Full text
PDF











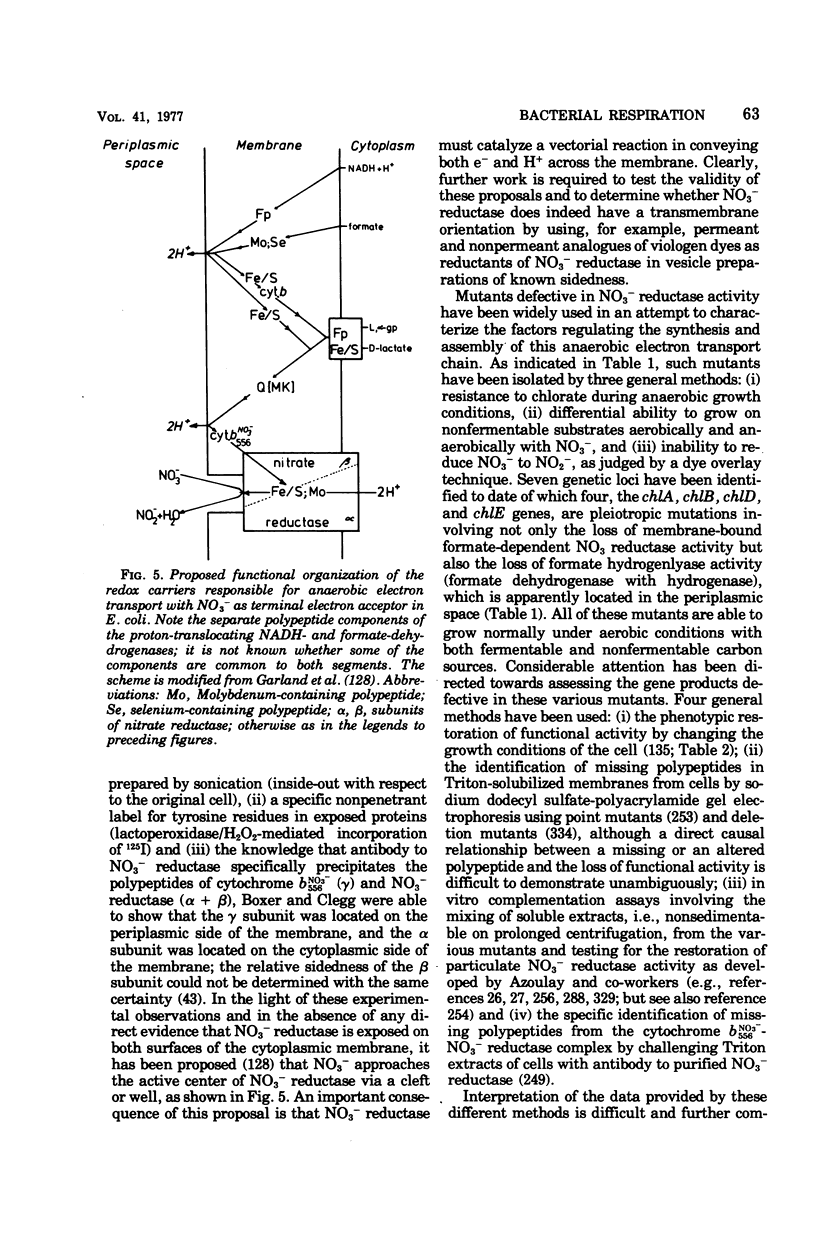



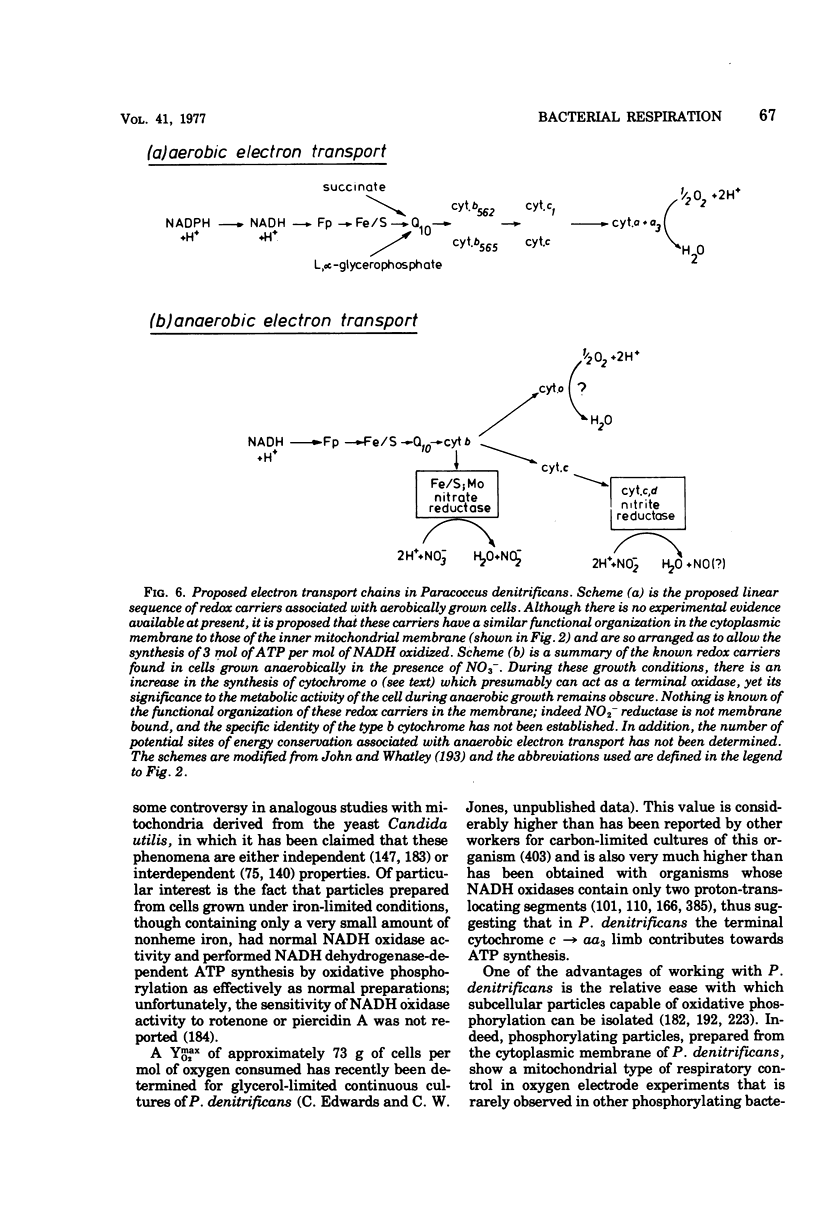

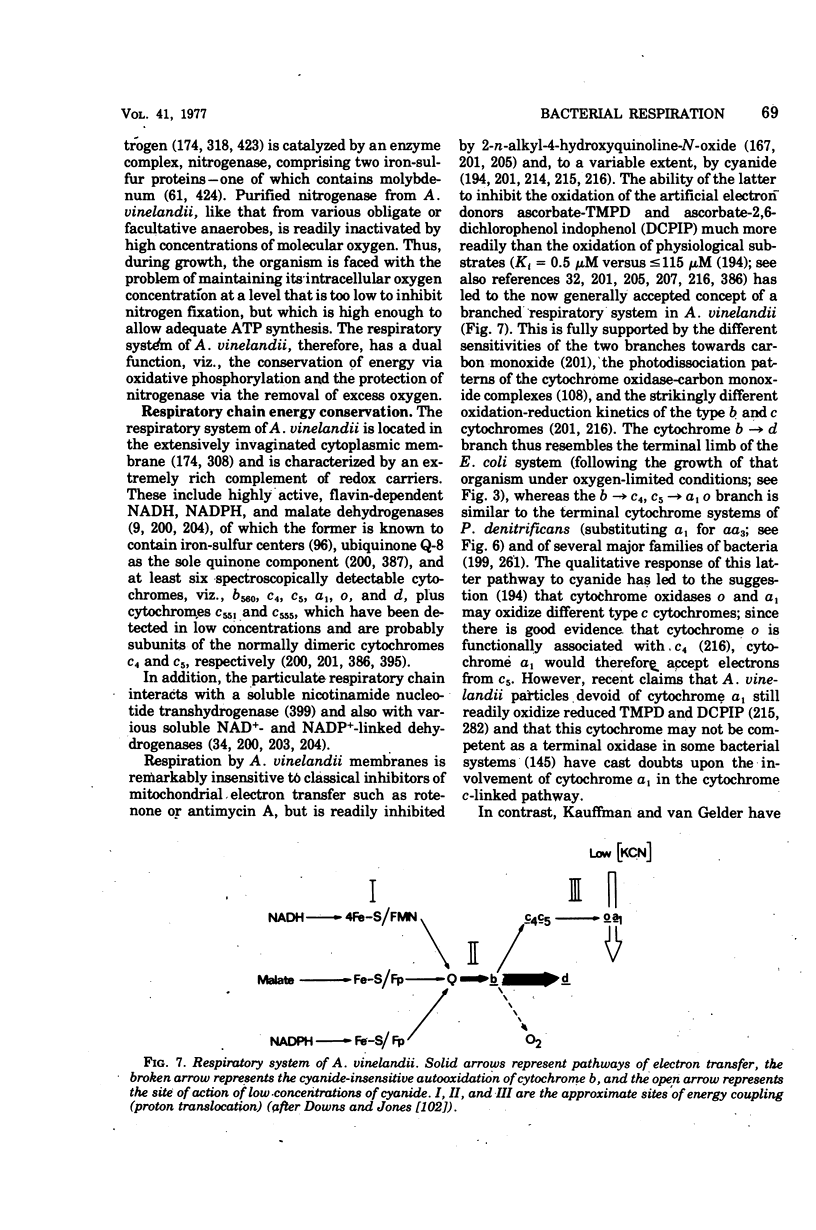




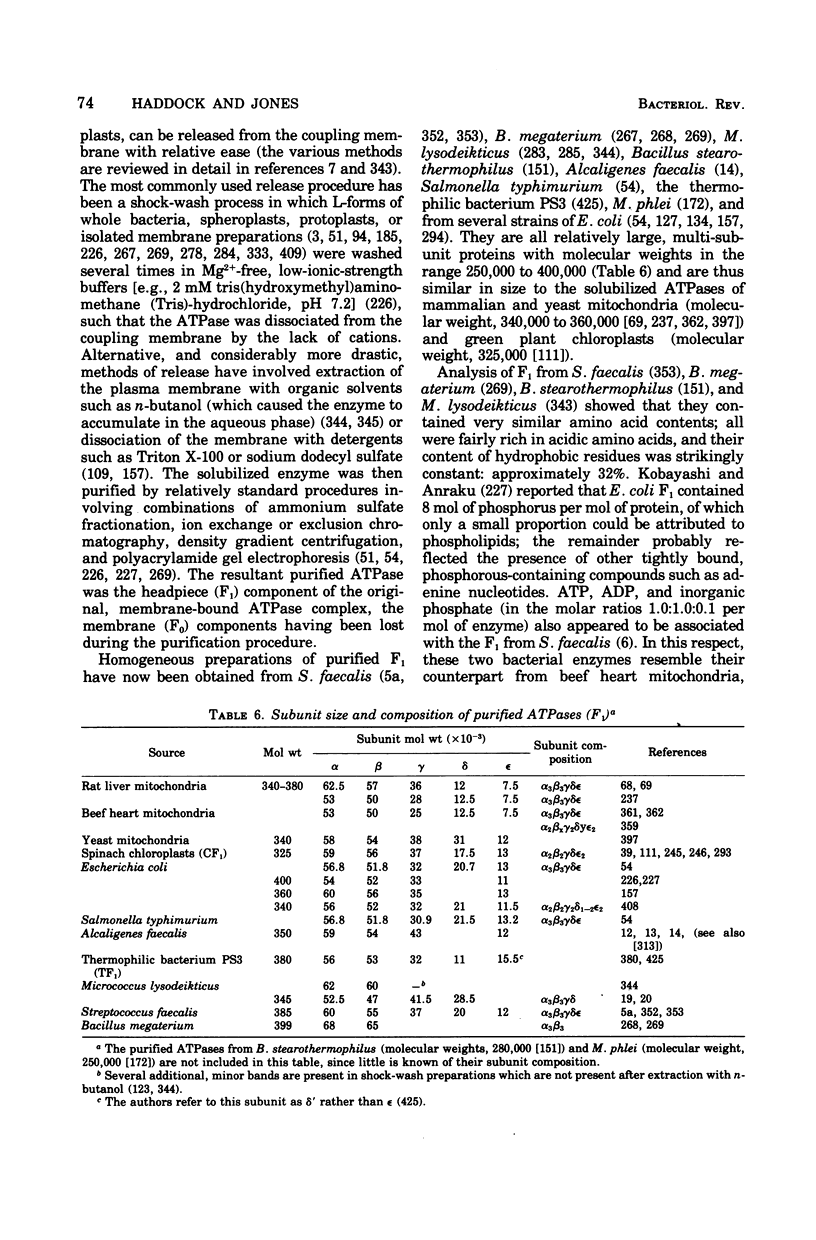
























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D. ELECTRON MICROSCOPE OBSERVATIONS ON INTACT CELLS, PROTOPLASTS, AND THE CYTOPLASMIC MEMBRANE OF BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Mar;89:855–873. doi: 10.1128/jb.89.3.855-873.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboderin A. A., Boedefeld E., Luisi P. L. Reaction of chicken egg white lysozyme with 7-chloro-4-nitrobenz-2-oxa-1,3-diazole. Biochim Biophys Acta. 1973 Nov 11;328(1):20–30. doi: 10.1016/0005-2795(73)90325-5. [DOI] [PubMed] [Google Scholar]
- Abrams A., Baron C. Inhibitory action of carbodiimides on bacterial membrane ATPase. Biochem Biophys Res Commun. 1970 Nov 25;41(4):858–863. doi: 10.1016/0006-291x(70)90162-2. [DOI] [PubMed] [Google Scholar]
- Abrams A., Baron C. The isolation and subunit structure of streptococcal membrane adenosine triphosphatase. Biochemistry. 1967 Jan;6(1):225–229. doi: 10.1021/bi00853a035. [DOI] [PubMed] [Google Scholar]
- Abrams A., Jensen C., Morris D. H. Role of Mg2+ ions in the subunit structure and membrane binding properties of bacterial energy transducing ATPase. Biochem Biophys Res Commun. 1976 Apr 5;69(3):804–811. doi: 10.1016/0006-291x(76)90946-3. [DOI] [PubMed] [Google Scholar]
- Abrams A., Nolan E. A., Jensen C., Smith J. B. Tightly bound adenine nucleotide in bacterial membrane ATPase. Biochem Biophys Res Commun. 1973 Nov 1;55(1):22–29. doi: 10.1016/s0006-291x(73)80054-3. [DOI] [PubMed] [Google Scholar]
- Abrams A., Smith J. B., Baron C. Carbodiimide-resistant membrane adenosine triphosphatase in mutants of Streptococcus faecalis. I. Studies of the mechanism of resistance. J Biol Chem. 1972 Mar 10;247(5):1484–1488. [PubMed] [Google Scholar]
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- Ackrell B. A., Erickson S. K., Jones C. W. The respiratory-chain NADPH dehydrogenase of Azotobacter vinelandii. Eur J Biochem. 1972 Apr 11;26(3):387–392. doi: 10.1111/j.1432-1033.1972.tb01778.x. [DOI] [PubMed] [Google Scholar]
- Ackrell B. A., Jones C. W. The respiratory system of Azotobacter vinelandii. 1. Properties of phosphorylating respiratory membranes. Eur J Biochem. 1971 May 11;20(1):22–28. doi: 10.1111/j.1432-1033.1971.tb01357.x. [DOI] [PubMed] [Google Scholar]
- Ackrell B. A., Jones C. W. The respiratory system of Azotobacter vinelandii. 2. Oxygen effects. Eur J Biochem. 1971 May 11;20(1):29–35. doi: 10.1111/j.1432-1033.1971.tb01358.x. [DOI] [PubMed] [Google Scholar]
- Adolfsen R., McClung J. A., Moudrianakis E. N. Electrophoretic microheterogeneity and subunit composition of the 13S coupling factors of oxidative and photosynthetic phosphorylation. Biochemistry. 1975 Apr 22;14(8):1727–1735. doi: 10.1021/bi00679a027. [DOI] [PubMed] [Google Scholar]
- Adolfsen R., Moudrianakis E. N. Purification and properties of 2 soluble coupling factors of oxidative phosphorylation from Alcaligenes faecalis. Biochemistry. 1971 Jun 8;10(12):2247–2253. doi: 10.1021/bi00788a010. [DOI] [PubMed] [Google Scholar]
- Alberty R. A. Effect of pH and metal ion concentration on the equilibrium hydrolysis of adenosine triphosphate to adenosine diphosphate. J Biol Chem. 1968 Apr 10;243(7):1337–1343. [PubMed] [Google Scholar]
- Altendorf K. H., Staehelin L. A. Orientation of membrane vesicles from Escherichia coli as detected by freeze-cleave electron microscopy. J Bacteriol. 1974 Feb;117(2):888–899. doi: 10.1128/jb.117.2.888-899.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altendorf K., Harold F. M., Simoni R. D. Impairment and restoration of the energized state in membrane vesicles of a mutant of Escherichia coli lacking adenosine triphosphatase. J Biol Chem. 1974 Jul 25;249(14):4587–4593. [PubMed] [Google Scholar]
- Altendorf K., Zitzmann W. Identification of the DCCD-reactive protein of the energy transducing adenosinetriphosphatase complex from Escherichia coli. FEBS Lett. 1975 Nov 15;59(2):268–272. doi: 10.1016/0014-5793(75)80390-5. [DOI] [PubMed] [Google Scholar]
- Andreu J. M., Albendea J. A., Munõz E. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Molecular properties of the purified enzyme unstimulated by trypsin. Eur J Biochem. 1973 Sep 3;37(3):505–515. doi: 10.1111/j.1432-1033.1973.tb03012.x. [DOI] [PubMed] [Google Scholar]
- Andreu J. M., Muñoz E. Micrococcus lysodeikticus ATPase. Purification by preparative gel electrophoresis and subunit structure studied by urea and sodium dodecylsulfate gel electrophoresis. Biochim Biophys Acta. 1975 May 15;387(2):228–233. doi: 10.1016/0005-2728(75)90105-x. [DOI] [PubMed] [Google Scholar]
- Asano A., Cohen N. S., Baker R. F., Brodie A. F. Orientation of the cell membrane in ghosts and electron transport particles of Mycobacterium phlei. J Biol Chem. 1973 May 25;248(10):3386–3397. [PubMed] [Google Scholar]
- Asano A., Hirata H., Brodie A. F. A factor(s) required for activation of oxidative phosphorylation in protoplast ghosts of Mycobacterium phlei. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1340–1346. doi: 10.1016/s0006-291x(72)80122-0. [DOI] [PubMed] [Google Scholar]
- Asano A., Imai K., Sato R. Oxidative phosphorylation in Micrococcus denitrificans. 3. ATP-supported reduction of NAD+ by succinate. J Biochem. 1967 Aug;62(2):210–214. doi: 10.1093/oxfordjournals.jbchem.a128650. [DOI] [PubMed] [Google Scholar]
- Asano A., Imai K., Sato R. Oxidative phosphorylation in Micrococcus dentrificans. II. The properties of pyridine nucleotide transhydrogenase. Biochim Biophys Acta. 1967;143(3):477–486. doi: 10.1016/0005-2728(67)90053-9. [DOI] [PubMed] [Google Scholar]
- Ashcroft J. R., Haddock B. A. Synthesis of alternative membrane-bound redox carriers during aerobic growth of Escherichia coli in the presence of potassium cyanide. Biochem J. 1975 May;148(2):349–352. doi: 10.1042/bj1480349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay E., Puig J., Couchoud-Beaumont P. Etude des mutants chlorate-résistants chez Escherichia coli K 12. I. Reconstitution in vitro de l'activité nitrate-réductase particulaire chez Escherichia coli K 12. Biochim Biophys Acta. 1969 Feb 11;171(2):238–252. doi: 10.1016/0005-2744(69)90157-0. [DOI] [PubMed] [Google Scholar]
- BELJANSKI M. Sur la formation d'enzymes respiratoires chez un mutant d'Escherichia coli streptomycino-résistant et auxotrophe pour l'hémine. Ann Inst Pasteur (Paris) 1957 Mar;92(3):396–412. [PubMed] [Google Scholar]
- BISHOP D. H., PANDYA K. P., KING H. K. Ubiquinone and vitamin K in bacteria. Biochem J. 1962 Jun;83:606–614. doi: 10.1042/bj0830606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURRIS R. H., TISSIERES A. Purification and properties of cytochromes C4 and C5 from Azotobacter vinelandii. Biochim Biophys Acta. 1956 May;20(2):436–437. doi: 10.1016/0006-3002(56)90331-6. [DOI] [PubMed] [Google Scholar]
- Baak J. M., Postma P. W. Oxidative phosphorylation in intact Azotobacter vinelandii. FEBS Lett. 1971 Dec 15;19(3):189–192. doi: 10.1016/0014-5793(71)80511-2. [DOI] [PubMed] [Google Scholar]
- Baillie R. D., Hou C., Bragg P. D. The preparation and properties of a solubilized respiratory complex from Escherichia coli. Biochim Biophys Acta. 1971 Apr 6;234(1):46–56. doi: 10.1016/0005-2728(71)90128-9. [DOI] [PubMed] [Google Scholar]
- Baltscheffsky H., Baltscheffsky M. Electron transport phosphorylation. Annu Rev Biochem. 1974;43(0):871–897. doi: 10.1146/annurev.bi.43.070174.004255. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Jr Multiple sites for coupling of glucose transport to the respiratory chain of membrane vesicles from Azotobacter vinelandii. J Biol Chem. 1973 Dec 10;248(23):8120–8124. [PubMed] [Google Scholar]
- Barnes E. M., Jr Respiration-coupled glucose transport in membrane vesicles from Azotobacter vinelandii. Arch Biochem Biophys. 1972 Oct;152(2):795–799. doi: 10.1016/0003-9861(72)90275-5. [DOI] [PubMed] [Google Scholar]
- Baron C., Abrams A. Isolation of a bacterial membrane protein, nectin, essential for the attachment of adenosine triphosphatase. J Biol Chem. 1971 Mar 10;246(5):1542–1544. [PubMed] [Google Scholar]
- Barrera C. R., Jurtshuk P. Characterization of the highly active isocitrate (NADP+) dehydrogenase of Azotobacter vinelandii. Biochim Biophys Acta. 1970 Dec 16;220(3):416–429. doi: 10.1016/0005-2744(70)90273-1. [DOI] [PubMed] [Google Scholar]
- Bartsch R. G. Bacterial cytochromes. Annu Rev Microbiol. 1968;22:181–200. doi: 10.1146/annurev.mi.22.100168.001145. [DOI] [PubMed] [Google Scholar]
- Berzborn R. J. Trennung von Untereinheiten des Kopplungsfaktors 1 der Chloroplasten (CF 1 ) und dern immunologische Charakterisierung. Hoppe Seylers Z Physiol Chem. 1972 May;353(5):693–693. [PubMed] [Google Scholar]
- Bhattacharyya P., Epstein W., Silver S. Valinomycin-induced uptake of potassium in membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1488–1492. doi: 10.1073/pnas.68.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra J., Huttunen M. T., Konings W. N. Anaerobic transport in Escherichia coli membrane vesicles. J Biol Chem. 1975 Sep 10;250(17):6792–6798. [PubMed] [Google Scholar]
- Boxer D. H., Clegg R. A. A transmembrane location for the proton-translocating reduced ubiquinone leads to nitrate reductase segment of the respiration chain of Escherichia coli. FEBS Lett. 1975 Dec 1;60(1):54–57. doi: 10.1016/0014-5793(75)80417-0. [DOI] [PubMed] [Google Scholar]
- Boyer P. D., Cross R. L., Momsen W. A new concept for energy coupling in oxidative phosphorylation based on a molecular explanation of the oxygen exchange reactions. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2837–2839. doi: 10.1073/pnas.70.10.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D., Davies P. L., Hou C. Function of energy-dependent transhydrogenase in Escherichia coli. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1248–1255. doi: 10.1016/0006-291x(72)90969-2. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Energization of energy-dependent transhydrogenase of Escherichia coli at a second site of energy conservation. Arch Biochem Biophys. 1974 Aug;163(2):614–616. doi: 10.1016/0003-9861(74)90521-9. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Purification of a factor for both aerobic-driven and ATP-driven energy-dependent transhydrogenases of Escherichia coli. FEBS Lett. 1972 Dec 15;28(3):309–312. doi: 10.1016/0014-5793(72)80738-5. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Reconstitution of energy-dependent transhydrogenase in ATPase-negative mutants of Escherichia coli. Biochem Biophys Res Commun. 1973 Feb 5;50(3):729–736. doi: 10.1016/0006-291x(73)91305-3. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Reduced nicotinamide adenine dinucleotide oxidation in Escherichia coli particles. I. Properties and cleavage of the electron transport chain. Arch Biochem Biophys. 1967 Mar;119(1):194–201. doi: 10.1016/0003-9861(67)90446-8. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Reduced nicotinamide adenine dinucleotide oxidation in Escherichia coli particles. II. NADH dehydrogenases. Arch Biochem Biophys. 1967 Mar;119(1):202–208. doi: 10.1016/0003-9861(67)90447-x. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Subunit composition, function, and spatial arrangement in the Ca2+-and Mg2+-activated adenosine triphosphatases of Escherichia coli and Salmonella typhimurium. Arch Biochem Biophys. 1975 Mar;167(1):311–321. doi: 10.1016/0003-9861(75)90467-1. [DOI] [PubMed] [Google Scholar]
- Bragg P. D. Reduction of nonheme iron in the respiratory chain of Escherichia coli. Can J Biochem. 1970 Jul;48(7):777–783. doi: 10.1139/o70-121. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Reynafarje B., Lehninger A. L. Stoichiometric relationship between energy-dependent proton ejection and electron transport in mitochondria. Proc Natl Acad Sci U S A. 1976 Feb;73(2):437–441. doi: 10.1073/pnas.73.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Vincent S. P., Lowe D. J., Clegg R. A., Garland P. B. Electron-paramagnetic-resonance studies on the molybdenum of nitrate reductase from Escherichia coli K12. Biochem J. 1976 Apr 1;155(1):201–203. doi: 10.1042/bj1550201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman R. L., Dobrogosz W. J. Stimulation of cytochrome synthesis in Escherichia coli by cyclic AMP. Arch Biochem Biophys. 1974 Jun;162(2):595–601. doi: 10.1016/0003-9861(74)90220-3. [DOI] [PubMed] [Google Scholar]
- Bryan-Jones D. G., Whittenbury R. Haematin-dependent oxidative phosphorylation in Streptococcus faecalis. J Gen Microbiol. 1969 Oct;58(2):247–260. doi: 10.1099/00221287-58-2-247. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., John P., Whatley F. R. The reversibility of active sulphate transport in membrane vesicles of Paracoccus denitrificans. Biochem J. 1975 Sep;150(3):527–536. doi: 10.1042/bj1500527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. C., Holsten R. D., Hardy R. W. Isolation by crystallization of the Mo-Fe protein of Azotobacter nitrogenase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):90–99. doi: 10.1016/0006-291x(70)90762-x. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K-12: the genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim Biophys Acta. 1973 Feb 22;292(2):366–375. doi: 10.1016/0005-2728(73)90043-1. [DOI] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- Carmeli C. Proton translocation induced by ATPase activity in chloroplasts. FEBS Lett. 1970 Apr 16;7(3):297–300. doi: 10.1016/0014-5793(70)80187-9. [DOI] [PubMed] [Google Scholar]
- Carreira J., Leal J. A., Rojas M., Muñoz E. Membrane ATPase of Escherichia coli K 12. Selective solubilization of the enzyme and its stimulation by trypsin in the soluble and membrane-bound states. Biochim Biophys Acta. 1973 May 25;307(3):541–556. doi: 10.1016/0005-2736(73)90299-x. [DOI] [PubMed] [Google Scholar]
- Cattell K. J., Knight I. G., Lindop C. R., Beechey R. B. The isolation of dicyclohexylcarbodi-imide-binding proteins from mitochondrial membranes. Biochem J. 1970 May;117(5):1011–1013. doi: 10.1042/bj1171011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell K. J., Lindop C. R., Knight I. G., Beechey R. B. The identification of the site of action of NN'-dicyclohexylcarbodi-imide as a proteolipid in mitochondrial membranes. Biochem J. 1971 Nov;125(1):169–177. doi: 10.1042/bj1250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Coty W. A., Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria. 3. Subunit composition. J Biol Chem. 1973 Nov 10;248(21):7427–7431. [PubMed] [Google Scholar]
- Catterall W. A., Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria. I. Purification, homogeneity, and physical properties. J Biol Chem. 1971 Aug 25;246(16):4987–4994. [PubMed] [Google Scholar]
- Cavari B. Z., Avi-Dor Y., Grossowicz N. Induction by oxygen of respiration and phosphorylation of anaerobically grown Escherichia coli. J Bacteriol. 1968 Sep;96(3):751–759. doi: 10.1128/jb.96.3.751-759.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg R. A., Garland P. B. Non-haem iron and the dissociation of piericidin A sensitivity from site 1 energy conservation in mitochondria from Torulopsis utilis. Biochem J. 1971 Aug;124(1):135–151. doi: 10.1042/bj1240135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg R. A. Purification and some properties of nitrate reductase (EC 1.7.99.4) from Escherichia coli K12. Biochem J. 1976 Mar 1;153(3):533–541. doi: 10.1042/bj1530533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobley J. G. Reduction of cytochromes by nitrite in electron-transport particles from Nitrobacter winogradskyi: proposal of a mechanism for H+ translocation. Biochem J. 1976 Jun 15;156(3):493–498. doi: 10.1042/bj1560493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobley J. G., Singer T. P., Beinert H., Grossman S. Piericiden A sensitivity, site 1 phosphorylation, and reduced nicotinamide adenine dinucleotide dehydrogenase during iron-limited growth of Candida utilis. J Biol Chem. 1975 Jan 10;250(1):211–217. [PubMed] [Google Scholar]
- Cole J. A., Wimpenny J. W. Metabolic pathways for nitrate reduction in Escherichia coli. Biochim Biophys Acta. 1968 Jul 16;162(1):39–48. doi: 10.1016/0005-2728(68)90212-0. [DOI] [PubMed] [Google Scholar]
- Cole J. S., 3rd, Aleem M. I. Electron transport-linked compared with proton-induced ATP generation in Thiobacillus novellus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3571–3575. doi: 10.1073/pnas.70.12.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., Luke R. K., Newton N. A., O'Brien I. G., Rosenberg H. Mutations affecting iron transport in Escherichia coli. J Bacteriol. 1970 Oct;104(1):219–226. doi: 10.1128/jb.104.1.219-226.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., McCann L. M., Butlin J. D., Crane F. L. Reconstitution of the energy-linked transhydrogenase activity in membranes from a mutant strain of Escherichia coli K12 lacking magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1973 Apr;132(4):689–695. doi: 10.1042/bj1320689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., McCann L. Oxidative phosphorylation in Escherichia coli K12. An uncoupled mutant with altered membrane structure. Biochem J. 1974 Feb;138(2):211–215. doi: 10.1042/bj1380211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., McCann L. Reconstitution of oxidative phosphorylation and the adenosine triphosphate-dependent transhydrogenase activity by a combination of membrane fractions from unCA- and uncB- mutant strains of Escherichia coli K12. Biochem J. 1973 Aug;134(4):1015–1021. doi: 10.1042/bj1341015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F. Studies on electron transport and energy-linked reactions using mutants of Escherichia coli. Biochim Biophys Acta. 1974 Apr 30;346(1):1–25. doi: 10.1016/0304-4173(74)90010-x. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Butlin J. D., Gibson F. The energy-linked transhydrogenase reaction in respiratory mutants of Escherichia coli K12. Biochem J. 1971 Nov;125(2):489–493. doi: 10.1042/bj1250489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. B., Quayle J. R. The autotrophic growth of Micrococcus denitrificans on Methanol. Biochem J. 1975 Sep;150(3):569–571. doi: 10.1042/bj1500569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Charles H. P. Porphyrin-accumulating mutants of Escherichia coli. J Bacteriol. 1973 Jan;113(1):122–132. doi: 10.1128/jb.113.1.122-132.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane R. T., Sun I. L., Crane F. L. Lipophilic chelator inhibition of electron transport in Escherichia coli. J Bacteriol. 1975 May;122(2):686–690. doi: 10.1128/jb.122.2.686-690.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creaghan I. T., Guest J. R. Amber mutants of the -ketoglutarate dehydrogenase gene of Escherichia coli K12. J Gen Microbiol. 1972 Jul;71(2):207–220. doi: 10.1099/00221287-71-2-207. [DOI] [PubMed] [Google Scholar]
- Czerwinski E. W., Mathews F. S. Location of the iron atom and the non-crystallographic symmetry elements in cytochrome b562. J Mol Biol. 1974 Jun 15;86(1):49–57. doi: 10.1016/s0022-2836(74)80006-9. [DOI] [PubMed] [Google Scholar]
- DEEB S. S., HAGER L. P. CRYSTALLINE CYTOCHROME B1 FROM ESCHERICHIA COLI. J Biol Chem. 1964 Apr;239:1024–1031. [PubMed] [Google Scholar]
- Dalton H., Postgate J. R. Effect of oxygen on growth of Azotobacter chroococcum in batch and continuous cultures. J Gen Microbiol. 1968 Dec;54(3):463–473. doi: 10.1099/00221287-54-3-463. [DOI] [PubMed] [Google Scholar]
- Daniel J., Roisin M. P., Burstein C., Kepes A. Mutants of Escherichia coli K12 unable to grow on non-fermentable carbon substrates. Biochim Biophys Acta. 1975 Feb 17;376(2):195–209. doi: 10.1016/0005-2728(75)90011-0. [DOI] [PubMed] [Google Scholar]
- Davies P. L., Bragg P. D. Properties of a soluble Ca 2+ - and Mg 2+ -activated ATPase released from Escherichia coli membranes. Biochim Biophys Acta. 1972 Apr 14;266(1):273–284. doi: 10.1016/0005-2736(72)90142-3. [DOI] [PubMed] [Google Scholar]
- Dervartanian D. V., Bramlett R. Electron paramagnetic resonance studies of 95Mo-enriched NADH dehydrogenase isolated from iron-deficient Azotobacter vinelandii. Biochim Biophys Acta. 1970 Dec 16;220(3):443–448. doi: 10.1016/0005-2744(70)90275-5. [DOI] [PubMed] [Google Scholar]
- Deters D. W., Racker E., Nelson N., Nelson H. Partial resolution of the enzymes catalyzing photophosphorylation. XV. Approaches to the active site of coupling factor I. J Biol Chem. 1975 Feb 10;250(3):1041–1047. [PubMed] [Google Scholar]
- Douglas M. W., Ward F. B., Cole J. A. The formate hydrogenlyase activity of cytochrome c552-deficient mutants of Escherichia coli K12. J Gen Microbiol. 1974 Feb;80(2):557–560. doi: 10.1099/00221287-80-2-557. [DOI] [PubMed] [Google Scholar]
- Downs A. J., Jones C. W. Energy conservation in Bacillus megaterium. Arch Microbiol. 1975 Oct 27;105(2):159–167. doi: 10.1007/BF00447131. [DOI] [PubMed] [Google Scholar]
- Downs A. J., Jones C. W. Respiration-linked proton translocation in Azotobacter vinelandii. FEBS Lett. 1975 Dec 1;60(1):42–46. doi: 10.1016/0014-5793(75)80414-5. [DOI] [PubMed] [Google Scholar]
- Drozd J., Postgate J. R. Effects of oxygen on acetylene reduction, cytochrome content and respiratory activity of Azotobacter chroococcum. J Gen Microbiol. 1970 Sep;63(1):63–73. doi: 10.1099/00221287-63-1-63. [DOI] [PubMed] [Google Scholar]
- Eilermann L. J. Oxidative phosphorylation in Azotobacter vinelandii. Atebrin as a fluorescent probe for the energized state. Biochim Biophys Acta. 1970 Aug 4;216(1):231–233. doi: 10.1016/0005-2728(70)90177-5. [DOI] [PubMed] [Google Scholar]
- Eilermann L. J., Pandit-Hovenkamp H. G., Kolk A. H. Oxidative phosphorylation in Azotobacter vinelandii particles. Phosphorylation sites and respiratory control. Biochim Biophys Acta. 1970 Jan 13;197(1):25–30. doi: 10.1016/0005-2728(70)90004-6. [DOI] [PubMed] [Google Scholar]
- Eilermann L. J., Pandit-Hovenkamp H. G., van Meer-Van Buren M., Kolk A. H., Feenstra M. Oxidative phosphorylation in Azotobacter vinelandii. Effect of inhibitors and uncouplers on P-O ratio, trypsin-induced ATPase and ADP-stimulated respiration. Biochim Biophys Acta. 1971 Sep 7;245(2):305–312. doi: 10.1016/0005-2728(71)90149-6. [DOI] [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. The purification and properties of formate dehydrogenase and nitrate reductase from Escherichia coli. J Biol Chem. 1975 Sep 10;250(17):6693–6705. [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. The role of a novel cytochrome b-containing nitrate reductase and quinone in the in vitro reconstruction of formate-nitrate reductase activity of E. coli. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1234–1241. doi: 10.1016/s0006-291x(74)80416-x. [DOI] [PubMed] [Google Scholar]
- Erickson S. K., Diehl H. The terminal oxidases of Azotobacter vinelandii. Biochem Biophys Res Commun. 1973 Jan 23;50(2):321–327. doi: 10.1016/0006-291x(73)90843-7. [DOI] [PubMed] [Google Scholar]
- Evans D. J. Membrane Mg-(Ca)-Activated Adenosine Triphosphatase of Escherichia coli: Characterization in the Membrane-Bound and Solubilized States. J Bacteriol. 1970 Dec;104(3):1203–1212. doi: 10.1128/jb.104.3.1203-1212.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEWSON C. A., NICHOLAS D. J. Respiratory enzymes in Micrococcus denitrificans. Biochim Biophys Acta. 1961 Mar 18;48:208–210. doi: 10.1016/0006-3002(61)90778-8. [DOI] [PubMed] [Google Scholar]
- Farmer I. S., Jones C. W. The energetics of Escherichia coli during aerobic growth in continuous culture. Eur J Biochem. 1976 Aug 1;67(1):115–122. doi: 10.1111/j.1432-1033.1976.tb10639.x. [DOI] [PubMed] [Google Scholar]
- Farron F. Isolation and properties of a chloroplast coupling factor and heat-activated adenosine triphosphatase. Biochemistry. 1970 Sep 15;9(19):3823–3828. doi: 10.1021/bi00821a023. [DOI] [PubMed] [Google Scholar]
- Faust P. J., Vandemark P. J. Phosphorylation coupled to NADH oxidation with fumarate in Streptococcus faecalis 10Cl. Arch Biochem Biophys. 1970 Apr;137(2):392–398. doi: 10.1016/0003-9861(70)90454-6. [DOI] [PubMed] [Google Scholar]
- Ferguson S. J., John P., Lloyd W. J., Radda G. K., Whatley F. R. Selective and reversible inhibition of the ATPase of Micrococcus denitrificans by 7-chloro-4-nitrobenzo-2-oxa-1,3 diazole. Biochim Biophys Acta. 1974 Sep 20;357(3):457–461. doi: 10.1016/0005-2728(74)90037-1. [DOI] [PubMed] [Google Scholar]
- Forget P., Dervartanian D. V. The bacterial nitrate reductases: EPR studies on nitrate reductase A from Micrococcus denitrificans. Biochim Biophys Acta. 1972 Feb 28;256(2):600–606. doi: 10.1016/0005-2728(72)90089-8. [DOI] [PubMed] [Google Scholar]
- Forget P. Les nitrate-réductases bactériennes. Solubilisation, purification et propriétés de l'enzyme A de Micrococcus denitrificans. Eur J Biochem. 1971 Feb 1;18(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01262.x. [DOI] [PubMed] [Google Scholar]
- Forget P. The bacterial nitrate reductases. Solubilization, purification and properties of the enzyme A of Escherichia coli K 12. Eur J Biochem. 1974 Mar 1;42(2):325–332. doi: 10.1111/j.1432-1033.1974.tb03343.x. [DOI] [PubMed] [Google Scholar]
- Fujita T. Studies on soluble cytochromes in Enterobacteriaceae. II. Cytochromes b-562 and c-550. J Biochem. 1966 Sep;60(3):329–334. doi: 10.1093/oxfordjournals.jbchem.a128440. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Salton M. R. Common peptides in Micrococcus lysodeikticus membrane proteins. Biochim Biophys Acta. 1972 Oct 23;288(1):65–72. doi: 10.1016/0005-2736(72)90223-4. [DOI] [PubMed] [Google Scholar]
- Fukuyama T., Ordal E. J. Induced Biosynthesis of Formic Hydrogenlyase in Iron-Deficient Cells of Escherichia coli. J Bacteriol. 1965 Sep;90(3):673–680. doi: 10.1128/jb.90.3.673-680.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M. Membrane D-lactate dehydrogenase from Escherichia coli. Purification and properties. Biochemistry. 1973 Jun 19;12(13):2468–2474. doi: 10.1021/bi00737a016. [DOI] [PubMed] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. doi: 10.1007/BF01870079. [DOI] [PubMed] [Google Scholar]
- Futai M., Sternweis P. C., Heppel L. A. Purification and properties of reconstitutively active and inactive adenosinetriphosphatase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2725–2729. doi: 10.1073/pnas.71.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Downie J. A., Haddock B. A. Proton translocation and the respiratory nitrate reductase of Escherichia coli. Biochem J. 1975 Dec;152(3):547–559. doi: 10.1042/bj1520547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Cox G. B. The use of mutants of Escherichia coli K12 in studying electron transport and oxidative phosphorylation. Essays Biochem. 1973;9:1–29. [PubMed] [Google Scholar]
- Giordano G., Riviere C., Azoulay E. Membrane reconstitution in chl-r mutants of Escherichia coli K 12. VII. Purification of the soluble ATPase of supernatant extracts and kinetics of incorporation into reconstituted particles. Biochim Biophys Acta. 1975 May 6;389(2):203–218. doi: 10.1016/0005-2736(75)90316-8. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., DeMoss J. A. Comparison of nitrate reductase mutants of Escherichia coli selected by alternative procedures. Mol Gen Genet. 1972;116(1):1–10. doi: 10.1007/BF00334254. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., DeMoss J. A. Phenotypic restoration by molybdate of nitrate reductase activity in chlD mutants of Escherichia coli. J Bacteriol. 1971 Nov;108(2):854–860. doi: 10.1128/jb.108.2.854-860.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorneva G. A., Ryabova I. D. Membrane orientation in vesicles from Micrococcus lysodeikticus cells. FEBS Lett. 1974 Jun 15;42(3):271–274. doi: 10.1016/0014-5793(74)80743-x. [DOI] [PubMed] [Google Scholar]
- Green D. E. The electromechanochemical model for energy coupling in mitochondria. Biochim Biophys Acta. 1974 Apr 30;346(1):27–78. doi: 10.1016/0304-4173(74)90011-1. [DOI] [PubMed] [Google Scholar]
- Grossman S., Cobley J. G., Singer T. P. Reduced nicotinamide adenine dinucleotide dehydrogenase, piericidin sensitivity, and site 1 phosphorylation in different growth phases of Candida utilis. J Biol Chem. 1974 Jun 25;249(12):3819–3826. [PubMed] [Google Scholar]
- Guest J. R. Biochemical and genetic studies with nitrate reductase C-gene mutants of Escherichia coli. Mol Gen Genet. 1969;105(4):285–297. doi: 10.1007/BF00277583. [DOI] [PubMed] [Google Scholar]
- Gutman M., Schejter A., Avi-Dor Y. The preparation and properties of the membranal DPNH dehydrogenase from Escherichia coli. Biochim Biophys Acta. 1968 Nov 26;162(4):506–517. doi: 10.1016/0005-2728(68)90057-1. [DOI] [PubMed] [Google Scholar]
- HIRSCH C. A., RASMINSKY M., DAVIS B. D., LIN E. C. A FUMARATE REDUCTASE IN ESCHERICHIA COLI DISTINCT FROM SUCCINATE DEHYDROGENASE. J Biol Chem. 1963 Nov;238:3770–3774. [PubMed] [Google Scholar]
- Hachimori A., Muramatsu N., Noso Y. Studies on an ATPase of thermophilic bacteria. I. Purification and properties. Biochim Biophys Acta. 1970 Jun 10;206(3):426–437. doi: 10.1016/0005-2744(70)90158-0. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Downie J. A., Garland P. B. Kinetic characterization of the membrane-bound cytochromes of Escherichia coli grown under a variety of conditions by using a stopped-flow dual-wavelength spectrophotometer. Biochem J. 1976 Feb 15;154(2):285–294. doi: 10.1042/bj1540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Downie J. A. The reconstitution of functional respiratory chains in membranes from electron-transport-deficient mutants of Escherichia coli as demonstrated by quenching of atebrin fluorescence. Biochem J. 1974 Sep;142(3):703–706. doi: 10.1042/bj1420703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Garland P. B. Effect of sulphate-limited growth on mitochondrial electron transfer and energy conservation between reduced nicotinamide-adenine dinucleotide and the cytochromes in Torulopsis utilis. Biochem J. 1971 Aug;124(1):155–170. doi: 10.1042/bj1240155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Kendall-Tobias M. W. Functional anaerobic electron transport linked to the reduction of nitrate and fumarate in membranes from Escherichia coli as demonstrated by quenching of atebrin fluorescence. Biochem J. 1975 Dec;152(3):655–659. doi: 10.1042/bj1520655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Schairer H. U. Electron-transport chains of Escherichia coli. Reconstitution of respiration in a 5-aminolaevulinic acid-requiring mutant. Eur J Biochem. 1973 May;35(1):34–45. doi: 10.1111/j.1432-1033.1973.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Haddock B. A. The reconstitution of oxidase activity in membranes derived from a 5-aminolaevulinic acid-requiring mutant of Escherichia coli. Biochem J. 1973 Dec;136(4):877–884. doi: 10.1042/bj1360877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafkenscheid J. C., Bonting S. L. Studies on (Na+-K+)-activated ATPase. 23. A Mg2+-ATPase in Escherichia coli, activated by monovalent cations. Biochim Biophys Acta. 1969 Mar 18;178(1):128–136. doi: 10.1016/0005-2744(69)90139-9. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Cox G. B., Looney F. D., Gibson F. Ubisemiquinone in membranes from Escherichia coli. Biochem J. 1970 Jan;116(2):319–320. doi: 10.1042/bj1160319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes G. G., Hilborn D. A. Steady state kinetics of soluble and membrane-bound mitochondrial ATPase. Biochim Biophys Acta. 1971 Jun 1;233(3):580–590. doi: 10.1016/0005-2736(71)90156-8. [DOI] [PubMed] [Google Scholar]
- Hampton M. L., Freese E. Explanation for the apparent inefficiency of reduced nicotinamide adenine dinucleotide in energizing amino acid transport in membrane vesicles. J Bacteriol. 1974 May;118(2):497–504. doi: 10.1128/jb.118.2.497-504.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. L., Kennedy E. P. Energy-transducing adenosine triphosphatase from Escherichia coli: purification, properties, and inhibition by antibody. J Bacteriol. 1973 May;114(2):772–781. doi: 10.1128/jb.114.2.772-781.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J. F., Olden K., Kennedy E. P. Heterogeneity of membrane vesicles from Escherichia coli and their subfractionation with antibody to ATPase. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4843–4846. doi: 10.1073/pnas.71.12.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J. F. Purification and characterization of a dicyclohexylcarbodiimide-sensitive adenosine triphosphatase complex from membranes of Escherichia coli. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1329–1337. doi: 10.1016/0006-291x(75)90505-7. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempfling W. P., Beeman D. K. Release of glucose repression of oxidative phosphorylation in Escherichia coli B by cyclic adenosine 3',5'-monophosphate. Biochem Biophys Res Commun. 1971 Nov;45(4):924–930. doi: 10.1016/0006-291x(71)90426-8. [DOI] [PubMed] [Google Scholar]
- Hempfling W. P., Mainzer S. E. Effects of varying the carbon source limiting growth on yield and maintenance characteristics of Escherichia coli in continuous culture. J Bacteriol. 1975 Sep;123(3):1076–1087. doi: 10.1128/jb.123.3.1076-1087.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempfling W. P. Studies of the efficiency of oxidative phosphorylation in intact Escherichia coli B. Biochim Biophys Acta. 1970;205(2):169–182. doi: 10.1016/0005-2728(70)90247-1. [DOI] [PubMed] [Google Scholar]
- Henderson P. J. Steady-state enzyme kinetics with high-affinity substrates or inhibitors. A statistical treatment of dose-response curves. Biochem J. 1973 Sep;135(1):101–107. doi: 10.1042/bj1350101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Burgess A. H. Fractionation of the electron-transport chain of Escherichia coli. Biochim Biophys Acta. 1974 Aug 23;357(2):215–230. doi: 10.1016/0005-2728(74)90062-0. [DOI] [PubMed] [Google Scholar]
- Hendler R. W., Burgess A. H. Respiration and protein synthesis in Escherichia coli membrane-envelope fragments. VI. Solubilization and characterization of the electron transport chain. J Cell Biol. 1972 Nov;55(2):266–281. doi: 10.1083/jcb.55.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Towne D. W., Shrager R. I. Redox properties of beta-type cytochromes in Escherichia coli and rat liver mitochondria and techniques for their analysis. Biochim Biophys Acta. 1975 Jan 31;376(1):42–62. doi: 10.1016/0005-2728(75)90203-0. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L., Hinkle P. C. Oxidative phosphorylation and proton translocation in membrane vesicles prepared from Escherichia coli. Biochem Biophys Res Commun. 1974 May 7;58(1):178–184. doi: 10.1016/0006-291x(74)90908-5. [DOI] [PubMed] [Google Scholar]
- Higashi T., Kalra V. K., Lee S. H., Bogin E., Brodie A. F. Energy-transducing membrane-bound coupling factor-ATPase from Mycobacterium phlei. I. Purification, homogeneity, and properties. J Biol Chem. 1975 Aug 25;250(16):6541–6548. [PubMed] [Google Scholar]
- Hinkle P. C., Horstman L. L. Respiration-driven proton transport in submitochondrial particles. J Biol Chem. 1971 Oct 10;246(19):6024–6028. [PubMed] [Google Scholar]
- Hinkle P., Mitchell P. Effect of membrane potential on equilibrium poise between cytochrome a and cytochrome c in rat liver mitochondria. J Bioenerg. 1970 Jun;1(1):45–60. doi: 10.1007/BF01516088. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Kaback H. R. Mutants of Salmonella typhimurium and Escherichia coli pleiotropically defective in active transport. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3336–3340. doi: 10.1073/pnas.69.11.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T., Kamen M. D. Bacterial cytochromes. II. Functional aspects. Annu Rev Microbiol. 1970;24:399–428. doi: 10.1146/annurev.mi.24.100170.002151. [DOI] [PubMed] [Google Scholar]
- Horstman L. L., Racker E. Partial resolution of the enzyme catalyzing oxidative phosphorylation. XXII. Interaction between mitochondrial adenosine triphosphatase inhibitor and mitochondrial adenosine triphosphatase. J Biol Chem. 1970 Mar 25;245(6):1336–1344. [PubMed] [Google Scholar]
- Houghton R. L., Fisher R. J., Sanadi D. R. Energy-linked and energy-independent transhydrogenase activities in Escherichia coli vesicles. Biochim Biophys Acta. 1975 Jul 8;396(1):17–23. doi: 10.1016/0005-2728(75)90185-1. [DOI] [PubMed] [Google Scholar]
- Imai K., Asano A., Sato R. Oxidative phosphorylation in Micrococcus denitrificans. I. Preparation and properties of phosphorylating membrane fragments. Biochim Biophys Acta. 1967;143(3):462–476. doi: 10.1016/0005-2728(67)90052-7. [DOI] [PubMed] [Google Scholar]
- Imai K., Asano A., Sato R. Oxidative phosphorylation in Micrococcus denitrificans. IV. Further characterization of electron-transfer pathway and phosphorylation activity in NADH oxidation. J Biochem. 1968 Feb;63(2):207–218. doi: 10.1093/oxfordjournals.jbchem.a128763. [DOI] [PubMed] [Google Scholar]
- Imai K., Asano A., Sato R. Oxidative phosphorylation in Micrococcus denitrificans. V. Effects of iron deficiency on respiratory components and oxidative phosphorylation. J Biochem. 1968 Feb;63(2):219–225. doi: 10.1093/oxfordjournals.jbchem.a128764. [DOI] [PubMed] [Google Scholar]
- Ishida M., Mizushima S. Membrane ATPase of Bacillus megaterium. I. Properties of membrane ATPase and its solubilized form. J Biochem. 1969 Jul;66(1):33–43. doi: 10.1093/oxfordjournals.jbchem.a129117. [DOI] [PubMed] [Google Scholar]
- Itagaki E., Hager L. P. Studies on cytochrome b-562 of Escherichia coli. I. Purification and crystallization of cytochrome b-562. J Biol Chem. 1966 Aug 25;241(16):3687–3695. [PubMed] [Google Scholar]
- Jeacocke R. E., Niven D. F., Hamilton W. A. The protonmotive force in Staphylococcus aureus. Biochem J. 1972 Apr;127(3):57P–58P. doi: 10.1042/bj1270057p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John P., Hamilton W. A. Release of respiratory control in particles from Micrococcus denitrificans by ion-translocating antibiotics. Eur J Biochem. 1971 Dec 10;23(3):528–532. doi: 10.1111/j.1432-1033.1971.tb01650.x. [DOI] [PubMed] [Google Scholar]
- John P., Whatley F. R. Oxidative phosphorylation coupled to oxygen uptake and nitrate reduction in Micrococcus denitrificans. Biochim Biophys Acta. 1970 Sep 1;216(2):342–352. doi: 10.1016/0005-2728(70)90225-2. [DOI] [PubMed] [Google Scholar]
- John P., Whatley F. R. Paracoccus denitrificans and the evolutionary origin of the mitochondrion. Nature. 1975 Apr 10;254(5500):495–498. doi: 10.1038/254495a0. [DOI] [PubMed] [Google Scholar]
- John Philip, Hamilton W. A. Respiratory control in membrane particles from Micrococcus denitrificans. FEBS Lett. 1970 Oct 16;10(4):246–248. doi: 10.1016/0014-5793(70)80639-1. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Ackrell B. A., Erickson S. K. Respiratory control in Azotobacter vinelandii membranes. Biochim Biophys Acta. 1971 Aug 6;245(1):54–62. doi: 10.1016/0005-2728(71)90007-7. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Brice J. M., Downs A. J., Drozd J. W. Bacterial respiration-linked proton translocation and its relationship to respiratory-chain composition. Eur J Biochem. 1975 Mar 17;52(2):265–271. doi: 10.1111/j.1432-1033.1975.tb03994.x. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Brice J. M., Wright V., Ackrell B. A. Respiratory protection of nitrogenase in Azotobacter vinelandii. FEBS Lett. 1973 Jan 15;29(2):77–81. doi: 10.1016/0014-5793(73)80530-7. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Erickson S. K., Ackrell B. A.C. Some parameters affecting respiratory control in Azotobacter vinelandii membranes. FEBS Lett. 1971 Feb 12;13(1):33–35. doi: 10.1016/0014-5793(71)80657-9. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. Electron transport in Azotobacter vinelandii. Biochim Biophys Acta. 1966 Mar 7;113(3):467–481. doi: 10.1016/s0926-6593(66)80005-x. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. Preparation of red and green electron transport particles from Azotobacter vinelandii. Biochim Biophys Acta. 1967 Sep 6;143(2):354–362. doi: 10.1016/0005-2728(67)90089-8. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. The cytochrome system of Azotobacter vinelandii. Biochim Biophys Acta. 1967 Sep 6;143(2):340–353. doi: 10.1016/0005-2728(67)90088-6. [DOI] [PubMed] [Google Scholar]
- Jones C. W. The inhibition of Azotobacter vinelandii terminal oxidases by cyanide. FEBS Lett. 1973 Nov 1;36(3):347–350. doi: 10.1016/0014-5793(73)80407-7. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Bednarz A. J., Zey P., Denton C. H. L-malate oxidation by the electron transport fraction of Azotobacter vinelandii. J Bacteriol. 1969 Jun;98(3):1120–1127. doi: 10.1128/jb.98.3.1120-1127.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Jr, Mueller T. J., Acord W. C. Bacterial terminal oxidases. CRC Crit Rev Microbiol. 1975 May;3(4):399–468. doi: 10.3109/10408417509108757. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., May A. K., Pope L. M., Aston P. R. Comparative studies on succinate and terminal oxidase activity in microbial and mammalian electron-transport systems. Can J Microbiol. 1969 Jul;15(7):797–807. doi: 10.1139/m69-139. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Old L. Cytochrome c oxidation by the electron transport fraction of Azotobacter vinelandii. J Bacteriol. 1968 May;95(5):1790–1797. doi: 10.1128/jb.95.5.1790-1797.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., COLLINS J. F., BIGLEY D. The influence of growth substrates on metabolic pathways in Micrococcus denitrificans. Biochim Biophys Acta. 1960 Mar 25;39:9–24. doi: 10.1016/0006-3002(60)90117-7. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. IX. Reconstruction of oligomycin-sensitive adenosine triphosphatase. J Biol Chem. 1966 May 25;241(10):2467–2474. [PubMed] [Google Scholar]
- Kamen M. D., Horio T. Bacterial cytochromes. I. Structural aspects. Annu Rev Biochem. 1970;39:673–700. doi: 10.1146/annurev.bi.39.070170.003325. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Gutnick D. L. Use of neomycin in the isolation of mutants blocked in energy conservation in Escherichia coli. J Bacteriol. 1972 Jul;111(1):287–289. doi: 10.1128/jb.111.1.287-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner B. I., Nelson N., Gutnick D. L. Differentiation between mutants of Escherichia coli K defective in oxidative phosphorylation. Biochim Biophys Acta. 1975 Sep 8;396(3):347–359. doi: 10.1016/0005-2728(75)90141-3. [DOI] [PubMed] [Google Scholar]
- Kauffman H. F., van Gelder B. F. The respiratory chain of Azotobacter vinelandii. I. Spectral properites of cytochrome d. Biochim Biophys Acta. 1973 May 30;305(2):260–267. doi: 10.1016/0005-2728(73)90174-6. [DOI] [PubMed] [Google Scholar]
- Kauffman H. F., van Gelder B. F. The respiratory chain of Azotobacter vinelandii. II. The effect of cyanide on cytochrome d. Biochim Biophys Acta. 1973 Sep 26;314(3):276–283. doi: 10.1016/0005-2728(73)90112-6. [DOI] [PubMed] [Google Scholar]
- Kim I. C., Bragg P. D. Properties of nonheme iron in a cell envelope fraction from Escherichia coli. J Bacteriol. 1971 Sep;107(3):664–670. doi: 10.1128/jb.107.3.664-670.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli: its genetic locus and its physiological role. J Bacteriol. 1971 Dec;108(3):1224–1234. doi: 10.1128/jb.108.3.1224-1234.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Purification and properties of the flavine-stimulated anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli. J Bacteriol. 1972 Oct;112(1):539–547. doi: 10.1128/jb.112.1.539-547.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- Knobloch K., Ishaque M., Aleem M. I. Oxidative phosphorylation in Micrococcus denitrificans under autotrophic growth conditions. Arch Mikrobiol. 1971;76(2):114–125. doi: 10.1007/BF00411785. [DOI] [PubMed] [Google Scholar]
- Knowles A. F., Penefsky H. S. The subunit structure of beef heart mitochondrial adenosine triphosphatase. Physical and chemical properties of isolated subunits. J Biol Chem. 1972 Oct 25;247(20):6624–6630. [PubMed] [Google Scholar]
- Knowles C. J., Smith L. Measurements of ATP levels of intact Azotobacter vinelandii under different conditions. Biochim Biophys Acta. 1970 Mar 3;197(2):152–160. doi: 10.1016/0005-2728(70)90026-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Anraku Y. Membrane-bound adenosine triphosphatase of Escherichia coli. I. Partial purification and properties. J Biochem. 1972 Mar;71(3):387–399. [PubMed] [Google Scholar]
- Kobayashi H., Anraku Y. Membrane-bound adenosinetriphosphatase of Escherichia coli. II. Physicochemical properties of the enzyme. J Biochem. 1974 Dec;76(6):1175–1182. doi: 10.1093/oxfordjournals.jbchem.a130670. [DOI] [PubMed] [Google Scholar]
- Kohn L. D., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. XV. Purification and properties of the membrane-bound D-lactate dehydrogenase from Escherichia coli. J Biol Chem. 1973 Oct 25;248(20):7012–7017. [PubMed] [Google Scholar]
- Konings W. N., Bisschop A., Veenhuis M., Vermeulen C. A. New procedure for the isolation of membrane vesicles of Bacillus subtilis and an electron microscopy study of their ultrastructure. J Bacteriol. 1973 Dec;116(3):1456–1465. doi: 10.1128/jb.116.3.1456-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N. Localization of membrane proteins in membrane vesicles of Bacillus subtilis. Arch Biochem Biophys. 1975 Apr;167(2):570–580. doi: 10.1016/0003-9861(75)90500-7. [DOI] [PubMed] [Google Scholar]
- Kozlov I. A., Mikelsaar H. N. On the subunit structure of soluble mitochondrial ATPase. FEBS Lett. 1974 Jul 15;43(2):212–214. doi: 10.1016/0014-5793(74)81002-1. [DOI] [PubMed] [Google Scholar]
- Lam Y., Nicholas D. J. A nitrate reductase from Micrococcus denitrificans. Biochim Biophys Acta. 1969 Apr 22;178(2):225–234. doi: 10.1016/0005-2744(69)90392-1. [DOI] [PubMed] [Google Scholar]
- Lam Y., Nicholas D. J. A nitrite reductase with cytochrome oxidase activity from Micrococcus denitrificans. Biochim Biophys Acta. 1969 Aug 5;180(3):459–472. doi: 10.1016/0005-2728(69)90025-5. [DOI] [PubMed] [Google Scholar]
- Lam Y., Nicholas D. J. Aerobic and anaerobic respiration in Micrococcus denitrificans. Biochim Biophys Acta. 1969 Apr 8;172(3):450–461. doi: 10.1016/0005-2728(69)90141-8. [DOI] [PubMed] [Google Scholar]
- Lambeth D. O., Lardy H. A. Purification and properties of rat-liver-mitochondrial adenosine triphosphatase. Eur J Biochem. 1971 Oct 14;22(3):355–363. doi: 10.1111/j.1432-1033.1971.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Langman L., Young I. G., Frost G. E., Rosenberg H., Gibson F. Enterochelin system of iron transport in Escherichia coli: mutations affecting ferric-enterochelin esterase. J Bacteriol. 1972 Dec;112(3):1142–1149. doi: 10.1128/jb.112.3.1142-1149.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastras M., Munõz E. Membrane adenosine triphosphatase of Micrococcus lysodeikticus: effect of millimolar Mg2+ in modulating the properties of the membrane-bound enzyme. J Bacteriol. 1974 Aug;119(2):593–601. doi: 10.1128/jb.119.2.593-601.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawford H. G., Cox J. C., Garland P. B., Haddock B. A. Electron transport in aerobically grown Paracoccus denitrificans: kinetic characterization of the membrane-bound cytochromes and the stoichiometry of respiration-driven proton translocation. FEBS Lett. 1976 May 1;64(2):369–374. doi: 10.1016/0014-5793(76)80330-4. [DOI] [PubMed] [Google Scholar]
- Lawford H. G., Haddock B. A. Respiration-driven proton translocation in Escherichia coli. Biochem J. 1973 Sep;136(1):217–220. doi: 10.1042/bj1360217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees H., Postgate J. R. The behaviour of Azotobacter chroococcum in oxygen- and phosphate-limited chemostat culture. J Gen Microbiol. 1973 Mar;75(1):161–166. doi: 10.1099/00221287-75-1-161. [DOI] [PubMed] [Google Scholar]
- Lester R. L., DeMoss J. A. Effects of molybdate and selenite on formate and nitrate metabolism in Escherichia coli. J Bacteriol. 1971 Mar;105(3):1006–1014. doi: 10.1128/jb.105.3.1006-1014.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien S., Berzborn R. J., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. IX. Studies on the subunit structure of coupling factor 1 from chloroplasts. J Biol Chem. 1972 Jun 10;247(11):3520–3524. [PubMed] [Google Scholar]
- Lo T. C., Rayman M. K., Sanwal B. D. Transport of succinate in Escherichia coli. I. Biochemical and genetic studies of transport in whole cells. J Biol Chem. 1972 Oct 10;247(19):6323–6331. [PubMed] [Google Scholar]
- Luke R. K., Gibson F. Location of three genes concerned with the conversion of 2,3-dihydroxybenzoate into enterochelin in Escherichia coli K-12. J Bacteriol. 1971 Aug;107(2):557–562. doi: 10.1128/jb.107.2.557-562.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H. Anaerobic cytochrome b1 in Escherichia coli: association with and regulation of nitrate reductase. J Bacteriol. 1975 Mar;121(3):1111–1116. doi: 10.1128/jb.121.3.1111-1116.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H. Biosynthesis of membrane-bound nitrate reductase in Escherichia coli: evidence for a soluble precursor. J Bacteriol. 1976 Apr;126(1):122–131. doi: 10.1128/jb.126.1.122-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A. Alterations in the cytoplasmic membrane proteins of various chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1971 Oct;108(1):564–570. doi: 10.1128/jb.108.1.564-570.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A., Normansell D. E. Purification and properties of nitrate reductase from Escherichia coli K12. J Biol Chem. 1974 Aug 25;249(16):5321–5327. [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A. Reconstitution of nitrate reductase activity and formation of membrane particles from cytoplasmic extracts of chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1164–1176. doi: 10.1128/jb.114.3.1164-1176.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H. Solubilization of Escherichia coli nitrate reductase by a membrane-bound protease. J Bacteriol. 1975 Mar;121(3):1102–1110. doi: 10.1128/jb.121.3.1102-1110.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H. Synthesis of nitrate reductase components in chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1975 Mar;121(3):1117–1121. doi: 10.1128/jb.121.3.1117-1121.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcot J., Azoulay E. Obtention et etude de doubles mutants chlorate-resistants chez Escherichia coli K12. FEBS Lett. 1971 Mar 5;13(3):137–139. doi: 10.1016/0014-5793(71)80219-3. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Jones C. W. Reactivity with oxygen of bacterial cytochrome oxidases a1, aa3 and o. FEBS Lett. 1973 Jun 15;33(1):101–105. doi: 10.1016/0014-5793(73)80169-3. [DOI] [PubMed] [Google Scholar]
- Mickelson M. N. Phosphorylation and the reduced nicotinamide adenine dinucleotide oxidase reaction in Streptococcus agalactiae. J Bacteriol. 1969 Nov;100(2):895–901. doi: 10.1128/jb.100.2.895-901.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Lin E. C. Anaerobic energy-yielding reaction associated with transhydrogenation from glycerol 3-phosphate to fumarate by an Escherichia coli system. J Bacteriol. 1975 Dec;124(3):1282–1287. doi: 10.1128/jb.124.3.1282-1287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Lin E. C. Electron transport chain from glycerol 3-phosphate to nitrate in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1288–1294. doi: 10.1128/jb.124.3.1288-1294.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Lin E. C. Enzyme complex which couples glycerol-3-phosphate dehydrogenation to fumarate reduction in Escherichia coli. J Bacteriol. 1973 May;114(2):767–771. doi: 10.1128/jb.114.2.767-771.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky R., Barlow V. Alternative purification of the membrane-bound ATPase from Bacillus megaterium KM, and some properties. Biochim Biophys Acta. 1972 Aug 9;274(2):556–562. doi: 10.1016/0005-2736(72)90202-7. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Barlow V. Molecular weight, amino acid composition and other properties of membrane-bound ATPase from Bacillus megaterium KM. Biochim Biophys Acta. 1973 Jan 26;291(2):480–488. doi: 10.1016/0005-2736(73)90499-9. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Barlow V. Purification and properties of ATPase from the cytoplasmic membrane of Bacillus megaterium KM. Biochim Biophys Acta. 1971 Sep 14;241(3):835–845. doi: 10.1016/0005-2736(71)90011-3. [DOI] [PubMed] [Google Scholar]
- Mitchell P. A chemiosmotic molecular mechanism for proton-translocating adenosine triphosphatases. FEBS Lett. 1974 Jul 15;43(2):189–194. doi: 10.1016/0014-5793(74)80997-x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Hypothesis: cation-translocating adenosine triphosphatase models: how direct is the participation of adenosine triphosphate and its hydrolysis products in cation translocation? FEBS Lett. 1973 Jul 15;33(3):267–274. doi: 10.1016/0014-5793(73)80209-1. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Activation and inhibition of mitochondrial adenosine triphosphatase by various anions and other agents. J Bioenerg. 1971 Feb;2(1):1–11. doi: 10.1007/BF01521319. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Proton translocation coupled to ATP hydrolysis in rat liver mitochondria. Eur J Biochem. 1968 May;4(4):530–539. doi: 10.1111/j.1432-1033.1968.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Proton-translocation phosphorylation in mitochondria, chloroplasts and bacteria: natural fuel cells and solar cells. Fed Proc. 1967 Sep;26(5):1370–1379. [PubMed] [Google Scholar]
- Mitchell P. The protonmotive Q cycle: a general formulation. FEBS Lett. 1975 Nov 15;59(2):137–139. doi: 10.1016/0014-5793(75)80359-0. [DOI] [PubMed] [Google Scholar]
- Monteil H., Schoun J., Guinard M. A Na+K+-activated Mg2+-dependent ATPase released from Proteus L-form membrane. Eur J Biochem. 1974 Feb 1;41(3):525–532. doi: 10.1111/j.1432-1033.1974.tb03293.x. [DOI] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. Proton translocation quotient for the adenosine triphosphatase of rat liver mitochondria. FEBS Lett. 1973 Mar 15;30(3):317–320. doi: 10.1016/0014-5793(73)80678-7. [DOI] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. The proton-translocating nicotinamide-adenine dinucleotide (phosphate) transhydrogenase of rat liver mitochondria. Biochem J. 1973 Mar;132(3):571–585. doi: 10.1042/bj1320571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz E., Freer J. H., Ellar D. J., Salton M. R. Membrane-associated ATPase activity from Micrococcus lysodeikticus. Biochim Biophys Acta. 1968 Apr 29;150(3):531–533. doi: 10.1016/0005-2736(68)90156-9. [DOI] [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Kamin H., Rosenthal D. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. II. Identification of a new class of heme prosthetic group: an iron-tetrahydroporphyrin (isobacteriochlorin type) with eight carboxylic acid groups. J Biol Chem. 1973 Apr 25;248(8):2801–2814. [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Tove S. R., Kamin H. Siroheme: a new prosthetic group participating in six-electron reduction reactions catalyzed by both sulfite and nitrite reductases. Proc Natl Acad Sci U S A. 1974 Mar;71(3):612–616. doi: 10.1073/pnas.71.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutaftschiev S., Azoulay E. Membrane reconstitution in chl-r mutants of Escherichia coli K 12. VI. Morphological study of membrane assembly during complementation between extracts of chl-r mutants. Biochim Biophys Acta. 1973 May 25;307(3):525–540. doi: 10.1016/0005-2736(73)90298-8. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Nachbar M. S., Schor M. T., Salton M. R. Adenosinetriphosphatase of Micrococcus lysodeikticus: selective release and relationship to membrane structure. Biochem Biophys Res Commun. 1968 Aug 13;32(3):539–546. doi: 10.1016/0006-291x(68)90696-7. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Salton M. R., Ng M. H., Schor M. T. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Purification, properties of the "soluble" enzyme and properties of the membrane-bound enzyme. Eur J Biochem. 1969 Feb;7(4):490–501. [PubMed] [Google Scholar]
- Mével-Ninio M. T., Valentine R. C. Energy requirements for biosynthesis of DNA in Escherichia coli. Role of membrane-bound energy-transducing ATPase (coupling factor). Biochim Biophys Acta. 1975 Mar 20;376(3):485–491. doi: 10.1016/0005-2728(75)90169-3. [DOI] [PubMed] [Google Scholar]
- Mével-Ninio M., Yamamoto T. Conversion of active transport vesicles of Escherichia coli into oxidative phosphorylation vesicles. Biochim Biophys Acta. 1974 Jul 25;357(1):63–66. doi: 10.1016/0005-2728(74)90112-1. [DOI] [PubMed] [Google Scholar]
- NICHOLAS D. J., WILSON P. W., HEINEN W., PALMER G., BEINERT H. Use of electron paramagnetic resonance spectroscopy in investigations of functional metal components in micro-organisms. Nature. 1962 Nov 3;196:433–436. doi: 10.1038/196433a0. [DOI] [PubMed] [Google Scholar]
- Nagai S., Aiba S. Reassessment of maintenance and energy uncoupling in the growth of Azotobacter vinelandii. J Gen Microbiol. 1972 Dec;73(3):531–538. doi: 10.1099/00221287-73-3-531. [DOI] [PubMed] [Google Scholar]
- Neijssel O. M., Tempest D. W. Bioenergetic aspects of aerobic growth of Klebsiella aerogenes NCTC 418 in carbon-limited and carbon-sufficient chemostat culture. Arch Microbiol. 1976 Mar 19;107(2):215–221. doi: 10.1007/BF00446843. [DOI] [PubMed] [Google Scholar]
- Neijssel O. M., Tempest D. W. The regulation of carbohydrate metabolism in Klebsiella aerogenes NCTC 418 organisms, growing in chemostat culture. Arch Microbiol. 1975 Dec 31;106(3):251–258. doi: 10.1007/BF00446531. [DOI] [PubMed] [Google Scholar]
- Nelson N., Deters D. W., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. 8. Properties of isolated subunits of coupling factor 1 from spinach chloroplasts. J Biol Chem. 1973 Mar 25;248(6):2049–2055. [PubMed] [Google Scholar]
- Nelson N., Kanner B. I., Gutnick D. L. Purification and properties of Mg2+-Ca2+ adenosinetriphosphatase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2720–2724. doi: 10.1073/pnas.71.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. XII. Purification and properties of an inhibitor isolated from chloroplast coupling factor 1. J Biol Chem. 1972 Dec 10;247(23):7657–7662. [PubMed] [Google Scholar]
- Newton N. A., Cox G. B., Gibson F. Function of ubiquinone in Escherichia coli: a mutant strain forming a low level of ubiquinone. J Bacteriol. 1972 Jan;109(1):69–73. doi: 10.1128/jb.109.1.69-73.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton N. A., Cox G. B., Gibson F. The function of menaquinone (vitamin K 2 ) in Escherichia coli K-12. Biochim Biophys Acta. 1971 Jul 20;244(1):155–166. doi: 10.1016/0304-4165(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Newton N. A soluble cytochrome containing c-type and a2-type haem groups from Micrococcus denitrificans. Biochem J. 1967 Oct;105(1):21C–23C. doi: 10.1042/bj1050021c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton N. The two-haem nitrite reductase of Micrococcus denitrificans. Biochim Biophys Acta. 1969;185(2):316–331. doi: 10.1016/0005-2744(69)90425-2. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis F. J., Kanner B. I., Gutnick D. L., Postma P. W., van Dam K. Energy conservation in membranes of mutants of Escherichia coli defective in oxidative phosphorylation. Biochim Biophys Acta. 1973 Oct 19;325(1):62–71. doi: 10.1016/0005-2728(73)90151-5. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis F. J., van der Drift J. A., Voet A. B., Van Dam K. Evidence for a naturally occurring ATPase-inhibitor in Escherichia coli. Biochim Biophys Acta. 1974 Dec 19;368(3):461–463. doi: 10.1016/0005-2728(74)90192-3. [DOI] [PubMed] [Google Scholar]
- Oki M. Correlation between metabolism of phosphatidylglycerol and membrane synthesis in Escherichia coli. J Mol Biol. 1972 Jul 21;68(2):249–264. doi: 10.1016/0022-2836(72)90212-4. [DOI] [PubMed] [Google Scholar]
- Oki M., Mitsui H. Defective membrane synthesis in an E. coli mutant. Nature. 1974 Nov 1;252(5478):64–66. doi: 10.1038/252064a0. [DOI] [PubMed] [Google Scholar]
- Onishi T. Mechanism of electron transport and energy conservation in the site I region of the respiratory chain. Biochim Biophys Acta. 1973 Dec 7;301(2):105–128. [PubMed] [Google Scholar]
- Oppenheim J. D., Salton M. R. Localization and distribution of Micrococcus lysodeikticus membrane ATPase determined by ferritin labeling. Biochim Biophys Acta. 1973 Mar 16;298(2):297–322. doi: 10.1016/0005-2736(73)90360-x. [DOI] [PubMed] [Google Scholar]
- Oppenheim J., Marcus L. Correlation of ultrastructure in Azotobacter vinelandii with nitrogen source for growth. J Bacteriol. 1970 Jan;101(1):286–291. doi: 10.1128/jb.101.1.286-291.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULLMAN M. E., MONROY G. C. A NATURALLY OCCURRING INHIBITOR OF MITOCHONDRIAL ADENOSINE TRIPHOSPHATASE. J Biol Chem. 1963 Nov;238:3762–3769. [PubMed] [Google Scholar]
- Palmieri F., Klingenberg M. Inhibition of respiration under the control of azide uptake by mitochondria. Eur J Biochem. 1967 Jun;1(4):439–446. doi: 10.1007/978-3-662-25813-2_60. [DOI] [PubMed] [Google Scholar]
- Pandya K. P., King H. K. Ubiquinone and menaquinone in bacteria: a comparative study of some bacterial respiratory systems. Arch Biochem Biophys. 1966 Apr;114(1):154–157. doi: 10.1016/0003-9861(66)90316-x. [DOI] [PubMed] [Google Scholar]
- Papa S., Guerrieri F., Rossi Bernardi L., Tager J. M. Effect of oligomygin on proton translocation in submitochondrial particles. Biochim Biophys Acta. 1970 Jan 13;197(1):100–103. doi: 10.1016/0005-2728(70)90016-2. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S., Warner R. C. Partial resolution of the enzymes catalyzing oxidative phosphorylation. VI. Studies on the mechanism of cold inactivation of mitochondrial adenosine triphosphatase. J Biol Chem. 1965 Dec;240(12):4694–4702. [PubMed] [Google Scholar]
- Peter H. W., Ahlers J. Phospholipid requirements of ATPase of Escherichia coli. Arch Biochem Biophys. 1975 Sep;170(1):169–178. doi: 10.1016/0003-9861(75)90108-3. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Haddock B. A. Effects of sulphate-limited growth in continuous culture on the electron-transport chain and energy conservation in Escherichia coli K12. Biochem J. 1975 Dec;152(3):537–546. doi: 10.1042/bj1520537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K., Haddock B. A. Energy-linked reduction of nicotinamide--adenine dinucleotide in membranes derived from normal and various respiratory-deficient mutant strains of Escherichia coli K12. Biochem J. 1974 Oct;144(1):77–85. doi: 10.1042/bj1440077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. A., Cox R., McConville M., Charles H. P. Mutations affecting porphyrin biosynthesis in Escherichia coli. Enzyme. 1973;16(1):65–73. doi: 10.1159/000459363. [DOI] [PubMed] [Google Scholar]
- Pudek M. R., Bragg P. D. Inhibition by cyanide of the respiratory chain oxidases of Escherichia coli. Arch Biochem Biophys. 1974 Oct;164(2):682–693. doi: 10.1016/0003-9861(74)90081-2. [DOI] [PubMed] [Google Scholar]
- Pudek M. R., Bragg P. D. Reaction of cyanide with cytochrome d in respiratory particles from exponential phase Escherichia coli. FEBS Lett. 1975 Feb 1;50(2):111–113. doi: 10.1016/0014-5793(75)80468-6. [DOI] [PubMed] [Google Scholar]
- Racker E., Horstman L. L. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 13. Structure and function of submitochondrial particles completely resolved with respect to coupling factor. J Biol Chem. 1967 May 25;242(10):2547–2551. [PubMed] [Google Scholar]
- Racker E. Resolution and reconstitution of the inner mitochondrial membrane. Fed Proc. 1967 Sep;26(5):1335–1340. [PubMed] [Google Scholar]
- Rainnie D. J., Bragg P. D. The effect of iron deficiency on respiration and energy-coupling in Escherichia coli. J Gen Microbiol. 1973 Aug;77(2):339–349. doi: 10.1099/00221287-77-2-339. [DOI] [PubMed] [Google Scholar]
- Redwood W. R., Gibbes D. C., Thompson T. E. Interaction of a solubilized membrane ATPase with lipid bilayer membranes. Biochim Biophys Acta. 1973 Aug 9;318(1):10–22. doi: 10.1016/0005-2736(73)90331-3. [DOI] [PubMed] [Google Scholar]
- Reeves J. P. Transient pH changes during D-lactate oxidation by membrane vesicles. Biochem Biophys Res Commun. 1971 Nov;45(4):931–936. doi: 10.1016/0006-291x(71)90427-x. [DOI] [PubMed] [Google Scholar]
- Riviere C., Giordano G., Pommier J., Azoulay E. Membrane reconstitution in chl-r mutants of Escherichia coli K 12. VIII. Purification and properties of the FA factor, the product of the chl B gene. Biochim Biophys Acta. 1975 May 6;389(2):219–235. doi: 10.1016/0005-2736(75)90317-x. [DOI] [PubMed] [Google Scholar]
- Robertson R. N., Boardman N. K. The link between charge separation, proton movement and ATPase reactions. FEBS Lett. 1975 Dec 1;60(1):1–6. doi: 10.1016/0014-5793(75)80405-4. [DOI] [PubMed] [Google Scholar]
- Roisin M. P., Kepes A. The membrane ATPase of Escherichia coli. I. Release into solution, allotopic properties and reconstitution of membrane-bound ATPase. Biochim Biophys Acta. 1973 May 30;305(2):249–259. doi: 10.1016/0005-2728(73)90173-4. [DOI] [PubMed] [Google Scholar]
- Rolfe B., Onodera K. Genes, enzymes and membrane proteins of the nitrate respiration system of Escherichia coli. J Membr Biol. 1972;9(2):195–207. [PubMed] [Google Scholar]
- Rosen B. P., Adler L. W. The maintenance of the energized membrane state and its relation to active transport in Escherichia coli. Biochim Biophys Acta. 1975 Apr 14;387(1):23–36. doi: 10.1016/0005-2728(75)90049-3. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Beta-galactoside transport and proton movements in an adenosine triphosphatase-deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1289–1296. doi: 10.1016/0006-291x(73)90605-0. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Restoration of active transport in an Mg2+-adenosine triphosphatase-deficient mutant of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1124–1129. doi: 10.1128/jb.116.3.1124-1129.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H., Cox G. B., Butlin J. D., Gutowski S. J. Metabolite transport in mutants of Escherichia coli K12 defective in electron transport and coupled phosphorylation. Biochem J. 1975 Feb;146(2):417–423. doi: 10.1042/bj1460417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of transmembrane electrochemical proton gradients. J Bioenerg. 1975 May;7(2):61–74. doi: 10.1007/BF01558427. [DOI] [PubMed] [Google Scholar]
- Ruiz-Herrera J., DeMoss J. A. Nitrate reductase complex of Escherichia coli K-12: participation of specific formate dehydrogenase and cytochrome b1 components in nitrate reduction. J Bacteriol. 1969 Sep;99(3):720–729. doi: 10.1128/jb.99.3.720-729.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J., Showe M. K., DeMoss J. A. Nitrate reductase complex of Escherichia coli K-12: isolation and characterization of mutants unable to reduce nitrate. J Bacteriol. 1969 Mar;97(3):1291–1297. doi: 10.1128/jb.97.3.1291-1297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R., Schor M. T. Release and purification of Micrococcus lysodeikticus ATPase from membranes extracted with n-butanol. Biochim Biophys Acta. 1974 Apr 12;345(1):74–82. doi: 10.1016/0005-2736(74)90247-8. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Schor M. T. Subunit structure and properties of two forms of adenosine triphosphatase released from Micrococcus lysodeikticus membranes. Biochem Biophys Res Commun. 1972 Oct 17;49(2):350–357. doi: 10.1016/0006-291x(72)90417-2. [DOI] [PubMed] [Google Scholar]
- Sapshead L. M., Wimpenny J. W. The influence of oxygen and nitrate on the formation of the cytochrome pigments of the aerobic and anaerobic respiratory chain of Micrococcus denitrificans. Biochim Biophys Acta. 1972 May 25;267(2):388–397. doi: 10.1016/0005-2728(72)90126-0. [DOI] [PubMed] [Google Scholar]
- Schairer H. U., Haddock B. A. -Galactoside accumulation in a Mg 2+ -,Ca 2+ -activated ATPase deficient mutant of E.coli. Biochem Biophys Res Commun. 1972 Aug 7;48(3):544–551. doi: 10.1016/0006-291x(72)90382-8. [DOI] [PubMed] [Google Scholar]
- Schnebli H. P., Abrams A. Membrane adenosine triphosphatase from Streptococcus faecalis. Preparation and homogeneity. J Biol Chem. 1970 Mar 10;245(5):1115–1121. [PubMed] [Google Scholar]
- Schnebli H. P., Vatter A. E., Abrams A. Membrane adenosine triphosphatase from Streptococcus faecalis. Molecular weight, subunit structure, and amino acid composition. J Biol Chem. 1970 Mar 10;245(5):1122–1127. [PubMed] [Google Scholar]
- Scholes P. B., Smith L. Composition and properties of the membrane-bound respiratory chain system of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):363–375. doi: 10.1016/0005-2728(68)90081-9. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Acid-base titration across the plasma membrane of Micrococcus denitrificans: factors affecting the effective proton conductance and the respiratory rate. J Bioenerg. 1970 Jun;1(1):61–72. doi: 10.1007/BF01516089. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P., Moyle J. The polarity of proton translocation in some photosynthetic microorganisms. Eur J Biochem. 1969 Apr;8(3):450–454. doi: 10.1111/j.1432-1033.1969.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Respiration-driven proton translocation in Micrococcus denitrificans. J Bioenerg. 1971 Sep;1(3):309–323. doi: 10.1007/BF01516290. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Brooks J. C. Studies on the mitochondrial oligomycin-insensitivt ATPase. I. An improved method of purification and the behavior of the enzyme in solutions of various depolymerizing agents. Arch Biochem Biophys. 1970 Sep;140(1):257–266. doi: 10.1016/0003-9861(70)90030-5. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Brooks J. C. The subunit composition of the mitochondrial oligomycin-insensitive ATPase. FEBS Lett. 1971 Oct 1;17(2):327–329. doi: 10.1016/0014-5793(71)80178-3. [DOI] [PubMed] [Google Scholar]
- Senior A. E. Mitochondrial adenosine triphosphatase. Location of sulfhydryl groups and disulfide bonds in soluble enzyme from beef heart. Biochemistry. 1975 Feb 25;14(4):660–664. doi: 10.1021/bi00675a002. [DOI] [PubMed] [Google Scholar]
- Senior A. E. The structure of mitochondrial ATPase. Biochim Biophys Acta. 1973 Dec 31;301(3):249–277. doi: 10.1016/0304-4173(73)90006-2. [DOI] [PubMed] [Google Scholar]
- Senior P. J., Beech G. A., Ritchie G. A., Dawes E. A. The role of oxygen limitation in the formation of poly- -hydroxybutyrate during batch and continuous culture of Azotobacter beijerinckii. Biochem J. 1972 Aug;128(5):1193–1201. doi: 10.1042/bj1281193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp W. S. Absorption bands of multiple b and c cytochromes in bacteria detected by numerical analysis of absorption spectra. Arch Biochem Biophys. 1972 Jun;150(2):482–488. doi: 10.1016/0003-9861(72)90065-3. [DOI] [PubMed] [Google Scholar]
- Shipp W. S. Cytochromes of Escherichia coli. Arch Biochem Biophys. 1972 Jun;150(2):459–472. doi: 10.1016/0003-9861(72)90063-x. [DOI] [PubMed] [Google Scholar]
- Shipp W. S., Piotrowski M., Friedman A. E. Apparent cytochrome gene dose effects in F-lac and F-gal heterogenotes of Escherichia coli. Arch Biochem Biophys. 1972 Jun;150(2):473–481. doi: 10.1016/0003-9861(72)90064-1. [DOI] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Hawkins T., Kohn L. D. Immunochemical properties of the membrane-bound D-lactate dehydrogenase from Escherichia coli. J Biol Chem. 1975 Jun 10;250(11):4285–4290. [PubMed] [Google Scholar]
- Showe M. K., DeMoss J. A. Localization and regulation of synthesis of nitrate reductase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1305–1313. doi: 10.1128/jb.95.4.1305-1313.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Davis P. S., Kamin H. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. 3. The Escherichia coli hemoflavoprotein: catalytic parameters and the sequence of electron flow. J Biol Chem. 1974 Mar 10;249(5):1572–1586. [PubMed] [Google Scholar]
- Siegel L. M., Davis P. S. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. IV. The Escherichia coli hemoflavoprotein: subunit structure and dissociation into hemoprotein and flavoprotein components. J Biol Chem. 1974 Mar 10;249(5):1587–1598. [PubMed] [Google Scholar]
- Siegel L. M., Murphy M. J., Kamin H. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. I. The Escherichia coli hemoflavoprotein: molecular parameters and prosthetic groups. J Biol Chem. 1973 Jan 10;248(1):251–264. [PubMed] [Google Scholar]
- Simoni R. D., Postma P. W. The energetics of bacterial active transport. Annu Rev Biochem. 1975;44:523–554. doi: 10.1146/annurev.bi.44.070175.002515. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Shallenberger M. K. Coupling of energy to active transport of amino acids in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2663–2667. doi: 10.1073/pnas.69.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Shandell A. Energy transduction in Escherichia coli. Genetic alteration of a membrane polypeptide of the (Ca2+,Mg2+)-ATPase. J Biol Chem. 1975 Dec 25;250(24):9421–9427. [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. Anaerobic transport of amino acids coupled to the glycerol-3-phosphate-fumarate oxidoreductase system in a cytochrome-deficient mutant of Escherichia coli. Biochim Biophys Acta. 1976 Mar 12;423(3):450–461. doi: 10.1016/0005-2728(76)90200-0. [DOI] [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. Reduced nicotinamide adenine dinucleotide dependent reduction of fumarate coupled to membrane energization in a cytochrome deficient mutant of Escherichia coli K12. Biochim Biophys Acta. 1975 Aug 11;396(2):229–241. doi: 10.1016/0005-2728(75)90037-7. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Sternweis P. C. Restoration of coupling factor activity to Escherichia coli ATPase missing the delta subunit. Biochem Biophys Res Commun. 1975 Feb 3;62(3):764–771. doi: 10.1016/0006-291x(75)90465-9. [DOI] [PubMed] [Google Scholar]
- Sone N., Yoshida M., Hirata H., Kagawa Y. Purification and properties of a dicyclohexylcarbodiimide-sensitive adenosine triphosphatase from a thermophilic bacterium. J Biol Chem. 1975 Oct 10;250(19):7917–7923. [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Isolation and properties of fumarate reductase mutants of Escherichia coli. J Bacteriol. 1973 May;114(2):563–570. doi: 10.1128/jb.114.2.563-570.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Proteins of the inner membrane of Escherichia coli: identification of succinate dehydrogenase by polyacrylamide gel electrophoresis with sdh amber mutants. J Bacteriol. 1974 Mar;117(3):947–953. doi: 10.1128/jb.117.3.947-953.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. W. Determination of the efficiency of oxidative phosphorylation in continuous cultures of Aerobacter aerogenes. Arch Microbiol. 1975 Mar 10;102(3):187–192. doi: 10.1007/BF00428367. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. A reevaluation of the method for the determination of ATP production by measuring molar growth yields. Biochim Biophys Acta. 1973 Feb 12;301(1):53–70. doi: 10.1016/0304-4173(73)90012-8. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H. Biochemistry and genetics of nitrate reductase in bacteria. Adv Microb Physiol. 1976;14(11):315–375. doi: 10.1016/s0065-2911(08)60230-1. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Burris R. H. Purification and properties of cytochromes c of Azotobacter vinelandii. Biochim Biophys Acta. 1969 Aug 5;180(3):473–489. doi: 10.1016/0005-2728(69)90026-7. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Burris R. H. Restoration by ubiquinone of Azotobacter vinelandii reduced nicotinamide adenine dinucleotide oxidase activity. J Bacteriol. 1969 Apr;98(1):311–313. doi: 10.1128/jb.98.1.311-313.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman A. J., Griffiths D. E. Studies on energy-linked reactions. Energy-linked reduction of oxidized nicotinamide-adenine dinucleotide by succinate in Escherichia coli. Biochem J. 1971 Jan;121(1):117–124. doi: 10.1042/bj1210117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman A. J., Griffiths D. E. Studies on energy-linked reactions. Energy-linked transhydrogenase reaction in Escherichia coli. Biochem J. 1971 Jan;121(1):125–130. doi: 10.1042/bj1210125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synthesis and sideedness of membrane-bound respiratory nitrate reductase (EC1.7.99.4) in Escherichia coli lacking cytochromes. Biochem J. 1975 May;148(2):329–333. [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Chartrand P., Proschek R., Desrochers M., Tardif D., Lapointe C. Uroporphyrin-accumulating mutant of Escherichia coli K-12. J Bacteriol. 1975 Dec;124(3):1205–1212. doi: 10.1128/jb.124.3.1205-1212.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Horodniceanu T. Locus determining the synthesis of delta-aminolevulinic acid in Escherichia coli K-12. J Bacteriol. 1968 Nov;96(5):1882–1884. doi: 10.1128/jb.96.5.1882-1884.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Szégli G., Horodniceanu T., Greceanu V., Dumitrescu A. Hemin-deficient mutants of Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):570–572. doi: 10.1128/jb.96.2.570-572.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANIGUCHI S., ITAGAKI E. Nitrate reductase of nitrate respiration type from E. coli. I. Solubilization and purification from the particulate system with molecular characterization as a metalloprotein. Biochim Biophys Acta. 1960 Nov 4;44:263–279. doi: 10.1016/0006-3002(60)91562-6. [DOI] [PubMed] [Google Scholar]
- Thayer W. S., Hinkle P. C. Stoichiometry of adenosine triphosphate-driven proton translocation in bovine heart submitochondrial particles. J Biol Chem. 1973 Aug 10;248(15):5395–5402. [PubMed] [Google Scholar]
- Thipayathasana P. Isolation and properties of Escherichia coli ATPase mutants with altered divalent metal specificity for ATP hydrolysis. Biochim Biophys Acta. 1975 Oct 10;408(1):47–57. doi: 10.1016/0005-2728(75)90157-7. [DOI] [PubMed] [Google Scholar]
- Thipayathasana P., Valentine R. The requirement for energy transducing ATPase for anaerobic motility in Escherichia coli. Biochim Biophys Acta. 1974 Jun 28;347(3):464–468. doi: 10.1016/0005-2728(74)90083-8. [DOI] [PubMed] [Google Scholar]
- Tucker A. N., Lillich T. T. Effect of the systemic fungicide carboxin on electron transport function in membranes of Micrococcus denitrificans. Antimicrob Agents Chemother. 1974 Nov;6(5):572–578. doi: 10.1128/aac.6.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assembly of the mitochondrial membrane system. V. Properties of a dispersed preparation of the rutamycin-sensitive adenosine triphosphatase of yeast mitochondria. J Biol Chem. 1971 Dec 10;246(23):7328–7336. [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assesmbly of the mitochondrial membrane system. VI. Mitochondrial synthesis of subunit proteins of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1972 Jan 25;247(2):594–603. [PubMed] [Google Scholar]
- VERNON L. P. Bacterial cytochromes. I. Cytochrome composition of Micrococcus denitrificans and Pseudomonas denitrificans. J Biol Chem. 1956 Oct;222(2):1035–1044. [PubMed] [Google Scholar]
- VERNON L. P., WHITE F. G. Terminal oxidases of Micrococcus denitrificans. Biochim Biophys Acta. 1957 Aug;25(2):321–328. doi: 10.1016/0006-3002(57)90475-4. [DOI] [PubMed] [Google Scholar]
- Van Verseveld H. W., Stouthamer A. H. Oxidative phosphorylation in Micrococcus denitrificans: calculation of the P/O ratio in growing cells. Arch Microbiol. 1976 Apr 1;107(3):241–247. doi: 10.1007/BF00425334. [DOI] [PubMed] [Google Scholar]
- Van den Broek W. J., Santema J. S., Wassink J. H., Veeger C. Pyridine-nucleotide transhydrogenase. 1. Isolation, purification and characterisation of the transhydrogenase from Azotobacter vinelandii. Eur J Biochem. 1971 Dec 22;24(1):31–45. doi: 10.1111/j.1432-1033.1971.tb19652.x. [DOI] [PubMed] [Google Scholar]
- Venables W. A. Genetic studies with nitrate reductase-less mutants of Escherichia coli. I. Fine structure analysis of the narA, narB and narE loci. Mol Gen Genet. 1972;114(3):223–231. doi: 10.1007/BF01788891. [DOI] [PubMed] [Google Scholar]
- Villarreal-Moguel E. I., Ibarra V., Ruíz-Herrera J., Gitler C. Resolution of the nitrate reductase complex from the membrane of Escherichia coli. J Bacteriol. 1973 Mar;113(3):1264–1267. doi: 10.1128/jb.113.3.1264-1267.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G., Steinhart R. ATPase of Escherichia coli: purification, dissociation, and reconstitution of the active complex from the isolated subunits. Biochemistry. 1976 Jan 13;15(1):208–216. doi: 10.1021/bi00646a032. [DOI] [PubMed] [Google Scholar]
- WEIBULL C., GREENWALT J. W., LOW H. The hydrolysis of adenosine triphosphate by cell fractions of Bacillus megaterium. I. Localization and general characteristics of the enzymic activities. J Biol Chem. 1962 Mar;237:847–852. [PubMed] [Google Scholar]
- West I. C., Mitchell P. The proton-translocating ATPase of Escherichia coli. FEBS Lett. 1974 Mar 15;40(1):1–4. doi: 10.1016/0014-5793(74)80880-x. [DOI] [PubMed] [Google Scholar]
- White D. C., Sinclair P. R. Branched electron-transport systems in bacteria. Adv Microb Physiol. 1971;5:173–211. doi: 10.1016/s0065-2911(08)60407-5. [DOI] [PubMed] [Google Scholar]
- White D. C., Tucker A. N., Kaback H. R. Relationship between amino acid transport and electron transport by membrane vesicles of Micrococcus denitrificans. Arch Biochem Biophys. 1974 Dec;165(2):672–680. doi: 10.1016/0003-9861(74)90296-3. [DOI] [PubMed] [Google Scholar]
- Wikström M. K. The different cytochrome b components in the respiratory chain of animal mitochondria and their role in electron transport and energy conservation. Biochim Biophys Acta. 1973 Dec 7;301(2):155–193. doi: 10.1016/0304-4173(73)90003-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. J., Morman M. R., White D. C. Phospholipid composition and metabolism of Micrococcus denitrificans. J Bacteriol. 1972 Dec;112(3):1288–1294. doi: 10.1128/jb.112.3.1288-1294.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. J. Proton-driven phosphorylation reactions in mitochondrial and chloroplast membranes. FEBS Lett. 1975 May 1;53(2):123–125. doi: 10.1016/0014-5793(75)80001-9. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W., Cole J. A. The regulation of metabolism in facultative bacteria. 3. The effect of nitrate. Biochim Biophys Acta. 1967 Oct 9;148(1):233–242. doi: 10.1016/0304-4165(67)90298-x. [DOI] [PubMed] [Google Scholar]
- Woodrow G. C., Young I. G., Gibson F. Mu-induced polarity in the Escherichia coli K-12 ent gene cluster: evidence for a gene (entG) involved in the biosynthesis of enterochelin. J Bacteriol. 1975 Oct;124(1):1–6. doi: 10.1128/jb.124.1.1-6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff D. L. Delta-aminolevulinic acid-requiring mutant from Escherichia coli. J Bacteriol. 1967 Apr;93(4):1473–1474. doi: 10.1128/jb.93.4.1473-1474.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguzhinsky L. S., Boguslavsky L. I., Volkov A. G., Rakhmaninova A. B. Synthesis of ATP coupled with action of membrane protonic pumps at the octane-water interface. Nature. 1976 Feb 12;259(5543):494–496. doi: 10.1038/259494a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. H., Mével-Ninio M., Valentine R. C. Essential role of membrane ATPase or coupling factor for anaerobic growth and anaerobic active transport in Escherichia coli. Biochim Biophys Acta. 1973 Sep 26;314(3):267–275. doi: 10.1016/0005-2728(73)90111-4. [DOI] [PubMed] [Google Scholar]
- Yates M. G., Planqué K. Nitrogenase from Azotobacter chroococcum. Purification and properties of the component proteins. Eur J Biochem. 1975 Dec 15;60(2):467–476. doi: 10.1111/j.1432-1033.1975.tb21025.x. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Sone N., Hirata H., Kagawa Y. A highly stable adenosine triphosphatase from a thermophillie bacterium. Purification, properties, and reconstitution. J Biol Chem. 1975 Oct 10;250(19):7910–7916. [PubMed] [Google Scholar]
- Yoshida M., Sone N., Hirata H., Kagawa Y. ATP synthesis catalyzed by purified DCCD-sensitive ATPase incorporated into reconstituted purple membrane vesicles. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1295–1300. doi: 10.1016/0006-291x(75)90167-9. [DOI] [PubMed] [Google Scholar]
- Young I. G. Biosynthesis of bacterial menaquinones. Menaquinone mutants of Escherichia coli. Biochemistry. 1975 Jan 28;14(2):399–406. doi: 10.1021/bi00673a029. [DOI] [PubMed] [Google Scholar]
- Young I. G., Langman L., Luke R. K., Gibson F. Biosynthesis of the iron-transport compound enterochelin: mutants of Escherichia coli unable to synthesize 2,3-dihydroxybenzoate. J Bacteriol. 1971 Apr;106(1):51–57. doi: 10.1128/jb.106.1.51-57.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Hachimi Z., Samuel O., Azerad R. Biochemical study on ubiquinone biosynthesis in Escherichia coli : I. Specificity of para hydroxybenzoate polyprenyltransferase. Biochimie. 1974;56(9):1239–1247. doi: 10.1016/s0300-9084(74)80017-9. [DOI] [PubMed] [Google Scholar]
- van Thienen G., Postma P. W. Coupling between energy conservation and active transport of serine in Escherichia coli. Biochim Biophys Acta. 1973 Oct 25;323(3):429–440. doi: 10.1016/0005-2736(73)90188-0. [DOI] [PubMed] [Google Scholar]
- van de Stadt R. J., Nieuwenhuis F. J., van Dam K. On the reversibility of the energy-linked transhydrogenase. Biochim Biophys Acta. 1971 Apr 6;234(1):173–176. doi: 10.1016/0005-2728(71)90143-5. [DOI] [PubMed] [Google Scholar]
- van der Beek E. G., Stouthamer A. H. Oxidative phosphorylation in intact bacteria. Arch Mikrobiol. 1973;89(4):327–339. doi: 10.1007/BF00408900. [DOI] [PubMed] [Google Scholar]



