Abstract
TGFBIp, also known as keratoepithelin and βig-h3, is among the most abundant proteins in the human cornea and approximately 60% is associated with the insoluble fraction following extraction in sodium dodecyl sulfate (SDS) sample buffer. TGFBIp is of particular interest because a wide range of mutations causes amyloid or fuchsinophilic crystalloid deposits in the cornea leading to visual impairment. In the present study, we show that the SDS-insoluble fraction of TGFBIp from porcine and human corneas is covalently linked to the NC3 domain of type XII collagen in a TGFBIp:type XII collagen stoichiometric ratio of 2:1 via a reducible bond. Since type XII collagen is anchored to striated collagen fibers of the extracellular matrix, its interaction with TGFBIp is likely to provide anchoring for cells to the extracellular matrix through the integrin-binding capability of TGFBIp. Furthermore, the TGFBIp-type XII collagen molecule will affect our understanding of the molecular pathogenesis of the TGFBI-linked corneal dystrophies.
Transforming growth factor beta induced protein (TGFBIp, also known as keratoepithelin and βig-h3; UniProt accession number Q15582) is an extracellular matrix (ECM) protein, which is found in several tissues of the human body(1–7). Significantly, TGFBIp is the second most abundant protein in the human cornea(8), yet its precise function in corneal homeostasis remains elusive. As part of the ECM of several tissues, it has been found to interact non-covalently with a number of matrix macromolecules including types I, II, and IV collagens(9), and laminin, fibronectin(10, 11), decorin, and biglycan(12). In addition, TGFBIp has been reported to interact covalently and non-covalently with type VI collagen in bovine nuchal ligament(13). Finally, recombinant TGFBIp has been shown to bind several sub-types of human integrins(14–20). Taken together, the interactions to the fibril-forming collagens (types I, II, and III) as well as to integrins indicate that TGFBIp may mediate contacts between the collagenous ECM matrix scaffold and the cells embedded therein. These molecular interactions may play important roles in the molecular pathogenesis of corneal dystrophies linked to mutations in the transforming growth factor beta induced (TGFBI) gene and the organization of connective tissues in certain types of cancers(21, 22).
The corneal stroma comprises roughly 90% of the corneal thickness and is composed mainly of collagens and proteoglycans(8) with relatively few embedded cells(23). The organization of the macromolecules and cells including the hexogonal arrangement of type I collagen fibers is essential for corneal stromal transparency(24, 25). This highly ordered collagenous organization is maintained by proteoglycans and smaller collagen types(26) which also interact with keratocytes in the collagen matrix. Elucidating the molecular interactions between TGFBIp and the ECM in the cornea may prove valuable in understanding corneal transparency and TGFBI-linked corneal dystrophy pathogeneses.
We have previously reported that approximately 60% of TGFBIp is covalently bound to the SDS-insoluble part of the ECM in both human and porcine corneas through a reducible cross-link(27). In the present study, we have identified trivalent Fibril-Associated Collagen with Interrupted Triple helices (FACIT) type XII collagen (UniProt accession number Q99715) as the covalently linked interaction partner in mammalian cornea. We propose that this novel interaction between TGFBIp and type XII collagen is important for understanding the homeostasis of the healthy cornea and the pathobiology of TGFBI-linked corneal dystrophies.
EXPERIMENTAL PROCEDURES
Materials
Chemicals were purchased from Sigma-Aldrich Co. Porcine eyes were obtained from Danish Crown (Horsens, Denmark). The corneas of 200 eyes were excised and the corneal stroma was isolated by scraping off the epithelial and endothelial cell layers. The corneas were dried in a vacuum desiccator and homogenized in liquid nitrogen using a tissue homogenizer (Ystral, Ballrechten-Dottingen, Germany). The resulting powder was stored at −20°C until use for further experiments.
Production of antiserum against full-length recombinant human TGFBIp (anti-FL-rhTGFBIp)
Rabbits were immunized three times subcutaneously with approximately 100 μg of the purified full-length human recombinant TGFBIp expressed in HEK293T cells and purified as described previously(28).
Solubilization of cross-linked TGFBIp from intact porcine corneas
Approximately 4 mg of homogenized porcine cornea was suspended in 1 mL alkylation buffer containing 50 mM iodoacetamide (IAA), 20 mM Tris-HCl, pH 8.0 and left rotating in the dark, for 1 hour at 23°C. After centrifugation at 10,000 g for 2 min, protein was extracted from the pellet by rotating 1 hour in 1 mL extraction buffer containing 1 M (NH4)2SO4, 20 mM Tris-HCl, pH 7.4 at 23°C. This extraction process was repeated three times on the tissue. Subsequently, the pellet was washed 6 times in 1 mL 1×phosphate buffered saline (PBS). All steps were performed at 23°C. The resulting pellet was solubilized by adding 300 μL 4 mg/mL Clostridium collagenase I (Life technologies, Carlsbad, CA) in 1×PBS supplemented with 3 mM CaCl2, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL fungizone (amphotericin B) (Sigma-Aldrich, St. Louis, MO), rotating at 37°C. At time points indicated in the figure legends the sample was centrifuged at 16,000 g for 2 min, and 50 μL was removed from the supernatant for analysis by non-reducing SDS-PAGE and immunoblotting. The remaining pellet was re-suspended and digested further at 37°C.
Purification of TGFBIp-containing complex from porcine corneal stroma
Approximately, 1 g of porcine corneal stroma was added to 50 mL extraction buffer and left rotating for 1 hour. This treatment was repeated and followed by alkylation in 15 mL alkylation buffer for 1 hour rotating in the dark. Subsequently, the sample was washed three times in 50 mL 20 mM Tris-HCl, pH 7.4 (buffer A). All steps were performed at 23°C. Between each washing step, the sample was centrifuged for 10 min at 1,000 × g and the supernatants were discarded. The resulting pellet was digested with 80 mg collagenase I in 50 mL 1×PBS containing 3 mM CaCl2, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL fungizone. This was left rotating for 6 hours at 37°C. The sample was then centrifuged as described above, and the supernatant was collected and stored at −20°C until used.
The supernatant from collagenase I-digested porcine corneal stroma was dialyzed two times against 5 L buffer A at 4°C and filtered using a 0.22 μm filter (Thermo Scientific, Rochester, NY) before TGFBIp was purified as previously described(28). Briefly, protein was purified by affinity chromatography using a heparin Sepharose column (1 mL HiTrap Heparin HP column, GE Healthcare, Buckinghamshire, England) connected to an FPLC system (GE Healthcare). The column was pre-equilibrated in buffer A and the sample was then applied at 1 mL/min. Bound proteins were eluted using a linear NaCl gradient (0.0–1.0 M). Selected fractions were analyzed by non-reducing SDS-PAGE followed by immunoblotting using the anti-FL-rhTGFBIp antiserum. Fractions containing the TGFBIp-complex were pooled and dialyzed two times against 5 L buffer A overnight. The resulting sample was then filtered and purification was continued by ion exchange chromatography using an anion exchange column (1 mL HiTrap Q HP column, GE Healthcare). Proteins were eluted by applying a linear gradient of NaCl (0.0–1.0 M) and analyzed by non-reducing SDS-PAGE and immunoblotting as described above.
Polyacrylamide gel electrophoresis (PAGE), densitometry, and immunoblotting
SDS-PAGE was performed on 5–15% gradient gels (10 cm × 10 cm × 1.5 mm) using the glycine/2-amino-2-methyl-1,3-propanediol/HCl system(29). Unless otherwise stated, samples were diluted/dissolved in 50 mM Tris-HCl, 150 mM NaCl, pH 7.4. Proteins were visualized using Coomassie or silver staining. Relative quantifications of protein amounts in SDS-PAGE were measured using ImageJ software (version 1.46, National Institutes of Health). Immunoblots were made by transferring proteins to polyvinylidene difluoride (PVDF) membranes (Immobilon-P, Millipore, Billerica, MA) in 10 mM N-cyclohexyl-3-aminopropanesulfonic acid (CAPS) and 10% (v/v) methanol (pH 11)(30) and TGFBIp-containing bands were subsequently detected using a polyclonal antiserum raised against full-length recombinant human TGFBIp.
Electro-elution of protein bands from polyacrylamide gels
Relevant bands from SDS-PAGE gels were excised and protein was extracted using an Electro-Eluter Concentrator (C.B.S. Scientific Co., Del Mar, CA) as described previously(31). Briefly, gel plugs were washed with water and placed in the electro-eluter cell separated from the reservoir by a dialysis membrane with a molecular weight cut-off (MWCO) of 12–14,000 Da, and swelled in 0.4 M NH4HCO3 containing 2% SDS. The electro-eluter reservoir was filled with 0.1% SDS, 50 mM NH4HCO3 and a current of 50 V was applied overnight. The next day the reservoir solution was changed to 10 mM NH4HCO3 containing 0.02% SDS and an 80 V current was applied overnight. Proteins were collected at the anode of the cell and concentrated using ethanol precipitation prior to gel electrophoresis.
Protein identification by matrix assisted laser desorption ionization-mass spectrometry (MALDI-MS)
Bands from Coomassie- and silver stained gels were excised and washed extensively with water and acetonitrile. Sequence grade trypsin from porcine (Promega, Madison, WI) at a concentration of 12.5 ng/μL in 50 mM NH4HCO3 (was added to the gel plugs and incubated at 37°C for 16–17 hours. Peptides were isolated using a C-18 ZipTip (Millipore, Billerica, MA) and samples were mixed with α-cyano-4-hydroxy-cinnamic acid at 2 mg/mL in 50% acetonitrile/0.3% trifluoroacetic acid. Subsequently, 0.5 μL of the mixture was spotted on MALDI targets and samples were analyzed by MALDI-MS and MALDI-MS/MS using a quadropole time-of-flight (QTOF) Ultima Global mass spectrometer (Micromass, Waters Corp., Manchester, United Kingdom). The resulting peak lists of peptides were used to query all entries of the NCBI non-redundant protein database or the Swiss-Prot protein database on a local Mascot server using the Mascot search engine (Matrix Sciences, London, United Kingdom). The searches were performed with a peptide mass tolerance of 10 parts per million (ppm) and a single missed tryptic cleavage was allowed. Only significant hits as defined by Mascot probability analysis were accepted.
Edman degradation for amino-terminal sequence identification
Proteins in TGFBIp-containing fractions were separated by reducing or non-reducing SDS-PAGE. To avoid amino-terminal derivatization, the stacking gel was allowed to polymerize overnight prior to electrophoresis, and samples were heated for 3 min at only 80°C before SDS-PAGE. Following electrophoresis, proteins were transferred to a PVDF membrane (Immobilon-P, Millipore) in 10 mM CAPS and 10% (v/v) methanol (pH 11)(30). Samples were then analyzed by automated Edman degradation using an Applied Biosystems PROCISE™ 494 HT sequencer with on-line HPLC (Model 120A, Applied Biosystems, Foster City, CA) for phenylthiohydantoin analysis.
Analysis of intermolecular covalent TGFBIp cross-link in human corneas
A healthy human cornea was obtained post mortem at the Department of Forensic Medicine, Aarhus University Hospital, Denmark as described previously(32). Two pieces of 0.5 mg cornea were dried in a SpeedVac concentrator. One piece was boiled in non-reducing SDS sample buffer for 3 × 10 min after which the supernatants were removed. The resulting pellet was then boiled in SDS sample buffer containing 30 mM dithiothreitol (DTT). The other piece of cornea was digested with collagenase I as described above. Equal amounts of sample were analyzed by SDS-PAGE and immunoblotting as described in the figure legend.
RESULTS
Digestion of collagen fibers releases a high-molecular-weight TGFBIp-containing complex from the cornea
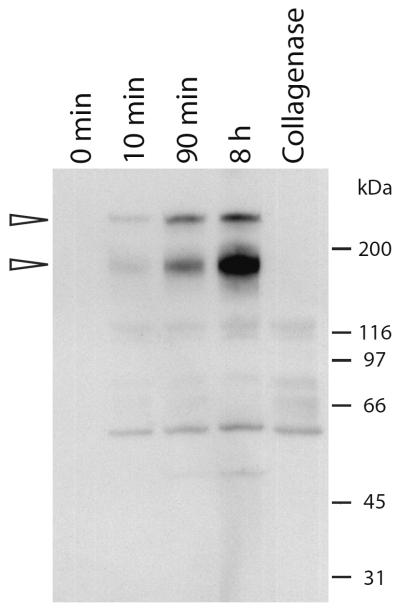
In a previous study, we showed that approximately 60% of TGFBIp in the human and porcine corneas is covalently bound to the SDS-insoluble fraction of the ECM through a reducible bond(27). To investigate the nature of the interaction we purified the TGFBIp-containing complex from porcine corneas. This was done by removing soluble ECM proteins through an extensive washing procedure after which, the insoluble collagenous pellet was subjected to collagenase I digestion (Figure 1). An immunoblot analysis following non-reducing SDS-PAGE of the resulting supernatants revealed a time-dependent release of a major 170-kDa and a minor 224-kDa TGFBIp-containing complex from the SDS-insoluble corneal fraction.
Figure 1. Collagenase I-digestion releases a 170-kDa TGFBIp-containing complex from the SDS-insoluble fraction of porcine cornea.
Porcine cornea powder was washed extensively and digested with collagenase I for different periods of time at 37°C. The supernatant from the first wash (0 min), and supernatants after digestion for 10 min, 90 min, and 8 hours were analyzed by anti-TGFBIp immunoblotting following non-reducing SDS-PAGE. Collagenase auto digest (no cornea powder) was loaded in the final lane (Collagenase). Molecular weights are indicated on the right. The immunoblot shows that there is a time-dependent release of a major 170-kDa and a minor 210-kDa TGFBIp-containing molecule when corneal tissue is incubated with collagenase I.
Purifying the TGFBIp-containing complex from corneas
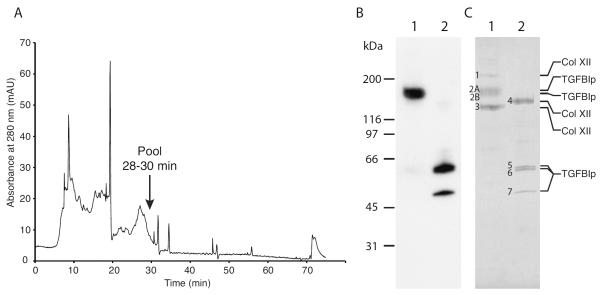
The major 170-kDa TGFBIp-containing complex was purified from the supernatant of collagenase I-digested porcine corneas by heparin affinity and anion exchange chromatography (Figure 2). The broad peak ranging from about 25 to 30 minutes in the anion exchange chromatogram (Figure 2A) contained only noncovalently bound TGFBIp in the first fractions (25–27), whereas fractions from 28–30 minutes contained primarily the high-molecular-weight TGFBIp-containing complex. Immunoblotting of the final pool showed that TGFBIp, under non-reducing conditions, was present in a 170-kDa complex, which was disrupted upon reduction with DTT to yield two TGFBIp species of about 62 kDa and 50 kDa (Figure 2B) indicating that TGFBIp is released from a molecule of higher molecular weight. In addition to the 170-kDa, a Coomassie blue stained SDS-PAGE gel of the same samples showed that the purified TGFBIp-containing complex preparation generate two additional protein bands of 210 kDa and 138 kDa (Figure 2C, protein bands 1 and 3, respectively) when separated under non-reducing conditions. An additional 151-kDa protein (Figure 2C, protein band 4) was observed under reducing conditions compared to the gel band pattern from the TGFBIp specific immunoblot (Figure 2C).
Figure 2. Purification of the high-molecular-weight TGFBIp-containing complex from porcine cornea.
(A) Anion exchange chromatogram from the final step of purification of the high-molecular-weight TGFBIp-containing complex. Collagenase I-digested cornea was initially applied to a heparin affinity column from which all bound protein was dialyzed and applied to an anion exchange column. Fractions 28–30 were pooled and further analyzed. (B) Immunoblot of non-reducing (lane 1) and reducing (lane 2) SDS-PAGE of the pooled fractions containing the 170-kDa TGFBIp-containing complex. (C) Coomassie-stained SDS-PAGE gel of non-reduced (lane 1) and reduced (lane 2) samples of the purified 170-kDa TGFBIp-containing complex. Protein bands that were analyzed by MALDI-MS and N-terminal sequencing are indicated with numbers 1 to 8 and the identity based on MS-analysis is shown on the right. The characterization of the TGFBIp-containing complex shows that non-covalently bound type XII collagen molecules migrating at 210 kDa and 138 kDa co-purifies with the covalent and reducible 170-kDa TGFBIp:collagen XII complex.
Establishing the identity and stoichiometry of the TGFBIp-containing complex components
To establish the identities of the subunits in the 170-kDa TGFBIp-containing complex, protein bands from non-reducing and reducing SDS-PAGE were analyzed by mass spectrometry (Table 1). Thus, protein bands 1, 3, and 4 were identified as type XII collagen and protein bands 2, 5, 6, and 7 were identified as TGFBIp (Figure 2C and Table 1). Edman degradation was utilized to determine the exact N-termini of the protein bands (Table 1).
Table 1.
Protein identifications*
| Band | ID | Accession no. | Peptide-range | % coverage | Mowse score (expect) | N-terminal |
|---|---|---|---|---|---|---|
| 1 | Collagen alpha-1 (XII) (Sus scrofa) | XP_001927071 | 284–1815 | 30 | 162 (4.3e–10) | |
| 2A | TGFBIp ( | O11780 | 28–602 | 45 | 200 (6.9e–14) | GPAKSPYQ L |
| 2B | TGFBIp (Sus scrofa) | O11780 | 28–588 | 42 | 134 (2.7e–7) | LVSNVNIEL LN |
| 3 | Collagen alpha-1 (XII) (Sus scrofa) | XP_001927071 | 284–1312 | 24 | 216 (1.7e–15) | ILSSGMECL TR |
| 4 | Collagen alpha-1 (XII) (Sus scrofa) | XP_001927071 | 284–1312 | 26 | 223 (3.4e–16) | ILSSGMECL TR |
| 5 | TGFBIp (Sus scrofa) | O11780 | 28–588 | 42 | 122 (4.3e–6) | |
| 6 | TGFBIp (Sus scrofa) | O11780 | 28–588 | 42 | 131 (5.4e–7) | |
| 7 | TGFBIp (Sus scrofa) | O11780 | 173–602 | 32 | 124 (2.7e–6) | LVSNVNIEL LN |
Search criteria: Database: NBCInr (Sus scrofa), Variable modifications: Carbamidomethyl (C), Propionamide (C), Oxidation (M). +/− 10 ppm.
Initially, only TGFBIp was identified in the non-reduced 170-kDa band (Figure 2) by mass spectrometry and N-terminal sequencing (Tables 1). The additional component of the 170-kDa TGFBIp-containing band was identified by electro-eluting the non-reduced 170-kDa band followed by separation using reducing SDS-PAGE (Figure 3). The major resulting 151-kDa band was identified by MALDI-MS as type XII collagen while the bands of 62 kDa and 50 kDa were identified as TGFBIp (data not shown).
Figure 3. Electro-elution of the 170-kDa TGFBIp-containing band.
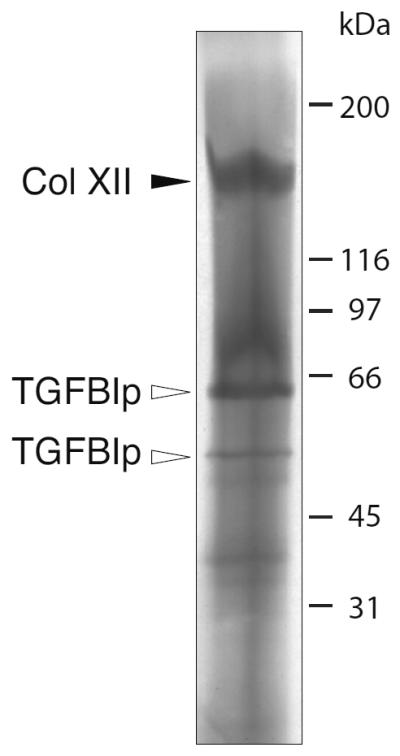
The 170-kDa TGFBIp-containing band observed by SDS-PAGE under non-reducing conditions was electro-eluted and analyzed by reducing SDS-PAGE. MS analysis of the resulting marked bands show that the 170-kDa TGFBIp-containing complex contains one isoform of type XII collagen of 151 kDa (solid arrowhead marked Col XII) and TGFBIp isoforms migrating at 62 kDa and 50 kDa (open arrowheads marked TGFBIp).
To determine the stoichiometry within the covalent reducible TGFBIp-type XII collagen complex (Figure 2C, protein band 2) the Coomassie blue stained gel bands were analyzed by densitometry (Table 2). When comparing the summed intensities of all bands in the non-reducing lane to those of the reducing lane (Figure 2C) the ratio was approximately 1:1 suggesting that all TGFBIp and type XII collagen of the purified sample entered the SDS gel. To obtain information about the stoichiometry between TGFBIp and type XII collagen, the intensities of the protein bands in the reducing lane were compared. The intensity-ratio between the 151-kDa type XII collagen band (band 4) and the summed intensities of the TGFBIp bands (bands 5–7) was determined as 1:0.8 (Table 2), assuming that the two molecules are stained equally well by Coomassie. When correcting for the mass differences between TGFBIp and type XII collagen, this results in an approximate molar ratio of two TGFBIp molecules per one type XII collagen molecule.
Table 2.
Densitometry
| Band | Raw data (AU) | Relative intensities* |
|---|---|---|
| 1 | 1842 | 0.25 |
| 2 | 11562 | 2 |
| 3 | 13780 | 1 |
| 4 | 13073 | 1 |
| 5–6 | 7678 | 0.6 |
| 7 | 2641 | 0.2 |
Approximate intensities relative to protein band 4; AU, arbitrary units.
To show that an equivalent covalent TGFBIp-ECM interaction is found in human corneas, a healthy human cornea was analyzed by anti-TGFBIp immunoblotting after reducing and non-reducing SDS-PAGE before and after collagenase I-digestion as was done for the porcine corneas (Figure 4). When comparing lanes of the non-reduced samples for the non-collagenase-digested and the collagenase-digested corneal stroma (lanes 1 and 3) it can be seen that the TGFBIp-containing complex of approximately 170 kDa is released by the collagenase treatment. Upon reduction with DTT, this complex dissociates to result in free TGFBIp migrating below the 66 kDa molecular weight marker (lane 4). Thus, these results confirm that TGFBIp and type XII collagen form a covalently linked complex in the mammalian cornea.
Figure 4. Collagenase I digestion releases a 170-kDa TGFBIp-containing complex in human corneas.
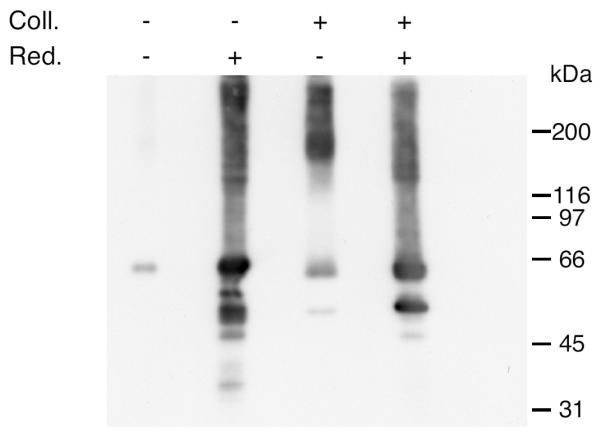
Dried human cornea was analyzed for the presence of high-molecular-weight TGFBIp-containing complexes before and after digestion with collagenase I. Collagenase digested (Coll.; +) and non-digested (Coll.; −) samples were analyzed by reducing (Red.; +) and non-reducing (Red.; −) conditions followed by immunoblotting to detect TGFBIp-containing bands. The immunoblot shows that in the non-digested cornea only little TGFBIp is visible on the gel without reducing agent present compared to the massive band observed in the reducing lane. When the collagenase I-digested sample is analyzed a TGFBIp-containing band of 170 kDa is observed in the non-reducing lane as seen for the porcine tissue (Figures 1 and 2). Upon reduction with DTT the most prominent signal migrates as a TGFBIp monomer at 66 kDa. Collectively, these data suggest that TGFBIp in the human cornea is also covalently linked to type XII collagen through a disulfide bridge.
DISCUSSION
TGFBIp (UniProt accession number Q15582) is a major protein in several connective tissues and is involved in different types of corneal dystrophies(33, 34) and cancers(21, 22). In the present study, we report a novel disulfide-mediated cross-link between TGFBIp and type XII collagen in mammalian corneas. Human type XII collagen (UniProt accession number Q99715) belongs to the FACIT collagens and exists as a long (330 kDa) and a short (203 kDa) splice variant, which can form both homo- or heterotrimers. The C-terminal collagenous region of type XII collagen interacts with the surface of banded type I collagen fibers, while the noncollagenous 3 (NC3) domains are exposed to the surrounding matrix allowing for additional intermolecular interactions(35). In vivo, type XII collagen has been shown to be associated with the assembly of the corneal matrix and to be present in regions of increased stability(36, 37) and it has been reported to be overexpressed during corneal scarring(38). Significantly, TGFBIp has been shown to co-localize with type XII collagen in rabbit corneas(39), and our recent data show that type XII collagen is a major component of the corneal proteome, suggesting that it plays an important function in the homeostasis of the human cornea(8).
Thus, based on the present results and the reported reducible covalent interaction between TGFBIp and type VI collagen in bovine nuchal ligament(13) we conclude that TGFBIp forms intermolecular disulfide bridges to different proteins of the ECM and that it may vary between connective tissues.
TGFBIp is cross-linked to the NC3 domain of type XII collagen in a 2 to 1 ratio
Collagenase cleavage of type XII collagen has previously been shown to result in the release of the NC3 domain (including the fibronectin type-III and von Willebrand factor A domains) as a non-covalently associated trimer(40). This is supported by the current study mainly showing identification of NC3 derived peptides following MALDI-MS analyses (Table 1). Thus, the purified complex is likely to consist of a trimeric type XII collagen NC3 domain linked to TGFBIp via a disulfide bond. Additional information about the TGFBIp-type XII collagen cross-link can be deduced from the mass spectrometry and N-terminal sequencing data (Tables 1 and 2). The N-terminus of porcine type XII collagen was found to be ILSSGMECLTR. This sequence is similar to the N-terminal sequence (EGMECLTR) of the human short splice-variant (UniProt accession no. Q99715) suggesting that the porcine sequence similarly is the short splice-variant (Figure 5).
Figure 5. Schematic illustration of type XII collagen, TGFBIp, and the species purified from porcine cornea.
Human type XII collagen exists as a long and a short splice-variant. Porcine type XII collagen (accession no. XP_001927071) has not been characterized at the protein level at this point, but the sequence of the predicted protein is more than 90% identical to that of human type XII collagen. The fragment of porcine type XII collagen purified in the present study (protein band 4 (151 kDa) in Figure 2) is shown. The C-terminal part of this fragment (indicated with dashed lines) was not identified by MALDI-MS but is likely to be present due to the size of the band in SDS-PAGE (151 kDa).
Densitometry of the protein bands following reducing SDS-PAGE suggested that there are on average two TGFBIp molecules associated with each collagen monomer in the 170-kDa complex. However, it is difficult to determine the exact number of TGFBIp molecules linked to each type XII collagen trimer. During non-reducing SDS-PAGE two of the observed bands only contained type XII collagen (Figure 2C, bands 1 and 3). This indicated that the 170-kDa band may contain more than two equivalents of TGFBIp per collagen molecule. As mentioned below, there are many potential binding sites for TGFBIp on the NC3 domain of type XII collagen, which contains tandem repeats of various subdomains.
The disulfide bond-pattern of TGFBIp remains unclear. However, 6 of the 11 Cys-residues in TGFBIp are found in the EMI domain and have been suggested to form three intra-domain disulfide bridges(41). The remaining 5 Cys-residues are found within the first three fasciclin (FAS1) domains. The two pairs of Cys-residues found in the FAS1-2 (Cys317 and Cys339) and FAS1-3 (Cys473 and Cys478) domains are in very close proximity in homology modeling from the FAS1-4 domain nuclear magnetic resonance (NMR) structure, suggesting that these might be involved in intra-domain disulfide bridges. Based on this it is most likely that Cys214 in the FAS1-1 domain is free to form the intermolecular disulfide bridge with type XII collagen.
The NC3 domain of porcine type XII collagen contains 16 Cys-residues. Two of these are positioned before the identified N-terminus and a total of 7 Cys-residues are found in the laminin G/thrombospondin N-terminal domain, which is likely absent from the type XII collagen fragment purified from porcine cornea (Figure 5). This leaves 7 Cys-residues as potential cross-linkers in type XII collagen. None of these have yet been reported to form intra- or inter-molecular bridges and are therefore all potential candidates for forming the disulfide bridge to TGFBIp.
Potential impact on TGFBIp function in normal and diseased cornea
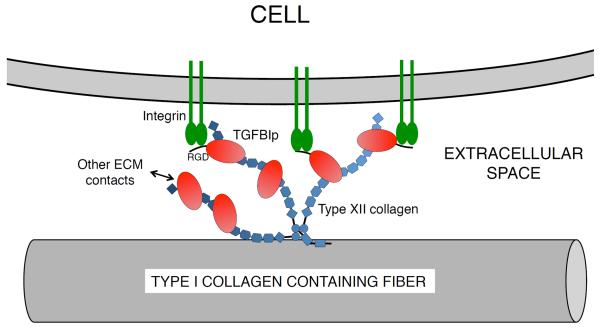
TGFBIp appears to exist both in a free soluble form and a covalently bound state anchored to the ECM suggesting a dual function; the immobilized molecules may serve as anchors for cells in the ECM, while the soluble molecules may serve a regulatory function. Being a flexible molecule, type XII collagen likely acts as a spacer between the collagen scaffold of the ECM and the cells embedded in it. While the collagenous domains of type XII collagen binds to the striated collagen fibers, the NC3 domain extends into the extracellular space with its von Willebrand factor A-, fibronectin-, and thrombospondin N-terminal domains accessible to other macromolecules of the ECM. TGFBIp will facilitate additional inter-molecular interactions and based on the present data we propose a model in which TGFBIp, with its ability to bind integrins, acts as a mediator of ECM-cell contacts (Figure 6).
Figure 6. Model for the proposed biological function of the covalent TGFBIp-type XII collagen interaction in the cornea.
Type XII collagen has been shown to interact noncovalently with type I collagen fibers of the corneal ECM. The non-collagenous domains of type XII collagen trimer extents into the extracellular space and binds TGFBIp. TGFBIp is consequently able to interact with other macromolecules of the ECM. The immobilized TGFBIp may function as an ECM attachment anchor for the cells of the cornea.
The observation that the majority of TGFBIp is covalently linked to the ECM of the cornea likely has implications for the understanding of the TGFBI-linked corneal dystrophies. Thus, despite the high content of TGFBIp in the human cornea, unwanted protein-protein contact, potentially leading to protein aggregation, may be limited by the anchoring to the ECM. In this regard, it is interesting to note that our recent proteomic analyses of TGFBI-linked corneal dystrophy patients did not identify type XII collagen as a major component in association with granular or amyloid TGFBIp deposits in the corneas of TGFBI-linked corneal dystrophy patients(42). This suggests that it is the soluble fraction of TGFBIp that aggregates and cause disease. The current results support the idea that the functional role of TGFBIp is to mediate the association of cells to the insoluble collagenous matrix of connective tissues.
ACKNOWLEDGEMENTS
We would like to thank Dr. Henrik Vorum for providing tissue for control samples.
Abbreviations
- TGFBIp
transforming growth factor beta induced protein
- ECM
extracellular matrix
- FACIT
Fibril-Associated Collagen with Interrupted Triple helices.
Footnotes
This work was supported by the National Eye Institute (R01EY012712) and the Danish National Research Foundation.
REFERENCES
- 1.Kitahama S, Gibson MA, Hatzinikolas G, Hay S, Kuliwaba JL, Evdokiou A, Atkins GJ, Findlay DM. Expression of fibrillins and other microfibril-associated proteins in human bone and osteoblast-like cells. Bone. 2000;27:61–67. doi: 10.1016/s8756-3282(00)00292-1. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson JW, Mikesh MF, Wheeler EF, LeBaron RG. Developmental expression patterns of Beta-ig (βIG-H3) and its function as a cell adhesion protein. Mechanisms of development. 2003;120:851–864. doi: 10.1016/s0925-4773(03)00165-5. [DOI] [PubMed] [Google Scholar]
- 3.Ohno S, Doi T, Tsutsumi S, Okada Y, Yoneno K, Kato Y, Tanne K. RGD-CAP (βig-h3) is expressed in precartilage condensation and in prehypertrophic chondrocytes during cartilage development. Biochimica et biophysica acta. 2002;1572:114–122. doi: 10.1016/s0304-4165(02)00286-6. [DOI] [PubMed] [Google Scholar]
- 4.Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Molecular human reproduction. 2002;8:871–879. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- 5.Sciandra F, Morlacchi S, Allamand V, De Benedetti G, Macchia G, Petrucci TC, Bozzi M, Brancaccio A. First molecular characterization and immunolocalization of keratoepithelin in adult human skeletal muscle. Matrix biology : journal of the International Society for Matrix Biology. 2008;27:360–370. doi: 10.1016/j.matbio.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Norris RA, Kern CB, Wessels A, Wirrig EE, Markwald RR, Mjaatvedt CH. Detection of βig-H3, a TGFβ induced gene, during cardiac development and its complementary pattern with periostin. Anatomy and embryology. 2005;210:13–23. doi: 10.1007/s00429-005-0010-z. [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Bae JS, Park SH, Lee BH, Park RW, Choi JY, Park JY, Ha SW, Kim YL, Kwon TH, Kim IS. Expression of TGF-β-induced matrix protein βig-h3 is up-regulated in the diabetic rat kidney and human proximal tubular epithelial cells treated with high glucose. Kidney international. 2003;64:1012–1021. doi: 10.1046/j.1523-1755.2003.00158.x. [DOI] [PubMed] [Google Scholar]
- 8.Dyrlund TF, Poulsen ET, Scavenius C, Nikolajsen CL, Thogersen IB, Vorum H, Enghild JJ. Human cornea proteome: identification and quantitation of the proteins of the three main layers including epithelium, stroma, and endothelium. Journal of proteome research. 2012;11:4231–4239. doi: 10.1021/pr300358k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto K, Noshiro M, Ohno S, Kawamoto T, Satakeda H, Akagawa Y, Nakashima K, Okimura A, Ishida H, Okamoto T, Pan H, Shen M, Yan W, Kato Y. Characterization of a cartilage-derived 66-kDa protein (RGD-CAP/β ig-h3) that binds to collagen. Biochimica et biophysica acta. 1997;1355:303–314. doi: 10.1016/s0167-4889(96)00147-4. [DOI] [PubMed] [Google Scholar]
- 10.Billings PC, Whitbeck JC, Adams CS, Abrams WR, Cohen AJ, Engelsberg BN, Howard PS, Rosenbloom J. The transforming growth factor-β-inducible matrix protein (beta)ig-h3 interacts with fibronectin. The Journal of biological chemistry. 2002;277:28003–28009. doi: 10.1074/jbc.M106837200. [DOI] [PubMed] [Google Scholar]
- 11.Kim JE, Park RW, Choi JY, Bae YC, Kim KS, Joo CK, Kim IS. Molecular properties of wild-type and mutant βIG-H3 proteins. Investigative ophthalmology & visual science. 2002;43:656–661. [PubMed] [Google Scholar]
- 12.Reinboth B, Thomas J, Hanssen E, Gibson MA. βig-h3 interacts directly with biglycan and decorin, promotes collagen VI aggregation, and participates in ternary complexing with these macromolecules. The Journal of biological chemistry. 2006;281:7816–7824. doi: 10.1074/jbc.M511316200. [DOI] [PubMed] [Google Scholar]
- 13.Hanssen E, Reinboth B, Gibson MA. Covalent and non-covalent interactions of βig-h3 with collagen VI. βig-h3 is covalently attached to the amino-terminal region of collagen VI in tissue microfibrils. The Journal of biological chemistry. 2003;278:24334–24341. doi: 10.1074/jbc.M303455200. [DOI] [PubMed] [Google Scholar]
- 14.Ohno S, Noshiro M, Makihira S, Kawamoto T, Shen M, Yan W, Kawashima-Ohya Y, Fujimoto K, Tanne K, Kato Y. RGD-CAP (βig-h3) enhances the spreading of chondrocytes and fibroblasts via integrin α(1)β(1) Biochimica et biophysica acta. 1999;1451:196–205. doi: 10.1016/s0167-4889(99)00093-2. [DOI] [PubMed] [Google Scholar]
- 15.Bae JS, Lee SH, Kim JE, Choi JY, Park RW, Yong Park J, Park HS, Sohn YS, Lee DS, Bae Lee E, Kim IS. βig-h3 supports keratinocyte adhesion, migration, and proliferation through α3beta1 integrin. Biochemical and biophysical research communications. 2002;294:940–948. doi: 10.1016/S0006-291X(02)00576-4. [DOI] [PubMed] [Google Scholar]
- 16.Kim JE, Kim SJ, Lee BH, Park RW, Kim KS, Kim IS. Identification of motifs for cell adhesion within the repeated domains of transforming growth factor-beta-induced gene, βig-h3. The Journal of biological chemistry. 2000;275:30907–30915. doi: 10.1074/jbc.M002752200. [DOI] [PubMed] [Google Scholar]
- 17.Nam JO, Kim JE, Jeong HW, Lee SJ, Lee BH, Choi JY, Park RW, Park JY, Kim IS. Identification of the αvβ3 integrin-interacting motif of βig-h3 and its anti-angiogenic effect. The Journal of biological chemistry. 2003;278:25902–25909. doi: 10.1074/jbc.M300358200. [DOI] [PubMed] [Google Scholar]
- 18.Kim JE, Jeong HW, Nam JO, Lee BH, Choi JY, Park RW, Park JY, Kim IS. Identification of motifs in the fasciclin domains of the transforming growth factor-beta-induced matrix protein βig-h3 that interact with the αvβ5 integrin. The Journal of biological chemistry. 2002;277:46159–46165. doi: 10.1074/jbc.M207055200. [DOI] [PubMed] [Google Scholar]
- 19.Kim MO, Yun SJ, Kim IS, Sohn S, Lee EH. Transforming growth factor-β-inducible gene-h3 (β(ig)-h3) promotes cell adhesion of human astrocytoma cells in vitro: implication of α6β4 integrin. Neuroscience letters. 2003;336:93–96. doi: 10.1016/s0304-3940(02)01260-0. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Kim IS. Transforming growth factor-beta-induced gene product, as a novel ligand of integrin αMβ2, promotes monocytes adhesion, migration and chemotaxis. The international journal of biochemistry & cell biology. 2008;40:991–1004. doi: 10.1016/j.biocel.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Zhao YL, Piao CQ, Hei TK. Downregulation of βig-h3 gene is causally linked to tumorigenic phenotype in asbestos treated immortalized human bronchial epithelial cells. Oncogene. 2002;21:7471–7477. doi: 10.1038/sj.onc.1205891. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, El-Gabry M, Hei TK. Loss of βig-h3 protein is frequent in primary lung carcinoma and related to tumorigenic phenotype in lung cancer cells. Molecular carcinogenesis. 2006;45:84–92. doi: 10.1002/mc.20167. [DOI] [PubMed] [Google Scholar]
- 23.Muller LJ, Pels L, Vrensen GF. Novel aspects of the ultrastructural organization of human corneal keratocytes. Investigative ophthalmology & visual science. 1995;36:2557–2567. [PubMed] [Google Scholar]
- 24.Maurice DM. Clinical physiology of the cornea. International ophthalmology clinics. 1962;2:561–572. doi: 10.1097/00004397-196210000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Ameen DB, Bishop MF, McMullen T. A lattice model for computing the transmissivity of the cornea and sclera. Biophysical journal. 1998;75:2520–2531. doi: 10.1016/S0006-3495(98)77697-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller LJ, Pels E, Schurmans LR, Vrensen GF. A new three-dimensional model of the organization of proteoglycans and collagen fibrils in the human corneal stroma. Experimental eye research. 2004;78:493–501. doi: 10.1016/s0014-4835(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 27.Andersen RB, Karring H, Moller-Pedersen T, Valnickova Z, Thogersen IB, Hedegaard CJ, Kristensen T, Klintworth GK, Enghild JJ. Purification and structural characterization of transforming growth factor beta induced protein (TGFBIp) from porcine and human corneas. Biochemistry. 2004;43:16374–16384. doi: 10.1021/bi048589s. [DOI] [PubMed] [Google Scholar]
- 28.Runager K, Garcia-Castellanos R, Valnickova Z, Kristensen T, Nielsen NC, Klintworth GK, Gomis-Ruth FX, Enghild JJ. Purification, crystallization and preliminary X-ray diffraction of wild-type and mutant recombinant human transforming growth factor β-induced protein (TGFBIp) Acta crystallographica. Section F, Structural biology and crystallization communications. 2009;65:299–303. doi: 10.1107/S1744309109005016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bury A. Analysis of protein and peptide mixtures : Evaluation of three sodium dodecyl sulphate-polyacrylamide gel electrophoresis buffer systems. Journal of Chromatography A. 1981;213:491–500. [Google Scholar]
- 30.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. The Journal of biological chemistry. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 31.Hunkapiller MW, Lujan E, Ostrander F, Hood LE. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods in enzymology. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- 32.Karring H, Runager K, Valnickova Z, Thogersen IB, Moller-Pedersen T, Klintworth GK, Enghild JJ. Differential expression and processing of transforming growth factor beta induced protein (TGFBIp) in the normal human cornea during postnatal development and aging. Experimental eye research. 2010;90:57–62. doi: 10.1016/j.exer.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klintworth GK, Valnickova Z, Enghild JJ. Accumulation of βig-h3 gene product in corneas with granular dystrophy. The American journal of pathology. 1998;152:743–748. [PMC free article] [PubMed] [Google Scholar]
- 34.Munier FL, Korvatska E, Djemai A, Le Paslier D, Zografos L, Pescia G, Schorderet DF. Kerato-epithelin mutations in four 5q31-linked corneal dystrophies. Nature genetics. 1997;15:247–251. doi: 10.1038/ng0397-247. [DOI] [PubMed] [Google Scholar]
- 35.Veit G, Hansen U, Keene DR, Bruckner P, Chiquet-Ehrismann R, Chiquet M, Koch M. Collagen XII interacts with avian tenascin-X through its NC3 domain. The Journal of biological chemistry. 2006;281:27461–27470. doi: 10.1074/jbc.M603147200. [DOI] [PubMed] [Google Scholar]
- 36.Marchant JK, Zhang G, Birk DE. Association of type XII collagen with regions of increased stability and keratocyte density in the cornea. Experimental eye research. 2002;75:683–694. doi: 10.1006/exer.2002.2058. [DOI] [PubMed] [Google Scholar]
- 37.Young BB, Zhang G, Koch M, Birk DE. The roles of types XII and XIV collagen in fibrillogenesis and matrix assembly in the developing cornea. Journal of cellular biochemistry. 2002;87:208–220. doi: 10.1002/jcb.10290. [DOI] [PubMed] [Google Scholar]
- 38.Massoudi D, Malecaze F, Soler V, Butterworth J, Erraud A, Fournie P, Koch M, Galiacy SD. NC1 long and NC3 short splice variants of type XII collagen are overexpressed during corneal scarring. Investigative ophthalmology & visual science. 2012;53:7246–7256. doi: 10.1167/iovs.11-8592. [DOI] [PubMed] [Google Scholar]
- 39.Leung EW, Rife L, Smith RE, Kay EP. Extracellular matrix components in retrocorneal fibrous membrane in comparison to corneal endothelium and Descemet's membrane. Molecular vision. 2000;6:15–23. [PubMed] [Google Scholar]
- 40.Lunstrum GP, Morris NP, McDonough AM, Keene DR, Burgeson RE. Identification and partial characterization of two type XII-like collagen molecules. The Journal of cell biology. 1991;113:963–969. doi: 10.1083/jcb.113.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Callebaut I, Mignotte V, Souchet M, Mornon JP. EMI domains are widespread and reveal the probable orthologs of the Caenorhabditis elegans CED-1 protein. Biochemical and biophysical research communications. 2003;300:619–623. doi: 10.1016/s0006-291x(02)02904-2. [DOI] [PubMed] [Google Scholar]
- 42.Karring H, Runager K, Thogersen IB, Klintworth GK, Hojrup P, Enghild JJ. Composition and proteolytic processing of corneal deposits associated with mutations in the TGFBI gene. Experimental eye research. 2012;96:163–170. doi: 10.1016/j.exer.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]