Abstract
A report on two homozygous twin girls affected by X linked hypophosphataemic rickets. They were examined due to short stature and genu varum of both tibias. They were treated with calcitriol and Joulie's solution, whereon it was observed that serum parathyroid hormone and phosphaturia decreased while phosphataemia increased. They underwent a tibial osteotomy (by means of the insertion of Kirchner needles) at 7.7 years of age for correction of genu varum and a normal consolidation was reached 1 month later. Nonetheless, height was percentile <1 after menarche, so both sisters asked for bone lengthening. Because of this, at 15 years of age femoral distraction was performed, but no bone callus was observed 14 months later. Consequently, they were treated with subcutaneous growth hormone, showing bone callus at 6 months. Finally, the external fixators were removed due to ossification in the lengthened segments.
Background
There are various forms of hypophosphataemic rickets (HF), characterised by a deficit in the reabsorption of inorganic phosphate in the proximal tubule of the kidney with a compromise in bone mineralisation. This can produce bone deformations and short stature, which are also accompanied by inappropriately normal concentrations of 1,25(OH)2D3 in the face of hypophosphataemia.
HF includes a group of forms that are dependent FGF23 (X linked hypophosphataemic rickets (XLH), autosomal dominant and autosomal recessive). Fibroblast growth factor 23 (FGF23; or FGF23 protein) belongs to the group of phosphatonins (protein factors or hormones responsible for regulating phosphorus) and, in fact, is currently the best characterised phosphatonin. These diseases are caused by mutations in various genes involved in the regulation of renal phosphate reabsorption (PHEX, FGF23, DMP1 and ENPP1), which induce a rise in the circulating levels of FGF23.
HF also includes a group of independent forms of FGF23, such as hereditary hypophosphataemic rickets with hypercalciuria, which is caused by mutations in a gene encoding a transporter of sodium dependent phosphate (SLC34A3).1
The most common form is XLH. The gene responsible is the PHEX located on the short arm of the X chromosome in the Xp22.1 region. There are also known forms of autosomal dominant hypophosphataemic rickets (produced by mutations in the FGF23 gene, located on the 12p13 chromosome) in addition to forms with sporadic onset.
Affected newborns have normal weight, but children may suffer growth retardation. Intellectual development is unaffected. Widened joint spaces and flaring at the knees may become apparent in infants during their first year of life, particularly in boys. When a child begins to stand and walk, bowing of the weight-bearing long bones quickly becomes clinically evident. Dentition may be absent or delayed in very young children; older children may experience multiple dental abscesses.
Conditions to consider in the differential diagnoses of hypophosphataemic rickets are: renal tubular acidosis, hereditary hypophosphataemic rickets with hypocalciuria, Fanconi syndrome (types I and II), vitamin D-dependent rickets (types I and II), vitamin D-deficient rickets and pseudohypoparathyroidism.2
Therapeutic resources include calcitriol, growth hormone (GH), phosphates and anticalciurics to promote the growth of healthy bone and to decrease the mineral loss associated with hypophosphataemic rickets. During therapy, acute hypercalcaemia can occur, which can produce irritability, mental confusion and even seizures. A more damaging toxicity is nephrocalcinosis, produced by prolonged treatment with calcitriol.3
A few clinical trials have been conducted, with relatively few patients, for the purpose of improving final height. However, the majority of these studies conclude that patients treated with GH, in conjunction with calcitriol and oral phosphates, demonstrate improvement in growth but not in body proportions, which can sometimes worsen.3–6 Also, there are two kinds of autosomal recessive hypophosphataemic rickets.
We report a case about the effect of GH treatment in homozygous twin sisters with XLH after bone distraction without bone callus formation.
Case presentation
Identical (homozygous) twin sisters who were seen at 3.9 years of age due to short stature and genu varum of both tibias. Family and personal histories were negative for other skeletal abnormalities. Birth weight: 2880 g (sister A), and 2890 g (sister B). Height: 88 and 87 cm (percentile <1) and weight 12 kg (percentile 3), respectively.
Radiological findings
Leg comparison radiography showed genu varum in both girls with signs of rickets on the physes and metaphyses (widening) of the femur, tibia and fibula. Bone age of both: 1.6 years. Bone density scan: normal; it was performed using a densitometer, Hologic QDR 1000 (Waltham, Massachusetts, USA).
Laboratory analysis
As shown in table 1, serum and urine bone biomarkers were measured in both sisters. The molecular study was carried out after DNA isolation from peripheral blood lymphocytes. The amplification of the PHEX gene (chromosome Xp22.12.9) confirmed heterozygous mutation c.1965+1G>T in both sisters. Molecular study was normal in both parents, so it suggested that these cases were de novo mutations.
Table 1.
Laboratory assays for bone biomarkers in each girl at time of diagnosis
| Sister A | Sister B | Normal levels | |
|---|---|---|---|
| Calcium, (mg/dL) | 9.2 | 9 | 8.4–10.2 |
| Phosphorus, (mg/dL) | 2.52 | 2.36 | 2.7–4.5 |
| PTH, (mg/dL) | 70 | 74 | 10–65 |
| Serum phosphorus/creatinine, (mg/dL) | 0.21 | 0.21 | 0.5–1.10 |
| Calcitonin, (pg/mL) | 11 | 9 | 0–11.4 |
| Osteocalcin, (ng/mL) | 7.2 | 5.9 | 5–25 |
| 25(OH)D | 68 ng/mL | 36 mg/mL | 30–100 ng/mL |
| 1,25(OH)2D, (pg/mL) | 19 | 19 | 15–60 |
| Total alkaline phosphatase, (UI/L) | 915 | 385 | 30–115 |
| Phosphate reabsorption rate, (%) | 80 | 83 | 90 |
| TmP/GFR*, (mg/dL) | 2.20 | 1.92 | 4–8 |
*TmP/GFR: theoretical renal phosphate threshold.
PTH, parathyroid hormone; GFR, glomerular filtration rate.
Treatment
The twins were treated with calcitriol 0.5/0.75 µg/24 h and Joulie's solution (sodium phosphate 13.6 g, phosphoric acid 85% 5.88 g, water 100 mL) administering 5 mL/4 h. Numerous checks were made of serum bone biomarkers, and 2 years later a normalisation of PTH and a moderate increase in phosphataemia were observed, although normal levels were not reached. Calcitriol and Joulie's solution are maintained until today, after gradually adjusting the dose according to body weight.
Disappearance of the radiological signs of rickets: At 7.7 years of age, the genu varum in both children was corrected by bilateral tibial and peroneal osteotomy and the insertion of Kirchner needles, which were removed 1 month later.
In both children, height was less than percentile 1 after menarche (sister A 143.3 cm, sister B 141.3 cm) so at 15 years of age, they requested a bone lengthening due to short stature and dissymmetry of the truck and lower limbs, once informed consent had been signed. The lengthening technique was a bilateral femoral osteotomy and use of dynamic axial fixators.
Outcome and follow-up
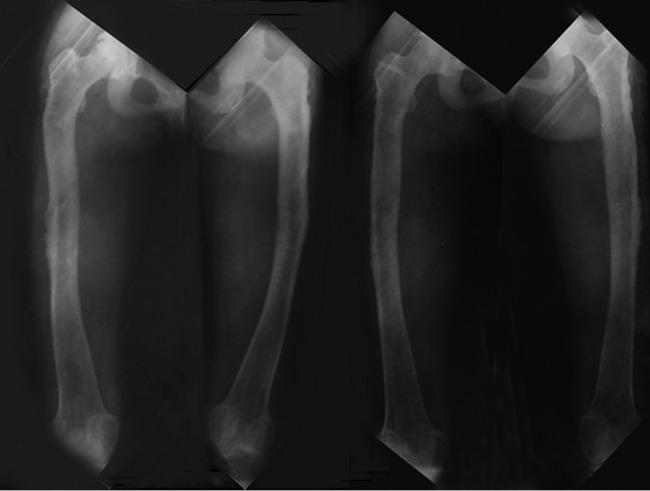
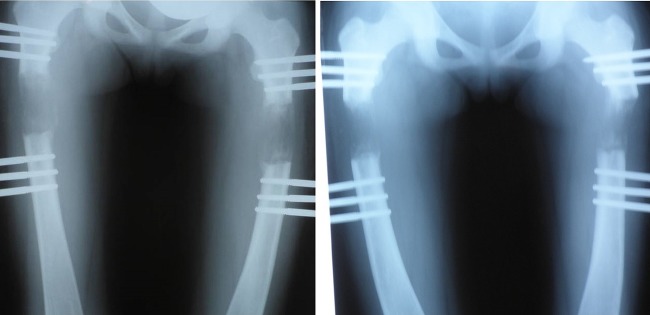
After 14 months of orthopaedic treatment, the absence of bone callus and the impossibility of removing the lengtheners were observed (figure 1), thus, treatment with GH at 0.035 mg/kg/24 h for 6 months was decided on, observing bone mineralisation of the osteoid zone and removing the orthopaedic fixators (figure 2).
Figure 1.
Child A and Child B, 14 months after bone distraction was initiated.
Figure 2.
Child A and Child B, right and left femurs of both children, after 6 months of treatment with growth hormone and subsequent removal of external fixators.
Discussion
The lack of consolidation or production of bone remodelling occurs in fewer than 5% of cases due to intrinsic factors of the patient such as malnutrition, infection, dysplasia secondary to metabolic changes, or endocrine, kidney, heart or liver disorders. These patients had XLH, which may have brought about the lack of consolidation of both fragments of the femur. Nonetheless, the lack of consolidation did not occur in the tibias, when correction of genu varum was performed at an earlier age. We say that bone age of both was 1.6 years knowing that when there are rickets, determining bone age is sometimes inaccurate. In hypophosphataemia, rickets typically occur as disharmonious growth, as noted in these twin sisters.
The mechanism of action of GH for ossification is not well known. Several studies in animal models indicate that systemic administration of recombinant homologous GH accelerates ossification of bone regenerate in distraction osteogenesis.7 8
It could also be due to the expression of other factors involved in growth, but increased expression of the GH receptor (GHR) has been found. The upregulation of the GHR gene expression in osteogenesis due to bone distraction may increase sensitivity to the endogenous GH systemic system and promote bone healing and regeneration. However, other authors believe that the effect of GH on the bone with distraction osteogenesis may be mediated by insulin-like growth factor-I. Results obtained in treatment of osteogenesis imperfecta in conjunction with bisphosphonates, versus those treated with bisphosphonates alone, led to short-term (6 months) treatment of these patients with GH. Testing with subcutaneous injections of GH in mice increased cortical bone layer of the femur, as well as increasing the linear growth and the biomechanical properties of this bone, without acting directly on collagen.9
Treatment with GH to improve stature in patients with XLH appears to improve the phosphorus state in the body and bone mineral density, which may have contributed to the consolidation of the bone.10
One might think that after distraction technique the fracture consolidation would arise spontaneously. However, based on the evolution of the two sisters it may have been a coincidence, which leads us to believe GH treatment was the reason that the distracted bone went on to mineralise (since no bone callus was formed previously).
Future studies are needed to explain the mechanisms of action of GH on bone remodelling and its possible contribution towards producing bone callus. It would be useful to evaluate GH as an adjuvant therapy in bone fractures.
Learning points.
Our results suggest that growth hormone has been shown to be useful in surgical bone fracture consolidation.
Based on these results, we propose the use of recombinant growth hormone (GH) for bone callus formation in fractures of other aetiologies.
This use of GH can be especially interesting in the healing of fractures in bone mineralisation disorders.
Acknowledgments
To Mª Dolores Cañete, for her technical assistance in laboratory analysis.
Footnotes
Contributors: FV-R carried out the orthopaedic treatment. RC, MA-Q prescribed the treatment and conducted the clinical control evolution. JC-V performed the laboratory analysis and wrote the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review : Not commissioned; externally peer reviewed.
References
- 1.Prié D, Friedlander G. Genetic disorders of renal phosphate transport. N Engl J Med 2010;362:2399–409 [DOI] [PubMed] [Google Scholar]
- 2.Santos F, Fuente R, Mejia N, et al. Hypophosphatemia and growth. Pediatr Nephrol 2013;28:595–603 [DOI] [PubMed] [Google Scholar]
- 3.Haffner D, Nissel R, Wühl E, et al. Effects of growth hormone treatment on body proportions and final height among small children with X-linked hypophosphatemic rickets. Pediatrics 2004;113:e593–6 [DOI] [PubMed] [Google Scholar]
- 4.Baroncelli GI, Bertelloni S, Ceccarelli C, et al. Effect of growth hormone treatment on final height, phosphate metabolism, and bone mineral density in children with X-linked hypophosphatemic rickets. J Pediatr 2001;138:236–43 [DOI] [PubMed] [Google Scholar]
- 5.Makitie O, Toiviainen-Salo S, Marttinen E, et al. Metabolic control and growth during exclusive growth hormone treatment in X-linked hypophosphatemic rickets. Horm Res 2008;69:212–20 [DOI] [PubMed] [Google Scholar]
- 6.Živičnjak M, Schnabel D, Staude H, et al. ; Hypophosphatemic Rickets Study Group of the Arbeitsgemeinschaft für Pädiatrische Endokrinologie and Gesellschaft für Pädiatrische Nephrologie. Three-year growth hormone treatment in short children with X-linked hypophosphatemic rickets: effects on linear growth and body disproportion. J Clin Endocrinol Metab 2011;96:E2097–105 [DOI] [PubMed] [Google Scholar]
- 7.Antoniazzi F, Monti E, Venturi G, et al. GH in combination with bisphosphonate treatment in osteogenesis imperfecta. Eur J Endocrinol 2010;163:479–87 [DOI] [PubMed] [Google Scholar]
- 8.Monti E, Mottes M, Fraschini P, et al. Current and emerging treatments for the management of osteogenesis imperfecta. Ther Clin Risk Manag 2010;6:367–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King D, Jarjoura D, McEwen HA, et al. Growth hormone injections improve bone quality in a mouse model of osteogenesis imperfecta. J Bone Miner Res 2005;20:987–93 [DOI] [PubMed] [Google Scholar]
- 10.Wilson DM. Growth hormone and hypophosphatemic rickets. J Pediatr Endocrinol Metab 2000;13(Suppl 2):993–8 [PubMed] [Google Scholar]