Summary
Background
Infantile neuronal ceroid lipofuscinosis (INCL) is a devastating neurodegenerative lysosomal storage disease caused by mutations in the CLN1 gene encoding palmitoyl-protein thioesterase-1 (PPT1). PPT1-deficiency causes lysosomal ceroid accumulation leading to INCL pathogenesis. Previously, we reported that phosphocysteamine and N-acetylcysteine mediated ceroid depletion in cultured cells from INCL patients. We conducted a pilot study to determine whether a combination of cysteamine bitartrate and N-acetylcysteine is beneficial for these patients.
Methods
Patients (6-month to 3-years old) with any combination of 2 of the 7 most lethal PPT1 mutations were admitted. All patients were recruited from physician referrals and the PPT1 mutations were analyzed prior to admission. Patients were evaluated by electroretinography(ERG), brain MRI and MRS, electroencephalography (EEG), and electron microscopic analyses of leukocytes for granular osmiophilic deposits (GRODs). Patients received oral cysteamine bitartrate (60mg/kg/day) and N-acetylcysteine (60mg/kg/day) and were evaluated every 6 to 12 months until they showed isoelectric EEG attesting to a vegetative state or were too sick to travel. Outcomes were compared with the reported INCL natural history. In two cases, the disease progression was compared with that of a sibling who was above the age limit for inclusion into the protocol.
Findings
Between March, 2001, and June, 2011, we recruited 10 children with INCL but one was lost to follow-up after the first visit. Thus, a total of 9 patients (5 females and 4 males) were studied. At the first follow-up visit, peripheral leukocytes in all 9 patients showed virtually complete depletion of GRODs and 7 of 9 patients manifested less irritability and/or improved alertness based upon parental and physician observations. Evaluation by Denver scale showed acquisition of no new developmental skills and retinal function assessed by ERG progressively declined. Most notably, average time to isoelectric EEG (indicating vegetative state) was significantly longer in our patients compared to that previously reported. MRI studies demonstrated signal abnormalities similar to previous reports. Brain volume and NAA declined steadily, but no published quantitative MRI or MRS studies of INCL patients are available for comparison on these measures. There were no adverse events related to therapy other than a mild gastrointestinal discomfort in 2 of 9 patients, which was eliminated when the liquid preparation of cysteamine bitatrate was replaced with capsules.
Interpretation
The objectively demonstrated benefits in our study are the depletion of GRODs and delay of isoelectric EEG in all patients; in addition, several subjective benefits were suggested, all of which warrant further study. Nevertheless, this report systematically and quantitatively documents the natural history of 9 INCL patients with the most lethal CLN1/PPT1 mutations and thereby provides a benchmark for evaluating future experimental therapies.
Funding
This study was supported in part by a Bench-to-Bedside Award from the Clinical Center of the NIH and by the Intramural Program of the Eunice Kennedy Shriver National Institutes of Child Health and Human Development, NIH.
Introduction
Background
The neuronal ceroid lipofuscinoses (NCLs), also known as Batten disease, constitute a group of the most common neurodegenerative lysosomal storage disorders (LSDs)1–3. NCLs are both clinically and genetically heterogeneous although most are autosomal recessive diseases predominantly affecting children1–3. Mutations in more than 10 different genes (CLNs) underlie various types of NCLs (http://www.ucl.ac.uk/ncl)4. Despite the age variability in the disease onset, the clinical features common to all NCLs include psychomotor retardation, seizures, visual loss, and premature death2–5. Pathological findings include rapidly progressive brain atrophy resulting from destruction of mostly cerebrocortical neurons and the presence of autofluorescent storage material both in neurons and in other cell types2–6.
Inactivating mutations in the CLN1 gene encoding palmitoyl-protein thioesterase-1 (PPT1) underlie infantile NCL (INCL) pathogenesis7. Children with this disease are phenotypically normal at birth but by 6–18 months of age they manifest psychomotor retardation. By two years of age, these children undergo complete retinal degeneration and blindness. At around four years of age, an isoelectric electroencephalogram (EEG) attests to a vegetative state for several more years before eventual death2–7. These grim facts underscore an urgent need for developing effective therapeutic strategies for INCL and for that matter, for all the NCL types8.
Palmitoylation (also called S-acylation) is the only reversible lipid modification in which a long-chain saturated fatty acid (predominantly palmitate) is attached to cysteine residues in polypeptides via thioester linkage9. PPT1 cleaves this thioester linkage in palmitoylated proteins (constituents of ceroid) required for degradation by lysosomal hydrolases. Thus, PPT1-deficiency causes ceroid accumulation in lysosomes10 leading to INCL. In time, these ceroids organize to form granular osmiophilic deposits (GRODs)11.
Objectives
Since the thioester linkage is labile12 and susceptible to nucleophilic attack13, we reasoned that nucleophilic (electron donor) small molecules may have therapeutic potential for INCL. Previously, we used both in vitro and in cellula experimental models to demonstrate that phosphocysteamine, a nucleophilic and lysosomotropic drug with anti-oxidant properties, cleaves the thioester linkage in palmitoyl-CoA (a model substrate of PPT1) as well as in palmitoylated proteins and mediates the depletion of GRODs in cultured cells from INCL patients and suppresses apoptosis14. Moreover, N-acetylcysteine also cleaved thioester linkage and is a potent antioxidant, which has beneficial effects on neurodegenerative diseases including myoclonus epilepsy15. These results prompted us to conduct a pilot study to determine whether there are any positive effects of orally administering a combination of cysteamine bitartrate and N-acetylcysteine to INCL patients.
Methods
Study design and participants
This pilot study (www.clinicaltrials.gov; NCT00028262) was approved by the Institutional Review Board (IRB) of the Eunice Kennedy-Shriver National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health (NIH). Continuous monitoring of this study for any adverse effects of the therapy was conducted by our institution’s Data Safety Monitoring Committee, which is independent of the IRB. A written informed consent was read, understood and signed by a parent or guardian before any patient was enrolled into our protocol. In order to assure that the study population was homogeneous, we recruited only those patients who carried a combination of two of the following CLN1/PPT1 mutations: L10X, R151X, R164X, W296X, R122W, c.169insA and E184K. These mutations were chosen because they have been reported to manifest a uniform phenotype16.
Initiation and discontinuation of Therapy
After initial evaluation (see below), oral cysteamine bitartrate therapy was initiated at a dose of 10 mg/kg/day in four divided doses given with food. The cysteamine bitartrate dose was gradually increased weekly, as tolerated, to a target dosage of 60 mg/kg/day. This dose was used because it has been previously reported that at this dose cysteamine is well tolerated, safe and efficacious in treating children with cystinosis17. After target dose of cysteamine bitartrate (60 mg/kg/day) was achieved, patients were then started on oral N-acetylcysteine titrated to 5% oral solution, with the dose incrementally escalated to a target dose of 60 mg/kg/day. N-acetylcysteine was also administered daily into four equal doses. Although N-acetylcysteine can be given in doses as high as 140mg/kg, we used a lower dose of because it is given in combination with cysteamine bitartrate, which is also an antioxidant. We used MRI and MRS evidence of advanced brain atrophy and/or isoelectric EEG attesting vegetative clinical status as the criteria to discontinue treatment.
Procedures
All patients were evaluated by brain MRI, MRS, ERG, visual evoked potential (VEP), EEG and transmission electron microscopic (TEM) analysis of the peripheral white blood cells (WBCs) for GRODs. Children also underwent physical and neuro-developmental assessments using the Denver scale18. The laboratory studies included blood counts, serum chemistries, prolactin, insulin-like growth factor (IGF-1) and thyroid function tests.
General anesthesia was required for most procedures for all patients except for some who were in the late stages of the disease in whom imaging and ERG examinations were performed without sedation. General anesthesia was administered by staff anesthesiologists as previously reported19. Upon completion of the procedures and emergence from anesthesia, children were observed in the post-anesthesia care unit until ready to be transferred to the pediatric ward.
Assessment of visual function and ocular motility were undertaken followed by recording a single channel flash visual evoked potential (VEP). Ganzfeld electroretinograms (ERGs) were obtained while patients were anesthetized with continuous infusion of propofol. ERG responses were recorded following the standards set forth by the International Society for Clinical Electrophysiology of Vision (ISCEV)20. All responses were recorded using a Gold Lens electrode and an Espion electroretinography console with a portable ColorBurst stimulator (Diagnosys, Lowell, MA). These responses were compared to a set obtained from age-matched controls who were children referred to the National Eye Institute for sedated ERG and had age-appropriate ERG responses. Additional specialized recordings included scotopic intensity-response function curves and photopic on off responses; data from these recordings will be presented in a separate publication. Follow-up recordings were obtained to study the rate of progression in individual patients. Cycloplegic refraction, fundoscopy, and fundus photography were also performed at the end of the procedure.
All patients were serially evaluated with MRI and MRS of the brain. MRI consisted of sagittal spin-echo T1-weighted images, axial T1-weighted images, axial T2-weighted images, axial 3D T1-weighted images, and axial diffusion-weighted images. Single voxel MR spectroscopy was performed, with short echo time (25 or 35 ms) and PRESS technique. Voxels were located in the left centrum ovale, midline parietal grey matter, left thalamus, pons, and left cerebellar white matter.
We also analyzed the GRODs in peripheral WBCs from all patients before and during treatment by TEM at 30000x as previously described14. Identification and counting of the GRODs was performed by two investigators working independently of each other and the results were averaged.
Monitoring for Adverse Effects of the Therapy
Parents were instructed to document any adverse effect(s) of therapy. They were also given instructions on how to stop the medications and told to contact the PI and the local physician taking care of the patient if any adverse effects were apparent. All adverse effects were recorded and reported to the Data Safety Monitoring Committee.
Statistical analysis
Linear mixed effects models were used to investigate the change over time after treatment of the average number of GRODs per cell and the average area of the GRODs. For each of the 9 patients at each of the treatment time windows, the average number of GRODs per cell was obtained by dividing the total number of GRODs by the total number of cells under consideration, and the average area of a GROD was computed as the total area divided by the total number of GRODs. Log-transformation was applied to average number of GRODs per cell, the average area of a GROD, and the number of days from pre-treatment blood draws to post-treatment blood draws before fitting the linear mixed effects models assuming first order auto-regressive correlation structure among the longitudinal measures.
Role of the funding source
The funding sources had no role in the study design, data collection, data analyses, data interpretation, writing of the manuscript, or decision to submit for publication. The corresponding author along with all co-authors had full access to all the data in this study and all authors reviewed the final draft of the manuscript prior to submission. The corresponding author (ABM) took final responsibility for the decision to submit the manuscript for publication.
Results
The natural history of our patient population was compared with that reported in the literature (Table 1). The duration of follow-up ranged from 8 to 75 months after initiation of therapy. The mean age of patients at the time of admission was 25.8 months. Of the nine subjects followed in this protocol, the average age of symptom onset was 12.7 months (range 4–18 months). Most of the subjects initially came to medical attention due to delayed acquisition of motor skills or developmental regression, with the exception of two patients whose INCL diagnosis was suspected because they had an affected older sibling. One of the patients (Pt#3) at 5 months of age developed strabismus before manifesting other developmental problems. In particular, four subjects demonstrated specific loss of ambulatory capability, and all subjects demonstrated language and/or cognitive decline as well as loss of other motor skills (Table 2). Only one subject presented with new onset seizures, although all patients had myoclonus. All subjects except patient 7 were on various anti-epileptic agents including sodium valproate, carbamazepine, benzodiazepines (nitrazepam, clonazepam) or levetiracetam prior to enrollment into the study.
Table 1.
Natural History of Infantile Neuronal Ceroid Lipofuscinosis
|
Per Literature (months) |
Present Study (months) |
|
|---|---|---|
| Deceleration of head growth46 | 5 | 36 (CI 27.3 – 45.5) |
| Developmental regression6, 26, 46, 46 | 6 to 18 | 15 (CI 10.3–18.8) |
| Myoclonic jerks and seizures6, 47 | 12 to 24 | 23 (CI 18.8–27.8) |
| Loss of vision6, 25, 47 | 12 to 24 | 25 (CI 11.2–38.8) |
| Brain atrophy per head imaging25, 49 | 12 to 24 | 19 (CI 14.9 – 22.4) |
| Electroencephalogram and/or electroretinogram extinguished26, 46, 48 | 36 | 52 (CI 41.6 – 62.2) |
| Visual evoked potential extinguished48 | 48 | 48 (CI 38.6 – 56.4) |
CI = confidence interval
Table 2.
Protocol Patients and Their CLN1/PPT1 Mutations
| Patient | Age of Presentation | Presenting Symptoms |
CLN1/PPT1 Mutations |
|---|---|---|---|
| 1 | 15 months | Verbal regression | 364 A>T (p.R122W) |
| 2 | 18 months | Stumbling & impaired vision | 169 A>i (stop at 101) |
| 3 | 5 months | Strabismus | 451 C>T (p.R151X) |
| 22 months | Poor balance & myoclonus | ||
| 4 | 9 months | Increased tone, inability to bear weight | 451 C>T (p.R151X) |
| 5 | 18 months | Stopped walking, irritability | 4 5 1 C > T (p .R 1 5 1 X) & 29 T>A (p.L10X) |
| 6 | 12 months | Stopped crawling, decreased tone | 451 C>T (p.R151X) & 29 T>A (p.L10X) |
| 7 | 18 months | Stopped walking, impaired vision | 4 5 1 C > T (p.R151X) & 29 T>A (p.L10X) |
| 8 | 4 months | Delayed motor skills | 451 C>T (p.R151X) |
| 16 months | Seizures | ||
| 9 | 15 months | Delayed motor skills | 451 C>T (p.R151X) & 798+2T>C splice site |
Two of the nine children initially experienced mild gastrointestinal discomfort with liquid cysteamine bitartrate, which was eliminated when the liquid preparation was replaced with cysteamine bitartrate capsules, the contents of which was added to the food. No other untoward side effects of cysteamine bitartrate or N-acetylcysteine were observed throughout the treatment period. Notably, only one patient showed substantial interval weight loss (between admissions at age 4 and 2/12years and age 4 and 10/12 years). All nine patients showed normal height at the 10th to 75th percentile for age. Five of the nine patients showed appreciable decrease in head circumference to measure at or below the 5th percentile for age. Six of the patients underwent gastrostomy tube placement to ease difficulty with feeding due to progressive loss of oral motor skills and ability to protect the airway.
Routine laboratory studies were performed in all patients at each visit (Table S1). Laboratory parameters remained within the normal range over the entire course of our study with the exception of one patient, (Pt#1), who had a single elevated erythrocyte sedimentation rate (ESR) of 51 mm/hr at the time she had herpes simplex lesions on her skin (proven by viral DNA study and treated with acyclovir). Serum prolactin levels were occasionally elevated for 8 of 9 patients (range: low 30’s to 109 µg/L) but otherwise within normal limits for all patients without persistently altered levels as has been previously reported in cystinosis patients treated with oral cysteamine21.
All anesthetic courses were uncomplicated and no patient displayed hemodynamic lability during or after procedures. During the course of anesthetics and imaging studies, two children developed hypothermia. In one of these cases, hypothermia was associated with bradycardia, which was successfully treated with atropine. The drop in temperature was observed despite the routine use of blankets during MRI and use of active warming measures (forced-air warming blankets) during ERGs as well as in the recovery room22. All children returned to baseline status within a few hours after recovery from anesthesia.
Because eight of the nine patients admitted to this protocol clinically manifested considerable neurological impairment prior to the initiation of therapy, we expected the pre-treatment brain MRI scans to demonstrate cerebral atrophy. We did not obtain scans on normal children as part of this study, but for comparison we used previously reported normalized total brain volumes for children of various ages23. Compared with this reference curve, our youngest (and only relatively asymptomatic) patient (Pt#7) had a total brain volume of +5% relative to the average volume for her age group at the time of admission to the study protocol. Follow-up measurements on this patient and all measurements on other patients were lower than the expected volume for the patient’s age, ranging from 11–36% below average with a mean of 23% below average. Progression of atrophy was observed in all patients. In the early stages, the atrophy primarily involved the cerebral hemispheres but eventually progressed to involve the entire brain (Figure 1). Initially the atrophy primarily involved the cortical gray matter, but in the later stages cerebellar gray matter, white matter and deep nuclei were also affected. As has been reported in other studies22, 24 signal abnormalities were observed in the deep nuclei on T2 weighted images. Signal abnormalities were also observed on the T1 weighted images; the pattern we observed was that first the thalamus became hyperintense relative to the white matter, then the globus pallidus, and finally, the putamen and caudate nucleus.
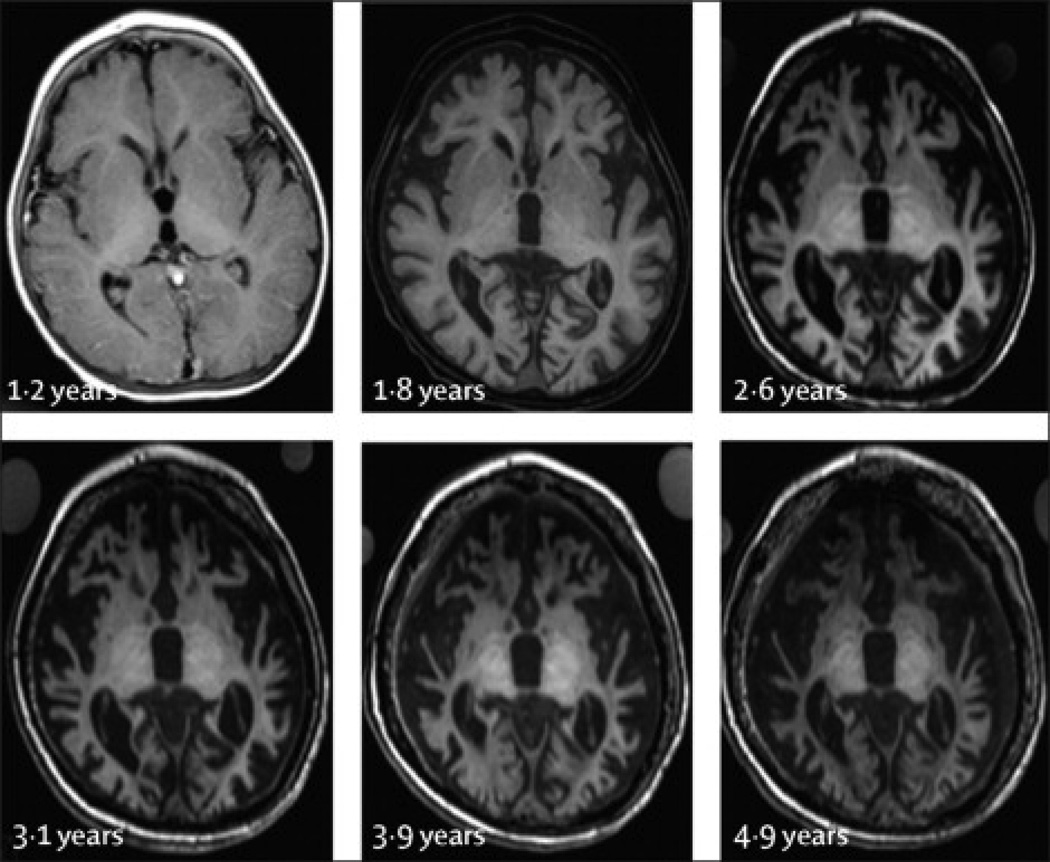
Figure 1. Progression of cerebral atrophy in a single subject (Patient 8).
All images are axial T1 weighted images acquired in the plane defined by the anterior commissure and posterior commissure. Age (in years) is noted adjacent to each image. Atrophy progressed rapidly between ages 1.2 yrs and 2.6 yrs, with gradual changes thereafter. This trajectory was typical for the patients in this study. Gray matter loss proceeded rapidly, and gray matter was not clearly identified after 2.6 yrs in this patient. Signal abnormalities in the deep nuclei progressed as follows: first the thalamus became hyperintense relative to white matter, then the globus pallidus, and finally the putamen and caudate.
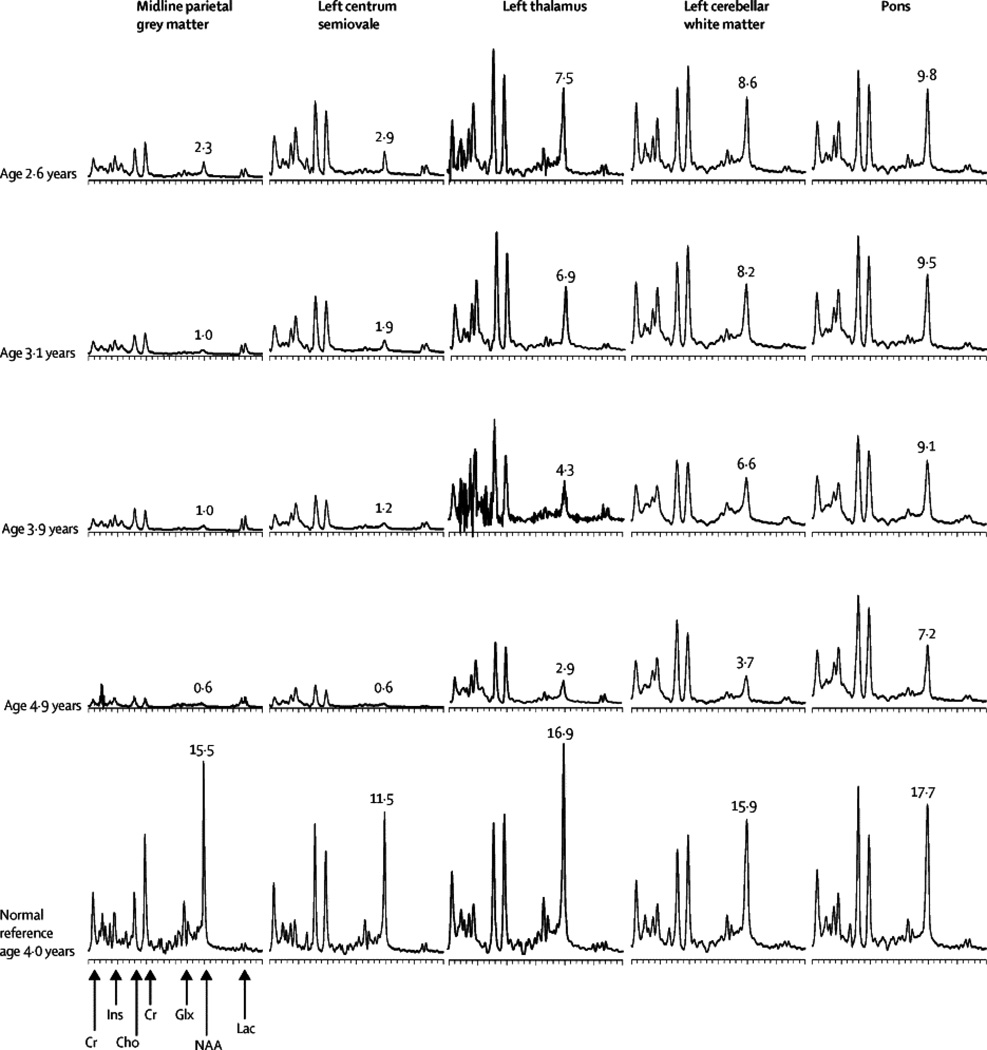
In all patients the progression of the disease was also monitored by MRS. Representative MRS results from one patient (pt#8) are shown (Figure 2). The most striking abnormality is the decline in N-acetyl aspartic acid (NAA), at all five voxel locations. NAA is a metabolite found only in neurons, and is usually taken as a measure of functional neuronal mass. The earliest deficit of NAA was seen in the midline parietal gray matter, followed in succession by the left centrum semiovale, left thalamus, left cerebellar white matter, and pons. This corroborates the imaging observations that the earliest abnormalities are seen in the gray matter, followed by changes in the white matter and basal ganglia, with relatively late involvement of the cerebellum and brain stem.
Figure 2. Progression of MRS abnormalities in a single subject (Patient 8).
All spectra are on the same scale (equal area for equal mM NAA), and have been corrected for T1 decay, T2 decay and CSF partial volume. NAA (mM) in tissue is noted next to the NAA peak. Cysteamine bitartrate-N-acetylcysteine combination treatment was initiated shortly after the examination at 2.6 yr. Abnormalities are most severe in the cerebral gray matter. Decline in the thalamus and cerebellum is slower than in the cerebrum. Progression of abnormalities is slowest in the pons. NAA demonstrates a steady decline at all locations. Cr = creatine, Ins = myo-inositol, Cho = choline, Glx = glutamine+glutamate+GABA, NAA = N-acetyl aspartic acid, Lac = lactate.
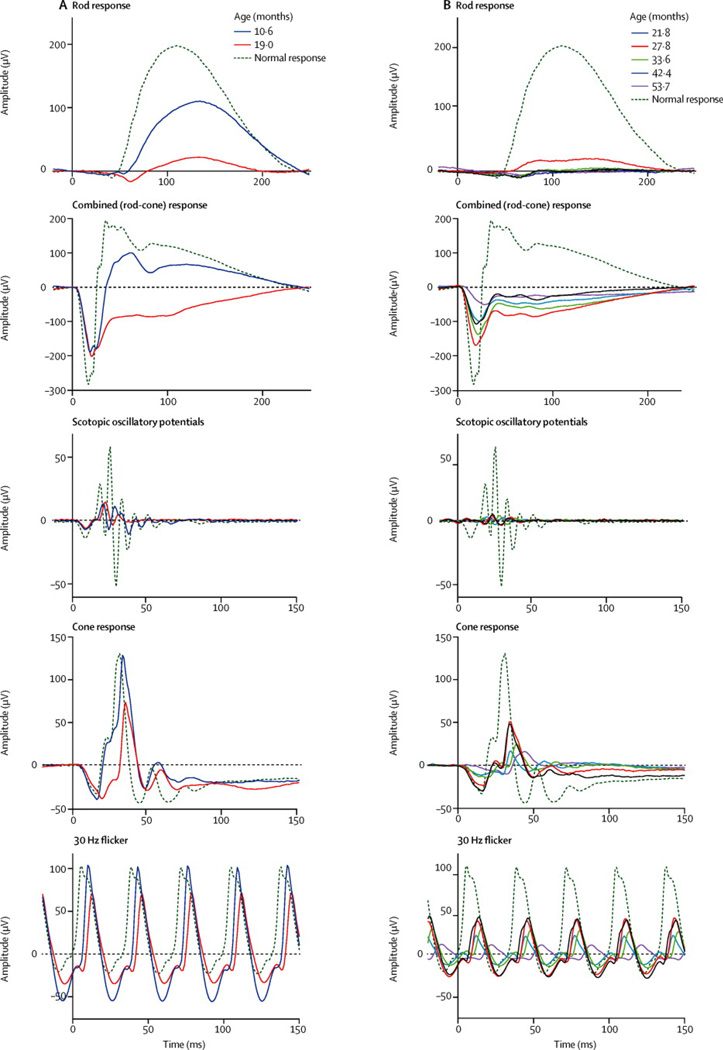
Ophthalmological findings and the decline of visual function were previously reported in a cohort of INCL patients25. All of our patients had a history of reduced visual function, nystagmus, or strabismus and for some, the ophthalmic manifestations were the initial findings that brought the patient to medical attention. The ophthalmic history and the findings on physical examination including those of fundoscopy (Figure S1) were documented, and clinical examination results are summarized in Table S2. On their initial ERG recordings, all patients demonstrated reduced b-wave amplitude on the dark-adapted rod and combined (rod-cone) response ERG. The combined (rod-cone) response b-wave amplitude (origin post-synaptic to photoreceptors with input from bipolar cells) was less than the a-wave amplitude (presumptive photoreceptor origin), which yielded an electronegative configuration. This was observed in all patients except for the initial recording of patient 7 who was admitted to our protocol at around 10 months of age and did not manifest this configuration. In contrast, initial a-wave amplitude in the 3.0 dark-adapted responses was normal in 4 cases; after age 2.6 years, a-wave amplitude was reduced in all patients. A progressive decline in ERG amplitude with age was consistently observed (Figure S2). The decline was precipitous with most patients’ ERG responses reaching noise level by 60 months of age (range 37 to 71 months). Some variability in amplitudes of scotopic ERG response was noted in four patients (Patient 1, 3, 4, and 9). In these patients, an increase in amplitude was noted on one visit following which the responses continued the trend of progressive decline (Figure 3). Eight out of 9 patients had noise level VEP responses through the treatment period. One patient (Pt#7) had a measurable VEP response that declined to noise level by the second visit. Cumulatively, our findings from all visits indicated poor visual function except for Patient #7, who continued to maintain a fix and follow response at 18 months of age. Progressively worsening retinal vascular attenuation and optic nerve head pallor were consistently documented for all patients.
Figure 3. Electroretinographic recordings from two representative study patients.
A. Patient 7 presented at the earliest age (10 m) and had the best preserved response amplitudes among all study patients at that visit. The follow-up visit for Patient 7 was at the age of 19.0 m and by that time her recordings showed the characteristic findings expected in INCL including significantly reduced b-wave amplitude on both the rod response and the scotopic combined (rod-cone) response. Note the electronegative waveform on the combined response at the 19.0 m follow-up visit (but not on initial presentation). B. Patient 1 had multiple study follow-ups with the ERG showing progressive reduction in amplitude across all recorded responses. Note that, for all study patients, the scotopic responses were more severely affected and showed earlier changes than the photopic responses.
EEG recordings were analyzed according to the criteria suggested for INCL diagnosis22. Only three of the nine patients developed new epileptiform foci during the course of our study. Representative EEG recordings from one patient (Pt#6) over several visits showed progressive deterioration (Figure S3). None of our nine patients displayed isoelectric EEG by three years of age as expected based on the previously reported natural history of INCL, and in fact, for 5 patients followed long enough to document isoelectric EEGs, the average age of patients with this finding was 59 ±13 months.
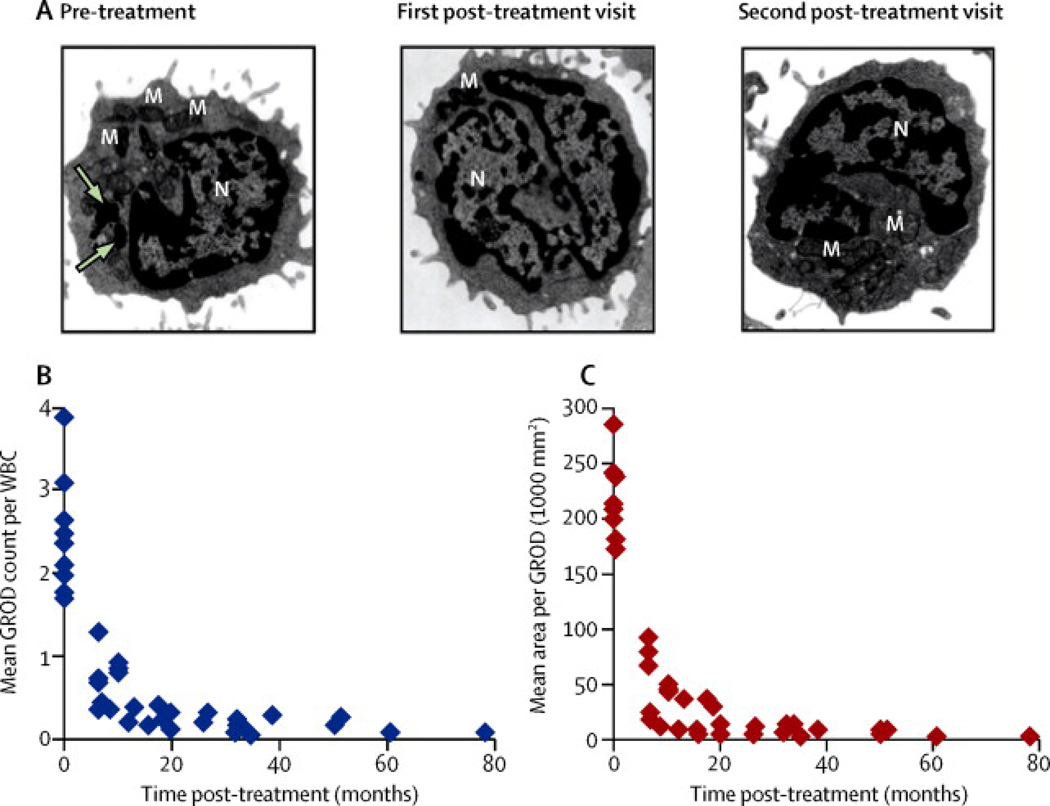
The most striking effect of the combination therapy was observed in WBCs of cysteamine bitartrate-N-acetylcysteine-treated INCL patients. Prior to initiation of treatment, more than 50% of all WBCs in all patients contained readily identifiable GRODs in electron micrographs. Electron micrographs of peripheral WBCs with GRODs from one representative patients (before and after oral cysteamine bitartrate-N-acetylcysteine combination) are presented (Figure 4A, arrows). Notably, from the first post-treatment follow-up the number of GRODs per WBC declined in all nine patients (Figure 4B) as did the average cross-sectional area of the GRODs (Figure 4C). The analysis showed significant decrease after treatment in average number of GRODs (Beta=−0.3317, 95% CI=[−0.3943, −0.2691]; P <0.0001), and in the average area of a GROD (Beta=−0.4379, 95%CI=[−0.5241, −0.3517]; P<0.0001). In summary, subsequent to the first follow-up examination after initiation of treatment, GRODs were virtually undetectable in terms of the number as well as size, and remained so throughout the study period.
Figure 4. Electron microscopic analysis of GRODs in leukocytes before and after treatment.
Transmission electron microscopic analyses of peripheral WBCs from all patients were performed at all patient visits, both pre-treatment and post-treatment. GRODs are irregularly-shaped dark extranuclear structures that are distinct from mitochondria, secretory granules, and other membrane–enclosed cellular organelles. Representative micrographs (A) from a patient are shown at 3 time points (pre-treatment and two post-treatment). Note that GRODs are readily detectable only in the pre-treatment image. Arrows indicate GRODs; M, mitochondria; N, nucleus. Number of GRODs per cell (B) and area of the GRODs (C) for all 9 patients together are shown as they evolved over time. For all patients, both the number and size of the GRODs declined over time, starting with the first visit after initiation of treatment.
For several patients (#2, 3, 4, 5, and 9), myoclonic jerks seemed to have improved with cysteamine bitartrate-N-acetylcysteine combination, but this effect may have been confounded by other anti-epileptic medications that were also being given to decrease myoclonus. However, Patient #2 needed less medication to control myoclonic jerking than was needed for an older affected sibling at the same age. Two patients (#1 and #4) were reported by parents to resume attempts to roll over from back or side after initiation of cysteamine bitartrate-N-acetylcysteine combination. Improved alertness and spontaneous smiling were also noticed as was the clinical observation that the patients seemed less irritable (Table 3). This effect may be a substantial benefit since the patients with INCL typically manifest extreme irritability at around age 2 years up to age 4 years22, 23, 27. The calming effects of oral cysteamine bitartrate and N-acetylcysteine combination were not only reported by parents and other caregivers, but were also documented during developmental assessments.
Table 3.
Summary of Neurodevelopmental Findings#
| Patient # |
*CA at Start of Intervention |
Skills at Start of Intervention |
CA at Final Assessment |
Skills at Final Assessment |
Potential Improvements/Subjective Changes |
|---|---|---|---|---|---|
| 1 | 22 months | 8 months | 53 months | Less than 2 months |
Improved alertness and irritability, regained ability to roll from back or side, spontaneous smiling |
| 2 | 25 months | 8 months | 100 months | Less than 2 months |
Improved alertness and irritability, good growth without need for G-tube, less medications to control myoclonic jerking than were needed for older affected brother at same age |
| 3 | 27 months | 4 months | 71 months | Less than 2 months |
Reduced irritability and myoclonic jerking |
| 4 | 27 months | 3 months | 71 months | 2 months | Reduced irritability and myoclonic jerking, regained ability to roll from side, spontaneous smiling |
| 5 | 32 months | 4 to 7 months |
58 months | 3 months | Improved alertness, decreased myoclonic jerking and stable developmental status through age 58 months |
| 6 | 29 months | 4 months | 58 months | Less than 2 months |
Reduced irritability and spontaneous smiling |
| 7 | 10 months | 6 to 9 months |
18 months | 9 months increased irritability over time with skills worse than those of older affected brother at same age |
None |
| 8 | 30 months | 4 months | 58 months | Less than 2 months |
None |
| 9 | 30 months | 4 months | 65 months | Less than 2 months |
Improved alertness and better sleeping, decreased myoclonic jerking |
CA = chronological age;
According to Denver developmental screening test.
Discussion
This study was conducted to determine whether a combination of oral cysteamine bitartrate and N-acetylcysteine is beneficial for INCL patients. We followed nine children with the most lethal CLN1/PPT1 gene mutations who were receiving the two drug combination. This pilot study was limited by the inability to conduct a randomized protocol with such a small number of patients. A combination of INCL being a rare disease (1 in >100,000 births) and the stringent nature of the criteria for entry into this pilot study made it difficult to recruit a large number of patients in a reasonable period of time. Also, for humanitarian reasons, all affected patients received the combination regimen, and the effects were gauged based upon the previously published natural history of INCL.
The present study represents the first report of systematic, long-term follow-up and monitoring of several parameters to evaluate INCL disease progression. Such systematic follow-up has already provided new insights into INCL including subdural effusions in several of our patients, which were unrelated to the treatment28. Thus, the results of our study, in addition to demonstrating some of the potentially beneficial effects of oral cysteamine bitartrate-N-acetylcysteine combination, provide additional comprehensive documentation of the progression of a subset of INCL patients with the most lethal CLN1/PPT1 mutations under therapy, and may be useful for comparison in evaluating potential therapeutic regimens in the future.
One subjective outcome of this study is the apparent reduction of irritability in 7 of 9 patients, which may be important from the standpoint of management of these patients. The mechanism of irritability in INCL patients is not clearly understood although it has been reported that in other neurodegenerative diseases such as Parkinson and Alzheimer's disease, elevated levels of reactive oxygen species (ROS) mediates neuroinflammation and may contribute to irritability29. Using postmortem brain tissues from an INCL patient as well as in those from Ppt1−/− mice30 that mimic INCL31, we previously reported elevation of superoxide dismutase (SOD) levels, most likely due to increased levels of ROS32. A recent report on gene expression profiling in Ppt1−/− mice has shown that increased expression of genes related to inflammation occur in the brains of these mice33. Consistent with this report, we also found that the receptor for advanced glycation end products (RAGE) signaling contributes to neuroinflammation in these mice34. Since both cysteamine bitartrate and N-acetylcysteine are potent anti-oxidants and scavengers of ROS, these drugs may reduce ROS levels in the brain, thereby suppressing oxidative stress-mediated neuroinflammation, irritability in our patients. Recently, it has been reported that anti-oxidants have dual effects on neurodegenerative diseases, as these agents manifest direct neuroprotective effects against oxidative-stress and indirectly protect the neurons by suppressing inflammation mediated by activated glia35.
A clear objective finding in the present study was the depletion of GRODs in peripheral WBCs of all 9 patients. At the first follow-up, both the fraction of WBCs containing GRODs and the number of GRODs per WBC declined in all nine patients. Notably, by the second follow-up examination, the GRODs were completely undetectable and remained so for the duration of the study. The results of our present study are consistent with those of our previous in vitro and in cellula experiments14. These results may also suggest that cysteamine bitartrate-N-acetylcysteine combination mediates efficient depletion of ceroid in vivo ameliorating some of the deleterious effects of ceroid accumulation. Another objective finding of our combination therapy was that the extinction of EEG (i.e. isoelectric EEG) in our patients took significantly longer than reported in the literature (Table 1).
Our study provides the largest series of sequential electrophysiological findings (recorded using ISCEV standards) to date in INCL patients. Previous longitudinal studies in INCL patients have used Karpe's method of electroretinography (a technique that is no longer used) under general anesthesia36. Weleber et al (2004)25 have reported the results of electrophysiological findings from a single visit of INCL patients with clinical-pathological correlations. The results of our serial evaluations of 9 INCL patients are consistent with those of Weleber et al25 that the onset of retinal degeneration in these patients occurs very early in life and has a rapid progression. Notably, the b-wave is affected much earlier than the a-wave in the dark-adapted combined (rod-cone) response indicating that the inner retinal responses are compromised earlier in life than those generated by photoreceptors37. Scotopic responses were affected earlier than photopic responses as previously reported25. Our data does not allow us to speculate on the natural course of the disease progression had these patients not received cysteamine bitartrate-N-acetylcysteine combination. However, the results of this study underscore the fact that like most neurodegenerative diseases, any therapeutic intervention must be instituted very early in life to prevent the progression of retinal degeneration and to preserve neuronal function in the retina.
Previously, MRI signal abnormalities in the basal ganglia of INCL patients have been described22, 24 and our findings concur with those reports. We observed that brain atrophy in all of our patients continued to progress even after initiation of the cysteamine bitartrate-N-acetylcysteine combination. However, due to the lack of quantative details in the published reports on brain volume in INCL patients, we are unable to determine if the treatment mitigated progression of atrophy in any way. Similarly, we observed a progressive deficit in the NAA concentration at each of the anatomical locations of the brain that we studied by MRS. However, due to the lack of detailed quantitative reports in the literature, we are unable to determine if there was any mitigation in the decline of this marker of neuronal injury and loss. Nevertheless, the detailed measurements in our present study may serve as a quantitative biomarker to which future therapies for INCL can be compared and evaluated.
Cysteamine, a lysosomotropic drug, has been used for the treatment of cystinosis for more than 25 years17 and it also crosses the blood-brain barrier38, 39. While both cysteamine bitartrate and N-acetylcysteine manifest nucleophilic properties, the efficiency of cleavage of the thioester linkage by cysteamine bitartrate may not be as efficient40 as previously reported14. Nevertheless, in all of our patients a combination of these drugs mediated depletion of GRODs. It is possible that some of these effects are due to the combination of nucleophilic and anti-oxidant effects of these drugs.
Currently, several strategies are being tested to develop an effective treatment for INCL patients8, 41. However, the blood-brain barrier (BBB) poses a formidable obstacle for macromolecular therapeutic agents to be transported from the blood to the brain. We previously reported that the BBB in Ppt1−/−mice is disrupted42, 43. Thus, it is possible that a macromolecular agent with therapeutic effectiveness may initially cross the leaky BBB, thereby allowing these agents to enter the brain. However, if these agents are effective in restoring the integrity of the BBB they may not reach the brain. Thus, non-toxic, thioesterase-mimetic small molecules may provide a more efficacious therapeutic strategy for INCL patients as these molecules would readily cross the BBB even when its integrity is restored. Towards this goal, we recently identified a small molecule, N-tert-(Butyl)-hydroxylamine, which protected neurons, and modestly extended lifespan in Ppt1−/−mice44.
Panel: Research in context
Systematic review
We searched PubMed for long-term studies of infantile neuronal ceroid lipofuscinosis (INCL) patients with a combination of cysteamine bitartrate and N-acetylcysteine to determine if this combination has any beneficial effects. We searched for reports published in the English language between May 1, 1980, and June 6, 2014, with the search terms “Infantile neuronal ceroid lipofuscinosis” AND “cysteamine bitartrate” AND “clinical trial” AND/OR “N-acetyl cysteine” OR “cysteamine bitartrate and N-acetylcysteine combination”. We did not identify any clinical studies meeting these criteria. We also searched using the terms “Infantile neuronal ceroid lipofuscinosis” AND “Cystagon” AND “clinical trial”. However, when we used the terms “infantile neuronal ceroid lipofuscinosis” AND “Cysteamine bitartrate” only one clinical study45 was listed in which 4 patients with juvenile-onset INCL patients with mild PPT1 genotypes who were above 3 years of age were given only cysteamine bitartrate at a dose of 50mg/kg body weight. In that study, the age of the patients was higher and the dose of cysteamine bitartrate was lower than those used in our study. In addition, only cysteamine bitartrate alone was used. The authors reported that disease progression may have been slower for patients taking cysteamine bitartrate, although it was not clear whether the slower progression was a continuation of an observation that began prior to the initiation of oral cysteamine bitartrate at a lower dose than that our patients received.
Interpretation
A pilot study using a combination of cysteamine bitartrate and N-acetylcysteine has shown that despite neurological deterioration prior to admission, this treatment had resulted in modest improvement in a homogeneous patient population carrying the most lethal CLN1 mutations. It is recommended that any experimental therapy targeting INCL patients must be started early. It remains unclear if our treatment, started earlier, might have had a better outcome. Nevertheless, this report systematically documents the natural history of 9 INCL patients carrying the most lethal CLN1 mutations and may provide a basis for evaluating future experimental therapies.
Supplementary Material
Acknowledgements
We thank the families of our patients for having their children participate in this clinical study. We also thank all the physicians who made referrals of their INCL patients to this protocol and the Batten Disease Support and Research Association (BDSRA) for making the patients’ families aware of our protocol. We are also grateful to the physicians, nurses and social workers on 9 West and 1NW Wards of the Clinical Center, NIH, for their compassion and dedicated patient care. We thank Dr. Shiyong Peng for calculating the confidence intervals in table 1. This study was supported in part by a Bench-to-Bedside Award from the Clinical Center of the NIH and by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and The National Eye Institute, NIH. We dedicate this paper to four of our colleagues who served as consultants to this protocol, Drs. James B. Sidbury, Jr., Pirkko Santavuori, Jean DeB. Butler and Krystyna E. Wisniewski who sadly passed away while this study was ongoing.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
A.B.M. conceived the project and received a bench-to-bedside award from the Clinical Center of the NIH. The detailed study design was jointly performed by A.B.M., S.W.L., E.H.B., R.C.C., W.M.Z., Z.Z., Z.M.N.Q., and A.G. In-patient admissions and patient care were provided by K.J.G., N.M., S.B., SWL and ABM. Image processing and statistical analysis of the GRODs were performed by G.C, A. L. and Z.Z. R.C.C and W.M.Z. performed ERGs and analyzed the data. O.I.K analyzed EEGs. All authors had access to all the data. All authors contributed in preparing and revising the manuscript. All the authors reviewed and approved the final version of the manuscript and approved its submission for publication.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
References
- 1.Rider JA, Rider DL. Batten disease: past present and future. Am J Med Genet. 1988;5(suppl):21–26. doi: 10.1002/ajmg.1320310606. [DOI] [PubMed] [Google Scholar]
- 2.Haltia M. The neuronal ceroid lipofuscinoses. Biochim Biophys Acta. 2006;1762:850–856. doi: 10.1016/j.bbadis.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Anderson GW, Goebel HH, Simonati A. Human pathology in NCL. Biochim Biophys Acta. 2013;1832:1807–1826. doi: 10.1016/j.bbadis.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Kousi M, Lehesjoki A-E, Mole SE. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. HumMutat. 2012;33:42–63. doi: 10.1002/humu.21624. [DOI] [PubMed] [Google Scholar]
- 5.Siintola E, Lehesjoki A-E, Mole SE. Molecular genetics of the NCLs—status and perspectives. Biochim Biophys Acta. 2006;1762:857–864. doi: 10.1016/j.bbadis.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Mole SE, Williams RE, Goebel HH. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenet. 2005;6:107–126. doi: 10.1007/s10048-005-0218-3. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann SL, Peltonen L. The neuronal ceroid lipofuscinoses. In: Scriver C, Beaudet A, Valle D, et al., editors. The metabolic & molecular bases of inherited disease. McGraw-Hill; 2001. pp. 3877–3894. [Google Scholar]
- 8.Kohan R, Cismondi IA, Oller-Ramirez AM, et al. Therapeutic approaches to the challenge of neuronal ceroid lipofuscinoses. Curr Pharm Biotechnol. 2011;12:867–883. doi: 10.2174/138920111795542633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aicart-Ramos C, Valero RA, Rodriguez-Crespo I. Protein palmitoylation and subcellular trafficking. Biochim Biophys Acta. 2011;1808:2981–2994. doi: 10.1016/j.bbamem.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Lu JY, Verkruyse LA, Hofmann SL. Lipid thioesters derived from acylated proteins accumulate in infantile neuronal ceroid lipofuscinosis: correction of the defect in lymphoblasts by recombinant palmitoyl-protein thioesterase. Proc Natl Acad Sci U S A. 1996;93:10046–10050. doi: 10.1073/pnas.93.19.10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goebel HH, Wisniewski KE. Current state of clinical and morphological features in human NCL. Brain Pathol. 2004;14:61–69. doi: 10.1111/j.1750-3639.2004.tb00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt MFG. Fatty acylation of proteins. Biochim Biophys Acta. 1989;988:411–426. doi: 10.1016/0304-4157(89)90013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jocelyn PC. in Biochemistry of the SH groups. New York: Academic Press; 1972. pp. 63–93. [Google Scholar]
- 14.Zhang Z, Butler JD, Levin SW, et al. Lysosomal ceroid depletion by drugs: therapeutic implications for a hereditary neurodegenerative disease of childhood. Nat. Med. 2001;7:478–484. doi: 10.1038/86554. [DOI] [PubMed] [Google Scholar]
- 15.Bavarsad Shahripour R, Harrigan MR, Alexandrov AV. N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain Behav. 2014;4:108–122. doi: 10.1002/brb3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das AK, Becerra CH, Yi W, et al. Molecular genetics of palmitoyl-protein thioesterase deficiency in the U.S. J Clin Invest. 1998;102:361–370. doi: 10.1172/JCI3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gahl WA, Reed GF, Thoene JG, et al. Cysteamine therapy for children with nephropathic cystinosis. N Engl J Med. 1987;316:971–977. doi: 10.1056/NEJM198704163161602. [DOI] [PubMed] [Google Scholar]
- 18.Frankenburg WK, Dobbs JB. The Denver Developmental Screening Test. J. Pediat. 1967;71:181–191. doi: 10.1016/s0022-3476(67)80070-2. [DOI] [PubMed] [Google Scholar]
- 19.Miao N, Levin SW, Baker EH, et al. Children with infantile neuronal ceroid lipofuscinosis have an increased risk of hypothermia and bradycardia during anesthesia. Anesth Analg. 2009;109:372–378. doi: 10.1213/ane.0b013e3181aa6e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marmor MF, Holder GE, Seeliger MW, Yamamoto S. Standard for clinical electroretinography (2004 update) Doc Ophthalmol. 2004;108:107–114. doi: 10.1023/b:doop.0000036793.44912.45. [DOI] [PubMed] [Google Scholar]
- 21.Gahl WA, Bercu BB. Blunted prolactin response to thyrotropin-releasing hormone stimulation in cystinotic children receiving cysteamine. J Clin EndocrinolMetab. 1985;60:793–796. doi: 10.1210/jcem-60-4-793. [DOI] [PubMed] [Google Scholar]
- 22.Santavuori P, Vanhanen S-L, Sainio K, et al. Infantile neuronal ceroid-lipofuscinosis (INCL): diagnosis criteria. J Inherit Metab Dis. 1993;16:227–229. doi: 10.1007/BF00710250. [DOI] [PubMed] [Google Scholar]
- 23.Shen EY, Wu KH, Lin MF, et al. Study of brain growth in children—a new approach to volume measurements using MRI-reconstructed 3D neuroimaging. Childs Nerv Syst. 2010;26:1619–1623. doi: 10.1007/s00381-010-1280-1. [DOI] [PubMed] [Google Scholar]
- 24.Vanhanen S-L, Raininko R, Autti T, et al. MRI evaluation of the brain in infantile neuronal ceroid lipofuscinosis. Part 2: MRI findings in 21 patients. J Child Neurol. 1995;10:444–450. doi: 10.1177/088307389501000604. [DOI] [PubMed] [Google Scholar]
- 25.Weleber RG, Gupta N, Trzupek KM, et al. Electroretinographic and clinicopathologic correlations of retinal dysfunction in infantile neuronal ceroid lipofuscinosis (infantile Batten disease) Mol Gene Metab. 2004;83:128–137. doi: 10.1016/j.ymgme.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Santavuori P, Lauronen L, Kirveskari E, et al. Neuronal ceroid lipofuscinoses in childhood. Neurol. Sci. 2000;21:S35–S41. doi: 10.1007/s100720070038. [DOI] [PubMed] [Google Scholar]
- 27.Santavuori P, Linnankivi T, Jaeken J, et al. Psychological symptoms and sleep disturbances in neuronal ceroid-lipofuscinosis (NCL) J Inherit Metab Dis. 1993;16:245–248. doi: 10.1007/BF00710255. [DOI] [PubMed] [Google Scholar]
- 28.Levin SW, Baker EH, Gropman A, et al. Subdural fluid accumulation in patients with infantile neuronal ceroid lipofuscinosis. Arch. Neurol. 2009;66:1567–1571. doi: 10.1001/archneurol.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 30.Gupta P, Soyombo AA, Atashband A, et al. Disruption of PPT1 or PPT2 causes neuronal ceroid lipofuscinosis in knockout mice. Proc Natl Acad Sci USA. 2001;98:13566–13571. doi: 10.1073/pnas.251485198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bible E, Gupta P, Hofmann SL, et al. Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2004;16:346–359. doi: 10.1016/j.nbd.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Wei H, Kim SJ, Zhang Z, et al. ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum Mol Genet. 2008;17:469–477. doi: 10.1093/hmg/ddm324. [DOI] [PubMed] [Google Scholar]
- 33.Qiao X, Lu JY, Hofmann SL. Gene expression profiling in a mouse model of infantile neuronal ceroid Lipofuscinosis reveals upregulation of immediate early genes and mediators of the inflammatory response. BMC Neurosci. 2007;8:95. doi: 10.1186/1471-2202-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saha A, Kim SJ, Zhang Z, et al. RAGE signaling contributes to neuroinflammation in infantile neuronal ceroid lipofuscinosis. FEBS Lett. 2008;582:3823–3831. doi: 10.1016/j.febslet.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ock J, Han HS, Hong SH, et al. Obovatol attenuates microglia-mediated neuroinflammation by modulating redox-regulation. Br J Pharmacol. 2010;159:1646–1662. doi: 10.1111/j.1476-5381.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raitta C, Santavuori P. Ophthalmological findings in infantile type of so-called neuronal ceroid lipofuscinosis. Acta Ophthalmol (Copenh) 1973;51:755–763. doi: 10.1111/j.1755-3768.1973.tb06044.x. [DOI] [PubMed] [Google Scholar]
- 37.Weleber RG. The dystrophic retina in multisystem disorders: the electroretinogram in neuronal ceroid lipofuscinoses. Eye (Lond) 1998;12:580–590. doi: 10.1038/eye.1998.148. [DOI] [PubMed] [Google Scholar]
- 38.Cazals X, Lauvin MA, Favelle O, et al. Cystinosis encephalopathy: MRI perivascular enhancement with micronodular T2* hypointensity. Diagn Interv Imaging. 2013;94:653–655. doi: 10.1016/j.diii.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Bousquet M, Gibrat C, Ouellet M, et al. Cystamine metabolism and brain transport properties: clinical implications for neurodegenerative diseases. J Neurochem. 2010;114:1651–1656. doi: 10.1111/j.1471-4159.2010.06874.x. [DOI] [PubMed] [Google Scholar]
- 40.Lu JY, Hofmann SL. Inefficient cleavage of palmitoyl-protein thioesterase (PPT) substrates by aminothiols: implications for treatment of infantile neuronal ceroid lipofuscinosis. J Inherit Metab Dis. 2006;29:119–126. doi: 10.1007/s10545-006-0225-z. [DOI] [PubMed] [Google Scholar]
- 41.Wong AM, Rahim AA, Waddington SN, et al. Current therapies for the soluble lysosomal forms of neuronal ceroid lipofuscinosis. Biochem Soc Trans. 2010;38:1484–1488. doi: 10.1042/BST0381484. [DOI] [PubMed] [Google Scholar]
- 42.Munasinghe J, Zhang Z, Kong E, et al. Evaluation of neurodegeneration in a mouse model of infantile Batten disease by magnetic resonance imaging and magnetic resonance spectroscopy. Neurodegener Dis. 2012;9:159–169. doi: 10.1159/000334838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saha A, Sarkar C, Singh SP, et al. The blood-brain barrier is disrupted in a mouse model of infantile neuronal ceroid lipofuscinosis: amelioration by resveratrol. Hum Mol Genet. 2012;21:2233–2244. doi: 10.1093/hmg/dds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarkar C, Chandra G, Peng S, et al. Neuroprotection and lifespan extension in Ppt1(−/−) mice by NtBuHA: therapeutic implications for INCL. Nat Neurosci. 2013;16:1608–1617. doi: 10.1038/nn.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gavin M, Wen GY, Messing J, et al. Substrate reduction therapy in four patients with milder CLN1 mutations and juvenile-onset Batten disease using Cysteamine bitartrate. JIMD Rep. 2013;11:87–92. doi: 10.1007/8904_2013_226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haltia M. The neuronal ceroid-lipofuccinoses. J Neuropathol Exp Neurol. 2003;62:1–13. doi: 10.1093/jnen/62.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Santorelli FM, Bertini E, Petruzzella V, et al. A Novel Insertion (A169) in the CLN1 Gene is Associated with Infantile Neuronal Ceroid Lipofuscinosis in an Italian patient. Biochim Biophys Res Commun. 1998;245:519–522. doi: 10.1006/bbrc.1998.8484. [DOI] [PubMed] [Google Scholar]
- 48.Vanhanen S-L, Saino K, Lappi M, et al. EEG and Evoked Potentials in Infantile Neuronal Ceroid-Lipofuscinosis. Dev Med Child Biol. 1997;39:456–463. doi: 10.1111/j.1469-8749.1997.tb07465.x. [DOI] [PubMed] [Google Scholar]
- 49.Santavuori P, Raininko R, Vanhanen S-L, et al. MRI of the Brain, EEG Sleep Spindles and SPECT in the Early Diagnosis of Infantile Neuronal Ceroid Lipofuscinosis. Neurol. 1992;34:61–79. doi: 10.1111/j.1469-8749.1992.tb08564.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.