Abstract
Background
Neurologic and autonomic presentation in multiple system atrophy (MSA) may predict early mortality. Quantification of early autonomic failure as mortality predictor is lacking.
Methods
Early neurologic and autonomic clinical features were retrospectively reviewed in 49 MSA cases (median age at onset, 56.1 years; 16 women) confirmed by autopsy at Mayo Clinic. When available, the 10-point composite autonomic severity score derived from the autonomic reflex screen provided quantification of the degree of autonomic failure and thermoregulatory sweat test quantitated body surface anhidrosis.
Results
Symptoms at onset were autonomic in 50%, parkinsonian in 30%, and cerebellar in 20% of cases. Survival (median [95% confidence interval]) was 8.6 [6.7–10.2] years. Survival was shorter in patients with early laboratory evidence of generalized (composite autonomic severity score ≥ 6) autonomic failure (7.0 [3.9–9.8] vs. 9.8 [4.6–13.8] years; P=0.036), and early requirement of bladder catheterization (7.3 [3.1–10.2] vs. 13.7 [8.5–14.9] years; P=0.003) compared to those without these clinical features. On Cox proportional analysis, prognostic indicators of shorter survival were older age at onset (hazard ratio [95% confidence interval], 1.04 [1.01–1.08]; P=0.03), early requirement of bladder catheterization (7.9 [1.88–38.63]; P=0.004) and early generalized (composite autonomic severity score ≥ 6) autonomic failure (2.8 [1.01–9.26]; P=0.047). Gender, phenotype, and early development of gait instability, aid-requiring ambulation, orthostatic symptoms, neurogenic bladder or significant anhidrosis (thermoregulatory sweat test ≥ 40%) were not indicators of shorter survival.
Conclusions
Our data suggests that early development of severe generalized autonomic failure more than triples the risk of shorter survival in patients with MSA.
Keywords: Autonomic failure, multiple system atrophy, prognosis, parkinsonism, neurogenic bladder
INTRODUCTION
Multiple system atrophy (MSA) is a progressive fatal neurodegenerative disorder that frequently starts with autonomic failure.1 Factors predicting survival in patients with MSA are not fully established and the role of early autonomic failure as a prognostic indicator remains controversial.2–5 The disagreement may be due to the use of different definitions based on patient awareness of a variety of urinary and orthostatic symptoms. Characterization of the severity and distribution of early autonomic failure using validated quantitative instruments for risk stratification is lacking in MSA.
We therefore conducted a retrospective study to determine (a) the median survival time in 49 patients with MSA confirmed by autopsy and (b) whether there is a difference in prognosis between subjects by age at onset of disease, gender, clinical phenotype, early motor disability, or early autonomic failure. Our hypothesis was that early generalized autonomic failure is an independent risk factor of shorter survival in patients with MSA.
METHODS
Standard protocol approvals, registrations, and patient consents
After approval from the appropriate institutional review board, consenting medical records were reviewed.
Patients
We performed a retrospective review of the medical records of all 49 patients who had autopsy-confirmed diagnosis of MSA at the Mayo Clinic according to established criteria.6, 7 All patients had undergone full neurologic evaluation. Clinical data were abstracted from systematic chart review in a standardized fashion by only one author (J.F.) and included disease onset, phenotype, progression to motor and autonomic disability endpoints and survival. Laboratory evidence of autonomic failure was abstracted from autonomic function tests when available. All patients had a negative family history for Parkinsonism, tremor or ataxia in first degree relatives. Two patients with medical history of well-controlled diabetes mellitus were not excluded as they did not have clinical evidence of end-organ damage or peripheral neuropathy.
Disease onset
The onset was defined as the time of first motor (i.e., parkinsonian or cerebellar) or autonomic (i.e., urinary incontinence, urinary retention, or orthostatic blood pressure decline) symptom as defined in the consensus criteria of possible MSA.6
Clinical phenotypes
Patients were divided into two clinical phenotypes according to consensus criteria based on the predominant motor involvement as those with predominant Parkinsonism (MSA-P) and those with predominant cerebellar involvement (MSA-C).6
Motor disability and stridor
The timing of gait instability and loss of ambulatory independence were used to assess the degree of early motor progression. Gait instability was defined as frequent falls. Frequent falls were defined as at least two falls per year or chart documentation of “frequent”, “several”, or “consistent” falls not attributed to presyncope or syncope as judged by physician. Loss of ambulatory independence (aid-requiring walking) was defined as use of cane, walker, or a companion’s arm for support at all times. Stridor not confirmed by polysomnogram was defined as a very loud, strained sound with higher pitch than snoring occurring during inspiration recognized by members of the same household when sound was imitated by physician.
Autonomic phenotype
Time of initial symptoms and signs of autonomic involvement were abstracted from medical records, when available. Orthostatic hypotension was defined as a drop of 30 mmHg or more in systolic blood pressure or 20 mmHg or more in the diastolic blood pressure.6 Neurogenic bladder was defined as symptoms of urinary incontinence or retention. Neurogenic urinary incontinence was defined as 1) urge incontinence not accounted for by prostate pathology; 2) stress incontinence that failed to improve with bladder suspension surgery; 3) new or worsening incontinence following urologic surgery; 4) unexplained incontinence with position changes such as from lying to standing; and 5) enuresis, i.e., full loss of urine, particularly during sleep. Neurogenic urinary retention was defined as any difficulty or inability to void 1) not accounted for by structural outflow obstruction; 2) not responding to prostate resection or other bladder outlet surgery; or 3) requiring placement of a bladder stimulator
Objective autonomic deficits were abstracted from available Autonomic Reflex Screen and Thermoregulatory Sweat Testing (TST) and summarized using the Composite Autonomic Severity Score (CASS). The Autonomic Reflex Screen is routinely used in clinical practice and comprises: 1) the Quantitative Sudomotor Axon Reflex Test at proximal and distal standard sites to assess sympathetic postganglionic sudomotor axon function;8 2) heart rate responses to deep breathing and the Valsalva maneuver to assess cardiovagal function;9 and 3) beat-to-beat blood pressure responses to the Valsalva maneuver and head-up tilt to assess sympathetic adrenergic function.10 The Thermoregulatory Sweat Testing assesses the integrity of central and peripheral sudomotor pathways involved in the thermoregulatory response to changes in environmental temperature by providing quantitative evaluation of the proportion of anterior body surface that does not sweat. TST ≥40% indicates moderate-to-severe anhidrosis and TST=100% indicates complete anhidrosis.11 The Composite Autonomic Severity Score is a validated instrument for laboratory quantitation of autonomic failure derived from the Autonomic Reflex Screen and Thermoregulatory Sweat Testing that has been normalized for the confounding factors of age and sex. It scores autonomic failure as follows: 1–3, mild and limited; 4–6, moderate; and 7–10, severe and generalized.10 CASS ≥ 6 indicates moderate-to-severe generalized autonomic failure.
Prognostic indicators
Survival was defined based on time to death from first symptom of disease. Potential predictors of survival that were analyzed included age at first symptom, gender, clinical phenotype, early motor disability, early stridor, early autonomic manifestations, and early objective evidence of severe autonomic failure. Early motor disability was defined as presence of gait instability (frequent falls) or loss of ambulatory independence (aid-required walking) within three years of disease onset. Stridor was categorized as early if present within three years of disease onset. Early autonomic manifestation was defined as development of orthostatism (presyncope, syncope or evidence of orthostatic hypotension) or neurogenic bladder (symptoms of urinary incontinence or retention) within one year of disease onset. Early evidence of severe autonomic failure was defined as presence of severe neurogenic bladder (need for bladder catheterization), moderate-to-severe widespread anhidrosis (TST ≥ 40%), or laboratory evidence of moderate-to-severe generalized autonomic failure (CASS ≥ 6) within three years of disease onset.
Statistical analyses
Data were entered into a database for statistical analysis. Continuous variables were expressed as median [interquantile range (IQR) or 95% confidence interval (CI)]. Wilcoxon rank-sum test was used to compare continuous variables for different subgroups. The frequency of clinical features between subgroups was analyzed with Fisher’s exact test. Kaplan-Meier curves were used to graphically analyze the interval in years from first symptom onset to death and expressed as median values. Log-rank test statistics were used to determine whether Kaplan-Meier transition curves differed among subgroups. Cox proportional hazards models were used to calculate univariate hazard ratios for shorter survival using age at disease onset as continuous variable and gender, clinical phenotype and early development of neurologic and autonomic manifestations as categorical variables. The hazard ratios of severe autonomic manifestations were adjusted for age at disease onset. Statistical significance was defined at P<0.05. Statistical analyses of data were performed with JMP version 9.0.1.
RESULTS
Patient characteristics
The group consisted of 33 men and 16 women (Table 1). Median age at onset was 56.1 [IQR, 50.5–60.9] years. Autonomic symptoms were the first manifestations in 50% (23/47) of patients and preceded motor onset by 3.3 [IQR, 1.0–6.6] years. Among those with motor onset, the latency from motor onset to autonomic symptoms was 1.9 [IQR, 0.9–3.3] years. The predominant clinical phenotype was MSA-P in 65% (32/49) and MSA-C in 35% (17/49). Table 1 shows baseline characteristics and the distribution of potential prognostic indicators. Latencies from consensus onset to autonomic manifestations according to early vs. non-early autonomic status were: orthostatism, 0.4 [IQR, 0.3–0.8] vs. 2.5 [IQR, 1.8–5.6] years; bladder symptoms, 0.1 [IQR, 0.1–0.5] vs. 2.8 [IQR, 1.6–3.9] years; bladder catheterization, 2.5 [IQR, 1.5–2.9] vs. 4.4 [IQR, 3.4–8.2] years.
Table 1.
Patient characteristics (n=49)
| Characteristic | Value |
|---|---|
| Male, % (n/n) | 67% (33/49) |
| Age at onset, median [IQR], years | 56.1 [50.5 – 60.9] |
| Age at death, median [IQR], years | 65.7 [58.9 – 70.4] |
| Disease duration, median [IQR], years | 8.6 [5.7 – 12.9] |
| Predominant motor phenotype | |
| MSA-P, % (n/n) | 65 (32/49) |
| MAS-C, % (n/n) | 35 (17/49) |
| Initial manifestation | |
| Autonomic, % (n/n) | 50 (23/47) |
| Parkinsonism, % (n/n) | 30 (14/47) |
| Cerebellar, % (n/n) | 20 (10/47) |
| Early motor disability | |
| Frequent falls (≤ 3 years from onset), n/n | 11/35 |
| Aid-requiring walking (≤ 3 years from onset), n/n | 7/28 |
| Early respiratory impairment | |
| Stridor (≤ 3 years from onset), n/n | 4/6 |
| Early autonomic failure | |
| Orthostatism (≤ 1 year from onset), n/n | 13/31 |
| Bladder symptoms (≤ 1 year from onset), n/n | 15/37 |
| Bladder catheterization (≤ 3 years from onset), n/n | 8/22 |
| TST anhidrosis ≥ 40% (≤ 3 years from onset), n/n | 8/15 |
| CASS ≥ 6 (≤ 3 years from onset), n/n | 13/21 |
Age at disease onset was available in 46 out of 49 patients. Frequent falls were defined as at least 2 falls per year or documentation of “frequent”, “several”, or “consistent” falls not attributed to orthostatism. Orthostatism was defined as history of presyncope/ syncope or evidence of orthostatic hypotension. CASS, composite autonomic severity score (CASS ≥ 6 indicates laboratory evidence of severe generalized autonomic failure); MSA-P, parkinsonian type MSA; MSA-C, cerebellar type MSA; TST, thermoregulatory sweat test (anhidrosis is reported as percentage of body surface not sweating; TST anhidrosis ≥ 40% indicates moderate-to-severe anhidrosis)
Outcome and risk factors
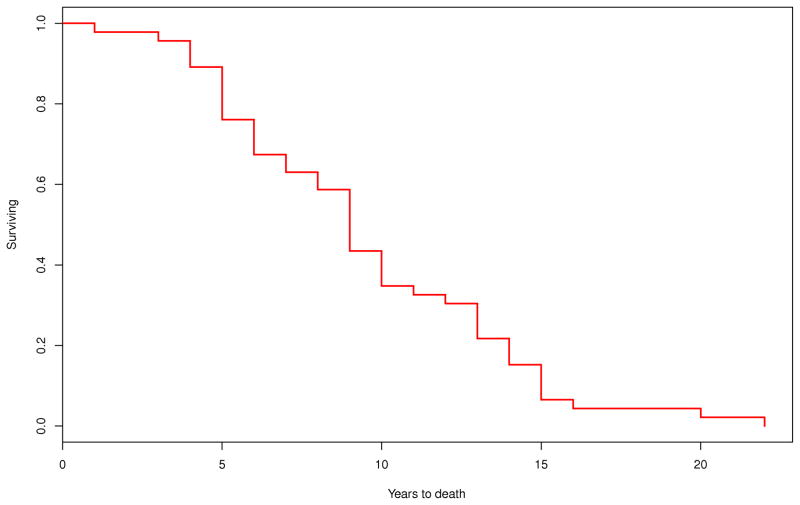
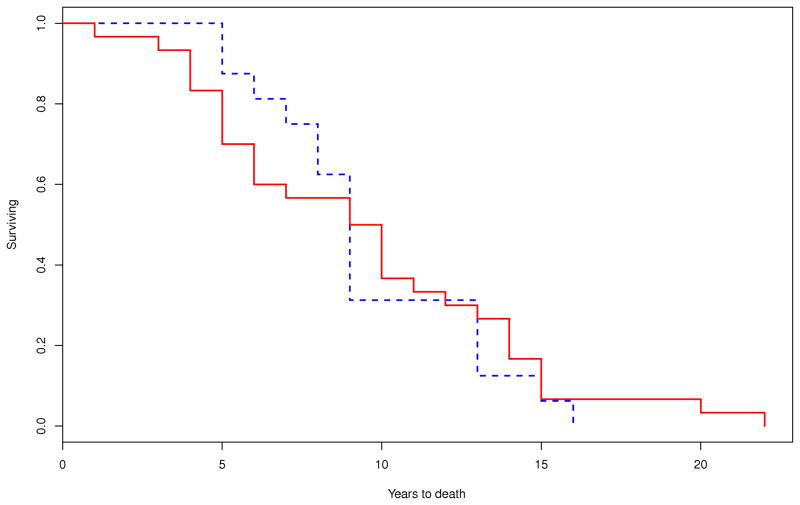
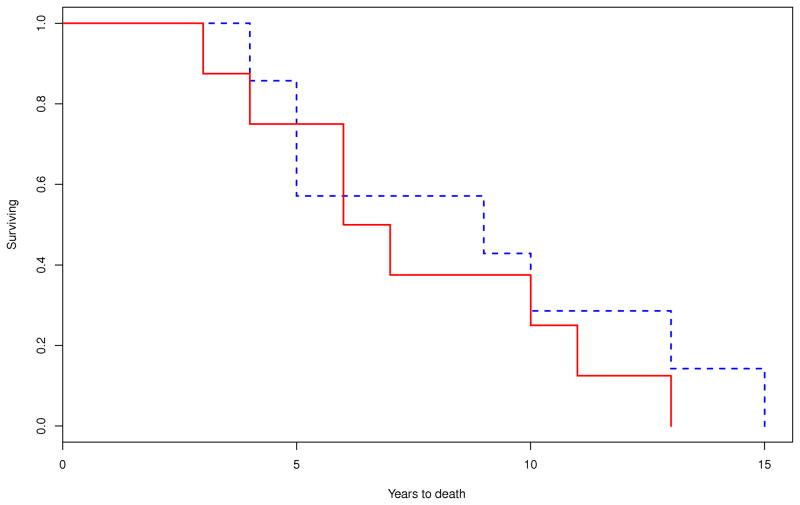
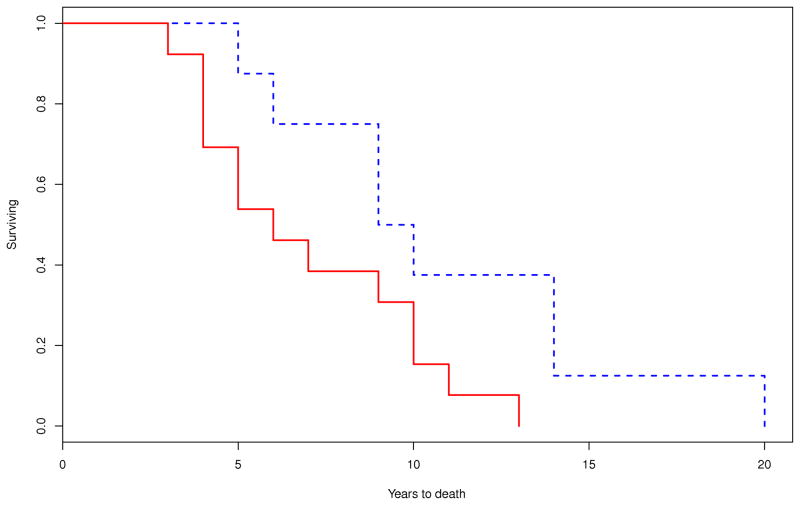
Survival was calculated on 46 out of 49 patients as age at disease onset was not available on the following three patients: one male with MSA-P, one male with MSA-C and one female with MSA-P. On Kaplan-Meier survival analysis, the median duration of illness from symptom onset to death was 8.6 (95% CI, 6.7–10.2) years. There were no differences in the median survival time between those with MSA-P (9.2 [95% CI, 5.7–11.6] years; n=30) and MSA-C (8.6 [95% CI, 7.5–12.9] years; n=16; P=0.708) (Figure 1). Patients with early laboratory evidence of severe generalized autonomic failure (CASS≥6) had shorter median survival time (5.7 [95% CI, 3.9–9.8] years, n=13) compared to those without this manifestation (9.8 [95% CI, 4.6–13.8] years; n=8; P=0.036). Median survival was also reduced among those who required bladder catheterization early in the disease (7.3 [95% CI, 3.1–10.2] years, n=8) compared to those who did not (13.7 [95% CI, 8.5–14.9] years, n=14; P=0.003) (Figure 2).
Figure 1.
Kaplan-Meier survival curves for probability of dying A) in all patients with MSA (n=49); and B) in patients with MSA-P (n=30) compared to those with MSA-C (n=16) (P=0.70) (– MSA-P; - - MSA-C).
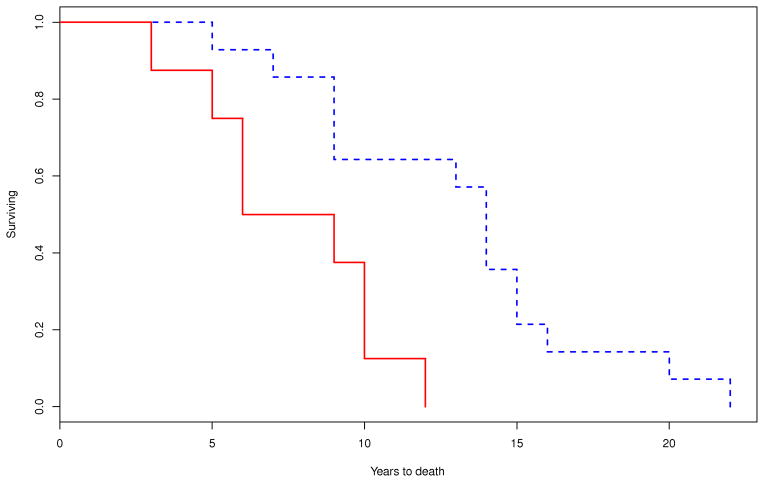
Figure 2.
Comparison of Kaplan-Meier curves for the probability of dying between patients who developed A) early requirement of bladder catheterization (within 3 years of disease onset; P=0.003) (– yes; - - no); B) early severe thermoregulatory anhidrosis (%TST > 40 anhidrosis within 3 years of disease onset; P=0.641) (– yes; - - no); and C) early laboratory evidence of generalized autonomic failure (CASS ≥ 6 within 3 years of disease onset; P=0.036) (– yes; - -no).
Survival was not affected by gender (females, 9.8 [95% CI, 4.6–12.5] years vs. males, 8.5 [95% CI, 5.7–10.2] years; P=0.812; [ n=15 vs. n=31]) or early development of frequent falls (7.5 [95% CI, 3.9–8.8] vs. 10.0 [95% CI, 8.2–12.9] years; P=0.066; [n=11 vs. n=24]); loss of walking independence (8.8 [95% CI, 3.1–13.5] vs. 8.2 [95% CI, 5.2–9.5] years; P=0.831; [n=7 vs. n=21]); manifestation of orthostatic hypotension (8.4 [95% CI, 5.2–11.3] vs. 8.6 [95% CI, 6.1–12.9] years; P=0.450; [n=13 vs. n=18]); bladder symptoms (8.5 [95% CI, 4.6–12.5] vs. 9.7 [95% CI, 7.5–13.5] years; P=0.481; [n=15 vs. n=22]); or moderate-to-severe anhidrosis (TST anhidrosis ≥40%) (6.4 [95% CI, 3.1–11.3] vs. 8.5 [95% CI, 3.9–12.9] years; P=0.641; [n=8 vs. n=7]. The median survival after onset of stridor (n=6) was 6.3 [IQR, 3.6–10.9] years and ranged from 2.1 to 16.6 years. The small number of patients in whom we were able to define presence or absence of stridor did not allow for comparison of Kaplan-Meier survival curves.
On Cox proportional survival analysis, the hazard ratio of shorter survival was significantly higher with increasing age at disease onset (P=0.03), early need for bladder catheterization (P=0.06) and early laboratory evidence of generalized autonomic failure (P=0.03) (Table 2). After adjusting for age at onset, hazard ratios of shorter survival remained significantly high for early need for bladder catheterization (P=0.02) and early laboratory evidence of generalized autonomic failure (P=0.047). There was no significant difference in survival by gender, clinical phenotype, and status for early manifestations of orthostatic hypotension, neurogenic bladder, gait instability, laboratory evidence of moderate-to-severe anhidrosis, and loss of ambulatory independence. The small number of patients in whom we were able to define presence or absence of stridor did not allow for Cox proportional survival analysis (Table 2).
Table 2.
Relative risk for shorter survival on various clinical features among patients with MSA
| Variable | No. of Patients | Hazard ratio (HR) | |||
|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | ||
| Age at onset | 46 | 1.04 (1.01–1.08) | 0.03 * | ||
| Gender | |||||
| Male | 15 | 1.08 (0.58 – 2.10) | 0.81 | 1.29 (0.68 – 2.56) | 0.44 |
| Female | 31 | 1.0 (reference) | 1.0 (reference) | ||
| Clinical phenotype | |||||
| MSA-P | 30 | 0.80 (0.48 – 1.69) | 0.71 | 0.98 (0.53 – 1.88) | 0.95 |
| MSA-C | 16 | 1.0 (reference) | 1.0 (reference) | ||
| Time to orthostatism | |||||
| ≤ 1 year | 13 | 1.32 (0.63 – 2.73) | 0.46 | 1.30 (0.61 – 2.70) | 0.48 |
| >1 year | 18 | 1.0 (reference) | 1.0 (reference) | ||
| Time of neurogenic bladder | |||||
| ≤ 1 year | 15 | 1.27 (0.64 – 2.45) | 0.49 | 1.21 (0.61 – 2.35) | 0.58 |
| >1 year | 22 | 1.0 (reference) | 1.0 (reference) | ||
| Time to TST ≥ 40% anhidrosis | |||||
| ≤ 3 years | 8 | 1.29 (0.44 – 3.95) | 0.64 | 1.08 (0.27 – 4.26) | 0.90 |
| > 3 years | 7 | 1.0 (reference) | 1.0 (reference) | ||
| Time to CASS ≥ 6 | |||||
| ≤ 3 years | 13 | 2.9 (1.07 – 9.18) | 0.03* | 2.8 (1.01 – 9.26) | 0.047* |
| > 3 years | 8 | 1.0 (reference) | 1.0 (reference) | ||
| Time to bladder catheterization | |||||
| ≤ 3 years | 8 | 4.81 (1.57 – 16.25) | 0.006* | 4.42 (1.29 – | 0.02* |
| > 3 years | 14 | 1.0 (reference) | 16 1.0 (reference) 55) | ||
| Time to frequent falls | |||||
| ≤ 3 years | 11 | 0.51 (0.24 – 1.10) | 0.09 | 1.57 (0.64 – 3.57) | 0.31 |
| > 3 years | 24 | 1.0 (reference) | 1.0 (reference) | ||
| Time to aid-requiring walking | |||||
| ≤ 3 years | 7 | 0.91 (0.35 – 2.08) | 0.83 | 0.74 (0.27 – 1.78) | 0.52 |
| > 3 years | 21 | 1.0 (reference) | 1.0 (reference) | ||
Statistically significant
Age at disease onset was available in 46 out of 49 patients. Orthostatism was defined as history of presyncope/ syncope or evidence of orthostatic hypotension. CASS, Composite Autonomic Severity Score; MSA-P, parkinsonian type MSA; MSA-C, cerebellar type MSA; TST, thermoregulatory sweat test
DISCUSSION
The most relevant findings are that: 1) autonomic involvement starts early in a substantial proportion of patients with MSA; and 2) both development of severe and generalized autonomic failure at early stages of disease and older age at onset predict shorter survival. Our study confirms that generalized autonomic failure is predictive of MSA diagnosis12 and provides evidence that the severity of early autonomic involvement might actually be an important predictor of survival. The frequency of early autonomic involvement and its impact on survival in MSA is in agreement with prior reports.2, 4, 5, 13–20 Differences in ascertaining autonomic failure may account for discrepancies in the role of the autonomic involvement as a prognostic indicator in MSA.17
Higher age at onset associated with shorter survival is consistent with previous reports.5, 13, 14, 16, 21 Gender, and clinical phenotypes (MSA-P vs. MSA-C) did not differentially affect survival in our patients, as previously reported in one study5 but contrasting with others.13, 14, 16, 21 Differences in study populations, diagnostic certainty (clinical vs. autopsy-based), and selection bias may account for these discrepancies. Early and severe motor involvement did not increase mortality in our study population. A similar study found that despite increase in morbidity, early motor progression did not result in premature death.5 Nocturnal stridor is considered a predictor of poor prognosis in MSA.22 Due to the retrospective nature of our study it was difficult to ascertain presence of stridor from chart review using our study definition. Hence, our data on stridor was limited to draw statistically and clinically significant conclusions regarding its prognostic role.
An association between early development of severe urinary retention and shorter survival was observed in our patients. In a prior study, up to 25% of MSA patients died of complications related to recurrent and intractable lower urinary tract infections.23 Early development of recurrent urinary infections with or without kidney damage due to vesicoureteral reflux could be one potential explanation for the increased mortality observed in our study. Among patients with MSA, urinary retention due to detrusor underactivity tends to follow development of urinary incontinence due to detrusor overactivity.24 An association between urinary incontinence due to detrusor overactivity, loss of medullary serotonergic neurons and nocturnal hypoxia has been reported in MSA.25 It is possible that detrusor overactivity might have preceded the early development of detrusor underactivity in our patients, and that the loss of medullary serotonergic neurons and development of nocturnal hypoxia, both associated with increased risk of sudden death, might have already occurred by the time they needed early bladder catheterization.
Early moderate-to-severe generalized autonomic failure as defined by CASS ≥ 6 was a risk factor for mortality. The mechanism of the relationship between generalized autonomic failure and mortality is not clear. Sudden death during sleep is a major mechanism of mortality in patients with MSA.17, 26, 27 Severe depletion of medullary serotonergic neurons is a distinct pathological finding in MSA patients with autonomic failure, neurogenic bladder and sleep apnea.28 An association between autonomic failure, medullary serotonergic loss and sudden death has been implicated as a mechanism of poor prognosis in MSA.4, 29 Among autopsy cases, a more severe depletion of serotonergic neurons has been reported in those who succumbed to sudden death.29 The medullary serotonergic neurons modulate the micturition reflex30 and are central respiratory chemosensors that participate in the regulation of arousal, respiratory rhythmogenesis and ventilatory response to hypoxemia and hypercarbia.31 They are also involved in sympathetically-mediated thermoregulation.32 Hence, early bladder dysfunction as well as early severity of autonomic failure may reflect a more severe loss of serotonergic neurons that participate in autonomic regulation and respiratory chemosensitivity. Early involvement of serotonergic systems, potentially increases the risk of sudden nocturnal death by impairing arousal, and respiratory and autonomic responses to nocturnal stressors to maintain tissue oxygenation during sleep
This study has several limitations. First, the use of data from autopsy cases may bias our analysis toward more severe cases. Second, much of the clinical data were obtained via the history recorded in the chart at the time of the neurologic evaluations. This may result in bias due to poor patient recall. Third, we did not have enough power to analyze stridor due to the retrospective nature of the study. Finally, data on causes of death were not available on most of our patients. However, reported causes could have been inaccurate as shown in a previous study on the quality of death certification in atypical parkinsonian disorders.33
In summary, as autonomic involvement is frequent during early stages of MSA, and its severity is a robust predictor of shorter survival, well standardized tests that allow quantification of severity and distribution of autonomic involvement early in the disease are useful for predicting survival prognosis and planning of novel neuroprotective treatment trials for MSA.
Acknowledgments
Funding Sources: This investigation was supported in part by National Institutes of Health (NS 32352 Autonomic Disorders Program Project, NS 44233 Pathogenesis and Diagnosis of Multiple System Atrophy, U54 NS065736 Autonomic Rare Disease Clinical Consortium and K23NS075141 Differential Approach to the Postural Tachycardia Syndrome), Mayo CTSA (UL1 TR000135), NIH Research Training Grant under Ruth L. Kirschstein National Research Service Award T32 HD07447, Shih Memorial, and Mayo Funds.
The Autonomic Diseases Consortium is a part of the NIH Rare Diseases Clinical Research Network (RDCRN). Funding and/or programmatic support for this project has been provided by U54 NS065736 from the National Institute of Neurological Diseases and Stroke (NINDS) and the NIH Office of Rare Diseases Research (ORDR).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Footnotes
Conflict of Interest: none.
Author’s Roles Author contributions:
Dr. Figueroa – study concept and design; acquisition of data; analysis and interpretation; and drafting the manuscript for content
Dr. Singer – study concept and design; analysis and interpretation; and critical revision of the manuscript
Dr. Parsaik – acquisition of data; analysis and interpretation
Dr. Benarroch – analysis and interpretation; and critical revision of the manuscript
Dr. Ahlskog – analysis and interpretation; and critical revision of the manuscript
Dr. Fealey – analysis and interpretation; and critical revision of the manuscript
Dr. Parisi – analysis and interpretation; critical revision of the manuscript
Dr. Sandroni – analysis and interpretation; critical revision of the manuscript
Dr. Mandrekar – statistical analysis and interpretation
Dr. Iodice – acquisition of data; critical revision of the manuscript
Dr. Low – study concept and design; analysis and interpretation; critical revision of the manuscript; and supervision
Dr. Bower – study concept and design; analysis and interpretation; critical revision of the manuscript for important intellectual content; and supervision
Financial Disclosures
The authors report no disclosures.
References
- 1.Stefanova N, Bucke P, Duerr S, Wenning GK. Multiple system atrophy: an update. Lancet Neurol. 2009;8:1172–1178. doi: 10.1016/S1474-4422(09)70288-1. [DOI] [PubMed] [Google Scholar]
- 2.O'Sullivan SS, Massey LA, Williams DR, et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. 2008;131:1362–1372. doi: 10.1093/brain/awn065. [DOI] [PubMed] [Google Scholar]
- 3.Petrovic IN, Ling H, Asi Y, et al. Multiple system atrophy-parkinsonism with slow progression and prolonged survival: a diagnostic catch. Mov Disord. 2012;27:1186–1190. doi: 10.1002/mds.25115. [DOI] [PubMed] [Google Scholar]
- 4.Tada M, Onodera O, Tada M, et al. Early development of autonomic dysfunction may predict poor prognosis in patients with multiple system atrophy. Arch Neurol. 2007;64:256–260. doi: 10.1001/archneur.64.2.256. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe H, Saito Y, Terao S, et al. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain. 2002;125:1070–1083. doi: 10.1093/brain/awf117. [DOI] [PubMed] [Google Scholar]
- 6.Gilman S, Wenning GK, Low PA, et al. Second consensus conference on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trojanowski JQ, Revesz T. Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol Appl Neurobiol. 2007;33:615–620. doi: 10.1111/j.1365-2990.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 8.Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. 1983;14:573–580. doi: 10.1002/ana.410140513. [DOI] [PubMed] [Google Scholar]
- 9.Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O'Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997;20:1561–1568. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Low PA. Autonomic nervous system function. J Clin Neurophysiol. 1993;10:14–27. doi: 10.1097/00004691-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Fealey RD, Low PA, Thomas JE. Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clin Proc. 1989;64:617–628. doi: 10.1016/s0025-6196(12)65338-5. [DOI] [PubMed] [Google Scholar]
- 12.Iodice V, Lipp A, Ahlskog JE, et al. Autopsy confirmed multiple system atrophy cases: Mayo experience and role of autonomic function tests. J Neurol Neurosurg Psychiatry. 2012;83:453–459. doi: 10.1136/jnnp-2011-301068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Shlomo Y, Wenning GK, Tison F, Quinn NP. Survival of patients with pathologically proven multiple system atrophy: a meta-analysis. Neurology. 1997;48:384–393. doi: 10.1212/wnl.48.2.384. [DOI] [PubMed] [Google Scholar]
- 14.Schulz JB, Klockgether T, Petersen D, et al. Multiple system atrophy: natural history, MRI morphology, and dopamine receptor imaging with 123IBZM-SPECT. J Neurol Neurosurg Psychiatry. 1994;57:1047–1056. doi: 10.1136/jnnp.57.9.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrag A, Wenning GK, Quinn N, Ben-Shlomo Y. Survival in multiple system atrophy. Mov Disord. 2008;23:294–296. doi: 10.1002/mds.21839. [DOI] [PubMed] [Google Scholar]
- 16.Testa D, Filippini G, Farinotti M, Palazzini E, Caraceni T. Survival in multiple system atrophy: a study of prognostic factors in 59 cases. J Neurol. 1996;243:401–404. doi: 10.1007/BF00868999. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Jeon BS, Lee JY, Yun JY. Survival of Korean patients with multiple system atrophy. Mov Disord. 2011;26:909–912. doi: 10.1002/mds.23580. [DOI] [PubMed] [Google Scholar]
- 18.Wenning GK, Ben Shlomo Y, Magalhaes M, Daniel SE, Quinn NP. Clinical features and natural history of multiple system atrophy. An analysis of 100 cases. Brain. 1994;117:835–845. doi: 10.1093/brain/117.4.835. [DOI] [PubMed] [Google Scholar]
- 19.Wenning GK, Geser F, Krismer F, et al. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol. 2013;12:264–274. doi: 10.1016/S1474-4422(12)70327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klockgether T, Ludtke R, Kramer B, et al. The natural history of degenerative ataxia: a retrospective study in 466 patients. Brain. 1998;121:589–600. doi: 10.1093/brain/121.4.589. [DOI] [PubMed] [Google Scholar]
- 21.Saito Y, Matsuoka Y, Takahashi A, Ohno Y. Survival of patients with multiple system atrophy. Intern Med. 1994;33:321–325. doi: 10.2169/internalmedicine.33.321. [DOI] [PubMed] [Google Scholar]
- 22.Silber MH, Levine S. Stridor and death in multiple system atrophy. Mov Disord. 2000;15:699–704. doi: 10.1002/1531-8257(200007)15:4<699::aid-mds1015>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Papapetropoulos S, Tuchman A, Laufer D, Papatsoris AG, Papapetropoulos N, Mash DC. Causes of death in multiple system atrophy. J Neurol Neurosurg Psychiatry. 2007;78:327–329. doi: 10.1136/jnnp.2006.103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakakibara R, Hattori T, Uchiyama T, et al. Urinary dysfunction and orthostatic hypotension in multiple system atrophy: which is the more common and earlier manifestation? J Neurol Neurosurg Psychiatry. 2000;68:65–69. doi: 10.1136/jnnp.68.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deguchi K, Ikeda K, Goto R, et al. The close relationship between life-threatening breathing disorders and urine storage dysfunction in multiple system atrophy. J Neurol. 2010;257:1287–1292. doi: 10.1007/s00415-010-5508-5. [DOI] [PubMed] [Google Scholar]
- 26.Munschauer FE, Loh L, Bannister R, Newson-Davis J. Abnormal respiration and sudden death during sleep in multiple system atrophy with autonomic failure. Neurology. 1990;40:677–679. doi: 10.1212/wnl.40.4.677. [DOI] [PubMed] [Google Scholar]
- 27.Shimohata T, Ozawa T, Nakayama H, Tomita M, Shinoda H, Nishizawa M. Frequency of nocturnal sudden death in patients with multiple system atrophy. J Neurol. 2008;255:1483–1485. doi: 10.1007/s00415-008-0941-4. [DOI] [PubMed] [Google Scholar]
- 28.Benarroch EE, Schmeichel AM, Low PA, Parisi JE. Involvement of medullary serotonergic groups in multiple system atrophy. Ann Neurol. 2004;55:418–422. doi: 10.1002/ana.20021. [DOI] [PubMed] [Google Scholar]
- 29.Tada M, Kakita A, Toyoshima Y, et al. Depletion of medullary serotonergic neurons in patients with multiple system atrophy who succumbed to sudden death. Brain. 2009;132:1810–1819. doi: 10.1093/brain/awp110. [DOI] [PubMed] [Google Scholar]
- 30.Ito T, Sakakibara R, Nakazawa K, et al. Effects of electrical stimulation of the raphe area on the micturition reflex in cats. Neuroscience. 2006;142:1273–1280. doi: 10.1016/j.neuroscience.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 31.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 32.Berner NJ, Grahn DA, Heller HC. 8-OH-DPAT-sensitive neurons in the nucleus raphe magnus modulate thermoregulatory output in rats. Brain Res. 1999;831:155–164. doi: 10.1016/s0006-8993(99)01426-2. [DOI] [PubMed] [Google Scholar]
- 33.Nath U, Thomson R, Wood R, et al. Population based mortality and quality of death certification in progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) J Neurol Neurosurg Psychiatry. 2005;76:498–502. doi: 10.1136/jnnp.2004.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]