Abstract
In postpartum dairy cows, lipopolysaccharide (LPS) derived from gram-negative bacteria such as Escherichia coli causes uterine inflammation and leads to ovarian dysfunction. The aim of this study was to determine the effect of LPS on steroid production in bovine theca cells at different stages of follicular development. Theca cells isolated from pre- and post-selection follicles (PRFs, <8.5 mm in diameter, and POFs, >8.5 mm in diameter, respectively) of bovine ovaries were exposed to LPS under luteinizing hormone (LH) conditions, estradiol (E2) conditions or both conditions in vitro. Bovine theca cells expressed the LPS receptor gene complex: Toll-like receptor 4 (TLR4), CD14 and MD2. LPS suppressed progesterone (P4) and androstenedione (A4) production with downregulation of steroidogenic enzyme transcripts when theca cells were stimulated with LH. By contrast, LPS did not affect P4 or A4 production when theca cells were stimulated with E2. P4 and A4 production in theca cells from PRFs was suppressed by LPS as early as at 48 h of culture, whereas the effect of LPS on theca cells from POFs was observed at 96 h of culture. The results demonstrate that LPS inhibits steroid production in theca cells under LH conditions. Moreover, theca cells from POFs showed a slower response to LPS compared with that of theca cells from PRFs, which might imply a distinct effect of LPS on follicles at different developmental stages. These findings suggest a possible mechanism of ovarian dysfunction and subsequent infertility in cows with endometritis.
Keywords: Androstenedione, Dairy cow, Lipopolysaccharide, Progesterone, Theca cells
Uterine bacterial infection commonly occurs in postpartum dairy cows and perturbs uterine and ovarian function [1]. It causes enormous economic losses related to compromised reproductive performance: conception rates are approximately 20% lower in cows with endometritis, the median calving to conception interval is 30 days longer, and 3% more animals are culled for failure to conceive [2, 3]. Cows with metritis display slower growth of the first postpartum dominant follicle and lower peripheral plasma estradiol (E2), and in ovulating animals, peripheral plasma progesterone (P4) concentrations are lower [4].
Escherichia coli is among the main types of bacteria causing endometritis, and much of the tissue pathology is associated with the bacterial endotoxin lipopolysaccharide (LPS) [5]. LPS has been detected in the follicular fluid (FF) of cows with endometritis [6], suggesting a relationship between uterine inflammation, LPS production and follicular function. LPS is recognized by its specific receptor, Toll-like receptor 4 (TLR4), in complex with co-receptors cluster of differentiation 14 (CD14) and myeloid differentiation factor 2 (MD2). Binding of LPS to TLR4 results in nuclear translocation of nuclear factor κB components, which leads to production of proinflammatory cytokines and chemokines [7, 8]. It has been shown that bovine and murine follicular granulosa cells express mRNA for the TLR4 receptor complex and respond to LPS via TLR4 to generate an inflammatory response [9,10,11], whereas the mRNA expression of TLR4 in theca cells has been reported only in hens [12].
The first step in follicular biosynthesis of steroid hormones occurs in theca cells; P4 is synthesized by the conversion of cholesterol and is later converted into androstenedione (A4). A4 diffuses through the basement membrane and is converted to E2 by granulosa cells [13]. In vitro studies have shown that LPS suppresses E2 production in granulosa cells from large and small follicles of bovine ovaries [6, 14]. Tumor necrosis factor-α, one of the major proinflammatory cytokines, reduces A4 production of bovine theca cells in vitro [15], indicating that not only granulosa cells but also bovine theca cells generate an inflammatory response that perturbs steroid production of follicles. However, the effect of LPS on steroid production in theca cells is somewhat controversial; Taylor and Terranova have shown that LPS perturbs P4 and A4 production in rat ovarian theca cells [16], whereas A4 production of bovine theca cells was reported to be unaffected by LPS [6]. This discrepancy between these two studies might be due to the difference in species or the presence of LH stimulation. In the study of Taylor and Terranova, steroid production of theca cells was stimulated by LH. After antrum formation, the steroidogenic functions of follicles are regulated by gonadotrophins [13] and locally produced factors such as E2 [17]. Therefore, we hypothesized LPS may suppress P4 and A4 production of bovine theca cells when theca cells are stimulated by LH or E2.
The objective of the present study was to determine the effect of LPS on steroid production of bovine theca cells under LH conditions, E2 conditions or both conditions. In addition, the distinct effect of LPS on theca cell function at different stages of follicular development was investigated.
Materials and methods
Materials
Dulbecco’s modified Eagle’s/F12 medium, kanamycin, streptomycin and phosphate-buffered saline (PBS) were purchased from Sigma Chemical (St. Louis, MO, USA). Fetal calf serum (FCS) was obtained from Biowest (Rue de la Caille, Nuaillé, France).
Sample collection and classification of the developmental stage of follicles
Ovaries of multiparous Holstein cows were obtained at a local slaughterhouse and placed in ice-cold PBS. All ovaries were collected from cows without any signs of uterine inflammation or from cows not within 3 weeks postpartum. Healthy developing follicles were assessed as described by Metcalf et al. [18] for a vascularized pink theca externa and amber follicular fluid without debris. Follicular fluid (FF) was aspirated using a syringe with a 22-gauge needle, and the follicle diameter was determined from the weight of the FF, as previously described by Murasawa et al. [19]. Follicles were classified into 2 categories based on follicle diameter [20], E2 concentration and the E2-P4 ratio (E/P) in the FF: pre-selection follicles (PRFs; <8.5 mm, mean E2 concentration 17.7 ng/ml, mean P4 concentration 12.2 ng/ml, mean E/P 1.4) and post-selection follicles (POFs; >8.5 mm, mean E2 concentration 481.3 ng/ml, mean P4 concentration 26.0 ng/ml, mean E/P 25.1). Theca cells were isolated from these follicles using the following method [21]. Briefly, follicles were opened by making a small incision on the surface, and theca cells were obtained by manually peeling the basal lamina. Granulosa cells were removed from the peeled theca cells by gentle scraping with a medicine spatula. The complete removal of granulosa cells was confirmed under a stereomicroscope. To determine the mRNA expression of LPS receptors, collected theca cells from PRFs (n = 5) and POFs (n = 5) were stored at – 80 C until total RNA extraction.
Culture of theca cells from PRFs and POFs
Theca cells isolated from PRFs and POFs using the methods described above were placed in PBS containing 2 mg collagenase (452 U/mg, type 1, Sigma Chemical), 1 mg hyaluronidase (391 U/mg, type VIII, Sigma Chemical), 1 mg protease (4.5 U/g, Sigma Chemical) and 0.4% (v/v) bovine serum albumin, and a dissociation reaction was performed for 50 to 60 min at 37 C. Centrifugal separation was carried out at 350 × g. Then, Tris–HCl Buffer (pH 8.0) was put into the tube for 1 min at 37 C. Dispersed cells were washed twice with PBS. Theca cells were suspended in culture medium in 1 ml of Dulbecco’s modified Eagle’s/F12 medium (DMEM/F12) containing 100 μg/ml streptomycin, 100 μg/ml kanamycin, and 5% FCS in 12-well culture plates (Nunc; Nalge Nunc International, New York, NY, USA) at 1 × 105 cells per well and cultured for 24 h at 37 C in 5% CO2 and 95% air. The wells were then washed twice with PBS to remove unattached cells.
Cell culture challenge
After an initial 24-h establishment period, the culture medium was replaced with a medium supplemented with 1% FCS. To induce and maintain P4 and A4 production by theca cells, we added a physiological concentration of LH (2.5 ng/ml; bovine LH, USDA-bLH-B6, National Institute of Diabetes and Digestive and Kidney Diseases, biopotency 2.3 units/mg), E2 (100 ng/ml; β-Estradiol, Sigma-Aldrich Japan, Tokyo, Japan) or both to the culture medium. These concentrations of LH and E2 were based on previous in vivo and in vitro studies [22,23,24,25,26]. Each medium contained 0, 0.1, 1 or 10 μg/ml of E. coli O55:B5 LPS (Sigma-Aldrich Japan). These concentrations are similar to those in the FF of animals with clinical disease [6]. Theca cells were cultured for 96 h to determine the effect of LPS on steroid production. After 48 h of treatment (term 1), the media were carefully removed and stored at – 20 C until the hormone assay. Then, the culture medium was replaced with fresh media containing 0, 0.1, 1 or 10 μg/ml LPS for an additional 48-h treatment period. At 96 h of treatment (term 2), the culture medium was removed and stored at – 20 C until the hormone assay. At the end of the culture period, theca cells were detached from the culture plates by treatment with 0.02% trypsin and 0.02% EDTA for 5 min at 37 C. After trypsin deactivation by the addition of DMEM/F12 supplemented with 5% FCS, cells were collected and centrifuged at 900 × g for 10 min at 4 C. The cells were then washed with PBS and resuspended in PBS. Cell suspensions were used for determination of the number of viable cells. The cell viability was measured with the trypan blue exclusion test. Theca cells were dyed with trypan blue, and the number of viable cells (without any uptake of trypan blue) was counted using a hemocytometer. After counting the cell number, the rest of cell suspensions were centrifuged at 1250 × g for 10 min at 4 C. Theca cells were collected for RNA isolation at 48 h (PRFs) or 96 h (POFs), at which time the maximum response was observed.
Hormone assay
The concentration of P4 and A4 in culture medium was measured using an enzyme immunoassay as previously described [27, 28]. The standard curve ranged from 50 to 50,000 pg/ml for P4 and 7.8 to 8000 pg/ml for A4. The culture medium was diluted with assay buffer when the P4 and A4 concentrations reached high levels. The intra- and interassay coefficients of variation averaged 7.0% and 5.7% for P4, and 9.0% and 5.8% for A4, respectively.
RNA extraction, reverse transcription (RT) and quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from cultured theca cells with TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions and frozen at – 80 C. Before RT, samples were treated with DNase, and single-strand complementary DNA was then reverse transcribed from total RNA using a commercial kit (PrimeScript RT Reagent Kit with gDNA Eraser; Takara Bio, Shiga, Japan). The RT conditions were as follows: 15 min of complementary DNA synthesis at 37 C and 5 sec of inactivation at 85 C. The mRNA levels of steroidogenic acute regulatory protein (StAR), 17β-hydroxylase/17,20-lyase (CYP17), LH receptor (LHr) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were quantified with real-time PCR using an iQcycler (Bio-Rad Laboratories, Tokyo, Japan) and a commercial kit (QuantiTectTM SYBR® Green PCR; QIAGEN, Hilden, Germany). Primers for real-time PCR were designed from bovine sequences using the Primer-3 software (Table 1). The amplification program included 15 min of activation at 95 C followed by 50 cycles of PCR (95 C for 15 sec, annealing temperature for 30 sec and 72 C for 30 sec). The starting quantity of mRNA from each sample was determined using standard curves, and expression levels of genes of interest were then normalized to the reference gene GAPDH.
Table 1. Primer pairs used for the detection of mRNA.
| Genes | Primer sequence (5′→ 3′) | Size (bp) | Annealing temp. (C) |
Genbank Accession No. | |
| LPS receptor | |||||
| TLR4 | For: CTT GCG TAC AGG TTG TTC CTA A | 153 | 56 | NM174198 | |
| Rev: CTG GGA AGC TGG AGA AGT TAT G | |||||
| CD14 | For: GGG TAC TCT CTG CTC AAG GAA C | 199 | 56 | NM174008 | |
| Rev: CTT GGG CAA TGT TCA GCA C | |||||
| MD2 | For: GGG AAG CCG TGG AAT ACT CTA T | 204 | 54 | DQ319076 | |
| Rev: CCC CTG AAG GAG AAT TGT ATT G | |||||
| Progesterone production | |||||
| StAR | For: GTG GAT TTT GCC AAT CAC CT | 203 | 58 | NM174189 | |
| Rev: TTA TTG AAA ACG TGC CAC CA | |||||
| Androstenedione production | |||||
| CYP17 | For: TGG ATC GTG GCC TAC CTC CT | 215 | 58 | M12547 | |
| Rev: AGG TCG CCA ATG CTG GAG TC | |||||
| Gonadotropin receptor | |||||
| LHr | For: AGG AAA ATG CAC GCC TGG AG | 202 | 58 | U20504 | |
| Rev: GTG GCA TCC AGG AGG TTG GT | |||||
| Internal standard | |||||
| GAPDH | For: CTC TCA AGG GCA TTC TAG GC | 120 | 58 | U85042 | |
| Rev: TGA GAA AGT CGT TGA GG | |||||
Statistical analysis
All data are presented as means ± standard error of the mean, with n = 3 per experiment. Statistical analysis was performed using StatView 5.0 (SAS Institute, Cary, NC, USA) and JMP 6 (SAS Institute). Due to the inherent variability in steroid concentrations between different cell cultures, the results are presented as the percent inhibition across three separate experiments, with the control set as 100%. The Shapiro-Wilk test was used to test for normal distribution of the data, and all the parameters showed a normal distribution. Homogeneity of variance was examined by F-test. The significance of differences in mRNA expression of LPS receptors was analyzed using the Student’s t-test (PRFs vs. POFs). For analysis of the number of viable cells, steroid production and mRNA expression of steroidogenic enzymes, one-way analysis of variance followed by the Tukey-Kramer test as a multiple comparison test was performed. All analyses were considered statistically significant at P < 0.05.
Results
Expression of LPS receptors in bovine theca cells
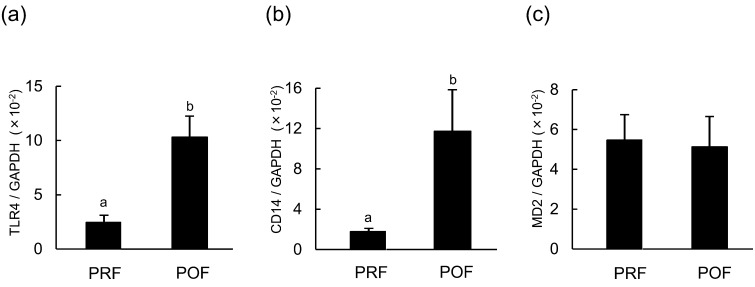
Bovine theca cells from PRFs and POFs expressed the LPS receptor complex: TLR4, CD14 and MD2 (Fig. 1). In POFs, the expression of TLR4 and CD14 was significantly higher than that in PRFs, whereas follicles at both stages showed similar expressions of MD2.
Fig. 1.
mRNA expression of (a) TLR4, (b) CD14 and (c) MD2 in the theca cells of pre-selection follicles (PRFs; <8.5 mm, n = 5) and post-selection follicles (POFs; >8.5 mm , n = 5). All values are means ± standard error of the mean. Values with different letters (a, b) are significantly different between groups (P < 0.05).
Effect of LPS on steroid production in bovine theca cells isolated from POFs
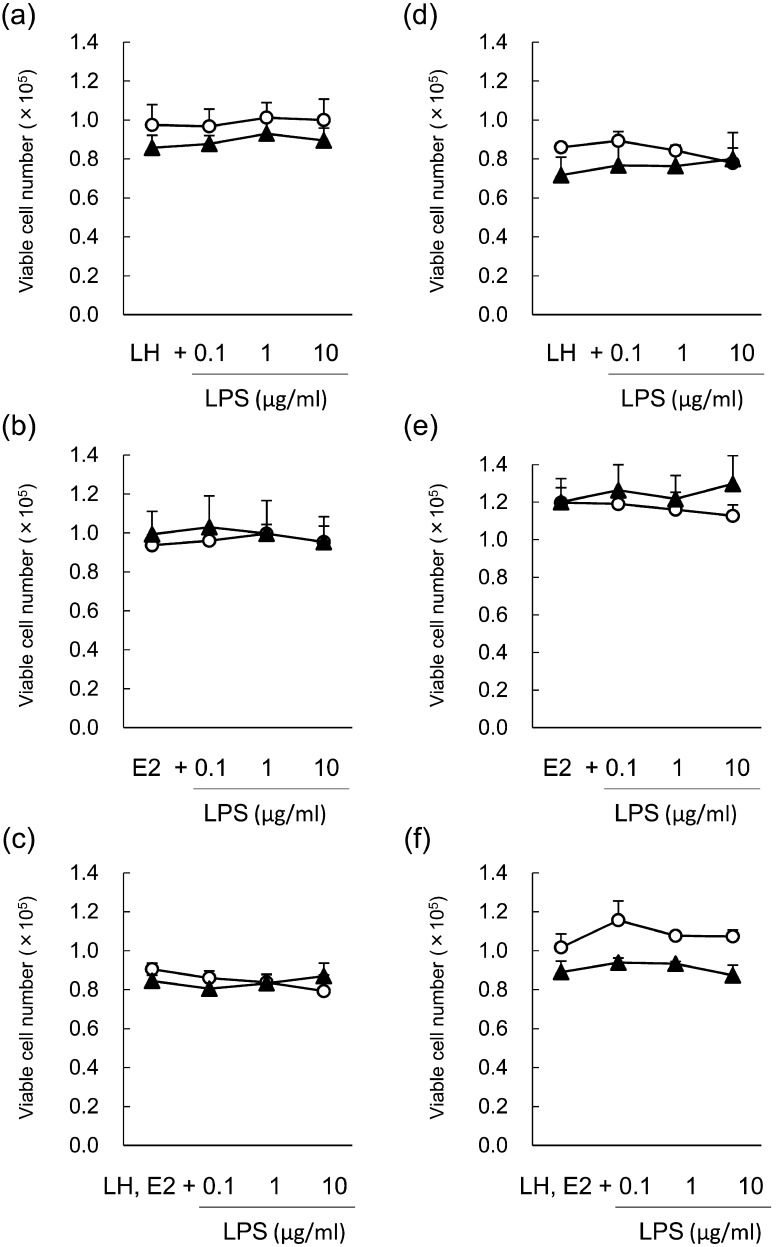
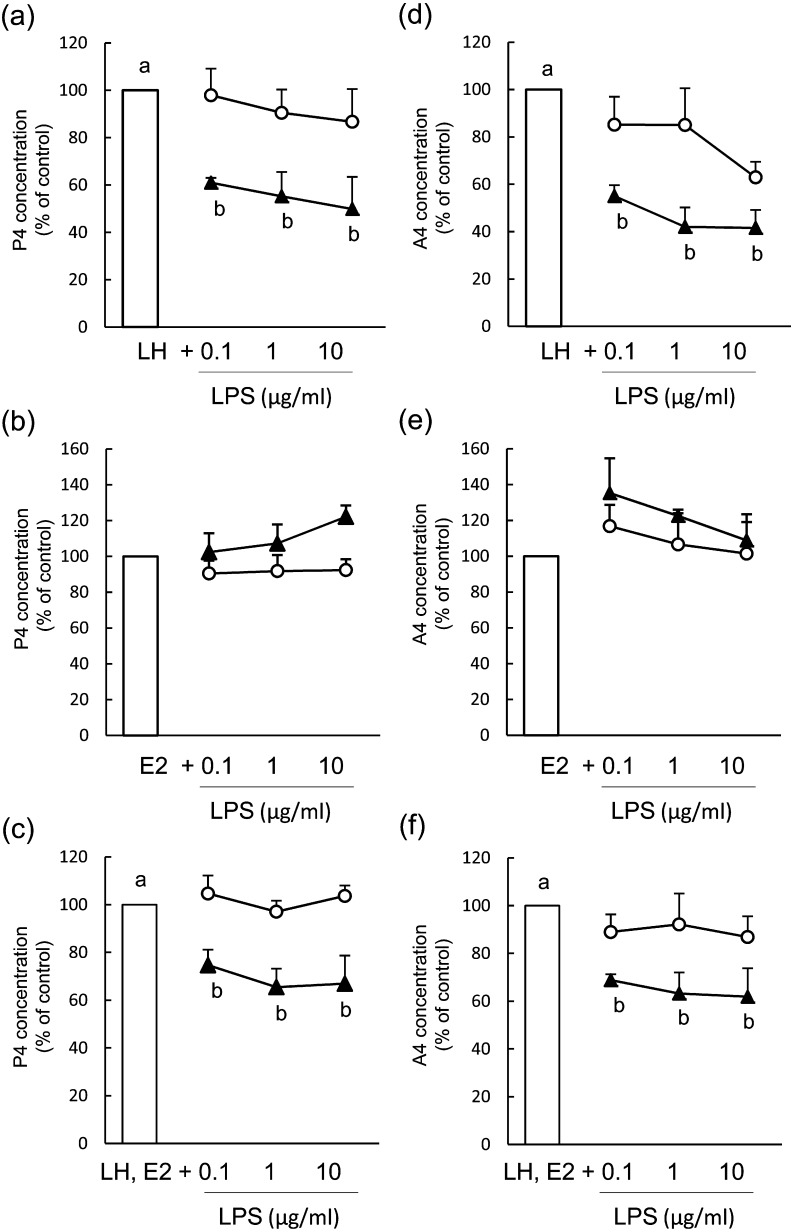
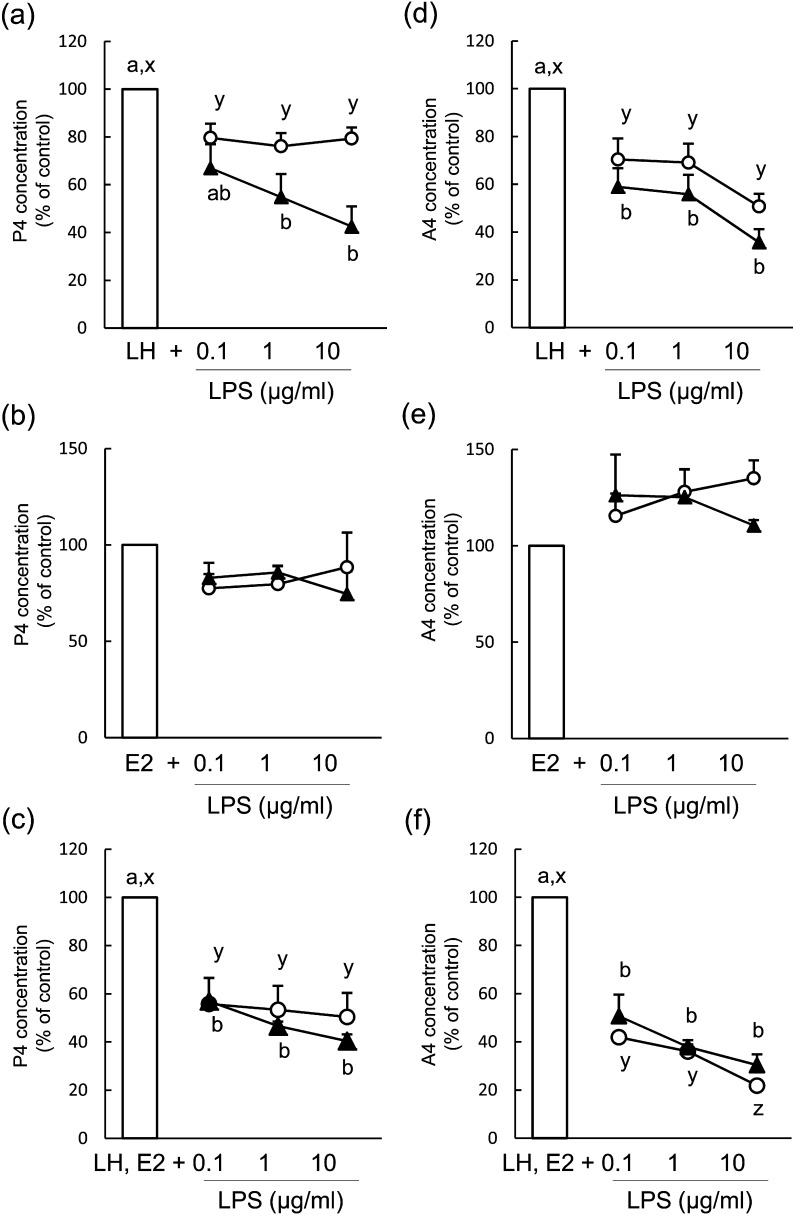
The number of viable cells was unaffected by LPS treatment at 48 h and 96 h of culture (Fig. 2a–c). During term 2 (48–96 h) of culture, LPS (0.1–10 μg/ml) inhibited P4 (Fig. 3a, mean concentration of the control group: 287.3 ± 53.8 ng/ml) and A4 (Fig. 3d, mean concentration of the control group: 1.3 ± 0.3 ng/ml) production when theca cells were stimulated with LH; however, P4 and A4 production was unaffected by LPS treatment during term 1 (0-48 h) of culture (mean concentration of the control group: 127.4 ± 15.6 ng/ml for P4 and 1.4 ± 1.4 ng/ml for A4). LPS did not affect P4 (Fig. 3b, mean concentration of the control group: 54.6 ± 4.9 ng/ml during term 1 and 43.6 ± 4.2 ng/ml during term 2) or A4 (Fig. 3e, mean concentration of the control group: 1.5 ± 1.7 ng/ml during term 1 and 0.4 ± 0.8 ng/ml during term 2) production during either term of the culture when theca cells were stimulated with E2. The production of P4 (Fig. 3c, mean concentration of the control group: 59.9 ± 9.4 ng/ml during term 1 and 133.7 ± 24.8 ng/ml during term 2) and A4 (Fig. 3f, mean concentration of the control group: 2.4 ± 0.2 ng/ml during term 1 and 1.7 ± 0.2 ng/ml during term 2) decreased during term 2 of culture in LH- and E2-treated theca cells.
Fig. 2.
Effect of lipopolysaccharide (LPS) on number of viable theca cells isolated from post-selection follicles (>8.5 mm; a–c) and pre-selection follicles (<8.5 mm; d–f) during term 1 (white circles, 0–48 h) and term 2 (black triangles, 48–96 h). Theca cells were stimulated with 2.5 ng/ml luteinizing hormone (LH; a, d), 100 ng/ml estradiol (E2; b, e) or LH and E2 (c, f). All values are means ± standard error of the mean of three independent experiments.
Fig. 3.
Effect of lipopolysaccharide (LPS) on the production of progesterone (P4; a–c) and androstenedione (A4; d–f) in bovine theca cells from post-selection follicles (>8.5 mm) during term 1 (white circles, 0–48 h) and term 2 (black triangles, 48–96 h). Theca cells were stimulated with 2.5 ng/ml luteinizing hormone (LH; a, d), 100 ng/ml estradiol (E2; b, e) or LH and E2 (c, f). Data are expressed as the percentage of control (100%) steroid accumulation in the culture medium. All values are means ± standard error of the mean of three independent experiments. Values with different letters (a, b) are significantly different between groups (P < 0.05).
Effect of LPS on the mRNA expression of steroidogenesis-related factors in bovine theca cells isolated from POFs
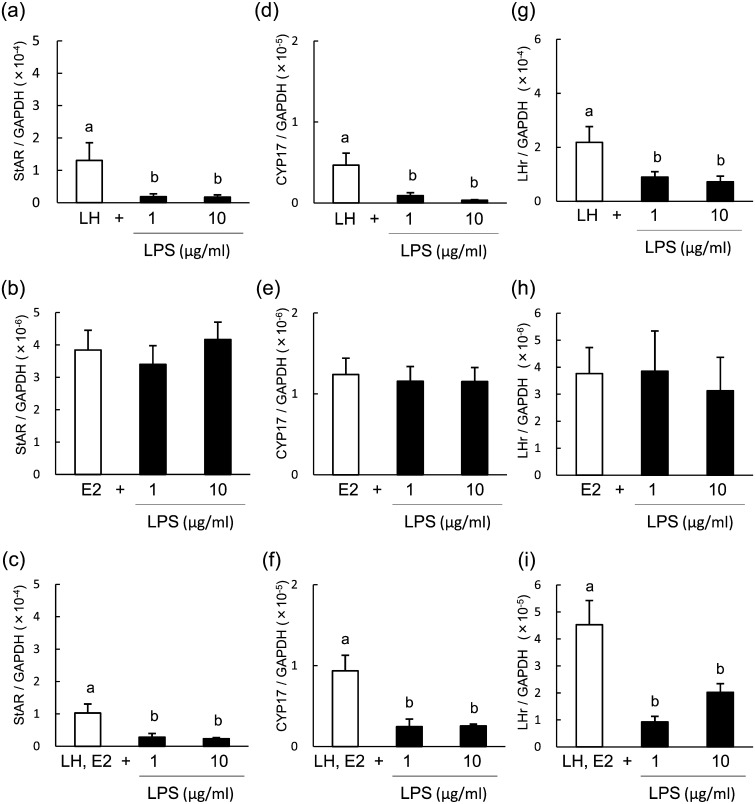
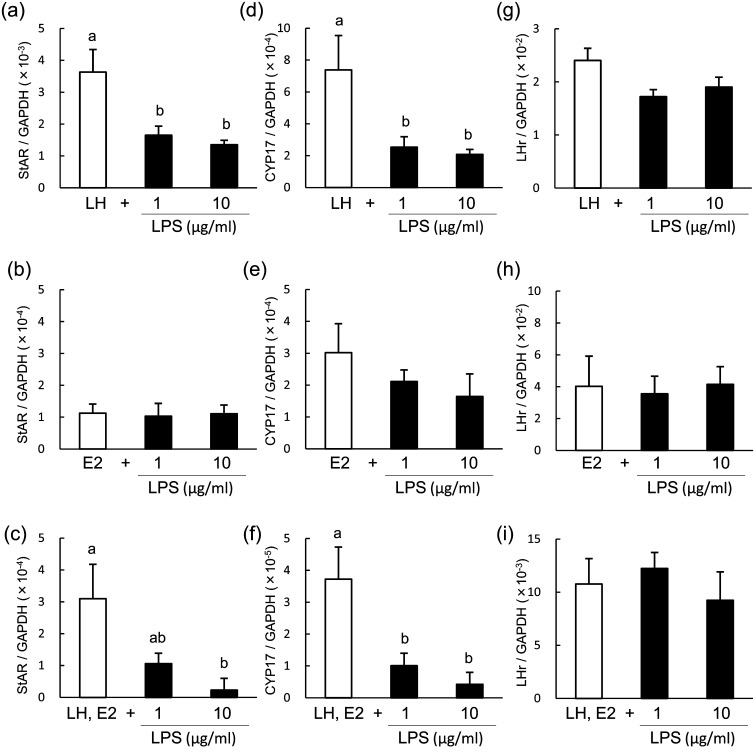
To determine the inhibitory effect of LPS on steroid production, we analyzed the mRNA expression of StAR, CYP17 and LHr in theca cells of POFs at 96 h of culture, at which time the maximum response was observed. LPS suppressed the mRNA expression of StAR (Fig. 4a and 4c), CYP17 (Fig. 4d and 4f) and LHr (Fig. 4g and 4i) in LH-treated theca cells and in LH- and E2-treated theca cells but not in E2-treated theca cells (Fig. 4b, 4e and 4h).
Fig. 4.
Effect of lipopolysaccharide (LPS) on the mRNA expression of StAR (a–c) CYP17 (d–f) and LHr (d–f) in bovine theca cells from post-selection follicles (>8.5 mm) at 96 h of culture. Theca cells were stimulated with 2.5 ng/ml luteinizing hormone (LH; a, d, g), 100 ng/ml estradiol (E2; b, e, h) or LH and E2 (c, f, i). All values are means ± standard error of the mean of three independent experiments. Values with different letters (a, b) are significantly different between groups (P < 0.05).
Effect of LPS on steroid production in bovine theca cells isolated from PRFs
The number of viable cells was unaffected by LPS treatment at 48 h and 96 h of culture (Fig. 2d–f). In theca cells of PRFs, LPS (0.1–10 μg/ml) inhibited the production of P4 (Fig. 5a, mean concentration of the control group: 228.2 ± 96.1 ng/ml during term 1 and 315.8 ± 32.6 ng/ml during term 2) and A4 (Fig. 5d, mean concentration of the control group: 1.5 ± 0.3 ng/ml during term 1 and 4.1 ± 0.2 ng/ml during term 2) during terms 1 and 2 of culture when theca cells were stimulated with LH. LPS did not affect the production of P4 (Fig. 5b, mean concentration of the control group: 37.7 ± 11.8 ng/ml during term 1 and 29.9 ± 1.3 ng/ml during term 2) or A4 (Fig. 5e, mean concentration of the control group: 0.4 ± 0.1 ng/ml during term 1 and 0.5 ± 0.2 ng/ml during term 2) during either term of the culture when theca cells were stimulated with E2. The production of P4 (Fig. 5c, mean concentration of the control group: 185.4 ± 29.3 ng/ml during term 1 and 275.2 ± 18.9 ng/ml during term 2) and A4 (Fig. 5f, mean concentration of the control group: 15.8 ± 4.9 ng/ml during term 1 and 6.0 ± 2.2 ng/ml during term 2) decreased during both terms of culture in LH- and E2-treated theca cells.
Fig. 5.
Effect of lipopolysaccharide (LPS) on the production of progesterone (P4; a-c) and androstenedione (A4; d-f) in bovine theca cells from pre-selection follicles (<8.5 mm) during term 1 (white circles, 0–48 h) and term 2 (black triangles, 48–96 h). Theca cells were stimulated with 2.5 ng/ml luteinizing hormone (LH; a, d), 100 ng/ml estradiol (E2; b, e) or LH and E2 (c, f). Data are expressed as the percentage of control (100%) steroid accumulation in the culture medium. All values are means ± standard error of the mean of three independent experiments. Values with different letters (a, b) are different between groups (P < 0.05)
Effect of LPS on the mRNA expression of steroidogenesis-related factors in bovine theca cells isolated from PRFs
The mRNA expression of StAR, CYP17 and LHr in theca cells of PRFs was analyzed at 48 h of culture, at which time the maximum response was observed. LPS suppressed mRNA expression of StAR (Fig. 6a and 6c) and CYP17 (Fig. 6d and 6f) but did not affect the expression of LHr (Fig. 6g and 6i) in LH-treated theca cells. LPS did not affect the mRNA expression of StAR, CYP17 or LHr in E2-treated theca cells (Fig. 6b, 6e and 6h).
Fig. 6.
Effect of lipopolysaccharide (LPS) on the mRNA expression of StAR (a–c), CYP17 (d–f) and LHr (d–f) in bovine theca cells from pre-selection follicles (<8.5 mm) at 48 h of culture. Theca cells were stimulated with 2.5 ng/ml luteinizing hormone (LH; a, d, g), 100 ng/ml estradiol (E2; b, e, h) or LH and E2 (c, f, i). All values are means ± standard error of the mean of three independent experiments. Values with different letters (a, b) are significantly different between groups (P < 0.05).
Discussion
This study investigated the effect of LPS on steroid production in bovine theca cells at different stages of follicular development under LH conditions, E2 conditions or both conditions. LPS suppressed P4 and A4 production with downregulation of StAR and CYP17 mRNA expression when theca cells were stimulated with LH. By contrast, LPS did not affect P4 or A4 production when theca cells were stimulated with E2. LPS is known to act in the hypothalamus or pituitary to suppress gonadotropin release, which perturbs follicular growth and function in cattle [29]. In addition to its indirect effects, LPS may directly affect follicular function, including steroid production. Bovine granulosa cells reportedly express TLR4, which recognizes LPS, and LPS suppresses E2 production in granulosa cells of bovine follicles [6, 14]. Similar to studies of bovine granulosa cells, the present study revealed that bovine theca cells from PRFs and POFs expressed TLR4, CD14 and MD2 mRNAs, which constitute the specific receptor for LPS [30], suggesting that bovine theca cells are capable of responding to LPS. Moreover, LPS suppressed the production of P4 and A4 in LH-stimulated theca cells, downregulating the transcription of steroidogenic enzymes StAR and CYP17. These findings indicate that LPS can act locally on bovine theca cells and suppress the steroidogenic function of theca cells as well as granulosa cells.
We recently reported that in follicles with a high level of LPS in follicular fluid, the follicular E2 concentration was lower compared with that in follicles with a low level of LPS [31]. In those follicles with a high level of LPS, mRNA expression of CYP17 in theca cells and P450 aromatase in granulosa cells was lower. These findings indicate that LPS in follicular fluid influenced the steroid production in follicles. In addition to these observations in vivo, an in vitro study has shown that LPS suppressed E2 production of granulosa cells [14]. Based on these reports and the results of the present study, it can be assumed that LPS acts on both theca cells and granulosa cells: in theca cells, LPS suppressed A4 production, leading to a shortage of substrate for E2 production, and in granulosa cells, E2 synthesis was further perturbed by LPS. This biphasic inhibitory effect of LPS might cause ovarian dysfunction and subsequent impaired fertility in cows with a postpartum uterine infection.
The mechanism by which LPS inhibits steroid production has been unclear. Herath et al. reported that LPS has no effect on A4 production in theca cells, regardless of the follicle size from which the cells were isolated [6]. This discrepancy might be related to the presence of LH stimulation. In the present study, steroid production in theca cells was inhibited by LPS when cells were stimulated with a physiological concentration of LH (2.5 ng/ml). By contrast, LPS did not affect P4 or A4 production when theca cells were stimulated with E2 (100 ng/ml). The steroidogenic functions of bovine theca cells are regulated by LH and E2 [32] via different mechanisms; LH stimulates the cyclic adenosine monophosphate (cAMP) signaling pathway, which upregulates the transcription of steroidogenic enzymes [13], whereas activated E2 receptors present in the nucleus bind to specific DNA sequences and stimulate E2-target gene transcription [33]. Taylor and Terranova [16] reported that LPS inhibits P4 and A4 production of LH-stimulated theca cells of the rat ovary. In that study, theca cells were insensitive to the inhibitory effects of LPS when stimulated with cAMP analog, indicating that the effect of LPS occurs at a site proximal to cAMP generation. Moreover, treatment of theca cells with herbimycin A, which blocked the effect of LPS, led to an increase in cAMP accumulation in culture medium. Based on these findings and results from the present study, it is assumed that the inhibitory effects of LPS might be involved in the cAMP signaling pathway activated by LH. No synergetic effect of LH and E2 stimulation was observed in the inhibitory effect of LPS on P4 and A4 production in theca cells.
P4 and A4 production in theca cells isolated from PRFs was suppressed by LPS as early as at 48 h of culture, whereas the effect of LPS on theca cells from POFs was observed at 96 h of culture. These results may indicate a quick response of PRF theca cells to LPS. However, the expression of TLR4 and CD14 was significantly lower in PRFs compared with that in POFs. Although we do not know whether the transcription level of the LPS receptor reflects the sensitivity or quickness of the response to LPS in theca cells, LPS might act on theca cells via different pathways in PRFs and POFs. TLR4 is known to activate two distinct signaling pathways: the MyD88-dependent and TRIF-dependent signaling pathways. The recruitment of Myd88 is associated with early phase activation of NF-κB, whereas the TRIF-dependent signaling pathway activates late-phase NF-κB [34]. The difference in the time required for LPS response observed in the present study might be associated with these distinct LPS signaling pathways. Moreover, the mRNA expression of LHr was decreased by LPS in theca cells from POFs but not in theca cells from PRFs, which may support the possibility of a distinct mechanism for LPS effects on theca cell function in PRFs and POFs. Further study is necessary to determine the detailed pathway of signal transduction by which LPS inhibits steroid production in theca cells.
Follicle selection is the mechanism whereby only one of the many available follicles becomes the ovulatory follicle. Averaged over several reports, follicular recruitment to selection takes approximately 60 h, and selection to ovulation takes approximately 120 h [20, 35]. In the present study, the amounts of time required for an LPS response in PRFs (48 h) and POFs (96 h) were similar to the term of follicular development before and after selection, respectively. In theca cells of PRFs, quick response to LPS might be necessary to obstruct selection of follicles that contain LPS in follicular fluid. In those follicles, it is speculated that LPS may inhibit follicular development by suppressing steroid production of theca cells. After follicle selection, LPS might continuously affect theca cells of POFs and cause disturb ovulation. It has been reported that postpartum uterine infections causes slower growth of dominant follicles [4] and delayed ovulation [36]. In the present study, LPS downregulated the mRNA expression of LHr in theca cells of POFs, which might cause insensitiveness to LH pulses or an LH surge. These findings in theca cells of PRFs and POFs may indicate the possibility that LPS causes improper follicular maturation and impaired ovarian activity during postpartum endometritis.
Although LPS suppressed the steroidogenic function of theca cells, cell survival was unaffected even after 96 h of LPS treatment in the present study. Similarly, Taylor and Terranova reported that LPS perturbs P4 and A4 production in LH-stimulated theca cells in the rat ovary without affecting the number of viable cells [16]. Moreover, LPS does not affect cell proliferation in bovine granulosa cells [14]. LPS might be associated with disturbance of follicular development by inhibiting steroid production rather than by inducing cell apoptosis.
In conclusion, LPS acts on ovarian theca cells and inhibits steroid production in LH-stimulated theca cells. Theca cells from POFs showed a response to LPS that was slower than that of theca cells from PRFs, which might imply a distinct effect of LPS on follicles at various developmental stages. These findings highlight a possible mechanism of ovarian dysfunction in cows with endometritis.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS), the Global COE Program, the Ministry of Education, Sports, Science and Technology, Japan, and the Ito Foundation. F.M. is supported by a JSPS Research Fellowship for Young Scientists.
References
- 1.Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth HJ. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol Reprod 2009; 81: 1025–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borsberry S, Dobson H. Periparturient diseases and their effect on reproductive performance in five dairy herds. Vet Rec 1989; 124: 217–219 [DOI] [PubMed] [Google Scholar]
- 3.LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, Johnson WH. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J Dairy Sci 2002; 85: 2223–2236 [DOI] [PubMed] [Google Scholar]
- 4.Williams EJ, Fischer DP, Noakes DE, England GC, Rycroft A, Dobson H, Sheldon IM. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 2007; 68: 549–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction 2002; 123: 837–845 [PubMed] [Google Scholar]
- 6.Herath S, Williams EJ, Lilly ST, Gilbert RO, Dobson H, Bryant CE, Sheldon IM. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction 2007; 134: 683–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 2004; 430: 257–263 [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140: 805–820 [DOI] [PubMed] [Google Scholar]
- 9.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robanya I, Richards JS. Induced expression of pattern recognition receptors in cumulus oocyte complexes: novel evidence for innate immune-like functions during ovulation. Mol Endocrinol 2006; 20: 3228–3239 [DOI] [PubMed] [Google Scholar]
- 10.Richards JS, Liu Z, Shimada M. Immune-like mechanisms in ovulation. Trends Endocrinol Metab 2008; 19: 191–196 [DOI] [PubMed] [Google Scholar]
- 11.Bromfield JJ, Sheldon IM. Lipopolysaccharide initiates inflammation in bovine granulosa cells via the TLR4 pathway and perturbs oocyte meiotic progression in vitro. Endocrinology 2011; 152: 5029–5040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subedi K, Isobe N, Nishibori M, Yoshimura Y. Changes in the expression of toll-like receptor mRNAs during follicular growth and in response to lipopolysaccharide in the ovarian follicles of laying hens. J Reprod Dev 2007; 53: 1227–1235 [DOI] [PubMed] [Google Scholar]
- 13.Magoffin DA. Ovarian theca cell. Int J Biochem Cell Biol 2005; 37: 1344–1349 [DOI] [PubMed] [Google Scholar]
- 14.Shimizu T, Miyauchi K, Shirasuna K, Bollwein H, Magata F, Murayama C, Miyamoto A. Effects of lipopolysaccharide (LPS) and peptidoglycan (PGN) on estradiol production in bovine granulosa cells from small and large follicles. Toxicol In Vitro 2012; 26: 1134–1142 [DOI] [PubMed] [Google Scholar]
- 15.Spicer LJ. Tumor necrosis factor-alpha (TNF-alpha) inhibits steroidogenesis of bovine ovarian granulosa and thecal cells in vitro. Involvement of TNF-alpha receptors. Endocrine 1998; 8: 109–115 [DOI] [PubMed] [Google Scholar]
- 16.Taylor CC, Terranova PF. Lipopolysaccharide inhibits rat ovarian thecal-interstitial cell steroid secretion in vitro. Endocrinology 1995; 136: 5527–5532 [DOI] [PubMed] [Google Scholar]
- 17.Roberts AJ, Skinner MK. Estrogen regulation of thecal cell steroidogenesis and differentiation: thecal cell-granulosa cell interactions. Endocrinology 1990; 127: 2918–2929 [DOI] [PubMed] [Google Scholar]
- 18.Metcalf MG. Estimation of viability of bovine granulosa cells. J Reprod Fertil 1982; 65: 425–429 [DOI] [PubMed] [Google Scholar]
- 19.Murasawa M, Takahashi T, Nishimoto H, Yamamoto S, Hamano S, Tetsuka M. Relationship between ovarian weight and follicular population in heifers. J Reprod Dev 2005; 51: 689–693 [DOI] [PubMed] [Google Scholar]
- 20.Ginther OJ. Selection of the dominant follicle in cattle and horses. Anim Reprod Sci 2000; 60-61: 61–79 [DOI] [PubMed] [Google Scholar]
- 21.Allegrucci C, Hunter MG, Webb R, Luck MR. Interaction of bovine granulosa and theca cells in a novel serum-free co-culture system. Reproduction 2003; 126: 527–538 [DOI] [PubMed] [Google Scholar]
- 22.Gilad E, Meidan R, Berman A, Graber Y, Wolfenson D. Effect of heat stress on tonic and GnRH-induced gonadotrophin secretion in relation to concentration of oestradiol in plasma of cyclic cows. J Reprod Fertil 1993; 99: 315–321 [DOI] [PubMed] [Google Scholar]
- 23.Wrathall JH, Knight PG. Effects of inhibin-related peptides and oestradiol on androstenedione and progesterone secretion by bovine theca cells in vitro. J Endocrinol 1995; 145: 491–500 [DOI] [PubMed] [Google Scholar]
- 24.Ginther OJ, Kot K, Kulick LJ, Wiltbank MC. Sampling follicular fluid without altering follicular status in cattle: oestradiol concentrations early in a follicular wave. J Reprod Fertil 1997; 109: 181–186 [DOI] [PubMed] [Google Scholar]
- 25.Spicer LJ. Effects of estradiol on bovine thecal cell function in vitro: dependence on insulin and gonadotropins. J Dairy Sci 2005; 88: 2412–2421 [DOI] [PubMed] [Google Scholar]
- 26.Murayama C, Miyazaki H, Miyamoto A, Shimizu T. Luteinizing hormone (LH) regulates production of androstenedione and progesterone via control of histone acetylation of StAR and CYP17 promoters in ovarian theca cells. Mol Cell Endocrinol 2012; 350: 1–9 [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto A, Okuda K, Schweigert FJ, Schams D. Effects of basic fibroblast growth factor, transforming growth factor-beta and nerve growth factor on the secretory function of the bovine corpus luteum in vitro. J Endocrinol 1992; 135: 103–114 [DOI] [PubMed] [Google Scholar]
- 28.Acosta TJ, Miyamoto A, Ozawa T, Wijayagunawardane MP, Sato K. Local release of steroid hormones, prostaglandin E2, and endothelin-1 from bovine mature follicles in vitro: effects of luteinizing hormone, endothelin-1, and cytokines. Biol Reprod 1998; 59: 437–443 [DOI] [PubMed] [Google Scholar]
- 29.Suzuki C, Yoshioka K, Iwamura S, Hirose H. Endotoxin induces delayed ovulation following endocrine aberration during the proestrous phase in Holstein heifers. Domest Anim Endocrinol 2001; 20: 267–278 [DOI] [PubMed] [Google Scholar]
- 30.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009; 458: 1191–1195 [DOI] [PubMed] [Google Scholar]
- 31.Magata F, Horiuchi M, Echizenya R, Miura R, Chiba S, Matsui M, Miyamoto A, Kobayashi Y, Shimizu T. Lipopolysaccharide in ovarian follicular fluid influences the steroid production in large follicles of dairy cows. Anim Reprod Sci 2014; 144: 6–13 [DOI] [PubMed] [Google Scholar]
- 32.Richards JS. Perspective: the ovarian follicle—a perspective in 2001. Endocrinology 2001; 142: 2184–2193 [DOI] [PubMed] [Google Scholar]
- 33.Klinge CM. Estrogen receptor interaction with co-activators and co-repressors. Steroids 2000; 65: 227–251 [DOI] [PubMed] [Google Scholar]
- 34.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011; 34: 637–650 [DOI] [PubMed] [Google Scholar]
- 35.Palhao MP, Beg MA, Rodrigues MT, Ginther OJ. Follicle and hormone dynamics in single versus double ovulating heifers. Reproduction 2009; 138: 561–570 [DOI] [PubMed] [Google Scholar]
- 36.Kadivar A, Ahmadi MR, Vatankhah M. Associations of prepartum body condition score with occurrence of clinical endometritis and resumption of postpartum ovarian activity in dairy cattle. Trop Anim Health Prod 2014; 46: 121–126 [DOI] [PubMed] [Google Scholar]