Abstract
Objective
Our objectives were to describe trajectories of depressive symptoms and pain at hospital discharge and six weeks later and to examine the relationship of persistent depressive symptoms to pain.
Methods
Before and six weeks after hospital discharge, 251 cardiac surgery patients (aged 67.3 ± 9.5 years; 73% male) completed the Beck Depression Inventory (BDI) and the Brief Pain Inventory (BPI). Patients were categorized into two groups based on the presence or absence of persistent depressive symptoms (BDI>10 at both times). Between-group differences in pain interference (BPI-INT) and pain severity (BPI-SEV) were evaluated using repeated measures analysis of variance. Linear regressions were performed to determine if changes in depressive symptoms were related to BPI-INT and BPI-SEV, controlling for demographic and clinical data.
Results
Persistent (16.3%) or worsening depressive symptoms (15.3%) from hospital discharge to six weeks were observed; many experienced at least some persistent pain (BPI-INT - 67.8%; BPI-SEV - 47.8%). From discharge to six weeks, patients with persistent depressive symptoms sustained higher levels of BPI-INT (p<.001) and BPI-SEV (P<.003). In multivariate analysis, only changes in depressive symptoms, not clinical and demographic variables, were related to BPI-INT (p<.001) and BPI-SEV (p=.001).
Conclusion
Persistent depressive symptoms are independently associated with continued pain up to six weeks after hospital discharge. Successful treatment of ongoing pain should include screening for depressive symptoms and initiation of appropriate treatment.
Keywords: cardiac surgery, depressive symptoms, pain
Introduction
Following cardiac surgery, many patients experience depression and its symptoms.(1, 2) Cardiac surgery patients with depressive symptoms experience more readmissions and hospitalizations, lower quality of life, increased mortality, and higher pain scores than those without depressive symptoms.(3-5) Unlike the effects of depressive symptoms on recovery, the effects of pain after cardiac surgery are less well known. Reports of the prevalence of persistent or chronic pain after cardiac surgery vary from 21 to 55%.(6, 7) An estimated 30-50% of coronary artery bypass patients experience persistent postoperative pain.(8-10) Studies of comorbid pain and depressive symptoms in primary care and psychiatric settings report a reciprocal relationship between pain and depressive symptoms, in that prevalence rates of each condition are higher when the other is also present.(11) For example, 65% of cardiac surgery patients with depression experience pain, and depression is present in up to 85% of these patients with pain conditions.(12)
In general surgical populations, postoperative pain has been associated with psychological distress,(7) but this relationship has not been consistent. For example, in spinal surgery patients, a reduction in pain intensity was associated with a reduction in depressive symptoms at 6 months postoperatively, but not at 3 months;(13) after breast cancer treatment, women with chronic pain were not more likely to be depressed than women without pain.(14) In this broader context, few investigators have reported on the phenomenon of comorbid pain and depressive symptoms in patients following cardiac surgery. Despite compelling evidence that both depressive symptoms and pain are both prevalent after cardiac surgery, only one study to date has investigated post-cardiac surgery comorbid pain and depressive symptoms.(15) Investigators found that after hospital discharge, 67% of depressed post-coronary bypass patients also had at least moderate pain. Further, depressed patients with at least moderate pain were less likely to respond to depression treatment.(15) These findings have not been confirmed by repeated study.
The present study examined the prevalence of depressive and pain symptoms in patients undergoing cardiac surgery from hospital discharge (baseline) to 6-week follow-up. We then tested whether changes in depressive symptoms were associated with changes in pain symptoms from baseline to 6 week follow-up.
Methods
Sample and Setting
This is a secondary analysis from a randomized controlled trial of cognitive behavioral therapy for depression in patients following cardiac surgery (coronary artery bypass grafting or valve replacement/repair) from July 2006 through October 2009. Institutional Review Board (IRB) approvals were obtained from five tertiary care centers in the Greater Los Angeles area from which cardiac surgery patients were recruited. Exclusion criteria for the parent study were: age < 30 years, residing outside of the greater Los Angeles area, presence of cognitive impairment (Mini Mental State Exam [MMSE] < 24) or major comorbid psychiatric condition (schizophrenia, bipolar disorder, substance abuse), and autoimmune disorder or malignancy. Of the 490 participants who completed questionnaires at hospital discharge, 251 also completed mailed 6-week follow-up questionnaires and are the subject of this report. There were no differences in age, gender, Charlson comorbidity score, or time from surgery to baseline data collection between those who did and did not complete follow-up questionnaires. Those who were married or partnered tended to be more likely to complete questionnaires at both time points (p = .06).
Procedures
Patients were approached after surgery and before hospital discharge; no preoperative symptom questionnaires were collected. After consent, a brief in-hospital screening interview was conducted to assess cognitive function. Individuals with a score of ≥ 24 on the MMSE(16) completed a brief questionnaire booklet to obtain baseline measures of sociodemographic (marital and work status) and psychobehavioral characteristics (depressive and anxiety symptoms, perceived control, and pain), which was completed prior to hospital discharge. Medical records were reviewed to obtain demographic data and to identify co-morbidities (Charlson Comorbidity Index(17)), pre-existing psychiatric conditions, and current medications. Patients with preoperative depression and those on antidepressants were included in the study. Six weeks after hospital discharge, a second questionnaire booklet was mailed to patients along with a self-addressed stamped envelope. Patients with Beck Depression Inventory scores ≥ 10 at both pre-discharge and post-discharge evaluations were considered to have persistent depressive symptoms. For this report, all data were collected prior to randomization in the parent study.
Instruments
Depressive symptoms were measured with the Beck Depression Inventory (BDI), a 21-item self-report measure used widely in cardiac patients.(18) (19) Applied in over 2,000 empirical studies, the BDI has sound internal consistency (i.e., mean Cronbach's alpha = 0.82) and concurrent validity (i.e. with the Hamilton Rating Scale for Depression (r = .75) for non-psychiatric populations.(20) In the current study, internal consistency of the BDI at baseline yielded an α-coefficient of 0.87. Persistent depressive symptoms was defined as BDI ≥ 10, indicating the presence of more than minimal depressive symptoms.(19, 21)
Pain was measured with the Brief Pain Inventory (BPI) short form, a well-established 9-item questionnaire that assesses a person's current pain state (BPI-SEV) and the degree to which pain interferes in daily living (BPI-INT).(22) The BPI consists of both dichotomous response, open-ended, and Likert scale (0 to 10) questions in which 0 equals “no pain” or “no interference” and 10 equals “pain as bad as you can imagine” and “complete interference”. In cardiac surgery patients, the BPI has demonstrated acceptable internal consistency (Cronbach's α from .84 to .94). (23) In this study, Cronbach's α for the pain interference (BPI-INT) and severity (BPI-SEV) scores was 0.93 and 0.86, respectively.
Anxiety, known to be highly comorbid with depressive symptoms,(24) was also assessed by the Brief Symptom Inventory (BSI) Anxiety Subscale, a 6-item questionnaire that measures psychological state anxiety symptoms. Each item is rated on a 5-point scale, with higher scores indicative of higher anxiety. The BSI has been used in related cardiac populations,(25) and has demonstrated strong internal consistency (Cronbach's α = .87) and criterion validity with the Spielberger Anxiety Index (r = .70).(26) Cronbach's α in this study was 0.87.
Perceived control over one's health was included because it is known to be inversely correlated with negative affective symptoms and because it is an indicator of cardiac patients' ability to engage in monitoring and management of their cardiac health. Perceived control was measured by the Control Attitudes Scale-Revised (CAS-R), an 8-item questionnaire designed to measure a person's attitude regarding control of his/her cardiac condition. Questions are asked using a 5-point Likert scale with higher scores indicating a higher degree of distress.(27) The CAS-R has established internal consistency, with Cronbach's α coeifficients of .72, .76, and .73 for coronary heart disease, acute myocardial infarction, and heart failure patients, respectively. (27) Cronbach's α in this study was 0.73.
Perceived social support was measured because it has been associated with chronic pain in other surgical populations.(28) Perceived social support was assessed using the Multidimensional Scale of Perceived Social Support (MSPSS).(29) The MSPSS assesses perceived social support adequacy from family, friends, or significant others. It consists of 12 items rated on a 7-point Likert scale. Each item is rated from 1 (very strongly disagree) to 7 (very strongly agree). The total score is the sum of the 12 items and ranges from 12 to 84. Higher scores indicate a higher level of perceived social support. Construct validity and reliability have been reported. Cronbach's alpha coefficients previously ranged from .85 to .90.(29) Cronbach's α in this study was .95.
Sleep quality was measured with the Pittsburgh Sleep Quality Index (PSQI). It is a 19-item self-rated questionnaire in which individuals are asked to consider seven subcomponents of their sleep over the last month (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction). Scores are derived by summing the subcomponents and range from 0-21, with higher scores indicative of poorer sleep quality.(30) The total summary score was used to measure overall sleep quality. Cronbach's α in this study was .85.
Analysis
All data analyses were conducted with IBM SPSS 21 (IBM, Somers, NY 2010). Baseline characteristics between patients with and without persistent depressive symptoms were compared using X2 analyses and Student's T-test. The trajectory of depressive symptoms was evaluated from two perspectives. First, changes in depressive symptom scores were described in four groups (low (BDI < 10 at both baseline and 6-week follow-up), worsening (BDI < 10 baseline, but BDI ≥ 10 at 6-week follow-up), improving (BDI ≥ 10 at baseline, but BDI < 10 at six weeks), and high (BDI ≥ 10 at both baseline and six weeks). Then, frequencies for persistent depressive symptoms (defined as BDI scores ≥ 10 at both baseline and 6-week follow-up) and persistent pain (defined as BPI-INT or BPI-SEV scores ≥ 1 at both time points) were calculated. For persistent depressive symptoms, the cut-point of BDI ≥ 10 was based on the recommendation for presence of greater than minimal symptoms.(19) For persistent pain, the cut-point of BPI-INT and BPI-SEV ≥ 1 was selected because it represented the presence of any pain and had been used in previous studies.(7)
The contribution of persistent depressive symptoms on 6-week measures of BPI-INT and BPI-SEV was evaluated using RM-ANOVA with stratification of the sample by persistent depressive symptoms or not, controlling for age and baseline pain symptoms. Two linear regressions were performed to quantify the relation between BPI-INT and BPI-SEV scores and changes in depressive symptom scores from baseline to 6-week follow-up. Simple forced entry was used, with variables entered in two blocks. Clinical variables, baseline BPI-INT or BPI-SEV scores and baseline variables of related psychobehavioral variables (anxiety, sleep quality, perceived control, and perceived social support) were entered into the first block, with depressive symptom change scores (6-week follow-up minus baseline) entered into the second block. Given the possibility of shared variance related to insomnia in the BDI and PSQI, we entered the PSQI in the first block to remove the influence of any overlapping variance. As a further check, we ran the regressions with and without the PSQI. Since results were similar, we present the analyses with the PSQI in the models. Significance was set at p < .05 for all analyses.
Results
A total of 251 cardiac surgery patients completed questionnaires at discharge and six weeks later (Table 1). The sample was predominately white (72%), male (73%), older (mean age = 67.3 ± 9.5 years), married (71%), and did not have a history of depression (90%).
Table 1. Characteristics of Patients with and without Persistent Depressive Symptoms.
| Characteristics | Persistent Depressive Symptoms (BDI ≥ 10) n=41 | No Persistent Depressive Symptoms (BDI < 10) n=210 | P valuea |
|---|---|---|---|
| N (%) or Mean (SD) | N (%) or Mean (SD) | ||
| Demographics | |||
| Age (years) | 63.5 (7.9) | 68.1 (9.7) | .005 |
| Female | 11 (26.8) | 57 (27.1) | .97 |
| Non-Hispanic White | 27 (65.9) | 153 (72.9) | .36 |
| > high school education | 33 (80.5) | 158 (76.3) | .56 |
| Married or cohabitating | 28 (68.3) | 150 (71.4) | .69 |
| Employed | 15 (38.5) | 84 (40.0) | .86 |
| Biomedical measures | |||
| Body Mass Index (kg/m2) | 28.9 (7.3) | 28.8 (5.6) | .88 |
| Charlson Comorbidity Index Total Score | 3.3 (1.6) | 3.7 (1.8) | .28 |
| Non-Elective Surgery | 14 (36.8) | 76 (38.2) | .88 |
| Type of Surgery – CABG | 25 (71.4) | 85 (54.5) | .14 |
| Post-op Complications (yes) | 14 (40.0) | 76 (45.8) | .53 |
| Number of grafts | 2.6 (1.2) | 2.2 (1.2) | .11 |
| Pump time (min) | 80.8 (33.3) | 96.7(44.6) | .033 |
| Cross clamp time (min) | 55.5 (26.1) | 72.0 (37.9) | .011 |
| Ejection Fraction | 52.0 (17.3) | 53.6 (12.8) | .53 |
| Time from surgery to enrollment (days) | 9.5 (13.7) | 9.2 (11.9) | .90 |
| Psychosocial measures | |||
| Mini Mental Exam Total Score | 27.4 (1.0) | 27.6 (0.8) | .15 |
| Baseline anxiety | 1.3 (1.1) | 0.7 (0.7) | <.001 |
| Baseline pain interference | 5.0 (3.5) | 3.3 (2.6) | <.001 |
| Baseline pain severity | 3.9 (2.4) | 3.1 (2.0) | .028 |
| Baseline perceived control | 27.2 (6.3) | 31.4 (7.0) | .001 |
| Baseline perceived social support | 70.8 (11.0) | 73.3 (12.9) | .24 |
| Baseline sleep quality | 10.7 (4.2) | 7.0 (4.0) | <.001 |
| Depression-related measures | |||
| History of Depression | 9 (22.0) | 17 (8.1) | .008 |
| On antidepressants | 5 (12.2) | 19 (9.0) | .53 |
| Baseline depressive symptoms | 15.7 (6.6) | 5.7 (4.1) | <.001 |
BDI = Beck Depression Inventory
using t tests or X2 tests
In the sample as a whole, mean depressive symptom scores did not change from baseline to 6-week follow-up (Table 2). At baseline, depressive symptoms were significantly correlated with both BPI-INT and BPI-SEV. Similarly, changes in depressives at six weeks were also significantly correlated with changes in BPI-INT and BPI-SEV (Table 3).
Table 2. Change in Depressive and Pain Symptoms from Hospital Discharge (Baseline) to Six-Week Follow-Up (n = 251).
| Hospital Discharge (Baseline) Mean (SD) | Six-week Follow-Up Mean (SD) | Change Score (Follow-Up Minus Baseline) Mean (SD) | p-valuea | |
|---|---|---|---|---|
| Beck Depression Inventory | 7.33 (5.93) | 7.49 (7.66) | .15 (7.16) | .75 |
| Brief Pain Inventory: Pain Interference | 3.54 (2.84) | 2.10 (2.35) | -1.44 (2.87) | <.001 |
| Brief Pain Inventory: Pain Severity | 3.26 (2.10) | 2.32 (1.92) | -0.94 (2.11) | <.001 |
using t tests
Table 3. Correlations of Baseline and Change Scores (Follow Up Minus Baseline) Among Depressive Symptoms, Pain Interference, and Pain Severity (n = 251).
| Baseline | Change Score (Follow up minus baseline) | |||
|---|---|---|---|---|
| Brief Pain Inventory Interference | Brief Pain Inventory Severity | Brief Pain Inventory Interference | Brief Pain Inventory Severity | |
| Baseline | ||||
| Beck Depression Inventory Total | .37* | .29* | -.07 | -.10 |
| Beck Depression Inventory Cognitive/Affective Symptoms | .29* | .24* | -.06 | -.06 |
| Beck Depression Inventory Somatic Symptoms | .33* | .23* | -.12 | -.12 |
| Change Score (Follow up minus baseline) | ||||
| Beck Depression Inventory Total | -.02 | -.002 | .35* | .22* |
| Beck Depression Inventory Cognitive/Affective Symptoms | -.01 | -.01 | .33* | .18* |
| Beck Depression Inventory Somatic Symptoms | -.03 | .01 | .28* | .20* |
p=.01, 2-tailed
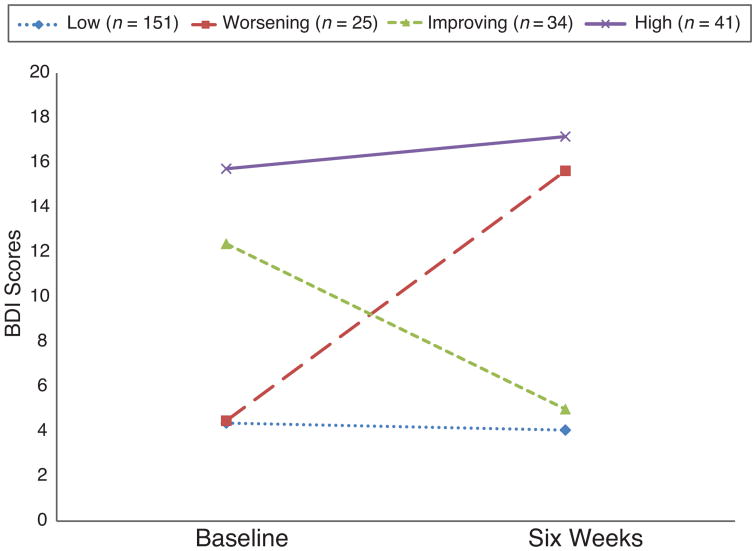
Forty-one (16.3%) patients reported persistent depressive symptoms (i.e. BDI score ≥ 10 at both time points). In 25 (10%) and 34 (13.5%) patients, BDI scores worsened or improved, respectively, from baseline to six weeks. The remaining 151 (60.2%) had BDI scores < 10 at both time points (Figure 2). Compared to patients without persistent depressive symptoms (n=210, 83.7%), patients with persistent depressive symptoms were younger, were more likely to have a history of depression, and had higher pain symptoms, higher anxiety scores, greater disturbance of sleep quality and lower perceived control scores at baseline (hospital discharge). There were no significant differences in race/ethnicity, educational level, marital or employment status, or other demographic variables between the groups.
Figure 2. Patterns of Change in Depressive Symptoms between Baseline and Six Weeks.
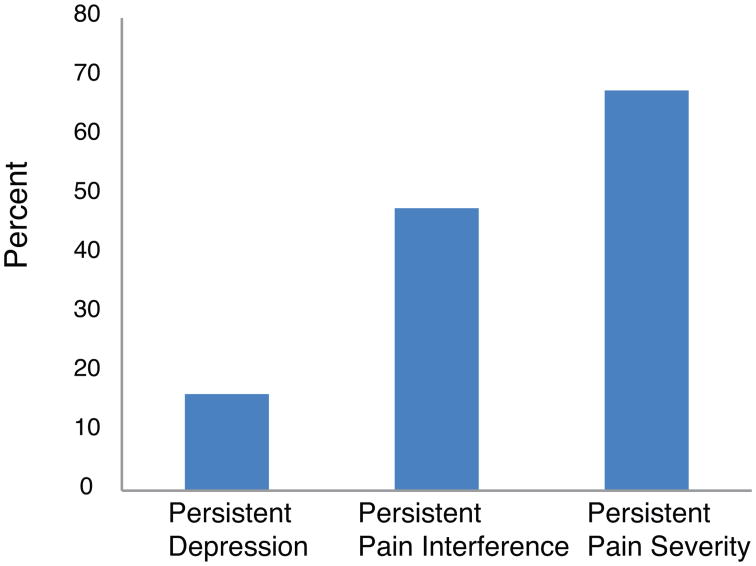
The most commonly reported locations of pain at baseline and 6-weeks were chest/sternum (baseline: 39.8%; 6-weeks: 40.2%), back (baseline: 17.1%; 6-weeks: 12.7%), and legs/knees (baseline: 12%; 6-weeks: 12.7%). Both pain interference and pain severity scores decreased over time (Table 2). Persistent pain interference (BPI-INT ≥ 1 at both time points) and persistent pain severity (BPI-SEV ≥ 1 at both time points) were common, ranging from 47.8% to 67.7% (Figure 1). Compared to patients without persistent pain interference, those with persistent pain interference were younger (69.3 ± 8.7 vs. 65.2 ± 9.9 years, respectively, p = .002) and had fewer co-morbid medical conditions (3.9 ± 1.7 vs. 3.3 ± 1.9, respectively, p = .02). No demographic differences were found between those with and without persistent pain severity.
Figure 1. Percentages of Persistent Depression and Persistent Pain.
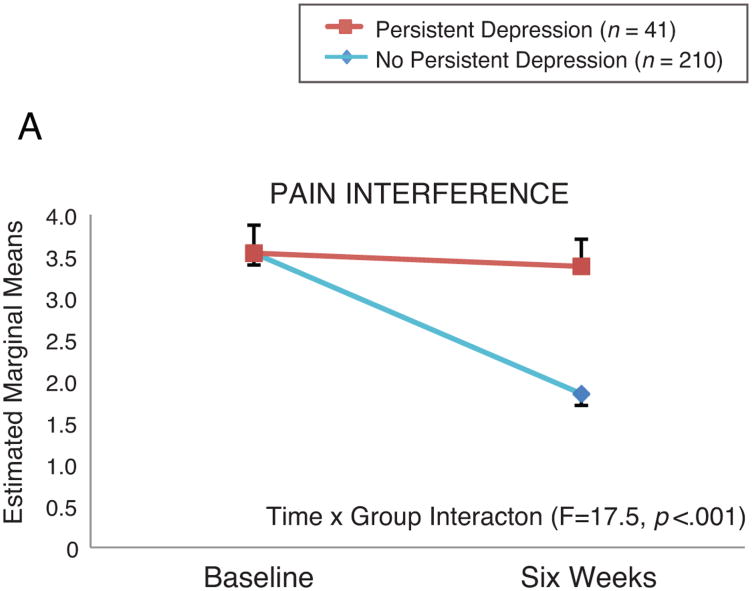
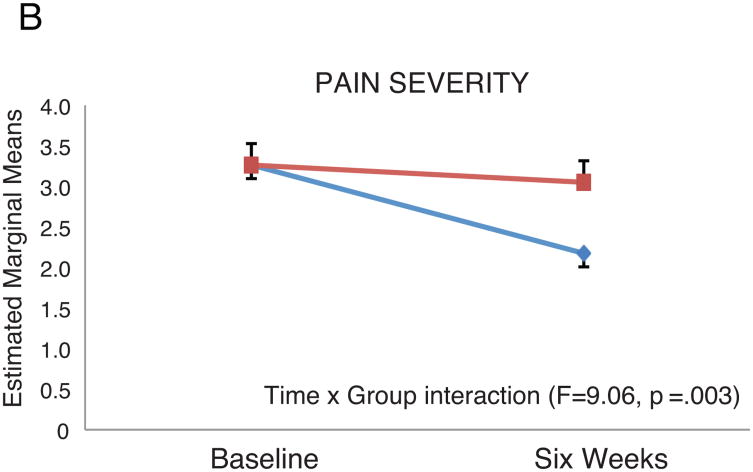
Patients with persistent depressive symptoms had higher BPI-INT and BPI-SEV scores at six weeks compared to patients without persistent depressive symptoms. In RM-ANOVA analysis, the group × time interaction remained significant in both the pain interference model (BPI-INT; F=17.5, p<.001) and the pain severity model (BPI-SEV; F = 9.06, p = .003) after controlling for age and baseline pain scores (Figure 3). In linear regression modeling, after controlling for other psychobehavioral variables (anxiety, perceived control, social support, global sleep quality), none of which were significant, changes in depressive symptoms were independently associated with higher BPI-INT (p<.001) and BPI-SEV (p=.001) (Tables 4-5). Alone, changes in depressive symptoms explained 17% of the variance in BPI-INT, but only 4% of the variance in BPI-SEV scores. When the cognitive/affective and somatic subscales of the BDI were considered separately in linear regression modeling, similar findings were observed.
Figure 3. Effect of Persistent Depression on Pain Interference and Pain Severity. Controlling for age and baseline pain interference (top panel) or pain severity (bottom panel); standard errors shown.
Table 4. Determinants of Pain Interference Six Weeks after Hospital Dischargea.
| Model | R2 | Adjusted R2 | R2 Change | F Change | F Change Sig | ||
|---|---|---|---|---|---|---|---|
| Step 1 | .22 | .17 | .22 | 4.39 | <.001 | ||
| Step 2 | .38 | .35 | .17 | 46.62 | <.001 | ||
| Variables in the equation at Step 2: | Standardized Beta | t | Sig. | 95.0% Confidence Interval for Beta | |||
| Lower | Upper | ||||||
| (Constant) | 1.91 | .058 | -.12 | 6.84 | |||
| Age in years | -.15 | -2.31 | .022 | -.07 | -.01 | ||
| Body mass index | .01 | .15 | .88 | -.05 | .05 | ||
| Pump time (minutes) | .04 | .20 | .84 | -.02 | .02 | ||
| Cross clamp time (minutes) | -.06 | -.34 | .73 | -.03 | .02 | ||
| Baseline pain interference | .27 | 4.19 | <.001 | .12 | .34 | ||
| Marital status (single/partnered) | .04 | .63 | .53 | -.44 | .85 | ||
| Baseline perceived control | .005 | .08 | .94 | -.04 | .05 | ||
| Baseline global sleep quality | .12 | 1.80 | .074 | -.01 | .14 | ||
| Baseline anxiety | .15 | 2.20 | .026 | .05 | .83 | ||
| Baseline social support | -.05 | -.76 | .45 | -.04 | .02 | ||
| Time from surgery to enrollment | .04 | .56 | .58 | -.02 | .03 | ||
| Change in depressive symptoms | .42 | 6.82 | <.001 | .09 | .17 | ||
linear regression, pain interference as dependent variable in analysis
Table 5. Determinants of Pain Severity Six Weeks after Hospital Dischargea.
| Model | R2 | Adjusted R2 | R2 Change | F Change | F Change Sig | |
|---|---|---|---|---|---|---|
| Step 1 | .26 | .22 | .26 | 5.64 | <.001 | |
| Step 2 | .31 | .26 | .04 | 10.60 | .001 | |
| Variables in the equation at Step 2: | Standardized Beta | t | Sig. | 95.0% Confidence Interval for Beta | ||
| Lower | Upper | |||||
| (Constant) | 1.61 | .11 | -.55 | 5.35 | ||
| Age in years | -.12 | -1.71 | .090 | -.05 | .004 | |
| Body mass index | -.01 | -.21 | .84 | -.05 | .04 | |
| Pump time (minutes) | .07 | .35 | .73 | -.01 | .02 | |
| Cross clamp time (minutes) | -.08 | -.41 | .68 | -.02 | .02 | |
| Baseline pain severity | .40 | 5.62 | <.001 | .24 | .50 | |
| Marital status (single/partnered) | .12 | 1.78 | .077 | -.05 | 1.05 | |
| Baseline perceived control | -.05 | -.61 | .54 | -.05 | .03 | |
| Baseline global sleep quality | .05 | .72 | .47 | -.04 | .09 | |
| Baseline anxiety | .09 | 1.26 | .21 | -.12 | .54 | |
| Baseline social support | .001 | .01 | .99 | -.02 | .02 | |
| Time from surgery to enrollment | .06 | .88 | .38 | -.01 | .03 | |
| Change in depressive symptoms | .21 | 3.26 | .001 | .02 | .09 | |
linear regression, pain severity as dependent variable in analysis
Discussion
Patterns of Depressive Symptoms
In the sample as a whole, depressive symptoms did not change from discharge to 6-week follow-up. While depressive symptoms have been reported to be highly prevalent generally after cardiac surgery,(2, 31) approximately 60% of our sample had low symptoms both at hospital discharge and at six weeks follow-up. An additional 10% had at least moderate symptoms at hospital discharge, but those symptoms had abated six weeks later. These findings are consistent with recent reports in which depression or its symptoms were reported to be more prevalent at the time of hospital discharge (21 to 24%) (5, 32, 33) than a month to 12 weeks later (13 – 16%).(34, 35) Morone et al found significantly higher rates of depression (up to 60%) two weeks after hospital discharge.(15) However, our findings and those of other investigators (34-36) suggest depressive symptoms may continue to evolve after the first two post-discharge weeks. Therefore, it seems likely that in some patients the presence of early postoperative depressive symptoms may be a reactive or adjustment response to surgery and hospitalization.
In approximately 30% of patients, depressive symptoms either remained above minimal levels or worsened after hospital discharge. These finding are consistent with other reports.(37, 38) Patients with persistent depressive symptoms (from discharge to six weeks) were more likely to have a history of depression (Table 1). This finding supports the role of depression history as an important clinical risk factor for depressive symptoms in this population.(39) Regarding worsening symptoms, some patients may experience a “honeymoon” period in the hospital and only experience depressive symptoms after they return home, which underscores the need for continued assessment of depressive symptoms in the post-discharge period after cardiac surgery.
Contribution of Depressive Symptoms to Pain Symptoms
From discharge to six weeks, cardiac surgery patients with persistent depressive symptoms sustained higher levels of pain interference and pain severity. In addition, clinical and demographic variables were not related to either later pain interference or pain severity in multivariate analysis (Tables 4-5). In these analyses, only baseline pain measures and changes in depressive symptoms were associated with 6-week pain measures when demographic, clinical, and baseline psychobehavioral variables were considered. Pain interference was more strongly related to changes in depressive symptoms than was pain severity. This finding may be explained by cognitive theory in that a negative bias in depressed individuals, i.e. “depressogenic” thoughts and assumptions, may be associated with a more negative interpretation of pain.(40) Baseline sleep quality did not contribute significantly to later pain interference or pain severity. Some studies have reported a direct relationship between sleep and pain, regardless of the effects of depressive symptoms (41, 42). Of note, in patients both with and without persistent depressive symptoms, sleep quality was poor; mean scores in both groups were above the level normally considered to indicate the presence of sleep impairment.(30) Thus, the absence of a relationship between sleep quality and pain may be due to the relative absence of variability in sleep impairment. Other studies found a direct relationship between depressive symptoms and pain (43), as we did. Overall, it is likely that there are multidirectional linkages among symptoms of pain, depression, and sleep disturbance which suggest common neurobiologic substrates.(44)
In a few previous reports of postoperative thoracotomy pain, other investigators have also shown that early postoperative pain is predictive of later chronic pain.(45) In another post-thoracotomy sample, Ochroh et al (46) reported greater postoperative pain in women after major thoracotomy, although we found no differences in pain severity or interference between men and women. In two reports of coronary artery bypass patients, investigators found that depressive symptoms early after hospital discharge was associated with greater pain one year later,(15) and that depressive symptoms and chronic pain correlated when both were measured over two years after surgery.(47)
A difficulty in synthesizing these data is that studies have measured depressive and pain symptoms with a wide variety of instruments and at many different time points following cardiac surgery. Nonetheless, our findings indicate that the association of persistent depressive symptoms and persistent pain begins much earlier after surgery than previously believed. An important consideration in understanding the relationship between persistent pain and depressive symptoms after cardiac surgery is its complexity. Current evidence suggests that this relationship is bidirectional.(11, 44) Multiple factors are likely to influence the relationship of persistent pain and depressive symptoms. Researchers hypothesize that shared neurobiology, genetics, and environmental factors play a role.(48) Cognitive processes (i.e. negative thinking) and behavioral factors (i.e. poor sleep hygiene) are associated with both pain and depressive symptoms.(11, 48) Psychosocial conditions, such as perceived control and social support, are likely to influence the severity of both pain and depressive symptoms.(48) With that in mind, further study is needed to elucidate how these factors interact with pain and depressive symptoms to influence their severity and chronicity.
Our study has several limitations. All of our patients resided in a single metropolitan area and were recruited from urban medical centers, so our findings may not be generalizable to other settings. We enrolled patients after surgery, so no preoperative questionnaires were obtained. We included patients having several types of cardiac surgery, so their pain experiences may have differed. We measured depressive symptoms using a well-established symptom instrument, so we cannot make inferences regarding clinical depression. Additionally, the potential circularity of pain-depression relationships, presence of other unmeasured contributors to pain, and possible active treatments occurring between baseline and six week measurements may limit interpretation of our data.
Despite these limitations, our findings have implications for clinical practice. First, depressive symptoms follow different, identifiable patterns after cardiac surgery. While many individuals may not have troublesome depressive symptoms after surgery or in the first months after hospital discharge, some patients will have either persistent depressive symptoms or increasing depressive symptoms after discharge. Thus, more routine depression screening, as recommended by the American Heart Association (49), could be beneficial. Second, in a majority of patients, persistent pain symptoms continue up to six weeks after cardiac surgery. Clinicians may find it helpful to evaluate both pain and depressive symptoms over time, appreciating that their complex relationship contributes to delayed recovery. Successful treatment of postoperative pain, which is an important goal in all clinical guidelines, may be enhanced with screening for depressive symptoms and initiation of treatment, if appropriate.
Acknowledgments
Source of Funding: The study was supported by NIH grant 5R01NR009228-02.
Acronyms
- BDI
Beck Depression Inventory
- BMI
body mass index
- BPI
Brief Pain Inventory
- BPI-INT
Brief Pain Inventory – Interference
- BPI-SEV
Brief Pain Inventory – Severity
- BSI
Brief Symptom Inventory
- CAS-R
Control Attitudes Scale – Revised
- IRB
Institutional Review Board
- MMSE
Mini Mental State Exam
- MSPSS
Multidimensional Scale of Perceived Social Support
- PSQI
Pittsburgh Sleep Quality Index
- RM-ANOVA
repeated measures analysis of variance
Footnotes
Conflicts of Interest: We have no conflicts of interest.
Trial Registration: Clinicaltrials.gov Identifier: NCT00522717
Contributor Information
Lynn V. Doering, University of California, Los Angeles, School of Nursing, Los Angeles, CA.
Belinda Chen, University of California, Los Angeles, School of Nursing, Los Angeles, CA.
Anthony McGuire, California State University, Long Beach, School of Nursing, Long Beach, CA.
Rebecca Cross Bodán, California State University, Fullerton, School of Nursing, Fullerton, CA.
Michael R. Irwin, Cousins Center for Psychoneuroimmunology, UCLA Semel Institute for Neuroscience and Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA.
References
- 1.Stroobant N, Vingerhoets G. Depression, anxiety, and neuropsychological performance in coronary artery bypass graft patients: a follow-up study. Psychosomatics. 2008;49:326–31. doi: 10.1176/appi.psy.49.4.326. [DOI] [PubMed] [Google Scholar]
- 2.Pignay-Demaria V, Lesperance F, Demaria RG, Frasure-Smith N, Perrault LP. Depression and anxiety and outcomes of coronary artery bypass surgery. Ann Thorac Surg. 2003;75:314–21. doi: 10.1016/s0003-4975(02)04391-6. [DOI] [PubMed] [Google Scholar]
- 3.Baker RA, Andrew MJ, Schrader G, Knight JL. Preoperative depression and mortality in coronary artery bypass surgery: preliminary findings. ANZ journal of surgery. 2001;71:139–42. doi: 10.1046/j.1440-1622.2001.02055.x. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Zur H, Rappaport B, Ammar R, Uretzky G. Coping strategies, life style changes, and pessimism after open-heart surgery. Health & social work. 2000;25:201–9. doi: 10.1093/hsw/25.3.201. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 5.Connerney I, Shapiro PA, McLaughlin JS, Bagiella E, Sloan RP. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet. 2001;358:1766–71. doi: 10.1016/S0140-6736(01)06803-9. Research Support, Non-U.S. Gov't Research Support, US Gov't, P.H.S. [DOI] [PubMed] [Google Scholar]
- 6.Cogan J. Pain management after cardiac surgery. Semin Cardiothorac Vasc Anesth. 2010;14:201–4. doi: 10.1177/1089253210378401. [DOI] [PubMed] [Google Scholar]
- 7.Johansen A, Romundstad L, Nielsen CS, Schirmer H, Stubhaug A. Persistent postsurgical pain in a general population: prevalence and predictors in the Tromso study. Pain. 2012;153:1390–6. doi: 10.1016/j.pain.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Bruce J, Drury N, Poobalan AS, Jeffrey RR, Smith WC, Chambers WA. The prevalence of chronic chest and leg pain following cardiac surgery: a historical cohort study. Pain. 2003;104:265–73. doi: 10.1016/s0304-3959(03)00017-4. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 9.Kalso E, Mennander S, Tasmuth T, Nilsson E. Chronic post-sternotomy pain. Acta anaesthesiologica Scandinavica. 2001;45:935–9. doi: 10.1034/j.1399-6576.2001.450803.x. [DOI] [PubMed] [Google Scholar]
- 10.Sharma AD, Parmley CL, Sreeram G, Grocott HP. Peripheral nerve injuries during cardiac surgery: risk factors, diagnosis, prognosis, and prevention. Anesthesia and analgesia. 2000;91:1358–69. doi: 10.1097/00000539-200012000-00010. Review. [DOI] [PubMed] [Google Scholar]
- 11.Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. The journal of pain : official journal of the American Pain Society. 2011;12:964–73. doi: 10.1016/j.jpain.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 13.Skolasky RL, Riley LH, 3rd, Maggard AM, Wegener ST. The relationship between pain and depressive symptoms after lumbar spine surgery. Pain. 2012;153:2092–6. doi: 10.1016/j.pain.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheridan D, Foo I, O'Shea H, Gillanders D, Williams L, Fallon M, Colvin L. Long-term follow-up of pain and emotional characteristics of women after surgery for breast cancer. Journal of pain and symptom management. 2012;44:608–14. doi: 10.1016/j.jpainsymman.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Morone NE, Weiner DK, Belnap BH, Karp JF, Mazumdar S, Houck PR, He F, Rollman BL. The impact of pain and depression on recovery after coronary artery bypass grafting. Psychosomatic medicine. 2010;72:620–5. doi: 10.1097/PSY.0b013e3181e6df90. Comparative Study Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folstein MF, Folsltein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:9. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Disease. 1987;40:10. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Frasure-Smith N, Lesperance F, Juneau M, Talajic M, Bourassa MG. Gender, depression, and one-year prognosis after myocardial infarction. Psychosomatic medicine. 1999;61:26–37. doi: 10.1097/00006842-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Beck AT, Steer RA. Psychometric properties of the Beck Depression inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:23. [Google Scholar]
- 20.Richter P, Werner J, Heerlein A, Kraus A, Sauer H. On the validity of the Beck Depression Inventory. A review. Psychopathology. 1998;31:160–8. doi: 10.1159/000066239. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behaviour research and therapy. 1997;35:785–91. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 22.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–18. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Gjeilo KH, Stenseth R, Wahba A, Lydersen S, Klepstad P. Validation of the brief pain inventory in patients six months after cardiac surgery. Journal of pain and symptom management. 2007;34:648–56. doi: 10.1016/j.jpainsymman.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychol Med. 2008;38:365–74. doi: 10.1017/S0033291707002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinley S, Moser DK, Dracup K. Treatment-seeking behavior for acute myocardial infarction symptoms in North America and Australia. Heart Lung. 2000;29:237–47. doi: 10.1067/mhl.2000.106940. [DOI] [PubMed] [Google Scholar]
- 26.Abu Ruz ME, Lennie TA, Riegel B, McKinley S, Doering LV, Moser DK. Evidence that the brief symptom inventory can be used to measure anxiety quickly and reliably in patients hospitalized for acute myocardial infarction. J Cardiovasc Nurs. 2010;25:117–23. doi: 10.1097/JCN.0b013e3181b56626. [DOI] [PubMed] [Google Scholar]
- 27.Moser DK, Riegel B, McKinley S, Doering LV, Meischke H, Heo S, Lennie TA, Dracup K. The Control Attitudes Scale-Revised: psychometric evaluation in three groups of patients with cardiac illness. Nurs Res. 2009;58:42–51. doi: 10.1097/NNR.0b013e3181900ca0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanley MA, Jensen MP, Ehde DM, Hoffman AJ, Patterson DR, Robinson LR. Psychosocial predictors of long-term adjustment to lower-limb amputation and phantom limb pain. Disability and rehabilitation. 2004;26:882–93. doi: 10.1080/09638280410001708896. Comparative Study Research Support, U.S. Gov't, P.H.S. [DOI] [PubMed] [Google Scholar]
- 29.Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. Journal of personality assessment. 1990;55:610–7. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 30.Smyth C. The Pittsburgh Sleep Quality Index (PSQI) Journal of gerontological nursing. 1999;25:10–1. doi: 10.3928/0098-9134-19991201-10. [DOI] [PubMed] [Google Scholar]
- 31.Tully PJ, Baker RA. Depression, anxiety, and cardiac morbidity outcomes after coronary artery bypass surgery: a contemporary and practical review. Journal of geriatric cardiology : JGC. 2012;9:197–208. doi: 10.3724/SP.J.1263.2011.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraguas Junior R, Ramadan ZB, Pereira AN, Wajngarten M. Depression with irritability in patients undergoing coronary artery bypass graft surgery: the cardiologist's role. General hospital psychiatry. 2000;22:365–74. doi: 10.1016/s0163-8343(00)00094-3. Comparative Study. [DOI] [PubMed] [Google Scholar]
- 33.Tully PJ, Baker RA, Turnbull D, Winefield H. The role of depression and anxiety symptoms in hospital readmissions after cardiac surgery. Journal of behavioral medicine. 2008;31:281–90. doi: 10.1007/s10865-008-9153-8. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 34.McKhann GM, Borowicz LM, Goldsborough MA, Enger C, Selnes OA. Depression and cognitive decline after coronary artery bypass grafting. Lancet. 1997;349:1282–4. doi: 10.1016/S0140-6736(96)09466-4. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell RH, Robertson E, Harvey PJ, Nolan R, Rodin G, Romans S, Abramson BL, Brister SJ, Ivanov J, Stewart DE. Sex differences in depression after coronary artery bypass graft surgery. American heart journal. 2005;150:1017–25. doi: 10.1016/j.ahj.2005.05.005. Comparative Study. [DOI] [PubMed] [Google Scholar]
- 36.Rafanelli C, Roncuzzi R, Milaneschi Y. Minor depression as a cardiac risk factor after coronary artery bypass surgery. Psychosomatics. 2006;47:289–95. doi: 10.1176/appi.psy.47.4.289. [DOI] [PubMed] [Google Scholar]
- 37.Murphy BM, Elliott PC, Higgins RO, Le Grande MR, Worcester MUC, Goble AJ, Tatoulis J. Anxiety and depression after coronary artery bypass graft surgery: most get better, some get worse. European Journal of Cardiovascular Prevention & Rehabilitation. 2008;15:434–40. doi: 10.1097/HJR.0b013e3282fbc945. [DOI] [PubMed] [Google Scholar]
- 38.Elliott PC, Murphy BM, Oster KA, Le Grande MR, Higgins RO, Worcester MUC. Changes in Mood States After Coronary Artery Bypass Graft Surgery. European Journal of Cardiovascular Nursing. 2010;9:188–94. doi: 10.1016/j.ejcnurse.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Andrew MJ, Baker RA, Kneebone AC, Knight JL. Mood state as a predictor of neuropsychological deficits following cardiac surgery. Journal of psychosomatic research. 2000;48:537–46. doi: 10.1016/s0022-3999(00)00089-1. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 40.Clark DA, Beck AT, Alford BA. Scientific Foundations of Cognitive Theory and Therapy of Depression. New York, New York: John Wiley & Sons, Inc.; 1999. [Google Scholar]
- 41.Nicassio PM, Wallston KA. Longitudinal relationships among pain, sleep problems, and depression in rheumatoid arthritis. Journal of abnormal psychology. 1992;101:514–20. doi: 10.1037//0021-843x.101.3.514. [DOI] [PubMed] [Google Scholar]
- 42.Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Fitzgerald JD, Ranganath VK, Nicassio PM. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. 2012;35:537–43. doi: 10.5665/sleep.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Brien EM, Waxenberg LB, Atchison JW, Gremillion HA, Staud RM, McCrae CS, Robinson ME. Negative mood mediates the effect of poor sleep on pain among chronic pain patients. Clin J Pain. 2010;26:310–9. doi: 10.1097/AJP.0b013e3181c328e9. [DOI] [PubMed] [Google Scholar]
- 44.Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep medicine reviews. 2013;17:173–83. doi: 10.1016/j.smrv.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pluijms WA, Steegers MA, Verhagen AF, Scheffer GJ, Wilder-Smith OH. Chronic post-thoracotomy pain: a retrospective study. Acta anaesthesiologica Scandinavica. 2006;50:804–8. doi: 10.1111/j.1399-6576.2006.01065.x. [DOI] [PubMed] [Google Scholar]
- 46.Ochroch EA, Gottschalk A, Troxel AB, Farrar JT. Women suffer more short and long-term pain than men after major thoracotomy. Clin J Pain. 2006;22:491–8. doi: 10.1097/01.ajp.0000208246.18251.f2. Clinical Trial Comparative Study Randomized Controlled Trial. [DOI] [PubMed] [Google Scholar]
- 47.Taillefer MC, Carrier M, Belisle S, Levesque S, Lanctot H, Boisvert AM, Choiniere M. Prevalence, characteristics, and predictors of chronic nonanginal postoperative pain after a cardiac operation: a cross-sectional study. The Journal of thoracic and cardiovascular surgery. 2006;131:1274–80. doi: 10.1016/j.jtcvs.2006.02.001. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 48.Goesling J, Clauw D, Hassett A. Pain and Depression: An Integrative Review of Neurobiological and Psychological Factors. Curr Psychiatry Rep. 2013;15:1–8. doi: 10.1007/s11920-013-0421-0. [DOI] [PubMed] [Google Scholar]
- 49.Lichtman JH, Bigger JT, Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lesperance F, Mark DB, Sheps DS, Taylor CB, Froelicher ES American Heart Association Prevention Committee of the Council on Cardiovascular N, American Heart Association Council on Clinical C, American Heart Association Council on E, Prevention, American Heart Association Interdisciplinary Council on Quality of C, Outcomes R, American Psychiatric A. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768–75. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]