Abstract
The ubiquitin-proteasome pathway regulates many basic cellular processes and has been proven to be a promising target for cancer therapy. Bortezomib is the first U.S. Food and Drug Administration (FDA) approved proteasome inhibitor used in the treatment of newly diagnosed multiple myeloma, relapsed/refractory multiple myeloma, and mantle cell lymphoma. The anti-cancer mechanisms of bortezomib elucidated by preclinical studies include: upregulation of proapoptotic proteins (e.g., Noxa, IκB), inhibition of NFκB and its anti-apoptotic target genes, suppression of several anti-apoptotic proteins (e.g., Bcl-XL, Bcl-2, and STAT-3), down-regulation of expression of several proteins involved in DNA repair pathways, and induction of endoplasmic reticulum (ER) stress and pro-apoptotic Unfolded Protein Response (UPR). Bortezomib has potent chemo-/radio-sensitizing effects and can overcome traditional drug resistance in tumors when used in combination with potential chemotherapies. Although bortezomib has been successful in improving clinical outcomes when used in hematological malignancies, relapse may occur in those patients who responded initially. Furthermore, some cytotoxicities (such as peripheral neuropathy) were found to be associated with bortezomib treatment. These observations have encouraged researchers to search for the next generation proteasome inhibitors (including carfilzomib and marizomib) that could overcome bortezomib resistance and have improved properties, reduced toxicities, and broader anticancer activities, based on the lessons learned from the mechanisms and use of bortezomib. This review summarizes the current status of bortezomib as well as several other proteasome inhibitors that are currently under clinical and preclinical investigation.
Introduction
Cellular homeostasis and regulation of cellular functions depend on finely orchestrated intracellular processes, such as systematic degradation of regulatory proteins. In eukaryotic cells, the ubiquitin-proteasome pathway (UPP) is primarily responsible for degrading the majority of cellular proteins and plays an essential role in many basic cellular processes (Adams, 2003). The UPP is comprised of a ubiquitin conjugating system and the proteasome (Figure 1). Cellular proteins that govern important functions including signal transduction, cell cycle control, transcriptional regulation, and apoptosis are substrates of the UPP. Furthermore, the UPP selectively eliminates mutant, misfolded, and damaged proteins and is indispensable for cellular housekeeping (Ciechanover, 1994).
Figure 1.
The ubiquitin-proteasome pathway. Target proteins of the proteasome are tagged with polyubiquitin molecules in an ATP-dependent process through E1, E2, and E3 ligases. Polyubiquitinated proteins are then recognized by the 19S regulatory complex of the 26S proteasome and fed into the 20S catalytic core for degradation and the ubiquitin molecules recycled. The 20S proteasome contains four stacked rings (αββα): α subunits serve as a gate to regulate protein entry while the β subunits possess the enzymatic activities.
Given the significance of the UPP, aberrations in this pathway have been implicated in the pathogenesis of various human diseases including cancer (Ciechanover, 1998). Cancer cells frequently harbor defects in some regulatory proteins whose degradation is either overabundant or insufficient. For instance, excessive degradation of the tumor suppressor p53 or IκB (inhibitor of NF-κB) in tumor cells could promote cancer cell growth. Thus, targeting key features of the UPP responsible for the mechanisms underlying tumorigenesis and progression in cancer cells has been the subject of intense investigation. This review summarizes the development and applications of bortezomib, the first U.S. FDA approved selective inhibitor of the proteasome, for the treatment of multiple myeloma and mantle cell lymphoma. Furthermore, the second generation proteasome inhibitors as anticancer drugs currently being tested in clinical trials and several other proteasome inhibitors currently being developed preclinically are also discussed here.
The Ubiquitin-Proteasome Pathway
A basic understanding of the proteasome structure and function in the UPP is necessary to understand the mechanism of action of bortezomib. Degradation of proteins by the UPP involves two discrete and important steps: (i) the activation of ubiquitin and subsequent covalent attachment of multiple moieties to a protein substrate by the E1, E2, and E3 enzymes, followed by (ii) degradation of the tagged protein by the 26S proteasome (Figure 1) (Adams, 2003). Structurally, the 26S proteasome is a proteolytic complex comprised of a hollow cylindrical multi-catalytic 20S core and two 19S regulatory caps (Groll et al., 1997). The 19S cap recognizes, binds, and cleaves the polyubiquitin chain of the target protein thereby directing it into the 20S catalytic core (Peters et al., 1993). The 20S catalytic core consists of two identical alpha subunit (α1-α7) rings and two identical beta subunit (β1-β7) rings. The catalytic cleavage of peptides is facilitated by three proteolytically active beta subunits, β1 (caspase-like activity), β2 (trypsin-like activity), and β5 (chymotrypsin-like activity). The catalytic site of β5 subunit is defined by several hydrophobic residues, and the hydroxyl group on the side chain of threonine 1 (Thr1) is responsible for catalyzing cleavage of peptides through nucleophilic attack (Groll et al., 1999). Inhibition of the proteasomal chymotrypsin-like activity significantly affects protein processing by the proteasome (Groll et al., 1999).
As discussed earlier, degradation of misfolded and unfolded proteins is a major function of the UPP. Misfolded or unfolded proteins are recognized by the endoplasmic reticulum (ER) stress or unfolded protein response (UPR) pathway and are guided to degradation by the 26S proteasome (Tsai et al., 2002). Disruption of proteasome activity causes accumulation of otherwise degradable proteins within the cell and constitutive ER stress blocks cellular growth and division, eventually leading to cell death (Obeng et al., 2006). Cells in general may not respond similarly to proteasome inhibition and several types of cancer cells have actually been found to be far more sensitive to proapoptotic effects of proteasome inhibition than normal cells (Dou and Li, 1999), and this provides the essential basis for proteasome inhibitors as anticancer drugs. Additionally, inhibition of the tumor cellular proteasome could potentially disrupt the mechanisms of de novo and acquired resistance, sensitizing them to chemo- or radiotherapy (Voorhees et al., 2003). Therefore, development of small molecule proteasome inhibitors has received considerable attention as an important therapeutic strategy for a range of cancers, including multiple myeloma, lymphoma, and some solid tumors (Meiners et al., 2008; Orlowski and Zeger, 2006).
Bortezomib: Biological Effects and Possible Mechanisms of Action
Bortezomib (also known as Velcade® and PS-341) was originally developed by Myogenics, and after successful completion of Phase I clinical trials in multiple myeloma patients it was later bought by Millennium Pharmaceuticals. After further clinical development, it had become a first-in-class U.S. FDA approved proteasome inhibitor for the treatment of multiple myeloma and mantle cell lymphoma (Kane et al., 2003; 2007; 2006). The molecular formula of bortezomib is C19H25BN4O4 (Figure 2) and its chemical IUPAC name is [3-methyl-1-(3-phenyl-2-pyrazin-2-ylcar-bonylamino-propanoyl) amino-butyl] boronic acid. It is a dipeptidyl boronic acid-based specific, reversible inhibitor of the chymotrypsin-like activity of the 20S proteasome (Jackson et al., 2005; Papandreou et al., 2004). The boronic acid moiety of bortezomib forms a (pseudo)covalent bond with the nucleophilic hydroxyl side chain of Thr1 in the S1 pocket of the β5 subunit (Groll et al., 2006a).
Figure 2.
Chemical structures of proteasome inhibitors bortezomib, carfilzomib, and marizomib.
Bortezomib has been reported to possess potent antitumor activity in a wide variety of cancer cell lines including multiple myeloma, prostate cancer, pancreatic cancer, renal cell carcinoma, and squamous cell carcinoma both in vitro and in various animal xenograft models (Adams, 2002; Jagannath et al., 2005; Kondagunta et al., 2004; Richardson et al., 2005; Shah et al., 2001; Sunwoo et al., 2001). In both solid tumor-and hematological malignancy-derived cell lines bortezomib has demonstrated equal potency in terms of anticancer effect (Frankel et al., 2000).
The mechanism of action of bortezomib was found to be unique when it was compared with 60,000 other compounds by the National Cancer Institute (NCI) (Adams et al., 1999). Though its detailed mechanism of action is still not completely understood some preclinical studies have proposed possible anticancer mechanisms of bortezomib. Proteasome inhibition by bortezomib induces ER stress and constitutive ER stress causes calcium release, which is subsequently taken up by the mitochondria, leading to cytochrome c release and subsequent activation of effector caspases, followed by cleavage of Bid to tBid, ultimately resulting in apoptosis (Landowski et al., 2005). Recently, Gu et al. (2008) reported that bortezomib-triggered apoptosis in multiple myeloma cells was dependent on caspase-2 activation. This study showed that caspase-2, which is associated with ER stress, acts as a proximal caspase and functions upstream of mitochondrial signaling and is required for breakdown of mitochondrial transmembrane potential, release of cytochrome c, and down-stream activation of caspase-9. As myeloma cells produce and secrete large amounts of immunoglobulin, their threshold for induction of ER stress and proapoptotic UPR following proteasome inhibition may be lower. Not surprisingly myeloma cells have been shown to be very sensitive to proteasome inhibition (Meister et al., 2007).
Mitsiades et al. (2002) described the molecular sequelae of proteasome inhibition by bortezomib in human multiple myeloma cells. In these cells bortezomib was reported to stimulate and up-regulate genes involved in proapoptotic cascades and down-regulate pro-survival genes. Inhibition of the proteasomal catalytic activity by bortezomib suppresses an important survival mechanism: the activation of nuclear factor-κB (NF-κB) pathway (Adams, 2004b; Palombella et al., 1994). NF-κB is a heterodimeric transcription factor found in the cytoplasm bound to its inhibitory counterpart protein IκB in its inactive state. Ubiquitination and degradation of IκB by the UPP leads to release and translocation of NF-κB to the nucleus (Karin et al., 2004). Once in the nucleus NF-κB can up-regulate the expression of genes that promote cell growth and survival [e.g., insulin-like growth factor 1 (IGF1) and its receptor IGF1R, NF-κB, Bcl-2 family members, and inhibitor-of-apoptosis proteins (IAPs)]. Therefore, it is thought that bortezomib prevents degradation of IκB and activation of NF-κB, in turn suppressing the production of survival factors. Recently it has been reported that NF-κB activity levels are different in drug-sensitive and drug-resistant multiple myeloma cells (Ma et al., 2003). Furthermore, the presence of elevated NF-κB activity levels has been observed in patients with relapsed multiple myeloma (Feinman et al., 1999). These studies suggest that NF-κB is a key target of bortezomib in multiple myeloma cells.
More recent studies have also identified other possible mechanisms involved in bortezomib’s anticancer activity. NOXA, a pro-apoptotic protein belonging to the Bcl-2 family, appears to be another key modulator of bortezomib’s anticancer effects (Qin et al., 2005). In p53-mediated apoptosis, up-regulation of p53 expression with subsequent Noxa gene expression has been observed. Tumor suppressor p53 has been shown to interact directly with and activate the promoter for Noxa gene expression (Oda et al., 2000). Induction of apoptosis by NOXA involves its direct interaction with anti-apoptotic proteins like Bcl-2 and Bcl-XL or by stimulating other apoptosis-promoting factors (Adams and Cory, 1998; Gross et al., 1999; Oda et al., 2000). In a variety of tumor cell lines with defective p53 signaling, bortezomib can still induce NOXA expression and block tumor growth (Adams, 2004a; Caravita et al., 2006; Nikiforov et al., 2007). Bortezomib causes 20- to 60-fold induction of NOXA expression selectively in cancer cells but not in normal cells (Fernandez et al., 2005; Qin et al., 2005). This selectivity of proteasome inhibitors is directly dependent on c-Myc binding sites in the Noxa promoter and depletion of c-Myc blocks the tumor cell-selective induction of NOXA by bortezomib (Nikiforov et al., 2007).
Hypoxia-inducible factor-1α (HIF-1α), which is known to support tumor growth, is one of the most studied and promising molecular targets for anti-cancer therapy. In both androgen-dependent and androgen-independent prostate cancer cell lines, bortezomib was reported to reduce HIF-1α protein synthesis through suppression of PI3K/Akt/mTOR and MAPK pathways (Befani et al., 2011). Very recently, studies in breast cancer cell lines by Ishii et al. (2006) reported Cyclin D1 levels to be an important marker or target for predicting the response to bortezomib treatment. Cyclin D1 is a short-lived protein that is regulated by the UPP (Diehl et al., 1998; Germain et al., 2000). Cyclin D1 was found to be over-expressed in approximately 25% and 90% of all multiple myeloma and mantle cell lymphoma patients, respectively (Lesage et al., 2005; O’Connor et al., 2005). Cyclin D1 expression levels correlated with the overall response rate to bortezomib treatment in each disease. This study showed that in a variety of human tumors including breast cancer cells, Cyclin D1 levels inversely correlated with the levels of anti-apoptotic transcription factor Signal Transducer and Activator of Transcription 3 (STAT3). STAT3 is known to regulate the expression of anti-apoptotic Bcl-XL and has been found to be overexpressed in many human cancers. Bortezomib treatment was more effective in Cyclin D1-overexpressing cells and therefore the authors proposed an additional mechanism of action for bortezomib’s anti-cancer activity which is the stabilization of Cyclin D1 protein levels in order to inhibit the STAT3/Bcl-XL survival axis (Ishii et al., 2006).
Bortezomib has also been tested in combination with other chemotherapeutic drugs. In doxorubicin-, mitoxantrone-, and melphalan-sensitive and -resistant RPMI-8226 human multiple myeloma cells, bortezomib was found to possess potent growth inhibitory effects in vitro (Hideshima et al., 2001). In these combinations bortezomib had a profound sensitization effect on cancer cells resistant to chemotherapeutic drugs such as melphalan and doxorubicin. The mechanism of sensitization was found to be down-regulation of expression of several proteins involved in DNA repair pathways (Mitsiades et al., 2003).
Bortezomib: Reports from Clinical Trials
The FDA approval of bortezomib as a front-line treatment for patients with newly diagnosed and relapsed/refractory multiple myeloma reinforces the UPP as a valid target in the treatment of malignant diseases. Bortezomib, either alone or in combination, has an overall positive response in the clinic. For example, in a Phase II clinical trial conducted by Oakervee and colleagues, relapsed multiple myeloma patients were treated with a combination therapy of bortezomib, doxorubicin, and dexamethasone. Nearly partial response (PR) was achieved in 20 of 21 patients (95%), including complete response (CR) in 43%, near CR in 14%, very good PR in 24%, and PR in 14% (Oakervee et al., 2005). In another Phase II trial conducted by Jagannath et al. (2005), 32 consecutive patients with untreated symptomatic multiple myeloma patients were given bortezomib in combination with dexamethasone, yielding a response rate (CR+PR) of 88%. Additionally, in the Phase II Study of Uncontrolled Multiple Myeloma Managed with Proteasome Inhibition Therapy (SUMMIT), 202 relapsed and refractory multiple myeloma patients were treated with 1.3 mg/m2 bortezomib for a 3-week cycle for up to eight cycles. The combined overall response rate was 35% with bortezomib treatment alone (Richardson et al., 2003). The most recent phase III VISTA trial, which included patients with previously treated multiple myeloma, reported that the combination of bortezomib with melphalan and prednisone improves overall patient survival (Mateos et al., 2010). Analysis of the data from this trial showed that bortezomib-based drugs as first-line treatments had a greater survival advantage over conventional drugs followed by bortezomib treatments for salvage (Mateos et al., 2010). It also showed that in patients treated with bortezomib plus melphalan-prednisone the rate of improvement of peripheral neuropathy was 79%, indicating this adverse side effect to be generally manageable and reversible in most patients with relapsed myeloma (Mateos et al., 2010).
Toxicity, Resistance, and Limitations of Bortezomib
In spite of its improved efficacy compared to traditional chemotherapies, bortezomib still possesses some toxicity in the clinic and about 60% of patients will eventually not respond to bortezomib due to the emergence of resistance. The average time between the start of bortezomib treatment and the occurrence of resistance to it is about one year (Richardson et al., 2003). The most frequent toxicities associated with bortezomib are gastrointestinal symptoms, anemia, thrombocytopenia, asthenia (fatigue, malaise, and weakness), elevated calcium levels, and peripheral neuropathy (Orlowski et al., 2005).
Some of the molecular mechanisms for the emergence of resistance have been found to be mutations in the bortezomib binding pocket of the β5 subunit, overexpression of the proteasomal β5 subunit (Oerlemans et al., 2008), over-expression of the anti-apoptotic protein Bcl-2 (Smith et al., 2011), high secretion of GRP-78, a chaperone protein of the unfolded protein response (Kern et al., 2009), and over-expression of heat shock proteins (HSPs) 27, 70, and 90 as well as T-cell factor 4 (Shringarpure et al., 2006). For example, de Wilt et al. (2011) showed that in non-small cell lung cancer (NSCLC) intrinsic bortezomib resistance correlated with high basal levels of proteasome activity, whereas acquired resistance was associated with proteasome subunit over-expression and emergence of mutant β5-subunits. Additionally, prevalence of proteasome inhibition- resistant, constitutive NF-κB activity in RPMI 8226 multiple myeloma cells and stem-like cells of mantle cell lymphoma has been reported (Jung et al., 2011; Yang et al., 2008). Furthermore, down-regulation of XBP1, a major regulator of UPR, has been observed in myeloma cells resistant to bortezomib (Ling et al., 2011).
Beyond bortezomib’s approved indications for the treatment of multiple myeloma and mantle cell lymphoma, it has been investigated for the treatment of solid tumors in clinical studies. It was found that bortezomib had decreased efficacy in solid tumors, possibly due to induction of stress granule formation by bortezomib. This involves phosphorylation of translation initiation factor eIF2α by heme-regulated inhibitor kinase (HRI) (Fournier et al., 2010).
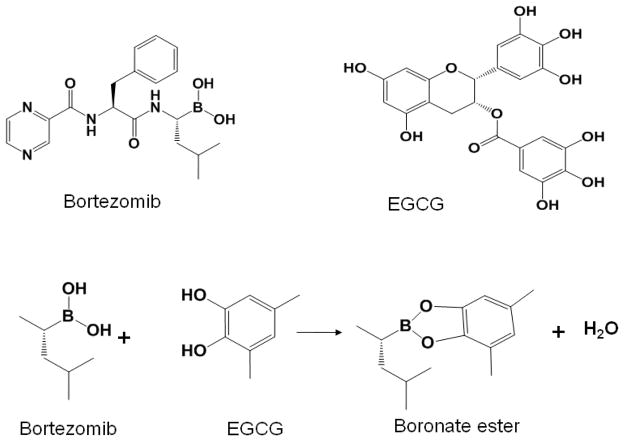
Recent preclinical studies have shown that green tea polyphenol epigallocatechin-3-gallate (EGCG) (Golden et al., 2009; Kim et al., 2009) and vitamin C (Perrone et al., 2009; Zou et al., 2006) have the potential to inhibit the cytotoxicity of bortezomib. These two natural compounds contain vicinal diols that can bind and inactivate boronic acid present in bortezomib, thereby inhibiting the proteasome-inhibitory and anticancer activities of bortezomib (Figure 3). Further clinical studies are needed for evidence-based recommendations on consumption of green tea and vitamin C for patients receiving bortezomib.
Figure 3.
Bortezomib interacts with certain natural compounds with vicinal diols. Postulated interaction between bortezomib and EGCG as an example is presented.
The Second Generation Proteasome Inhibitors: Carfilzomib and Marizomib
The acquired resistance to bortezomib therapy seen in patients encouraged researchers to search for other new proteasome inhibitors as well as novel natural compounds with proteasome-inhibitory activity. Carfilzomib (Figure 2), a peptide epoxyketone derived from epoxomicin, was first developed by Proteolix, Inc. and currently by Onyx Pharmaceuticals. Carfilzomib binds irreversibly to the proteasome and preferentially inhibits the chymotrypsin-like activity over the caspase-like or trypsin-like activities (Kuhn et al., 2007). Results from preclinical studies demonstrate that carfilzomib induced higher levels of apoptosis than bortezomib in primary plasma cell models, and was able to overcome resistance to bortezomib. In animal models, carfilzomib had anti-cancer activity in both dose- and time-dependent manners, and again the anti-cancer efficacy of carfilzomib was stronger than that of bortezomib when tested on its clinical dosing schedule (Demo et al., 2007). Carfilzomib is currently being investigated in both Phase II and III clinical trials for the treatment of recurrent multiple myeloma and solid tumors (Kuhn et al., 2011). Phase II clinical trials in patients with previously treated multiple myeloma showed response rates in the range of 25–54% (Yang et al., 2009). Another clinical trial has been initiated to evaluate the efficacy and safety of carfilzomib with lenalidomide and dexamethasone in patients with relapsed multiple myeloma (NCT00603447, Proteolix).
Another second generation proteasome inhibitor is marizomib (Figure 2) which is currently being developed by Nereus Pharmaceuticals, Inc. (Potts et al., 2011). It is a natural product derivative resembling lactacystin, the first identified natural proteasome inhibitor. In contrast to bortezomib and carfilzomib, marizomib irreversibly targets all three active sites of the proteasome (Joazeiro et al., 2006). Marizomib showed significantly stronger and more durable effects on the proteasomal chymotrypsin-like and trypsin-like activities than bortezomib in preclinical studies (Chauhan et al., 2005; Groll et al., 2006b). The increased potency of marizomib may be related to its specificity toward caspase 8-mediated apoptosis compared to bortezomib (Chauhan et al., 2005). Consistent with these properties, marizomib was able to overcome bortezomib resistance and work synergistically with conventional therapy in multiple myeloma and chronic lymphocytic leukemia (CLL) cell models (Chauhan et al., 2008; 2010; Sterz et al., 2008). Phase I studies aimed at establishing optimal dosing of marizomib against advanced solid tumors or refractory lymphomas and multiple myeloma have been conducted (Yang et al., 2009). Furthermore, clinical studies investigating marizomib in combination with vorinostat are ongoing in a Phase Ib open-label study in patients with advanced non-small lung cancer (NCT00667082, Nereus Pharmaceuticals).
In addition, CEP-18770 (developed by Cephalon) and MLN-9708 (developed by Millennium Pharmaceutics) are two other reversible, peptide boronic acid-based proteasome inhibitors (Dick and Fleming, 2010; Kupperman et al., 2010). CEP-18770 is being investigated in Phase I and II clinical trials for the treatment of recurrent, advanced stage solid tumors, lymphoblastic leukemia and non-Hodgkin’s lymphoma, while MLN-9708 is in Phase I and II clinical trials for lymphoma and solid tumors (www.cancer.gov/clinicaltrials/search; www.clinicaltrials.gov). Furthermore, ONX-0912 (by Onyx Pharmaceuticals), another peptide epoxyketone proteasome inhibitor, is currently in Phase I and II clinical trials for patients with solid tumors and hematological cancers (www.cancer.gov/clinicaltrials/search; www.clinicaltrials.gov).
Further clinical studies are needed to define the profiles of toxicity and anticancer efficacy of these second generation proteasome inhibitor drugs.
Other Proteasome Inhibitors Currently Being Developed
The immunoproteasome (20Si and 26Si) is a cytokine-inducible form of the constitutive proteasome, with the replacement of β1, β2, and β5 subunits with the immunoproteasome-specific β1i, β2i, and β5i subunits, respectively. It has been found that myeloma cells express increased levels of the immunoproteasome complex. Also, lower levels of the immunoproteasome and increased levels of the constitutive proteasome are associated with relapsed myeloma and bortezomib resistance (Kuhn et al., 2011). IPSI-001, an immunoproteasome-specific inhibitor, has been investigated and found to preferably inhibit immunoproteasome 20Si activity over constitutive 20S proteasome activity. The main mechanism involves binding of IPSI-001 to the β1i subunit. This inhibition leads to enhanced apoptotic cell death in human cancer cells generated from a hematologic origin. Other IPSIs, PR-924, PR-957, and different β1i specific inhibitors, are also currently being developed. It has been shown that IPSIs were able to overcome bortezomib-resistance in the preclinical setting, suggesting that they may provide an alternative therapeutic option for cancer patients resistant to bortezomib (Dou, 2011; Kuhn et al., 2011).
In addition to the proteasome inhibitors mentioned above, some other specific proteasome inhibitors (e.g., TMC-95A and PR39) have also been developed, which, however, have not yet been tested clinically. Some natural flavonoids (e.g., EGCG, apigenin) and medicinal compounds (e.g., celastrol, withaferin A) have shown to possess tumor proteasome-inhibitory capabilities in vitro and in vivo and these compounds have already been used in various human studies. Also, some old drugs that can bind to copper or zinc (e.g., 5-amino-8-hydroxyquinoline, clioquinol, and disulfiram) and inhibit the tumor proteasome in preclinical settings have been used in various clinical studies. The above mentioned proteasome inhibitors could also chemo-/radio-sensitize human cancer cells, but whether they could overcome bortezomib-resistance needs to be studied (Dou, 2010; Ruschak et al., 2011).
Conclusions and Future Perspectives
Most targeted therapies are designed with the intent of inhibiting a single protein target or signaling pathway to dampen tumor progression and have shown limited anti-cancer efficacy. Alternatively, targeting the UPP, which regulates multiple events and involves many protein targets, represents one of the most promising anticancer strategies. Bortezomib is the first U.S. FDA approved proteasome inhibitor in clinical use. It possesses potent antitumor activity and acts as a chemo-/radio-sensitizing agent when combined with conventional therapy or radiation. However, side effects and the eventual emergence of drug-resistance in a significant portion of cancer patients encouraged the development of second generation proteasome inhibitors like carfilzomib, marizomib, CEP-18770, MLN-9708, and ONX-0912 that are currently being investigated in clinical trials. Furthermore, IPSIs were shown to overcome bortezomib-resistance in the preclinical setting, and are ready to move to clinical studies. Natural products EGCG, apigenin, celastrol, and withaferin A also possess tumor proteasome-inhibitory effects in vitro and in vivo and these compounds have already been investigated in various human studies. Moreover, some copper- or zinc-binding drugs (e.g., 5-amino-8-hydroxyquinoline, clioquinol, and disulfiram) inhibit the tumor proteasome in both preclinical and clinical studies. However, all the new proteasome inhibitors’ efficacy, their ability to overcome bortezomib-resistance, and whether they themselves develop resistance remain to be investigated and elucidated. Finally, the importance of the UPP has incited researchers to target other aspects of the pathway such as E1, E2, and, especially E3 enzymes, as well as the ubiquitin receptors and deubiquinating enzymes (DUBs) toward the goal of improving cancer therapy. There are high hopes in the field that the discovery of novel anticancer agents targeting the ubiquitin-proteasome pathway will help illuminate the future of cancer treatment.
Acknowledgments
The authors thank Daniela Buac and Sara Schmitt for critical reading of this manuscript. This work was partially supported by grants from the National Cancer Institute (1R01CA120009, 3R01CA120009-04S1, and 5R01CA127258-05 to Q.P.D.).
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- Adams J. Preclinical and clinical evaluation of proteasome inhibitor PS-341 for the treatment of cancer. Curr Opin Chem Biol. 2002;6(4):493–500. doi: 10.1016/s1367-5931(02)00343-5. [DOI] [PubMed] [Google Scholar]
- Adams J. The proteasome: structure, function, and role in the cell. Cancer Treat Rev. 2003;29(Suppl 1):3–9. doi: 10.1016/s0305-7372(03)00081-1. [DOI] [PubMed] [Google Scholar]
- Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004a;5(5):417–421. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004b;4(5):349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59(11):2615–2622. [PubMed] [Google Scholar]
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Befani CD, Vlachostergios PJ, Hatzidaki E, Patrikidou A, Bonanou S, Simos G, Papandreou CN, Liakos P. Bortezomib represses HIF-1alpha protein expression and nuclear accumulation by inhibiting both PI3K/Akt/TOR and MAPK pathways in prostate cancer cells. J Mol Med (Berl) 2011 Sep 10; doi: 10.1007/s00109-011-0805-8. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Caravita T, De Fabritiis P, Palumbo A, Amadori S, Boccadoro M. Bortezomib: efficacy comparisons in solid tumors and hematologic malignancies. Nat Clin Pract Oncol. 2006;3(7):374–387. doi: 10.1038/ncponc0555. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Catley L, Li G, Podar K, Hideshima T, Velankar M, Mitsiades C, Mitsiades N, Yasui H, Letai A, Ovaa H, Berkers C, Nicholson B, Chao TH, Neuteboom ST, Richardson P, Palladino MA, Anderson KC. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8(5):407–419. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Singh A, Brahmandam M, Podar K, Hideshima T, Richardson P, Munshi N, Palladino MA, Anderson KC. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2008;111(3):1654–1664. doi: 10.1182/blood-2007-08-105601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D, Singh AV, Ciccarelli B, Richardson PG, Palladino MA, Anderson KC. Combination of novel proteasome inhibitor NPI-0052 and lenalidomide trigger in vitro and in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2010;115(4):834–845. doi: 10.1182/blood-2009-03-213009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79(1):13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17(24):7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wilt LH, Jansen G, Assaraf YG, Van Meerloo J, Cloos J, Schimmer AD, Chan ET, Kirk CJ, Peters GJ, Kruyt FA. Proteasome-based mechanisms of intrinsic and acquired bortezomib resistance in non-small cell lung cancer. Biochem Pharmacol. 2011 Oct 18; doi: 10.1016/j.bcp.2011.10.009. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Demo SD, Kirk CJ, Aujay MA, Buchholz TJ, Dajee M, Ho MN, Jiang J, Laidig GJ, Lewis ER, Parlati F, Shenk KD, Smyth MS, Sun CM, Vallone MK, Woo TM, Molineaux CJ, Bennett MK. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67(13):6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- Dick LR, Fleming PE. Building on bortezomib: second-generation proteasome inhibitors as anti-cancer therapy. Drug Discov Today. 2010;15(5–6):243–249. doi: 10.1016/j.drudis.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12(22):3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou QP. Pharmaceutical design of novel anticancer agents: a lesson from nature. Curr Pharm Des. 2010;16(16):1799–1800. doi: 10.2174/138161210791208965. [DOI] [PubMed] [Google Scholar]
- Dou QP. Targeting tumor ubiquitin-proteasome pathway with new and old drugs. Curr Cancer Drug Targets. 2011;11(3):236–238. doi: 10.2174/156800911794519789. [DOI] [PubMed] [Google Scholar]
- Dou QP, Li B. Proteasome inhibitors as potential novel anticancer agents. Drug Resist Updat. 1999;2(4):215–223. doi: 10.1054/drup.1999.0095. [DOI] [PubMed] [Google Scholar]
- Feinman R, Koury J, Thames M, Barlogie B, Epstein J, Siegel DS. Role of NF-kappaB in the rescue of multiple myeloma cells from glucocorticoid-induced apoptosis by bcl-2. Blood. 1999;93(9):3044–3052. [PubMed] [Google Scholar]
- Fernandez Y, Verhaegen M, Miller TP, Rush JL, Steiner P, Opipari AW, Jr, Lowe SW, Soengas MS. Differential regulation of noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer Res. 2005;65(14):6294–6304. doi: 10.1158/0008-5472.CAN-05-0686. [DOI] [PubMed] [Google Scholar]
- Fournier MJ, Gareau C, Mazroui R. The chemotherapeutic agent bortezomib induces the formation of stress granules. Cancer Cell Int. 2010;10:12. doi: 10.1186/1475-2867-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A, Man S, Elliott P, Adams J, Kerbel RS. Lack of multicellular drug resistance observed in human ovarian and prostate carcinoma treated with the proteasome inhibitor PS-341. Clin Cancer Res. 2000;6(9):3719–3728. [PubMed] [Google Scholar]
- Germain D, Russell A, Thompson A, Hendley J. Ubiquitination of free cyclin D1 is independent of phosphorylation on threonine 286. J Biol Chem. 2000;275(16):12074–12079. doi: 10.1074/jbc.275.16.12074. [DOI] [PubMed] [Google Scholar]
- Golden EB, Lam PY, Kardosh A, Gaffney KJ, Cadenas E, Louie SG, Petasis NA, Chen TC, Schonthal AH. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009;113(23):5927–5937. doi: 10.1182/blood-2008-07-171389. [DOI] [PubMed] [Google Scholar]
- Groll M, Berkers CR, Ploegh HL, Ovaa H. Crystal structure of the boronic acid-based proteasome inhibitor bortezomib in complex with the yeast 20S proteasome. Structure. 2006a;14(3):451–456. doi: 10.1016/j.str.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, Bartunik HD, Huber R. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature. 1997;386(6624):463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- Groll M, Heinemeyer W, Jager S, Ullrich T, Bochtler M, Wolf DH, Huber R. The catalytic sites of 20S proteasomes and their role in subunit maturation: a mutational and crystallographic study. Proc Natl Acad Sci U S A. 1999;96(20):10976–10983. doi: 10.1073/pnas.96.20.10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groll M, Huber R, Potts BC. Crystal structures of Salinosporamide A (NPI-0052) and B (NPI-0047) in complex with the 20S proteasome reveal important consequences of beta-lactone ring opening and a mechanism for irreversible binding. J Am Chem Soc. 2006b;128(15):5136–5141. doi: 10.1021/ja058320b. [DOI] [PubMed] [Google Scholar]
- Gross A, Mcdonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13(15):1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Gu H, Chen X, Gao G, Dong H. Caspase-2 functions upstream of mitochondria in endoplasmic reticulum stress-induced apoptosis by bortezomib in human myeloma cells. Mol Cancer Ther. 2008;7(8):2298–2307. doi: 10.1158/1535-7163.MCT-08-0186. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61(7):3071–3076. [PubMed] [Google Scholar]
- Ishii Y, Pirkmaier A, Alvarez JV, Frank DA, Keselman I, Logothetis D, Mandeli J, O’connell MJ, Waxman S, Germain D. Cyclin D1 overexpression and response to bortezomib treatment in a breast cancer model. J Natl Cancer Inst. 2006;98(17):1238–1247. doi: 10.1093/jnci/djj334. [DOI] [PubMed] [Google Scholar]
- Jackson G, Einsele H, Moreau P, Miguel JS. Bortezomib, a novel proteasome inhibitor, in the treatment of hematologic malignancies. Cancer Treat Rev. 2005;31(8):591–602. doi: 10.1016/j.ctrv.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, Lutzky J, Mckinley M, Gabayan E, Mazumder A, Schenkein D, Crowley J. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129(6):776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- Joazeiro CA, Anderson KC, Hunter T. Proteasome inhibitor drugs on the rise. Cancer Res. 2006;66(16):7840–7842. doi: 10.1158/0008-5472.CAN-06-2033. [DOI] [PubMed] [Google Scholar]
- Jung HJ, Chen Z, Fayad L, Wang M, Romaguera J, Kwak LW, Mccarty N. Bortezomib-resistant nuclear factor kappa B expression in stem like cells in mantle cell lymphoma (MCL) Exp Hematol. 2011 Oct 21; doi: 10.1016/j.exphem.2011.10.004. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8(6):508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- Kane RC, Dagher R, Farrell A, Ko CW, Sridhara R, Justice R, Pazdur R. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13(18 Pt 1):5291–5294. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- Kane RC, Farrell AT, Sridhara R, Pazdur R. United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res. 2006;12(10):2955–2960. doi: 10.1158/1078-0432.CCR-06-0170. [DOI] [PubMed] [Google Scholar]
- Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3(1):17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- Kern J, Untergasser G, Zenzmaier C, Sarg B, Gastl G, Gunsilius E, Steurer M. GRP-78 secreted by tumor cells blocks the antiangiogenic activity of bortezomib. Blood. 2009;114(18):3960–3967. doi: 10.1182/blood-2009-03-209668. [DOI] [PubMed] [Google Scholar]
- Kondagunta GV, Drucker B, Schwartz L, Bacik J, Marion S, Russo P, Mazumdar M, Motzer RJ. Phase II trial of bortezomib for patients with advanced renal cell carcinoma. J Clin Oncol. 2004;22(18):3720–3725. doi: 10.1200/JCO.2004.10.155. [DOI] [PubMed] [Google Scholar]
- Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, Demo SD, Bennett MK, Van Leeuwen FW, Chanan-Khan AA, Orlowski RZ. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110(9):3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DJ, Orlowski RZ, Bjorklund CC. Second generation proteasome inhibitors: carfilzomib and immunoproteasome-specific inhibitors (IPSIs) Curr Cancer Drug Targets. 2011;11(3):285–295. doi: 10.2174/156800911794519725. [DOI] [PubMed] [Google Scholar]
- Kupperman E, Lee EC, Cao Y, Bannerman B, Fitzgerald M, Berger A, Yu J, Yang Y, Hales P, Bruzzese F, Liu J, Blank J, Garcia K, Tsu C, Dick L, Fleming P, Yu L, Manfredi M, Rolfe M, Bolen J. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70(5):1970–1980. doi: 10.1158/0008-5472.CAN-09-2766. [DOI] [PubMed] [Google Scholar]
- Landowski TH, Megli CJ, Nullmeyer KD, Lynch RM, Dorr RT. Mitochondrial-mediated disregulation of Ca2+ is a critical determinant of Velcade (PS-341/bortezomib) cytotoxicity in myeloma cell lines. Cancer Res. 2005;65(9):3828–3836. doi: 10.1158/0008-5472.CAN-04-3684. [DOI] [PubMed] [Google Scholar]
- Lesage D, Troussard X, Sola B. The enigmatic role of cyclin D1 in multiple myeloma. Int J Cancer. 2005;115(2):171–176. doi: 10.1002/ijc.20907. [DOI] [PubMed] [Google Scholar]
- Ling SC, Lau EK, Al-Shabeeb A, Nikolic A, Catalano A, Iland H, Horvath N, Ho PJ, Harrison S, Fleming S, Joshua DE, Allen JD. Response of myeloma to proteasome inhibitor bortezomib is correlated with unfolded protein response regulator XBP-1. Haematologica. 2011 Oct 11; doi: 10.3324/haematol.2011.043331. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma MH, Yang HH, Parker K, Manyak S, Friedman JM, Altamirano C, Wu ZQ, Borad MJ, Frantzen M, Roussos E, Neeser J, Mikail A, Adams J, Sjak-Shie N, Vescio RA, Berenson JR. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res. 2003;9(3):1136–1144. [PubMed] [Google Scholar]
- Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Esseltine DL, Liu K, Cakana A, Van De Velde H, San Miguel JF. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28(13):2259–2266. doi: 10.1200/JCO.2009.26.0638. [DOI] [PubMed] [Google Scholar]
- Meiners S, Ludwig A, Stangl V, Stangl K. Proteasome inhibitors: poisons and remedies. Med Res Rev. 2008;28(2):309–327. doi: 10.1002/med.20111. [DOI] [PubMed] [Google Scholar]
- Meister S, Schubert U, Neubert K, Herrmann K, Burger R, Gramatzki M, Hahn S, Schreiber S, Wilhelm S, Herrmann M, Jack HM, Voll RE. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67(4):1783–1792. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA, Treon SP, Munshi NC, Richardson PG, Hideshima T, Anderson KC. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 2002;99(22):14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades N, Mitsiades CS, Richardson PG, Poulaki V, Tai YT, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA, Schlossman R, Munshi NC, Hideshima T, Anderson KC. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101(6):2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- Nikiforov MA, Riblett M, Tang WH, Gratchouck V, Zhuang D, Fernandez Y, Verhaegen M, Varambally S, Chinnaiyan AM, Jakubowiak AJ, Soengas MS. Tumor cell-selective regulation of NOXA by c-MYC in response to proteasome inhibition. Proc Natl Acad Sci U S A. 2007;104(49):19488–19493. doi: 10.1073/pnas.0708380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor OA, Wright J, Moskowitz C, Muzzy J, Macgregor-Cortelli B, Stubblefield M, Straus D, Portlock C, Hamlin P, Choi E, Dumetrescu O, Esseltine D, Trehu E, Adams J, Schenkein D, Zelenetz AD. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23(4):676–684. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Oakervee HE, Popat R, Curry N, Smith P, Morris C, Drake M, Agrawal S, Stec J, Schenkein D, Esseltine DL, Cavenagh JD. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol. 2005;129(6):755–762. doi: 10.1111/j.1365-2141.2005.05519.x. [DOI] [PubMed] [Google Scholar]
- Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288(5468):1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- Oerlemans R, Franke NE, Assaraf YG, Cloos J, Van Zantwijk I, Berkers CR, Scheffer GL, Debipersad K, Vojtekova K, Lemos C, Van Der Heijden JW, Ylstra B, Peters GJ, Kaspers GL, Dijkmans BA, Scheper RJ, Jansen G. Molecular basis of bortezomib resistance: proteasome subunit beta5 (PSMB5) gene mutation and overexpression of PSMB5 protein. Blood. 2008;112(6):2489–2499. doi: 10.1182/blood-2007-08-104950. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Voorhees PM, Garcia RA, Hall MD, Kudrik FJ, Allred T, Johri AR, Jones PE, Ivanova A, Van Deventer HW, Gabriel DA, Shea TC, Mitchell BS, Adams J, Esseltine DL, Trehu EG, Green M, Lehman MJ, Natoli S, Collins JM, et al. Phase 1 trial of the proteasome inhibitor bortezomib and pegylated liposomal doxorubicin in patients with advanced hematologic malignancies. Blood. 2005;105(8):3058–3065. doi: 10.1182/blood-2004-07-2911. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Zeger EL. Targeting the proteasome as a therapeutic strategy against haematological malignancies. Expert Opin Investig Drugs. 2006;15(2):117–130. doi: 10.1517/13543784.15.2.117. [DOI] [PubMed] [Google Scholar]
- Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78(5):773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X, Pien CS, Millikan RE, Tu SM, Pagliaro L, Kim J, Adams J, Elliott P, Esseltine D, Petrusich A, Dieringer P, Perez C, Logothetis CJ. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol. 2004;22(11):2108–2121. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- Peters JM, Cejka Z, Harris JR, Kleinschmidt JA, Baumeister W. Structural features of the 26 S proteasome complex. J Mol Biol. 1993;234(4):932–937. doi: 10.1006/jmbi.1993.1646. [DOI] [PubMed] [Google Scholar]
- Potts BC, Albitar MX, Anderson KC, Baritaki S, Berkers C, Bonavida B, Chandra J, Chauhan D, Cusack JC, Fenical W, Ghobrial IM, Groll M, Jensen PR, Lam KS, Lloyd GK, Mcbride W, McConkey DJ, Miller CP, Neuteboom STC, Oki Y, et al. Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011;11(3):254–284. doi: 10.2174/156800911794519716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin JZ, Ziffra J, Stennett L, Bodner B, Bonish BK, Chaturvedi V, Bennett F, Pollock PM, Trent JM, Hendrix MJ, Rizzo P, Miele L, Nickoloff BJ. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 2005;65(14):6282–6293. doi: 10.1158/0008-5472.CAN-05-0676. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- Ruschak AM, Slassi M, Kay LE, Schimmer AD. Novel proteasome inhibitors to overcome bortezomib resistance. J Natl Cancer Inst. 2011;103(13):1007–1017. doi: 10.1093/jnci/djr160. [DOI] [PubMed] [Google Scholar]
- Shah JJ, Orlowski RZ. Proteasome inhibitors in the treatment of multiple myeloma. Leukemia. 2009;23(11):1964–1979. doi: 10.1038/leu.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SA, Potter MW, Mcdade TP, Ricciardi R, Perugini RA, Elliott PJ, Adams J, Callery MP. 26S proteasome inhibition induces apoptosis and limits growth of human pancreatic cancer. J Cell Biochem. 2001;82(1):110–122. doi: 10.1002/jcb.1150. [DOI] [PubMed] [Google Scholar]
- Shringarpure R, Catley L, Bhole D, Burger R, Podar K, Tai YT, Kessler B, Galardy P, Ploegh H, Tassone P, Hideshima T, Mitsiades C, Munshi NC, Chauhan D, Anderson KC. Gene expression analysis of B-lymphoma cells resistant and sensitive to bortezomib. Br J Haematol. 2006;134(2):145–156. doi: 10.1111/j.1365-2141.2006.06132.x. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Dai H, Correia C, Takahashi R, Lee SH, Schmitz I, Kaufmann SH. Noxa/Bcl-2 protein interactions contribute to bortezomib resistance in human lymphoid cells. J Biol Chem. 2011;286(20):17682–17692. doi: 10.1074/jbc.M110.189092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterz J, Von Metzler I, Hahne JC, Lamottke B, Rademacher J, Heider U, Terpos E, Sezer O. The potential of proteasome inhibitors in cancer therapy. Expert Opin Investig Drugs. 2008;17(6):879–895. doi: 10.1517/13543784.17.6.879. [DOI] [PubMed] [Google Scholar]
- Sunwoo JB, Chen Z, Dong G, Yeh N, Crowl Bancroft C, Sausville E, Adams J, Elliott P, Van Waes C. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res. 2001;7(5):1419–1428. [PubMed] [Google Scholar]
- Tsai B, Ye Y, Rapoport TA. Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat Rev Mol Cell Biol. 2002;3(4):246–255. doi: 10.1038/nrm780. [DOI] [PubMed] [Google Scholar]
- Voorhees PM, Dees EC, O’neil B, Orlowski RZ. The proteasome as a target for cancer therapy. Clin Cancer Res. 2003;9(17):6316–6325. [PubMed] [Google Scholar]
- Yang DT, Young KH, Kahl BS, Markovina S, Miyamoto S. Prevalence of bortezomib-resistant constitutive NF-kappaB activity in mantle cell lymphoma. Mol Cancer. 2008;7:40. doi: 10.1186/1476-4598-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zonder JA, Dou QP. Clinical development of novel proteasome inhibitors for cancer treatment. Expert Opin Investig Drugs. 2009;18(7):957–971. doi: 10.1517/13543780903002074. [DOI] [PMC free article] [PubMed] [Google Scholar]