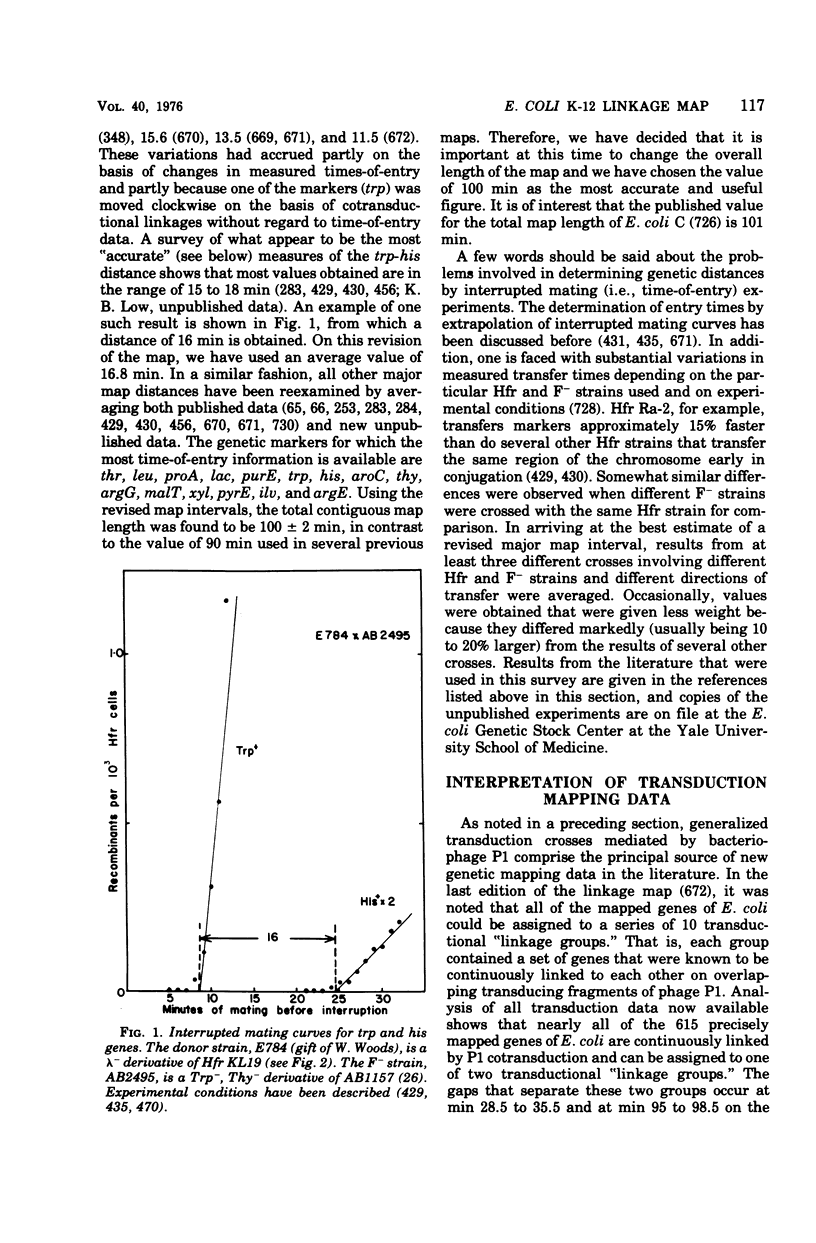
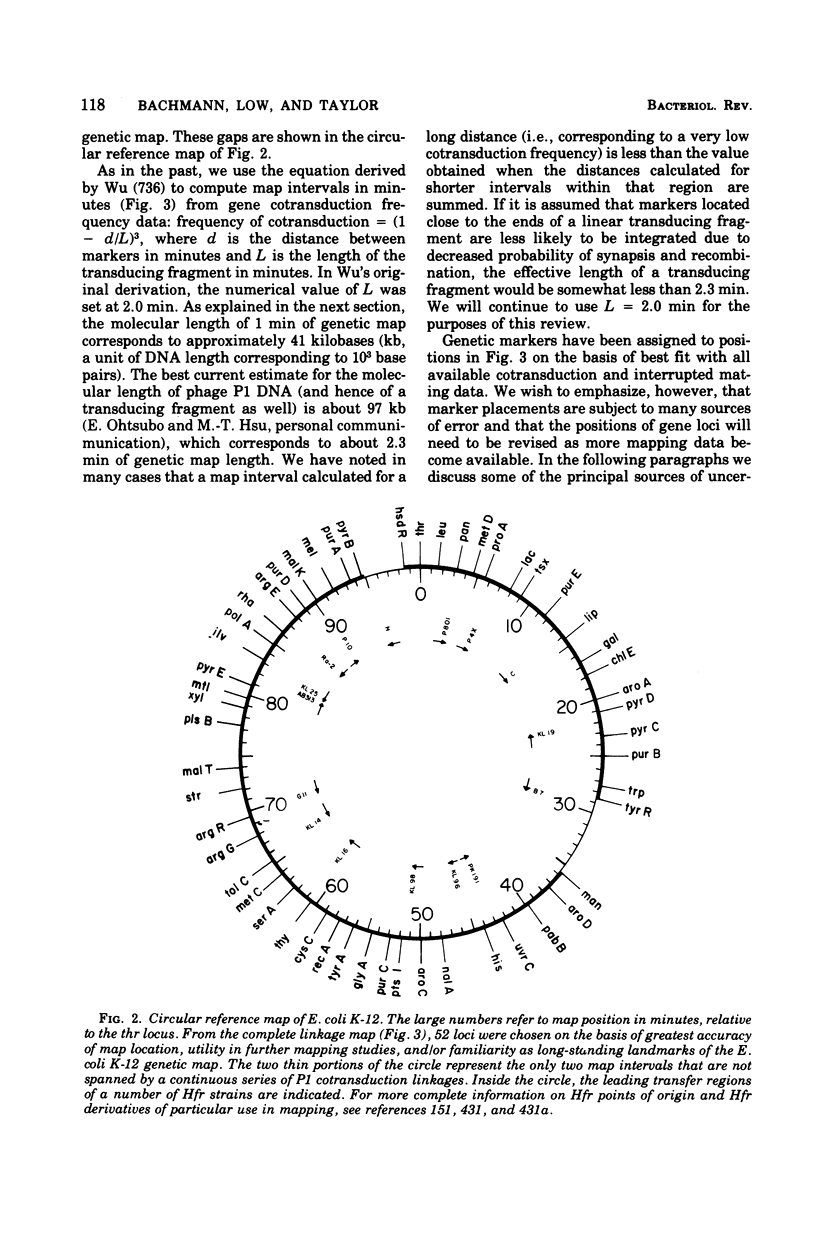
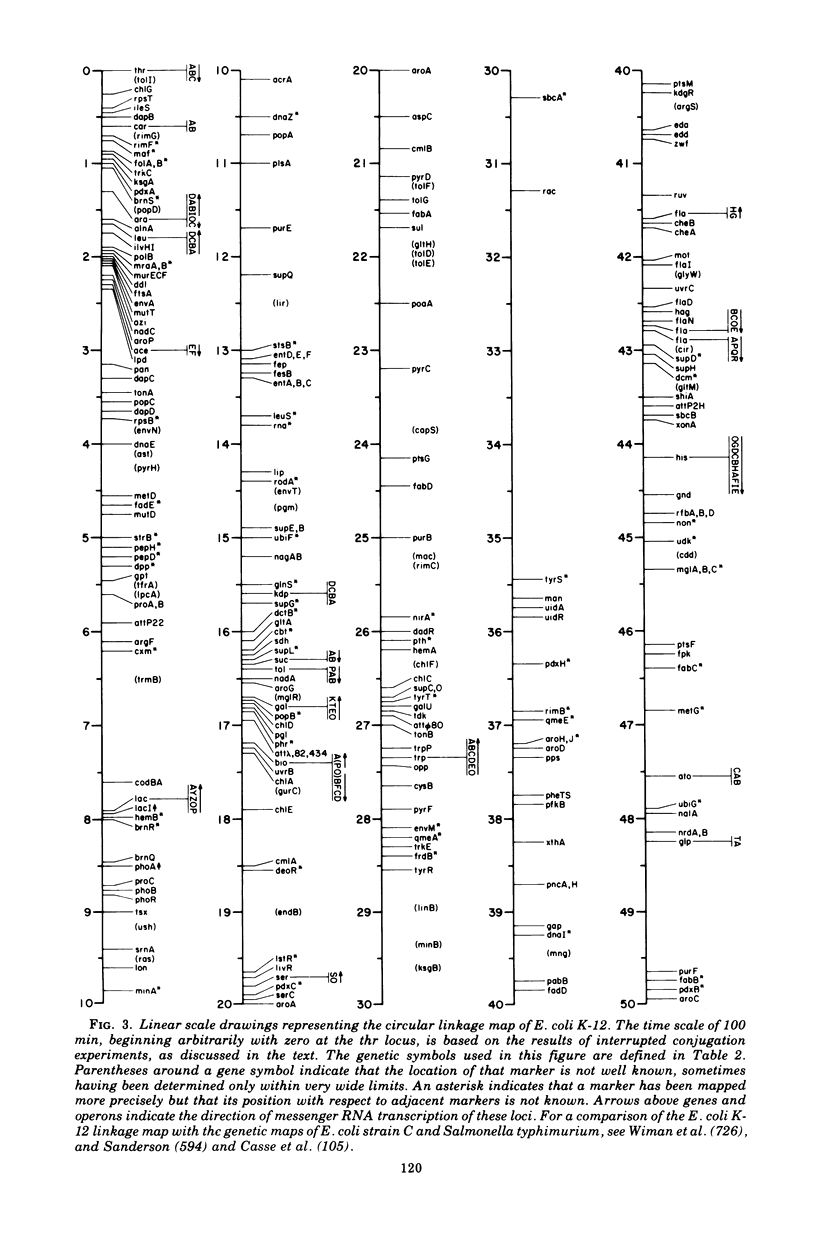
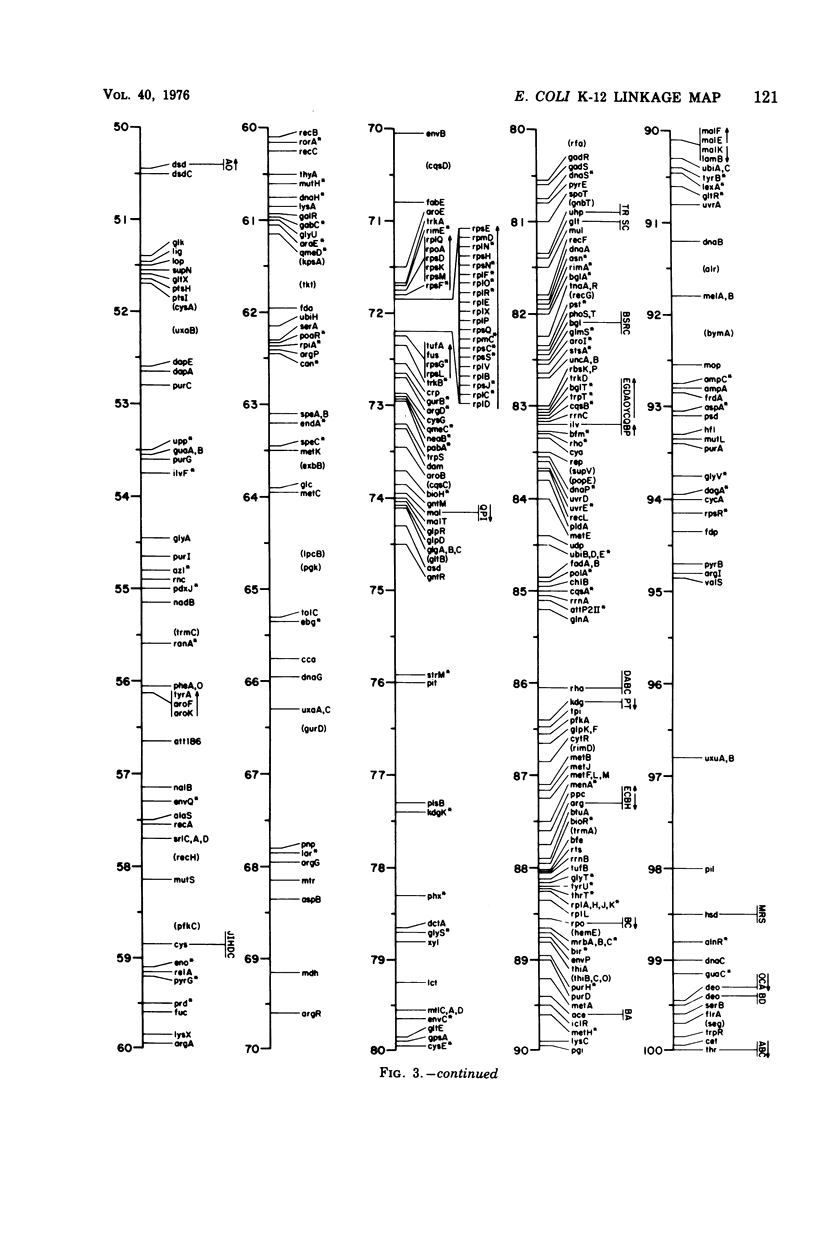
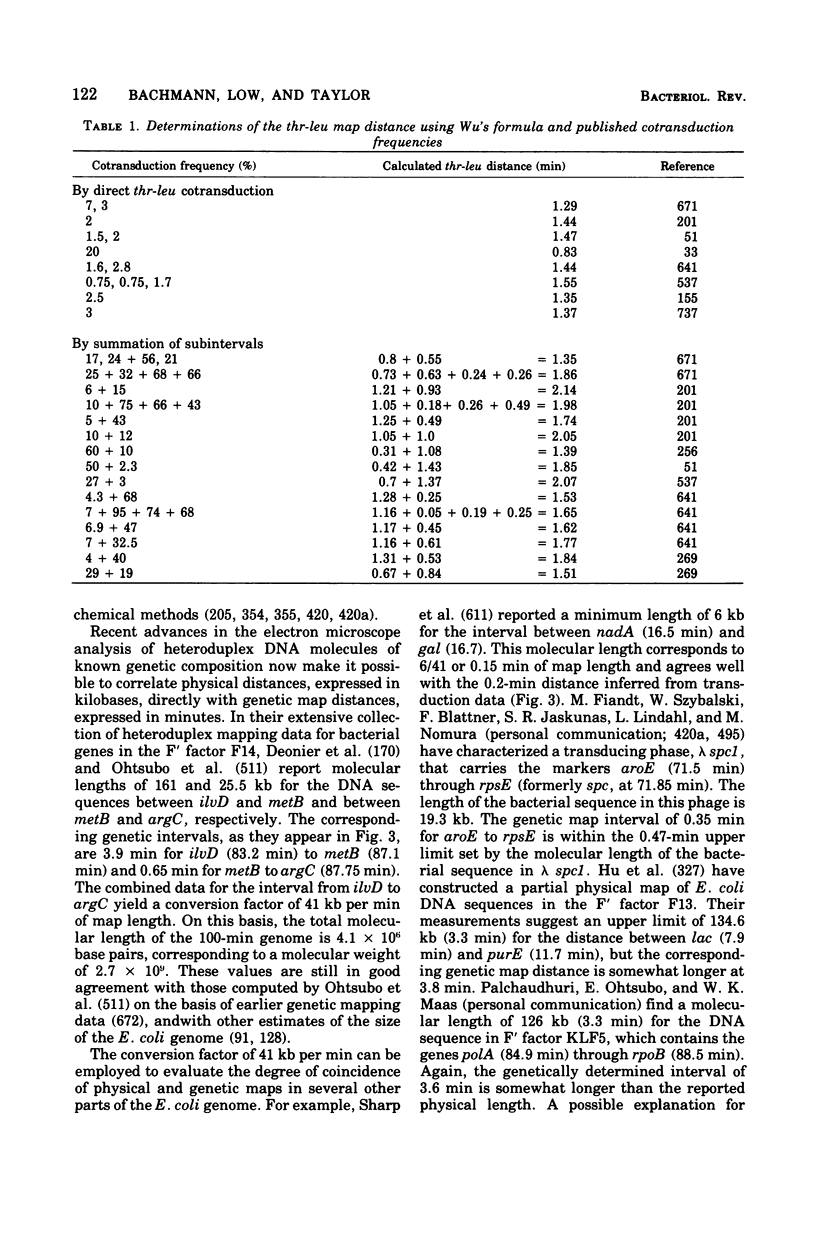
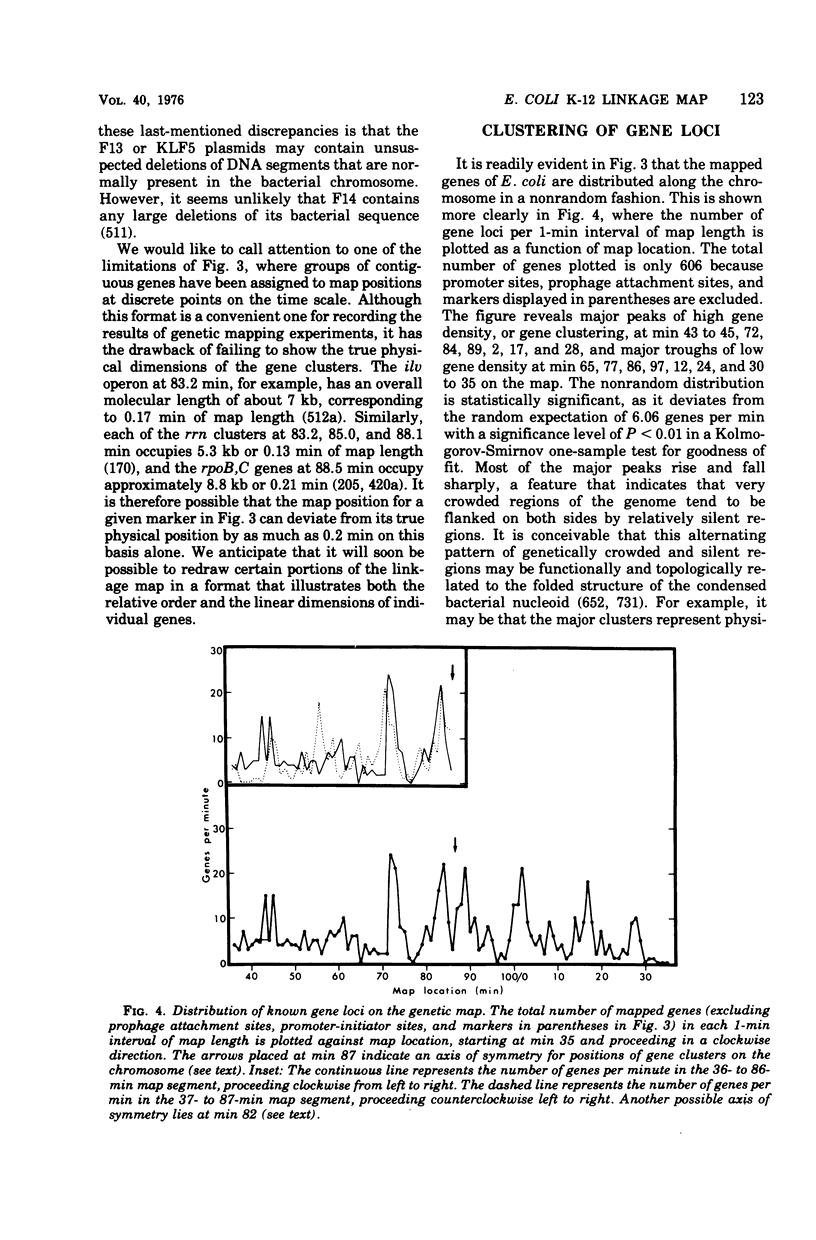
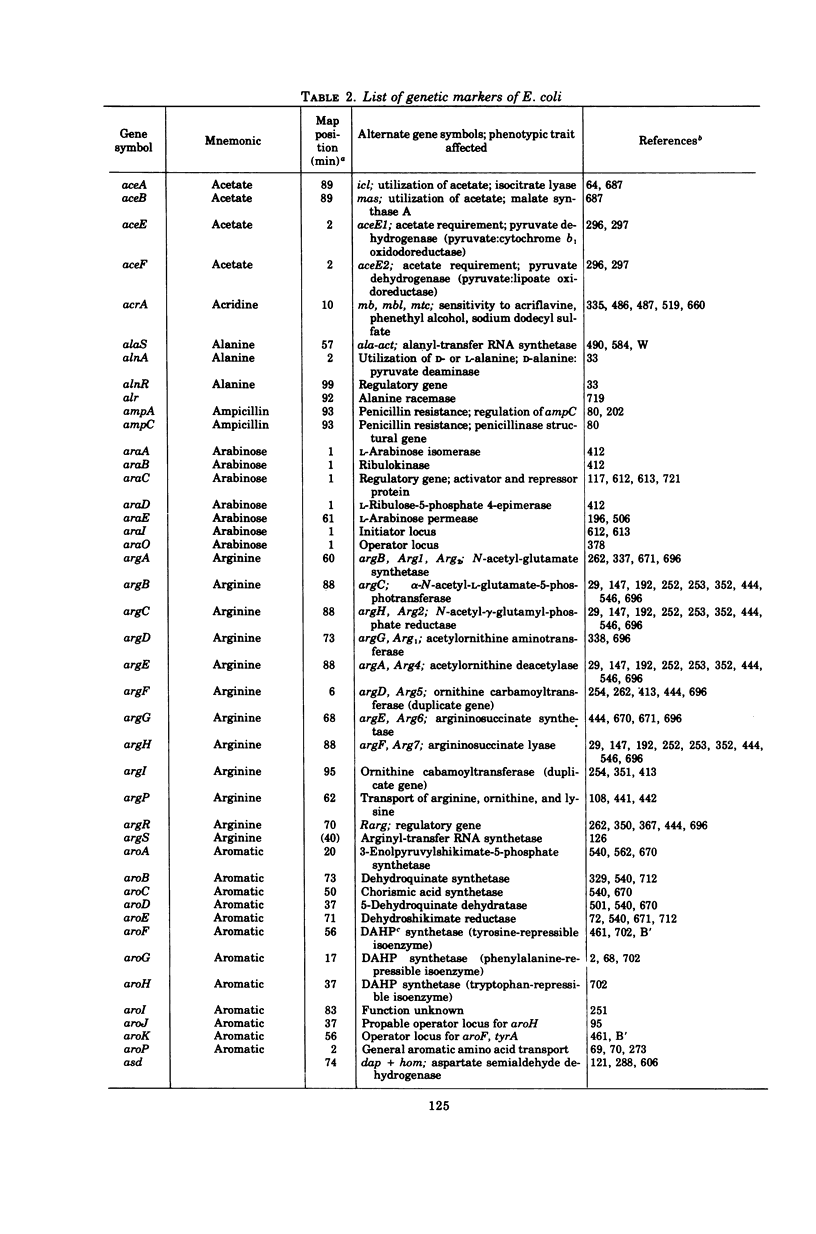
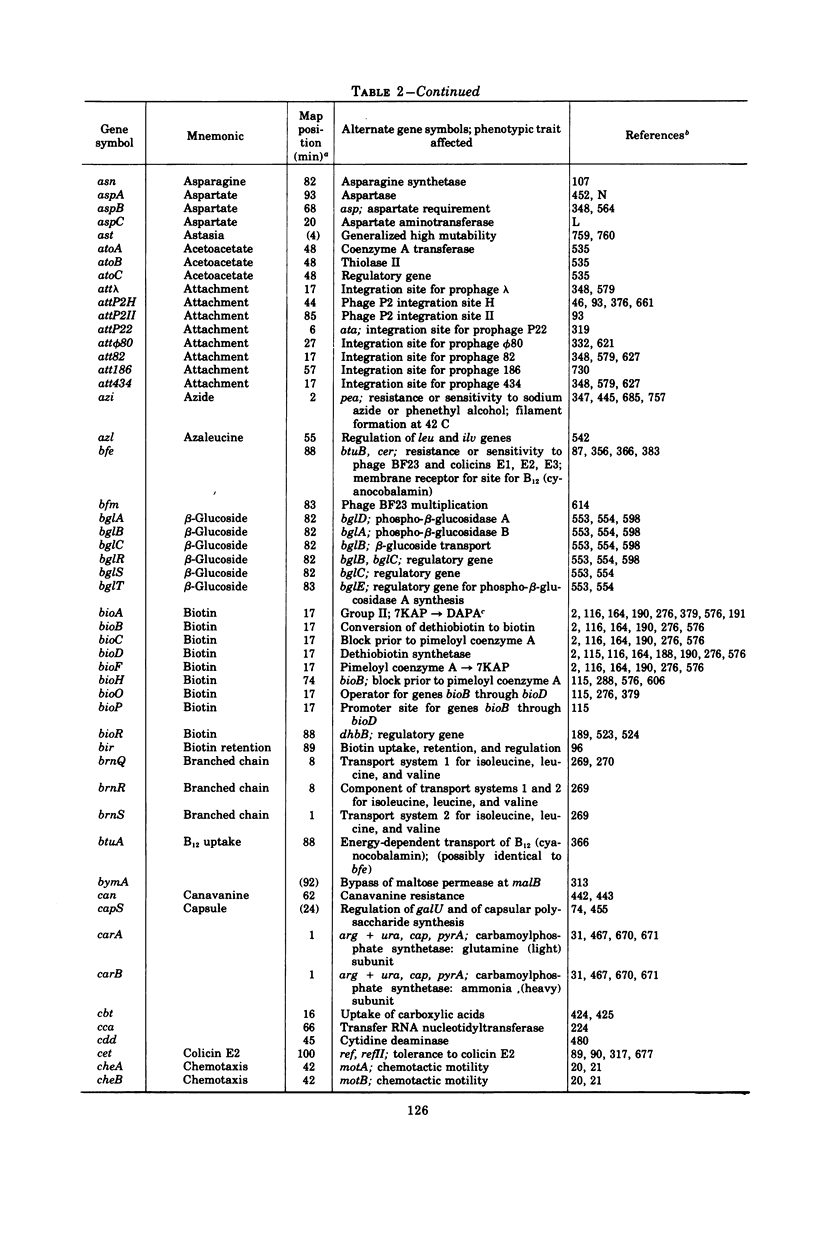
Full text
PDF







































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER J., KAISER A. D. Mapping of the galactose genes of Escherichia coli by transduction with phage P1. Virology. 1963 Feb;19:117–126. doi: 10.1016/0042-6822(63)90001-1. [DOI] [PubMed] [Google Scholar]
- ALFOLDI L., STENT G. S., CLOWES R. C. The chromosomal site of the RNA control (RC) locus in Escherichia coli. J Mol Biol. 1962 Sep;5:348–355. doi: 10.1016/s0022-2836(62)80077-1. [DOI] [PubMed] [Google Scholar]
- ASHWORTH J. M., KORNBERG H. L., NOTHMANN D. L. LOCATION OF THE STRUCTURAL GENE FOR CITRATE SYNTHASE ON THE CHROMOSOME OF ESCHERICHIA COLI K12. J Mol Biol. 1965 Mar;11:654–657. doi: 10.1016/s0022-2836(65)80021-3. [DOI] [PubMed] [Google Scholar]
- Abe M., Okamoto N., Doi O., Nojima S. Genetic mapping of the locus for detergent-resistant phospholipase A(pldA) in Escherichia coli K-12. J Bacteriol. 1974 Aug;119(2):543–546. doi: 10.1128/jb.119.2.543-546.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhya S., Cleary P., Campbell A. A deletion analysis of prophage lambda and adjacent genetic regions. Proc Natl Acad Sci U S A. 1968 Nov;61(3):956–962. doi: 10.1073/pnas.61.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhya S., Schwartz M. Phosphoglucomutase mutants of Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):621–626. doi: 10.1128/jb.108.2.621-626.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S. I., Pritchard R. H. A map of four genes specifying enzymes involved in catabolism of nucleosides and deoxynucleosides in Escherichia coli. Mol Gen Genet. 1969 Aug 15;104(4):351–359. doi: 10.1007/BF00334234. [DOI] [PubMed] [Google Scholar]
- Ahmad S. I., Pritchard R. H. A regulatory mutant affecting the synthesis of enzymes involved in the catabolism of nucleosides in Escherichia coli. Mol Gen Genet. 1971;111(1):77–83. doi: 10.1007/BF00286556. [DOI] [PubMed] [Google Scholar]
- Ahmad S. I., Pritchard R. H. An operator constitutive mutant affecting the synthesis of two enzymes involved in the catabolism of nucleosides in Escherichia coli. Mol Gen Genet. 1973 Aug 28;124(4):321–328. doi: 10.1007/BF00267661. [DOI] [PubMed] [Google Scholar]
- Alikhanian S. I., Iljina T. S., Kaliaeva E. S., Kameneva S. V., Sukhodolec V. V. Mutants of Escherichia coli K12 lacking thymine. Nature. 1965 May 22;206(4986):848–849. doi: 10.1038/206848a0. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Russell R. M., Murray K. N. Characterization of an Escherichia coli mutant deficient in dihydrolipoyl dehydrogenase activity. J Bacteriol. 1973 Jul;115(1):1–8. doi: 10.1128/jb.115.1.1-8.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A., Cooper R. A. Biochemical and genetical studies on ribose catabolism in Escherichia coli K12. J Gen Microbiol. 1970 Aug;62(3):335–339. doi: 10.1099/00221287-62-3-335. [DOI] [PubMed] [Google Scholar]
- Anderson A., Cooper R. A. Genetic mapping of a locus for triosephosphate isomerase on the genome of Escherichia coli K12. J Gen Microbiol. 1970 Aug;62(3):329–334. doi: 10.1099/00221287-62-3-329. [DOI] [PubMed] [Google Scholar]
- Anderson P. Sensitivity and Resistance to Spectinomycin in Escherichia coli. J Bacteriol. 1969 Nov;100(2):939–947. doi: 10.1128/jb.100.2.939-947.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono H., Otsuji N. Genetic mapping of regulator gene phoS for alkaline phosphatase in Escherichia coli. J Bacteriol. 1968 Mar;95(3):1182–1183. doi: 10.1128/jb.95.3.1182-1183.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apirion D., Schlessinger D. Mapping and complementation of three genes specifying 30S ribosomal components in Escherichia coli. J Bacteriol. 1968 Oct;96(4):1431–1432. doi: 10.1128/jb.96.4.1431-1432.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apirion D. Three genes that affect Escherichia coli ribosomes. J Mol Biol. 1967 Dec 14;30(2):255–275. [PubMed] [Google Scholar]
- Apirion D., Watson N. Mapping and characterization of a mutation in Escherichia coli that reduces the level of ribonuclease III specific for double-stranded ribonucleic acid. J Bacteriol. 1975 Oct;124(1):317–324. doi: 10.1128/jb.124.1.317-324.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber W., Linn S. DNA modification and restriction. Annu Rev Biochem. 1969;38:467–500. doi: 10.1146/annurev.bi.38.070169.002343. [DOI] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J. Genetics of motility in Escherichia coli: complementation of paralysed mutants. Genetics. 1967 Jul;56(3):363–373. doi: 10.1093/genetics/56.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J. Location of genes for motility and chemotaxis on the Escherichia coli genetic map. J Bacteriol. 1969 Jan;97(1):156–161. doi: 10.1128/jb.97.1.156-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay E., Puig J. Reconstitution of enzymatically active particles from inactive soluble elements in Escherichia coli K 12. Biochem Biophys Res Commun. 1968 Dec 30;33(6):1019–1024. doi: 10.1016/0006-291x(68)90415-4. [DOI] [PubMed] [Google Scholar]
- BECKWITH J. R., PARDEE A. B., AUSTRIAN R., JACOB F. Coordination of the synthesis of the enzymes in the pyrimidine pathway of E. coli. J Mol Biol. 1962 Dec;5:618–634. doi: 10.1016/s0022-2836(62)80090-4. [DOI] [PubMed] [Google Scholar]
- BOYER H. GENETIC CONTROL OF RESTRICTION AND MODIFICATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Dec;88:1652–1660. doi: 10.1128/jb.88.6.1652-1660.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTIN G. M'ECANISMES R'EGULATEURS DANS LA BIOSYNTH'ESE DES ENZYMES DU M'ETABOLISME DU GALACTOSE CHEZ ESCHERICHIA COLI K12. II. LE D'ETERMINISME G'EN'ETIQUE DE LA R'EGULATION. J Mol Biol. 1963 Aug;7:183–205. doi: 10.1016/s0022-2836(63)80045-5. [DOI] [PubMed] [Google Scholar]
- BUTTIN G. [On the structure of the galactose operon in Escherichia coli K12]. C R Hebd Seances Acad Sci. 1962 Aug 13;255:1233–1235. [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Z., Gilvarg C. Triornithine-resistant strains of Escherichia coli. Isolation, definition, and genetic studies. J Biol Chem. 1974 Jan 10;249(1):143–148. [PubMed] [Google Scholar]
- Barbour S. D., Nagaishi H., Templin A., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. II. Rec+ revertants caused by indirect suppression of rec- mutations. Proc Natl Acad Sci U S A. 1970 Sep;67(1):128–135. doi: 10.1073/pnas.67.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberg S., Ashcroft E. Absence of polar effect of frameshift mutations in the E gene of the Escherichia coli argECBH cluster. J Gen Microbiol. 1971 Dec;69(3):365–373. doi: 10.1099/00221287-69-3-365. [DOI] [PubMed] [Google Scholar]
- Beacham I. R., Kahana R., Levy L., Yagil E. Mutants of Escherichia coli K-12 "cryptic," or deficient in 5'-nucleotidase (uridine diphosphate-sugar hydrolase) and 3'-nucleotidase (cyclic phosphodiesterase) activity. J Bacteriol. 1973 Nov;116(2):957–964. doi: 10.1128/jb.116.2.957-964.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith J. R., Signer E. R., Epstein W. Transposition of the Lac region of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:393–401. doi: 10.1101/sqb.1966.031.01.051. [DOI] [PubMed] [Google Scholar]
- Beelen R. H., Feldmann A. M., Wijsman H. J. A regulatory gene and a structural gene for alaninase in Escherichia coli. Mol Gen Genet. 1973 Mar 19;121(4):369–374. doi: 10.1007/BF00433235. [DOI] [PubMed] [Google Scholar]
- Belfort M., Wulff D. L. Genetic and biochemical investigation of the Escherichia coli mutant hfl-1 which is lysogenized at high frequency by bacteriophage lambda. J Bacteriol. 1973 Jul;115(1):299–306. doi: 10.1128/jb.115.1.299-306.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich M. A. A glutamate-dependent phenotype in E. coli K12: the result of two mutations. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1498–1503. doi: 10.1016/0006-291x(72)90242-2. [DOI] [PubMed] [Google Scholar]
- Berg C. M. Auxotroph accumulation in deoxyribonucleic acid polymeraseless strains of Escherichia coli K-12. J Bacteriol. 1971 Jun;106(3):797–801. doi: 10.1128/jb.106.3.797-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman-Kurtz M., Lin E. C., Richey D. P. Promoter-like mutant with increased expression of the glycerol kinase operon of Escherichia coli. J Bacteriol. 1971 Jun;106(3):724–731. doi: 10.1128/jb.106.3.724-731.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein A., Rolfe B., Onodera K. Pleiotropic properties and genetic organization of the tolA,B locus of Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):74–83. doi: 10.1128/jb.112.1.74-83.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein A. The E. coli cell surface: on the genetic organization of the tolPAB cluster. Mol Gen Genet. 1973;123(2):111–121. doi: 10.1007/BF00267328. [DOI] [PubMed] [Google Scholar]
- Beyersmann D., Messer W., Schlicht M. Mutants of Escherichia coli B-r defective in deoxyribonucleic acid initiation: dnaI, a new gene for replication. J Bacteriol. 1974 Jun;118(3):783–789. doi: 10.1128/jb.118.3.783-789.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum L. S., Kaplan S. Localization of a portion of the ribosomal RNA genes in Escherichia coli. Proc Natl Acad Sci U S A. 1971 May;68(5):925–929. doi: 10.1073/pnas.68.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R., Isaksson L. A. Isolation of mutants of Escherichia coli lac king 5-methyluracil in transfer ribonucleic acid or 1-methylguanine in ribosomal RNA. J Mol Biol. 1970 Jul 14;51(1):83–100. doi: 10.1016/0022-2836(70)90272-x. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Neidhardt F. C. Analysis of 5-methyluridine function in the transfer RNA of Escherichia coli. Cancer Res. 1971 May;31(5):706–709. [PubMed] [Google Scholar]
- Bloom F. R., McFall E., Young M. C., Carothers A. M. Positive control in the D-serine deaminase system of Escherichia coli K-12. J Bacteriol. 1975 Mar;121(3):1092–1101. doi: 10.1128/jb.121.3.1092-1101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T. P1 transduction: formation of heterogenotes upon cotransduction of bacterial genes with a P2 prophage. Virology. 1972 Jan;47(1):76–93. doi: 10.1016/0042-6822(72)90241-3. [DOI] [PubMed] [Google Scholar]
- Bollen A., Faelen M., Lecocq J. P., Herzog A., Zengel J., Kahan L., Nomura M. The structural gene for the ribosomal protein S18 in Escherichia coli. I. Genetic studies on a mutant having an alteration in the protein S18. J Mol Biol. 1973 Jun 5;76(4):463–472. doi: 10.1016/0022-2836(73)90485-3. [DOI] [PubMed] [Google Scholar]
- Bonney R. J., Weinfeld H. Regulation of thymidine metabolism in Escherichia coli K-12: studies on the inducer and the coordinateness of induction of the enzymes. J Bacteriol. 1971 Jun;106(3):812–818. doi: 10.1128/jb.106.3.812-818.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W., Sarvas M. O. Close linkage between a galactose binding protein and the beta-methylgalactoside permease in Escherichia coli. Eur J Biochem. 1970 Apr;13(3):526–533. doi: 10.1111/j.1432-1033.1970.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Boos W. Structurally defective galactose-binding protein isolated from a mutant negative in the -methylgalactoside transport system of Escherichia coli. J Biol Chem. 1972 Sep 10;247(17):5414–5424. [PubMed] [Google Scholar]
- Bourd G. I., Erlagaeva R. S., Bolshakova T. N., Gershanovitch V. N. Glucose catabolite repression in Escherichia coli K12 mutants defective in methyl-alpha-d-glucoside transport. Eur J Biochem. 1975 May 6;53(2):419–427. doi: 10.1111/j.1432-1033.1975.tb04082.x. [DOI] [PubMed] [Google Scholar]
- Bracha M., Yagil E. Location of the genes controlling alkaline phosphatase on the F13 episome of Escherichia coli. J Bacteriol. 1974 Nov;120(2):970–973. doi: 10.1128/jb.120.2.970-973.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A., Grossman L. An endonuclease from Escherichia coli that acts preferentially on UV-irradiated DNA and is absent from the uvrA and uvrB mutants. Proc Natl Acad Sci U S A. 1974 May;71(5):1838–1842. doi: 10.1073/pnas.71.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge L., Gorini L. Genetic analysis of streptomycin resistance in Escherichia coli. Genetics. 1970 May;65(1):9–25. doi: 10.1093/genetics/65.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeze A. S., Sims P., Stacey K. A. Trimethoprim-resistant mutants of E. coli K12: preliminary genetic mapping. Genet Res. 1975 Jun;25(3):207–214. doi: 10.1017/s0016672300015640. [DOI] [PubMed] [Google Scholar]
- Brice C. B., Kornberg H. L. Genetic control of isocitrate lyase activity in Escherichia coli. J Bacteriol. 1968 Dec;96(6):2185–2186. doi: 10.1128/jb.96.6.2185-2186.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice C. B., Kornberg H. L. Location on the chromosome of Escherichia coli of a gene specifying phosphopyruvate synthase activity. Biochim Biophys Acta. 1967 Mar 22;136(2):412–414. doi: 10.1016/0304-4165(67)90094-3. [DOI] [PubMed] [Google Scholar]
- Broda P., Collins J. F. Gross map distances and Hfr transfer times in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):747–752. doi: 10.1128/jb.117.2.747-752.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broda P. Modified map positions for lac and the pro markers in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):741–746. doi: 10.1128/jb.117.2.741-746.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman J. H., Hoekstra W. P. Mapping of fabC, a locus for the biosynthesis of unsaturated fatty acids in Escherichia coli K12. Mol Gen Genet. 1973 Jul 31;124(1):65–67. doi: 10.1007/BF00267165. [DOI] [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Maintenance and exchange of the aromatic amino acid pool in Escherichia coli. J Bacteriol. 1971 Apr;106(1):70–81. doi: 10.1128/jb.106.1.70-81.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Regulation of aromatic amino acid biosynthesis Escherichia coli K12. Genetics. 1968 Sep;60(1):31–48. doi: 10.1093/genetics/60.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Somerville R. L. Repression of aromatic amino acid biosynthesis in Escherichia coli K-12. J Bacteriol. 1971 Oct;108(1):386–399. doi: 10.1128/jb.108.1.386-399.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. E., Apirion D. Mapping a cluster of ribosomal genes in Escherichia coli. Mol Gen Genet. 1974;133(4):317–327. doi: 10.1007/BF00332707. [DOI] [PubMed] [Google Scholar]
- Bryant R. E., Sypherd P. S. Genetic analysis of cold-sensitive ribosome maturation mutants of Escherichia coli. J Bacteriol. 1974 Mar;117(3):1082–1092. doi: 10.1128/jb.117.3.1082-1092.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Markovitz A. Derepression of uridine diphosphate-glucose pyrophosphorylase (galU) in capR(lon), capS, and capT mutants and studies on the galU repressor. J Bacteriol. 1973 Sep;115(3):1011–1020. doi: 10.1128/jb.115.3.1011-1020.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Taylor A. L. Genetic analysis of diaminopimelic acid- and lysine-requiring mutants of Escherichia coli. J Bacteriol. 1971 Mar;105(3):844–854. doi: 10.1128/jb.105.3.844-854.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Taylor A. L. Mutants of Escherichia coli with a growth requirement for either lysine or pyridoxine. J Bacteriol. 1971 Mar;105(3):988–998. doi: 10.1128/jb.105.3.988-998.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore V., Harris M. H., Schlesinger S. Properties of tyrosyl transfer ribonucleic acid synthetase from two tyrS mutants of Escherichia coli K-12. J Biol Chem. 1972 Aug 10;247(15):4843–4849. [PubMed] [Google Scholar]
- Burman L. G., Nordström K. Colicin tolerance induced by ampicillin or mutation to ampicillin resistance in a strain of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):1–13. doi: 10.1128/jb.106.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Park J. T., Lindström E. B., Boman H. G. Resistance of Escherichia coli to penicillins: identification of the structural gene for the chromosomal penicillinase. J Bacteriol. 1973 Oct;116(1):123–130. doi: 10.1128/jb.116.1.123-130.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister M. Chromosomal Location of a Gene Involved in Potassium Ion Uptake in Escherichia coli B. J Bacteriol. 1969 Nov;100(2):796–802. doi: 10.1128/jb.100.2.796-802.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush J. W., Markovitz A. The Genetic Basis for Mucoidy and Radiation Sensitivity in capR (lon) Mutants of E. coli K-12. Genetics. 1973 Jun;74(2):215–225. doi: 10.1093/genetics/74.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K-12: the genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim Biophys Acta. 1973 Feb 22;292(2):366–375. doi: 10.1016/0005-2728(73)90043-1. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R. S. Genetic analysis of Escherichia coli K12 mutants resistant to bacteriophage BF23 and the E-group colicins. Mol Gen Genet. 1971;113(2):154–156. doi: 10.1007/BF00333188. [DOI] [PubMed] [Google Scholar]
- Buxton R. S. Genetic analysis of thymidine-resistant and low-thymine-requiring mutants of Escherichia coli K-12 induced by bacteriophage Mu-1. J Bacteriol. 1975 Feb;121(2):475–484. doi: 10.1128/jb.121.2.475-484.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R. S., Holland I. B. Genetic studies of tolerance to colicin E2 in Escherichia coli K-12. I. Re-location and dominance relationships of cet mutations. Mol Gen Genet. 1973 Dec 14;127(1):69–88. doi: 10.1007/BF00267784. [DOI] [PubMed] [Google Scholar]
- Buxton R. S., Holland I. B. Genetic studies of tolerance to colicin E2 in Escherichia coli K-12. II. Multiple mutations as a cause of the various phenotypic properties of cet minus mutants. Mol Gen Genet. 1974;131(2):159–171. doi: 10.1007/BF00266151. [DOI] [PubMed] [Google Scholar]
- Böck A., Faiman L. E., Neidhardt F. C. Biochemical and genetic characterization of a mutant of Escherichia coli with a temperature-sensitive valyl ribonucleic acid synthetase. J Bacteriol. 1966 Oct;92(4):1076–1082. doi: 10.1128/jb.92.4.1076-1082.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Genetic mapping of phenylalanyl-sRNA synthetase in Escherichia coli. Science. 1967 Jul 7;157(3784):78–79. doi: 10.1126/science.157.3784.78. [DOI] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Isolation of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase Activity. J Bacteriol. 1966 Aug;92(2):464–469. doi: 10.1128/jb.92.2.464-469.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Location of the structural gene for glycyl ribonucleic acid synthetase by means of a strain of Escherichia coli possessing an unusual enzyme. Z Vererbungsl. 1966;98(3):187–192. doi: 10.1007/BF00888946. [DOI] [PubMed] [Google Scholar]
- Böck A., Ruffler D., Piepersberg W., Wittmann H. G. Genetic analysis of an alteration of ribosomal protein S20 in revertants of an alanyl-tRNA-synthetase mutant of Escherichia coli. Mol Gen Genet. 1974;134(4):325–332. doi: 10.1007/BF00337467. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calendar R., Lindahl G. Attachment of prophage P2: gene order at different host chromosomal sites. Virology. 1969 Dec;39(4):867–881. doi: 10.1016/0042-6822(69)90023-3. [DOI] [PubMed] [Google Scholar]
- Calendar R., Lindqvist B., Sironi G., Clark A. J. Characterization of REP- mutants and their interaction with P2 phage. Virology. 1970 Jan;40(1):72–83. doi: 10.1016/0042-6822(70)90380-6. [DOI] [PubMed] [Google Scholar]
- Camakaris H., Pittard J. Regulation of tyrosine and phenylalanine biosynthesis in Escherichia coli K-12: properties of the tyrR gene product. J Bacteriol. 1973 Sep;115(3):1135–1144. doi: 10.1128/jb.115.3.1135-1144.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camakaris J., Pittard J. Repression of 3-deoxy-D-arabinoheptulosonic acid-7-phosphate synthetase (trp) and enzymes of the tryptophan pathway in Escherichia coli K-12. J Bacteriol. 1971 Aug;107(2):406–414. doi: 10.1128/jb.107.2.406-414.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A., Del Campillo-Campbell A., Chang R. A mutant of Escherichia coli that requires high concentrations of biotin. Proc Natl Acad Sci U S A. 1972 Mar;69(3):676–680. doi: 10.1073/pnas.69.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. H., Lengyel J. A., Langridge J. Evolution of a second gene for beta-galactosidase in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1841–1845. doi: 10.1073/pnas.70.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L., Shizuya H., Richardson C. C. Mapping of a mutation, polB100, affecting deoxyribonucleic acid polymerase II in Escherichia coli K-12. J Bacteriol. 1974 Aug;119(2):494–499. doi: 10.1128/jb.119.2.494-499.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L., Soll L., Richardson C. C. Isolation and partial characterization of a mutant of Escherichia coli deficient in DNA polymerase II. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2090–2094. doi: 10.1073/pnas.69.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M., Cabezon T., Bollen A. Mapping of neamine resistance: identification to two genetic loci, nea A and nea B. Mol Gen Genet. 1974 Jun 27;130(4):321–326. doi: 10.1007/BF00333871. [DOI] [PubMed] [Google Scholar]
- Carbon J., Squires C. Studies on genetically altered transfer RNA species in Escherichia coli. Cancer Res. 1971 May;31(5):663–666. [PubMed] [Google Scholar]
- Cardelli J., Konisky J. Isolation and characterization of an Escherichia coli mutant tolerant to colicins Ia and Ib. J Bacteriol. 1974 Aug;119(2):379–385. doi: 10.1128/jb.119.2.379-385.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl P. L. Escherichia coli mutants with temperature-sensitive synthesis of DNA. Mol Gen Genet. 1970;109(2):107–122. doi: 10.1007/BF00269647. [DOI] [PubMed] [Google Scholar]
- Casse F., Pascal M. C., Chippaux M. Comparison between the chromosomal maps of Escherichia coli and Salmonella typhimurium. Length of the inverted segment in the trp region. Mol Gen Genet. 1973 Aug 17;124(3):253–257. doi: 10.1007/BF00293096. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Cattanéo J., Damotte M., Sigal N., Sanchez-Medina F., Puig J. Genetic studies of Escherichia coli K 12 mutants with alterations in glycogenesis and properties of an altered adenosine diphosphate glucose pyrophosphorylase. Biochem Biophys Res Commun. 1969 Mar 10;34(5):694–701. doi: 10.1016/0006-291x(69)90794-3. [DOI] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. The asparagine synthetase of Escherhic coli. I. Biosynthetic role of the enzyme, purification, and characterization of the reaction products. J Biol Chem. 1969 Aug 10;244(15):4112–4121. [PubMed] [Google Scholar]
- Celis T. F., Rosenfeld H. J., Maas W. K. Mutant of Escherichia coli K-12 defective in the transport of basic amino acids. J Bacteriol. 1973 Nov;116(2):619–626. doi: 10.1128/jb.116.2.619-626.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelala C. A., Margolin P. Effects of deletions on cotransduction linkage in Salmonella typhimurium: evidence that bacterial chromosome deletions affect the formation of transducing DNA fragments. Mol Gen Genet. 1974;131(2):97–112. doi: 10.1007/BF00266146. [DOI] [PubMed] [Google Scholar]
- Clarke S. J., Low B., Konigsberg W. H. Close linkage of the genes serC (for phosphohydroxy pyruvate transaminase) and serS (for seryl-transfer ribonucleic acid synthetase) in Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1091–1095. doi: 10.1128/jb.113.3.1091-1095.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. J., Low B., Konigsberg W. Isolation and characterization of a regulatory mutant of an aminoacyl-transfer ribonucleic acid synthetase in Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1096–1103. doi: 10.1128/jb.113.3.1096-1103.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary P. P., Campbell A., Chang R. Location of promoter and operator sites in the biotin gene cluster of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2219–2223. doi: 10.1073/pnas.69.8.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary P. P., Campbell A. Deletion and complementation analysis of biotin gene cluster of Escherichia coli. J Bacteriol. 1972 Nov;112(2):830–839. doi: 10.1128/jb.112.2.830-839.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary P. P., Englesberg E. Transcriptional control in the L-arabinose operon of Escherichia coli B-r. J Bacteriol. 1974 Apr;118(1):121–128. doi: 10.1128/jb.118.1.121-128.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. A., Ward F. B. Nitrite reductase-deficient mutants of Escherichia coli K12. J Gen Microbiol. 1973 May;76(1):21–29. doi: 10.1099/00221287-76-1-21. [DOI] [PubMed] [Google Scholar]
- Colson C., Glover S. W., Symonds N., Stacey K. A. The location of the genes for host-controlled modification and restriction in Escherichia coli K-12. Genetics. 1965 Nov;52(5):1043–1050. doi: 10.1093/genetics/52.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine H. Mutants des voies de biosynthèse et de dégradation de la proline chez E. coli K 12. Ann Inst Pasteur (Paris) 1971 Jan;120(1):9–22. [PubMed] [Google Scholar]
- Condamine H. Sur la régulation de la production de proline chez E. Coli K 12. Ann Inst Pasteur (Paris) 1971 Feb;120(2):126–143. [PubMed] [Google Scholar]
- Cooper P. H., Hirshfield I. N., Maas W. K. Map location of arginyl-tRNA synthetase mutations in Escherichia coli K-12. Mol Gen Genet. 1969 Aug 15;104(4):383–390. doi: 10.1007/BF00334238. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Cooper S. Utilization of d-Methionine by Escherichia coli. J Bacteriol. 1966 Aug;92(2):328–332. doi: 10.1128/jb.92.2.328-332.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F., Takahashi H. Abortive bacteriophage T4 head assembly in mutants of Escherichia coli. J Mol Biol. 1973 May 5;76(1):61–87. doi: 10.1016/0022-2836(73)90081-8. [DOI] [PubMed] [Google Scholar]
- Cosloy S. D. D-serine transport system in Escherichia coli K-12. J Bacteriol. 1973 May;114(2):679–684. doi: 10.1128/jb.114.2.679-684.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E. C., Degnen G. E., Scheppe M. L. Mutator gene studies in Escherichia coli: the mutS gene. Genetics. 1972 Dec;72(4):551–567. doi: 10.1093/genetics/72.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E. C., Yanofsky C. Mutator gene studies in Escherichia coli. J Bacteriol. 1969 Oct;100(1):390–397. doi: 10.1128/jb.100.1.390-397.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., Luke R. K., Newton N. A., O'Brien I. G., Rosenberg H. Mutations affecting iron transport in Escherichia coli. J Bacteriol. 1970 Oct;104(1):219–226. doi: 10.1128/jb.104.1.219-226.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., Pittard J. Mutant strains of Escherichia coli K-12 unable to form ubiquinone. J Bacteriol. 1968 May;95(5):1591–1598. doi: 10.1128/jb.95.5.1591-1598.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Young I. G., McCann L. M., Gibson F. Biosynthesis of ubiquinone in Escherichia coli K-12: location of genes affecting the metabolism of 3-octaprenyl-4-hydroxybenzoic acid and 2-octaprenylphenol. J Bacteriol. 1969 Aug;99(2):450–458. doi: 10.1128/jb.99.2.450-458.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Charles H. P. Porphyrin-accumulating mutants of Escherichia coli. J Bacteriol. 1973 Jan;113(1):122–132. doi: 10.1128/jb.113.1.122-132.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Lin E. C. Chromosomal location of the structural gene for glycerol kinase in Escherichia coli. J Bacteriol. 1966 May;91(5):1763–1766. doi: 10.1128/jb.91.5.1763-1766.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P. Gene rearrangements in the evolution of the tryptophan synthetic pathway. Bacteriol Rev. 1975 Jun;39(2):87–120. doi: 10.1128/br.39.2.87-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Preiss J. Distribution of closely linked markers following intragenic recombination in Escherichia coli. J Mol Biol. 1972 Nov 28;71(3):717–733. doi: 10.1016/s0022-2836(72)80034-2. [DOI] [PubMed] [Google Scholar]
- Creaghan I. T., Guest J. R. Amber mutants of the -ketoglutarate dehydrogenase gene of Escherichia coli K12. J Gen Microbiol. 1972 Jul;71(2):207–220. doi: 10.1099/00221287-71-2-207. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: mapping of sn-glycerol 3-phosphate acyltransferase Km mutants. J Bacteriol. 1974 Oct;120(1):227–233. doi: 10.1128/jb.120.1.227-233.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: mapping of the structural gene for L-glycerol 3-phosphate dehydrogenase. J Bacteriol. 1974 May;118(2):598–605. doi: 10.1128/jb.118.2.598-605.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Birge C. H., Vagelos P. R. Evidence for two genes specifically involved in unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1969 Nov;100(2):601–604. doi: 10.1128/jb.100.2.601-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Godson G. N. Mutants of Escherichia coli with temperature-sensitive lesions in membrane phospholipid synthesis: genetic analysis of glycerol-3-phosphate acyltransferase mutants. Mol Gen Genet. 1972;116(3):199–210. doi: 10.1007/BF00269765. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Silbert D. F., Wulff D. L. Mapping of the fabA locus for unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1972 Oct;112(1):206–211. doi: 10.1128/jb.112.1.206-211.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunin R., Elseviers D., Sand G., Freundlich G., Glandsdorff N. On the functional organization of the arg ECBH cluster of genes in Escherichia coli K-12. Mol Gen Genet. 1969;106(1):32–47. doi: 10.1007/BF00332819. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles S., Maas W. K. Isolation, characterization, and mapping of Escherichia coli mutants blocked in the synthesis of ornithine decarboxylase. J Bacteriol. 1975 Nov;124(2):791–799. doi: 10.1128/jb.124.2.791-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Berg C. M., Harris P. E. Kinetic and genetic analyses of D-cycloserine inhibition and resistance in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1238–1250. doi: 10.1128/jb.90.5.1238-1250.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOY C. H., RIVERA A., Jr, SRINIVASAN P. R. Evidence for the enzymatic synthesis of N-(5'-phosphoribosyl) anthranilic acid, a new intermediate in tryptophan biosynthesis. Biochem Biophys Res Commun. 1961 Jan 25;4:83–88. doi: 10.1016/0006-291x(61)90261-3. [DOI] [PubMed] [Google Scholar]
- Dahl R., Wang R. J., Morse M. L. Effect of pleiotropic carbohydrate mutations (ctr) on tryptophan catabolism. J Bacteriol. 1971 Aug;107(2):513–518. doi: 10.1128/jb.107.2.513-518.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Jacob F. Genetic mapping of the regulator and operator genes of the lac operon. J Mol Biol. 1968 Sep 28;36(3):413–417. doi: 10.1016/0022-2836(68)90165-4. [DOI] [PubMed] [Google Scholar]
- De Felice M., Guardiola J., Esposito B., Iaccarino M. Structural genes for a newly recognized acetolactate synthase in Escherichia coli K-12. J Bacteriol. 1974 Dec;120(3):1068–1077. doi: 10.1128/jb.120.3.1068-1077.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M., Guardiola J., Lamberti A., Iaccarino M. Escherichia coli K-12 mutants altered in the transport systems for oligo- and dipeptides. J Bacteriol. 1973 Nov;116(2):751–756. doi: 10.1128/jb.116.2.751-756.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M., Guardiola J., Malorni M. C., Klopotowski T., Iaccarino M. Regulation of the pool size of valine in Escherichia coli K-12. J Bacteriol. 1974 Dec;120(3):1058–1067. doi: 10.1128/jb.120.3.1058-1067.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan P. G., Hoekstra W. P., Verhoef C., Felix H. S. Recombination in Escherichia coli. 3. Mapping by the gradient of transmission. Mutat Res. 1969 Nov-Dec;8(3):505–512. doi: 10.1016/0027-5107(69)90067-0. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- De Wilde M. Identification de la substitution d'acide aminé responsable de la résistance à la Spectinomycine chez un mutant d'Escherichia coli. Arch Int Physiol Biochim. 1973 May;81(2):369–369. [PubMed] [Google Scholar]
- DeWilde M., Michel F., Broman K. The structural gene for ribosomal protein S18 in Escherichia coli. III. Mapping outside the ribosomal protein gene cluster at minute 84 on the genome. Mol Gen Genet. 1974;133(4):329–333. doi: 10.1007/BF00332708. [DOI] [PubMed] [Google Scholar]
- Degnen G. E., Cox E. C. Conditional mutator gene in Escherichia coli: isolation, mapping, and effector studies. J Bacteriol. 1974 Feb;117(2):477–487. doi: 10.1128/jb.117.2.477-487.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekio S. Genetic studies of the ribosomal proteins in Escherichia coli. 7. Mapping of several ribosomal protein components by transduction experiments between different strains of Escherichia coli. Mol Gen Genet. 1971;113(1):20–30. [PubMed] [Google Scholar]
- Del Campillo-Campbell A., Kayajanian G., Campbell A., Adhya S. Biotin-requiring mutants of Escherichia coli K-12. J Bacteriol. 1967 Dec;94(6):2065–2066. doi: 10.1128/jb.94.6.2065-2066.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B. Characterization of pyridoxine auxotrophs of Escherichia coli: chromosomal position of linkage group I. J Bacteriol. 1969 Oct;100(1):295–300. doi: 10.1128/jb.100.1.295-300.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B., Ito H. Characterization of pyridoxine auxotrophs of Escherichia coli: serine and pdxF mutants. J Bacteriol. 1970 Nov;104(2):658–667. doi: 10.1128/jb.104.2.658-667.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Iwaya M., Larison L. L. The rep mutation. II. Its effect on Escherichia coli and on the replication of bacteriophage phi X174. Virology. 1972 Aug;49(2):486–496. doi: 10.1016/0042-6822(72)90500-4. [DOI] [PubMed] [Google Scholar]
- Deonier R. C., Otsubo E., Lee H. J., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. VII. Mapping the ribosomal RNA genes of plasmid F14. J Mol Biol. 1974 Nov 15;89(4):619–629. doi: 10.1016/0022-2836(74)90039-4. [DOI] [PubMed] [Google Scholar]
- Dickinson E. S., Sundaram T. K. Chromosomal location of a gene defining nicotinamide deamidase in Escherichia coli. J Bacteriol. 1970 Mar;101(3):1090–1091. doi: 10.1128/jb.101.3.1090-1091.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donch J. J., Chung Y. S., Green M. H., Greenberg J., Warren G. Genetic analysis of sul mutants of Escherichia coli B. Genet Res. 1971 Jun;17(3):185–193. doi: 10.1017/s0016672300012210. [DOI] [PubMed] [Google Scholar]
- Donch J., Greenberg J. Genetic analysis of lon mutants of strain K-12 of Escherichia coli. Mol Gen Genet. 1968;103(2):105–115. doi: 10.1007/BF00427138. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F., Pace N. R. Transcriptional organization of the ribosomal RNA cistrons in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1786–1790. doi: 10.1073/pnas.68.8.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M. W., Ward F. B., Cole J. A. The formate hydrogenlyase activity of cytochrome c552-deficient mutants of Escherichia coli K12. J Gen Microbiol. 1974 Feb;80(2):557–560. doi: 10.1099/00221287-80-2-557. [DOI] [PubMed] [Google Scholar]
- Dover S., Halpern Y. S. Genetic analysis of the gamma-aminobutyrate utilization pathway in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):494–501. doi: 10.1128/jb.117.2.494-501.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- Egan A. F., Russell R. R. Conditional mutations affecting the cell envelope of Escherichia coli K-12. Genet Res. 1973 Apr;21(2):139–152. doi: 10.1017/s001667230001332x. [DOI] [PubMed] [Google Scholar]
- Eggertsson G., Adelberg E. A. Map positions and specificities of suppressor mutations in Escherichia coli K-12. Genetics. 1965 Aug;52(2):319–340. doi: 10.1093/genetics/52.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggertsson G. Mapping of ochre suppressors in Escherichia coli. Genet Res. 1968 Feb;11(1):15–20. doi: 10.1017/s0016672300011150. [DOI] [PubMed] [Google Scholar]
- Eggertsson G. Suppressors causing temperature sensitivity of growth in Escherichia coli. Genetics. 1968 Oct;60(2):269–280. doi: 10.1093/genetics/60.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M. A., Krell K. Dethiobiotin synthesis from 7,8-diaminolargonic acid in cell-free extracts of a biotin auxotroph of Escherichia coli K-12. J Biol Chem. 1969 Oct 25;244(20):5503–5509. [PubMed] [Google Scholar]
- Eisenberg M. A., Star C. Synthesis of 7-oxo-8-aminopelargonic acid, a biotin vitamer, in cell-free extracts of Escherichia coli biotin auxotrophs. J Bacteriol. 1968 Oct;96(4):1291–1297. doi: 10.1128/jb.96.4.1291-1297.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M. A., Stoner G. L. Biosynthesis of 7,8-diaminopelargonic acid, a biotin intermediate, from 7-keto-8-aminopelargonic acid and S-adenosyl-L-methionine. J Bacteriol. 1971 Dec;108(3):1135–1140. doi: 10.1128/jb.108.3.1135-1140.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenburg M. A., Mee B., Prakash O., Eisenburg M. R. Properties of alpha-dehydrobiotin-resistant mutants of Escherichia coli K-12. J Bacteriol. 1975 Apr;122(1):66–72. doi: 10.1128/jb.122.1.66-72.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elseviers D., Cunin R., Glansdorff N. Control regions within the argECBH gene cluster of Escherichia coli K12. Mol Gen Genet. 1972;117(4):349–366. doi: 10.1007/BF00333028. [DOI] [PubMed] [Google Scholar]
- Elseviers D., Gorini L. Direct selection of mutants restricting efficiency of suppression and misreading levels in E. coli B. Mol Gen Genet. 1975;137(4):277–287. doi: 10.1007/BF00703254. [DOI] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Irr J., Power J., Lee N. Positive control of enzyme synthesis by gene C in the L-arabinose system. J Bacteriol. 1965 Oct;90(4):946–957. doi: 10.1128/jb.90.4.946-957.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Davies M. Potassium-dependant mutants of Escherichia coli K-12. J Bacteriol. 1970 Mar;101(3):836–843. doi: 10.1128/jb.101.3.836-843.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Fox C. F. Mapping of a locus for unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1970 Jul;103(1):273–274. doi: 10.1128/jb.103.1.273-274.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Jewett S., Fox C. F. Isolation and mapping of phosphotransferase mutants in Escherichia coli. J Bacteriol. 1970 Nov;104(2):793–797. doi: 10.1128/jb.104.2.793-797.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Kim B. S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Nordström K. Genetics and physiology of a tolE mutant of Escherichia coli K-12 and phenotypic suppression of its phenotype by galactose. J Bacteriol. 1973 Sep;115(3):1219–1222. doi: 10.1128/jb.115.3.1219-1222.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. II. An improved mapping of the ampA gene. Genet Res. 1968 Oct;12(2):147–156. doi: 10.1017/s0016672300011769. [DOI] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. R., Nordström K., Englund P. Resistance of Escherichia coli to penicillins. IX. Genetics and physiology of class II ampicillin-resistant mutants that are galactose negative or sensitive to bacteriophage C21, or both. J Bacteriol. 1971 Dec;108(3):1210–1223. doi: 10.1128/jb.108.3.1210-1223.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington L., Glass R. E., Hayward R. S., Scaife J. G. Structure and orientation of an RNA polymerase operon in Escherichia coli. Nature. 1974 Jun 7;249(457):519–522. doi: 10.1038/249519a0. [DOI] [PubMed] [Google Scholar]
- FALKOW S., SCHNEIDER H., BARON L. S., FORMAL S. B. VIRULENCE OF ESCHERICHIA-SHIGELLA GENETIC HYBRIDS FOR THE GUINEA PIG. J Bacteriol. 1963 Dec;86:1251–1258. doi: 10.1128/jb.86.6.1251-1258.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDERICQ P., BETZ-BAREAU M. Recombinants génétiques de souches marquées par résistance aux colicines et aux bactériophages. Ann Inst Pasteur (Paris) 1952 Sep;83(3):283–294. [PubMed] [Google Scholar]
- Faik P., Kornberg H. L. Isolation and properties of E. coli mutants affected in gluconate uptake. FEBS Lett. 1973 Jun 1;32(2):260–264. doi: 10.1016/0014-5793(73)80847-6. [DOI] [PubMed] [Google Scholar]
- Faik P., Kornberg H. L., McEvoy-Bowe E. Isolation and properties of Escherichia coli mutants defective in 2-keto 3-deoxy 6-phosphogluconate aldolase activity. FEBS Lett. 1971 Dec 15;19(3):225–228. doi: 10.1016/0014-5793(71)80519-7. [DOI] [PubMed] [Google Scholar]
- Falcoz-Kelly F., Janin J., Saari J. C., Véron M., Truffa-Bachi P., Cohen G. N. Revised structure of aspartokinase I-homoserine dehydrogenase I of Escherichia coli K12. Evidence for four identical subunits. Eur J Biochem. 1972 Aug 4;28(4):507–519. doi: 10.1111/j.1432-1033.1972.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Fangman W. L., Novick A. Mutant bacteria showing efficient utilization of thymidine. J Bacteriol. 1966 Jun;91(6):2390–2391. doi: 10.1128/jb.91.6.2390-2391.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas W., Gilvarg C. The reduction step in diaminopimelic acid biosynthesis. J Biol Chem. 1965 Dec;240(12):4717–4722. [PubMed] [Google Scholar]
- Ferenci T., Kornberg H. L., Smith Janet. Isolation and properties of a regulatory mutant in the hexose phosphate transport system of Escherichia coli. FEBS Lett. 1971 Mar 5;13(3):133–136. doi: 10.1016/0014-5793(71)80218-1. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Kornberg H. L. The utilization of fructose by Escherichia coli. Properties of a mutant defective in fructose 1-phosphate kinase activity. Biochem J. 1973 Feb;132(2):341–347. doi: 10.1042/bj1320341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feunteun J., Monier R., Vola C., Rosset R. Ribosomal assembly defective mutants of Escherichia coli. Nucleic Acids Res. 1974 Jan;1(1):149–169. doi: 10.1093/nar/1.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feunteun J., Rosset R., Ehresmann C., Stiegler P., Fellner P. Abnormal maturation of precursor 16S RNA in a ribosomal assembly defective mutant of E. coli. Nucleic Acids Res. 1974 Jan;1(1):141–147. doi: 10.1093/nar/1.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiil N., Friesen J. D. Isolation of "relaxed" mutants of Escherichia coli. J Bacteriol. 1968 Feb;95(2):729–731. doi: 10.1128/jb.95.2.729-731.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C. C., Allen J. S., Gustafson R. A., Allen R. G., Walker J. R. Bacterial cell division regulation: characterization of the dnaH locus of Escherichia coli. J Bacteriol. 1974 Aug;119(2):443–449. doi: 10.1128/jb.119.2.443-449.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaks J. G., Leboy P. S., Birge E. A., Kurland C. G. Mutations and genetics concerned with the ribosome. Cold Spring Harb Symp Quant Biol. 1966;31:623–631. doi: 10.1101/sqb.1966.031.01.081. [DOI] [PubMed] [Google Scholar]
- Fleck E. W., Carbon J. Multiple gene loci for a single species of glycine transfer ribonucleic acid. J Bacteriol. 1975 May;122(2):492–501. doi: 10.1128/jb.122.2.492-501.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk W. R., Berg P. Isolation and partial characterization of Escherichia coli mutants with altered glycyl transfer ribonucleic acid synthetases. J Bacteriol. 1970 Apr;102(1):193–203. doi: 10.1128/jb.102.1.193-203.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J., Barrett C. Characterization of Escherichia coli mutants tolerant to bacteriocin JF246: two new classes of tolerant mutants. J Bacteriol. 1973 Nov;116(2):885–892. doi: 10.1128/jb.116.2.885-892.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. Chromosomal location of the tolG locus for tolerance to bacteriocin JF246 in Escherichia coli K-12. J Bacteriol. 1974 Mar;117(3):1354–1355. doi: 10.1128/jb.117.3.1354-1355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J., Hilderman R. H., Deutscher M. P. Mapping of the locus for Escherichia coli transfer ribonucleic acid nucleotidyltransferase. J Bacteriol. 1974 May;118(2):628–632. doi: 10.1128/jb.118.2.628-632.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Carter J. R., Kennedy E. P. GENETIC CONTROL OF THE MEMBRANE PROTEIN COMPONENT OF THE LACTOSE TRANSPORT SYSTEM OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):698–705. doi: 10.1073/pnas.57.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradkin J. E., Fraenkel D. G. 2-keto-3-deoxygluconate 6-phosphate aldolase mutants of Escherichia coli. J Bacteriol. 1971 Dec;108(3):1277–1283. doi: 10.1128/jb.108.3.1277-1283.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G., Banerjee S. Deletion mapping of zwf, the gene for a constitutive enzyme, glucose 6-phosphate dehydrogenase in Escherichia coli. Genetics. 1972 Aug;71(4):481–489. doi: 10.1093/genetics/71.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. Genetic mapping of mutations affecting phosphoglucose isomerase and fructose diphosphatase in Escherichia coli. J Bacteriol. 1967 May;93(5):1582–1587. doi: 10.1128/jb.93.5.1582-1587.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G., Horecker B. L. Fructose-1, 6-diphosphatase and acid hexose phosphatase of Escherichia coli. J Bacteriol. 1965 Oct;90(4):837–842. doi: 10.1128/jb.90.4.837-842.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G., Parola A. "Up-promoter" mutations of glucose 6-phosphate dehydrogenase in Escherichia coli. J Mol Biol. 1972 Oct 28;71(1):107–111. doi: 10.1016/0022-2836(72)90405-6. [DOI] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Production, properties and utility of bacterial minicells. Curr Top Microbiol Immunol. 1975;69:1–84. doi: 10.1007/978-3-642-50112-8_1. [DOI] [PubMed] [Google Scholar]
- Freedberg W. B., Lin E. C. Three kinds of controls affecting the expression of the glp regulon in Escherichia coli. J Bacteriol. 1973 Sep;115(3):816–823. doi: 10.1128/jb.115.3.816-823.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen J. D., Fiil N. P., Parker J. M., Haseltine W. A. A new relaxed mutant of Escherichia coli with an altered 50S ribosomal subunit. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3465–3469. doi: 10.1073/pnas.71.9.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. A., Karlström H. O., Warner H. R., Reichard P. Defective gene product in dnaF mutant of Escherichia coli. Nat New Biol. 1972 Jul 19;238(81):69–71. doi: 10.1038/newbio238069a0. [DOI] [PubMed] [Google Scholar]
- GLANSDORFF N. TOPOGRAPHY OF COTRANSDUCIBLE ARGININE MUTATIONS IN ESCHERICHIA COLI K-12. Genetics. 1965 Feb;51:167–179. doi: 10.1093/genetics/51.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., GUNDERSEN W., BURGER M. Genetics of regulation of enzyme synthesis in the arginine biosynthetic pathway of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:173–182. doi: 10.1101/sqb.1961.026.01.022. [DOI] [PubMed] [Google Scholar]
- Gallucci E., Garen A. Suppressor genes for nonsense mutations. II. The su-4 and su-5 suppressor genes of Escherichia coli. J Mol Biol. 1966 Jan;15(1):193–200. doi: 10.1016/s0022-2836(66)80220-6. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K., Rotman B. Transport systems for galactose and galactosides in Escherichia coli. I. Genetic determination and regulation of the methyl-galactoside permease. J Mol Biol. 1966 Mar;16(1):42–50. doi: 10.1016/s0022-2836(66)80261-9. [DOI] [PubMed] [Google Scholar]
- Gardner J. F., Smith O. H., Fredricks W. W., McKinney M. A. Secondary-site attachment of coliphage lambda near the thr operon. J Mol Biol. 1974 Dec 25;90(4):613–631. doi: 10.1016/0022-2836(74)90528-2. [DOI] [PubMed] [Google Scholar]
- Garen A., Garen S., Wilhelm R. C. Suppressor genes for nonsense mutations. I. The Su-1, Su-2 and Su-3 genes of Escherichia coli. J Mol Biol. 1965 Nov;14(1):167–178. doi: 10.1016/s0022-2836(65)80238-8. [DOI] [PubMed] [Google Scholar]
- Garrick-Silversmith L., Hartman P. E. Histidine-requiring mutants of Escherichia coli K12. Genetics. 1970 Oct;66(2):231–244. doi: 10.1093/genetics/66.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin R. T., Gorini L. A new gene for ribosomal restriction in Escherichia coli. Mol Gen Genet. 1975;137(1):73–78. doi: 10.1007/BF00332540. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Wulff D. L. Fine structure mapping, complementation, and physiology of Escherichia coli hfl mutants. Genetics. 1974 Jul;77(3):435–448. doi: 10.1093/genetics/77.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. P. Bacterial mutants in which the gene N function of bacteriophage lambda is blocked have an altered RNA polymerase. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2977–2981. doi: 10.1073/pnas.68.12.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Casjens S. R., Kaiser A. D. Host participation in bacteriophage lambda head assembly. J Mol Biol. 1973 May 5;76(1):45–60. doi: 10.1016/0022-2836(73)90080-6. [DOI] [PubMed] [Google Scholar]
- Gholson R. K., Tritz G. J., Matney T. S., Andreoli A. J. Mode of nicotinamide adenine dinucleotide utilization by Escherichia coli. J Bacteriol. 1969 Sep;99(3):895–896. doi: 10.1128/jb.99.3.895-896.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A., Celis J. E. Joint transcription of two tRNA1Tyr genes from Escherichia coli. Nature. 1974 May 31;249(456):418–421. doi: 10.1038/249418a0. [DOI] [PubMed] [Google Scholar]
- Ghysen A., Pironio M. Relationship between the N function of bacteriophage lambda and host RNA polymerase. J Mol Biol. 1972 Mar 28;65(2):259–272. doi: 10.1016/0022-2836(72)90281-1. [DOI] [PubMed] [Google Scholar]
- Gibson F., Pittard J. Pathways of biosynthesis of aromatic amino acids and vitamins and their control in microorganisms. Bacteriol Rev. 1968 Dec;32(4 Pt 2):465–492. [PMC free article] [PubMed] [Google Scholar]
- Glansdorff N. Pseudoinversions in the chromosome of Escherichia coli K-12. Genetics. 1967 Jan;55(1):49–61. doi: 10.1093/genetics/55.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glansdorff N., Sand G., Verhoef C. The dual genetic control of ornithine transcarbamylase synthesis in Escherichia coli K12. Mutat Res. 1967 Nov-Dec;4(6):743–751. doi: 10.1016/0027-5107(67)90083-8. [DOI] [PubMed] [Google Scholar]
- Glanville E V, Demerec M. Threonine, Isoleucine, and Isoleucine-Valine Mutants of Salmonella Typhimurium. Genetics. 1960 Oct;45(10):1359–1374. doi: 10.1093/genetics/45.10.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser J. H., DeMoss J. A. Comparison of nitrate reductase mutants of Escherichia coli selected by alternative procedures. Mol Gen Genet. 1972;116(1):1–10. doi: 10.1007/BF00334254. [DOI] [PubMed] [Google Scholar]
- Glickman B. W., Zwenk H., van Sluis C. A., Rörsch A. The isolation and characterization of an X-ray-sensitive ultraviolet-resistant mutant of Escherichia coli. Biochim Biophys Acta. 1971 Nov 29;254(1):144–154. doi: 10.1016/0005-2787(71)90121-3. [DOI] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. Purification and properties of the recBC DNase of Escherichia coli K-12. J Biol Chem. 1972 Mar 25;247(6):1849–1860. [PubMed] [Google Scholar]
- Goldschmidt E. P., Cater M. S., Matney T. S., Butler M. A., Greene A. Genetic analysis of the histidine operon in Escherichia coli K12. Genetics. 1970 Oct;66(2):219–229. doi: 10.1093/genetics/66.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M., Abelson J., Landy A., Brenner S., Smith J. D. Amber suppression: a nucleotide change in the anticodon of a tyrosine transfer RNA. Nature. 1968 Mar 16;217(5133):1019–1024. doi: 10.1038/2171019a0. [DOI] [PubMed] [Google Scholar]
- Gordon J., Baron L. S., Schweiger M. Chromosomal localization of the structural genes of the polypeptide chain elongation factors. J Bacteriol. 1972 Apr;110(1):306–312. doi: 10.1128/jb.110.1.306-312.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Hicks M. L., Gellert M. Genetics and function of DNA ligase in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):531–547. doi: 10.1016/0022-2836(73)90221-0. [DOI] [PubMed] [Google Scholar]
- Gratia J. P. Studies on defective lysogeny due to chromosomal deletion in Escherichia coli. I. Single lysogens. Biken J. 1966 Jun;9(2):77–87. [PubMed] [Google Scholar]
- Greene R. C., Su C. H., Holloway C. T. S-Adenosylmethionine synthetase deficient mutants of Escherichia coli K-12 with impaired control of methionine biosynthesis. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1120–1126. doi: 10.1016/0006-291x(70)90355-4. [DOI] [PubMed] [Google Scholar]
- Griffith G. R., Chandler J. L., Gholson R. K. Studies on the de novo biosynthesis of NAD in Escherichia coli. The separation of the nadB gene product from the nadA gene product and its purification. Eur J Biochem. 1975 May;54(1):239–245. doi: 10.1111/j.1432-1033.1975.tb04133.x. [DOI] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Iaccarino M. Mutant of Escherichia coli K-12 missing acetolactate synthase activity. J Bacteriol. 1974 Oct;120(1):536–538. doi: 10.1128/jb.120.1.536-538.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Klopotowski T., Iaccarino M. Mutations affecting the different transport systems for isoleucine, leucine, and valine in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):393–405. doi: 10.1128/jb.117.2.393-405.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola J., Iaccarino M. Escherichia coli K-12 mutants altered in the transport of branched-chain amino acids. J Bacteriol. 1971 Dec;108(3):1034–1044. doi: 10.1128/jb.108.3.1034-1044.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Model for regulation of Escherichia coli DNA repair functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2330–2334. doi: 10.1073/pnas.72.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J. R. Biochemical and genetic studies with nitrate reductase C-gene mutants of Escherichia coli. Mol Gen Genet. 1969;105(4):285–297. doi: 10.1007/BF00277583. [DOI] [PubMed] [Google Scholar]
- Guest J. R., Creaghan I. T. Further studies with lipoamide dehydrogenase mutants of Escherichia coli K12. J Gen Microbiol. 1974 Mar;81(1):237–245. doi: 10.1099/00221287-81-1-237. [DOI] [PubMed] [Google Scholar]
- Guest J. R., Creaghan I. T. Gene-protein relationships of the alpha-keto acid dehydrogenase complexes of Escherichia coli K12: isolation and characterization of lipoamide dehydrogenase mutants. J Gen Microbiol. 1973 Mar;75(1):197–210. doi: 10.1099/00221287-75-1-197. [DOI] [PubMed] [Google Scholar]
- Guest J. R. Gene-protein relationships of the alpha-keto acid dehydrogenase complexes of Escherichia coli K12: Chromosomal location of the lipoamide dehydrogenase gene. J Gen Microbiol. 1974 Feb;80(2):523–532. doi: 10.1099/00221287-80-2-523. [DOI] [PubMed] [Google Scholar]
- Guha A. Divergent orientation of transcription from the biotin locus of Escherichia coli. J Mol Biol. 1971 Feb 28;56(1):53–62. doi: 10.1016/0022-2836(71)90083-0. [DOI] [PubMed] [Google Scholar]
- Guterman S. K., Dann L. Excretion of enterochelin by exbA and exbB mutants of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1225–1230. doi: 10.1128/jb.114.3.1225-1230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman S. K. Inhibition of colicin B by enterochelin. Biochem Biophys Res Commun. 1971 Sep;44(5):1149–1155. doi: 10.1016/s0006-291x(71)80206-1. [DOI] [PubMed] [Google Scholar]
- HENNING U., HERZ C. EIN STRUKTURGEN-KOMPLEX FUER DEN PYRUVAT-DEHYDROGENASE-KOMPLEX VON ESCHERICHIA COLI K 12. Z Vererbungsl. 1964 Nov 11;95:260–275. [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi S. M., Yuan R. Complementation in vitro by mutant restriction enzymes from Escherichia coli K. J Biol Chem. 1974 Jul 25;249(14):4580–4586. [PubMed] [Google Scholar]
- Hammer-Jespersen K., Munch-Petersen A. Mutants of Escherichia coli unable to metabolize cytidine: isolation and characterization. Mol Gen Genet. 1973 Nov 2;126(2):177–186. doi: 10.1007/BF00330992. [DOI] [PubMed] [Google Scholar]
- Hammer-Jespersen K., Munch-Ptersen A. Multiple regulation of nucleoside catabolizing enzymes: regulation of the deo operon by the cytR and deoR gene products. Mol Gen Genet. 1975;137(4):327–335. doi: 10.1007/BF00703258. [DOI] [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder M. E., Ladenson R. C., Schimmel S. D., Silbert D. F. Mutants of Escherichia coli with temperature-sensitive malonyl coenzyme A-acyl carrier protein transacylase. J Biol Chem. 1974 Dec 10;249(23):7468–7475. [PubMed] [Google Scholar]
- Harriman P. D. A single-burst analysis of the production of P1 infectious and transducing particles. Virology. 1972 May;48(2):595–600. doi: 10.1016/0042-6822(72)90071-2. [DOI] [PubMed] [Google Scholar]
- Hatfield D., Hofnung M., Schwartz M. Genetic analysis of the maltose A region in Escherichia coli. J Bacteriol. 1969 May;98(2):559–567. doi: 10.1128/jb.98.2.559-567.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D., Hofnung M., Schwartz M. Nonsense mutations in the maltose A region of the genetic map of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1311–1315. doi: 10.1128/jb.100.3.1311-1315.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway B. G., Bergquist P. L. Temperature-sensitive mutations affecting the replication of F-prime factors in Escherichia coli K 12. Mol Gen Genet. 1973 Dec 31;127(4):297–306. doi: 10.1007/BF00267100. [DOI] [PubMed] [Google Scholar]
- Hawrot E., Kennedy E. P. Biogenesis of membrane lipids: mutants of Escherichia coli with temperature-sensitive phosphatidylserine decarboxylase. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1112–1116. doi: 10.1073/pnas.72.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard J. T., Jr, Butler M. A., Baptist J. N., Matney T. S. Chromosomal location of mutations affecting the electrophoretic mobility of malate dehydrogenase in Escherichia coli K-12. J Bacteriol. 1975 Apr;122(1):329–331. doi: 10.1128/jb.122.1.329-331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil A., Zillig W. Reconstitution of bacterial DNA-dependent RNA-polymerase from isolated subunits as a tool for the elucidation of the role of the subunits in transcription. FEBS Lett. 1970 Dec;11(3):165–168. doi: 10.1016/0014-5793(70)80519-1. [DOI] [PubMed] [Google Scholar]
- Helling R. B. Selection of a mutant of Escherichia coli which has high mutation rates. J Bacteriol. 1968 Oct;96(4):975–980. doi: 10.1128/jb.96.4.975-980.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Mechanism of kasugamycin resistance in Escherichia coli. Nat New Biol. 1972 Jan 5;235(53):6–9. doi: 10.1038/newbio235006a0. [DOI] [PubMed] [Google Scholar]
- Henning U., Dennert G., Hertel R., Shipp W. S. Translation of the structural genes of the E. coli pyruvate dehydrogenase complex. Cold Spring Harb Symp Quant Biol. 1966;31:227–234. doi: 10.1101/sqb.1966.031.01.031. [DOI] [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Biochemical and genetic studies with lysine+methionine mutants of Escherichia coli: lipoic acid and alpha-ketoglutarate dehydrogenase-less mutants. J Gen Microbiol. 1968 Oct;53(3):363–381. doi: 10.1099/00221287-53-3-363. [DOI] [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Studies with alpha-ketoglutarate dehydrogenase mutants of Escherichia coli. Mol Gen Genet. 1969 Oct 13;105(2):182–190. doi: 10.1007/BF00445687. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Combriato G., Dolph W. Three different missense suppressor mutations affecting the tRNA GGG Gly species of Escherichia coli. J Bacteriol. 1974 Feb;117(2):351–359. doi: 10.1128/jb.117.2.351-359.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman J. D., Fraenkel D. G. Glyceraldehyde 3-phosphate dehydrogenase mutants of Escherichia coli. J Bacteriol. 1975 Jun;122(3):1175–1179. doi: 10.1128/jb.122.3.1175-1179.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Ito K., Matsuyama T., Ozaki H., Yura T. 5-methyltryptophan-resistant mutations lniked with the arginine G marker in Escherichia coli. J Bacteriol. 1968 Nov;96(5):1880–1881. doi: 10.1128/jb.96.5.1880-1881.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Gefter M., Mindich L. A mutant of Escherichia coli defective in DNA polymerase II activity. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3238–3242. doi: 10.1073/pnas.69.11.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Hoekstra W. P., Storm P. K., Zuidweg E. M. Recombination in Escherichia coli. VI. Characterization of a recombination-deficient mutation with unusual properties. Mutat Res. 1974 Jun;23(3):319–326. doi: 10.1016/0027-5107(74)90105-5. [DOI] [PubMed] [Google Scholar]
- Hoess R. H., Herman R. K. Isolation and characterization of mutator strains of Escherichia coli K-12. J Bacteriol. 1975 May;122(2):474–484. doi: 10.1128/jb.122.2.474-484.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Wilhelm R. C. Genetic mapping and dominance of the amber suppressor, Su1 (supD), in Escherichia coli K-12. J Bacteriol. 1970 Jul;103(1):32–36. doi: 10.1128/jb.103.1.32-36.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Wilhelm R. C., Konigsberg W., Katze J. R. A structural gene for seryl-tRNA synthetase in Escherichia coli K12. J Mol Biol. 1970 Feb 14;47(3):619–625. doi: 10.1016/0022-2836(70)90332-3. [DOI] [PubMed] [Google Scholar]
- Hofnung M. Divergent operons and the genetic structure of the maltose B region in Escherichia coli K12. Genetics. 1974 Feb;76(2):169–184. doi: 10.1093/genetics/76.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofnung M., Hatfield D., Schwartz M. malB region in Escherichia coli K-12: characterization of new mutations. J Bacteriol. 1974 Jan;117(1):40–47. doi: 10.1128/jb.117.1.40-47.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofnung M., Schwartz M., Hatfield D. Complementation studies in the maltose-A region of the Escherichia coli K12 genetic map. J Mol Biol. 1971 Nov 14;61(3):681–694. doi: 10.1016/0022-2836(71)90072-6. [DOI] [PubMed] [Google Scholar]
- Hofnung M., Schwartz M. Mutations allowing growth on maltose of Escherichia coli K 12 strains with a deleted malT gene. Mol Gen Genet. 1971;112(2):117–132. doi: 10.1007/BF00267490. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Vielmetter W. Bidirectional growth of the E. coli chromosome. Nat New Biol. 1973 Apr 4;242(118):130–132. doi: 10.1038/newbio242130a0. [DOI] [PubMed] [Google Scholar]
- Holland I. B., Threlfall E. J. Identification of closely linked loci controlling ultraviolet sensitivity and refractivity to colicin E2 in Escherichia coli. J Bacteriol. 1969 Jan;97(1):91–96. doi: 10.1128/jb.97.1.91-96.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. P., Russell R. R. Mutations affecting amino sugar metabolism in Escherichia coli K-12. J Bacteriol. 1972 Jul;111(1):290–291. doi: 10.1128/jb.111.1.290-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe I., Roth J. Specialized transducing phages derived from salmonella phage P22. Genetics. 1974 Apr;76(4):633–654. doi: 10.1093/genetics/76.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Horiuchi T., Nagata T. Lethality of the Escherichia coli K12 cell doubly deficient in DNA polymerase I and DNA strand-joining activity. Mol Gen Genet. 1974;128(2):105–115. doi: 10.1007/BF02654484. [DOI] [PubMed] [Google Scholar]
- Horiuchi T., Nagata T. Mutations affecting growth of the Escherichia coli cell under a condition of DNA polymerase I-deficiency. Mol Gen Genet. 1973;123(1):89–110. doi: 10.1007/BF00282992. [DOI] [PubMed] [Google Scholar]
- Horiuchi T., Sato T., Nagata T. DNA degradation in an amber mutant of Escherichia coli K12 affecting DNA ligase and viability. J Mol Biol. 1975 Jun 25;95(2):271–287. doi: 10.1016/0022-2836(75)90395-2. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Ohtsubo E., Davidson N. Electron microscopic heteroduplex studies of sequence relations among plasmids of Escherichia coli: structure of F13 and related F-primes. J Bacteriol. 1975 May;122(2):749–763. doi: 10.1128/jb.122.2.749-763.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S. S., Markovitz A. Multiple regulation of the galactose operon-genetic evidence for a distinct site in the galactose operon that responds to capR gene regulation in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1974 Feb;71(2):507–511. doi: 10.1073/pnas.71.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Pittard J. Genetic analysis of mutant strains of Escherichia coli requiring p-aminobenzoic acid for growth. J Bacteriol. 1967 Jun;93(6):1938–1942. doi: 10.1128/jb.93.6.1938-1942.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J. S., Greene R. C., Su C. H. Genetic characterization of the metK locus in Escherichia coli K-12. J Bacteriol. 1975 Jun;122(3):1144–1152. doi: 10.1128/jb.122.3.1144-1152.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIBASHI M., SUGINO Y., HIROTA Y. CHROMOSOMAL LOCATION OF THYMINE AND ARGININE GENES IN ESCHERICHIA COLI AND AN F' INCORPORATING THEM. J Bacteriol. 1964 Mar;87:554–561. doi: 10.1128/jb.87.3.554-561.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino M., Berg P. Isoleucine auxotrophy as a consequence of a mutationally altered isoleucyl-transfer ribonucleic acid synthetase. J Bacteriol. 1971 Feb;105(2):527–537. doi: 10.1128/jb.105.2.527-537.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Hiraga S., Yura T. A deoxythymidine kinase deficient mutant of Escherichia coli. II. Mapping and transduction studies with phage phi 80. Genetics. 1967 Nov;57(3):643–654. doi: 10.1093/genetics/57.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S. W., Pittard J. Phenylalanine biosynthesis in Escherichia coli K-12: mutants derepressed for chorismate mutase P-prephenate dehydratase. J Bacteriol. 1971 Jun;106(3):784–790. doi: 10.1128/jb.106.3.784-790.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imae Y. Mitomycin C-sensitive mutant of Eschericha coli K-12. J Bacteriol. 1968 Mar;95(3):1191–1192. doi: 10.1128/jb.95.3.1191-1192.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani M., Maitra P. K. Isolation and characterization of Escherichia coli mutants defective in enzymes of glycolysis. Biochem Biophys Res Commun. 1974 Jan;56(1):127–133. doi: 10.1016/s0006-291x(74)80324-4. [DOI] [PubMed] [Google Scholar]
- Itikawa H., Baumberg S., Vogel H. J. Enzymic basis for a genetic suppression: accumulation and deacylation of N-acetylglutamic gamma-semialdehyde in enterobacterial mutants. Biochim Biophys Acta. 1968 Jul 9;159(3):547–550. doi: 10.1016/0005-2744(68)90142-3. [DOI] [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Hiraga S., Yura T. Temperature-sensitive repression of the tryptophan operon in Escherichia coli. J Bacteriol. 1969 Jul;99(1):279–286. doi: 10.1128/jb.99.1.279-286.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Hiraga S., Yura T. Tryptophanyl transfer RNA synthetase and expression of the tryptophan operon in the trpS mutants of Escherichia coli. Genetics. 1969 Mar;61(3):521–538. doi: 10.1093/genetics/61.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K. Regulatory mechanism of the tryptophan operon in Escherichia coli: possible interaction between trpR and trpS gene products. Mol Gen Genet. 1972;115(4):349–363. doi: 10.1007/BF00333173. [DOI] [PubMed] [Google Scholar]
- Iwakura Y., Ishihama A., Yura T. RNA polymerase mutants of Escherichia coli. Streptolydigin resistance and its relation to rifampicin resistance. Mol Gen Genet. 1973 Mar 1;121(2):181–196. doi: 10.1007/BF00277531. [DOI] [PubMed] [Google Scholar]
- Iyehara H., Otsuji N. Location of the Escherichia coli K-12 ruv gene affecting septum formation after inhibition of deoxyribonucleic acid synthesis. J Bacteriol. 1975 May;122(2):791–793. doi: 10.1128/jb.122.2.791-793.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., ULLMAN A., MONOD J. LE PROMOTEUR, 'EL'EMENT G'EN'ETIQUE N'ECESSAIRE 'A L'EXPRESSION D'UN OP'ERON. C R Hebd Seances Acad Sci. 1964 Mar 16;258:3125–3128. [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Genetic and physical determinations of chromosomal segments in Escherichia coli. Symp Soc Exp Biol. 1958;12:75–92. [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Localization of two functions of the phosphoribosyl anthranilate transferase of Escherichia coli to distinct regions of the polypeptide chain. J Bacteriol. 1974 Feb;117(2):502–508. doi: 10.1128/jb.117.2.502-508.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A., Gillespie D. Genetic complementation between Escherichia coli RNA polymerase mutants. Biochem Biophys Res Commun. 1971 Sep;44(5):1030–1040. doi: 10.1016/s0006-291x(71)80189-4. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Control of the argECBH cluster in Escherichia coli. Mol Gen Genet. 1972;117(4):337–348. doi: 10.1007/BF00333027. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Mapping the gene determining ornithine transcarbamylase and its operator in Escherichia coli B. J Bacteriol. 1971 Nov;108(2):645–651. doi: 10.1128/jb.108.2.645-651.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarry B., Rosset R. Further mapping of 5S RNA cistrons in Escherichia coli. Mol Gen Genet. 1973 Oct 16;126(1):29–35. doi: 10.1007/BF00333479. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Burgess R. R., Nomura M. Identification of a gene for the alpha-subunit of RNA polymerase at the str-spc region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5036–5040. doi: 10.1073/pnas.72.12.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskunas S. R., Burgess R. R., Nomura M. Identification of a gene for the alpha-subunit of RNA polymerase at the str-spc region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5036–5040. doi: 10.1073/pnas.72.12.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Identification of two copies of the gene for the elongation factor EF-Tu in E. coli. Nature. 1975 Oct 9;257(5526):458–462. doi: 10.1038/257458a0. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Isolation of polar insertion mutants and the direction of transcription of ribosomal protein genes in E. coli. Nature. 1975 Jul 17;256(5514):183–187. doi: 10.1038/256183a0. [DOI] [PubMed] [Google Scholar]
- Jasper P., Whitney E., Silver S. Genetic locus determining resistance to phage BF23 and colicins E 1 , E 2 and E 3 in Escherichia coli. Genet Res. 1972 Jun;19(3):305–312. doi: 10.1017/s0016672300014555. [DOI] [PubMed] [Google Scholar]
- Jenkins S. J., Sparkes C. A., Jones-Mortimer M. C. A gene involved in lysine excretion in Escherichia coli K12. Heredity (Edinb) 1974 Jun;32(3):409–412. doi: 10.1038/hdy.1974.51. [DOI] [PubMed] [Google Scholar]
- Johnson B. F., Greenberg J. Mapping of sul, the suppressor of lon in Escherichia coli. J Bacteriol. 1975 May;122(2):570–574. doi: 10.1128/jb.122.2.570-574.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Kornberg H. L. Genetical analysis of fructose utilization by Escherichia coli. Proc R Soc Lond B Biol Sci. 1974 Sep 17;187(1087):121–131. doi: 10.1098/rspb.1974.0066. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Mapping of structural genes for the enzymes of cysteine biosynthesis in Escherichia coli K12 and Salmonella typhimurium LT2. Heredity (Edinb) 1973 Oct;31(2):213–221. doi: 10.1038/hdy.1973.76. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Positive control of sulphate reduction in Escherichia coli. Isolation, characterization and mapping oc cysteineless mutants of E. coli K12. Biochem J. 1968 Dec;110(3):589–595. doi: 10.1042/bj1100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson B. L., Fraenkel D. G. Transketolase mutants of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1289–1295. doi: 10.1128/jb.100.3.1289-1295.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY B. Localization of P2 prophage in two strains of Escherichia coli. Virology. 1963 Jan;19:32–39. doi: 10.1016/0042-6822(63)90021-7. [DOI] [PubMed] [Google Scholar]
- Kadner R. J. Genetic Control of the Transport of Hexose Phosphates in Escherichia coli: Mapping of the uhp Locus. J Bacteriol. 1973 Nov;116(2):764–770. doi: 10.1128/jb.116.2.764-770.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Liggins G. L. Transport of vitamin B12 in Escherichia coli: genetic studies. J Bacteriol. 1973 Aug;115(2):514–521. doi: 10.1128/jb.115.2.514-521.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Maas W. K. Regulatory gene mutations affecting arginine biosynthesis in Escherichia coli. Mol Gen Genet. 1971;111(1):1–14. doi: 10.1007/BF00286549. [DOI] [PubMed] [Google Scholar]
- Kadner R. J. Transport systems for L-methionine in Escherichia coli. J Bacteriol. 1974 Jan;117(1):232–241. doi: 10.1128/jb.117.1.232-241.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Watson W. J. Methionine transport in Escherichia coli: physiological and genetic evidence for two uptake systems. J Bacteriol. 1974 Aug;119(2):401–409. doi: 10.1128/jb.119.2.401-409.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Winkler H. H. Isolation and characterization of mutations affecting the transport of hexose phosphates in Escherichia coli. J Bacteriol. 1973 Feb;113(2):895–900. doi: 10.1128/jb.113.2.895-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan L., Zengel J., Nomura M., Bollen A., Herzog A. The structural gene for the ribosomal protein S18 in Escherichia coli. II. Chemical studies on the protein S18 having an altered electrophoretic mobility. J Mol Biol. 1973 Jun 5;76(4):473–483. doi: 10.1016/0022-2836(73)90486-5. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Gutnick D. L. Use of neomycin in the isolation of mutants blocked in energy conservation in Escherichia coli. J Bacteriol. 1972 Jul;111(1):287–289. doi: 10.1128/jb.111.1.287-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Kondo S. Genetic and molecular characteristics of X-ray-sensitive mutants of Escherichia coli defective in repair synthesis. J Bacteriol. 1970 Nov;104(2):871–881. doi: 10.1128/jb.104.2.871-881.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Nakata T., Nose Y. Genetic mapping with a thiamine-requiring auxotroph of Escherichia coli K-12 defective in thiamine phosphate pyrophosphorylase. J Bacteriol. 1968 Apr;95(4):1483–1485. doi: 10.1128/jb.95.4.1483-1485.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Nose Y. Thiamine regulatory mutants in Escherichia coli. J Biochem. 1969 Mar;65(3):417–425. doi: 10.1093/oxfordjournals.jbchem.a129029. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Kornberg H. L. Genetic control of the uptake of C(4)-dicarboxylic acids by Escherichia coli. FEBS Lett. 1969 Apr;3(2):93–96. doi: 10.1016/0014-5793(69)80105-5. [DOI] [PubMed] [Google Scholar]
- Kemper J. Gene order and co-transduction in the leu-ara-fol-pyrA region of the Salmonella typhimurium linkage map. J Bacteriol. 1974 Jan;117(1):94–99. doi: 10.1128/jb.117.1.94-99.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D. P., Englesberg E. Arabinose-leucine deletion mutants of Escherichia coli B-r. J Bacteriol. 1969 Jun;98(3):1159–1169. doi: 10.1128/jb.98.3.1159-1169.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketner G., Campbell A. A deletion mutation placing the galactokinase gene of Escherichia coli under control of the biotin promoter. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2698–2702. doi: 10.1073/pnas.71.7.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khmel'nitskii M. I., Zlotnikov K. M. Mutatornye svoistva i kartirovanie mutatsii prv u Escherichia coli K-12, povyshaiushchikh chastotu vnutrigennykh rekombinatsii. Genetika. 1974 Dec;10(12):110–114. [PubMed] [Google Scholar]
- Kida S. The biological function of the R2a regulatory gene for alkaline phosphatase in Escherichia coli. Arch Biochem Biophys. 1974 Jul;163(1):231–237. doi: 10.1016/0003-9861(74)90473-1. [DOI] [PubMed] [Google Scholar]
- Kirschbaum J. B., Claeys I. V., Nasi S., Molholt B., Miller J. H. Temperature-sensitive RNA polymerase mutants with altered subunit synthesis and degradation. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2375–2379. doi: 10.1073/pnas.72.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum J. B., Konrad E. B. Isolation of a specialized lambda transducing bacteriophage carrying the beta subunit gene for Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1973 Nov;116(2):517–526. doi: 10.1128/jb.116.2.517-526.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum J. B., Scaife J. Evidence for a lambda transducing phage carrying the genes for the beta and beta' subunits of Escherichia coli RNA polymerase. Mol Gen Genet. 1974;132(3):193–201. doi: 10.1007/BF00269392. [DOI] [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli: its genetic locus and its physiological role. J Bacteriol. 1971 Dec;108(3):1224–1234. doi: 10.1128/jb.108.3.1224-1234.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Kline E. L., Brown C. S., Umbarger H. E. Effect of a leu-linked mutation on the valine sensitivity of acetohydroxy acid synthase activity in Escherichia coli. J Bacteriol. 1975 Feb;121(2):491–496. doi: 10.1128/jb.121.2.491-496.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline E. L. New amino acid regulatory locus having unusual properties in heterozygous merodiploids. J Bacteriol. 1972 Jun;110(3):1127–1134. doi: 10.1128/jb.110.3.1127-1134.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad E. B., Lehman I. R. Novel mutants of Escherichia coli that accumulate very small DNA replicative intermediates. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2150–2154. doi: 10.1073/pnas.72.6.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Jones-Mortimer M. C. PtsX: a gene involved in the uptake of glucose and fructose by Escherichia coli. FEBS Lett. 1975 Mar 1;51(1):1–4. doi: 10.1016/0014-5793(75)80840-4. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Genetic control of glucose uptake by Escherichia coli. FEBS Lett. 1972 Feb 15;20(3):270–272. doi: 10.1016/0014-5793(72)80084-x. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Genetic control of hexose phosphate uptake by Escherichia coli. Nature. 1969 Dec 27;224(5226):1261–1262. doi: 10.1038/2241261a0. [DOI] [PubMed] [Google Scholar]
- Kuhn J., Somerville R. L. Mutant strains of Escherichia coli K12 that use D-amino acids. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2484–2487. doi: 10.1073/pnas.68.10.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupor S. R., Fraenkel D. G. 6-phosphogluconolactonase mutants of Escherichia coli and a maltose blue gene. J Bacteriol. 1969 Dec;100(3):1296–1301. doi: 10.1128/jb.100.3.1296-1301.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Clark A. J. Indirect suppression of recB and recC mutations by exonuclease I deficiency. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1366–1370. doi: 10.1073/pnas.69.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Clark A. J. Isolation of exonuclease VIII: the enzyme associated with sbcA indirect suppressor. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3593–3597. doi: 10.1073/pnas.71.9.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano M., Schlessinger D. G factor mutants of Escherichia coli: map location and properties. Biochem Biophys Res Commun. 1971 Feb 5;42(3):441–444. doi: 10.1016/0006-291x(71)90390-1. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Schlessinger D., Morse D. E. Loss of dispensable endonuclease activity in relief of polarity by suA. Nat New Biol. 1971 Jun 16;231(24):214–217. doi: 10.1038/newbio231214a0. [DOI] [PubMed] [Google Scholar]
- Kvetkas M. J., Krisch R. E., Zelle M. R. Genetic analysis of a large-cell, radiation-resistant strain of Escherichia coli. J Bacteriol. 1970 Aug;103(2):393–399. doi: 10.1128/jb.103.2.393-399.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner A., Magee B. B., Liska B., Low K. B., Adelberg E. A., Söll D. Isolation and partial characterization of a temperature-sensitive Escherichia coli mutant with altered glutaminyl-transfer ribonucleic acid synthetase. J Bacteriol. 1974 Oct;120(1):154–158. doi: 10.1128/jb.120.1.154-158.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE N., ENGLESBERG E. Dual effects of structural genes in Escherichia coli. Proc Natl Acad Sci U S A. 1962 Mar 15;48:335–348. doi: 10.1073/pnas.48.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Drabble W. T. The gua operon of Escherichia coli K-12: evidence for polarity from guaB to guaA. J Bacteriol. 1973 Sep;115(3):992–1002. doi: 10.1128/jb.115.3.992-1002.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. E., Denhardt D. T. The rep mutation. III. Altered structure of the replicating Escherichia coli chromosome. J Bacteriol. 1974 Nov;120(2):805–814. doi: 10.1128/jb.120.2.805-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langman L., Young I. G., Frost G. E., Rosenberg H., Gibson F. Enterochelin system of iron transport in Escherichia coli: mutations affecting ferric-enterochelin esterase. J Bacteriol. 1972 Dec;112(3):1142–1149. doi: 10.1128/jb.112.3.1142-1149.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge J. Characterization and intragenic position of mutations in the gene for galactoside permease of Escherichia coli. Aust J Biol Sci. 1974 Jun;27(3):331–340. doi: 10.1071/bi9740331. [DOI] [PubMed] [Google Scholar]
- Lapointe J., Delcuve G. Thermosensitive mutants of Escherichia coli K-12 altered in the catalytic Subunit and in a Regulatory factor of the glutamy-transfer ribonucleic acid synthetase. J Bacteriol. 1975 May;122(2):352–358. doi: 10.1128/jb.122.2.352-358.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark K. G. Genetic control over the initiation of the synthesis of the short deoxynucleotide chains in E. coli. Nat New Biol. 1972 Dec 20;240(103):237–240. doi: 10.1038/newbio240237a0. [DOI] [PubMed] [Google Scholar]
- Lawrence J., Cox G. B., Gibson F. Biosynthesis of ubiquinone in Escherichia coli K-12: biochemical and genetic characterization of a mutant unable to convert chorismate into 4-hydroxybenzoate. J Bacteriol. 1974 Apr;118(1):41–45. doi: 10.1128/jb.118.1.41-45.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg S. Genetics of host-controlled restriction and modification of deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1966 Mar;91(3):1029–1036. doi: 10.1128/jb.91.3.1029-1036.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain C., Halleux P., Stalon V., Glansdorff N. The dual genetic control of ornithine carbamolytransferase in Escherichia coli. A case of bacterial hybrid enzymes. Eur J Biochem. 1972 May;27(1):93–102. doi: 10.1111/j.1432-1033.1972.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Lengeler J., Lin E. C. Reversal of the mannitol-sorbitol diauxie in Escherichia coli. J Bacteriol. 1972 Nov;112(2):840–848. doi: 10.1128/jb.112.2.840-848.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennette E. T., Apirion D. Genetic analysis of an Escherichia coli syndrome. J Bacteriol. 1971 Dec;108(3):1322–1328. doi: 10.1128/jb.108.3.1322-1328.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberfarb R. M., Bryson V. Isolation, characterization, and genetic analysis of mutator genes in Escherichia coli B and K-12. J Bacteriol. 1970 Oct;104(1):363–375. doi: 10.1128/jb.104.1.363-375.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C. The genetics of bacterial transport systems. Annu Rev Genet. 1970;4:225–262. doi: 10.1146/annurev.ge.04.120170.001301. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Jaskunas S. R., Dennis P. P., Nomura M. Cluster of genes in Escherichia coli for ribosomal proteins, ribosomal RNA, and RNA polymerase subunits. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2743–2747. doi: 10.1073/pnas.72.7.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D. M., Hinkle D. C., Richardson C. C. Deoxyribonucleic acid polymerase III of Escherichia coli. Purification and properties. J Biol Chem. 1975 Jan 25;250(2):461–469. [PubMed] [Google Scholar]
- Lloyd R. G., Barbour S. D. The genetic location of the sbcA gene of Escherichia coli. Mol Gen Genet. 1974;134(2):157–171. doi: 10.1007/BF00268417. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G. The segregation of the SbcA and Rac phenotypes in an Escherichia coli recB mutant. Mol Gen Genet. 1974;134(3):249–259. doi: 10.1007/BF00267719. [DOI] [PubMed] [Google Scholar]
- Lo T. C., Rayman M. K., Sanwal B. D. Transport of succinate in Escherichia coli. I. Biochemical and genetic studies of transport in whole cells. J Biol Chem. 1972 Oct 10;247(19):6323–6331. [PubMed] [Google Scholar]
- Lomax M. S., Greenberg G. R. Characteristics of the deo operon: role in thymine utilization and sensitivity to deoxyribonucleosides. J Bacteriol. 1968 Aug;96(2):501–514. doi: 10.1128/jb.96.2.501-514.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louarn J. M. Size distribution and molecular polarity of nascent DNA in a temperature-sensitive dna G mutant of Escherichia coli. Mol Gen Genet. 1974;133(3):193–200. doi: 10.1007/BF00267668. [DOI] [PubMed] [Google Scholar]
- Louarn J., Funderburgh M., Bird R. E. More precise mapping of the replication origin in Escherichia coli K-12. J Bacteriol. 1974 Oct;120(1):1–5. doi: 10.1128/jb.120.1.1-5.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B., Gates F., Goldstein T., Söll D. Isolation and partial characterization of temperature-sensitive Escherichia coli mutants with altered leucyl- and seryl-transfer ribonucleic acid synthetases. J Bacteriol. 1971 Nov;108(2):742–750. doi: 10.1128/jb.108.2.742-750.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Inversion of transfer modes and sex factor-chromosome interactions in conjugation in Escherichia coli. J Bacteriol. 1967 Jan;93(1):98–106. doi: 10.1128/jb.93.1.98-106.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Restoration by the rac locus of recombinant forming ability in recB - and recC - merozygotes of Escherichia coli K-12. Mol Gen Genet. 1973 Apr 12;122(2):119–130. doi: 10.1007/BF00435185. [DOI] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutant of Escherichia coli K-12 with an impaired D-alanine:D-alanine ligase. J Bacteriol. 1973 Jan;113(1):96–104. doi: 10.1128/jb.113.1.96-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutants of Escherichia coli K-12 with low activities of the L-alanine adding enzyme and the D-alanyl-D-alanine adding enzyme. J Bacteriol. 1972 Apr;110(1):35–40. doi: 10.1128/jb.110.1.35-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutants of Escherichia coli K-12 with low activity of the diaminopimelic acid adding enzyme. J Bacteriol. 1972 Apr;110(1):41–46. doi: 10.1128/jb.110.1.41-46.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke R. K., Gibson F. Location of three genes concerned with the conversion of 2,3-dihydroxybenzoate into enterochelin in Escherichia coli K-12. J Bacteriol. 1971 Aug;107(2):557–562. doi: 10.1128/jb.107.2.557-562.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo M., Halpern Y. S. Gene controlling L-glutamic acid decarboxylase synthesis in Escherichia coli K-12. J Bacteriol. 1970 Aug;103(2):382–386. doi: 10.1128/jb.103.2.382-386.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAAS W. K., MAAS R., WIAME J. M., GLANSDORFF N. STUDIES ON THE MECHANISM OF REPRESSION OF ARGININE BIOSYNTHESIS IN ESCHERICHIA COLI. I. DOMINANCE OF REPRESSIBILITY IN ZYGOTES. J Mol Biol. 1964 Mar;8:359–364. doi: 10.1016/s0022-2836(64)80199-6. [DOI] [PubMed] [Google Scholar]
- MATSUSHIRO A., SATO K., ITO J., KIDA S., IMAMOTO F. ON THE TRANSCRIPTION OF THE TRYTOPHAN OPERON IN ESCHERICHIA COLI. I. THE TRYPTOPHAN OPERATOR. J Mol Biol. 1965 Jan;11:54–63. doi: 10.1016/s0022-2836(65)80170-x. [DOI] [PubMed] [Google Scholar]
- Maas W. K. Genetic defects affecting an arginine permease and repression of arginine synthesis in Escherichia coli. Fed Proc. 1965 Sep-Oct;24(5):1239–1242. [PubMed] [Google Scholar]
- Maas W. K. Mapping of genes involved in the synthesis of spermidine in Escherichia coli. Mol Gen Genet. 1972;119(1):1–9. doi: 10.1007/BF00270439. [DOI] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A. Alterations in the cytoplasmic membrane proteins of various chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1971 Oct;108(1):564–570. doi: 10.1128/jb.108.1.564-570.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie G., Wilson D. B. Regulation of the gal operon of Escherichia coli by the capR gene. J Biol Chem. 1972 May 25;247(10):2973–2978. [PubMed] [Google Scholar]
- Mamelak L., Boyer H. W. Genetic control of the secondary modification of deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1970 Oct;104(1):57–62. doi: 10.1128/jb.104.1.57-62.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M., Halpern Y. S. Genetic analysis of glutamate transport and glutamate decarboxylase in Escherichia coli. J Bacteriol. 1967 Apr;93(4):1409–1415. doi: 10.1128/jb.93.4.1409-1415.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M., Halpern Y. S. Genetic analysis of the glutamate permease in Escherichia coli K-12. J Bacteriol. 1969 Mar;97(3):1118–1128. doi: 10.1128/jb.97.3.1118-1128.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M., Halpern Y. S. Genetic and physiological analysis of glutamate decarboxylase in Escherichia coli K-12. J Bacteriol. 1969 Mar;97(3):1509–1510. doi: 10.1128/jb.97.3.1509-1510.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M., Halpern Y. S. Mapping of the aspartase gene in Escherichia coli K-12. Isr J Med Sci. 1969 May-Jun;5(3):413–415. [PubMed] [Google Scholar]
- Marinus M. G. Location of DNA methylation genes on the Escherichia coli K-12 genetic map. Mol Gen Genet. 1973 Dec 14;127(1):47–55. doi: 10.1007/BF00267782. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R., Söll D., Kwong T. C. Isolation and partial characterization of three Escherichia coli mutants with altered transfer ribonucleic acid methylases. J Bacteriol. 1975 Apr;122(1):257–265. doi: 10.1128/jb.122.1.257-265.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz A., Lieberman M. M., Rosenbaum N. Derepression of phosphomannose isomerase by regulator gene mutations involved in capsular polysaccharide synthesis in Escherichia coli K-12. J Bacteriol. 1967 Nov;94(5):1497–1501. doi: 10.1128/jb.94.5.1497-1501.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh N. J., Duggan D. E. Ordering of mutant sites in the isoleucine-valine genes of Escherichia coli by use of merogenotes derived from F 14 : a new procedure for fine-structure mapping. J Bacteriol. 1972 Feb;109(2):730–740. doi: 10.1128/jb.109.2.730-740.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara M., Takata R., Osawa S. Chromosomal loci for 16S ribosomal RNA in Escherichia coli. Mol Gen Genet. 1972;117(4):311–317. doi: 10.1007/BF00333025. [DOI] [PubMed] [Google Scholar]
- Matsuzawa H., Hayakawa K., Sato T., Imahori K. Characterization and genetic analysis of a mutant of Escherichia coli K-12 with rounded morphology. J Bacteriol. 1973 Jul;115(1):436–442. doi: 10.1128/jb.115.1.436-442.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern I. E., Pittard J. Regulation of tyrosine biosynthesis in Escherichia coli K-12: isolation and characterization of operator mutants. J Bacteriol. 1971 Jul;107(1):8–15. doi: 10.1128/jb.107.1.8-15.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E. P., Smith O. H., Fredricks W. W., McKinney M. A. The isolation and characterization of glutamine-requiring strains of Escherichia coli K12. Mol Gen Genet. 1975;137(2):131–142. doi: 10.1007/BF00341679. [DOI] [PubMed] [Google Scholar]
- Mayuga C., Meier D., Wang T. Escherichia coli: the K 12 ribosomal protein and the streptomycin region of the chromosome. Biochem Biophys Res Commun. 1968 Oct 24;33(2):203–206. doi: 10.1016/0006-291x(68)90768-7. [DOI] [PubMed] [Google Scholar]
- McFadden G., Denhardt D. T. Mechanism of replication of phi x174 single-stranded DNA. IX. Requirement for the Escherichia coli dnaG protein. J Virol. 1974 Nov;14(5):1070–1075. doi: 10.1128/jvi.14.5.1070-1075.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall E. Escherichia coli K-12 mutant forming a temperature-sensitive D-serine deaminase. J Bacteriol. 1975 Mar;121(3):1074–1077. doi: 10.1128/jb.121.3.1074-1077.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall E. Mapping of the d-serine deaminase region in Escherichia coli K-12. Genetics. 1967 Jan;55(1):91–99. doi: 10.1093/genetics/55.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menninger J. R., Walker C., Tan P. F. Studies on the metabolic role of peptidyl-tRNA hydrolase. I. Properties of a mutant E. coli with temperature-sensitive peptidyl-tRNA hydrolase. Mol Gen Genet. 1973 Mar 19;121(4):307–324. doi: 10.1007/BF00433230. [DOI] [PubMed] [Google Scholar]
- Mergeay M., Gigot D., Beckmann J., Glansdorff N., Piérard A. Physiology and genetics of carbamoylphosphate synthesis in Escherichia coli K12. Mol Gen Genet. 1974;133(4):299–316. doi: 10.1007/BF00332706. [DOI] [PubMed] [Google Scholar]
- Milcarek C., Weiss B. Preliminary mapping of mutations affecting exonuclease 3 in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):1086–1088. doi: 10.1128/jb.113.2.1086-1088.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Beckwith J., Muller-Hill B. Direction of transcription of a regulatory gene in E. coli. Nature. 1968 Dec 28;220(5174):1287–1290. doi: 10.1038/2201287a0. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Ippen K., Scaife J. G., Beckwith J. R. The promoter-operator region of the lac operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):413–420. doi: 10.1016/0022-2836(68)90395-1. [DOI] [PubMed] [Google Scholar]
- Mindlin S. Z., Ilyina T. S., Voeykova T. A., Velkov V. V. Genetical analysis of rifampicin resistant mutants of E. coli K 12. Mol Gen Genet. 1972;115(2):115–121. doi: 10.1007/BF00277290. [DOI] [PubMed] [Google Scholar]
- Miyakawa T., Matsuzawa H., Matsuhashi M., Sugino Y. Cell wall peptidoglycan mutants of Escherichia coli K-12: existence of two clusters of genes, mra and mrb, for cell wall peptidoglycan biosynthesis. J Bacteriol. 1972 Nov;112(2):950–958. doi: 10.1128/jb.112.2.950-958.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H., Schlesinger M. J., Bracha M., Yagil E. Pleiotropic effects of mutations involved in the regulation of Escherichia coli K-12 alkaline phosphatase. J Bacteriol. 1974 Aug;119(2):583–592. doi: 10.1128/jb.119.2.583-592.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey A. T., Fraenkel D. G. Chromosomal location of a gene for fructose 6-phosphate kinase in Escherichia coli. J Bacteriol. 1969 Nov;100(2):1108–1109. doi: 10.1128/jb.100.2.1108-1109.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Guertin M. Amber suA mutations which relieve polarity. J Mol Biol. 1972 Feb 14;63(3):605–608. doi: 10.1016/0022-2836(72)90453-6. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Primakoff P. Relief of polarity in E. coli by "suA". Nature. 1970 Apr 4;226(5240):28–31. doi: 10.1038/226028a0. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Kosel C. Ultraviolet light-induced mutation in UV-resistant, thermosensitive derivatives of lexA-strains of Escherichia coli K-12. Mol Gen Genet. 1975;136(2):95–106. doi: 10.1007/BF00272033. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Low K. B., Edmiston S. J. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet lght-induced mutations. J Bacteriol. 1972 Nov;112(2):886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch-Petersen A., Nygaard P., Hammer-Jespersen K., Fiil N. Mutants constitutive for nucleoside-catabolizing enzymes in Escherichia coli K12. Isolation, charactrization and mapping. Eur J Biochem. 1972 May 23;27(2):208–215. doi: 10.1111/j.1432-1033.1972.tb01828.x. [DOI] [PubMed] [Google Scholar]
- Murgola E. J., Adelberg E. A. Mutants of Escherichia coli K-12 with an altered glutamyl-transfer ribonucleic acid synthetase. J Bacteriol. 1970 Jul;103(1):178–183. doi: 10.1128/jb.103.1.178-183.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T., Horiuchi T. An amber dna mutant of Escherichia coli K12 affecting DNA ligase. J Mol Biol. 1974 Aug 5;87(2):369–373. doi: 10.1016/0022-2836(74)90156-9. [DOI] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Zwaig N., Istúriz T., Sánchez R. S. Mutations affecting gluconate metabolism in Escherichia coli. J Bacteriol. 1973 May;114(2):463–468. doi: 10.1128/jb.114.2.463-468.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Genetic determination of resistance to acriflavine, phenethyl alcohol, and sodium dodecyl sulfate in Escherichia coli. J Bacteriol. 1968 Oct;96(4):987–996. doi: 10.1128/jb.96.4.987-996.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Tojo T., Greenberg J. Interaction of the expression of two membrane genes, acrA and plsA, in Escherichia coli K-12. J Bacteriol. 1975 Jun;122(3):874–879. doi: 10.1128/jb.122.3.874-879.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Yura T. Localization of the structural gene for the ' subunit of RNA polymerase in Escherichia coli. Biochem Biophys Res Commun. 1973 Jul 17;53(2):645–652. doi: 10.1016/0006-291x(73)90710-9. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestmann E. R., Hill R. F. Mutagenesis by mutator gene mutH1 in continuous cultures of Escherichia coli. J Bacteriol. 1974 Jul;119(1):33–35. doi: 10.1128/jb.119.1.33-35.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton N. A., Cox G. B., Gibson F. The function of menaquinone (vitamin K 2 ) in Escherichia coli K-12. Biochim Biophys Acta. 1971 Jul 20;244(1):155–166. doi: 10.1016/0304-4165(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J., De Haan P. G. Genetic and biochemical studies of the guanosine 5'-monophosphate pathway in Escherichia coli. Biochim Biophys Acta. 1967 Aug 22;145(1):31–40. doi: 10.1016/0005-2787(67)90651-x. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J., Oskamp A. A. Regulation of the biosynthesis of guanosine 5'-monophosphate: evidence for one operon. J Mol Biol. 1968 Jul 14;35(1):103–109. doi: 10.1016/s0022-2836(68)80040-3. [DOI] [PubMed] [Google Scholar]
- Nomura M., Witten C. Interaction of colicins with bacterial cells. 3. Colicin-tolerant mutations in Escherichia coli. J Bacteriol. 1967 Oct;94(4):1093–1111. doi: 10.1128/jb.94.4.1093-1111.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Marker-specific effects in genetic recombination. J Mol Biol. 1970 Aug;51(3):633–655. doi: 10.1016/0022-2836(70)90013-6. [DOI] [PubMed] [Google Scholar]
- Normark S., Boman H. G., Matsson E. Mutant of Escherichia coli with anomalous cell division and ability to decrease episomally and chromosomally mediated resistance to ampicillin and several other antibiotics. J Bacteriol. 1969 Mar;97(3):1334–1342. doi: 10.1128/jb.97.3.1334-1342.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S. Genetics of a chain-forming mutant of Escherichia coli. Transduction and dominance of the envA gene mediating increased penetration to some antibacterial agents. Genet Res. 1970 Aug;16(1):63–78. doi: 10.1017/s0016672300002287. [DOI] [PubMed] [Google Scholar]
- Normark S. Mutation in Escherichia coli K-12 mediating spherelike envelopes and changes tolerance to ultraviolet irradiation and some antibiotics. J Bacteriol. 1969 Jun;98(3):1274–1277. doi: 10.1128/jb.98.3.1274-1277.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel G., Novel M., Didier-Fichet M. L., Stoeber F. Etude génétique de mutants du système de dégradation des hexuronides chez Escherichia coli K 12. C R Acad Sci Hebd Seances Acad Sci D. 1970 Jul 27;271(4):457–460. [PubMed] [Google Scholar]
- Novel G., Novel M. Mutants d'Escherichia coli K 12 affectés pour leur croissance sur méthyl-beta-D-glucuronide: localisation of gène de structure de la beta-D-glucuronidase (uid A. Mol Gen Genet. 1973;120(4):319–335. [PubMed] [Google Scholar]
- Novel G., Stoeber F. Individualité de la D-glucuronate-cétol isomerase d'Escherichia coli K 12. Biochimie. 1973;55(9):1057–1070. doi: 10.1016/s0300-9084(73)80444-4. [DOI] [PubMed] [Google Scholar]
- Novel M., Novel G. Mutants d'Escherichia coli K-12, capables de croître sur méthyl-beta-D-galacturonide: mutants simples constitutifs pour la synthèse de la beta-glucuronidase et mutants doubles déréprimés aussi pour la synthèse de deux enzymes d'utilisation du glucuronate. C R Acad Sci Hebd Seances Acad Sci D. 1974 Aug 19;279(8):695–698. [PubMed] [Google Scholar]
- Novotny C. P., Englesberg E. The L-arabinose permease system in Escherichia coli B/r. Biochim Biophys Acta. 1966 Mar 28;117(1):217–230. doi: 10.1016/0304-4165(66)90169-3. [DOI] [PubMed] [Google Scholar]
- Ogawa H. Genetic locations of uvrD and pol genes of E. coli. Mol Gen Genet. 1970;108(4):378–381. doi: 10.1007/BF00267777. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- Okada T. Mutational Site of the Gene Controlling Quantitative Thymine Requirement in ESCHERICHIA COLI K-12. Genetics. 1966 Dec;54(6):1329–1336. doi: 10.1093/genetics/54.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi Y. Genetic analysis of an Escherichia coli mutant with a lesion in stable RNA turnover. Genetics. 1974 Feb;76(2):185–194. doi: 10.1093/genetics/76.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Adler J. Isolation and complementation of mutants in galactose taxis and transport. J Bacteriol. 1974 Feb;117(2):509–516. doi: 10.1128/jb.117.2.509-516.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Adler J. Properties of mutants in galactose taxis and transport. J Bacteriol. 1974 Feb;117(2):517–526. doi: 10.1128/jb.117.2.517-526.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orias E., Gartner T. K., Lannan J. E., Betlach M. Close linkage between ochre and missense suppressors in Escherichia coli. J Bacteriol. 1972 Mar;109(3):1125–1133. doi: 10.1128/jb.109.3.1125-1133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Nyman K. Genetic mapping of the antigenic determinants of two polysaccharide K antigens, K10 and K54, in Escherichia coli. J Bacteriol. 1974 Oct;120(1):43–51. doi: 10.1128/jb.120.1.43-51.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsubo E., Lee H. J., Deonier R. C., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. VI. Mapping of F14 sequences homologous to phi 80dmetBJF and phi 80dargECBH bacteriophages. J Mol Biol. 1974 Nov 15;89(4):599–618. doi: 10.1016/0022-2836(74)90038-2. [DOI] [PubMed] [Google Scholar]
- Otsubo E., Nishimura Y., Hirota Y. Transfer-defective mutants of sex factors in Escherichia coli. I. Defective mutants and complementation analysis. Genetics. 1970 Feb;64(2):173–188. doi: 10.1093/genetics/64.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsubo E. Transfer-defective mutants of sex factors in Escherichia coli. II. Deletion mutants of an F-prime and deletion mapping of cistrons involved in genetic transfer. Genetics. 1970 Feb;64(2):189–197. doi: 10.1093/genetics/64.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuji N., Higashi T., Kawamata J. Genetic and physiological analysis of mitomycin C-sensitive mutants of Escherichia coli K12. Biken J. 1972 Jun;15(2):49–59. [PubMed] [Google Scholar]
- Otsuji N., Iyehara H., Hideshima Y. Isolation and characterization of an Escherichia coli ruv mutant which forms nonseptate filaments after low doses of ultraviolet light irradiation. J Bacteriol. 1974 Feb;117(2):337–344. doi: 10.1128/jb.117.2.337-344.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuji N. Properties of mitomycin C-sensitive mutants of Escherichia coli K-12. J Bacteriol. 1968 Feb;95(2):540–545. doi: 10.1128/jb.95.2.540-545.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- PITTARD J. EFFECT OF INTEGRATED SEX FACTOR ON TRANSDUCTION OF CHROMOSOMAL GENES IN ESCHERICHIA COLI. J Bacteriol. 1965 Mar;89:680–686. doi: 10.1128/jb.89.3.680-686.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J., LOUTIT J. S., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. I. DELAY IN INITIATION OF CHROMOSOME TRANSFER. J Bacteriol. 1963 Jun;85:1394–1401. doi: 10.1128/jb.85.6.1394-1401.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESTIDGE L. S., PARDEE A. B. A SECOND PERMEASE FOR METHYL-THIO-BETA-D-GALACTOSIDE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 May 4;100:591–593. doi: 10.1016/0304-4165(65)90029-2. [DOI] [PubMed] [Google Scholar]
- Pai C. H. Biochemical and genetic characterization of dehydrobiotin resistant mutants of Escherichia coli. Mol Gen Genet. 1974;134(4):345–357. doi: 10.1007/BF00337469. [DOI] [PubMed] [Google Scholar]
- Pai C. H. Mutant of Escherichia coli with derepressed levels of the biotin biosynthetic enzymes. J Bacteriol. 1972 Dec;112(3):1280–1287. doi: 10.1128/jb.112.3.1280-1287.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panny S. R., Heil A., Mazus B., Palm P., Zillig W., Mindlin S. Z., Ilyina T. S., Khesin R. B. A temperature sensitive mutation of the beta'-subunit of DNA-dependent RNA polymerase from E. coli T 16. FEBS Lett. 1974 Nov 15;48(2):241–245. doi: 10.1016/0014-5793(74)80477-1. [DOI] [PubMed] [Google Scholar]
- Pardo D., Rosset R. Genetic studies of erythromycin resistant mutants of Escherichia coli. Mol Gen Genet. 1974;135(3):257–268. doi: 10.1007/BF00268620. [DOI] [PubMed] [Google Scholar]
- Pascal M. C., Puig J., Lepelletier M. Etude génétique d'une mutation affectant l'activité L-actate-déshydrogénase chez escherichia coli K 12. C R Acad Sci Hebd Seances Acad Sci D. 1969 Jan 27;268(4):737–739. [PubMed] [Google Scholar]
- Patel N., Moyed H. S., Kane J. F. Xanthosine-5'-phosphate amidotransferase from Escherichia coli. J Biol Chem. 1975 Apr 10;250(7):2609–2613. [PubMed] [Google Scholar]
- Patte J. C., Cohen G. N. Isolement et propriétés d'un mutant d'Escherichia coli depourvu d'aspartokinase sensible à la lysine. Biochim Biophys Acta. 1965 Jun 22;99(3):561–563. [PubMed] [Google Scholar]
- Patte J. C., Le Bras G., Cohen G. N. Regulation by methionine of the synthesis of a third aspartokinase and of a second homoserine dehydrogenase in Escherichia coli K 12. Biochim Biophys Acta. 1967 Mar 22;136(2):245–247. doi: 10.1016/0304-4165(67)90069-4. [DOI] [PubMed] [Google Scholar]
- Patterson D., Gillespie D. Deductive analysis of a protein-synthesis mutant of Escherichia coli. Biochem Genet. 1973 Feb;8(2):205–230. doi: 10.1007/BF00485547. [DOI] [PubMed] [Google Scholar]
- Paul A. V., Inouye M. Temperature-sensitive modification and restriction phenotypes of an Escherichia coli dnaD mutant. J Bacteriol. 1974 Sep;119(3):907–912. doi: 10.1128/jb.119.3.907-912.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli G., Overath P. ato Operon: a highly inducible system for acetoacetate and butyrate degradation in Escherichia coli. Eur J Biochem. 1972 Sep 25;29(3):553–562. doi: 10.1111/j.1432-1033.1972.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Peyru G., Fraenkel D. G. Genetic mapping of loci for glucose-6-phosphate dehydrogenase, gluconate-6-phosphate dehydrogenase, and gluconate-6-phosphate dehydrase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1272–1278. doi: 10.1128/jb.95.4.1272-1278.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Gene controlling the uptake of shikimic acid by Escherichia coli. J Bacteriol. 1966 Oct;92(4):1070–1075. doi: 10.1128/jb.92.4.1070-1075.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piérard A., Glansdorff N., Mergeay M., Wiame J. M. Control of the biosynthesis of carbamoyl phosphate in Escherichia coli. J Mol Biol. 1965 Nov;14(1):23–36. doi: 10.1016/s0022-2836(65)80226-1. [DOI] [PubMed] [Google Scholar]
- Piérard A., Glansdorff N., Yashphe J. Mutations affecting uridine monophosphate pyrophosphorylase or the argR gene in Escherichia coli. Effects on carbamoyl phosphate and pyrimidine biosynthesis and on uracil uptake. Mol Gen Genet. 1972;118(3):235–245. doi: 10.1007/BF00333460. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XXI. Mutations affecting derepression and valine resistance. J Bacteriol. 1973 Apr;114(1):183–194. doi: 10.1128/jb.114.1.183-194.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XXII. A pleiotropic mutation affecting induction of isomeroreductase activity. J Bacteriol. 1973 Apr;114(1):195–207. doi: 10.1128/jb.114.1.195-207.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portalier R. C., Robert-Baudouy J. M., Némoz G. M. Etudes de mutations affectant les gènes de structure de l'isomerase uronique et de l'oxydoreductase altronique chez Escherichia coli K 12. Mol Gen Genet. 1974;128(4):301–319. doi: 10.1007/BF00268518. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Robert-Baudouy J. M., Stoeber F. R. Localisation génétique et caractérisation biochimique de mutations affectant le gène de structure de l'hydrolyase altronique chez Escherichia coli K 12. Mol Gen Genet. 1972;118(4):335–350. doi: 10.1007/BF00333569. [DOI] [PubMed] [Google Scholar]
- Pouwels P. H., Cunin R., Glansdorff N. Letter: Divergent transcription in the argECBH cluster of genes in Escherichia coli K12. J Mol Biol. 1974 Mar;83(3):421–424. doi: 10.1016/0022-2836(74)90288-5. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J. M. Localisation génétique de mutations 2-céto-3-désoxy-6-P-gluconate aldolase négatives chez E. coli K 12. Mol Gen Genet. 1971;113(1):31–42. [PubMed] [Google Scholar]
- Pouyssegur J., Stoeber F. Genetic control of the 2-keto-3-deoxy-d-gluconate metabolism in Escherichia coli K-12: kdg regulon. J Bacteriol. 1974 Feb;117(2):641–651. doi: 10.1128/jb.117.2.641-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Lagarde A. Système de transport du 2-céto-3-désoxy-gluconate chez E. coli K 12: localisation d'un gène de structure et de son opérateur. Mol Gen Genet. 1973 Mar 1;121(2):163–180. doi: 10.1007/BF00277530. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Stoeber F. Mutations affectant le gène de structure de la 2-céto-3-désoxy-6-P-gluconate aldolase chez E. coli K 12. Mol Gen Genet. 1972;114(4):305–311. doi: 10.1007/BF00267499. [DOI] [PubMed] [Google Scholar]
- Powell K. A., Cox R., McConville M., Charles H. P. Mutations affecting porphyrin biosynthesis in Escherichia coli. Enzyme. 1973;16(1):65–73. doi: 10.1159/000459363. [DOI] [PubMed] [Google Scholar]
- Power J. The L-rhamnose genetic system in Escherichia coli K-12. Genetics. 1967 Mar;55(3):557–568. doi: 10.1093/genetics/55.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad I., Schaefler S. Regulation of the beta-glucoside system in Escherchia coli K-12. J Bacteriol. 1974 Nov;120(2):638–650. doi: 10.1128/jb.120.2.638-650.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad I., Young B., Schaefler S. Genetic determination of the constitutive biosynthesis of phospho- -glucosidase A in Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):909–915. doi: 10.1128/jb.114.3.909-915.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard R. H., Ahmad S. I. Fluorouracil and the isolation of mutants lacking uridine phosphorylase in Escherichia coli: location of the gene. Mol Gen Genet. 1971;111(1):84–88. doi: 10.1007/BF00286557. [DOI] [PubMed] [Google Scholar]
- Puig J., Azoulay E. Etude génétique et biochimique des mutants résistant au Clo minus 3 (gènes chl A, chl B, chl C) C R Acad Sci Hebd Seances Acad Sci D. 1967 Apr 10;264(15):1916–1918. [PubMed] [Google Scholar]
- Puig J., Azoulay E., Gendre J., Richard E. Etude génétique des mutants de la région chl A chez l'Escherichia coli k12. C R Acad Sci Hebd Seances Acad Sci D. 1969 Jan 6;268(1):183–184. [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. 3. MAP ORDER OF THE STRUCTURAL GENES AND OPERATOR GENES. J Bacteriol. 1965 Mar;89:661–664. doi: 10.1128/jb.89.3.661-664.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWBURY R. J., WOODS D. D. O-SUCCINYLHOMOSERINE AS AN INTERMEDIATE IN THE SYNTHESIS OF CYSTATHIONINE BY ESCHERICHIA COLI. J Gen Microbiol. 1964 Sep;36:341–358. doi: 10.1099/00221287-36-3-341. [DOI] [PubMed] [Google Scholar]
- Radke K. L., Siegel E. C. Mutation preventing capsular polysaccharide synthesis in Escherichia coli K-12 and its effect on bacteriophage resistance. J Bacteriol. 1971 May;106(2):432–437. doi: 10.1128/jb.106.2.432-437.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve E. C., Doherty P. Linkage relationships of two genes causing partial resistance to chloramphenicol in Escherichia coli. J Bacteriol. 1968 Oct;96(4):1450–1451. doi: 10.1128/jb.96.4.1450-1451.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves P. Mutants resistant to colicin CA42-E2: cross resistance and genetic mapping of a special class of mutants. Aust J Exp Biol Med Sci. 1966 Jun;44(3):301–315. doi: 10.1038/icb.1966.29. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Genetic locus for ribonuclease I in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1522–1523. doi: 10.1128/jb.97.3.1522-1523.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M. Isolation and mapping of polynucleotide phosphorylase mutants of Escherichia coli. J Bacteriol. 1969 Mar;97(3):1431–1436. doi: 10.1128/jb.97.3.1431-1436.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P., Grimley C., Lowery C. Transcription termination factor rho activity is altered in Escherichia coli with suA gene mutations. Proc Natl Acad Sci U S A. 1975 May;72(5):1725–1728. doi: 10.1073/pnas.72.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere C., Giordano G., Pommier J., Azoulay E. Membrane reconstitution in chl-r mutants of Escherichia coli K 12. VIII. Purification and properties of the FA factor, the product of the chl B gene. Biochim Biophys Acta. 1975 May 6;389(2):219–235. doi: 10.1016/0005-2736(75)90317-x. [DOI] [PubMed] [Google Scholar]
- Roback E. R., Friesen J. D. A temperature-sensitive glycyl-transfer ribonucleic acid synthetase mutant of Escherichia coli. Can J Microbiol. 1973 Apr;19(4):421–426. doi: 10.1139/m73-070. [DOI] [PubMed] [Google Scholar]
- Robbins J. C., Oxender D. L. Transport systems for alanine, serine, and glycine in Escherichia coli K-12. J Bacteriol. 1973 Oct;116(1):12–18. doi: 10.1128/jb.116.1.12-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Baudouy J. M., Portalier R. C. Mutations affectant le catabolisme du glucuronate chez Escherichia coli K12. Mol Gen Genet. 1974;131(1):31–46. doi: 10.1007/BF00269385. [DOI] [PubMed] [Google Scholar]
- Robert-Baudouy J. M., Portalier R. C., Stoeber F. R. Localisation génétique et caractérisation biochimique de mutations affectant le gène de structure de l'hydrolyase mannonique chez Escherichia coli K 12. Mol Gen Genet. 1972;118(4):351–362. doi: 10.1007/BF00333570. [DOI] [PubMed] [Google Scholar]
- Roberts L. M., Reeve E. C. Two mutations giving low-level streptomycin resistance in Escherichia coli K 12. Genet Res. 1970 Dec;16(3):359–365. doi: 10.1017/s0016672300002640. [DOI] [PubMed] [Google Scholar]
- Rodolakis A., Casse F., Starka J. Morphological mutants of Escherichia coli K12. Mapping of the env C mutation. Mol Gen Genet. 1974 May 21;130(2):177–181. doi: 10.1007/BF00269088. [DOI] [PubMed] [Google Scholar]
- Rodolakis A., Thomas P., Starka J. Morphological mutants of Escherichia coli. Isolation and ultrastructure of a chain-forming envC mutant. J Gen Microbiol. 1973 Apr;75(2):409–416. doi: 10.1099/00221287-75-2-409. [DOI] [PubMed] [Google Scholar]
- Rolfe B., Eisenberg M. A. Genetic and biochemical analysis of the biotin loci of Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):515–524. doi: 10.1128/jb.96.2.515-524.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld I. S., D'Agnolo G., Vagelos P. R. Synthesis of unsaturated fatty acids and the lesion in fab B mutants. J Biol Chem. 1973 Apr 10;248(7):2452–2460. [PubMed] [Google Scholar]
- Rosset R., Gorini L. A ribosomal ambiguity mutation. J Mol Biol. 1969 Jan 14;39(1):95–112. doi: 10.1016/0022-2836(69)90336-2. [DOI] [PubMed] [Google Scholar]
- Rothman J. L. Transduction studies on the relation between prophage and host chromosome. J Mol Biol. 1965 Jul;12(3):892–912. doi: 10.1016/s0022-2836(65)80336-9. [DOI] [PubMed] [Google Scholar]
- Rotman B., Ganesan A. K., Guzman R. Transport systems for galactose and galactosides in Escherichia coli. II. Substrate and inducer specificities. J Mol Biol. 1968 Sep 14;36(2):247–260. doi: 10.1016/0022-2836(68)90379-3. [DOI] [PubMed] [Google Scholar]
- Ruffler D., Buckel P., Piepersberg W., Böck A. Alanyl-tRNA synthetase of Escherichia coli: genetic analysis of the structural gene and of suppressor mutations. Mol Gen Genet. 1974;134(4):313–323. doi: 10.1007/BF00337466. [DOI] [PubMed] [Google Scholar]
- Ruffler D., Böck A. Location of the structural gene for fructose-1,6-diphosphate aldolase in Escherichia coli. J Bacteriol. 1973 Nov;116(2):1054–1055. doi: 10.1128/jb.116.2.1054-1055.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J., Showe M. K., DeMoss J. A. Nitrate reductase complex of Escherichia coli K-12: isolation and characterization of mutants unable to reduce nitrate. J Bacteriol. 1969 Mar;97(3):1291–1297. doi: 10.1128/jb.97.3.1291-1297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. L., Abelson J. N., Landy A., Gefter M. L., Brenner S., Smith J. D. Duplicate genes for tyrosine transfer RNA in Escherichia coli. J Mol Biol. 1970 Jan 14;47(1):1–13. doi: 10.1016/0022-2836(70)90397-9. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Close linkage of prd and rel genes in Escherichia coli K-12. Mol Gen Genet. 1973 Aug 28;124(4):369–370. doi: 10.1007/BF00267666. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Mapping of a D-cycloserine resistance locus in escherichia coli K-12. J Bacteriol. 1972 Aug;111(2):622–624. doi: 10.1128/jb.111.2.622-624.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R., Pittard A. J. Mutants of Escherichia coli unable to make protein at 42 C. J Bacteriol. 1971 Nov;108(2):790–798. doi: 10.1128/jb.108.2.790-798.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R., Pittard A. J. New suppresor in Escherichia coli. J Bacteriol. 1971 Sep;107(3):736–740. doi: 10.1128/jb.107.3.736-740.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R. Temperature-sensitive osmotic remedial mutants of Escherichia coli. J Bacteriol. 1972 Nov;112(2):661–665. doi: 10.1128/jb.112.2.661-665.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMERVILLE R. L., YANOFSKY C. STUDIES ON THE REGULATION OF TRYPTOPHAN BIOSYNTHESIS IN ESCHERICHIA COLI. J Mol Biol. 1965 Apr;11:747–759. doi: 10.1016/s0022-2836(65)80032-8. [DOI] [PubMed] [Google Scholar]
- Saedler H., Gullon A., Fiethen L., Starlinger P. Negative control of the galactose operon in E. coli. Mol Gen Genet. 1968;102(1):79–88. doi: 10.1007/BF00341872. [DOI] [PubMed] [Google Scholar]
- Sakai H., Hashimoto S., Komano T. Replication of deoxyribonucleic acid in Escherichia coli C mutants temperature sensitive in the initiation of chromosome replication. J Bacteriol. 1974 Sep;119(3):811–820. doi: 10.1128/jb.119.3.811-820.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S., Maas W. K. Inducible system for the utilization of beta-glucosides in Escherichia coli. II. Description of mutant types and genetic analysis. J Bacteriol. 1967 Jan;93(1):264–272. doi: 10.1128/jb.93.1.264-272.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schairer H. U., Gruber D. Mutants of Escherichia coli K12 defective in oxidative phosphorylation. Eur J Biochem. 1973 Aug 17;37(2):282–286. doi: 10.1111/j.1432-1033.1973.tb02986.x. [DOI] [PubMed] [Google Scholar]
- Schleif R. Isolation and characterization of streptolydigin resistant RNA polymerase. Nature. 1969 Sep 6;223(5210):1068–1069. doi: 10.1038/2231068a0. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Nester E. W. Mutants of Escherichia coli with an altered tyrosyl-transfer ribonucleic acid synthetase. J Bacteriol. 1969 Oct;100(1):167–175. doi: 10.1128/jb.100.1.167-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. Genetical studies on the lipopolysaccharide structure of Escherichia coli K12. J Gen Microbiol. 1973 Jul;77(1):151–160. doi: 10.1099/00221287-77-1-151. [DOI] [PubMed] [Google Scholar]
- Schmitt R. Analysis of melibiose mutants deficient in alpha-galactosidase and thiomethylgalactoside permease II in Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):462–471. doi: 10.1128/jb.96.2.462-471.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubach W. H., Whitmer J. D., Davern C. I. Genetic control of DNA initiation in Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):205–221. doi: 10.1016/0022-2836(73)90107-1. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Location of the maltose A and B loci on the genetic map of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1083–1089. doi: 10.1128/jb.92.4.1083-1089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. Sur l'existence chez Escherichia coli K 12 d'une régulation commune à la biosynthèse des récepteurs du bactériophage et au métabolisme du maltose. Ann Inst Pasteur (Paris) 1967 Nov;113(5):685–704. [PubMed] [Google Scholar]
- Semple K. S., Silbert D. F. Mapping of the fabD locus for fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1975 Mar;121(3):1036–1046. doi: 10.1128/jb.121.3.1036-1046.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A., Adhya S. L. The galactose operon of E. coli K-12. II. A deletion analysis of operon structure and polarity. Genetics. 1969 Jun;62(2):249–264. doi: 10.1093/genetics/62.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. Chromosomal location of the gene determining uridine diphoglucose formation in Escherichia coli K-12. J Bacteriol. 1966 Aug;92(2):518–520. doi: 10.1128/jb.92.2.518-520.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Sheppard D. E., Englesberg E. Further evidence for positive control of the L-arabinose system by gene araC. J Mol Biol. 1967 May 14;25(3):443–454. doi: 10.1016/0022-2836(67)90197-0. [DOI] [PubMed] [Google Scholar]
- Sheppard D., Englesberg E. Positive control in the L-arabinose gene-enzyme complex of Escherichia coli B/r exhibited with stable merodiploids. Cold Spring Harb Symp Quant Biol. 1966;31:345–347. doi: 10.1101/sqb.1966.031.01.044. [DOI] [PubMed] [Google Scholar]
- Shinozawa T. A mutant of Escherichia coli K12 unable to support the multiplication of bacteriophage BF23. Virology. 1973 Aug;54(2):427–440. doi: 10.1016/0042-6822(73)90154-2. [DOI] [PubMed] [Google Scholar]
- Siegel E. C., Bryson V. Mutator gene of Escherichia coli B. J Bacteriol. 1967 Jul;94(1):38–47. doi: 10.1128/jb.94.1.38-47.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. C. Genetic location in Escherichia coli K-12 of the ultraviolet-sensitive mutation uvrD3. J Bacteriol. 1970 Oct;104(1):604–605. doi: 10.1128/jb.104.1.604-605.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. C., Ivers J. J. mut-25, a mutation to mutator linked to purA in Escherichia coli. J Bacteriol. 1975 Feb;121(2):524–530. doi: 10.1128/jb.121.2.524-530.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. C. Ultraviolet-sensitive mutator mutU4 of Escherichia coli inviable with polA. J Bacteriol. 1973 Jan;113(1):161–166. doi: 10.1128/jb.113.1.161-166.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. C. Ultraviolet-sensitive mutator strain of Escherichia coli K-12. J Bacteriol. 1973 Jan;113(1):145–160. doi: 10.1128/jb.113.1.145-160.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signer E. R., Beckwith J. R., Brenner S. Mapping of suppressor loci in Escherichia coli. J Mol Biol. 1965 Nov;14(1):153–166. doi: 10.1016/s0022-2836(65)80237-6. [DOI] [PubMed] [Google Scholar]
- Signer E. R. Interaction of prophages at the att80 site with the chromosome of Escherichia coli. J Mol Biol. 1966 Jan;15(1):243–255. doi: 10.1016/s0022-2836(66)80224-3. [DOI] [PubMed] [Google Scholar]
- Silver S., Johnseine P., Whitney E., Clark D. Manganese-resistant mutants of Escherichia coli: physiological and genetic studies. J Bacteriol. 1972 Apr;110(1):186–195. doi: 10.1128/jb.110.1.186-195.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Genetic analysis of bacteriophage Mu-induced flagellar mutants in Escherichia coli. J Bacteriol. 1973 Oct;116(1):114–122. doi: 10.1128/jb.116.1.114-122.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Genetic analysis of flagellar mutants in Escherichia coli. J Bacteriol. 1973 Jan;113(1):105–113. doi: 10.1128/jb.113.1.105-113.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Positioning flagellar genes in Escherichia coli by deletion analysis. J Bacteriol. 1974 Jan;117(1):73–79. doi: 10.1128/jb.117.1.73-79.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar P. D. A BINARY MUTABILITY SYSTEM IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1956 May;42(5):245–249. doi: 10.1073/pnas.42.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner A. J., Cooper R. A. Genetic studies on ribose 5-phosphate isomerase mutants of Escherichia coli K-12. J Bacteriol. 1974 Jun;118(3):1183–1185. doi: 10.1128/jb.118.3.1183-1185.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov G. B., Filkova E. V., Skavronskaya A. G. The mutator property of uvr502 mutation affecting UV sensitivity of Escherichia coli. Mol Gen Genet. 1972;118(1):51–56. doi: 10.1007/BF02428332. [DOI] [PubMed] [Google Scholar]
- Smirnov G. B., Skavronskaya A. G. Location of uvr502 mutation on the chromosome of Escherichia coli K-12. Mol Gen Genet. 1971;113(3):217–221. doi: 10.1007/BF00339541. [DOI] [PubMed] [Google Scholar]
- Smith D. A., Childs J. D. Methionine genes and enzymes of Salmonella typhimurium. Heredity (Edinb) 1966 May;21(2):265–286. doi: 10.1038/hdy.1966.22. [DOI] [PubMed] [Google Scholar]
- Sokolova E. V., Ovadis M. I., Gorlenko Z. M., Khesin R. B. Localization of streptolydigin resistant mutation in E. coli chromosome and effect of streptolydigin on T2 phage development in stl-r and stl-s strains of E. coli. Biochem Biophys Res Commun. 1970 Nov 25;41(4):870–876. doi: 10.1016/0006-291x(70)90164-6. [DOI] [PubMed] [Google Scholar]
- Soll L., Berg P. Recessive lethals: a new class of nonsense suppressors in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jun;63(2):392–399. doi: 10.1073/pnas.63.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll L. Mutational alterations of tryptophan-specific transfer RNA that generate translation suppressors of the UAA, UAG and UGA nonsense codons. J Mol Biol. 1974 Jun 25;86(2):233–243. doi: 10.1016/0022-2836(74)90015-1. [DOI] [PubMed] [Google Scholar]
- Solomon E., Lin E. C. Mutations affecting the dissimilation of mannitol by Escherichia coli K-12. J Bacteriol. 1972 Aug;111(2):566–574. doi: 10.1128/jb.111.2.566-574.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers J. M., Amzallag A., Middleton R. B. Genetic fine structure of the leucine operon of Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1268–1272. doi: 10.1128/jb.113.3.1268-1272.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Blackman E. Mutation to erythromycin dependence in Escherichia coli K-12. J Bacteriol. 1973 Oct;116(1):74–83. doi: 10.1128/jb.116.1.74-83.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Ikeya Y., Elliot D. Two genetic loci for resistance to kasugamycin in Escherichia coli. J Bacteriol. 1973 Feb;113(2):704–710. doi: 10.1128/jb.113.2.704-710.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F. Kasugamycin resistance: 30S ribosomal mutation with an unusual location on the Escherichia coli chromosome. Science. 1970 Jan 2;167(3914):56–58. doi: 10.1126/science.167.3914.56. [DOI] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Isolation and properties of fumarate reductase mutants of Escherichia coli. J Bacteriol. 1973 May;114(2):563–570. doi: 10.1128/jb.114.2.563-570.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Proteins of the inner membrane of Escherichia coli: changes in composition associated with anaerobic growth and fumarate reductase amber mutation. J Bacteriol. 1974 Mar;117(3):954–959. doi: 10.1128/jb.117.3.954-959.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperl G. T., DeMoss J. A. chlD gene function in molybdate activation of nitrate reductase. J Bacteriol. 1975 Jun;122(3):1230–1238. doi: 10.1128/jb.122.3.1230-1238.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C., Carbon J., Hill C. W. Glycine transfer RNA of Escherichia coli. I. Structural genes for two glycine tRNA species. J Mol Biol. 1970 Sep 28;52(3):557–569. doi: 10.1016/0022-2836(70)90419-5. [DOI] [PubMed] [Google Scholar]
- Stadler D., Kariya B. Marker effects in the genetic transduction of tryptophan mutants of E. coli. Genetics. 1973 Nov;75(3):423–439. doi: 10.1093/genetics/75.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamminger G., Lazzarini R. A. Analysis of the RNA of defective VSV particles. Cell. 1974 Sep;3(1):85–93. doi: 10.1016/0092-8674(74)90044-0. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L., Olsen W. L., Hofschneider P. H. Analysis of bacteriophage-M 13-DNA replication in an Escherichia coli mutant thermosensitive in DNA polymerase 3. Eur J Biochem. 1973 Jan 15;32(2):247–253. doi: 10.1111/j.1432-1033.1973.tb02604.x. [DOI] [PubMed] [Google Scholar]
- Sternberg N. Properties of a mutant of Escherichia coli defective in bacteriophage lambda head formation (groE). I. Initial characterization. J Mol Biol. 1973 May 5;76(1):1–23. doi: 10.1016/0022-2836(73)90078-8. [DOI] [PubMed] [Google Scholar]
- Stetson H., Somerville R. L. Expression of the tryptophan operon in merodiploids of Escherichia coli. I. Gene dosage, gene position and marker effects. Mol Gen Genet. 1971;111(4):342–351. doi: 10.1007/BF00569786. [DOI] [PubMed] [Google Scholar]
- Stoeber F., Lagarde A., Nemoz G., Novel G., Novel M., Portalier R., Pouyssegur J., Robert-Baudouy J. Le métabolisme des hexuronides et des hexuronates chez Escherichia coli K 12: aspects physiologiques et et génétiques de sa régulation. Biochimie. 1974;56(2):199–213. doi: 10.1016/s0300-9084(74)80379-2. [DOI] [PubMed] [Google Scholar]
- Stonington O. G., Pettijohn D. E. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci U S A. 1971 Jan;68(1):6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm P. K., Hoekstra W. P., de Haan P. G., Verhoef C. Genetic recombination in Escherichia coli. IV. Isolation and characterization of recombination-deficiency mutants of Escherichia coli K12. Mutat Res. 1971 Sep;13(1):9–17. doi: 10.1016/0027-5107(71)90121-7. [DOI] [PubMed] [Google Scholar]
- Storm P. K., Zaunbrecher W. M. A new radiation sensitive mutant of Escherichia coli K-12. Mol Gen Genet. 1972;115(1):89–92. doi: 10.1007/BF00272221. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., de Haan P. G., Nijkamp H. J. Mapping of purine markers in Escherichia coli K 12. Genet Res. 1965 Nov;6(3):442–453. doi: 10.1017/s0016672300004328. [DOI] [PubMed] [Google Scholar]
- Stretton A. O., Kaplan S., Brenner S. Nonsense codons. Cold Spring Harb Symp Quant Biol. 1966;31:173–179. doi: 10.1101/sqb.1966.031.01.025. [DOI] [PubMed] [Google Scholar]
- Stroobant P., Young I. G., Gibson F. Mutants of Escherichia coli K-12 blocked in the final reaction of ubiquinone biosynthesis: characterization and genetic analysis. J Bacteriol. 1972 Jan;109(1):134–139. doi: 10.1128/jb.109.1.134-139.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Genetic mapping of a mutation that causes ribonucleases III deficiency in Escherichia coli. J Bacteriol. 1975 Oct;124(1):307–316. doi: 10.1128/jb.124.1.307-316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. H., Greene R. C. Regulation of methionine biosynthesis in Escherichia coli: mapping of the metJ locus and properties of a metJ plus-metJ minus diploid. Proc Natl Acad Sci U S A. 1971 Feb;68(2):367–371. doi: 10.1073/pnas.68.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino Y. Mutants of Escherichia coli sensitive to methylene blue and acridines. Genet Res. 1966 Feb;7(1):1–11. doi: 10.1017/s0016672300009423. [DOI] [PubMed] [Google Scholar]
- Sunshine M. G., Kelly B. Extent of host deletions associated with bacteriophage P2-mediated eduction. J Bacteriol. 1971 Nov;108(2):695–704. doi: 10.1128/jb.108.2.695-704.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland B. M., Court D., Chamberlin M. J. Studies on the DNA photoreactivating enzyme from Escherichia coli. I. Transduction of the phr gene by bacteriophage lambda. Virology. 1972 Apr;48(1):87–93. doi: 10.1016/0042-6822(72)90116-x. [DOI] [PubMed] [Google Scholar]
- Svenningsen B. A. Regulated in vitro synthesis of the enzymes of the deo operon of Escerichia coli. properties of the DNA directed system. Mol Gen Genet. 1975;137(4):289–304. doi: 10.1007/BF00703255. [DOI] [PubMed] [Google Scholar]
- Sánchez-Anzaldo F. J., Bastarrachea F. Genetic characterization of streptomycin-resistant and -dependent mutants of Escherichia coli K12. Mol Gen Genet. 1974 Apr 9;130(1):47–64. doi: 10.1007/BF00270518. [DOI] [PubMed] [Google Scholar]
- Săsărman A., Chartrand P., Proschek R., Desrochers M., Tardif D., Lapointe C. Uroporphyrin-accumulating mutant of Escherichia coli K-12. J Bacteriol. 1975 Dec;124(3):1205–1212. doi: 10.1128/jb.124.3.1205-1212.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Horodniceanu T. Locus determining the synthesis of delta-aminolevulinic acid in Escherichia coli K-12. J Bacteriol. 1968 Nov;96(5):1882–1884. doi: 10.1128/jb.96.5.1882-1884.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Szégli G., Horodniceanu T., Greceanu V., Dumitrescu A. Hemin-deficient mutants of Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):570–572. doi: 10.1128/jb.96.2.570-572.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A. L., THOMAN M. S. THE GENETIC MAP OF ESCHERICHIA COLI K-12. Genetics. 1964 Oct;50:659–677. doi: 10.1093/genetics/50.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T. Bacterial mutants defective in plasmid formation: requirement for the lon + allele. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1469–1473. doi: 10.1073/pnas.68.7.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Kakefuda T. Involvement of a bacterial factor in morphogenesis of bacteriophage capsid. Nat New Biol. 1972 Sep 13;239(89):34–37. doi: 10.1038/newbio239034a0. [DOI] [PubMed] [Google Scholar]
- Takata R., Osawa S., Tanaka K., Teraoka H., Tamaki M. Genetic studies of he ribosoml proteis in Escherichaoli. V. Mapp-ing of erythromycin resistance mutations which lea to alteration of a 50s ribosomal protein component. Mol Gen Genet. 1970;109(2):123–130. doi: 10.1007/BF00269648. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., Kawano G., Kinoshita T. Chromosomal location of a fusidic acid resistant marker in Escherichia coli. Biochem Biophys Res Commun. 1971 Feb 5;42(3):564–567. doi: 10.1016/0006-291x(71)90408-6. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templin A., Kushner S. R., Clark A. J. Genetic analysis of mutations indirectly suppressing recB and recC mutations. Genetics. 1972 Oct;72(2):105–115. [PMC free article] [PubMed] [Google Scholar]
- Thirion J. P., Hofnung M. On some genetic aspects of phage lambda resistance in E. coli K12. Genetics. 1972 Jun;71(2):207–216. doi: 10.1093/genetics/71.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall E. J., Holland I. B. Co-transduction with serB of a pleiotropic mutation affecting colicin E2 refractivity, ultraviolet sensitivity, recombination proficiency and surface properties of Escherichia coli K12. J Gen Microbiol. 1970 Aug;62(3):383–398. doi: 10.1099/00221287-62-3-383. [DOI] [PubMed] [Google Scholar]
- Thèze J., Margarita D., Cohen G. N., Borne F., Patte J. C. Mapping of the structural genes of the three aspartokinases and of the two homoserine dehydrogenases of Escherichia coli K-12. J Bacteriol. 1974 Jan;117(1):133–143. doi: 10.1128/jb.117.1.133-143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thèze J., Saint-Girons I. Threonine locus of Escherichia coli K-12: genetic structure and evidence for an operon. J Bacteriol. 1974 Jun;118(3):990–998. doi: 10.1128/jb.118.3.990-998.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingle M. A., Neidhardt F. C. Mapping of a structural gene for valyl-transfer ribonucleic acid synthetase in Escherichia coli by transduction. J Bacteriol. 1969 May;98(2):837–839. doi: 10.1128/jb.98.2.837-839.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa H. Structural genes of ATP-dependent deoxyribonuclease of Escherichia coli. Nat New Biol. 1972 Sep 6;239(88):14–16. doi: 10.1038/newbio239014a0. [DOI] [PubMed] [Google Scholar]
- Tritz G. J., Matney T. S., Chandler J. L., Gholson R. K. Chromosomal location of the C gene involved in the biosynthesis of nicotinamide adenine dinucleotide in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):45–49. doi: 10.1128/jb.104.1.45-49.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritz G. J., Matney T. S., Chandler J. L., Gholson R. K. Identification of the purI locus in Escherichia coli K-12. J Bacteriol. 1970 Jun;102(3):881–883. doi: 10.1128/jb.102.3.881-883.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritz G. J., Matney T. S., Gholson R. K. Mapping of the nadB locus adjacent to a previously undescribed purine locus in Escherichia coli K-12. J Bacteriol. 1970 May;102(2):377–381. doi: 10.1128/jb.102.2.377-381.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B., Wishnow R., Loomis W. F., Jr, Magasanik B. Catabolite repression gene of Escherichia coli. J Bacteriol. 1969 Nov;100(2):809–816. doi: 10.1128/jb.100.2.809-816.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., UMBARGER M. A., SIU P. M. BIOSYNTHESIS OF SERINE IN ESCHERICHIA COLI AND SALMONELLA TYPHIMURIUM. J Bacteriol. 1963 Jun;85:1431–1439. doi: 10.1128/jb.85.6.1431-1439.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DE PUTTE P., VAN DILLEWIJN, ROERSCH A. THE SELECTION OF MUTANTS OF ESCHERICHIA COLI WITH IMPAIRED CELL DIVISION AT ELEVATED TEMPERATURE. Mutat Res. 1964 Jul;106:121–128. doi: 10.1016/0027-5107(64)90014-4. [DOI] [PubMed] [Google Scholar]
- Van Pel A., Colson C. DNA restriction and modification systems in Salmonella. II. Genetic complementation between the K and B systems of Escherichia coli and the Salmonella typhimurium system SB, with the same chromosomal location. Mol Gen Genet. 1974;135(1):51–60. doi: 10.1007/BF00433901. [DOI] [PubMed] [Google Scholar]
- Vanderwinkel E., De Vlieghere M. Physiologie et génétique de l'isocitritase et des malate synthases chez Escherichia coli. Eur J Biochem. 1968 Jun;5(1):81–90. doi: 10.1111/j.1432-1033.1968.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Venables W. A. Genetic studies with nitrate reductase-less mutants of Escherichia coli. I. Fine structure analysis of the narA, narB and narE loci. Mol Gen Genet. 1972;114(3):223–231. doi: 10.1007/BF01788891. [DOI] [PubMed] [Google Scholar]
- Venables W. A., Guest J. R. Transduction of nitrate reductase loci of Escherichia coli by phages P-1 and lambda. Mol Gen Genet. 1968;103(2):127–140. doi: 10.1007/BF00427140. [DOI] [PubMed] [Google Scholar]
- Verhoef C., de Haan P. G. Genetic recombination in Escherichia coli. I. Relation between linkage of unselected markers and map distance. Mutat Res. 1966 Apr;3(2):101–110. doi: 10.1016/0027-5107(66)90023-6. [DOI] [PubMed] [Google Scholar]
- Vinopal R. T., Clifton D., Fraenkel D. G. PfkA locus of Escherichia coli. J Bacteriol. 1975 Jun;122(3):1162–1171. doi: 10.1128/jb.122.3.1162-1171.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinopal R. T., Fraenkel D. G. PfkB and pfkC loci of Escherichia coli. J Bacteriol. 1975 Jun;122(3):1153–1161. doi: 10.1128/jb.122.3.1153-1161.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinopal R. T., Fraenkel D. G. Phenotypic suppression of phosphofructokinase mutations in Escherichia coli by constitutive expression of the glyoxylate shunt. J Bacteriol. 1974 Jun;118(3):1090–1100. doi: 10.1128/jb.118.3.1090-1100.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackernagel W., Winkler U. A mutation in Escherichia coli enhancing the UV-mutability of phage lambda but not of its infectious DNA in a spheroplast assay. Mol Gen Genet. 1972;114(1):68–79. doi: 10.1007/BF00268748. [DOI] [PubMed] [Google Scholar]
- Wada C., Yura T. Phenethyl alcohol resistance in Escherichia coli. 3. A temperature-sensitive mutation(dnaP) affecting DNA replication. Genetics. 1974 Jun;77(2):199–220. doi: 10.1093/genetics/77.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada C., Yura T. Phenethyl alcohol resistance in Escherichia coli. II. Replication of F factor in the resistant strain C600. Genetics. 1971 Nov;69(3):257–287. [PMC free article] [PubMed] [Google Scholar]
- Walker J. R. Defective excision repair of pyrimidine dimers in the ultraviolet-sensitive Escherichia coli ras- mutant. J Bacteriol. 1970 Sep;103(3):552–559. doi: 10.1128/jb.103.3.552-559.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R. Escherichia coli ras locus: its involvement in radiation repair. J Bacteriol. 1969 Sep;99(3):713–719. doi: 10.1128/jb.99.3.713-719.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Genetic and biochemical analysis of the isoenzymes concerned in the first reaction of aromatic biosynthesis in Escherichia coli. J Bacteriol. 1967 Jan;93(1):237–244. doi: 10.1128/jb.93.1.237-244.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Regulator gene controlling enzymes concerned in tyrosine biosynthesis in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1234–1241. doi: 10.1128/jb.97.3.1234-1241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Newton A. Iron transport in Escherichia coli: relationship between chromium sensitivity and high iron requirement in mutants of Escherichia coli. J Bacteriol. 1969 Jun;98(3):1135–1141. doi: 10.1128/jb.98.3.1135-1141.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. J., Morse H. G., Morse M. L. Carbohydrate Accumulation and Metabolism in Escherichia coli: Characteristics of the Reversions of ctr Mutations. J Bacteriol. 1970 Dec;104(3):1318–1324. doi: 10.1128/jb.104.3.1318-1324.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. J., Morse H. G., Morse M. L. Carbohydrate accumulation and metabolism in Escherichia coli: the close linkage and chromosomal location of ctr mutations. J Bacteriol. 1969 May;98(2):605–610. doi: 10.1128/jb.98.2.605-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wann M., Mahajan S. K., Wood T. H. Genetic mapping in Escherichia coli K-12 by radiation-induced crossing-over. J Bacteriol. 1970 Sep;103(3):601–606. doi: 10.1128/jb.103.3.601-606.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargel R. J., Hadur C. A., Neuhaus F. C. Mechanism of D-cycloserine action: transport mutants for D-alanine, D-cycloserine, and glycine. J Bacteriol. 1971 Mar;105(3):1028–1035. doi: 10.1128/jb.105.3.1028-1035.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Parker J., Fiil N. P., Flaks J. G., Friesen J. D. New chromosomal location for structural genes of ribosomal proteins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2765–2769. doi: 10.1073/pnas.72.7.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J. A. Genetic and phenotypic characterization of dnaC mutations. J Bacteriol. 1975 Feb;121(2):594–599. doi: 10.1128/jb.121.2.594-599.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Davies J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol Rev. 1968 Dec;32(4 Pt 2):493–528. [PMC free article] [PubMed] [Google Scholar]
- Westling-Häggström B., Normark S. Genetic and physiological analysis of an envB spherelike mutant of Escherichia coli K-12 and characterization of its transductants. J Bacteriol. 1975 Jul;123(1):75–82. doi: 10.1128/jb.123.1.75-82.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney E. N. The tolC locus in Escherichia coli K12. Genetics. 1971 Jan;67(1):39–53. doi: 10.1093/genetics/67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Berkower I., Wright M., Hurwitz J. Studies on in vitro DNA synthesis: purification of dna C gene product containing dna D activity from Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2369–2373. doi: 10.1073/pnas.70.8.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Wright M., Hurwitz J. Association of DNA-dependent and -independent ribonucleoside triphosphatase activities with dnaB gene product of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Mar;71(3):783–787. doi: 10.1073/pnas.71.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman H. J. A genetic map of several mutations affecting the mucopeptide layer of Escherichia coli. Genet Res. 1972 Aug;20(1):65–74. doi: 10.1017/s0016672300013598. [DOI] [PubMed] [Google Scholar]
- Wijsman H. J. Mutation affecting plasmolysis in Escherichia coli. J Bacteriol. 1972 May;110(2):789–790. doi: 10.1128/jb.110.2.789-790.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman H. J., Pafort H. C. Pleiotropic mutations in Escherichia coli conferring tolerance to glycine and sensitivity to penicillin. Mol Gen Genet. 1974;128(4):349–357. doi: 10.1007/BF00268522. [DOI] [PubMed] [Google Scholar]
- Wijsman H. J. The characterization of an alanine racemase mutant of Escherichia coli. Genet Res. 1972 Dec;20(3):269–277. doi: 10.1017/s001667230001380x. [DOI] [PubMed] [Google Scholar]
- Wilcox G., Boulter J., Lee N. Direction of transcription of the regulatory gene araC in Escherichia coli B-r. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3635–3639. doi: 10.1073/pnas.71.9.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., Bennett R. L., Malamy M. H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973 Feb;113(2):529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman M., Bertani G., Kelly B., Sasaki I. Genetic map of Escherichia coli strain C. Mol Gen Genet. 1970;107(1):1–31. doi: 10.1007/BF00433220. [DOI] [PubMed] [Google Scholar]
- Wolf B. The characteristics and genetic map location of a temperature sensitive DNA mutant of E. coli K12. Genetics. 1972 Dec;72(4):569–593. doi: 10.1093/genetics/72.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. H. Effects of temperature, agitation, and donor strain on chromosome transfer in Escherichia coli K-12. J Bacteriol. 1968 Dec;96(6):2077–2084. doi: 10.1128/jb.96.6.2077-2084.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Woods W. H., Egan J. B. Integration stie of noninducible coliphage 186. J Bacteriol. 1972 Aug;111(2):303–307. doi: 10.1128/jb.111.2.303-307.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Wright M., Buttin G., Hurwitz J. The isolation and characterization from Escherichia coli of an adenosine triphosphate-dependent deoxyribonuclease directed by rec B, C genes. J Biol Chem. 1971 Nov;246(21):6543–6555. [PubMed] [Google Scholar]
- Wu H. C., Wu T. C. Isolation and characterization of a glucosamine-requiring mutant of Escherichia coli K-12 defective in glucosamine-6-phosphate synthetase. J Bacteriol. 1971 Feb;105(2):455–466. doi: 10.1128/jb.105.2.455-466.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Davidson N., Carbon J. Physical mapping of the transfer RNA genes on lambda-h80dglytsu+36. J Mol Biol. 1973 Jun 25;78(1):23–34. doi: 10.1016/0022-2836(73)90425-7. [DOI] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. Locus determining P1 phage restriction in Escherichia coli. J Bacteriol. 1969 Apr;98(1):314–314. doi: 10.1128/jb.98.1.314-314.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C., CARLTON B. C., GUEST J. R., HELINSKI D. R., HENNING U. ON THE COLINEARITY OF GENE STRUCTURE AND PROTEIN STRUCTURE. Proc Natl Acad Sci U S A. 1964 Feb;51:266–272. doi: 10.1073/pnas.51.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]
- Yagil E., Bracha M., Lifshitz Y. The regulatory nature of the phoB gene for alkaline phosphatase synthesis in Escherichia coli. Mol Gen Genet. 1975;137(1):11–16. doi: 10.1007/BF00332537. [DOI] [PubMed] [Google Scholar]
- Yagil E., Bracha M., Silberstein N. Further genetic mapping of the phoA-phoR region for alkaline phosphatase synthesis in Escherichia coli K 12. Mol Gen Genet. 1970;109(1):18–26. doi: 10.1007/BF00334043. [DOI] [PubMed] [Google Scholar]
- Yajko D. M., Valentine M. C., Weiss B. Mutants of Escherichia coli with altered deoxyribonucleases. II. Isolation and characterization of mutants for exonuclease I. J Mol Biol. 1974 May 15;85(2):323–343. doi: 10.1016/0022-2836(74)90367-2. [DOI] [PubMed] [Google Scholar]
- Yajko D. M., Weiss B. Mutations simultaneously affecting endonuclease II and exonuclease III in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Feb;72(2):688–692. doi: 10.1073/pnas.72.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Chromosomal mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jan 28;63(2):281–294. doi: 10.1016/0022-2836(72)90375-0. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Folk W. R., Berg P., Soll L. A single mutational modification of a tryptophan-specific transfer RNA permits aminoacylation by glutamine and translation of the codon UAG. J Mol Biol. 1974 Jun 25;86(2):245–260. doi: 10.1016/0022-2836(74)90016-3. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Jacob F., Gros F. Mutations thermosensibles des systèmes activant la valine chez E. coli. Bull Soc Chim Biol (Paris) 1965;47(8):1609–1626. [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]
- Yokota T., Gots J. S. Requirement of adenosine 3', 5'-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 Aug;103(2):513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G., Langman L., Luke R. K., Gibson F. Biosynthesis of the iron-transport compound enterochelin: mutants of Escherichia coli unable to synthesize 2,3-dihydroxybenzoate. J Bacteriol. 1971 Apr;106(1):51–57. doi: 10.1128/jb.106.1.51-57.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G., Leppik R. A., Hamilton J. A., Gibson F. Biochemical and genetic studies on ubiquinone biosynthesis in Escherichia coli K-12:4-hydroxybenzoate octaprenyltransferase. J Bacteriol. 1972 Apr;110(1):18–25. doi: 10.1128/jb.110.1.18-25.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G., McCann L. M., Stroobant P., Gibson F. Characterization and genetic analysis of mutant strains of Escherichia coli K-12 accumulating the biquinone precursors 2-octaprenyl-6-methoxy-1,4-benzoquinone and 2-octaprenyl-3-methyl-6-methoxy-1,4-benzoquinone. J Bacteriol. 1971 Mar;105(3):769–778. doi: 10.1128/jb.105.3.769-778.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G., Stroobant P., Macdonald C. G., Gibson F. Pathway for ubiquinone biosynthesis in Escherichia coli K-12: gene-enzyme relationships and intermediates. J Bacteriol. 1973 Apr;114(1):42–52. doi: 10.1128/jb.114.1.42-52.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M. T., Kaney A. R., Atwood K. C. Genetic mapping off fructose-1,6-diphosphatase in Escherichia coli. J Bacteriol. 1965 Oct;90(4):1150–1152. doi: 10.1128/jb.90.4.1150-1152.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M. T., Vermeulen C. W., Atwood K. C. Location of the genes for 16S and 23S ribosomal RNA in the genetic map of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Sep;67(1):26–31. doi: 10.1073/pnas.67.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura T., Igarashi K. RNA polymerase mutants of Escherichia coli. I. Mutants resistant to streptovaricin. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1313–1319. doi: 10.1073/pnas.61.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura T., Wada C. Phenethyl alcohol resistance in Escherichia coli. I. Resistance of strain C600 and its relation to azide resistance. Genetics. 1968 Jun;59(2):177–190. doi: 10.1093/genetics/59.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamenhof P. J. A genetic locus responsible for generalized high mutability in Escherichia coli. Proc Natl Acad Sci U S A. 1966 Sep;56(3):845–852. doi: 10.1073/pnas.56.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamenhof P. J. On the identity of two bacterial mutator genes: effects of antimutagens. Mutat Res. 1969 May-Jun;7(3):463–465. doi: 10.1016/0027-5107(69)90117-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann R. A., Garvin R. T., Gorini L. Alteration of a 30S ribosomal protein accompanying the ram mutation in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2263–2267. doi: 10.1073/pnas.68.9.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R. A., Ikeya Y., Sparling P. F. Alteration of ribosomal protein S4 by mutation linked to kasugamycin-resistance in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jan;70(1):71–75. doi: 10.1073/pnas.70.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaig N., Nagel de Zwaig R., Istúriz T., Wecksler M. Regulatory mutations affecting the gluconate system in Escherichia coli. J Bacteriol. 1973 May;114(2):469–473. doi: 10.1128/jb.114.2.469-473.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan P. G., Felix H. S., Peters R. Mapping of the gene for cytosine deaminase on the Escherichia coli chromosome. Antonie Van Leeuwenhoek. 1972;38(3):257–263. doi: 10.1007/BF02328097. [DOI] [PubMed] [Google Scholar]
- de Haan P. G., Verhoef C. Genetic recombination in Escherichia coli. II. Calculation of incorporation frequency and relative map distance by recombinant analysis. Mutat Res. 1966 Apr;3(2):111–117. doi: 10.1016/0027-5107(66)90024-8. [DOI] [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]
- van de Putte P., van Sluis C. A., van Dillewijn J., Rörsch A. The location of genes controlling radiation sensitivity in Escherichia coli. Mutat Res. 1965 Apr;2(2):97–110. doi: 10.1016/0027-5107(65)90041-2. [DOI] [PubMed] [Google Scholar]


