Abstract
In this paper we use the CAP principles to consider the impact of common clinical problems on action. We focus on three major syndromes: paresis, apraxia and ataxia. We also review mechanisms that could account for spontaneous recovery, using what is known about the best studied clinical dysfunction, paresis, and also ataxia. Together, this and the previous paper lay the groundwork for the third paper in this series, which reviews the relevant rehabilitative interventions.
I. Paresis
Phenomenology
The most common motor disorder experienced by individuals after central nervous system damage is paresis. In the strictest sense, paresis is the reduced ability to voluntarily activate the spinal motor neurons. Total paresis is called plegia, reflecting a complete inability to voluntarily activate the motor neurons. In the human experience however, paresis is more appropriately viewed as a syndrome, i.e. a collection of impairments that co-exist in most patients.1 The impairments that typically make up the paretic syndrome are weakness, spasticity, a decreased ability to fractionate movement, and an often subtle, higher-order planning deficit. Paresis can result from a wide range of neurological diseases and conditions such as stroke, multiple sclerosis, cerebral palsy, amyotrophic lateral sclerosis, traumatic brain injury, and spinal cord injury. The disease or condition may define the distribution of the paresis, such as hemiparesis seen after stroke and paraparesis seen after spinal cord injury.2
When a person with paresis reaches out to grasp a cup or pick up a key, the resulting movement differs from that performed by a person with an intact nervous system. Paretic movements are slower and less accurate and not as efficient as normal movements. A person with paresis often makes multiple attempts to position the hand near the desired object and to open the fingers wide enough to grasp it. Particularly when grasping a small object such as a key, the fingers may touch and retouch the object multiple times to accomplish a successful precision grasp. Forces produced by the fingers to lift an object are not as well coordinated in people with paresis compared to neurologically intact individuals. Fingertip forces can be poorly timed and of inappropriate magnitude and direction such that, even if successfully lifted off the table, a cup may tilt as it is raised or a key may fall from the fingers. Once the object is in hand, a person with paresis has difficulty moving it to some locations. Lifting the cup to the mouth, where the arm movement is close to and directly in front of the body, is usually much easier than lifting the cup to a shoulder-height shelf on the opposite side of the body. Reaching movements where the hand moves to locations further away from the body are often accomplished via compensatory trunk movements, rather than the normal rotations at the shoulder and elbow. Because of the difficulty controlling the reaching movement, the hand may not be appropriately oriented to grasp or to release an object. Letting go of a grasped object is often as difficult as grasping an object for people with paresis. It can take multiple attempts and extra time to open the hand and leave the object on the table. Lastly, increased efforts to move, especially in those individuals with more severe paresis, often results in associated movements of other body parts. For example, when a person with stroke tries to grasp the key with the paretic hand, the ipsilesional, non-paretic hand may also extend at the wrist and flex at the fingers, or the patient may even extend the knee. As will be discussed later, this reflects activation of the hemisphere opposite the lesion when the weak limb tries to move.
Mechanisms of paresis
Paresis can be largely considered a problem of movement execution. The primary mechanism underlying paresis is damage to the corticospinal system, i.e., cortical motor areas and the corticospinal tract that connects the cerebral cortex to the spinal cord. Figure 1 illustrates how the disruption of corticospinal system input alters the activation of motor units, the activation of muscles and sets of muscles, and the ability to move. Paresis reflects a problem in transferring the motor commands from cortex to spinal cord (red line from motor cortex to hand). Together, these changes in the ability to volitionally activate motor units, muscles, and sets of muscles can explain much of the impairments of weakness and reduced fractionation of movement. Spasticity is largely a result of loss of supraspinal inhibition to the spinal cord, causing the response to afferent input (e.g. input from muscle spindles or cutaneous receptors) to be abnormally large.
Figure 1.
Effects of damage to the corticospinal system on movement generation and feed-forward computations. A. In the intact system, the motor command for elbow flexion, for example, computed in the motor areas is sent to the spinal cord motorneurons (MN) and interneurons (red line). The command results in agonist contraction (biceps), antagonist relaxation (triceps), and the generation of force. A copy of the motor command is input to an internal model of limb position computed centrally (for details, see Frey et al., this volume). B. After damage to the corticospinal system, the generation of movement is compromised both by reduced descending commands to the spinal cord (dotted red line) and by interference with feed-forward computations of the internal model. Some descending effects illustrated here are: reduced spinal MN (and muscle motor units) activation, decreased agonist drive, increased co-activation of antagonist muscles, and altered sensory input due to loss of supraspinal inhibition of sensory afferents. As a consequence, activation and termination of muscle activity are delayed, force production and its rate are decreased, and muscle activation is less selective. Movements are slower and less accurate, leading to repeated attempts or compensatory strategies and, overall, less functionality. Centrally, compensatory increase in movement plans (or effort), and irregular sensory feedback and proprioception may lead to the formation of an abnormal internal model and erroneous feed-forward computations.
It is important to note that higher-order planning deficits can be superimposed on impairments of motor execution. Excessive activation of the motor cortex to try to overcome the interruption of motor commands can lead to abnormalities of the motor command (efference) copy and thus of the forward model, causing inappropriate estimation of the movement parameters and joint positions needed to efficiently execute the movement (refer to Figure 1). These include difficulties with anticipating the correct force with which to grip an object or anticipating the consequences of a selected movement.
A clinical example of an abnormal forward model is the phenomenon of past-pointing after oculomotor palsy. Patients with a partial sixth nerve palsy and weakness of abduction on lateral gaze tend to overshoot a target when pointing to it with the hand while looking. The explanation is that excessive activation of oculomotor plans to overcome the partial paralysis of eye muscles ‘spills over’ to motor commands for the hand that incorrectly predict the new hand position despite normal visual information. Paresis and difficulty with fractionation can be also made worse by concurrent deficits in the perception of limb position (proprioception) or tactile properties of objects (touch, surface texture, etc) or by pain. Abnormal sensory feedback (blue line from hand to sensory cortex, and position feedback (Figure 1) can worsen an already abnormal forward model.
Examination of the paretic patient
Items from the normal clinical exam are used to determine if the patient has a paretic syndrome and to rule out other disorders of movement. Weakness is one of the more salient impairments of paresis and is the one most easily tested. Standard muscle strength testing using the Medical Research Council rating scale (0 – 5) allows clinicians to determine the severity and distribution of the weakness. If patients are unable to maintain a position against gravity (< 3 on the scale), then an alternate means to quantify weakness is to measure the active range of motion (AROM) at that joint. AROM and strength can be considered indirect measures of the ability to volitionally activate the spinal motor neuron pools. AROM measures may be better able to capture deficits at the lower end of spectrum, i.e. whether the muscles can be activated enough to move the joint through the range. Strength measures may be better able to capture deficits at the higher end of the spectrum, i.e. whether the muscles can be activated sufficiently to produce force against externally imposed loads.
Spasticity is traditionally defined as a velocity-dependent resistance to movement. Spasticity can be assessed by passively moving the affected limb through its available range of motion and then by varying the speed at which it is moved. Spasticity is present if there is increasing resistance as the limb is moved faster. The resistance is often stronger in one direction than the other (e.g. greater during passive elbow extension versus flexion), typically being most pronounced in anti-gravity muscles. Spasticity is differentiated from rigidity: the latter is not velocity-dependent (resistance is the same regardless of the speed of passive movement) and is less likely to be directionally dependent (feels the same during flexion and extension). Unlike spasticity, rigidity is believed to stem from altered basal ganglia function (for review of rigidity see Dewailde 2001 or Hallet 2003).3,4 The underlying neuropathology affects the severity and pattern of spasticity. For example, people with spinal cord injury often experience greater levels of spasticity than people with stroke. Following stroke, the severity of the spasticity matches reasonably well to the severity of weakness. Spasticity may contribute to problems such as being unable to release a grasped object.
Fractionation of movement is a critical part of our ability to use our upper extremities for so many different movements. Along with spasticity and weakness, there is frequently a reduced ability to fractionate movement in people with paresis. The ability to fractionate movement can be assessed by asking the patient to move one segment in isolation and keep other, adjacent segments still. Assessment of fractionation is most common at the fingers, where patients are asked to touch the tip of the thumb to the tip of each of the other fingertips. Loss of fractionated movement also occurs at more proximal segments such as when asking a patient to flex the shoulder alone. The reduced fractionating can be seen as the patient flexes and abducts the shoulder while simultaneously flexing the elbow and wrist, and pronating the forearm. This reduction in fractionated movement, particularly in patients with stroke, is the same concept as the abnormal movement synergies originally observed by Twitchell and categorized by Brunnstrom.1,5 Like spasticity, the degree of loss in movement fractionation is related to the degree of weakness. Patients with more severe paresis and spasticity typically have less ability to fractionate movement, while those with milder paresis and minimal spasticity can make well-fractionated movements. (For experimental data on weakness, spasticity, and fractionation, and their relationship to hand function post-stroke, see Lang & Beebe, 2007).6
Higher-order planning deficits (including those attributable to the apraxia syndrome) can be superimposed on the impairments discussed above. They include difficulties with anticipating the correct force with which to grip an object or anticipating the consequences of a selected movement. Whether or not higher-order planning deficits are present in an individual patient is difficult to assess early after neurological injury, as the deficit can be masked by weakness and loss of fractionation. Additionally, one should always test for deficits in limb position or abnormal tactile discrimination that can amplify difficulty with planning more complex movements. For patients with severe paresis, the presence or absence of higher-order planning deficits may be harder to detect because of the diminished ability to execute volitional action. For those with milder paresis, planning deficits are usually identified by patients’ reports of difficulties with challenging activities requiring high levels of dexterity (e.g. skilled tool use, typing) as they return to jobs and other daily activities. Specific testing of the higher-order deficits may best be done within the evaluation for apraxia (see section below).
Lastly, it is important to evaluate secondary impairments that may arise from paresis. The most common secondary impairments in the upper extremity are contracture and atrophy. The presence and severity of secondary impairments may affect the process of selecting the most appropriate treatment for an individual patient and the anticipated prognosis.
Quantitative Measurement
Quantitative measures7 of motor impairment, motor function, and motor disability seen with paresis are provided in Table 1. For the upper extremity function measures, data from people with stroke indicates that the measures are strongly related to one another. Quantitative scales are important to initially measure impairment, function, and disability; they should be repeated regularly (e.g. monthly) for clinical decisions to continue, switch, or interrupt a treatment, ideally by personnel not involved in the patients’ rehabilitation (to avoid bias). Scales with continuous variables (e.g. active range of motion in degrees) are preferable to interval scales (e.g. MRC scale for motor strength).
Table 1.
Quantitative measures for the assessment of paresis, its associated impairments, and upper extremity function
| TESTS | ADDITIONAL INFORMATION | |
|---|---|---|
| Weakness | Muscle strength testing | Clinical muscle testing provides an easy to follow measure of strength. Assessment by dynamometers (in unit of weight) are more sensitive, but also more variable. Force was thought to be an important function of motor cortex, but more recent studies indicate the measures of dexterity or speed may be more functionally meaningful. |
| Active range of motion | Quantitative assessment of range of motion (in degrees). It has been shown to be associated with motor outcome. Testing two joints (one proxiumal e.g. shoulder flexion and one distal e.g. finger extension) captures around 75% variance in upper extremity motor function at 3 months post stroke | |
| Motricity Index | This is a standardized scale that combines muscle strength scores (above) from 3 movements from each limb. For the upper extremity, the 3 movements are shoulder abduction, elbow flexion, and pinch force on a cube. For the lower extremity, the 3 movements are hip flexion, knee extension, and ankle dorsiflexion. The total score (0- 100, 100 = normal) provides a single value that quantifies paresis in a way that is easy to understand by patients and families. | |
| Spasticity | Modified Ashworth Scale | Interval assessment of spasticity. Spasticity is thought to limit function but in the overwhelming majority of subjects treatment of spasticity does not improve function. Conversely, improvement of voluntary control of movement also decreases spasticity. Treatment of spasticity should not precede or limit training of voluntary movements. |
| Fractionation | Fugl-Meyer Scale | This scale is commonly used in research studies to measure global motor impairment in each limb. Items are rated on quality of movement. Compared to the motricity index, this instrument takes longer to administer and has more variability in scoring. |
| Higher order planning deficits | Naturalistic Action test ADL-observation test Florida Apraxia Battery |
Clinical scales for apraxia. Unknown their long-term sensitivity in measuring motor outcome. |
| Upper extremity function | Action Research Arm Test | Popular criterion scale of motor function that has strong psychometric properties and is used widely around the world. Test kits can be built from published references for low cost. Quick to administer, particularly for very low-functioning and very high-functioning patients |
| Wolf Motor Function Test | Popular timed and criterion-rated scale of motor function developed for CIMT research studies. Measures same construct of upper extremity function as the test above, takes slightly longer to administer. | |
| 9 Hole Peg Test | Timed test to specifically assess finger dexterity. Published age- and gender-specific norms are available for comparison. Less appropriate for lower functioning patients. Factor analyses indicates it measures the same construct of upper extremity function as the tests above | |
| Box & Block Test | Timed test to grasp and release 1 inch cube blocks. Used for a variety of patient populations to assess upper extremity function. Less common now than in earlier decades. | |
| Stroke impact scale Hand function and activity subscales |
Self- report measure of impairment, function, and disability after stroke. Highly valuable for its ability to assess stroke-specific problems and outcomes. Can be done in waiting rooms as a traditional questionnaire, via interview, telephone, mail, and/or by caregivers. Hand Function subscale scores are correlated to above measures of upper extremity function. |
II. Apraxia
Phenomenology
Apraxia is a common clinical disorder affecting complex, skilled movements that may result from stroke, traumatic brain injury or degenerative dementias including Alzheimer’s disease and corticobasal ganglionic degeneration (CBD). It is particularly common after dominant (usually left) hemisphere stroke, and can be observed in both contralesional and ipsilesional limbs. There are several major apraxia syndromes, all of which frequently co-occur with the components of the paretic syndrome: weakness, spasticity, and impaired fractionation (see above sections), rendering diagnosis difficult, particularly in the contralesional limb.
When a person with apraxia attempts to use a key (even with the ipsilesional hand), there may be inaccuracy in the direction, amplitude and timing of movement and/or posture of the arm and hand. For example, rather than exhibiting a clear turning movement at the wrist, the patient may attempt to slide the wrist to the side, or may attempt to turn at the shoulder rather than wrist joint. Alternatively (and less frequently) the patient may attempt to use the key as if it were another object.
Early anecdotal observations suggested that apraxia is evoked only in the context of specialized, abstract tests, with little functional impact. It is now widely recognized that, to the contrary, apraxia is a major predictor of poor functional performance on everyday tasks and of increased dependency on caregivers. However, for the purpose of diagnosis, the disorder is most clearly evoked on tests of gesture to the sight of objects and on so-called “multiple objects tests”. We will describe apraxic patterns of performance on these tests next.
Subtypes and Mechanisms of Apraxia
The taxonomy of apraxia subtypes is confused by a number of different conventions in labeling and diagnosis. Recent investigations indicate that there are reliable differences in two major subtypes of apraxia.
Ideational/conceptual apraxia
Ideational/conceptual apraxia8 refers to the impaired ability to carry out multiple-step actions with objects, such as making a sandwich or lighting a candle. Patients with ideational/conceptual apraxia may substitute inappropriate actions, mis-sequence actions, or omit action steps. For example, an ideational apraxic might spread butter on bread before placing it in the toaster, or use a spoon to cut food in the close proximity of a visible knife.
Ideational/conceptual apraxia may be conceptualized as an inability to select or sequence the appropriate motor programs for completing a temporally-extended sequence of meaningful actions. This problem can be exacerbated by problems of executive control, loss of knowledge about objects (object semantics), and impairments in arousal and attention.
Ideational apraxia is most frequently induced by large strokes, moderate-to-severe traumatic brain injury, or degenerative dementia. It tends to diminish over time after stroke or brain injury. Although ideational apraxia was previously associated with frontal-subcortical lesions, recent investigations indicate that large lesions at many brain loci may result in the disorder.8
Ideomotor apraxia
Ideomotor apraxia (IMA)9 refers to the impaired ability to plan and recognize complex motor actions especially when they rely on stored (semantic) knowledge.10 Patients with IMA can have trouble in carrying out skilled, object-related motor actions with either hand, and may also be deficient with actions that convey a symbolic meaning, like saluting. Recent investigations suggest that IMA reflects damage at two major levels (see below). The complexity of the underlying mechanisms is a major cause of the confusion that has characterized the literature in this area for many years.9
One computational deficit in IMA is reduced ability to program movements using an “intrinsic” spatial coordinate frame, with relative preservation of coding in “extrinsic” spatial coordinates. An intrinsic spatial coding scheme is used to calculate the positions of body parts with respect to one another, possibly partly in terms of joint angles. For example, while reaching to a cup, calculation of the positions of the hand and fingers with respect to the shoulder is an intrinsic computation. This computation corresponds to the development of a forward model based on estimation of body state described in the accompanying paper by Frey et al. Extrinsic spatial coding is used to calculate the positions of the body and its parts with respect to the external world. For example, calculation of the positions of the hand and fingers with respect to a cup is an extrinsic computation. Clearly, most actions in the world require both types of calculation. However, pantomimed actions (see examination) are actions performed without any external referent, and this is thought to explain why pantomimes are so difficult for ideomotor apraxics.
The second major deficit in IMA is reduced activation of stored representations of skilled object-related actions to the sight of objects (i.e., with visual input) or to command (i.e., with auditory input). Instead, there is over-reliance upon somatosensory information—particularly information gleaned from object structure (shape, size, weight, and possibly texture). This helps to explain why many ideomotor apraxics perform better when actually holding objects rather than mimicking their function (see examination).
Unlike ideational apraxia, IMA is a syndrome with relatively clear brain localization, most frequently due to left inferior parietal and left premotor cortical damage following middle cerebral artery stroke, Alzheimer’s disease, or CBD. Frequently, both ideational and ideomotor apraxia may occur in the same patient; however, these subtypes also dissociate.
Examination of the apraxic patient
Ideational/conceptual apraxia is diagnosed on the basis of action errors in everyday activities, including omissions of key steps (e.g., wrapping a gift while the wrapping paper is still on the roll), substitutions of erroneous objects (use of juice rather than milk in cereal), reversal of steps (spreading butter and jelly, then toasting bread), and other more flagrant errors (using a yellow marking pen to color bread rather than spreading yellow mustard on bread). Few formal tests for ideational apraxia have been developed. One is the Naturalistic Action Test, which assesses the ability to perform a number of everyday tasks such as packing a lunchbox and making toast while ignoring distractor objects.10 Errors of a number of types are quantified, and performance compared to normative scores. The Test for Upper Limb Apraxia (TULIA) is a brief bedside examination with good reliability.11
Testing for IMA frequently includes gesture to command or imitation of the examiner for transitive (object-related) movements such as hammering, cutting, brushing teeth and the like, and intransitive (symbolic) movements such as waving goodbye or signaling stop. Also useful is asking the patient to gesture in response to seeing and holding actual tools. The Florida Apraxia Battery assesses gesture production in a number of different conditions and provides standardized guidelines for scoring.12 Kinematic analyses have revealed that IMA patients pantomime skilled tool-use movements with abnormal joint angles and limb trajectories, and uncoupling of the spatial and temporal aspects of movement. Spatiotemporal errors persist to a lesser degree with actual tool use.
III. Ataxia
Phenomenology
Ataxia results from damage to the cerebellum, its input and output pathways in the brainstem, the spinocerebellar tracts or posterior columns in the spinal cord, or large fibers in peripheral sensory nerves. For the sake of brevity, the term “cerebellum” will be used to include the cerebellum and its brainstem connections. The term ataxia is sometimes used in a specific sense to refer to impaired spatial and temporal coordination of movements or sometimes more generally as a catch-all term for poor coordination, inaccurate and variable movements, dysmetria and intention tremor.
A patient with ataxia may present with balance and gait problems, depending on what component of the input/output pathways to/from the cerebellum are compromised. Patients are unable to maintain a standing and/or a sitting posture, and may show violent oscillations of the trunk, laterally, or anteriorly and posteriorly, as if trying to catch themselves. Balance and posture difficulties are typically exacerbated while standing up. Walking abnormalities are characterized by difficulties in coordinating a correct stepping sequence with one foot hitting the ground either early or late, typically with inaccurate force and placement. The result is a stepping gait in which the legs seem to advance chaotically forward while the patient’s trunk may be leaning in the wrong direction. In severe bilateral ataxia, patients cannot walk if not fully supported. There can be also problems in the use or coordination of the arms and hands. Patients may reach inaccurately and may be unable to grasp with precision because tremor interferes with a smooth action. Tremor typically increases when the hand is approaching the target. Speech can be also affected by tremor, which makes it sound scanning, in a very characteristic manner. Finally, cognitive deficits in verbal production, timing, and non-verbal decision-making have been also reported (see below).
Mechanisms of ataxia
Current theories lead to the idea that the cerebellum provides predictive state estimates that allow feed-forward coordination between agonist and antagonist muscles and between limb segments. When there is cerebellar damage, patients are forced to rely more on delayed sensory feedback and respond with reactive rather than predictive control. Some of the jerkiness and slowness in movements (ataxia) can represent this attempt at catch-up with feedback adjustments, but errors accumulate because of feedback delays. Prediction here does not seem to be based on some internal clock that anticipates the timing of events but instead on an internal simulation or model of the limb system that receives an efference copy of the command and can predict the consequence of that command on kinematic and dynamic variables (state variables); predicted states can then be sent to motor areas to send anticipatory commands for feed-forward control.13–15 For an illustration of this model, please refer to Figure 1 in the paper by Frey et al. This is illustrated in the model in Figure 1 of the paper by Frey et al.
If cerebellar patients have persistent inability to anticipate the consequences of their motor commands then it must mean that they are unable to learn the internal model (forward model in the CAP framework) of their limb needed to anticipate errors. This leads to a very interesting question – can cerebellar patients be rehabilitated if they can’t learn? We address this later in this consensus paper.
Examination of the ataxic patient
It is important that ataxia be distinguished from weakness, loss of fractionated movements, hyperreflexia or hypertonia, or higher-order motor abnormalities such as apraxia. Unfortunately this can be difficult when the cerebellum and non-cerebellar structures are damaged together. For example, in certain brainstem strokes, cerebellar connections and the corticospinal tract can both be affected. In addition, damage to the corticospinal tract can also lead to poor multi-joint coordination and inaccurate movements, leading to overlap in clinical signs and psychophysical findings. Nevertheless, careful examination of limb movements will usually reveal cerebellar involvement, or its absence, with a good deal of accuracy.
Hypermetria
From the Greek hypermetros, meaning “beyond measure”, hypermetria refers to the tendency for patients with cerebellar disease to fail to properly terminate fast movements and overshoot the target. Hypermetria is usually assessed in two ways. (1) Finger-nose-finger test: The patient first holds up the arm so that the elbow and shoulder are in more or less the same horizontal plane. The patient is instructed to make rapid out-and-back movements touching the forefinger to the examiner’s forefinger, and then back to his nose. Rotation of both shoulder and elbow joints is required here to make the pointing movement, because multi-joint movements accentuate cerebellar abnormalities. Speed is important because the errors are velocity-dependent and patients often slow down to compensate for their errors. Patients tend to overshoot the examiner’s finger and then attempt to correct the overshoot. The initial overshoot and subsequent wayward corrections are collectively called dysmetria. (2) Finger chase: here the patient holds the forefinger in front of and in near contact with the examiner’s forefinger. The examiner then moves his finger rapidly up, down and sideways with a pause between each movement, instructing the patient to track his finger with his own finger. The cerebellar patient will show marked overshoots with each movement. This test is more challenging and can elicit dysmetria in those with milder ataxia.
Failure of check
This test, like those above, unmasks the inability to properly decelerate the limb. (1) The patient is asked to flex the elbow against the examiner’s attempt to extend it. The examiner then lets go suddenly. The ataxic patient is unable to decelerate the upper limb and could hit himself in the face – so it is important for the examiner to use the other arm to prevent this. (2) The patient is asked to raise either one or both arms above the head and then bring them down fast with palms down, stopping abruptly when the arms subtend 90° around the shoulder joint, i.e, arms out in front. The ataxic patient is unable to stop abruptly and overshoots.
Excessive rebound
The patient is asked to hold the arms out in front, palms down. The examiner then quickly and gently displaces one arm downwards, looking for excessive upward displacement, termed rebound.
Dysdiadochokinesis
Patients are asked to rapidly tap one hand on the other, alternating between palm-up and palm-down. Patients with cerebellar disease are slower, are unable to maintain a steady rhythm, and show a variable contact point (slippage between palms). This test unmasks acceleration and deceleration abnormalities, as well as timing abnormalities (see later).
Tremor
The archetypal cerebellar tremor is an oscillation of 3–5Hz that tends to be accentuated at the endpoint of a movement, for example during the deceleration phase of the finger-nose-finger test.
Hypotonia
The traditional teaching is that hypotonia is a classic cerebellar sign. Transient hypotonia may be seen after large acute cerebellar lesions and some of the spinocerebellar ataxias. However, otherwise tone tends to be normal in cerebellar disease and this is therefore neither a sensitive nor specific finding. Hypotonia can be tested for by shaking the limb and noting excessive “floppiness” about the wrist.
Two tests mistakenly thought to test the cerebellum
Rapid alternating or sequential finger movements. Slowing may be due to cerebellar disease but is much more common after corticospinal tract damage and it is hard to distinguish between them. Thus this test should be avoided for determining cerebellar involvement.
Past-pointing. Here patients are asked to place the extended index finger on the examiner’s forefinger, then raise the arm above the head and then, with the eyes closed, bring the forefinger down (without bending the arm) onto the examiner’s finger. The abnormality that is sought is a systematic directional bias. This is not a particularly sensitive test because it can be compensated for and corrected after a few attempts. The important point is that this tests for vestibular imbalance due to unilateral vestibular disease, with a directional error towards the side of the vestibular lesion. This is not seen with cerebellar lesions.
IV. Recovery of Function, Its Neural Basis and Implications for Rehabilitation
Recovery from paresis
Predictors of recovery
After a central nervous system injury, recovery of paresis occurs along a fairly predictable time course. Figure 4 illustrates the time course of recovery from paresis at the impairment and at the functional level, as derived from epidemiological data after stroke. In general, most motor recovery occurs within the first 3 months, with stronger recovery occurring in the first 4–8 weeks and eventually reaching a plateau at around 12 weeks. Several large longitudinal studies show that initial severity is one of the best predictors of final impairment and function. For example, 50–80% of patients with mild motor deficits (MRC scale 4–5) can expect full recovery, while only 10–25% with severe deficits (MRC scale 1–2) can expect full recovery at 3 months Another way to express the same concept is that patients with mild paresis are 2–20 times more likely to recover than patients with severe paralysis. Another sensitive measure of chronic recovery at the acute stage is the presence/absence of motor evoked potentials (MEPs) measured by magnetically stimulating the affected hemisphere and recording muscle responses. Patients with MEPs present in the first few days are 20–100 times are more likely to recover than patients with absent MEPs. A third important concept is that patients with more mild deficits recover more quickly and completely than patients with more severe deficits. For the purpose of predicting recovery of individual patients, it is important to appreciate that this is the general pattern of recovery and that most, but not all, patients follow similar time courses. There are several consistent predictors of poor outcomes post-stroke that are useful to look for when evaluating individual patients. First, the more non-motor impairments (e.g. somatosensory loss or visual field loss) that accompany the motor deficits, the less likely a person is to return to functional independence. Second, earlier improvements in motor deficits indicate that a person is more likely to reach higher levels of independence. And, third, individuals with minimal grip strength and/or minimal active movement at the shoulder at 30 days post-injury are unlikely to regain functional use of the hand and arm. Recovery of function typically lags recovery of motor deficits by about one week, but the shapes of the two recovery curves are very similar. The reason for the lag and the similar shape may be because as the motor ability emerges, movement practice is required to capitalize on the motor recovery and incorporate it into daily function. These principles are important to decide whether and when to switch the emphasis of rehabilitation from restorative to compensatory techniques. For instance in the case of a patient with a non-functional hand at 3 months, the chance to regain function is very small even with prolonged restorative intervention, at least based on current methods.
Figure 4.
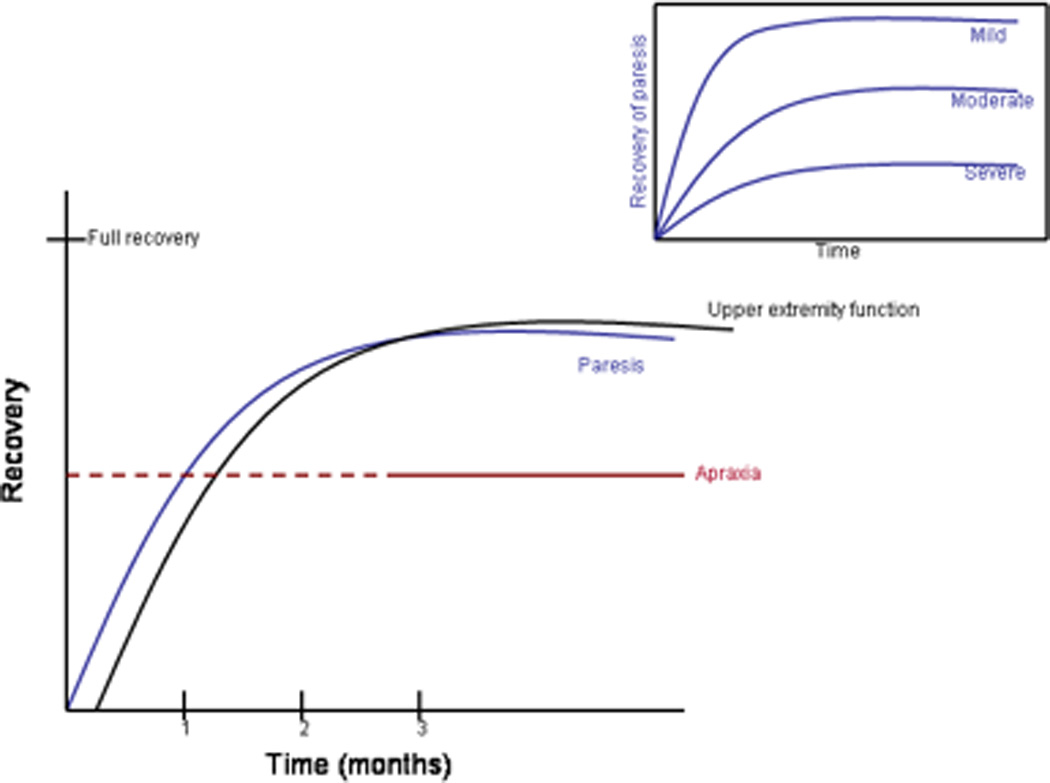
Schematic of time course of recovery after injury. Inset illustrates different recovery rates of paresis based on initial severity. The dashed line for apraxia represents the current lack of information on recovery within the first 3 months post-injury.
Mechanisms of recovery from paresis
It is important to recognize that the brain regions mediating performance of a particular task are not, as often taught, discrete or unique single anatomical sites. Any complex motor behavior is the consequence of activity in a distributed network of regions or nodes, often in both cerebral hemispheres that contribute, perhaps in differentiated but also possibly in overlapping ways to motor control. Understanding of this principle is important because it explains the relative similarity of clinical signs elicited by lesions in different locations, as well as the difficulties in trying to assign unique brain regions to single behaviors. From a neurorehabilitative point of view, it raises the possibility of accessing a specific network through different nodes, as discussed below.
The process of recovery of motor function after stroke represents a relative continuum from the initial hours after the actual event to the chronic stage years later. Early recovery in the hours or first few days following a stroke is likely to reflect improvement of hemodynamic and metabolic factors in the area of injury or surrounding tissue. For instance, early recanalization of an obstructed blood vessel or relative increases in blood pressure can improve the metabolic function of an ischemic area, and therefore its neuronal function. At the cellular level in the first few weeks following a stroke, a number of genetic, cellular, and neuronal changes occur both near the lesion and in the regions connected to it. For instance, increases in excitability in contralateral homologous zones and decreases in excitability near the lesion have been described. Synaptic sprouting from the contralateral homologous cortex to the site of a lesion has been demonstrated to occur in rats. In monkeys, changes in physiological organization of cellular responses near the lesion as well as sprouting of new connections between distant cortical areas have been demonstrated. Interestingly, some of these changes are modulated by rehabilitation. Whether and how these neural changes are relevant to patients remains unclear and deserves study.
In humans, neuroimaging and neurophysiological studies are beginning to provide a few general principles on neurological re-organization after stroke. First, after subcortical lesions involving descending fibers from the primary motor cortex, cortical activity moves toward anterior premotor areas likely involved in driving brainstem and spinal mechanisms through different parts of the corticospinal tract. Second, patients after stroke tend to recruit more areas than healthy subjects when moving their paretic limb. This relative over-activation seems to be inversely correlated with level of function, i.e. more spread of activation across cortical regions equals lower function, and longitudinal normalization of activation patterns seems to correlate with better recovery. Third, an important emerging physiological principle is the importance of balanced activity between the two hemispheres for normal function. Unbalanced activation or excitability seems to be associated with greater impairment. This principle has important rehabilitative applications (see Box 3).
Box 3. Interhemispheric interactions in motor control.
Neuroimaging studies have consistently documented enhanced activity in motor areas of both cerebral hemispheres, associated with movements of the paretic hand. Patients with stroke experience changes in motor cortical excitability and an abnormally high interhemispheric inhibition from contralesional to ipsilesional M1, when attempting to move the paretic hand. These changes are more prominent in those with more substantial motor impairment. Interestingly, these abnormalities may be task-dependent, since they may not be present when patients are at rest. These findings raise the hypothesis that targeted modulation of excitability in motor regions of the intact and affected hemisphere, through either somatosensory or motor cortical stimulation, could potentially contribute to improvements in motor function.21
Somatosensory input is required for accurate motor performance and skill acquisition. Reduction of such input by local anesthesia impairs motor control, as shown in patients with large-fiber sensory neuropathy who display characteristically abnormal motor behavior. In stroke patients, somatosensory deficits are associated with slower recovery of motor function. It has been proposed that somatosensory stimulation, which enhances motor cortical excitability, could operate as an adjuvant to rehabilitative treatments. Indeed, application of somatosensory stimulation to an affected body part can facilitate motor function in patients with stroke. Interestingly, anaesthesia of body parts contralateral to the paretic side can influence motor function in the paretic hand, perhaps through modulation of interhemispheric inhibitory interactions. For example, anesthesia of one hand results in facilitated motor function in the other hand in healthy subjects and in stroke patients.
Noninvasive brain stimulation represents a useful tool to modulate human brain function.22 Three techniques have been the most commonly tested in the framework of recent neurorehabilitative studies. Transcranial magnetic stimulation (TMS), a procedure that modulates cortical excitability, has contributed significantly to the understanding of mechanisms underlying cognitive and sensorimotor processes. Depending on stimulation parameters, TMS can enhance or decrease excitability in the neural structures under the stimulating coil. Transcranial DC stimulation (tDCS) is a procedure used to polarize brain regions through the noninvasive, transcranial application of weak direct currents that can also enhance or decrease cortical excitability depending on the polarity with which is applied. Both techniques, recently tested after stroke, are able to modulate brain function, are painless in addition to being noninvasive, and can be used in the setting of double-blind experimental designs. Additionally, recent studies attempted to facilitate motor function after stroke by stimulating perilesional areas on the cortex through epidural electrodes, based on studies in animal models. Cortical stimulation has been applied with the purpose of either facilitating activity in ipsilesional M1 or downregulating activity in contralesional M1, consistent with the notion of interhemispheric inhibitory interactions between motor cortical regions.
Illustrative Case A: Hemiparesis
R.V. is a 70 year old, right handed female with a left hemisphere stroke with damage extending subcortically into the corona radiata. Her clinical presentation includes some voluntary activation of the contralesional shoulder, elbow and wrist (grade 2), but no individuated movements of the digits. Mild tactile and proprioceptive deficits are noted on the affected side, as well as some weakness in the contralesional face and leg. She has no aphasia or neglect, and ipsilesional hand function is unimpaired. R.V.’s ability to imagine movements was evaluated by asking her to determine whether images depicted left or right hands appearing in different orientations. Despite the paralysis, her performance on this task was within the normal range and did not differ between hands. Though by no means atypical, this case illustrates several key concepts. Involvement of the contralesional face and leg, in addition to the hand, reflects damage to adjacent regions of the descending motor fibers organized in a somatotopic fashion. In terms of the model discussed, this hemiparesis can be thought of as a difficulty computing the proper motor command. This may be due either to the direct insult to regions of the left prefrontal, premotor and/or primary motor cortex, or to damage to the descending white matter tracts that carry commands from these regions to the brainstem and spinal cord. The ability of R.V. to shrug the shoulder may reflect contributions from the motor areas of the intact cerebral hemisphere to control of proximal muscles via uncrossed pathways. Depending on the posterior extent of the lesion within the internal capsule, parietal output to the spinal cord may also still be intact. A challenge will be how to engage these routes. One possibility may be to augment standard therapies with motor imagery (mental rehearsal exercises). As noted above, R.V. appears to be capable of these behaviors and evidence from neuroimaging indicates that such tasks consistently increase activity within parietal as well as frontal regions.
Illustrative Case B: Apraxia
B.O. is a 52 year old, right-handed male who suffered a CVA affecting the territory of the left MCA and resulting in a large fronto-temporo-parietal lesion. His speech production and comprehension are mildly impaired, and he has a distal hemiparesis on the contralesional side (intact shoulder shrug only). When reaching to grasp objects, the patient has no difficulties bringing the hand to the correct location in space, or in shaping, orienting and preshaping the grip. However, apraxia testing revealed significant difficulties with common skills. When asked to demonstrate how to use a spoon to eat a bowl of soup, B.O. instead pantomimed the action of brushing his teeth (content error). When asked to prepare a letter for mailing, he sealed the envelope before inserting the note (sequencing error). B.O. is, however, able to correctly recognize and name familiar objects, and also identify their common uses and functions. Likewise, he performs at low-normal levels on both tests of working memory and executive function.
The difficulties that this apraxic patient is experiencing may be due to deficits in the integrity or selection of stored representations of functional use actions. His ability to produce coordinated reaching-to-grasp movements with the intact hand suggests that he can form an accurate internal model of prehensile actions. Yet, it appears that he has difficulties retrieving the correct functional use action program in response to the object stimulus. Testing with an action recognition task requiring distinctions between correctly- and incorrectly-performed functional actions would help to distinguish deficits in the integrity of functional use representations versus action selection/retrieval deficits.
Recovery from apraxia
There have been few investigations of recovery from apraxia. The available evidence suggests that IMA after left hemisphere stroke is persistent, with only mild improvement over time. Patients with less severe apraxia at initial testing, not surprisingly, are most likely to recover.16
Recovery from ataxia
Compared to paresis, far less is known about the time course of recovery from ataxia and the factors that influence this recovery. In humans, substantial recovery can occur in the first three months after cerebellar stroke and proceeds through a series of stages that can be mapped onto characteristic changes in the triphasic electromyographic (EMG) response as it converges towards the normal pattern.17 Recovery from hypermetria in humans can be unmasked by increasing the inertial load of the moving hand with weights.18 This interesting finding suggests that spontaneous recovery from hypermetria is incomplete and may not be mediated through learning.
Mechanisms of ataxia recovery
Recovery of ataxia after a stroke affecting the cortex or the output nuclei can occur quickly within 2–3 weeks in monkeys due to adjustment of activity in the opposite normal cerebellum.19 This is also commonly observed in human patients in whom the prognosis after a single cerebellar stroke is generally good. However, a second lesion in the opposite cerebellum reinstates the original deficits and produces novel deficits in the other arm. Recovery in monkeys in this case is much longer and incomplete, and is likely dependent on other regions like the somatosensory cortex. In fact damage to the somatosensory cortex can also reinstate deficits that have recovered after a single cerebellar lesion.20
These results have important implications for neurorehabilitation and are further discussed in the accompanying paper by Pomeroy et al. First, in the monkey experiments, recovery occurred in the setting of daily practice, which means that recovery required interaction of a learning process with spontaneous biological recovery processes. It is doubtful that similar degrees of recovery would have been seen if the monkeys had not been made to practice. Second, faster and more complete recovery occurs early (within one month), which means that practice protocols may need to be initiated early after injury. Third, the anatomical loci and physiological processes mediating recovery are multiple and each may be targeted in different ways. For example, non-invasive cortical stimulation over sensory cortex might enhance recovery from ataxia and should be studied.
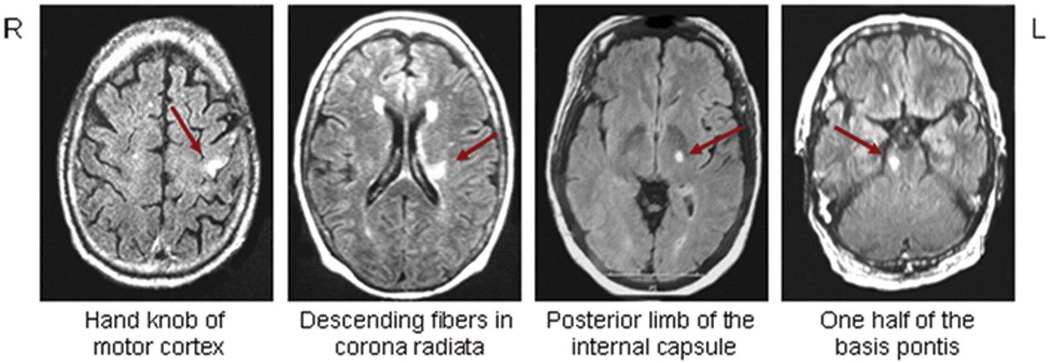
Figure 2.
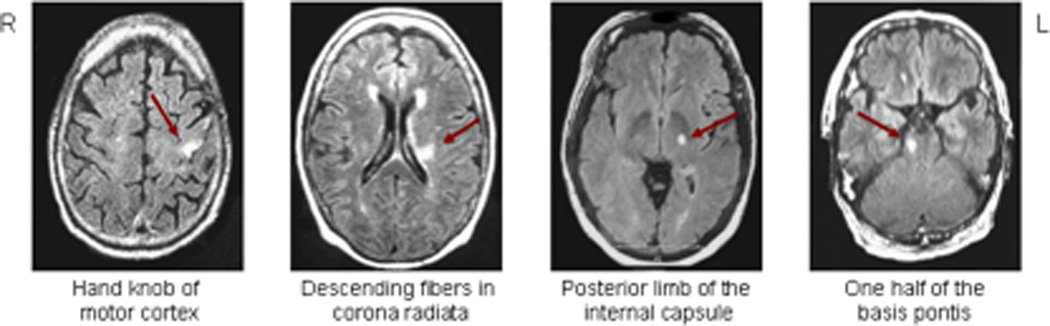
Lesions to the corticospinal system causing paresis. The small lesion in the motor cortex (leftmost panel) resulted in paresis only to the contralateral upper limb, whereas the other 3 lesions resulted in paresis affecting contralateral face, upper limb and lower limb.
Figure 3.
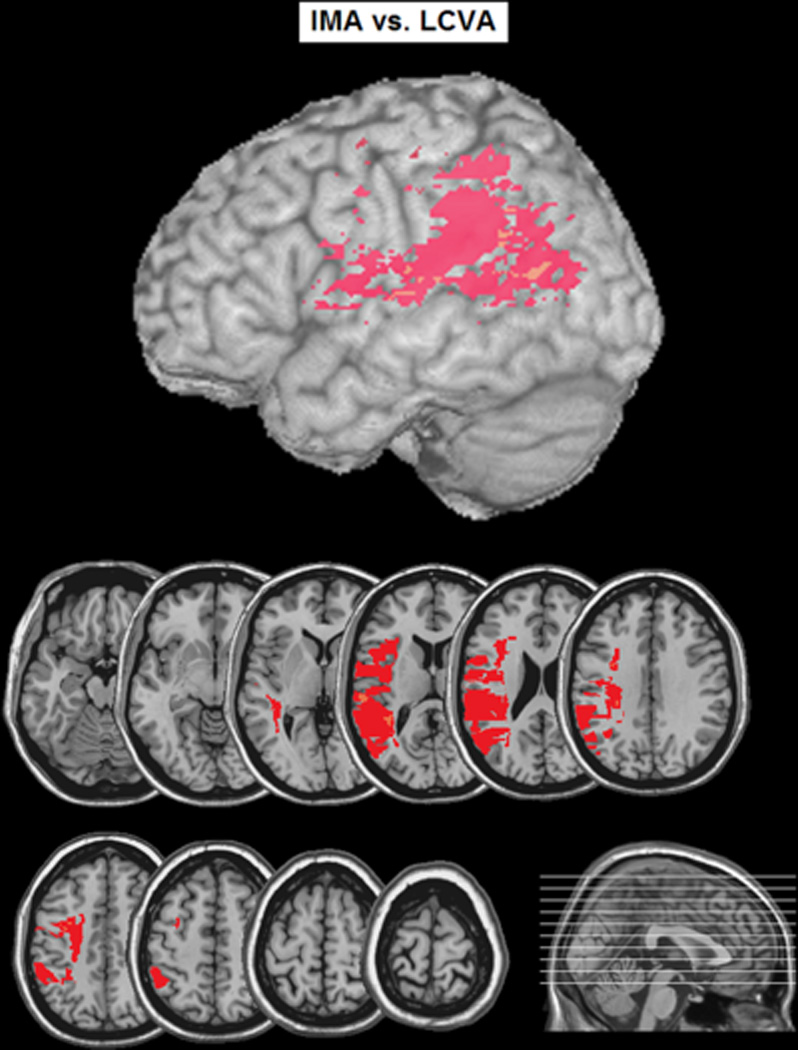
Voxel-based lesion analysis comparing left-hemisphere stroke patients with ideomotor apraxia (IMA) to left hemisphere stroke patients without IMA (control). Colored voxels indicate areas where the percentage of participants with damage to a given voxel was greater in the IMA than control group.
Box 1: Distinct use and grasp systems.
An important contrast in patients with IMA concerns the relative integrity of prehensile actions made to objects on the basis of their structure (e.g., size, shape, and weight) as compared with actions related to skilled object use. Thus, when attempting to reach out and grasp a cup, an apraxic will perform nearly normally. In contrast, apraxic patients are impaired in their ability to show “how to use” familiar objects. Many apraxics are deficient in detailed knowledge of skilled use actions (as evidenced by poor recognition of those actions when performed by others), while their ability to plan the same movements for sensory-driven activities, e.g. catch a ball or grasp a cup, are not affected. Referring back to the different fronto-parietal circuits described in Frey et al., this issue, apraxia is associated with lateral and ventral damage of parietal and frontal cortices, regions that are involved in storage of the spatial parameters for skilled actions. This may correspond to the circuit linking IPL (area PFG) with areas PMv (F5) and area 44. In contrast, the superior parietal lobule-to-dorsal premotor cortex (SPL-PMd) circuit is intact.
Box 2. Motor neglect.
One relatively common impairment of movement not directly related to motor execution or planning deficits is motor neglect. Patients tend to ignore their affected arm and appear weak or clumsy on examination; surprisingly, however, their strength and dexterity improve dramatically when they are cued to pay attention to the arm. This failure in attending to and moving the affected arm is often present in the absence of any sign of spatial neglect, or of impaired attention to sensory stimuli. Motor neglect can reflect a problem of motor activation without specific spatial impairment, or it can also take the form of a specific deficit in moving toward contralesional space (directional hypokinesia). Lesions that cause motor neglect are typically anteriorly located in the frontal white matter or the basal ganglia.
References
- 1.Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- 2.Beebe JA, Lang CE. Absence of a proximal to distal gradient of motor deficits in the upper extremity early after stroke. Clin Neurophysiol. 2008;119:2074–2085. doi: 10.1016/j.clinph.2008.04.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delwaide PJ. Parkinsonian Rigidity. Funct Neurol. 2001;16:147–156. [PubMed] [Google Scholar]
- 4.Hallet M. Parkinson revisited: pathophysiology of motor signs. Adv Neurol. 2003;91:19–28. [PubMed] [Google Scholar]
- 5.Brunnström S. Movement Therapy in Hemiplegia. New York, NY: Harper & Row; 1970. [Google Scholar]
- 6.Lang CE, Beebe JA. Relating movement control at 9 upper extremity segments to loss of hand function in people with chronic hemiparesis. Neurorehabil Neural Repair. 2007;21:279–291. doi: 10.1177/1545968306296964. [DOI] [PubMed] [Google Scholar]
- 7.Finch E, Brooks D, Stratford PW, Mayo NE. Physical Rehabilitation Outcome Measures: A guide to enhanced clinical decision making. 2nd ed. Ontario, Canada: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 8.Buxbaum LJ, Schwartz MF, Montgomery MW. Ideational apraxia and naturalistic action. Cognitive Neuropsychology. 1998;15:617–643. doi: 10.1080/026432998381032. [DOI] [PubMed] [Google Scholar]
- 9.Buxbaum LJ. Ideomotor apraxia: A call to action. Neurocase. 2001;7:445–458. doi: 10.1093/neucas/7.6.445. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz MF, Segal M, Veramonti T, Ferraro M, Buxbaum LJ. The Naturalistic Action Test. Neuropsychol Rehabil. 2002;12(4):311–339. [Google Scholar]
- 11.Vanbellingen T, Kersten B, Van de Winckel A, Bellion M, Baronti F, Müri R, Bohlhalter S. A new bedside test of gestures in stroke: the apraxia screen of TULIA (AST) J Neurol Neurosurg Psychiatry. 2010 doi: 10.1136/jnnp.2010.213371. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Rothi LJG, Raymer AM, Ochipa C, Maher LM, Greenwald ML, Heilman KM. Florida Apraxia Battery (Experimental Edition) 1992 Unpublished. [Google Scholar]
- 13.Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol. 2006;16:645–649. doi: 10.1016/j.conb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Diedrichsen J, Criscimagna-Hemminger SE, Shadmehr R. Dissociating timing and coordination as functions of the cerebellum. J Neurosci. 2007;27:6291–6301. doi: 10.1523/JNEUROSCI.0061-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donkervoort M, Dekker J, Deelman B. The course of apraxia and ADL functioning in left hemisphere stroke patients treated in rehabilitation centres and nursing homes. Clin Rehabil. 2006;20:1085–1093. doi: 10.1177/0269215506071257. [DOI] [PubMed] [Google Scholar]
- 17.Manto M, Jacquy J, Hildebrand J, Godaux E. Recovery of hypermetria after a cerebellar stroke occurs as a multistage process. Ann Neurol. 1995b;38:437–445. doi: 10.1002/ana.410380314. [DOI] [PubMed] [Google Scholar]
- 18.Manto M, Godaux E, Jacquy J. Detection of silent cerebellar lesions by increasing the inertial load of the moving hand. Ann Neurol. 1995a;37:344–350. doi: 10.1002/ana.410370310. [DOI] [PubMed] [Google Scholar]
- 19.Amrani K, Dykes RW, Lamarre Y. Bilateral contributions to motor recovery in the monkey following lesions of the deep cerebellar nuclei. Brain Res. 1996;740:275–284. doi: 10.1016/s0006-8993(96)00899-2. [DOI] [PubMed] [Google Scholar]
- 20.Mackel R. The role of the monkey sensory cortex in the recovery from cerebellar injury. Exp Brain Res. 1987;66:638–652. doi: 10.1007/BF00270696. [DOI] [PubMed] [Google Scholar]
- 21.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Floel A, Cohen LG. Recovery of function in humans: Cortical stimulation and pharmacological treatments after stroke. Neurobiol Dis. 2010:243–251. doi: 10.1016/j.nbd.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Frey SH, Fogassi L, Grafton S, et al. Neurological principles and rehabilitation of action disorders: computation, anatomy & physiology (CAP) model. Neurorehabil Neural Repair. doi: 10.1177/1545968311410940. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Pomeroy V, Aglioti SM, Mark VM, et al. Neurological principles and rehabilitation of action disorders: rehabilitation interventions. Neurorehabil Neural Repair. doi: 10.1177/1545968311410942. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


