Abstract
A 55-year-old woman with metastatic human epidermal growth factor receptor 2 (HER-2) positive breast cancer (BC) to the lungs and bones was diagnosed with central nervous system (CNS) metastases in November 2011. The MRI showed a right parietal lobe mass with adjacent leptomeningeal disease and several small bilateral cerebellar metastases. She was treated with whole brain irradiation (WBI), followed by capecitabine and lapatinib (December 2011-March 2013) and trastuzumab and lapatinib (May 2013-August 2013). Then, the brain MRI showed progression. In the absence of significant neurological symptoms, we postponed WBI and closely monitored for the development of neurological symptoms. Systemic treatment with trastuzumab emtansine (T-DM1), an antibody-drug conjugate composed of the cytotoxic agent DM1 conjugated to trastuzumab, was initiated in September 2013 to control systemic disease. Unexpectedly, after two cycles of treatment the brain MRI showed a decrease in size of CNS metastases. This case report suggests possible activity of T-DM1 in HER-2 positive BC with CNS metastases.
Background
One-third of patients with human epidermal growth factor receptor 2 (HER-2) positive breast cancer develop central nervous system (CNS) metastases during the course of their disease. The activity of anti-HER-2 systemic targeted antibody-based therapies in the CNS is suggested to be limited by their inability to cross the blood-brain barrier. While trastuzumab is considered too large to cross the blood-brain barrier, the combination of lapatinib and capecitabine has shown activity in CNS metastases. In the absence of systemic therapies with good activity in the CNS, local therapies consisting of surgery and/or radiotherapy (whole brain radiotherapy or stereotactic radiosurgery) are the standard of care for the management of CNS breast cancer metastases. The activity of T-DM1 (an antibody-drug conjugate composed of the cytotoxic agent DM1 conjugated to trastuzumab) in CNS metastases is not clearly defined. This case report suggests that T-DM1 is active in CNS metastases.
Case presentation
In November 2011, a 55-year-old woman with metastatic HER-2 positive breast cancer to the bones and lungs developed symptoms of frontal headache, photophobia and dizziness. The MRI of the brain showed metastatic CNS disease involving the brain and leptomeninges.
She had been initially diagnosed 7 years earlier (May 2003) with locally advanced HER-2 positive invasive ductal carcinoma of the left breast. She received six cycles of neoadjuvant chemotherapy with cyclophosphamide, epirubicin and 5-fluorouracil, followed by a left modified radical mastectomy. Pathology showed a 5 cm residual invasive ductal carcinoma, micropapillary type, grade 2, with lymphovascular invasion; 9 of 24 axillary lymph nodes were involved, with extranodal extension and tumour emboli in the lymphatics and blood vessels. The residual tumour did not express oestrogen or progesterone receptors but the HER-2/neu oncoprotein was overexpressed. Owing to the extensive residual disease in the mastectomy specimen, she received four cycles of adjuvant docetaxel prior to radiotherapy to the left chest wall and supraclavicular area (5000 cGy in 25 fractions). One year of adjuvant trastuzumab was started later, in April 2006, on approval of adjuvant trastuzumab based on the results presented in 2005.
The patient remained disease-free until May 2010, when she developed extensive metastatic disease to the bones and lungs and was enrolled in a phase II trial with nabpaclitaxel and trastuzumab. On disease progression to the CNS in November 2011, with a 2.9 cm right parietal lobe mass with adjacent leptomeningeal disease and several small bilateral cerebellar metastases, she was treated with whole brain irradiation (20 Gy in 5 fractions, from 2 December to 8 December 2011). Chemotherapy was switched to capecitabine and lapatinib on 13 December 2011. Although CNS disease remained under control, systemic treatment was changed to trastuzumab and lapatinib in May 2013, after documentation of disease progression in the lungs and pleura.
In the Summer of 2013, the patient reported of extreme tiredness and felt off-balance. New scans were obtained that showed disease progression in the bone and brain. Whole brain reirradiation was considered, but since the neurological symptoms were not significantly affecting the patient's quality of life, we decided to monitor the patient closely for the development of significant symptoms and repeat the brain MRI 1 month later. Systemic treatment was switched to T-DM1 on 18 September 2013.
Investigations
The brain MRI with intravenous contrast, prior to the beginning of treatment with T-DM1, showed innumerable supratentorial and infratentorial metastases with evidence of leptomeningeal disease. Comparing with previous imaging, there was an increase in size of several lesions, particularly the right parietal and right cerebellar metastasis (measuring 10×6 mm), right and left thalamic metastases and an increase in the extent of the leptomeningeal disease around the right parietal lesion.
After two cycles of T-DM1, the brain MRI showed an interval decrease in the size of some of the metastases and leptomeningeal disease. After seven cycles of treatment, a further decrease in the size of the lesions was observed, with stability of the other lesions (figures 1–3).
Figure 1.
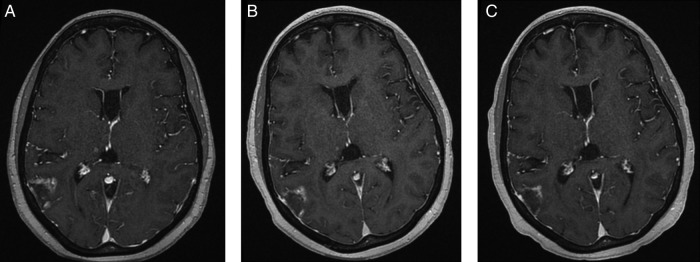
Gadolinium-enhanced FSPGR T1 (TR/TE/FA 8.5/4.2/20) postcontrast images demonstrate interval decrease in nodular enhancement around the posterior right temporal metastasis from A (pretreatment) to B (1-month post-T-DM1) and C (4 months post-T-DM1).
Figure 2.
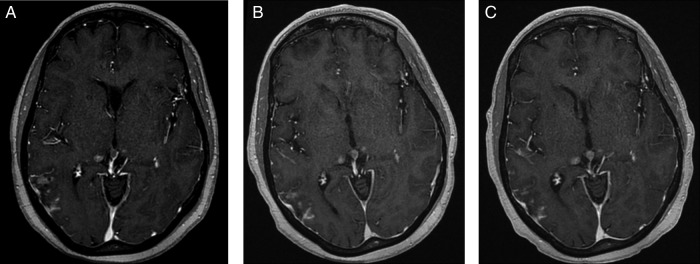
Gadolinium-enhanced FSPGR T1 (TR/TE/FA 8.5/4.2/20) postcontrast images from A (pretreatment) to B (1-month post-T-DM1) and C (4 months post-T-DM1) demonstrate interval decrease in leptomeningeal enhancement just inferior to mass demonstrated in figure 1. There is no growth of the right and left thalamic metastases. Also, note interval growth of pineal gland metastasis.
Figure3.
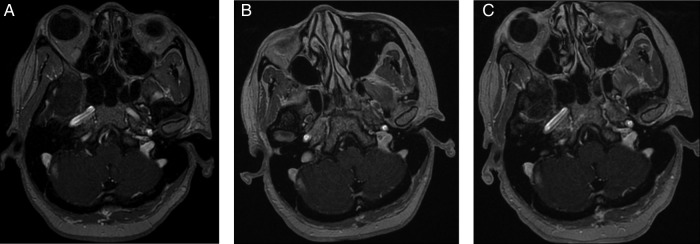
Gadolinium-enhanced FSPGR T1 (TR/TE/FA 8.5/4.2/20) postcontrast images from A (pretreatment) to B (1-month post-T-DM1) and C (4 months post-T-DM1) demonstrate stable appearance of the right cerebellar metastasis.
Treatment
Single agent T-DM1, 3.6 mg/kg intravenously every 21 days.
Outcome and follow-up
Patient remains on treatment with T-DM1, having completed the 13th cycle of T-DM1 on 27 May 2014, with no significant side effects from the treatment. She is asymptomatic, with stable systemic and CNS disease.
Discussion
Patients with HER-2 positive metastatic breast cancer are at increased risk of CNS disease. One-third of patients develop CNS metastases during the course of their disease, even in the setting of well-controlled systemic disease.1 Surgery and/or radiation (whole brain radiotherapy or stereotactic radiosurgery) is the standard of care for the initial management of CNS metastases.
The activity of anti-HER-2 antibody based targeted therapies in the CNS is limited by the antibody's inability to cross the blood-brain barrier. Trastuzumab is a large molecule that does not cross the blood-brain barrier, but when the blood-brain barrier is impaired (in the setting of meningeal carcinomatosis, brain metastases or whole-brain radiotherapy), trastuzumab levels were reported to be increased in the cerebrospinal fluid.2 On the contrary, lapatinib, a smaller molecule, seems to cross the blood-brain barrier and is active in CNS disease when combined with capecitabine.3
T-DM1 is an antibody drug conjugate consisting of a highly potent microtubule polymerisation inhibitor (DM1) linked to trastuzumab via a stable linker. It was approved for the treatment of metastatic HER-2 positive breast cancer based on the results of the EMILIA trial.4 In this phase III study, T-DM1 significantly prolonged progression-free and overall survival compared with lapatinib and capecitabine in previously treated patients with metastatic HER-2 positive breast cancer. Patients with symptomatic CNS disease at baseline were excluded from this study. Data from a retrospective study using the patients enrolled in the EMILIA trial showed that the rate of CNS progression was low and similar in both treatment arms. In the subset of patients with CNS metastases at baseline, T-DM1 was associated with significantly improved overall survival.5
Although there is no data from prospective studies supporting that T-DM1 can produce CNS tumour shrinkage or stabilisation, this case report suggests that T-DM1 is active in CNS disease. Recently, other authors also reported a case of CNS metastases responding to systemic treatment with T-DM1.6
Learning points.
Patients with human epidermal growth factor receptor 2 (HER-2) positive breast cancer frequently develop central nervous system (CNS) metastases, even in the setting of well-controlled systemic disease.
Local therapies (surgery and/or radiation) are usually the initial options for the management of CNS disease.
The activity of systemic antibody-based therapies in the CNS is limited by the antibody's inability to cross the blood-brain barrier intact’.
This case suggests that T-DM1 is active in patients with HER-2 positive CNS metastases.
Footnotes
Contributors: SV is the primary doctor, responsible for medical decisions. He reviewed the draft of the case report and he is the guarantor. ST collected information, drafted the case report and revised the final paper. She also participated in the patient's care. PM reported the brain imaging, chose and reported the pictures that illustrate the case and also reviewed the paper.
Competing interests: Dr Sunil Verma: Advisory Board and Honoraria from Roche.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol 2009;27:5278–86 [DOI] [PubMed] [Google Scholar]
- 2.Stemmler HJ, Schmitt M, Willems A, et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer with brain metastases and impaired blood-brain barrier. Anticancer Drugs 2007;18:23–8 [DOI] [PubMed] [Google Scholar]
- 3.Lin NU, Dieras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from Her2-positive breast cancer. Clin Cancer Res. 2009;15:1452–9 [DOI] [PubMed] [Google Scholar]
- 4.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krop I, Lin N, Blackwell K, et al. Efficacy and safety of trastuzumab emtansine (T-DM1) vs lapatinib plus capecitabine in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer and central nervous system metastases: results from a retrospective exploratory analysis of EMILIA. Poster Presentation, SABCS, 2013 [DOI] [PMC free article] [PubMed]
- 6.Bartsch R, Berghoff AS, Preusser M. Breast cancer brain metastases responding to primary systemic therapy with T-DM1. J Neurooncol 2014;116:205–6 [DOI] [PubMed] [Google Scholar]


