Abstract
Using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), we have developed a simple method to isolate myosin heavy chain (MHC) and actin from small (60–80 mg) human skeletal muscle samples for the determination of their fractional synthesis rates. The amounts of MHC and actin isolated are adequate for the quantification of [13C]leucine abundance by gas chromatography-combustion-isotope ratio mass spectrometry (GC-C-IRMS). Fractional synthesis rates of mixed muscle protein (MMP), MHC, and actin were determined in six healthy young subjects (27 ± 1 yr) after they received a 14-h intravenous infusion (prime = 7.58 μmol/kg body wt, constant infusion = 7.58 μmol·kg body wt−1·h−1) of [1-13C]leucine. The fractional synthesis rates of MMP, MHC, and actin were found to be 0.0468 ± 0.0048, 0.0376 ± 0.0033, and 0.0754 ± 0.0078%/h, respectively. Overall, the synthesis rate of MHC was 20% lower (P = 0.012), and the synthesis rate of actin was 61% higher (P = 0.060, not significant) than the MMP synthesis rate. The isolation of these proteins for isotope abundance analysis by GC-C-IRMS provides important information about the synthesis rates of these specific contractile proteins, as opposed to the more general information provided by the determination of MMP synthesis rates.
Keywords: muscle protein synthesis, amino acid metabolism, protein metabolism, stable isotope tracers, mass spectrometry
MOST STUDIES that have used stable isotope tracer methodology to determine the fractional synthesis rate of human muscle protein have reported the rate of “mixed” muscle protein (MMP) synthesis (14, 15, 23, 24, 26, 27). A problem with the measurement of MMP synthesis is that it reflects the average rate of synthesis of several muscle proteins in the sample (i.e., contractile, enzymatic, mitochondrial). Changes in the synthesis rate of individual contractile proteins could be confounded by corresponding or opposing changes in the synthesis rates of other proteins in the MMP sample. In particular, Rooyackers et al. (19) found that the rate of mitochondrial protein synthesis was ~95% higher than that of MMP in young subjects. Therefore, isolating specific contractile proteins from human muscle samples obtained during a stable isotopically labeled amino acid (e.g., [1-13C]leucine) administration protocol, and measuring the amount of labeled amino acid incorporated into these specific contractile proteins by use of gas isotope ratio mass spectrometry (IRMS) would provide a more refined approach to in vivo studies of muscle contractile protein metabolism.
Recent efforts have focused on isolating the contractile proteins of interest from a small human muscle sample obtained during stable-label amino acid infusion studies. Balagopal et al. (3) have described a method to isolate myosin heavy chain (MHC) from rat and human skeletal muscle tissue by elution gel electrophoresis. They reported that the fractional synthesis rate of MHC for one human subject was 0.0206%/h, which is about one-half that of rates previously reported for human skeletal MMP (14, 15, 24, 26, 27). More recently, Balagopal et al. (2) reported higher MHC synthesis rates for 10 young human subjects, 0.0299 ± 0.0043%/h. This technique has provided valuable information about the fractional synthesis rate of MHC, the major contractile protein found in the muscle cell. However, the approach requires relatively large muscle samples (100–150 mg), is time consuming (13–15 h), and involves the cumbersome procedure of eluting a single muscle protein from a large polyacrylamide gel column. Furthermore, actin, which is another major contractile protein in skeletal muscle, has never been isolated from small human muscle samples for the purpose of determining its fractional rate of synthesis by use of stable isotope tracer methodology. Because MHC and actin may have different synthesis rates (9, 11) and their rates may respond differently to various interventions, it would be useful to isolate actin as well as MHC for the purpose of determining their fractional synthesis rates.
In this study we describe a simple sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) method to isolate MHC and actin from small (60–80 mg) human muscle samples for the quantification of [13C]leucine abundance by gas chromatography-combustion-isotope ratio mass spectrometry (GC-C-IRMS). The fractional synthesis rates of MHC and actin are compared with the rate of MMP determined in the same subjects. Additionally, the synthesis rates of these proteins are examined in terms of whole body protein synthesis and breakdown rates.
MATERIALS AND METHODS
Subjects and study protocol
Six healthy young subjects (Table 1) participated in this study, which was approved by the Human Studies Review Board at Washington University School of Medicine. None of the subjects had participated in any type of weight-lifting exercise for ≥1 yr. Informed consent was obtained after the purpose and procedures were described. Before enrollment, all subjects were screened for cardiovascular, metabolic, and neuromuscular disease. This included a physical examination, a medical history, a blood chemistry profile (sequential metabolic analysis or SMA-9), a complete blood count, and a graded exercise test. Each subject’s body composition was assessed using dual-energy X-ray absorptiometry (Hologic QDR-2000 system, Waltham, MA). The Hologic enhanced whole body analysis software (V 5.71) was used to process the images and determine body fat mass, fat-free mass, and percent body fat.
Table 1.
Subject data
| Subject No. |
Gender | Age, yr |
Weight, kg |
Height, cm |
Body Fat, % |
FFM, kg |
|---|---|---|---|---|---|---|
| 1 | Female | 30 | 52.4 | 152 | 34.1 | 34.6 |
| 2 | Male | 23 | 80.0 | 186 | 24.8 | 60.1 |
| 3 | Female | 30 | 63.0 | 173 | 32.8 | 42.3 |
| 4 | Male | 25 | 68.7 | 179 | 13.8 | 59.2 |
| 5 | Male | 25 | 73.2 | 186 | 8.7 | 66.8 |
| 6 | Female | 30 | 66.2 | 170 | 23.0 | 51.0 |
| Mean±SE | 27±1 | 67.3±3.8 | 174±5.2 | 22.9±4.1 | 52.3±4.9 |
FFM, fat-free mass.
These tests were followed by a 3-day meat-free controlled protein meal plan used for the estimation of myofibrillar protein breakdown and muscle mass by determining 24-h urinary excretion of 3-methylhistidine and creatinine, respectively (4, 8). The weight maintenance meal plan consisted of 1.5 g protein·kg body wt−1·day−1 and 30–36 kcal·kg body wt−1·day−1. It was designed by a research dietician and was prepared in the research kitchen of the General Clinical Research Center (GCRC). On the last day of the diet all subjects were admitted to the GCRC, where they received an overnight 14-h [1-13C]leucine (Mass Trace, Woburn, MA) intravenous infusion (prime = 7.58 μmol/kg body wt, constant infusion = 7.58 μmol·kg body wt−1·h−1) in the fasted condition. Blood samples (5 ml) were collected before and at half-hour intervals during the last 2 h of the [13C]leucine infusion for the determination of plasma [1-13C]ketoisocaproic acid (KIC) enrichment (20). Additionally, breath samples were collected into 20-ml evacuated tubes before and during the last hour of the tracer infusion for 13CO2/12CO2 determination by IRMS. This measurement was used to calculate whole body leucine oxidation rates.
The plasma [13C]KIC enrichment and tissue fluid [13C]leucine enrichment were used as surrogate measures for muscle [13C]leucyl-tRNA enrichment to calculate rates of muscle protein synthesis (2, 3, 5, 14, 19, 23). The percutaneous needle muscle biopsy technique was used to remove a sample (80–120 mg) of muscle tissue from the vastus lateralis ~1.5 h after the [1-13C]leucine infusion was started. A second muscle sample was removed from the contralateral vastus lateralis muscle 13–14 h after the infusion was started. The tissue samples were rinsed and blotted in normal saline and were cleared of any fat and connective tissue. They were immediately frozen in liquid nitrogen and stored at −80°C until analysis.
Sample analyses and calculations
Plasma KIC was isolated, prepared as the trimethylsilyl quinoxalinol derivative (13, 20), and analyzed for [13C] abundance by use of gas chromatography-electron impact quadrupole-mass spectrometry (GC-MS) (HP 5890 Series II and GC/HP 5970 Series MS, Hewlett-Packard, Avondale, PA). Tissue fluid-free amino acids were extracted by homogenization in 10% TCA. The N-heptafluorobutyryl propyl esters were formed (6), and the [13C]leucine abundance was determined using electron capture-negative chemical ionization GC-MS (HP 5988A Series II and GC/HP 5970 Series MS, Hewlett-Packard). The plasma [13C]KIC enrichment and the tissue fluid [13C]leucine enrichment [in atom percent excess (APE)] were used to represent the precursor pool (leucyl tRNA) enrichment (5, 14, 23) for the calculation of protein synthesis rate (Ks; in %/h). The following equation was used to calculate Ks (14)
where (t2 − t1) was the time (h) between muscle biopsies.
Analysis of MMP synthesis rates
To determine the [13C]leucine abundance in MMP, 15- to 30-mg muscle samples were homogenized in 1 ml of 10% TCA (Tissumizer, Tekmar, Cincinnati, OH) and hydrolyzed in 6 N HCl at 110°C for 24 h. The n-acetyl n-propyl (NAP) esters of the component amino acids were formed (1), and the [13C]leucine abundance in the hydrolyzed MMP was determined using GC-C-IRMS, as previously described (25).
Isolation of MHC and actin for analysis of synthesis rates
All procedures for the MHC and actin extractions were performed on ice or at 4°C. Frozen muscle samples (60–80 mg) were homogenized in 1 ml of a 250 mM sucrose buffer (in mM: 250 sucrose, 100 KCl, 5 EDTA, and 20 imidazole, pH 6.8). The homogenate was centrifuged at 1,200 g for 10 min, and the supernatant was discarded. The pellet was suspended in 1 ml of a 0.5% Triton X-100 solution (175 mM KCl, 0.5% Triton X-100, pH 6.8), which is a modification of the solution described by Solaro et al. (21). The suspension was homogenized and centrifuged as before. This step was performed to remove many of the soluble matrix proteins. The resultant pellet was rinsed (i.e., homogenized and centrifuged) with 1 ml wash buffer (150 mM KCl and 20 mM Tris, pH 7.0) to remove excess Triton X-100 solution. The pellet was then suspended in 1 ml of the KCl wash buffer. This suspension was diluted 1:1 in SDS buffer (62.5 mM Tris, 2% SDS, 10% glycerol, 0.001% bromophenol blue, and 5% β-mercaptoethanol), as previously described (10), and boiled for 2 min.
These extracts of myofibrillar protein (containing ~1 mg protein each) were separated by SDS-PAGE. All electrophoresis chemicals were purchased from Bio-Rad Laboratories (Hercules, CA) except Tricine, which was obtained from Sigma Chemical (St. Louis, MO). Each extract was separated on an individual gel with a single wide lane, utilizing a 7% T-2.5% C polyacrylamide slab gel with a 4% T-2.5% C stacking gel. A Bio-Rad Protean II xi vertical slab electrophoresis unit with a Bio-Rad PowerPac 1000 power supply was used. The proteins were separated (150 V, ~3.5 h) using a discontinuous buffer system. The cathode buffer was a Tris-Tricine buffer (1.6 mM Tris, 16 mM Tricine, and 0.01% SDS), and the anode buffer was a Tris buffer (2.5 mM Tris and 0.01% SDS, pH 6.4).
The separated proteins were visualized by Coomassie staining (0.1% Coomassie brilliant blue R 250, 45.0% methanol, and 10.0% glacial acetic acid) for 10–15 min. After overnight destaining (30.0% methanol and 10.0% glacial acetic acid), the bands corresponding to the molecular masses of MHC and actin were identified. These protein bands were carefully cut out within their distinctly stained boundaries and minced into separate tubes. The samples were hydrolyzed in 3–4 ml of concentrated HCl (110°C for 48 h), and the NAP esters of the amino acids were prepared (1) for analysis of [13C]leucine abundance using GC-C-IRMS (25).
Identification of MHC and actin SDS-PAGE bands
The locations of the MHC and actin bands were confirmed by using SDS-PAGE of rat hindlimb muscle samples [soleus and extensor digitorum longus (EDL); 30–50 mg wet wt]. These muscle samples were prepared and separated in the same manner as above. MHC and actin were identified with a molecular weight standard (Amersham RPN 756, Arlington Heights, IL) under identical gel conditions. These bands were further positively identified through Western blot analyses. Briefly, gels of rat hindlimb muscle preparations (soleus and EDL) were transferred to Millipore Immobilon P transfer membranes. The blots were blocked with powdered milk and sequentially incubated with rabbit anti-skeletal myosin (Sigma M-7523) or with rabbit anti-actin (Sigma A-2066). These membranes were then incubated with peroxidaseconjugated anti-rabbit IgG (Sigma A-0545), followed by enhanced chemiluminescence (ECL) reagent (1 min). Subsequently, the proteins were visualized by exposure of the blots to Hyperfilm ECL (Amersham RPN 2106) for 10 s.
Protein quantity and quality of MHC and actin bands
To quantify the protein yield from the Triton X-100 myofibrillar protein extraction used in this study, we determined the protein content of aliquots of rat hindlimb muscle (soleus, n = 8; EDL, n = 8) using the Coomassie Protein Assay Reagent (Pierce 23200, Rockford, IL).
Gel densitometry was utilized to determine the quality of the protein extracts loaded onto the gels. Approximately 30 μg of protein were loaded onto individual lanes of a 12-lane 10% T-2.5% C polyacrylamide slab gel with a 4% T-2.5% C stacking gel. For comparative purposes, 30 μg of myosin (Sigma M-1636) and MHC (Sigma M-7659) from rabbit muscle were run in two of the lanes, and MW standards were run in the outside two lanes. Separate Triton X-100 preparations of soleus (n = 4) and EDL (n = 4) muscles were loaded in the remaining lanes. Gel electrophoresis was done as previously described, until the tracking dye just reached the bottom of the gel (~3 h). The protein bands were visualized by Coomassie staining for 1 h. After overnight destaining, the gel was scanned with a densitometer to quantify the intensity of each protein band. The relative proportions of the intensity of the individual protein bands were determined with a computerized program (Sigma Scan-Pro, V 3.0; SPSS, Chicago, IL). Two identical gels were run in the same manner, and the results from the two gels were averaged.
A total of eight separate single-lane polyacrylamide gels were run under the above conditions with four different acrylamide percentages (5% T-2.5% C, 7% T-2.5% C, 10% T-2.5% C, and 12% T-2.5% C) and two different protein concentrations (200 and 400 μg) of the Triton X-100 extracts of rat muscle (soleus and EDL). This was done to further resolve the protein bands on the gel, so that any proteins that might comigrate with MHC and actin could be visualized.
Reliability of MHC and actin isotopic enrichment measurements
To test the reproducibility of the muscle sample preparation, contractile protein isolation, and [13C]leucine analysis, we performed the entire procedure on four pieces (60–70 mg each) of a single human vastus lateralis muscle sample (277 mg) obtained at the end of a [13C]leucine infusion experiment. Each piece of muscle was individually extracted with Triton X-100 and separated by SDS-PAGE. The gel bands of MHC and actin were carefully cut out, and the proteins were hydrolyzed, chemically derivatized, and analyzed by GC-C-IRMS. For each piece of tissue, enough protein was provided for five MHC and three actin measurements of [13C]leucine on the GC-C-IRMS. These replicate determinations were averaged and compared, and the coefficient of variation was calculated.
Calculation of muscle mass, myofibrillar protein breakdown, and whole body protein synthesis
The whole body muscle mass of each subject was estimated from the mean of three 24-h creatinine excretion measures on the basis of the assumption that 1.0 g/day of urinary creatinine is equivalent to 20.0 kg muscle (8). Total myofibrillar protein breakdown rate was estimated from the mean of three 24-h urinary 3-methylhistidine excretion measures. Myofibrillar protein breakdown calculations were based on the assumption that 3.63 μmol of urinary 3-methylhistidine represent 1 g of degraded myofibrillar protein (4).
The absolute rate of whole body muscle protein synthesis was calculated as the product of the MMP fractional synthesis rate and the whole body muscle mass, with the assumption that muscle protein constitutes 20% of muscle mass (14). This calculation assumes that the synthesis rate of skeletal muscle protein is uniform throughout the body. The whole body protein synthesis rate was calculated as the difference between whole body leucine turnover rate and leucine oxidation rate (12–14), with the assumption that the mean leucine content of skeletal muscle is 7.8% (12).
Estimates of MHC and actin breakdown rates were made with the assumption that the fractional synthesis rate is equivalent to the fractional breakdown rate (i.e., no net protein loss or gain). The total MHC and actin protein weights were calculated with the assumptions that muscle protein constitutes 20% of muscle mass (14), MHC accounts for 25% of the muscle protein mass (17, 28), and that actin comprises 14% of the muscle protein mass (28). Therefore, the product of the fractional synthesis rate and the respective protein mass gives us an estimation of the total MHC and actin breakdown rates.
Statistics
Means ± SE are reported for each contractile protein synthesis rate measured. The fractional synthesis rates of MHC and actin were compared with the rate of MMP by use of a paired Student’s t-test. Regression analyses were performed to evaluate the correlations between the synthesis rates of MHC, actin, and MMP. A significance level of P ≤ 0.05 was chosen for all analyses.
RESULTS
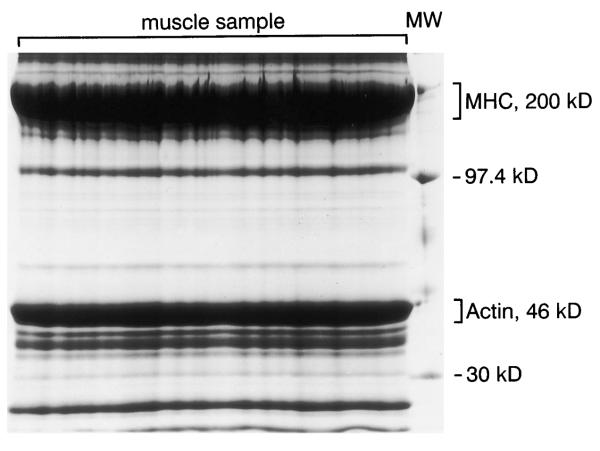
A typical single-lane 7% T-2.5% C gel after SDS-PAGE of ~1 mg protein from human vastus lateralis muscle (70 mg original muscle sample) is shown in Fig. 1. This gel shows large single bands that correspond to the molecular mass markers of 200 and 46 kDa, the approximate molecular masses of MHC and actin, respectively. The MHC and actin bands are easily distinguished from all other protein bands. Two small bands are typically present at a slightly lower molecular mass than the large MHC protein band. These two protein bands were also present in the early eluent fractions of MHC peak during continuous elution gel electrophoresis by Balagopal et al. (3). Using rat myofibrillar protein extracts (200 and 400 μg), we were able to resolve and separately visualize these two small bands from the MHC protein on the 5% T, 7% T, 10% T, and 12% T (2.5% C) polyacrylamide slab gels. However, when larger volumes of protein (as found in our human myofibrillar extracts, ~1 mg protein) were loaded, gels of lower percentages (i.e., 5% T-2.5% C or 7% T-2.5% C) would provide better separation of these high molecular mass (100–200 kDa) proteins (7). No protein bands were found to comigrate with the actin band when 200-or 400-μg rat myofibrillar protein extracts were separated on the 7% T, 10% T, or 12% T (2.5% C) gels. However, because of its low molecular mass, the actin migrated with the buffer front on the 5% T-2.5% C gel. Therefore, we chose the 7% T-2.5% C polyacrylamide gel for optimal separation and resolution of both MHC and actin proteins.
Fig. 1.
A single wide lane 7% T-2.5% C polyacrylamide gel after SDS-PAGE of human vastus lateralis muscle (~1 mg protein; 70 mg original muscle sample weight). A molecular mass (MW) standard was run in the narrow lane at far right. Myosin heavy chain (MHC) and actin bands can be visualized with Coomassie staining at ~200 and 46 kDa, respectively.
All protein bands, though broader, were still separated and well resolved on the 7% T-2.5% C gel after SDS-PAGE with ~1 mg of human myofibrillar protein extract. However, it is possible that trace amounts of contaminating proteins were present in the MHC and actin bands. Balagopal et al. (3) reported that a 140 kDa protein accounted for 1–2% of the protein eluted in their MHC fractions after continuous elution gel electrophoresis.
Protein analyses of rat skeletal muscle showed that the Triton X-100 myofibrillar protein extraction yielded 13.6 ± 1.1 (soleus) and 15.3 ± 1.3 (EDL) μg protein/mg wet weight. The gel densitometric analyses revealed that 28.0 ± 0.8% (soleus) and 32.9 ± 0.6% (EDL) of the extracted protein were MHC, and that 24.8 ± 1.2% (soleus) and 25.4 ± 1.3% (EDL) were actin. By calculation, a 70-mg muscle sample, extracted using the described procedures, would yield ~1,008 μg myofibrillar protein (~14.4 μg protein/mg wet weight) that would be loaded and separated by SDS-PAGE. Because the total protein extracted was ~30.5% MHC and ~25.1% actin, 1,008 μg myofibrillar protein should consist of 307 μg MHC and 253 μg actin. When the MHC and actin bands are cut from the gel and hydrolyzed, the MHC should yield 31 μg leucine and the actin should yield 20 μg leucine, with the assumption that MHC and actin are 10 and 7.8% leucine by weight, respectively (12, 22). We have found these amounts of leucine to be adequate for about five MHC and three actin determinations of [13C]leucine by GC-C-IRMS.
Typical GC-C-IRMS chromatograms for CO2 gas (mass-to-charge ratio, or m/z, 44 ion), obtained from the combustion of NAP-leucine and NAP-isoleucine isolated from human MHC and actin, are shown in Figs. 2 and 3. These figures demonstrate that adequate CO2 is evolved from the combustion of 6–7 μg leucine isolated from human MHC and actin. Table 2 shows the high reproducibility of this technique for isolating MHC and actin for measurement of [13C]leucine isotope enrichment using GC-C-IRMS. When four samples taken from a single human muscle sample were individually extracted, separated, derivatized, and analyzed, the coefficients of variation were 0.013 for MHC [13C]leucine enrichment (~0.052 APE) and 0.016 for actin [13C]leucine enrichment (~0.076 APE).
Fig. 2.
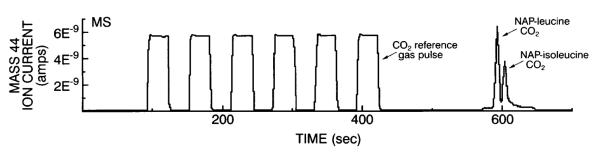
Typical mass spectrometer tracings for CO2 gas (mass 44) obtained from the combustion of n-acetyl n-propyl (NAP)-leucine and NAP-isoleucine isolated from human vastus lateralis muscle myosin heavy chain (MHC) protein hydrolysates. Six reference gas (CO2) square-wave pulses are also shown.
Fig. 3.
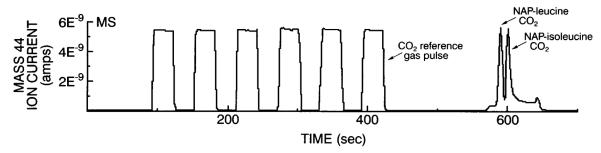
Typical mass spectrometer tracings for CO2 gas (mass 44) obtained from the combustion of NAP-leucine and NAP-isoleucine isolated from human vastus lateralis muscle actin protein hydrolysates. Six reference gas (CO2) square-wave pulses are also shown.
Table 2.
Reproducibility of MHC and actin enrichment measurements
| MHC (n=5) | Actin (n=3) | |
|---|---|---|
| Sample 1 | 1.0846±0.0001 | 1.0869±0.0003 |
| Sample 2 | 1.0846±0.0001 | 1.0870±0.0005 |
| Sample 3 | 1.0849±0.0002 | 1.0871±0.0002 |
| Sample 4 | 1.0847±0.0002 | 1.0867±0.0002 |
| Mean±SE | 1.0847±0.00007 | 1.0869±0.00008 |
| CV, % | 0.013 | 0.016 |
[13C]leucine enrichment values (means±SE) are expressed in atom % excess (APE). MHC, myosin heavy chain; CV, coefficient of variation.
The plasma [13C]KIC and tissue fluid [13C]leucine enrichments are given in Table 3. The mean tissue fluid [13C]leucine was 75.3% of the plasma [13C]KIC, and they were significantly correlated (R2 = 0.803, P = 0.016). Although the tissue fluid [13C]leucine enrichments were not significantly different between the first and second muscle biopsies of each subject (P = 0.561), the SE was considerably higher than that of the plasma [13C]KIC enrichments. Therefore, because of the greater sample number of plasma KIC measurements and the greater reliability compared with tissue fluid leucine measurements, we chose to use the plasma [13C]KIC enrichment for the calculation of muscle protein fractional synthesis rates that are shown in Tables 4–6. With the present study design, the use of tissue fluid [13C]leucine enrichment would increase the muscle protein synthesis rates but would not affect the relative comparisons among MMP, MHC, and actin synthesis rates (Table 6).
Table 3.
13C enrichment of plasma KIC and tissue fluid leucine
| Subject No. |
Plasma [13C]KIC Enrichment (n=5) |
Tissue Fluid [13C]Leucine Enrichment (n=2) |
|---|---|---|
| 1 | 7.11±0.14 | 6.02±0.82 |
| 2 | 7.10±0.17 | 5.93±0.32 |
| 3 | 7.09±0.14 | 5.72±0.24 |
| 4 | 6.81±0.15 | 4.45±1.38 |
| 5 | 6.85±0.08 | 4.55±1.05 |
| 6 | 7.06±0.14 | 4.92±0.24 |
| Mean±SE | 7.00±0.14 | 5.27±0.29 |
Values are means±SE expressed in APE. [13C]KIC, [1-13C]ketoisocaproic acid.
Table 4.
[13C]leucine enrichment increment in human MMP, MHC, and actin
| Subject No. |
[13C]leucine Enrichment Increment |
||
|---|---|---|---|
| MMP | MHC | Actin | |
| 1 | 0.0407 | 0.0275 | 0.0495 |
| 2 | 0.0286 | 0.0264 | 0.0770 |
| 3 | 0.0550 | 0.0451 | 0.0495 |
| 4 | 0.0407 | 0.0308 | 0.0715 |
| 5 | 0.0462 | 0.0352 | 0.0539 |
| 6 | 0.0308 | 0.0297 | 0.0902 |
| Mean±SE | 0.0403±0.0040 | 0.0325±0.0028 | 0.0653±0.0069 |
Increment values are expressed in APE. MMP, mixed muscle protein; MHC, myosin heavy chain.
Table 6.
Mean fractional synthesis rates of MMP, MHC, and actin by calculation with different surrogate measures of [13C]leucyl-tRNA precursor pool
| Precursor Pool | MMP (n=6) |
MHC (n=6) |
Actin (n=6) |
|---|---|---|---|
| Plasma KIC | 0.0468±0.0048 | 0.0376±0.0033 | 0.0754±0.0078 |
| Tissue fluid leucine |
0.0633±0.0073 | 0.0508±0.0052 | 0.1027±0.0132 |
Values are means ± SE expressed in %/h. Values calculated with tissue fluid [13C]leucine enrichment were ~35% greater than rates calculated with plasma [13C]KIC enrichment (P=0.004).
The increments in [13C]leucine abundance in MMP, MHC, and actin during the [13C]leucine infusion are shown in Table 4. The fractional synthesis rates (%/h) for MMP, MHC, and actin for the six young subjects are shown in Table 5. If tissue fluid [13C]leucine enrichment was used in place of plasma [13C]KIC enrichment to represent the precursor pool enrichment in the calculations of muscle protein fractional synthesis, the rates of MMP, MHC, and actin were ~35% higher (Table 6).
Table 5.
Fractional synthesis rates for human MMP, MHC, and actin by calculation with plasma [13C]KIC as precursor pool
| Subject No. | MMP | MHC | Actin |
|---|---|---|---|
| 1 | 0.0463 | 0.0313 | 0.0563 |
| 2 | 0.0321 | 0.0297 | 0.0866 |
| 3 | 0.0629 | 0.0516 | 0.0566 |
| 4 | 0.0490 | 0.0371 | 0.0861 |
| 5 | 0.0552 | 0.0421 | 0.0644 |
| 6 | 0.0350 | 0.0337 | 0.1025 |
| Mean±SE | 0.0468±0.0048 | 0.0376±0.0033 | 0.0754±0.0078 |
Fractional synthesis rates are expressed in %/h.
For this limited number of subjects, no gender differences were observed for the synthesis rates of MMP [P = 0.826, nonsignificant (NS)], MHC (P = 0.629, NS), or actin (P = 0.912, NS). For all subjects the MHC synthesis rate was less than the MMP rate (P = 0.012), and for five of the six subjects the actin synthesis rate was greater than the MMP (P = 0.060, NS). On average, the fractional synthesis rate of MHC was 20% lower and the synthesis rate of actin was 61% higher than that of MMP. A significant correlation existed between MHC and MMP synthesis rates (R2 = 0.793; P = 0.017), but the correlation between actin and MMP synthesis rates did not reach significance (R2 = 0.572; P = 0.081, NS).
Table 7 gives the estimated whole body muscle mass and the estimated breakdown rates of myofibrillar protein, MHC, and actin for each subject. We found that the estimated breakdown rate of MHC was ~28% of the myofibrillar protein breakdown rate, and that the estimated actin breakdown rate accounted for ~33% of the myofibrillar protein breakdown rate. Together, estimated MHC and actin breakdown rates were ~61% of the myofibrillar protein breakdown rate that was estimated from urinary 3-methylhistidine excretion. If tissue fluid [13C]leucine values were used in place of plasma [13C]KIC values to represent the precursor pool in the calculations of muscle protein fractional synthesis, the estimated breakdown rates of MHC and actin were ~35% higher: 11.73 ± 1.22 and 13.63 ± 2.22 mg·kg body wt−1·h−1. In this case, the estimated breakdown rates of MHC and actin would account for 84% of the total protein breakdown rate (39 and 45%, respectively).
Table 7.
Estimated muscle mass and breakdown rates of myofibrillar protein, MHC, and actin
| Breakdown Rates, mg·kg body wt−1·h−1 |
||||
|---|---|---|---|---|
| Subject No. |
Muscle Mass, kg |
Myofibrillar protein |
MHC | Actin |
| 1 | 21.2 | 30.0 | 6.33 | 6.38 |
| 2 | 39.8 | 33.4 | 7.38 | 12.08 |
| 3 | 23.8 | 22.9 | 10.46 | 6.42 |
| 4 | 33.5 | 36.6 | 9.67 | 12.54 |
| 5 | 31.1 | 33.5 | 9.54 | 8.17 |
| 6 | 30.6 | 25.0 | 8.33 | 14.17 |
| Mean±SE | 30.0±2.7 | 30.3±2.2 | 8.62±0.64 | 9.96±1.38 |
Whole body leucine kinetics data and the whole body muscle protein synthesis rate are given in Table 8. The mean whole body muscle protein synthesis rate was significantly greater than the mean myofibrillar protein breakdown rate, 40.7 ± 3.1 vs. 30.3 ± 2.2 mg·kg body wt−1·h−1 (P = 0.031). If the whole body muscle protein synthesis rates were calculated from the fractional synthesis rate of MMP with tissue fluid [13C]leucine used as the precursor pool rather than plasma [13C]KIC, the mean value for whole body muscle protein synthesis was even greater, 54.1 ± 4.1 mg·kg body wt−1·h−1.
Table 8.
Whole body leucine kinetics and whole body muscle protein synthesis
| Subject No. |
Leucine Flux |
Leucine Oxidation |
Nonoxidative Portion of Leucine Flux |
Whole Body Protein Synthesis |
Whole Body Muscle Protein Synthesis |
|---|---|---|---|---|---|
| 1 | 97.7 | 27.4 | 70.3 | 118.9 | 37.4 |
| 2 | 97.2 | 25.2 | 72.0 | 121.8 | 31.9 |
| 3 | 105.1 | 23.3 | 81.8 | 138.5 | 47.5 |
| 4 | 108.5 | 26.2 | 82.3 | 139.3 | 47.8 |
| 5 | 109.3 | 24.2 | 85.1 | 143.9 | 46.9 |
| 6 | 104.0 | 22.0 | 82.0 | 138.7 | 32.4 |
| Mean±SE | 103.6±2.1 | 24.7±0.8 | 78.9±2.5 | 133.5±4.3 | 40.7±3.1 |
Leucine flux, leucine oxidation, and nonoxidative portion of leucine flux are given in μmol·kg body wt−1·h−1; whole body protein synthesis and muscle protein synthesis rates are given in mg·kg body wt−1·h−1.
With the assumption that MHC accounts for ~25% of total skeletal muscle protein (17, 28), the absolute rate of whole body MHC synthesis would be 8.4 ± 0.5 mg·kg body wt−1·h−1. With the assumption that actin comprises 14% of the total muscle protein mass (28), the absolute rate of whole body actin synthesis would be 9.4 ± 0.7 mg·kg body wt−1·h−1. As with the whole body muscle protein synthesis rate, these calculations are based on the assumption that the synthesis rates of MHC and actin are uniform throughout the body. When the fractional synthesis rates obtained with tissue fluid [13C]leucine are used as the precursor pool, the whole body MHC and actin synthesis rates increase to 11.2 ± 0.7 and 12.5 ± 0.9 mg·kg body wt−1·h−1, respectively.
DISCUSSION
We have developed a highly reliable method for isolating MHC and actin from small (60–80 mg) human skeletal muscle samples for determination of their fractional synthesis rates. The fractional synthesis rate of MHC when this methodology was used was 0.0376 ± 0.0033%/h, which was somewhat greater than the value of 0.0299 ± 0.0043%/h previously reported (2). Balagopal et al. (2) found the MHC fractional synthesis rates to vary greatly among individual subjects, with a range of 0.0123 to 0.0497%/h. However, the MHC synthesis rates of our subjects were not quite as variable, ranging from 0.0297 to 0.0516%/h. We also found the fractional synthesis rate of MMP to be higher than previously reported (2): 0.0468 ± 0.0048 vs. 0.0408 ± 0.0032%/h. Because the range of synthesis rates of both MMP and MHC showed considerable overlap between the two studies, individual subject variation could have accounted for these differences. Similarly, Balagopal et al. (2) reported that the fractional synthesis rate of MHC was 72% that of MMP, and we found the percentage to be ~80%.
No previous studies have examined the fractional synthesis rate of actin in human muscle tissue by using stable isotope tracer methodology and mass spectrometric detection. Animal studies of actin synthesis rates have provided contradictory information (9, 11, 16). It has been reported that the synthesis rates of actin and MHC are similar in the longissimus dorsi and hindlimb muscles of piglets (16), that the synthesis rate of actin is lower than that of MHC in rabbit skeletal muscle (muscles not specified) (9), but that the synthesis rate of actin is higher than that of MHC in the rat diaphragm (11). In the vastus lateralis muscle of healthy young adults, we found the fractional synthesis rate of actin to be approximately twofold that of MHC.
Balagopal et al. (2) previously reported that tissue fluid [13C]leucine values were ~67% of plasma [13C]KIC values. Similarly, we found that the tissue fluid [13C]leucine values were ~75% of the [13C]KIC values for this group of subjects. Therefore, the fractional synthesis rates of MMP, MHC, and actin were ~35% higher if tissue fluid [13C]leucine values were used in place of plasma [13C]KIC values to represent the precursor pool. We found the measurement of plasma [13C]KIC to be more reliable than the measurement of tissue fluid [13C]leucine, although it has been suggested that tissue fluid [13C]leucine may be a better surrogate measure of [13C]leucyl-tRNA enrichment than plasma [13C]KIC (2). Both precursor pools have been shown to be reliable predictors of [13C]leucyl-tRNA enrichment in the skeletal muscle of the swine (5). Even though the choice of precursor pool measurement alters the absolute values, the relative relationship between the fractional synthesis rates of these proteins remains the same. The choice of precursor pool is probably not crucial when relative differences in protein synthesis rates are examined.
However, the absolute rate of protein fractional synthesis becomes important when the contribution of the various proteins to whole body protein metabolism is calculated. For example, the estimated breakdown rates of MHC and actin accounted for 61% of the estimated myofibrillar protein breakdown rate when plasma [13C]KIC was used as the precursor pool enrichment for the calculation of MHC and actin fractional synthesis rates. However, this percentage increased to 84% of the myofibrillar protein breakdown rate when tissue fluid [13C]leucine was used as the precursor pool enrichment.
Similarly, when tissue fluid [13C]leucine enrichment was used for the precursor pool, the calculated whole body synthesis rates of muscle protein, MHC, and actin increased by ~35%. For this group of subjects, the contribution of whole body muscle protein synthesis to whole body protein synthesis was ~30% when the fractional synthesis rate of MMP was calculated with plasma [13C]KIC values. This is similar to the previous finding of Nair et al. (14) that the whole body muscle protein synthesis rate accounted for 27% of the whole body protein synthesis rate. However, if the MMP fractional synthesis rate based on tissue fluid [13C]leucine is used in our calculations, the whole body muscle protein synthesis rate represents ~40% of the whole body protein synthesis rate. This more closely corresponds to the findings of Rennie et al. (18), who reported that the fasting whole body muscle protein synthesis rate represents 44% of the whole body synthesis rate. Both of these previous studies (14, 18) used plasma [13C]KIC as the precursor pool enrichment in the MMP synthesis rate calculations.
We found that the whole body synthesis rates of MHC and actin represented 21 and 23% of the whole body muscle protein synthesis rate. This is in close agreement with Balagopal et al. (2), who found that whole body MHC synthesis contributed 18% to whole body muscle protein synthesis. Using the MHC and actin fractional synthesis rates based on plasma [13C]KIC, we found that the whole body synthesis rates of MHC and actin represented 6 and 7% of whole body protein synthesis, respectively. This is also in agreement with the previous finding (2) that whole body MHC synthesis contributed 5% to the whole body protein synthesis. When tissue fluid [13C]leucine was used in our calculations of fractional synthesis rates, the contribution of MHC and actin to whole body protein synthesis increased to 8 and 9%.
In conclusion, we have developed a simple and reliable method to isolate MHC and actin from small human skeletal muscle samples by utilizing SDS-PAGE. The amount of MHC and actin isolated are adequate for GC-C-IRMS detection of the [13C]leucine abundance and for the calculation of MHC and actin fractional synthesis rates. This information is valuable, because muscle contractile proteins have different synthesis rates that are most likely differentially regulated. In the future, the isolation of these proteins for isotope abundance analysis by GC-C-IRMS could provide more information about the effects of various clinical conditions (e.g., cachexia, diabetes, obesity, muscular dystrophy) and suitable interventions (e.g., exercise, hormone administration, nutritional therapy, gene therapy) on the synthesis rates of these specific contractile proteins, as opposed to the more general information provided by MMP synthesis rates.
Acknowledgments
The authors gratefully acknowledge Bingbing Li, Jina Pak, Jan Crowley, Jennifer Gischler, and Dr. Mickey Latour for their skilled technical assistance, and the dietetic and nursing staff of the General Clinical Research Center of Washington University School of Medicine for their support.
This work was supported by the Claude Pepper Older Americans Independence Center Grant AG-13629 and National Institutes of Health (NIH) Grants DK-49393, RR-00954, and RR-00036. D. L. Hasten was supported by NIH National Research Service Award AG-05771.
REFERENCES
- 1.Adams RF. Determination of amino acid profiles in biological samples by gas chromatography. J. Chromatogr. 1974;95:189–212. doi: 10.1016/s0021-9673(00)84078-9. [DOI] [PubMed] [Google Scholar]
- 2.Balagopal P, Ljungqvist O, Nair KS. Skeletal muscle myosin heavy-chain synthesis rate in healthy humans. Am. J. Physiol. 1997;272:E45–E50. doi: 10.1152/ajpendo.1997.272.1.E45. (Endocrinol. Metab. 35) [DOI] [PubMed] [Google Scholar]
- 3.Balagopal P, Nair KS, Stirewalt WS. Isolation of myosin heavy chain from small skeletal muscle samples by preparative continuous elution gel electrophoresis; application to measurement of synthesis rate in human and animal tissue. Anal. Biochem. 1994;221:72–77. doi: 10.1006/abio.1994.1381. [DOI] [PubMed] [Google Scholar]
- 4.Ballard FJ, Tomas FM. 3-Methylhistidine as a measure of skeletal muscle protein breakdown in human subjects: the case for its continued use. Clin. Sci. (Colch.) 1983;65:209–215. doi: 10.1042/cs0650209. [DOI] [PubMed] [Google Scholar]
- 5.Baumann PQ, Stirewalt WS, O’Rourke BD, Howard D, Nair KS. Precursor pools of protein synthesis: a stable isotope study in a swine model. Am. J. Physiol. 1994;267:E203–E209. doi: 10.1152/ajpendo.1994.267.2.E203. (Endocrinol. Metab. 30) [DOI] [PubMed] [Google Scholar]
- 6.Crowley JR, Yarasheski K, Leeuwenburgh C, Turk J, Heinecke JW. Isotope dilution mass spectrometric quantification of 3-nitrotyrosine in proteins and tissues is facilitated by reduction to 3-aminotyrosine. Anal. Biochem. 1998;259:127–135. doi: 10.1006/abio.1998.2635. [DOI] [PubMed] [Google Scholar]
- 7.Hames BD. An introduction to polyacrylamide gel electrophoresis. In: Hames BD, Rickwood D, editors. Gel Electrophoresis of Proteins. IRL Press; Oxford: 1981. pp. 12–15. [Google Scholar]
- 8.Heymsfield SB, Artega C, McManus C, Smith J, Moffit S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am. J. Clin. Nutr. 1983;37:478–494. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 9.Koizumi T. Turnover rates of structural proteins of rabbit skeletal muscle. J. Biochem. 1974;76:431–439. doi: 10.1093/oxfordjournals.jbchem.a130585. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond.) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Low RB, Goldberg AL. Nonuniform rates of turnover of myofibrillar proteins in rat diaphragm. J. Cell Biol. 1973;56:590–595. doi: 10.1083/jcb.56.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews DE, Motil KJ, Rohrbaugh DK, Burke JF, Young VR, Bier DM. Measurement of leucine metabolism in man from a primed, continuous infusion of l-[113C]leucine. Am. J. Physiol. 1980;238:E473–E479. doi: 10.1152/ajpendo.1980.238.5.E473. (Endocrinol. Metab. 1) [DOI] [PubMed] [Google Scholar]
- 13.Matthews DE, Schwarz HP, Yang RD, Motil KJ, Young VR, Bier DM. Relationship of plasma leucine and alpha-ketoisocaproate during a l-[13C]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism. 1982;31:1105–1112. doi: 10.1016/0026-0495(82)90160-3. [DOI] [PubMed] [Google Scholar]
- 14.Nair KS, Halliday D, Griggs RC. Leucine incorporation into mixed skeletal muscle protein in humans. Am. J. Physiol. 1988;254:E208–E213. doi: 10.1152/ajpendo.1988.254.2.E208. (Endocrinol. Metab. 17) [DOI] [PubMed] [Google Scholar]
- 15.Nair KS, Welle SL, Halliday D, Campbell RG. Effect of β-hydroxybutyrate on whole-body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. J. Clin. Invest. 1988;82:198–205. doi: 10.1172/JCI113570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry BN. Protein turnover in skeletal muscle of piglets. Br. J. Nutr. 1974;31:35–45. doi: 10.1079/bjn19740006. [DOI] [PubMed] [Google Scholar]
- 17.Pollard TD. Cytoplasmic contractile proteins. J. Cell Biol. 1981;91:156s–165s. doi: 10.1083/jcb.91.3.156s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rennie MJ, Edwards RHT, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin. Sci. (Colch.) 1982;63:519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- 19.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc. Natl. Acad. Sci. USA. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarz HP, Karl IE, Bier DM. The α-keto acids of branched-chain amino acids: simplified derivatization for physiological samples and complete separation as quinoxalinols by packed column gas chromatography. Anal. Biochem. 1980;108:360–366. doi: 10.1016/0003-2697(80)90600-4. [DOI] [PubMed] [Google Scholar]
- 21.Solaro RJ, Pang DC, Briggs FN. The purification of cardiac myofibrils with Triton X-100. Biochim. Biophys. Acta. 1971;245:259–262. doi: 10.1016/0005-2728(71)90033-8. [DOI] [PubMed] [Google Scholar]
- 22.Tada M, Bailin G, Barany K, Barany M. Proteolytic fragmentation of bovine heart heavy meromyosin. Biochemistry. 1969;8:4842–4850. doi: 10.1021/bi00840a029. [DOI] [PubMed] [Google Scholar]
- 23.Watt PW, Lindsay Y, Scrimgeour CM, Chien PAF, Gibson JNA, Taylor DJ, Rennie MJ. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: use in studies of human tissue protein synthesis. Proc. Natl. Acad. Sci. USA. 1991;88:5892–5896. doi: 10.1073/pnas.88.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yarasheski KE, Campbell JA, Smith K, Rennie MJ, Holloszy JO, Bier DM. Effect of growth hormone and resistance exercise on muscle growth in young men. Am. J. Physiol. 1992;262:E261–E267. doi: 10.1152/ajpendo.1992.262.3.E261. (Endocrinol. Metab. 25) [DOI] [PubMed] [Google Scholar]
- 25.Yarasheski KE, Smith K, Rennie MJ, Bier DM. Measurement of muscle protein fractional synthetic rate by capillary gas chromatography combustion isotope ratio mass spectrometry. Biol. Mass Spectrom. 1982;21:486–490. doi: 10.1002/bms.1200211004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yarasheski KE, Zachwieja JJ, Angelopoulos TJ, Bier DB. Short-term growth hormone treatment does not increase muscle protein synthesis in experienced weight lifters. J. Appl. Physiol. 1993;74:3073–3076. doi: 10.1152/jappl.1993.74.6.3073. [DOI] [PubMed] [Google Scholar]
- 27.Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am. J. Physiol. 1993;265:E210–E214. doi: 10.1152/ajpendo.1993.265.2.E210. (Endocrinol. Metab. 28) [DOI] [PubMed] [Google Scholar]
- 28.Yates LD, Greaser ML. Quantitative determination of myosin and actin in rabbit skeletal muscle. J. Mol. Biol. 1983;168:123–141. doi: 10.1016/s0022-2836(83)80326-x. [DOI] [PubMed] [Google Scholar]