;150:844–849. [PubMed] [Google Scholar]
- Falsetti P, Acciai C, Palilla R, Bosi M, Carpinteri F, Zingarelli A, Pedace C, Lenzi L. Oropharyngeal dysphagia after stroke: incidence, diagnosis, and clinical predictors in patients admitted to a neurorehabilitation unit. J Stroke Cerebrovasc Dis. 2009;18:329–35. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Boninsegna C, Beltramello A. Foix-Chavany syndrome: CT study and clinical report of three cases. Neuroradiology. 1979;18:41–42. doi: 10.1007/BF00346210. [DOI] [PubMed] [Google Scholar]
- Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, Spencer DD. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 1991;11:3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. Functional organization of inferior area 6 in the macaque monkey I. somatotopy and the control of proximal movements. Exp Brain Res. 1988;71:475–490. doi: 10.1007/BF00248741. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Dutschmann M, Obled S, Bianchi AL. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir Physiol Neurobiol. 2005;147:159–176. doi: 10.1016/j.resp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Ghezzi A, Baldini S. A simple method for recording motor evoked potentials of lingual muscles to transcranial magnetic and peripheral electrical stimulation. Electroencephalo Clin Neurophysiol. 1998;109:114–118. doi: 10.1016/s0924-980x(98)00008-3. [DOI] [PubMed] [Google Scholar]
- Ghika J, Bogousslavsky J, Regli F. Infarcts in the territory of the deep perforators from the carotid system. Neurology. 1989;39:507–512. doi: 10.1212/wnl.39.4.507. [DOI] [PubMed] [Google Scholar]
- Godschalk M, Mitz AR, van Duin B, van der Berg H. Somatotopy of monkey premotor cortex examined with microstimulation. Neurosci Res. 1995;23:269–279. doi: 10.1016/0168-0102(95)00950-7. [DOI] [PubMed] [Google Scholar]
- Gong S, DeCuypere M, Zhao Y, LeDoux MS. Cerebral cortical control of orbicularis oculi motoneurons. Brain Res. 2005;1047:177–193. doi: 10.1016/j.brainres.2005.04.045. [DOI] [PubMed] [Google Scholar]
- Grabski K, Lamalle L, Vilain C, Schwartz JL, Vallée N, Tropres I, Baciu M, Le Bas JF, Sato M. Functional MRI assessment of orofacial articulators: neural correlates of lip, jaw, larynx, and tongue movements. Hum Brain Mapp. 2012;33:2306–2321. doi: 10.1002/hbm.21363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan-Smith PJ, Hopkins IJ, Shield LK, Boldt DW. Acute pseudobulbar palsy due to bilateral focal cortical damage: the opercular syndrome of Foix-Chavany-Marie. J Child Neurol. 1989;4:131–136. doi: 10.1177/088307388900400213. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Green HD, Walker AE. The effects of ablation of the cortical motor face are in monkeys. J Neurophysiol. 1938;1:262–280. [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Crone R, Hughes D, Tallis RC, Thompson DG. Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. Lancet. 1997;350:686–692. doi: 10.1016/S0140-6736(97)02068-0. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol. 1999;277:G219–225. doi: 10.1152/ajpgi.1999.277.1.G219. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Rothwell JC, Aziz Q, Thompson DG. Organization and reorganization of human swallowing motor cortex: implications for recovery after stroke. Clin Sci. 2000;99:151–157. [PubMed] [Google Scholar]
- Hannig S, Jürgens U. Projections of the ventrolateral pontine vocalization area in the squirrel monkey. Exp Brain Res. 2005;169:92–105. doi: 10.1007/s00221-005-0128-5. [DOI] [PubMed] [Google Scholar]
- Hatanaka N, Tokuno H, Nambu A, Inoue T, Takada M. Input-output organization of jaw movement-related areas in monkey frontal cortex. J Comp Neurol. 2005;492:401–425. doi: 10.1002/cne.20730. [DOI] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci. 1993;13:952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the medial surface of the hemisphere. J Neurosci. 1995;15:3284–3306. doi: 10.1523/JNEUROSCI.15-05-03284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G. Brainstem-spinal cord projections in the cat, related to control of head and axial movements. In: Büttner-Ennever JA, editor. Neuroanatomy of the occulomotor system. Amsterdam: Elsevier Science Publishers; 1988. pp. 431–470. [PubMed] [Google Scholar]
- Holstege G, Kuypers HGJM. Fibre connections to the trigeminal, facial and hypoglossal motor nuclei in the cat. A lesion-degeneration study. Brain. 1977;100:239–264. doi: 10.1093/brain/100.2.239. [DOI] [PubMed] [Google Scholar]
- Holstege G, Graveland G, Bijker-Biemond C, Schuddeboom I. Location of motoneurons innervating soft palate, pharynx and upper esophagus, anatomical evidence for a possible swallowing center in the pontine reticular formation: an HRP and autoradiography tracing study. Brain Behav Evol. 1983;23:47–62. doi: 10.1159/000121488. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HGJM, Dekker JJ. The organization of the bulbar fibre connections to trigeminal, facial and hypoglossal motor nuclei. Brain. 1977;100:265–286. [PubMed] [Google Scholar]
- Horner J, Massey EW, Riski JE, Lathrop DL, Chase KN. Aspiration following stroke: clinical correlates and outcome. Neurology. 1988;38:1359–1362. doi: 10.1212/wnl.38.9.1359. [DOI] [PubMed] [Google Scholar]
- Horsley V, Schäffer EA. A record of experiments upon the functions of the cerebral cortex. Philos Trans R Soc Lond, Ser B. 1888;179:1–45. [Google Scholar]
- Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr Opin Neurobiol. 2007;17:234–242. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Huang CS, Hiraba H, Murray GM, Sessle BJ. Organization of the primate face motor cortex as revealed by intracortical microstimulation and electrophysiological identification of afferent inputs and corticobulbar projections. J Neurophysiol. 1988;59:796–818. doi: 10.1152/jn.1988.59.3.796. [DOI] [PubMed] [Google Scholar]
- Humbert IA, Robbins J. Normal swallowing and functional magnetic resonance imaging: a systematic review. Dysphagia. 2007;22:266–275. doi: 10.1007/s00455-007-9080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins KD, Martino AM, Strick PL. Corticospinal projections from the medial wall of the hemisphere. Exp Brain Res. 1988;71:667–672. doi: 10.1007/BF00248761. [DOI] [PubMed] [Google Scholar]
- Jean A. Brainstem organization of the swallowing network. Brain Behav Evol. 1984;25:109–116. doi: 10.1159/000118856. [DOI] [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Jenny AB, Saper CB. Organization of the facial nucleus and corticofacial projection in the monkey: a reconsideration of the upper motor neuron facial palsy. Neurology. 1987;37:930–939. doi: 10.1212/wnl.37.6.930. [DOI] [PubMed] [Google Scholar]
- Jürgens U. The efferent and afferent connections of the supplementary motor area. Brain Res. 1984;300:63–81. doi: 10.1016/0006-8993(84)91341-6. [DOI] [PubMed] [Google Scholar]
- Jürgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Jürgens U, Alipour M. A comparative study on the cortico-hypoglossal connections in primates, using biotin dextranamine. Neurosci Lett. 2002;328:245–248. doi: 10.1016/s0304-3940(02)00525-6. [DOI] [PubMed] [Google Scholar]
- Kazui S, Sawada T, Naritomi H, Kuriyama Y, Yamaguchi T. Angiographic evaluation of brain infarction limited to the anterior cerebral artery territory. Stroke. 1993;24:549–553. doi: 10.1161/01.str.24.4.549. [DOI] [PubMed] [Google Scholar]
- Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol. 2001;280:G531–538. doi: 10.1152/ajpgi.2001.280.4.G531. [DOI] [PubMed] [Google Scholar]
- Krings T, Buchbinder BR, Butler WE, Chiappa KH, Jiang HJ, Cosgrove GR, Rosen BR. Functional magnetic resonance imaging and transcranial magnetic stimulation: complementary approaches in the evaluation of cortical motor function. Neurology. 1997;48:1406–1416. doi: 10.1212/wnl.48.5.1406. [DOI] [PubMed] [Google Scholar]
- Kumral E, Bayulkem G, Evyapan D, Yunten N. Spectrum of anterior cerebral artery territory infarction: clinical and MRI findings. Eur J Neurol. 2002;9:615–624. doi: 10.1046/j.1468-1331.2002.00452.x. [DOI] [PubMed] [Google Scholar]
- Künzle H. An autoradiographic analysis of the efferent connections from premotor and adjacent prefrontal regions (areas 6 and 9) in Macaca fascicularis. Brain Behav Evol. 1978;15:185–234. doi: 10.1159/000123779. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. Cortical projections to the pons and medulla oblongata in the cat and man. Anat Rec. 1956;124:322–323. [Google Scholar]
- Kuypers HGJM. Some projections from the peri-central cortex to the pons and lower brain stem in monkey and chimpanzee. J Comp Neurol. 1958a;110:221–251. doi: 10.1002/cne.901100205. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. Corticobulbar connexions to the pons and lower brain-stem in man: An anatomical study. Brain. 1958b;81:364–388. doi: 10.1093/brain/81.3.364. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. An anatomical analysis of cortico-bulbar connexions to the pons and lower brain stem in cat. J Anat. 1958c;92:198–218. [PMC free article] [PubMed] [Google Scholar]
- Kuypers HGJM, Lawrence DG. Cortical projections to the red nucleus and the brain stem in the rhesus monkey. Brain Res. 1967;4:151–188. doi: 10.1016/0006-8993(67)90004-2. [DOI] [PubMed] [Google Scholar]
- Larson CR, Byrd KE, Garthwaite CR, Luschei ES. Alterations in the pattern of mastication after ablations of the lateral precentral cortex in rhesus macaques. Exp Neurol. 1980;70:638–651. doi: 10.1016/0014-4886(80)90189-2. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR. Afferent and efferent connections of the dorsolateral precentral gyrus (area 4, hand/arm region) in the macaque monkey, with comparisons to area 8. J Comp Neurol. 1986;254:460–492. doi: 10.1002/cne.902540403. [DOI] [PubMed] [Google Scholar]
- Leyton ASF, Sherrington CS. Observations on the excitable cortex of the chimpanzee, orang-utan and gorilla. Q J Exp Physiol. 1917;11:135–222. [Google Scholar]
- Lin LD, Murray GM, Sessle BJ. Effects on non-human primate mastication of reversible inactivation by cooling of the face primary somatosensory cortex. Arch Oral Biol. 1998;43:133–141. doi: 10.1016/s0003-9969(97)00101-5. [DOI] [PubMed] [Google Scholar]
- Lowe AA. The neural regulation of tongue movements. Prog Neurobiol. 1981;15:295–344. doi: 10.1016/0301-0082(80)90008-8. [DOI] [PubMed] [Google Scholar]
- Lund JP, Kolta A. Brainstem circuits that control mastication: do they have anything to say during speech? J Commun Disord. 2006;39:381–390. doi: 10.1016/j.jcomdis.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda RM, Gallese V, Rizzolati G. Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: an intracortical microstimulation study in the macaque monkey. J Comp Neurol. 1991;311:463–482. doi: 10.1002/cne.903110403. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticospinal projections from mesial frontal and cingulate areas in the monkey. Neuroreport. 1994;5:2545–2548. doi: 10.1097/00001756-199412000-00035. [DOI] [PubMed] [Google Scholar]
- Luschei ES, Garthwaite CR, Armstrong ME. Relationship of firing patterns of units in face area of monkey precentral cortex to conditioned jaw movements. J Neurophysiol. 1971;34:552–561. doi: 10.1152/jn.1971.34.4.552. [DOI] [PubMed] [Google Scholar]
- Luschei ES, Goodwin GM. Patterns of mandibular movement and jaw muscle activity during mastication in the monkey. J Neurophysiol. 1974;37:954–966. doi: 10.1152/jn.1974.37.5.954. [DOI] [PubMed] [Google Scholar]
- Luschei ES, Goodwin GM. Role of the monkey precentral cortex in control of voluntary jaw movements. J Neurophysiol. 1975;38:146–157. doi: 10.1152/jn.1975.38.1.146. [DOI] [PubMed] [Google Scholar]
- Lowe AA. The neural regulation of tongue movements. Prog Brain Res. 1981;15:295–344. doi: 10.1016/0301-0082(80)90008-8. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Marangoz C, Miles TS, Wiesendanger M. Microstimulation of the supplementary motor area (SMA) in the awake monkey. Exp Brain Res. 1982;45:410–416. doi: 10.1007/BF01208601. [DOI] [PubMed] [Google Scholar]
- Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum Brain Mapp. 2009;30:3209–3226. doi: 10.1002/hbm.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RE, Sessle BJ. The role of the cerebral cortex in swallowing. Dysphagia. 1993;8:195–202. doi: 10.1007/BF01354538. [DOI] [PubMed] [Google Scholar]
- Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85:938–950. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, Menon RS. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol. 2004;92:2428–2443. doi: 10.1152/jn.01144.2003. [DOI] [PubMed] [Google Scholar]
- Martino AM, Strick PL. Corticospinal projections originate from the arcuate premotor area. Brain Res. 1987;404:307–312. doi: 10.1016/0006-8993(87)91384-9. [DOI] [PubMed] [Google Scholar]
- Matelli M, Camarda R, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol. 1986;251:281–298. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- McGuinness E, Sivertsen D, Allman JM. Organization of the face representation in macaque motor cortex. J Comp Neurol. 1980;193:591–608. doi: 10.1002/cne.901930302. [DOI] [PubMed] [Google Scholar]
- McNeal DW, Darling WG, Ge J, Stilwell-Morecraft KS, Solon KM, Hynes SM, Pizzimenti MA, Rotella DL, Vanadurongvan T, Morecraft RJ. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J Comp Neurol. 2010;518:586–621. doi: 10.1002/cne.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows J. Dysphagia in unilateral cerebral lesions. J Neurol Neurosurg Psychiat. 1973;36:853–860. doi: 10.1136/jnnp.36.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Liebsch R, Röricht S. Tongue motor responses following transcranial magnetic stimulation of the motor cortex and proximal hypoglossal nerve in man. Electroencephalogr Clin Neurophysiol. 1997;105:15–23. doi: 10.1016/s0924-980x(96)96598-4. [DOI] [PubMed] [Google Scholar]
- Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17:166–171. doi: 10.1097/MOO.0b013e32832b255e. [DOI] [PubMed] [Google Scholar]
- Miller AJ. The neuroscientific principles of swallowing and dysphagia. San Diego: Singular Publishing Group; 1999. [Google Scholar]
- Miller AJ. Oral and pharyngeal reflexes in the mammalian nervous system: their diverse range in complexity and the pivotal role of the tongue. Crit Rev Oral Biol Med. 2002;13:409–425. doi: 10.1177/154411130201300505. [DOI] [PubMed] [Google Scholar]
- Miller AJ. The neurobiology of swallowing. Dev Disabil Res Rev. 2008;14:77–86. doi: 10.1002/ddrr.12. [DOI] [PubMed] [Google Scholar]
- Mitz AR, Wise SP. The somatotopic organization of the supplementary motor area: intracortical microstimulation mapping. J Neurosci. 1987;7:1010–1021. doi: 10.1523/JNEUROSCI.07-04-01010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Cipolloni PB, Stilwell-Morecraft KS, Gedney MT, Pandya DN. Cytoarchitecture and cortical connections of the posterior cingulate and adjacent somatosensory fields in the rhesus monkey. J Comp Neurol. 2004;469:37–69. doi: 10.1002/cne.10980. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Pizzimenti MA, Darling WG. Terminal Distribution of the Corticospinal Projection from the Hand/Arm Region of the Primary Motor Cortex to the Cervical Enlargement in Rhesus Monkey. J Comp Neurol. 2013a;521:4205–4235. doi: 10.1002/cne.23410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Herrick JL, Stilwell-Morecraft KS, Louie JL, Schroeder CM, Ottenbacher JG, Schoolfield MW. Localization of arm representation in the corona radiata and internal capsule in the non-human primate. Brain. 2002;125:176–198. doi: 10.1093/brain/awf011. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Louie JL, Herrick JL, Stilwell-Morecraft KS. Cortical innervation of the facial nucleus in the non-human primate: a new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain. 2001;124:176–208. doi: 10.1093/brain/124.1.176. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Dvanajscak Z, Ge J, Schneider P. Localization of arm representation in the cerebral peduncle of the non-human primate. J Comp Neurol. 2007b;504:149–167. doi: 10.1002/cne.21438. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Gedney M, Ge J, Schroeder CM, Van Hoesen GW. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J Comp Neurol. 2007a;500:134–165. doi: 10.1002/cne.21165. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Schroeder CM, Keifer J. Organization of face representation in the cingulate gyrus of the rhesus monkey. NeuroReport. 1996;7:1343–1348. doi: 10.1097/00001756-199605310-00002. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Cipolloni PB, Ge J, McNeal DW, Pandya DN. Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Res Bull. 2012;87:457–497. doi: 10.1016/j.brainresbull.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft, Rossing WR. The motor cortex and facial expression: new insights from neuroscience. The Neurologist. 2004;10:235–249. doi: 10.1097/01.nrl.0000138734.45742.8d. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Ugollini G, Lanciego JL, Wouterlood FG, Pandya DN. Classic and Contemporary Neural Tract Tracing Techniques. In: Johansen-Berg H, Behrens TE, editors. Diffusion MRI: from quantitative measurement to in-vivo neuroanatomy. 2. Oxford: Oxford University Press; 2013b. pp. 359–399. [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. J Comp Neurol. 1992;322:471–489. doi: 10.1002/cne.903220403. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Frontal granular input to the cingulate (M3), supplementary (M2) and primary (M1) motor cortices in the rhesus monkey. J Comp Neurol. 1993;337:669–689. doi: 10.1002/cne.903370411. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Convergence of limbic input to the cingulate motor cortex in rhesus monkey. Brain Res Bull. 1998;45:209–232. doi: 10.1016/s0361-9230(97)00344-4. [DOI] [PubMed] [Google Scholar]
- Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res. 2001;140:280–289. doi: 10.1007/s002210100813. [DOI] [PubMed] [Google Scholar]
- Mosier KM, Liu WC, Maldjian JA, Shah R, Modi B. Lateralization of cortical function in swallowing: a functional MR imaging study. Am J Neuroradiol. 1999;20:1520–1526. [PMC free article] [PubMed] [Google Scholar]
- Moustafa EM, Lin LD, Murray GM, Sessle BJ. An electromyographic analysis of orofacial motor activities during trained tongue-protrusion and biting tasks in monkeys. Arch Oral Biol. 1994;39:955–965. doi: 10.1016/0003-9969(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Muakkassa KF, Strick PL. Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized ‘premotor’ areas. Brain Res. 1979;177:176–182. doi: 10.1016/0006-8993(79)90928-4. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Artner C, Mamoli B. Motor evoked potentials in unilateral lingula paralysis after monosynaptic ischaemia. J Neurol Neurosurg Psychiatry. 1998;65:755–761. doi: 10.1136/jnnp.65.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellbacher W, Artner C, Mamoli B. The role of the intact hemisphere in recovery of midline muscles after recent monohemispheric stroke. J Neurol. 1999;246:250–256. doi: 10.1007/s004150050343. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Boroojerdi B, Ziemann U, Hallett M. Analogous corticocortical inhibition and facilitation in ipsilateral and contralateral human motor cortex representations of the tongue. J Clin Neurophysiol. 2001;18:550–558. doi: 10.1097/00004691-200111000-00005. [DOI] [PubMed] [Google Scholar]
- Murray EA, Coulter JD. Organization of corticospinal neurons in the monkey. J Comp Neurol. 1981;195:339–365. doi: 10.1002/cne.901950212. [DOI] [PubMed] [Google Scholar]
- Murray GM, Lin LD, Moustafa EM, Sessle BJ. Effects of reversible inactivation by cooling of the primate face motor cortex on the performance of a trained tongue-protrusion task and a trained biting tas. J Neurophysiol. 1991;65:511–530. doi: 10.1152/jn.1991.65.3.511. [DOI] [PubMed] [Google Scholar]
- Murray GM, Sessle BJ. Functional properties of single neurons in the face primary motor cortex of the primate: I. input and output features of tongue motor cortex. J Neurophysiol. 1992;67:747–758. doi: 10.1152/jn.1992.67.3.747. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol. 1991;66:705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- Nisipeanu P, Rieder I, Blumen S, Korczyn AD. Pure congenital Foix-Chavany-Marie syndrome. Dev Med Child Neurol. 1997;39:696–698. doi: 10.1111/j.1469-8749.1997.tb07366.x. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldery E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Penfield W, Welch K. Instability of response to stimulation of the sensorimotor cortex in man. J Physiol. 1949;109:358–365. doi: 10.1113/jphysiol.1949.sp004399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Welch K. The supplementary motor area of the cerebral cortex. A clinical and experimental study. Arch Neurol Psychiatry. 1951;66:289–317. doi: 10.1001/archneurpsyc.1951.02320090038004. [DOI] [PubMed] [Google Scholar]
- Rathelot J-A, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci. 2006;103:8257–8262. doi: 10.1073/pnas.0602933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathelot J-A, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci. 2009;106:918–923. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J, Levin RL. Swallowing after unilateral stroke of the cerebral cortex: preliminary experience. Dysphagia. 1988;3:11–17. doi: 10.1007/BF02406275. [DOI] [PubMed] [Google Scholar]
- Robbins J, Levine RL, Maser A, Rosenbek JC, Kempster GB. Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil. 1993;74:1295–1300. doi: 10.1016/0003-9993(93)90082-l. [DOI] [PubMed] [Google Scholar]
- Rödel RM, Laskawi R, Markus H. Tongue representation in the lateral cortical motor region of the human brain as assessed by transcranial magnetic stimulation. Ann Otol Rhinol Laryngol. 2003;112:71–76. doi: 10.1177/000348940311200114. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Courtine G, Jindrich DL, Brock JH, Ferguson AR, Strand SC, Nout YS, Roy RR, Miller DM, Beattie MS, Havton LA, Bresnahan JC, Edgerton VR, Tuszynski MH. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nature Neurosci. 2010;13:1505–1510. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanides F. The architecture of the cortical taste nerve areas in squirrel monkey (Saimiri Sciureus) and their relationships to insular, sensorimotor and prefrontal regions. Brain Res. 1968;8:97–124. doi: 10.1016/0006-8993(68)90174-1. [DOI] [PubMed] [Google Scholar]
- Sanides F. Functional architecture of motor and sensory cortices in primates in the light of a new concept of neocortical evolution. In: Noback CR, Montagna W, editors. The primate brain: advances in primatology. New York: Appleton-Century-Crofts; 1970. pp. 137–208. [Google Scholar]
- Sawczuk A, Mosier KM. Neural control of tongue movement with respect to respiration and swallowing. Crit Rev Oral Biol Med. 2001;12:18–37. doi: 10.1177/10454411010120010101. [DOI] [PubMed] [Google Scholar]
- Sessle BJ, Wiesendanger M. Structural and functional definition of the motor cortex in the monkey (Macaca fascicularis) J Physiol. 1982;323:245–265. doi: 10.1113/jphysiol.1982.sp014071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Jürgens U. Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Res. 2003;974:43–59. doi: 10.1016/s0006-8993(03)02548-4. [DOI] [PubMed] [Google Scholar]
- Singh S, Hamdy S. Dysphagia in stroke patients. Postgrad Med J. 2006;82:383–391. doi: 10.1136/pgmj.2005.043281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff AJ, Deacon TW. Musculotopic organization of the hypoglossal nucleus in the cynomolgus monkey, Macaca fascicularis. J Comp Neurol. 1992;324:81–93. doi: 10.1002/cne.903240107. [DOI] [PubMed] [Google Scholar]
- Sörös P, Inamoto Y, Martin RE. Functional brain imaging of swallowing: an activation likelihood estimation meta-analysis. Hum Brain Mapp. 2009;30:2426–2439. doi: 10.1002/hbm.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh PA, Deepa C. Congenital suprabulbar palsy: a distinct clinical syndrome of heterogeneous aetiology. Dev Med Child Neurol. 2004;46:617–625. doi: 10.1017/s0012162204001045. [DOI] [PubMed] [Google Scholar]
- Symon L, Dorsch NW, Crockard HA. The production and clinical features of chronic stroke model in experimental primates. Stroke. 1975;6:476–481. doi: 10.1161/01.str.6.5.476. [DOI] [PubMed] [Google Scholar]
- Talairach J, Bancaud J. The supplementary motor area in man: anatomo-functional findings by stereo-electroencephalography in epilepsy. Int J Neurol. 1966;5:330–347. [Google Scholar]
- Tanji J, Kurata K. Contrasting neuronal activity in supplementary and precentral motor cortex of monkeys. I. Responses to instructions determining motor responses to forthcoming signals of different modalities. J Neurophysiol. 1985;53:129–141. doi: 10.1152/jn.1985.53.1.129. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Takada M, Nambu A, Inase M. Reevaluation of ipsilateral corticocortical inputs to the orofacial region of the primary motor cortex in the macaque monkey. J Comp Neurol. 1997;389:34–48. doi: 10.1002/(sici)1096-9861(19971208)389:1<34::aid-cne3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Umapathi T, Venketasubramanian N, Leck KJ, Tan CB, Lee WL, Tjia H. Tongue deviation in acute ischaemic stroke: a study of supranuclear twelfth cranial nerve palsy in 300 stroke patients. Cerebrovasc Dis. 2000;10:462–465. doi: 10.1159/000016108. [DOI] [PubMed] [Google Scholar]
- Urban PP, Heimgärtner I, Hopf HC. Transkranielle stimulation der zungenmuskulatur bei gesunden und patienten mit encephalomyelitis disseminate. Z Elektroenzephal org Elektromyogr Verwandte Geb. 1994;25:254–258. [Google Scholar]
- Urban PP, Hopf HC, Connemann B, Hundemer HP, Koehler J. The course of cortico-hyopglossal projections in the human brainstem: functional testing using transcranial magnetic stimulation. Brain. 1996;119:1031–1038. doi: 10.1093/brain/119.3.1031. [DOI] [PubMed] [Google Scholar]
- Urban PP, Hopf HC, Fleischer S, Zorowka PG, Müller-Forell W. Impaired cortico-bulbar tract function in dysarthria due to hemispheric stroke: functional testing using transcranial magnetic stimulation. Brain. 1997;120:1077–1084. doi: 10.1093/brain/120.6.1077. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW. The differential distribution, diversity and sprouting of cortical projections to the amygdala in the rhesus monkey. In: Ben-Ari Y, editor. The amygdaloid complex. Amsterdam: Elsevier/North-Holland Biomedical Press; 1981. pp. 77–90. [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey. II. Cortical afferents. J Comp Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Walker AE, Green HD. Electrical excitability of the motor face area: a comparative study in primates. J Neurophysiol. 1938;1:152–165. [Google Scholar]
- Wang Y, Shima K, Sawamura H, Tanji J. Spatial distribution of cingulate cells projecting to the primary, supplementary, and pre-supplementary motor areas: a retrograde multiple labeling study in the macaque monkey. Neurosci Res. 2001;39:39–49. doi: 10.1016/s0168-0102(00)00198-x. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Abe S, Ishikawa T, Yamada Y, Yamane GY. Cortical regulation during the early stage of initiation of voluntary swallowing in humans. Dysphagia. 2004;19:100–108. doi: 10.1007/s00455-003-0509-5. [DOI] [PubMed] [Google Scholar]
- Wei C-C, Huang S-W, Hsu S-L, Chen H-C, Chen J-S. Analysis of using tongue deviation angle as a warning sign of a stroke. BioMed Eng Online. 2012;11:53, 1–12. doi: 10.1186/1475-925X-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby EW, Anderson NE. Lower cranial nerve motor function in unilateral vascular lesions of the cerebral hemisphere. Br Med J. 1984;289:791–794. doi: 10.1136/bmj.289.6448.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey CN, Erickson TC, Gilson WE. Localization in somatic sensory and motor areas of human cerebral cortex as determined by direct recording of evoked potentials and electrical stimulation. J Neurosurg. 1979;51:476–506. doi: 10.3171/jns.1979.51.4.0476. [DOI] [PubMed] [Google Scholar]
- Woolsey CN, Settlage PH, Meyer DR, Sencer W, Pinto Hamuy T, Travis AM. Patterns of localization in precentral and “supplementary” motor areas and their relation to the concept of a premotor area. Res Publ Assoc Res Nerv Ment Dis. 1952;30:238–264. [PubMed] [Google Scholar]
- Yamada Y, Yamamura K, Inoue M. Coordination of cranial motoneurons during mastication. Respir Physiol Neurobiol. 2005;147:177–189. doi: 10.1016/j.resp.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. The functional neuroanatomy of voluntary swallowing. Ann Neurol. 1999;46:281–286. [PubMed] [Google Scholar]