Abstract
CONTEXT
Dental sealants and composite filling materials containing bisphenol A (BPA) derivatives are increasingly used in childhood dentistry. Evidence is accumulating that BPA and some BPA derivatives can pose health risks attributable to their endocrine-disrupting, estrogenic properties.
OBJECTIVES
To systematically compile and critically evaluate the literature characterizing BPA content of dental materials; to assess BPA exposures from dental materials and potential health risks; and to develop evidence-based guidance for reducing BPA exposures while promoting oral health.
METHODS
The extant toxicological literature and material safety data sheets were used as data sources.
RESULTS
BPA is released from dental resins through salivary enzymatic hydrolysis of BPA derivatives, and BPA is detectable in saliva for up to 3 hours after resin placement. The quantity and duration of systemic BPA absorption is not clear from the available data. Dental products containing the bisphenol A derivative glycidyl dimethacrylate (bis-GMA) are less likely to be hydrolyzed to BPA and have less estrogenicity than those containing bisphenol A dimethacrylate (bis-DMA). Most other BPA derivatives used in dental materials have not been evaluated for estrogenicity. BPA exposure can be reduced by cleaning and rinsing surfaces of sealants and composites immediately after placement.
CONCLUSIONS
On the basis of the proven benefits of resin-based dental materials and the brevity of BPA exposure, we recommend continued use with strict adherence to precautionary application techniques. Use of these materials should be minimized during pregnancy whenever possible. Manufacturers should be required to report complete information on the chemical composition of dental products and encouraged to develop materials with less estrogenic potential.
Keywords: pit and fissure sealants, dental sealants, dental composites, bisphenol A, endocrine disruptors, oral health, pediatric dentistry, children’s environmental health
Bisphenol A (BPA) is a synthetic chemical resin used worldwide in the production of plastic products, notably polycarbonate plastic food-storage containers, some water bottles, bottle tops, and epoxy resin lacquer linings of metal food cans.1 More than 2 million tons of BPA are currently produced per year, and there is an anticipated 6% to 10% annual growth in future demand.2 BPA derivatives are components of resin-based dental sealants and composites that are increasingly used in both preventive and restorative oral health care. Use of composite resin dental materials for fillings was stimulated by demand for aesthetic restorations and by concern about potential neurotoxic and nephrotoxic effects of mercury-containing amalgam fillings. Although exposure to elemental mercury from amalgams has been documented, the level of exposure has proven to be low, and several careful prospective epidemiologic studies using resin-based dental materials as a control group have found no adverse effects on neurodevelopment or kidney function.3–6
Resin-based dental sealants, the protective coating applied most often to permanent molars, have demonstrated effectiveness in preventing cavities (evidentiary level 1a)7,8 and arresting progression of caries.9,10 Stimulated in part by federal programs at the Centers for Disease Control and Prevention and the Maternal and Child Health Bureau, a steadily increasing number of children are receiving sealants during routine pediatric dental care and through school-based dental programs.11 Increasing sealant prevalence is included in Healthy People objectives 21-8a and 21-8b, the target of which is to reach 50% of 8--year-old and 14-year-old children by 2010.1,9,12,13 In the United States during the period 1999–2004, the percentage of children with sealants varied with age from 20.3% to 41.0%. The average number of sealed permanent teeth per child was ~3 for 6- to 11-year-olds and 5 for 12- to 19-year-olds.11
BPA was synthesized ~100 years ago and recognized to have estrogenic properties as early as the 1930s. However, only recently have data begun to emerge to indicate that BPA exposures, even those in the range generally experienced by the US population, may have adverse effects on human health and especially on infant development. 1,14 The failure to test BPA for toxic potential is part of a larger problem, in that many of the 3000 high-volume synthetic chemicals currently in commerce in the United States have been subject to little toxicological examination. 15 Even today, most toxicological research on BPA has been conducted in experimental systems, and there is debate within the literature regarding the extent to which rodent study results can be extrapolated to humans because of different toxicokinetics between species.16 Although comprehensive reports such as the European Union risk assessment14 and US National Toxicology Program Center for the Evaluation of Risks to Human Reproduction expert panel report1 have documented these data and estimated minimal to some human risk potential, there has been relatively little primary research on patterns of human exposure or on potential effects on human health.
In this review we examine the benefits and potential risks to health, especially to child health, of dental materials that contain BPA derivatives. We present information on the BPA content of dental sealants and composites, on patterns of human exposure to BPA and its derivatives from dental materials, and on the potential estrogenicity of BPA derivatives. The goal is to develop evidence-based guidance for reducing BPA exposures while promoting oral health.
RISE OF RESIN-BASED DENTAL MATERIALS
Modern resin-based dental sealants for preventing tooth decay on the biting surfaces of teeth were first marketed in the mid-1960s.12 They are introduced into occlusal pits and fissures of high-risk teeth and create a protective layer that denies cariogenic bacteria access to nutrients necessary for continued progression of tooth decay.13 A review of sealant clinical trials revealed repair, replacement, or restoration rates to be between 5% and 10% each year.17 Before the advent of resin-based sealants, dentistry had much less satisfactory methods of preventing biting-surface cavities in molar teeth that are critical to function and tooth alignment. Earlier efforts to address this issue either failed outright or damaged the tooth irreparably.18–21
Dental composite resins are also used routinely for restoration of decayed, fractured, and poorly formed teeth. The fact that dental composite materials continue to improve in strength, resistance, ease of application, translucency, and polishability rapidly increased their use in the first decade after being introduced and continues to increase their popularity.22,23
BPA IN DENTAL SEALANTS AND COMPOSITES
Dental resins are composed primarily of BPA derivatives rather than pure BPA. These derivatives are liquid monomers that polymerize into a solid after either chemical or light curing. BPA may be found as an impurity in dental resins but is not used in their formulation because moisture from saliva inhibits its polymerization by causing hydrolysis of the 2 end hydroxyl groups.24,25 Thus, BPA glycidyl dimethacrylate (bis-GMA), the derivative used most frequently as the base of the resin, has methyl methacrylate groups added to the hydroxyl groups of BPA via a glycidyl spacer. Other BPA derivatives traditionally used in dental resins include BPA dimethacrylate (bis-DMA) and BPA diglycidylether (BADGE) (Fig 1) as well as BPA ethoxylate dimethacrylate (bis-EMA) and urethane-modified bis-GMA. Other monomers such as triethylene glycol dimethacrylate (TEGDMA) and urethane dimethacrylate (UDMA) are frequently added to the resin to maximize viscosity.24
FIGURE 1.
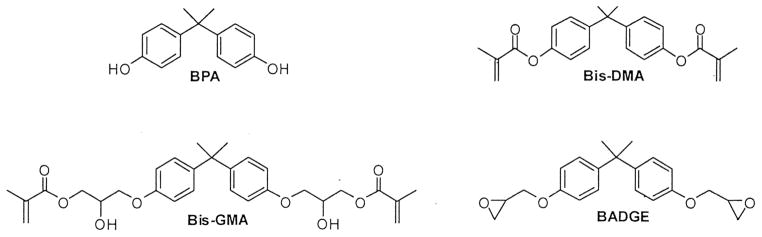
Chemical structures of BPA, bis-GMA, bis-DMA, and BPA diglycidylether (BADGE). These BPA derivatives are modified forms of BPA synthesized to maximize polymerization of dental resins. Currently, Bis-GMA seems to be the most commonly used.
Although pure BPA is not a component of dental resin, it has been detected in saliva after resin placement as a result of hydrolysis of bis-DMA by salivary esterases. 26,27 Although bis-DMA has been shown to hydrolyze into BPA, bis-GMA does not undergo this reaction, presumably because the chemical structure prevents hydrolysis at the ester linkage.24,26,27 To our knowledge, no studies have addressed the potential for other BPA derivatives used in dental materials to hydrolyze to BPA.
BPA-DERIVATIVE COMPOSITION OF DENTAL MATERIALS
Several top-selling sealants and composites in the United States do not disclose the specific monomer composition of their resins in their material safety data sheets (MSDSs) (Tables 1 and 2).28 Manufacturers who do provide data on product composition often use unique monomer structures (eg, urethane modified) that have not been tested for estrogenicity or use generic descriptions of their monomer contents. Furthermore, although other dental material monomers TEGDMA and urethane dimethacrylate are not BPA-based and may not be estrogenic, limited toxicological testing results have suggested that these 2 compounds are cytotoxic.29–31
TABLE 1.
Sealants Representing >90% of the US Market Share in 200828 and Corresponding MSDS Data
| Company | Sealant Name | Market Share, % | MSDS Monomer Composition |
|---|---|---|---|
| Ultradent | Ultraseal | 30.0a | Bis-GMA (CAS No. 1565-94-2); diurethane dimethacrylate (CAS No. 41137-60-4) |
| 3M ESPE | Clinpro Sealant | 22.6 | Bis-GMA (CAS No. 1565-94-2); TEGDMA (CAS No. 109-16-0) |
| Dentsply Preventive Care | Delton | 10.0 | “Aromatic and aliphatic dimethacrylate monomers” |
| Pulpdent Corporation | Embrace | 8.5 | “Acrylate resins” |
| Premier Dental Products Company | Enamel Loc | 6.0 | Urethane dimethacrylate (CAS No. 72869-86-4); bis-GMA (CAS No. 1565-94-2); TEGDMA (CAS No. 109-16-0); “methacrylated phosphoric acid esters” (CAS No. 32435-46-4) |
| Dentsply Preventive Care | Delton Plus | 3.49 | “Resin blend” |
| Dentsply Caulk | FluroShield | 4.2 | Urethane-modified bis-GMA dimethacrylate |
| Kerr Corporation | OptiGuard | 2.8 | “Uncured methacrylate ester monomers” (CAS No. 109-16-0) |
| Ivoclar North America | Helioseal | 2.8 | Bis-GMA (CAS No. 1565-94-2); TEGDMA (CAS No. 109-16-0) |
| Pulpdent Corporation | Seal-Rite | 2.7 | “Methacrylate resins” |
Generic terms in the MSDSs indicate the presence of nonspecific monomer blends. CAS indicates Chemical Abstracts Service.
Privately sold but estimated by Strategic Marketing, Inc to comprise the largest portion (~30%) of the total market share.
TABLE 2.
Composites Representing >60% of the US Market Share in 200828 and Corresponding MSDS Data
| Company | Composite Name | Market Share, % | MSDS Monomer Composition |
|---|---|---|---|
| 3M ESPE | Filtek Supreme Plus | 16.60 | BPA ethoxylate dimethacrylate (CAS No. 41637-38-1); diurethane dimethacrylate (CAS No. 72869-86-4); bis-GMA (CAS No. 1565-94-2); TEGDMA (CAS No. 109-16-0) |
| Dentsply Caulk | TPH 3 | 9.70 | Urethane-modified bis-GMA dimethacrylate (CAS No. 126646-17-1); “polymerizable dimethacrylate resin” (CAS-109-16-0 and 24448-20-2) |
| Dentsply Caulk | Esthet-X | 8.20 | Urethane-modified bis-GMA dimethacrylate (CAS No. 126646-17-1) |
| Kerr Corporation | Premise Indirect | 5.70 | “Uncured methacrylate ester monomers” (CAS No. 109-16-0) |
| Kerr Corporation | Herculite XR | 5.30 | “Uncured methacrylate ester monomers” (CAS No. 109-16-0) |
| 3M ESPE | Filtek Z250 | 5.00 | BPA ethoxylate dimethacrylate (CAS No. 41637-38-1); diurethane dimethacrylate (CAS No. 72869-86-4); bis-GMA (CAS No. 1565-94-2); TEGDMA (CAS No. 109-16-0) |
| 3M ESPE | Z100 | 3.80 | Bis-GMA (CAS No. 1565-94-2); TEGDMA (CAS No. 109-16-0) |
| Heraeus | VENUS | 3.10 | “Methacrylates” |
| 3M ESPE | Filtek Supreme Plus flowable restorative | 3.10 | Bis-GMA (CAS No. 1565-94-2); TEGDMA (CAS No. 109-16-0); BPA ethoxylate dimethacrylate (CAS No. 41637-38-1) |
| Kerr Corporation | Point 4 | 2.70 | “Uncured methacrylate ester monomers” (CAS No. 109-16-0) |
Generic terms in the MSDSs indicate the presence of nonspecific monomer blends. CAS indicates Chemical Abstracts Service.
EXPOSURE TO BPA AND DERIVATIVES AFTER DENTAL RESIN PLACEMENT
Over the past decade, results of studies that evaluated the content of BPA, bis-GMA, and bis-DMA in saliva after application of dental sealants and composites have been mixed. In some studies, both in vitro27,32,33 and in vivo,26,34–36 BPA or BPA derivatives were detected, whereas in other in vitro studies there was no detection over observation periods as long as 10 days after sealant placement.37–39
This variability in findings seems to reflect differences in methodologies and in limits of analytical detection. Some studies assayed BPA and BPA derivatives by using gas chromatography/mass spectroscopy, whereas others used high-performance liquid chromatography (HPLC). HPLC studies have varied according to medium (aqueous versus acetonitrile), which influenced whether monomers could be separated for reliable identification.24,40 On the basis of the method used, levels of detection varied,14 and the lower limit of detection was not published for some studies.32,36,37
Within the published in vitro studies, only 1 used human saliva27 rather than water or ethanol mixtures. In addition, some studies used more than 1 brand of sealant or composite and found variations in BPA leaching across different products.26,27,35,41,42 Several studies revealed leaching of BPA and bis-DMA but not of bis-GMA,26,27,32–36,41,42 which is likely a reflection of varying bis-DMA composition in different products, because bis-GMA has not been shown to hydrolyze to BPA.26,27
Among the in vivo studies in which BPA was found to leach from polymerized dental sealants, 3 studies measured trends in BPA and BPA-derivative levels over time after sealant placement.26,34,35 Salivary BPA levels decreased over time across studies; the highest exposures (range: 0.3–2.8 ppm) occurred immediately after sealant placement25 and lower exposures (range: 5.8–105.6 ppb) occurred 1 hour after placement.33 The longest duration of salivary detection in any of the studies was 3 hours after sealant placement, 34 although none of the studies took measurements at time points between 3 and 24 hours.26,34,35 Joskow et al35 found that in sealants containing bis-DMA, salivary BPA levels increased to 88 times baseline (mean: 26.5 ng/mL) immediately after treatment and were 17 times baseline (mean: 5.12 ng/mL) 1 hour after treatment, although there were no salivary measurements beyond 1 hour after sealant placement. Thus, BPA exposure after sealant placement is most likely an acute event. Presumably, at a certain time after application, the unpolymerized monomer is completely absorbed into saliva, posing little risk of chronic low-dose BPA exposure. It is possible, however, that these studies were not sufficiently sensitive to detect extremely low doses of BPA that could chronically leach from the resin over longer periods of time.
Two studies measured systemic absorption of BPA after dental resin placement. Fung et al34 found BPA in saliva but not in blood when they took measurements up to 5 days after sealant placement. Joskow et al35 found that urinary BPA levels peaked to 6.4 times baseline 1 hour after sealant placement and then decreased significantly by 24 hours. Without measurement of other urinary metabolites such as those of bis-DMA, findings from this study may represent an underestimate of the total systemic absorption of compounds with estrogenic activity.43
PRIMARY SOURCES OF BPA EXPOSURE TO THE US POPULATION
National surveys conducted by the Centers for Disease Control and Prevention have revealed measurable levels of BPA metabolites in the urine of >95% of US residents, despite the short half-life of the compound.43,44 These findings indicate that population exposure is repeated and frequent.
Contaminated food has conventionally been thought to account for a majority of BPA intake. However, recent data from adults who did not have a substantial change in urinary BPA levels after fasting indicate that other sources of BPA, such as drinking water from polyvinyl chloride pipes, copy paper, and dental materials, may account for more exposure than previously realized.45 However, to our knowledge, there have been no studies that have directly quantified the contribution of dental sealants and resin-based composites to total BPA intake.
ESTROGENIC ACTIVITY OF BPA AND BPA DERIVATIVES
Compared with estradiol, BPA binds weakly to nuclear estrogen receptors, 46 but there is evidence to suggest more potent activity at nonclassical membrane receptors.47 However, this includes the majority of data currently available. Animal studies have revealed increased uterine weight, premature vaginal opening,48 and increased differentiation and proliferation of mammary epithelial cells in female rats exposed to BPA.49 Increased prostate weight was seen in male rats exposed prenatally.50 Other animal studies have revealed effects directly related to hormonal disruption, including decreased number of offspring and decreased birth weight after high concentration prenatal exposure, 51,52 delayed onset of puberty, 53,54 and increased aggression.55,56 There has been some debate in the literature regarding the extractability of BPA-exposure data from rodents to humans because of different toxicokinetics between species.16
Although the estrogenicity of BPA has been demonstrated in vitro and in vivo, only a few studies have examined the estrogenicity of BPA derivatives. These studies have primarily been performed in vitro and have found bis-DMA but not bis-GMA (the more common ingredient of most dental resins) to be estrogenic. For instance, BPA, bis-DMA, and high-concentration BPA diglycidylether, but not bis-GMA, induced proliferation of a breast cancer cell line.36 In another study, BPA, but neither bis-GMA nor TEGDMA, showed estrogenic activity.57 Other studies have revealed that only BPA and bis-DMA, but not bis-GMA, interacted with the estrogen receptor.25 More recently, BPA and bis-DMA were shown to be estrogen agonists, androgen antagonists, and inhibitors of aromatase activity at the receptor level.58
To our knowledge, only 1 study has examined the estrogenic activity of a BPA-derivative in vivo. Mariotti et al59 found that high-dose, but not low-dose, bis-GMA injected subcutaneously into mice increased uterine weight and collagen content. No other BPA derivatives were tested in this study. There was no change in uterine cell size or number. Thus, the increased uterine weight may have resulted from a trophic rather than estrogenic effect. Even if there was a true estrogenic effect, the formulation of bis-GMA used in this study is known to contain BPA as an impurity, and the subcutaneous doses were thought to be supraphysiologic. 14,36 This study’s results contradict the in vitro data in which bis-GMA was not shown to be estrogenic, which highlights the need for additional in vivo studies examining the estrogenicity of bis-GMA and other BPA derivatives.
HUMAN HEALTH RISKS OF BPA
The US Environmental Protection Agency (EPA) reference range for acceptable daily BPA exposure is set at <50 μg/kg body weight per day. This level was extrapolated from studies that revealed high-dose BPA in animals (50–500 μg/kg body weight per day) to be associated with adverse effects such as reduced number offspring, reduced birth weight, and delayed puberty. However, since this reference level was established, low-dose BPA levels on the order of 10 μg/kg body weight per day have been associated with changes in behavior and prostate and urinary tract development and with early-onset puberty.1 Therefore, the EPA reference dose for BPA may need to be revised downward.
There is a paucity of direct human epidemiologic studies examining clinical effects of BPA exposure. Some studies have revealed an association between BPA and low follicle-stimulating hormone level in occupationally exposed men,60 high testosterone levels in men and women,61,62 polycystic ovary syndrome in women,61,62 and recurrent miscarriage in women.63 The latter study has been criticized because the 2 cohorts (n = 77) had similar median BPA levels, but the average BPA level of those with recurrent miscarriages was significantly higher on the basis of a few individuals with extremely high levels.64 Prenatal exposure to BPA has been associated with chromosomal defects65 and increased externalizing behaviors.66 There has also been suggestion of a gender-specific effect toward lower birth weights in boys exposed to certain phenols prenatally.67 The only large (n = 1455) cross-sectional study to date of human exposure to BPA took into account a number of potentially confounding variables, including BMI, and revealed an association between BPA and cardiovascular disease, elevated liver enzyme levels, and diabetes but not between BPA and insulin, glucose, cholesterol, or triglyceride levels.68 However, the underlying mechanistic explanation for these findings is not yet clear. Also, this study has been challenged because of a large number of potential confounders and because it analyzed a discrete amount of data from a large data set, which allowed for errors in modeling that could have increased the possibility of false-positive results.69
To assess possible risks to human health in relation to widespread population exposures, the US National Toxicology Program Center for the Evaluation of Risks to Human Reproduction risk analysis for BPA, based largely on extrapolation from the animal literature, concluded that there is “some” concern for neural and behavioral effects in fetuses, infants, and children at current human exposures. The authors concluded further that “there is negligible concern that exposure to BPA causes reproductive effects in nonoccupationally exposed adults and minimal concern for workers exposed to higher levels in occupational settings.”1
EPIDEMIOLOGIC STUDIES OF DENTAL MATERIALS
A randomized controlled trial of dental amalgam versus resin-based materials in US children found that children with resin-based composites had worse psychosocial outcomes on some measures of neurodevelopment 5 years after placement than those with amalgam as assessed by the parent-completed Child Behavior Checklist.70 This finding was not replicated in a similar randomized controlled trial in Portugal that assessed outcomes 7 years after composite placement via specific neurobehavioral tests of memory, attention/concentration, and motor function.4
RECOMMENDED APPLICATION TECHNIQUES
Three randomized controlled trials have examined techniques to limit BPA and derivative exposure from dental resins.41,42,71 These studies noted that 20% to 45% of monomer remains unpolymerized after curing and has potential to leach into saliva. This unreacted monomer is typically found in a liquid layer on the outer surface of the material, where exposure to oxygen inhibits polymerization. Immediate removal of this unpolymerized layer after sealant placement dramatically reduces the levels of available monomer. Gargling water for 30 seconds after composite placement has been shown to decrease salivary BPA levels to nearly baseline for 9 different composite resins.42 In another study, pumice on a cotton ball or in a rotating rubber dental prophylaxis cup was highly effective in eliminating absorption of bis-DMA, bis-GMA, and TEGDMA compared with rubbing the layer with dry cotton or wet cotton or using an air/water spray.71
DISCUSSION
We acknowledge the benefits in preventive and restorative dentistry of plastic resins that contain BPA derivatives, and we discuss the potential risks to health associated with BPA exposure.
The evidence is strong that resin-based dental sealants improve children’s oral health. Also, BPA exposure from dental materials seems transient and can potentially be controlled. However, BPA has been shown to be estrogenic in vitro, in animal models, and in preliminary human studies. These findings provide an incentive to minimize human exposure to BPA in dental materials as a health precaution.
Until recently, little information was available on possible human health consequences of exposure to BPA. Recently emerging data suggest that BPA and other phenols have potential to negatively affect infant development, especially when exposures to these compounds occur during prenatal and early postnatal life.51,52,65–67 It is important, therefore, to reduce these exposures to the greatest extent possible. Particular efforts to minimize exposures may be warranted during pregnancy.
A fundamental legal and policy problem that underlies the questions about BPA exposure is a profound lack of toxicological and exposure information on BPA and BPA derivatives. This lack of information reflects the fact that the Toxic Substances Control Act (TSCA) of 1976, the major federal law that pertains to the evaluation and regulation of chemicals for potential toxicity, has been a substantial failure; more than one-third of high-production-volume chemicals have no toxicity data publicly available. In the past 30 years, only 5 of the 80 000 chemicals currently in commerce have been removed from the market because of their documented toxicity under the provisions of the TSCA. The TSCA does not require companies to release information on ingredients of synthetic products to the public if these ingredients are claimed to be trade secrets, and it does not require companies to perform biomonitoring to understand patterns of exposure or health effects of product components. The US Congress is currently planning to consider legislation entitled the Kids, Worker, and Consumer Safe Chemicals Act (HR 6100, S 3040), which is expected to be more forceful than the TSCA in requiring industry to undertake more rigorous toxicity testing of chemical products.
In the absence of complete toxicological information on the potential adverse effects of dental materials made from BPA derivatives, we offer the following recommendations for prudent practice. Note that these recommendations come from our multidisciplinary team of authors and have not been specifically endorsed by the American Academy of Pediatrics. Revisions to these recommendations may be needed as new data on BPA emerge.
Recommendations for Product Choice
Although the literature is sparse, evidence-based recommendations for choice of dental resin products can begin to be made on the basis of the available data on monomer estrogenicity. Bis-GMA–based resins, which seem to be used most commonly on the basis of current MSDSs, are preferable to bis-DMA–based resins in terms of estrogenicity. Also, studies have consistently shown that bis-DMA, but not bis-GMA, hydrolyzes to BPA on contact with salivary esterases. However, product choice remains difficult, because many products combine bis-GMA with other potentially estrogenic monomers (eg, bisphenol A ethoxylate dimethacrylate and urethane-modified bis-GMA) that have not been as well tested, as well as with monomers such as TEGDMA and urethane dimethacrylate, for which there has also been a paucity of toxicological testing. To enable dentists and consumers to make informed product choices, more-complete data on chemical composition of sealant and composite products need to be made available by manufacturers of dental products on their MSDSs.
Recommendations for Resin Application
There are precautionary measures that can be taken to reduce BPA exposure during application of resin-based dental sealants and composites.41,42,71 These measures are particularly relevant, because the most significant window of potential exposure to BPA is in the period immediately surrounding application.
A precautionary application technique recommended on the basis of these findings is removal of residual monomer by rubbing the monomer layer with pumice on a cotton roll or having the patient gargle for 30 seconds and spit immediately after application of the dental sealant or composite. Because rinsing and spitting can be challenging for many children, thorough rinsing with an air-water syringe may be a suitable substitute. As with all dental operative procedures, use of a rubber dam to control the operative field would further limit potential exposures.
It is prudent to control and limit exposure to unpolymerized dental resin materials during pregnancy, given the sensitivity of the fetus to endocrine-disrupting compounds including BPA and the emerging data indicating adverse developmental effects after prenatal exposure to BPA.65–67 Resin placement during pregnancy should therefore be minimized, and in cases of necessary application, there should be scrupulous control of the operative field and adherence to all other procedures that have been shown to minimize exposure.
Recommendations for Additional Study
An initial research need is to assess all of the dental resins in current or contemplated use for estrogenicity, salivary absorption, and tendency for hydrolysis to BPA. Additional studies are also needed to further assess the potential systemic absorption of BPA and BPA derivatives from saliva at concentrations released by dental materials and the potential to control these exposures through clinical techniques. Large-scale studies could also determine if there is chronic, low-level leaching of BPA from dental materials as they wear over time, because no current in vivo study has tested for leaching >5 days after polymerization. 34 Additional epidemiologic studies may also determine if there is a correlation between sealant exposure and clinical and subclinical end points associated with BPA exposure, such as cardiovascular disease, diabetes, or neurodevelopmental effects.68
Recommendation for Product Development
Although there has been a recent increase in government funding toward BPA research and there are several on-going studies under supervision of the US Food and Drug Administration,72 the dental-materials industry will continue to benefit from exploring alternative materials for use in sealants and composites. BPA- and BPA-derivative–free dental materials should be a priority for development. Furthermore, thorough toxicological testing should be performed to ensure safety of these alternatives as they are developed.
Overall Recommendation
On the basis of the substantiated preventive benefits of resin-based dental sealants and given the brevity of elevated exposure to BPA after sealant application, we recommend continuing application of resin-based sealants in dental practice and in school--based/school-linked dental-sealant programs. Adherence to the precautionary recommendations described above on product choice and application techniques should be a high priority, because it can reduce avoidable exposures. Elective use of resin-containing dental sealants and composite restorations during pregnancy should be minimized, and nonelective use should be carefully managed to prevent exposure to unpolymerized monomer. Manufacturers should provide specific information on the chemical structures of monomers in resin dental products and should be encouraged to develop alternatives.
Acknowledgments
Dr Sheffield is supported by a grant from the National Institutes of Health Research Training Program in Environmental Pediatrics.
We thank Elizabeth S. Choi, Kristen Donohue, Jeannette R. Jakus, MD, MBA, and Christopher Moore, MPH, for help compiling the MSDS data and initial background information on BPA. We appreciate helpful discussions with Maida Galvez, MD, MPH, and Joel Forman, MD. We are also grateful to David D. Manning, PhD (Albany Molecular Research, Inc) for assistance with depicting the chemical structures in Fig 1. We also acknowledge Strategic Marketing, Inc, which provided a list of sealants and composites comprising the top US market share.
Funded by the National Institutes of Health (NIH).
ABBREVIATIONS
- BPA
bisphenol A
- bis-GMA
bisphenol A glycidyl dimethacrylate
- bis-DMA
bisphenol A dimethacrylate
- TEGDMA
triethylene glycol dimethacrylate
- MSDS
material safety data sheet
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.National Toxicology Program. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. NTP CERHR MON. 2008;(22):i–III1. [PubMed] [Google Scholar]
- 2.Burridge E. Bisphenol A: product profile. Eur Chem News. 2003;78(2048):17. [Google Scholar]
- 3.Bellinger DC, Trachtenberg F, Barregard L, et al. Neuropsychological and renal effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295(15):1775–1783. doi: 10.1001/jama.295.15.1775. [DOI] [PubMed] [Google Scholar]
- 4.DeRouen TA, Martin MD, Leroux BG, et al. Neurobehavioral effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295(15):1784–1792. doi: 10.1001/jama.295.15.1784. [DOI] [PubMed] [Google Scholar]
- 5.Factor-Litvak P, Hasselgren G, Jacobs D, et al. Mercury derived from dental amalgams and neuropsychologic function. Environ Health Perspect. 2003;111(5):719–723. doi: 10.1289/ehp.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauterbach M, Martins IP, Castro-Caldas A, et al. Neurological outcomes in children with and without amalgam-related mercury exposure: seven years of longitudinal observations in a randomized trial. J Am Dent Assoc. 2008;139(2):138–145. doi: 10.14219/jada.archive.2008.0128. [DOI] [PubMed] [Google Scholar]
- 7.Ahovuo-Saloranta A, Hiiri A, Nordblad A, Makela M, Worthington HV. Pit and fissure sealants for preventing dental decay in the permanent teeth of children and adolescents. Cochrane Database Syst Rev. 2008;(4):CD001830. doi: 10.1002/14651858.CD001830.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Truman BI, Gooch BF, Sulemana I, et al. Reviews of evidence on interventions to prevent dental caries, oral and pharyngeal cancers, and sports-related craniofacial injuries. Am J Prev Med. 2002;23(1 suppl):21–54. doi: 10.1016/s0749-3797(02)00449-x. [DOI] [PubMed] [Google Scholar]
- 9.Beauchamp J, Caufield PW, Crall JJ, et al. American Dental Association Council on Scientific Affairs. Evidence-based clinical recommendations for the use of pit-and-fissure sealants: a report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2008;139(3):257–268. doi: 10.14219/jada.archive.2008.0155. [DOI] [PubMed] [Google Scholar]
- 10.Griffin SO, Oong E, Kohn W, et al. The effectiveness of sealants in managing caries lesions. J Dent Res. 2008;87(2):169–174. doi: 10.1177/154405910808700211. [DOI] [PubMed] [Google Scholar]
- 11.Dye BA, Tan S, Smith V, et al. Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital Health Stat. 2007;11(248):1–92. [PubMed] [Google Scholar]
- 12.Cueto EI, Buonocore MG. Sealing of pits and fissures with an adhesive resin: its use in caries prevention. J Am Dent Assoc. 1967;75(1):121–128. doi: 10.14219/jada.archive.1967.0205. [DOI] [PubMed] [Google Scholar]
- 13.Oong EM, Griffin SO, Kohn WG, Gooch BF, Caufield PW. The effect of dental sealants on bacteria levels in caries lesions: a review of the evidence. J Am Dent Assoc. 2008;139(3):271–278. doi: 10.14219/jada.archive.2008.0156. quiz 357–278. [DOI] [PubMed] [Google Scholar]
- 14.European Commission Joint Research Center. [Accessed July 21, 2010];European Union risk assessment report: 4,4′-isopropylidenediphenol (bisphenol-A) Available at: http://ecb.jrc.it/DOCUMENTS/Existing-Chemicals/RISK_ASSESSMENT/REPORT/bisphenolareport325.pdf.
- 15.Goldman LR, Koduru S. Chemicals in the environment and developmental toxicity to children: a public health and policy perspective. Environ Health Perspect. 2000;108(suppl 3):443–448. doi: 10.1289/ehp.00108s3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekant W, Volkel W. Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol Appl Pharmacol. 2008;228(1):114–134. doi: 10.1016/j.taap.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Feigal RJ. Sealants and preventive restorations: review of effectiveness and clinical changes for improvement. Pediatr Dent. 1998;20(2):85–92. [PubMed] [Google Scholar]
- 18.Bodecker CF. Eradication of enamel fissures. Dental Items. 1929;51:859–866. [Google Scholar]
- 19.Hyatt T. Prophylactic odontotomy: the cutting into the tooth for the prevention of disease. Dental Cosmos. 1923;65:234–241. [PMC free article] [PubMed] [Google Scholar]
- 20.Klein H, Knutson JW. Studies on dental caries XIII: effect of ammoniacalsilver nitrate on caries in the first permanent molar. J Am Dent Assoc. 1942;29:1420–1426. [Google Scholar]
- 21.Wilson IP. Preventive dentistry. Dent Dig. 1895;1:70–72. [PMC free article] [PubMed] [Google Scholar]
- 22.Beltrán-Aguilar ED, Barker LK, Canto MT, et al. Centers for Disease Control and Prevention. Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis: United States, 1988–1994 and 1999–2002. MMWR Surveill Summ. 2005;54(3):1–43. [PubMed] [Google Scholar]
- 23.Ferracane JL. Current trends in dental composites. Crit Rev Oral Biol Med. 1995;6(4):302–318. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- 24.Soderholm KJ, Mariotti A. BIS-GMA–based resins in dentistry: are they safe? J Am Dent Assoc. 1999;130(2):201–209. doi: 10.14219/jada.archive.1999.0169. [DOI] [PubMed] [Google Scholar]
- 25.Tarumi H, Imazato S, Narimatsu M, Matsuo M, Ebisu S. Estrogenicity of fissure sealants and adhesive resins determined by reporter gene assay. J Dent Res. 2000;79(11):1838–1843. doi: 10.1177/00220345000790110401. [DOI] [PubMed] [Google Scholar]
- 26.Arenholt-Bindslev D, Breinholt V, Preiss A, Schmalz G. Time-related bisphenol-A content and estrogenic activity in saliva samples collected in relation to placement of fissure sealants. Clin Oral Investig. 1999;3(3):120–125. doi: 10.1007/s007840050089. [DOI] [PubMed] [Google Scholar]
- 27.Schmalz G, Preiss A, Arenholt-Bindslev D. Bisphenol-A content of resin monomers and related degradation products. Clin Oral Investig. 1999;3(3):114–119. doi: 10.1007/s007840050088. [DOI] [PubMed] [Google Scholar]
- 28.Dental Market Sales Data. Rochelle Park, NJ: Strategic Data Marketing; 2008. [Accessed July 14, 2008]. Available at: www.sdmdata.com/rpt_cat_merch.html. [Google Scholar]
- 29.Bakopoulou A, Papadopoulos T, Garefis P. Molecular toxicology of substances released from resin-based dental restorative materials. Int J Mol Sci. 2009;10(9):3861–3899. doi: 10.3390/ijms10093861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geurtsen W. Biocompatibility of resin-modified filling materials. Crit Rev Oral Biol Med. 2000;11(3):333–355. doi: 10.1177/10454411000110030401. [DOI] [PubMed] [Google Scholar]
- 31.Schweikl H, Spagnuolo G, Schmalz G. Genetic and cellular toxicology of dental resin monomers. J Dent Res. 2006;85(10):870–877. doi: 10.1177/154405910608501001. [DOI] [PubMed] [Google Scholar]
- 32.Nathanson D, Lertpitayakun P, Lamkin MS, Edalatpour M, Chou LL. In vitro elution of leachable components from dental sealants. J Am Dent Assoc. 1997;128(11):1517–1523. doi: 10.14219/jada.archive.1997.0091. [DOI] [PubMed] [Google Scholar]
- 33.Pulgar R, Olea-Serrano MF, Novillo-Fertrell A, et al. Determination of bisphenol A and related aromatic compounds released from bis-GMA-based composites and sealants by high performance liquid chromatography. Environ Health Perspect. 2000;108(1):21–27. doi: 10.1289/ehp.0010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fung EY, Ewoldsen NO, St Germain HA, Jr, et al. Pharmacokinetics of bisphenol A released from a dental sealant. J Am Dent Assoc. 2000;131(1):51–58. doi: 10.14219/jada.archive.2000.0019. [DOI] [PubMed] [Google Scholar]
- 35.Joskow R, Barr DB, Barr JR, Calafat AM, Needham LL, Rubin C. Exposure to bisphenol A from bis-glycidyl dimethacrylate-based dental sealants. J Am Dent Assoc. 2006;137(3):353–362. doi: 10.14219/jada.archive.2006.0185. [DOI] [PubMed] [Google Scholar]
- 36.Olea N, Pulgar R, Perez P, et al. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996;104(3):298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geurtsen W, Spahl W, Leyhausen G. Variability of cytotoxicity and leaching of substances from four light-curing pit and fissure sealants. J Biomed Mater Res. 1999;44(1):73–77. doi: 10.1002/(sici)1097-4636(199901)44:1<73::aid-jbm8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 38.Hamid A, Hume WR. A study of component release from resin pit and fissure sealants in vitro. Dent Mater. 1997;13(2):98–102. doi: 10.1016/s0109-5641(97)80018-8. [DOI] [PubMed] [Google Scholar]
- 39.Lewis JB, Rueggeberg FA, Lapp CA, Ergle JW, Schuster GS. Identification and characterization of estrogen-like components in commercial resin-based dental restorative materials. Clin Oral Investig. 1999;3(3):107–113. doi: 10.1007/s007840050087. [DOI] [PubMed] [Google Scholar]
- 40.Noda M, Komatsu H, Sano H. HPLC analysis of dental resin composites components. J Biomed Mater Res. 1999;47(3):374–378. doi: 10.1002/(sici)1097-4636(19991205)47:3<374::aid-jbm12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 41.Komurcuoglu E, Olmez S, Vural N. Evaluation of residual monomer elimination methods in three different fissure sealants in vitro. J Oral Rehabil. 2005;32(2):116–121. doi: 10.1111/j.1365-2842.2004.01405.x. [DOI] [PubMed] [Google Scholar]
- 42.Sasaki N, Okuda K, Kato T, et al. Salivary bisphenol-A levels detected by ELISA after restoration with composite resin. J Mater Sci Mater Med. 2005;16(4):297–300. doi: 10.1007/s10856-005-0627-8. [DOI] [PubMed] [Google Scholar]
- 43.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113(4):391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Völkel W, Colnot T, Csanády GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol. 2002;15(10):1281–1287. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- 45.Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ Health Perspect. 2009;117(5):784–789. doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 47.Alonso-Magdalena P, Laribi O, Ropero AB, et al. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect. 2005;113(8):969–977. doi: 10.1289/ehp.8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashby J, Tinwell H. Uterotrophic activity of bisphenol A in the immature rat. Environ Health Perspect. 1998;106(11):719–720. doi: 10.1289/ehp.98106719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colerangle JB, Roy D. Profound effects of the weak environmental estrogen-like chemical bisphenol A on the growth of the mammary gland of Noble rats. J Steroid Biochem Mol Biol. 1997;60(1–2):153–160. doi: 10.1016/s0960-0760(96)00130-6. [DOI] [PubMed] [Google Scholar]
- 50.Nagel SC, vom Saal FS, Thayer KA, Dhar MG, Boechler M, Welshons WV. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ Health Perspect. 1997;105(1):70–76. doi: 10.1289/ehp.9710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JC, Shin HC, Cha SW, Koh WS, Chung MK, Han SS. Evaluation of developmental toxicity in rats exposed to the environmental estrogen bisphenol A during pregnancy. Life Sci. 2001;69(22):2611–2625. doi: 10.1016/s0024-3205(01)01341-8. [DOI] [PubMed] [Google Scholar]
- 52.Tyl RW, Myers CB, Marr MC, et al. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol Sci. 2002;68(1):121–146. doi: 10.1093/toxsci/68.1.121. [DOI] [PubMed] [Google Scholar]
- 53.Tan BL, Kassim NM, Mohd MA. Assessment of pubertal development in juvenile male rats after sub-acute exposure to bisphenol A and nonylphenol. Toxicol Lett. 2003;143(3):261–270. doi: 10.1016/s0378-4274(03)00172-3. [DOI] [PubMed] [Google Scholar]
- 54.Tinwell H, Haseman J, Lefevre PA, Wallis N, Ashby J. Normal sexual development of two strains of rat exposed in utero to low doses of bisphenol A. Toxicol Sci. 2002;68(2):339–348. doi: 10.1093/toxsci/68.2.339. [DOI] [PubMed] [Google Scholar]
- 55.Farabollini F, Porrini S, Della Seta D, Bianchi F, Dessi-Fulgheri F. Effects of perinatal exposure to bisphenol A on sociosexual behavior of female and male rats. Environ Health Perspect. 2002;110(suppl 3):409–414. doi: 10.1289/ehp.02110s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawai K, Nozaki T, Nishikata H, Aou S, Takii M, Kubo C. Aggressive behavior and serum testosterone concentration during the maturation process of male mice: the effects of fetal exposure to bisphenol A. Environ Health Perspect. 2003;111(2):175–178. doi: 10.1289/ehp.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hashimoto Y, Moriguchi Y, Oshima H, Nishikawa J, Nishihara T, Nakamura M. Estrogenic activity of chemicals for dental and similar use in vitro. J Mater Sci Mater Med. 2000;11(8):465–468. doi: 10.1023/a:1013009006522. [DOI] [PubMed] [Google Scholar]
- 58.Bonefeld-Jørgensen EC, Long M, Hofmeister MV, Vinggaard AM. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-noctylphenol in vitro: new data and a brief review. Environ Health Perspect. 2007;115(suppl 1):69–76. doi: 10.1289/ehp.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mariotti A, Soderholm KJ, Johnson S. The in vivo effects of bisGMA on murine uterine weight, nucleic acids and collagen. Eur J Oral Sci. 1998;106(6):1022–1027. doi: 10.1046/j.0909-8836.1998.eos106607.x. [DOI] [PubMed] [Google Scholar]
- 60.Hanaoka T, Kawamura N, Hara K, Tsugane S. Urinary bisphenol A and plasma hormone concentrations in male workers exposed to bisphenol A diglycidyl ether and mixed organic solvents. Occup Environ Med. 2002;59(9):625–628. doi: 10.1136/oem.59.9.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takeuchi T, Tsutsumi O. Serum bisphenol A concentrations showed gender differences, possibly linked to androgen levels. Biochem Biophys Res Commun. 2002;291(1):76–78. doi: 10.1006/bbrc.2002.6407. [DOI] [PubMed] [Google Scholar]
- 62.Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J. 2004;51(2):165–169. doi: 10.1507/endocrj.51.165. [DOI] [PubMed] [Google Scholar]
- 63.Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod. 2005;20(8):2325–2329. doi: 10.1093/humrep/deh888. [DOI] [PubMed] [Google Scholar]
- 64.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 65.Yamada H, Furuta I, Kato EH, et al. Maternal serum and amniotic fluid bisphenol A concentrations in the early second trimester. Reprod Toxicol. 2002;16(6):735–739. doi: 10.1016/s0890-6238(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 66.Braun JM, Yolton K, Dietrich KN, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117(12):1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolff MS, Engel SM, Berkowitz GS, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116(8):1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lang IA, Galloway TS, Scarlett A, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300(11):1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 69.Young SS, Yu M. Association of bisphenol A with diabetes and other abnormalities. JAMA. 2009;301(7):720–721. doi: 10.1001/jama.2009.122. author reply 721–722. [DOI] [PubMed] [Google Scholar]
- 70.Bellinger DC, Trachtenberg F, Zhang A, Tavares M, Daniel D, McKinlay S. Dental amalgam and psychosocial status: the New England Children’s Amalgam Trial. J Dent Res. 2008;87(5):470–474. doi: 10.1177/154405910808700504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rueggeberg FA, Dlugokinski M, Ergle JW. Minimizing patients’ exposure to uncured components in a dental sealant. J Am Dent Assoc. 1999;130(12):1751–1757. doi: 10.14219/jada.archive.1999.0132. [DOI] [PubMed] [Google Scholar]
- 72.US Food and Drug Administration. [Accessed July 28, 2010];Update on bisphenol A for use in food contact applications. 2010 Jan; www.fda.gov/NewsEvents/PublicHealthFocus/ucm197739.


