Abstract
Introduction
Malaria due to Plasmodium knowlesi is reported throughout South-East Asia, and is the commonest cause of it in Malaysia. P. knowlesi replicates every 24 h and can cause severe disease and death. Current 2010 WHO Malaria Treatment Guidelines have no recommendations for the optimal treatment of non-severe knowlesi malaria. Artemisinin-combination therapies (ACT) and chloroquine have each been successfully used to treat knowlesi malaria; however, the rapidity of parasite clearance has not been prospectively compared. Malaysia's national policy for malaria pre-elimination involves mandatory hospital admission for confirmed malaria cases with discharge only after two negative blood films; use of a more rapidly acting antimalarial agent would have health cost benefits. P. knowlesi is commonly microscopically misreported as P. malariae, P. falciparum or P. vivax, with a high proportion of the latter two species being chloroquine-resistant in Malaysia. A unified ACT-treatment protocol would provide effective blood stage malaria treatment for all Plasmodium species.
Methods and analysis
ACT KNOW, the first randomised controlled trial ever performed in knowlesi malaria, is a two-arm open-label trial with enrolments over a 2-year period at three district sites in Sabah, powered to show a difference in proportion of patients negative for malaria by microscopy at 24 h between treatment arms (clinicaltrials.gov #NCT01708876). Enrolments started in December 2012, with completion expected by September 2014. A total sample size of 228 is required to give 90% power (α 0.05) to determine the primary end point using intention-to-treat analysis. Secondary end points include parasite clearance time, rates of recurrent infection/treatment failure to day 42, gametocyte carriage throughout follow-up and rates of anaemia at day 28, as determined by survival analysis.
Ethics and dissemination
This study has been approved by relevant institutional ethics committees in Malaysia and Australia. Results will be disseminated to inform knowlesi malaria treatment policy in this region through peer-reviewed publications and academic presentations.
Trial registration number
Keywords: INFECTIOUS DISEASES, PARASITOLOGY
Strengths and limitations of this study.
All age demographics known to acquire Plasmodium knowlesi malaria will be eligible for enrolment, at relevant district referral sites where treatment is normally given.
The primary intention-to-treat analysis will appropriately evaluate rapidity of parasite clearance and associated health economic benefits; however, secondary analyses may not show a clinically useful difference in treatment failure at 42 days in the absence of known resistance to the study drugs.
This trial addresses a knowledge gap in knowlesi malaria, with no previous randomised controlled trials in P. knowlesi infection to determine optimal treatment. If ACT is shown to be superior to chloroquine, it would support a national policy change to a unified ACT treatment protocol which would provide effective blood stage malaria treatment for all Plasmodium species.
Introduction
Naturally acquired infections with Plasmodium knowlesi, the fifth human malaria, are growing.1 Since 2004, increasing numbers of cases have been reported from residents and returned travellers predominantly from Malaysia and throughout South-East Asia.2–11 Cases coincide with the geographic distribution of its natural simian hosts (long-tailed and pig-tailed macaques) and Anopheles leucosphyrus group mosquito vector.12–14 Despite the increase in reported cases, difficulties with microscopic diagnosis and a lack of PCR-based epidemiological surveillance studies throughout South-East Asia mean the true disease burden is underestimated. P. knowlesi is microscopically misidentified as P. malariae and P. falciparum due to morphological similarities in the late trophozoite and early ring stages, respectively,14 and is also misreported as P. vivax.15 In Eastern Malaysia, multiple studies have shown that for cases microscopically reported as ‘P. malariae’, <1% of these are confirmed when tested by PCR, with the vast majority being P. knowlesi.6 16–21 The rise in zoonotic P. knowlesi cases has occurred at the same time that notifications of P. falciparum and P. vivax are decreasing due to an effective national Malaria Control Program in Malaysia.22 P. knowlesi is now the most common cause of malaria in Malaysia, with minimum estimates of around 2268 cases (including those reported as ‘P. malariae’ in Eastern Malaysia) from public health microscopy notifications in 2012 alone.23
Misdiagnosis of P. knowlesi has concerning treatment implications, as unlike P. malariae, knowlesi malaria has a rapid 24 h replication rate and can cause hyperparasitaemia, severe complications and fatal outcomes.21 24–26 The inadvertent use of chloroquine (CQ) for widely CQ-resistant P. falciparum or P. vivax in this region may lead to treatment failure, with a consequent higher risk of severe disease or death. In addition, misreporting with P. vivax may have implications for the correct initiation of liver-stage P. vivax eradication.
Treatment of non-severe knowlesi malaria
Initial observational and retrospective studies have suggested that both CQ and artemisinin-combination therapy (ACT) are effective therapies for non-severe P. knowlesi infection.16 21 25 27 28 Case reports predominantly from returned travellers to South-East Asia also document non-severe knowlesi malaria responding well to conventional antimalarials such as CQ, mefloquine (MQ), atovaquone with proguanil, doxycycline and quinine.2 4 29–35 However, to date there have been no prospective randomised trials to determine the optimal treatment of non-severe P. knowlesi infection in Malaysia, and there are no current recommendations on how to treat non-severe P. knowlesi infection in the current 2010 WHO Malaria Treatment Guidelines.36
Artesunate-mefloquine
Artesunate (AS)-MQ is a common and widely available ACT, and along with artemether-lumefantrine (A-L) is one of only two first-line WHO recommended options for the treatment of non-severe P. falciparum infection that are registered in Malaysia and produced according to international good manufacturing practice standards. ACTs are the current mainstay of malaria elimination efforts,36 with a mechanism of action resulting both in a rapid reduction in parasite mass and resolution of clinical features, while the long-acting component eliminates residual parasites and delays the development of de novo resistance.37 38 Safety and tolerability of all ACTs are dependent on their partner drug39 and while gastrointestinal and self-limiting neuropsychiatric adverse events (AESs) have been reported with MQ, multiple safety and efficacy trials have recommended its use in adults and children for non-severe falciparum malaria.40–42 Owing to concerns over the safety of MQ in the first trimester of pregnancy43 and its use in patients with pre-existing psychiatric disorders or those who have previously had cerebral malaria, it is currently not advised for these groups.39
The initial reported use of ACT for knowlesi malaria was from our retrospective study at a tertiary referral hospital in Sabah, where a small sample of 8 of 34 patients with PCR-confirmed non-severe P. knowlesi infection were treated with oral A-L. Median microscopic parasite clearance time was 1 day (range 0–3), which was significantly faster than those receiving CQ (median 2.5 days, range 1–3, p=0.01).25 Our subsequent prospective study at the same site documented 109 knowlesi malaria patients successfully treated with A-L, with no recurrences identified. Unpublished data from this study showed that of the patients with non-severe P. knowlesi malaria enrolled, 51 received A-L monotherapy, with a median parasite clearance time of 2 days.21 There are no published series of other ACTs used in the treatment of P. knowlesi infection, although currently both AS-MQ and A-L are being used in Sabah for non-severe P. knowlesi and P. falciparum malaria, including in children >5 kg, as recommended by local guidelines. In vitro studies using MQ have not been conclusive.44 Clinically, however, MQ as a single agent has been used in the successful treatment of a Swedish traveller returning from Sarawak, Malaysia with PCR-confirmed non-severe P. knowlesi malaria and 0.1% infected erythrocytes in 2009.29 However, oral MQ was not effective when used as an initial monotherapy in a case reported from Peninsular Malaysia with severe disease and 1% infected erythrocytes.45 Current WHO 2013 management of severe malaria guidelines recommend intravenous AS and not oral therapy (with any drug) for patients meeting severe malaria criteria due to any Plasmodium species.46 The long half life of MQ of around 14 days also means when used as the partner drug in an ACT for non-severe malaria caused by other Plasmodium species such as P. vivax, there is a significant reduction compared to A-L in the day 42 treatment failure rate.44 45
Chloroquine
CQ with primaquine was initially suggested to have favourable treatment outcomes for non-severe P. knowlesi human infections after a retrospective review of patients from Kapit Hospital in Sarawak in 2004.16 Following this, a prospective observational study conducted at the same site between 2006 and 2007 administered CQ as a total base dose of 25 mg/kg and primaquine as a gametocidal agent to 73 patients with non-severe PCR-confirmed P. knowlesi malaria. This resulted in a median fever clearance of 26 h, mean times to 50% and 90% microscopic parasite clearance of 3.1 and 10.3 h, respectively, and a median PCR-adjusted clearance time of 3 days. None of the 60 patients who completed the 28-day follow-up demonstrated any evidence of resistance, reinfection or recrudescence.27 A further 11 patients with non-severe P. knowlesi malaria confirmed by PCR (including seven microscopically misdiagnosed initially as P. vivax) from a prospective study at a tertiary site in Sabah were successfully treated with CQ with or without primaquine.21
Rationale for the trial
While ACT and CQ have each been successfully used to treat knowlesi malaria, the rapidity of parasite clearance has not been prospectively compared. Malaysian Ministry of Health national policy in the malaria pre-elimination phase involves mandatory hospital admission for all patients with confirmed malaria with hospital discharge only permitted after two negative blood films.47 Use of a more rapidly acting antimalarial agent would therefore allow earlier hospital discharge and have health cost benefits. A switch to a unified ACT-treatment protocol would avoid ineffectual treatment with CQ in the event of P. knowlesi being misdiagnosed as P. falciparum or P. vivax, both CQ-resistant in Malaysia. As ACTs are already being used for treating P. falciparum and are recommended for the increasingly CQ-resistant P. vivax found in South-East Asia,48 the potential benefit of a unified treatment policy to facilitate prompt and effective treatment of all Plasmodium species requires evidence for ACT also being optimal treatment for P. knowlesi infection.
Aim and objectives
We aim to test whether the fixed combination of AS-MQ is superior to CQ in order to define the optimal treatment for non-severe P. knowlesi infection in adults and children.
The primary end point is the therapeutic efficacy of AS-MQ versus CQ, as defined by the assessment of microscopic P. knowlesi parasite clearance 24 h after initiation of treatment. Secondary end points will include parasite clearance time, including at days 2 and 42. True treatment failure rates require a 42-day follow-up period as recommended by WHO antimalarial efficacy and resistance monitoring guidelines.49 Prevalence of anaemia at day 28 will also be assessed in order to enable comparisons over a similar time frame to studies looking at the use of ACT for other malaria species and the impact on anaemia-associated morbidity.42 Presence of gametocytes at follow-up will also be assessed to determine any post-treatment transmission benefit as seen in other malarial species treated with ACT.50
Specific aims include:
Parasite clearance time of P. knowlesi infection.
Length of hospital inpatient stay and estimated cost to health sector.
Rates of recurrent infection/treatment failure at day 42.
Occurrence of anaemia at day 28 when using AS-MQ versus CQ.
P. knowlesi gametocyte carriage throughout follow-up when using AS-MQ versus CQ.
Frequency of complications throughout follow-up when using AS-MQ versus CQ.
Rates of P. knowlesi recurrence in a 1-year follow-up period.
Utility of rapid diagnostic tests (RDTs) in the diagnosis of P. knowlesi infection.
Trial design
Design and study sites
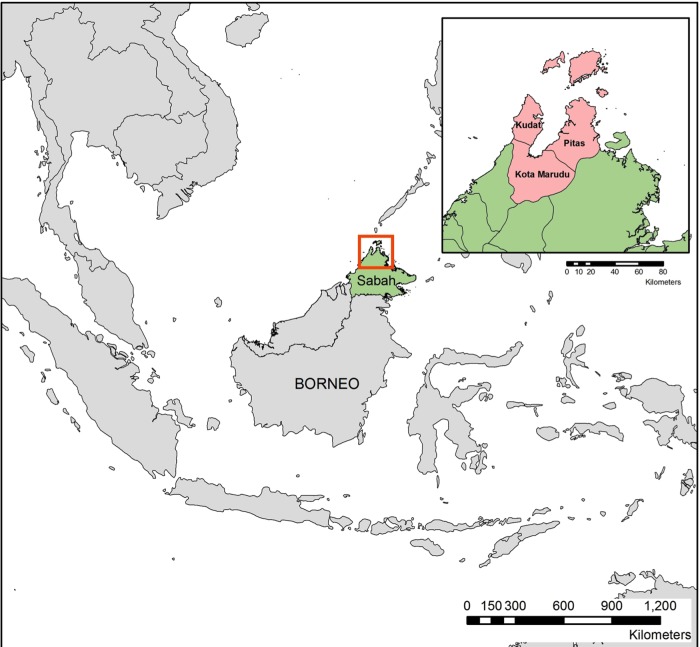
This is a two-arm, randomised, open-label trial to be conducted at Kudat, Kota Marudu and Pitas District Hospitals in Sabah, Malaysia (see figure 1) over a 3- year period, which will be powered to determine the optimal treatment for non-severe P. knowlesi infection.
Figure 1.
Map of study sites.
Trial population
Male and female patients with acute, non-severe P. knowlesi malaria who present to Kudat, Kota Murudu and Pitas District Hospitals.
Inclusion criteria
Male and female patients at least 1 year of age and weighing more than 10 kg.
Microscopic diagnosis of P. knowlesi (including microscopy result of P. malariae).
Negative P. falciparum malaria RDT (histidine-rich protein 2).
Fever (temperature ≥37.5°C) or history of fever in the past 48 h.
Able to participate in the trial and comply with the clinical trial protocol.
Written informed consent to participate in the trial.
Subsequent PCR confirmation of P. knowlesi monoinfection; including patients initially enrolled and randomised in concurrent P. vivax randomised controlled trial using same study design (clinicaltrials.gov #NCT01708876).
Exclusion criteria
· Clinical or laboratory criteria for severe malaria, including warning signs, requiring parenteral treatment according to modified WHO 2010 criteria.21
· Parasitaemia >20 000/μL, a parasite threshold associated with increased risk of severe malaria in P. knowlesi infection.21
· Inability to tolerate oral treatment.
· Concomitant infection with any other malaria species.
· Pregnancy or lactation.
· Unable or unwilling to use contraception during study period.
· Known hypersensitivity or allergy to artemisinin derivatives.
· Serious underlying disease (cardiac, renal or hepatic).
· Received antimalarials in previous 2 months.
· Previous psychiatric illness or epilepsy.
· Previous episode of cerebral malaria.
Sample size
We expect that 55% of study participants treated with CQ will remain parasitaemic 24 h after the start of treatment in contrast to 33% of study participants treated with AS-MQ.25 27 A trial that will have 90% power to falsify the Null hypothesis: “there is no significant difference in parasite clearance between artesunate-mefloquine and chloroquine at 24 hours” with a significance level (α) of 0.05 requires 114 participants in each arm or 228 trial participants. It is assumed that 10% of study participants cannot be followed up for 42 days. Therefore, we plan to recruit 256 trial participants.
Duration and size of trial
Total duration of the trial is 36 months, with an enrolment period of 24 months. Individual participation requires follow-up clinic visits after discharge from hospital on days 7, 14, 28 and 42 post-treatment, and telephone contact, when possible, to assess for malaria recurrence up to a total period of 12 months. The number of trial participants required is 256 (each arm=128).
Treatment allocation
An independent statistician will use computer-generated randomisation to generate blocks of patient numbers for each treatment in the separate arms. Each code will be given a sealed opaque envelope which will contain that patient's treatment group and which will only be opened by a study nurse when a patient has been allocated a code number.
Test drugs
Both study drugs are currently registered and available in Malaysia, and approved for use by the Malaysian Ministry of Health. The choice of drugs, including their administration and dosing have all been chosen to mimic existing local protocols and conditions in order to ensure the data gathered are relevant to the clinical setting in which the drugs will be used.
- Artequin (Mepha Ltd, Aesch, Switzerland) consisting of two different fixed dose tablet blister packs:
- 600/1500–three tablets of AS 200 mg, six tablets of MQ hydrochloride 275 mg equivalent to MQ 250 mg base;
- 300/750–three tablets of AS 100 mg, three tablets of MQ 250 mg base.
· Artequin Paediatric: a fixed dose formulation of granules containing: AS 50 mg and MQ 125 mg per dose.
· CQ: consisting of 155 mg tablets.
AS-MQ dosing
The safety and efficacy of AS-MQ in the treatment of non-severe malaria due to other Plasmodium species is based on a dose of AS 4 mg/kg/day for 3 days51 and a total MQ dose of 25 mg/kg split over either days 2 and 3, or equally over all 3 days, as this leads to increased bioavailability and reduces risk of vomiting.41 52 The dosages of the AS-MQ combinations to be administered in this study are based on these target weight dependent calculations and are consistent with current WHO36 and Malaysian Ministry of Health treatment guidelines (see figure 2).47
Figure 2.
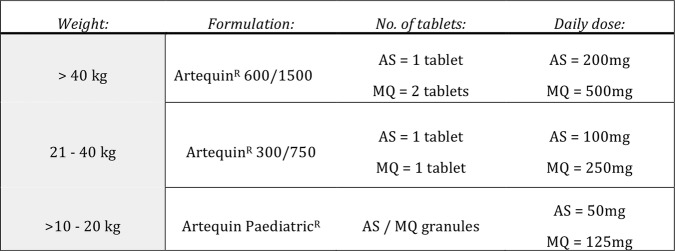
Artesunate-mefloquine (AS-MQ) dosing guide.
CQ dosing
The total dose of CQ is 25 mg base/kg body weight over 3 days, as used for non-severe knowlesi malaria in other studies27 and is also consistent with WHO and Malaysian Ministry of Health guidelines for non-severe vivax malaria. Each tablet contains 155 mg of CQ base.
Adult dose (≥35 kg): 620 mg (4 tablets) at 0 h, and 310 mg (2 tablets) at 6, 24 and 48 h.
Child dose (<35 kg): 10 mg/kg at 0 h, and 5 mg/kg at 6–8, 24 and 48 h.
Dosing schedule
The day a patient is enrolled and receives the first dose of medicine is designated ‘day 0’ (figure 3). All antimalarial treatment administration will be supervised and witnessed by a study team member. Enrolled patients will be observed for at least 30 min after treatment to ensure they do not vomit the medicine. Any concomitant medications will be documented in the case record form (CRF).
Figure 3.
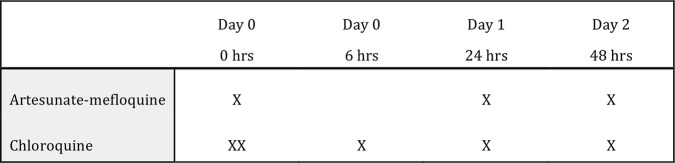
Dosing schedule.
Rescue medication
Should the patient vomit the first dose of each treatment within 1 h, this dose will be repeated. If a patient vomits again within 1 h, or for any patient subsequently meeting modified WHO 2010 criteria for severe malaria21 or early treatment failure,36 the trial medication will be discontinued and rescue medication will be initiated. Patients will receive intravenous AS 2.4 mg/kg initially, and again at 12 and 24 h. A full course of subsequent oral rescue treatment will be given that will be opposite to the trial medication administered initially.
Trial procedures
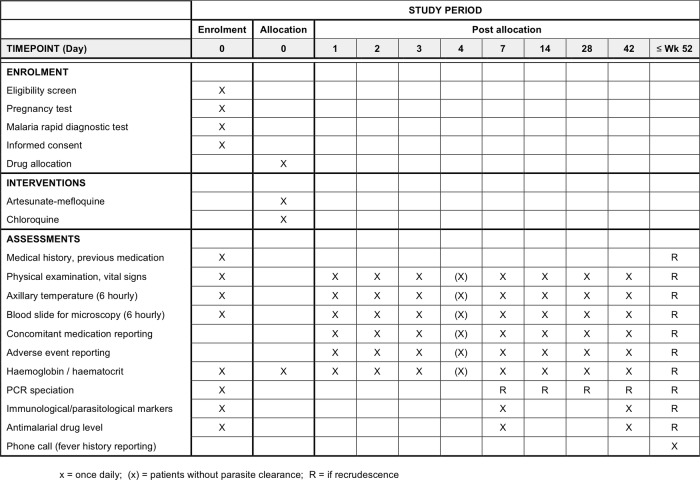
Appropriately qualified research medical staff will conduct screening and enrolment of participants. This will include clinical examination, observations and blood sampling for laboratory investigations during admission, and at scheduled clinic follow-up visits at 7, 14, 28 and 42 days post-treatment (see figure 4). Research staff will also ensure patients receive proper allocated medication dosages, monitor for adherence and AEs and initiate rescue medication where appropriate. A standardised CRF will be used to collect clinical, biochemical and parasitological data including AEs until the end of the individual's study period.
Figure 4.
Timeline of activities.
Temperature
Temperature will be measured six hourly during admission and then at all follow-up visits using a consistent method in order to evaluate fever clearance.
Parasite counts
Thick and thin blood films for parasite counts will be obtained via finger prick six hourly postadministration of study medications until negative by microscopy consecutively over a minimum 24 h period, then at follow-up on days 7, 14, 28 and 42. A qualified microscopist will read all the slides independently and be blinded to treatment. Slides will be cross-checked by a second expert microscopist at a later date and parasite densities quantitated by the expert microscopist will be used. Blood smears with discordant results (differences between the two microscopists in species diagnosis, in parasite density of >50% or in the presence of parasites) will be re-examined by a third microscopist, and parasite density will be calculated by averaging the two closest counts.
Blood sampling
Up to 65 mL of an adult participant's blood, or up to 50 mL for children, will be taken in total for research purposes during the standard study duration and schedule of activities. This will include initial samples processed for thick and thin blood films, assessment of glucose-6-phosphate dehydrogenase (G6PD) activity (Beutler fluorescent spot test), malaria RDTs, serological assays and other parasitological and immunological parameters. Haemoglobin will be assessed daily during admission and at follow-up visits. Confirmatory PCR speciation will be performed on all samples using established methods.53–55 Drug levels for CQ and MQ using high-pressure liquid chromatography with ultraviolet detection will be performed on samples from days 0, 7 and 28. Blood cultures will be done on all patients in order to exclude any concurrent blood stream infection. All samples will be labelled anonymously, and may be stored for a period of up to 10 years after publication of the study. Results of blood sampling, including any other standard laboratory or radiological investigations ordered by the study site hospital during admission, will be documented in the CRF.
Malaria RDTs
Pretreatment blood will be used for different malaria RDTs in order to assess for P. falciparum histidine-rich protein 2 (HRP2) as an exclusion criterion and to evaluate different monoclonal antibodies against Plasmodium parasite-lactate dehydrogenase (LDH) for P. knowlesi detection.56–58 RDTs will include: CareStart Pf/VOM, Humasis Pf/Pv Ag, Bionote Pf/Pv Ag, OnSite Pf/Pv Ag, CareStart PAN. RDTs will be transported, stored and conducted according to manufacturer’s instructions.
Pregnancy testing
Female patients of childbearing age, defined as those who menstruate or are aged over 12 years, will be asked to take a screening urine pregnancy test before enrolment in the study. CQ has limited prospective published data in the first trimester but is generally regarded as safe.36 However, due to previous concerns from studies of MQ of monotherapy in pregnant women in Thailand,43 AS-MQ is currently not recommended throughout pregnancy and any patients found to be pregnant will be excluded from the study.
Safety evaluation
Treatment failure classification and management
While no treatment failures have been reported for non-severe P. knowlesi infection, as prospective data is limited, the conservative framework modelled on WHO definitions for treatment failure in P. falciparum infection will be used.36 These treatment outcomes are classified on the basis of an assessment of both the parasitological and clinical outcome of antimalarial treatment. All patients will be classified as having early treatment failure, late clinical failure, late parasitological failure or an adequate clinical and parasitological response.36
Adverse events
AEs and serious AEs (SAEs) are defined according to International Conference on Harmonisation (ICH) guidelines for Good Clinical Practice (GCP).59 At each patient encounter, a standardised system for assessing, reporting and following up AEs and SAEs will be adhered to as per guidelines developed by the World Wide Antimalarial Resistance Network (WWARN).60 All SAEs occurring during the study will have a separate notification form and be reported by the principal investigator to the sponsor and the study monitor.
Removal of patients from trial
Participants will be removed from the trial if informed consent is withdrawn, or if they fail to complete treatment (ie, inability to tolerate oral medication, non-adherence to treatment regime, or develop one or more SAEs necessitating cessation of study medicine before completion).
Suspension or termination of the trial
The safety of both these medications is well established and they are currently first-line recommended Malaysian Ministry of Health treatments.47 In addition, as these are not new administration regimens, a safety and monitoring board will not be used. Strict reporting of AEs and SAEs will be done, with an experienced study monitor to conduct visits before, during and at the end of the trial. Any decision on suspending or terminating the trial will be based on an evaluation of SAE reporting and monitoring visits.
Analysis
Clinical, biochemical and parasitological information from standardised CRFs will be double entered into an electronic database (EpiData, V.2.2, Denmark) to ensure accuracy. The data will be transferred and analysed in STATA, V.12 (StataCorp Ltd, Texas, USA).
Primary variable
The difference in the proportion of patients who are parasite free at 24 h after treatment with AS-MQ compared with CQ. This will be compared by intention-to-treat analysis.
Secondary variables
Days 28 and 42 cure rate (with and without PCR correction).
Proportion of patients with a negative slide at days 2 and 3.
Parasite clearance: proportion of parasites/μL at days 1, 2 or 3 after treatment compared with baseline.
Time to resolution of fever, other symptoms and clinical signs.
Haematological recovery as shown by incremental change in haematocrit or haemoglobin from days 0 to 28 and days 0 to 42.
Gametocyte carriage throughout 42-day study period.
Progression to severe malaria (modified WHO 2010 criteria21).
Sensitivity and specificity of RDTs for the diagnosis of P. knowlesi malaria.
Recurrence of any species of malaria during the 12-month follow-up period.
Overall cure rates at day 42 will be evaluated by survival analysis with cumulative incidences calculated by Kaplan-Meier method and also compared by the Mantel-Haensel log-rank test. No interim analysis will be performed.
Other data included in final analyses
(1) A description of all patients screened and reasons for non-inclusion; (2) clinical, epidemiological and laboratory features of all patients included in the study; (3) the proportion of AEs and SAEs in all the patients included in the study; (4) the proportion of patients lost to follow-up or withdrawn, with reasons for withdrawal and (5) length of hospital inpatient stay and estimated cost to health sector.
Withdrawal or censoring in analysis
Patients will be withdrawn from the analysis if PCR results are unclassifiable or shown not to be P. knowlesi monoinfection. Patient's results will be censored in the analysis if PCR genotyping indicates any treatment failures are due to reinfection with P. knowlesi or new infection with another Plasmodium species, or if concomitant treatments with antimalarial activity are taken during the study period, including the initiation of rescue medication. Any patients withdrawn from the trial may still have data collected to that time point included in the analysis. The extent of missing data will be assessed and either consistent censoring of these patients results or multiple imputation methods will be considered.
Ethics and dissemination
Institutional Review Board approval
The study has been registered under http://www.clinicaltrials.gov (#NCT01708876). Any subsequent protocol amendments will be submitted to the ethics committees and made available to the study monitor.
Informed consent
Patients will be included in the study only if they or parents or guardians of participants <18 years of age give written informed consent. Assent for those from ages 7 to 17 will be obtained in addition, as per Malaysian Medical Research Ethics Committee (MREC) guidelines. Informed consent will be obtained by trained study nurses in Bahasa Malaysia or English. If the patient is illiterate, then thumbprint will be obtained on the consent form in accordance with Malaysian GCP guidelines. A consent statement for pregnancy testing is also required for female participants of childbearing age who are sexually active. Informed consent may be withdrawn at any time during the study period and will have no effect on the patient's clinical management at the study site. Biospecimen storage for a period of up to 10 years at the participating institutions will be documented on the patient information sheet and consent form. Samples will only be used for MREC approved assays.
Confidentiality
All information on patients will remain confidential and be shared only within the research team. Unique identifiers will be used for computer-based data entry and blood samples. Screening forms, the case report form and the completed identification code list will be kept in locked files.
Healthcare services
Any person who decides not to participate or who cannot be enrolled into the study because he or she does not meet the criteria will be referred for standard Malaysian Ministry of Health management. It will be stated clearly that a decision not to participate in the trial will not affect subsequent care.
Reimbursement of transport
Participants in the study will be reimbursed for their transport to attend all follow-up visits to the study sites. Patients will be paid MYR30 for each follow-up visit. No other gifts or payments will be made.
Trial-related injury
This is an investigator-initiated trial evaluating current first-line recommended standard therapies. As there is minimal additional risk from a normal standard of care, a trial Data Safety and Monitoring Board was not deemed necessary. Individuals will only agree to participate after being fully informed of inherent risks. There is no clinical trial insurance and patients will not receive financial compensation for any trial-related injury; however, patients will receive full access to standard Malaysian Ministry of Health hospital and outpatient care.
Dissemination of results
At the end of the study, the principal investigator will finalise a report on the study and its main outcome. This report will be shared with the national malaria control programme and the Malaysian Ministry of Health. Results will be published in peer-reviewed journal articles, presented at academic meetings, and relayed to communities at Kudat, Kota Murudu and Pitas through public meetings. All investigators will have access to the full trial data set and be included in relevant publications.
Acknowledgments
The authors thank the Malaysian Ministry of Health and the relevant Hospital Directors and staff at the study sites for support, as well as the research nurses, laboratory and administrative staff involved in the study.
Footnotes
Contributors: TW, NMA and TWY conceived and with MJG, LvS and RNP designed the study. TW, PD, MJG, NMA and TWY assisted in the logistics of setting up the study sites. MJG wrote the manuscript. All authors reviewed the manuscript.
Funding: Malaysian Ministry of Health Grant #BP00500420, AusAlD APMEN, Grant #108-07, and Australian National Health Medical Research Council: program grant 1037304 and project grant 1045156 (Fellowships to NMA, TWY, scholarship to MJG). Study sponsor: Clinical Research Centre, Queen Elizabeth Hospital, Sabah, Malaysia.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: Malaysian Medical Research Ethics Committee (#NMRR-12-89-11005) and Human Research Ethics Committee, Menzies School of Health Research, Darwin, Australia (#HREC-2012-1815).
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
References
- 1.Cox-Singh J, Davis TM, Lee KS, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis 2008;46:165–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jongwutiwes S, Putaporntip C, Iwasaki T, et al. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerg Infect Dis 2004;10:2211–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchand RP, Culleton R, Maeno Y, et al. Co-infections of Plasmodium knowlesi, P. falciparum, and P. vivax among humans and anopheles dirus mosquitoes, Southern Vietnam. Emerg Infect Dis 2011;17:1232–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng OT, Ooi EE, Lee CC, et al. Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg Infect Dis 2008;14:814–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luchavez J, Espino F, Curameng P, et al. Human infections with Plasmodium knowlesi, the Philippines. Emerg Infect Dis 2008;14:811–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox-Singh J, Singh B. Knowlesi malaria: newly emergent and of public health importance? Trends Parasitol 2008;24:406–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ninan T, Nalees K, Newin M, et al. Plasmodium knowlesi malaria infection in human. Brunei Int Med J 2012;8:358–61 [Google Scholar]
- 8.Jiang N, Chang Q, Sun X, et al. Co-infections with Plasmodium knowlesi and other malaria parasites, Myanmar. Emerg Infect Dis 2010;16:1476–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khim N, Siv S, Kim S, et al. Plasmodium knowlesi infection in humans, Cambodia, 2007–2010. Emerg Infect Dis 2011;17:1900–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyagi RK, Das MK, Singh SS, et al. Discordance in drug resistance-associated mutation patterns in marker genes of Plasmodium falciparum and Plasmodium knowlesi during coinfections. J Antimicrob Chemother 2013;68:1081–8 [DOI] [PubMed] [Google Scholar]
- 11.Zhu HM, Li J, Zheng H. [Human natural infection of Plasmodium knowlesi]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2006;24:70–1 [PubMed] [Google Scholar]
- 12.Vythilingam I. Plasmodium knowlesi in humans: a review on the role of its vectors in Malaysia. Trop Biomed 2010;27:1–12 [PubMed] [Google Scholar]
- 13.Baird JK. Malaria zoonoses. Travel Med Infect Dis 2009;7:269–77 [DOI] [PubMed] [Google Scholar]
- 14.Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev 2013;26:165–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barber BE, William T, Grigg MJ, et al. Limitations of microscopy to differentiate Plasmodium species in a region co-endemic for Plasmodium falciparum, Plasmodium vivax and Plasmodium knowlesi. Malar J 2013;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh B, Kim Sung L, Matusop A, et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 2004;363:1017–24 [DOI] [PubMed] [Google Scholar]
- 17.Anderios FEA. Detection of malaria parasites in Sabah by nested PCR: a focus of naturally acquired P knowlesi infections. Sains Malaysiana 2008;32:137–41 [Google Scholar]
- 18.Naing DKS, Anderios F, Lin Z. Geographic and ethnic distribution of P knowlesi infection in Sabah, Malaysia. Int J Collaborative Res Int Med Public Health 2011;3:391–400 [Google Scholar]
- 19.Singh B, Daneshvar C. Plasmodium knowlesi malaria in Malaysia. Med J Malaysia 2010;65:166–72 [PubMed] [Google Scholar]
- 20.Joveen-Neoh WF, Chong KL, Wong CM, et al. Incidence of malaria in the interior division of Sabah, Malaysian Borneo, based on nested PCR. J Parasitol Res 2011;2011:104284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber BE, William T, Grigg MJ, et al. A prospective comparative study of knowlesi, falciparum, and vivax malaria in Sabah, Malaysia: high proportion with severe disease from plasmodium knowlesi and plasmodium vivax but no mortality with early referral and artesunate therapy. Clin Infect Dis 2013;56:383–97 [DOI] [PubMed] [Google Scholar]
- 22.Ministry of Health Malaysia, V. B. D. C. P. Vector control in Malaysia. Consultative meeting/workshop on management of vector control programs, 11–15 August 2008; Siem Reap, Cambodia. vc_policy/vc_malaysia.pdf. http://www.actmalaria.net/files/vector_control/ (accessed 15 Jul 2012).
- 23.APMEN V: Vivax Working Group Meeting. 2013. http://apmen.org/storage/apmen-v/apmen-v-powerpoint-presentations-all/day-1/APMENV_S6_Artesunate-mefloquine%20vs.%20chloroquine%20in%20patients%20with%20P.vivax%20-%20a%20randomized%20open%20label%20trial%20Sabah_Tim%20William1.pdf (accessed 4 Jan 2014).
- 24.Daneshvar C, Davis TM, Cox-Singh J, et al. Clinical and laboratory features of human Plasmodium knowlesi infection. Clin Infect Dis 2009;49:852–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.William T, Menon J, Rajahram G, et al. Severe Plasmodium knowlesi malaria in a tertiary care hospital, Sabah, Malaysia. Emerg Infect Dis 2011;17:1248–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajahram GS, Barber BE, William T, et al. Deaths due to Plasmodium knowlesi malaria in Sabah, Malaysia: association with reporting as Plasmodium malariae and delayed parenteral artesunate. Malar J 2012;11:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daneshvar C, Davis TM, Cox-Singh J, et al. Clinical and parasitological response to oral chloroquine and primaquine in uncomplicated human Plasmodium knowlesi infections. Malar J 2010;9:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barber BE, William T, Jikal M, et al. Plasmodium knowlesi malaria in children. Emerg Infect Dis 2011;17:814–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bronner U, Divis PC, Farnert A, et al. Swedish traveller with Plasmodium knowlesi malaria after visiting Malaysian Borneo. Malar J 2009;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Figtree M, Lee R, Bain L, et al. Plasmodium knowlesi in human, Indonesian Borneo. Emerg Infect Dis 2010;16:672–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoosen A, Shaw MTM. Plasmodium knowlesi in a traveller returning to New Zealand. Travel Med Infect Dis 2011;9:144–8 [DOI] [PubMed] [Google Scholar]
- 32.Berry A, Iriart X, Wilhelm N, et al. Imported Plasmodium knowlesi malaria in a French tourist returning from Thailand. Am J Trop Med Hyg 2011;84:535–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ta Tang T-H, Ali-Tammam M, Martínez Mdel C, et al. First case of detection of Plasmodium knowlesi in Spain by real time PCR in a traveller from Southeast Asia. Malar J 2010;9:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CE, Adeeba K, Freigang G. Human Plasmodium knowlesi infections in Klang Valley, Peninsula Malaysia: a case series. Med J Malaysia 2010;65:63–5 [PubMed] [Google Scholar]
- 35.Kuo M-C, Chiang T-Y, Chan C-W, et al. A case report of Simian Malaria, Plasmodium knowlesi, in a Taiwanese Traveler from Palawan Island, the Philippines. Epidemiol Bull 2009;25: 178–91 [Google Scholar]
- 36.WHO. WHO malaria treatment guidelines. Geneva 2010 [Google Scholar]
- 37.White NJ. Clinical pharmacokinetics and pharmacodynamics of artemisinin and derivatives. Trans R Soc Trop Med Hyg 1994;88(Suppl 1):S41–3 [DOI] [PubMed] [Google Scholar]
- 38.White N. Antimalarial drug resistance and combination chemotherapy. Philos Trans R Soc Lond B Biol Sci 1999;354:739–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg 2007;77:181–92 [PubMed] [Google Scholar]
- 40.Ambler MT, Dubowitz LM, Arunjerdja R, et al. The neurological assessment in young children treated with artesunate monotherapy or artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria. Malar J 2009;8:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krudsood S, Looareesuwan S, Silachamroon U, et al. Artesunate and mefloquine given simultaneously for three days via a prepacked blister is equally effective and tolerated as a standard sequential treatment of uncomplicated acute Plasmodium falciparum malaria: randomized, double-blind study in Thailand. Am J Trop Med Hyg 2002;67:465–72 [DOI] [PubMed] [Google Scholar]
- 42.Sinclair D, Zani B, Donegan S, et al. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst Rev 2009;(3):CD007483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nosten F, Vincenti M, Simpson J, et al. The effects of mefloquine treatment in pregnancy. Clin Infect Dis 1999;28:808–15 [DOI] [PubMed] [Google Scholar]
- 44.Fatih FA, Staines HM, Siner A, et al. Susceptibility of human Plasmodium knowlesi infections to anti-malarials. Malar J 2013;12:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lau YL, Tan LH, Chin LC, et al. Plasmodium knowlesi reinfection in human. Emerg Infect Dis 2011;17:1314–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO. WHO management of severe malaria Geneva 2013:1–89 [Google Scholar]
- 47.Ministry of Health Malaysia, V. B. D. C. P. Management guidelines of malaria in Malaysia. Malaysia: Ministry of Health, 2013. http://www.moh.gov.my/index.php [Google Scholar]
- 48.Douglas NM, Anstey NM, Angus BJ, et al. Artemisinin combination therapy for vivax malaria. Lancet Infect Dis 2010;10:405–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.WHO. Methods for surveillance of antimalarial drug efficacy. WHO, 2009 [Google Scholar]
- 50.Price RN, Nosten F, Luxemburger C, et al. Effects of artemisinin derivatives on malaria transmissibility. Lancet 1996;347: 1654–8 [DOI] [PubMed] [Google Scholar]
- 51.Angus BJ, Thaiaporn I, Chanthapadith K, et al. Oral artesunate dose-response relationship in acute falciparum malaria. Antimicrob Agents Chemother 2002;46:778–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price R, Simpson JA, Teja-Isavatharm P, et al. Pharmacokinetics of mefloquine combined with artesunate in children with acute falciparum malaria. Antimicrob Agents Chemother 1999;43:341–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Padley D, Moody AH, Chiodini PL, et al. Use of a rapid, single-round, multiplex PCR to detect malarial parasites and identify the species present. Ann Trop Med Parasitol 2003;97:131–7 [DOI] [PubMed] [Google Scholar]
- 54.Imwong M, Tanomsing N, Pukrittayakamee S, et al. Spurious amplification of a Plasmodium vivax small-subunit RNA gene by use of primers currently used to detect P. knowlesi. J Clin Microbiol 2009;47:4173–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Divis PC, Shokoples SE, Singh B, et al. A TaqMan real-time PCR assay for the detection and quantitation of Plasmodium knowlesi. Malar J 2010;9:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCutchan TF, Piper RC, Makler MT. Use of malaria rapid diagnostic test to identify Plasmodium knowlesi infection. Emerg Infect Dis 2008;14:1750–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barber BE, William T, Grigg MJ, et al. Evaluation of the sensitivity of a pldh-based and an aldolase-based rapid diagnostic test for diagnosis of uncomplicated and severe malaria caused by PCR-confirmed plasmodium knowlesi, Plasmodium falciparum, and plasmodium vivax. J Clin Microbiol 2013;51:1118–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grigg MJ, William T, Barber BE, et al. Combining parasite lactate dehydrogenase-based and histidine rich protein-2 rapid tests to improve specificity for the diagnosis of malaria due to Plasmodium knowlesi and other Plasmodium species in Sabah, Malaysia. J Clin Microbiol 2014;52:2053–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Good Clinical Practice: ICH guidelines. http://www.ich.org/products/guidelines/efficacy/efficacy-single/article/good-clinical-practice.html.
- 60.World Wide Anti-malarial Resistance Network (WWARN). http://www.wwarn.org (accessed 21 Jul 2012).