Abstract
Background
Numerous brain imaging studies have demonstrated structural changes in the basal ganglia, thalamus, sensorimotor cortex and cerebellum across different forms of primary dystonia. However, our understanding of brain abnormalities contributing to the clinically well-described phenomenon of task-specificity in dystonia remained limited.
Methods
We used high-resolution MRI with voxel-based morphometry and diffusion tensor imaging with tract-based spatial statistics of fractional anisotropy to examine gray and white matter organization in two task-specific dystonia forms, writer’s cramp and laryngeal dystonia, and two non-task-specific dystonia forms, cervical dystonia and blepharospasm.
Results
A direct comparison between the both dystonia forms revealed that characteristic gray matter volumetric changes in task-specific dystonia involve the brain regions responsible for sensorimotor control during writing and speaking, such as primary somatosensory cortex, middle frontal gyrus, superior/inferior temporal gyrus, middle/posterior cingulate cortex, occipital cortex as well as the striatum and cerebellum (lobules VI-VIIa). These gray matter changes were accompanied by white matter abnormalities in the premotor cortex, middle/inferior frontal gyrus, genu of the corpus callosum, anterior limb/genu of the internal capsule, and putamen. Conversely, gray matter volumetric changes in non-task-specific group were limited to the left cerebellum (lobule VIIa) only, while white matter alterations were found to underlie the primary sensorimotor cortex, inferior parietal lobule and middle cingulate gyrus.
Conclusion
Distinct microstructural patterns in task-specific and non-task-specific dystonias may represent neuroimaging markers and provide evidence that these two dystonia subclasses likely follow divergent pathophysiological mechanisms precipitated by different triggers.
Keywords: brain imaging, task specificity, focal dystonia
Introduction
Task-specificity in primary focal dystonia is a clinically well-described but poorly understood phenomenon. While brain abnormalities in the basal ganglia, sensorimotor cortex and cerebellum are identified across different forms of dystonia1–3, it is unknown what specific alterations in these brain regions may differentiate between task-specific (TSD) and non-task-specific (NTSD) dystonias.
Generally, in patients with task-specific focal hand dystonia, writer’s cramp, changes in gray matter volume (GMV) are reported in the hand region of the sensorimotor cortex, putamen, thalamus and cerebellum4–7 with white matter integrity changes in the posterior limb of the internal capsule and adjacent structures8. In another form of TSD, laryngeal dystonia or spasmodic dysphonia, we recently identified decreased white matter integrity in the genu of the internal capsule as well as increased GMV in the laryngeal sensorimotor cortex, inferior frontal, superior/middle temporal and supramarginal gyri, putamen, and cerebellum9, 10. In NTSD forms, such as cervical dystonia, increased GMV has been found in the motor cortex, globus pallidus and cerebellum11, whereas white matter abnormalities were identified in the genu and body of the corpus callosum and the basal ganglia12. In addition, a recent study found reduced basal ganglia GMV in the unaffected familial relatives of patients with cervical dystonia13. Studies in patients with non-task-specific blepharospasm reported GMV increases in the putamen14, 15 and reductions in the inferior parietal lobule15 as well as reduced left corticobulbar tract volume and connectivity16. Although, collectively, these studies are highlighting the basal ganglia, sensorimotor cortex and cerebellum as the main brain regions altered across different forms of dystonia, the lack of knowledge about the disorder-specific changes in patients with TSD and NTSD limits our understanding of the pathophysiology of these disorders and, consequently, our ability to identify their neuroimaging biomarkers.
The aim of this comparative study was to examine gray and white matter alterations in patients with the two forms of TSD (writer’s cramp and laryngeal dystonia) and two forms of NTSD (cervical dystonia and blepharospasm) using high-resolution MRI with voxel-based morphometry (VBM) and diffusion weighted imaging with tract-based spatial statistics. We hypothesized that abnormalities in TSD would affect the focal segments of brain circuits, which are critical for planning and execution of highly learned tasks in humans, such as writing and speaking, respectively, while brain changes in NTSD patients would be more uniform and symmetrically involving both hemispheres. We also expected that abnormalities in the basal ganglia and cerebellum would represent common changes across different types of dystonia, both with and without task-specificity.
Materials and Methods
Participants
We recruited 45 patients with focal dystonias, who had been diagnosed with TSD (12 writer’s cramp and 12 laryngeal dystonia) or NTSD (11 cervical dystonia and 10 blepharospasm) as well as 24 healthy controls. NTSD patients did not show preferential specificity to different tasks at the time of the recruitment, thus validating their dystonia type as NTSD. There were no statistical differences in age and gender between either the patient groups or each patient group and controls (all p > 0.15) (Table 1). The participants had no neurological (except focal dystonia in the patient group), psychiatric or laryngeal problems based on history, physical and neurological examinations. All participants were right-handed and fully symptomatic at the time of study participation. Those who received botulinum toxin treatment were recruited at the end of their treatment cycle at least 3 months post injection. The mean duration of the disorder was 16.5±12.9 years in writer’s cramp, 10.2±6.3 years in laryngeal dystonia, 12.3±8.9 years in cervical dystonia and 7.3±4.5 years in blepharospasm. Duration of the disorder was not significantly different between the patient groups (all p > 0.05). Clinical neuroradiological evaluation of MRI in all subjects showed normal brain structure without any gross abnormalities.
Table 1.
Demographic and clinical data
| Cervical dystonia | Blepharospasm | Laryngeal dystonia | Writer’s cramp | Controls | p-value* | |
|---|---|---|---|---|---|---|
| Number of subjects | 11 | 10 | 12 | 12 | 24 | n/a |
| Age (mean), [range] | 57.28 [29–77] | 59.2 [45–69] | 54.75 [44–66] | 52.75 [21–72] | 52.13 [38–71] | all between-patient p > 0.16; all patient-control p > 0.05 |
| Gender (Female/Male) | 7/4 | 9/1 | 8/4 | 6/5 | 12/12 | all between-patient p > 0.15; all patient-control p > 0.05 |
| Disease Duration (yrs) mean ± standard deviation | 12.3±8.9 | 7.3±4.5 | 10.2±6.3 | 16.5±12.9 | n/a | all between-patient p > 0.05 |
Comparisons were made between each patient group and controls as well as between the patient groups using two-sample t-test or Fisher’s exact test, wherever appropriate. n/a – not applicable
All participants provided written informed consent, which was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai.
Image Acquisition
High-resolution T1-weighted images were acquired on a 3T Phillips scanner equipped with an eight-channel receive-only coil using 3D magnetization prepared rapid acquisition gradient echo (3D-MPRAGE) sequence with TI = 450 ms; TE = 2.9 ms; FA = 10 degrees; FOV = 240 mm; matrix = 256 × 256 mm; 172 contiguous axial slices; slice thickness = 0.9 mm. Diffusion-weighted images were acquired using a single-shot spin-echo echo-planar imaging sequence with paired gradient pulses positioned 180° around the refocusing pulse for diffusion weighting (TR = 13000 ms, FOV = 240 mm; matrix = 96 × 96 mm zero-filled to 256 × 256 mm; 54 contiguous axial slices with slice thickness of 2.4 mm). Diffusion was measured along 33 non-collinear directions with a b-factor of 1000 s/mm2. One reference image was acquired with no diffusion gradients applied (b0 scan).
Voxel Based Morphometry
Using SPM8 software with VBM8 toolbox, raw images were bias corrected for MRI inhomegeneities and noise; tissue-classified, co-registered and normalized to a standard MNI space using DARTEL (diffeomorphic anatomical registration using exponeniated lie algebra)17. Tissue probability maps were warped on to the image non-linearly allowing for tissue segmentation. Jacobian determinants were used to modulate GMV through multiplication of nonlinear components derived during normalization to preserve tissue volume after warping. The spatially normalized images were smoothed with an isotropic 8-mm Gaussian kernel.
In order to examine structural brain differences in relation to clinical phenomenology (TSD vs. NTSD) and estimate the main effect, one-way analysis of variance (ANOVA) with one factor (GMV) and 3 levels (controls, TSD, NTSD) was carried out at an FWE-corrected p ≤ 0.05. The FWE rate was determined using Monte Carlo simulations18, 19, which resulted in identification of a minimum cluster size of 528 mm3 at a voxelwise threshold of 0.001.
The follow-up post hoc two-sample t-tests with age, gender, total intracranial volume and duration of the disorder as nuisance covariates were performed to determine statistical differences of GMV changes between TSD/NTSD and controls as well as between TSD and NTSD directly. The severity of disorder was not included as a covariate because these data were not available in all patients. The overall statistical significance for between-group comparisons was set at a corrected p ≤ 0.016 to account for multiple group comparisons. In addition, voxelwise correction for multiple comparisons was achieved by applying FWE correction to each comparison separately using Monte Carlo simulations (minimum cluster size 150 mm3, voxelwise threshold 0.001).
Diffusion Weighted Imaging
Using FSL software, images were brain extracted and corrected for movement artifacts and eddy current distortions; the fractional anisotropy (FA) maps were calculated and registered to a standard MNI space using nonlinear registration. Following the creation of a mean FA skeleton and registration of each individual FA map to the skeleton, whole-brain voxelwise statistical analyses were conducted using tract-based spatial statistics (TBSS)20. One-way ANOVA with one factor (white matter skeleton) and 3 levels (controls, TSD, NTSD) was used to estimate the main effect at an FWE-corrected p ≤ 0.05 (Monte-Carlo simulations: voxelwise threshold 0.001, minimum cluster size 50 mm3). The follow-up post hoc two-sample t-tests with age, gender and disorder duration examined differences in TSD/NTSD vs. controls and TSD vs. NTSD at a TFCE-corrected p ≤ 0.01621.
In addition, we examined the statistical dependence of VBM/TBSS measures with disorder duration in TSD and NTSD using voxelwise Spearman’s rank correlation coefficients at an FWE-corrected p ≤ 0.05 (voxelwise threshold 0.001, minimum cluster size 18 mm3).
Results
Voxel-Based Morphometry
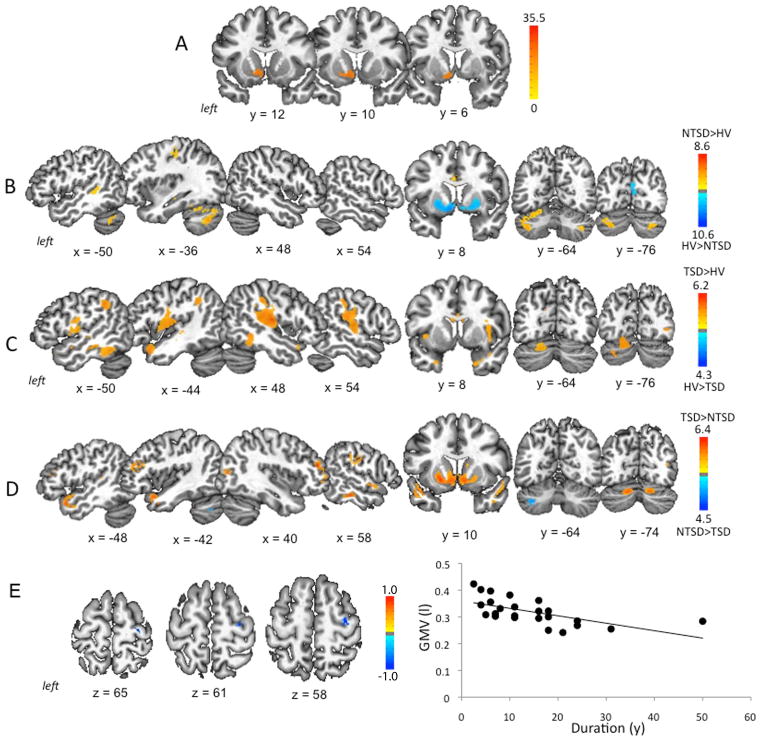
Initial ANOVA between all groups found a significant main effect of GMV changes in the left anterior dorsal putamen, caudate nucleus and ventral striatum (FWE-corrected p ≤ 0.05). (Fig. 1A, Table 2).
Figure 1. Gray matter volumetric abnormalities in TSD and NTSD.
(A) Results of initial analysis of variance (ANOVA) comparing gray matter volume (GMV) between TSD, NTSD and healthy controls. (B) GMV differences between NTSD patients and healthy controls. (C) GMV differences between TSD patients and healthy controls. (D) Direct comparison of GMV differences between TSD and NTSD groups. (E) Significant inverse correlation between GMV (in liters) in the right premotor cortex and disorder duration (in years) in the TSD group. Brain abnormalities are shown on a series of axial, sagittal and coronal sections in the standard MNI space. The corresponding coordinates of peak changes are given in the Table 1. The color bars represent F score (A), t scores (B, C, D) and rs score (E), respectively.
Table 2.
Gray matter changes between task-specific dystonia (TSD), non-task-specific dystonia (NTSD) and controls
| Brain region | F-score | x,y,z | ||
|---|---|---|---|---|
| Initial ANOVA | Left | Striatum | 14.37 | −3, 10, −14 |
| GMV changes in NTSD/TSD vs. controls | ||||
| Brain Region | T-score | x,y,z | ||
| NTSD vs. Controls | Left | Primary Sensorimotor Cortex | 3.82 | −33, −27, 48 |
| Left | Middle Temporal Gyrus | 3.06 | −54, −41, 3 | |
| Left | Thalamus | 4.02 | −9, −26, 12 | |
| Left | Cerebellum (lobule VIIa) | 5.58 | −36, −69, −44 | |
| Right | Cerebellum (lobule VIIa) | 5.32 | 34, −71, −45 | |
| Right | Middle Frontal Gyrus | −4.88 | 39, 54, −5 | |
| Right | Inferior Temporal Gyrus | −4.71 | 60, −33, −23 | |
| Right | Occipital Cortex | −4.20 | 4, −78, 10 | |
| Right | Striatum | −6.72 | 21, 12, −7 | |
| Left | Striatum | −5.26 | −21, 10, −6 | |
| TSD vs. Controls | Left | Premotor Cortex | 3.26 | −36, −14, 60 |
| Left | Inferior Parietal Lobule | 2.77 | −51, −50, 38 | |
| Left | Cuneus | 4.32 | −18, −54, 27 | |
| Left | Cerebellum (lobules VI and VIIa) | 3.79 | −18, −80, −24 | |
| Right | Primary sensorimotor Cortex | 2.86 | 45, −24, 32 | |
| Right | Superior Temporal Gyrus | 4.53 | 48, −17, 10 | |
| Right | Supramarginal Gyrus | 3.27 | 49, −27, 36 | |
| Left | Operculum/Insula | 3.44 | −45, −9, 9 | |
| Right | Operculum/Insula | 3.11 | 40, 5, 2 | |
| Left | Middle Temporal Gyrus | 3.55 | −50, −33, −14 | |
| Left | Inferior Temporal Gyrus | 4.13 | −47, −45, 37 | |
| Right | Middle/Inferior Temporal Gyrus | 3.01 | 49, −47, −12 | |
| GMV changes in TSD vs. NTSD | T-score | x,y,z | ||
| TSD > NTSD | Left | Middle/Inferior Frontal Gyrus | 4.25 | −44, 31, 20 |
| Right | Middle Frontal Gyrus | 6.42 | 39, 44, 16 | |
| Left | Middle/Posterior Cingulate cortex | 3.30 | −1, −30, 32 | |
| Right | Middle/Posterior Cingulate cortex | 4.57 | 4, −35, 36 | |
| Left | Inferior Temporal Gyrus | 4.71 | −57, −38, −19 | |
| Right | Inferior Temporal Gyrus | 5.34 | 57, −29, −21 | |
| Left | Striatum | 5.24 | −21, 10, −6 | |
| Right | Striatum | 3.86 | 23, 13, −5 | |
| Left | Cerebellum (lobule VI-VIIa) | 4.85 | −18, −71, −30 | |
| Right | Cerebellum (lobule VI-VIIa) | 4.39 | 16, −74, −29 | |
| Right | Primary Somatosensory Cortex | 3.94 | 58, −15, 27 | |
| Right | Superior Temporal Gyrus | 4.23 | 60, −1, −8 | |
| Right | Occipital Cortex | 5.32 | 42, −78, 7 | |
| NTSD > TSD | Left | Cerebellum (lobule VIIa) | 3.39 | −38, −59, −44 |
Compared to healthy controls, NTSD showed increased GMV in the left primary sensorimotor cortex, middle temporal gyrus, thalamus, and bilateral cerebellum (lobule VIIa), while reduced GMV was found in the right middle frontal gyrus, inferior temporal gyrus, occipital cortex as well as anterior dorsal putamen and ventral striatum bilaterally at a corrected p ≤ 0.016 (Fig. 1B, Table 2). TSD patients, compared to controls, demonstrated increased GMV in the left premotor cortex, inferior parietal lobule, cuneus, cerebellum (lobules VI and VIIa); right primary sensorimotor cortex, superior temporal gyrus, supramarginal gyrus; bilateral operculum/insula and middle/inferior temporal gyrus at a corrected p ≤ 0.016 (Fig. 1C, Table 2).
A direct comparison of TSD with NTSD revealed GMV changes in the bilateral middle frontal gyrus, middle/posterior cingulate cortex, inferior temporal gyrus, anterior dorsal putamen and caudate nucleus, ventral striatum, cerebellum (lobules VI-VIIa); right primary somatosensory cortex, superior temporal gyrus, and occipital cortex in TSD. Gray matter differences in the left cerebellum (lobule VIIa) were specific to NTSD (Fig. 1D, Table 2).
GMV in the right premotor cortex was negatively correlated with TSD duration (rs = −0.69) (Fig. 1E). No statistically significant relationships were found between NTSD duration and GMV at an FWE-corrected p ≤ 0.05.
Diffusion Weighted Imaging
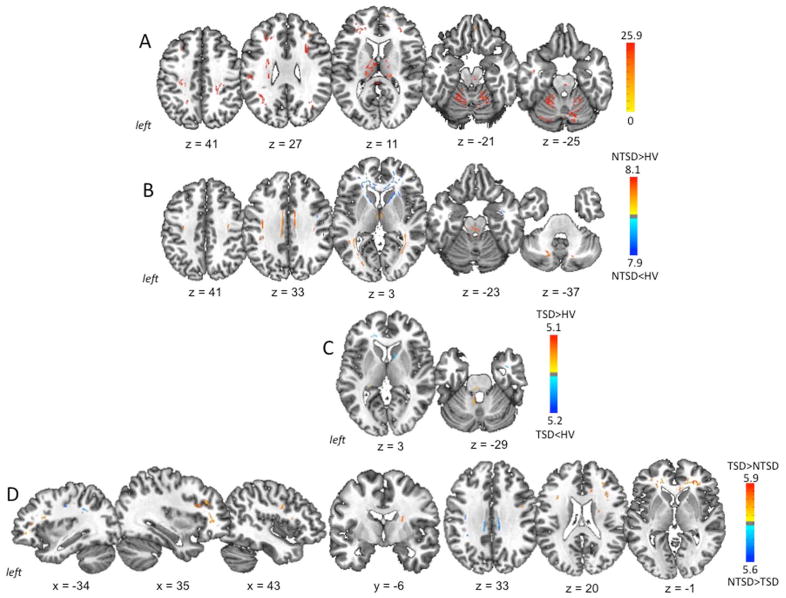
ANOVA showed significant main effect for between-groups differences in the white matter underlying the bilateral middle frontal gyrus, primary sensorimotor cortices, occipital cortex, genu and splenium of the corpus callosum, thalamus, midbrain/pons, cerebellum (lobules VI/VIIa), as well as the left operculum/insula, inferior parietal lobule/supramarginal gyrus and middle/inferior temporal gyrus at an FWE-corrected p ≤ 0.05 (Fig. 2A, Table 3).
Figure 2. White matter abnormalities in TSD and NTSD.
(A) Initial analysis of variance (ANOVA) comparing fractional anisotropy (FA) as a measure of white matter integrity and coherence between TSD, NTSD and healthy controls. (B) Abnormal FA in the NTSD group compared to healthy subjects. (C) Abnormal FA in the TSD group compared to healthy subjects. (D) Differences in FA between TSD and NTSD groups. Brain abnormalities are shown on a series of axial, sagittal and coronal sections in the standard MNI space. The corresponding coordinates of peak changes are given in the Table 2. The color bars represent F score (A) and t scores (B, C, D), respectively.
Table 3.
White matter changes between task-specific dystonia (TSD), non-task-specific dystonia (NTSD) and controls
| Brain region | F-score | x,y,z | ||
|---|---|---|---|---|
| Initial ANOVA | Left | Middle Frontal Gyrus | 15.65 | −25, 34, 2 |
| Right | Middle Frontal Gyrus | 15.04 | 34, 36, 1 | |
| Left | Primary Sensorimotor Cortex | 17.66 | −24, −31, 47 | |
| Right | Primary Sensorimotor Cortex | 15.82 | 23, −32, 48 | |
| Left | Occipital Cortex | 13.20 | −34, −73, 3 | |
| Right | Occipital Cortex | 11.39 | 34, −71, −7 | |
| Left | Corpus Callosum (Genu) | 23.51 | −8, 29, 1 | |
| Right | Corpus Callosum (Genu) | 22.02 | 4, 26, 1 | |
| Left | Corpus Callosum (Splenium) | 23.30 | −4, −33, 20 | |
| Right | Corpus Callosum (Splenium) | 25.22 | 1, −39, 13 | |
| Left | Thalamus | 17.24 | −12, −6, 13 | |
| Right | Thalamus | 16.97 | 15, −15, 17 | |
| Midbrain/Pons | 13.79 | −4, −21, −8 | ||
| Left | Cerebellum (lobules VI/VIIa) | 11.62 | −17, −57, −24 | |
| Right | Cerebellum (lobules VI/VIIa) | 11.26 | 14, −49, −25 | |
| Left | Operculum/Insula | 13.11 | −27, −1, 28 | |
| Left | Inferior Parietal Lobule/Supramarginal Gyrus | 12.97 | −42, −47, 31 | |
| Left | Middle Temporal Gyrus | 17.91 | −40, −44, −2 | |
| Left | Inferior Temporal Gyrus | 14.29 | −43, −7, −25 | |
| White matter changes in NTSD/TSD vs. controls | ||||
| Decreased Fractional Anisotropy | T-score | x,y,z | ||
| NTSD vs. Controls | Left | Middle/Inferior Frontal Gyri | −7.46 | −31, 43, −2 |
| Right | Middle/Inferior Frontal Gyri | −5.41 | 31, 42, −2 | |
| Left | Corpus Callosum (Genu/Body) | −7.95 | −16, 36, 0 | |
| Right | Corpus Callosum (Genu/Body) | −7.24 | 13, 25, 18 | |
| Left | Internal Capsule (Anterior limb/Genu) | −4.21 | −18, 16, 2 | |
| Right | Internal Capsule (Anterior limb/Genu) | −5.54 | 11, 5, −5 | |
| Right | Primary Sensorimotor Cortex | −3.99 | 48, 2, 28 | |
| Right | Inferior Temporal gyrus | −5.69 | 44, 1, −32 | |
| Increased Fractional Anisotropy | ||||
| Corpus Callosum (Splenium) | 4.82 | 0, −37, 17 | ||
| Left | Cingulate Gyrus | 7.38 | −9, −8, 33 | |
| Right | Cingulate Gyrus | 4.62 | 9, −9, 34 | |
| Left | Occipital cortex | 5.72 | −24, −80, 4 | |
| Right | Occipital cortex | 6.40 | 37, −47, 1 | |
| Midbrain/Pons | 3.99 | −9, −28, −26 | ||
| Left | Cerebellum (lobule VIIa) | 6.17 | −21, −68, −39 | |
| Right | Cerebellum (lobule VIIa) | 6.07 | 15, −74, −36 | |
| Right | Ventral Thalamus | 5.22 | 3, −13, 3 | |
| Decreased Fractional Anisotropy | ||||
| TSD vs. Controls | Left | Middle Frontal Gyrus | −4.45 | −27, 42, −3 |
| Right | Middle Frontal Gyrus | −3.04 | 33, 42, −2 | |
| Right | Internal capsule (Anterior limb/Genu) | −4.30 | 10, 5, 1 | |
| Left | Corpus Callosum (Genu) | −3.79 | −15, 37, 1 | |
| Increased Fractional Anisotropy | ||||
| Left | Cerebellum | 3.97 | −9, −51, −30 | |
| White Matter Changes in TSD vs. NTSD | ||||
| T-score | x,y,z | |||
| TSD > NTSD | Left | Middle/Inferior Frontal Gyrus | 5.15 | −15, 34, −6 |
| Right | Middle/Inferior Frontal Gyrus | 5.92 | 23, 37, −3 | |
| Left | Corpus Callosum (Genu) | 5.42 | −6, 28, 5 | |
| Right | Corpus Callosum (Genu) | 5.37 | 13, 31, 8 | |
| Left | Putamen | 3.24 | −22, −5, 15 | |
| Right | Putamen | 3.86 | 23, −6, 14 | |
| Right | Premotor Cortex | 4.17 | 45, 0, 28 | |
| Right | Internal Capsule (Anterior Limb/Genu) | 3.91 | 23, −4, 16 | |
| NTSD > TSD | Left | Middle Cingulate Cortex | 4.58 | −10, −31, 32 |
| Right | Middle Cingulate Cortex | 4.67 | 10, −28, 34 | |
| Left | Primary Sensorimotor cortex | 4.38 | −37, −14, 31 | |
| Left | Inferior Parietal Lobule | 4.27 | −35, −43, 32 | |
Whole-brain analysis between NTSD and controls showed reduced FA in the genu and body of the corpus callosum, anterior limb/genu of the internal capsule as well as in the white matter skeleton underlying bilateral middle/inferior gyrus, right primary sensorimotor cortex and inferior temporal gyrus at a corrected p ≤ 0.016 (Fig. 2B, Table 3). In addition, NTSD exhibited increased FA in the splenium of the corpus callosum, bilateral cingulate gyrus, occipital cortex, midbrain/pons, cerebellum (lobule VIIa) and the right ventral thalamus.
White matter changes were more localized in TSD compared to controls and included sparse FA reductions in the bilateral middle frontal gyrus, right anterior limb/genu of the internal capsule and the left genu of the corpus callosum at a corrected p ≤ 0.016. Increased FA was found in the cerebellum (lobule IX) (Fig. 2C, Table 3).
When comparing NTSD and TSD directly, TSD-specific differences in fiber tract integrity were found in the bilateral middle/inferior frontal gyrus, genu of the corpus callosum and putamen; as well as in the right premotor cortex and anterior limb/genu of the internal capsule. The NTSD group demonstrated changes in the white matter underlying bilateral middle cingulate gyrus, left primary sensorimotor cortex, and left inferior parietal lobule at a corrected p ≤ 0.016 (Fig. 2D, Table 3).
No statistical differences were found between white matter abnormalities and disorder duration in either TSD or NTSD group.
Discussion
Our results are the first to describe differential brain abnormalities in two classes of adult-onset primary focal dystonia, TSD and NTSD, and thus address potential microstructural alterations underlying the task-specificity in dystonia. A direct comparison between the TSD and NTSD groups revealed TSD-specific diffuse GMV changes in the brain regions controlling different levels of sensorimotor processing, such as the primary somatosensory, middle frontal, superior/inferior temporal gyri, middle/posterior cingulate cortex, occipital cortex, striatum and cerebellum (lobules VI-VIIa). These GMV changes were accompanied by fiber tract aberrations in the genu of the corpus callosum and anterior limb/genu of the internal capsule as well as the white matter changes underlying the premotor cortex, middle/inferior frontal and inferior temporal gyri. Conversely, NTSD-specific GMV changes were limited to the left cerebellum (lobule VIIa) only with fiber tract abnormalities underlying the primary sensorimotor, middle cingulate cortex and inferior parietal lobe. The presence of two distinct microstructural patterns in TSD and NTSD provides evidence that these two dystonia subclasses likely follow divergent pathophysiological mechanisms possibly precipitated by different triggers. These findings also point to multi-level, complex alterations of the sensorimotor integration and control for production of a highly learned task, such as writing or speaking.
Common change in TSD and NTSD
Compared to healthy controls, both TSD and NTSD groups showed similarly increased GMV in the primary sensorimotor cortex (albeit right in TSD and left in NTSD), which may relate to abnormalities of the motor control leading to involuntary muscle contractions. Another common feature shared by both focal dystonia classes was the involvement of the cerebellum, which gives further credence, from a structural perspective, to its role in dystonia pathophysiology22–24. Specifically, gray matter changes in the cerebellar lobules VI-VII were shared by both TSD and NTSD when compared to controls. Importantly, the anatomical/lobular location and laterality of these changes (bilateral in TSD and left in NTSD) was different between TSD and NTSD. It has been postulated that activation of the cerebellar cortex and dentate nucleus is important in consolidating learning25. In particular, efferent fibers from the lobules VI-VII project to the association cortices, such as prefrontal, parietal and superior temporal regions26. Since the cerebello-thalamo-cortical tract facilitates intracortical inhibition via the projections to interneurons in the sensorimotor cortices27, reduction in its fiber tract integrity has been shown to correlate with cortical activation and hyperexcitability28, 29. Neuronal plastic changes have also been associated with cerebellar input30, raising the possibility of a morphometric relationship between the cerebellum and sensorimotor cortex. Whether this circuit causes excessive neuronal plasticity or triggers the dystonic cascade is unknown; but the presence of microstructural changes provides further support to the possibility that the cerebello-thalamo-cortical system is involved in dystonia as an endophenotypic trait31, 32 and likely plays an important role in the generation of task-related behaviors.
The GMV decreases in the anterior dorsal putamen and ventral striatum were observed in NTSD compared to controls but not in TSD possibly due to the variability in striatal volumes and topographic location of abnormalities among patients with writer’s cramp and laryngeal dystonia, which may have induced a cancellation effect during the aggregate analysis. However, a direct comparison between NTSD and TSD clearly revealed the striatal differences between these groups in the anterior dorsal putamen and caudate nucleus as well as ventral striatum. Our findings are in line with the well-known predilection for striatal lesions to trigger dystonia33, 34 as well as previous reports of striatal GMV changes in all four focal dystonias investigated here6, 7, 10, 11, 14, 15, 35–37. An inverse correlation between symptom severity and putamen volume in cervical dystonia has also been described36. In considering this, we postulate that, from a microstructural aspect, the striato-pallido-thalamo-cortical network may differentially change based on the nature and/or extent of motor tasks. The order of evolution of these changes and their relationship to abnormal metabolic activity, aberrant cortical inhibition and hyperexcitabilty, or neuroplastic changes need to be investigated in future studies.
Distinct changes in TSD and NTSD
In contrast to NTSD, the comparison between TSD vs. controls found wider spread GMV abnormalities, involving the left premotor cortex and operculum/insula. This may stem from the left hemispheric dominance of sensorimotor planning of speech and writing in right-handed individuals. In addition, a negative correlation between symptom duration and right premotor cortex GMV in TSD speaks to a cortical reorganization of the contralateral motor circuitry and possible ‘wearing off’ of some aspects of motor planning as the disorder persists. GMV increases in the left inferior parietal cortex and right supramarginal and superior temporal gyri may have further impact on sensorimotor processing, such as integration of spatial orientation and attention to task-relevant cognitive, sensory and motor information38, 39. The fiber tract abnormalities in the middle/inferior frontal gyri, premotor cortex, corpus callosum and internal capsule in TSD appear to be tied to the broader cortical changes found in this group. Thus, the argument can be made that increased GMV in these regions may reflect a primary pathophysiological process specific to TSD.
The presence of increased GMV in the bilateral cerebellum and left thalamus as well as decreased GMV in the occipital cortex was specific to NTSD but not TSD when compared to controls. These changes have been previously described in morphometric studies involving separate blepharospasm and cervical dystonia patients35,16, 11, 35. However, the direct comparisons between NTSD and TSD revealed GMV abnormalities in the left cerebellum only. As all of the cortical changes in the NTSD group failed to segregate out in TSD vs. NTSD comparisons, we suggest that limited cortical gray matter alterations may be a characteristic feature of NTSD due, in part, to lesser requirement for higher sensorimotor and cognitive control for task production as in case of TSD.
On the other hand, diffuse changes of axonal coherence observed in NTSD, when compared to controls, highlight a more epigenetic etiology causing direct and/or compensatory changes in the cortical, subcortical, and cerebellar fiber tracts. Paradoxically, the between group comparisons revealed diffuse FA differences specific to TSD compared to focal changes seen in NTSD, further reflecting possible underlying inherent differences in the nature of white matter abnormalities among these dystonia subclasses.
Limitations
The number of subjects with each dystonia form was relatively small. We previously showed that increasing the number of subjects resulted in wider spread of GMV changes when pre-processing was held constant3. This was especially apparent in the cortical regions. But, since the primary aim of this study was to investigate the microstructural changes between TSD and NTSD, the composite n for each group was 24 and 23, respectively. Therefore, it is unlikely that our results lack significance.
As it is a case with neuroimaging studies in general, our study did not allow for sorting out cause from consequence in the pathophysiology of TSD and NTSD, as these techniques are usually unable to directly differentiate between primary and secondary brain changes. However, our study is the first to examine the extent of brain differences between TSD and NTSD and by this adds valuable information for future investigation, such as targeted evaluation of postmortem tissue from these patients and cross-disciplinary studies to distinguish between the primary and secondary causes of this disorder.
In summary, the current study provides new insights into the structural brain changes in TSD and NTSD as it reveals the existence of unique microstructural phenotypes for both classes. Future studies will need to elucidate the extent of relationships between these changes and divergent pathophysiological triggers in both TSD and NTSD.
Acknowledgments
We thank Winona Tse, MD, Catherine Cho, MD, and Ann Hunt, MD, for patient referral, and Frank Macaluso, Charles Adapoe, Heather Alexander, and Manjula Khubchandani, PhD, for help with data acquisition. Supported by the grants from the Bachmann-Strauss Dystonia and Parkinson Foundation and the National Institute on Deafness and Other Communication Disorders, NIH (R01DC011805) to K. Simonyan, and the National Center for Advancing Translational Sciences, NIH (UL1TR000067) to Mount Sinai Clinical Research Center.
Footnotes
Financial Disclosure/Conflict of Interest concerning the research related to the manuscript: None
Authors’ Roles
Ritesh A. Ramdhani: Research project: Execution; Statistical Analysis: Design, Execution; Manuscript: Writing of the first draft, Review and Critique. Veena Kumar: Research project: Execution; Statistical Analysis: Design, Execution; Manuscript: Writing of the first draft, Review and Critique. Miodrag Velickovic: Research project: Organization; Manuscript: Review and Critique. Steven J. Frucht: Research project: Organization; Manuscript: Review and Critique. Michele Tagliati: Research project: Organization; Manuscript: Review and Critique. Kristina Simonyan: Research project: Execution; Statistical Analysis: Design, Execution; Manuscript: Writing of the first draft, Review and Critique.
Relevant conflicts of interest/financial disclosures
Ritesh A. Ramdhani, MD, has nothing to disclose. Veena Kumar has nothing to disclose. Miodrag Velickovic, MD, has nothing to disclose. Steven J. Frucht, MD, has received consulting fees from Merz and Impax Laboratories, Inc., unrelated to the research in this article. Michele Tagliati, MD, has received speaker honoraria from Medtronic, Inc., and consultation fees from St. Jude Medical, Inc. (formerly Advanced Neuromodulation Systems), Abbvie, Allergan, Boston Scientific and Impax Laboratories, Inc., unrelated to the research in this article. Kristina Simonyan, MD, PhD, has nothing to disclose.
References
- 1.Zoons E, Booij J, Nederveen AJ, Dijk JM, Tijssen MA. Structural, functional and molecular imaging of the brain in primary focal dystonia--a review. Neuroimage. 2011;56(3):1011–1020. doi: 10.1016/j.neuroimage.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 2.Neychev VK, Gross RE, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiology of disease. 2011;42(2):185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramdhani RA, Simonyan K. Primary dystonia: conceptualizing the disorder through a structural brain imaging lens. Tremor and Hyperkinetic Movements. 2013 doi: 10.7916/D8H70DJ7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garraux G, Bauer A, Hanakawa T, Wu T, Kansaku K, Hallett M. Changes in brain anatomy in focal hand dystonia. Ann Neurol. 2004;55(5):736–739. doi: 10.1002/ana.20113. [DOI] [PubMed] [Google Scholar]
- 5.Delmaire C, Vidailhet M, Elbaz A, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer’s cramp. Neurology. 2007;69(4):376–380. doi: 10.1212/01.wnl.0000266591.49624.1a. [DOI] [PubMed] [Google Scholar]
- 6.Granert O, Peller M, Jabusch HC, Altenmuller E, Siebner HR. Sensorimotor skills and focal dystonia are linked to putaminal grey-matter volume in pianists. Journal of neurology, neurosurgery, and psychiatry. 2011;82(11):1225–1231. doi: 10.1136/jnnp.2011.245811. [DOI] [PubMed] [Google Scholar]
- 7.Egger K, Mueller J, Schocke M, et al. Voxel based morphometry reveals specific gray matter changes in primary dystonia. Mov Disord. 2007;22(11):1538–1542. doi: 10.1002/mds.21619. [DOI] [PubMed] [Google Scholar]
- 8.Delmaire C, Vidailhet M, Wassermann D, et al. Diffusion abnormalities in the primary sensorimotor pathways in writer’s cramp. Archives of neurology. 2009;66(4):502–508. doi: 10.1001/archneurol.2009.8. [DOI] [PubMed] [Google Scholar]
- 9.Simonyan K, Tovar-Moll F, Ostuni J, et al. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain. 2008;131(Pt 2):447–459. doi: 10.1093/brain/awm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonyan K, Ludlow CL. Abnormal structure-function relationship in spasmodic dysphonia. Cereb Cortex. 2012;22(2):417–425. doi: 10.1093/cercor/bhr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Draganski B, Thun-Hohenstein C, Bogdahn U, Winkler J, May A. “Motor circuit” gray matter changes in idiopathic cervical dystonia. Neurology. 2003;61(9):1228–1231. doi: 10.1212/01.wnl.0000094240.93745.83. [DOI] [PubMed] [Google Scholar]
- 12.Colosimo C, Pantano P, Calistri V, Totaro P, Fabbrini G, Berardelli A. Diffusion tensor imaging in primary cervical dystonia. J Neurol Neurosurg Psychiatry. 2005;76(11):1591–1593. doi: 10.1136/jnnp.2004.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh RA, Whelan R, O’Dwyer J, et al. Striatal morphology correlates with sensory abnormalities in unaffected relatives of cervical dystonia patients. J Neurol. 2009;256(8):1307–1313. doi: 10.1007/s00415-009-5119-1. [DOI] [PubMed] [Google Scholar]
- 14.Black KJ, Ongur D, Perlmutter JS. Putamen volume in idiopathic focal dystonia. Neurology. 1998;51(3):819–824. doi: 10.1212/wnl.51.3.819. [DOI] [PubMed] [Google Scholar]
- 15.Etgen T, Muhlau M, Gaser C, Sander D. Bilateral grey-matter increase in the putamen in primary blepharospasm. Journal of neurology, neurosurgery, and psychiatry. 2006;77(9):1017–1020. doi: 10.1136/jnnp.2005.087148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horovitz SG, Ford A, Najee-Ullah MA, Ostuni JL, Hallett M. Anatomical correlates of blepharospasm. Translational neurodegeneration. 2012;1(1):12. doi: 10.1186/2047-9158-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 19.Ward BD. Simultaneous inference for fMRI data. 2000 [Google Scholar]
- 20.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 22.Eidelberg D, Moeller JR, Antonini A, et al. Functional brain networks in DYT1 dystonia. Annals of neurology. 1998;44(3):303–312. doi: 10.1002/ana.410440304. [DOI] [PubMed] [Google Scholar]
- 23.Ceballos-Baumann AO, Sheean G, Passingham RE, Marsden CD, Brooks DJ. Botulinum toxin does not reverse the cortical dysfunction associated with writer’s cramp. A PET study Brain : a journal of neurology. 1997;120 (Pt 4):571–582. doi: 10.1093/brain/120.4.571. [DOI] [PubMed] [Google Scholar]
- 24.Galardi G, Perani D, Grassi F, et al. Basal ganglia and thalamo-cortical hypermetabolism in patients with spasmodic torticollis. Acta neurologica Scandinavica. 1996;94(3):172–176. doi: 10.1111/j.1600-0404.1996.tb07049.x. [DOI] [PubMed] [Google Scholar]
- 25.Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(2):1017–1022. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(23):8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molinari M, Filippini V, Leggio MG. Neuronal plasticity of interrelated cerebellar and cortical networks. Neuroscience. 2002;111(4):863–870. doi: 10.1016/s0306-4522(02)00024-6. [DOI] [PubMed] [Google Scholar]
- 28.Argyelan M, Carbon M, Niethammer M, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(31):9740–9747. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niethammer M, Carbon M, Argyelan M, Eidelberg D. Hereditary dystonia as a neurodevelopmental circuit disorder: Evidence from neuroimaging. Neurobiology of disease. 2011;42(2):202–209. doi: 10.1016/j.nbd.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doyon J, Owen AM, Petrides M, Sziklas V, Evans AC. Functional anatomy of visuomotor skill learning in human subjects examined with positron emission tomography. The European journal of neuroscience. 1996;8(4):637–648. doi: 10.1111/j.1460-9568.1996.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 31.Quartarone A, Pisani A. Abnormal plasticity in dystonia: Disruption of synaptic homeostasis. Neurobiology of disease. 2011;42(2):162–170. doi: 10.1016/j.nbd.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Quartarone A, Morgante F, Sant’angelo A, et al. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. Journal of neurology, neurosurgery, and psychiatry. 2008;79(9):985–990. doi: 10.1136/jnnp.2007.121632. [DOI] [PubMed] [Google Scholar]
- 33.Marsden CD, Obeso JA, Zarranz JJ, Lang AE. The anatomical basis of symptomatic hemidystonia. Brain : a journal of neurology. 1985;108 (Pt 2):463–483. doi: 10.1093/brain/108.2.463. [DOI] [PubMed] [Google Scholar]
- 34.Vitek JL. Pathophysiology of dystonia: a neuronal model. Movement disorders : official journal of the Movement Disorder Society. 2002;17 (Suppl 3):S49–62. doi: 10.1002/mds.10142. [DOI] [PubMed] [Google Scholar]
- 35.Obermann M, Yaldizli O, De Greiff A, et al. Morphometric changes of sensorimotor structures in focal dystonia. Movement disorders : official journal of the Movement Disorder Society. 2007;22(8):1117–1123. doi: 10.1002/mds.21495. [DOI] [PubMed] [Google Scholar]
- 36.Draganski B, Schneider SA, Fiorio M, et al. Genotype-phenotype interactions in primary dystonias revealed by differential changes in brain structure. NeuroImage. 2009;47(4):1141–1147. doi: 10.1016/j.neuroimage.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pantano P, Totaro P, Fabbrini G, et al. A transverse and longitudinal MR imaging voxel-based morphometry study in patients with primary cervical dystonia. AJNR American journal of neuroradiology. 2011;32(1):81–84. doi: 10.3174/ajnr.A2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottlieb J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53(1):9–16. doi: 10.1016/j.neuron.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Gottlieb J, Balan P, Oristaglio J, Suzuki M. Parietal control of attentional guidance: the significance of sensory, motivational and motor factors. Neurobiology of learning and memory. 2009;91(2):121–128. doi: 10.1016/j.nlm.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]