Abstract
Recently, a novel mode of sulphur oxidation was described in marine sediments, in which sulphide oxidation in deeper anoxic layers was electrically coupled to oxygen reduction at the sediment surface. Subsequent experimental evidence identified that long filamentous bacteria belonging to the family Desulfobulbaceae likely mediated the electron transport across the centimetre-scale distances. Such long-range electron transfer challenges some long-held views in microbial ecology and could have profound implications for sulphur cycling in marine sediments. But, so far, this process of electrogenic sulphur oxidation has been documented only in laboratory experiments and so its imprint on the seafloor remains unknown. Here we show that the geochemical signature of electrogenic sulphur oxidation occurs in a variety of coastal sediment environments, including a salt marsh, a seasonally hypoxic basin, and a subtidal coastal mud plain. In all cases, electrogenic sulphur oxidation was detected together with an abundance of Desulfobulbaceae filaments. Complementary laboratory experiments in intertidal sands demonstrated that mechanical disturbance by bioturbating fauna destroys the electrogenic sulphur oxidation signal. A survey of published geochemical data and 16S rRNA gene sequences identified that electrogenic sulphide oxidation is likely present in a variety of marine sediments with high sulphide generation and restricted bioturbation, such as mangrove swamps, aquaculture areas, seasonally hypoxic basins, cold sulphide seeps and possibly hydrothermal vent environments. This study shows for the first time that electrogenic sulphur oxidation occurs in a wide range of marine sediments and that bioturbation may exert a dominant control on its natural distribution.
Keywords: bioturbation, Desulfobulbaceae, electrobiogeochemistry, electrogenic sulphur oxidation, sulphur oxidizing bacteria
Introduction
Sulphate reduction is the dominant mineralization pathway in coastal marine sediments, and leads to an accumulation of sulphide in deeper sediment horizons (Jørgensen, 1982). Various respiratory pathways of microbial sulphur oxidation have evolved as a means of harvesting this stored chemical energy (Jørgensen and Nelson, 2004). Recently, a novel sulphur oxidation process, herein called electrogenic sulphur oxidation, was discovered whereby the oxidation of free sulphide in deeper sediment horizons was observed to be directly coupled to reduction of oxygen near the sediment surface (Nielsen et al., 2010). Such a spatial separation of redox half-reactions indicates there must be a direct pathway by which electrons originating from sulphide are conducted across centimetre-scale distances to oxygen (Nielsen et al, 2010; Risgaard-Petersen et al., 2012). Experimental manipulation revealed that this electrical connection responded immediately (that is, within minutes) to oxygen depletion in the overlying water, and so the connection was too fast to be explained by diffusion of redox-active shuttles (Nielsen et al., 2010). Metal-reducing bacteria are known to transport electrons externally via cell surface cytochromes, redox shuttles or conductive pili (Reguera et al., 2005; Gorby et al., 2006; Lovley, 2008; Clarke et al., 2011), and such extracellular electron transport can bridge ∼100 micrometer distances in electrode biofilms (Reguera et al., 2006; Logan and Rabaey, 2012). The electron transport associated with this electrogenic sulphur oxidation, by contrast, operates on scales of centimetres, thereby extending the known length scale of microbially mediated electron transport by two orders of magnitude.
Electrogenic sulphur oxidation creates a unique geochemical signal in surface sediments, which is detectable by microsensor profiling. A key feature is that the cathodic half-reaction (that is, O2+4e−+4H+→2H2O) consumes protons, thereby creating a pH maximum in the oxic zone. Deeper in the sediment, protons are released by an anodic half-reaction (that is, electrogenic sulphide oxidation; such as ½H2S+2H2O→½SO42−+4e−+5H+) creating a broad pH minimum near the sulphide horizon, separated from the oxic zone by a suboxic zone ranging from several millimetres to approximately 2 cm wide. In laboratory experiments, the development of this characteristic geochemical fingerprint has been used as a sign of electrogenic sulphur oxidation activity (Nielsen et al., 2010; Pfeffer et al., 2012; Risgaard-Petersen et al., 2012).
Compelling evidence indicates that electrogenic sulphur oxidation is likely carried out by long filamentous bacteria. First, in sediments with the geochemical signature of this process, detailed microscopic investigation revealed high densities of long multicellular filamentous bacteria, belonging to the family Desulfobulbaceae, which were spanning the entire length of the suboxic zone (Pfeffer et al., 2012). Second, when a thin wire was passed horizontally through the suboxic zone, the electric circuitry was effectively ‘cut', as indicated by an immediate decrease in sediment oxygen consumption by a factor of 2, and a decrease in the signature pH maximum. This experiment identified that the electron conduction occurred along a continuous structure spanning the suboxic zone (Pfeffer et al., 2012). Thirdly, when sediment was experimentally incubated with filters of various pore sizes which were inserted horizontally, the geochemical signature of electrogenic sulphur oxidation developed only in cores that had filters with pore sizes large enough for Desulfobulbaceae filaments to penetrate (Pfeffer et al., 2012). Taken together, these experiments provide strong evidence that electrogenic sulphur oxidation is carried out by Desulfobulbaceae filaments.
The capacity of Desulfobulbaceae filaments to oxidize sulphur by long-range electron transport represents an entirely novel microbial lifestyle (Pfeffer et al., 2012). By conducting electrons across centimetre-scale distances, these filamentous bacteria appear capable of enabling a previously unrecognized strategy for resource competition in marine sediments (Nielsen et al., 2010; Risgaard-Petersen et al., 2012). Yet until now, electrogenic sulphur oxidation has only been induced in laboratory experiments that have used sieved and homogenized sediment from a deep sediment horizon obtained from one particular location in the Baltic Sea (Nielsen et al., 2010; Pfeffer et al., 2012; Risgaard-Petersen et al., 2012). Our objective here is to identify whether electrogenic sulphur oxidation, together with Desulfobulbaceae filaments, are found in natural marine sediments. We further aim to identify major environmental constraints on the occurrence and distribution of electrogenic sulphur oxidation in marine sediments. Given the high growth rate and densities of these bacteria observed in laboratory experiments (Pfeffer et al., 2012; Risgaard-Petersen et al., 2012), we hypothesize that electrogenic sulphur oxidation is a competitively successful strategy in natural marine sediments and therefore may be widespread in nature.
Materials and methods
Site descriptions
The laboratory experiments of Nielsen et al. (2010) and Pfeffer et al. (2012) suggested that electrogenic sulphur oxidation may be competitively successful in coastal marine sediments with high rates of sulphide generation linked to high rates of organic matter mineralization. Our site selection therefore targeted four different coastal seafloor habitats in the southern North Sea area, three of which were in depositional areas and were particularly rich in organic matter. We examined intact sediments collected from these sites for geochemical evidence of electrogenic sulphur oxidation and for the presence of Desulfobulbaceae filaments. Our first site was Rattekaai Salt Marsh (RSM), an intertidal area heavily loaded with organic matter, including large periodic deposits of macroalgal detritus, which supports high rates of sulphate reduction (Oenema, 1988; Pallud and Van Cappellen, 2006; Table 1). We sampled the un-vegetated sediments in an intertidal drainage channel. At low tide, sulphide-rich porewater seeps out from these creek sediments. Large bioturbating animals were absent from these seepage areas, likely excluded by high levels of porewater sulphide. Our second site was Station 130 in the Belgian Coastal Zone (BCZ), a subtidal site at 12 m water depth (Table 1). This site is characterized by high accumulation rates of muddy deposits which generate intense sulphate reduction and free sulphide was previously detected below a suboxic zone of several centimetres (Gao et al., 2009). A monthly survey in 2011 revealed that large bioturbating macrofauna is generally absent from this site (Braeckman et al., 2014), and no macrofauna was detected in our collected sediment cores. Our third site was Marine Lake Grevelingen (MLG), a seasonally stratified coastal marine system in the Delta area of The Netherlands which experiences bottom water hypoxia and anoxia in the summer in its deep basins (Table 1). We collected sediments from the deepest area of two steep-sided basins (Holpervoet basin at 23 m; Den Osse basin at 35 m water depth). These basins rapidly accumulate organic rich deposits (>2 cm per year) due to focussing of detrital material. At the time of sampling (winter), the water column was fully oxygenated, but even so, macrofauna is never abundant in these seasonally anoxic sediments and no macrofauna was present in our retrieved sediment cores. The fourth site was an intertidal sand flat in the Oosterschelde tidal inlet (Delta area, The Netherlands). Unlike the first three sites, the Oosterschelde Sand Flats (OSF) support high rates of bioturbation, most notably by the lugworm Arenicola marina (Coosen et al., 1994). Free sulphide concentrations are low to non-detectable (<20 μmol l−1) in at least the top 2.5 cm of the sediment. All sites were visited on two or three occasions (Table 1).
Table 1. The geochemical fingerprint of electrogenic sulphur oxidation was observed at three sites in the coastal North Sea under field conditions.
| Site name | Coordinates | Dates of sampling | OPD (mm) | DOU (mmol m−2 d−1) | Current density (mA m−2) | O2 consumed by cathodic reaction (%) |
|---|---|---|---|---|---|---|
| RSM, Oosterschelde, The Netherlands | N 51° 26′ 21′′ E 04° 10′ 11′′ | 22 June 2011 25 February 2013 | 0.7±0.1 1.3±0.4 | 30.3±3.6 19.9±2.2 | 29.9±10.7 7.3±4.0 | 22.7±10.1 8.2±4.3 |
| Station 130, BCZ | N 51° 16′ 13′′ E 02° 54′ 19′′ | 10 October 2011 1 March 2013 | 0.7±0.1 1.2±0.8 | 32.3±15.0 24.8±20.9 | 25.2±2.6 4.6±1.8 | 19.1±7.1 5.3±2.9 |
| MLG, Holpervoet and Den Osse basins, The Netherlands | N 51° 46′ 24′′ E 03° 56′ 16′′ | 3 November 2011 22 January 2012 24 February 2012 | 0.9±0.2 1.4±0.4 1.8±0.4 | 44.1±16.1 13.9±4.1 22.2±9.5 | 24.8±5.5 16.1±10.4 27.0±16.2 | 14.0±7.9 29.5±25.3 33.7±30.6 |
| OSF, The Netherlands | N 51° 26′ 52′′ E 04° 05′ 47′′ | 17 May 2011 15 August 2011 | 1.6±0.3 1.5±0.3 | 4.8±1.6 3.5±0.5 | NA | NA |
Abbreviations: BCZ, Belgian Coastal Zone; DOU, diffusive oxygen uptake across the sediment–water interface; MLG, Marine Lake Grevelingen; OPD, Oxygen Penetration Depth; OSF, Oosterschelde sand flats; RSM, Rattekaai Salt Marsh.
Values indicate means of between 2 and 4 replicate cores. Reported error is standard deviation between replicate cores.
The intertidal sediment samples (that is, RSM and OSF) were collected by manually inserting core tubes (4 cm diameter) in the sediment at low tide. The sediment from MLG was retrieved with a Uwitec gravity corer (6 cm diameter). On the first occasion, sediment from the BCZ was collected with a Reineck box corer followed by careful subsampling with a 10 cm diameter core tube. On the second occasion, sediment from this site was collected with a Uwitec gravity corer. Retrieved cores were closely inspected and only cores with an undisturbed sediment surface were kept for the study. Microprofiling was either conducted immediately on-board the sailing vessel (BCZ and MLG in 2011 only), or sediment cores were returned to a nearby laboratory where microprofiling was conducted under climate-controlled conditions. Microprofiling was conducted between 2 and 12 h after core collection under darkened conditions. The intertidal sediments from RSM and OSF were kept in the dark for at least 6 h prior to microprofiling to ensure there was no effect of photosynthesis on the pH profiles.
Microelectrode profiling and reactive transport modelling
Sediment profiling was performed using commercial microsensors operated with a motorized micromanipulator (Unisense A.S., Aarhus, Denmark). Sediment was brought level to the core liner surface. Cores were placed in an aquarium containing water collected from the study site and held at in situ temperature and constantly bubbled with air. During microsensor profiling, an airstream was additionally provided at the water surface upstream of the sediment cores to ensure constant water flow over the sediment, estimated at 2–4 cm s−1 at 0.5 cm above the sediment surface. Oxygen and H2S microelectrodes had a tip diameter of 50 μm, and pH microsensors had a tip diameter of 100 or 200 μm. Vertical oxygen microprofiles were recorded at 100 μm steps, beginning at least 1 mm above the sediment–water interface. A two-point O2 calibration was made in air-saturated seawater and the anoxic zone of the sediment (100% and 0%, respectively). Vertical H2S and pH microprofiles were made concurrently, recorded at 200 μm steps in the oxic zone, and 200 or 400 μm steps below. For H2S, a five-point standard curve was made using Na2S standards. ΣH2S was calculated from H2S based on pH measured at the same depth using the R package AquaEnv (Hofmann et al., 2010). For pH, a three-point calibration using NBS standard buffers was made, followed by a salt correction with TRIS buffer (Dickson et al., 2007). pH measurements were performed with a Ag/AgCl reference electrode and values are reported on the total scale. Porosity was determined from water content and dry density measurements upon drying to constant weight at 60 °C.
Reactive transport modelling was used to calculate reaction rates from the microsensor data (Table 1). The diffusive oxygen uptake (DOU) of the sediment was calculated from the oxygen depth profile near the sediment–water interface using Fick's first law as below:
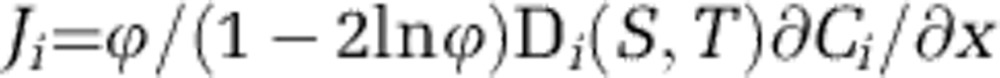 |
where ϕ is the measured porosity, the term (1−2ln ϕ) is a correction for sediment tortuosity (Boudreau, 1996), and Di is the diffusion coefficient for oxygen calculated at salinity (S) and temperature (T) calculated using the R package marelac (Soetaert et al., 2012). The maximum concentration gradient, ∂Ci/∂x, was calculated as the linear slope immediately below the sediment–water interface.
The cathodic proton consumption (CPC) is the proton consumption corresponding to the cathodic half-reaction, O2+4e−+4H+→2H2O. The CPC was calculated as the sum of the upward and downward alkalinity fluxes immediately above and below the pH maximum in the oxic zone. Alkalinity fluxes were calculated as the sum of the fluxes of the major individual porewater compounds that contribute to the alkalinity:
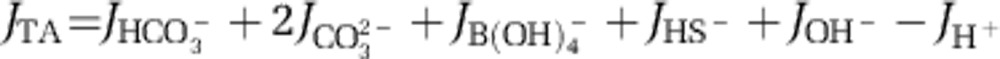 |
The speciation of individual ions (carbonate, borate, sulphide and water equilibria) were calculated from H2S and pH microsensor data using the R package AquaEnv (Hoffman et al., 2010). For this calculation, we assumed a constant depth profile for dissolved inorganic carbon and borate. The contribution of other macronutrients (ammonium, silicate) to alkalinity fluxes are likely small and were not considered in this study.
The concentration gradient ∂Ci/∂x for each individual species was estimated by a noise robust numerical differentiation using the Savitsky–Golay algorithm, optimized with a N=21 point stencil (Savitsky and Golay, 1964). The current density was calculated from the CPC using a conversion factor of one electron per proton (1A=1.036 × 10−5 mol e− s−1). The percentage, p, of oxygen consumed electrochemically was calculated as P=25 (CPC/DOU), based on a reaction stoichiometry that four moles of H+ are consumed per mole of O2 by the cathodic reaction. The remaining part of the oxygen consumption (1−p) is attributed to aerobic respiration, which does not consume or release alkalinity. This simplified model assumes that no other biogeochemical reactions are releasing or consuming alkalinity within the oxic zone. In reality, some alkalinity consumption will take place, associated with nitrification, ferrous iron oxidation, calcium carbonate precipitation or direct sulphur oxidation by oxygen (Risgaard-Petersen et al., 2012). The rates of these reactions cannot be estimated from microsensor profiles. However, correcting for these reactions would increase the value of p. Accordingly, the p estimates derived here should be interpreted as conservative, lower bounds on the oxygen that is consumed electrochemically.
Identification of Desulfobulbaceae filaments
Microscopic identification of Desulfobulbaceae filaments was achieved by catalysed reporter deposition–fluorescence in situ hybridization (CARD–FISH; Pernthaler et al., 2002), using a Desulfobulbaceae-specific oligonucleotide probe (DSB706; 5′-ACC CGT ATT CCT CCC GAT-3′) labelled with horseradish peroxidase (Lücker et al., 2007). Sediment samples were fixed with a 1:1 (vol/vol) ethanol solution and stored at −20 °C until analysis. Hundred microlitres of the preserved sample sediment slurry was pipetted onto polycarbonate membrane filters (0.2 μm pore size). The retained filamentous cells were permeabilized with lysozyme (1 h at 37 °C) and treated with achromopeptidase (30 min at 37 °C). Endogenous peroxidases were inactivated with a methanol and peroxide solution (30 min at 20 °C), and subsequently hybridized in standard hybridization buffer (46 °C) with 45% formamide. CARD–FISH was also performed with probes EUB388-I and NONEUB388 (with 10% formamide) as positive and negative controls, respectively. Subsequently, hybridized cells were visualized using epifluorescence microscopy (Axioplan II Imaging, Zeiss, Jena, Germany). Desulfobulbaceae filaments were additionally quantified from suboxic zone (0.3–0.8 cm) sediment sections obtained from RSM, BCZ and OSF, using the FISH probe as above. Filament length was measured in 200 random microscopic fields. Sediment samples from MLG enabled filament identification, but could not be used for enumeration.
Specimens for scanning electron microscopy (SEM) were prepared by isolating filaments with a hook made from flame-drawn pasteur pipettes under a light microscope ( × 40) and transferring these filaments to polycarbonate filters (pore size 5 μm). Filaments retained on filters were then transferred through a gradual ethanol gradient (25–100%), critical point dried with liquid CO2, mounted on stubs and coated with gold. Images were made with a JEOL 5600 SEM under low-vacuum, operated at 30 kV.
Partial 16S rRNA gene sequences of the Desulfobulbaceae filaments were recovered by constructing 16S rRNA cDNA clone libraries using suboxic zone sections of sediments (0.3–0.8 cm) in which the geochemical fingerprint of electrogenic sulphide oxidation was detected. RNA was extracted with a ZR Fungal/Bacterial RNA Miniprep kit (Zymo Research, Irvine, CA, USA) and reverse-transcribed using random primers. The 16S rRNA gene was amplified by PCR using general bacterial primers (Bac F968.60 and Bac R140.10), cloned into Escherichia coli by one shot chemical transformation and sequenced using a genetic analyzer (3130 Genetic Analyzer, Applied Biosystems, Grand Island, NY, USA). Sequences were aligned with the software ARB (Ludwig et al., 2004) against the SILVA database. A phylogenetic tree was constructed in ARB by maximum parsimony, with 1000 bootstrap iterations. The software Pintail was used to check for chimeras (Ashelford et al., 2005). Related 16S rRNA gene sequences were obtained by BLAST searches of GenBank.
Experiments with bioturbated sediments
In addition to the field study, we performed two laboratory experiments to investigate the role of bioturbation on the natural distribution of electrogenic sulphur oxidation in coastal marine sediments. Sediments collected from the heavily bioturbated OSF field site did not show evidence of electrogenic sulphur oxidation or a detectable presence of Desulfobulbaceae filaments on repeated visits (details in Results). In a first experiment, we tested whether the geochemical signature of electrogenic sulphur oxidation could develop in these sediments when bioturbation is removed. Sediments from the sand flats differed from the other three study sites in several ways, including a coarser grain size and lower water content, lower organic C content, lower oxygen consumption rates and lower free sulphide content to at least 2.5 cm depth. We first tested if these differences, independent of mechanical mixing by bioturbation, inhibited the development of the geochemical signature of electrogenic sulphur oxidation. Sediment was collected in May 2011, homogenized and defaunated by passing it through a 500 μm sieve. The sediments were then repacked into core tubes (4 cm diameter), with the sediment surface adjusted to be level with the top of the core liner. The cores were placed in an aquarium with water drawn from the study site and kept at 16 °C in a climate-controlled room. The aquarium was continuously bubbled to ensure full aeration, and water was replaced weekly. The potential development of electrogenic sulphur oxidation in these sediments was tracked by recording microsensor profiles in triplicate sediment cores every few days.
The first experiment confirmed that these sediments could develop the geochemical signature of electrogenic sulphur oxidation (see Results). Consequently, we then designed a second manipulation experiment to test if bioturbation specifically could halt electrogenic sulphur oxidation. Intact sediment cores were collected intact from OSF in August 2011. To accommodate bioturbating macrofauna, these cores were 15 cm diameter and had ∼20 cm sediment depth with 15 cm overlying water. Cores were incubated in a climate controlled room at 16 °C throughout the experiment. Cores were first defaunated by asphyxia, which was achieved by closing the cores with an air-tight sealing. After 10 days, the cores were re-opened and dead macrofauna (mostly the lugworm A. marina) was carefully removed from the sediment surface. These defaunated sediment cores were then incubated with fully aerated water, and we refer to this as Day 1 of the experiment. On Day 8, microsensor profiles were recorded, and revealed the geochemical signature of electrogenic sulphur oxidation. On Day 10, the dominant bioturbating animal (the lugworm A. marina) was collected from the OSF. Two lugworms were re-introduced to each sediment core, achieving approximately natural densities (∼100 m−2). Extensive microsensor profiling was conducted again on Day 15 (referred to as ‘re-faunated' cores). Vertical microsensor profiles were measured both in areas where the sediment was not visibly disturbed, and in areas where the sediment was heavily disturbed by the activities of the lugworms (that is, around the feeding pocket and the fecal casts). Extended experimental details are available in Rao et al. (in review).
Results
A geochemical signal of long-range electron transport in natural sediments
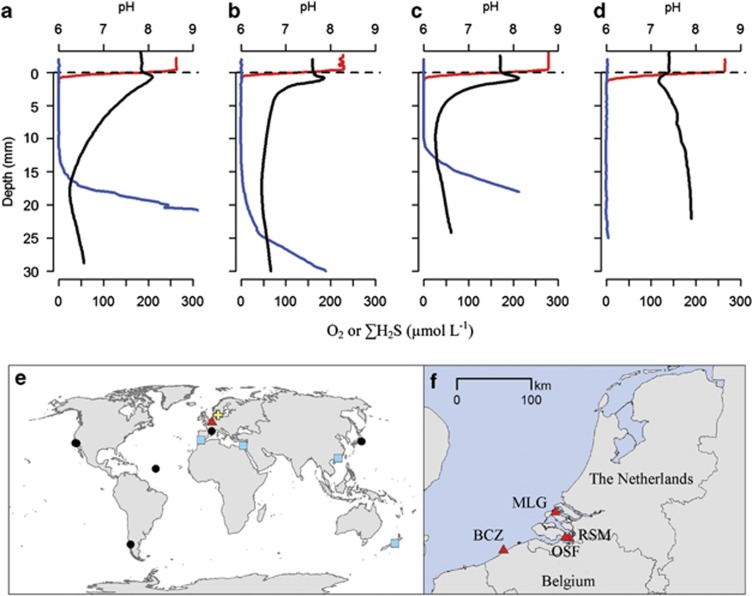
The geochemical signature of electrogenic sulphide oxidation was present at three of the four coastal zone habitats investigated in the North Sea area. Representative microsensor profiles are shown for the three sites where the geochemical signature was found (RSM, BCZ and MLG; Figures 1a–c), and the one site where it was not found (OSF; Figure 1d; all site locations are shown in Figures 1e and f). Furthermore, the geochemical signature of electrogenic sulphur oxidation was evident on multiple visits at the three sites (RSM, BCZ and MLG), identifying that electrogenic sulphide oxidation occurs regularly at these sites. These three sites were characterized by high DOU rates (range: 13.9–44.1 at RSM, BCZ and MLG vs 3.5–4.8 mmol O2 m−2 d−1 at OSF; Table 1). The first three sites had suboxic zones between 5 and 40 mm wide and typically showed a steep increase in the ΣH2S concentration below the suboxic zone. At these three sites, at least 5.3–33.7% of the DOU was attributable to electrogenic sulphide oxidation (Table 1). Current densities ranged from 4.6±1.8 mA m−2 at BCZ in March 2013 up to 29.9±10.7 mA m−2 at RSM in June 2011.
Figure 1.
Microsensor depth profiles of O2 (red), ΣH2S (blue) and pH (black) show the characteristic biogeochemical fingerprint of electrogenic sulphur oxidation in surface sediments from three coastal sites in the North Sea: (a) RSM, (b) BCZ and (c) MLG. In contrast, microsensor depth profiles from (d) OSF do not show the characteristic fingerprint. Representative microprofile curves are illustrated. (e) World map indicating Aarhus Harbour, the location where the electrogenic signature and Desulfobulbaceae filaments were first found in laboratory incubations (yellow cross), and the present study region (Dutch Delta area; red triangle). (f) Detailed map of individual study site locations (red triangles); full site details are given in Table 1. The world map also indicates locations where the geochemical signature of long distance electron transport have been found in literature reports (black circles) and locations where highly similar (⩾97%) 16S rRNA gene sequences to the Desulfobulbaceae filaments were retrieved from GenBank (blue squares); see Discussion for details.
Long Desulfobulbaceae filaments in natural sediments
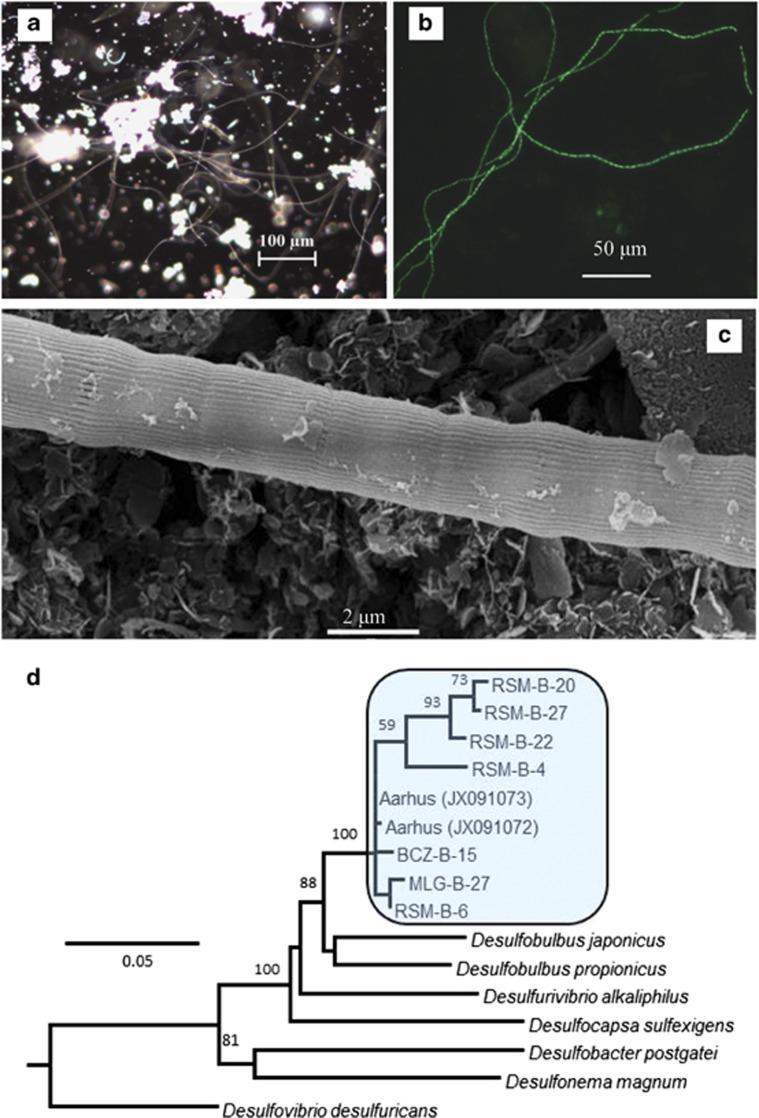
At all sites with geochemical evidence of electrogenic sulphide oxidation, microscopic examination of the sediments revealed long, unbranched, filamentous bacteria (Figure 2a), which were subsequently identified by CARD–FISH as Desulfobulbaceae (Figure 2b; Supplementary Figures S1 and S2). Enumeration using FISH revealed densities of 82.0 and 122.8 m of filament per cubic centimetre of bulk sediment at RSM and BCZ, respectively. No filaments were detected at the OSF reference site, where there was no geochemical evidence of electrogenic sulphide oxidation. Filament fragments up to 8 mm in length (∼4000 cells) were retrieved from MLG sediments by gently dissecting sediment under a microscope. SEM images revealed a pattern of evenly spaced external ridges running in parallel along the length of the filaments isolated from all three sites (Figure 2c; Supplementary Figures S1 and S2). Cell diameters were 1.25–3.04 μm at RSM, 0.89–1.37 μm in the BCZ and 2.18–2.36 μm in MLG based on measurements of 2–5 filaments measured using SEM per site. Filaments with larger cell diameters had more ridges and we estimated across all samples between 16 and 58 ridges per filament.
Figure 2.
Identification of filamentous Desulfobulbaceae bacteria present in intact sediment from sites described in Figure 1 and Table 1. (a) Abundant long filamentous bacteria taken from near the surface of sediment and gently teased apart with forceps. (b) Identification of the filaments belonging to Desulfobulbaceae using CARD–FISH (DSB 706 probe). (c) SEM image of a bacterial filament isolated from the sediment. All images shown here are from MLG. See Supplementary material for images from RSM and BCZ. (d) Phylogenetic tree of Desulfobulbaceae 16S rRNA sequences recovered from intact sediment described in Figure 1. Closely related sequences of filamentous Desulfobulbaceae from Aarhus Bay and other isolated bacteria are also included. Scale bar shows 5% sequence divergence and bootstrap support is indicated on tree branches.
Desulfobulbaceae clones with ⩾97% sequence similarity to the 16S rRNA gene sequences reported from the laboratory incubations of Pfeffer et al. (2012), were found in all field samples that showed the electrogenic sulphur oxidation signature (RSM, BCZ and MLG; Figure 2d). A summary of the full clone library composition is given in Supplementary Figure 4. Sequences have been deposited in GenBank under accession numbers BankIt1707352:KJ562723-KJ562819. A clone library was not constructed from OSF sediments, where the geochemical signature of electrogenic sulphur oxidation was not observed. However, a previous study of similar bioturbated sediments in the Oosterschelde found no Desulfobulbaceae sequences in either the surface-oxidized sediment or the deeper sulphate-reducing sediment (Miyatake et al., 2013).
Effects of bioturbation on geochemical signal
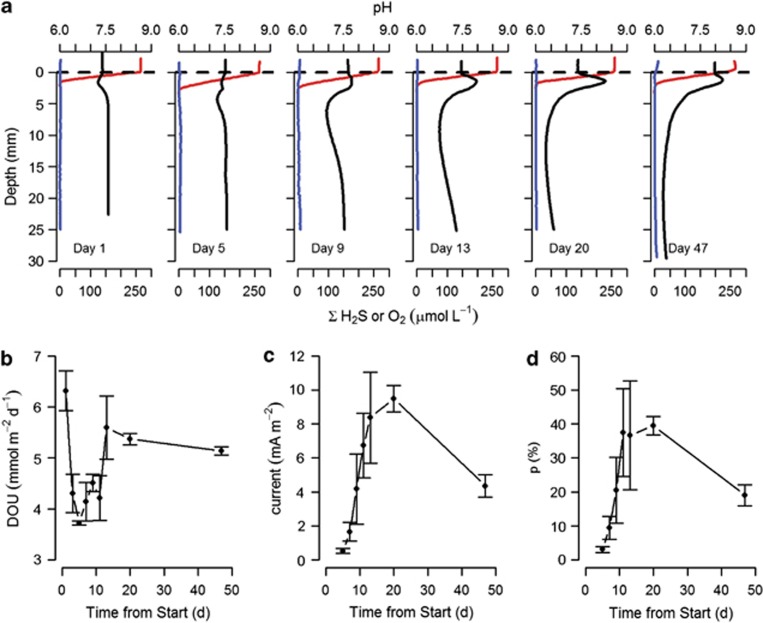
The geochemical fingerprint of electrogenic sulphur oxidation was not present in intact sediment cores collected from the highly bioturbated sandy sediment at OSF collected in May and August 2011. However, sediments from OSF that were defaunated by sieving and incubated under darkened laboratory conditions in aerated seawater developed the pH profile indicative of electrogenic sulphur oxidation (Figure 3). The characteristic pH profile indicative of cathodic oxygen consumption was first evident on Day 5 after homogenization, whereby porewater pH was just beginning to increase at the base of the oxic zone (Figure 3). This pH peak progressively increased over the days of incubation, with a maximum subsurface pH observed on Day 20. At the start of the incubation, before the onset of cathodic oxygen consumption, the DOU declined from 6.3±0.7 at Day 1 to 3.7±0.1 mmol O2 m−2 d−1 at Day 5. After Day 5, in concert with the development of a pH maximum in the oxic zone, there was an increase in DOU. Between Day 5 and Day 20, DOU increased from 3.7±0.1 to 5.4±0.2 mmol O2 m−2 d−1, representing a 51% increase in oxygen uptake. Over this same period (Days 5–20), the estimated electrical current density increased from 0.5±0.3 up to 9.5±1.4 mA m−2. The percentage of sediment DOU attributed to cathodic reactions increased from 0% on Day 1 to 22.4±1.2% on Day 5, and up to 51.6±3.7% on Day 20. Although CARD–FISH was not performed during this particular experiment, subsequent analysis of sediment collected from OSF and sieved and incubated following the same procedures, confirmed the presence of Desulfobulbaceae filaments whenever the geochemical signature of electrogenic sulphur oxidation was present (Supplementary Figure 3).
Figure 3.
(a) A subset of microsensor profiles from sediment collected from a heavily bioturbated sediment from an OSF that has been artificially defaunated and incubated with well-oxygenated overlying water. Despite a low concentration of free sulphide, development of a geochemical signature of long-range electron transport is evident from Day 5 onwards. (b) DOU during the incubations, average of n=3, plotted with standard error about the mean. (c) Current density calculated from these sediments and (d) percentage, p, of oxygen flux that is attributable to cathodic oxygen uptake.
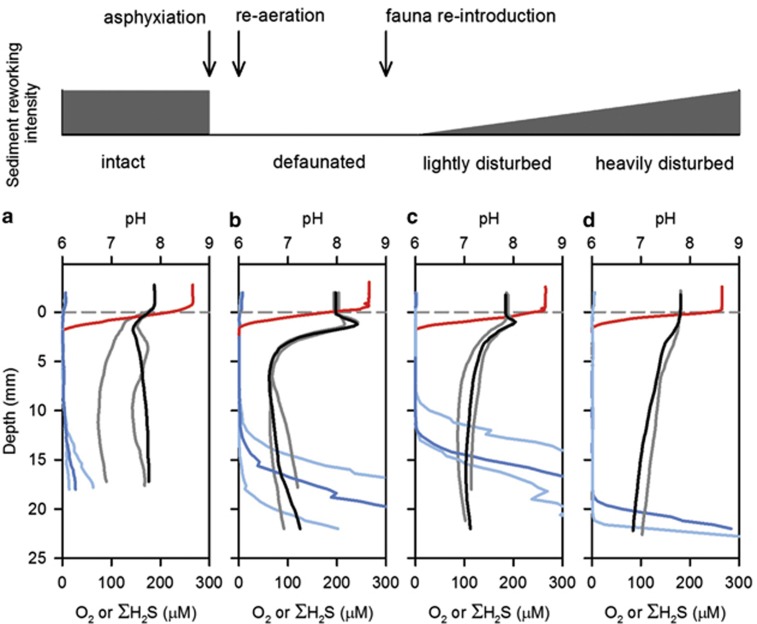
To test if infauna specifically can inhibit the geochemical signature of electrogenic sulphide oxidation, we performed a second manipulation experiment, where fauna were first removed by asphyxiation and subsequently reintroduced. When intact sediment cores were retrieved from the OSF field site and immediately profiled, these sediments did not exhibit evidence of electrogenic sulphur oxidation (Figure 4a). In defaunated sediments, the geochemical signature of electrogenic sulphur oxidation again developed within 8 days of exposure to aerated seawater (Figure 4b). The deep burrowing lugworm A. marina was then re-introduced and caused extensive sediment overturning, removing sediment from the feeding pocket and expelling sediment and organic waste as fecal casts on the sediment surface. In sediment patches that were not mechanically disrupted by the burrowing lugworms, the electrogenic signature persisted, though with attenuated pH extremes (Figure 4c). In sediment patches that were directly overturned by lugworm bioturbation, the electrogenic signature disappeared entirely (Figure 4d).
Figure 4.
Effect of sediment disturbance by fauna on electrogenic sulphur oxidation. Microprofiles of O2 (red), ΣH2S (blue) and pH (black and grey) are shown from sediment cores collected from the intertidal OSF. Different shades are used for replicate profiles for pH and ΣH2S for image clarity, whereas a single median profile is shown for O2. (a) Profiles from intact sediment cores. (b) Profiles from defaunated sediment cores. (c) Profiles from re-faunated sediment cores, in areas that were not visibly disrupted by mechanical disturbance caused by the re-introduced lugworms. (d) Profiles from re-faunated sediment cores, in areas that were not visibly disrupted by mechanical disturbance caused by the re-introduced lugworms.
Discussion
Our results provide evidence that electrogenic sulphur oxidation, previously reported from laboratory incubations (Nielsen et al., 2010; Pfeffer et al., 2012; Risgaard-Petersen et al., 2012), occurs under natural conditions in the seafloor. We observed evidence of electrogenic sulphur oxidation, together with Desulfobulbaceae filaments, in a variety of coastal marine sediments (that is, a salt marsh drainage channel, a subtidal site recovering from seasonal hypoxia, a coastal area of high mud deposition). These sediments were characterized by high DOU and high organic matter content. In the narrow oxic zone (0.7–2.0 mm depth), a pH maximum was observed under dark conditions, indicative of cathodic oxygen consumption. Below this depth, there was a suboxic zone where oxygen and sulphide were not detectable, which was typically 5–15 mm deep (although sometimes extending deeper). Below the suboxic zone, these sediments exhibited a steep sulphide gradient, indicating high sulphide production. Repeated visits to these three study sites identified that electrogenic sulphur oxidation is a regularly occurring, although not necessarily permanent, feature at these field sites.
Our observation of the geochemical signature of electrogenic sulphur oxidation always coincided with the presence of Desulfobulbaceae filaments that were closely related (that is, ⩾97% 16S rRNA gene sequence similarity) to those previously reported from the laboratory incubations of Pfeffer et al. (2012). Desulfobulbaceae filaments from the suboxic zone of our study sites revealed densities of 82.0 and 122.8 m filament per cubic centimetre sediment at RSM and BCZ, respectively, and no filaments were found at the reference site OSF. These densities are similar to those reported in Pfeffer et al. (2012) (127 m cm−3) and support the hypothesis that the long-range electron transport associated with electrogenic sulphur oxidation is mediated by the Desulfobulbaceae filaments (Pfeffer et al., 2012).
The filamentous bacteria retrieved from the three electrogenic sulphur oxidation study sites (RSM, BCZ and MLG) were long and unbranched, and retrieved fragments could exceed eight millimetres in length. The unusual morphological feature of raised ridges running in parallel along the length of the filaments, observed previously in laboratory incubations, were also prominent in the Desulfobulbaceae filaments isolated in this study. Pfeffer et al. (2012) reported filament diameters between 0.4 and 0.7 μm with fewer than 20 ridges per filament, whereas we herein report filament diameters between 0.9 and 3.0 μm with 16–58 ridges per filament. It remains to be seen if these morphological differences represent genotypic or phenotypic variation.
A cosmopolitan distribution of electrogenic sulphur oxidation is supported by a number of published datasets in which the geochemical fingerprint of this process is evident, but has not been formally recognized (Figure 1d). Shallow subsurface pH maxima, characteristic of cathodic oxygen consumption, have been reported from in situ microsensor profiling in the Santa Barbara (Cai et al., 2000) and Santa Monica Basins (Reimers et al., 1996). These basins are permanently hypoxic, support high rates of sulphate reduction, and are devoid of bioturbating fauna. Similar pH profiles were also reported from a site situated within an oyster aquaculture park in the Thau lagoon (Mediterranean Sea, France; Dedieu, 2005) and from sediments near a fish farm in Pillan fjord (Chile; Mulsow et al., 2006), both of which experience high organic matter loading and having little or no burrowing infauna. Seasonally hypoxic sediments from Tokyo Bay have also demonstrated microprofile evidence of cathodic oxygen consumption (Sayama, 2011), similar to those reported here from MLG. At a site characterized by high sulphide fluxes and devoid of large bioturbating infauna on the Mid-Atlantic ridge, high rates of sulphide oxidation were detected which could not be attributed to any other known sulphide removal process, such as sulphur oxidation by large nitrate-accumulating bacteria (Schauer et al., 2011). One explanation is that electrogenic sulphur oxidations is responsible for the observed sulphide removal, however, direct evidence is needed to confirm this possibility.
Gene sequence archives also support a cosmopolitan distribution of the conductive filamentous bacteria in locations with high rates of sulphide generation, via high organic matter loading or sulphide seepage, and a paucity of bioturbating animals. 16S rRNA gene sequences that were highly similar (⩾97%) to the Desulfobulbaceae at our field sites have been detected at cold seeps on the Hikurangi Margin (Baco et al., 2010) and the Nile Deep Sea Fan (Grünke et al., 2011) in areas devoid of fauna; in organic rich cohesive sediment of a subtropical mangrove swamp (Liang et al., 2007); and at a sewage impacted site in the Bay of Cadiz (Köchling et al., 2011).
Previous laboratory experiments have shown that sulphur oxidation by long-range electron transport can play a dominant role in oxygen consumption and can exert a strong imprint on the overall sulphur cycling and pH dynamics in sediments (Nielsen et al., 2010; Risgaard-Petersen et al., 2012). Our observations show that this process likely has a similarly profound effect on mineral cycling in some natural coastal sediments. We observed pH values as high as 8.8 in the oxic zone, and as low as 6.1 in the suboxic zone (Figure 1). These pH extremes lead to dissolution of metal sulphides and calcium carbonate within the deep suboxic zone, followed by re-precipitation of iron (hydr)oxides and carbonate near the sediment–water interface (Risgaard-Petersen et al., 2012). High alkalinity effluxes have been reported from muddy sites on the BCZ, including Station 130 (Braeckman et al., 2014), and such effluxes are consistent with cathodic oxygen consumption and elevated carbonate dissolution induced by electrogenic sulphur oxidation. Current densities estimated at the three field sites demonstrating electrogenic sulphur oxidation (15–23 mA m−2) are comparable with the values observed in previous laboratory experiments (Nielsen et al., 2010; Risgaard-Petersen et al., 2012) and are similar to those reported from the anodes of sediment batteries colonized by natural microbial communities (Tender et al., 2002; Ryckelynck et al., 2006). We estimated that a minimum of 5.3–33.7% of the DOU in these sediments was due to electrogenic sulphur oxidation, substantiating that this process can be a dominant biogeochemical process in natural marine sediments.
Our data further suggest that bioturbation may exert a major control on the natural distribution of electrogenic sulphur oxidation in coastal marine sediments. In heavily bioturbated sediment from OSF, the geochemical signature of electrogenic sulphide oxidation was not detected under field conditions, but could be induced in laboratory experiments, as long as bioturbation was excluded. In these sediments, a seed population of Desulfobulbaceae bacteria must have existed, from which these filaments were able to proliferate when burrowing animals are removed. We observed that sediment overturning by bioturbation caused a clear disruption of the electrochemical signal. This may arise from a direct breakage of the bacterial filaments, in a manner similar to the previously described cutting experiments of Pfeffer et al. (2012). In addition, sediment overturning may halt electrogenic sulphur oxidation by depositing an anoxic layer of sediment on top of the sediment surface, thereby depriving the resident filamentous bacteria from access to electron acceptors.
In summary, we have shown that electrogenic sulphur oxidation is found in intact coastal marine sediments under natural conditions. We found this process, together with the Desulfobulbaceae filaments, in three depositional sediment areas (a salt marsh, a subtidal coastal mud plain and a hypoxic marine basin). These sediments were collectively characterized as being rich in organic matter, and consequently supporting high rates of sulphate reduction, and having an oxygenated overlying water column. These sediments were furthermore subject to little biomechanical mixing by macrofauna, which appears to be a key control on the distribution of electrogenic sulphur oxidation in natural settings. Until now, the occurrence of electrogenic sulphur oxidation has not been accounted for in studies of natural marine sediments. Given its widespread distribution, electrogenic sulphur oxidation may be a key process in the biogeochemistry and microbial ecology of the seafloor.
Acknowledgments
We thank U Braeckman for providing data on the macrofauna distribution in BCZ, R Dasseville (U Gent) for help with SEM, V Confurius for assistance with clone libraries and S Engelhard (NIOZ) for help with CARD–FISH images. We thank three anonymous reviewers for their comments. This research was financially supported by the Brussels Institute for Research and Innovation (HoloFlow project: SYM, EMZ), the Darwin Center for Biogeosciences (DS, DV-C, HTSB), the Research Foundation – Flanders (FWO-Odysseus grant FJRM), the Netherlands Organisation for Scientific Research (NWO-VIDI grant FJRM) and the European Research Council (ERC Starting grant FJRM).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Ashelford KE, Chuzhanova NA, Fry JC, Jones AJ, Weightman AJ. At least 1 in 20 16S rRNA sequence records currently held in public repositories is estimated to contain substantial anomalies. Appl Environ Microbiol. 2005;71:7724–7736. doi: 10.1128/AEM.71.12.7724-7736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baco AR, Rowden AA, Levin L, Smith CR, Bowden DA. Initial characterization of cold seep faunal communities on the New Zealand Margin. Mar Geol. 2010;272:251–259. [Google Scholar]
- Boudreau BP. The diffusive tortuosity of fine-grained unlithified sediments. Geochim Cosmochim Acta. 1996;60:3139–3142. [Google Scholar]
- Braeckman U, Foshtomi MY, Van Gansbeke D, Meysman F, Soetaert K, Vincx M, et al. 2014Variable importance of macrofaunal functional biodiversity for biogeochemical cycling in temperate coastal sediment Ecosystems(in press) doi: 10.1007/s10021-014-9755-7 [DOI]
- Clarke TA, Edwards MJ, Gates AJ, Hall A, White GF, Bradley J, et al. Structure of a bacterial cell surface decaheme electron conduit. Proc Nat Acad Sci. 2011;108:9384–9389. doi: 10.1073/pnas.1017200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W-J, Zhao P, Wang Y. pH and pCO2 microelectrode measurements and the diffusive behavior of carbon dioxide species in coastal marine sediments. Mar Chem. 2000;70:133–148. [Google Scholar]
- Coosen J, Seys J, Meire PM, Craeymeersch J. Effect of sedimentological and hydrodynamical changes in the intertidal areas of the Oosterschelde estuary (SW Netherlands) on distribution, density and biomass of five common macrobenthic species: Spio martinensis (Mesnil), Hydrobia ulvae (Pennant), Arenicola marina (L.), Scoloplos armiger (Muller) and Bathyporeia sp. Hydrobiologia. 1994;282/283:235–249. [Google Scholar]
- Dedieu K.2005. Biogeochemical dynamics of oxygen in coastal sediments: study by micro-electrodes and models. Ph.D. thesis. Université Pierre et Marie Curie (Paris), France. National thesis number: 2005PA066019, 2005
- Dickson AG, Sabine CL, Christian JR.(eds.). (2007Guide to Best Practices For Ocean CO2 Measurements PICES Special Publication; 3191 [Google Scholar]
- Gao Y, Lesven L, Gillan D, Sabbe K, Billon G, De Galan S, et al. Geochemical behavior of trace elements in sub-tidal marine sediments of the Belgian coast. Mar Chem. 2009;117:88–96. [Google Scholar]
- Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci. 2006;103:11358–11363. doi: 10.1073/pnas.0604517103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünke S, Felden J, Lichtschlag A, Girnth A-C, De Beer D, Wenzhöfer F, et al. Niche differentiation among mat-forming, sulfide-oxidizing bacteria at cold seeps of the Nile Deep Sea Fan (Eastern Mediterranean Sea) Geobiol. 2011;9:300–348. doi: 10.1111/j.1472-4669.2011.00281.x. [DOI] [PubMed] [Google Scholar]
- Hofmann AF, Soetaert K, Middelburg JJ, Meysman FJR. AquaEnv: an aquatic acid–base modelling environment in R. Aquat Geochem. 2010;16:507–546. [Google Scholar]
- Jørgensen BB. Mineralization of organic matter in the sea bed – the role of sulphate reduction. Nature. 1982;296:643–645. [Google Scholar]
- Jørgensen BB, Nelson DC.2004Sulfide oxidation in marine sediments: geochemistry meets microbiologyIn: Amend JP, Edwards K, Lyons TW (eds).Sulfur Biogeochemistry—Past and Presentvol 379Special Paper, Geological Society of America; 63–81. [Google Scholar]
- Köchling T, Lara-Martin P, Gonzalez-Mazo E, Amils R, Sanz JL. Microbial community composition of anoxic marine sediments in the Bay of Cadiz (Spain) Internat Microbiol. 2011;14:143–154. doi: 10.2436/20.1501.01.143. [DOI] [PubMed] [Google Scholar]
- Liang J-B, Chen Y-Q, Lan C-Y, Tam NFY, Zan Q-J, Huang L-N. Recovery of novel bacterial diversity from mangrove sediment. Mar Biol. 2007;150:739–747. [Google Scholar]
- Logan BE, Rabaey K. Conversion of wastes in bioelectricity and chemicals using microbial electrochemical technologies. Science. 2012;337:686–690. doi: 10.1126/science.1217412. [DOI] [PubMed] [Google Scholar]
- Lovley DR. The microbe electric: conversion of organic matter to electricity. Curr Opin Biotech. 2008;19:564–571. doi: 10.1016/j.copbio.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Lücker S, Steger D, Kjeldsen KU, MacGregor BJ, Wagner M, Loy A. Improved 16S rRNA-targeted probe set for analysis of sulfate-reducing bacteria by fluorescence in situ hybridization. J Microbiol Methods. 2007;69:523–528. doi: 10.1016/j.mimet.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, et al. ARB: a software environment for sequence data. Nucleic Acids Res. 2004;32:1362–1371. doi: 10.1093/nar/gkh293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake T, MacGregor BJ, Boschker HTS. Depth-related differences in organic substrate utilization by major microbial groups in intertidal marine sediment. Appl Environ Microbiol. 2013;79:389–392. doi: 10.1128/AEM.02027-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulsow S, Krieger Y, Kennedy R. Sediment profile imaging (SPI) and micro-electrode technologies in impact assessment studies: example from two fjords in Southern Chile used for fish farming. J Mar Syst. 2006;62:152–163. [Google Scholar]
- Nielsen LP, Risgaard-Petersen N, Fossing H, Christensen PB, Sayama M. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature. 2010;463:1071–1074. doi: 10.1038/nature08790. [DOI] [PubMed] [Google Scholar]
- Oenema O.1988. Early diagenesis in recent fine-grained sediments in the Eastern Scheldt, Ph.D. thesis, Utrecht University: Utrecht, The Netherlands.
- Pallud C, Van Cappellen P. Kinetics of microbial sulfate reduction in estuarine sediments. Geochim Cosmochim Acta. 2006;70:1148–1162. [Google Scholar]
- Pernthaler A, Pernthaler J, Amann R. Fluorescence in situ hybridization and catalyzed reported deposition for the identification of marine bacteria. App Environ Microbiol. 2002;68:3094–3101. doi: 10.1128/AEM.68.6.3094-3101.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer C, Larsen S, Song J, Dong M, Besenbacher F, Meyer RL, et al. Filamentous bacteria transport electrons over centimeter distances. Nature. 2012;491:218–221. doi: 10.1038/nature11586. [DOI] [PubMed] [Google Scholar]
- Rao AMF, Malkin SY, Meysman FJR.in review. Enhanced alkalinity production in intertidal sands from the Oosterschelde (The Netherlands) induced by the lugworm Arenicola marina. Estuarine Coast Shelf Sci (ECSS-S-13-00698; pp 46).
- Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. Extracellular electron transfer via microbial nanowires. Nature. 2005;435:1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, Lovley DR. Biofilm and nanowire production leads to increased current in Geobacter sulphurreducens fuel cells. Appl Environ Microbiol. 2006;72:7345–7348. doi: 10.1128/AEM.01444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers CE, Ruttenberg KC, Canfield DE, Christiansen MB, Martin JB. Porewater pH and authigenic phases formed in the uppermost sediments of the Santa Barbara Basin. Geochem Cosmo Acta. 1996;60:4037–4057. [Google Scholar]
- Risgaard-Petersen N, Revil A, Meister P, Nielsen LP. Sulphur, iron and calcium cycling associated with natural electric currents running through marine sediments. Geochim Cosmochim Acta. 2012;92:1–13. [Google Scholar]
- Ryckelynck N, Stecher HA, III, Reimers CE. Understanding the anodic mechanism of a seafloor fuel cell: interactions between geochemistry and microbial activity. Biogeochem. 2006;76:113–139. [Google Scholar]
- Savitzky A, Golay MJE. Smoothing and differentiation of data by simplified least squares procedures. Anal Chem. 1964;36:1627–1639. [Google Scholar]
- Sayama M.2011. Seasonal dynamics of sulphide oxidation processes in Tokyo Bay dead zone sediments. Goldschmidt Conference Abstracts.
- Schauer R, Røy H, Augustin N, Gennerich H-H, Peters M, Wenzhoefer F, et al. Bacterial sulphur cycling shapes microbial communities in surface sediments of an ultramafic hydrothermal vent field. Environ Microbiol. 2011;13:2633–2648. doi: 10.1111/j.1462-2920.2011.02530.x. [DOI] [PubMed] [Google Scholar]
- Soetaert K, Petzoldt T, Meysman FJR.2012. Package ‘marelac': Tools for aquatic sciences. v.2.1.2.
- Tender LM, Reimers CE, Stecher HA, III, Holmes DE, Bond DR, Lowy DA, et al. Harnessing microbially generated power on the seafloor. Nat Biotechnol. 2002;20:821–825. doi: 10.1038/nbt716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.