Abstract
Juvenile neuronal ceroid lipofuscinoses (JNCL) or Batten disease is the most common type of NCL in the United States and Europe. This devastating disorder presents with vision failure and progresses to include seizures, motor dysfunction, and dementia. Death usually occurs in the third decade, but some patients die before age twenty. Though the mechanism of visual failure remains poorly understood, recent advances in molecular genetics have improved diagnostic testing and suggested possible therapeutic strategies. The ophthalmologist plays a crucial role in both early diagnosis and documentation of progression of JNCL. We update Batten disease research, particularly as it relates to the eye, and present various theories on the pathophysiology of retinal degeneration.
Keywords: autofluorescence, Batten disease, ceroid, CLN3, neuronal ceroid ipofuscinosis Ceroid, retinal degeneratio
I. Introduction
The ophthalmologist plays a crucial role in the early diagnosis of juvenile neuronal ceroid lipofuscinosis (JNCL). There has been substantial progress in understanding the molecular genetics of JNCL, in the development of new animal models and less invasive diagnostic tests, and, most importantly, in a better understanding of JNCL pathogenesis. The ophthalmologist’s role is not limited to diagnosis, as an understanding of the pathophysiology of vision loss may shed light on the systemic manifestations of the disease.
A. BACKGROUND
The neuronal ceroid lipofuscinoses (NCLs) are a group of inherited lysosomal storage diseases that together constitute the most common neurodegenerative disorders of childhood. They are clinically and genetically heterogeneous and are characterized by intracellular accumulation of autofluorescent material, neurodegeneration, and blindness. The stored material, ceroid, shares a similar composition to lipofuscin and is present in most tissues, including brain and retina.31, 32, 79, 85 Historically, four major NCL subtypes (infantile, late-infantile, juvenile, and adult) have been delineated based on age of onset and the ultrastructure of the storage material. With the advent of molecular genetics, the NCLs have been further classified into eight genetically distinct forms, independent of age of onset (Table 1). All forms of NCL are recessively inherited, with the exception of an adult variant (Parry type).65
Table 1.
Genes known to cause NCL (adapted from Ramirez-Montealegre D et al. Brain. 2006; 129(6):1353-6).
| Disease | Chromosome Location |
Gene Affected |
Protein |
|---|---|---|---|
| Congenital NCL (CNCL) | 11p15.5 | CTSD | Cathepsin D |
| Santavuori-Haltia (INCL) | 1p32 | CLN1 | Palmitoyl protein thioesterase I |
| Jansky-Bielschowsky (LINCL) | 11p15 | CLN2 | Tripeptidyl peptidase protein I |
| Batten, Spielmeyer-Sjogren (JNCL) | 16p12 | CLN3 | Unknown |
| Kufs (ANCL) | Unknown | CLN4* | Unknown |
| Finnish LINCL (vfinLINCL) | 13q31-32 | CLN5 | Unknown |
| Costa Rican LINCL (vLINCL) | 15q21-23 | CLN6 | Unknown |
| Turkish LINCL (vturkLINCL) | 4q28.1-q28.2 |
CLN7 (MFSD8) |
Unknown |
| Northern Epilepsy/Epilepsy with Progressive Mental Retardation |
8p23 | CLN8 | Unknown |
INCL (infantile neuronal ceroid lipofuscinoses), LINCL (late infantile neuronal ceroid lipofuscinoses), JNCL (juvenile neuronal ceroid lipofuscinoses), CNCL (congenital neuronal ceroid lipofuscinoses), ANCL (adult neuronal ceroid lipofuscinoses).
Note : Adult onset NCLs have also been associated to mutations in CLN1.
JNCL is the most common type of NCL in the United States and Europe.26 Visual loss is almost invariably the presenting symptom, though even at an early stage slight mental decline may be detected on neuropsychological evaluation.5, 12, 13, 22, 56, 86, 91 The disease progresses to include seizures, motor deterioration, problems in speech, mental decline, and finally death.40 Cardiac problems are also common, especially at late stages of the disease.40 In advanced disease retinal degeneration is widespread, with neuronal depletion and subsequent atrophy and severe gliosis, similar to that found in advanced retinitis pigmentosa (RP).12, 13, 82, 91
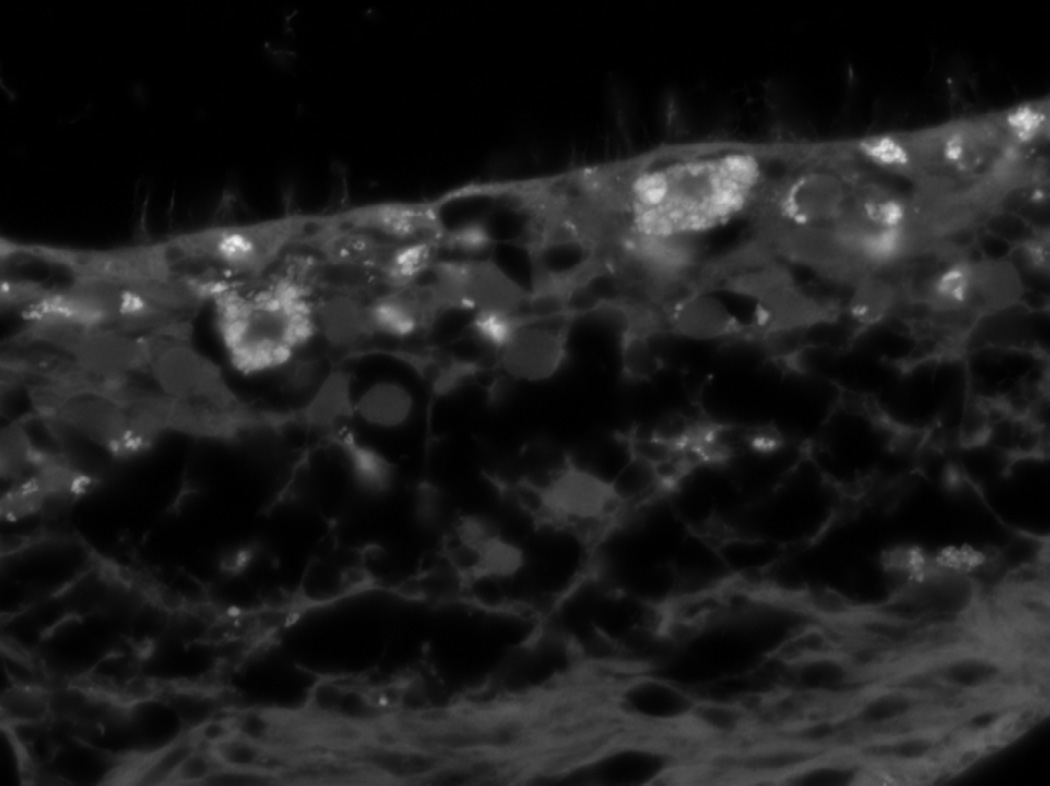
The pathophysiology of retinal degeneration in JNCL remains poorly understood; however, it appears likely that the primary defect affects different levels of the visual pathway. Retinal degeneration may be caused by accumulation of storage material in the ganglion cell layer, which may be a primary microglial defect76, 78 or an upstream insult in the dorsal lateral geniculate nucleus (LGNd), leading to optic atrophy and a retrograde retinal degeneration.96 The JNCL retina illustrated in Fig. 1 demonstrates accumulations of autofluorescent material predominantly in the photoreceptor cell layer, but also in the ganglion cell layer.
Figure 1.
Autofluorescence in JNCL retina. PR layer is on top, RGC layer bottom. The filter used for the fluorescence is the Zeiss Filter Set 43HE Excitation BP 550/25 Beam Splitter FT 570 Emission BP 605/70.
Historically, diagnosis required characterization of the autofluorescent storage material by electron microscopy of rectal, conjunctival, or skin biopsies, peripheral blood, or post-mortem tissue. Current genetic techniques applied to a DNA sample from blood or a buccal swab allow the physician to make an accurate diagnosis and to counsel patients and families more quickly and effectively. Details can be obtained through contacting the Batten Disease Diagnostic and Clinical Research Center at http://dbb.urmc.rochester.edu/labs/pearce/bddcrc/index.htm
JNCL was first described by Stengel (1826) and was amplified by Batten (1903), Spielmeyer (1905), Vogt (1909), and Sjogren (1931). As a result the disease has had various eponyms: Vogt-Spielmeyer disease, Spielmeyer-Sjogren disease, Spielmeyer-Vogt-Sjogren disease, and Batten disease. JNCL may affect patients of any ethnic background. Its incidence has been estimated at 2 to 4 in 100,000 live births in the United States,23 and may be highest in Scandinavia, with reports as high as 7 per 100,000 live births.94
II. Pathogenesis
Neuropathologically, JNCL has two major features: widespread neuronal degeneration-- including retinal atrophy and massive loss of brain substance--and accumulation of intracellular lipopigments. The role of the latter in the disease process is still uncertain. Lipopigments stain bright red with PAS and blue with Luxol-fast blue, are autofluorescent, and display enhanced acid phosphatase activity. This autofluorescence is visible mainly in the perikarya of nerve and glial cells and often expands into the cell body. The complete chemical composition of this autofluorescent lipopigment, often termed ceroid, is not known due to its obvious heterogeneity.85 Immunohistochemistry has shown that a predominant component is the subunit c of mitochondrial ATP synthase.68, 98
Reactive gliosis is characteristic of all forms of NCL, but it is unclear whether glial activation precedes or is triggered by neuronal loss. Activation of microglia and astroglia, before neuronal degeneration, has been postulated.78 Neuroimmune and inflammatory components of the disease are clearly evident. Studies of CLN3-mouse models for JNCL have revealed autoantibodies to many proteins, several of which are known to be expressed in the CNS,17, 18, 20, 21 and suggest that this may underlie the apparent neuroimmune response.52, 53
A. OCULAR HISTOPATHOLOGY
Histopathology of autopsy eyes demonstrate severe retinal degeneration resembling that of advanced RP.11–13, 33, 82, 91, 93 No eyes with early disease have been studied.
1. Light Microscopy
Light microscopy reveals normal conjunctiva, cornea, iris, angle, ciliary body, and lens.33, 93 There is near complete loss of photoreceptor cells, outer nuclear layer, and outer plexiform layer. Some of the retinal periphery may be spared.33 There is marked atrophy of the nerve fiber layer, ganglion cell layer, and optic nerve.33, 93 Glial fibrosis is pronounced, mostly in the nerve fiber layer, and especially around vessels.33 The remaining ganglion cells and some inner nuclear layer neurons are engorged with cytoplasmic granules. No granules are seen in remaining photoreceptor cells.11 In areas there is retinal pigment epithelial atrophy as well as hypertrophy. The remaining retinal pigment epithelium (RPE) contains fewer lipofuscin granules than controls and is devoid of melanin granules.11, 33, 93 RPE also contains PAS-positive material, confirmed by electron microscopy (EM) to be typical curvilinear inclusions.
2. Electron Microscopy
EM study of the conjunctiva discloses inclusions with multimembranous material and dense granular material in vascular endothelium, pericytes, fibrocytes, and Schwann cells. Inferior oblique muscle contains intracytoplasmic curvilinear inclusions between myofibrils. Iris fibrocytes, ciliary body pericytes, optic nerve head fibrous astrocytes, central and peripheral retinal ganglion cells, other retinal cells, macrophages in the retina, pericytes and endothelial cells of macular blood vessels, and RPE all contain atypical intracytoplasmic curvilinear or fingerprint inclusions. The corneal epithelial and endothelial cells, as well as the lens, do not contain inclusions.11, 33
B. GENETICS
Mutations in CLN3, a gene localized to chromosome 16p12 that encodes a 438-amino-acid protein of unknown function, underlie JNCL.23 To date over 30 CLN3 mutations have been reported, the most common (over 80% of cases) being a 1.02-kb deletion leading to skipping of exons 7 and 8 with early truncation of the CLN3 protein (www.ucl.ac.uk/ncl/).60–62 Studies have implicated CLN3, or its yeast homolog Btn1p, with lysosomal and/or vacuolar pH regulation, arginine transport defects, nitric oxide signaling, BMP synthesis, endocytic pathways, apoptosis, and autophagy.16, 27, 34, 39, 55, 66, 67, 71–74 Nevertheless, it appears likely that CLN3 is a lysosomal/endosomal protein that is trafficked through the endoplasmic reticulum and Golgi apparatus.69, 75 The only study addressing CLN3 localization in the retina utilized a peptide antibody raised to a portion of CLN3 in mice. The resulting immunohistochemistry pointed to a mitochondrial localization, predominantly in Müller cells and in neurons in the inner nuclear layer, with only moderate amounts in photoreceptor inner segment mitochondria.44 However, specificity of this antibody to CLN3 was not demonstrated. It is therefore also likely that CLN3 is indeed lysosomal/endosomal in retinal cell types.
Systematic characterization of CLN3-mice has revealed that murine models, particularly the Cln3−/− mouse,58 recapitulate the human disease. The clinical manifestations of vision loss, deteriorating motor function, and seizures likely result from specific biochemical and molecular disruptions in the neuronal and glial cell populations associated with these functions.50, 95, 96
While the function of CLN3 remains elusive, its loss may result in tissue-specific or cell-specific disease manifestations. While the exact sequence of cellular events in JNCL still requires further clarification, the clinical progression of the disease may be measured by the Unified Batten Disease Rating Scale (UBDRS).57 Neuropsychological examinations have added to our understanding.4, 5 Integrating these clinical findings with those from the mouse models for JNCL will be crucial in determining the precise pathophysiology.
C. ANIMAL MODELS
Model systems such as yeast, worms, flies and mice are providing invaluable information on the pathogenesis and molecular mechanisms underlying all the NCLs. Mammalian models exist in the form of sheep (CLN6),15 cow (CLN5),42 dog (CLN2 and CLN6),8, 28 and mouse (CTSD, CLN1, CLN2, CLN3, CLN5, CLN6 and CLN8).14, 19, 30, 36, 38, 45, 47, 48, 51, 54, 58, 77, 88–90 We will focus on mouse models of JNCL (CLN3) and summarize those studies that pertain to the visual system.
1. Cln3 Mice Models and Vision
Four different CLN3-mouse models have been generated.
a. Cln3−/− Loss of Function (LOF) Mouse
In 1999 Mitchison et al developed the first JNCL mouse model, which, being the first, has also been the most studied. Targeted replacement of exons 1-6 of CLN3 with a neo-cassette in reverse orientation in embryonic stem cells generated a null-allele. Accumulation of autofluorescent material, carbohydrate storage material, and apoptotic cell death were detected in these mice.36, 59 MRI studies have revealed global changes in the brains.37 These mice do not manifest retinal degeneration, suggesting that the presence of autofluorescent materials alone may not be sufficient to affect retinal function.87 The optic nerves are affected.84 A subsequent study of retinorecipient neurons revealed a specific loss of large projection neurons in the LGNd.96 These observations suggest that optic nerve degeneration and the loss of the LGNd large projection neurons may underlie or contribute to deterioration in retinal function.
b. Cln3Δex7-8 Knock-in Mouse Model
A second mouse model resembles the most common mutation in JNCL, the 1.02kb deletion.24 In this model a Lox-P flanked PGK neo-cassette was inserted between exons 7 and 8. These mice also recapitulate the ubiquitous presence of autofluorescent storage material. Interestingly, in older mice (10-17 months old), the Cln3Δex7-8 knock-in model demonstrates hypopigmentation of retinas and a significant decrease in the number of cone photoreceptors that directly correlates with the level of hypopigmentation.24
c. Cln3 Knock-out (Cln3−/−) Mouse Model
A third mouse model used a targeting vector to replace most of exon 7 and all of exon 8 of CLN3 with a neo cassette.47 These mice also show the characteristic autofluorescent storage material in the CNS. Electrophysiologic testing of 12-14-month-old mice showed a significant reduction in the scotopic b-wave, with no difference in the average a-wave amplitude, resulting in a decrease of the b-wave/a-wave ratio. The average photopic ERG in 12-month-old mice was significantly decreased when compared to normal controls. The 24-month-old Cln3−/− mice exhibited slower pupillary light reflex responses at threshold light levels. At maximal light levels, a biphasic response was present, with rapid initiation followed by continued slow constriction that persisted after the light stimulus had ended.
Decreased mean photoreceptor cell density and substantial loss of cells within the inner nuclear layer (INL) were present in the retinas of the Cln3−/− mice as compared to age-matched controls. Considerable axonal loss, along with decrease in optic nerve cross-sectional area, were present, similar to that seen in the Cln3−/− mice.46
d. Cln3 Knock-in Reporter Mouse Model
Eliason et al30 recently reported the generation of a CLN3 knock-in mouse model. The nuclear localized beta-galactosidase and a 3’ simian virus 40 polyadenylation sequence, followed by a neomycin coding sequence driven by the thymidine kinase promoter and exons 9 –13, were ligated into the targeting plasmid just downstream of the neomycin cassette. This mouse model identifies the endogenous expression of CLN3 in the different tissues. The authors report clear expression in the retina; however, further studies are required to determine the extent, if any, of the damage produced by loss of the functional protein.
III. Clinical Presentation
A. CLINICAL HISTORY
Vision loss, including night blindness, photophobia, and loss of peripheral and color vision, is the most common presenting symptom of JNCL.56, 64, 92 The mean age of onset of visual loss has been estimated to be between 6.4-6.6 years,13, 22 with presentation to an ophthalmologist at 5.5-8.5 years (mean 7.3 years).22
Onset of further neurological decline is variable, being from 1 to 9 years from the initial presentation of deteriorating vision.13, 86 Typically, the disease is characterized by increasing frequency of seizures and a slow decline in cognitive and motor function. JNCL is invariably fatal.40 MRI of the brain is usually normal under the age of 10 years; however, during the subsequent years, variable cerebral and cerebellar atrophy appears, the thalamus may show abnormally low signal intensity and periventricular white matter may show high signal intensity on T2-weighted images.82, 99
B. CLINICAL EXAM
A precipitous decline in vision is a characteristic feature of JNCL with legal blindness diagnosed within 1-2 years of presentation.12, 13, 22, 82, 91 There are no anterior segment abnormalities described in JNCL.43, 93
1. Ocular Motility
Children have been noted to have eccentric viewing or ‘overlooking,’ holding the eyes in a raised position when attempting to fixate.22, 91 This feature is attributed to relative preservation of the superior peripheral retina with loss of central and inferior visual field, perhaps due to phototoxicity.35 Rotary nystagmus may be present on all fields of gaze.43
2. Retina and Optic Nerve
Fundus examination at presentation may range from normal to a severe pigmentary retinopathy. Early in the disease, subtle granularity of the retinal pigment epithelium in the central macula may be present, although a bull’s eye maculopathy is classically described. Later in the disease, optic nerve atrophy, vascular attenuation, and pigment accumulation in the peripheral retina develops. The rate of progression is extremely rapid in comparison to other retinal degenerations.12, 13, 22, 29, 33, 43, 56, 86, 91 .
IV. Differential Diagnosis
The differential diagnosis of JNCL should include all diseases that produce bilateral visual loss in childhood, including Stargardt disease, fundus albipunctatus, retinitis punctata albescens, retinitis pigmentosa, drusen, cone or cone-rod dystrophy, chloroquine/hydroxychloroquine maculopathy, and medically-unexplained visual loss. It is important to note that initial, erroneous diagnoses by ophthalmologists have included many of the above.13, 22
V.Diagnostic Testing
A. ELECTROPHYSIOLOGIC TESTING
Characteristic EEG findings include large amplitude and slow wave and spike complexes.22 Electroretinogram (ERG) generally shows profound loss of amplitude at presentation, particularly under scotopic conditions. The loss of b-wave amplitude is greater than the decrease in a-wave amplitude, creating an electronegative configuration.22, 97 In early cases, when the visual acuity is normal, ERG may show a normal a-wave and a reduced b-wave; however, repeat testing shows rapidly progressive loss of b-wave amplitude.41 The relative sparing of the a-wave, indicating that the primary lesion of the retina may be in the inner layers, is consistent with the inner retinal localization of gene product CLN3 found in animal models.44
B. LABORATORY TESTING
Vacuolated lymphocytes are found in the peripheral blood of all patients with JNCL.91 In addition, the deposited autofluorescent ceroid material accumulates in many tissue types and has a characteristic ultrastructural appearance when seen with electron microscopy (see Histopathology above).
C. OTHER CLINICAL TESTING
Fluorescein angiography may define pigmentary disturbances more clearly. In vivo autofluorescence levels have been described to be very low.56 While not truly diagnostic, variable cerebral and cerebellar atrophy may appear, typically after the age of 10. The thalamus may show abnormally low signal intensity, and periventricular white matter may show high signal intensity on T2-weighted images.6, 7, 82, 99 There may also be evidence of bilateral mild optic nerve and tract atrophy without cortical involvement.13, 22
VI. Diagnosis
Advances in genetic and biochemical understanding of JNCL have lead to less invasive and more efficient methods of diagnosis. Before the common disease alleles were identified, diagnosis relied on the presenting clinical features along with histopathologic examination of peripheral blood and tissue biopsied from either the conjunctiva or rectum (see Histopathology above). DNA tests for common disease alleles are now well established, enabling robust carrier and patient diagnosis. Prenatal diagnosis has also been performed using PCR to identify an allele-specific intragenic microsatellite marker in a chorionic villus sample.63 Over 80% of affected individuals are homozygotes for the 1.02 kb deletion,62 and nearly all of the remainder are compound heterozygotes for the 1.02 kb deletion and another missense or nonsense disease-causing mutation. A homogeneous PCR nucleobase quenching assay has been developed to detect the 1.02 kb deletion.81 In this method, DNA isolated from blood samples or patient fibroblasts is probed with a 6-FAM fluorophore, which distinguishes between normal CLN3 and the common deletion of the CLN3 gene. The 6-FAM fluorophore decreases signal when paired to the amplicon by quenching of guanosine residues. Upon denaturation, the fluorescent signal increases and is detectible.25 This technique offers fast, accurate, and relatively inexpensive determination of the 1.02 kb mutation. Detection of other, less common mutations requires nucleotide sequencing. The homogenous PCR nucleobase quenching assay technique should be an early step in determining the genotype and diagnosis of patients suspected of having JNCL. Refinement of these techniques has now enabled rapid diagnosis of JNCL, independent of the mutation, using a DNA sample obtained from a buccal swab.
VII. Treatment
Currently, all treatment for JNCL is symptomatic. Psychotic, affective, and schizophreniform features are managed with citalopram, risperidone, olanzapine, or quetiapine.9 Reports of decreased D1 dopamine transporter density in JNCL by PET, 1, 80 along with similar findings in one of the Cln3−/− mouse models,95 suggest that JNCL patients may have a dysregulation of the dopamine system, offering a rationale for a trial of dopaminergic medications.3
Seizures are treated with anticonvulsants, including, but not limited to, valproic acid, carbamazepine, lamotrigine and clonazepam.1, 2, 3, 83 Regardless of the specific anticonvulsant used, many JNCL patients’ seizures are well controlled.
Murine models may point to new treatment strategies. The autoimmune response first reported in the mouse models is recapitulated in humans, which suggests that targeted immunosuppression might have some benefits.52, 53, 70 Treating Cln3−/− mice with a non-competitive AMPA receptor inhibitor ameliorated motor coordination deficits, and these drugs might also have the potential to slow human disease.49
A standardized method to evaluate JNCL patients is crucial, particularly as potential new therapies emerge.10 Marshall et al have developed and tested a multimodal clinical rating instrument, the Unified Batten Disease Rating Scale (UBDRS).57 This tool will allow for better monitoring of the evolution of the disease and assessment of the effects of therapeutic interventions.
VIII. Genetic Counseling
The risk of a second child being affected in families with one affected child is 25%. Timely recognition of the diagnosis is therefore critical for the genetic counseling. In addition, early detection and definition of the gene and mutations responsible for JNCL may become important as new therapeutic strategies emerge and clinical trials are undertake. Most mutations can be rapidly identified through a simple PCR reaction, and the rest are defined by sequencing DNA obtained from a buccal swab.
IX. Conclusion and Future Directions
No unifying hypothesis presently exists to explain the molecular mechanisms leading to JNCL. It is not known if storage of lipopigment is a cause or effect of disease.
The development of visual failure in JNCL is poorly understood. It is possible that the accumulation of lipofuscin-like pigment contributes to the progression of disease and ‘overwhelms’ the RPE, whose job it is to renew photoreceptor outer segments. It is also possible that the accumulations are cytotoxic to neighboring cells. Perhaps the primary site of damage is not in the outer retina, as suggested by the electronegative ERG97 and the inner retinal localization of gene product CLN3,44 but rather a primary degeneration of the LGNd and cortical lesions with retrograde degeneration.96 JNCL may involve neurodegenerative processes similar to those seen in glaucoma patients. In addition, the role of a neuroimmune response needs further definition.
To understand the mechanisms underlying retinal degeneration in JNCL, it is necessary to better characterize retinal changes in the early stages of disease. Research into the composition and time course of appearance of retinal lipofuscin-like accumulations will provide further clues to pathogenesis of vision failure. Much of the research will rely on animal models that should allow characterization of the role played by the CLN3 protein. The use of non-invasive imaging techniques, such as adaptive optics imaging, to assess patients early in the disease course, may clarity the primary site of retinal disease. Further areas of study include the role of the autoimmune response in vision failure and the role of activated microglia in diseased retinas. With help from ophthalmologists, children will be diagnosed earlier, and clues to the pathogenesis of vision failure may be uncovered.
X. Method of Literature Search
The PubMed database was searched from 1950 to 2007 using the search terms “Batten disease and eye”, “Juvenile neuronal ceroid lipofuscinosis and eye”, and “Neuronal ceroid lipofuscinosis and eye”. English abstracts of non-English articles of significant clinical interest were included where relevant. Additional references of clinical relevance were taken from selected articles.
Acknowledgments
The authors wish to thank Dr. Richard Libby for preparing the retinal image of autofluorescence in JNCL and Amanda Getty for help in preparing the manuscript. Tissue was supplied from the Los Angeles Brain Bank. Supported by R01 NS43310.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Aberg L, Liewendahl K, Nikkinen P, et al. Decreased striatal dopamine transporter density in jncl patients with parkinsonian symptoms. Neurology. 2000;54(5):1069–1074. doi: 10.1212/wnl.54.5.1069. [DOI] [PubMed] [Google Scholar]
- 2.Aberg LE, Backman M, Kirveskari E, et al. Epilepsy and antiepileptic drug therapy in juvenile neuronal ceroid lipofuscinosis. Epilepsia. 2000;41(10):1296–1302. doi: 10.1111/j.1528-1157.2000.tb04608.x. [DOI] [PubMed] [Google Scholar]
- 3.Aberg LE, Rinne JO, Rajantie I, et al. A favorable response to antiparkinsonian treatment in juvenile neuronal ceroid lipofuscinosis. Neurology. 2001;56(9):1236–1239. doi: 10.1212/wnl.56.9.1236. [DOI] [PubMed] [Google Scholar]
- 4.Adams H, de Blieck EA, Mink JW, et al. Standardized assessment of behavior and adaptive living skills in juvenile neuronal ceroid lipofuscinosis. Dev Med Child Neurol. 2006;48(4):259–264. doi: 10.1017/S0012162206000570. [DOI] [PubMed] [Google Scholar]
- 5.Adams HR, Kwon J, Marshall FJ, et al. Neuropsychological symptoms of juvenile-onset batten disease: Experiences from 2 studies. J Child Neurol. 2007;22(5):621–627. doi: 10.1177/0883073807302603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Autti T, Raininko R, Santavuori P, et al. Mri of neuronal ceroid lipofuscinosis. Ii. Postmortem mri and histopathological study of the brain in 16 cases of neuronal ceroid lipofuscinosis of juvenile or late infantile type. Neuroradiology. 1997;39(5):371–377. doi: 10.1007/s002340050427. [DOI] [PubMed] [Google Scholar]
- 7.Autti T, Raininko R, Vanhanen SL, et al. Mri of neuronal ceroid lipofuscinosis. I. Cranial mri of 30 patients with juvenile neuronal ceroid lipofuscinosis. Neuroradiology. 1996;38(5):476–482. doi: 10.1007/BF00607283. [DOI] [PubMed] [Google Scholar]
- 8.Awano T, Katz ML, O'Brien DP, et al. A frame shift mutation in canine tpp1 (the ortholog of human cln2) in a juvenile dachshund with neuronal ceroid lipofuscinosis. Mol Genet Metab. 2006;89(3):254–260. doi: 10.1016/j.ymgme.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Backman ML, Aberg LE, Aronen ET, et al. New antidepressive and antipsychotic drugs in juvenile neuronal ceroid lipofuscinoses--a pilot study. Eur J Paediatr Neurol. 2001;5(Suppl A):163–166. doi: 10.1053/ejpn.2000.0455. [DOI] [PubMed] [Google Scholar]
- 10.Backman ML, Santavuori PR, Aberg LE, et al. Psychiatric symptoms of children and adolescents with juvenile neuronal ceroid lipofuscinosis. J Intellect Disabil Res. 2005;49(Pt 1):25–32. doi: 10.1111/j.1365-2788.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 11.Bensaoula T, Shibuya H, Katz ML, et al. Histopathologic and immunocytochemical analysis of the retina and ocular tissues in batten disease. Ophthalmology. 2000;107(9):1746–1753. doi: 10.1016/s0161-6420(00)00264-5. [DOI] [PubMed] [Google Scholar]
- 12.Birch DG. Retinal degeneration in retinitis pigmentosa and neuronal ceroid lipofuscinosis: An overview. Mol Genet Metab. 1999;66(4):356–366. doi: 10.1006/mgme.1999.2829. [DOI] [PubMed] [Google Scholar]
- 13.Bohra LI, Weizer JS, Lee AG, et al. Vision loss as the presenting sign in juvenile neuronal ceroid lipofuscinosis. J Neuroophthalmol. 2000;20(2):111–115. doi: 10.1097/00041327-200020020-00010. [DOI] [PubMed] [Google Scholar]
- 14.Bronson RT, Lake BD, Cook S, et al. Motor neuron degeneration of mice is a model of neuronal ceroid lipofuscinosis (batten's disease) Ann Neurol. 1993;33(4):381–385. doi: 10.1002/ana.410330408. [DOI] [PubMed] [Google Scholar]
- 15.Broom MF, Zhou C, Broom JE, et al. Ovine neuronal ceroid lipofuscinosis: A large animal model syntenic with the human neuronal ceroid lipofuscinosis variant cln6. J Med Genet. 1998;35(9):717–721. doi: 10.1136/jmg.35.9.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Y, Espinola JA, Fossale E, et al. Autophagy is disrupted in a knock-in mouse model of juvenile neuronal ceroid lipofuscinosis. J Biol Chem. 2006;281(29):20483–20493. doi: 10.1074/jbc.M602180200. [DOI] [PubMed] [Google Scholar]
- 17.Castaneda JA, Lim MJ, Cooper JD, et al. Immune system irregularities in lysosomal storage disorders. Acta Neuropathol. 2008 Feb;115(2):159–174. doi: 10.1007/s00401-007-0296-4. [DOI] [PubMed] [Google Scholar]
- 18.Castaneda JA, Pearce DA. Identification of alpha-fetoprotein as an autoantigen in juvenile batten disease. Neurobiol Dis. 2008;29(1):92–102. doi: 10.1016/j.nbd.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Chang B, Bronson RT, Hawes NL, et al. Retinal degeneration in motor neuron degeneration: A mouse model of ceroid lipofuscinosis. Invest Ophthalmol Vis Sci. 1994;35(3):1071–1076. [PubMed] [Google Scholar]
- 20.Chattopadhyay S, Ito M, Cooper JD, et al. An autoantibody inhibitory to glutamic acid decarboxylase in the neurodegenerative disorder batten disease. Hum Mol Genet. 2002;11(12):1421–1431. doi: 10.1093/hmg/11.12.1421. [DOI] [PubMed] [Google Scholar]
- 21.Chattopadhyay S, Pearce DA. Interaction with btn2p is required for localization of rsglp: Btn2p-mediated changes in arginine uptake in saccharomyces cerevisiae. Eukaryot Cell. 2002;1(4):606–612. doi: 10.1128/EC.1.4.606-612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins J, Holder GE, Herbert H, et al. Batten disease: Features to facilitate early diagnosis. Br J Ophthalmol. 2006;90(9):1119–1124. doi: 10.1136/bjo.2006.091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Consortium BD. Isolation of a novel gene underlying batten disease, cln3. The international batten disease consortium. Cell. 1995;82(6):949–957. doi: 10.1016/0092-8674(95)90274-0. [DOI] [PubMed] [Google Scholar]
- 24.Cotman SL, Vrbanac V, Lebel LA, et al. Cln3(deltaex7/8) knock-in mice with the common jncl mutation exhibit progressive neurologic disease that begins before birth. Hum Mol Genet. 2002;11(22):2709–2721. doi: 10.1093/hmg/11.22.2709. [DOI] [PubMed] [Google Scholar]
- 25.Crockett AO, Wittwer CT. Fluorescein-labeled oligonucleotides for real-time pcr: Using the inherent quenching of deoxyguanosine nucleotides. Anal Biochem. 2001;290(1):89–97. doi: 10.1006/abio.2000.4957. [DOI] [PubMed] [Google Scholar]
- 26.de los Reyes E, Dyken PR, Phillips P, et al. Profound infantile neuroretinal dysfunction in a heterozygote for the cln3 genetic defect. J Child Neurol. 2004;19(1):42–46. doi: 10.1177/08830738040190010703. [DOI] [PubMed] [Google Scholar]
- 27.Dhar S, Bitting RL, Rylova SN, et al. Flupirtine blocks apoptosis in batten patient lymphoblasts and in human postmitotic cln3- and cln2-deficient neurons. Ann Neurol. 2002;51(4):448–466. doi: 10.1002/ana.10143. [DOI] [PubMed] [Google Scholar]
- 28.Drogemuller C, Wohlke A, Distl O. Characterization of candidate genes for neuronal ceroid lipofuscinosis in dog. J Hered. 2005;96(7):735–738. doi: 10.1093/jhered/esi088. [DOI] [PubMed] [Google Scholar]
- 29.Eksandh LC, Ponjavic V, Ayyagari R, et al. Phenotypic expression of juvenile x-linked retinoschisis in swedish families with different mutations in the xlrs1 gene. Arch Ophthalmol. 2000;118(8):1098–1104. doi: 10.1001/archopht.118.8.1098. [DOI] [PubMed] [Google Scholar]
- 30.Eliason SL, Stein CS, Mao Q, et al. A knock-in reporter model of batten disease. J Neurosci. 2007;27(37):9826–9834. doi: 10.1523/JNEUROSCI.1710-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goebel HH. The neuronal ceroid-lipofuscinoses. Semin Pediatr Neurol. 1996;3(4):270–278. doi: 10.1016/s1071-9091(96)80031-3. [DOI] [PubMed] [Google Scholar]
- 32.Goebel HH, Braak H, Seidel D, et al. Morphologic studies on adult neuronal-ceroid lipofuscinosis (ncl) Clin Neuropathol. 1982;1(4):151–162. [PubMed] [Google Scholar]
- 33.Goebel HH, Fix JD, Zeman W. The fine structure of the retina in neuronal ceroid-lipofuscinosis. Am J Ophthalmol. 1974;77(1):25–39. doi: 10.1016/0002-9394(74)90601-1. [DOI] [PubMed] [Google Scholar]
- 34.Golabek AA, Kida E, Walus M, et al. Cln3 protein regulates lysosomal ph and alters intracellular processing of alzheimer's amyloid-beta protein precursor and cathepsin d in human cells. Mol Genet Metab. 2000;70(3):203–213. doi: 10.1006/mgme.2000.3006. [DOI] [PubMed] [Google Scholar]
- 35.Good WV, Crain LS, Quint RD, et al. Overlooking: A sign of bilateral central scotomata in children. Dev Med Child Neurol. 1992;34(1):69–73. doi: 10.1111/j.1469-8749.1992.tb08566.x. [DOI] [PubMed] [Google Scholar]
- 36.Greene ND, Bernard DL, Taschner PE, et al. A murine model for juvenile ncl: Gene targeting of mouse cln3. Mol Genet Metab. 1999;66(4):309–313. doi: 10.1006/mgme.1999.2828. [DOI] [PubMed] [Google Scholar]
- 37.Greene ND, Lythgoe MF, Thomas DL, et al. High resolution mri reveals global changes in brains of cln3 mutant mice. Eur J Paediatr Neurol. 2001;5(Suppl A):103–107. doi: 10.1053/ejpn.2000.0444. [DOI] [PubMed] [Google Scholar]
- 38.Gupta P, Soyombo AA, Atashband A, et al. Disruption of ppt1 or ppt2 causes neuronal ceroid lipofuscinosis in knockout mice. Proc Natl Acad Sci U S A. 2001;98(24):13566–13571. doi: 10.1073/pnas.251485198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hobert JA, Dawson G. A novel role of the batten disease gene cln3: Association with bmp synthesis. Biochem Biophys Res Commun. 2007;358(1):111–116. doi: 10.1016/j.bbrc.2007.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofman IKA, Santavuori P, Gottlob I, et al. The neuronal ceroid lipofuscinoses (batten disease) Amsterdam, The Netherlands: IOS Press; 1999. [Google Scholar]
- 41.Horiguchi M, Miyake Y. Batten disease--deteriorating course of ocular findings. Jpn J Ophthalmol. 1992;36(1):91–96. [PubMed] [Google Scholar]
- 42.Houweling PJ, Cavanagh JA, Palmer DN, et al. Neuronal ceroid lipofuscinosis in devon cattle is caused by a single base duplication (c.662dupg) in the bovine cln5 gene. Biochim Biophys Acta. 2006;1762(10):890–897. doi: 10.1016/j.bbadis.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Inan C, Wong D, Wisniewski KE, et al. First african-american child with juvenile neuronal ceroid lipofuscinosis. Am J Med Genet. 1998;79(5):335–336. doi: 10.1002/(sici)1096-8628(19981012)79:5<335::aid-ajmg1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 44.Katz ML, Gao CL, Prabhakaram M, et al. Immunochemical localization of the batten disease (cln3) protein in retina. Invest Ophthalmol Vis Sci. 1997;38(11):2375–2386. [PubMed] [Google Scholar]
- 45.Katz ML, Johnson GS. Mouse gene knockout models for the cln2 and cln3 forms of ceroid lipofuscinosis. Eur J Paediatr Neurol. 2001;5(Suppl A):109–114. doi: 10.1053/ejpn.2000.0445. [DOI] [PubMed] [Google Scholar]
- 46.Katz ML, Johnson GS, Tullis GE, et al. Phenotypic characterization of a mouse model of juvenile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2008;29(2):242–253. doi: 10.1016/j.nbd.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katz ML, Shibuya H, Liu PC, et al. A mouse gene knockout model for juvenile ceroid-lipofuscinosis (batten disease) J Neurosci Res. 1999;57(4):551–556. [PubMed] [Google Scholar]
- 48.Kopra O, Vesa J, von Schantz C, et al. A mouse model for finnish variant late infantile neuronal ceroid lipofuscinosis, cln5, reveals neuropathology associated with early aging. Hum Mol Genet. 2004;13(23):2893–2906. doi: 10.1093/hmg/ddh312. [DOI] [PubMed] [Google Scholar]
- 49.Kovacs AD, Pearce DA. Attenuation of ampa receptor activity improves motor skills in a mouse model of juvenile batten disease. Exp Neurol. 2008;209(1):288–291. doi: 10.1016/j.expneurol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kovacs AD, Weimer JM, Pearce DA. Selectively increased sensitivity of cerebellar granule cells to ampa receptor-mediated excitotoxicity in a mouse model of batten disease. Neurobiol Dis. 2006;22(3):575–585. doi: 10.1016/j.nbd.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 51.Lee RL, Johnson KR, Lerner TJ. Isolation and chromosomal mapping of a mouse homolog of the batten disease gene cln3. Genomics. 1996;35(3):617–619. doi: 10.1006/geno.1996.0410. [DOI] [PubMed] [Google Scholar]
- 52.Lim MJ, Alexander N, Benedict JW, et al. Igg entry and deposition are components of the neuroimmune response in batten disease. Neurobiol Dis. 2007;25(2):239–251. doi: 10.1016/j.nbd.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Lim MJ, Beake J, Bible E, et al. Distinct patterns of serum immunoreactivity as evidence for multiple brain-directed autoantibodies in juvenile neuronal ceroid lipofuscinosis. Neuropathol Appl Neurobiol. 2006;32(5):469–482. doi: 10.1111/j.1365-2990.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 54.Lonka L, Kyttala A, Ranta S, et al. The neuronal ceroid lipofuscinosis cln8 membrane protein is a resident of the endoplasmic reticulum. Hum Mol Genet. 2000;9(11):1691–1697. doi: 10.1093/hmg/9.11.1691. [DOI] [PubMed] [Google Scholar]
- 55.Luiro K, Yliannala K, Ahtiainen L, et al. Interconnections of cln3, hook1 and rab proteins link batten disease to defects in the endocytic pathway. Hum Mol Genet. 2004;13(23):3017–3027. doi: 10.1093/hmg/ddh321. [DOI] [PubMed] [Google Scholar]
- 56.Mantel I, Brantley MA, Jr, Bellmann C, et al. Juvenile neuronal ceroid lipofuscinosis (batten disease) cln3 mutation (chrom 16p11.2) with different phenotypes in a sibling pair and low intensity in vivo autofluorescence. Klin Monatsbl Augenheilkd. 2004;221(5):427–430. doi: 10.1055/s-2004-812819. [DOI] [PubMed] [Google Scholar]
- 57.Marshall FJ, de Blieck EA, Mink JW, et al. A clinical rating scale for batten disease: Reliable and relevant for clinical trials. Neurology. 2005;65(2):275–279. doi: 10.1212/01.wnl.0000169019.41332.8a. [DOI] [PubMed] [Google Scholar]
- 58.Mitchison HM, Bernard DJ, Greene ND, et al. Targeted disruption of the cln3 gene provides a mouse model for batten disease. The batten mouse model consortium [corrected] Neurobiol Dis. 1999;6(5):321–334. doi: 10.1006/nbdi.1999.0267. [DOI] [PubMed] [Google Scholar]
- 59.Mitchison HM, Lim MJ, Cooper JD. Selectivity and types of cell death in the neuronal ceroid lipofuscinoses. Brain Pathol. 2004;14(1):86–96. doi: 10.1111/j.1750-3639.2004.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mole SE, Mitchison HM, Munroe PB. Molecular basis of the neuronal ceroid lipofuscinoses: Mutations in cln1, cln2, cln3, and cln5. Hum Mutat. 1999;14(3):199–215. doi: 10.1002/(SICI)1098-1004(1999)14:3<199::AID-HUMU3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 61.Mole SE, Zhong NA, Sarpong A, et al. New mutations in the neuronal ceroid lipofuscinosis genes. Eur J Paediatr Neurol. 2001;5(Suppl A):7–10. doi: 10.1053/ejpn.2000.0427. [DOI] [PubMed] [Google Scholar]
- 62.Munroe PB, Mitchison HM, O'Rawe AM, et al. Spectrum of mutations in the batten disease gene, cln3. Am J Hum Genet. 1997;61(2):310–316. doi: 10.1086/514846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munroe PB, Rapola J, Mitchison HM, et al. Prenatal diagnosis of batten's disease. Lancet. 1996;347(9007):1014–1015. doi: 10.1016/s0140-6736(96)90148-8. [DOI] [PubMed] [Google Scholar]
- 64.Nardocci N, Verga ML, Binelli S, et al. Neuronal ceroid-lipofuscinosis: A clinical and morphological study of 19 patients. Am J Med Genet. 1995;57(2):137–141. doi: 10.1002/ajmg.1320570205. [DOI] [PubMed] [Google Scholar]
- 65.Nijssen PC, Ceuterick C, van Diggelen OP, et al. Autosomal dominant adult neuronal ceroid lipofuscinosis: A novel form of ncl with granular osmiophilic deposits without palmitoyl protein thioesterase 1 deficiency. Brain Pathol. 2003;13(4):574–581. doi: 10.1111/j.1750-3639.2003.tb00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Osorio NS, Carvalho A, Almeida AJ, et al. Nitric oxide signaling is disrupted in the yeast model for batten disease. Mol Biol Cell. 2007;18(7):2755–2767. doi: 10.1091/mbc.E06-11-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Padilla-Lopez S, Pearce DA. Saccharomyces cerevisiae lacking btn1p modulate vacuolar atpase activity to regulate ph imbalance in the vacuole. J Biol Chem. 2006;281(15):10273–10280. doi: 10.1074/jbc.M510625200. [DOI] [PubMed] [Google Scholar]
- 68.Palmer DN, Bayliss SL, Westlake VJ. Batten disease and the atp synthase subunit c turnover pathway: Raising antibodies to subunit c. Am J Med Genet. 1995;57(2):260–265. doi: 10.1002/ajmg.1320570230. [DOI] [PubMed] [Google Scholar]
- 69.Pearce DA. Localization and processing of cln3, the protein associated to batten disease: Where is it and what does it do? J Neurosci Res. 2000;59(1):19–23. [PubMed] [Google Scholar]
- 70.Pearce DA, Atkinson M, Tagle DA. Glutamic acid decarboxylase autoimmunity in batten disease and other disorders. Neurology. 2004;63(11):2001–2005. doi: 10.1212/01.wnl.0000145836.72059.3b. [DOI] [PubMed] [Google Scholar]
- 71.Pearce DA, Carr CJ, Das B, et al. Phenotypic reversal of the btn1 defects in yeast by chloroquine: A yeast model for batten disease. Proc Natl Acad Sci U S A. 1999;96(20):11341–11345. doi: 10.1073/pnas.96.20.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pearce DA, Ferea T, Nosel SA, et al. Action of btn1, the yeast orthologue of the gene mutated in batten disease. Nat Genet. 1999;22(1):55–58. doi: 10.1038/8861. [DOI] [PubMed] [Google Scholar]
- 73.Pearce DA, Nosel SA, Sherman F. Studies of ph regulation by btn1p, the yeast homolog of human cln3p. Mol Genet Metab. 1999;66(4):320–323. doi: 10.1006/mgme.1999.2819. [DOI] [PubMed] [Google Scholar]
- 74.Persaud-Sawin DA, Boustany RM. Cell death pathways in juvenile batten disease. Apoptosis. 2005;10(5):973–985. doi: 10.1007/s10495-005-0733-6. [DOI] [PubMed] [Google Scholar]
- 75.Phillips SN, Benedict JW, Weimer JM, et al. Cln3, the protein associated with batten disease: Structure, function and localization. J Neurosci Res. 2005;79(5):573–583. doi: 10.1002/jnr.20367. [DOI] [PubMed] [Google Scholar]
- 76.Pontikis CC, Cella CV, Parihar N, et al. Late onset neurodegeneration in the cln3−/− mouse model of juvenile neuronal ceroid lipofuscinosis is preceded by low level glial activation. Brain Res. 2004;1023(2):231–242. doi: 10.1016/j.brainres.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 77.Ranta S, Zhang Y, Ross B, et al. The neuronal ceroid lipofuscinoses in human epmr and mnd mutant mice are associated with mutations in cln8. Nat Genet. 1999;23(2):233–236. doi: 10.1038/13868. [DOI] [PubMed] [Google Scholar]
- 78.Read WK, Bridges CH. Neuronal lipodystrophy. Occurrence in an inbred strain of cattle. Pathol Vet. 1969;6(3):235–243. doi: 10.1177/030098586900600305. [DOI] [PubMed] [Google Scholar]
- 79.Rider JA, Rider DL. Batten disease: Past, present, and future. Am J Med Genet. 1988;(Suppl 5):21–26. doi: 10.1002/ajmg.1320310606. [DOI] [PubMed] [Google Scholar]
- 80.Rinne JO, Ruottinen HM, Nagren K, et al. Positron emission tomography shows reduced striatal dopamine d1 but not d2 receptors in juvenile neuronal ceroid lipofuscinosis. Neuropediatrics. 2002;33(3):138–141. doi: 10.1055/s-2002-33677. [DOI] [PubMed] [Google Scholar]
- 81.Rothberg PG, Ramirez-Montealegre D, Frazier SD, et al. Homogeneous polymerase chain reaction nucleobase quenching assay to detect the 1-kbp deletion in cln3 that causes batten disease. J Mol Diagn. 2004;6(3):260–263. doi: 10.1016/S1525-1578(10)60519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruther K, Gal A, Kohlschutter A. [the role of the ophthalmologist in the management of juvenile neuronal ceroid lipofuscinosis] Klin Monatsbl Augenheilkd. 2006;223(6):542–544. doi: 10.1055/s-2005-859019. [DOI] [PubMed] [Google Scholar]
- 83.Santavuori P, Lauronen L, Kirveskari E, et al. Neuronal ceroid lipofuscinoses in childhood. Neurol Sci. 2000;21(3 Suppl):S35–S41. doi: 10.1007/s100720070038. [DOI] [PubMed] [Google Scholar]
- 84.Sappington RM, Pearce DA, Calkins DJ. Optic nerve degeneration in a murine model of juvenile ceroid lipofuscinosis. Invest Ophthalmol Vis Sci. 2003;44(9):3725–3731. doi: 10.1167/iovs.03-0039. [DOI] [PubMed] [Google Scholar]
- 85.Seehafer SS, Pearce DA. You say lipofuscin, we say ceroid: Defining autofluorescent storage material. Neurobiol Aging. 2006;27(4):576–588. doi: 10.1016/j.neurobiolaging.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 86.Seeliger M, Ruther K, Apfelstedt-Sylla E, et al. [juvenile neuronal ceroid lipofuscinosis (batten-mayou) disease. Ophthalmologic diagnosis and findings] Ophthalmologe. 1997;94(8):557–562. doi: 10.1007/s003470050158. [DOI] [PubMed] [Google Scholar]
- 87.Seigel GM, Lotery A, Kummer A, et al. Retinal pathology and function in a cln3 knockout mouse model of juvenile neuronal ceroid lipofuscinosis (batten disease) Mol Cell Neurosci. 2002;19(4):515–527. doi: 10.1006/mcne.2001.1099. [DOI] [PubMed] [Google Scholar]
- 88.Shacka JJ, Klocke BJ, Young C, et al. Cathepsin d deficiency induces persistent neurodegeneration in the absence of bax-dependent apoptosis. J Neurosci. 2007;27(8):2081–2090. doi: 10.1523/JNEUROSCI.5577-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shacka JJ, Roth KA. Cathepsin deficiency as a model for neuronal ceroid lipofuscinoses. Am J Pathol. 2005;167(6):1473–1476. doi: 10.1016/S0002-9440(10)61233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sleat DE, Wiseman JA, El-Banna M, et al. A mouse model of classical late-infantile neuronal ceroid lipofuscinosis based on targeted disruption of the cln2 gene results in a loss of tripeptidyl-peptidase i activity and progressive neurodegeneration. J Neurosci. 2004;24(41):9117–9126. doi: 10.1523/JNEUROSCI.2729-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spalton DJ, Taylor DS, Sanders MD. Juvenile batten's disease: An ophthalmological assessment of 26 patients. Br J Ophthalmol. 1980;64(10):726–732. doi: 10.1136/bjo.64.10.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taratuto AL, Saccoliti M, Sevlever G, et al. Childhood neuronal ceroid-lipofuscinoses in argentina. Am J Med Genet. 1995;57(2):144–149. doi: 10.1002/ajmg.1320570207. [DOI] [PubMed] [Google Scholar]
- 93.Traboulsi EI, Green WR, Luckenbach MW, et al. Neuronal ceroid lipofuscinosis. Ocular histopathologic and electron microscopic studies in the late infantile, juvenile, and adult forms. Graefes Arch Clin Exp Ophthalmol. 1987;225(6):391–402. doi: 10.1007/BF02334164. [DOI] [PubMed] [Google Scholar]
- 94.Uvebrant P, Hagberg B. Neuronal ceroid lipofuscinoses in scandinavia. Epidemiology and clinical pictures. Neuropediatrics. 1997;28(1):6–8. doi: 10.1055/s-2007-973654. [DOI] [PubMed] [Google Scholar]
- 95.Weimer JM, Benedict JW, Elshatory YM, et al. Alterations in striatal dopamine catabolism precede loss of substantia nigra neurons in a mouse model of juvenile neuronal ceroid lipofuscinosis. Brain Res. 2007;1162:98–112. doi: 10.1016/j.brainres.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weimer JM, Custer AW, Benedict JW, et al. Visual deficits in a mouse model of batten disease are the result of optic nerve degeneration and loss of dorsal lateral geniculate thalamic neurons. Neurobiol Dis. 2006;22(2):284–293. doi: 10.1016/j.nbd.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weleber RG. The dystrophic retina in multisystem disorders: The electroretinogram in neuronal ceroid lipofuscinoses. Eye. 1998;12(pt 3b):580–590. doi: 10.1038/eye.1998.148. [DOI] [PubMed] [Google Scholar]
- 98.Westlake VJ, Jolly RD, Bayliss SL, et al. Immunocytochemical studies in the ceroid-lipofuscinoses (batten disease) using antibodies to subunit c of mitochondrial atp synthase. Am J Med Genet. 1995;57(2):177–181. doi: 10.1002/ajmg.1320570214. [DOI] [PubMed] [Google Scholar]
- 99.Williams RE, Aberg L, Autti T, et al. Diagnosis of the neuronal ceroid lipofuscinoses: An update. Biochim Biophys Acta. 2006;1762(10):865–872. doi: 10.1016/j.bbadis.2006.07.001. [DOI] [PubMed] [Google Scholar]