Abstract
MicroRNAs (miRs) are noncoding RNAs (17–25 nt) that control translation and/or mRNA degradation. Using Northern blot analysis, we identified that miR-1 is specifically expressed in growth plate cartilage in addition to muscle tissue, but not in brain, intestine, liver, or lung. We obtained the first evidence that miR-1 is highly expressed in the hypertrophic zone of the growth plate, with an 8-fold increase compared with the proliferation zone; this location coincides with the Ihh and Col X expression regions in vivo. MiR-1 significantly induces chondrocyte proliferation and differentiation. We further identified histone deacetylase 4 (HDAC4) as a target of miR-1. HDAC4 negatively regulates chondrocyte hypertrophy by inhibiting Runx2, a critical transcription factor for chondrocyte hypertrophy. MiR-1 inhibits both endogenous HDAC4 protein by 2.2-fold and the activity of a reporter gene bearing the 3′-untranslated region (UTR) of HDAC4 by 3.3-fold. Conversely, knockdown of endogenous miR-1 relieves the repression of HDAC4. Deletion of the miR-1 binding site in HDAC4 3′-UTR or mutated miR-1 abolishes miR-1-mediated inhibition of the reporter gene activity. Overexpression of HDAC4 reverses miR-1 induction of chondrocyte differentiation markers Col X and Ihh. HDAC4 inhibits Runx2 promoter activity in a dosage-dependent manner. Thus, miR-1 plays an important role in the regulation of the chondrocyte phenotype during the growth plate development via direct targeting of HDAC4. — Li, P., Wei, X., Guan, Y., Chen, Q., Zhao, T., Sun, C., Wei, L. MicroRNA-1 regulates chondrocyte phenotype by repressing histone deacetylase 4 during growth plate development.
Keywords: differentiation, HDAC4, Runx2, collagen X
MicroRNAs (miRNAs) are a growing class of small, noncoding RNAs (17–25 nt) with a regulatory function. Regulation occurs post-transcriptionally in collaboration with protein counterparts forming RNA-induced silencing complexes (RISCs) (1, 2). These complexes repress target gene expression by interfering with the translation of—or by accelerating the degradation of—mRNA through direct binding to partially complementary sequences in the 3′-untranslated region (3′-UTR) of the target mRNA (2–6). miRNAs are emerging as important modulators in cellular pathways, such as growth and proliferation, apoptosis, and developmental timing (7–9) and have been targeted in miRNA-based therapeutics for human diseases (8, 10). Certain miRNAs are specifically expressed in cartilage tissue; however, their distinct roles in chondrocyte proliferation and differentiation are yet to be identified.
MiR-1 has distinct roles in modulating skeletal muscle proliferation and differentiation in cultured myoblasts in vitro. MiR-1 promotes myogenesis by targeting histone deacetylase 4 (HDAC4) (11) and targeted deletion of the muscle-specific miRNA, miR-1–2 in vivo resulted in 50% lethality by weaning due to abnormal cardiac morphogenesis, and dysregulation of electrical conduction and cell cycle (12). However, the spatio-temporal distribution of miRNA-1 and its role during growth plate development are not clear. Although Kobayashi and colleagues found that miR-1 is expressed in chondrocytes, they could not exclude the possibility of muscle contamination in their study (13).
Histone acetylation promotes gene transcription by relaxing chromation structure, thereby facilitating access of the transcriptional machinery to DNA target sequences (14). The transcription-activating effect of histone acetylation is counterbalanced by histone deacetylation, which favors chromatin condensation and transcriptional repression. In mammalian cells, the two major classes of histone deacetylases (HDACs) are called class I and II HDACs (15). Class I HDACs (HDAC1, HDAC2, HDAC3, and HDAC8) are widely expressed, while class II HDACs (HDAC4, HDAC5, HDAC7, and HDAC9) have cell type-restricted expression patterns. Both HDAC5- and HDAC9-knockout mice display a cardiac hypertrophy (16), which suggests general roles for class II HDACs in the control of cellular hypertrophy.
Studies have demonstrated that HDAC4 is a negative regulator of growth plate maturation and works by binding to and inhibiting the activity of Runt-related transcription factor 2 (Runx2) to suppress chondrocyte hypertrophy (17). Runx2 is a critical transcription factor for chondrocyte hypertrophy (17) and proliferation (18). Recently, we noticed the expression of HDAC4 was significantly decreased in hypertrophic chondrocytes in the growth plates of newborn mice (19). Although these studies indicate that HDAC4 plays a critical role for chondrocyte proliferation and differentiation, it is not clear what regulates activity and expression of HDAC4 itself. MiR-1 promotes myogenesis by targeting HDAC4 (11); it is, therefore, possible that the lack of HDAC4 observed in the hypertrophic chondrocytes of the growth plate may have been due to the repression of HDAC4 translation by miRNA-1. We hypothesize that HDAC4's inhibiting effects on Runx2 and subsequent chondrocyte hypertrophy are also miR-1 dependent.
In this report, we provide the evidence that miR-1 is a critical mediator to regulate growth plate development. Specifically, miR-1 promotes chondrocyte differentiation by targeting the 3′-UTR of HDAC4 mRNA and suppressing the protein levels of HDAC4.
MATERIALS AND METHODS
miRNA-1 mimic, miRNA-1 inhibitor, and expression plasmid
miR-1 mimic and negative control miRNA (ConmiRNA) mimic were purchased from Qiagen (Valencia, CA, USA). These mimics are double-stranded RNA oligonucleotides, chemically modified with the Qiagen ON-TARGET modifications, which can effectively mimic the function of endogenous mature miRNA. miR-1 inhibitor (anti-miR-1) or control microRNA inhibitor (Control) was purchased from Qiagen. These inhibitors are RNA oligonucleotides chemically modified with the 2′-OMe modifications, which are designed to inhibit the function of endogenous miRNA-1. TargetScan (http://www.targetscan.org) and miRanda (http://www.microrna.org) were utilized to identify targets genes for miR-1. Sequence analysis indicates that miR-1 is conserved in mammalian species (miRBas accession number: MIMAT0000416), and there are three miR-1 target sites in the UTR of HDAC4 in different species (2334–2340, 3514–3520, and 3547–3553 of HDAC4 3′-UTR). Five luciferase reporter plasmids containing WT and deleted miR-1 seed sites within the human HDAC4 3′-UTR sequence were purchased: 1) wild-type (covering all three binding sites), 2) binding sites 1 and 2 deleted; 3) binding sites 1 and 3 deleted; and 4) binding sites 2 and 3 deleted; and 5) all three binding sites deleted (GengCopoeia, Rockville, MD, USA). pGL3-HDAC4 3′-UTR luciferase reporter, miR-1, and miR-1 mutation were kindly provided by Dr. D.-Z. Wang (Harvard University Medical School, Boston, MA, USA) (11).
Northern blot and in situ hybridization analyses
Total miRNA was isolated from mouse brain, heart, lung, intestine, liver, human articular cartilage, and tibia growth plate of 17-d chicken embryos using miRNeasy Mini Kit (Qiagen) following the manufacturer's instructions. Three zones (proliferating, maturation/prehypertrophy, and hypertrophy) of the growth plate were separated under dissection microscope, as described previously (20). The distribution of miR-1 was detected in the proliferating and hypertrophy zones. Northern blot was performed using MiRNA Northern blot assay kit (Signosis, Santa Clara, CA, USA). Five micrograms of RNA was separated on 15% urea–polyacrylamide gel and then transferred to nylon membrane using Trans-Blot Cell (Bio-Rad Laboratories, Hercules, CA, USA). RNAs were immobilized by UV cross-linking for 2 min and baked at 80°C for 1 h. Blots were hybridized with biotin-labeled miR-1 and RNU48 probes overnight at 42°C and then washed with wash buffer. After the reaction between the streptavidin-HRP conjugate and the substrates, the membrane was exposed using hyperfilm for 2 min, and the signals of the blots were detected with AGFA CP1000 (AGFA, Madison, WI, USA). To detect the distribution of miR-1, the proximal tibia growth plate from 1 d postpartum mice was used to detect miR-1 following the manufacture instructions (miRCURY LNA microRNA ISH kit, no. 9001, EXIQON, Woburn, MA, USA).
Primary chicken chondrocyte isolation and culture
Primary cultures of chicken embryonic chondrocytes were established, as described previously (19, 21). Briefly, chondrocytes were isolated from the cephalic part of the sternum growth plate of 17-d-old embryonic chicks and digested with 0.1% trypsin (Sigma, St. Louis, MO, USA), 0.3% collagenase (Worthington, Freehold, NJ, USA), and 0.1% type 1 testicular hyaluronidase (dissociation medium; Sigma) for 30 min at 37°C with shaking. After digestion and removal of the supernatant, the fresh dissociation medium was replaced and incubated at 37°C for an additional hour. Chondrocytes were resuspended in plating medium plus 0.01% testicular hyaluronidase (plating medium: 10% FBS, l-glutamine (Invitrogen, Carlsbad, CA, USA), and antibiotics (penicillin and streptomycin) in DMEM-F-12 1:1 medium (Life Technologies, Grand Island, NY, USA). After culturing overnight, the medium of the chondrocyte culture was replaced with fresh medium without hyaluronidase. Medium was changed every other day.
Transfection
Transfections were performed using GenMute siRNA and DNA transfection reagent (SignaGen Laboratories, Ijamsville, MD, USA). Cells were plated overnight prior to transfection and the monolayer cell density reached the optimal 70–80% confluency at the time of transfection. Fresh complete medium (0.75 ml) was added into each well of 12-well plates with serum and antibiotics 30–60 min before transfection. MiR-1 and its inhibitor (120 nM) or/and 0.375 μg plasmid DNA were transfected with GenMute following the manufacturer's instructions. Gene levels were measured 24 h post-transfection for mRNA and 48 h post-transfection for proteins.
Cell proliferation assays
The number of living cells was detected using a cell counting kit-8 (CCK-8) (Beyotime, Beijing, China). Chondrocytes were seeded in 96-well plates at a density of 8000 cells/well in DMEM/F-12 medium overnight and then transfected with miR-1 mimic, miR control, miR-1 inhibitor, and miR-1 inhibitor control. The number of viable cells was measured by recording the optical density at 450 nm and generating cell growth curves at 15, 36, and 48 h after transfection (22). Three repeated experiments were performed, and 6 multiple wells were set up each time. Cell proliferation was also detected by 5-ethynyl-2′-deoxyuridine (EdU) labeling of cultured cells. Chondrocytes were grown on glass coverslips in DMEM/F12 supplemented with 10% FBS. EdU (Cell-Light EDU Nascent RNA detection kit; Ribobio, Guangzhou, China) was added to the culture medium at a concentration of 50 μM for 2 h (23). The cells were fixed by 4% paraformaldehyde in PBS for 30 min at room temperature after labeling. The cells were then incubated with glycine at a concentration of 2 mg/ml in PBS for 5 min to quench the fixation effects of paraformaldehyde, permeated by 0.5% Triton X-100 in PBS for 10 min, and subsequently stained with fluorescent Cy3-azide and DAPI after rinsing 3 times with PBS. Slides were mounted in FluorSave reagent (Calbiochem-Novabiochem Corp., La Jolla, CA, USA), and positive cells (red) were calculated as percentage under a fluorescent microscope (E800; Nikon, Tokyo, Japan). Approximately 300 cells from 3 independent experiments were scored.
Real-time RT-PCR
Real-time RT-PCR was performed as described previously (24, 25). Total RNA was isolated from chondrocytes with RNeasy isolation kit (Qiagen) and quantified by NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). One microgram of RNA was reverse-transcribed to obtain first-strand cDNA (PrimeScript RT Master Mix kit; Takara, Tokyo, Japan). Forty nanograms per microliter of the resulting cDNA was used as the template to quantify the relative content of mRNA using QuantiTect SYBR Green PCR kit (Qiagen) with DNA Engine Opticon 2 Continuous Fluorescence Detection System (MJ Research, Waltham, MA, USA). Gene changes, including type X collagen (Col X), Indian hedgehog (Ihh), and Runx2, were qualified by real-time PCR, as described in our previous publications (25, 26). Primers used are as follows: The forward and reverse primers for Col X (chicken) were 5′-AGTGCTGTCATTGATCTCATGGA-3′ and 5′-TCAGAGGAATAGAGACCATTGGATT-3′, respectively; Ihh (chicken) were 5′-AAGTCAGAGCACTCGGCTGCC-3′ and 5′-GTTGTCGGCCACGAAGAGCA-3′, respectively; Runx2 (chicken) were 5′-CTTCGCCGTCCATTCACTCC-3′ and 5′-GTGCATTCGTGGGTTGGAGA-3′, respectively; actin-α 1 (ACTA1; a skeletal muscle marker; chicken) were 5′-GCTGGGATCCATGAGACAA-3′ and 5′-GCATAGAGGTCCTTCCTGAT-3′, respectively; myogenic differentiation 1 (MYOD1; a skeletal muscle marker; chicken) were 5′-TCAACGAGGCCTTTGAGACC-3′ and 5′-CCTGCAGGCTCTCGATGTAG-3′, respectively; Col 2 (chicken) were 5′-GCTGGGATCCATGAGACAA-3′ and 5′-GCATAGAGGTCCTTCCTGAT-3′, respectively; 18S were 5′-CGGCTACCACATCCAAGGAA-3′ and 5′-GCTGGAATTACCGCGGCT-3′, respectively. The 18S RNA was amplified at the same time and used as an internal control. Amplification conditions were as follows: 2 min of preincubation at 50°C, 10 min at 95°C for enzyme activation, and 40 cycles at 95°C denaturation for 10 s, 55°C annealing for 30 s, and 72°C extension for 30 s. The cycle threshold (Ct) values for 18S RNA and target genes were measured and calculated by computer software (Bio-Rad). Relative transcript levels were calculated as x = 2 − ΔΔCt, in which ΔΔCt = ΔE − ΔC, and ΔE = Ctexp − Ct18s; ΔC = Ctctl − Ct18s.
Total microRNA was extracted from chondrocytes using miRNeasy Mini Kit (Qiagen) by following the manufacturer's instructions. The concentration, purity, and integrity of total RNA were determined by NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). MiScript reverse transcription kit (Qiagen) was used to synthesize cDNA. MiScript SYBR Green PCR kit was used for quantitative determination of the expression of miRNA-1. U6 was used as an endogenous control. MiRNA-1 and U6 primers were purchased from Qiagen.
Western blot analysis
Chondrocytes were transfected with expression HDAC4 vector, and the cells were washed in ice-cold PBS and lysed in RIPA buffer (50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 5 mM EDTA, 1% Nonidet P-40) at 4°C for 30 min 48 h after transfection. Lysates were cleared by centrifugation for 20 min at 4°C and equal amounts of cell lysates were used for electrophoretic analysis on SDS-PAGE and transferred to nitrocellulouse membrane. Immunoblotting was coupled with fluorescent signal detection with an Odyssey fluorescence scanner (LI-COR Biosciences, Lincoln, NE, USA). The following antibodies were used for this study: anti-HDAC4 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-Actin (Cell Signaling Technology, Danvers, MA, USA); anti-mouse-IRDye800 (Roche, Rockland Immunochemicals, Gilbertsville, PA, USA) and anti-rabbit-Alexa Fluor 680 (Molecular Probes, Eugene, OR, USA). The relative intensities of HDAC4 from Western blot bands were semiquantified by densitometry after normalized to β-actin using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA), as previously published (19) 48 h after transfection with 120 nM miR-1 or anti-miR-1.
Luciferase reporter gene assays
Chondrocytes were transiently transfected with 0.4 μg of Runx2 promoter luciferase reporter plasmid using GenMute siRNA and DNA transfection reagent (SignaGen) in 12-well plates. Cotransfections with promoters were performed with expression constructs for HDAC4. Cells were harvested in 1× reporter lysis buffer 48 h after transfection. Runx2 luciferase activities were read using the dual-luciferase reporter assay system and normalized to Renilla activity (Dual-Luciferase Reporter Assay; Promega, Madison, WI, USA).
There are three putative seed regions of miR-1 in the HDAC4 3′-UTR. To identify the miR-1 target region in the HDAC4 3′-UTR mRNA, we deleted the three binding sites from the putative seed regions in the HDAC4 3′-UTR, respectively. COS-1 cells were transfected with 0.4 μg of five different 3′-UTRs of human HDAC4 luciferase reporter plasmids respectively using GenMute siRNA and DNA Transfection Reagent (SignaGen) in 12-well plates. miRNA-1 mimic was added into the well at the same time (120 nM). Cells were harvested in 1× reporter lysis buffer 48 h after transfection. HDAC4 3′-UTR luciferase reporter gene activities were read using the Luc-Pair miR Luciferase assay and normalized to Renilla activity (GeneCopoeia, Rockville, MD, USA).
Statistical analysis
The data represented the means ± sd. Statistical analysis was carried out using two-way ANOVA and Turkey's test with significance set as P < 0.05.
RESULTS
Expression of miR-1 in different tissues
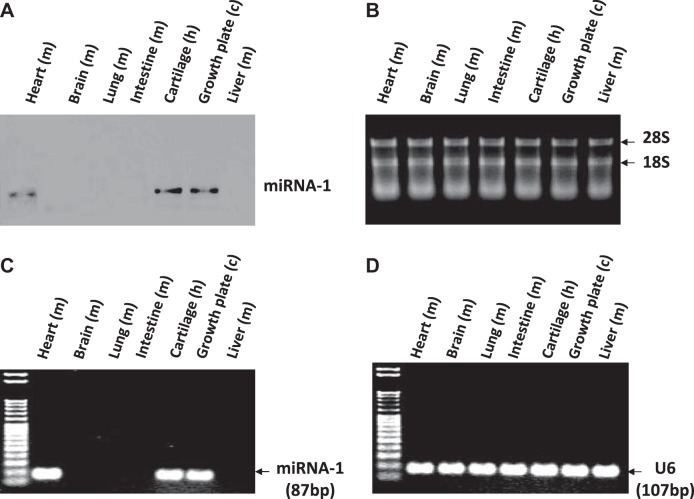
Northern blot detected miR-1 in mouse heart, human articular cartilage, and chicken growth plate from d 17 embryos, but not in mouse brain, intestine, liver, or lung (Fig. 1A). The stained gel of total RNA showed 28S/18S ladder as loading control (Fig. 1B). RT-PCR also detected miR-1 in mouse heart, human cartilage, and chicken growth plate, but not in other tissues (Fig. 1C), and U6 was used as loading control (Fig. 1D).
Figure 1.
Expression of miR-1 in different tissues. A) Northern blot detected miR-1 in mouse heart, human articular cartilage, and chicken growth plate from d 17 embryos, but not in mouse brain, lung, intestine, or liver. B) The stained gel of total RNA showed 28S/18S ladder as loading control. C) RT-PCR detected miR-1 in mouse heart, human articular cartilage, and chicken growth plate from d 17 embryos, but not in other tissues. D) U6 was used as a loading control.
MiR-1 highly expressed in hypertrophic chondrocytes
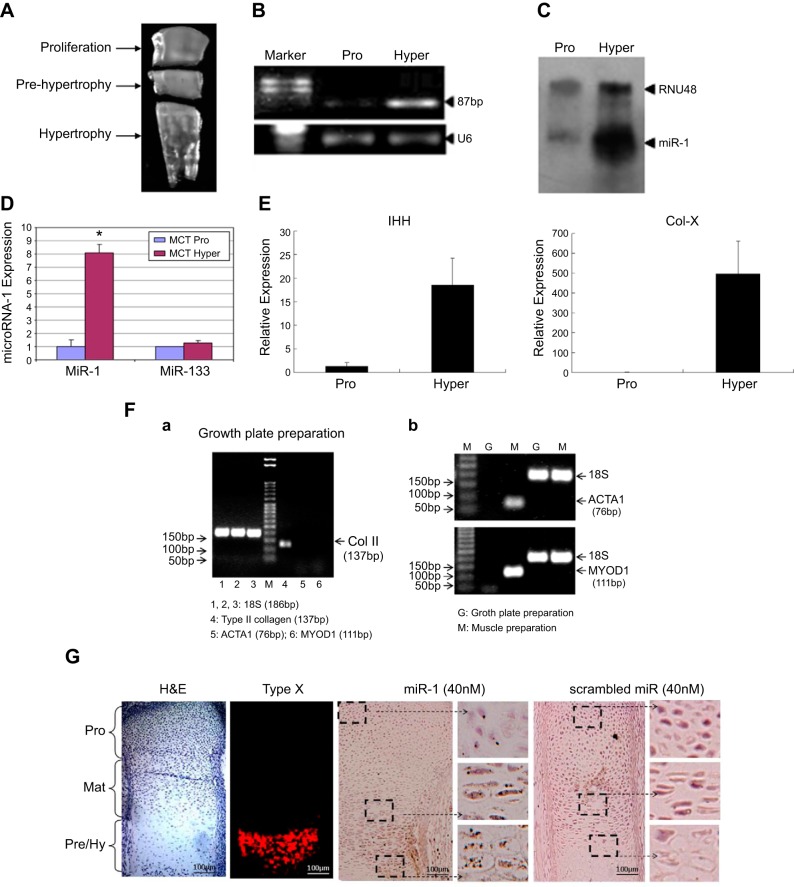
Proliferative and hypertrophic zones of tibia growth plate from d 17 chicken embryos were collected, and the middle part was discarded (Fig. 2A). RT-PCR results indicated that the level of miR-1 was increased in the hypertrophic zones compared to proliferation zones (Fig. 2B) (Pro: proliferation zone; Hyper: hypertrophic zone). Northern blot analysis confirmed strong expression of miR-1 in the hypertrophic cells compared with proliferation cells (RNU48 as control) (Fig. 2C). We also quantified the expression levels of miR-1 and miR-133 using a murine chondrocyte (MCT) cell line. Strong expression of miR-1 and similar expression of miR-133 were found in the hypertrophic chondrocytes, compared with proliferation chondrocytes (Fig. 2D). The mRNA levels of Ihh and Col X increased 15- and 466-fold, respectively, in the hypertrophic zone compared to the proliferative zone (Fig. 2E). Col 2 but not ACTA1 and MYOD1 was detected in the growth plate preparation (Fig. 2Fa). The ACTA1 and MYOD1 only were detected in the skeletal muscle preparation (Fig. 2Fb). miR-1 was strongly expressed in the prehypertrophic and hypertrophic zones compared with the proliferation zone detected by ISH (Fig. 2G).
Figure 2.
MiR-1 is highly expressed in hypertrophic chondrocytes. A) Proliferative and hypertrophic zones were isolated from d 17 chicken tibia growth plates, and the middle part was discarded. B, C) Total RNA was isolated from the proliferative (pro) and hypertrophic (hyper) zones of tibia growth plate from d 17 chicken embryos. The expression levels of miR-1 were qualified by RT-PCR and Northern blot. U6 and RNU48 were used as internal control. D) The expression levels of miR-1 were quantified by real-time RT-PCR in the mouse proliferative and hypertrophic chondrocyte cell line (MCT cells). miR-133 in hypertrophic cells maintained a similar level of expression compared to proliferation chondrocytes. E) Real-time RT-PCR confirmed a higher expression of Col X and Ihh in the hypertrophic zone compared with the proliferative zone. Values represent the mean ± sd from 3 independent experiments. *P < 0.05. F) RT-PCR confirmed a high expression of Col 2 but not ACTA1 and MYOD1 (markers for skeletal muscle) was detected from the growth plate preparations. G) A high expression of miR-1 was detected in the prehypertrophic and hypertrophic zones compared with the proliferation zone by ISH.
MiR-1 stimulates chondrocyte proliferation
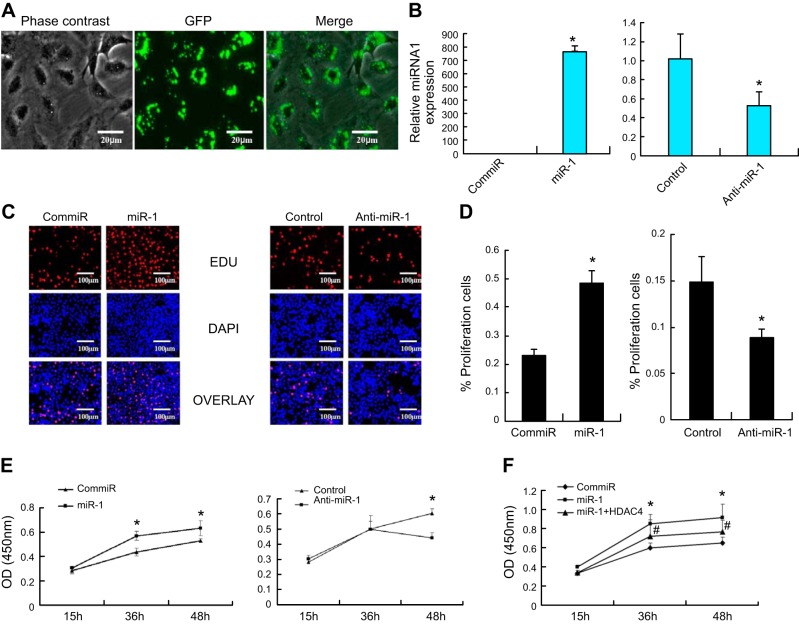
To determine whether miR-1 affects chondrocyte proliferation, miR-1 mimic, and anti-miR-1 were transfected into primary chicken chondrocytes. microRNA transfection efficiency was determined by immunofluorescence microscopy. More than 88% of chondrocytes were transfected 24 h after transfection (Fig. 3A). Real-time PCR result showed that overexpression of miR-1 dramatically increases the level of miR-1, while knockdown miR-1 (anti-miR-1) decreases its expression compared to control miRs (Fig. 3B). MiR-1 significantly enhanced chondrocyte proliferation at 48 h post-transfection, as revealed by EDU cell proliferation staining (Fig. 3C). Approximately 300 cells from 3 independent experiments were scored (Fig. 3D). Cell proliferation was further validated using CCK-8 cell proliferation assay. Overexpression of miR-1 increased chondrocyte proliferation at 36 and 48 h post-transfection (Fig. 3E). while the cells were cotransfected with HDAC4 and miR-1 mimic decreases the cell proliferation induced by miR-1 mimic (Fig. 3F).
Figure 3.
MiR-1 stimulates chondrocyte proliferation. A) More than 88% of chondrocytes were transfected with miR-1, as determined by immunofluorescence microscopy 24 h after transfection in the primary chicken chondrocytes. B) Real-time RT-PCR result showed that overexpression of miR-1 (120 nM) increases the level of miR-1 while knockdown miR-1 by anti-miR-1 decreases its expression compared to control miRNA-1 in the primary chicken chondrocytes 24 h post-transfection. Values are presented as mean ± sd (n=3). *P < 0.05. C, D) MiR-1 stimulates chondrocyte proliferation. Primary chicken chondrocytes were transfected with a miR-1 mimic (miR-1) or negative control mimic (ConmiR), and miR-1 inhibitor (anti-miR-1) or a control miRNA inhibitor (control) at 120 nM, respectively. Forty-eight hours post-transfection, cell growth was measured by an EDU-based cell proliferation assay. Data are presented as means ± sd (n=5). *P < 0.05. E) Proliferation of chicken chondrocytes was also measured by a CCK-8-based cell proliferation assay. Values are means ± sd (n=5). *P < 0.05. F) miR-1 mimic increases cell proliferation, while cells were cotransfected with miR-1 mimic and HDAC4 has an opposite effect. Data are presented as means ± sd (n=3). *P < 0.05, compared to ConmiR. #P < 0.05, compared to miR-1.
MiR-1 enhances chondrocyte differentiation
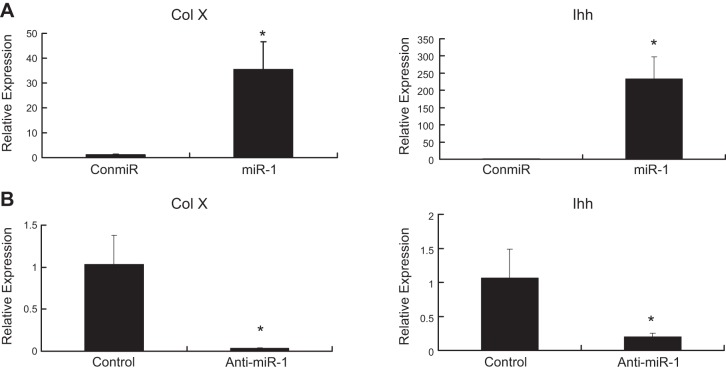
Real-time PCR results indicated that the mRNA levels of Ihh, a marker of prehypertrophic chondrocytes, and Col X, a marker of hypertrophic chondrocytes, increased 24 h after transfection of a miR-1 mimic (Fig. 4A), while transfection of anti-miR-1 decreased expression of both genes (Fig. 4B).
Figure 4.
MiR-1 induces chondrocyte differentiation. A) miR-1 increases the mRNA levels of Col X and Ihh, markers for chondrocyte differentiation. Primary chicken chondrocytes were transfected with a miR-1 mimic (miR-1) or control miRNA (ConmiR) at 120 nmol, respectively. The mRNA levels of Col X and Ihh were quantified by real-time RT-PCR 24 h post-transfection. Data are presented as mean ± sd (n=3) *P < 0.05. B) Inhibition of miR-1 down-regulates mRNA levels of Col X and Ihh. Primary chicken chondrocytes were transfected with a miR-1 inhibitor (anti-miR-1) or a control microRNA inhibitor (Control) at 120 nmol, respectively. The mRNA levels of Ihh and Col X were quantified by real-time RT-PCR 24 h post-transfection. Data are presented as means ± sd (n=3; 3 independent cell culture experiments). *P < 0.05. All transfections were performed in triplicate.
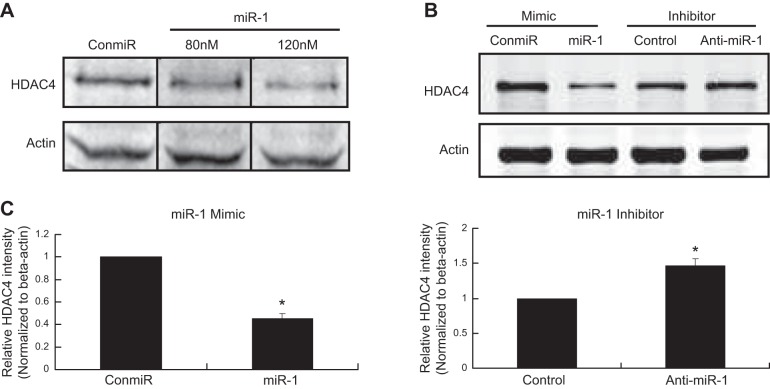
MiR-1 down-regulates HDAC4 protein in a dosage-dependent manner
Cell lysates were subjected to Western blot analysis for HDAC4 protein 48 h after miR-1 transfection in chicken chondrocytes. Western blot analysis results demonstrate that miR-1 inhibits HDAC4 protein production in a dosage-dependent manner (Fig. 5A). The relative intensities of HDAC4 from Western blot bands were semiquantified by densitometry (Fig. 5C). Conversely, knockdown miR-1 using anti-miR-1 has opposite effects (Fig. 5B, C).
Figure 5.
MiR-1 down-regulates HDAC4 protein in chondrocytes. A) MiR-1 down-regulates HDAC4 protein in dosage-dependent manner. Chondrocytes from d 17 embryos of chicken growth plate were transfected with a miR-1 mimic (miR-1) at 80 and 120 nmol or with a control miRNA (ConmiR) at 120 nmol. Western blot analysis was performed with HDAC4 antibody 48 h after transfection. B) Chicken growth plate chondrocytes from d 17 embryos were transfected with a miR-1 mimic (miR-1) or control microRNA (ConmiR), and a miR-1 inhibitor (anti-miR-1) or a control microRNA inhibitor (Control) at 120 nmol, respectively. Western blot analysis was performed with an antibody against HDAC4 48 h after transfection. miR-1 (120 nM) reduces the level of HDAC4, while antimiR-1 increases the level of HDAC4 compared to the control. C) Band intensities obtained from 3 individual Western blots were quantified using ImageJ software after normalize to β-actin to generate a bar graph of the relative difference in HDAC4 protein levels. Error bars represent ±1 sd of the mean. *P < 0.05.
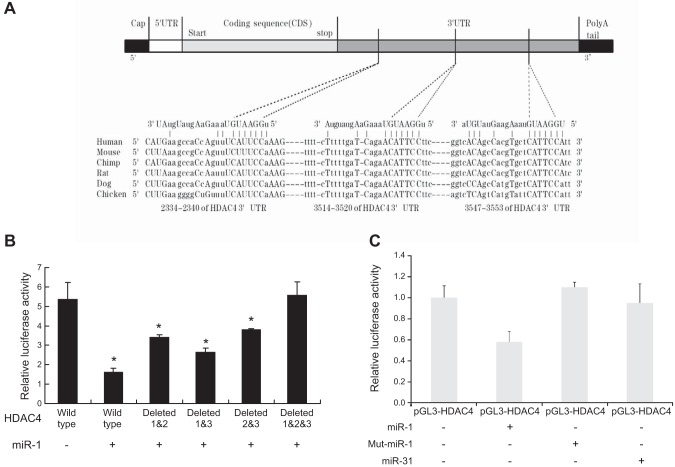
Identification of three miR-1 targeting sites of HDAC4 3′-UTRs
Sequence analysis indicates that miR-1 is conserved in different mammalian species, and there are three putative seed regions of miR-1 in the HDAC4 3′-UTR. The predicted seeding sites were shown in HDAC4 3′-UTR (position: 5′ 2334–2340 3′, 5′ 3514–3520 3′, 5′ 3547–3553 3′) (Fig. 6A). Luciferase reporter assay indicated that miR-1 mimic suppresses the luciferase activity of the wild-type HDAC4 3′-UTR reporter containing three binding sites at 120 nM, this suppression is abolished partially in the single deleted HDAC4 3′-UTR reporter and completely in the three binding sites deleted HDAC4 3′-UTR reporter (Fig. 6B). These results suggest that all of three binding sites are required for miR-1 inhibiting HDAC4 production. COS-1 cells were also cotransfected with mouse HDAC4 3′-UTR luciferase report and miR-1 or mutated miR-1 expression vectors. Relative luciferase activity results indicated that transfection of miR-1 suppresses the luciferase activity of the HDAC4 3′-UTR reporter, and this suppression is abolished in the mut-miR-1. miR-31 mimic was used as negative control because there is no binding site for miR-31 in the HDAC4 3′-UTR (Fig. 6C).
Figure 6.
Repression of HDAC4 3′-UTR by miR-1. A) Sequences of the miR-1 target sites in the 3′-UTR of HDAC4 in different species. MiR-1 is conserved in different mammalian species. The predicted seeding sites were shown in HDAC4 3′-UTR (position: 2334–2340, 3514–3520, 3547–3553). B) COS-1 cell was cotransfected with different deleted binding sites of HDAC4 3′-UTR plasmid and/or miR-1 mimic: 1) wild type containing 3 binding sites, 2) wild type+miR-1 (120 nM), 3) binding sites 1 and 2 deleted + miR-1 (120 nM), 4) binding sites 1 and 3 deleted + miR-1 (120 nM), 5) binding sites 2 and 3 deleted + miR-1 (120 nM), and 6) all three binding sites deleted + miR-1 (120 nM). Forty-eight hours after transfection, the cells were harvested for quantification of dual-luciferase activities. Data are the means of 3 independent experiments, with sd indicated. *P < 0.05, compared with HDAC4 wild type. C) COS-1 cell was cotransfected with pGL3-HDAC4 3′-UTR luciferase report and miR-1 or miR-31 or miR-1mut. Luciferase activity was determined 48 h after transfection. Data are the means of 3 independent experiments, with sd indicated. *P < 0.05.
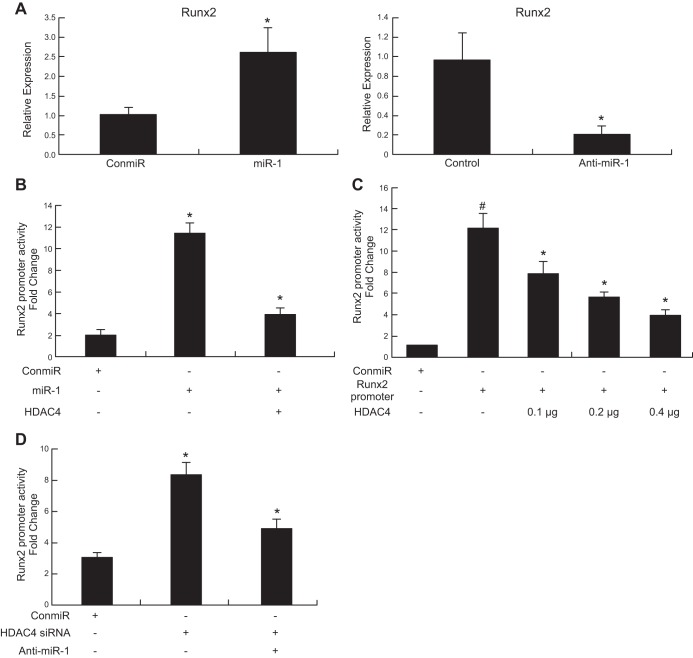
MiR-1 induction of Runx2
To determine whether miR-1 regulates Runx2, a target gene of HDAC4, we quantified Runx2 mRNA levels and Runx2 promoter activities in chondrocytes treated with miR-1 and anti-miR-1. Real-time PCR results showed that overexpression of miR-1 increases the mRNA level of Runx2 while knockdown miR-1 (anti-miR-1) decreases its expression compared with control miRNA (Fig. 7A). Luciferase activity results indicated that cotransfection of HDAC4 overcomes the induction of Runx2 promoter activities by miR-1 (Fig. 7B), and HDAC4 significantly inhibits the Runx2 promoter activities in a dosage-dependent manner (Fig. 7C). Knockdown of HDAC4 by HDAC4 siRNA increases Runx2 promoter activity, while anti-miR-1 overcomes the Runx2 promoter activity induced by HDAC4 siRNA (Fig. 7D). These data indicate that miR-1 regulates Runx2 through inhibiting HDAC4 pathway.
Figure 7.
MiR-1 increases Runx2 gene expression and Runx2 luciferase reporter activity. A) Chicken growth plate chondrocytes from d 17 embryos were transfected with a miR-1 mimic (miR-1) or a control miRNA (ConmiR), and a miR-1 inhibitor (anti-miR-1) or a control miRNA inhibitor (Control) at 120 nmol. Total RNA was extracted, and the mRNA level of Runx2 was quantified by real-time RT-PCR 24 h post-transfection. Data are the means of 3 independent experiments, with sd indicated. *P < 0.05, compared with control miR. B) Chondrocytes were transiently transfected with Runx2 promoter and expression vectors encoding HDAC4, as indicated in the presence or absence of control miRNA (ConmiR) or miR-1 mimic (miR-1). The cells were harvested for quantification of dual-luciferase activities 48 h after transfection. miR-1 increases Runx2 promoter activity while HDAC4 overcomes the Runx2 promoter activity induced by miR-1. Data are the means of 3 independent experiments, with sd indicated. *P < 0.05, compared with Con-miR; #P < 0.05, compared with miR-1 alone. C) HDAC4 inhibits Runx2 promoter activity in a dose-dependent manner. Data are the means of 3 independent cell culture experiments, with sd indicated. #P < 0.05, compared to control; *P < 0.05, compared to Runx 2 promoter alone. D) Knockdown HDAC4 by HDAC4 siRNA increases Runx2 promoter activity, while anti-miR-1 overcomes the Runx2 promoter activity induced by HDAC4 siRNA. Data are the means of 3 independent cell culture experiments, with sd indicated. *P < 0.05, compared to ConmiR; #P < 0.05, compared to HDAC4 siRNA alone.
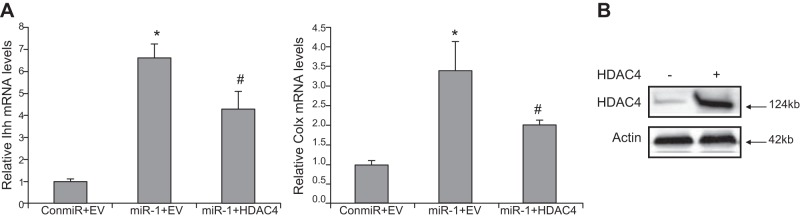
miR-1 induction of Ihh and Col X expression
To determine whether HDAC4 is involved in miR-1-inducing chondrocyte differentiation, we quantified the mRNA levels of Ihh and Col X by real-time PCR. miR-1 increases the mRNA levels of Ihh and Col X, while cotransfection of HDAC4 abolishes this induction (Fig. 8). Thus, overexpression of HDAC4 provides evidence that miR-1 is able to mediate the induction of chondrocyte differentiation via targeting HDAC4 (Fig. 8).
Figure 8.
HDAC4 inhibiting miR-1 induction of Ihh and Col X expression. A) Chicken growth plate chondrocytes from d 17 embryo were transfected with a control miRNA (ConmiR) or miR-1 or miR-1 plus HDAC4 expression vector. The mRNA levels of Ihh and Col X were quantified by real-time RT-PCR 24 h after transfection. Data are the means of 3 independent experiments, with sd indicated. *P < 0.05, compared with ConmiR. #P < 0.05, compared with miR-1. B) Overexpression of HDAC4 increased HDAC4 protein levels. Western blot analysis was performed with cell lysates collected 48 h post-transfection by an antibody against HDAC4.
DISCUSSION
MiR-1 plays an important role in muscle cell proliferation and differentiation (11). Targeted deletion of the muscle-specific miR-1–2 results in 50% lethality by weaning due to an effect on regulation of cardiac morphogenesis, electrical conduction, and cell cycle (12). These studies indicate that miR-1 plays a critical role in muscle tissue development. Little is known, however, about the spatio-temporal distribution and the function of miRNA-1 during growth plate development.
Recently, Kobayashi and his colleagues found that miR-1 is expressed in chondrocytes, but they could not exclude the possibility of muscle contamination (13). To test whether miR-1 is expressed in cartilage and to avoid a similar problem, we dissected the tibia growth plate from 17-d-old chicken embryos under dissection microscopy. These tissues did not contain any muscle (Fig. 2A). We found that miR-1 is, indeed, expressed in the cartilage in addition to the muscle, but not in liver, intestine, brain, or lung (Fig. 1). We also tested for miR-133, which is abundantly expressed in muscle cells (11), but found that miR-133 was not expressed in the chondrocytes. This suggests that miR-1 expression was not due to contaminating muscle tissue. Since miR-1 is conserved across species, it will be interesting to examine whether miR-1 has similar functions in chondrocytes in addition to muscle tissue.
We further demonstrated that miR-1 is more strongly expressed in hypertrophic chondrocytes than in proliferative chondrocytes (Fig. 2B). The result was further validated by Northern blot analysis (Fig. 2C). A similar result was also found in the MCT cell line (Fig. 2D). The same mouse chondrocyte cell line has been used for chondrocyte proliferation and differentiation studies (27–29). The distribution of miR-1 coincides with Ihh and type X expression region in vivo, markers for hypertrophic chondrocytes (Fig. 2E). Interestingly, the decrease of HDAC4 is also observed in the hypertrophic zone (21).
HDAC4 inhibits chondrocyte hypertrophy by interacting with and suppressing the activity of Runx2, a master transcription factor necessary for chondrocyte hypertrophy (17, 19). However, one important question is what regulates HDAC4 expression and how chondrocyte hypertrophy proceeds despite HDAC4. Our Western blot results demonstrate that miR-1 inhibits HDAC4 protein production and increases Runx2, Col X, and Ihh expression. Inhibiting miR-1 has an opposite effect. This is consistent with the notion that miRNAs control target gene translation and/or mRNA degradation (30). Our data strongly suggest that miR-1 stimulates chondrocyte differentiation through inhibiting HDAC4. The decrease in HDAC4 releases the HDAC4 inhibition of Runx2, which results in increased Ihh and Col X and subsequent chondrocyte hypertrophy. Consistent with this hypothesis, the mRNA levels of Ihh and Runx2 are greatly increased in HDAC4−/− mice (17). It has also been shown previously that Runx2 strongly induced Col X and Ihh promoter activities by binding to the promoter region of the Col X and Ihh genes directly (18, 31, 32). Taken together, our data suggest a model in which miR-1 stimulates chondrocyte differentiation by inhibiting HDAC4 and inducing Runx2 and Col X expression.
It has been demonstrated that miRNAs act through the 3′-UTRs of targeted transcripts (33). Sequences analysis indicates that miR-1 is conserved in mammalian species (miRBas accession number: MIMAT0000416) (http://microrna.sanger.ac.uk/sequences/). We identified a new miR-1 binding site (2334–2340) of HDAC4 3′-UTR in addition to binding sites 3512-20 and 3547-53 (11). Our data demonstrate that miR-1 mimic inhibits the activity of the reporter gene harboring the three binding sites of HDAC4 3′-UTR, it fails to do so to the reporter gene harboring the three binding sites deleted in the target region and partially fails to do so to the reporter gene harboring single binding site deletion. This indicates that all three binding regions in the 3′-UTR of the HDAC4 mRNA are required for miR-1 inhibition of HDAC4 translation. We further showed that the expression plasmids encoding miR-1 inhibit the activity of the report gene harboring the wild-type 3′-UTR of the HDAC4, while the expression plasmid of the mutant miR-1 fails to do so to the reporter gene harboring the wild-type 3′-UTR of the HDAC4.
Interestingly, we noticed that miR-1 also induces chondrocyte proliferation. Recently, Kobayashi et al. (13) has shown that, in a growth plate, deficiency of Dicer, an essential component of biogenesis of miRNAs, leads to progressive reduction in the proliferating pool of chondrocytes. Our data indicate that inhibition of miR-1 also decreases chondrocyte proliferation, which is consistent with Kobayashi's study (13), although Kobayashi's study represents the end result of inhibiting all miRNAs through knocking out Dicer in cartilage. Our data indicate that the expression of miR-1 is sufficient for inducing Runx2 and Ihh, while the inhibition of miR-1 leads to suppression of Runx2 and Ihh. Runx2−/−3−/− mice did not express Ihh and adenoviral introduction of Runx2 in Runx2−/− chondrocyte cultures strongly induced Ihh expression (18). Moreover, Runx2 directly bound to the promoter region of the Ihh gene and strongly induced expression of the reporter gene driven by the Ihh promoter (18). Ihh is a well-known gene that plays a key role for chondrocyte proliferation and maturation (18, 34, 35). Thus, miR-1 induces chondrocyte proliferation, at least in part, by inhibiting HDAC4, resulting in activity of Runx2/Ihh signaling in the chondrocytes.
In summary, our data indicate for the first time that miR-1 is able to regulate chondrocyte proliferation and differentiation. MiR-1 functions by inhibiting its direct target HDAC4 at the post-transcription level and inducing expression of Runx2, Col X, and Ihh, key regulators of endochondral bone formation. These findings provide new insights into miR-1 regulating growth plate development.
Acknowledgments
The project was supported by grants R01AR059142 from U.S. National Insitutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases and P20GM104937 from NIH/National Institute of General Medical Sciences, National Science Foundation of China grants 81071495, 81171676 and 31271033, SXNSF 2011011042.
The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. The authors gratefully acknowledge Dr. Ericka M. Bueno for help with the manuscript preparation and editorial services.
Footnotes
- ACTA1
- actin-α 1
- anti-miR-1
- microRNA-1 inhibitor
- CCK-8
- cell counting kit-8
- Col X
- type X collagen
- ConmiR
- control microRNA
- DAPI
- 4′,6-diamidino-2-phenylindole
- EdU
- 5-ethynyl-2′-deoxyuridine
- HDAC
- histone deacetylase
- Ihh
- Indian hedgehog
- MCT
- murine chondrocyte
- miR-1
- microRNA-1
- miR
- microRNA
- mut-miR-1
- mutated microRNA-1
- MYOD1
- myogenic differentiation 1
- RISC
- RNA-induced silencing complex
- Runx2
- Runt-related transcription factor 2
- UTR
- untranslated region
REFERENCES
- 1. Redfern A. D., Colley S. M., Beveridge D. J., Ikeda N., Epis M. R., Li X., Foulds C. E., Stuart L. M., Barker A., Russell V. J., Ramsay K., Kobelke S. J., Li X., Hatchell E. C., Payne C., Giles K. M., Messineo A., Gatignol A., Lanz R. B., O'Malley B. W., Leedman P. J. (2013) RNA-induced silencing complex (RISC) proteins PACT, TRBP, and Dicer are SRA binding nuclear receptor coregulators. Proc. Natl. Acad. Sci. U. S. A. 110, 6536–6541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Valencia-Sanchez M. A., Liu J., Hannon G. J., Parker R. (2006) Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 20, 515–524 [DOI] [PubMed] [Google Scholar]
- 3. Bagga S., Bracht J., Hunter S., Massirer K., Holtz J., Eachus R., Pasquinelli A. E. (2005) Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 122, 553–563 [DOI] [PubMed] [Google Scholar]
- 4. Eulalio A., Rehwinkel J., Stricker M., Huntzinger E., Yang S.-F., Doerks T., Dorner S., Bork P., Boutros M., Izaurralde E. (2007) Target-specific requirements for enhancers of decapping in miRNA-mediated gene silencing. Genes Dev. 21, 2558–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee R. C., Ambros V. (2001) An extensive class of small RNAs in Caenorhabditis elegans. Science 294, 862–864 [See comment] [DOI] [PubMed] [Google Scholar]
- 6. Li S., Czubryt M. P., McAnally J., Bassel-Duby R., Richardson J. A., Wiebel F. F., Nordheim A., Olson E. N. (2005) Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc. Natl. Acad. Sci. U. S. A. 102, 1082–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin Z., Murtaza I., Wang K., Jiao J., Gao J., Li P.-F. (2009) miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc. Natl. Acad. Sci. U. S. A. 106, 12103–12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y., Liang Y., Lu Q. (2008) MicroRNA epigenetic alterations: predicting biomarkers and therapeutic targets in human diseases. Clin. Genet. 74, 307–315 [DOI] [PubMed] [Google Scholar]
- 9. Yan L.-X., Huang X.-F., Shao Q., Huang M.-Y., Deng L., Wu Q.-L., Zeng Y.-X., Shao J.-Y. (2008) MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA 14, 2348–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Rooij E., Sutherland L. B., Thatcher J. E., DiMaio J. M., Naseem R. H., Marshall W. S., Hill J. A., Olson E. N. (2008) Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. U. S. A. 105, 13027–13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen J.-F., Mandel E. M., Thomson J. M., Wu Q., Callis T. E., Hammond S. M., Conlon F. L., Wang D.-Z. (2006) The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 38, 228–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao Y., Ransom J. F., Li A., Vedantham V., von Drehle M., Muth A. N., Tsuchihashi T., McManus M. T., Schwartz R. J., Srivastava D. (2007) Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1–2. Cell 129, 303–317 [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi T., Lu J., Cobb B. S., Rodda S. J., McMahon A. P., Schipani E., Merkenschlager M., Kronenberg H. M. (2008) Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. Proc. Natl. Acad. Sci. U. S. A. 105, 1949–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jenuwein T., Allis C. D. (2001) Translating the histone code. Science 293, 1074–1080 [See comment] [DOI] [PubMed] [Google Scholar]
- 15. Grozinger C. M., Schreiber S. L. (2002) Deacetylase enzymes: biological functions and the use of small-molecule inhibitors. Chem. Biol. 9, 3–16 [DOI] [PubMed] [Google Scholar]
- 16. Chang S., McKinsey T. A., Zhang C. L., Richardson J. A., Hill J. A., Olson E. N. (2004) Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol. Cell. Biol. 24, 8467–8476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vega R. B., Matsuda K., Oh J., Barbosa A. C., Yang X., Meadows E., McAnally J., Pomajzl C., Shelton J. M., Richardson J. A., Karsenty G., Olson E. N. (2004) Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 119, 555–566 [See comment] [DOI] [PubMed] [Google Scholar]
- 18. Yoshida C. A., Yamamoto H., Fujita T., Furuichi T., Ito K., Inoue K.-I., Yamana K., Zanma A., Takada K., Ito Y., Komori T. (2004) Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 18, 952–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guan Y.-J., Yang X., Wei L., Chen Q. (2011) MiR-365: a mechanosensitive microRNA stimulates chondrocyte differentiation through targeting histone deacetylase 4. FASEB J. 25, 4457–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y., Chen Q. (2000) Changes of matrilin forms during endochondral ossification. Molecular basis of oligomeric assembly. J. Biol. Chem. 275, 32628–32634 [DOI] [PubMed] [Google Scholar]
- 21. Guan Y., Chen Q., Yang X., Haines P., Pei M., Terek R., Wei X., Zhao T., Wei L. (2012) Subcellular relocation of histone deacetylase 4 regulates growth plate chondrocyte differentiation through Ca2+/calmodulin-dependent kinase IV. Am. J. Physiol. Cell Physiol. 303, C33–C40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu X., Lv Y., Xie Y., Hong Q., Cai G., Zhang S., Liu W., Chen X. (2011) Change of MAX interactor 1 expression in an anti-Thy1 nephritis model and its effect on mesangial cell proliferation. Cell. Physiol. Biochem. 27, 391–400 [DOI] [PubMed] [Google Scholar]
- 23. Qu D., Wang G., Wang Z., Zhou L., Chi W., Cong S., Ren X., Liang P., Zhang B. (2011) 5-Ethynyl-2′-deoxycytidine as a new agent for DNA labeling: detection of proliferating cells. Anal. Biochem. 417, 112–121 [DOI] [PubMed] [Google Scholar]
- 24. Wei L., Kanbe K., Lee M., Wei X., Pei M., Sun X., Terek R., Chen Q. (2010) Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation. Dev. Biol. 341, 236–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wei L., Fleming B. C., Sun X., Teeple E., Wu W., Jay G. D., Elsaid K. A., Luo J., Machan J. T., Chen Q. (2010) Comparison of differential biomarkers of osteoarthritis with and without posttraumatic injury in the Hartley guinea pig model. J. Orthop. Res. 28, 900–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wei F., Zhou J., Wei X., Zhang J., Fleming B. C., Terek R., Pei M., Chen Q., Liu T., Wei L. (2012) Activation of Indian hedgehog promotes chondrocyte hypertrophy and upregulation of MMP-13 in human osteoarthritic cartilage. Osteoarthritis Cartilage 20, 755–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beier F., Lee R. J., Taylor A. C., Pestell R. G., LuValle P. (1999) Identification of the cyclin D1 gene as a target of activating transcription factor 2 in chondrocytes. Proc. Natl. Acad. Sci. U. S. A. 96, 1433–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beier F., Taylor A. C., LuValle P. (1999) The Raf-1/MEK/ERK pathway regulates the expression of the p21(Cip1/Waf1) gene in chondrocytes. J. Biol. Chem. 274, 30273–30279 [DOI] [PubMed] [Google Scholar]
- 29. Correa D., Hesse E., Seriwatanachai D., Kiviranta R., Saito H., Yamana K., Neff L., Atfi A., Coillard L., Sitara D., Maeda Y., Warming S., Jenkins N. A., Copeland N. G., Horne W. C., Lanske B., Baron R. Zfp521 is a target gene and key effector of parathyroid hormone-related peptide signaling in growth plate chondrocytes. Dev. Cell 19, 533–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Emiliani S., Fischle W., Van Lint C., Al-Abed Y., Verdin E. (1998) Characterization of a human RPD3 ortholog, HDAC3. Proc. Natl. Acad. Sci. U. S. A. 95, 2795–2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng Q., Zhou G., Morello R., Chen Y., Garcia-Rojas X., Lee B. (2003) Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J. Cell. Biol. 162, 833–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li F., Lu Y., Ding M., Napierala D., Abbassi S., Chen Y., Duan X., Wang S., Lee B., Zheng Q. (2011) Runx2 contributes to murine Col10a1 gene regulation through direct interaction with its cis-enhancer. J. Bone Miner. Res. 26, 2899–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chin L. J., Ratner E., Leng S., Zhai R., Nallur S., Babar I., Muller R.-U., Straka E., Su L., Burki E. A., Crowell R. E., Patel R., Kulkarni T., Homer R., Zelterman D., Kidd K. K., Zhu Y., Christiani D. C., Belinsky S. A., Slack F. J., Weidhaas J. B. (2008) A SNP in a let-7 microRNA complementary site in the KRAS 3′ untranslated region increases non-small cell lung cancer risk. Cancer Res. 68, 8535–8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lai L. P., Mitchell J. (2005) Indian hedgehog: its roles and regulation in endochondral bone development. J. Cell. Biochem. 96, 1163–1173 [DOI] [PubMed] [Google Scholar]
- 35. St-Jacques B., Hammerschmidt M., McMahon A. P. (1999) Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 13, 2072–2086; erratum, 2617 [DOI] [PMC free article] [PubMed] [Google Scholar]