Abstract
The incidence and severity of Crohn’s disease (CD) are increased in female patients. Using SAMP1/YitFc (SAMP) mice, a spontaneous model of chronic intestinal inflammation that displays histologic and pathogenic similarities to human CD, we investigated the potential mechanism(s) contributing to sex differences observed in CD. Similar to gender differences observed in CD patients, SAMP female (SAMP-F) mice displayed an earlier onset and more severe ileitis compared to SAMP males (SAMP-M). Further, T regulatory cells (Tregs) from gut-associated lymphoid tissue (GALT) of SAMP-F were reduced in frequency and impaired in their in vitro and in vivo suppressive functions compared to SAMP-M Tregs. Given the interaction between sex hormones and Treg function, we investigated the possible role of estrogen (E2) in SAMP ileitis. SAMP-M responded to exogenous E2 administration by expanding Treg frequency and reducing ileal inflammation, whereas SAMP-F were resistant. Conventional T cells (Tconv) and Tregs responded differentially to estrogen signaling, leading to distinct immunoprotective effects mediated by distinct estrogen receptor (ER) isoforms. These mechanisms were impaired in T cells from SAMP-F mice. Thus, hormone signaling influences the expansion and function of GALT Tregs in an ER-dependent manner and contributes to gender-based differences in experimental CD.
Introduction
Autoimmune diseases collectively affect more than twenty million people, with a disproportionate occurrence in the female population1, 2. The extent of gender bias is disease specific, reaching as high as 50:1 (female to male ratio) in Hashimoto’s thyroiditis3. Gender disparity has been reported for the occurrence of inflammatory bowel disease (IBD), particularly for CD, with a modestly increased incidence in females compared to males4, 5. In addition to a bias in favor of adult females, emerging evidence indicates that the severity of intestinal inflammation greatly differs between sexes, such that female CD patients are likely to experience more severe disease manifestations compared to males6. Changes in clinical symptoms, both relapsing and remitting, are often associated with hormonal surges during puberty, pregnancy and the post-partum period7.
Several factors contribute to the pathogenesis of IBD. Although the precise etiology is currently unknown, it is generally accepted that IBD results from a dysregulated immune response to exogenous factors, such as pathogen associated molecular patterns (PAMPs) (e.g., gut microflora), in genetically predisposed individuals. Genome-wide association studies in IBD cohorts have identified several candidate susceptibility loci, including the HLA and TNF regions of chromosome 6, the NOD region of chromosome 16, and additional loci on chromosome X (ChrX), including FOXP38. These regions have been associated with a genetic predisposition to IBD, and high frequency haplotypes on ChrX have recently been shown to be associated with female IBD patients9. Interestingly, ChrX contains susceptibility loci linked to several other autoimmune diseases, including Wiskott-Aldrich Syndrome and an associated colitis phenotype in both humans and mice10. Despite the linkage associations on ChrX, little is known regarding the specific candidate genes involved in IBD sex differences, or the mechanisms by which the mucosal immune system may interact with sex hormones and their receptors.
E2 signaling is generally immunoprotective, and despite their increased susceptibility to autoimmune disorders, females have been reported to exhibit more robust immune responses against infectious agents8. Although E2 signaling may lead to divergent effects in different disease settings11, 12, anti-inflammatory effects of E2 and ER agonists in rodent models of Th1-mediated colitis have been described13, 14, supporting a protective role for E2 in chronic intestinal inflammation. Estrogen exerts its regulatory effects through two nuclear receptors, ER-α and -β, which initiate intracellular signaling, activate transcription of ER-dependent genes, and serve as co-factors for other transcriptional elements15. Although ERs are expressed by a variety of immune cells within the gastrointestinal mucosa, including lymphocytes, macrophages and dendritic cells16, specific mechanism(s) by which estrogen-ER interactions exert their immunoprotective effects in the gut have not been fully elucidated.
The frequency and/or function of CD4+CD25+Foxp3+ Tregs are impaired in many human autoimmune diseases and experimental inflammatory models, including SAMP ileitis17. Tregs play a critical role in maintaining immune tolerance to self-antigens and dampening deleterious immune responses18, 19, and are particularly important in the prevention of autoimmune gastritis and IBD20–22. Seminal work demonstrated that CD4+CD45RBlow T cells, which primarily encompass a subset of T cells with regulatory function, have the ability to downregulate experimental colitis in recipient mice when adoptively co-transferred with CD4+CD45RBhi pathogenic T cells23. Importantly, adoptive transfer experiments have also shown that CD4+CD25+Foxp3hi naturally occurring Tregs (nTregs) are able to maintain normal gut homeostasis by dampening memory effector T cell (Teff) expansion during mucosal immune responses to bacterial infection through production of IL-10 and TGFβ, and expression of functional molecules including PD1, CTLA-4, and LAG324.
In order to mechanistically determine pathogenic factors contributing to gender disparity observed in CD and the potential interaction between sex hormones and Treg function, we utilized the SAMP mouse strain, a spontaneous murine model of human CD (reviewed in25). The ileitis phenotype in SAMP mice occurs spontaneously without chemical, genetic, or immunologic manipulation, and recapitulates the human condition for disease location (terminal ileum), discontinuous nature of inflammatory lesions, and response to standard therapies used for treating CD. Inflammation is fully developed by 10 wks of age, with Th1-type immune responses predominating early during the inductive phase, and a mixed Th1/Th2 phenotype emerging as ileitis develops into chronic, established disease. Mesenteric lymph nodes (MLNs) of SAMP display increased size and cellularity compared to AKR/J mice (AKR, control parental strain), with SAMP-derived conventional CD4+ T cells capable of adoptively transferring ileitis to naive SCID recipients26. A global increase in Treg frequency is observed in SAMP compared to AKR MLN, although Tregs from SAMP (CD4+CD25+) are functionally impaired in preventing colitis when adoptively co-transferred into SCID recipients with pathogenic, Treg-depleted Tconv cells17. These observations have never been evaluated with regard to potential sex differences.
Herein, we report that SAMP mice, similar to Crohn’s patients, display a gender bias for intestinal inflammation, with SAMP-F showing an earlier onset and increased disease severity compared to SAMP-M. We confirmed these findings in TNFΔARE/+ mice, a second model of chronic ileitis displaying Crohn’s-like pathology27. Tregs from SAMP-F display reduced Foxp3 expression and reduced suppressive function, both in vivo and in vitro. This gender disparity appears to be dependent on signaling through different ER isoforms and their downstream functional effects on Treg differentiation, including E2-mediated induction of Treg markers in naive T cells from SAMP-M, but not SAMP-F; E2-mediated suppression of T cell proliferation in SAMP-M, but not –F; and enhancement of Treg function in SAMP-M, but not –F. Taken together, our data demonstrate that estrogen signaling is immunoprotective via direct effects on dampening Tconv proliferation and inducing conversion to Treg cells. These normally protective mechanisms are compromised in SAMP-F, leading to alterations in T cell homeostasis. Thus, differential responses to E2 among male and female T cells likely contribute to the increased severity of intestinal inflammation observed in SAMP-F mice and female CD patients.
Results
Early onset and increased severity of ileitis in SAMP-F
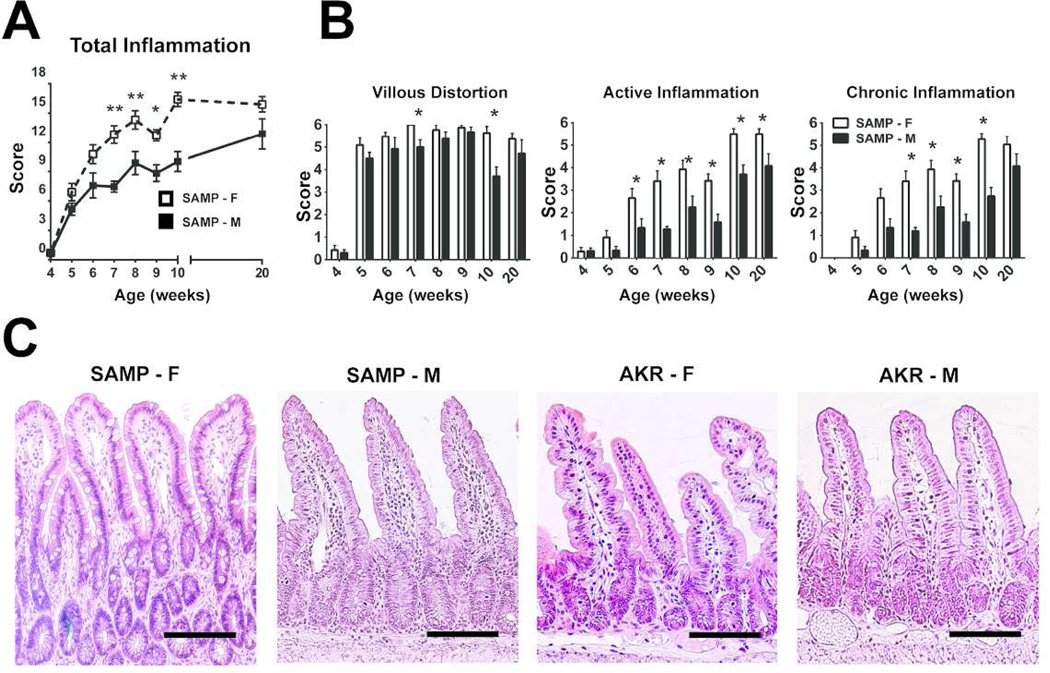
Ileal inflammation in age-matched cohorts of SAMP-M and –F mice was analyzed using a detailed time course (Figure 1A). At 5 wks, both SAMP-M and -F showed minimal inflammation. By 6 wks, corresponding to the onset of puberty, SAMP-F displayed increased ileal inflammation, which became more severe and disparate from SAMP-M up to 10 wks of age. AKR mice had a total inflammatory score ≤1 for all ileal specimens evaluated (data not shown). Both active (acute) and chronic inflammation were significantly increased in SAMP-F compared to SAMP-M from 7–10 wks of age, with villous distortion significantly elevated in SAMP-F at 7 and 10 wks (Figure 1B). At 6 weeks of age, SAMP-F (Figure 1C, left panel) showed histologic features of epithelial crypt hypertrophy and villous blunting, dense inflammatory infiltrates throughout the villi and submucosa, and Paneth and goblet cell hyperplasia compared to age- and sex-matched SAMP-M and AKR (Figure 1C, middle and right panels). These data strongly show an earlier onset of ileitis with increased disease severity in SAMP-F vs. -M, similar to that reported in CD patients.
Figure 1. SAMP-F mice exhibit early, severe ileitis compared to SAMP-M.
(A) Time course of ileitis in age-matched cohorts of SAMP-F and –M. Composite inflammatory scores are represented as mean ± SEM (*p<0.05, **p<0.01, n=12/group). (B) Time course of histologic indices representing active inflammation, chronic inflammation, and villous distortion expressed as mean ± SEM (*p≤0.05, n=12/group). (C) Representative photomicrographs of H&E stained ilea (n=12/group) from 6-wk-old SAMP and AKR. Scale bar = 100 µm; X20 + 1.25 original magnification.
SAMP-F display impaired frequency and in vitro function of CD25+Foxp3+ Tregs
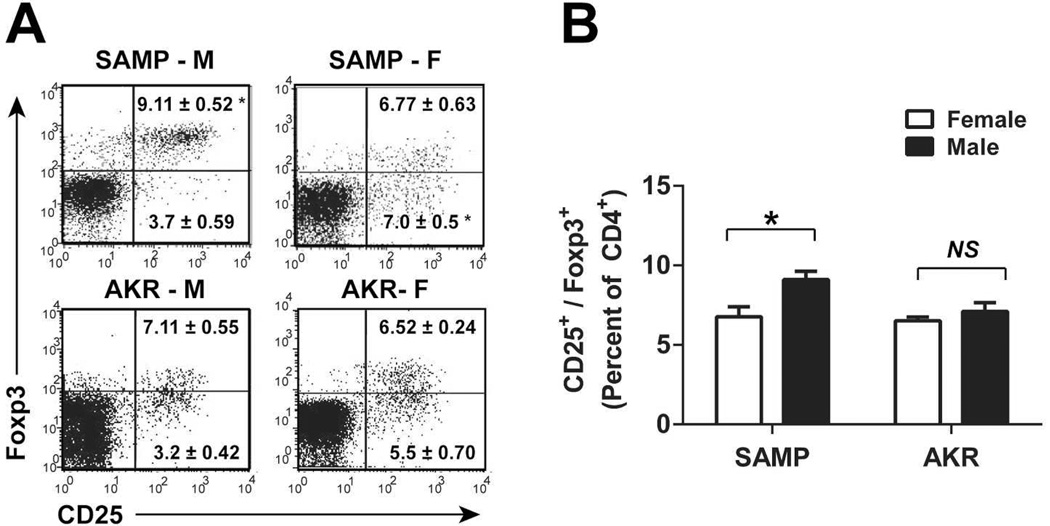
Given the increased frequency of gut-infiltrating lymphocytes in SAMP, we hypothesized that regulatory mechanisms, such as Treg function, may be reduced in SAMP leading to ileitis. To determine whether alterations in intestinal Treg cells contribute to gender differences in SAMP, MLN cells were collected from >10-wk-old SAMP and AKR mice and analyzed for expression of CD25 and Foxp3. SAMP-M showed an increased percentage of Treg cells (CD4+CD25+Foxp3hi) relative to SAMP-F and AKR controls (Figure 2A). Conversely, MLN from SAMP-F contained an elevated percentage of CD4+ T cells expressing CD25 that were Foxp3low, representing memory-Teff cells, reported to be pathogenic in colitis transfer models28. Cumulative data (n=6–14 animals/group) show that the mean percentage of CD25+Foxp3+ cells in SAMP females is reduced compared to males (Figure 2B).
Figure 2. Treg frequency is decreased in SAMP-F compared to SAMP-M.
(A) Representative FACS histograms show expression of CD25 and Foxp3 among CD4-gated lymphocytes from MLN (mice ≥10 wks). (B) Quantitation of results from (A). Percentages of CD25+/Foxp3+ T cells are expressed as mean ± SEM (*p<0.01, n=8–14/group).
Gender differences in experimental CD and the critical role of Tregs in this process were confirmed by observations made in the TNFΔARE/+ model of Crohn’s-like ileitis (Supplemental Figure S1). These mice possess a genetic deletion of the TNF AU-rich regulatory elements, resulting in post-transcriptional TNF de-regulation and chronic TNF overproduction that ultimately leads to the development of a CD-like pathology in the ileum27. Similar to SAMP-F, female TNFΔARE/+ mice show increased disease severity with an associated reduction in the overall percentage and suppressive ability of GALT Tregs compared to age-matched TNFΔARE/+ males (Figure S1). These data establish that observations regarding sex differences in experimental ileitis do not appear to be model-specific and further support the gender-dependent role of Tregs in influencing the severity of disease in CD patients.
SAMP-F Tregs are dysfunctional in vivo
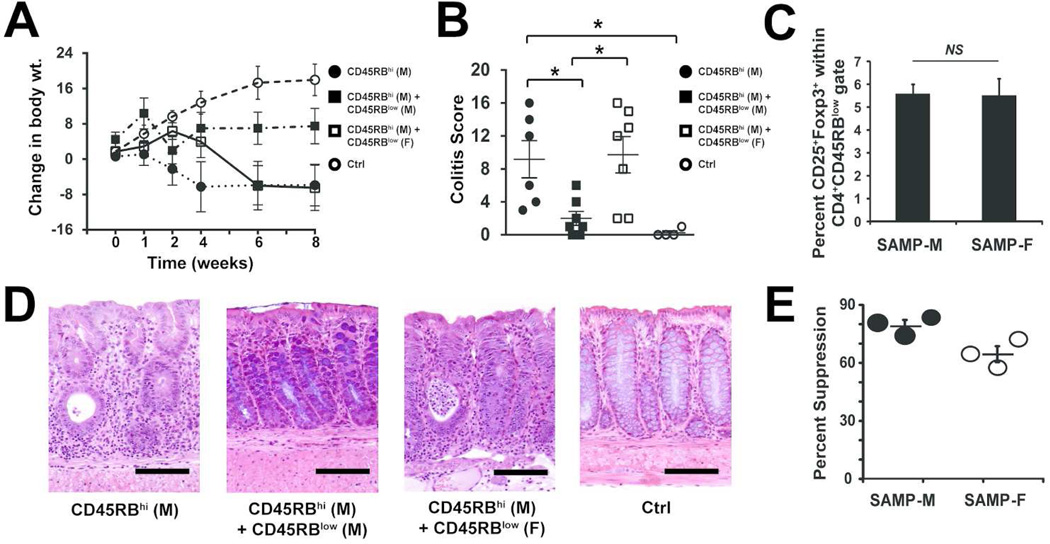
Adoptive transfer of CD4+CD45RBhi T cells to immunodeficient SCID mice results in progressive colitis23. Importantly, when CD4+CD45Blow cells (enriched for Tregs) are co-transferred with the RBhi cells, recipients are protected from colitis23. To determine if SAMP Tregs express gender-specific differences in suppressive function, we reconstituted SCID mice with RBhi T cells from SAMP-M, with or without RBlow cells from either SAMP-M or SAMP-F. As expected, eight weeks post-transfer, SCIDs reconstituted with pathogenic MLN CD4+CD45RBhi T cells from SAMP-M alone (RBhi) developed a colitis phenotype (diarrhea with bloody stools, accompanied by a dramatic body weight loss) (Figure 3A). SCIDs receiving co-transfer of RBhi/RBlow cells (both from SAMP-M) showed no signs of colitis and exhibited less weight loss relative to recipients receiving RBhi cells alone. Interestingly, SCIDs receiving mixed gender RBhi-M/RBlow-F SAMP cells developed colitis with severity similar to recipients of RBhi cells alone (Figure 3B). The inability of female RBlow cells to reverse colitis was not due to a decrease in number of Tregs transferred, as comparable percentages of CD25+Foxp3+ Tregs were found within the CD4+CD45RBlow population regardless of gender (5.57 ± 0.42% in SAMP-F cells versus 5.50 ± 0.75% in SAMP-M cells) (Figure 3C). The FACS-based gating strategy for isolation of CD4+CD45RBlow and CD4+CD45RBhi T cells is shown in Supplemental Figure S2.
Figure 3. Treg cells from SAMP-F are dysfunctional in vivo and in vitro.
6-wk-old SCID mice were reconstituted with FACS-sorted MLN CD45RBhi CD4+ T cells from SAMP-M alone (● RBhi) or co-transferred with MLN CD45RBlow CD4+ T cells from either SAMP-M (■ RBlow-M) or SAMP-F (□ RBlow-F); vehicle, mock-transferred SCIDs served as controls (○ Ctrl). (A) Changes in body weight are shown in weeks post-transfer and (B) histologic evaluation of colitis is shown at 8 wks post-transfer. Percent change in body weight and composite inflammatory scores are represented as mean ± SEM (*p≤0.05, n=16/group). (C) Mean percentage of CD25+/Foxp3+ cells within the RBlow T cell population of SAMP-M and –F is expressed ± SEM. (D) Representative photomicrographs of colon from indicated mice (scale bar=100 µm; original magnification 20× + 1.25NA). (E) In vitro Treg suppression activity. CD4+CD25− Tconv and CD4+CD25+ Treg cells were isolated from SAMP-M or SAMP-F mice. Tconv were cultured alone or with a 1:1 ratio of Treg for 3 days in the presence of αCD3/αCD28. Percent suppression is expressed as the percentage of Tconv proliferation that was suppressed by co-culture with Treg cells. Each dot represents one experiment (3 pooled mice per experiment) ± SEM.
Histologically, colon tissues from recipients of CD45RBhi cells alone (Figure 3D, left panel) displayed significant villous and submucosal inflammation, epithelial hypertrophy, and villous blunting. Co-transfer of RBlow cells from SAMP-M, but not from SAMP-F, mice led to a reduction of inflammation and hypertrophy (Figure 3D, middle panels), whereas control (mock-transferred) mice had minimal inflammation (Figure 3D, right panel).
Colonic inflammation in recipient mice was further characterized by examining myeloperoxidase (MPO) activity in local colonic tissues, as well as cytokine secretion from αCD3/αCD28-activated MLN cells 8 weeks after transfer. MPO, a marker of neutrophil-mediated inflammation, was elevated in colon tissues from SCID mice transferred with CD45RBhi cells alone, compared to tissues from control mice. Co-transfer of CD45RBlow cells from SAMP-M, but not SAMP-F, was able to significantly abrogate MPO activity (Supplemental Figure S3). Similar to the effects seen in native SAMP mice, proinflammatory cytokine secretion from activated MLN cells was increased in mice with severe colitis (RBhi alone and RBhi/RBlow-F recipients) compared to RBhi/RBlow-M recipients and control mice (Figure S3). Secretion of Th17-associated IL-17 and IL-22 was also increased in cells from RBhi and RBhi/RBlow-F recipients, and correlated to colitis severity in this model. In contrast, levels of proinflammatory Th1, Th2, and Th17 cytokines were decreased, and anti-inflammatory IL-10 was increased, in activated MLN cells from RBhi/RBlow-M recipients compared to cells from RBhi and RBhi/RBlow-F recipients. Interestingly, cells from RBhi/RBlow-M recipients also expressed higher levels of IL-2, a critical growth factor for Tregs.
In addition to testing the in vivo function of Treg cells using the CD45RB adoptive transfer model, we also investigated the in vitro suppressive capacity of gender-specific SAMP Tregs using CFSE suppression assays. CD4+CD25− Tconv and CD4+CD25+ Treg cells were isolated by FACS sorting (Supplemental Figure S4). Tconv were labeled with eFluor 670 (CFSE analog) and cultured with αCD3/αCD28, with or without Treg cells for 3 days. Suppression, expressed as the percentage of proliferating cells (eFluor 670 “low”) inhibited by co-culture with Tregs, was calculated for male versus female SAMP. We found that Tregs from SAMP-F were less effective at suppressing the proliferation of Tconv than Treg from SAMP-M (Figure 3E). Together, these data confirm the in vivo findings and demonstrate that Tregs from SAMP-M are functionally more effective than their SAMP-F counterparts.
Estrogen signaling differentially affects male and female T cells in SAMP ileitis
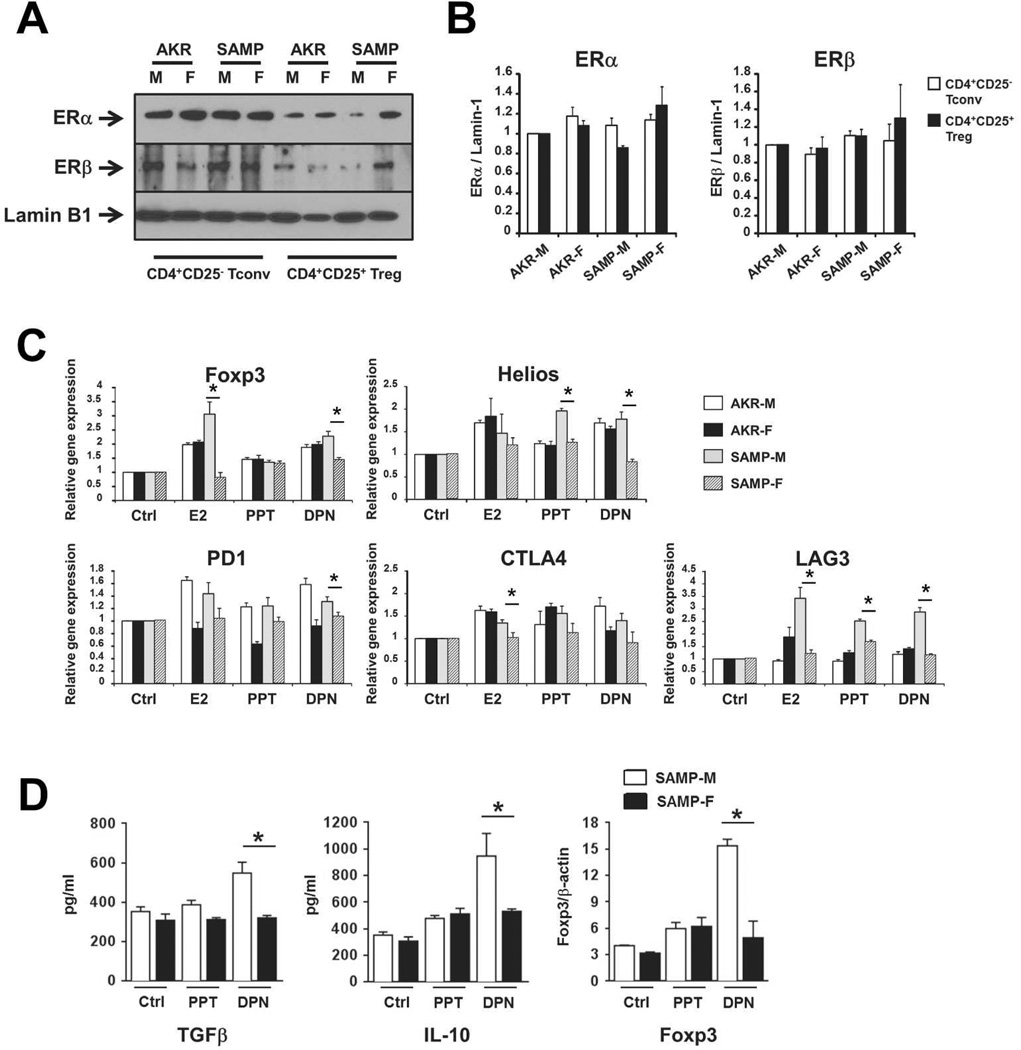
Previous investigators have reported that estrogen (17-β-estradiol or E2) mediates conversion of conventional naive T cells (CD4+CD25− Tconv) to Tregs in WT mice29. Given our observed decrease in Tregs of SAMP-F vs –M mice, we hypothesized that SAMP-F may demonstrate aberrant E2-mediated conversion of Treg cells. We first characterized the expression of ER isoforms ERα and ERβ in primary T cells from 10-wk-old SAMP and AKR male and female mice. Purified Tconv and Treg cells from all four cohorts expressed detectable ERα and ERβ in nuclear protein extracts, with no significant differences detected among male and female T cells. A representative Western blot is shown in Figure 4A and mean densitometric analysis is shown in Figure 4B (±SEM, n=3–5 mice/group).
Figure 4. Estrogen signaling differentially affects Treg induction in SAMP-M vs. SAMP-F mice.
(A, B) CD4+CD25− Tconv and CD4+CD25+ Treg cells were isolated from spleens of indicated mice (10 weeks of age). Nuclear protein lysates were probed for ERα, ERβ, and Lamin-1 by Western blot. Representative images (A) and semi-quantitative densitometric analysis (B) are shown (n=3 total experiments, 3–5 pooled mice per group). (C) CD4+CD25− T cells were isolated from untreated, 10 week old mice and cultured in vitro with vehicle (Ctrl), E2, PPT, or DPN (10nM). mRNA specific for indicated genes was normalized to GAPDH and mean fold changes are expressed ± SEM relative to vehicle-treated samples (*p≤0.05, n=3/group). (D) CD4+CD25+ Treg were isolated from MLN of untreated, 10 week old mice and cultured in vitro in the presence of vehicle (Ctrl), PPT, or DPN (10nM). Cell supernatants were analyzed for IL-10 and TGFβ protein by ELISA (left and middle) and nuclear protein lysates were probed for Foxp3 and β-actin protein by Western blot (semi-quantitative densitometric analysis is shown at left). All results are expressed as mean ± SEM (*p<0.05, **p<0.01, n=6/group).
To directly test the ability of naive T cells to acquire a Treg phenotype in response to E2, Tconv (CD4+CD25−) were isolated from 10-wk-old AKR and SAMP male and female mice and stimulated ex vivo with DMSO/PBS (vehicle Ctrl), E2, or the ER agonists propyl pyrazole triol (PPT, specific for ERα) or diarylpropionitrile (DPN, specific for ERβ). In support of the hypothesis that E2 signaling contributes to the induction of Tregs, T cells from AKR controls and SAMP-M showed an increase in mRNA transcripts specific for the Treg-associated molecules Foxp3, Helios, PD1, CTLA-4 and LAG3 (Figure 4C). Interestingly, T cells from SAMP-F did not induce Treg-associated genes as robustly as cells from SAMP-M.
To study the effects of E2 signaling on fully differentiated Treg cells, MLN Tregs were isolated from 10-wk-old SAMP-M and –F and stimulated ex vivo with either vehicle Ctrl, PPT or DPN. Interestingly, only SAMP-M responded to ERβ signaling via enhancement of anti-inflammatory TGFβ and IL-10 and upregulation of Foxp3 (Figure 4D). Notably, and in contrast to effects observed in SAMP-M, cells from SAMP-F did not upregulate TGFβ, IL-10, or Foxp3 in response to ER agonist treatment (Figure 4D).
Differential effect of estrogen on ileitis and Treg expansion
To determine whether differences in physiologic levels of estrogen may be responsible for modulating disease severity, serum estrogen was measured during post-pubertal development in SAMP and AKR mice (>10 weeks of age). Although circulating estrogen levels were elevated in female compared to male mice, E2 levels were comparable between female SAMP and AKR, as well as between male SAMP and AKR (Supplemental Figure S5). Together, these results indicate that physiologic estrogen levels do not likely represent an independent risk factor for sex differences observed in SAMP ileitis.
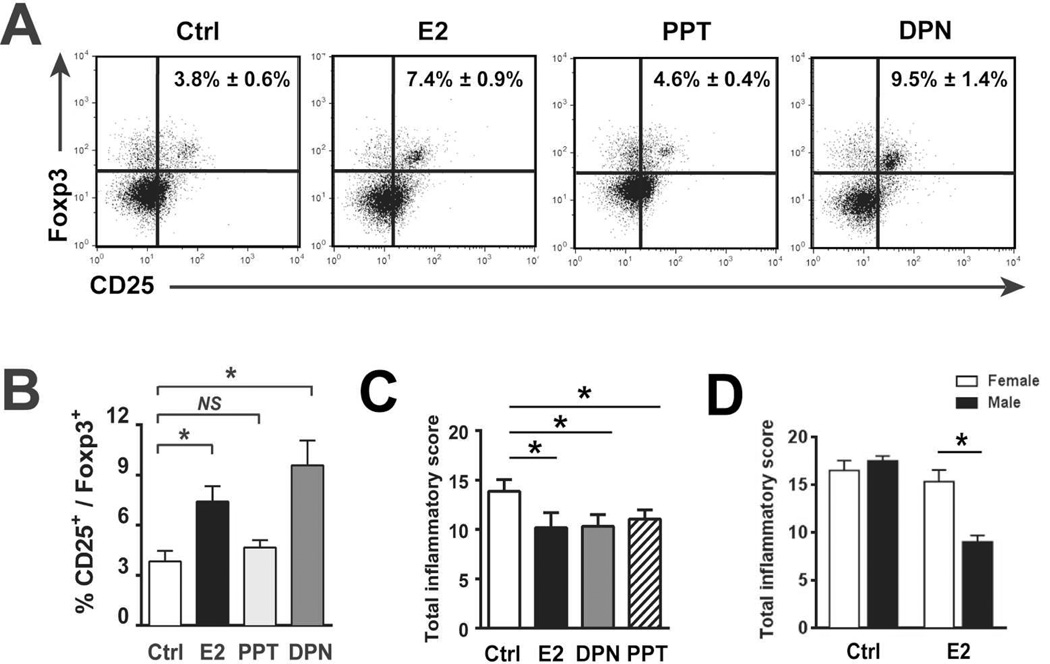
To study the in vivo effects of E2 signaling in the absence of endogenous hormonal effects, 10-week-old SAMP-M mice were gonadectomized (GDX). These “gender-neutral” GDX-SAMP were then treated with i.p. injections of E2, PPT, DPN, or vehicle Ctrl. Following 6 weeks of hormone treatment, MLN cells from GDX-SAMP-M were examined. MLN T cells from GDX-SAMP mice showed an increase in the percentage and absolute numbers of CD4+CD25+Foxp3+ T cells following in vivo treatment with E2 and DPN (Figure 5A and B). This was in contrast to PPT- and vehicle-treated mice, which did not show an expansion of CD25+Foxp3+ cells. These observations suggest that in SAMP-M, activation of ERβ-specific pathways results in conversion of GALT-derived Tregs.
Figure 5. E2 signaling is protective against ileitis in SAMP-M mice.
(A,B,C) 10-wk-old GDX-SAMP mice were administered vehicle (Ctrl), E2 (10 ug/kg), PPT (10 mg/kg), or DPN (10 mg/kg) by i.p injection. Following 6 weeks of treatment, MLNs were harvested and cells stained for flow cytometry. Representative histograms are shown in (A) and percentages of CD25+Foxp3+ cells among CD4-gated lymphocytes are expressed as mean ± SEM (*p<0.05, **p<0.01, n=6/group) (B). (C) Total Inflammatory scores are represented as mean ± SEM (*p≤0.05, n=8–14/group). (D) 10-wk-old GDX-SAMP were implanted with subcutaneous estrogen for 6 wks (see Materials & Methods). Total inflammatory scores following 6 weeks of indicated hormone treatment (expressed as mean ± SEM, *p<0.05, n=6/group).
Given our previous observation that DPN preferentially augments Foxp3, IL-10, and TGFβ in Treg cells (Figure 4D), and in vivo evidence of DPN-mediated expansion of GALT Tregs (Figure 5A), we asked whether clinical responses to exogenous E2 were mediated through ER-dependent pathways, as both ERβ and ERα are widely distributed throughout the gastrointestinal tract and expressed in gut mucosal cells30. 6-week in vivo treatment of GDX SAMP-M with E2, PPT and DPN resulted in decreased ileitis compared to vehicle control (Figure 5C), suggesting that the immunoprotective effects of E2 may be mediated by diverse mechanisms to both ER isoforms, and not solely through ERβ-mediated induction of Tregs.
Given our findings of decreased ileitis in E2-treated GDX-SAMP-M, we asked whether GDX-SAMP-F are similarly protected from ileitis via estrogen signaling. 10-wk-old GDX-SAMP mice were exposed to continuous-released E2 by subcutaneously placed implants for 6 weeks as previously described31. Interestingly, although GDX-SAMP-M demonstrated a partial resolution of ileitis following E2 treatment, similar to effects observed in E2-injected animals (Figure 5C), GDX-SAMP-F did not improve in response to estrogen (Figure 5D).
SAMP-F Tregs are resistant to E2-mediated immunosuppression
Given our observation that in vivo E2 treatment reduces ileitis selectively in SAMP-M mice, but not SAMP-F (Figure 5D), we investigated the potential mechanism(s) for this differential effect. We focused our studies on the role of E2 modulation of Treg function, since we observed gender-based differences in Treg phenotype (Figure 2) and function (Figures 3, 4). We initially asked whether E2 can directly affect the suppressive capacity of Treg cells by adding exogenous E2 (10nM) to in vitro T cell suppression assays. Interestingly, we found that the suppressive function of Treg cells from both SAMP-M and –F were unchanged by the addition of E2 or the ER agonists PPT and DPN (10nM, data not shown).
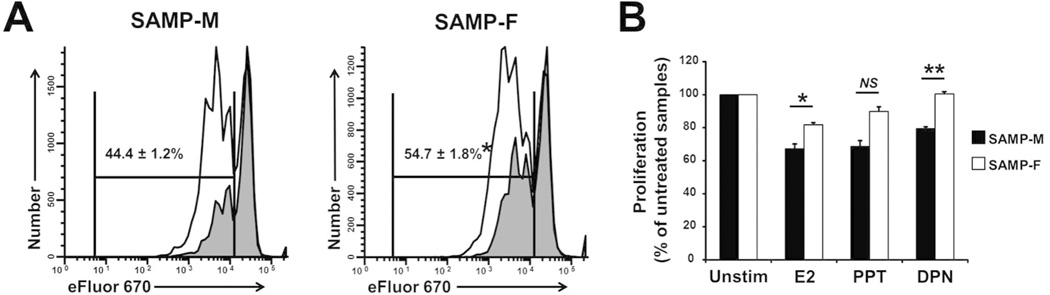
Since E2 did not appear to change the suppressive capacity of Tregs, we asked whether E2 mediates immunosuppressive effects through direct signaling to naïve T cells. We cultured CD4+CD25− Tconv cells with E2, PPT, or DPN (10nM) for 3 days in CFSE proliferation assays. We found that the percentage of dividing cells (reduced CFSE) was significantly lower in SAMP-M cultures compared to SAMP-F (44.4±1.2% vs 54.7±1.8%, respectively) with the addition of E2 (Figure 6A), suggesting that SAMP-M T cells are more susceptible to E2-mediated immunosuppression. When the proliferation of E2-stimulated T cells was calculated with respect to that of unstimulated T cells from matched donors, both E2 and DPN treatment resulted in a significant reduction in proliferation in SAMP-M T cells compared to SAMP-F (Figure 6B). Collectively, this data suggests that SAMP-F T cells are not as responsive to regulation by E2 signaling as SAMP-M T cells, likely contributing to their heightened activation in vivo. Further, the heightened activation status of these cells may partially explain the observed Treg dysfunction in female vs. male SAMP mice.
Figure 6. SAMP-F Tregs are resistant to E2-mediated immunosuppression.
(A, B) CD4+CD25− Tconv cells were isolated from 10-wk-old SAMP mice by FACS sorting. Sorted cells were labeled with eFluor 670 cell proliferation dye and cultured in vitro with αCD3/αCD28 in the presence or absence of E2, PPT, or DPN (10nM) for 3 days. Proliferation was assessed by e670 dye dilution. (A) Representative histograms show the proliferation of viable, CD4-gated cells in the absence (white peaks) and presence (gray peaks) of E2. Cumulative statistics show the percentage of dividing cells in the presence of E2 (*p<0.002, n=3/group). (B) Percent proliferation of E2-, PPT-, or DPN-treated cells was calculated relative to percent proliferation of untreated cells using dye dilution and gating strategy shown in (A). Results are expressed as mean ± SEM (*p<0.001, n=3/group).
DISCUSSION
Immunological, epidemiological, and clinical evidence indicates that female sex hormones and susceptibility genes on the X chromosome are capable of inducing differences in the etiology and pathophysiology of chronic immune/inflammatory diseases32. In IBD, specifically CD, emerging evidence supports clear gender disparity, particularly in disease severity; however, the mechanism(s) of sex differences in the pathogenesis of chronic intestinal inflammation remain poorly understood. Specifically, females often experience dichotomous alterations in symptoms during changes in hormonal levels, such as puberty, pregnancy and menopause. This correlation strongly suggests a role for hormones in modulating disease, yet these mechanisms have not been fully studied.
The present study reveals that the SAMP model of CD-like ileitis recapitulates several aspects of human CD, including an accelerated and more severe disease course in females. Interestingly, genetic outcross studies and linkage analysis of SAMP mice have identified loci on several chromosomes with polymorphic markers that segregate with the ileitis phenotype, including X-linked susceptibility loci33. SAMP therefore represents a unique tool to examine cellular and molecular mechanisms underlying gender bias in IBD. Our study reveals several novel observations regarding the interplay of hormone signaling and T cell function in the context of mucosal inflammation that begin to unravel the mechanisms behind gender differences in autoimmunity.
Our results show that SAMP-F mice display a dense infiltrate of leukocytes in the inflamed ileum beginning at approximately 6 weeks of age, corresponding to the onset of puberty (Figure 1B). Infiltrating cells drain to the MLN and comprise a heterogeneous population of lymphocytes and myeloid cells, including Tregs. The nTreg population in mice generally comprises 5–10% of CD4+ cells, and the majority of CD4+CD25+ cells co-express Foxp334. Interestingly, our results indicate that fewer than 50% of the CD4+CD25+ cells from SAMP-F MLN express Foxp3, compared to the majority of CD4+CD25+ cells expressing Foxp3 in SAMP-M and AKR control mice (Figure 2). Loss of Foxp3 expression in mature Treg cells has been reported to attenuate Treg suppressive functions and is associated with autoimmune disease35; of particular relevance to GALT-resident T cells, the adjuvant nature of commensal flora has been reported to drive phenotypic conversion of T cells and to divert them away from regulatory lineages36. Whether CD4+CD25+Foxp3low lymphocytes retain suppressive function in vivo is unknown, since Foxp3-GFP or –RFP reporter mice do not exist on the SAMP background; however, future backcrossing of these Foxp3 reporter mice onto SAMP may address this question. Given the importance of bacterial flora to mucosal immune outcomes, it is also intriguing that there appears to be gender-associated differences in the composition of the intestinal microbiome itself, such that a predominance of different species may alter innate or adaptive immune cell function37.
Homeostasis between regulatory and effector T cell populations is a key determinant of the in vivo balance between self-tolerance and autoimmunity. Using an in vivo adoptive transfer model, previous studies have shown that GALT-derived Tregs from SAMP are functionally impaired17. Our current work builds upon that observation to show that CD4+CD25+ Tregs isolated from SAMP-F show increased functional impairment, both in vivo and in vitro, compared to Tregs from SAMP-M (Figure 3). This finding is in agreement with previous work showing phenotypic and functional defects in Tregs from female autoimmune patients38, as well as from female rodents in experimental models of autoimmunity39, 40. Of note, a strength of the CD45RBhigh adoptive transfer model is that it is designed to test the suppressive function of Treg cells on the same population of pathogenic Teff cells isolated from SAMP-M MLN. This eliminates potential variation in effector cell proliferation, and thus allows us to infer that differences in in vivo suppression are derived from inherent differences in Treg suppressive function, rather than differences in Teff proliferation.
Our CD45RBhigh adoptive transfer experiments also demonstrate that Tregs derived from SAMP-F are less effective than those from SAMP-M in downregulating proinflammatory cytokine production in the gut (Figure S3). Protein expression of both Th1- and Th2-associated cytokines was significantly higher in mice receiving co-transfer of SAMP-F Tregs, compared to those receiving SAMP-M Tregs. Interestingly, IL-17A and IL-22 expression were also dampened by co-transfer of SAMP-M, but not –F, Treg cells, suggesting that CD45RBlow GALT-derived Treg cells maintain the capacity to suppress Th17 responses in SAMP-M mice.
Given that female mice exhibit accelerated ileitis, we sought to determine whether estrogen signaling plays a mechanistic role in this process by examining the relative contribution of signaling through the two E2 receptor isoforms. Signaling through both ERα and ERβ is able to downregulate ileal inflammation in vivo when administered continuously for 6 weeks to GDX mice, beginning at 10 weeks of age (Figure 5C). However, isoform-specific effects on T cell differentiation and function suggest that the in vivo mechanism-of-action for estrogen is multi-factorial.
Both Tconv and Treg cells express ER isoforms ERα and ERβ (Figure 4A, B). However, our findings suggest that signaling through these receptors leads to distinct immune outcomes depending on the phenotype of the T cell. Specifically, signaling through ERβ, but not ERα, reinforces Treg phenotype and function by enhancing expression of Foxp3 in CD4+CD25+ Treg cells, as well as secretion of IL-10 and TGFβ by these cells (Figure 4D). Fully differentiated Treg cells do not similarly respond to ERα signaling (Figure 4D). Tconv cells, conversely, respond to both ERα and ERβ signaling by downregulation of proliferation in response to αCD3/αCD28 (Figure 6). Tconv also upregulate expression of Treg-associated markers, including Foxp3, Helios, PD1, CTLA4, and LAG3 in response to E2 or ER agonists (Figure 4C). E2 has been previously reported to upregulate PD1 on Treg, leading to enhanced suppression41; this is likely an important mechanism for E2’s immunosuppressive action. Collectively, our data suggests that E2 signals to both ER isoforms on naïve T cells, diverting them away from pathogenic effector cells and toward tolerogenic inducible Treg (iTreg) cells through induction of Treg-associated genes29, 41. Interestingly, whereas naïve T cells respond to E2 through both ER isoforms, mature Tregs respond preferentially through ERβ signaling. Functional outcomes of this signaling include enhanced expression of Foxp3, IL-10, and TGFβ (Figure 4D).
Moreover, and in agreement with previous work suggesting an immunosuppressive function for E242, 43, in vivo administration of DPN significantly increases the percentage of GALT-derived CD4+CD25+Foxp3+ Treg cells (Figure 5A, B). PPT signaling through ERα shows an inability to expand the mucosal Treg population, and in fact, Treg frequency is reduced in PPT-treated animals compared to those receiving E2 (Figure 5A).
Among its beneficial effects, signaling through ERβ has previously been shown to maintain intestinal barrier function44. Although SAMP mice display a permeability defect compared to AKR controls at an early age, we found no differences in permeability between SAMP-M and –F mice (Supplemental Figure S6A), suggesting that the SAMP permeability defect does not influence gender-related pathologies, including accelerated disease course or changes to Treg function. We also examined intestinal permeability following in vivo E2/PPT/DPN administration to GDX-SAMP mice, and found that none of the E2 ligands resulted in a loss of permeability (Supplemental Figure S6B). In fact, treatment with ERβ-specific DPN resulted in a modest yet statistically significant increase in permeability, suggesting that the beneficial effects of ERβ signaling are likely due to direct effects on immune cells rather than to changes in permeability. Importantly, our results do not address the potential for ERβ to confer protection against permeability defects and/or gender bias in the SAMP model if administered prior to the onset of ileitis; this is an important area for future study, particularly in light of evidence that ERβ stimulation can promote proper intestinal barrier function44.
The present study describes possible mechanisms that contribute to gender disparity characteristic of both SAMP ileitis and patients with CD. Our data suggest that estrogen signaling may confer immunoprotection through both ERα and ERβ, operating in tandem mechanisms on conventional as well as regulatory T cells. Ultimately, E2 signaling in both isoforms enhances tolerance, via upregulation of Treg suppressive function, as well as induction of iTregs from Tconv precursors. These combined mechanisms confer some level of immunoprotection for SAMP-M through expansion of mucosal Tregs, whereas SAMP-F T cells are resistant to E2’s beneficial effects. These findings, combined with the observation that SAMP-M specifically undergo ERβ-mediated expansion of Tregs in vivo and IL-10/TGFβ production in vitro, support the concept that GALT-derived Tregs from SAMP-M are more functionally effective than SAMP-F at ameliorating chronic ileitis. Collectively, our data suggest that the development of therapeutics selectively designed to enhance immunoprotective ER signaling on T cells may hold promise in the treatment of chronic inflammatory disorders such as CD.
MATERIALS AND METHODS
Mice
SAMP, AKR/J, and TNFΔARE/+ mice were propagated at the University of Virginia (UVA) and Case Western Reserve University (CWRU), with SAMP founders provided by S. Matsumoto (Yakult Central Institute for Microbiological Research, Tokyo, Japan)45 and TNFTARE/+ founders provided by G. Kollias (Biomedical Sciences Research Center “Alexander Fleming,” Vari, Greece)27. Mice were bred and maintained under SPF conditions, fed standard laboratory chow (Harlan Teklad, Indianapolis, IN), and kept on a 12h light/dark cycle. C3Smn.CB17-Prkdcscid/J (SCID) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All procedures were approved by the IACUC at UVA and CWRU.
Histologic evaluation of intestinal inflammation
Distal 10 cm of ilea or entire length of colon from experimental mice were removed, flushed of fecal contents, opened longitudinally, and placed in Bouin’s fixative. Tissues were embedded in paraffin, cut to 3 µm, and stained with H&E. Disease severity was evaluated by a trained pathologist (James R. Mize) in a blinded fashion using established histologic scoring systems for ileitis and colitis46. Images were obtained on an Axiophot microscope, captured on an Axiocam and assembled using Axiovision Release 4.5 (Carl Zeiss, Inc., Thornwood, NY).
Flow cytometry
Cell suspensions were prepared from MLNs and spleens as previously described46. One million (106) cells/sample were stained with the following antibody combinations: Alexa-488-, PE-, and APC-labeled rat anti–mouse CD4 (RM4-5), CD25 (PC61) (BD), and Foxp3 (140D) (Biolegend, San Diego, CA). For intracellular staining, cells were fixed after cell surface staining, followed by permeabilization and staining for Foxp3 according to manufacturer’s instructions (Biolegend). Samples were analyzed with a FACSCalibur flow cytometer and CellQuest Software (both Becton Dickinson, San Jose, CA).
Colitis adoptive transfer model
MLN CD4+ cells were negatively selected by magnetic beads (Miltenyi Biotec, Auburn, CA) and stained with anti-CD4 and anti-CD45RB antibodies (BD PharMingen, San Diego, CA). FACS-sorted CD4+CD45RBhi (3–5×105) and CD4+CD45RBlow (105) cells were transferred i.p. into 6-wk-old MHC-matched SCID mice. CD45RBhi and CD45RBlow were defined as the top- and bottom- 30% of CD45RB-expressing cells within the CD4+ lymphocyte gate. 8 wks post-transfer, mice were sacrificed and colons processed for histologic evaluation26. MLN cells were collected for in vitro stimulation assays and cytokine production as described above.
In vitro T cell proliferation and suppression assays
FACS-purified CD4+CD25− Tconv and CD4+CD25+ Treg cells were isolated from 10-week old mice. Tconv were labeled with eFluor 670 (CFSE analog. 0.5 µM) and cultured with or without a 1:1 ratio of Treg cells. α-CD3 (1 µg/ml) and α-CD28 (1 µg/ml)-coated beads (Miltenyi Biotec) were added to cultures at 1:4 bead:Tconv ratio. Proliferation was determined after 3 days of culture by flow cytometric dye dilution assay.
Immunoblots
Western blots for ERα, ERβ, and Lamin-B1 were performed using nuclear extracts (Pierce NE-PER nuclear extraction kit, Thermo Scientific, Rockford, IL) of FACS-sorted Tconv and Treg cells. Lysates were immunoblotted as previously described47 using polyclonal Abs specific for ERα (Santa Cruz Biotechnology, Santa Cruz, CA), ERβ (Santa Cruz Biotechnology), and Lamin-B1 (Cell Signaling Technology, Danvers, MA), followed by goat anti-rabbit HRP (Santa Cruz Biotechnology). Chemiluminescent signals were developed using SuperSignal West Substrate (Thermo Scientific) and detected on Kodak BioMax MR film (Kodak, Rochester, NY). Densitometry was performed using a VersaDoc imaging system and QuantityOne software (both BioRad, Hercules, CA) comparing mean adjusted volume. Western blots for Foxp3 were performed using total cell lysates probed with primary Ab specific for Foxp3 (Santa Cruz Biotechnology, Inc.), followed by goat anti-mouse HRP (EMD Millipore, Billerica, MA).
RNA extraction and Quantitative PCR analysis
FACS-purified CD4+CD25− Tconv from 10-wk-old mice were treated ex vivo with vehicle (Ctrl), E2, PPT, or DPN (all Tocris Bioscience, Ellisville, MO, used at 10nM) for 2 hrs. Total RNA was isolated from cultured cells using the High Pure RNA Isolation Kit (Roche Applied Science, Indianapolis, IN) as described by the manufacturer. Total RNA (1 µg) was reverse transcribed using reverse transcriptase. The resulting cDNA was diluted to 100 µl and used in subsequent real time PCR reactions. Gene expression was assessed using the Taqman system from Applied Biosystems (Life Technologies, Carlsbad, CA).
In vitro cell stimulation assays
Cell suspensions were prepared from MLNs and spleens as previously described46. MACS-enriched CD4+CD25+ T cells (CD4+CD25+ T regulatory T cell isolation kit; Miltenyi Biotec), were cultured in 96-well plates coated with α–mouse CD3 (1 µg/ml, clone 145-2C11) and soluble α-CD28 (0.5 µg/ml, clone 37.51) (both BD Bioscience). PPT and DPN were added at 10 nM (Tocris Bioscience).
Hormone/ER agonist treatment and estrogen detection
Hormones (Sigma, St. Louis, MO) were continuously delivered to 10-wk-old GDX SAMP for 6 wks by s.c. placement of E2-filled hormone implants (1 cm length) prepared in Silastic® tubing (5 mm, 1.02 mm ID × 2.16 mm OD) as described previously48; empty implant (blank) served as Ctrl. PPT, DPN (both 10 mg/kg, i.p.), E2 (10 µg/kg, i.p.) or vehicle Ctrl (DMSO:PBS, vol/vol) was delivered by daily 200 µl injections for 6 wks. Serum estrogen levels were detected using an estradiol estimation kit (Alpco Diagnostics, Salem, NH).
Cytokine protein measurements
IL-6, IL-12, TNFα, IFNγ, IL-2, and IL-4 were measured from activated MLN cell supernatants by cytokine array (protein~Q-Plex™ Mouse Cytokine-Screen IR 16-Plex kit, Quansys Biosciences, Logan, UT). IL-10, TGFβ, IL-17A, and IL-22 were detected by ELISA (eBioscience) according to manufacturer’s instructions. Colon samples from adoptive transfer experiments were processed and assayed for MPO activity as previously described49.
Permeability assays
Intestinal epithelial permeability to solutes was measured using urinary FE ratio of lactulose to mannitol (Lac/Man excretion ratio), as previously described50.
Statistical analysis
Two-tailed Student’s t-test with Welch’s correction and Bonferroni’s test were performed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). P-values ≤0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank R. Michael Sramkoski, Muhammadreza Sachedina, Michael Whitecar, Mary Riggins, Jonathan “Adam” Alford, Jason D’Antuono, William Ross, Sharon Hoang, and James “Rusty” Mize, and for their technical support and expertise. We also acknowledge continued support from the National Institutes of Health: AI102269, DK091222, and DK056762 (TTP); DK083251 NRSA T32 (WAG); and a Research Fellowship Award from the Crohn’s & Colitis Foundation of America (RRG).
Abbreviations used in this paper
- Chr
chromosome
- CD
Crohn’s disease
- DPN
diarylpropionitrile
- E2
estrogen/17-β-estradiol
- ER
estrogen receptor
- F
female
- GALT
gut-associated lymphoid tissue
- GDX
gonadectomized
- IBD
inflammatory bowel disease
- iTreg
inducible regulatory T cell
- M
male
- MLN
mesenteric lymph node
- MPO
myeloperoxidase
- nTregs
naturally occurring regulatory T cells
- PPT
propyl pyrazoletriol
- SAMP
SAMP1/YitFc
- SCID
severe combined immune deficiency
- Tconv
conventional T cell
- Teff
effector T cell
- Treg
regulatory T cell
- UC
ulcerative colitis
Footnotes
The authors have no conflicting financial interests.
REFERENCES
- 1.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clinical immunology and immunopathology. 1997;84(3):223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 2.Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283(5406):1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 3.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2(9):777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 4.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(Suppl 1):S3–S9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery SM, Wakefield AJ, Ekbom A. Sex-specific risks for pediatric onset among patients with Crohn's disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2003;1(4):303–309. [PubMed] [Google Scholar]
- 6.Saibeni S, Cortinovis I, Beretta L, Tatarella M, Ferraris L, Rondonotti E, et al. Gender and disease activity influence health-related quality of life in inflammatory bowel diseases. Hepatogastroenterology. 2005;52(62):509–515. [PubMed] [Google Scholar]
- 7.Mogadam M, Korelitz BI, Ahmed SW, Dobbins WO, 3rd, Baiocco PJ. The course of inflammatory bowel disease during pregnancy and postpartum. Am J Gastroenterol. 1981;75(4):265–269. [PubMed] [Google Scholar]
- 8.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nature reviews Immunology. 2008;8(9):737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saruta M, Targan SR, Mei L, Ippoliti AF, Taylor KD, Rotter JI. High-frequency haplotypes in the X chromosome locus TLR8 are associated with both CD and UC in females. Inflamm Bowel Dis. 2009;15(3):321–327. doi: 10.1002/ibd.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen DD, Wurbel MA, Goettel JA, Eston MA, Ahmed OS, Marin R, et al. Wiskott-Aldrich syndrome protein deficiency in innate immune cells leads to mucosal immune dysregulation and colitis in mice. Gastroenterology. 2012;143(3):719–729. e711–e712. doi: 10.1053/j.gastro.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan GS, Brenner D, Tusche MW, Brustle A, Knobbe CB, Elia AJ, et al. 2-Methoxyestradiol inhibits experimental autoimmune encephalomyelitis through suppression of immune cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(51):21034–21039. doi: 10.1073/pnas.1215558110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu WF, Tan XJ, Dai YB, Krishnan V, Warner M, Gustafsson JA. Targeting estrogen receptor beta in microglia and T cells to treat experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(9):3543–3548. doi: 10.1073/pnas.1300313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harnish DC, Albert LM, Leathurby Y, Eckert AM, Ciarletta A, Kasaian M, et al. Beneficial effects of estrogen treatment in the HLA-B27 transgenic rat model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2004;286(1):G118–G125. doi: 10.1152/ajpgi.00024.2003. [DOI] [PubMed] [Google Scholar]
- 14.Verdu EF, Deng Y, Bercik P, Collins SM. Modulatory effects of estrogen in two murine models of experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2002;283(1):G27–G36. doi: 10.1152/ajpgi.00460.2001. [DOI] [PubMed] [Google Scholar]
- 15.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19(4):833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clinical reviews in allergy & immunology. 2011;40(1):66–73. doi: 10.1007/s12016-010-8203-5. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa D, Okazawa A, Corridoni D, Jia LG, Wang XM, Guanzon M, et al. Tregs are dysfunctional in vivo in a spontaneous murine model of Crohn's disease. Mucosal immunology. 2013;6(2):267–275. doi: 10.1038/mi.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunological reviews. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 19.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, Levings MK. The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology. 2008;125(2):145–153. doi: 10.1111/j.1365-2567.2008.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annual review of immunology. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 22.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128(7):1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5(11):1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 24.Nagler-Anderson C, Bhan AK, Podolsky DK, Terhorst C. Control freaks: immune regulatory cells. Nat Immunol. 2004;5(2):119–122. doi: 10.1038/ni0204-119. [DOI] [PubMed] [Google Scholar]
- 25.Pizarro TT, Pastorelli L, Bamias G, Garg RR, Reuter BK, Mercado JR, et al. SAMP1/YitFc mouse strain: a spontaneous model of Crohn's disease-like ileitis. Inflamm Bowel Dis. 2011;17(12):2566–2584. doi: 10.1002/ibd.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson TS, Bamias G, Naganuma M, Rivera-Nieves J, Burcin TL, Ross W, et al. Expanded B cell population blocks regulatory T cells and exacerbates ileitis in a murine model of Crohn disease. J Clin Invest. 2004;114(3):389–398. doi: 10.1172/JCI20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10(3):387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 28.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, et al. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214(2):456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 30.Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001;61(2):632–640. [PubMed] [Google Scholar]
- 31.Kudwa AE, Harada N, Honda SI, Rissman EF. Effects of organisational oestradiol on adult immunoreactive oestrogen receptors (alpha and beta) in the male mouse brain. J Neuroendocrinol. 2007;19(10):767–772. doi: 10.1111/j.1365-2826.2007.01585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, et al. A role for sex chromosome complement in the female bias in autoimmune disease. J Exp Med. 2008;205(5):1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozaiwa K, Sugawara K, Smith MF, Jr, Carl V, Yamschikov V, Belyea B, et al. Identification of a quantitative trait locus for ileitis in a spontaneous mouse model of Crohn's disease: SAMP1/YitFc. Gastroenterology. 2003;125(2):477–490. doi: 10.1016/s0016-5085(03)00876-x. [DOI] [PubMed] [Google Scholar]
- 34.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 35.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445(7129):766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 36.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29(4):637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 38.Barreto M, Ferreira RC, Lourenco L, Moraes-Fontes MF, Santos E, Alves M, et al. Low frequency of CD4+CD25+ Treg in SLE patients: a heritable trait associated with CTLA4 and TGFbeta gene variants. BMC Immunol. 2009;10:5. doi: 10.1186/1471-2172-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lapierre P, Beland K, Martin C, Alvarez F, Jr, Alvarez F. Forkhead box p3+ regulatory T cell underlies male resistance to experimental type 2 autoimmune hepatitis. Hepatology. 2010;51(5):1789–1798. doi: 10.1002/hep.23536. [DOI] [PubMed] [Google Scholar]
- 40.Pop SM, Wong CP, Culton DA, Clarke SH, Tisch R. Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med. 2005;201(8):1333–1346. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1) Int Immunol. 2007;19(3):337–343. doi: 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- 42.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173(4):2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 43.Prieto GA, Rosenstein Y. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology. 2006;118(1):58–65. doi: 10.1111/j.1365-2567.2006.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Looijer-van Langen M, Hotte N, Dieleman LA, Albert E, Mulder C, Madsen KL. Estrogen receptor-beta signaling modulates epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2011;300(4):G621–G626. doi: 10.1152/ajpgi.00274.2010. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto S, Okabe Y, Setoyama H, Takayama K, Ohtsuka J, Funahashi H, et al. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43(1):71–78. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bamias G, Okazawa A, Rivera-Nieves J, Arseneau KO, De La Rue SA, Pizarro TT, et al. Commensal bacteria exacerbate intestinal inflammation but are not essential for the development of murine ileitis. J Immunol. 2007;178(3):1809–1818. doi: 10.4049/jimmunol.178.3.1809. [DOI] [PubMed] [Google Scholar]
- 47.Goodman WA, Young AB, McCormick TS, Cooper KD, Levine AD. Stat3 phosphorylation mediates resistance of primary human T cells to regulatory T cell suppression. J Immunol. 2011;186(6):3336–3345. doi: 10.4049/jimmunol.1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bodo C, Rissman EF. The androgen receptor is selectively involved in organization of sexually dimorphic social behaviors in mice. Endocrinology. 2008;149(8):4142–4150. doi: 10.1210/en.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boughton-Smith NK, Wallace JL, Whittle BJ. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Actions. 1988;25(1–2):115–123. doi: 10.1007/BF01969102. [DOI] [PubMed] [Google Scholar]
- 50.Olson TS, Reuter BK, Scott KG, Morris MA, Wang XM, Hancock LN, et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203(3):541–552. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.