Abstract
Tropomodulin1 (Tmod1) is an actin-capping protein that plays an important role in actin filament pointed-end dynamics and length in striated muscle. No mechanisms have been identified to explain how Tmod1's functional properties are regulated. The purpose of this investigation was to explore the functional significance of the phosphorylation of Tmod1 at previously identified Thr54. Rat cardiomyocytes were assessed for phosphorylation of Tmod1 using Pro-Q Diamond staining and 32P labeling. Green fluorescent protein-tagged phosphorylation-mimic (T54E) and phosphorylation-deficient (T54A) versions of Tmod1 were expressed in cultured cardiomyocytes, and the ability of these mutants to assemble and restrict actin lengths was observed. We report for the first time that Tmod1 is phosphorylated endogenously in cardiomyocytes, and phosphorylation at Thr54 causes a significant reduction in the ability of Tmod1 to assemble to the pointed end compared with that of the wild type (WT; 48 vs. 78%, respectively). In addition, overexpression of Tmod1-T54E restricts actin filament lengths by only ∼3%, whereas Tmod1-WT restricts the lengths significantly by ∼8%. Finally, Tmod1-T54E altered the actin filament-capping activity in polymerization assays. Taken together, our data suggest that pointed-end assembly and Tmod1's thin filament length regulatory function are regulated by its phosphorylation state.—Bliss, K. T., Tsukada, T., Novak, S. M., Dorovkov, M. V., Shah, S. P., Nworu, C., Kostyukova, A. S., Gregorio, C. C. Phosphorylation of tropomodulin1 contributes to the regulation of actin filament architecture in cardiac muscle.
Keywords: post-translational modification, sarcomere
Regulation of actin filament organization and dynamics is important for cellular architecture and numerous biological functions including muscle contraction. The functional and structural properties of actin filaments are precisely regulated by the polymerization and depolymerization of actin monomers at both fast-growing (barbed) and slow-growing (pointed) ends. Actin monomer dynamics at thin filament ends are regulated by actin filament-capping proteins (for reviews, see refs. 1–3).
Tropomodulin1 (Tmod1) is an actin thin filament pointed-end capping protein in striated muscle (4). Tmod1 is 1 of 4 isoforms in the tropomodulin family (Tmod1−4) that are ∼70% similar in amino acid sequence with different expression profiles (5). Tmod1 is the only isoform expressed in cardiac muscle where it interacts with the thin filament components actin and tropomyosin (6, 7). Each protein in the tropomodulin family contains an N-terminal unstructured domain and a C-terminal domain consisting of 5 leucine-rich repeat motifs (8, 9). The functional regions within Tmod1 that have been identified include 2 actin-binding regions (aa 48−92 and 344–359) and 2 tropomyosin-binding regions (aa 1–38 and 109–144; refs. 10–13).
Functional studies have revealed that Tmod1 plays important roles in thin filament regulation and myofibrillogenesis. For example, studies in primary cultures of cardiomyocytes showed that either decreased or increased expression of Tmod1 resulted in thin filament elongation or shortening, respectively (14–16). In addition, overexpression of Tmod1 in mouse hearts results in dilated cardiomyopathy and degenerating myofibrils (17). Furthermore, Tmod1 null cardiomyocytes derived from mouse embryonic stem cells display fewer and more immature myofibrils (18), and Tmod1-knockout mice die at embryonic day 10.5 because of lethal defects in the myocardium (19–21).
Despite the substantial data reported on the structure and function of Tmod1, the mechanisms that regulate Tmod1 remain unclear. This is surprising because several mechanisms that regulate other known capping proteins, such as CapZ, the actin filament barbed-end capping protein in striated muscle, have been identified (for review, see ref. 1). Phosphorylation is one of the major post-translational modifications responsible for the regulation of cytoskeletal protein function in striated muscle (for reviews, see refs. 22–24). With respect to Tmod, a previous study using an anti-threonine antibody demonstrated phosphorylation of Tmod1 after activation with protein kinase Cα (PKCα); phosphorylated Tmod1 associated more readily with the rabbit lens epithelial cytoskeleton (25).
Transient receptor potential cation channel, subfamily M, member 7 (TRPM7) is a bifunctional protein containing a divalent cation channel fused to an intracellular α-kinase domain (26–28). The α-kinase family (29) contains proteins known for their ability to phosphorylate serine and threonine residues (for review, see ref. 30). TRPM7 is essential for cell survival and is believed to play an important role in Ca2+ and Mg2+ homeostasis, in regulation of cell growth and proliferation, in cell death, and in cell adhesion and motility (27, 31–36). It was found previously that the kinase domain of TRPM7 phosphorylates Tmod1 at Thr54 (37). To date, there have been no studies demonstrating the role of altering the phosphorylation state of Tmod1 in striated muscle. Here, we provide the first evidence that Tmod1 is phosphorylated endogenously in cardiac cells and determine that the modulation of Tmod1 Thr54 phosphorylation in cardiac myocytes is an important aspect of actin filament pointed-end capping activity.
MATERIALS AND METHODS
Protein expression and purification
Tmod1 was expressed in Escherichia coli BL21(DE3) pLysS using the method described in ref. 38 and purified according to ref. 9. G-actin was purified on a Sephacryl S-300 column. To detect actin polymerization using fluorescence, G-actin was labeled with pyrenyl-iodoacetamide, and the labeling ratios were calculated according to refs. 39 and 40). Protein purity was evaluated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; ref. 41). Concentrations of proteins were determined using a BCA protein assay kit (Thermo Scientific, Waltham, MA, USA) or by measuring their difference spectra in 6 M guanidine-HCl between pH 12.5 and 7.0 (42), using the extinction coefficients of 2357 per tyrosine and 830 per tryptophan (43).
To study the tropomyosin-Tmod1 interaction, model chimeric tropomyosin peptide, αTM1aZip, was synthesized by the Tufts University Core Facility (Boston, MA, USA). The peptide contains 14 N-terminal residues of long muscle α-tropomyosin [striated muscle α-tropomyosin (stTM)] and 19 C-terminal residues of the GCN4 leucine zipper domain, which was designed previously (44). N-acetylated stTM was a gift from Dr. Sarah Hitchcock-DeGregori (Robert Wood Johnson Medical School, Piscataway, NJ, USA). Recombinant human gelsolin was a gift from Dr. John Hartwig (Brigham and Women's Hospital and Hematology Division, Boston, MA, USA).
Plasmid construction
Site-directed mutagenesis was performed using a QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). The plasmids were amplified by PCR using PfuTurbo DNA polymerase and 2 complementary sets of oligonucleotides, which contain changed triplets, according to the manufacturer's instructions with one modification. For PCR, instead of mixing all components together, 2 solutions were made, each of which contained only one of the complementary oligonucleotides. After 4 cycles, the solutions were mixed and then 18 additional cycles were performed. The plasmids for expression of chicken Tmod1 (9) and mouse Tmod1 (7) were used as the templates. After PCR, the original plasmid was digested using DpnI, and the mixture was used to transform E. coli (Max Efficiency DH5α; Invitrogen, Carlsbad, CA, USA). After plasmid purification, the presence of mutations was confirmed by DNA sequencing. For transfection experiments, the mouse Tmod1 mutants were subcloned into the pEGFP-C1 vector (Clontech, Mountain View, CA, USA). Synthesis of all oligonucleotides and DNA sequencing were done at the University of Medicine and Dentistry of New Jersey DNA Synthesis and Sequencing Facility (Robert Wood Johnson Medical School Medical School, Piscataway, NJ, USA).
Functional experiments
Tropomyosin/Tmod1 binding was assayed by circular dichroism analysis using an model 400 spectropolarimeter (Aviv, Lakewood, NJ, USA) as described previously (12). The rates of actin polymerization were determined by the change in pyrene actin fluorescence using a fluorimeter (excitation, 366 nm; and emission, 387 nm; PTI, Lawrenceville, NJ, USA) as described previously (45). To measure the polymerization of actin at the pointed end, short filaments were capped at the barbed ends with gelsolin and prepared according to ref. 11. Polymerization was monitored by the increase in fluorescence when the filaments were diluted 5-fold with G-actin (10% pyrenyl actin) in polymerizing buffer (100 mM KCl, 2 mM MgCl2, 1 mM EGTA, 0.5 mM dithiothreitol, 0.2 mM ATP, 0.2 mM CaCl2, 1 mM NaN3, and 10 mM imidazole; pH 7.0) containing tropomyosin and Tmod1.
Cell culture and transfection procedures
Cardiac myocytes were isolated from 1- to 3-d-old neonatal rats and maintained as described previously (46). Isolated cells were plated in 35-mm tissue culture dishes containing 12-mm round glass coverslips for staining (∼1×106 cells/dish). Transfection was performed using Effectene (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. In brief, 24 h after plating, cultured myocytes were placed in 1.5 ml of prewarmed culture medium and returned to the incubator while DNA-Effectene complexes were formed. Then 0.5 μg of plasmid was mixed with 1.6 μl of Enhancer in 50 μl of optimized buffer and incubated at room temperature for 5 min. Next, 5 μl of Effectene reagent was added, and the mixture was incubated at room temperature for 10 min. After the incubation, the mixture was brought to a 0.5-ml volume using prewarmed culture medium and then added dropwise to the culture dish. The transfection efficiencies were ≤5% in this cell type.
Immunofluorescence microscopy
At 4 d after transfection, myocytes were fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min. Coverslips were washed and stored in PBS at 4°C until staining. Transfected cardiac myocytes were stained as described previously (47). The fixed cells were permeabilized in 0.2% Triton X-100-PBS for 15 min, incubated in blocking solution (2% bovine serum albumin plus 1% normal donkey serum in PBS) for 1 h and then incubated for 1 h with primary antibodies diluted in the blocking solution. The primary antibodies included rabbit anti-green fluorescent protein (GFP) antibodies (1:2000, ab290; Abcam, Cambridge, MA, USA) and monoclonal antisarcomeric α-actinin antibodies (1:3000, EA-53; Sigma-Aldrich, St. Louis, MO, USA). Texas Red-conjugated phalloidin (1:50, T7471, Invitrogen) was used to stain F-actin. The cells were washed and then were incubated with secondary antibodies in PBS for 45 min. Secondary antibodies obtained from Invitrogen included Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:1000, A11034) and Alexa Fluor 350-conjugated goat anti-mouse IgG (1:350, A21049). All coverslips were mounted on slides using Aqua-Poly/Mount (Polysciences, Warrington, PA, USA) and subsequently analyzed on an Axiovert microscope using a ×100 (numerical aperture 1.3) objective (Zeiss, Thornwood, NY, USA), and micrographs were recorded as digital images on a Photonics ORCA-ER digital camera (Hamamatsu, Shizuoka, Japan). Images were prepared for presentation using Photoshop CS (Adobe Systems, San Jose, CA, USA) and were minimally processed for brightness and contrast, applying these changes equally to the entire image. Actin filament lengths were measured from images of cells stained with Texas Red-conjugated phalloidin using ImageJ 1.41 software (U.S. National Institutes of Health, Bethesda, MD, USA). A paired Student's t test was used to test significance in the length restriction experiments. When determining “consistent” vs. “inconsistent/diffuse” Tmod1 assembly categories, consistent refers to clear and well-defined striated staining (>90% of sarcomeres within the cell contain GFP-positive striations), and inconsistent/diffuse refers to faint and partial or no detectable striated staining (<90% of sarcomeres contain GFP striations).
Immunoprecipitation (IP) and phosphodetection of GFP-Tmod1
Rat cardiomyocytes at a density of 7.5 × 105 cells/35-mm tissue culture dish were transduced 48 h after plating with either 20 multiplicity of viral infection (MOI) of GFP or GFP-mTmod1 adenovirus in serum-free conditions. After 48 h, the cells were treated with 1 μM okadaic acid (EMD Millipore, Billerica, MA, USA) or vehicle [dimethylsulfoxide (DMSO)] control for 1 h before collection. Cells were harvested in a buffer containing 20 mM Tris-HCl (pH 8.0), 137 mM NaCl, 10% glycerol, 1% Nonidet P-40, and 2 mM EDTA with 1× Halt protease inhibitor cocktail (Thermo Scientific,), 20 mM NaF, and 2 mM sodium orthovanadate. IP was performed using a llama GFP-binding protein fused to the Fc domain of human IgG, tagged with His6, and cross-linked to protein A resin (48). The IP was run on a 12% precast SDS-PAGE gel (Bio-Rad, Hercules, CA, USA). Total phosphoprotein was stained using Pro-Q Diamond gel stain (Invitrogen), following the manufacturer's instructions, and Coomassie blue for total protein. Images of the Pro-Q Diamond and Coomassie blue staining were obtained using a G:BOX Chemi XL1.4 imaging system (Syngene, Cambridge, UK).
Adenoviral (Adv) production of Tmod1
A replication-defective Adv vector expressing GFP-Tmod1 was constructed using the AdEasy Adenoviral Vector System (Stratagene. In brief, GFP-Tmod1 cDNA was subcloned into a pShuttle-CMV plasmid and linearized according to the manufacturer's instructions before transformation of BJ5183 cells containing the pAdEasy-1 vector. After homologous recombination, the purified pAdEasy-1 vector containing GFP-Tmod1 was then transfected into human embryonic kidney (HEK) 293 cells for adenovirus propagation. In each experiment, a replication-defective adenovirus expressing GFP was used to control for the nonspecific effects of Adv infection. All adenoviruses were propagated in HEK293 cells and purified by CsCl gradient centrifugation. The MOI was determined by a viral dilution assay in HEK293 cells grown in 96-well clusters. At an MOI of 5–10, >95% of the cells were infected, as determined by GFP-positive cells. There were no cytotoxic effects during the 24 h after Adv infection.
32P protein phosphorylation analysis
Embryonic chicken cardiomyocytes were cultured for 4 d in a 10-cm tissue culture dish and washed with phosphate-free Dulbecco's modified Eagle's medium (DMEM; Invitrogen), and the medium was replaced with phosphate-free DMEM supplemented with 5% fetal bovine serum. 32P was added at a concentration of 0.25 mCi/ml, and the cells were incubated at 37°C for 18 h. The cells were then rinsed twice in PBS to remove residual free 32P, and a lysate was harvested in SDS IP buffer: 300 mM NaCl, 10 mM NaHPO4 (pH 7.5), 5 mM EGTA, 0.2 mM EDTA, 1 mM MgCl2, 0.4% SDS, 20 mM NaF, calyculin, and 1× Halt protease inhibitor cocktail. Samples were boiled for 3 min and sonicated, and Triton X-100 was added to a final concentration of 2% to quench the SDS. Endogenous Tmod1 was immunoprecipitated using a polyclonal anti-Tmod1 antibody (1844; Santa Cruz Biotechnology, Dallas, TX, USA) bound to protein A/G agarose beads (Santa Cruz Biotechnology) overnight at 4°C. The samples were then run on a 10% acrylamide gel, stained with Coomassie blue, and then exposed to X-ray film. The identity of the prominent protein bands in the IP, i.e., the Tmod1 band at ∼42 kDa and the band at ∼35 kDa (found to be GAPDH), was determined using liquid chromatography-tandem mass spectrometry at the Arizona Proteomics Consortium (University of Arizona, Tucson, AZ, USA).
RESULTS
Regulation of the actin filament-capping activity of Tmod1 is important for cytoskeleton formation and for the dynamics of actin filaments. To date, no reports have been published on the mechanisms that regulate Tmod1's function in actin thin filament length regulation. Based on what is known about the mechanisms that regulate other actin filament-capping proteins, it is likely that there are factors that directly govern the functional properties of Tmod1 by binding to it and/or modifying it. A potential mechanism is post-translational modification of Tmod and, in particular, phosphorylation, which has been found to regulate cytoskeletal function in many cell types, including cardiac muscle (for review, see ref. 23). Therefore, we decided to investigate whether Tmod1 can be phosphorylated endogenously in cardiac cells. GFP-Tmod1 was expressed in rat cardiomyocytes in culture via Adv delivery. GFP-Tmod1 was precipitated from a lysate of cardiomyocytes in the presence of the phosphatase inhibitor okadaic acid and analyzed using Pro-Q Diamond staining. A band corresponding to the molecular weight of GFP-Tmod1 was found to be labeled, suggesting that Tmod1 can be phosphorylated by endogenous kinases in live cardiac cells (Fig. 1). Tmod1 phosphorylation was further investigated using 32P labeling in chick cardiomyocytes. In brief, myocytes were cultured in phosphate-free medium containing 32P. Tmod1 was immunoprecipitated, and its phosphorylation state was assessed using autoradiography. A band corresponding to endogenous Tmod1 was detected on the autoradiograph, indicating that Tmod1 is phosphorylated in cardiac myocytes (Supplemental Fig. S1).
Figure 1.
GFP-Tmod1 is phosphorylated in cardiomyocytes in culture. Phosphorylation of GFP-Tmod1 is detected by Pro-Q Diamond staining. GFP-Tmod1 immunoprecipitated from transduced rat cardiomyocytes treated with okadaic acid (+OA) show an increased level of total phosphorylation compared with that for vehicle (DMSO) control (−OA) and GFP transduced controls. The Coomassie blue–stained gel (left) shows the presence of immunoprecipitated GFP and GFP-Tmod1 (asterisks in respective lanes). The same gel after Pro-Q Diamond staining (right) indicates that GFP-Tmod1 has a detectable level of total phosphorylation when treated with okadaic acid. Phosphorylated ovalbumin (45 kDa) present in the prestained ladder is used as a positive control for the Pro-Q Diamond stain.
Previously it was demonstrated that Tmod1 can be phosphorylated in vitro; specifically Thr54 was determined to be a phosphorylation site for TRPM7 kinase within the N terminus of Tmod1 (37). Since we discovered that Tmod1 is phosphorylated in cardiac cells (Fig. 1 and Supplemental Fig. S1), we sought to determine whether altering the phosphorylation state of residue Thr54 would have functional consequences on Tmod1. GFP-tagged phosphorylation-mimic (T54E) or phosphorylation-deficient (T54A) Tmod1 was expressed in rat cardiomyocytes, and their assembly was compared with the assembly patterns of wild-type (WT) GFP-Tmod1. Notably, whereas ∼78% of WT cells displayed consistent, crisp, and brightly stained pointed end localization, only ∼48% of the Tmod1-T54E (phosphorylation-mimic)-expressing cells demonstrated consistent staining; the majority of the mutant cells presented with faint and inconsistent pointed end assembly with high levels of diffuse (nonassembled) protein in the cytoplasm (Figs. 2 and 3). On the other hand, cells expressing the Tmod1-T54A (phosphorylation-deficient) mutant assembled to the pointed end of the actin filament more similarly to GFP-WT Tmod1 than to the T54E phosphorylation mimic (Figs. 2 and 3; 78 and 66% respectively). These data reveal that phosphorylation of threonine at position 54 on Tmod1 reduced its pointed end assembly and could be an important contributor in the regulation of Tmod1 functional properties.
Figure 2.
Expression of a phosphorylation-mimic mutation at position 54 (GFP-Tmod1-T54E) within Tmod1 perturbs pointed-end assembly. Neonatal rat cardiomyocytes expressing GFP-Tmod1 (WT and mutant) or GFP alone were stained with anti-GFP antibodies, Texas Red phalloidin, and anti-α-actinin antibodies (to stain the Z discs). Right panels: merged images of the triple staining (GFP, green; F-actin, red; α-actinin, blue). Clear and consistent striated assembly of GFP-Tmod1 was observed at thin filament pointed ends in the majority of cells expressing the GFP-Tmod1 WT and the GFP-Tmod1-T54A mutant. However, faint and inconsistent pointed-end assembly was observed in cells expressing the phosphorylation-mimic mutation at threonine position 54. No significant differences in α-actinin staining were observed in cells expressing any of the Tmod1 proteins. Scale bar = 10 μm.
Figure 3.

Cardiac myocytes expressing GFP-Tmod1-T54E display inconsistent pointed-end assembly. The graph shows the percentage of myocytes demonstrating diffuse and/or inconsistent or consistent thin filament pointed-end assembly. Transfected cells were fixed 4 d after transfection and stained for GFP, with Texas Red-conjugated phalloidin, and for sarcomeric α-actinin. Only GFP-positive cardiomyocytes were analyzed. Data from 3 cultures were analyzed, and the values are presented as percentages (means ± sd from each culture); <50% of cells expressing GFP-Tmod1-T54E display faint, inconsistent pointed-end assembly, whereas >66% of the cells expressing GFP-Tmod1 WT and GFP-Tmod1-T54A have clear, consistent pointed-end assembly. These data indicate that the addition of the T54E mutation in Tmod1 perturbs its assembly at the pointed ends of thin filaments.
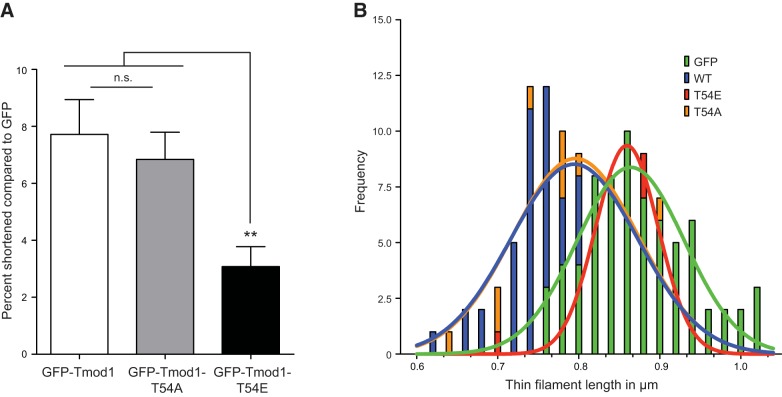
Because the presence of the T54E mutation perturbed the pointed-end assembly of GFP-Tmod1, the effect of expression of this mutant on thin filament lengths was examined. In rat cardiac cells having clear and consistent assembly of WT GFP-Tmod1, thin filament lengths were significantly shorter (7.7%) than those cells expressing GFP alone (Fig. 4) and as reported previously (14). The thin filaments were also similarly shorter (6.8%) in myocytes expressing GFP-Tmod1-T54A. However, myocytes expressing GFP-Tmod1-T54E demonstrated only a slight alteration in thin filament lengths with an average shortening of only 3.1% (Fig. 4). The GFP-Tmod1-T54E length restriction was significantly different (P≤0.005) than either the WT GFP-Tmod1- or the GFP-Tmod1-T54A-expressing cells (WT and T54A were not significantly different from each other). These data show that T54 phosphorylation-deficient (T54A) Tmod1 can regulate thin filament lengths similarly to WT Tmod1, but mimicking phosphorylation of Tmod1 at residue T54 (as in the T54E mutant) renders the protein unable to control thin filament lengths as efficiently as WT Tmod1. These results suggest that proper thin filament length maintenance may be affected by the phosphorylation status of Tmod1 at T54.
Figure 4.
Expression of phosphorylation-mimic GFP-Tmod1-T54E does not shorten thin filament lengths in an way analogous to that of WT Tmod1. Transfected cells were fixed 4 d after transfection and stained with Texas Red-conjugated phalloidin to measure thin filament lengths. Only GFP-positive cardiomyocytes were analyzed. A) Data are from 3 cultures, and the values are presented as percentage thin filament length shortened compared with that of cells expressing GFP alone (means ± sd). It is documented that the expression of WT GFP-Tmod1 will restrict (shorten) thin filament lengths (14). Indeed, expression of GFP-Tmod1 WT and GFP-Tmod1-T54A shortened thin filaments compared with GFP alone (7.7 and 6.8%, respectively). However, cells expressing GFP-Tmod1-T54E had a significant reduction in the ability to shorten thin filament lengths compared with that of controls (3.1%). n.s., not significant. **P ≤ 0.005. B) Histogram of actin length measurements from a representative experiment (y axis, frequency; x axis, thin filament length). A gaussian curve fit of the length measurements shows nearly identical thin filament length distribution and frequency in cells expressing GFP-Tmod1 WT and GFP-Tmod1-T54A, which are shorter than those in cells expressing GFP alone. The length frequency and distribution in cells expressing GFP-Tmod1-T54E are similar to those observed in cells expressing GFP alone.
We next sought to determine whether the phosphorylation state of Tmod1 at T54 had an effect on its in vitro actin-capping activity in the presence of tropomyosin, because the ability of Tmod1 to cap actin is tropomyosin dependent (4). The T54E mutation in Tmod1 had a slight, but detectable reduction on the inhibition of polymerization of actin filaments (i.e., actin filament capping; Fig. 5). This observation is consistent with the poor assembly and length restriction properties of the GFP-Tmod1-T54E mutant in cultured cardiomyocytes (Figs. 2–4).
Figure 5.

Tmod1-T54E displays a modest disruption in actin filament-capping activity in the presence of long muscle α-tropomyosin in vitro. Influence of the T54E mutation on inhibition of the pointed-end elongation of gelsolin-capped actin filaments (5.6 nM) was examined using full-length Tmod1 in the presence of 1 μM stTM. A reduction in actin filament-capping activity with Tmod1-T54E compared with that for WT Tmod1 was observed.
To determine whether the T54E mutation had any effect on binding of stTM, we used circular dichroism. When Tmod11–92-T54E was mixed with αTM1aZip (fragment of stTM), the helical content and melting temperature increased (Fig. 6), indicating complex formation. The dissociation constant calculated from the curves was close to that determined for complex formation of WT Tmod11–92 with αTM1aZip (45). Therefore, the reduction in the capping ability of the T54E mutant does not appear to be connected with the loss of tropomyosin binding. The fact that Tmod1 has 2 actin-capping sites and 2 tropomyosin-binding sites probably accounts for why the T54E mutation has just a minor effect on actin polymerization (Fig. 5) when this mutation is within full-length Tmod1 in vitro.
Figure 6.

Loss of actin filament capping activity is not due to loss of tropomyosin-binding activity. Binding of N-terminal Tmod1 fragment Tmod1-T54E to a muscle αtropomyosin peptide (αTM1aZip) was determined by circular dichroism analysis. The temperature dependence of the ellipticity at 222 nm was measured using circular dichroism spectroscopy, and the dissociation constant was calculated from the curve. Complex formation of Tmod1-T54E and αTM1aZip was observed (see increase in the helical content and melting temperature). However, there is no significant difference in the dissociation constant between Tmod1-T54E and WT N-terminal Tmod1; this indicates that T54E mutation did not influence the interaction with the long muscle α-tropomyosin in vitro.
Taken together, our data indicate that the phosphorylation state of Tmod1 at residue T54 is important for regulation of the Tmod1 assembly and its length-regulating properties.
DISCUSSION
The major filament systems comprising the cardiac sarcomere are actin-containing thin filaments, myosin-containing thick filaments, and the giant bidirectional spring protein, titin. Parallel arrays and interactions between these filament systems are precisely controlled by numerous cytoskeletal and regulatory proteins. Post-translational modifications of these proteins critically regulate myocardial function during normal physiological processes. Phosphorylation of integral sarcomeric proteins, for example, is a major mechanism that controls sarcomeric shortening and force development. Major kinase substrates include the thin filament proteins (troponin I, troponin T, and tropomyosin; the thick filament-associated proteins myosin-binding protein C (MyBP-C) and myosin regulatory light chain; and finally the giant muscle filament titin (for reviews, see refs. 23, 49–51). For example, cardiac troponin I is well-known to be phosphorylated by protein kinase A under the control of β-adrenergic stimulation, and its phosphorylation on specific serine resides is a key regulator of the length dependence of force generation in striated muscle (52, 53). Cardiac titin contains multiple phosphorylation sites that regulate passive tension (54–57). Cardiac MyBP-C is a prominent heart structural protein that modulates actomyosin interactions and has a multitude of identified phosphorylation sites with various physiological functions; 17 in vivo sites have been described to date (for review, see ref. 58). Notably, the cardiac MyBP-C interaction with the thin filament is reduced by phosphorylation of its N terminus (59, 60). Finally, the intermediate filament protein desmin is also phosphorylated during the cell cycle and development, and mutation of these phosphorylation sites interferes with cardiac development (61–63). Here, we provide data indicating that the functional properties of Tmod1, the actin filament pointed-end capping protein, can also be regulated by phosphorylation. Regulation of the phosphorylation status of several integral sarcomeric components appears to be critical for proper cardiac function through multiple kinase signaling cascades, and it appears that Tmod1 is no exception.
Previous studies have demonstrated Tmod phosphorylation in vitro; phosphorylation of Tmod was first determined by an anti-phospho-threonine antibody in PKCα-activated rabbit lens epithelial cells (25). In addition, Tmod2 and Tmod3 were found to coprecipitate with TRPM7 kinase in lysates of N1E-115 neuroblastoma cells (F. N. van Leeuwen, Radboud University, Nijmegen, The Netherlands; personal communication). Subsequent in vitro studies showed that Thr54 within Tmod1 was a phosphorylation site for TRPM7 (37). Notably, although the precise site of phosphorylation within Tmod1 for PKCα was not identified in the experiment involving rabbit lens epithelial cells (25), Scansite3 phosphorylation prediction software (http://scansite3.mit.edu) indicates that Thr54, Thr311, and Ser230 could be possible targets for PKCα. Therefore, TRPM7 may not be the only kinase to have activity at the Thr54 site in Tmod1.
It is important to note that there are other possible target residues for TRPM7 in Tmod1, and, thus, the global effect of TRPM7 phosphorylation of Tmod1 may differ from the individual function of Thr54 phosphorylation alone. Specifically, Ser2 has also been demonstrated to be phosphorylated in vitro by TRPM7. Because no effect of a Ser2 phosphorylation-mimic on actin-capping was observed (37), it was not explored in our current study. Certainly, the phosphorylation of Ser2 or other as of yet unidentified residues in Tmod1 is probably important to its functions. The potential combination of phosphorylation events on Tmod1 (i.e., multiple, concurrent phosphorylated residues) warrants further investigation.
In the present study, we determined that Tmod1 can be phosphorylated in vivo in cardiomyocytes. Because Thr54 is a target of phosphorylation by TRPM7 (37), we investigated a possible functional role for phosphorylation of the Thr54 residue within Tmod1. Our data suggest that the phosphorylation status of this site is important for Tmod1's thin filament pointed-end assembly and length regulation in cardiac myocytes.
To evaluate the functional significance of the phosphorylation state of Tmod1 in cardiac muscle, we expressed GFP-Tmod1 mutants that resemble phosphorylation-mimic (T54E) and phosphorylation-deficient (T54A) Tmod1. When GFP-Tmod1-T54E was expressed in neonatal rat cardiac myocytes, it was significantly inhibited from assembling at thin filament pointed ends, whereas GFP-Tmod1-T54A assembled more similarly, albeit not as consistently, as WT Tmod1. The fact that T54A did not behave identically to WT Tmod1 could indicate that a more precise modulation of the phosphorylation status of Tmod1 (“on” and “off”) is necessary for proper function. Notably, Thr54 is conserved among all Tmod isoforms (Tmod1–4), and, therefore, it is possible that other Tmod isoforms can be phosphorylated at Thr54 as well.
Tmod1 contains 2 identified actin filament-capping sites (aa 48–92 within its N-terminal half and another site including aa 344–359 within its C-terminal half). In the absence of tropomyosin, the capping affinity of the first actin-capping site (within the N-terminal region of Tmod1) is weaker (Kd=∼1.8 μM) than that of the second actin-capping site (Kd=∼0.4 μM) within C-terminal Tmod1 (10). The capping activity of the first actin-capping site is regulated by tropomyosin; therefore, Tmod's affinity for the pointed end increases 1000-fold in the presence of tropomyosin. The second actin-capping site does not appear to be regulated by tropomyosin (10). Results from our in vitro actin polymerization assays showed that the presence of the phosphorylation-mimic Thr54 mutation in Tmod1 affected its actin-capping activity in the presence of tropomyosin. Because the first actin-capping site is tropomyosin-dependent (10), we also evaluated the effect of Tmod1-T54E directly on Tmod1's tropomyosin binding activity using circular dichroism analysis, and no effect was detected. This result is consistent with the report that the phosphorylation state of Tmod did not appear to affect its tropomyosin-binding activity in PKCα-activated lens epithelial cells (25). Taken together, these data suggest that the phosphorylation state of Thr54 directly regulates Tmod1's thin filament-capping activity via its interaction with the actin filament not via its interaction with the tropomyosin filament.
In summary, this study demonstrates that the efficient pointed-end assembly of Tmod1 and its thin filament regulatory roles can be altered by phosphorylation status. We hypothesize a novel model of Tmod1's functional association with thin filament pointed ends. In this model, phosphorylation of the Thr54 residue within Tmod1 interferes with its pointed-end assembly and thin filament length regulation. Thus, phosphorylation/dephosphorylation of Tmod1 probably plays a fundamental role in the regulation of actin filament dynamics at cardiac muscle thin filament pointed ends.
Supplementary Material
Acknowledgments
The authors thank Ellen Taylor, Marcus DiMarco, Verena Koenning, Katrina Garvey, and Laura Arguedas for generating rat cardiomyocyte cultures and for technical assistance, Lucy Kotlyanskaya for assistance with biochemistry, Dr. Miensheng Chu and Marcela Berumen for generating the GFP-Tmod1 adenovirus, Dr. Gregory Rogers and Dr. Daniel Buster for assistance with the 32P phosphorylation analysis experiments and for providing llama GFP-binding protein conjugated beads, and the Rutgers Aresty Research Center for Undergraduates (New Brunswick, NJ, USA) that allowed S.P.S. to work in the laboratory of A.S.K.
This work was supported by the U.S. National Institutes of Health (grants HL083146 and HL108625 to C.C.G., HL081386 to the late Dr. Jeffrey Walker, GM081688 to A.S.K., and predoctoral fellowship 1F31HL 117520 to S.M.N.) and the American Heart Association (grant 0825870G to T.T. and predoctoral fellowships 10PRE3780013 to K.T.B. and 12PRE11900038 to S.M.N.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- Adv
- adenoviral
- DMEM
- Dulbecco's modified Eagle's medium
- DMSO
- dimethyl sulfoxide
- GFP
- green fluorescent protein
- HEK
- human embryonic kidney
- IP
- immunoprecipitation
- MOI
- multiplicity of viral infection
- MyBP-C
- myosin-binding protein C
- PBS
- phosphate-buffered saline
- SDS-PAGE
- sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- Tmod1
- tropomodulin1
- PKC
- protein kinase C
- stTM
- striated muscle α-tropomyosin
- TRPM7
- transient receptor potential cation channel, subfamily M, member 7
REFERENCES
- 1. Cooper J. A., Sept D. (2008) New insights into mechanism and regulation of actin capping protein. Int. Rev. Cell Mol. Biol. 267, 183–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Littlefield R. S., Fowler V. M. (2008) Thin filament length regulation in striated muscle sarcomeres: pointed-end dynamics go beyond a nebulin ruler. Semin. Cell Dev. Biol. 19, 511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gokhin D. S., Fowler V. M. (2011) Tropomodulin capping of actin filaments in striated muscle development and physiology. J. Biomed. Biotechnol. 2011, 103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weber A., Pennise C. R., Babcock G. G., Fowler V. M. (1994) Tropomodulin caps the pointed ends of actin filaments. J. Cell Biol. 127, 1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cox P. R., Zoghbi H. Y. (2000) Sequencing, expression analysis, and mapping of three unique human tropomodulin genes and their mouse orthologs. Genomics 63, 97–107 [DOI] [PubMed] [Google Scholar]
- 6. Almenar-Queralt A., Gregorio C. C., Fowler V. M. (1999) Tropomodulin assembles early in myofibrillogenesis in chick skeletal muscle: evidence that thin filaments rearrange to form striated myofibrils. J. Cell Sci. 112(Pt. 8), 1111–1123 [DOI] [PubMed] [Google Scholar]
- 7. Ito M., Swanson B., Sussman M. A., Kedes L., Lyons G. (1995) Cloning of tropomodulin cDNA and localization of gene transcripts during mouse embryogenesis. Dev. Biol. 167, 317–328 [DOI] [PubMed] [Google Scholar]
- 8. Krieger I., Kostyukova A., Yamashita A., Nitanai Y., Maeda Y. (2002) Crystal structure of the C-terminal half of tropomodulin and structural basis of actin filament pointed-end capping. Biophys. J. 83, 2716–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kostyukova A., Maeda K., Yamauchi E., Krieger I., Maeda Y. (2000) Domain structure of tropomodulin: distinct properties of the N-terminal and C-terminal halves. Eur. J. Biochem. 267, 6470–6475 [DOI] [PubMed] [Google Scholar]
- 10. Fowler V. M., Greenfield N. J., Moyer J. (2003) Tropomodulin contains two actin filament pointed end-capping domains. J. Biol. Chem. 278, 40000–40009 [DOI] [PubMed] [Google Scholar]
- 11. Greenfield N. J., Kostyukova A. S., Hitchcock-DeGregori S. E. (2005) Structure and tropomyosin binding properties of the N-terminal capping domain of tropomodulin 1. Biophys. J. 88, 372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kostyukova A. S., Choy A., Rapp B. A. (2006) Tropomodulin binds two tropomyosins: a novel model for actin filament capping. Biochemistry 45, 12068–12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kostyukova A. S., Rapp B. A., Choy A., Greenfield N. J., Hitchcock-DeGregori S. E. (2005) Structural requirements of tropomodulin for tropomyosin binding and actin filament capping. Biochemistry 44, 4905–4910 [DOI] [PubMed] [Google Scholar]
- 14. Littlefield R., Almenar-Queralt A., Fowler V. M. (2001) Actin dynamics at pointed ends regulates thin filament length in striated muscle. Nat. Cell Biol. 3, 544–551 [DOI] [PubMed] [Google Scholar]
- 15. Sussman M. A., Baque S., Uhm C. S., Daniels M. P., Price R. L., Simpson D., Terracio L., Kedes L. (1998) Altered expression of tropomodulin in cardiomyocytes disrupts the sarcomeric structure of myofibrils. Circ. Res. 82, 94–105 [DOI] [PubMed] [Google Scholar]
- 16. Gregorio C. C., Weber A., Bondad M., Pennise C. R., Fowler V. M. (1995) Requirement of pointed-end capping by tropomodulin to maintain actin filament length in embryonic chick cardiac myocytes. Nature 377, 83–86 [DOI] [PubMed] [Google Scholar]
- 17. Sussman M. A., Welch S., Cambon N., Klevitsky R., Hewett T. E., Price R., Witt S. A., Kimball T. R. (1998) Myofibril degeneration caused by tropomodulin overexpression leads to dilated cardiomyopathy in juvenile mice. J. Clin. Invest. 101, 51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ono Y., Schwach C., Antin P. B., Gregorio C. C. (2005) Disruption in the tropomodulin1 (Tmod1) gene compromises cardiomyocyte development in murine embryonic stem cells by arresting myofibril maturation. Dev. Biol. 282, 336–348 [DOI] [PubMed] [Google Scholar]
- 19. Fritz-Six K. L., Cox P. R., Fischer R. S., Xu B., Gregorio C. C., Zoghbi H. Y., Fowler V. M. (2003) Aberrant myofibril assembly in tropomodulin1 null mice leads to aborted heart development and embryonic lethality. J. Cell Biol. 163, 1033–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKeown C. R., Nowak R. B., Moyer J., Sussman M. A., Fowler V. M. (2008) Tropomodulin1 is required in the heart but not the yolk sac for mouse embryonic development. Circ. Res. 103, 1241–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chu X., Chen J., Reedy M. C., Vera C., Sung K. L., Sung L. A. (2003) E-Tmod capping of actin filaments at the slow-growing end is required to establish mouse embryonic circulation. Am. J. Physiol. Heart Circ. Physiol. 284, H1827–H1838 [DOI] [PubMed] [Google Scholar]
- 22. Solaro R. J., Kobayashi T. (2011) Protein phosphorylation and signal transduction in cardiac thin filaments. J. Biol. Chem. 286, 9935–9940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solaro R. J. (2008) Multiplex kinase signaling modifies cardiac function at the level of sarcomeric proteins. J. Biol. Chem. 283, 26829–26833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kobayashi T., Jin L., de Tombe P. P. (2008) Cardiac thin filament regulation. Pflügers Arch. 457, 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wagner L. M., Fowler V. M., Takemoto D. J. (2002) The interaction and phosphorylation of tropomodulin by protein kinase Cα in N/N 1003A lens epithelial cells. Mol. Vis. 8, 394–406 [PubMed] [Google Scholar]
- 26. Runnels L. W., Yue L., Clapham D. E. (2001) TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043–1047 [DOI] [PubMed] [Google Scholar]
- 27. Nadler M. J., Hermosura M. C., Inabe K., Perraud A. L., Zhu Q., Stokes A. J., Kurosaki T., Kinet J. P., Penner R., Scharenberg A. M., Fleig A. (2001) LTRPC7 is a Mg · ATP-regulated divalent cation channel required for cell viability. Nature 411, 590–595 [DOI] [PubMed] [Google Scholar]
- 28. Yamaguchi H., Matsushita M., Nairn A. C., Kuriyan J. (2001) Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol. Cell 7, 1047–1057 [DOI] [PubMed] [Google Scholar]
- 29. Ryazanov A. G., Pavur K. S., Dorovkov M. V. (1999) α-Kinases: a new class of protein kinases with a novel catalytic domain. Curr. Biol. 9, R43–45 [DOI] [PubMed] [Google Scholar]
- 30. Middelbeek J., Clark K., Venselaar H., Huynen M. A., van Leeuwen F. N. (2010) The alpha-kinase family: an exceptional branch on the protein kinase tree. Cell. Mol. Life Sci. 67, 875–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Su L. T., Liu W., Chen H. C., Gonzalez-Pagan O., Habas R., Runnels L. W. (2011) TRPM7 regulates polarized cell movements. Biochem. J. 434, 513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmitz C., Perraud A. L., Johnson C. O., Inabe K., Smith M. K., Penner R., Kurosaki T., Fleig A., Scharenberg A. M. (2003) Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114, 191–200 [DOI] [PubMed] [Google Scholar]
- 33. Clark K., Langeslag M., van Leeuwen B., Ran L., Ryazanov A. G., Figdor C. G., Moolenaar W. H., Jalink K., van Leeuwen F. N. (2006) TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 25, 290–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Su L. T., Agapito M. A., Li M., Simonson W. T., Huttenlocher A., Habas R., Yue L., Runnels L. W. (2006) TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J. Biol. Chem. 281, 11260–11270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sah R., Mesirca P., Van den Boogert M., Rosen J., Mably J., Mangoni M. E., Clapham D. E. (2013) Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc. Natl. Acad. Sci. U. S. A. 110, E3037–E3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim T. Y., Shin S. K., Song M. Y., Lee J. E., Park K. S. (2012) Identification of the phosphorylation sites on intact TRPM7 channels from mammalian cells. Biochem. Biophys. Res. Commun. 417, 1030–1034 [DOI] [PubMed] [Google Scholar]
- 37. Dorovkov M. V., Beznosov S. N., Shah S., Kotlianskaia L., Kostiukova A. S. (2008) Effect of mutations imitating the phosphorylation by TRPM7 kinase on the function of the N-terminal domain of tropomodulin. Biofizika 53, 943–949 [PubMed] [Google Scholar]
- 38. Studier F. W. (2005) Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 41, 207–234 [DOI] [PubMed] [Google Scholar]
- 39. Cooper J. A., Walker S. B., Pollard T. D. (1983) Pyrene actin: documentation of the validity of a sensitive assay for actin polymerization. J. Muscle Res. Cell Motil. 4, 253–262 [DOI] [PubMed] [Google Scholar]
- 40. Kouyama T., Mihashi K. (1981) Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur. J. Biochem. 114, 33–38 [PubMed] [Google Scholar]
- 41. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 42. Edelhoch H. (1967) Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry 6, 1948–1954 [DOI] [PubMed] [Google Scholar]
- 43. Fasman G. D. (1989) Protein conformational prediction. Trends Biochem. Sci. 14, 295–299 [DOI] [PubMed] [Google Scholar]
- 44. Greenfield N. J., Huang Y. J., Palm T., Swapna G. V., Monleon D., Montelione G. T., Hitchcock-DeGregori S. E. (2001) Solution NMR structure and folding dynamics of the N terminus of a rat non-muscle α-tropomyosin in an engineered chimeric protein. J. Mol. Biol. 312, 833–847 [DOI] [PubMed] [Google Scholar]
- 45. Kostyukova A. S., Hitchcock-DeGregori S. E. (2004) Effect of the structure of the N terminus of tropomyosin on tropomodulin function. J. Biol. Chem. 279, 5066–5071 [DOI] [PubMed] [Google Scholar]
- 46. Gustafson T. A., Bahl J. J., Markham B. E., Roeske W. R., Morkin E. (1987) Hormonal regulation of myosin heavy chain and α-actin gene expression in cultured fetal rat heart myocytes. J. Biol. Chem. 262, 13316–13322 [PubMed] [Google Scholar]
- 47. Pappas C. T., Bhattacharya N., Cooper J. A., Gregorio C. C. (2008) Nebulin interacts with CapZ and regulates thin filament architecture within the Z-disc. Mol. Biol. Cell 19, 1837–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buster D. W., Daniel S. G., Nguyen H. Q., Windler S. L., Skwarek L. C., Peterson M., Roberts M., Meserve J. H., Hartl T., Klebba J. E., Bilder D., Bosco G., Rogers G. C. (2013) SCFSlimb ubiquitin ligase suppresses condensin II-mediated nuclear reorganization by degrading Cap-H2. J. Cell Biol. 201, 49–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. LeWinter M. M., Granzier H. (2010) Cardiac titin: a multifunctional giant. Circulation 121, 2137–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Layland J., Solaro R. J., Shah A. M. (2005) Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc. Res. 66, 12–21 [DOI] [PubMed] [Google Scholar]
- 51. Barefield D., Sadayappan S. (2010) Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J. Mol. Cell. Cardiol. 48, 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hanft L. M., Biesiadecki B. J., McDonald K. S. (2013) Length dependence of striated muscle force generation is controlled by phosphorylation of cTnI at serines 23/24. J. Physiol. 591, 4535–4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wijnker P. J., Foster D. B., Tsao A. L., Frazier A. H., dos Remedios C. G., Murphy A. M., Stienen G. J., van der Velden J. (2013) Impact of site-specific phosphorylation of protein kinase A sites Ser23 and Ser24 of cardiac troponin I in human cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 304, H260–H268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anderson B. R., Bogomolovas J., Labeit S., Granzier H. (2010) The effects of PKCα phosphorylation on the extensibility of titin's PEVK element. J. Struct. Biol. 170, 270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hidalgo C., Hudson B., Bogomolovas J., Zhu Y., Anderson B., Greaser M., Labeit S., Granzier H. (2009) PKC phosphorylation of titin's PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ. Res. 105, 631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kotter S., Gout L., Von Frieling-Salewsky M., Muller A. E., Helling S., Marcus K., Dos Remedios C., Linke W. A., Kruger M. (2013) Differential changes in titin domain phosphorylation increase myofilament stiffness in failing human hearts. Cardiovasc. Res. 99, 648–656 [DOI] [PubMed] [Google Scholar]
- 57. Yamasaki R., Wu Y., McNabb M., Greaser M., Labeit S., Granzier H. (2002) Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ. Res. 90, 1181–1188 [DOI] [PubMed] [Google Scholar]
- 58. Kooij V., Holewinski R. J., Murphy A. M., Van Eyk J. E. (2013) Characterization of the cardiac myosin binding protein-C phosphoproteome in healthy and failing human hearts. J. Mol. Cell. Cardiol. 60, 116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shaffer J. F., Kensler R. W., Harris S. P. (2009) The myosin-binding protein C motif binds to F-actin in a phosphorylation-sensitive manner. J. Biol. Chem. 284, 12318–12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weith A., Sadayappan S., Gulick J., Previs M. J., Vanburen P., Robbins J., Warshaw D. M. (2012) Unique single molecule binding of cardiac myosin binding protein-C to actin and phosphorylation-dependent inhibition of actomyosin motility requires 17 amino acids of the motif domain. J. Mol. Cell. Cardiol. 52, 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hollrigl A., Hofner M., Stary M., Weitzer G. (2007) Differentiation of cardiomyocytes requires functional serine residues within the amino-terminal domain of desmin. Differentiation 75, 616–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Huang X., Li J., Foster D., Lemanski S. L., Dube D. K., Zhang C., Lemanski L. F. (2002) Protein kinase C-mediated desmin phosphorylation is related to myofibril disarray in cardiomyopathic hamster heart. Exp. Biol. Med. 227, 1039–1046 [DOI] [PubMed] [Google Scholar]
- 63. Kawajiri A., Yasui Y., Goto H., Tatsuka M., Takahashi M., Nagata K., Inagaki M. (2003) Functional significance of the specific sites phosphorylated in desmin at cleavage furrow: Aurora-B may phosphorylate and regulate type III intermediate filaments during cytokinesis coordinately with Rho-kinase. Mol. Biol. Cell 14, 1489–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.