Abstract
Approximately 70% of all newly diagnosed breast cancers express estrogen receptor (ER)-α. Although inhibiting ER action using targeted therapies such as fulvestrant (ICI) is often effective, later emergence of antiestrogen resistance limits clinical use. We used antiestrogen-sensitive and -resistant cells to determine the effect of antiestrogens/ERα on regulating autophagy and unfolded protein response (UPR) signaling. Knockdown of ERα significantly increased the sensitivity of LCC1 cells (sensitive) and also resensitized LCC9 cells (resistant) to antiestrogen drugs. Interestingly, ERα knockdown, but not ICI, reduced nuclear factor (erythroid-derived 2)-like (NRF)-2 (UPR-induced antioxidant protein) and increased cytosolic kelch-like ECH-associated protein (KEAP)-1 (NRF2 inhibitor), consistent with the observed increase in ROS production. Furthermore, autophagy induction by antiestrogens was prosurvival but did not prevent ERα knockdown–mediated death. We built a novel mathematical model to elucidate the interactions among UPR, autophagy, ER signaling, and ROS regulation of breast cancer cell survival. The experimentally validated mathematical model explains the counterintuitive result that knocking down the main target of ICI (ERα) increased the effectiveness of ICI. Specifically, the model indicated that ERα is no longer present in excess and that the effect on proliferation from further reductions in its level by ICI cannot be compensated for by increased autophagy. The stimulation of signaling that can confer resistance suggests that combining autophagy or UPR inhibitors with antiestrogens would reduce the development of resistance in some breast cancers.—Cook, K. L., Clarke, P. A. G., Parmar, J., Hu, R., Schwartz-Roberts, J. L., Abu-Asab, M., Wärri, A., Baumann, W. T., Clarke, R. Knockdown of estrogen receptor-α induces autophagy and inhibits antiestrogen-mediated unfolded protein response activation, promoting ROS-induced breast cancer cell death.
Keywords: fulvestrant, ICI 182780, apoptosis, reactive oxygen species
Breast cancer is one of the most prevalent female cancers, with ∼240,000 new cases diagnosed each year in the United States (1). About 70% of all breast cancers express the estrogen receptor (ER)-α protein. Endocrine-targeted therapies, such as the selective estrogen receptor modulator tamoxifen (TAM), the selective estrogen receptor down-regulator fulvestrant (Faslodex; ICI 182780; referred to herein as ICI), and aromatase inhibitors, have been developed to treat this breast cancer subtype. Although antiestrogen therapy remains one of the most frequently prescribed adjuvant chemotherapies, de novo and acquired resistance often limits drug effectiveness. Recurrent breast cancer remains, for the most part, an incurable disease, emphasizing the need for further understanding of the molecular mechanisms of antiestrogen resistance (2–4).
Both the unfolded protein response (UPR) and autophagy are implicated in mediating resistance to endocrine therapies (5–9). The UPR is an endoplasmic reticulum stress pathway comprising 3 signaling arms, each stimulated by the accumulation of unfolded proteins within the organelle. In the presence of unfolded proteins, glucose-regulated protein (GRP)-78 (also known as HSPA5 and BiP) is released from inositol-requiring enzyme (IRE)-1 (also known as ERN1), PKR-like endoplasmic reticulum kinase (PERK; also known as EIF2AK3), and activating transcription factor (ATF)-6, allowing activation of each of the 3 signaling arms. ATF6 translocates to the Golgi complex where it is cleaved to form an active transcription factor. PERK activation phosphorylates elongation initiation factor (eIF)-2α, thereby inhibiting cap-dependent protein translation and promoting ATF4 transcription. Activation of IRE1 results in the unconventional splicing of X-box-binding protein (XBP)-1 to form the active transcription factor XBP1-S. UPR signaling promotes the transcription of various protein chaperones to help correct misfolded proteins and stimulates a nuclear factor (erythroid-derived 2)-like 2 (NRF2) mediated antioxidant response; however, apoptosis is triggered if the UPR remains highly active for an extended duration (10–12). A key role of the UPR signaling component XBP1 is implicated in the development of antiestrogen resistance (13–16). A central role for GRP78, a master regulator of UPR signaling in controlling autophagy and apoptosis, has also been established recently in antiestrogen resistance (5, 9, 17). Autophagy, a cellular process of “self-eating,” results in the formation of a double-membraned vesicle that supplements cellular metabolism by digesting damaged organelles or misfolded proteins. Induction of autophagy by antiestrogens can protect breast cancer cells and promote their survival, implicating autophagy in therapeutic resistance (6, 8, 18–22).
In the current study, ICI stimulated UPR signaling in LCC1 and -9 breast cancer cells, and the ICI-mediated induction of UPR signaling depended on the presence of ERα. Moreover, antiestrogen treatment and ERα knockdown promoted autophagy, suggesting that endocrine interventions stimulate UPR and autophagy through different mechanisms of ERα regulation.
The initial results offer several possible alternative explanations for how signaling may affect cellular function. Since it was difficult to interpret the data intuitively, we built a mathematical model that reproduced the experimental data from signaling and biological outcomes measurements. The model explained several experimental results and was used to predict the qualitative changes in cell proliferation resulting from the proposed overexpression and knockdown experiments. The experiments proposed by the model were subsequently performed, and the predictions were validated. Together, the model and experimental data showed how ERα knockdown in antiestrogen-resistant cells can induce mitochondrial dysfunction and inhibit the UPR-induced antioxidant pathway, promoting the formation of reactive oxygen species (ROS) and apoptosis.
MATERIALS AND METHODS
Materials
The following materials were obtained as indicated: ICI 182780 (Tocris Bioscience, Ellisville, MO, USA); improved minimal essential medium (IMEM; Invitrogen-Life Technologies, Inc. Carlsbad, CA, USA); charcoal-stripped calf serum (CCS) (Equitech-Bio Inc., Kerrville, TX, USA); Lipofectamine RNAiMax reagent (Invitrogen); ERα shRNA plasmid (Trueclone cDNA; Origene, Rockville, MD, USA); autophagy-related gene (ATG)-7 siRNA (Cell Signaling Technology, Danvers, MA, USA); mouse IgG-negative control antibody (Dako, Glostrup, Denmark); and crystal violet (Fisher Scientific, Fairlawn, NJ, USA). Antibodies were obtained from the following sources: GRP78, GRP94, NRF2, kelch-like ECH-associated protein 1 (KEAP1), microtubule-associated protein light chain (LC)-3B, ATG7, PERK, C/EBP homologous protein [CHOP; also known as DNA damage inducible transcript 3 (DDIT3)], IRE1α, eIF2α, phospho-eIF2α, XBP1-S, and cleaved caspase-7 (Cell Signaling Technology); p62 (BD Biosciences, Franklin Lakes, NJ, USA); ATF6 (Sigma-Aldrich, St. Louis, MO, USA); phospho-IRE1 (ThermoScientific, Lafayette, CO, USA); XBP1-S (GenWay, San Diego, CA, USA); ERα (Vector Laboratories, Burlingame, CA, USA), β-tubulin (Sigma-Aldrich), p62 [for immunohistochemistry (IHC); MBL International, Woburn, MA, USA], GRP78 (for IHC), ATF4 (CREB2), Histone H1, and polyclonal and HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). LC3-GFP (Addgene, Cambridge MA, USA) and LysoTracker (Invitrogen) were used for confocal microscopy. Autophagosome determination, ROS determination, and annexin V apoptosis kits were from Enzo Life Sciences (Ann Arbor, MI, USA).
Cell culture
LCC1 and -9 breast carcinoma cells, previously derived in this laboratory (23, 24), were grown in phenol red–free IMEM, containing 5% CCS, defined as basal growth conditions. In brief, LCC1 cells are estrogen-independent, antiestrogen-sensitive breast cancer cells, derived from MCF7 cells grown in ovariectomized female athymic mice and explanted back into cell culture, whereas LCC9 cells are ER+, estrogen-independent, ICI-resistant, TAM cross-resistant cells, derived from LCC1 cells by stepwise selection against ICI. The cells were grown at 37°C in a humidified, 5% CO2:95% air atmosphere.
Crystal violet cell density assays
Human breast cancer cells (5×103 cells/mL) in IMEM containing 5% CCS were transfected with control or ERα shRNA and/or ATG7 siRNA and plated in 24-well tissue culture plates. On d 1 after plating, the cells were treated with various doses of ICI (10–1000 nM), 17β-estradiol (E2; 10 nM), or both. After 72 h, the media were aspirated, and the cells were stained with crystal violet and permeabilized with citrate buffer. Absorbance was read at 660 nm on a plate reader.
Luciferase assay
pGL3-CRE-luc (ERE-luc) or pGL3-basic and pRL-TK constructs were transfected into the breast cancer cells, together with control or ERα shRNA constructs. Twenty-four hours after transfection, the cells were treated with 100 nM ICI or ethanol control for an additional 72 h and lysed in passive lysis buffer for dual luciferase assays, according to the manufacturer's protocol (Promega Corp., Madison, WI, USA).
Western blot hybridization
As described previously, treated cell monolayers were solubilized in RIPA lysis buffer, protein was measured in a standard bicinchoninic acid assay, size fractionated by polyacrylamide gel electrophoresis, and transferred to nitrocellulose membrane. Nonspecific binding was blocked by incubation with Tris-buffered saline containing 5% powdered milk and 1% Triton X-100. The membranes were incubated overnight at 4°C with primary antibodies, followed by incubation with polyclonal horseradish peroxidase (HRP)–conjugated secondary antibodies (1:2000) for 1 h at room temperature. Immunoreactive products were visualized by chemiluminescence (SuperSignal Femto West; Pierce Biotechnology, Rockford, IL, USA) and quantified by densitometry using ImageJ digital densitometry software (National Institutes of Health). The membranes were stripped with Western blot stripping buffer (ThermoScientific) for 15 min and reprobed with various antibodies. Protein loading was visualized by incubation of the stripped membranes with a monoclonal antibody to β-tubulin (1:1000).
Flow cytometry
LCC1 and -9 cells were transfected with control (sequence-specific scrambled oligonucleotide) or ERα shRNA and/or ATG7 siRNA and treated with ICI or TAM (100 nM) for 72 h. To measure apoptosis, the cells were stained as described in the Annexin V-FITC Apoptosis Detection Kit (Enzo Life Sciences) and counted by flow cytometry [Flow Cytometry Shared Resource; Lombardi Comprehensive Cancer Center (LCCC), Georgetown University]. To measure autophagy and ROS generation, the cells were stained as described in the Autophagosome Detection Kit or the Total ROS Detection kit (both from Enzo Life Sciences) and counted by flow cytometry (LCCC Flow Cytometry Shared Resource). In brief, the cells were transfected with ERα shRNA, treated with ICI for 72 h, trypsinized, and resuspended in buffer containing ROS or a modified monodansylcadaverine stain before FACS analysis.
Confocal microscopy
LCC1 and -9 cells were transfected with GFP-LC3B (Addgene) and either control or ERα shRNA before treatment with 0.1% v/v ethanol vehicle, 10 nM E2, or 500 nM ICI for 24 h; permeabilized; and incubated with an ERα antibody. ERα localization, a propidium iodide (PI)/LC3II-GFP–positive punctate pattern, and PI/ROS staining were evaluated by confocal microscopy. Autophagosome and lysosome colocalization was determined by 24 h incubation with LysoTracker Red, a specific marker for the integral lysosomal membrane protein (LAMP)-1 (Invitrogen), and the formation of LC3-GFP puncta. Confocal microscopy was performed using an Olympus IX-70 (Olympus America Imaging, Hauppauge, NY, USA) confocal microscope (LCCC Imaging Shared Resource).
Electron microscopy (EM)
EM was performed as described previously (8, 25). In brief, LCC9 cells treated with vehicle control, 500 nM ICI, or ERα shRNA for 24 h were fixed with 2.5% glutaraldehyde, postfixed with 0.5% osmium tetroxide, dehydrated, and embedded in Spurr's epoxy resin. Ultrathin sections (90 nm) were double stained with uranyl acetate and lead citrate. The sections were viewed with a transmission electron microscope (CM10; Phillips Electronics, Eindhoven, The Netherlands).
Mathematical modeling
A mathematical model of LCC9 cells was created that contains the key molecular components measured in the experiments but models the UPR, autophagy, ROS, and cellular stress in a phenomenological manner that subsumes many individual processes for simplicity (see Fig. 7A). For example, stress is modeled as a single entity that is increased by the accumulation of ERα aggregates in the cytoplasm (26), is decreased by autophagy, and activates the UPR. The UPR is also modeled as a single entity that, when activated, phosphorylates eIF2α, cleaves ATF6, and splices XBP1-U mRNA. We exclude the intermediate pathways involving PERK, ATF6, and IRE1. The 3 downstream UPR pathways we modeled all led to increased GRP78 expression, which reduced UPR activation by increasing the protein-folding capacity of the endoplasmic reticulum and reducing stress therein.
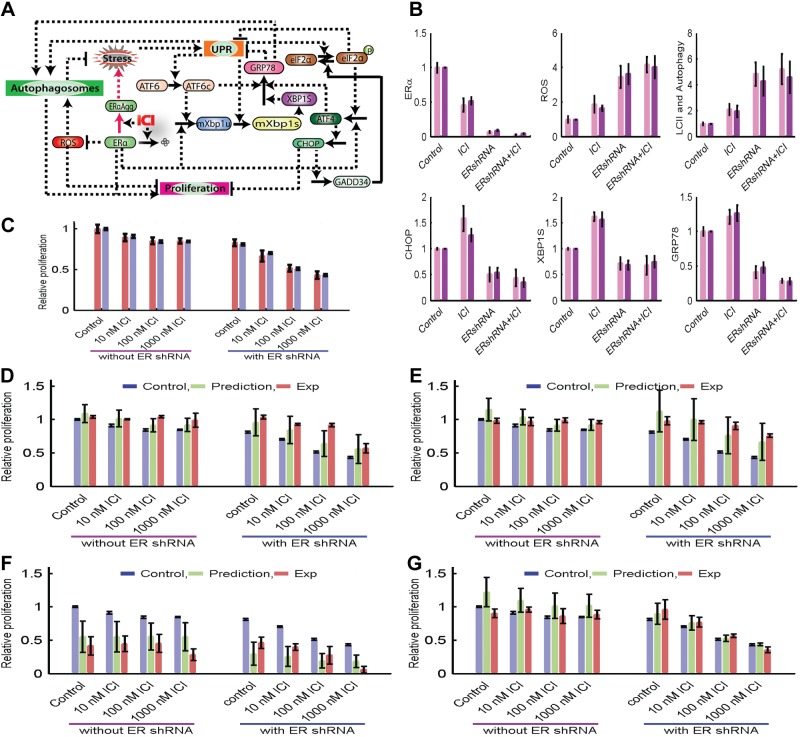
Figure 7.
Mathematical modeling of ER regulation of the UPR and autophagy in LCC9 cells to control breast cancer cell fate. A) Wiring diagram of the modeled interactions. Solid arrows represent reactions such as synthesis, degradation, and phosphorylation; dashed arrows represent activation; dashed lines with blunt ends represent inhibition. B) Levels of molecular components at 144 h after no treatment, 100 nM ICI, ERα knockdown, or 100 nM ICI plus ERα knockdown. C) Relative proliferation in control and ERα-knockdown cases in response to ICI treatment. See Materials and Methods for modeling details. D–G) The original experimental proliferation, model prediction of proliferation in response to perturbation, and experimental proliferation in response to perturbation for XBP1 overexpression (D), GRP78 overexpression (E), CHOP overexpression (F), and CHOP knockdown (G). The predictions plotted are from 454 parameter sets that matched the experimental data in panels B and C, providing an indication of how tightly the experimental data constrained the predictions.
To account for the increased ROS and autophagy responses to ERα knockdown, we modeled a direct inhibition of ROS production by ERα that subsumes the many possible indirect interactions through, for example, NRF2 (27), NRF1, and manganese superoxide dismutase (MnSOD) (28, 29) and regulation of mitochondrial enzymes. ROS can increase autophagy by activating JNK and inhibiting mTORC1 (28, 30, 31). We subsumed these interactions into a direct activation of autophagy by ROS, and similarly included direct activation terms from the UPR and GRP78.
Given the possibility that ERα can regulate ATF4 and XBP1 expression (32, 33), we added ERα regulatory terms to the equations for ATF4 and XBP1. ERα knockdown then reduced ATF4 and XBP1 expression, which in turn decreased the expression of CHOP and GRP78, as observed in the current experiments.
Finally, to model the proliferation rate, we created a scalar proliferation index that is an increasing function of ERα and autophagy and a decreasing function of ROS and CHOP and includes a constant term to represent growth factor signaling. These choices are supported by the following observations: ERα is pro growth even in LCC9 (resistant) cells; autophagy is prosurvival in most cases, not only degrading unfolded proteins and damaged organelles, but also providing energy, amino acids, nucleotides, and other intermediate metabolites (5); ROS can induce cell death by activating JNK and PKCδ (28); and CHOP regulates the transcription of proteins responsible for apoptosis (34). Although we modeled a direct positive effect of ERα on proliferation, this effect subsumes the up-regulation of BCL2 by ERα, which inhibits apoptosis (not explicitly incorporated in the model). Thus, a reduction in ERα can both kill cells by increased apoptosis and reduce the proliferation rate of surviving cells, but the high level of the model combines these individual effects.
A set of parameters was chosen that enabled the model to approximately reproduce the experimental data in Fig. 7B, C. From this initial parameter set, we considered perturbations of up to ±30% in each parameter to create 454 parameter sets that also match the experiment. Simulations were performed using all parameter sets and the mean and standard deviation (error bars) are reported.
RESULTS
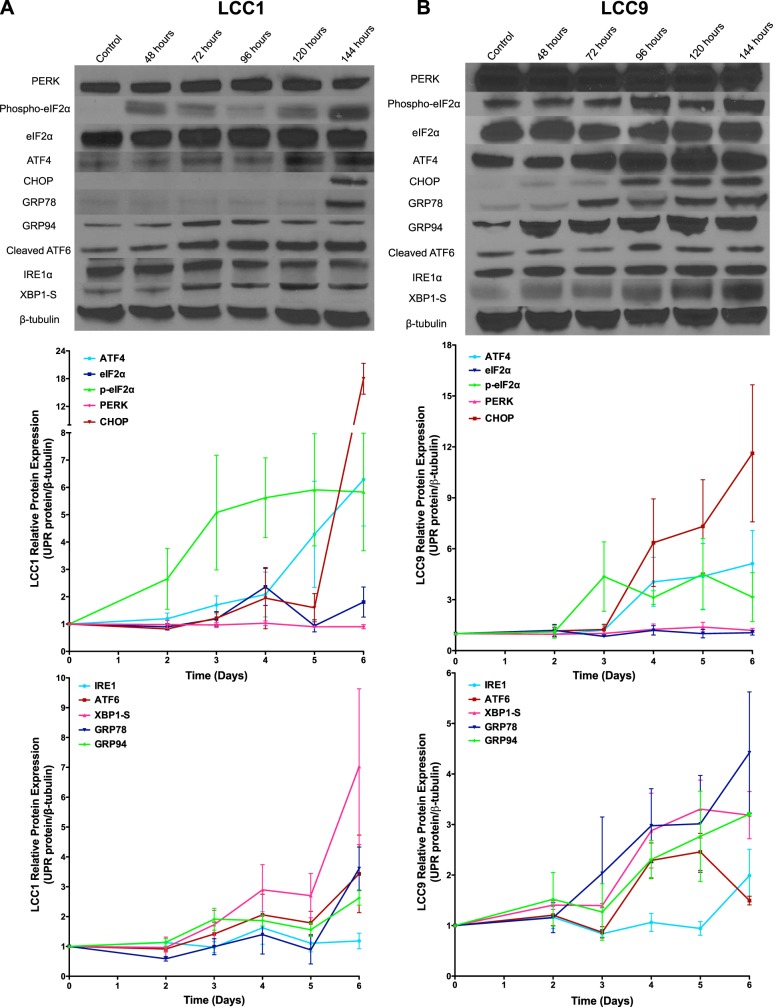
Fulvestrant induced UPR signaling in both antiestrogen-sensitive LCC1 (Fig. 1A) and antiestrogen-resistant LCC9 (Fig. 1B) breast cancer cells. The cells were treated with vehicle (control) or 100 nM ICI for 48, 72, 96, 120, or 144 h, and total protein was harvested. Levels of the UPR-related proteins GRP78, GRP94, IRE1α, PERK, eIF2α, phospho-eIF2α, ATF4, cleaved ATF6, and CHOP, and XBP1-S were each measured by Western blot hybridization. Activation of UPR signaling occurred ∼144 h after treatment initiation in LCC1 breast cancer cells and after ∼72 h in the ICI-resistant LCC9 cells, as observed by increased expression of GRP78, XBP1-S, ATF4, cleaved ATF6, and CHOP. These data suggest a differential activation of UPR signaling in antiestrogen-sensitive vs. -resistant ER+ breast cancer cell lines.
Figure 1.
ICI differentially activates UPR signaling in antiestrogen-sensitive LCC1 and antiestrogen-resistant LCC9 cells. Representative Western blot images of PERK, phospho-eIF2α, eIF2α, ATF4, CHOP, IRE1α, XBP1-S, cleaved-ATF6, GRP78, GRP94, and β-tubulin (loading control) in LCC1 (A) and LCC9 (B) breast cancer cells treated with 100 nM ICI for 6 d. Biological replicate Western blots (n = 3) for each time course were quantified with ImageJ, and data are reported as relative protein expression.
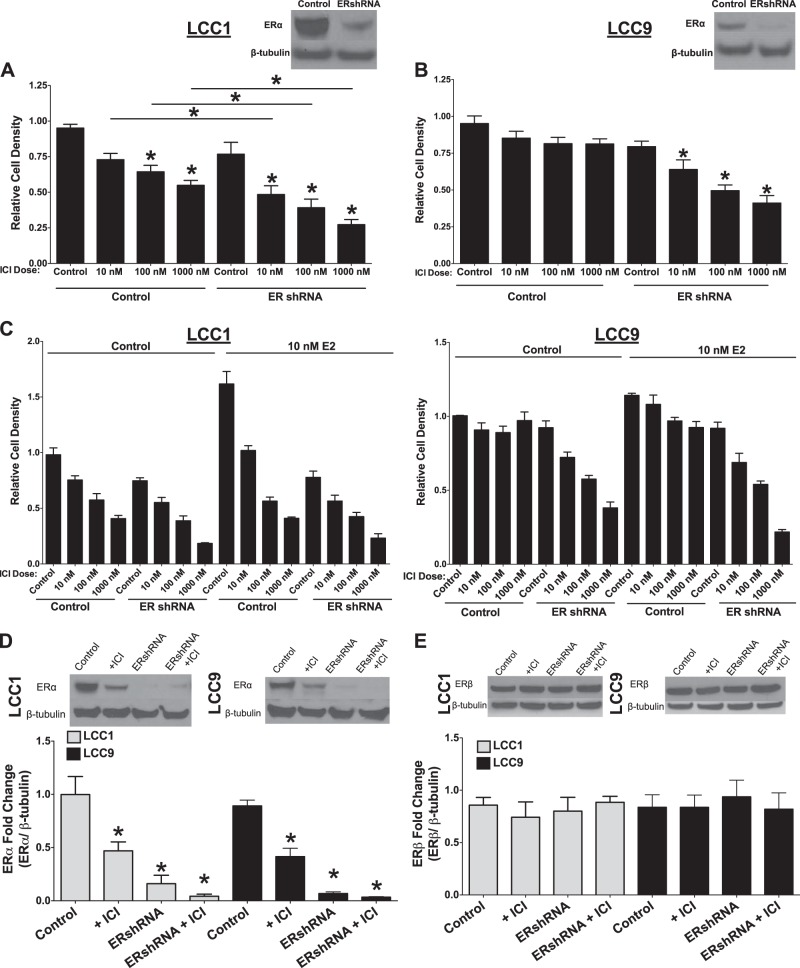
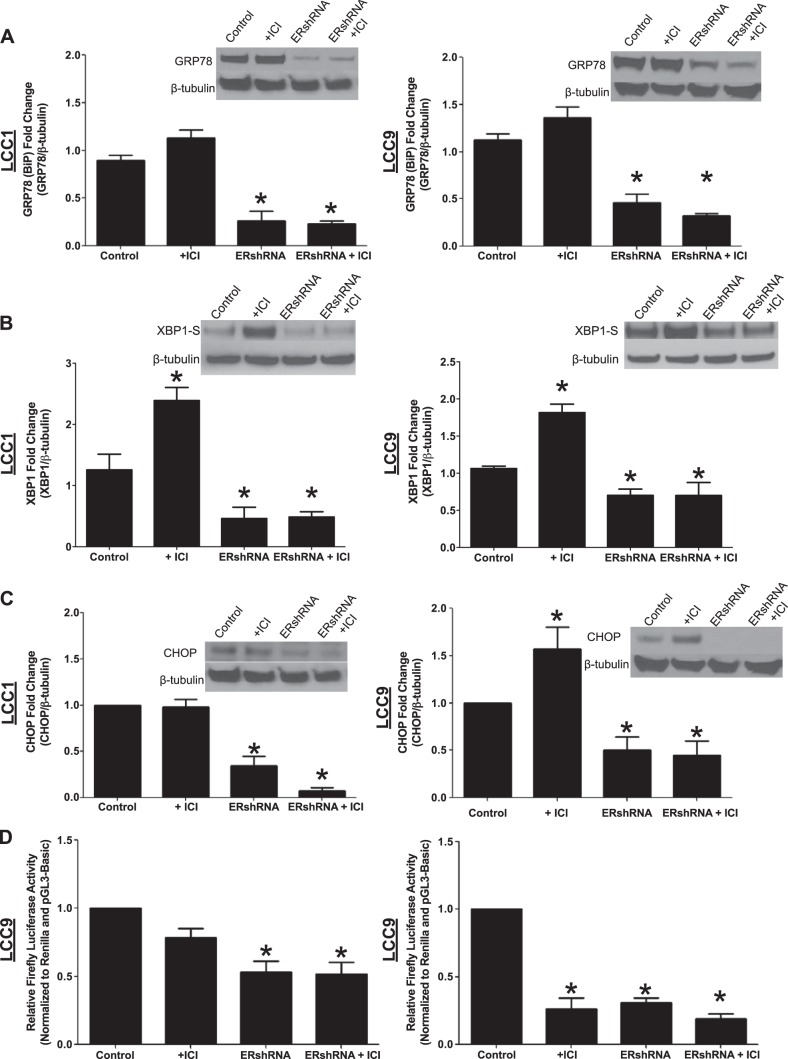
The effect of ERα knockdown on ICI-mediated down-regulation of cell density was determined by the crystal violet assay. LCC1 (Fig. 2A) and -9 (Fig. 2B) cells were transfected with ERα shRNA and treated with vehicle or ICI (10, 100, or 1000 nM) for 72 h. Inhibition of ERα expression enhanced ICI-mediated effects on the LCC1 cells and resensitized the LCC9 cells to antiestrogen therapy, the latter effect being consistent with findings in our earlier report (35). The cells were also treated with 10 nM E2 (Fig. 2C) to determine whether transfection with ERα shRNA successfully inhibits estrogen signaling. E2 treatment did not rescue the cells from an ICI-mediated decrease in cell density, suggesting that ligand activation of estrogen signaling is either inhibited or already maximal but is insufficient for full biological activity in these cells. LCC1 and -9 breast cancer cells were transfected with control or ERα shRNA and treated with vehicle or 100 nM ICI for 72 h. ERα expression was determined by Western blot hybridization, to confirm ERα knockdown. As shown in Fig. 2D, transfection with ERα shRNA produced a greater inhibition of ERα expression than did treatment with ICI, with no overall significant effect on ER-β (Fig. 2E). The effect of ERα knockdown, with or without concurrent antiestrogen treatment, on GRP78 (Fig. 3A), XBP1-S (Fig. 3B), and CHOP (Fig. 3C) expression was determined. Knockdown of ERα inhibited ICI-stimulated UPR signaling in both LCC1 and -9 cells. Knockdown of ERα alone was sufficient to inhibit XBP1-S, CHOP, and GRP78 expression in LCC1 and -9 cells. XBP1-S activity in LCC9 cells was determined with a CRE-luc system (Fig. 3D). Knockdown of ERα resulted in decreased XBP1 activity. ERE activity was also determined by CRE-luc as an internal control, to show inhibition of estrogen signaling by ICI and ERα knockdown.
Figure 2.
A, B) LCC1 (A) and LCC9 (B) cells were transfected with ERα shRNA or control pcDNA and treated with 0.1% v/v ethanol vehicle or 10, 100, or 1000 nM ICI for 72 h, and cell density was measured by crystal violet staining. C) LCC1 and -9 cells were transfected with ERα shRNA or control pcDNA and treated with 0.1% v/v ethanol vehicle or 10, 100, or 1000 nM ICI and/or 10 nM E2 for 72 h, and the cell density was measured by crystal violet staining. An average of 3 biological replicates were performed in triplicate technical replicates (n=3); LCC1 and -9 cells were transfected with ERα shRNA or control pcDNA and treated with or without 100 nM ICI for 72 h. D, E) Western blot hybridization of treated protein homogenates was used to measure ERα (D) or ERβ (E). *P < 0.05; 1-way ANOVA with Bonferroni post hoc analysis.
Figure 3.
Knockdown of ERα inhibits ICI-induced UPR signaling. LCC1 and -9 cells were transfected with ERα shRNA or control pcDNA and treated with or without 100 nM ICI for 72 h. A–C) Western blot hybridization of treated protein homogenates was used to measure GRP78 (A), XBP1-S (B), and CHOP (C) expression. D) XBP1 and ERE activity in LCC9 cells was determined by using a CRE-luc system (n = 3). *P < 0.05; 1-way ANOVA with Bonferroni post hoc analysis.
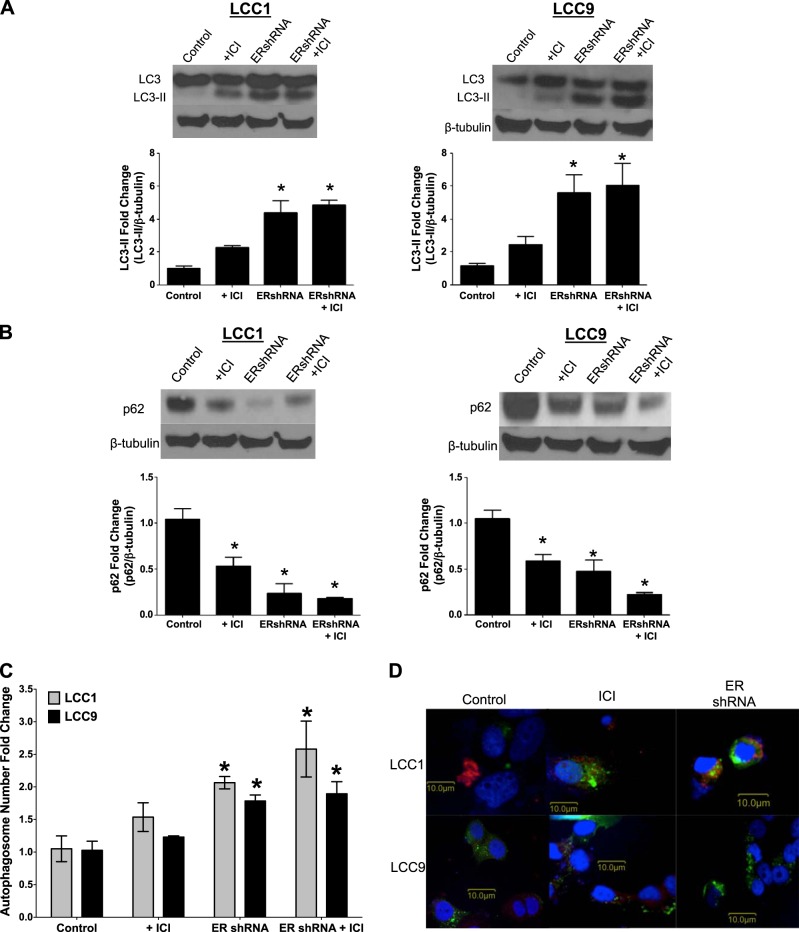
The effect of antiestrogens and ERα inhibition on autophagy in endocrine therapy–sensitive and –resistant cell lines was explored. LCC1 and -9 cells were transfected with control or ERα shRNA and treated with vehicle or 100 nM ICI for 72 h. Total protein was isolated and LC3-II (the lipidated form of LC3 found in autophagosome membranes), and p62 (protein marker for the target cargo of autophagosomes; degraded p62 is a marker of increased autophagy) expression was determined by Western blot hybridization (Fig. 4A, B). Both antiestrogen therapy and ERα knockdown increased LC3-II formation and reduced p62 expression, strongly implicating stimulation of autophagy. These cells were also stained with a modified monodansylcadaverine dye (an autophagosome stain) and the number of autophagosomes was counted by flow cytometry (Fig. 4D). The knockdown of ERα and treatment with antiestrogen drugs increased the monodansylcadaverine-stained cells, indicating increased autophagosome formation. LCC1 and -9 cells were cotransfected with control or ERα shRNA and LC3-GFP cDNA and treated with vehicle or 500 nM ICI and LysoTracker Red for 24 h to determine LC3 puncta formation. The cells treated with ICI or transfected with ERα shRNA showed high levels of LC3-GFP puncta formation (Fig. 4C).
Figure 4.
Knockdown of ERα stimulates autophagy. A, B) Knockdown of ERα resulted in increased LC3-II formation (A) and decreased p62 expression (B) in LCC1 and -9 breast cancer cells. LCC1 cells were transfected with LC3-GFP and treated with ICI and/or ERshRNA. C) A modified monodansylcadaverine stain was used to determine the number of autophagosomes by flow cytometry in the LCC1 and -9 breast cancer cells (n = 3). D) LC3 puncta formation was observed through confocal microscopy. *P < 0.05; 1-way ANOVA with Bonferroni post hoc analysis.
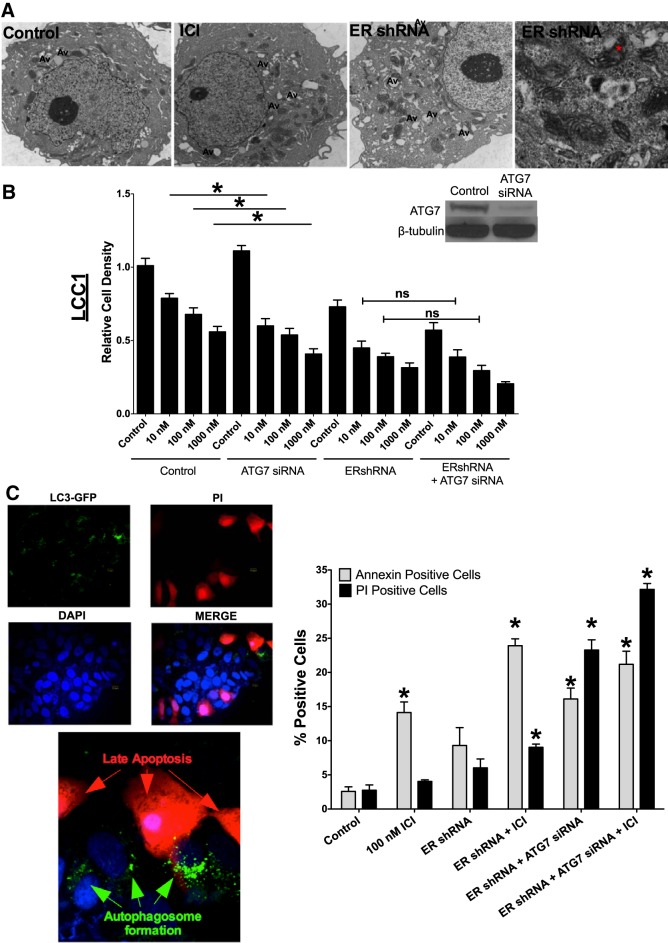
Cell morphology was determined by EM. LCC9 cells were treated with vehicle or 100 nM ICI or transfected with ERα shRNA for 72 h before they were fixed and examined by EM (Fig. 5A). The images of LCC9 transfected with ERα shRNA indicate accumulation of lipids within mitochondria, suggesting that ERα knockdown results in mitochondrial dysfunction.
Figure 5.
ERα-mediated autophagy is prosurvival. A) EM of LCC9 breast cancer cells indicated elevated autophagy and mitochondrial lipid deposition with ERα knockdown. LCC9 breast cancer cells were treated with vehicle, ICI, or ERshRNA and were used to determine cellular morphology by EM. Asterisk: lipid deposition in the mitochondria. Av, autophagic vacuoles. B) LCC1 cells were transfected with control (control siRNA + control shRNA) or ERα shRNA and/or ATG7 siRNA and treated with 0.1% v/v ethanol vehicle or 10, 100, and 1000 nM ICI for 72 h, and cell density was measured with crystal violet. Western blot hybridization of treated protein homogenates was used to measure ATG7 and β-tubulin. C) LCC1 cells were transfected with GFP-LC3 and ERα shRNA and treated with 100 nM ICI for 72 h. The cells were stained with PI and counterstained with DAPI. LC3 puncta and PI positivity were confirmed by confocal microscopy. D) LCC1 cells were transfected with control siRNA or ERα shRNA and/or ATG7 siRNA and treated with 100 nM ICI for 72 h. Annexin V-FITC and PI-stained cells were counted by using flow cytometry (n = 3). *P < 0.05; 1-way ANOVA with the Bonferroni post hoc analysis.
Autophagy is generally reported to be either a prosurvival or a prodeath process. LCC1 cells were transfected with ERα shRNA and/or ATG7 siRNA, to inhibit autophagosome formation (Fig. 5B), and treated with vehicle or various doses of ICI (10, 100, or 1000 nM) for 72 h. ATG7 and ERα knockdown was confirmed by Western blot hybridization. Inhibition of autophagy (through ATG7 knockdown) had no protective effect against ICI-mediated reduction of cell density in ERα knockdown cells, suggesting that autophagy is prosurvival in this context. Moreover, cotransfection of ERα shRNA and ATG7 siRNA significantly increased PI staining of LCC1 cells, indicating elevated levels of late apoptosis and further supporting a prosurvival role for autophagy (Fig. 5D). LCC1 cells were cotransfected with ERα shRNA and LC3-GFP, treated with 500 nM ICI for 24 h, and stained with PI and 4′,6-diamidino-2-phenylindole (DAPI). As shown in Fig. 5C, cells that stained positive for LC3-GFP puncta did not stain positive for PI, further suggesting that cells stimulate autophagy to promote cell survival.
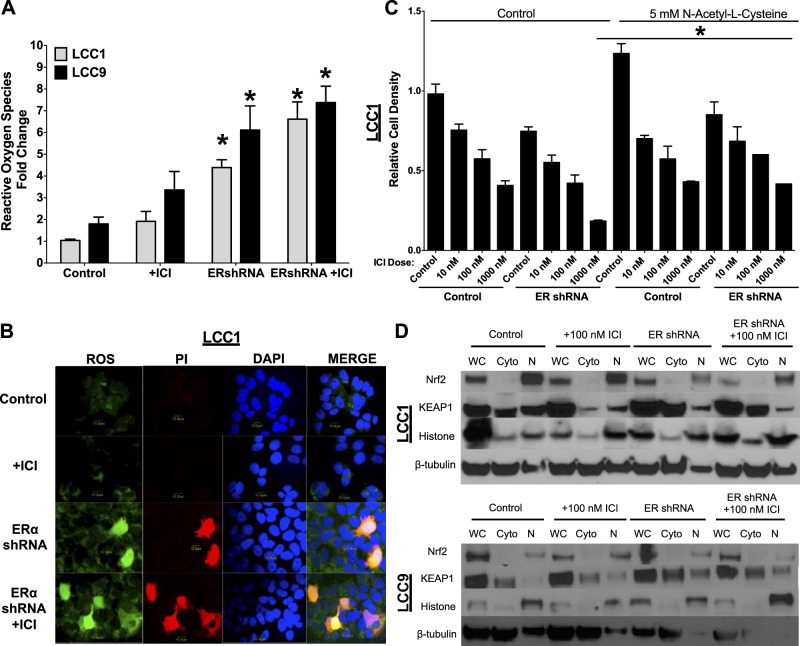
Since the EM images indicated dysfunctional mitochondria, we investigated the effect of antiestrogen therapy and ERα knockdown on generation of ROS. LCC1 and -9 cells were transfected with control or ERα shRNA and treated with 100 nM ICI for 72 h. The cells were harvested and stained for ROS. ROS-positive cells were quantified by flow cytometry (Fig. 6A). Knockdown of ERα, with or without concurrent antiestrogen treatment, resulted in a significant increase in ROS-positive cells when compared with counts of control-transfected cells. LCC1 cells were transfected with control or ERα shRNA and treated with 100 nM ICI for 72 h. The cells were stained for ROS (green), PI (red), or DAPI (blue). As shown in Fig. 6B, RNAi inhibition of ERα expression concurrently increased staining for both ROS and PI. Thus, the high levels of ROS induced by ERα knockdown are most likely proapoptotic. To test this role of ROS in cell fate, LCC1 cells were pretreated with 5 mM N-acetyl-l-cysteine (an antioxidant) and transfected with control or ERα shRNA and treated with vehicle or increasing doses of ICI (10, 100, and 1000 nM) for 72 h (Fig. 6C). Relative cell density was measured in a crystal violet assay. Antioxidant treatment had no effect on control-transfected LCC1 cells. However, N-acetyl-l-cysteine treatment significantly reduced the ICI-mediated inhibition of cell density at 1000 nM ICI in the ERα-ablated LCC1 cells, suggesting an important role of ROS-induced cell death in ERα knockdown–mediated antiestrogen sensitivity.
Figure 6.
ERα knockdown results in the formation of ROS and promotes ICI sensitivity. A) LCC1 and -9 cells were transfected with ERα shRNA or control siRNA and treated with 100 nM ICI for 72 h. Cells that stained positive for ROS were counted by flow cytometry. B) LCC1 cells were transfected with ERα shRNA and treated or not with 100 nM ICI for 72 h. The cells were stained for ROS and apoptosis (PI) and counterstained with DAPI. The presence of ROS was confirmed by confocal microscopy. C) LCC1 cells were transfected with ERα shRNA or control siRNA and treated or not with 5 mM N-acetyl-l-cysteine (an antioxidant) and with 0.1% v/v ethanol vehicle (control) or 10, 100, or 1000 ICI for 72 h, and cell density was measured by crystal violet staining. D) LCC1 and -9 cells were transfected with ERα shRNA or control pcDNA and treated with 100 nM ICI for 72 h or not treated. Western blot hybridization of treated protein homogenates was used to measure whole-cell, cytoplasmic, and nuclear levels of the UPR-induced antioxidant NRF2 and the NRF2 inhibitor KEAP1.
UPR signaling has been shown to play a role in ROS generation and antioxidant signaling (36). LCC1 and -9 cells were transfected with control or ERα shRNA and treated with vehicle or 100 nM ICI for 72 h. Whole cell, cytoplasmic, and nuclear protein concentrated fractions were extracted and the NRF2 and KEAP1 protein levels measured by Western blot hybridization (Fig. 6D). Knockdown of ERα significantly reduced the nuclear (active) expression of NRF2 and increased cytosolic KEAP1 levels, suggesting a role of the UPR-mediated antioxidant NRF2 in stimulating ERα knockdown–induced ROS generation.
Mathematical models of ER signaling
A mathematical model of LCC9 cells was created that contains the key molecular components measured in the experiments described above (Fig. 7A). Figure 7B shows a comparison of the results of model simulations of these components at 72 h with the corresponding experimental measurements in cells treated with vehicle, 100 nM ICI, ERα shRNA, or ICI+ERα shRNA. We also compared the autophagy level in our model with the experimentally measured level of LC3-II. In all cases the model was a good match for the experimental observations, confirming that the pathways that we used are sufficient to explain the results. In the model, ICI treatment activated UPR via an increase in ERα protein aggregates, resulting in an increase in XBP1-S, CHOP, and GRP78. ERα knockdown significantly increased ROS levels, activated autophagy, and reduced stress, contributing to a decrease in UPR activity. This decrease in UPR reduced the levels of XBP1-S, GRP78, and CHOP, a reduction that was enhanced by the decrease of direct signaling from ERα to XBP1 and ATF4. Treatment with ICI and ERα shRNA slightly decreased ERα and GRP78 and increased ROS, compared with the effects of ERα shRNA treatment alone.
Figure 7C shows that the proliferation index replicated the independence of relative proliferation observed experimentally when LCC9 cells were subject to increasing doses of ICI. The explanation for this independence, in the case of the model, is that the amount of ERα was in excess of that needed for normal proliferation. Thus, although the level of ERα decreased significantly with ICI, the decrease in proliferation was not dramatic and was mostly compensated for by an increase in autophagy. In the ERα shRNA case, proliferation decreased with increasing doses of ICI, which is somewhat counterintuitive because knocking down the main target of ICI (ERα) might be expected to impair the effectiveness of ICI. In the model, ERα was no longer present in excess and continued to decrease further with ICI. The increasing level of autophagy did not compensate for the greater reduction in ERα, allowing proliferation to decrease further with the addition of ICI.
To test whether such a simple model, based on a small amount of data, might be useful for predicting and interpreting the wet laboratory results, we predicted the effects of ICI on proliferation when XBP1-U, GRP78, or CHOP were overexpressed 3-fold, or CHOP was knocked down by 70%, both alone and in combination with ERα knockdown.
Validating mathematical model predictions
We show the original experimental proliferation results, our predictions for the proliferation under the new conditions, and the new experimental data obtained to test these predictions. Figure 7D shows that, for XBP1-U overexpression, we predicted a small increase in proliferation relative to the controls, and the result is seen in the new experiments. The additional knockdown of ERα produced a slightly greater increase in proliferation in both the predictions and experiments, although there was a significant difference in the size of the increase with the 100 nM ICI treatment. Figure 7E shows that, for GRP78 overexpression alone, the model predicted a slight increase in proliferation, while in conjunction with ERα shRNA it predicted a substantial increase. Both predictions were borne out by the experiments. Figure 7F shows that a large decrease in proliferation was predicted when CHOP was overexpressed either alone or in conjunction with ERα knockdown. The experiments also showed a large decrease, although the decrease was somewhat greater than that predicted in the cases without shRNA and less than predicted in the cases with ERα shRNA. Figure 7G shows that the model predicted an increase in proliferation for the control case when CHOP was knocked down, but the experiments showed no change. In the ERα shRNA cases, the model predicted no significant change, and the prediction was borne out in the subsequent laboratory experiments.
Taken together, the experiments qualitatively confirmed the model, in that the proliferation varied in the same direction as predicted, although the magnitude of variation differed. Quantitatively accurate predictions will require more detailed models based on substantially more data. Nonetheless, even simple models, such as the one we created in this study, are useful for predicting trends, creating informative experimental designs, and clarifying the interactions among cellular systems.
DISCUSSION
Resistance to endocrine therapies remains a significant clinical challenge. Understanding the mechanisms of resistance could greatly influence how we treat ER+ breast cancers. In the current study, the selective ER down-regulator fulvestrant (Faslodex; ICI 182780; ICI) stimulated the UPR in both antiestrogen-sensitive LCC1 (Fig. 1A) and antiestrogen-resistant LCC9 (Fig. 1B) breast cancer cells.
Although the earlier activation of GRP78 could lead to cell survival, CHOP expression was induced 48 h earlier in the LCC9 cells than in the LCC1 cells, suggesting that LCC9 cells are relatively resistant to proapoptotic CHOP signaling. Other studies in hyperoxia-induced lung injury models also exibited resistance to CHOP-mediated cell death (37). Supplemental Fig. S1A, B shows that CHOP overexpression led to a potent cell death response. However, transfection with CHOP cDNA produced ∼2- to 4-fold higher levels of CHOP expression than ICI-induced CHOP expression did, suggesting a dose–response relationship between CHOP and cell death. Moreover, LCC9 cells were less sensitive to a CHOP overexpression–mediated reduction in cell density.
ERα knockdown in breast cancer cells can potentiate ICI-mediated cell killing, but the mechanism is unknown (40). We confirmed this observation in LCC9 cells (Fig. 2A) and also showed that ERα shRNA increased the dose-dependent inhibition of cell density by ICI in LCC1 cells (Fig. 2B). This finding poses an interesting conundrum: how does knockdown of the primary molecular target for ICI (ERα) increase drug responsiveness?
We hypothesize that changes in other activities of ERα, such as UPR signaling or autophagy, result in the sensitization of breast cancer cells to ERα knockdown and antiestrogen treatment. LCC1 breast cancer cells respond to estrogen, whereas LCC9 cells are insensitive to estrogen stimulation, suggesting differential regulation of signals that mediate the respective sensitization of these cells to ERα activities (35). Transfection of ERα shRNA reduced ERα expression more than did treatment with the selective ER down-regulator ICI (Fig. 2D). We knocked down ERα in LCC1 and -9 cells and then treated them with vehicle or 10 nM E2 and ICI (Fig. 2C). In ERα-knockdown cells, estrogen had no effect on the ICI-mediated down-regulation of cell density, suggesting that transfection of ERα shRNA sufficiently inhibits ERα to prevent adequate prosurvival signaling by E2. Estrogen treatment stimulated cell growth in vehicle-treated LCC1 cells, and estrogen treatment inhibited the low-dose ICI (10 nM)-mediated down-regulation of cell density observed in the LCC1 control-transfected cells. Thus, these breast cancer cells are functionally estrogen independent for survival and proliferation but retain some estrogen responsiveness.
ICI induced UPR signaling in both the LCC1 and -9 breast cancer cells. However, when ERα expression was inhibited by RNAi, UPR signaling was affected differentially. Knockdown of ERα reduced GRP78 expression and inhibited ICI-mediated GRP78 induction (Fig. 3A) in both the LCC1 and -9 cells. Since we have recently established a critical role for GRP78 in mediating antiestrogen responsiveness (5), it is likely that the inhibition of GRP78, mediated by ERα knockdown, enhances ICI efficacy. Moreover, inhibition of ERα in LCC1 and -9 cells regulated XBP1-S (Fig. 3B). ERα knockdown decreased XBP1-S protein levels and prevented ICI-induced XBP1-S in LCC1 and -9 cells. Increased XBP1-S expression can drive antiestrogen resistance, suggesting that the inhibition of XBP1-S by ERα knockdown sensitizes these cells to antiestrogen-mediated cell death (13, 15). In an investigation of XBP1 activity with a CRE-luc system (Fig. 3D), ER knockdown significantly reduced XBP1 activity. Thus, we observed a change in XBP1-S protein levels in ER-ablated LCC9 cells, and XBP1 activity was inhibited, consistent with the reported interaction between ERα and XBP1 (38).
Although we observed a decrease in UPR signaling mediated by ERα knockdown that may affect antiestrogen responsiveness in breast cancer cells, we also observed an inhibition of the proapoptotic protein CHOP (Fig. 3C). This observed decrease in CHOP may reflect altered activation of a putative estrogen response element located in the promoter region of its gene (as determined by promoter gene sequence analysis) and implies that CHOP does not play a major role in cell death induced by ERα knockdown. Supporting this idea, E2 treatment of LCC1 and -9 breast cancer cells induced CHOP expression (Supplemental Fig. S1C).
Autophagy can be either prosurvival or prodeath (6, 18, 39). GRP78-mediated autophagy is critical for desensitizing LCC1 cells to antiestrogens (5, 9). Moreover, MCF7 endocrine-sensitive breast cancer cells serially treated with increasing doses of TAM exhibit increased prosurvival autophagy (21). Antiestrogen-resistant cells also express higher basal levels of autophagy, and inhibiting autophagy using siRNA or chemical inhibitors restores endocrine responsiveness (5, 19). These data indicate that autophagy is prosurvival and its induction can promote endocrine resistance in some ER+ breast cancers. Both ICI and RNAi-knockdown of ERα stimulated autophagy (Figs. 4 and 5), as measured by changes in LC3-II expression, LC3-GFP puncta formation, p62 degradation, modified monodansylcadaverine staining, and EM analysis of cell morphology. In sensitive cells, autophagy is stimulated by ICI through the drug's primary action—down-regulation of ERα protein by ubiquitin-mediated degradation (40)—and this autophagy is prosurvival (Fig. 5). Although ERα knockdown stimulates prosurvival autophagy, it is not sufficient to compensate for the prodeath activity of ERα loss in either antiestrogen-sensitive or -resistant cells.
EM showed increased mitochondrial lipid deposition in LCC9 breast cells transfected with ERα shRNA (Fig. 5A), suggesting a direct role of the ER in modulating mitochondrial homeostasis. Functional ERs have been described in the mitochondria of MCF7 breast cancer cells, where they can prevent mitochondria-generated ROS through the stimulation of MnSOD activity (28). Inhibition of ERα by RNAi increased formation of ROS in both the LCC1 and -9 cells (Fig. 6A). Moreover, increased ROS generation from ERα knockdown was associated with apoptosis and cell death (Fig. 6B). Pretreatment with the antioxidant N-acetyl-l-cysteine attenuated ICI-mediated down-regulation of cell proliferation in LCC1 cells transfected with ERα shRNA at the highest dose (1000 nM) of ICI, with no effect on control-transfected cells (Fig. 6C).
A possible mechanism of ROS generation after ERα knockdown is through inhibition of the UPR signaling that lowers the cellular response to stress. ERα regulates a mitochondrial UPR signaling mechanism in MCF7 breast cancer cells (41), and UPR signaling can activate and promote the transcription of NRF2, a potent cellular antioxidant signaling molecule. KEAP1 binds to NRF2, segregating NRF2 in the cytosol. UPR activation of PERK disrupts the KEAP1/NRF2 interaction, enabling NRF2 to enter the nucleus and induce the transcription of antioxidant-related genes (42). Transfection with ERα shRNA in LCC1 and -9 cells reduced the nuclear localization (active form) of NRF2 while concurrently stimulating cytoplasmic accumulation of KEAP1 (Fig. 6D). Accumulation of cytosolic KEAP1 would bind any remaining NRF2, thereby preventing a UPR-induced antioxidant response and allowing ROS generation. Moreover, treatment with the antioxidant SOD, or the potent oxidant hydrogen peroxide, showed differential activation of GRP78 between LCC1 and -9 breast cancer cells (Supplemental Fig. S2), suggesting a key role of ROS in mediating UPR signaling. These data show that knockdown of ERα mediates ICI-stimulated cell death through both the inhibition of UPR signaling and the formation of sufficiently high levels of ROS, for which activation of prosurvival autophagy cannot compensate.
The simple mathematical model that we created shows that it is possible to explain the LCC9 experiments in a consistent and quantitative manner using our current understanding of the relevant cellular systems. Perhaps the most useful result of the model is its explanation of the decrease in proliferation with ICI treatment in resistant cells when ERα is already knocked down by RNAi. We were then able to validate experimentally that the model can make qualitatively accurate predictions of the effects of overexpressing or knocking down certain key proteins. Now that we have investigated ERα and autophagy as survival mechanisms in the current model of proliferation, we plan to determine contributions from other components, such as AKT, ERK, and NFκB, in future modeling. For example, we cannot exclude the possibility that the overproduction of ROS also activates AKT (41), ERK (30), and NFκB (reviewed in ref. 43). Measuring these components after ERα knockdown may help further elucidate the crosstalk among the ER, ROS, and growth factor–signaling pathways. In addition, while decreased expression of ATF4 and XBP1 caused lower CHOP and GRP78 expression in the model, a direct role of ERα in the regulation of CHOP and GRP78 cannot be excluded and will be examined experimentally in future studies.
CONCLUSIONS
Antiestrogen resistance affects up to 50% of all patients with ER+ breast cancer; effectively ∼35% of all breast cancers. Understanding endocrine therapy–related molecular signaling may increase our ability to target ERα without stimulating resistance pathways. This study showed the critical prosurvival actions of antiestrogen-induced UPR signaling and autophagy that may lead to drug resistance. We showed that ICI stimulates autophagy through down-regulation of ERα, whereas ICI most likely promotes UPR signaling through the accumulation of cytoplasmic ERα. Inhibiting UPR signaling through ERα ablation resulted in the stimulation of prodeath ROS generation, promoting antiestrogen-mediated cell death in both endocrine-sensitive and -resistant breast cancers. Mathematical modeling was used to explain several aspects of the experimental data and to make subsequently validated qualitative predictions of the effects of model-proposed overexpression experiments. Our data suggest that combining UPR signaling inhibitors or autophagy inhibitors with antiestrogen drug therapy would be beneficial in the treatment of ER+ breast cancer and reduce the occurrence of drug resistance.
Supplementary Material
Acknowledgments
This study was supported by a U. S. Department of Defense Breast Cancer Research Program Postdoctoral Fellowship (BC112023; to K.C.) and in part by awards from the U.S. Department of Health and Human Services (U54-CA149147 and R01-CA131465) (to R.C.).
The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ATF
- activating transcription factor
- ATG
- autophagy-related gene
- CCS
- charcoal-stripped calf serum
- CHOP
- C/EBP homologous protein (also known as DDIT3, DNA damage-inducible transcript 3)
- DAPI
- 4′,6-diamidino-2-phenylindole
- E2
- 17β-estradiol
- eIF
- elongation initiation factor
- ER
- estrogen receptor
- GRP
- glucose-regulated protein
- ICI
- Faslodex, fulvestrant, or ICI 182780
- IHC
- immunohistochemistry
- IRE
- inositol-requiring enzyme
- KEAP1
- kelch-like ECH-associated protein 1
- LAMP
- lysosome-associated membrane protein
- LC3
- microtubule-associated protein light chain 3
- MnSOD
- manganese superoxide dismutase
- NRF2
- nuclear factor (erythroid-derived 2)-like 2
- PERK
- PKR-like endoplasmic reticulum kinase
- PI
- propidium iodide
- ROS
- reactive oxygen species
- TAM
- tamoxifen
- UPR
- unfolded protein response
- XBP
- X-box-binding protein
REFERENCES
- 1. DeSantis C., Siegel R., Bandi P., Jemal A. (2011) Breast cancer statistics, 2011. CA Cancer J. Clin. 61, 409–418 [DOI] [PubMed] [Google Scholar]
- 2. Clarke R., Skaar T. C., Bouker K. B., Davis N., Lee Y. R., Welch J. N., Leonessa F. (2001) Molecular and pharmacological aspects of antiestrogen resistance. J. Steroid Biochem Mol. Biol. 76, 71–84 [DOI] [PubMed] [Google Scholar]
- 3. Clarke R., Leonessa F., Welch J. N., Skaar T. C. (2001) Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol. Rev. 53, 25–71 [PubMed] [Google Scholar]
- 4. Riggins R. B., Bouton A. H., Liu M. C., Clarke R. (2005) Antiestrogens, aromatase inhibitors, and apoptosis in breast cancer. Vitam. Horm. 71, 201–237 [DOI] [PubMed] [Google Scholar]
- 5. Clarke R., Cook K. L., Hu R., Facey C. O., Tavassoly I., Schwartz J. L., Baumann W. T., Tyson J. J., Xuan J., Wang Y., Warri A., Shajahan A. N. (2012) Endoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fate. Cancer Res. 72, 1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cook K. L., Shajahan A. N., Clarke R. (2011) Autophagy and endocrine resistance in breast cancer. Expert Rev. Anticancer Ther. 11, 1283–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cook K. L., Clarke P. A., Clarke R. (2013) Targeting GRP78 and antiestrogen resistance in breast cancer. Future Medicin. Chem. 5, 1047–1057 [DOI] [PubMed] [Google Scholar]
- 8. Cook K. L., Soto-Pantoja D. R., Abu-Asab M., Clarke P. A., Roberts D. D., Clarke R. (2014) Mitochondria directly donate their membrane to form autophagosomes during a novel mechanism of parkin-associated mitophagy. Cell Biosci. 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parmar J. H., Cook K. L., Shajahan-Haq A. N., Clarke P. A., Tavassoly I., Clarke R., Tyson J. J., Baumann W. T. (2013) Modelling the effect of GRP78 on anti-oestrogen sensitivity and resistance in breast cancer. Interface Focus 3, 20130012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clarke R., Shajahan A. N., Wang Y., Tyson J., Riggins R. B., Weiner L. M., Baumann W. T., Xuan J., Zhang B., Facey C. O., Aiyer H., Cook K. L., Hickman F. E., Tavassoly I., Verdugo A., Chen C., Zwart A., Warri A., Hilakivi-Clarke L. A. (2011) Endoplasmic reticulum stress, the unfolded protein response, and gene network modeling in antiestrogen resistant breast cancer. Horm. Mol. Biol. Clin. Investig. 5, 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verfaillie T., Garg A. D., Agostinis P. (2010) Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 33, 249–264 [DOI] [PubMed] [Google Scholar]
- 12. Verfaillie T., Salazar M., Velasco G., Agostinis P. (2010) Linking ER stress to autophagy: potential implications for cancer therapy. Int. J. Cell. Biol. 2010, 930509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gomez B. P., Riggins R. B., Shajahan A. N., Klimach U., Wang A., Crawford A. C., Zhu Y., Zwart A., Wang M., Clarke R. (2007) Human X-box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. FASEB J. 21, 4013–4027 [DOI] [PubMed] [Google Scholar]
- 14. Scriven P., Coulson S., Haines R., Balasubramanian S., Cross S., Wyld L. (2009) Activation and clinical significance of the unfolded protein response in breast cancer. Br. J. Cancer 101, 1692–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shajahan A. N., Riggins R. B., Clarke R. (2009) The role of X-box binding protein-1 in tumorigenicity. Drug News Perspect. 22, 241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gu Z., Lee R. Y., Skaar T. C., Bouker K. B., Welch J. N., Lu J., Liu A., Zhu Y., Davis N., Leonessa F., Brunner N., Wang Y., Clarke R. (2002) Association of interferon regulatory factor-1, nucleophosmin, nuclear factor-kappaB, and cyclic AMP response element binding with acquired resistance to Faslodex (ICI 182780). Cancer Res. 62, 3428–3437 [PubMed] [Google Scholar]
- 17. Cook K. L., Clarke R. (2012) Heat shock 70 kDa protein 5/glucose-regulated protein 78 “AMP”ing up autophagy. Autophagy 8, 1827–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clarke R., Shajahan A. N., Riggins R. B., Cho Y., Crawford A., Xuan J., Wang Y., Zwart A., Nehra R., Liu M. C. (2009) Gene network signaling in hormone responsiveness modifies apoptosis and autophagy in breast cancer cells. J. Steroid Biochem. Mol. Biol. 114, 8–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crawford A. C., Riggins R. B., Shajahan A. N., Zwart A., Clarke R. (2010) Co-inhibition of BCL-W and BCL2 restores antiestrogen sensitivity through BECN1 and promotes an autophagy-associated necrosis. PloS One 5, e8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qadir M. A., Kwok B., Dragowska W. H., To K. H., Le D., Bally M. B., Gorski S. M. (2008) Macroautophagy inhibition sensitizes tamoxifen-resistant breast cancer cells and enhances mitochondrial depolarization. Breast Cancer Res. Treat. 112, 389–403 [DOI] [PubMed] [Google Scholar]
- 21. Samaddar J. S., Gaddy V. T., Duplantier J., Thandavan S. P., Shah M., Smith M. J., Browning D., Rawson J., Smith S. B., Barrett J. T., Schoenlein P. V. (2008) A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antiestrogen resistance. Mol. Cancer Ther. 7, 2977–2987 [DOI] [PubMed] [Google Scholar]
- 22. Schoenlein P. V., Periyasamy-Thandavan S., Samaddar J. S., Jackson W. H., Barrett J. T. (2009) Autophagy facilitates the progression of ERalpha-positive breast cancer cells to antiestrogen resistance. Autophagy 5, 400–403 [DOI] [PubMed] [Google Scholar]
- 23. Brunner N., Boulay V., Fojo A., Freter C. E., Lippman M. E., Clarke R. (1993) Acquisition of hormone-independent growth in MCF-7 cells is accompanied by increased expression of estrogen-regulated genes but without detectable DNA amplifications. Cancer Res. 53, 283–290 [PubMed] [Google Scholar]
- 24. Brunner N., Boysen B., Jirus S., Skaar T. C., Holst-Hansen C., Lippman J., Frandsen T., Spang-Thomsen M., Fuqua S. A., Clarke R. (1997) MCF7/LCC9: an antiestrogen-resistant MCF-7 variant in which acquired resistance to the steroidal antiestrogen ICI 182780 confers an early cross-resistance to the nonsteroidal antiestrogen tamoxifen. Cancer Res. 57, 3486–3493 [PubMed] [Google Scholar]
- 25. Soto-Pantoja D. R., Miller T. W., Pendrak M. L., DeGraff W. G., Sullivan C., Ridnour L. A., Abu-Asab M., Wink D. A., Tsokos M., Roberts D. D. (2012) CD47 deficiency confers cell and tissue radioprotection by activation of autophagy. Autophagy 8, 1628–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ishii Y., Papa L., Bahadur U., Yue Z., Aguirre-Ghiso J., Shioda T., Waxman S., Germain D. (2011) Bortezomib enhances the efficacy of fulvestrant by amplifying the aggregation of the estrogen receptor, which leads to a proapoptotic unfolded protein response. Clin. Cancer Res. 17, 2292–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen C. S., Tseng Y. T., Hsu Y. Y., Lo Y. C. (2012) Nrf2-Keap1 antioxidant defense and cell survival signaling are upregulated by 17beta;estradiol in homocysteine-treated dopaminergic SH-SY5Y cells. Neuroendocrinology 97, 232–241 [DOI] [PubMed] [Google Scholar]
- 28. Pedram A., Razandi M., Wallace D. C., Levin E. R. (2006) Functional estrogen receptors in the mitochondria of breast cancer cells. Mol. Biol. Cell 17, 2125–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mattingly K. A., Ivanova M. M., Riggs K. A., Wickramasinghe N. S., Barch M. J., Klinge C. M. (2008) Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol. Endocrinol. 22, 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong C. H., Iskandar K. B., Yadav S. K., Hirpara J. L., Loh T., Pervaiz S. (2010) Simultaneous induction of non-canonical autophagy and apoptosis in cancer cells by ROS-dependent ERK and JNK activation. PloS One 5, e9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alexander A., Cai S. L., Kim J., Nanez A., Sahin M., MacLean K. H., Inoki K., Guan K. L., Shen J., Person M. D., Kusewitt D., Mills G. B., Kastan M. B., Walker C. L. (2010) ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc. Natl. Acad. Sci. U. S. A. 107, 4153–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kiewlich D. (2009) Exploration of the co-regulation of the potential positive feedback partners estrogen receptor alpha and X-Box binding protein 1. Ph.D. thesis University of California, Davis [Google Scholar]
- 33. Bourdeau V., Deschenes J., Metivier R., Nagai Y., Nguyen D., Bretschneider N., Gannon F., White J. H., Mader S. (2004) Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol. Endocrinol. 18, 1411–1427 [DOI] [PubMed] [Google Scholar]
- 34. Marciniak S. J., Yun C. Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H. P., Ron D. (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuske B., Naughton C., Moore K., Macleod K. G., Miller W. R., Clarke R., Langdon S. P., Cameron D. A. (2006) Endocrine therapy resistance can be associated with high estrogen receptor alpha (ERalpha) expression and reduced ERalpha phosphorylation in breast cancer models. Endocr. Relat. Cancer 13, 1121–1133 [DOI] [PubMed] [Google Scholar]
- 36. Jager R., Bertrand M. J., Gorman A. M., Vandenabeele P., Samali A. (2012) The unfolded protein response at the crossroads of cellular life and death during endoplasmic reticulum stress. Biol. Cell 104, 259–270 [DOI] [PubMed] [Google Scholar]
- 37. Lozon T. I., Eastman A. J., Matute-Bello G., Chen P., Hallstrand T. S., Altemeier W. A. (2011) PKR-dependent CHOP induction limits hyperoxia-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L422–L429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ding L., Yan J., Zhu J., Zhong H., Lu Q., Wang Z., Huang C., Ye Q. (2003) Ligand-independent activation of estrogen receptor alpha by XBP-1. Nucleic Acids Res. 31, 5266–5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. He C., Klionsky D. J. (2009) Regulation mechanisms and signaling pathways of autophagy. Ann. Rev. Genet. 43, 67–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Long X., Nephew K. P. (2006) Fulvestrant (ICI 182780)-dependent interacting proteins mediate immobilization and degradation of estrogen receptor-alpha. J. Biol. Chem. 281, 9607–9615 [DOI] [PubMed] [Google Scholar]
- 41. Papa L., Germain D. (2011) Estrogen receptor mediates a distinct mitochondrial unfolded protein response. J. Cell Sci. 124, 1396–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cullinan S. B., Zhang D., Hannink M., Arvisais E., Kaufman R. J., Diehl J. A. (2003) Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 23, 7198–7209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gloire G., Legrand-Poels S., Piette J. (2006) NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 72, 1493–1505 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.