Abstract
Background:
Metallo-beta-lactamase (MBL) producing Pseudomonas aeruginosa in the burn patients is a leading cause of morbidity and mortality and remains a serious health concern among the clinicians.
Objectives:
The aim of this study was to detect MBL-producing P. aeruginosa in burn patients and determine multidrug-resistant (MDR) strains, and respective resistance patterns.
Patients and Methods:
In this cross-sectional study, 270 strains of P. aeruginosa were isolated from the burn patients referred to Ghotbeddin Burn Hospital, Shiraz, Iran. Among them, 55 MBL-producing P. aeruginosa strains were isolated from 55 patients hospitalized in burn unit. Minimum inhibitory concentrations (MICs) and MBLs were determined by the E-test method.
Results:
Of the 55 burn cases, 29 (53%) were females and 26 (47%) males. Injured burn patients’ ages ranged from 16 to 87 years, with maximum number of cases in the age group of 16 to 36 years (n, 40; 72.7%). Overall, 32 cases were accidental (60%), and 22 were suicidal burns (40%). Of the 55 burn patients, 17 cases were expired (30%). All deaths were due to chemical exposures. In antibiotic susceptibility testing by E-test method, ceftazidime was the most effective one and 35 isolates (63.5%) were resistant to all the 11 tested antibiotics.
Conclusions:
Routine microbiological surveillance and careful in vitro testing of antibiotics prior to prescription and strict adherence to hospital antibiotic policy may help to prevent, treat, and control MDR and pandrug-resistant (PDR) P. aeruginosa strains in burn units.
Keywords: Burn patients, Pseudomonas aeruginosa, Metallo-Beta-Lactamase, Drug Resistance
1. Background
Pseudomonas aeruginosa is an obligate aerobic, motile, rod-shaped, Gram-negative bacterium, which is able to grow and survive in almost any environment and is resistant to temperature extremes (1). It is an opportunistic pathogen causing severe acute and chronic nosocomial infections, especially in immunocompromised hosts and patients with serious underlying medical conditions (2, 3). Pseudomonas aeruginosa generally exhibits intrinsic resistance against many antibiotics and is associated with a high mortality rate (4). Almost all the clinical cases of P. aeruginosa infections are associated with the compromised host defense as seen in burn patients (5). Bacterial infections following severe thermal injuries can be most simplistically attributed to the extensive breaches in the skin barrier (6). P. aeruginosa is a ubiquitous bacterium; hence, the risk of encounter this microorganism before the burns can heal is extremely high in sever burn patients (5, 7). Presently, more patients with burns die of pneumonia than of burn wound infection; however, burn wound sepsis remains an important infectious complication in such patients (8, 9). Various beta-lactam antibiotics, aminoglycosides, fluoroquinolones, and polymyxins have been used to treat burn patients infected with P. aeruginosa (10, 11). All the strains are prone to become resistant by mutations. Burn hospitals often harbor multidrug resistant P. aeruginosa (MDRPA) isolates that can serve as the source of infection for other patients hospitalized in burn ward (12).
Although systemic or topical antibiotic therapy has considerably improved the management of infectious diseases in the burn patients, many infections are not fully treated or eradicated by the application of antipseudomonal drugs and can, thus, become chronic infections (13, 14). For instance, burn patients can become colonized with antibiotic-resistant, nosocomial P. aeruginosa strains that are not easily eliminated by antibiotic therapy (15). Selection of an efficient antibiotic therapy regimen should be based on the ability of drug to inhibit the bacteria isolated from burn wound, periodic bacterial cultures, and monitoring the nosocomial infections in the burn wards (16). Outbreaks of cross-colonization and infection are a major challenge to the patients hospitalized in burn units (17). In almost all cases, the colonized patient is considered as a major reservoir for the epidemic strains (5, 17). It has been estimated that at least 50% of all deaths caused by burns are the result of infection, and untreatable infections have become a tragically frequent morbidity in patients infected with P. aeruginosa (18). Eradication of MDRPA from hospital burn wards is a demanding task; therefore, the detection of metallo-beta-lactamase (MBL) producing P. aeruginosa is necessary for controlling the spread of resistant strains as well as developing new therapeutic guidelines and prophylactic strategies to control the bacterial infection in patients with burn wounds.
2. Objectives
Given the existing limited data on MBL-producing P. aeruginosa strains in Shiraz, this study aimed to detect MBL-producing P. aeruginosa in burn patients, and determine the MDRPA strains in such isolates.
3. Patients and Methods
In this cross-sectional study, 270 strains of P. aeruginosa were isolated from the burn patients referred to Ghotbeddin Burn Hospital, Shiraz, Iran, during 2009-2010. Data concerning gender, age, duration of hospitalization, cause, site, degree, and types of burns (accidental or suicidal) were collected from the patients infected with MBL-producing P. aeruginosa through questionnaires filled by skilled nurses. This study was performed in accordance with the ethical standards laid down in the 1964 Helsinki declaration. All the patients were assigned code numbers. The design and protocol of the study were approved by the Ethics Committee of Professor Alborzi Clinical Microbiology Research Center (PACMRC), Shiraz, Iran.
3.1. Isolation and Identification of Metallo-Beta-Lactamase-Producing Pseudomonas aeruginosa
During a one-year period, 55 MBL-producing P. aeruginosa strains were isolated from 55 burn patients hospitalized in the burn unit. The isolation and identification of P. aeruginosa from wound specimens were performed by the following conventional procedures. Specimens were collected by sterile swabs after the removal of dressing and cleansing the wound surface by 70% alcohol. The burn samples were cultured on nutrient agar (NA; Oxoid Ltd, London, UK) and incubated at 35℃ to 37℃ overnight. Then any suspicious colony was subcultured and purified. The isolates were identified as P. aeruginosa, based on Gram staining, catalase test, oxidase test, triple sugar iron (TSI) fermentation, motility, color, pyocyanin pigment production, and odor. For final confirmation, biochemical tests embedded in the API-20E biochemical kit system (Bio-Merieux, France) and manual biochemical tests were used in accordance with the manufacturer’s instructions. Strains were preserved at -20℃ on tryptic soy broth (TSB; Oxoid Ltd, London, UK) containing 20% (v/v) glycerol. MBL E-test strips (AB BIODISK, Solna, Sweden) were used to screen class B beta-lactamase. Tests were performed and interpreted according to the manufacturer's instructions.
3.2. Antibiotic Susceptibility and Resistance Patterns
Minimum inhibitory concentrations (MICs) of the 11 antimicrobial agents routinely prescribed in burn centers against the 55 isolates of MBL-producing P. aeruginosa were determined by the E-test method (AB BIODISK, Solna, Sweden), as recommended by the National Committee For Clinical Laboratory Standard Institute (CLSI) (19). A bacterial suspension from growth in a tryptic soy agar (TSA) plate was prepared in 2 mL of Mueller-Hinton broth (MHB), and the turbidity was adjusted so that it was equivalent to that of a 0.5 McFarland standard. The bacterial suspension was streaked onto a 150-mm-diameter plate containing Mueller-Hinton agar (MHA); the plate was later incubated at 35 to 37℃ in ambient air for 16 to 18 hours. The MIC was read on the basis of the interception of the elliptical zone of growth inhibition with the graded E-test strip according to the manufacturer’s instructions. The antibiotics (Mast Co., UK) consisted of imipenem (10 μg), meropenem (10 μg), cefepime (30 μg), ceftazidime (30 μg), piperacillin/tazobactam (110 μg), ciprofloxacin (5 μg), tobramycin (10 μg), amikacin (30 μg), gentamicin (10 μg), ampicillin (10 μg), and aztreonam (30 μg). American typing collection (ATCC 27853) of P. aeruginosa was used as a control strain to determine antibacterial susceptibility.
4. Results
Among 270 strains, 55 MBL-producing P. aeruginosa strains were isolated from patients hospitalized in burn unit. Of the 55 burn cases, 29 (53%) were females and 26 (47%) males. the age of the burn patients ranged from 16 to 87 years, with maximum number of cases in the age group of 16 to 36 years (n = 40; 72.7%) (Table 1). Fourteen burn-injured patients (25.5%) were from urban areas and 41 (74.5%) were from rural. Thirty-three cases (60%) were accidental, and 22 (40%) were suicidal. Overall, 51 patients (93%) had chemical injuries, and 4 (7%) had electrical injuries. In contrast to males, burns due to chemical exposures were more frequent in females (52.7%). Of the 55 burn patients, 17 patients (30%) died. Females showed a high incidence of mortality (females, 70.5%; males, 29.5%; and female to male ratio, 2.4). All deaths were due to chemical exposures (Table 2).
Table 1. Demographic Features of the Burn Patients a.
| Category | Results |
|---|---|
| Age, y | 35 ± 17 |
| 16-36 | 40 (72.7) |
| 37-56 | 9 (16.3) |
| > 56 | 6 (11) |
| Residence | |
| Rural | 41 (74.5) |
| Urban | 14 (25.5) |
| Hospitalization, d | 25 ± 12 |
| 1-30 | 34 (61.8) |
| 31-60 | 15 (27.2) |
| 61-90 | 5 (9) |
| 91-120 | 1 (1) |
a Data are presented as Mean ± SD or No. (%).
Table 2. Distribution of the Burn Patients in Terms of the Gender, Burn Cause, and Exposure Types.
| Results | |
|---|---|
| Gender | |
| Male (n = 26) | 8 urban |
| 18 rural | |
| Female (n = 29) | 6 urban |
| 23 rural | |
| Burn cause | |
| Accidental (n = 33) | 23 males |
| 10 females | |
| Suicidal (n = 22) | 3 males |
| 19 females | |
| Exposure | |
| Electricity (n = 4) | 0 male |
| 4 females | |
| Chemical (n = 51) | 22 males |
| 29 females | |
| Death (n = 17) | whole body except head (n = 7) |
| whole body (n = 9) | |
| Legs (n = 1) |
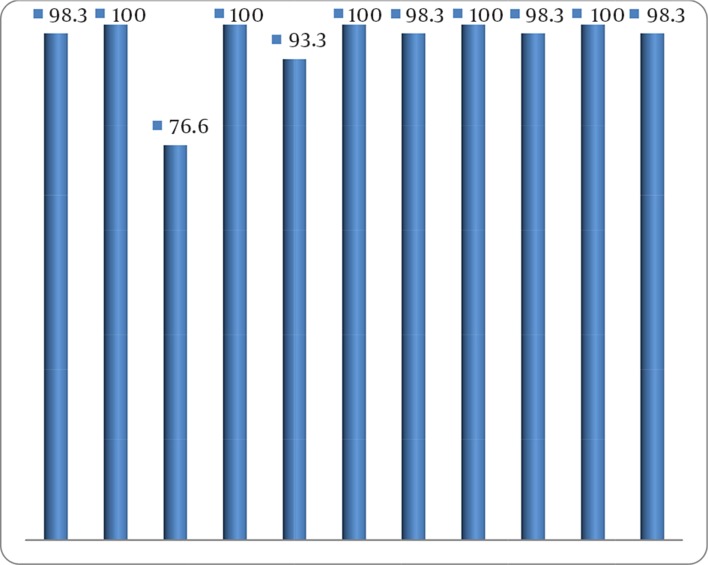
According to the in vitro antibiotic susceptibility testing by E-test method, ceftazidime was the most effective antibiotic (Figure 1). Thirty-five isolates (63.5%) were resistant to all the 11 tested antibiotics (Table 3).
Figure 1. Antibiotic Resistance Profile in Metallo-Beta-Lactamase-Producing Pseudomonas aeruginosa Isolates (n = 55).
Table 3. Antibiotic Resistance Patterns of Metallo-Beta-Lactamase-Producing Pseudomonas aeruginosa Strains Isolated From Burn Patients a, b.
| Antibiotic Resistance Patterns | Frequency (n = 55) |
|---|---|
| IMI, MEM, CAZ, ATM, TN, GM, PTZ, CPM, AK, CIP, AP | 35 (63.6) |
| IMI, MEM, ATM, TN, GM, PTZ, CPM, AK, CIP, AP | 8 (14.5) |
| IMI, MEM, ATM, TN, GM, PTZ, CPM, AK, AP | 5 (9.1) |
| IMI, MEM, CAZ, ATM, TN, PTZ, CPM, AK, CIP, AP | 1 (1.8) |
| MEM, CAZ, ATM, TN, GM, PTZ, CPM, AK, CIP | 1 (1.8) |
| IMI, MEM, CAZ, ATM, PTZ, CPM, AP | 1 (1.8) |
| IMI, MEM, CAZ, ATM, PTZ, CPM, AK, AP | 1 (1.8) |
| IMI, CAZ, ATM, TN, GM, CPM, AK, AP | 1 (1.8) |
| IMI, MEM, AK, AP | 1 (1.8) |
| IMI, AK, CIP, AP | 1 (1.8) |
aAbbreviations: AK, amikacin; AP, ampicillin; ATM, aztreonam; CAZ, ceftazidime; CIP, ciprofloxacin; CPM, cefepime; GM, gentamicin; IMI, imipenem; MEM, meropenem; PTZ, piperacillin/tazobactam; TN, tobramycin.
bData are presented as No. (%).
5. Discussion
P. aeruginosa is an opportunistic pathogen, which is commonly associated with nosocomial infections (20). Antimicrobial resistance during therapy occurs frequently among the initially susceptible P. aeruginosa isolates, resulting in the emergence of resistance to multiple antibiotics (21). MBL-producing P. aeruginosa strains have been identified from clinical isolates worldwide with increasing frequency over the past several years, and these isolates are responsible for protracted nosocomial infections in different countries (22). The rising frequency of MDRPA strains is a matter of concern as effective antimicrobial options are significantly limited (23). In this study, females were affected more than males (53% vs. 47%). This may be due to more frequent involvement of females in household chores, which demand more exposure to fire (e.g. in cooking and heating). The results indicated that the mortality in our burn cases was 30%, which is lower than the rates in some other reports (24) but higher than the rates in most of other countries (25). The total body surface area burned (whole body in 50% and whole body except head in 41% of cases) was the most frequent factor related to mortality. The increasing severity of injuries caused by more skin loss, exposes the largest burns to more complications, thus, rising mortality rate. Another prominent finding in the present study was the high level of antibacterial resistance profile detected in the isolates of P. aeruginosa. Resistance to aztreonam, piperacillin/tazobactam, meropenem, imipenem, and ampicillin was seen in all the isolates. Therefore, when administering empirical treatment in burn patients with nosocomial infections with P. aeruginosa, MBL should be considered in case the patients are not responsive to carbapenem therapy. The level of MBL production among MDRPA strains seems to be greater than that estimated in Iran. Reports on MBL-producing P. aeruginosa isolates are increasing globally due to the increased beta-lactam usage and emergence of resistant bacteria under antibiotic pressure. Currently, CLSI document has no guidelines for detecting MBLs in P. aeruginosa. This alarming antibiotic resistance trend was seen for P. aeruginosa strains, as seen in previous studies (26). A similar report of MDRPA was also reported by other researchers. Although ceftazidime was found to be the most effective drug in the present study, resistance to this antibiotic was high (76.6%). In one study from Iran, higher resistance to ceftazidime was reported (27); this could be due to the reason that these are reserve drugs and are used as the last resort for MDRPA in hospital burn settings in Shiraz. In the current study, the bacteria isolated from only nine patients receiving empirical antibiotic therapy were sensitive to the prescribed antibiotics according to MIC method. The changes in the bacterial resistance patterns, as observed in the burn wards, could have important implications for both clinical settings and epidemiological purposes. Such a high antibiotic resistance in P. aeruginosa isolates is probably due to the selective pressure exerted on the bacteria because of the factors such as poor adherence to hospital antibiotic policy and excessive as well as indiscriminate use of broad-spectrum antimicrobial agents including beta-lactams, carbapenems, aminoglycosides, and quinolones. These MDRPA strains establish themselves in the hospital environment and, thereby, spread from one patient to another, from medical personnel to the patients, or among different units in the hospital. More recently, MDRPA and pandrug-resistant P. aeruginosa isolates have emerged in hospital burn units. Hence, there is hardly any effective antibiotic against pandrug-resistant strains, in which an outer membrane barrier of low permeability and an array of efficient multidrug efflux pumps are combined with multitudes of specific antibiotic resistance mechanisms. Treatment of the infections caused by these so-called “superbugs” remains challenging because the pool of effective antibiotics is shrinking and few new antipseudomonal antibiotics are in development. In burn patients, an effective and continuous surveillance for infection control and accordingly, regular tissue culturing for control purposes at least twice a week are recommended. Routine microbiological surveillance and careful in vitro testing of antibiotics prior to prescription and strict adherence to the hospital antibiotic policy may be helpful in the prevention, treatment, and control of MDRPA in the patients hospitalized in burn units. Further investigations should be done to determine the changing sensitivity profiles of the different gram-positive and Gram-negative bacteria and respective antimicrobial susceptibility patterns (28-30). In this regard, the hospitals should formulate an effective antibiotic policy. When the wound bacterial counts are more than 105 microorganisms per gram of tissue, the risk of wound infection is great, skin graft survival is poor, and wound healing is delayed (31). Bacterial counts of less than 103 organisms per gram, are not usually invasive and allow skin graft survival rates of more than 90%. The goals of the local wound management should be the prevention of viable tissues desiccation and the control of bacteria.
Acknowledgments
Our thanks go to Hassan Khajehei for copy editing of the manuscript.
Footnotes
Implication for health policy/practice/research/medical education:Routine microbiological surveillance and careful in vitro testing of sensitivity to antibiotics prior to prescription and strict adherence to hospital antibiotic policy may help to prevent, treat, and control the multidrug-resistant (MDR) and pandrug-resistant (PDR) Pseudomonas aeruginosa in patients hospitalized in burn units.
Authors' Contributions:All authors listed have contributed sufficiently to the project to be included as authors, and all those who are qualified to be authors are listed in the author byline. To the best of our knowledge, no conflict of interest, financial or other, exists.
Funding/Support:This work was supported by research grant from the Professor Alborzi Clinical Microbiology Research Center (PACMRC).
References
- 1.Hamud S, Abida A. Pseudomonas aeruginosa resistance to tetracycline and triclosan. Growth. 2004;50:0.256. [Google Scholar]
- 2.Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67(3):159–73. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 3.Markou P, Apidianakis Y. Pathogenesis of intestinal Pseudomonas aeruginosa infection in patients with cancer. Front Cell Infect Microbiol. 2014;3:115. doi: 10.3389/fcimb.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowers DR, Liew YX, Lye DC, Kwa AL, Hsu LY, Tam VH. Outcomes of appropriate empiric combination versus monotherapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2013;57(3):1270–4. doi: 10.1128/AAC.02235-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19(2):403–34. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita Y, Tomida J, Kawamura Y. Responses of to antimicrobials. Front Microbiol. 2014;4:422. doi: 10.3389/fmicb.2013.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budzik JM, Rosche WA, Rietsch A, O'Toole GA. Isolation and characterization of a generalized transducing phage for Pseudomonas aeruginosa strains PAO1 and PA14. J Bacteriol. 2004;186(10):3270–3. doi: 10.1128/JB.186.10.3270-3273.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heggers JP, Haydon S, Ko F, Hayward PG, Carp S, Robson MC. Pseudomonas aeruginosa exotoxin A: its role in retardation of wound healing: the 1992 Lindberg Award. J Burn Care Rehabil. 1992;13(5):512–8. [PubMed] [Google Scholar]
- 9.Mozingo DW, Pruitt Jr BA. Infectious complications after burn injury. DTIC Document; 1994. [Google Scholar]
- 10.Abbas Poor SH, Mardaneh J, Dehbashi S, Jasemi SS. Profile of antimicrobial susceptibility isolated microorganisms from hospitalized patients in PICU ward and detection of Methicillin-resistant Staphylococcus aureus and ESBLproducing bacteria by phenotypic methods. ISMJ . 2013 [Google Scholar]
- 11.Coetzee E, Rode H, Kahn D. Pseudomonas aeruginosa burn wound infection in a dedicated paediatric burns unit. S Afr J Surg. 2013;51(2):50–3. doi: 10.7196/sajs.1134. [DOI] [PubMed] [Google Scholar]
- 12.Branski LK, Al-Mousawi A, Rivero H, Jeschke MG, Sanford AP, Herndon DN. Emerging infections in burns. Surg Infect (Larchmt). 2009;10(5):389–97. doi: 10.1089/sur.2009.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma BR, Singh VP, Bangar S, Gupta N. Septicemia: The Principal Killer of Burns Patients. American Journal of Infectious Diseases. 2005;1(3):132–38. [Google Scholar]
- 14.Ho SA, Lee TW, Denton M, Conway SP, Brownlee KG. Regimens for eradicating early Pseudomonas aeruginosa infection in children do not promote antibiotic resistance in this organism. J Cyst Fibros. 2009;8(1):43–6. doi: 10.1016/j.jcf.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Dai T, Huang YY, Sharma SK, Hashmi JT, Kurup DB, Hamblin MR. Topical antimicrobials for burn wound infections. Recent Pat Antiinfect Drug Discov. 2010;5(2):124–51. doi: 10.2174/157489110791233522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Japoni A, Farshad S, Alborzi A. Pseudomonas aeruginosa: Burn Infection, Treatment and Antibacterial Resistance. IRCMJ. 2009;11(3) [Google Scholar]
- 17.Mayhall CG. The epidemiology of burn wound infections: then and now. Clin Infect Dis. 2003;37(4):543–50. doi: 10.1086/376993. [DOI] [PubMed] [Google Scholar]
- 18.McVay CS, Velasquez M, Fralick JA. Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob Agents Chemother. 2007;51(6):1934–8. doi: 10.1128/AAC.01028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirs M, Andlovic A, Cerar T, Zohar-Cˇretnik T, Kobola L, Kolman J, et al. A case of OXA-48 carbapenemase-producing Klebsiella pneumoniae in a patient transferred to Slovenia from Libya, November 2011. Euro Surveill. 2011;16:20042. [PubMed] [Google Scholar]
- 20.Cardoso T, Ribeiro O, Aragao IC, Costa-Pereira A, Sarmento AE. Additional risk factors for infection by multidrug-resistant pathogens in healthcare-associated infection: a large cohort study. BMC Infect Dis. 2012;12:375. doi: 10.1186/1471-2334-12-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mardaneh J, Ahmadi K, Jahan Sepas A. Determination antimicrobial resistance profile of Pseudomonas aeruginosa strains isolated from hospitalized patients in Taleghani Hospital (Ahvaz, Iran) from 2011-2012. JFUMS. 2013;3(3):188–93. [Google Scholar]
- 22.Pagani L, Mantengoli E, Migliavacca R, Nucleo E, Pollini S, Spalla M, et al. Multifocal detection of multidrug-resistant Pseudomonas aeruginosa producing the PER-1 extended-spectrum beta-lactamase in Northern Italy. J Clin Microbiol. 2004;42(6):2523–9. doi: 10.1128/JCM.42.6.2523-2529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obritsch MD, Fish DN, MacLaren R, Jung R. Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy. 2005;25(10):1353–64. doi: 10.1592/phco.2005.25.10.1353. [DOI] [PubMed] [Google Scholar]
- 24.Al-Ibran E, Rao MH, Fatima K, Irfan S, Iqbal M, Khan M. Current Bacteriological profile in Fire-burn victims and their associated mortality at the Burns Centre, Karachi-Pakistan. Pakistan Journal of Medical Sciences. 2011;27(4) [Google Scholar]
- 25.Keen EF, 3rd, Robinson BJ, Hospenthal DR, Aldous WK, Wolf SE, Chung KK, et al. Incidence and bacteriology of burn infections at a military burn center. Burns. 2010;36(4):461–8. doi: 10.1016/j.burns.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Hsueh PR, Tseng SP, Teng LJ, Ho SW. Pan-drug-resistant Pseudomonas aeruginosa causing nosocomial infection at a university hospital in Taiwan. Clin Microbiol Infect. 2005;11(8):670–3. doi: 10.1111/j.1469-0691.2005.01196.x. [DOI] [PubMed] [Google Scholar]
- 27.Kalantar E, Torabi V, Salimizand H, Soheili F, Ramezanzadeh R. Incidence and Susceptibility Pattern of Metallo-Beta-Lactamase Producers Among Pseudomonas aeruginosa Isolated From Burn Patients at Kurdistan Province. Jundishapur Journal of Microbiology. 2012;5(3):507–10. doi: 10.5812/jjm.3664. [DOI] [Google Scholar]
- 28.Rosenberger LH, Hranjec T, Politano AD, Swenson BR, Metzger R, Bonatti H, et al. Effective cohorting and "superisolation" in a single intensive care unit in response to an outbreak of diverse multi-drug-resistant organisms. Surg Infect (Larchmt). 2011;12(5):345–50. doi: 10.1089/sur.2010.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahid M, Hussain A, Bridgeman P, Bose D. Clinical outcomes of the Ilizarov method after an infected tibial non union. Arch Trauma Res. 2013;2(2):71–5. doi: 10.5812/atr.11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaghaghian S, Pourabbas B, Alborzi A, Askarian M, Mardaneh J. Vancomycin-Resistant Entrococci colonization in chronic hemodialysis patients and its risk factors in southern Iran (2005-2006). Iran Red Crescent Med J. 2012;14(10):686–91. [PMC free article] [PubMed] [Google Scholar]
- 31.Orban C. Diagnostic criteria for sepsis in burn patients. Chirurgia (Bucur). 2012;107(6):697–700. [PubMed] [Google Scholar]