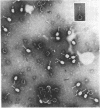
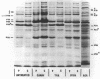
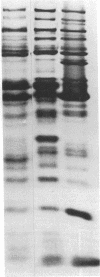
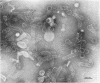
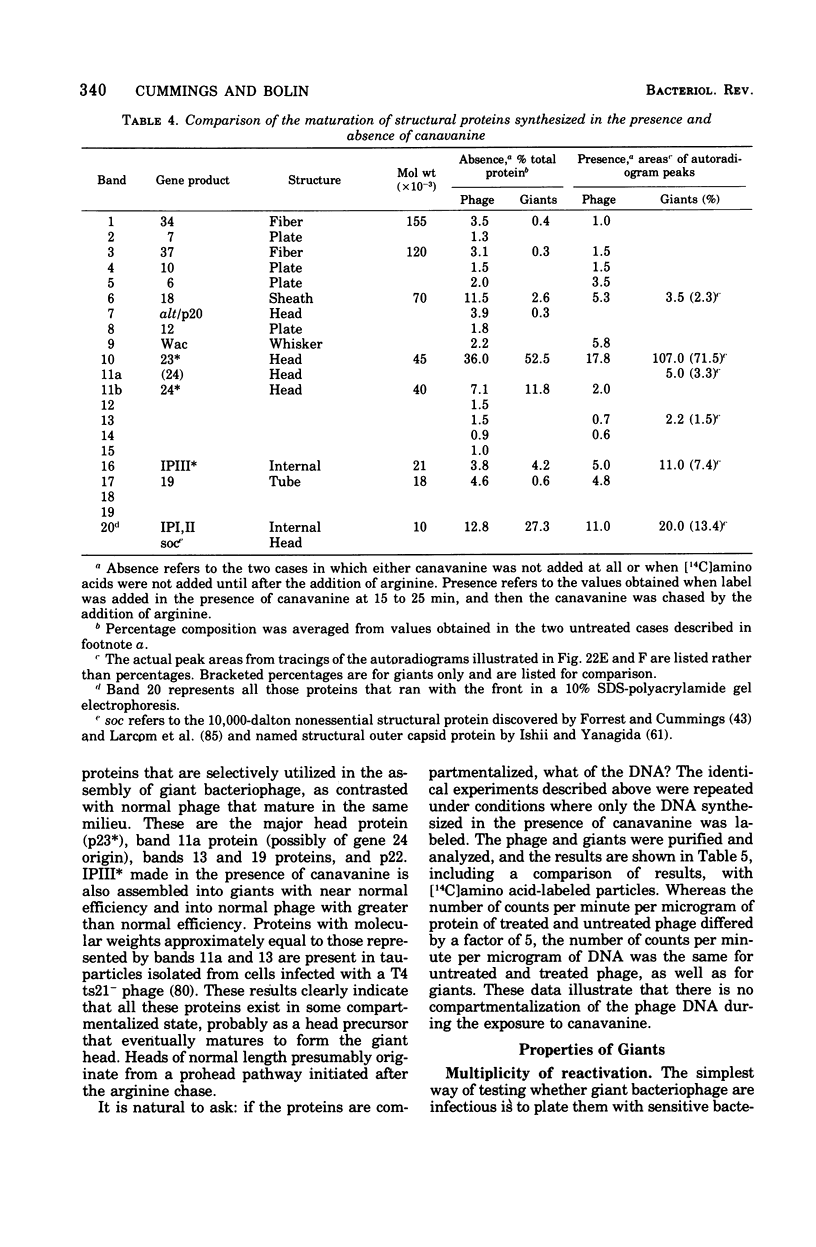
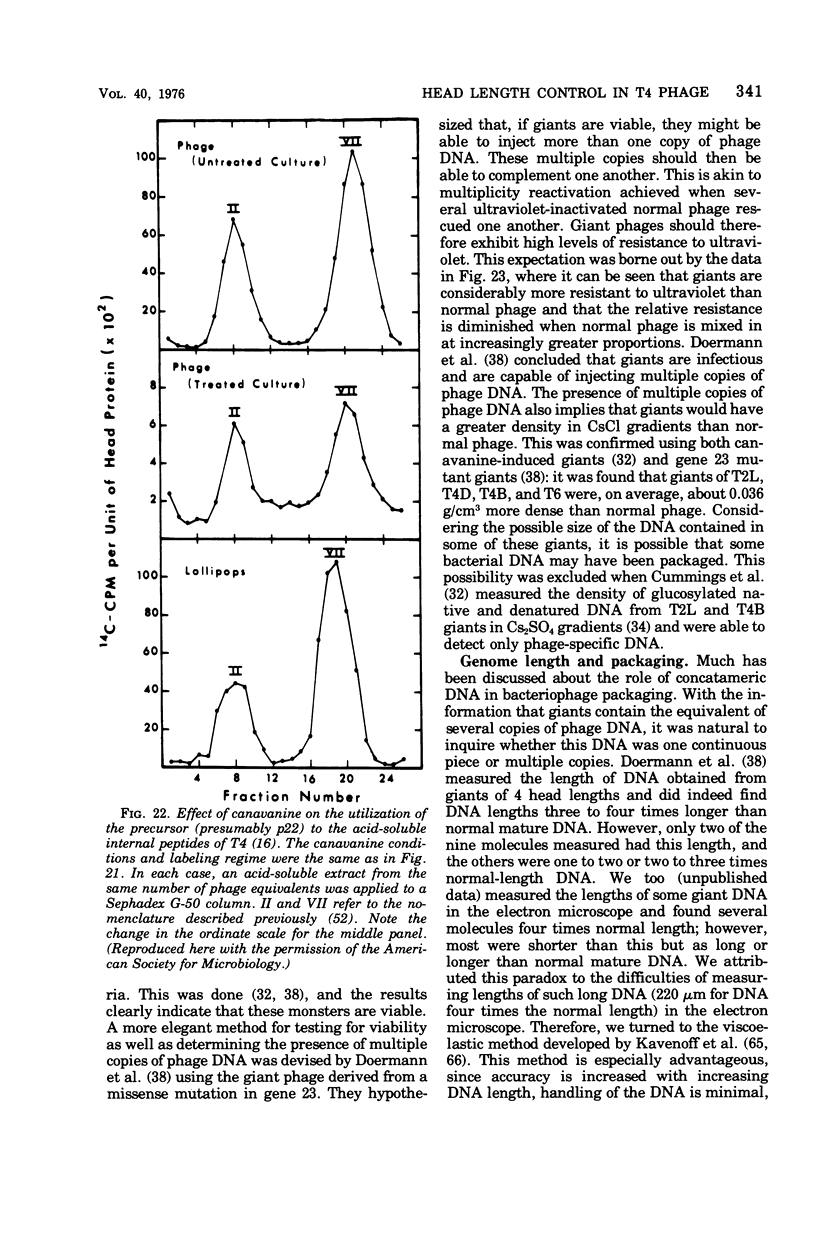
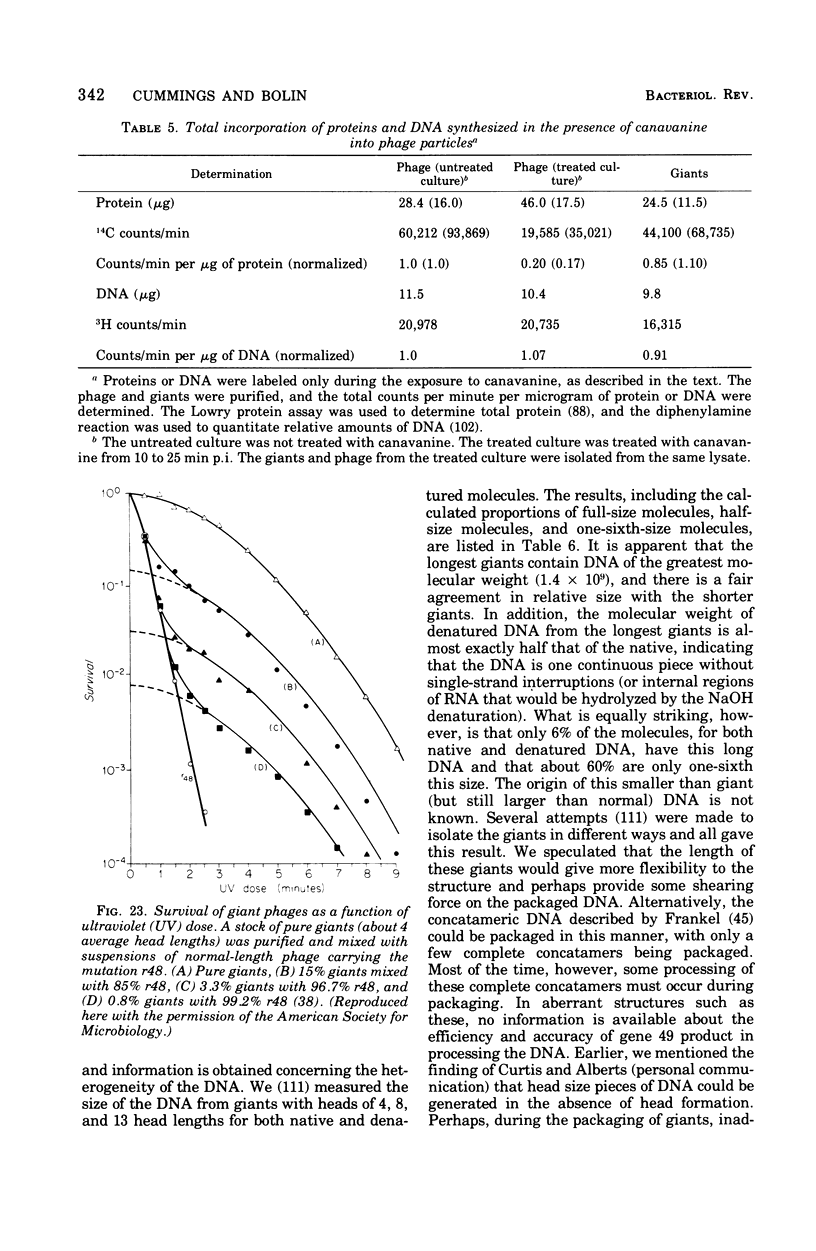
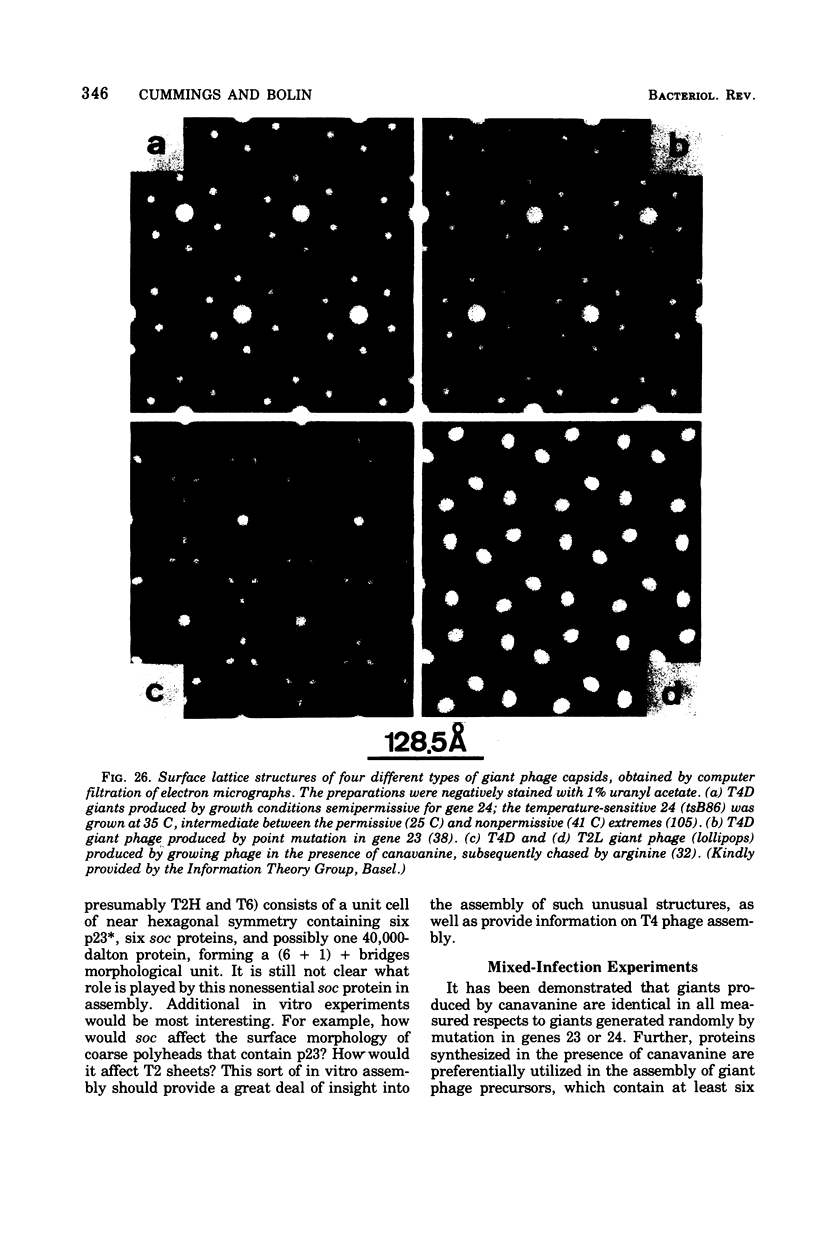
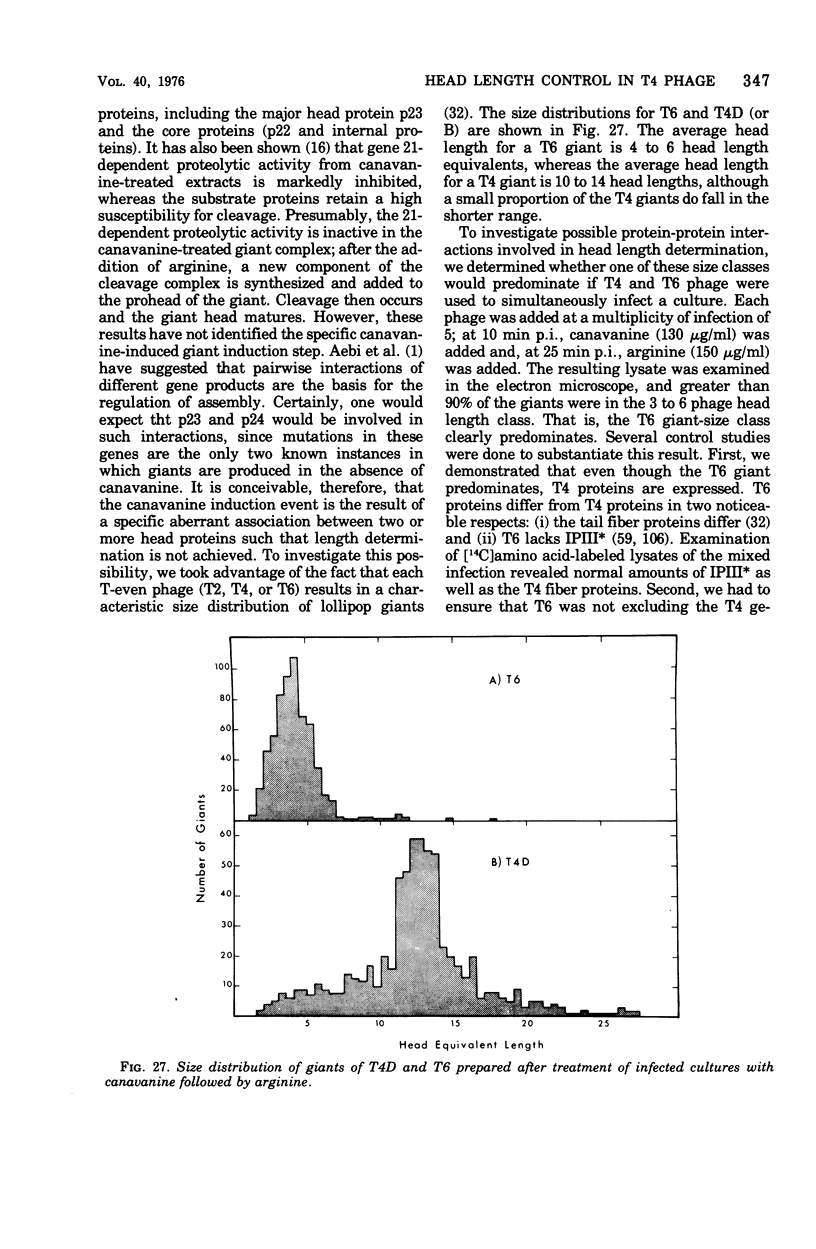
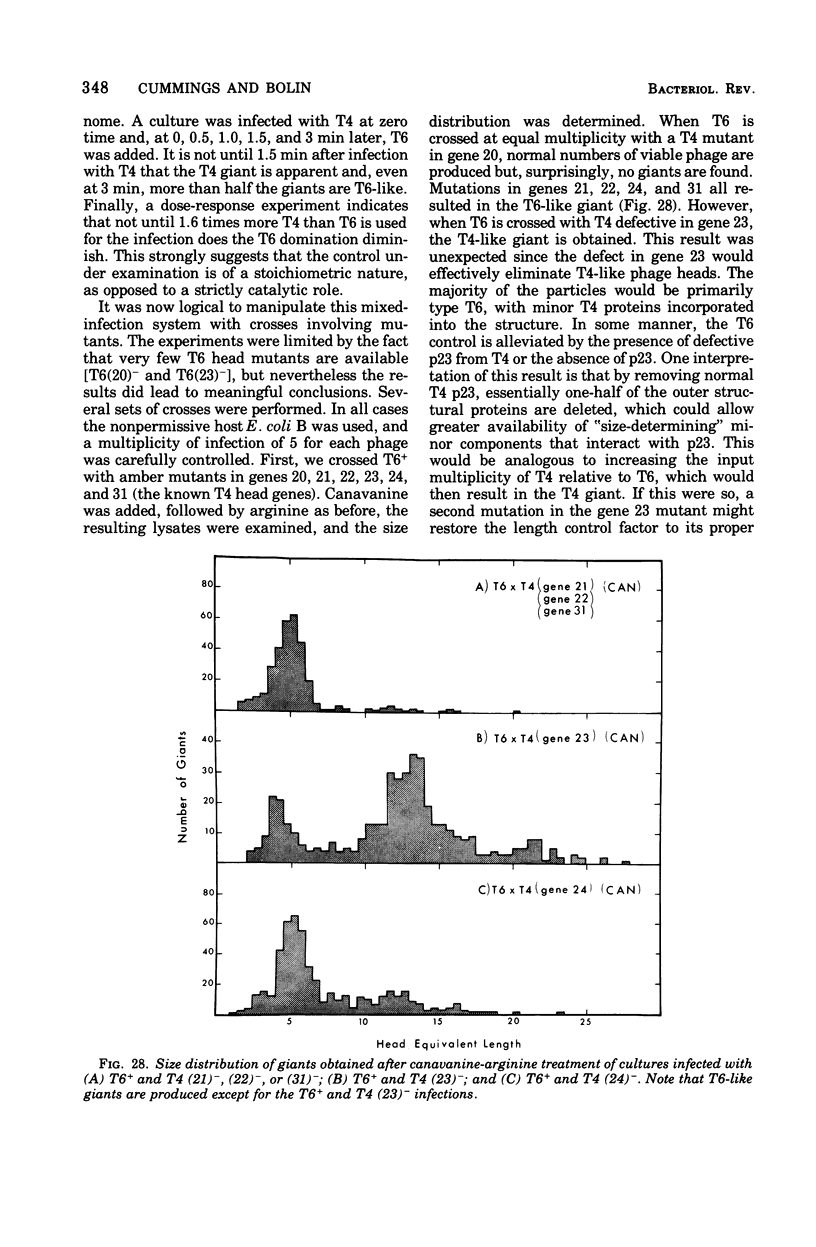
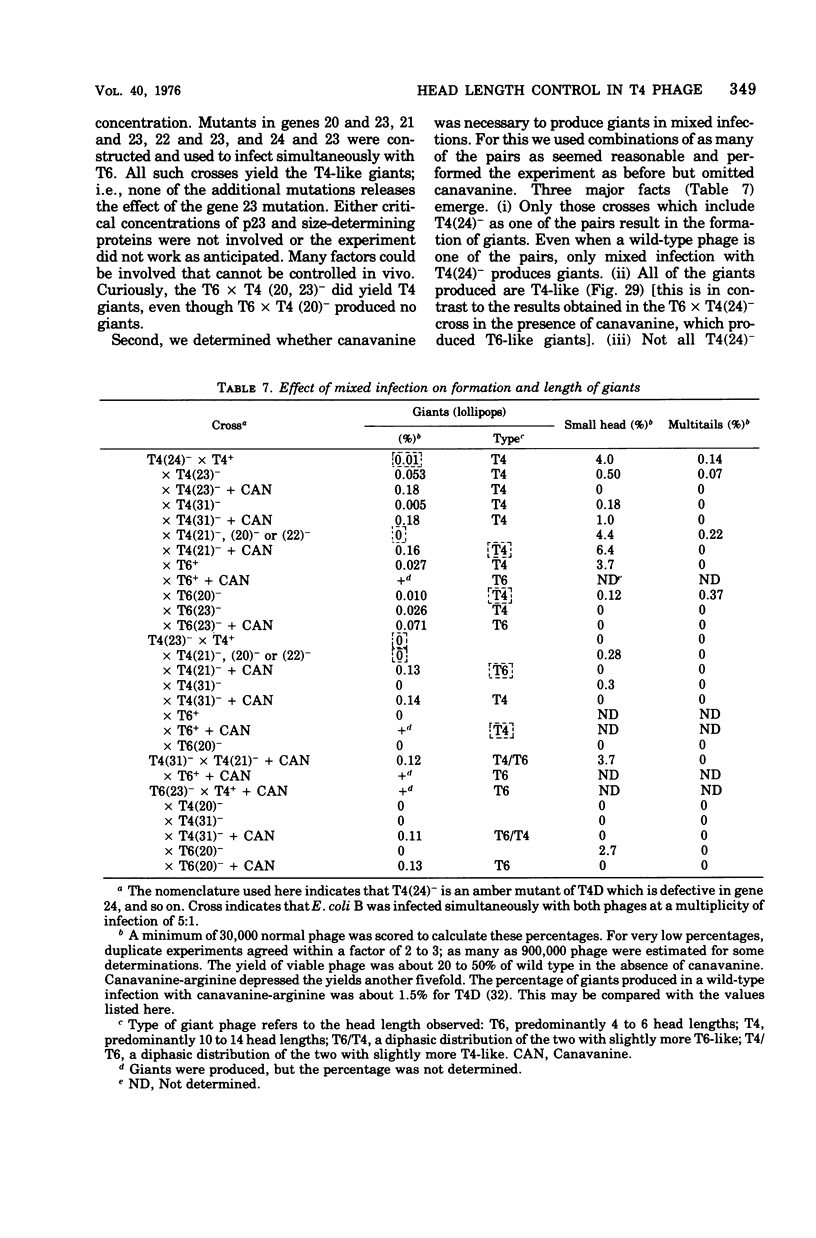
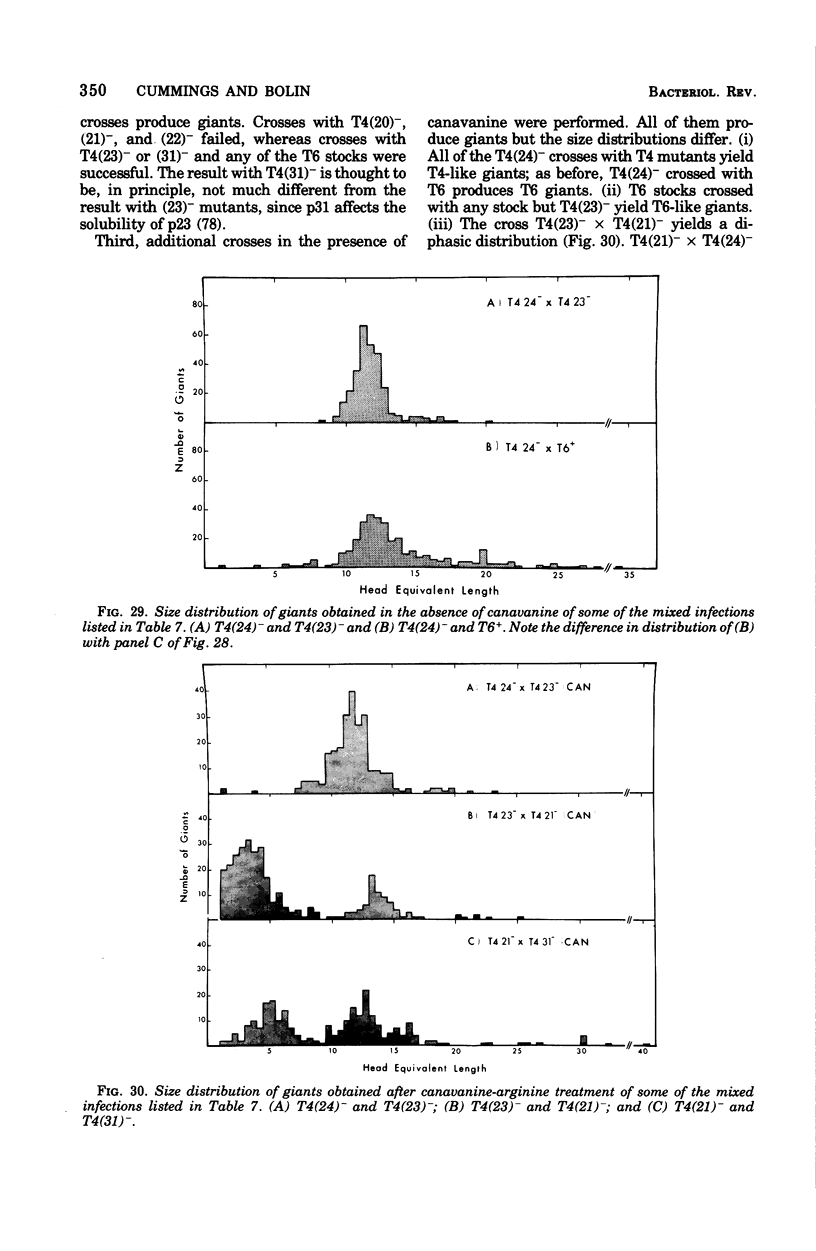
Full text
PDF



























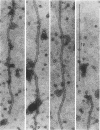
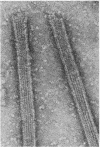
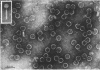
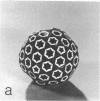
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Aebi U., Bijlenga R., v d Broek J., v d Broek H., Eiserling F., Kellenberger C., Kellenberger E., Mesyanzhinov V., Müller L., Showe M. The transformation of tau particles into T4 heads. II. Transformations of the surface lattice and related observations on form determination. J Supramol Struct. 1974;2(2-4):253–275. doi: 10.1002/jss.400020218. [DOI] [PubMed] [Google Scholar]
- Altman S., Lerman L. S. Kinetics and intermediates in the intracellular synthesis of bacteriophage T4 deoxyribonucleic acid. J Mol Biol. 1970 Jun 14;50(2):235–261. doi: 10.1016/0022-2836(70)90190-7. [DOI] [PubMed] [Google Scholar]
- Anderson T. F., Stephens R. Decomposition of T6 bacteriophage in alkaline solutions. Virology. 1964 May;23(1):113–117. doi: 10.1016/s0042-6822(64)80017-9. [DOI] [PubMed] [Google Scholar]
- Bancroft J. B. The self-assembly of spherical plant viruses. Adv Virus Res. 1970;16:99–134. doi: 10.1016/s0065-3527(08)60022-6. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Remsen C. C. Bacteriophage T2 as seen with the freeze-etching technique. Virology. 1970 Mar;40(3):703–718. doi: 10.1016/0042-6822(70)90215-1. [DOI] [PubMed] [Google Scholar]
- Bijlenga R. K., Scraba D., Kellenberger E. Studies on the morphopoiesis of the head of T-even phage. IX. Gamma-particles: their morphology, kinetics of appearance and possible precursor function. Virology. 1973 Nov;56(1):250–267. doi: 10.1016/0042-6822(73)90304-8. [DOI] [PubMed] [Google Scholar]
- Bishop R. J., Conley M. P., Wood W. B. Assembly and attachment of bacteriophage T4 tail fibers. J Supramol Struct. 1974;2(2-4):196–201. doi: 10.1002/jss.400020214. [DOI] [PubMed] [Google Scholar]
- Black L. W. Bacteriophage T4 internal protein mutants: isolation and properties. Virology. 1974 Jul;60(1):166–179. doi: 10.1016/0042-6822(74)90374-2. [DOI] [PubMed] [Google Scholar]
- Bolin R. W., Cummings D. J. Canavanine-mediated depletion of polyamine pools in Escherichia coli: effect of head morphogenesis and DNA synthesis. J Virol. 1975 Feb;15(2):232–237. doi: 10.1128/jvi.15.2.232-237.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin R. W., Cummings D. J. Structural aberrations in T-even bacteriophage. IV. Parameters of induction and formation of lollipops. J Virol. 1974 Jun;13(6):1368–1377. doi: 10.1128/jvi.13.6.1368-1377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin R. W., Cummings D. J. Structural aberrations in T-even bacteriophage. V. Effects of canavanine on the maturation and utilization of specific gene products. J Virol. 1974 Jun;13(6):1378–1391. doi: 10.1128/jvi.13.6.1378-1391.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin R. W., Cummings D. J. Structural aberrations in T-even bacteriophage. VII. In vitro analysis of the canavanine-mediated inhibition of proteolytic cleavage. J Virol. 1975 Nov;16(5):1273–1281. doi: 10.1128/jvi.16.5.1273-1281.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D., Klug A. Capsid geometry of bacteriophage T2: a freeze-etching study. J Mol Biol. 1975 Mar 15;92(4):559–565. doi: 10.1016/0022-2836(75)90309-5. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- CRICK F. H., WATSON J. D. Structure of small viruses. Nature. 1956 Mar 10;177(4506):473–475. doi: 10.1038/177473a0. [DOI] [PubMed] [Google Scholar]
- Casjens S., Hohn T., Kaiser A. D. Morphological proteins of phage lambda: identification of the major head protein as the product of gene E. Virology. 1970 Oct;42(2):496–507. doi: 10.1016/0042-6822(70)90293-x. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Chao J., Chao L., Speyer J. F. Bacteriophage T4 head morphogenesis: host DNA enzymes affect frequency of petite forms. J Mol Biol. 1974 May 5;85(1):41–50. doi: 10.1016/0022-2836(74)90127-2. [DOI] [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F., Takahashi H. Abortive bacteriophage T4 head assembly in mutants of Escherichia coli. J Mol Biol. 1973 May 5;76(1):61–87. doi: 10.1016/0022-2836(73)90081-8. [DOI] [PubMed] [Google Scholar]
- Couse N. L., Cummings D. J., Chapman V. A., DeLong S. S. Structural aberrations in T-even bacteriophage. I. Specificity of induction of aberrations. Virology. 1970 Nov;42(3):590–602. doi: 10.1016/0042-6822(70)90305-3. [DOI] [PubMed] [Google Scholar]
- Couse N. L., Haworth P., Moody W., Cummings D. J. Intracellular events in canavanine-treated, T4-infected Escherichia coli. Virology. 1972 Dec;50(3):765–771. doi: 10.1016/0042-6822(72)90430-8. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., De Long S. S., Mondale L. Induced structural defects in T-even bacteriophage. J Virol. 1967 Feb;1(1):193–204. doi: 10.1128/jvi.1.1.193-204.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S., Couse N. L. Structural aberrations in T-even bacteriophage. 3. Induction of "lollipops" and their partial characterization. Virology. 1973 Jul;54(1):245–261. doi: 10.1016/0042-6822(73)90134-7. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S. Disruption of T-even bacteriophages by dimethyl sulfoxide. J Virol. 1968 Jun;2(6):610–620. doi: 10.1128/jvi.2.6.610-620.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., Kusy A. R., DeLong S. S. Structural aberrations in T-even bacteriophage. II. Characterization of the proteins contained in aberrant heads. Virology. 1971 May;44(2):425–442. doi: 10.1016/0042-6822(71)90273-x. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Couse N. L., Forrest G. L. Structural defects of T-even bacteriophages. Adv Virus Res. 1970;16:1–41. doi: 10.1016/s0065-3527(08)60020-2. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Mondale L. Density-gradient banding of denatured deoxyribonucleic acid in cesium sulphate. Biochim Biophys Acta. 1966 Jul 13;120(3):448–453. doi: 10.1016/0926-6585(66)90311-6. [DOI] [PubMed] [Google Scholar]
- DE MARS R. I., LURIA S. E., FISHER H., LEVINTHAL C. The production of incomplete bacteriophage particles by the action of proflavine and the properties of the incomplete particles. Ann Inst Pasteur (Paris) 1953 Jan;84(1):113–128. [PubMed] [Google Scholar]
- Dickson R. C., Barnes S. L., Eiserling F. A. Structural proteins of bacteriophage T4. J Mol Biol. 1970 Nov 14;53(3):461–474. doi: 10.1016/0022-2836(70)90077-x. [DOI] [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamines in the synthesis of bacteriophage deoxyribonucleic acid. II. Requirement for polyamines in T4 infection of a polyamine auxotroph. J Virol. 1972 Mar;9(3):423–430. doi: 10.1128/jvi.9.3.423-430.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doermann A. H., Eiserling F. A., Boehner L. Genetic control of capsid length in bacteriophage T4. I. Isolation and preliminary description of four new mutants. J Virol. 1973 Aug;12(2):374–385. doi: 10.1128/jvi.12.2.374-385.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserling F. A., Geiduschek E. P., Epstein R. H., Metter E. J. Capsid size and deoxyribonucleic acid length: the petite variant of bacteriophage T4. J Virol. 1970 Dec;6(6):865–876. doi: 10.1128/jvi.6.6.865-876.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINCH J. T., KLUG A., STRETTON A. O. THE STRUCTURE OF THE "POLYHEADS" OF T4 BACTERIOPHAGE. J Mol Biol. 1964 Dec;10:570–575. doi: 10.1016/s0022-2836(64)80082-6. [DOI] [PubMed] [Google Scholar]
- Favre R., Boy de la Tour E., Segrè N., Kellenberger E. Studies on the morphopoiesis of the head of phage T-even. I. Morphological, immunological, and genetic characterization of polyheads. J Ultrastruct Res. 1965 Oct;13(3):318–342. doi: 10.1016/s0022-5320(65)80080-6. [DOI] [PubMed] [Google Scholar]
- Forrest G. L., Cummings D. J. Head proteins from T-even bacteriophage. I. Molecular weight characterization. J Virol. 1970 Mar;5(3):398–405. doi: 10.1128/jvi.5.3.398-405.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel-Conrat H., Williams R. C. RECONSTITUTION OF ACTIVE TOBACCO MOSAIC VIRUS FROM ITS INACTIVE PROTEIN AND NUCLEIC ACID COMPONENTS. Proc Natl Acad Sci U S A. 1955 Oct 15;41(10):690–698. doi: 10.1073/pnas.41.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R., Batcheler M. L., Clark C. K. The role of gene 49 in DNA replication and head morphogenesis in bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):439–463. doi: 10.1016/0022-2836(71)90147-1. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. DNA replication after T4 infection. Cold Spring Harb Symp Quant Biol. 1968;33:485–493. doi: 10.1101/sqb.1968.033.01.056. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P. Bacterial mutants in which the gene N function of bacteriophage lambda is blocked have an altered RNA polymerase. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2977–2981. doi: 10.1073/pnas.68.12.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. P., Eisen H. Bacterial mutants which block phage assembly. J Supramol Struct. 1974;2(2-4):349–359. doi: 10.1002/jss.400020224. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Casjens S. R., Kaiser A. D. Host participation in bacteriophage lambda head assembly. J Mol Biol. 1973 May 5;76(1):45–60. doi: 10.1016/0022-2836(73)90080-6. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Kaiser A. D., Wood W. B. Role of the host cell in bacteriophage morphogenesis: effects of a bacterial mutation on T4 head assembly. Nat New Biol. 1972 Sep 13;239(89):38–41. doi: 10.1038/newbio239038a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J., Champe S. P. T4-induced activity required for specific cleavage of a bacteriophage protein in vitro. J Virol. 1974 Feb;13(2):419–427. doi: 10.1128/jvi.13.2.419-427.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R., Lengyel J., Pruss G., Barrett K., Calendar R., Six E. Head size determination and the morphogenesis of satellite phage P4. Curr Top Microbiol Immunol. 1974;(68):59–75. doi: 10.1007/978-3-642-66044-3_3. [DOI] [PubMed] [Google Scholar]
- Hershko A., Fry M. Post-translational cleavage of polypeptide chains: role in assembly. Annu Rev Biochem. 1975;44:775–797. doi: 10.1146/annurev.bi.44.070175.004015. [DOI] [PubMed] [Google Scholar]
- Hohn B., Wurtz M., Klein B., Lustig A., Hohn T. Phage lambda DNA packaging, in vitro. J Supramol Struct. 1974;2(2-4):302–317. doi: 10.1002/jss.400020220. [DOI] [PubMed] [Google Scholar]
- Hohn T., Hohn B. Structure and assembly of simple RNA bacteriophages. Adv Virus Res. 1970;16:43–98. doi: 10.1016/s0065-3527(08)60021-4. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R. Bacteriophage T4 mutants deficient in alteration and modification of the Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):739–750. doi: 10.1016/0022-2836(74)90537-3. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Cone R. Analysis of T4 phage proteins. I. Conversion of precursor proteins into lower molecular weight peptides during normal capsid formation. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1275–1281. doi: 10.1073/pnas.66.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G. W., Jr, Wolin M. L., Champe S. P. Diversity of phage internal components among members of the T-even group. Trans N Y Acad Sci. 1972 Jan;34(1):36–51. doi: 10.1111/j.2164-0947.1972.tb02659.x. [DOI] [PubMed] [Google Scholar]
- Howatson A. F., Kemp C. L. The structure of tubular head forms of bacteriophage lambda; relation to the capsid structure of petit lambda and normal lambda heads. Virology. 1975 Sep;67(1):80–84. doi: 10.1016/0042-6822(75)90405-5. [DOI] [PubMed] [Google Scholar]
- Ishii T., Yanagida M. Molecular organization of the shell of the Teven bacteriophage head. J Mol Biol. 1975 Oct 5;97(4):655–660. doi: 10.1016/s0022-2836(75)80065-9. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D., Syvanen M., Masuda T. Processing and assembly of the head of bacteriophage lambda. J Supramol Struct. 1974;2(2-4):318–328. doi: 10.1002/jss.400020221. [DOI] [PubMed] [Google Scholar]
- Katsura I., Kühl P. W. A regulator protein for the length determination of bacteriophage lambda tail. J Supramol Struct. 1974;2(2-4):239–253. doi: 10.1002/jss.400020217. [DOI] [PubMed] [Google Scholar]
- Kavenoff R., Klotz L. C., Zimm B. H. One the nature of chromosome-sized DNA molecules. Cold Spring Harb Symp Quant Biol. 1974;38:1–8. doi: 10.1101/sqb.1974.038.01.003. [DOI] [PubMed] [Google Scholar]
- Kavenoff R., Zimm B. H. Chromosome-sized DNA molecules from Drosophila. Chromosoma. 1973;41(1):1–27. doi: 10.1007/BF00284071. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Der Kamp C. K.-V. On a modification of the gene product P23 according to its use as subunit of either normal capsids of phage T4 or of polyheads. FEBS Lett. 1970 Jun 1;8(3):140–144. doi: 10.1016/0014-5793(70)80247-2. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Eiserling F. A., Boy de la Tour E. Studies on the morphopoiesis of the head of phage T-even. 3. The cores of head-related structures. J Ultrastruct Res. 1967 Dec 12;21(3):335–360. doi: 10.1016/s0022-5320(67)80099-6. [DOI] [PubMed] [Google Scholar]
- Kellenberger E. Studies on the morphopoiesis of the head of phage T-even. V. The components of the T4 capsid and of other, capsid-related structures. Virology. 1968 Mar;34(3):549–561. doi: 10.1016/0042-6822(68)90074-3. [DOI] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- King J. Bacteriophage T4 tail assembly: four steps in core formation. J Mol Biol. 1971 Jun 28;58(3):693–709. doi: 10.1016/0022-2836(71)90034-9. [DOI] [PubMed] [Google Scholar]
- King J., Mykolajewycz N. Bacteriophage T4 tail assembly: proteins of the sheath, core and baseplate. J Mol Biol. 1973 Apr 5;75(2):339–358. doi: 10.1016/0022-2836(73)90025-9. [DOI] [PubMed] [Google Scholar]
- Kirschner M. W., Williams R. C. The mechanism of microtubule assembly in vitro. J Supramol Struct. 1974;2(2-4):412–428. doi: 10.1002/jss.400020229. [DOI] [PubMed] [Google Scholar]
- Klug A. The polymorphism of tobacco mosaic virus protein and its significance for the assembly of the virus. Ciba Found Symp. 1972;7:207–215. doi: 10.1002/9780470719909.ch12. [DOI] [PubMed] [Google Scholar]
- Kushner D. J. Self-assembly of biological structures. Bacteriol Rev. 1969 Jun;33(2):302–345. doi: 10.1128/br.33.2.302-345.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Beguin F., Gujer-Kellenberger G. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J Mol Biol. 1970 Jan 14;47(1):69–85. doi: 10.1016/0022-2836(70)90402-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Johnson R. A. Maturation of the head of bacteriophage T4. II. Head-related, aberrant tau-particles. J Mol Biol. 1973 Nov 15;80(4):601–611. doi: 10.1016/0022-2836(73)90199-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Mölbert E., Showe M., Kellenberger E. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970 Apr 14;49(1):99–113. doi: 10.1016/0022-2836(70)90379-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Paulson J. R., Hitchins V. Maturation of the head of bacteriophage T4. V. A possible DNA packaging mechanism: in vitro cleavage of the head proteins and the structure of the core of the polyhead. J Supramol Struct. 1974;2(2-4):276–301. doi: 10.1002/jss.400020219. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Quittner S. F. Maturation of the head of bacteriophage T4. IV. The proteins of the core of the tubular polyheads and in vitro cleavage of the head proteins. Virology. 1974 Dec;62(2):483–499. doi: 10.1016/0042-6822(74)90409-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Teaff N., D'Ambrosia J. Maturation of the head of bacteriophage T4. III. DNA packaging into preformed heads. J Mol Biol. 1974 Oct 5;88(4):749–765. doi: 10.1016/0022-2836(74)90397-0. [DOI] [PubMed] [Google Scholar]
- Larcom L. L., Bendet I. J., Mumma S. Subunits of T4 head structures. Virology. 1970 May;41(1):1–11. doi: 10.1016/0042-6822(70)90048-6. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Lundh N. P. Bacteriophage T4 head morphogenesis. Isolation, partial characterization, and fate of gene 21-defective tau-particles. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1636–1640. doi: 10.1073/pnas.70.6.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Wood W. B., Okinaka R. Bacteriophage T4 head morphogenesis. On the nature of gene 49-defective heads and their role as intermediates. J Mol Biol. 1971 May 14;57(3):555–573. doi: 10.1016/0022-2836(71)90109-4. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. Studies on repression of arginine biosynthesis in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:183–191. doi: 10.1101/sqb.1961.026.01.023. [DOI] [PubMed] [Google Scholar]
- MacHattie L. A., Ritchie D. A., Thomas C. A., Jr, Richardson C. C. Terminal repetition in permuted T2 bacteriophage DNA molecules. J Mol Biol. 1967 Feb 14;23(3):355–363. doi: 10.1016/s0022-2836(67)80110-4. [DOI] [PubMed] [Google Scholar]
- Martuscelli J., Taylor A. L., Cummings D. J., Chapman V. A., DeLong S. S., Cañedo L. Electron microscopic evidence for linear insertion of bacteriophage MU-1 in lysogenic bacteria. J Virol. 1971 Oct;8(4):551–563. doi: 10.1128/jvi.8.4.551-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody M. F. The shape of the T-even bacteriophage head. Virology. 1965 Aug;26(4):567–576. doi: 10.1016/0042-6822(65)90319-3. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Pardee A. B. Multiple pathways of putrescine biosynthesis in Escherichia coli. J Biol Chem. 1966 Jul 10;241(13):3129–3135. [PubMed] [Google Scholar]
- Mosig G., Carnighan J. R., Bibring J. B., Cole R., Bock H. G., Bock S. Coordinate variation in lengths of deoxyribonucleic acid molecules and head lengths in morphological variants of bacteriophage T4. J Virol. 1972 May;9(5):857–871. doi: 10.1128/jvi.9.5.857-871.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onorato L., Showe M. K. Gene 21 protein-dependent proteolysis in vitro of purified gene 22 product of bacteriophage T4. J Mol Biol. 1975 Mar 5;92(3):395–412. doi: 10.1016/0022-2836(75)90288-0. [DOI] [PubMed] [Google Scholar]
- Peyru G. M., Maas W. K. Inhibition of Escherichia coli B by homoarginine. J Bacteriol. 1967 Sep;94(3):712–718. doi: 10.1128/jb.94.3.712-718.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poglazov B. F., Nikolskaya T. I. Self-assembly of the protein of bacteriophage T2 tail cores. J Mol Biol. 1969 Jul 14;43(1):231–233. doi: 10.1016/0022-2836(69)90094-1. [DOI] [PubMed] [Google Scholar]
- Pruss G., Barrett K., Lengyel J., Goldstein R., Calendar R. Phage head size determination and head protein cleavage in vitro. J Supramol Struct. 1974;2(2-4):337–348. doi: 10.1002/jss.400020223. [DOI] [PubMed] [Google Scholar]
- SARABHAI A. S., STRETTON A. O., BRENNER S., BOLLE A. CO-LINEARITY OF THE GENE WITH THE POLYPEPTIDE CHAIN. Nature. 1964 Jan 4;201:13–17. doi: 10.1038/201013a0. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Black L. W. Assembly core of bacteriophage T4: an intermediate in head formation. Nat New Biol. 1973 Mar 21;242(116):70–75. doi: 10.1038/newbio242070a0. [DOI] [PubMed] [Google Scholar]
- Simon L. D. Infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope: T4 head morphogenesis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):907–911. doi: 10.1073/pnas.69.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. R., Cummings D. J. Comparison of the internal proteins of the T-even bacteriophages. J Mol Biol. 1972 Mar 14;64(3):651–669. doi: 10.1016/0022-2836(72)90089-7. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Emrich J., Stahl M. M. Chromosome structure in phage t4, iii. Terminal redundancy and length determination. Proc Natl Acad Sci U S A. 1967 Feb;57(2):292–295. doi: 10.1073/pnas.57.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Takano T., Kakefuda T. Involvement of a bacterial factor in morphogenesis of bacteriophage capsid. Nat New Biol. 1972 Sep 13;239(89):34–37. doi: 10.1038/newbio239034a0. [DOI] [PubMed] [Google Scholar]
- Uhlenhopp E. L., Zimm B. H., Cummings D. J. Structural aberrations in T-even bacteriophage. VI. Molecular weight of DNA from giant heads. J Mol Biol. 1974 Nov 15;89(4):689–702. doi: 10.1016/0022-2836(74)90045-x. [DOI] [PubMed] [Google Scholar]
- Unger L., DeMoss R. D. Action of a proline analogue, l-thiazolidine-4-carboxylic acid, in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1556–1563. doi: 10.1128/jb.91.4.1556-1563.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderslice R. W., Yegian C. D. The identification of late bacteriophage T4 proteins on sodium dodecyl sulfate polyacrylamide gels. Virology. 1974 Jul;60(1):265–275. doi: 10.1016/0042-6822(74)90384-5. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. C., FRASER D. Morphology of the seven T-bacteriophages. J Bacteriol. 1953 Oct;66(4):458–464. doi: 10.1128/jb.66.4.458-464.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS R. C., SMITH K. M. The polyhedral form of the Tipula iridescent virus. Biochim Biophys Acta. 1958 Jun;28(3):464–469. doi: 10.1016/0006-3002(58)90507-9. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Richards K. E. Letter: Capsid structure of bacteriophage lambda. J Mol Biol. 1974 Sep 15;88(2):547–550. doi: 10.1016/0022-2836(74)90501-4. [DOI] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Cleavage of head and tail proteins during bacteriophage T5 assembly: selective host involvement in the cleavage of a tail protein. J Mol Biol. 1973 Nov 5;80(3):505–518. doi: 10.1016/0022-2836(73)90418-x. [DOI] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Structural proteins of bacteriophage T5. Virology. 1973 Feb;51(2):443–453. doi: 10.1016/0042-6822(73)90443-1. [DOI] [PubMed] [Google Scholar]