Abstract
It has been demonstrated that continuous exposure to amodiaquine (AQ) alone elicits in vitro antischistosomal activities at concentrations of 1–10 μg/ml. However, orally administered drugs reach a peak blood concentration within one or two hours and then gradually decrease. The blood concentration does not remain at a constant level over several days as in vitro concentration of continuous drug exposure. In vitro activities by one day exposure to AQ better reflect the actual antischistosomal activities after oral administration than those elicited by continuous exposure.
The objective of the present study is to compare the antischistosomal potential of one-day exposure to AQ with that to praziquantel (PZQ), a current antischistosomal drug. Schistosoma mansoni adult worm pairs were incubated with 0 (control), 1, 2, 5 and 10 μg/ml AQ as well as 0.01, 0.02, 0.05 and 0.1 μg/ml PZQ for the first day, and were subsequently incubated in drug-free media for a period of 14 days. The one-day exposure to AQ significantly reduced the daily egg output of the worm pairs at 1–10 μg/ml. The inhibitory effect on egg production continued at 5 and 10 μg/ml but proved temporary at 1 and 2 μg/ml. Furthermore, AQ-induced specific morphological alterations (severe swelling and/or localization of hemozoin) were observed in the worms at 5 and 10 μg/ml. The AQ-specific appearance of the male worms gradually faded during subsequent incubation in drug-free media, although the female worms showed elongation. Meanwhile, PZQ inhibited the egg output of adult worm pairs at concentrations of 0.01–0.1 μg/ml during exposure. The inhibitory effect on egg production continued at 0.05 and 0.1 μg/ml but proved temporary at 0.01 and 0.02 μg/ml. Furthermore, PZQ induced a visible contraction and shortening of the male and female worms at 0.05 and 0.1 μg/ml during exposure, but the PZQ-specific alterations quickly disappeared during subsequent incubation in drug-free media. To our knowledge, this is the first report showing that one-day exposure to AQ inhibits the egg production of adult worm pairs at 1–10 μg/ml and induces specific morphological alterations in the worms at 5 and 10 μg/ml. The present findings have important implications for the evaluation of the therapeutic effects of both AQ monotherapy and combination therapy with artesunate on schistosomiasis in clinical field trials.
Keywords: antischistosomal drug, amodiaquine, praziquantel, schistosomes, Schistosoma mansoni, antimalarial drug
Introduction
Schistosomiasis remains one of the most prevalent parasitic diseases in the world [1]. In spite of sustained efforts to date, an estimated 779 million people are still at risk of the disease [1], and 207 million people are currently infected. Praziquantel (PZQ) has been cited as the only current drug of choice for the treatment of schistosomiasis [2], and mass chemotherapy using PZQ remains the main strategy for schistosomiasis control. However, the extensive use of PZQ in treatment programs has raised concern about the possible emergence of PZQ-tolerant and/or resistant strains of schistosomes [3–5]. Thus, the study and development of new antischistosomal drugs that can serve as an alternative are urgently needed.
Bouglanger et al. reported a high cure rate of 100% after combination therapy using artesunate (ART) and amodiaquine (AQ) in Schistosoma haematobium-infected children [6], while Keiser et al. showed that ART monotherapy yielded a low cure rate of only 21% [7]. These findings suggested that AQ exerted antischistosomal effects synergistically with ART. Mitsui and Aoki, and Kato et al. demonstrated that continuous exposure to AQ exerted antischistosomal effects in vitro at concentrations of 1–10 μg/ml and proposed that the drug was a promising candidate for the treatment of schistosomiasis [8, 9]. However, orally administered AQ reaches the blood stream within one or two hours, achieves a peak blood concentration and then gradually decreases [10]. The blood concentration of the drug does not remain at a constant level as in vitro concentration of continuous drug exposure. In vitro activities by one day exposure to AQ better reflect the actual antischistosomal activities after oral administration than those elicited by continuous exposure. In preparation for clinical field trials of the drug, therefore, the in vitro antischistosomal effects of one-day exposure to AQ provide information that is more useful than those observed in continuous exposure.
The objective of the present study is to compare the in vitro antischistosomal effects of one-day exposure to AQ and PZQ at concentrations of 1–10 μg/ml and 0.01–0.1 μg/ml, respectively. The in vitro antischistosomal effects of AQ were examined as compared with those of PZQ regarding 1) daily egg output of S. mansoni adult worm pairs, 2) their survival and 3) the gross morphological alterations caused by the drugs and observed under a dissecting microscope.
Materials and Methods
Chemicals and medium
AQ · 2HCl and PZQ were purchased from MP Biomedicals Inc. (Fountain Parkway Solon, Ohio, USA) and Alexis Corporation (San Diego, CA, USA), respectively. AQ was diluted in deionized water (DW) at a concentration of 5 mg/ml (free base) as a stock solution. PZQ was dissolved in DW containing 1% ethanol to a concentration of 50 μg/ml as a stock solution. Each stock AQ or PZQ solution was then added to NCTC 135 medium (Sigma, St. Louis, Missouri, USA) containing a 0.5% solution of antibiotics (penicillin 10,000 units and streptomycin 10,000 μg/mL, Gibco, Langley, Oklahoma, USA). In the experiment using PZQ, ethanol was contained at a maximum concentration of 0.002% in media supplemented with 0.1 μg/ml PZQ. The ethanol concentration did not affect the viability or morphology of S. mansoni adult worms in vitro culture as compared with those in ethanol-free media.
Parasite strain
A Puerto Rican strain of S. mansoni (NIH-Sm-PR-1) had been routinely maintained by passage through Mongolian jirds (Meriones unguiculatus; MGS/Sea, Kyudo Co., Ltd., Saga, Japan) and Biomphalaria glabrata snails (Newton’s NIH Puerto Rican/Brazilian M-line) in the Animal Research Centre at the Institute of Tropical Medicine, Nagasaki University. At eight weeks post-infection, adult worms were obtained by the perfusion technique as previously described by Smithers and Terry [11] and washed twice with NCTC 135 medium.
Ethical Statement
The present investigation was approved by the Ethics Review Committee for Animal Experimentation of Nagasaki University School of Medicine, and all animal experiments were performed in accordance with the animal experimentation guidelines of the Animal Research Centre for Tropical infections in the Institute of Tropical Medicine, Nagasaki University.
Schistosomes incubation
The incubation was conducted as previously described by Mitsui et al. [12], except for the differences in the test drugs and incubation period. Before exposure to the drugs, each S. mansoni adult worm pair was preincubated for one day in a single well of a 24-well multi-well plate (Sumitomo Bakelite Co. Ltd., Osaka, Japan) with 0.5 ml of NCTC 135 medium alone in 5% CO2 incubator at a temperature of 37°C. Subsequently, 54 adult worm pairs were randomly assigned to nine groups: 0 (control), 1, 2, 5 and 10 μg/ml AQ and 0.01, 0.02, 0.05 and 0.1 μg/ml PZQ. Then, each worm pair was transferred into a well containing 0.5 ml of NCTC 135 medium alone for control or supplemented with 1, 2, 5 and 10 μg/ml AQ and 0.01, 0.02, 0.05 and 0.1 μg/ml PZQ. The plates were incubated in the 5% CO2 incubator at 37°C on the first day and subsequently with daily replacement of the media alone over a period of 14 days. The number of eggs produced daily by each adult worm pair was counted, and the worm appearance was also observed visually under a Nikon SMZ 800 stereoscopic microscope (Nikon Corporation, Tokyo, Japan) equipped with a digital Senamal C-mount camera (Microscope Network, Kawaguchi, Saitama, Japan). The “dead or alive” status of adult worms was determined according to the criteria of Mitsui et al. [12].
Data analysis
The data were analyzed using Epi-Info software (Centers for Disease Control and Prevention, Atlanta, Georgia, USA). The daily egg output per female adult worm was expressed as the arithmetic mean (± standard error). The Mann-Whitney test was used to compare daily egg output between the treated and control groups.
Results
Effects of one-day exposure to amodiaquine and praziquantel on the daily egg production of Schistosoma mansoni adult worm pairs
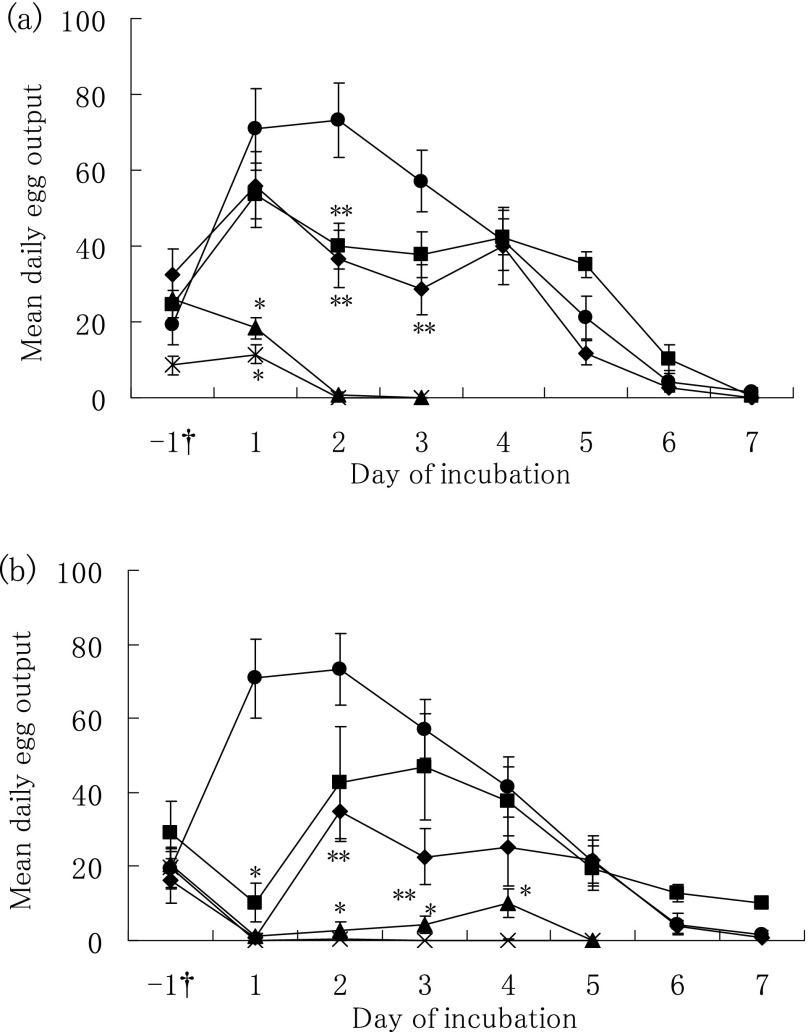
The in vitro effects of one-day exposure to AQ and PZQ on the daily egg output of S. mansoni adult worm pairs are shown in Fig. 1. After the adult worm pairs were exposed to AQ at 5 or 10 μg/ml for one day (Fig. 1-a), the mean daily egg output was continuously reduced to zero on day two. The daily egg output also showed a significant decrease on day two or three in the 1 and 2 μg/ml AQ groups as compared to that in the control group. Subsequently, the egg output gradually recovered to a level similar to that in the control group. Moreover, one-day exposure to PZQ significantly reduced the daily egg output at concentrations of 0.01–0.1 μg/ml on the first day of exposure as compared to that of the control group (Fig. 1-b). The daily egg output immediately recovered after incubation in drug-free media in the 0.01 and 0.02 μg/ml PZQ groups, but it remained depressed to zero or near zero in the 0.05 and 0.1 μg/ml PZQ groups.
Fig. 1.
Effects of one-day exposure to amodiaquine (AQ) and praziquantel (PZQ) on daily egg output of Schistosoma mansoni adult worm pairs. (a) Paired adult worms were incubated for one day with zero for control (closed circle), 1 (closed square), 2 (closed diamond), 5 (closed triangle), or 10 (cross) μg/ml AQ, or (b) with zero for control (closed circle), 0.01 (closed square) 0.02 (closed diamond), 0.05 (closed triangle), or 0.1 (cross) μg/ml PZQ, and subsequently incubated with daily replacement of media on the other days. Each bar shows the arithmetic mean ± standard error of daily egg output. *P < 0.01, **P < 0.05, compared with the corresponding control group by the Mann-Whitney test. †one day pre-incubation before worms were exposed to the drugs.
Effects of one-day exposure to amodiaquine and praziquantel on the survival of Schistosoma mansoni adult worm pairs
When S. mansoni adult worm pairs were incubated with the drugs and also continuously incubated in drug-free media over a period of 14 days, all the male and female worms survivedin all the 1, 2, 5, 10 μg/ml AQ, 0.01, 0.02, 0.05, 0.1 μg/ml PZQ and control groups.
Gross morphological alterations of Schistosoma mansoni adult worms caused by the one-day exposure to amodiaquine and praziquantel
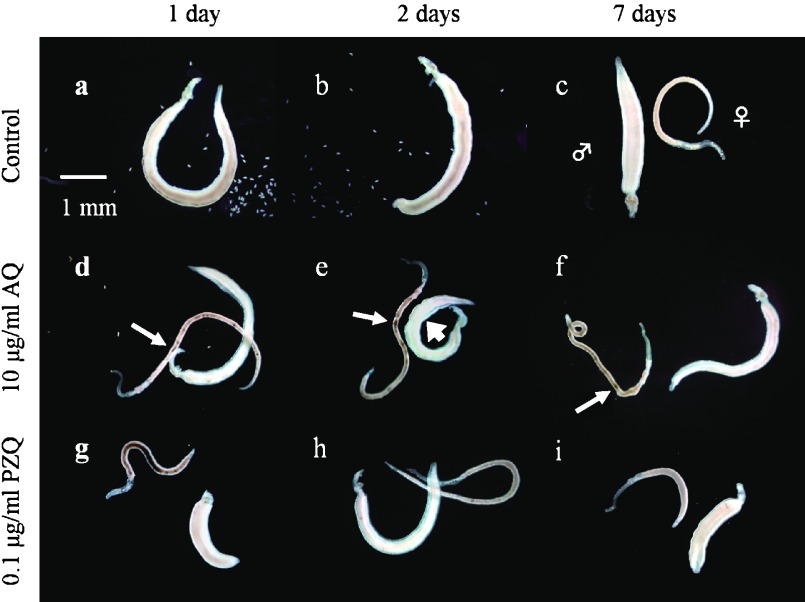
When adult worm pairs were continuously cultivated in media alone (control group), the black content, namely hemozoin [13], in the body of all male and female worms gradually disappeared over a period of three days (Fig. 2-a and -b). However, one-day exposure to AQ induced a severe swelling and/or localization of hemozoin in male worms, and a localization of hemozoin in female worms at 10 μg/ml on day one or two (Fig. 2-d and -e). Subsequently, the male worms gradually regained a normal appearance similar to that of the control group (Fig. 2-c and -f), while the localization of hemozoin was still observed in the female worms. The AQ-specific morphological alterations were also observed in all of the male and female worms incubated at 5 μg/ml but not at 1 and 2 μg/ml.
Fig. 2.
Effects of one-day exposure to amodiaquine (AQ) and praziquantel (PZQ) on the gross appearance of Schistosoma mansoni adult worm pairs. Paired adult worms were incubated drug free (control) or with 10 μg/ml AQ or 0.1 μg/ml PZQ on the first day and subsequently incubated with daily replacement of drug-free media on the other days. The male and female signs indicate male and female worms, respectively. Small and large arrows show the localization of hemozoin in female worms and the swelling in male worms, respectively.
During exposure to PZQ at 0.1 μg/ml, the male and female adult worms showed a visible contraction and shortening (Fig. 2-g). The PZQ-specific morphological alterations immediately disappeared with subsequent incubation in media alone over a period of one day (Fig. 2-h). One-day exposure to 0.05 μg/ml AQ also slightly induced the PZQ-specific alterations. However, the PZQ-specific alterations were not observable at 0.01 and 0.02 μg/ml.
Discussion
When S. mansoni adult worm pairs were exposed to AQ for the first one day and subsequently incubated in drug-free media, the inhibitory effect of one-day exposure to AQ on the daily egg output continued at 5 and 10 μg/ml but proved temporary at 1 and 2 μg/ml. Thus, the minimal concentration of AQ that inflicts irreversible damage on the egg production of female adult worm is presumed to lie between 2 and 5 μg/ml. Furthermore, the one-day exposure to AQ also induced the specific alterations (severe swelling and/or localization of hemozoin) in the male and female adult worms at 5 and 10 μg/ml. The alterations were the same as those caused by continuous exposure to AQ [9]. With subsequent incubation in drug-free media, all the female worms maintained the AQ-specific morphological damage during the 14-day incubation period, while the male worms gradually regained their normal appearance. The female worms were apparently more susceptible to AQ than the male worms. This result was consistent with experimental evidence that the treatment of S. mansoni-infected mice with chloroquine, which is similar in structure to AQ [14], caused morphological damage in female worms but not in male worms. In addition, the present study revealed that more than 2 μg/ml was required for AQ to inflict continuous damage on the female adult worms. However, after oral administration of a regular dose of AQ for the treatment of malaria in humans (10 mg/kg of body weight, once daily for three days) [15], the blood concentration of AQ and the major metabolite of N-desethylamodiaquine (DEAQ) may reach a level close to 1 μg/ml [10, 16]. Thus, it is surmised that the standard dose of AQ for the treatment of malaria may not be sufficient to produce the desired therapeutic effect in the treatment of schistosomiasis.
PZQ inhibited the daily egg output of adult worm pairs and induced specific morphological alterations in adult worms at lower concentrations than AQ. As shown by Pica-Mattoccina and Cioli [17], one-day exposure to PZQ also induced a visible contraction and shortening of worms at 0.1 μg/ml during the period of exposure. One day after subsequent incubation in drug-free media, the specific alterations quickly disappeared. All the male and female worms survived the 14-day period of incubation. The morphological alterations of the worms induced by PZQ occurred and disappeared more quickly than those caused by AQ. Pica-Mattoccina and Cioli also showed that all the adult worms exposed in vitro to PZQ overnight at a concentration of 0.125 μg/ml survived for seven days [17]. Thus, it is surmised that one-day exposure to PZQ also fails to inflict irreversible damage on adult worms at a concentration of 0.1 μg/ml. In addition, Shaw and Erasmus demonstrated that although PZQ caused damages to the reproductive system of in vivo paired female worms, the damaged reproductive system completely redeveloped and eventually resumed egg production [18]. The in vitro inhibitory effect of PZQ on egg production observed in the present study may not imply irreversible damage to the reproductive system of paired female worms.
Although the mechanism of PZQ activity has not been elucidated in detail, experimental evidence suggests that the drug induces a rapid contraction of schistosomes, accompanied by an increasingly rapid permeability of the cell membrane towards calcium ions [19]. On the other hand, Oliver et al. showed that chloroquine [14], one of the 4-aminoquinoline derivatives similar in structure to AQ, exerted antischistosomal activities by inhibiting the formation of hemozoin, a detoxification product of free haem metabolized from haemoglobin. Similarly, it has been surmised that the free haem caused by AQ may be responsible for damaging the reproductive system of female worms and inducing the specific morphological alterations of male and female worms [9]. The differences in the mode of action of AQ and PZQ are thought to contribute to differences in the specific morphological alterations caused by the two drugs, as well as in the onset of morphological alterations. The great differences in the mode of action shown by the two dugs suggest that AQ may be effective on PZQ-tolerant and/or resistant strains of schistosomes. Therefore, AQ deserves further investigation as a promising new candidate for the treatment of schistosomiasis.
Some field trials have revealed the strong antischistosomal effects of artemisinin-based combination therapies (ACTs) and underlined the antischistosomal activity of the current antimalarial drugs used in those trials [6, 7, 20, 21]. The in vitro antischistosomal effects of mefloquine, AQ, primaquine and so on have also been investigated, evidence that the current antimalarial drugs are attracting attention as promising new antischistosomal drugs [8, 22, 23]. Since the distribution of both malaria and schistosomiasis exhibits a large geographical overlap in sub-Saharan Africa [24], the use of AQ alone or ACT with AQ for the treatment of malaria may produce an additional therapeutic effect on schistosomiasis. Thus, the present findings are noteworthy and can be expected to encourage further research and evaluations regarding the therapeutic effect of AQ on schistosomiasis in field trials. However, it is possible that the extensive use of AQ may raise concerns about the emergence of AQ-tolerant and/or resistant strains of schistosomes, as has been seen in the use of PZQ [3–5]. Therefore, the use of AQ for the treatment of schistosomiasis should be carried out with ample caution regarding the emergence of AQ-tolerant and/or resistant strains of schistosomes.
Acknowledgements
I would like to thank Prof. Yoshiki Aoki, Graduate School of International Health Development, Nagasaki University for assisting in the revision of the manuscript. I also thank Mr. Mitsumasa Miura, Department of Parasitology, Institute of Tropical Medicine, Nagasaki University for technical support in the present experiments.
References
- 1.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 2006; 6: 411–425 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: Report of a WHO expert committee. WHO Tech Rep Ser No 912. Geneva: WHO; 2006 [PubMed]
- 3.Fallon PG, Doenhoff MJ. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am J Trop Med Hyg 1994; 51: 83–88 [DOI] [PubMed] [Google Scholar]
- 4.Guisse F, Polman K, Stelma FF, Mbaye A, Talla I, Niang M, Deelder AM, Ndir O, Gryseels B. Therapeutic evaluation of two different dose regimens of praziquantel in a recent Schistosoma mansoni focus in Northern Senegal. Am J Trop Med Hyg 1997; 56: 511–514 [DOI] [PubMed] [Google Scholar]
- 5.Ismail M, Botros S, Metwally A, William S, Farghally A, Tao LF, Day TA, Bennett JL. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg 1999; 60: 932–935 [DOI] [PubMed] [Google Scholar]
- 6.Boulanger D, Dieng Y, Cisse B, Remoue F, Capuano F, Dieme JL, Ndiaye T, Sokhna C, Trape JF, Greenwood B, Simondon F. Antischistosomal efficacy of artesunate combination therapies administered as curative treatments for malaria attacks. Trans R Soc Trop Med Hyg 2007; 101: 113–116 [DOI] [PubMed] [Google Scholar]
- 7.Keiser J, N’Guessan NA, Adoubryn KD, Silué KD, Vounatsou P, Hatz C, Utzinger J, N’Goran EK. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin Infect Dis 2010; 50: 1205–1213 [DOI] [PubMed] [Google Scholar]
- 8.Mitsui Y, Aoki Y. In vitro effects of current antimalarial drugs on the survival of paired Schistosoma mansoni adult worms and their egg production. Trop Med Health 2010; 38: 69–73 [Google Scholar]
- 9.Kato K, Miura M, Mitsui Y. In vitro effects of amodiaquine on paired Schistosoma mansoni adult worms at concentrations of less than 5 μg/ml. Mem Inst Oswaldo Cruz 2013; 108: 192–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winstanley P, Edwards G, Orme M, Breckenridge A. The disposition of amodiaquine in man after oral administration. Br J Clin Pharmacol 1987; 23: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smithers SR, Terry RJ. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology 1965; 55: 695–700 [DOI] [PubMed] [Google Scholar]
- 12.Mitsui Y, Miura M, Aoki Y. In vitro effects of artesunate on the survival of worm pairs and egg production of Schistosoma mansoni. J Helminthol 2009; 83: 7–11 [DOI] [PubMed] [Google Scholar]
- 13.Oliveira MF, Kycia SW, Gomez A, Kosar AJ, Bohle DS, Hempelmann E, Menezes D, Vannier-Santos MA, Oliveira PL, Ferreira ST. Structural and morphological characterization of hemozoin produced by Schistosoma mansoni and Rhodnius prolixus. FEBS Lett 2005; 579: 6010–6016 [DOI] [PubMed] [Google Scholar]
- 14.Oliveira MF, d’Avila JC, Tempone AJ, Soares JB, Rumjanek FD, Ferreira-Pereira A, Ferreira ST, Oliveira PL. Inhibition of heme aggregation by chloroquine reduces Schistosoma mansoni infection. J Infect Dis 2004; 190: 843–852 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. The use of antimalarial drugs: Report of an informal consultation. WHO (WHO/CDS/RBM/2001.33). Geneva: WHO; 2001
- 16.Gitau EN, Muchohi SN, Ogutu BR, Githiga IM, Kokwaro GO. Selective and sensitive liquid chromatographic assay of amodiaquine and desethylamodiaquine in whole blood spotted on filter paper. J Chromatography B Analyt Technol Biomed Life Sci 2004; 799: 173–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pica-Mattoccia L, Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int J Parasitol 2004; 34: 527–533 [DOI] [PubMed] [Google Scholar]
- 18.Shaw MK, Erasmus DA. Schistosoma mansoni: praziquantel-induced changes to the female reproductive system. Exp Parasitol 1988; 65: 31–42 [DOI] [PubMed] [Google Scholar]
- 19.Ali BH Review: a short review of some pharmacological, therapeutic and toxicological properties of praziquantel in man and animals. Pak J Pharm Sci 2006; 19: 170–175 [PubMed] [Google Scholar]
- 20.Mohamed AA, Mahgoub HM, Magzoub M, Gasim GI, Eldein WN, Ahmed Ael A, Adam I. Artesunate plus sulfadoxine/pyrimethamine versus praziquantel in the treatment of Schistosoma mansoni in eastern Sudan. Trans R Soc Trop Med Hyg 2009; 103: 1062–1064 [DOI] [PubMed] [Google Scholar]
- 21.Sissoko MS, Dabo A, Traoré H, Diallo M, Traoré B, Konaté D, Niaré B, Diakité M, Kamaté B, Traoré A, Bathily A, Tapily A, Touré OB, Cauwenbergh S, Jansen HF, Doumbo OK. Efficacy of artesunate + sulfamethoxypyrazine/pyrimethamine versus praziquantel in the treatment of Schistosoma haematobium in children. PLoS One 2009; 4: e6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao SH, Mei JY, Jiao PY. The in vitro effect of mefloquine and praziquantel against juvenile and adult Schistosoma japonicum. Parasitol Res 2009; 106: 237–246 [DOI] [PubMed] [Google Scholar]
- 23.Holtfreter MC, Loebermann M, Klammt S, Sombetzki M, Bodammer P, Riebold D, Kinzelbach R, Reisinger EC. Schistosoma mansoni: schistosomicidal effect of mefloquine and primaquine in vitro. Exp Parasitol 2001; 127: 270–276 [DOI] [PubMed] [Google Scholar]
- 24.Kabatereine NB, Standley CJ, Sousa-Figueiredo JC, Fleming FM, Stothard JR, Talisuna A, Fenwick A. Integrated prevalence mapping of schistosomiasis, soil-transmitted helminthiasis and malaria in lakeside and island communities in Lake Victoria, Uganda. Parasit Vectors 2011; 4: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]