Full text
PDF









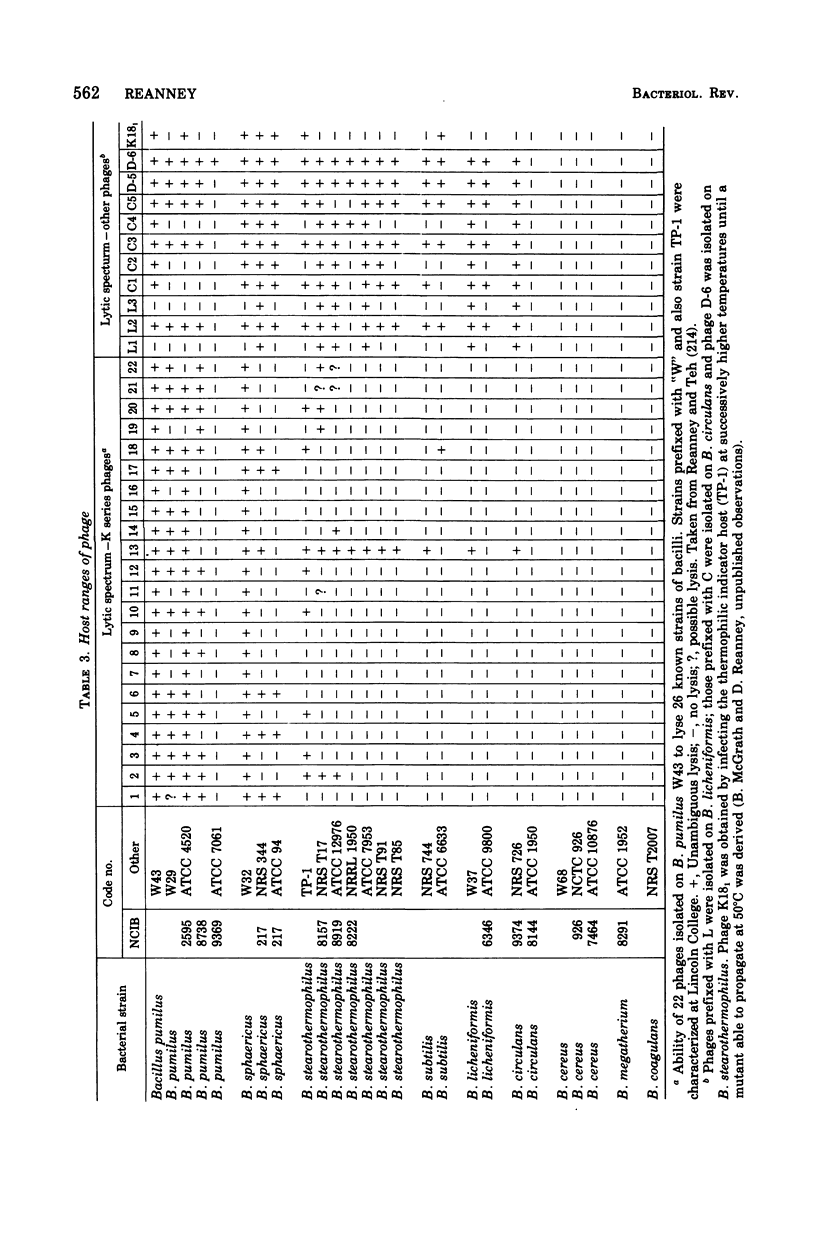

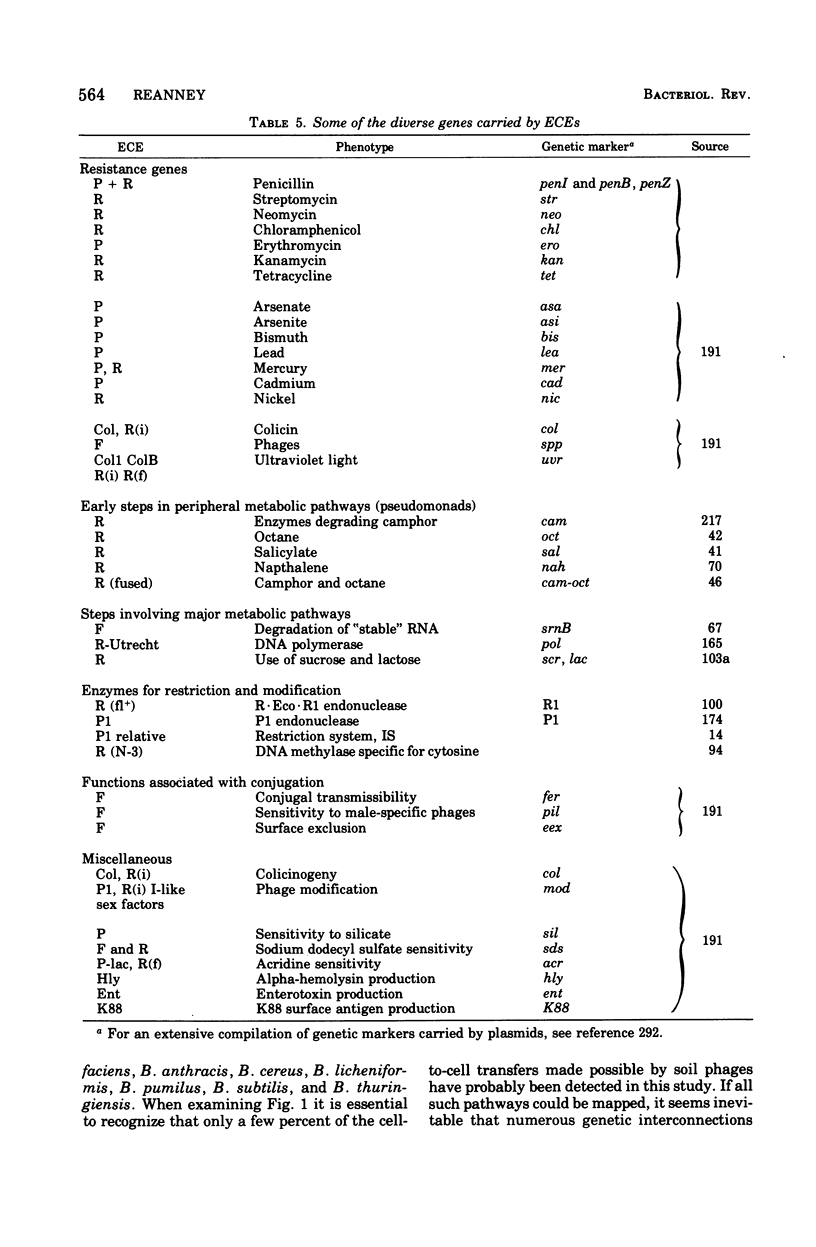
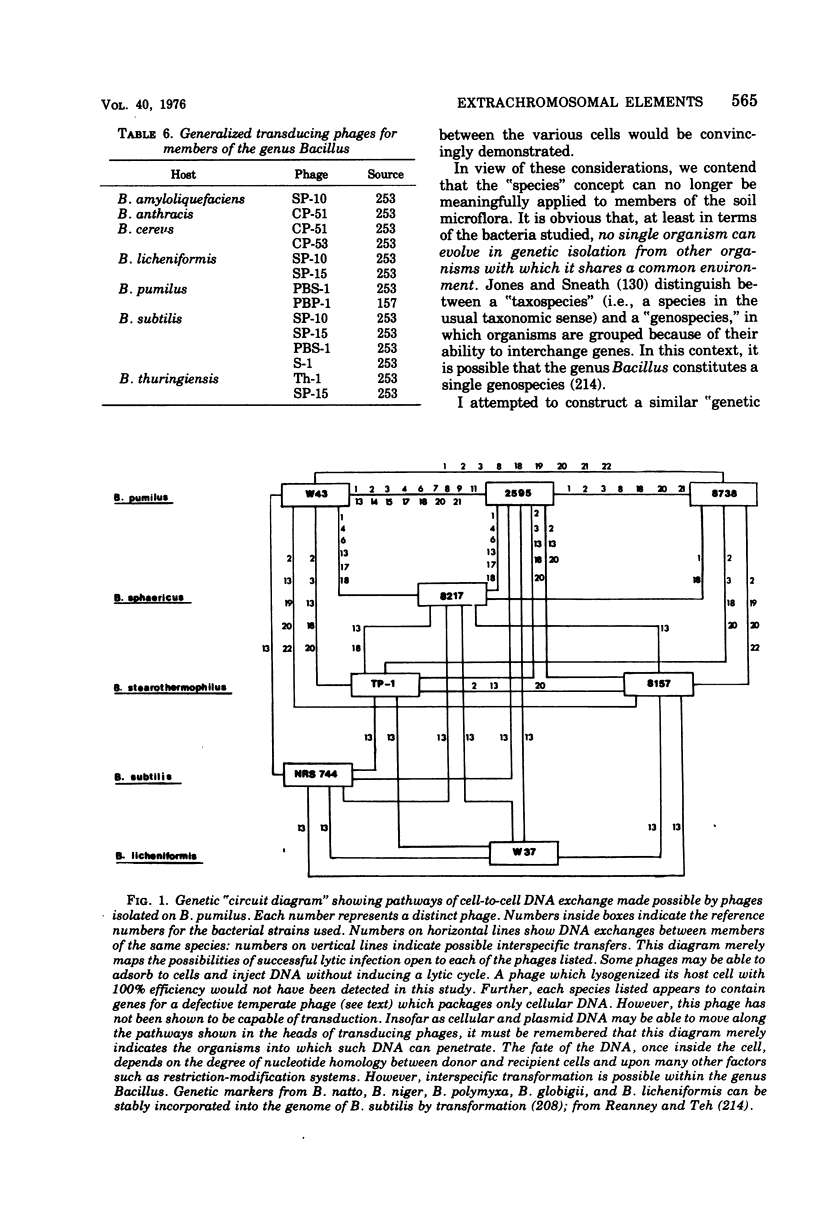
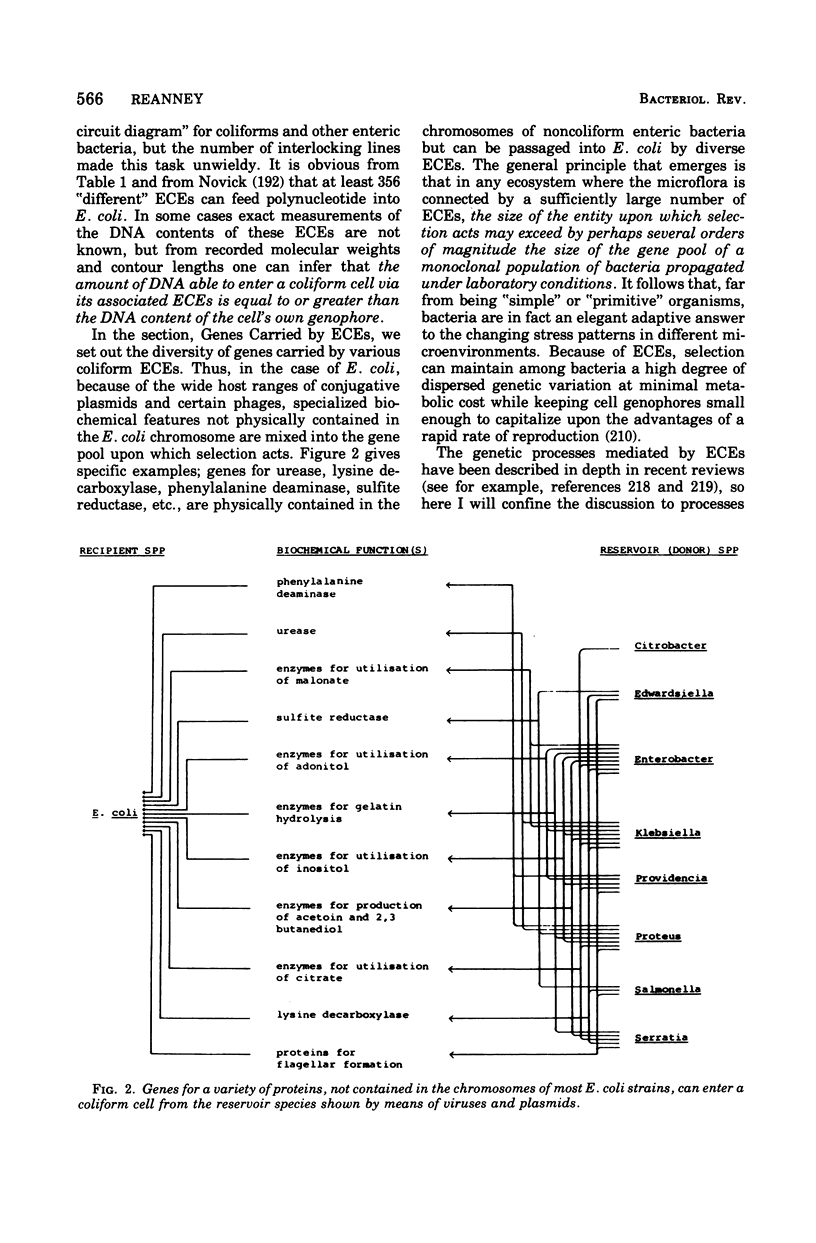






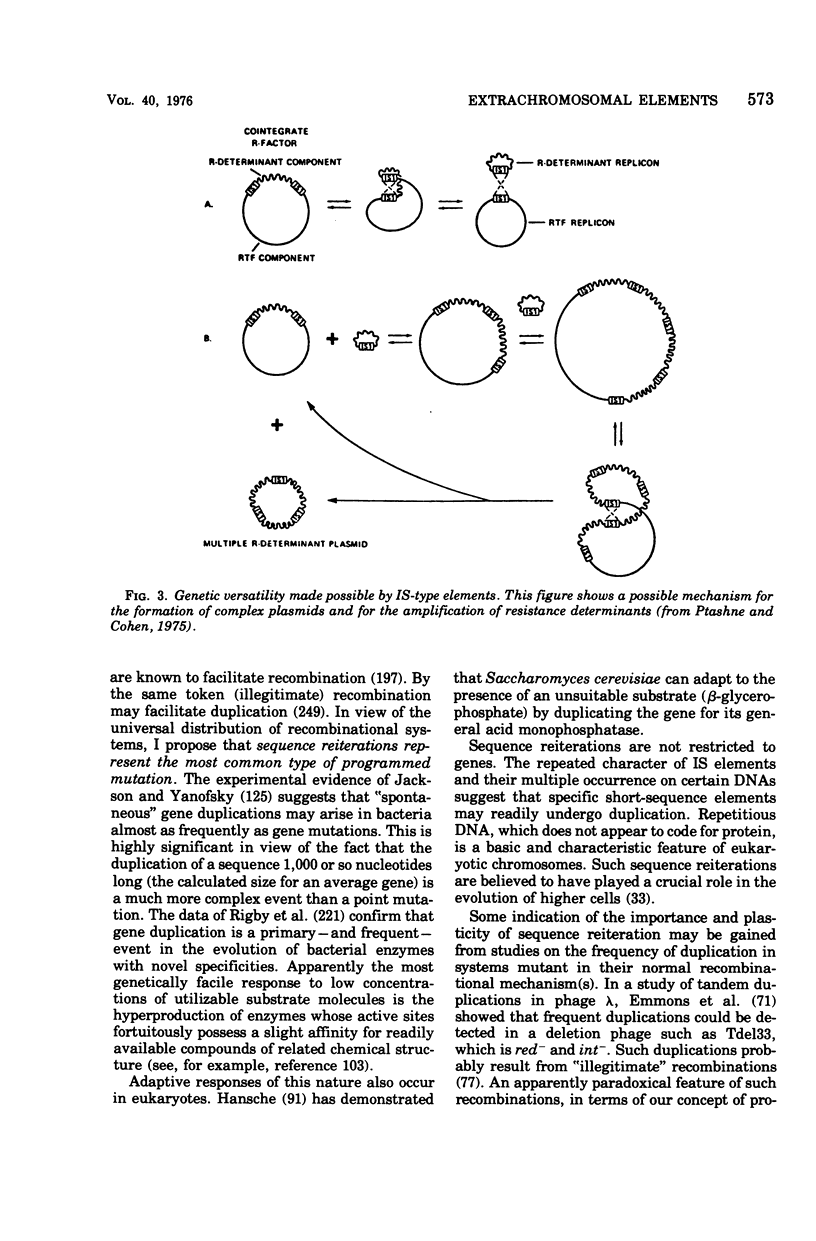

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., HARTMAN P. E., JACOB F. Chromosomal alterations affecting the regulation of histidine biosynthetic enzymes in Salmonella. J Mol Biol. 1963 Jul;7:23–42. doi: 10.1016/s0022-2836(63)80016-9. [DOI] [PubMed] [Google Scholar]
- Adelberg E. A., Bergquist P. The stabilization of episomal integration by genetic inversion: a general hypothesis. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2061–2065. doi: 10.1073/pnas.69.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A., Holloman W. K., Holliday R. Nuclease that preferentially inactivates DNA containing mismatched bases. Nature. 1975 Nov 6;258(5530):54–56. doi: 10.1038/258054a0. [DOI] [PubMed] [Google Scholar]
- Alff-Steinberger C. The genetic code and error transmission. Proc Natl Acad Sci U S A. 1969 Oct;64(2):584–591. doi: 10.1073/pnas.64.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allet B., Jeppesen P. G., Katagiri K. J., Delius H. Mapping the DNA fragments produced by cleavage by lambda DNA with endonuclease RI. Nature. 1973 Jan 12;241(5385):120–123. doi: 10.1038/241120a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S. Possible importance of transfer factors in bacterial evolution. Nature. 1966 Feb 5;209(5023):637–638. doi: 10.1038/209637a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S. The ecology of transferable drug resistance in the enterobacteria. Annu Rev Microbiol. 1968;22:131–180. doi: 10.1146/annurev.mi.22.100168.001023. [DOI] [PubMed] [Google Scholar]
- Anderson N. G. Evolutionary significance of virus infection. Nature. 1970 Sep 26;227(5265):1346–1347. doi: 10.1038/2271346a0. [DOI] [PubMed] [Google Scholar]
- Arber W. DNA modification and restriction. Prog Nucleic Acid Res Mol Biol. 1974;14(0):1–37. doi: 10.1016/s0079-6603(08)60204-4. [DOI] [PubMed] [Google Scholar]
- Arber W., Wauters-Willems D. Host specificity of DNA produced by Escherichia coli. XII. The two restriction and modification systems of strain 15T-. Mol Gen Genet. 1970;108(3):203–217. doi: 10.1007/BF00283350. [DOI] [PubMed] [Google Scholar]
- Artzt K., Bennett D. Analogies between embryonic (T/t) antigens and adult major histocompatibility (H-2) antigens. Nature. 1975 Aug 14;256(5518):545–547. doi: 10.1038/256545a0. [DOI] [PubMed] [Google Scholar]
- Astier-Manifacier S., Cornuet P. RNA-dependent RNA polymerase in Chinese cabbage. Biochim Biophys Acta. 1971 Mar 25;232(3):484–493. doi: 10.1016/0005-2787(71)90602-2. [DOI] [PubMed] [Google Scholar]
- BOTT K., STRAUSS B. THE CARRIER STATE OF BACILLUS SUBTILIS INFECTED WITH THE TRANSDUCING BACTERIOPHAGE SP10. Virology. 1965 Feb;25:212–225. doi: 10.1016/0042-6822(65)90200-x. [DOI] [PubMed] [Google Scholar]
- Ball L. A. Implications of secondary structure in messenger RNA. J Theor Biol. 1972 Aug;36(2):313–320. doi: 10.1016/0022-5193(72)90100-2. [DOI] [PubMed] [Google Scholar]
- Barksdale L., Arden S. B. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu Rev Microbiol. 1974;28(0):265–299. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of C-type viral genes: inheritance of exogenously acquired viral genes. Nature. 1974 Dec 6;252(5483):456–459. doi: 10.1038/252456a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: preservation of ancestral murine type C viral sequences in pig cellular DNA. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4090–4094. doi: 10.1073/pnas.72.10.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2276–2280. doi: 10.1073/pnas.70.8.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz J. L., Brown P. R., Smyth M. J., Clarke P. H. Evolution in action. Nature. 1974 Feb 1;247(5439):261–264. doi: 10.1038/247261a0. [DOI] [PubMed] [Google Scholar]
- Blundell T. L., Wood S. P. Is the evolution of insulin Darwinian or due to selectively neutral mutation? Nature. 1975 Sep 18;257(5523):197–203. doi: 10.1038/257197a0. [DOI] [PubMed] [Google Scholar]
- Bonner J., Wu J. R. A proposal for the structure of the Drosophila genome. Proc Natl Acad Sci U S A. 1973 Feb;70(2):535–537. doi: 10.1073/pnas.70.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boram W., Abelson J. Bacteriophage Mu integration: on the orientation of the prophage. Virology. 1973 Jul;54(1):102–108. doi: 10.1016/0042-6822(73)90119-0. [DOI] [PubMed] [Google Scholar]
- Botstein D., Herskowitz I. Properties of hybrids between Salmonella phage P22 and coliphage lambda. Nature. 1974 Oct 18;251(5476):584–589. doi: 10.1038/251584a0. [DOI] [PubMed] [Google Scholar]
- Bray D., Robbins P. W. Mechanism of epsilon-15 conversion studies with bacteriphage mutants. J Mol Biol. 1967 Dec 28;30(3):457–475. doi: 10.1016/0022-2836(67)90362-2. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Brockman W. W., Lee T. N., Nathans D. The evolution of new species of viral DNA during serial passage of simian virus 40 at high multiplicity. Virology. 1973 Aug;54(2):384–397. doi: 10.1016/0042-6822(73)90151-7. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3' leads to 5' exonuclease activity in deoxyribonucleic acid polymerases. J Biol Chem. 1972 Jan 10;247(1):241–248. [PubMed] [Google Scholar]
- Bukhari A. I., Metlay M. Genetic mapping of prophage Mu. Virology. 1973 Jul;54(1):109–116. doi: 10.1016/0042-6822(73)90120-7. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M., Chou G., Gunsalus I. C. Genetic regulation of octane dissimilation plasmid in Pseudomonas. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1137–1140. doi: 10.1073/pnas.70.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. Genetic basis of the biodegradation of salicylate in Pseudomonas. J Bacteriol. 1972 Nov;112(2):815–823. doi: 10.1128/jb.112.2.815-823.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty M. Induction and repression of L-arabinose isomerase in bacteriophage-infected Salmonella typhimurium. J Virol. 1970 May;5(5):541–547. doi: 10.1128/jvi.5.5.541-547.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalcroft J. P., Matthews R. E. Role of virus strains and leaf ontogeny in the production of mosaic patterns by turnip yellow mosaic virus. Virology. 1967 Dec;33(4):659–673. doi: 10.1016/0042-6822(67)90066-9. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. E., Britten R. J., Davidson E. H. Sequence organization in Xenopus DNA studied by the electron microscope. J Mol Biol. 1975 Aug 5;96(2):317–333. doi: 10.1016/0022-2836(75)90351-4. [DOI] [PubMed] [Google Scholar]
- Chou G. I., Katz D., Gunsalus I. C. Fusion and compatibility of camphor and octane plasmids in Pseudomonas. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2675–2678. doi: 10.1073/pnas.71.7.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen C., Christiansen G., Bak A. L., Stenderup A. Extrachromosomal deoxyribonucleic acid in different enterobacteria. J Bacteriol. 1973 Apr;114(1):367–377. doi: 10.1128/jb.114.1.367-377.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B. J., Axelrad A. A., Shreeve M. M., McLeod D. L. Erythroid colony induction without erythropoietin by Friend leukemia virus in vitro. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3556–3560. doi: 10.1073/pnas.72.9.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Recircularization and autonomous replication of a sheared R-factor DNA segment in Escherichia coli transformants. Proc Natl Acad Sci U S A. 1973 May;70(5):1293–1297. doi: 10.1073/pnas.70.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasito D. J., Rogoff M. H. Characterization of temperate bacteriophages of Bacillus thuringiensis. J Gen Virol. 1969 Sep;5(2):275–281. doi: 10.1099/0022-1317-5-2-275. [DOI] [PubMed] [Google Scholar]
- Cox E. C., Yanofsky C. Altered base ratios in the DNA of an Escherichia coli mutator strain. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1895–1902. doi: 10.1073/pnas.58.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. A minute circular DNA from Escherichia coli 15. Proc Natl Acad Sci U S A. 1968 Jul;60(3):992–999. doi: 10.1073/pnas.60.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. The origin of the genetic code. J Mol Biol. 1968 Dec;38(3):367–379. doi: 10.1016/0022-2836(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Crick F. General model for the chromosomes of higher organisms. Nature. 1971 Nov 5;234(5323):25–27. doi: 10.1038/234025a0. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Host ranges of R factors. J Gen Microbiol. 1972 May;70(3):453–460. doi: 10.1099/00221287-70-3-453. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli D., Fareed G. C. Amplification of a circular segment of SV40 DNA. Nature. 1974 Sep 13;251(5471):153–155. doi: 10.1038/251153a0. [DOI] [PubMed] [Google Scholar]
- Dewees A. A. Genetic modification of recombination rate in Tribolium castaneum. Genetics. 1975 Nov;81(3):537–552. doi: 10.1093/genetics/81.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson E., Prensky W., Robertson H. D. Comparative studies of two viroids: analysis of potato spindle tuber and citrus exocortis viroids by RNA fingerprinting and polyacrylamide-gel electrophoresis. Virology. 1975 Dec;68(2):309–316. doi: 10.1016/0042-6822(75)90274-3. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Potato spindle tuber viroid. 8. Correlation of infectivity with a UV-absorbing component and thermal denaturation properties of the RNA. Virology. 1972 Nov;50(2):606–609. doi: 10.1016/0042-6822(72)90412-6. [DOI] [PubMed] [Google Scholar]
- Doermann A. H. T4 and the rolling circle model of replication. Annu Rev Genet. 1973;7:325–341. doi: 10.1146/annurev.ge.07.120173.001545. [DOI] [PubMed] [Google Scholar]
- Donini P. Turnover of ribosomal RNA during the stringent response in Escherichia coli. J Mol Biol. 1972 Dec 30;72(3):553–569. doi: 10.1016/0022-2836(72)90174-x. [DOI] [PubMed] [Google Scholar]
- Dube S. K., Pragnell I. B., Kluge N., Gaedicke G., Steinheider G., Ostertag W. Induction of endogenous and of spleen focus-forming viruses during dimethylsulfoxide-induced differentiation of mouse erythroleukemia cells transformed by spleen focus-forming virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1863–1867. doi: 10.1073/pnas.72.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn N. W., Gunsalus I. C. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J Bacteriol. 1973 Jun;114(3):974–979. doi: 10.1128/jb.114.3.974-979.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons S. W., MacCosham V., Baldwin R. L. Tandem genetic duplications in phage lambda. III. The frequency of duplication mutants in two derivatives of phage lambda is independent of known recombination systems. J Mol Biol. 1975 Jan 15;91(2):133–146. doi: 10.1016/0022-2836(75)90154-0. [DOI] [PubMed] [Google Scholar]
- Feldman D. An electron microscopic study of virus particles in rhesus monkey placenta. Proc Natl Acad Sci U S A. 1975 Jan;72(1):118–121. doi: 10.1073/pnas.72.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiandt M., Szybalski W., Malamy M. H. Polar mutations in lac, gal and phage lambda consist of a few IS-DNA sequences inserted with either orientation. Mol Gen Genet. 1972;119(3):223–231. doi: 10.1007/BF00333860. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Duerinck F., Haegeman G., Iserentant D., Merregaert J., Min Jou W., Molemans F., Raeymaekers A., Van den Berghe A. Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene. Nature. 1976 Apr 8;260(5551):500–507. doi: 10.1038/260500a0. [DOI] [PubMed] [Google Scholar]
- Fincham J. R., Sastry G. R. Controlling elements in maize. Annu Rev Genet. 1974;8:15–50. doi: 10.1146/annurev.ge.08.120174.000311. [DOI] [PubMed] [Google Scholar]
- Garro A. J., Law M. F. Relationship between lysogeny, spontaneous induction, and transformation efficiencies in Bacillus subtilis. J Bacteriol. 1974 Dec;120(3):1256–1259. doi: 10.1128/jb.120.3.1256-1259.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Uchida T., Singer R. A. Expression of diphtheria toxin genes carried by integrated and nonintegrated phage beta. Virology. 1972 Dec;50(3):664–668. doi: 10.1016/0042-6822(72)90420-5. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Gallo R. C. RNA processing and RNA tumor virus origin and evolution. Science. 1975 May 23;188(4190):802–811. doi: 10.1126/science.47650. [DOI] [PubMed] [Google Scholar]
- Goldberg I. D., Bryan T. Productive infection of Bacillus subtilis 168, with bacteriophage SP-10, dependent upon inducing treatments. J Virol. 1968 Aug;2(8):805–812. doi: 10.21236/ad0686354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M., Moore G. W., Matsuda G. Darwinian evolution in the genealogy of haemoglobin. Nature. 1975 Feb 20;253(5493):603–608. doi: 10.1038/253603a0. [DOI] [PubMed] [Google Scholar]
- Gross L., Schidlovsky G., Feldman D., Dreyfuss Y., Moore L. A. C-type virus particles in placenta of normal healthy Sprague-Dawley rats. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3240–3244. doi: 10.1073/pnas.72.8.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Rous sarcoma virus activates embryonic globin genes in chicken fibroblasts. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4464–4468. doi: 10.1073/pnas.72.11.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerineau M., Slonimski P. P., Avner P. R. Yeast episome: oligomycin resistance associated with a small covalently closed non-mitochondrial circular DNA. Biochem Biophys Res Commun. 1974 Nov 27;61(2):462–469. doi: 10.1016/0006-291x(74)90979-6. [DOI] [PubMed] [Google Scholar]
- Gyles C., So M., Falkow S. The enterotoxin plasmids of Escherichia coli. J Infect Dis. 1974 Jul;130(1):40–49. doi: 10.1093/infdis/130.1.40. [DOI] [PubMed] [Google Scholar]
- HARADA K., KAMEDA M., SUZUKI M., EGAWA R., MITSUHASHI S. Studies on the drug-resistance of enteric bacteria. 10. Relation between transmissible drug-resistance (R) factor and fertility (F) factor in E. coli strain K-12. Jpn J Exp Med. 1961 Aug;31:291–299. [PubMed] [Google Scholar]
- HOLLOWAY B. W., COOPER G. N. Lysogenic conversion in Pseudomonas aeruginosa. J Bacteriol. 1962 Dec;84:1321–1324. doi: 10.1128/jb.84.6.1321-1324.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C., Wepprich R. K., Davies J. W., Weathers L. G., Semancik J. S. Functional distinctions between the ribonucleic acids from citrus exocortis viroid and plant viruses: cell-free translation and aminoacylation reactions. Virology. 1974 Oct;61(2):486–492. doi: 10.1016/0042-6822(74)90284-0. [DOI] [PubMed] [Google Scholar]
- Hansche P. E. Gene duplication as a mechanism of genetic adaptation in Saccharomyces cerevisiae. Genetics. 1975 Apr;79(4):661–674. doi: 10.1093/genetics/79.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattman S., Gold E., Plotnik A. Methylation of cytosine residues in DNA controlled by a drug resistance factor (host-induced modification-R factors-N 6 -methyladenine-5-methylcytosine). Proc Natl Acad Sci U S A. 1972 Jan;69(1):187–190. doi: 10.1073/pnas.69.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Detection of avian tumor virus RNA in uninfected chicken embryo cells. J Virol. 1973 Feb;11(2):157–167. doi: 10.1128/jvi.11.2.157-167.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Kontomichalou P., Smith J. T. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1974 Jan;117(1):56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E., Datta N., Coetzee J. N. Properties of plasmids produced by recombination between R factors of groups J and FII. Mol Gen Genet. 1975 Oct 22;140(4):289–302. doi: 10.1007/BF00267320. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E. Transposition of ampicillin resistance from RP4 to other replicons. Mol Gen Genet. 1974;132(1):31–40. doi: 10.1007/BF00268228. [DOI] [PubMed] [Google Scholar]
- Hedgpeth J., Goodman H. M., Boyer H. W. DNA nucleotide sequence restricted by the RI endonuclease. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3448–3452. doi: 10.1073/pnas.69.11.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D., Rosenberg S. L. The evolution of bacterial enzyme systems. Annu Rev Microbiol. 1970;24:429–462. doi: 10.1146/annurev.mi.24.100170.002241. [DOI] [PubMed] [Google Scholar]
- Henneberry R. C., Carlton B. C. Characterization of the polydisperse closed circular deoxyribonucleic acid molecules of Bacillus megaterium. J Bacteriol. 1973 May;114(2):625–631. doi: 10.1128/jb.114.2.625-631.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoess R. H., Herman R. K. Isolation and characterization of mutator strains of Escherichia coli K-12. J Bacteriol. 1975 May;122(2):474–484. doi: 10.1128/jb.122.2.474-484.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J. J. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R. D. Models of genetic recombination. Annu Rev Microbiol. 1974;28(0):445–468. doi: 10.1146/annurev.mi.28.100174.002305. [DOI] [PubMed] [Google Scholar]
- Hourcade D., Dressler D., Wolfson J. The amplification of ribosomal RNA genes involves a rolling circle intermediate. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2926–2930. doi: 10.1073/pnas.70.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe M. M., Bade E. G. Molecular biology of bacteriophage mu. Science. 1975 Nov 14;190(4215):624–632. doi: 10.1126/science.1103291. [DOI] [PubMed] [Google Scholar]
- Howe M. M. Prophage deletion mapping of bacteriophage Mu-1. Virology. 1973 Jul;54(1):93–101. doi: 10.1016/0042-6822(73)90118-9. [DOI] [PubMed] [Google Scholar]
- Hsiung G. D., Fong C. K., Evans C. H. Prevalence of endogenous oncornavirus in guinea pigs. Intervirology. 1974;3(5-6):319–331. doi: 10.1159/000149769. [DOI] [PubMed] [Google Scholar]
- Hsu M. T., Davidson N. Electron microscope heteroduplex study of the heterogeneity of Mu phage and prophage DNA. Virology. 1974 Mar;58(1):229–239. doi: 10.1016/0042-6822(74)90157-3. [DOI] [PubMed] [Google Scholar]
- Hu S., Ohtsubo E., Davidson N. Electron microscopic heteroduplex studies of sequence relations among plasmids of Escherichia coli: structure of F13 and related F-primes. J Bacteriol. 1975 May;122(2):749–763. doi: 10.1128/jb.122.2.749-763.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Otsubo E., Davidson N., Saedler H. Electron microscope heteroduplex studies of sequence relations among bacterial plasmids: identification and mapping of the insertion sequences IS1 and IS2 in F and R plasmids. J Bacteriol. 1975 May;122(2):764–775. doi: 10.1128/jb.122.2.764-775.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Ptashne K., Cohen S. N., Davidson N. alphabeta sequence of F is IS31. J Bacteriol. 1975 Aug;123(2):687–692. doi: 10.1128/jb.123.2.687-692.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Marmur J. Characterization of inducible bacteriophages in Bacillus licheniformis. J Virol. 1970 Feb;5(2):237–246. doi: 10.1128/jvi.5.2.237-246.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Kelloff G. J., Sarma P. S., Lane W. T., Turner H. C., Gilden R. V., Oroszlan S., Meier H., Myers D. D., Peters R. L. Group-specific antigen expression during embryogenesis of the genome of the C-type RNA tumor virus: implications for ontogenesis and oncogenesis. Proc Natl Acad Sci U S A. 1970 Sep;67(1):366–376. doi: 10.1073/pnas.67.1.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa Y., Ross J., Leder P. An association between globin messenger RNA and 60S RNA derived from Friend leukemia virus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1154–1158. doi: 10.1073/pnas.71.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Mitsuhashi S. A bacteriophage S1 derivative that transduces tetracycline resistance to Staphylococcus aureus. Virology. 1975 Dec;68(2):544–546. doi: 10.1016/0042-6822(75)90295-0. [DOI] [PubMed] [Google Scholar]
- Ioannou P. General model for the replication of double stranded DNA molecules. Nat New Biol. 1973 Aug 29;244(139):257–260. doi: 10.1038/newbio244257a0. [DOI] [PubMed] [Google Scholar]
- JACOB F. Transduction of lysogeny in Escherichia coli. Virology. 1955 Jul;1(2):207–220. doi: 10.1016/0042-6822(55)90017-9. [DOI] [PubMed] [Google Scholar]
- JONES L. M., McDUFF C. R., WILSON J. B. Phenotypic alterations in the colonial morphology of Brucella abortus due to a bacteriophage carrier state. J Bacteriol. 1962 Apr;83:860–866. doi: 10.1128/jb.83.4.860-866.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Duplication-translocations of tryptophan operon genes in Escherichia coli. J Bacteriol. 1973 Oct;116(1):33–40. doi: 10.1128/jb.116.1.33-40.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Grinter N. J. Plasmid RP4 as a vector replicor in genetic engineering. Nature. 1975 Jun 5;255(5508):504–506. doi: 10.1038/255504a0. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Wohlhieter J. A., Placek B. P., Sleet R. B., Baron L. S. Plasmid-determined ability of a Salmonella tennessee strain to ferment lactose and sucrose. J Bacteriol. 1976 Jan;125(1):385–386. doi: 10.1128/jb.125.1.385-386.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D., Sneath P. H. Genetic transfer and bacterial taxonomy. Bacteriol Rev. 1970 Mar;34(1):40–81. doi: 10.1128/br.34.1.40-81.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D., Dworkin M. Gene transfer to myxobacterium by Escherichia coli phage P1. Science. 1975 Feb 21;187(4177):653–654. doi: 10.1126/science.803710. [DOI] [PubMed] [Google Scholar]
- Kalter S. S., Helmke R. J., Heberling R. L., Panigel M., Fowler A. K., Strickland J. E., Hellman A. Brief communication: C-type particles in normal human placentas. J Natl Cancer Inst. 1973 Apr;50(4):1081–1084. doi: 10.1093/jnci/50.4.1081. [DOI] [PubMed] [Google Scholar]
- Kalter S. S., Helmke R. J., Panigel M., Heberling R. L., Felsburg P. J., Axelrod L. R. Observations of apparent C-type particles in baboon (Papio cynocephalus) placentas. Science. 1973 Mar 30;179(4080):1332–1333. doi: 10.1126/science.179.4080.1332. [DOI] [PubMed] [Google Scholar]
- Kerszman G., Glover S. W., Aronovitch J. The restriction of bacteriophage lambda in Escherichia coli strain w. J Gen Virol. 1967 Jul;1(3):333–347. doi: 10.1099/0022-1317-1-3-333. [DOI] [PubMed] [Google Scholar]
- Khoury G., Fareed G. C., Berry K., Martin M. A., Lee T. N., Nathans D. Characterization of a rearrangement in viral DNA: mapping of the circular simian virus 40-like DNA containing a triplication of a specific one-third of the viral genome. J Mol Biol. 1974 Aug 5;87(2):289–301. doi: 10.1016/0022-2836(74)90150-8. [DOI] [PubMed] [Google Scholar]
- Kimura M., Ohta T. Mutation and evolution at the molecular level. Genetics. 1973 Apr;73(Suppl):19–35. [PubMed] [Google Scholar]
- Kimura M. The rate of molecular evolution considered from the standpoint of population genetics. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1181–1188. doi: 10.1073/pnas.63.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. L., Jukes T. H. Non-Darwinian evolution. Science. 1969 May 16;164(3881):788–798. doi: 10.1126/science.164.3881.788. [DOI] [PubMed] [Google Scholar]
- King M. C., Wilson A. C. Evolution at two levels in humans and chimpanzees. Science. 1975 Apr 11;188(4184):107–116. doi: 10.1126/science.1090005. [DOI] [PubMed] [Google Scholar]
- Kitajima E. W., Lauritis J. A. Plant virions in plasmodesmata. Virology. 1969 Apr;37(4):681–685. doi: 10.1016/0042-6822(69)90288-8. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Chan R. K., Tye B. K., Botstein D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J Mol Biol. 1975 Oct 5;97(4):561–575. doi: 10.1016/s0022-2836(75)80059-3. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Miller C. A mechanism for keeping mutations in check. J Theor Biol. 1965 Jan;8(1):71–80. doi: 10.1016/0022-5193(65)90093-7. [DOI] [PubMed] [Google Scholar]
- Kolata G. B. Evolution of DNA: Changes in Gene Regulation. Science. 1975 Aug 8;189(4201):446–447. doi: 10.1126/science.189.4201.446. [DOI] [PubMed] [Google Scholar]
- Kramer F. R., Mills D. R., Cole P. E., Nishihara T., Spiegelman S. Evolution in vitro: sequence and phenotype of a mutant RNA resistant to ethidium bromide. J Mol Biol. 1974 Nov 15;89(4):719–736. doi: 10.1016/0022-2836(74)90047-3. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J. Cell genetics and hereditary symbiosis. Physiol Rev. 1952 Oct;32(4):403–430. doi: 10.1152/physrev.1952.32.4.403. [DOI] [PubMed] [Google Scholar]
- LI K., BARKSDALE L., GARMISE L. Phenotypic alterations associated with the bacteriophage carrier state of Shigella dysenteriae. J Gen Microbiol. 1961 Mar;24:355–367. doi: 10.1099/00221287-24-3-355. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., FRASER D. K., ADAMS J. N., BURROUS J. W. Lysogenization, transduction and genetic recombination in bacteria. Cold Spring Harb Symp Quant Biol. 1958;23:71–82. doi: 10.1101/sqb.1958.023.01.010. [DOI] [PubMed] [Google Scholar]
- Lanks K. W., Kitchin F. D. Conservative mutations in homologous proteins. Nature. 1970 May 23;226(5247):753–754. doi: 10.1038/226753a0. [DOI] [PubMed] [Google Scholar]
- Lanni Y. T. First-step-transfer deoxyribonucleic acid of bacteriophage T5. Bacteriol Rev. 1968 Sep;32(3):227–242. doi: 10.1128/br.32.3.227-242.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Otsubo E., Deonier R. C., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. V. ilv+ Deletion mutants of F14. J Mol Biol. 1974 Nov 15;89(4):585–597. doi: 10.1016/0022-2836(74)90037-0. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J., Benveniste R. E., Callahan R., Coon H. G. Isolation from the asian mouse Mus caroli of an endogenous type C virus related to infectious primate type C viruses. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2315–2319. doi: 10.1073/pnas.72.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. An N-glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3649–3653. doi: 10.1073/pnas.71.9.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. New class of enzymes acting on damaged DNA. Nature. 1976 Jan 1;259(5538):64–66. doi: 10.1038/259064a0. [DOI] [PubMed] [Google Scholar]
- Lovett P. S., Bramucci M. G. Biochemical studies of two Bacillus pumilus plasmids. J Bacteriol. 1974 Oct;120(1):488–494. doi: 10.1128/jb.120.1.488-494.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Bramucci M. G. Plasmid deoxyribonucleic acid in Bacillus subtilis and Bacillus pumilus. J Bacteriol. 1975 Oct;124(1):484–490. doi: 10.1128/jb.124.1.484-490.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Burdick B. D. Cryptic plasmid in Bacillus pumilus ATCC 7065. Biochem Biophys Res Commun. 1973 Sep 5;54(1):365–370. doi: 10.1016/0006-291x(73)90931-5. [DOI] [PubMed] [Google Scholar]
- Lovett P. S. PBPI: a flagella specific bacteriophage mediating transduction in Bacillus pumilus. Virology. 1972 Mar;47(3):743–752. doi: 10.1016/0042-6822(72)90564-8. [DOI] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhee D. G. DNA polymerase activity determined by the ultraviolet-protecting plasmid, R-Utrecht. Nature. 1974 Oct 4;251(5474):432–434. doi: 10.1038/251432a0. [DOI] [PubMed] [Google Scholar]
- Majumdar C., Dewey M., Frankel F. R. Bacteriophage-directed association of DNA polymerase 1 with host membrane: a dispensable function. Virology. 1972 Jul;49(1):134–144. doi: 10.1016/s0042-6822(72)80015-1. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M., Barrell B. G., Donelson J. Sequence of a repressor-binding site in the DNA of bacteriophage lamda. Nature. 1974 Aug 2;250(465):394–397. doi: 10.1038/250394a0. [DOI] [PubMed] [Google Scholar]
- Martuscelli J., Taylor A. L., Cummings D. J., Chapman V. A., DeLong S. S., Cañedo L. Electron microscopic evidence for linear insertion of bacteriophage MU-1 in lysogenic bacteria. J Virol. 1971 Oct;8(4):551–563. doi: 10.1128/jvi.8.4.551-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Barksdale L. System for the investigation of the bacteriophage-directed synthesis of diphtherial toxin. J Bacteriol. 1967 Feb;93(2):722–730. doi: 10.1128/jb.93.2.722-730.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Uetake H. Chromosomal locations of Salmonella conversion phages: mapping of prophages g 341, 15, and 34 in Salmonella anatum. Virology. 1972 Aug;49(2):359–367. doi: 10.1016/0042-6822(72)90488-6. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R., Heywood J. Restriction and modification of DNA. Annu Rev Biochem. 1972;41:447–466. doi: 10.1146/annurev.bi.41.070172.002311. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. R., Kramer F. R., Spiegelman S. Complete nucleotide sequence of a replicating RNA molecule. Science. 1973 Jun 1;180(4089):916–927. doi: 10.1126/science.180.4089.916. [DOI] [PubMed] [Google Scholar]
- Min Jou W., Haegeman G., Ysebaert M., Fiers W. Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein. Nature. 1972 May 12;237(5350):82–88. doi: 10.1038/237082a0. [DOI] [PubMed] [Google Scholar]
- Monahan J. J., Hall R. H. Chromatin, and gene regulation in eukaryotic cells at the transcriptional level. CRC Crit Rev Biochem. 1974 Jan;2(1):67–112. doi: 10.3109/10409237409105444. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Berg P. Cleavage of Simian virus 40 DNA at a unique site by a bacterial restriction enzyme. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3365–3369. doi: 10.1073/pnas.69.11.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. F., Cohen S. N., Chang A. C., Boyer H. W., Goodman H. M., Helling R. B. Replication and transcription of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1974 May;71(5):1743–1747. doi: 10.1073/pnas.71.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourant A. E. Transduction and skeletal evolution. Nature. 1971 Jun 18;231(5303):466–467. doi: 10.1038/231466a0. [DOI] [PubMed] [Google Scholar]
- Mukherjee B. B., Mobry P. M. Variations in hybridization of RNA from different mouse tissues and embryos to endogenous C-type virus DNA transcripts. J Gen Virol. 1975 Jul;28(1):129–135. doi: 10.1099/0022-1317-28-1-129. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Murray K. Manipulation of restriction targets in phage lambda to form receptor chromosomes for DNA fragments. Nature. 1974 Oct 11;251(5475):476–481. doi: 10.1038/251476a0. [DOI] [PubMed] [Google Scholar]
- Muzyczka N., Poland R. L., Bessman M. J. Studies on the biochemical basis of spontaneous mutation. I. A comparison of the deoxyribonucleic acid polymerases of mutator, antimutator, and wild type strains of bacteriophage T4. J Biol Chem. 1972 Nov 25;247(22):7116–7122. [PubMed] [Google Scholar]
- Mäkelä P. H. Genetic determination of the O antigens of Salmonella groups B (4,5,12) and C1(6,7). J Bacteriol. 1966 Mar;91(3):1115–1125. doi: 10.1128/jb.91.3.1115-1125.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK A., SZILARD L. Virus strains of identical phenotype but different genotype. Science. 1951 Jan 12;113(2924):34–35. doi: 10.1126/science.113.2924.34. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Bacterial ribosome. Bacteriol Rev. 1970 Sep;34(3):228–277. doi: 10.1128/br.34.3.228-277.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Morse S. I. In vivo transmission of drug resistance factors between strains of Staphylococcus aureus. J Exp Med. 1967 Jan 1;125(1):45–59. doi: 10.1084/jem.125.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T. Slightly deleterious mutant substitutions in evolution. Nature. 1973 Nov 9;246(5428):96–98. doi: 10.1038/246096a0. [DOI] [PubMed] [Google Scholar]
- Olivera R. M., Bonhoeffer E. Replication of Escherichia coli requires DNA polymerase I. Nature. 1974 Aug 9;250(5466):513–514. doi: 10.1038/250513a0. [DOI] [PubMed] [Google Scholar]
- Olsen R. H., Shipley P. Host range and properties of the Pseudomonas aeruginosa R factor R1822. J Bacteriol. 1973 Feb;113(2):772–780. doi: 10.1128/jb.113.2.772-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi Y. F factor promotes turnover of stable RNA in escherichia coli. Science. 1975 Jan 24;187(4173):257–258. doi: 10.1126/science.1089310. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Roesler G., Krieg C. J., Kind J., Cole T., Crozier T., Gaedicke G., Steinheider G., Kluge N., Dube S. Induction of endogenous virus and of thymidine kinase by bromodeoxyuridine in cell cultures transformed by Friend virus. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4980–4985. doi: 10.1073/pnas.71.12.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota T. Mutational pressure as the main cause of molecular evolution and polymorphism. Nature. 1974 Nov 29;252(5482):351–354. doi: 10.1038/252351a0. [DOI] [PubMed] [Google Scholar]
- Otsubo E., Deonier R. C., Lee H. J., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. IV. The F sequences in F14. J Mol Biol. 1974 Nov 15;89(4):565–584. doi: 10.1016/0022-2836(74)90036-9. [DOI] [PubMed] [Google Scholar]
- Palchoudhury S. R., Iyer V. N. Non-essentiality of the recA- mutation in the phenomenon of bacteriophage M13-induced elimination of F' factors. J Bacteriol. 1971 Jun;106(3):1040–1042. doi: 10.1128/jb.106.3.1040-1042.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Livingston D. M., Todaro G. J., Benveniste R. E., Scolnick E. M. Radioimmunoassay of mammalian type C viral proteins. 3. Detection of viral antigen in normal murine cells and tissues. J Exp Med. 1973 Mar 1;137(3):622–635. doi: 10.1084/jem.137.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne K., Cohen S. N. Occurrence of insertion sequence (IS) regions on plasmid deoxyribonucleic acid as direct and inverted nucleotide sequence duplications. J Bacteriol. 1975 May;122(2):776–781. doi: 10.1128/jb.122.2.776-781.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS P. W., KELLER J. M., WRIGHT A., BERNSTEIN R. L. ENZYMATIC AND KINETIC STUDIES ON THE MECHANISM OF O-ANTIGEN CONVERSION BY BACTERIOPHAGE EPSILON-15. J Biol Chem. 1965 Jan;240:384–390. [PubMed] [Google Scholar]
- Reanney D. C. A regulatory role for viral RNA in eukaryotes. J Theor Biol. 1975 Feb;49(2):461–492. doi: 10.1016/0022-5193(75)90186-1. [DOI] [PubMed] [Google Scholar]
- Reanney D. C. On the origin of prokaryotes. J Theor Biol. 1974 Nov;48(1):243–251. doi: 10.1016/0022-5193(74)90194-5. [DOI] [PubMed] [Google Scholar]
- Reanney D. C. Viruses and evolution. Int Rev Cytol. 1974;37(0):21–52. doi: 10.1016/S0074-7696(08)61356-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M. S., Matthews R. E. On the origin of the mosaic induced by turnip yellow mosaic virus. Virology. 1966 Apr;28(4):563–570. doi: 10.1016/0042-6822(66)90241-8. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Chakrabarty A. M., Gunsalus I. C. A transmissible plasmid controlling camphor oxidation in Pseudomonas putida. Proc Natl Acad Sci U S A. 1973 Mar;70(3):885–889. doi: 10.1073/pnas.70.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond R. C. Non-Darwinian evolution: a critique. Nature. 1970 Mar 14;225(5237):1025–1028. doi: 10.1038/2251025a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Burleigh B. D., Jr, Hartley B. S. Gene duplication in experimental enzyme evolution. Nature. 1974 Sep 20;251(5472):200–204. doi: 10.1038/251200a0. [DOI] [PubMed] [Google Scholar]
- Romig W. R. Infectivity of Bacillus subtilis bacteriophage deoxyribonucleic acids extracted from mature particles and from lysogenic hosts. Bacteriol Rev. 1968 Dec;32(4 Pt 1):349–357. [PMC free article] [PubMed] [Google Scholar]
- Russell R. L., Abelson J. N., Landy A., Gefter M. L., Brenner S., Smith J. D. Duplicate genes for tyrosine transfer RNA in Escherichia coli. J Mol Biol. 1970 Jan 14;47(1):1–13. doi: 10.1016/0022-2836(70)90397-9. [DOI] [PubMed] [Google Scholar]
- SEAMAN E., TARMY E., MARMUR J. INDUCIBLE PHAGES OF BACILLUS SUBTILIS. Biochemistry. 1964 May;3:607–613. doi: 10.1021/bi00893a001. [DOI] [PubMed] [Google Scholar]
- Saunders G. F., Campbell L. L. Characterization of a thermophilic bacteriophage for Bacillus stearothermophilus. J Bacteriol. 1966 Jan;91(1):340–348. doi: 10.1128/jb.91.1.340-348.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwesinger M. D., Novick R. P. Prophage-dependent plasmid integration in Staphylococcus aureus. J Bacteriol. 1975 Aug;123(2):724–738. doi: 10.1128/jb.123.2.724-738.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seman G., Levy B. M., Panigel M., Dmochowski L. Type-C virus particles in placenta of the cottontop marmoset (Saguinus oedipus). J Natl Cancer Inst. 1975 Jan;54(1):251–252. doi: 10.1093/jnci/54.1.251. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Geelen J. L. Detection of DNA complementary to pathogenic viroid RNA in exocortis disease. Nature. 1975 Aug 28;256(5520):753–756. doi: 10.1038/256753a0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Signer E. R. Lysogeny: the integration problem. Annu Rev Microbiol. 1968;22:451–488. doi: 10.1146/annurev.mi.22.100168.002315. [DOI] [PubMed] [Google Scholar]
- Sprague G. F., McKinney H. H. Aberrant ratio: an anomaly in maize associated with virus infection. Genetics. 1966 Dec;54(6):1287–1296. doi: 10.1093/genetics/54.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., McKinney H. H. Further evidence on the genetic behavior of ar in maize. Genetics. 1971 Apr;67(4):533–542. doi: 10.1093/genetics/67.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Stodolsky M. Recipient gene duplication during generalized transduction. Genetics. 1974 Nov;78(3):809–822. doi: 10.1093/genetics/78.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. S. Induction by mutagens of tandem gene duplications in the glyS region of the Escherichia coli chromosome. Genetics. 1974 Nov;78(3):823–830. doi: 10.1093/genetics/78.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Richmond M. H. Intergeneric transfer of a beta-lactamase gene between Ps. aeruginosa and E. coli. Nature. 1970 Jun 6;226(5249):952–954. doi: 10.1038/226952a0. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUTNER T. A., SWARTZ M. N., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. X. Influence of bromouracil substitutions on replication. Proc Natl Acad Sci U S A. 1962 Mar 15;48:449–455. doi: 10.1073/pnas.48.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. On the origin of RNA tumor viruses. Annu Rev Genet. 1974;8:155–177. doi: 10.1146/annurev.ge.08.120174.001103. [DOI] [PubMed] [Google Scholar]
- Todaro G. J. Evolution and modes of transmission of RNA tumor viruses. Parke-Davis Award lecture. Am J Pathol. 1975 Dec;81(3):590–606. [PMC free article] [PubMed] [Google Scholar]
- Uetake H., Hagiwara S. Phenotypic mixing as a mechanism of conversion gene transfer. Virology. 1972 Jan;47(1):48–53. doi: 10.1016/0042-6822(72)90237-1. [DOI] [PubMed] [Google Scholar]
- Vasil I. K., Vasil V. Totipotency and embryogenesis in plant cell and tissue cultures. In Vitro. 1972 Nov-Dec;8(3):117–127. doi: 10.1007/BF02619487. [DOI] [PubMed] [Google Scholar]
- Wallace B., Kass T. L. On the structure of gene control regions. Genetics. 1974 Jul;77(3):541–558. doi: 10.1093/genetics/77.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West D. A. Does virus infection have evolutionary significance? Nature. 1971 Feb 26;229(5287):637–637. doi: 10.1038/229637a0. [DOI] [PubMed] [Google Scholar]
- Wilson A. C., Maxson L. R., Sarich V. M. Two types of molecular evolution. Evidence from studies of interspecific hybridization. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2843–2847. doi: 10.1073/pnas.71.7.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Palindromes in chromosomes. J Mol Biol. 1974 Mar 25;84(1):115–138. doi: 10.1016/0022-2836(74)90216-2. [DOI] [PubMed] [Google Scholar]
- Winocour E. Some aspects of the interaction between polyoma virus and cell DNA. Adv Virus Res. 1969;14:153–200. doi: 10.1016/s0065-3527(08)60559-x. [DOI] [PubMed] [Google Scholar]
- Wright A. Mechanism of conversion of the salmonella O antigen by bacteriophageepsilon 34. J Bacteriol. 1971 Mar;105(3):927–936. doi: 10.1128/jb.105.3.927-936.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiki T., Mellors R. C., Hardy W. D., Jr Common cell-surface antigen associated with murine and feline C-type RNA leukemia viruses. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1878–1882. doi: 10.1073/pnas.70.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourno J., Kohno T., Roth J. R. Enzyme evolution: generation of a bifunctional enzyme by fusion of adjacent genes. Nature. 1970 Nov 28;228(5274):820–824. doi: 10.1038/228820a0. [DOI] [PubMed] [Google Scholar]
- Zhdanov V. M., Tikchonenko T. I. Viruses as a factor of evolution: exchange of genetic information in the biosphere. Adv Virus Res. 1974;19:361–394. doi: 10.1016/s0065-3527(08)60664-8. [DOI] [PubMed] [Google Scholar]
- van der Vijver J. C., van Es-Boon M., Michel M. F. Lysogenic conversion in Staphylococcus aureus to leucocidin production. J Virol. 1972 Aug;10(2):318–319. doi: 10.1128/jvi.10.2.318-319.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


