Abstract
A series of new hexadentate and pentadentate chelators were designed and synthesized as chelators of 64Cu. The new pentadentate and hexadentate chelators contain different types of donor groups and are expected to form neutral complexes with Cu(II). The new chelators were evaluated for complex kinetics and stability with 64Cu. The new chelators instantly bound to 64Cu with high labeling efficiency and maximum specific activity. All 64Cu-radiolabeled complexes in human serum remained intact for 2 days. The 64Cu-radiolabeled complexes were further challenged by EDTA in a 100-fold molar excess. Among the 64Cu-radiolabeled complexes evaluated, 64Cu-complex of the new chelator E was well tolerated with a minimal transfer of 64Cu to EDTA. 64Cu-radiolabeled complex of the new chelator E was further evaluated for biodistribution studies using mice and displayed rapid blood clearance and low organ uptake. 64Cu-chelator E produced a favorable in vitro and in vivo complex stability profiles comparable to 64Cu complex of the known hexadentate NOTA chelator. The in vitro and in vivo data highlight strong potential of the new chelator E for targeted PET imaging application.
Keywords: 64Cu, Chelating Agents, PET imaging, NOTA
1. Introduction
64Cu (t1/2 = 12.7 h, Emaxβ+ = 656 keV; Emaxβ− = 573 keV; Emaxγ = 511 keV) is one of the most useful radioisotopes for positron emission tomography (PET) imaging.1–2 Various 64Cu-radiolabeled antibody or peptide conjugates have been employed for PET imaging of cancers in preclinical and clinical settings.1–2 A bifunctional chelator that can rapidly complex Cu(II) with high kinetic inertness and thermodynamic stability is a critical component of clinically viable 64Cu-based radiopharmaceuticals for targeted PET imaging. Many antibodies and proteins are not tolerant of heating and radiolysis, and effective radiolabeling of a bifunctional chelator with a short-lived 64Cu under mild condition is required to minimize radiolytic damage of an antibody conjugated with the chelator. Bifunctional DTPA (diethylene triamine pentaacetic acid), TETA (1,4,8,11-tetraazacyclotetradecane-N,N′,N″,N′-tetraacetic acid), DOTA (1,4,7,10-tetraazacyclododecane-N,N′, N″, N′″-tetraacetic acid), and CB-TE2A (1,4,8,11-tetraazabicyclo[6.6.2]hexadecane-4,11-diacetic acid) have been investigated as chelators of 64Cu.2 Although acyclic DTPA is known to rapidly complex with Cu(II), 64Cu-DTPA was reported be kinetically labile and dissociated in serum.2 The macrocyclic chelators, DOTA (log K = 22.3) and TETA (log K = 21.7) form fairly stable complexes with Cu(II) using the four nitrogens in the macrocyclic ring and two pendant carboxylate oxygens as demonstrated by X-ray crystallography.3 In particular, TETA conjugated to an antibody was found to display high binding selectivity to Cu(II) in the presence of other metals with an oxidation state of +2 including Zn(II), Mg(II), and Ca(II).4 The 64Cu complexes of DOTA and TETA remained intact with a minimal loss of 64Cu in serum.2 However, in vivo biodistribution data suggested that 64Cu-DOTA and 64Cu-TETA analogues conjugated to a biomolecule including octreotide were dissociated in mice, and high liver uptake was observed with the 64Cu-radiolabeled complexes.5–7 64Cu complexes of cross-bridged cyclam (CB-TE2A) analogues were shown to possess improved kinetic in vivo stability compared to 64Cu-DOTA and 64Cu-TETA complexes.7–8 However, radiolabeling of CB-TE2A with 64Cu required a complicated and harsh condition due to slow complexation kinetics of the chelator with 64Cu.8 NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid) is a hexadentate macrocyclic chelator containing aminocarboxylate donors and binds to Cu(II) with high thermodynamic stability (log K = 21.6).3 64Cu-radiolabeled NOTA complexes have produced encouraging in vitro and in vivo profiles.6,9–10 We previously reported that 64Cu-NOTA complex remained stable in both serum and mice and displayed fast blood clearance and low uptake in normal organs.10
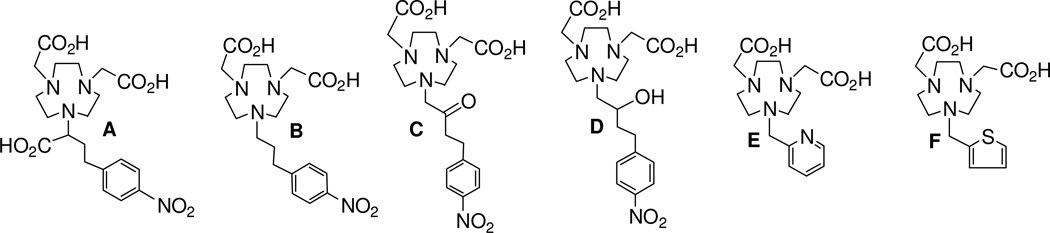
In our continued effort on improved chelation chemistry for development of radiopharmaceuticals,10–14 we synthesized and evaluated new hexadentate and pentadentate NOTA analogues (Figure 1) as potential chelators of 64Cu. The new chelators were evaluated for radiolabeling with 64Cu to determine the maximum specific activity and radiolabeling kinetics with 64Cu under mild condition. 64Cu-radiolabeled complexes of the promising new chelator were evaluated for complex stability in human serum and EDTA (ethylenediaminetetraacetic acid) challenge and in vivo biodistribution using mice.
Figure 1.
Potential Chelators for PET imaging using 64Cu
2. Material and Methods
Instruments and reagents
1H, 13C, and DEPT NMR spectra were obtained using a Bruker 300 NMR instrument, and chemical shifts are reported in ppm on the δ scale relative to TMS. Electro spray ionization (ESI) high resolution mass spectra (HRMS) were obtained on JEOL double sector JMS-AX505HA mass spectrometer (University of Notre Dame, IN). 64Cu was prepared on CS-15 cyclotron at Washington University Medical School, St. Louis, MO according to the previous reported method.10 Radioactivity was counted with a Beckman Gamma 8000 counter containing a NaI crystal (Beckman Instruments, Inc., Irvine, CA). Analytical and semi-prep HPLC were performed on Agilent 1200 (Agilent, Santa Clara, CA) equipped with a diode array detector (λ= 254 and 280 nm), thermostat set at 35 °C and a Zorbax Eclipse XDB-C18 column (4.6 × 150 mm, 80Å, Agilent, Santa Clara, CA). The mobile phase of a binary gradient (0–100%B/40 min; solvent A = 0.1% TFA in water; solvent B = 0.1% TFA in acetonitrile for method 1; 0–100%B/15 min; solvent A: H2O, solvent B: MeOH for method 2) at a flow rate of 1 mL/min was used for analytical HPLC. The mobile phase of a binary gradient (0–100%B/40 min; solvent A: H2O, solvent B: MeOH, flow rate: 3 mL/min) was used for semi-prep HPLC (method 3).
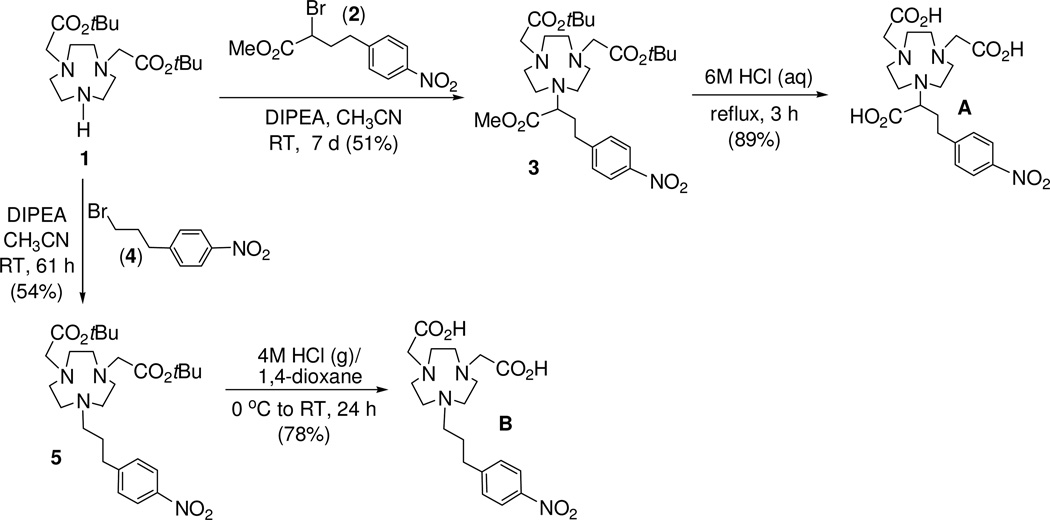
tert-butyl 2-{4,7-bis[2-(tert-butoxy)-2-oxoethyl]-1,4,7-triazanonan-1-yl}-4-(4-nitrophenyl)-butanoate (3)
To a solution of 112 (272 mg, 0.761mmol) in CH3CN (1 mL) was added portionwise compound 215 (230 mg, 0.761 mmol) and DIPEA (295 mg, 2.284 mmol) in CH3CN (1 mL). The resulting mixture was stirred for 7 days at room temperature. The reaction mixture was concentrated to dryness in vacuo. 0.1M HCl aqueous solution (20 mL) was added to the residue, and the resulting mixture was extracted with CH2Cl2 (2 × 20 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated to the dryness in vacuo. The residue was purified via column chromatography on silica gel (220–440 mesh) eluting with 15% CH3OH in CH2Cl2 to afford pure product 3 (240 mg, 51%). 1H NMR (CDCl3, 300 MHz) δ 1.42 (s, 18H), 1.96–1.99 (m, 2H), 2.67–2.98 (m, 15H), 3.23–3.29 (m, 5H), 3.65 (s, 3H), 7.36 (d, J = 8.5 Hz, 2H), 8.12 (d, J = 8.5 Hz, 2H); 13C NMR (CDCl3, 75 MHz) δ 28.2 (q), 31.8 (t), 32.7 (t), 51.2 (q), 53.5 (t), 55.5 (t), 56.0 (t), 59.4 (t), 66.4 (d), 80.8 (s), 123.7 (d), 129.4 (d), 146.4 (s), 149.7 (s), 171.4 (s), 173.7 (s). HRMS (positive ion ESI) Calcd for C29H47N4O8 [M + H]+ m/z 521.3388. Found: [M + H]+ m/z 521.3391.
2-[4,7-bis(carboxymethyl)-1,4,7-triazanonan-1-yl]-4-(4-nitrophenyl)butanoic acid (A)16
Compound 3 (20 mg, 0.032 mmol) was treated with 6M HCl solution (2 mL), and the resulting solution was refluxed for 3 h. The reaction mixture was cooled to room temperature, and the resulting solution was filtered, and the filtrate was concentrated in vacuo to provide chelator A (16 mg, 89%) as a yellow solid. 1H NMR (D2O, 300 MHz) δ 1.90–1.92 (m, 1H), 2.06–2.10 (m, 1H), 2.59–2.84 (m, 2H), 2.95–3.18 (m, 12H), 3.49–3.53 (m, 1H), 3.79 (s, 4H), 7.30 (d, J = 8.4 Hz, 2H), 7.99 (d, J = 8.4 Hz, 2H); 13C NMR (D2O, 75 MHz) δ 29.7 (t), 32.1 (t), 45.7 (t), 49.2 (t), 50.6 (t), 55.3 (t), 63.7 (d), 123.7 (d), 129.4 (d), 146.4 (s), 149.7 (s), 172.2 (s), 175.6 (s). The data of 1H and 13C NMR data were essentially identical to those previously reported.16 Analytical HPLC (tR = 7.6 min, method 1).
tert-butyl 2-{4-[2-(tert-butoxy)-2-oxoethyl]-7-[3-(4-nitrophenyl)propyl]-1,4,7-triazanonan- 1-yl}acetate (5)
To a solution of 112 (72 mg, 0.29 mmol) in CH3CN (2 mL) at 0 °C was added dropwise 411 (105 mg, 0.29 mmol) in CH3CN (1 mL) and DIPEA (112 mg, 0.87 mmol). The resulting mixture was stirred for 61 h at room temperature. The resulting mixture was concentrated to dryness in vacuo. Water (10 mL) and 0.1M HCl aqueous solution (1 mL) were added to the residue, and the resulting mixture was extracted with CHCl3 (3 × 10 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated to the dryness in vacuo The residue was purified via column chromatography on silica gel (60–220 mesh) eluting with 20% MeOH in CH2Cl2 to afford 5 (81 mg, 54%) as an oil. 1H NMR (CDCl3, 300 MHz) δ 1.41 (s, 18H), 2.21 (m, 2H), 2.65–2.90 (m, 8H), 3.01–3.18 (m, 5H), 3.37–3.70 (m, 8H), 7.41 (d, J = 6.0 Hz, 2H), 8.12 (d, J = 6.0 Hz, 2H); 13C NMR (CDCl3, 75 MHz) 5 25.9 (t), 28.1 (q), 32.8 (t), 49.8 (t), 52.3 (t), 53.5 (t), 55.3 (t), 58.2 (t), 81.7 (s), 123.9 (d), 129.4 (d), 146.7 (s), 147.9 (s), 170.7 (s). HRMS (positive ion ESI) Calcd for C27H45N4O6 [M + H]+ m/z 521.3334. Found: [M + H]+ m/z 521.3309.
2-[4-(carboxymethyl)-7-[3-(4-nitrophenyl)propyl]-1,4,7-triazanonan-1-yl]acetic acid (B)
Compound 5 (20 mg, 0.038 mmol) at 0~5 oC was treated dropwise with 4M HCl (g) in 1,4-dioxane (2 mL) over 10 min. The resulting mixture was gradually warmed to room temperature and stirred for 24 h. Diethyl ether (20 mL) was added to the reaction mixture which was stirred for 10 min. The resulting mixture was capped and placed in the freezer for 1 h. The solid formed was filtered, washed with ether, and quickly dissolved in deionized water. The resulting aqueous solution was concentrated in vacuo to provide chelator B (14 mg, 78%). 1H NMR (D2O, 300 MHz) δ 2.02–2.17 (m, 2H), 2.71–2.79 (m, 2H), 3.18 (m, 14H), 3.75–3.82 (m, 4H), 7.37 (d, J = 8.4 Hz, 2H), 8.07 (d, J = 8.7 Hz, 2H); 13C NMR (D2O, 75 MHz) 5 24.9 (t), 31.7 (t), 49.6 (t), 50.7 (t), 51.0 (t), 56.9 (t), 57.3 (t), 123.9 (d), 129.5 (d), 146.4 (s), 148.7 (s), 172.9 (s). HRMS (positive ion ESI) Calcd for C19H29N4O6 [M + H]+ m/z 409.2082. Found: [M + H]+ m/z 409.2087. Analytical HPLC (tR = 7.3 min, method 1).
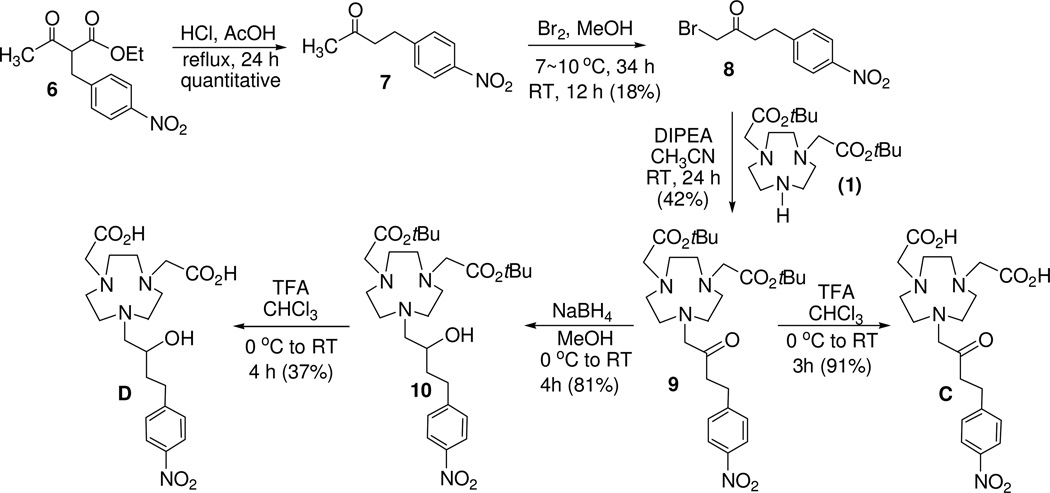
Ethyl 2-[(4-nitrophenyl)methyl]-3-oxobutanoate (6)17
Ethyl acetoacetate (10 g, 76.84 mmol) was added dropwise to NaH (1.84 g, 76.84 mmol) in the THF (220 ml). p-nitro benzyl bromide (16.6 g, 76.84 mmol) was added portionwise over 1 h. The reaction mixture was stirred for 1.5 h. After evaporation of the solvent, the residue was treated with H2O (100 mL) and extracted with ethyl acetate (2 × 100 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated to the dryness in vacuo. The residue was recrystallized with ethanol to provide pure product 6 (10 g, 49%). 1H NMR (CDCl3, 300 MHz) δ 1.21 (t, 3H, J = 7.2 Hz), δ 2.23 (s, 3H), δ 3.23–3.27 (m, 2H), δ 3.79 (t, 3H, J = 7.5 Hz), δ 4.15–4.17 (m, 2H), δ 7.36 (d, 2H, J = 8.4 Hz), δ 8.13 (d, 2H, J = 8.4 Hz). 13C NMR (CDCl3, 75 MHz) δ 14.0(q), 29.5 (q), 33.4 (t), 60.6 (d), 61.9 (d), 123.8 (d), 129.8 (d), 146.1 (s), 148.9 (s), 168.5 (s), 201.2 (s).
4-(4-nitrophenyl)butan-2-one (7)18
Compound 6 (10 g, 37.7 mmol) was dissolved in the mixture of acetic acid (85 mL) and conc. HCl solution (30 mL), and the resulting solution was refluxed for 24 h after which the reaction mixture was allowed to room temperature and concentrated to dryness in vacuo. The residue was treated with H2O (100 ml) and extracted with ethyl acetate (2 × 100 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated in vacuo to provide pure 7 (7.3 g, 100%). 1H NMR (CDCl3, 300 MHz) δ 2.09 (s, 3H), 2.77 (t, 2H, J = 9 Hz), 2.92 (t, 2H, J = 9 Hz), 7.29 (d, 2H, J = 9 Hz), 8.01 (d, 2H, J = 9 Hz); 13C NMR (CDCl3, 75 MHz) δ 29.3 (t), 30.1 (q), 44.2 (t), 123.7 (d), 129.3 (d), 146.5 (s), 149.0 (s), 206.8 (s). The data of 1H and 13C NMR data were essentially identical to those of 7 as previously reported.16
1-bromo-4-(4-nitrophenyl)butan-2-one (8)
To a solution of 7 (5.0 g, 25.88 mmol) in anhydrous methanol (10 mL) was added dropwise bromine (4.14 mg, 25.88 mmol) in anhydrous methanol (10 mL) at 7~9 °C over 1 h. The mixture was stirred for 34 h in same temperature. DI water (30 mL) was added to the mixture which was warmed to room temperature and continuously stirred for 12 h. The reaction mixture was extracted with CH2Cl2 (2 × 30 mL). The combined organic layers were dried over MgSO4, filtered, and the filtrate was concentrated in vacuo. The residue was purified via column chromatography on silica gel (60–220 mesh) and eluted with 10% ethyl acetate in hexanes to afford 8 (1.26 g, 18%) as a solid. 1H NMR (CDCl3, 300 MHz) δ 3.05 (s, 4H), 3.87 (s, 2H), 7.35 (d, 2H, J = 7.8 Hz), 8.12 (d, 2H, J = 8.1 Hz); 13C NMR (CDCl3, 75 MHz) δ 29.4 (t), 33.9 (t), 40.4 (t), 123.8 (d), 129.3 (d), 146.6 (s), 148.2 (s), 200.4 (s). HRMS (positive ion ESI) Calcd for C10H11BrNO3 [M + H]+ m/z 271.9917. Found: [M + H]+ m/z 271.9924.
tert-butyl 2-{4-[2-(tert-butoxy)-2-oxoethyl]-7-[4-(4-nitrophenyl)-2-oxobutyl]-1,4,7-triazanonan-1-yl}acetate (9)
Compound 8 (114 mg, 0.420 mmol) was added portionwise to a solution of 112 (150 mg, 0.420 mmol) in CH3CN (5 mL) at 0 °C. DIPEA (163 mg, 1.259 mmol) in CH3CN (2 mL) was added portionwise, and the resulting mixture was allowed to room temperature and stirred for 24 h. The reaction mixture was concentrated to dryness in vacuo. The residue was treated with deionized water (10 mL) and extracted with CHCl3 (2 × 10 mL). The combined organic layer was concentrated in vacuo. Then the resulting mixture was dissolved with 0.1M HCl solution (10 mL) and washed with CHCl3 (2 × 10 mL). The aqueous layer was neutralized using 0.1M NaOH (10 mL) and extracted with CHCl3 (3 × 20 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated to the dryness in vacuo. The residue was purified via column chromatography on silica gel (60–220mesh) eluting with 30% CH3OH in dichloromethane containing Et3N to provide pure 9 (96.8 mg, 42%). 1H NMR (CDCl3, 300 MHz) δ 1.38 (s, 18H), 2.89–3.57 (m, 21H), 4.47 (s, 1H), 7.31 (d, 2H, J = 7.5 Hz), 8.05 (d, 2H, J = 7.5 Hz).(This is Yongliang’s proton data.) 13C NMR (CDCl3, 75 MHz) δ 28.1 (q), 28.8 (t), 40.8 (t), 48.4 (t), 50.3 (t), 51.5 (t), 55.8 (t), 62.9 (t), 82.0 (s), 123.7 (d), 129.3 (d), 146.5 (s), 148.4 (s), 169.4 (s), 203.4 (s) HRMS (positive ion ESI) Calcd for C28H44N4O7 [M + H]+ m/z 549.6716. Found: [M + H]+ m/z 549.3403.
tert-butyl 2-{4-[2-(tert-butoxy)-2-oxoethyl]-7-[2-hydroxy-4-(4-nitrophenyl)butyl]-1,4,7-triazanonan-1-yl}acetate (10)
A solution of 9 (33 mg, 0.060 mmol) in anhydrous methanol (1 mL) at 0 °C was added portionwise NaBH4 (10 mg, 0.264 mmol) over 1 h. The mixture was then warmed to room temperature and stirred for 3 h. The reaction mixture was concentrated to dryness and treated with H2O (10 mL) and extracted with ethyl acetate (2 × 15 mL). The combined organic layers were dried over MgSO4, filtered, and the filtrated was concentrated in vacuo to provide pure 10 (26.5 mg, 81%). 1H NMR (CDCl3, 300 MHz) δ 1.43 (s, 18H), 1.59–1.70 (m, 2H), 2.36 (m, 1H), 2.69–3.05 (m, 15H), 3.29 (s, 4H), 3.51–3.69 (m, 1H), 7.35 (d, 2H, J = 8.4 Hz), 8.11 (d, 2H, J = 8.4 Hz). 13C NMR (D2O, 75 MHz) δ 28.2 (q), 32.2 (t), 35.8 (t), 55.8(t), 56.0 (t), 56.4 (t), 58.9 (t), 63.8 (t), 68.0 (d), 80.8 (s), 123.6 (d), 129.3 (d), 146.3 (s), 150.6 (s), 171.5 (s). HRMS (positive ion ESI) Calcd for C28H47N4O7 [M + H]+ m/z 551.3439. Found: [M + H]+ m/z 551.3463.
2-[4-(carboxymethyl)-7-[4-(4-nitrophenyl)-2-oxobutyl]-1,4,7-triazanonan-1-yl]acetic acid (C)
TFA (800 µl) was added dropwise to compound 9 (22.6 mg 0.041 mmol) at 0 °C, and the resulting solution was stirred for 2 h at 0 °C. The resulting mixture was allowed to room temperature and stirred for additional 1 h. The reaction mixture was concentrated to dryness in vacuo and treated with ether (2 mL), and the ether layer was decanted. The residue was dissolved in H2O (2 mL) and washed by CHCl3 (2 × 5 mL). The aqueous layer was concentrated to dryness in vacuo to provide pure product C (16.4 mg, 91%). 1H NMR (D2O, 300 MHz) δ 2.85 (m, 4H), 3.24–3.28 (m, 12H), 3.82 (s, 4H), 4.20 (s, 2H), 7.30 (d, 2H, J = 9.0 Hz), 8.00 (d, 2H, J = 9.0 Hz); 13C NMR (D2O, 75 MHz) δ 28.3 (t), 39.8 (t), 50.0 (t), 50.4 (t), 51.3 (t), 56.3 (t), 64.2 (t), 123.6 (d), 129.3 (d), 146.0 (s), 148.8 (s), 171.8 (s), 205.8 (s). HRMS (positive ion ESI) Calcd for C20H29N4O7 [M + H]+ m/z 437.2031. Found: [M + H]+ m/z 437.2041. Analytical HPLC (tR = 7.8 min, method 1)
2-[4-(carboxymethyl)-7-[2-hydroxy-4-(4-nitrophenyl)butyl]-1,4,7-triazanonan-1-yl]acetic acid (D)
TFA (1 mL) was added dropwise to compound 10 (24 µg 0.045 mmol) at 0 °C, the resulting mixture was stirred for 3 h at 0 °C. The resulting mixture was allowed to room temperature and stirred for additional 1 h. The reaction mixture was concentrated to dryness and treated with ether (3 mL), and the ether layer was decanted. The residue was dissolved in H2O (2 mL) and washed by CHCl3 (2 × 5 mL). The aqueous layer was concentrated to dryness in vacuo, and the residue was purified by semi-prep HPLC (method 3, tR = ~21 min) to provide pure product D (7.2 mg, 37%). 1H NMR (D2O, 300 MHz) δ 1.69 (m, 2H), 2.66–2.78 (m, 2H), 3.01–3.49 (m, 18H), 3.86 (m, 1H), 7.31 (d, 2H, J = 8.1 Hz), 8.01 (d, 2H, J = 8.4 Hz). 13C NMR (D2O, 75 MHz) δ 30.7 (t), 35.1 (t), 49.8 (t), 50.5 (t), 58.5(t), 62.3 (t), 65.5 (d), 123.7 (d), 129.4 (d), 145.9 (s), 150.0 (s), 175.5 (s). HRMS (positive ion ESI) Calcd for C10H31N4O7 [M + H]+ m/z 439.2187. Found: [M + H]+ m/z 439.2203. Analytical HPLC (tR = 8.0 min, method 2)
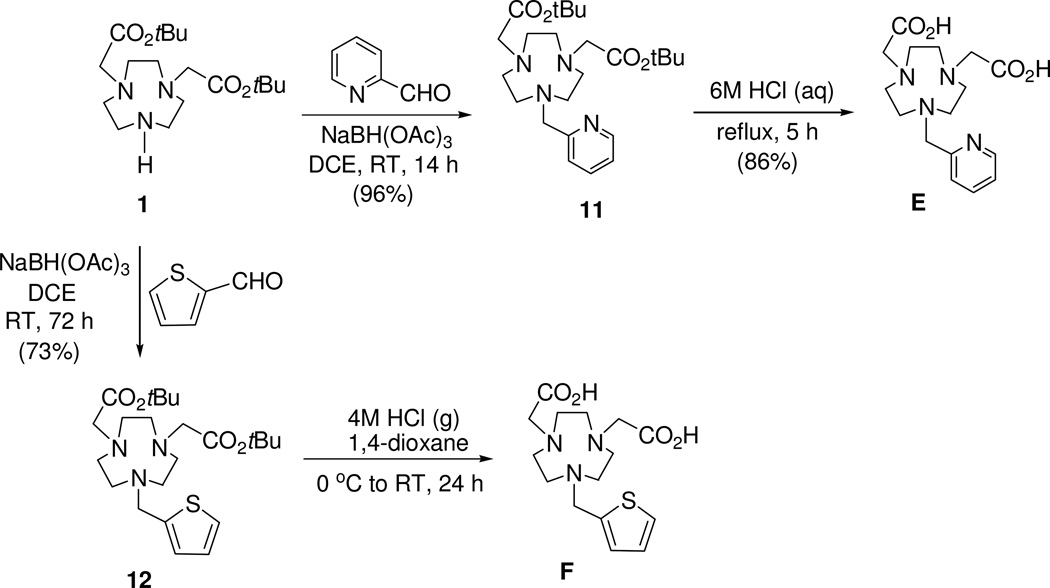
tert-butyl 2-{4-[2-(tert-butoxy)-2-oxoethyl]-7-(pyridin-2-ylmethyl)-1,4,7-triazanonan-1-yl}acetate (11)
To a solution of 112 (50 mg, 0.140 mmol) in 1,2-dichloroethane (1 mL) was added 2-pyridinecarboxaldehyde (15 mg, 0.140 mmol). The resulted solution was stirred for 10 min and then added with sodium triacetoxyborohydride (44.5 mg, 0.210 mmol) portionwise over 10 min. The mixture was stirred at room temperature for overnight. The reaction mixture was quenched by adding saturated NaHCO3 (15 mL), and the resulting solution was extracted with ethyl acetate (3 × 15 mL). The combined organic layers were concentrated to dryness in vacuo. The residue was dissolved in 0.1M HCl solution (10 mL) and washed with CHCl3 (2 × 10 mL). The aqueous layer was treated with saturated NaHCO3 (10 mL) and extracted with ethyl acetate (3 × 20 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated in vacuo to provide pure 11 (60 mg, 96%) as a yellowish oil. 1H NMR (CDCl3, 300 MHz) δ 1.44 (s, 18H), 2.75–2.90 (m, 12H), 3.31–3.36 (m, 4H), 3.87 (s, 2H), 7.13 (t, J = 5.4 Hz, 1H), 7.54 (d, J = 7.8 Hz, 1H), 7.61–7.67 (m, 1H), 8.50 (d, J = 3.9 Hz, 1H); 13C NMR (CDCl3, 75 MHz) δ 28.2 (q), 55.3 (t), 55.6 (t, 2C), 59.8 (t), 64.1 (t), 80.6 (s), 121.7 (d), 123.1 (d), 136.2 (d), 148.8 (d), 160.6 (s), 171.5 (s). HRMS (positive ion ESI) Calcd for C24H41N4O4 [M + H]+ m/z 449.3122. Found: [M + H]+ m/z 449.3118.
2-[4-(carboxymethyl)-7-(pyridin-2-ylmethyl)-1,4,7-triazanonan-1-yl]acetic acid (E)
Compound 11 (16 mg, 0.036 mmol) was treated with 6M HCl solution (3 mL), and the resulting solution was refluxed for 5 h. The reaction mixture was gradually cooled to room temperature, filtered, and concentrated to dryness in vacuo to provide chelator E (15 mg, 86%) as a yellow solid. 1H NMR (D2O, 300 MHz) δ 2.59–2.80 (m, 4H), 3.01 (m, 4H), 3.08 (s, 4H), 3.72 (s, 4H), 4.19 (s, 2H), 7.84 (t, J = 7.2 Hz, 2H), 7.98 (d, J = 7.8 Hz, 1H), 8.39 (dt, J = 7.5 Hz, J = 1.8 Hz 1H), 8.51 (d, J = 5.4 Hz, 1H). 13C NMR (D2O, 75 MHz) δ 47.5 (t), 48.9 (t), 50.5 (t), 55.4 (t), 56.4 (t), 126.4 (d), 127.9 (d), 141.4 (d), 147.3 (d), 153.0 (s), 173.1 (s). HRMS (positive ion ESI) Calcd for C16H25N4O4 [M + H]+ m/z 337.1870. Found: [M + H]+ m/z 337.1863. Analytical HPLC (tR = 4.2 min, method 1)
tert-butyl 2-{4-[2-(tert-butoxy)-2-oxoethyl]-7-(thiophen-2-ylmethyl)-1,4,7-triazanonan-1-yl}acetate (12)
To a solution of 112 (50.0 mg, 0.140 mmol) in 1,2-dichloroethane (1 mL) was added 2-thiophenecarboxaldehyde (15.7 mg, 0.140 mmol). The resulted solution was stirred for 10 min and treated with sodium triacetoxyborohydride (44.5 mg, 0.210 mmol) portionwise over 10 min. The reaction mixture was stirred at room temperature for 3 d. The reaction mixture was quenched by adding saturated NaHCO3 (15 mL), and the resulting solution was extracted with ethyl acetate (3 × 15 mL). The combined organic layer was concentrated in vacuo. The residue was treated with 0.1M HCl solution (10 mL) and extracted with CHCl3 (3 × 10 mL). The combined organic layers were dried over MgSO4, filtered, and the filtrate was concentrated to the dryness in vacuo. The residue was purified via column chromatography on silica gel (60–220 mesh) eluting with 15% CH3OH in CH2Cl2 to afford pure 12 (46 mg, 73%). 1H NMR (CDCl3, 300 MHz) δ 1.45 (s, 18H), 2.82–2.96 (m, 12H), 3.31 (s, 4H), 3.87 (s, 2H), 6.89–6.93 (m, 2H), 7.19 (dd, J = 1.2 Hz, J = 4.8 Hz, 1H); 13C NMR (CDCl3, 75 MHz) δ 28.2 (q), 55.0 (t), 55.2 (t), 55.3 (t), 56.8 (t), 59.9 (t), 80.7 (s), 124.6 (d), 125.3 (d), 126.3 (d), 160.6 (s), 171.5 (s). HRMS (positive ion ESI) Calcd for C23H40N3O4S [M + H]+ m/z 454.2734. Found: [M + H]+ m/z 454.2723.
2-[4-(carboxymethyl)-7-(thiophen-2-ylmethyl)-1,4,7-triazanonan-1-yl]acetic acid (F)
Compound 12 (17 mg, 0.037 mmol) at 0–5 °C was treaeted dropwise with 4M HCl (g) in 1,4-dioxane (2.5 mL) over 10 min. The resulting mixture was warmed to room temperature and stirred for 24 h. Diethyl ether (40 mL) was added to the mixture which was continued to stir for 10 min. The solid formed was filtered, washed with ether, and quickly dissolved in DI water. The aqueous solution was concentrated in vacuo to provide chelator F (13.5 mg, 80%) as a yellow solid. 1H NMR (D2O, 300 MHz) δ 2.90–3.61 (m, 18H), 6.91–7.08 (m, 1H), 7.27 (d, J = 2.4 Hz, 1H), 7.49 (d, J = 4.8 Hz, 1H). 13C NMR (D2O, 75 MHz) δ 49.0 (t), 50.2 (t), 50.3 (t), 54.3 (t), 56.9 (t), 128.0 (d), 129.8 (s), 130.1 (d), 132.7 (d), 173.95 (s). HRMS (positive ion ESI) Calcd for C15H24N3O4S [M + H]+ m/z 342.1482. Found: [M + H]+ m/z 342.1469. Analytical HPLC (tR = 5.8 min, method 1)
Preparation and characterization of Cu(II) complexes
Cu(II)-complexes of the chelators A-F were prepared by reaction of each chelator (5 µl, 10 mM) with CuCl2 (5 µl, 10 mM) in 0.25M NH4OAc buffer (pH 5.5) for 24 h at room temperature and 300 rpm. Each of Cu(II)-complex was purified by semi-prep HPLC (solvent A: 0.1% TFA in H2O, solvent B: 0.1% TFA in CH3CN, 0–60% B/40 min, flow rate: 3 mL/min). The purified Cu(II)-complex was characterized by analytical HPLC (method 1, Supporting Information)
Determination of maximum specific activity (MSA)
Whatman C18 silica gel TLC plates (KC18F, 60 Å) were purchased from Fisher Scientific (Pittsburgh, PA). Radio-TLCs were developed with 10% NH4OAc:MeOH (3:7) and analyzed using a Bioscan 200 imaging scanner (Bioscan, Inc., Washington, DC). 64CuCl2 was diluted with a 10-fold excess of 0.1M NH4OAc (pH 5.5) for radiolabeling. The maximum specific activities were determined experimentally via titrating 64CuCl2 in 0.1M NH4OAc (pH 5.5) with the chelators. Briefly, for each chelator, six reaction vials were prepared in 0.1M NH4OAc (pH 5.5) via dilution to give final chelator masses in the range 0.001 to 0.1 µg. 3.7 MBq (100 µCi) of 64Cu in 0.1M NH4OAc (pH ~5.5) was added to each vial and adjusted the final volume to 100 µL (final pH 5.5) and vortexed for 10–15 seconds. The reactions were incubated on a rotator at 37 °C for 1 h. After incubation, 1 µL aliquots were withdrawn from reaction vials an alyzed by TLC (C-18) with a mixture of 10% NH4OAc/MeOH (3:7) as a mobile phase for labeling percentage. All reactions were done in triplicate. The data were plotted as % labeling vs. amount of chelator used in the reaction and fitted using sigmoidal dose response equation in GraphPad Prism (La Jolla, CA). Based on this, the amount of mass required to achieve 50% labeling was determined, and this mass was multiplied by 2 to obtain the minimal mass for 100% labeling to determine the maximum specific activity.
Complexation formation kinetics of chelators with 64Cu
All HCl solutions were prepared from ultra-pure HCl (Fisher Scientific, #A466–500). For metal-free radiolabeling, plasticware including pipette tips, tubes, and caps was soaked in 0.1M HCl (aq) overnight and washed thoroughly with Milli-Q (18 MΩ) water, and air-dried overnight. 0.25M NH4OAc buffer solution (pH 5.5) was prepared using ultra-pure ammonium acetate and pH of the solution was adjusted using 0.1M and 1M HCl solution. The resulting buffer solution was treated with Chelex-100 resin (Biorad, #142–2842), shaken overnight at room temperature, and filtered through 0.22 µm filter (Corning, #430320) prior to use. TLC plates (6.6 × 1 cm or 6.6 × 2 cm, Silica gel 60 F254, EMD Chemicals Inc., #5554–7) with the origin line drawn at 0.6 cm from the bottom were prepared. To a buffer solution (11~18 µL, 0.25M NH4OAc, pH 5.5) in a capped microcentrifuge tube (1.5 mL, Fisher Scientific, #05–408–129) was sequentially added a solution of the chelator (6.7 µg, 1–7 µL) in the buffer and 64Cu in 0.05M HCl (20 µCi, 1~3 µL). The total volume of the resulting solution was 20 µL, and the concentration of each chelator in the reaction mixture was 0.6 mM. The reaction mixture was agitated on the thermomixerset at 1,000 rpm at room temperature for 30 min. The labeling efficiency was determined by ITLC eluted with 20mM EDTA in 0.15M NH4OAc as the mobile phase. A solution of radiolabeled complexes (2.0 µL) was withdrawn at the designated time points (1 min, 10 min, and 30 min), spotted on a TLC plate, and then eluted with the mobile phase. After completion of elution, the TLC plate was warmed and dried on the surface of a hot plate maintained at 35 °C and scanned using TLC scanner (Bioscan). Unbound and bound radioisotope appeared 30~35 mm (Rf = 0.6) and 50~55 mm (Rf = 1.0) from the bottom of the TLC plate, respectively.
In vitro serum stability of 64Cu-radiolabeled complexes
Human serum was purchased from Gemini Bioproducts (#100110). 64Cu-radiolabeled complexes were prepared by reaction of the bifunctional chelators (30 µg) with 64Cu (100 µCi) in 0.25M NH4OAc buffer (pH 5.5) for 1 h at room temperature, and labeling efficiency of the radiolabeled complexes were found to be ~100% as determined by ITLC (20mM EDTA in 0.15M NH4OAc). The freshly prepared radiolabeled complexes of chelators were directly used for serum stability studies without further purification. 64Cu-radiolabeled complex (85 µCi, 10 µL) was added to human serum (90 µL) in a microcentrifuge tube, and concentration of chelator in the resulting mixture was 0.5 mM. The stability of 64Cu-radiolabeled complexes in human serum was evaluated at 37 °C for 2 days. A solution of the radiolabeled complex in serum was withdrawn at the designated time points and evaluated by ITLC as described above.
EDTA Challenge19
64Cu-radiolabeled complexes were prepared by reaction of each chelator (20 µg) with 64Cu (60 µCi) in 0.25M NH4OAc buffer (40 µL, pH 5.5) for 2 h at room temperature. The labeling efficiency of the 64Cu-radiolabeled complexes was found to be ~100% as determined by ITLC (20mM EDTA in 0.15M NH4OAc). The 64Cu-radiolabeled complexes were directly used for EDTA challenge experiments. A solution of the 64Cu-radiolabeled complex containing each chelator (~1 mM) in 0.25M NH4OAc buffer (40 µL) was mixed with a solution of EDTA (~ 100 mM, 40 µL, H2O, pH 5.0) at a 100-fold molar excess. The resulting mixture was incubated for 24 h at 37 °C. The stability of 64Cu-radiolabeled complexes in the solution was evaluated using ITLC (20 mM EDTA in 0.15M NH4OAc). A solution of the radiolabeled complex in serum (3~20 µL) was withdrawn at the designated time points and evaluated by ITLC as described above. Stability of the complexes was also evaluated at 25 h time point by HPLC (solvent A: 0.1% TFA in H2O, solvent B: 0.1% TFA in CH3CN, 0–100% B/15 min, flow rate: 1 mL/min). 64Cu-EDTA complex was eluted early (tR = ~ 2.5 min), while 64Cu-radiolabeled complexes of chelators A-D, E, and F have the respective retention time (tR = 7~8 min, tR = 4.6 min, and tR = 6.2 min).
In vivo biodistribution
All animal studies were performed in compliance with the Guidelines for Care and Use of Research Animals established by Washington University’s Animal Studies Committee. Biodistribution studies were carried out in female CD-1 mice (Charles River Labs). The tissue uptakes of 64Cu-chelator A and 64Cu-chelator E were evaluated in mice (n = 4) that were injected via the tail vain with 0.74 MBq (20 µCi) per animal (100 µL). At 1, 4, and 24 h post-injection, mice were anesthetized with 1–2% isoflurane and sacrificed by cervical dislocation. Subsequently, the tissues of interest were harvested, weighed, and measured in a gamma counter. Samples were corrected for radioactive decay to calculate percent injected-dose per gram (%ID/g) of tissue.
3. Results and Discussion
3.1. Design of new chelators
The new chelators (Figure 1) were designed based on various factors including donor number (denticity), donor type, charge of the complex, compatibility between metal ion and donor group that can influence coordination chemistry of a metal. Cu(II) has a relatively small ionic radius of 73 ppm for coordination number 6 and is known to display a high affinity for nitrogen, oxygen, and sulfur donors.1 A bifunctional version of the known parent NOTA chelator (A)16 was prepared and evaluated for comparison to the new NOTA analogues. A new bifunctional chelator B contains five donor groups and the p-NO2-Bn group that is linked to the macrocyclic backbone by a relatively long propyl chain. Hexadentate chelators C and D contain a carbonyl donor group and a hydroxyl group in addition to the donor groups attached to the macrocyclic backbone, respectively. It would be interesting to evaluate if P-carbonyl or less flexible secondary hydroxyl group can efficiently cooperate with the other donor for rapid and tight complexation with Cu(II). The new hexadentate chelators E and F differ from NOTA wherein one of the aminocarboxylate donors is replaced with a pyridyl (E) or a thiophenyl (F) group. The hexadentate NOTA chelator A can form an anionic complex with Cu(II), while all new pentadentate and hexadentate chelators B-F are expected to form a neutral complex that would have an advantage of less protein interaction and a potentially more favorable in vivo tissue distribution over charged complexes.
3.2. Synthesis of new chelators and their Cu(II) complexes
Synthesis of chelators A and B is shown in Scheme 1. Bisubstituted 1,4,7-triazacyclononane (TACN) analogue 112 was reacted with an alkylating agent 215 and 411 to provide the key precursor molecules 3 and 5, respectively. Removal of tert-butyl groups in 3 and 5 was accomplished by treatment of 3 and 5 with HCl (aq) to afford the respective bifunctional chelators A16 and B. Synthesis of chelators C and D is outlined in Scheme 2. Hydrolysis followed by decarboxylation of 617 under acidic condition provided compound 718 which was subjected to α-bromination using Br2 to produce 8. Base-promoted reaction of 8 with 112 at room temperature for 24 h provided substitution product 9 which was further treated with TFA to provide chelator C. Compound 9 containing the carbonyl group was reduced to alcohol 10 using NaBH4. tert-butyl groups in 10 was removed by treatment of 10 with TFA in CHCl3 to furnish chelator D. The chelators E and F containing the heteroaromatic rings were synthesized as outlined in Scheme 3. Reductive amination of 2-pyridyl aldehyde and 2-thiophenyl aldehyde with bisubstituted TACN analogue 112 provided compounds 11 and 12, respectively. Compounds 11 and 12 were treated with 6M HCl (aq) and heated to reflux for 5 h to afford the desired chelators E and F, respectively.
Scheme 1.
Synthesis of TACN-Based Chalators A and B
Scheme 2.
Synthesis of Chelators C and D
Scheme 3.
Synthesis of TACN-Based Chalators E and F Containing a Heteroaromatic Ring
Cold Cu(II) complexes of the chelators A-F were prepared and characterized by HPLC. A solution of each chelator was reacted with CuCl2 in an equal molar concentration at room temperature for 24 h to provide the corresponding Cu(II) complexes. The Cu(II) complexes were purified using semi-prep HPLC and characterized by analytical HPLC (Supporting Information). The Cu(II) complexes of chelator E and F containing pyridyl and thiophenyl ring were eluted earlier with +/− 2 min window (tR = 4 min and tR = 6 min, respectively) as compared to Cu(II) complexes of chelators A-D (tR = 7~8 min).
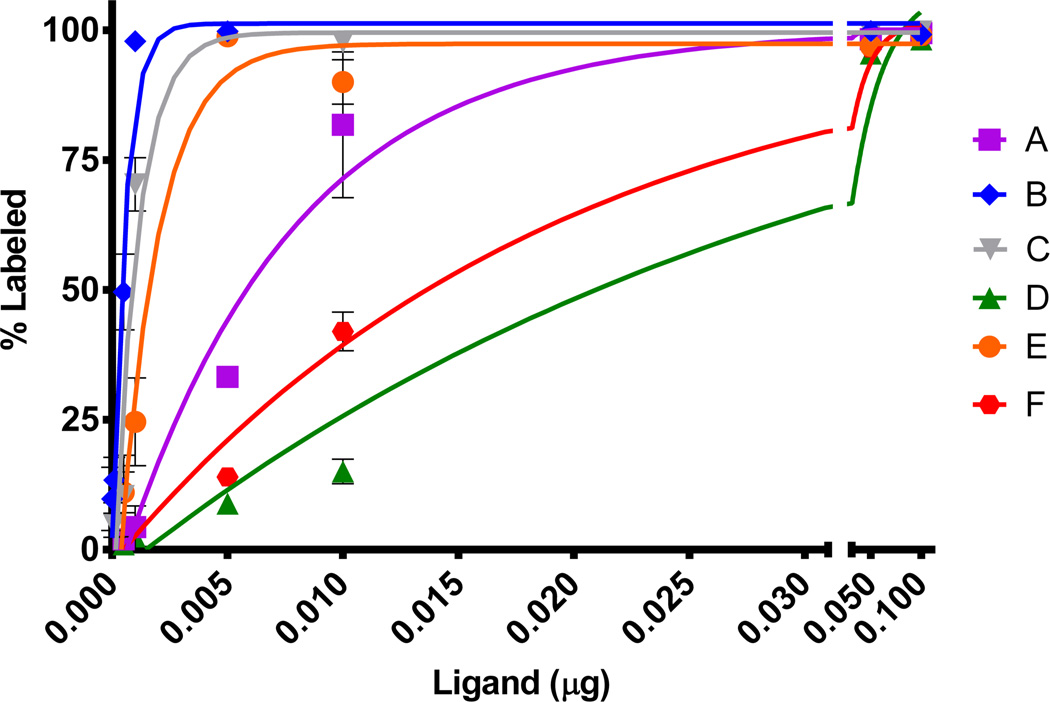
3.3. Maximum specific activity
The new chelators were evaluated for radiolabeling with 64Cu to determine the maximum specific activity. The specific activity was determined by titrating chelators with 64Cu, and the result is shown in Figure 2. The chelators in different concentrations (0.0001 µg to 0.1 µg) were labeled with 64Cu (0.1 M NH4OAc, pH 5.5, 37 °C). All chelators studied bound to 64Cu with high labeling efficiency (> 98%, 1 h). The respective maximum specific activity (Ci/µmol) of 4.87, 51.73, 27.45, 1.18, 14.69, and 1.82 was determined for chelators A-F. It is noteworthy that the pentadentate chelator B bound to 64Cu with the highest maximum specific activity (51.73 Ci/µmol). A TACN analogue substituted with two N-carboxymethyl groups were known to effectively complex with Cu(II).3 Introduction of a functional linker to the TACN backbone appears to have little impact on complexation of the donor groups with Cu(II). The relatively lower maximum specific activity was observed with the hexadentate chelators D (1.18 Ci/µmol) and F (1.82 Ci/µmol) containing a hydroxyl group and a thiophenyl group. As compared to the known NOTA bifunctional chelator A, significantly higher specific activity was observed with chelators B, C, and E
Figure 2.
Radiolabeling (%) of chelators in different concentration (0.0001 to 0.1 µg chelator) with 64Cu (100 µCi).
3.4. Radiolabeling reaction kinetics
The new chelators were evaluated for radiolabeling reaction kinetics with 64Cu at room temperature (Table 1 and Supporting Information). Each chelator (6.7 µg) in 0.25M NH4OAc (pH 5.5) was radiolabeled with 64Cu (20 µCi) at room temperature. During the reaction time (30 min), the components were withdrawn at the designated time points (1 min, 10 min, and 30 min), and the radiolabeling efficiency (%) was determined using ITLC (20 mM EDTA in 0.15M NH4OAc). The bifunctional NOTA chelator (A) was employed for comparison and displayed rapid complexation with 64Cu as expected.10 All new chelators instantly bound to 64Cu with excellent radiolabeling efficiency (>99%) at room temperature. Radiolabeling of the chelators with 64Cu was nearly complete within 1 min as determined by ITLC. 64Cu-EDTA migrated with the solvent front on TLC (Rf = 1.0), while 64Cu-radiolabeled chelator complexes travel slower on the TLC (Rf = 0.6). The 64Cu-radiolabeled complexes of the chelators and 64Cu-EDTA were well separated on the ITLC. All 64Cu-radiolabeled complexes were shown to be stable against EDTA present in the eluent of TLC.
Table 1.
Radiolabeling kinetics of chelators with 64Cu (0.25M NH4OAC, pH 5.5, RT)#
| Time (min) |
Bound complex (%) |
|||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| 1 | 99.8 ± 0.1 | 99.7 ± 0.1 | 99.3 ± 0.3 | 99.0 ± 0.0 | 99.9 ± 0.1 | 99.8 ± 0.2 |
| 10 | 99.8 ± 0.2 | 99.6 ± 0.4 | 99.2 ± 0.9 | 99.6 ± 0.2 | 99.9 ± 0.1 | 99.9 ± 0.2 |
| 30 | 99.6 ± 0.3 | 100 ± 0.0 | 99.5 ± 0.5 | 99.5 ± 0.4 | 99.9 ± 0.1 | 99.8 ± 0.2 |
Radiolabeling efficiency (mean ± standard deviation%) was measured in triplicate using ITLC (eluent: 20mM EDTA in 0.15M NH4OAc).
In vitro serum stability
In vitro serum stability of the radiolabeled complexes was performed to determine if the chelators radiolabeled with 64Cu remained stable without loss of the radioactivity in human serum. This was assessed by measuring the transfer of 64Cu from the complex to human serum proteins using ITLC (20 mM EDTA in 0.15M NH4OAc, Table 2 and Supporting Information). 64Cu-radioabeled chelators were readily prepared from the reactions of the chelators with 64Cu at room temperature. Essentially no unbound 64Cu was detected in the reaction mixture at 2 h time point after the reaction at room temperature as determined by ITLC. The 64Cu-radioabeled chelators were directly used for serum stability studies (pH 7, 37 °C) without further purification. All 64Cu-radiolabeled complexes of chelator A-F remained intact in human serum for 2 days as evidenced by ITLC analysis (Supporting Information). 64Cu-chelator C was found to be least stable in serum, and ~3% of 64Cu was dissociated from the complex containing the carbonyl group over 2 days. No measurable radioactivity was released from 64Cu-chelator E over 2 days. A tiny amount of 64Cu (<0.3%) was detected from other 64Cu-complexes of chelators B, D-F
Table 2.
Complex stability of 64Cu-radiolabeled complexes in human serum (pH 7, 37 °C)#
| Time (day) |
Bound complex (%) |
|||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| 0 | 99.9 ± 0.0 | 99.9 ± 0.1 | 99.9 ± 0.1 | 100 ± 0.1 | 99.9 ± 0.0 | 99.9 ± 0.1 |
| 1 | 100.0 ± 0.0 | 100 ± 0.1 | 98.7 ± 0.5 | 100 ± 0.0 | 100.0 ± 0.0 | 99.9 ± 0.1 |
| 2 | 99.9 ± 0.1 | 99.7 ± 0.4 | 97.8 ± 1.1 | 99.9 ± 0.1 | 100.0 ± 0.0 | 99.9 ± 0.0 |
Bound complex (mean ± standard deviation%) was measured in triplicate using ITLC.
3.5. Stability of 64Cu-radiolabeled complexes in EDTA solution
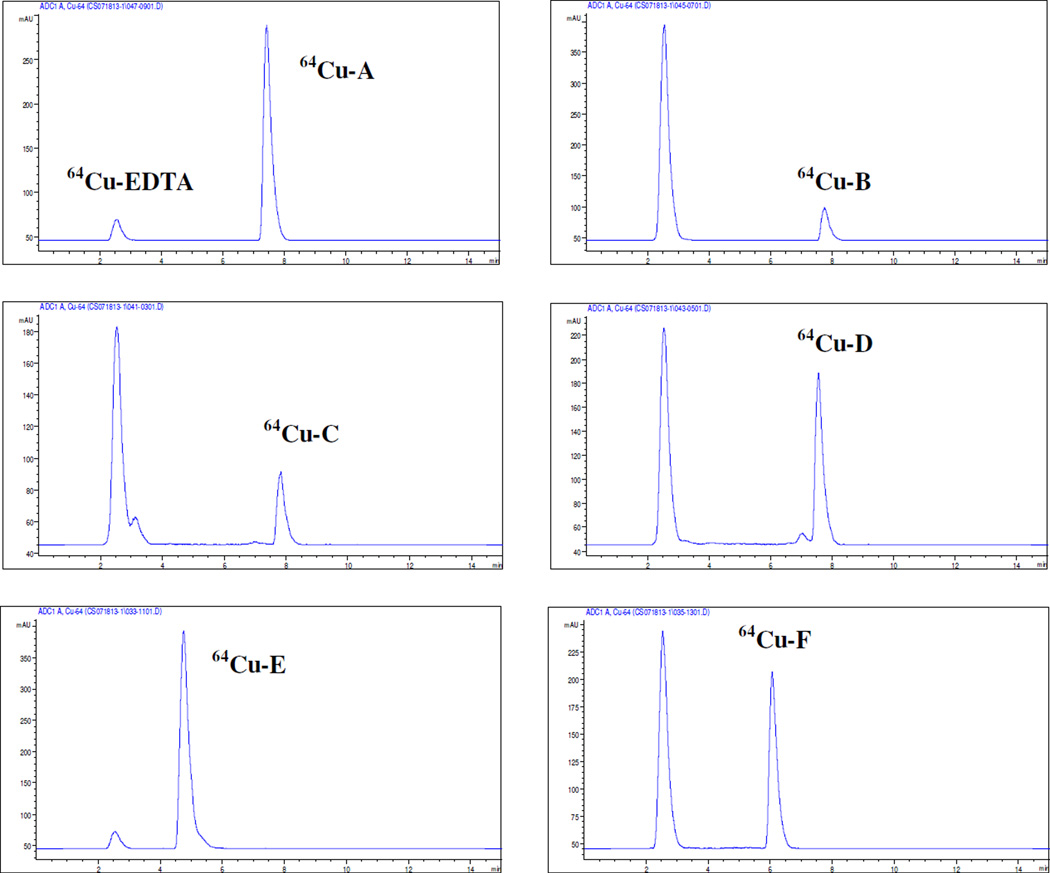
64Cu-radiolabeled complexes were further evaluated for complex stability based on EDTA challenge. 64Cu-radiolabeled complexes were freshly prepared and treated with a solution of EDTA at a 100-fold molar excess, and the resulting solution (pH 5.5) was incubated at 37 °C for 24 h. A sample was withdrawn at different time points (0 h, 1 h, 4 h, and 24 h) and analyzed using both ITLC and HPLC (Table 3 and Supporting Information). 64Cu-radiolabeled complexes of chelators A and E remained intact against EDTA challenge, and a small portion of the activity (~5%) was transferred from the complexes to EDTA at 24 h time point (ITLC). Among the complexes tested, 64Cu-radiolabeled pentadentate chelator B was least tolerant of EDTA treated, and most of 64Cu was dissociated from the complex (~80%) at 24 h time point. 64Cu-radiolabeled chelator C containing the carbonyl donor group was found dissociated rapidly in the presence of the excess EDTA, and 50% of the activity was transchelated by EDTA at 1 h time point. 64Cu-radiolabeled chelators D and F with the respective hydroxyl and thiophenyl donor group were slower in dissociation than 64Cu-radiolabeled chelators B and C and released >45% of 64Cu at 24 h time point. Dissociation of the activity from the 64Cu-radiolabeled chelators was also measured using radio-HPLC at 25 h time point (Figure 3). The peak related to 64C u-EdTA (tR = 2.5 min) was clearly separated from bound 64Cu complex of the chelators. 64Cu-radiolabeled complex of polar chelators E and F have the respective retention time at 4.8 min and 6.2 min, while other 64Cu-radiolabeled complexes have similar retention time (tR = 7~8 min). The 64Cu-radiolabeled chelators C and D gave a small peak (tR = ~3 min and tR = ~7 min, respectively). It is speculated that the less stable complexes interact with mobile phase during HPLC to give the minor unbound peaks.
Table 3.
Stability of 64Cu-radiolabeld complexes in EDTA solution (37 °C, pH 5.5)#
| Time (h) |
Bound complex (%) |
|||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| 0 | 99.9 ± 0.1 | 99.9 ± 0.1 | 97.7 ± 0.2 | 99.5 ± 0.3 | 99.7 ± 0.1 | 100 ± 0.1 |
| 1 | 98.6 ± 0.6 | 90.4 ± 0.1 | 50.4 ± 0.4 | 86.8 ± 0.7 | 98.2 ± 0.3 | 95.7 ± 0.5 |
| 4 | 96.8 ± 0.0 | 69.2 ± 0.3 | 45.8 ± 1.5 | 79.1 ± 0.4 | 92.6 ± 0.3 | 87.5 ± 0.4 |
| 19 | 95.3 ± 0.5 | 22.9 ± 2.1 | 41.0 ± 0.4 | 59.3 ± 0.0 | 93.6 ± 0.1 | 59.5 ± 0.6 |
| 24 | 94.9 ± 0.0 | 19.4 ± 1.1 | 39.0 ± 0.2 | 55.0 ± 0.6 | 94.3 ± 0.6 | 53.4 ± 1.7 |
| 25* | 90.3 ± 0.3 | 11.9 ± 0.6 | 21.7 ± 1.3 | 41.0 ± 2.6 | 93.1 ± 0.1 | 42.3 ± 0.3 |
Bound complex (mean ± standard deviation%) was measured in duplicate using ITLC.
Bound complex (mean ± standard deviation%) was measured in duplicate using radio-HPLC.
Figure 3.
HPLC chromatograms of 64Cu-radiolabeled complex against EDTA challenged for 25 h (37 °C, pH 5.5).
In summary, the in vitro complexation kinetic and stability data indicate that substitution of the N-carboxymethyl group in the NOTA chelating backbone with a different donor group including heteroaromatic ring, carbonyl group, or hydroxyl group gave no measurable effect on radiolabeling efficiency of the chelators and stability of the corresponding complexes in serum. It appears that the donor type in the chelators has little impact on complexation with Cu(II) since the chelators possess the adequate macrocyclic cavity and denticity for effective complexation of Cu(II). All new chelators were found to be highly effective in binding 64Cu. It is noteworthy that pentadentate chelator B rapidly bound to 64Cu and the complex remained intact in serum. However, when rigorously challenged by EDTA at a 100-fold molar excess, 64Cu-radioabeled complexes produced different complex stability profiles. The bifunctional NOTA chelator (A) and pyridyl-containing chelator (E) were well tolerant of EDTA challenge, and only a small amount of the activity was transferred to EDTA. Other chelators B-D and F radiolabeled with 64Cu were slowly or rapidly dissociated to produce 64Cu transchelated to EDTA, although the complexes remained stable in human serum at 37 oC over 2 days. The results of EDTA challenge experiment indicate that among the new chelators B-F tested, the chelator E containing the pyridyl group was the best in forming a stable complex with 64Cu that was comparable to the 64Cu complex of the known chelator A. The pentadentate chelator B was less effective in tightly binding 64Cu than other hexadentate chelators C - F.
3.6. Biodistribution of 64Cu-radiolabeled complexes
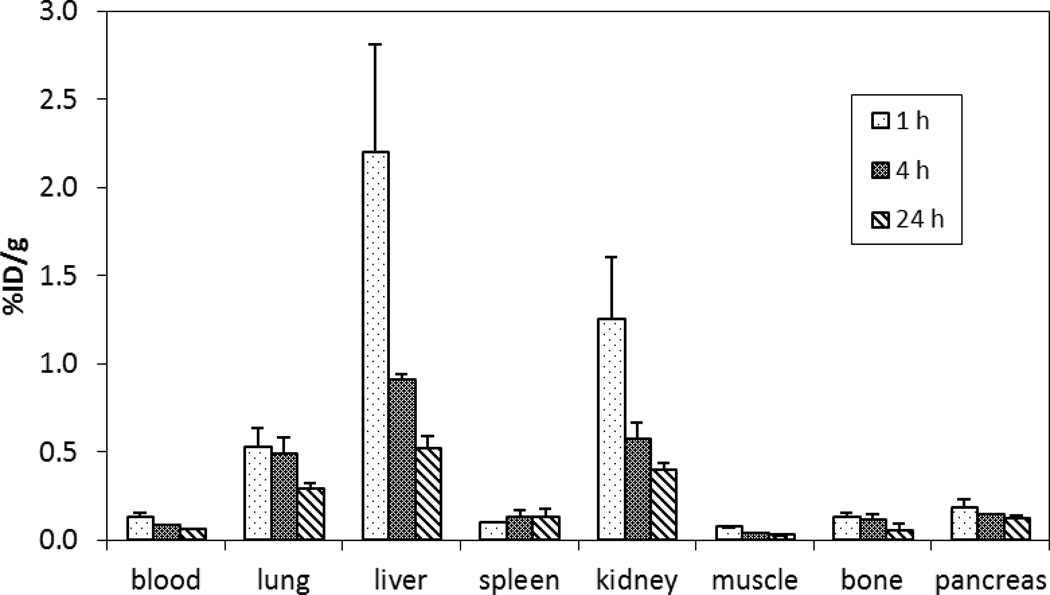
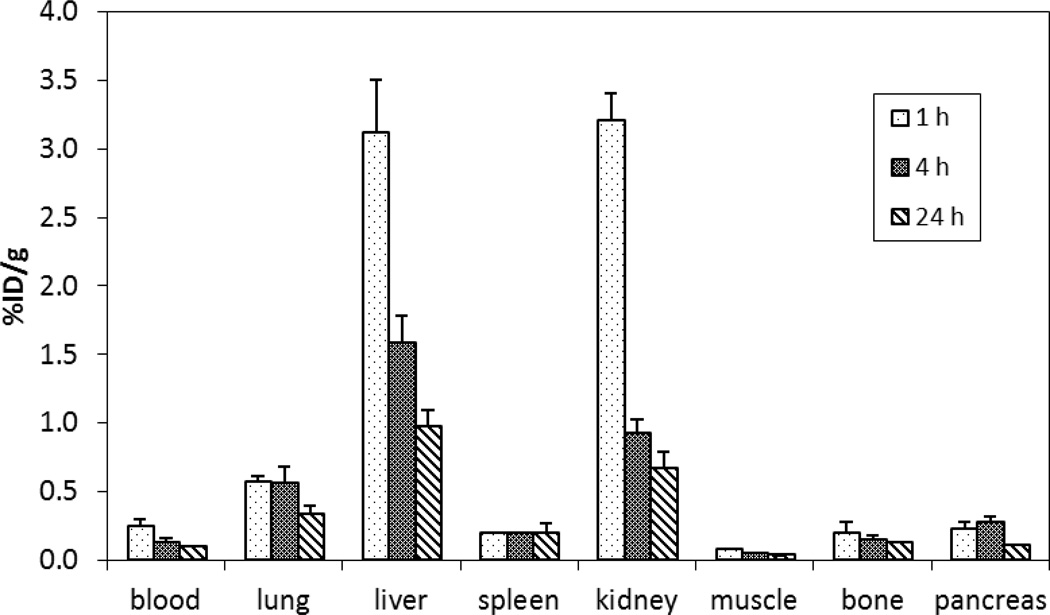
64Cu-radiolabeled complexes of chelators A and E which produced excellent in vitro complexation kinetic and stability data were further evaluated for a biodistribution study using non-tumor bearing mice (iv injection, n = 4). The 64Cu-radiolabeled complexes were independently prepared from reaction of the chelators with 64Cu at 37 °C for 1 h for in vivo biodistribution studies. 64Cu–radiolabeled complexes were evaluated for in vivo stability by measuring radioactivity that accumulated in selected organs and cleared from the blood of mice at three time points, 1 h, 4 h, and 24 h post-injection (n = 4). The results of the biodistribution studies for 64Cu-chelator A and 64Cu-chelator E are shown in Figures 4 and 5, respectively. Both 64Cu-chelator A and 64Cu-chelator E displayed a rapid blood clearance and low uptake in the normal organs. 64Cu-chelator A and 64Cu-chelator E exhibited the highest radioactivity level in the blood at 1 h (0.13 ± 0.03 %ID/g and 0.25 ± 0.05 %ID/g, respectively) which decreased over the time points. The radioactivity accumulated in the blood at 24 h was negligible for both 64Cu-chelator A (0.06 ± 0.00% ID/g) and 64Cu-chelator E (0.10 ± 0.01% ID/g). The 64Cu-radiolabeled complexes exhibited low uptake in the lung, muscle, bone, and pancreas (<0.57% ID/g) which peaked at 1 h and decreased at 24 h. A very low radioactivity level in the spleen over the time points was observed with 64Cu-chelator A (~0.2% ID/g) and 64Cu-chelator E (~0.1% ID/g). 64Cu-chelator E displayed higher renal and liver uptake as compared to 64Cu-chelator A over the course of the study. 64Cu-chelator A exhibited the highest radioactivity level in the liver (2.20 ± 0.62% ID/g) and kidney (1.25 ± 0.35% ID/g) at 1 h that declined to (0.52 ± 0.07% ID/g) and (0.40 ± 0.04% ID/g) at 24 h. A higher radioactivity level at the liver and kidney relative to other normal organs was measured with 64Cu-chelator E. Renal and liver uptake of 64Cu-chelator E peaked at 1 h (3.20 ± 0.21% ID/g and 3.11 ± 0.39% ID/g, respectively) and decreased over the time points (0.67 ±0.12% ID/g and 0.98 ± 0.12% ID/g at 24 h, respectively). The in vivo biodistribution data suggest that 64Cu-chelator E displayed favorable in vivo stability as evidenced by rapid blood clearance and low organ uptake and produced a biodistribution profile comparable to the 64Cu complex of the known NOTA-based chelator A.
Figure 4.
Biodistribution of 64Cu-radiolabeled chelator A in female CD-1 mice following iv injection.
Figure 5.
Biodistribution of 64Cu-radiolabeled chelator E in female CD-1 mice following iv injection.
4. Conclusion
The novel pentadentate or hexadentate NOTA analogues with different donor groups were prepared and evaluated as chelators of 64Cu. The radiolabeling efficiency data indicate that all new chelators instantly and almost completely bound to 64Cu at room temperature. All chelators were efficiently radiolabeled with 64Cu in a broad range of maximum specific activity. The corresponding 64Cu-radiolabeled complexes remained intact in human serum for 2 days. No obvious effect of donor atom and denticity on complexation kinetics and serum stability with 64Cu was observed with the chelators studied. However, the 64Cu-radiolabeled chelators B-D and F when challenged by EDTA lost a significant amount of 64Cu, while 64Cu-radiolabeled complexes of two hexadentate chelators (A and E) were quite inert against the rigorous EDTA challenge and released a minimal amount of the activity for 24 h. The results of EDTA challenge experiments indicate that the pyridyl donor (E) in the chelating backbone is more effective in tightly holding 64Cu than the thiophenyl, carbonyl, and hydroxyl group. The pentadentate chelator B was shown to form a thermodynamically less stable complex with 64Cu and not tolerant of the EDTA challenge. The 64Cu-chelator E containing a pyridyl group produced a biodistribution profile comparable to the known NOTA chelator A and remained stable in mice and displayed low radioactivity level in the blood and the normal organs. The results of the in vitro and in vivo evaluations indicate that the new chelator E is effective in binding 64Cu and will be further modified for use in targeted PET imaging.
Supplementary Material
Acknowledgement
The authors thank for the financial support from the National Institutes of Health (R01 CA136695). Dr. Nilantha Bandara was supported by a DOE Integrated Research Training Program of Excellence in Radiochemistry (DE-SC0002032).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data. HPLC and ITLC chromatograms for assessment of radiolabeling reaction kinetics and serum stability and EDTA challenge and in vivo biodistribution data. This material is available free of charge via the Internet.
References
- 1.Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Curr. Pharm. Design. 2007;13:3–16. doi: 10.2174/138161207779313768. [DOI] [PubMed] [Google Scholar]
- 2.Smith SVJ. Inorg. Biochem. 2004;98:1874–1901. doi: 10.1016/j.jinorgbio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Chem Rev. 2010;110:2858–2902. doi: 10.1021/cr900325h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kukis DL, Li M, Meares CF. Inorg. Chem. 1993;32:3981–3982. [Google Scholar]
- 5.Bass LA, Wang M, Welch MJ, Anderson CJ. Bioconjugate Chem. 2000;11:527–532. doi: 10.1021/bc990167l. [DOI] [PubMed] [Google Scholar]
- 6.Kukis DL, Diril H, Greiner DP, DeNardo SJ, DeNardo GL, Salako QA, Meares CF. Cancer Suppl. 1994;73:779–786. doi: 10.1002/1097-0142(19940201)73:3+<779::aid-cncr2820731306>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Sun X, Wuest M, Weisman GR, Wong EH, Reed DP, Boswell CA, Motekaitis R, Martell AE, Welch MJ, Anderson CJ. J. Med. Chem. 2002;45:469–477. doi: 10.1021/jm0103817. [DOI] [PubMed] [Google Scholar]
- 8.Boswell CA, Sun X, Niu W, Weisman GR, Wong EH, Rheingold AL, Anderson CJ. J. Med. Chem. 2004;47:1465–1474. doi: 10.1021/jm030383m. [DOI] [PubMed] [Google Scholar]
- 9.Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MR, Garrison JC, Hoffman TJ, Sieckman GL, Figueroa SD, Smith CJ. Proc. Natl. Acad. Sci. USA. 2007;104:12462–12467. doi: 10.1073/pnas.0705347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Silva RA, Jain S, Lears KA, Chong HS, Kang CS, Sun X, Rogers BE. Nucl. Med. Biol. 2012;39:1099–1104. doi: 10.1016/j.nucmedbio.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong HS, Song HA, Kang CS, Le T, Sun X, Dadwal M, Lee H, Lan X, Chen Y, Dai A. Chem. Commun. 2011;47:5584–5586. doi: 10.1039/c0cc05707j. [DOI] [PubMed] [Google Scholar]
- 12.Chong HS, Song HA, Birch N, Le T, Lim SY, Ma X. Efficient Synthesis and evaluation of bimodal ligand NETA. Bioorg. Med. Chem. Lett. 2008;18:3436–3439. doi: 10.1016/j.bmcl.2008.03.084. [DOI] [PubMed] [Google Scholar]
- 13.Chong HS, Sun X, Dong P, Kang CS. Eur. J. Org. Chem. 2011;33:6641–6648. doi: 10.1002/ejoc.201101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang CS, Sun X, Jia F, Song HA, Chen Y, Lewis M, Chong HS. Bioconjugate Chem. 2012;23:1775–1782. doi: 10.1021/bc200696b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruper WJ, Rudolf PR, Langhoff CA. J. Org. Chem. 1993;58:3869–3876. [Google Scholar]
- 16.Brechbiel MW, McMurry TJ, Gansow OA. Tetrahedron Lett. 1993;34:3691–3694. [Google Scholar]
- 17.Terent’ev AO, Borisov DA, Yaremenko IA, Chernyshev VV, Nikishin GI. J. Org. Chem. 2010;75:5065–5071. doi: 10.1021/jo100793j. [DOI] [PubMed] [Google Scholar]
- 18.Cao JJ, Zhou F, Zhou J. Angew. Chem. Int. Ed. 2010;49:4976–4980. doi: 10.1002/anie.201000896. [DOI] [PubMed] [Google Scholar]
- 19.Ma D, Lu F, Overstreet T, Milenic DE, Brechbiel MW. Nucl. Med. Biol. 2002;29:91–105. doi: 10.1016/s0969-8051(01)00287-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.