Abstract
etv2 is an endothelial-specific ETS transcription factor that is essential for vascular differentiation and morphogenesis in vertebrates. While recent data suggest that Etv2 is dynamically regulated during vascular development, little is known about the mechanisms involved in this process. Here, we find that etv2 transcript and protein expression are highly dynamic during zebrafish vascular development, with both apparent during early somitogenesis and subsequently down-regulated as development proceeds. Inducible knockdown of Etv2 in zebrafish embryos prior to mid-somitogenesis stages, but not later, caused severe vascular defects, suggesting a specific role in early commitment of lateral mesoderm to the endothelial linage. Accordingly, Etv2-overexpressing cells showed an enhanced ability to commit to endothelial lineages in mosaic embryos. We further find that the etv2 3’ untranslated region (UTR) is capable of repressing an endothelial autonomous transgene and contains binding sites for members of the let-7 family of microRNAs. Ectopic expression of let-7a could repress the etv2 3’UTR in sensor assays and was also able to block endogenous Etv2 protein expression, leading to concomitant reduction of endothelial genes. Finally, we observed that Etv2 protein levels persisted in maternal-zygotic dicer1 mutant embryos, suggesting that microRNAs contribute to its repression during vascular development. Taken together, our results suggest that etv2 acts during early development to specify endothelial lineages and is then down-regulated, in part through post-transcriptional repression by microRNAs, to allow normal vascular development.
Keywords: Etv2, Let-7, endothelial, angioblast, zebrafish, post-transcriptional regulation, microRNA
Introduction
The vertebrate circulatory system serves as an essential conduit for the systemic distribution of oxygenated blood, nutrients, hormones, immunological factors and the removal of metabolic waste. The formation of a patent and functional circulatory system begins before the onset of blood circulation with the specification of endothelial progenitors, or angioblasts, from the lateral mesoderm. As angioblasts differentiate and express an endothelial gene program, they migrate and coalesce to form vascular cords through a process called vasculogenesis (Cleaver and Krieg, 1999; Risau and Flamme, 1995). This initial vascular plexus is subsequently remodeled and extended into a system of patent blood vessels through a process referred to as angiogenesis (Cleaver and Krieg, 1999). While the morphological events that define vasculogenesis and angiogenesis are relatively well-defined, the transcriptional regulatory networks that control angioblast specification and subsequent endothelial differentiation are poorly understood.
Multiple transcription factor families have been implicated in the activation and maintenance of endothelial gene expression, including members of the Sox, Forkhead, GATA, and Krüppel-like families (De Val and Black, 2009). Among the most prevalent transcription factors involved in endothelial biology are members of the ETS family. ETS transcription factors are defined by the presence of a conserved, approximately 85-amino acid DNA-binding domain, referred to as the ETS domain, which consists of a winged helix-turn-helix motif that binds a core DNA sequence of 5’-GGA(A/T)-3’ (Sharrocks, 2001). There are numerous ETS factors expressed in vertebrate endothelial cells including ETS1, ETS2, ETV2 (etsrp/ER71), ETV6 (TEL), FLI1, ERG and ELK3 (NET/SAP2; De Val and Black, 2009; Dejana et al., 2007; Lelievre et al., 2001). Most characterized endothelial gene promoters or enhancers contain essential ETS-binding sites (De Val et al., 2008; Dejana et al., 2007; Hollenhorst et al., 2004; Liu and Patient, 2008) and it has been proposed that nearly every endothelial gene may be regulated by ETS factors in some manner (Dejana et al., 2007). Indeed, the founding member of the ETS family, Ets1, which is highly expressed in endothelial cells in multiple species (Maroulakou et al., 1994; Pham et al., 2007; Stiegler et al., 1993), is capable of directly binding to elements flanking genes encoding receptors important for vascular morphogenesis, including Vegf receptors−1 and −2 (Flt1 and Kdr, respectively; Kappel et al., 2000; Wakiya et al., 1996), Tie−2 (Iljin et al., 1999), and neuropilin-1 (Teruyama et al., 2001). Targeted deletion or knockdown of individual ETS factors can cause specific developmental defects in embryonic vascular morphogenesis or function, although in many cases mice bearing single gene deletions are viable. For example, mouse embryos lacking fli1 alone die at E12.5 due to poor blood vessel integrity and cranial hemorrhage (Spyropoulos et al., 2000). By contrast, Ets1-deficient mice are viable with no overt vascular defects (Barton et al., 1998) and only mild defects have been noted following knockdown of ets1 in zebrafish (Pham et al., 2007). The highly conserved DNA binding domain shared between ETS factors and their overlapping expression in endothelial cells likely contributes to some degree of functional redundancy that reduces the severity of vascular defects in these cases. Indeed, ETS factors share significant consensus DNA-binding specificity (Wei et al., 2010) and can bind to and transactivate the same consensus sequences in some promoters (Hollenhorst et al., 2004; Hollenhorst et al., 2011). Analysis of double knockout mice further supports at least partially overlapping functions among some ETS factors. For example, mouse embryos lacking either Ets1 or Ets2 alone display relatively normal vascular development. However, combined loss of both Ets1 and 2 leads to embryonic lethality between E11.5 and E15.5 due in part to defects in vessel remodeling and diminished angiogenic branching (Wei et al., 2009). Similarly, combined reduction of related ETS factors in zebrafish results in a higher penetrance of defects and a block in angiogenesis (Pham et al., 2007).
In contrast to the milder vascular phenotypes associated with loss of some endothelial ETS factors, mouse or zebrafish embryos lacking Ets-variant protein 2 (Etv2; also known as Ets-related protein/Etsrp and ER71) show profound defects at the earliest stages of vascular development. Etv2-deficient mouse embryos fail to specify hematopoietic and endothelial cell lineages leading to embryonic lethality at E9.5 due to a failure to develop a functional circulatory system (Ferdous et al., 2009; Lee et al., 2008). Zebrafish etv2 mutants and morphants exhibit severe reduction in the expression of most endothelial genes, including, kdrl, flt4, cdh5, and plxnd1 and display defects in the morphogenesis of the major trunk blood vessels (Pham et al., 2007; Sumanas and Lin, 2006). The severe early vascular defects and global effects on endothelial gene expression in both mouse and zebrafish embryos suggests that etv2 plays an early role in specifying endothelial cell lineages. Consistent with this possibility, overexpression Etv2 in both zebrafish embryos and mouse embryoid bodies can expand endothelial cell lineages and induce concomitant expression of hundreds of vascular genes (Gomez et al., 2012; Koyano-Nakagawa et al., 2012; Sumanas and Lin, 2006; Wong et al., 2009). Furthermore, recent studies demonstrate that Etv2 is an essential component, along with Fli1 and Erg, during direct endothelial reprogramming of human amniotic cells (Ginsberg et al., 2012). Together, these studies suggest a central role for Etv2 in the early commitment of mesodermal cells to the endothelial lineage during the initial stages of vascular development.
Despite the importance of Etv2 during early vascular development, its role during later stages is unclear. Evidence suggests that Etv2 may only be expressed in endothelial progenitors early during mouse development (E9.5), while expression in the zebrafish is evident in angioblasts but appears to be down-regulated by 36 hpf in endothelial cells of the axial vasculature (Ferdous et al., 2009; Lee et al., 2008; Sumanas and Lin, 2006). Interestingly, mouse embryos are viable following conditional endothelial ablation of etv2 using a Kdr:Cre driver (Wareing et al., 2012), suggesting that its function is restricted to very early stages of vascular development prior to the onset of kdr expression. Although these studies suggest dynamic control and function of etv2 expression during embryogenesis, carefully quantified and staged studies in this regard are still lacking. Furthermore, the mechanisms that exist to downregulate Etv2 during development have not been investigated.
In this work, we assessed the expression levels of etv2 transcript and protein during early zebrafish vascular development. Both mRNA quantification and whole mount immunostaining revealed that Etv2 is expressed during early and mid-somitogenesis and subsequently downregulated as endothelial cells differentiate and form the major trunk blood vessels. Conditional knockdown of Etv2 using a caged morpholino demonstrated that it is required only during endothelial cell specification and appears to be largely dispensable for subsequent vascular morphogenesis and function. We further find that the etv2 3’ UTR is subjected to negative regulation in endothelial cells and that this effect can be mediated by members of the let-7 microRNA family. Finally, we observe that Etv2 protein levels persist in endothelial cells of embryos lacking maternal and zygotic dicer1, which is required for microRNA maturation. Together, our results demonstrate that etv2 is required during a defined developmental window for angioblast specification and is actively downregulated, in part, through microRNA-mediated post-transcriptional regulation.
Results
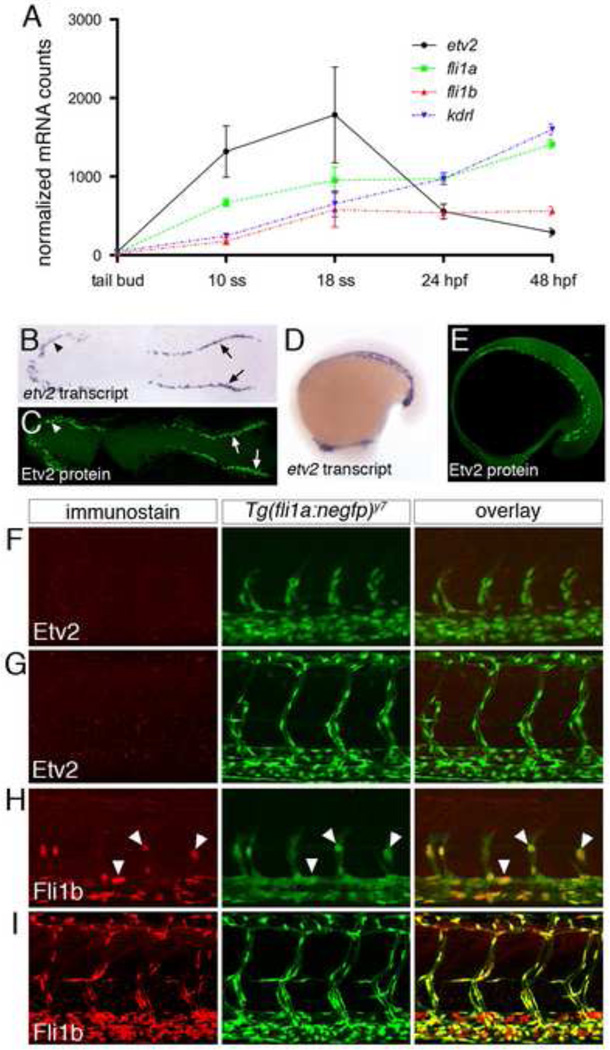
Based on previous studies that suggested etv2 levels might be dynamically regulated during embryogenesis, we carefully investigated its expression during zebrafish vascular development. We first applied the NanoString nCounter gene expression assay to quantitatively measure etv2 transcript levels at different stages of development in comparison to other endothelial genes. Using this approach, we observed that etv2 transcript increases between tail bud and 10 somite stage (ss) and peaks at 18 ss, at which time it is expressed nearly 2 fold greater than endothelial transcripts encoding Fli1a and Fli1b, and the zebrafish Vegf receptor-2 ortholog, Kdrl (Fig. 1A). Subsequently, etv2 transcript decreases between 18 ss and 48 hours post fertilization (hpf), when it is expressed at levels five fold below that of kdrl (Fig. 1Asame time points). By contrast, fli1a, fli1b, and kdrl transcripts continued to modestly increase from 10 ss until 48 hpf (Fig. 1A). Thus, the etv2 transcript displays an initial burst of expression during the time in which endothelial commitment and vasculogenesis are taking place and is subsequently downregulated. We next raised an antibody that recognized the divergent N-terminal domain of Etv2 and used this to perform whole mount immunostaining on zebrafish embryos. The Etv2 antibody did not cross react with other abundant Ets transcription factors normally found in endothelial cells (e.g. Ets1a, Fli1a, Fli1b; Supplementary Fig 1A) and was not detected in embryos injected with an Etv2 Morpholino (Supplementary Fig. 1B). Similar to etv2 transcript, we observed Etv2 protein in the anterior and posterior lateral mesoderm within nuclei of presumptive endothelial progenitors at 5 ss (Fig. 1B,C) and during initial formation of the trunk blood vessels at 18 ss (Fig. 1D, E). However, we did not observe vascular expression of Etv2 protein at 24 hpf or 48 hpf, while an endothelial-expressed nuclear localized EGFP (Tg(fli1a:negfp)y7) was easily detectable at both stages in the same embryos (Fig. 1F, G). By contrast, we observed robust expression of Fli1b protein in endothelial nuclei of Tg(fli1a:negfp)y7 embryos at the same time points (Figs. 1H, I). Interestingly, etv2 and fli1b transcript are expressed at similar levels at 24 hpf (Fig. 1A). Despite its down-regulation in endothelial cells, Etv2 protein was still detected at 24 hpf in a subset of cells posterior to the caudal vein plexus, which may comprise hematopoietic precursors (Bertrand et al., 2007; Supplementary Fig. 1C). We also detected a small population of weakly stained Etv2-positive cells in circulation at 48 hpf, while Fli1b was expressed in the majority of blood cells at this time point (Supplementary Fig. 1D). Taken together, these observations demonstrate that etv2 transcript and protein are expressed in angioblasts during vasculogenesis, but are subsequently downregulated in endothelial cells as vascular development proceeds.
Fig. 1.
Etv2 is down-regulated during vascular development. (A) Graph of nCounter quantification for etv2, fli1a, fli1b, and kdrl at the indicated developmental stages. Values are normalized to actb2 (beta-actin) and eef1a1l1(ef1alpha). (B, D) Whole mount in situ hybridization using an antisense etv2 riboprobe at 5ss and 18ss. (C, E) Embryos at 5ss and 18ss immunostained with Etv2 antibody and anti-rabbit Alexa-488. (B, C) Dorsal views of flat-mounted embryos, anterior to the left. (D, E) Lateral views, anterior to the left. (F–I) Two-photon micrographs of trunk vessels in fixed Tg(fli1a:negfp)y7 embryos immunostained with antibodies against (F, G) Etv2 or (H, I) Fli1b. Left panels, immunostained protein detected with Alexa-568 secondary antibody. Middle panels, transgenic expression of nuclear localized EGFP. Right panels, overlay of Alexa-568 and EGFP signals. Embryos at (F, H) 25 hpf or (G, I) 48 hpf.
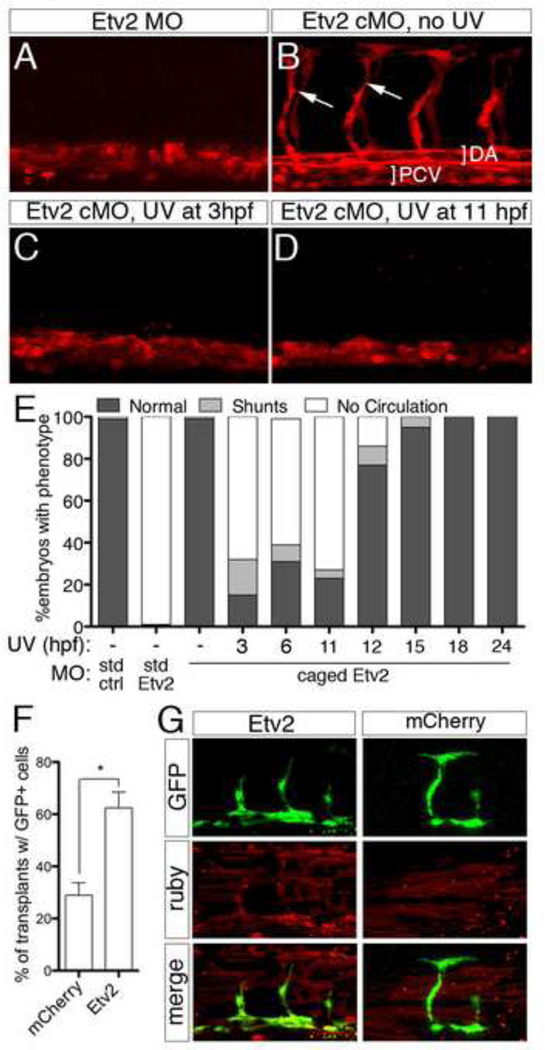
The dynamic expression of etv2 suggested that its function might only be required during early stages of vascular development. To investigate this possibility, we utilized a caged Morpholino (cMO) that is activated by exposure to UV light to conditionally block Etv2 translation at different developmental stages (Ouyang et al., 2009; Shestopalov et al., 2007). We injected Etv2 cMO into 1-cell stage Tg(fli1a.ep:DsRedex)um13 zebrafish embryos, exposed them to UV light at distinct developmental stages, and subsequently assessed vascular morphology and function. As has been shown previously (Pham et al., 2007; Sumanas and Lin, 2006), embryos injected with a standard Morpholino targeting Etv2 exhibited loss of intersegmental vessels (ISV) and a poorly formed dorsal aorta (DA) at 30 hpf and did not display circulation at 48 hpf (Fig. 2A, E; Supplementary Movie 1). By contrast, Tg(fli1a.ep:DsRedex)um13 embryos injected with Etv2 cMO that were not exposed to UV light, or those that were uninjected and exposed to UV, were phenotypically normal (Fig. 2B, E; Supplementary Movie 2 and data not shown). Likewise, Tg(fli1a.ep:DsRedex)um13 embryos injected with scrambled control morpholino (MO) exhibited normal vascular morphology at 30 hpf and normal circulation at 48 hpf (Fig. 2E and data not shown, Supplementary Movie 3). However, most embryos injected with Etv2 cMO and exposed to UV light at 11 hpf or earlier exhibited defects in vascular morphology and loss of circulation (Fig. 2C–E, Supplementary Movie 4), similar to embryos injected with an uncaged Etv2 MO (Fig. 2A, E). In all cases, we did not observe any overt effects on general morphology (data not shown). Many fewer Etv2 cMO-injected embryos exposed to UV light at 12 hpf displayed defects in circulation and UV illumination at later time points did not cause severe defects in trunk blood vessels or loss of circulation (Fig. 2E, Supplementary Movie 5). Given that Etv2 protein appears to be absent only 1.5 hours following activation of the Etv2 cMO (Supplementary Fig. 1E), these results suggest that Etv2 is required in a precisely defined early window during vascular development. Such an early requirement would be consistent with a role for Etv2 during commitment of lateral mesodermal precursors to an endothelial cell fate. If this were the case we would expect exogenous Etv2 to increase the potential of cells to contribute to the vascular lineage. Indeed, mosaic analysis revealed that donor cells derived from Tg(fli1a:egfp)y1 embryos injected with etv2 mRNA were much more likely to contribute to trunk blood vessels than donor embryos injected with mRNA encoding mCherry (Fig. 2F, G). Together, these observations demonstrate that Etv2 plays an essential role during endothelial cell specification but is likely dispensable for later aspects of vascular development.
Fig. 2.
Etv2 is required only during early stages of vascular development. (A–D) Confocal images of trunk blood vessels in Tg(fli1a.ep:DsRedex)um13 embryos at 30 hpf. Lateral views, dorsal is up, anterior to the left. Embryos injected with (A) 5 ng standard Etv2 Morpholino (MO) or (B) 2 ng Etv2 caged MO (cMO), but not illuminated with UV light. ISVs (arrows), dorsal aorta (DA; bracket) and posterior cardinal vein (PCV; bracket) are indicated. (C, D) Embryos injected with Etv2 cMO exposed to UV light at (C) 3 hpf or (D) 11 hpf. (E) Penetrance of indicated circulatory defects in embryos at 48 hpf following injection with MO and UV exposure as indicated. (F) Percentage of mosaic miniRuby-positive host embryos showing successful transplantation of Tg(fli1:EGFP)y1donor cells. Donor embryos were injected with 100 pg of mcherry or etv2 mRNA. *p < 0.05. (G) Representative confocal images of wild type hosts with contribution to both vascular (green) and non-vascular (red) tissue; donor embryos were injected with mRNA encoding either Etv2 or mCherry.
Recent studies in mouse demonstrate that persistent transgenic expression of Etv2 leads to defects in vascular morphology and causes a block in endothelial maturation (Hayashi et al., 2012). Coupled with our observations that Etv2 is robustly downregulated at early stages of vascular development, these results suggest the existence of mechanisms that actively reduce Etv2 expression. Furthermore, the discrepancy between Etv2 and Fli1b protein expression compared to their relative levels of transcript at 24 hpf (compare Fig. 1A, F, and H) suggested that a post-transcriptional mechanism might contribute to reduction in Etv2 protein. To investigate this possibility, we tested the ability of the etv2 3’UTR to repress reporter gene expression. In the process of cloning the appropriate regulatory sequences for this assay, we observed evidence of alternative 3’ UTRs encoded by the etv2 locus. In ENSEMBL (version 69, Zv9), the annotated etv2 3’UTR spans only 298 nucleotides, while two separate expressed sequence tags (ESTs) identified in the NCBI database extend past this sequence by an additional 315 nucleotides (Supplementary Fig. 2A). To further characterize expressed etv2 3’ UTR sequences, we performed 3’ rapid amplification of cDNA ends (RACE) from 24 hpf zebrafish embryos. Sequence analysis of cloned 3’RACE products and subsequent RT-PCR confirmed the expression of both the ENSEMBL- and EST-annotated 3’UTRs (hereafter referred to as Short and EST 3’ UTR, respectively) as well as a third isoform encoding a 3’ UTR of approximately 1030 nucleotides (Long etv2 3’ UTR; Supplementary Fig. 2A–C). RT-PCR analysis suggests that all three isoforms are coordinately down-regulated during development (Supplementary Fig. 2C, D) To determine the regulatory potential of these UTRs, we employed an endothelial cell autonomous transient transgenic reporter assay in which a 3’ UTR of interest is placed downstream of a red fluorescent protein (mCherry; Supplementary Fig. 2E; (Nicoli et al., 2010). This reporter construct also contains a separately expressed enhanced green fluorescent protein (EGFP) reporter fused to a control 3’UTR as an internal reference (Supplementary Fig. 2E). We cloned each etv2 3’UTR downstream of mCherry and assessed their effect on reporter expression in endothelial cells in vivo compared to the internal EGFP cassette. In embryos injected with a transgenic construct encoding mCherry fused with a control 3’UTR, we observed robust co-expression of both mCherry and EGFP in endothelial cells within trunk blood vessels (Fig. 3A). By contrast, both the EST and Long etv2 3’UTRs caused significant reduction of mCherry expression when compared to the co-expressed EGFP control (Fig. 3B, C), while the Short etv2 3’UTR did not appear to alter expression reporter expression (Fig. 3C and data not shown). These results suggest that post-transcriptional control of alternative 3’UTRs may contribute to etv2 regulation during vascular development.
Fig. 3.
The etv2 3’UTR represses a heterologous reporter in endothelial cells. (A, B) Representative confocal micrographs of 48 hpf wild type embryos co-injected with 25 pg of transposase mRNA and 25 pg of a Tol2 bis-cistronic endothelial cell autonomous sensor construct encoding mCherry fused to a (A) control 3’UTR or the (B) EST etv2 3’UTR sensor. Top, endothelial expression of the control EGFP transgene. Middle, endothelial expression of the mCherry sensor transgene. Bottom, merge of green and red channels. Lateral views, dorsal is up, anterior to the left. (C) Quantification of relative mCherry fluorescence levels compared to EGFP following 3’ UTR sensor injection. *p< 0.05, N. S. = Not significant.
microRNAs (miRNA) are short non-coding RNAs that can repress gene expression through interaction with target sequences usually located in the 3’UTR of transcripts (Du and Zamore, 2005). Since the EST and Long 3’UTRs repressed reporter gene expression, we investigated whether this may be due to microRNA regulation. Analysis of the etv2 3’UTR sequence revealed 5 putative binding sites for members of the let-7 family of microRNAs in the longest defined etv2 3’UTR, with the short and EST 3’UTRs having two and three binding sites, respectively (Supplementary Fig. 2A). Additionally, let-7 binding sites were also evident in the mouse and human etv2 transcripts (data not shown), suggesting a conserved role for let-7 in regulating Etv2 levels. To test the possibility that Iet-7 regulates the etv2 3’UTR we co-injected zebrafish embryos with mRNA encoding EGFP fused to the EST or short 3’UTR with either let-7a or a control duplex RNA, as well as mCherry mRNA with a control 3’UTR and subsequently compared levels of green and red fluorescence. Coinjection of the EST 3’UTR sensor with let-7a duplex decreased EGFP expression compared to control duplex without an effect on mCherry levels (Fig. 4A, B). Quantification of EGFP and mCherry in the same embryos by Western analysis demonstrated that let-7a led to significant and potent repression of the EST 3’UTR (Fig. 4E, F). We also observed modest but significant repression of the Short 3’ UTR by let-7a (Fig. 4C – F). Consistent with the highly conserved sequence within let-7 microRNAs, we observed similar repression of the EST 3’UTR by other let-7 family members (Supplementary Fig. 3), which have been reported to be expressed in zebrafish endothelial cells at 24 hpf (Nicoli et al., 2012). Furthermore, deletion of the five putative let-7 binding sites in the Long 3’ UTR resulted in a significant increase in mCherry reporter expression in the endothelial autonomous sensor assay compared to the wild type Long 3’UTR (Fig. 3C, data not shown). These observations suggest that let-7 microRNAs can contribute to the negative regulation of Etv2 in endothelial cells.
Fig. 4.
let-7a negatively regulates the etv2 3’UTR. (A–D) Transmitted light (left column), green fluorescence (middle column) and red fluorescence (right column) images of embryos injected with sensor mRNAs. (A, B) Embryos co-injected with 25 pg gfp-est-etv2-3’ UTR and 25 pg mcherry mRNAs and (A) control or (B) let-7a duplex. (C, D) Embryos co-injected with 25 pg gfp-short-etv2-3’ UTR and 25 pg mcherry mRNAs and (C) control or (D) let-7a duplex. (E) Western analysis for GFP and mCherry protein on embryo lysates at 24 hpf following injection with est- or short-etv2 3’ UTR sensor mRNA, mcherry mRNA, and indicated duplex. (F) Quantification of Western analysis from three independent experiments. Bars represent the average ratio of GFP band intensities from embryos injected with control duplex compared to let-7a duplex from either the EST or Short GFP-etv2 3’ UTR sensor. Significance was calculated using the student t-test. *p < 0.05
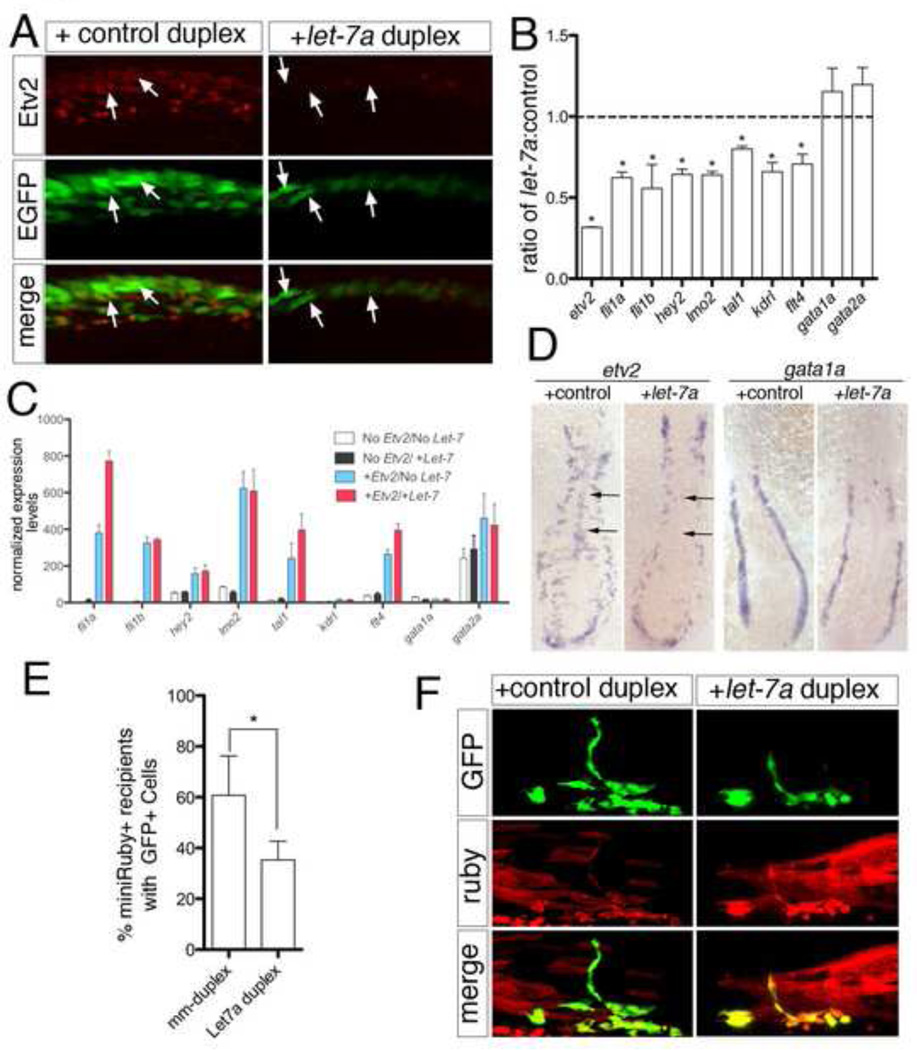
To determine if let-7 could repress endogenous etv2, we injected let-7a or control duplex into zebrafish embryos and assessed Etv2 protein and transcript levels. While Etv2 protein was apparent at low levels in Tg(fli1a:egfp)y1-positive cells lining the nascent dorsal aorta at 15 ss following injection with control duplex, we failed to detect it in embryos injected with let-7a duplex (Fig. 5A). We further noted reduced expression of the fli1a:egfp transgene in let-7a duplex injected embryos (Fig. 5A), which is likely due to endothelial differentiation defects as a result of reduced Etv2 function (Sumanas and Lin, 2006). We also observed that endogenous etv2 transcript was significantly reduced at 15 ss following injection of the let-7a duplex compared to embryos injected with control duplex (Fig. 5B). Furthermore, we noted concomitant reduction in fli1a, fli1b, hey2, lmo2, tal1, kdrl, and flt4 in let-7a duplex-injected embryos (Fig. 5B), consistent with the observation that ectopic Etv2 can induce expression of these endothelial genes in zebrafish embryos (Gomez et al., 2012; Gomez et al., 2009; Wong et al., 2009). Co-injection of etv2 mRNA containing a heterologous 3’UTR along with let-7a duplex rescued the expression of these Etv2-responsive genes, suggesting that they are not directly targeted by let-7a (Fig. 5C). While we observed repression of several endothelial genes, there was no change in early hematopoietic markers such as gata1a and gata2a following injection of let-7a duplex (Fig. 5B). Analysis of etv2 expression by whole mount in situ hybridization in let-7a injected embryos at 15 ss revealed a slight down-regulation in etv2 expression in the lateral mesoderm. More strikingly, we observed loss of midline-positioned etv2-positive cells, which normally comprise the forming aorta (Fig. 5D). This defect is also known to be associated with Etv2 deficiency (Sumanas and Lin, 2006) and is consistent with reduced Etv2 function as a consequence of ectopic let-7a expression. We did not note any obvious changes in gata1a (Fig. 5D), similar to the Nanostring gene expression data (Fig. 5B). Together these results demonstrate that let-7a can potently repress expression of the Etv2 protein and lead to reduction in endothelial gene expression.
Fig. 5.
Endogenous Etv2 is repressed by let-7a. (A) Two photon images of Tg(fli1a:egfp)y1 embryos injected with control or let-7a duplex and immunostained with Etv2 polyclonal serum and Alexa-568 secondary antibody. Lateral view, dorsal is up, anterior to the left. Arrows denote Etv2/GFP-positive cells (left panels) or Etv2-negative/GFP-positive cells in the forming dorsal aorta (right panels). (B) Histogram showing fold change in expression of indicated transcripts at 15 ss in embryos injected with let-7a compared to those injected with control duplex measured by the nCounter system. Genes normalized to actb2 (beta-actin) and eef1a1l1 (ef1alpha). *p<0.05. (C) Histogram of relative nCounter expression counts normalized as in (B) for indicated transcripts following injection with mRNA encoding Etv2 (+Etv2) or Etv2 lacking the DNA binding domain (no Etv2) and mismatch (no let-7) or let-7a duplex (+ let-7). (D) Whole mount in situ hybridization using riboprobes against etv2 (left) or gata1a (right) at 15 ss in embryos injected control or let-7a duplex RNA. Angioblasts that have migrated to the midline, or lack thereof, are indicated by arrows. Dorsal view of flat mounted embryo, anterior is up. (E) Histogram showing percentage of successfully transplanted wild type host embryos (miniRuby-positive) that display contribution to vascular tissue, as indicated by presence of Tg(fli1a:egfp)y1-positive cells. Donors were injected with control or let-7a duplex as above. Data are from three independent experiments and significance was calculated using the Fisher’s exact test; *p < 0.05. (F) Confocal micrographs showing contribution of Tg(fli1a:egfp)y1 positive cells (green channel) from donors that were injected with control or let-7 RNA duplex. miniRuby-positive cells (red channel) indicate overall contribution of donor cells in the trunk.
To investigate the effect of let-7a over-expression on endothelial cell commitment, we transplanted cells from Tg(fli1a:egfp)y1 embryos injected with let-7a into wild type embryos and assessed the frequency of successfully transplanted host embryos with EGFP-positive donor cells. Consistent with our observation that let-7a can repress endogenous etv2, significantly fewer host embryos transplanted with let-7a–overexpressing donor cells displayed contribution to vascular tissue compared to control duplex-injected embryos (Fig. 5E). Despite the negative effect of let-7a over-expression on endothelial cell contribution, let-7a duplex-injected donor cells were otherwise able to contribute to other cell types (Fig. 5F). Taken together these data suggest that let-7 family members can act to limit the ability of Etv2 to induce endothelial commitment during development.
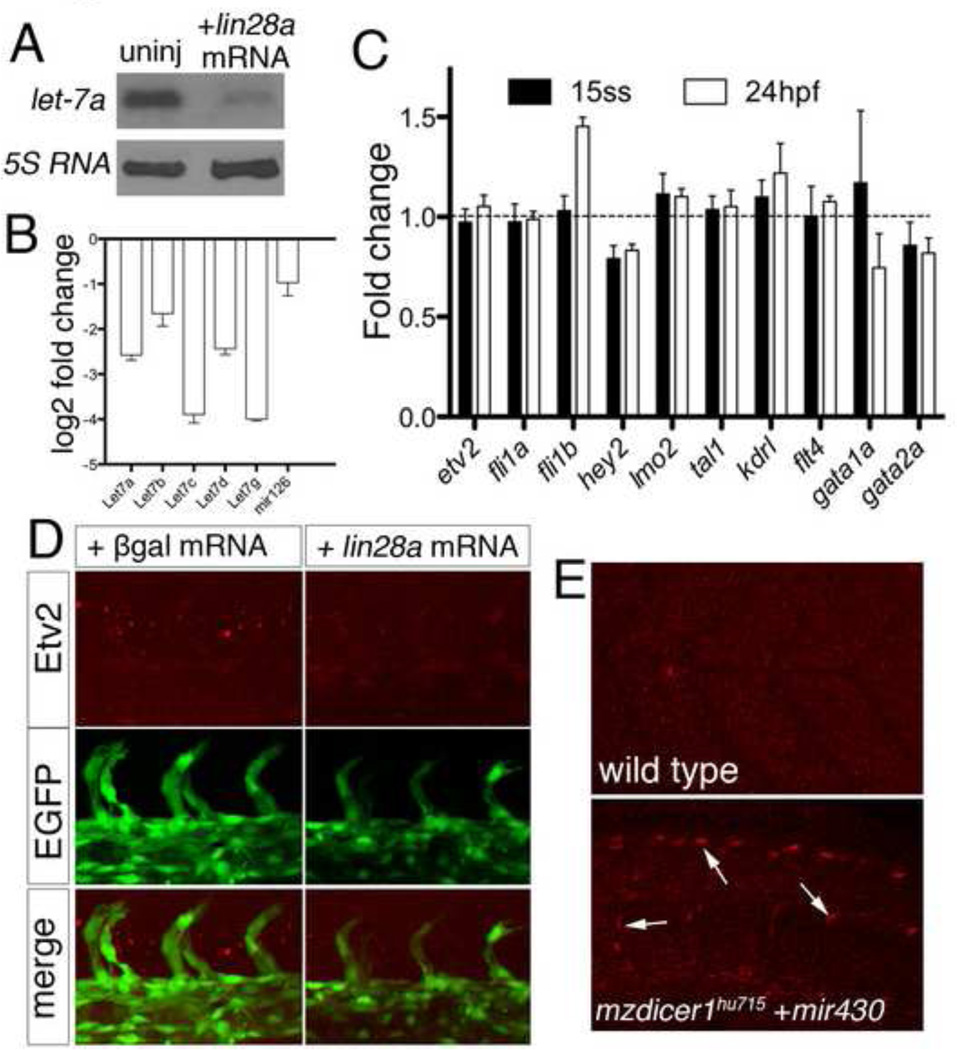
Determining the effect of let-7 deficiency is a challenge due to the large number of related family members. Indeed, let-7a itself can be expressed from at least 6 distinct loci in the zebrafish genome and, including duplication events, there are a total of 18 different let-7 family genes (ENSEMBL, Zv9). As mentioned above, several of these are expressed in endothelial cells and can repress the Etv2 3’UTR. Therefore, to reduce let-7 function we over-expressed lin28a, which uridylates let-7 miRNAs and causes their rapid degradation (Heo et al., 2008; Nam et al., 2011; Viswanathan et al., 2008). We find that injection of mRNA encoding Lin28a causes a significant decrease in let-7a expression in zebrafish embryos at 24 hpf (Fig. 6A). This effect was similar for other let-7 family members (Fig. 6B), while mir-126, an unrelated miRNA expressed specifically in endothelial cells, was not significantly reduced. Despite reduction in let-7 miRNAs, we did not observe any significant changes in endothelial gene expression at 15 ss or 24 hpf (Fig. 6C). Furthermore, neither etv2 transcript or protein levels appeared to change significantly in Lin28a over-expressing embryos and vascular development proceeded normally (Fig. 6C, D). These results raise the possibility that other miRNAs may contribute to post-transcriptional repression of Etv2 in the absence of let-7. Alternatively, sufficient levels of let-7 remain to repress Etv2, even in the presence of increased lin28a. Therefore, we further investigated whether miRNAs contribute to Etv2 down-regulation by determining its expression in embryos lacking maternal and zygotic (MZ) dicer function. dicer1 encodes an essential nuclease required for miRNA maturation and MZdicer mutant embryos are devoid of mature miRNAs (Giraldez et al., 2005). Wild type embryos at 48 hpf did not exhibit Etv2 expression in endothelial cells (Fig. 6E). By contrast, Etv2 protein expression was apparent at this stage in embryos lacking both maternal and zygotic dicer1 (MZdicer1) that had been injected with miR-430 to rescue early developmental defects associated with a lack of dicer1 (Fig. 6E; (Giraldez et al., 2005). Despite the persistence of Etv2 protein, down-regulation of etv2 transcript seemed to occur normally in wild type and MZdicer1 mutant embryos (Supplementary Fig. 2C). These results suggest that microRNAs may play a role in post-transcriptionally regulating levels of Etv2 protein during vascular development.
Fig. 6.
Contribution of let-7a and other microRNAs to Etv2 repression. (A) Northern analysis of RNA isolated from 24 hpf embryos left uninjected or injected with 1 ng lin28a mRNA. Blots were hybridized with using DIG labeled probes against let-7a and 5s RNA. (B) Histogram showing log2 fold change comparison of let-7 family members at 15 ss assessed by miScript qPCR quantification between embryos injected with 1 ng lin28a mRNA and those left uninjected, quantification from triplicate experiments. (C) Histogram showing fold change comparison of indicated genes assessed by nCounter quantification between embryos injected with 1 ng lin28a and 1 ng pgalactosidase mRNA. Genes normalized to actb2 (beta-actin) and eef1a1l1 (ef1alpha). (D) Two-photon micrographs of trunk blood vessels in Tg(fli1a:egfp)y1 embryos immunostained with Etv2 polyclonal antiserum and Alexa-568 secondary antibody at 24 hpf following injection with 1ng of β-galactosidase (left panels) or lin28a mRNA (right panels). (E) Confocal micrographs of embryos immnuostained with Etv2 polyclonal antiserum and Alexa-568 secondary antibody at 48hpf. Top, wild type embryo. Bottom, MZdicer1hu715 mutant embryos injected with mir-430 duplex RNA. Etv2-positive nuclei in the endothelial cells of trunk blood vessels are denoted by arrows (bottom).
Discussion
The ETS transcription factor Etv2 is essential for vascular development, but less is known about its dynamic regulation or functional requirements during different stages of vascular development. Using the zebrafish as a model system, we find that both etv2 transcript and protein are expressed during angioblast specification and vasculogenesis, but are subsequently downregulated as development proceeds. This expression pattern is mirrored by its functional requirement, which we find is restricted to early stages that correspond to angioblast emergence from the lateral mesoderm. We further provide evidence that post-transcriptional control of Etv2 levels likely contributes to its down-regulation, and this regulation occurs, in part, through let-7 miRNAs.
The phenotypes of etv2-deficient zebrafish and mouse embryos suggest that it should be considered as a master regulator of endothelial cell fates. In both species, loss of etv2 leads to profound defects in vascular morphogenesis and a global loss of endothelial gene expression (Lee et al., 2008; Pham et al., 2007; Sumanas and Lin, 2006). Conversely, exogenous Etv2 expression can precociously and ectopically induce an endothelial gene program (Gomez et al., 2009; Wong et al., 2009). Accordingly, we find that Etv2-overexpression can increase the commitment of cells to the endothelial lineage in mosaic embryos, similar to its effect in mouse embryoid bodies (Koyano-Nakagawa et al., 2012). While our results indicate that Etv2 is essential for endothelial cell commitment, it appears to be dispensable for later steps of vascular development. Conditional knockdown of Etv2 at early, but not later stages, severely perturbed vascular morphogenesis, demonstrating that its function is only required during an early window of development in which the first endothelial progenitors are known to emerge. This early functional restriction is likely due to the highly dynamic expression of etv2, which peaks during somitogenesis but is barely detectable by 24 hpf in the zebrafish embryo. Our results are consistent with recent studies in mouse embryonic stem cells where Etv2 expression can be detected in Brachyury-positive mesodermal cells that have not yet initiated expression of endothelial markers, such as kdr/vegfr-2 (Wareing et al., 2012). Furthermore, etv2 expression also appears to be reduced in mouse embryos as development proceeds (Ferdous et al., 2009). Finally, conditional ablation of etv2 in mouse embryos using a Kdr:Cre driver does not effect endothelial differentiation or vascular morphogenesis (Wareing et al., 2012), suggesting that etv2 is also dispensable for later steps in vascular development in mammals as well. Taken together with our studies, these results suggest that Etv2 plays an essential and conserved role to commit early lateral mesoderm progenitors to an endothelial cell lineage. Despite these observations, we still do not understand the precise molecular mechanisms by which Etv2 acts in this regard. While Etv2 is capable of directly activating functional downstream targets, such as Kdr {Ishitobi, 2011 #47;Lee, 2008 #36}, a more comprehensive identification of Etv2 target genes, and a better understanding of how Etv2 induces their expression, is essential to understand the role of this transcription factor in endothelial commitment.
In many cases, ETS transcription factors are essential throughout the ontogeny of a particular cell lineage, often acting reiteratively during commitment and differentiation. For example, Spi1 (also known as pu.1) is essential for development of the myeloid lineage (McKercher et al., 1996; Scott et al., 1994), where it is required for early expression of receptors for macrophage and granulocyte specific growth factors on progenitor cells (Anderson et al., 1998a). Subsequently, spi1 plays an essential role in the terminal differentiation and function of both macrophages and neutrophils, where it continues to be expressed (Anderson et al., 1998a; Anderson et al., 1998b). By contrast, Etv2 function is restricted to early endothelial commitment and its persistent expression has deleterious effects on differentiation and vascular morphogenesis. Continued endothelial expression of a Cre-activated conditional ROSA26:Etv2 transgene in mouse embryos leads to abnormal yolk sack vascular morphology and a failure to induce expression of genes involved in vascular maturation (Hayashi et al., 2012). A similar effect is observed during direct endothelial reprogramming of human amniotic cells, which requires ETV2, along with ERG and FLI1 (Ginsberg et al., 2012). While ETV2 is essential for direct reprogramming in this context, it must be subsequently down-regulated for normal endothelial differentiation to occur (Ginsberg et al., 2012). Together with our findings, these studies underscore the need to actively repress etv2 expression to allow normal endothelial differentiation.
Our results suggest that miRNA-mediated post-transcriptional repression plays an important role in reducing the levels of Etv2 to allow normal differentiation. The etv2 3’UTR is capable of mediating repression in endothelial cells and Etv2 protein persists in MZdicer1 mutant embryos, which lack miRNAs. We further find that post-transcriptional repression of etv2 is mediated, in part, by members of the let-7 family of microRNAs. Our results are consistent with the role of let-7 microRNAs in other animal species, where they are known to promote cellular differentiation or block transformation by negatively regulating genes associated with growth and proliferation, such as RAS and MYC (Chang et al., 2009; Johnson et al., 2005; Thornton and Gregory, 2012; Yu et al., 2007). We also find evidence for multiple etv2 transcript isoforms with different length 3’UTRs, which varied in their potential to repress reporter expression and their responsiveness to let-7 over-expression. While only the shortest 3’UTR did not appreciably repress reporter gene expression in our endothelial autonomous sensor assay, it retained two let-7 binding sites and could still be partially repressed by let-7a. Importantly, let-7 potently repressed endogenous Etv2 protein levels, suggesting an important role for this miRNA in regulating etv2 in vivo. However, the precise role of alternative 3’UTR usage in etv2 regulation is not clear at this time. Interestingly, previous studies demonstrate that shortened 3’UTRs, which can presumably evade microRNA regulation, are prevalent in proliferating or transformed cells (Mayr and Bartel, 2009; Sandberg et al., 2008). Furthermore, 3’UTRs generally lengthen during zebrafish emrbyogenesis as cells differentiate (Ulitsky et al., 2012). These observations suggest that there may be a transition in etv2 3’UTR usage as lateral mesodermal progenitors commit to the endothelial lineage and begin to differentiate, allowing let-7 to contribute to Etv2 down-regulation during this process.
Despite the effect of let-7a on endogenous Etv2 expression, we did not note any vascular defects caused by over-expressing lin28a, which drastically reduced let-7a levels. In addition, lin28a over-expression did not appear to cause Etv2 to persist at later stages. There could be several reasons for these results. First, the degree of let-7a knockdown caused by exogenous lin28a expression, while significant based on our quantification, may not be sufficient to mimic a true genetic null given the number and diversity of let-7 microRNAs expressed in endothelial cells at this time point (Nicoli et al., 2012). Thus, sufficient levels of let-7 microRNAs may remain to repress Etv2 even in the presence of high levels of Lin28a. Future application of lin28a transgenes that would allow persistent high level expression throughout development are likely required to fully address this issue. Second, other miRNAs may contribute to Etv2 repression in the absence of let-7 family members. While we are currently not able to distinguish these possibilities, the persistent expression of Etv2 protein in MZdicer1 mutant embryos underscores the importance of miRNAs in mediating its repression during development. Finally, there are likely to be additional regulatory mechanisms that contribute to Etv2 down-regulation and may be sufficient to allow normal vascular development in the absence of let-7. Indeed, while Etv2 protein persisted in MZdicer1 mutant embryos, etv2 mRNA was reduced as development proceeded similar to wild type siblings. Although exogenous let-7a appeared to reduce etv2 transcript following whole embryo analysis, comparison of in situ results and immunostaining suggested a much more potent effect on Etv2 protein rather than mRNA levels. Furthermore, the observed decrease in etv2 mRNA was likely due to embryos displaying fewer angioblasts, a phenotype associated with Etv2 deficiency (Pham et al., 2007; Sumanas and Lin, 2006) and consistent with the observed reduction in Etv2 protein following let-7a over-expression. Together, these observations suggest that down-regulation of etv2 mRNA may also occur through transcriptional mechanisms. Consistent with this possibility, a recent study has identified an enhancer element in the zebrafish etv2 locus that drives expression in endothelial cells only during early stages of embryonic development but not later (Veldman and Lin, 2012). The activity of this enhancer mirrors that which we observe for the endogenous transcript, suggesting that transcriptional mechanisms can contribute to the dynamic expression of etv2. Thus, while our work suggests that miRNA-mediated post-transcriptional repression contributes to Etv2 down-regulation, it is likely that other mechanisms also play an important role in this process.
Whether let-7 or other miRNAs contribute to the repression of Etv2 in mammals is unknown. We did observe that human and mouse ETV2 3’ UTRs also contain let-7 binding sites (data not shown). Moreover, zebrafish and human endothelial cells highly express several let-7 family members (Kuehbacher et al., 2007), but do not express appreciable amounts of ETV2 (Djebali et al., 2012). These observations suggest that let-7-mediated repression of etv2 may be a conserved aspect of its regulation. Based on its powerful ability to induce endothelial gene programs and block differentiation, along with the evidence that it is normally actively repressed, we would further speculate that Etv2 is likely to play a role in the pathogenesis of syndromes associated with dysregulated endothelial growth. In this regard, it will be of interest to determine if etv2 expression is persistent in cases of infantile hemangioma or angiosarcoma. At the same time, further investigation into the mechanisms that contribute to etv2 regulation will likely provide novel insights into how this master regulator contributes to endothelial lineage specification.
Material and Methods
Zebrafish Handling and Maintenance
Zebrafish and their embryos were handled according to standard protocols (Westerfield, 1993) and in accordance with the University of Massachusetts Medical School IACUC guidelines. Tg(fli1a:egfp)y1, Tg(fli1a:negfp)y7 and Tg(fli1a.ep:DsRedex)um13 lines have been described (Covassin et al., 2009; Lawson and Weinstein, 2002; Roman et al., 2002). Maternal zygotic (MZ) dicer1 embryos were made using the germline replacement technique as previously described (Ciruna et al., 2002; Giraldez et al., 2005) using dicer1hu715 donors (Wienholds et al., 2003).
Etv2 caged Morpholino injections
The Etv2 caged morpholino (cMO) used in this study has been previously reported (Ouyang et al., 2009). 230 fmol (2 ng) of Etv2 cMO was injected into Tg(fli1a.ep:DsRedEx)um13 embryos at 1-cell stage. Embryos were subjected to UV illumination for 10 seconds at indicated stages using a Zeiss Axioskop2 Plus compound microscope with a DAPI filter and an Achroplan (Zeiss) 20× water immersion objective. Following photoactivation, embryos were grown in egg water at 28.5 C. Control embryos were left in the dark. 5 ng of scrambled control or 5 ng Etv2 MO (Sumanas and Lin, 2006) were injected as negative and positive controls, respectively. Vascular morphology was assessed at 30 hpf. Embryos were imaged using an MZFLIII fluorescent dissection microscope or using a using a Leica DMIRE2 confocal microscope (Objective: HC PL APO 20×/0.70CS). Circulatory defects were observed at 48 hpf using a MZ12 stereomicroscope (Leica) and captured with a DMK21F04 camera (Imagesource) using Quicktime Pro or iMovie.
Plasmid Construction
The etv2 open reading frame was amplified from 24 hpf whole embryo cDNA and used in a BP recombination reaction with plasmid pDONR221 (Invitrogen) to make pME-etv2. The zebrafish lin28a open reading frame was amplified from a full-length Zebrafish Gene Collection (ZGC) clone (Clone ID: 2643384; Thermo Scientific; see Table S1 for primers), then subjected to BP recombination with plasmid pDONR221 to generate pME-lin28a. pME-etv2, pME-lin28a, or pME-mcherry (Nicoli et al., 2010) were used in LR reactions with pCSDest or pCSMTDest (Villefranc et al., 2007) to generate pCS-etv2, pCSMT-etv2, pCS-lin28a, and pCS-mCherry. Alternative etv2 3’ UTRs were cloned through PCR amplification using attB2 and attB3 primers (see Table S1) followed by BP recombination into pDONRP2r–P3 (Invitrogen) to give p3E–EST etv2 3’UTR, p3E–short etv2 3’UTR and p3E–long etv2 3’UTR. let-7 binding sites were identified by miRANDA, RNAhybrid, and a perl script. Bases 1, 3, 4, 5, 6 were mutated to adenines within 5 identified let-7 binding site seed sequences (Ramachandran et al., 2010) identified by all three methods. The mutant let-7 etv2 3’ UTR fragment was synthesized by Genewiz (pUC57-kan-etv2_3putr_mut_let7) followed by subcloning into p3E–mcs1 with AscI and XhoI to give p3E–mutlet-7 etv2 3’ UTR. To generate mRNA sensors constructs, p3E–ESTetv2 3’UTR or p3E–shortetv2 3’UTR were recombined with pCSDEST2 and pENTR-EGFP2 (Villefranc et al., 2007) to yield pCS2-egfp-ESTetv2 3’UTR and pCS2-egfp-shortetv2 3’UTR. Endothelial 3’ UTR sensor constructs were generated by performing an LR Gateway recombination reaction between pTolBasPegfpfliEPmcherryR2-R3 and one of the following 3’ entry clones: p3E–mcs1, p3E–shortEtv2–3’UTR, p3E–ESTEtv2–3’UTR, p3E–longEtv2–3’UTR, p3E–mut-let7-Etv2–3’UTR.
mRNA synthesis and injections
Capped mRNA was synthesized from pCS plasmids that had been linearized with NotI using the SP6 mMessage mMachine kit (Ambion). mRNAs were injected into 1-cell stage embryos according to standard protocols (Westerfield, 1993).
3’UTR Sensor assays
For whole embryo sensor assay, 50 pg of mCherry mRNA and 50 pg of indicated gfp etv2 3’ UTR mRNA was co-injected along with 2 nl of a 50µM stock of indicated miRNA duplexes into 1-cell stage zebrafish embryos. Embryos were visualized at 24 hpf using an MZFLIII dissection microscope equipped with epifluorescence and digital images were captured using an AxioCam mRC (Zeiss). Alternatively, equal numbers of dechorionated embryos were lysed by boiling in 2× Laemmli buffer. Lysates were run on an SDS-PAGE gel and transferred to Western blots, which were probed with antibodies against EGFP (Invitrogen) and mCherry (Clontech). Blots were stripped in between each antibody detection. Expression levels were quantified by measuring the optical density of bands using ImageJ following incubation with a horseradish peroxidase conjugated secondary antibody and chemiluminscence detection. For endothelial autonomous sensor assays, 25 pg of indicated pTol sensor construct was co-injected with 25pg transposase mRNA into one-cell stage wild type embryos. Individual 3’UTR constructs were always injected with control sensor in parallel. At 24 hpf, embryos were transferred to egg water containing 0.2mM 1-phenyl-2-thiourea (PTU) to inhibit the pigment formation. At 48–50hpf, approximately five embryos from each group per experiment displaying robust transgenesis were imaged by confocal microscopy. Gain settings were set using embryos injected with the control sensor and remained constant throughout the experiment. Quantification of fluorescence levels was performed using Imaris by creating a surface based on GFP fluorescence and examining the average values intensity sum of green and red channels. The red/green ratio of an experimental embryo was normalized against the red/green ratio of a control embryo imaged on the same day. All sensor experiments were done and quantified in quadruplicate using at least 5 embryos per experiment except the EST-3’UTR which was done in triplicate). Significance was calculated by a Welsh test and significance determined by a p value < 0.03.
Antibody production
A fragment encoding the N-terminal 218 amino acids of zebrafish Etv2 was amplified from 24hpf zebrafish cDNA (see Table S1 for primers), cloned into pCR2.1 by TOPO cloning (Invitrogen), and verified by sequencing. The etv2 fragment was subcloned into pGEX-6P-1 using BamHI and XhoI sites. pGEX-Etv2 was transformed into BL21(DE3) and glutathione S-transferase (GST) fusion protein expression was induced with IPTG. Expressing bacteria were lysed using Bug Buster (Novagen), and proteins were purified using Glutathione Sepharose 4B(GE Healthcare), followed by release of the Etv2 fragment and removal of the GST using PreScission Protease (GE Healthcare). Purified Etv2 protein was used for rabbit polyclonal antibody production (Caprologics, Gilbertville, MA). Etv2 antiserum was validated using Western analysis of lysates from HEK293T over-expressing myc-tagged zebrafish Etv2. The myc epitope was detected using a 1:10,000 dilution of 9E10 (Sigma) and Etv2 protein was detected using a 1:5,000 dilution of anti-Etv2 polyclonal antibody serum.
Whole mount immunostaining
Staged zebrafish embryos were fixed overnight at 4°C in 2% paraformaldehyde (w/v) dissolved in phosphate buffered saline containing 0.1% Tween-20 (PBSTw). Embryos were washed 4 times for 5 minutes at room temperature in PBSTw and in PBS containing 0.5% TritonX-100 (PBSTr) at room temperature for 30 minutes. Embryos were blocked for a minimum of 2 hrs in blocking solution (PBSTw, 0.1% TritonX-100, 10% normal goat serum, 1% BSA, 0.01% sodium azide) at room temperature. Fli1b and Etv2 rabbit polyclonal serum was diluted 1:1000 and 1:500, respectively, in blocking solution and embryos incubated over night at 4°C. Embryos were washed 6 times in PBSTw for at least 4 hrs at room temperature and then incubated overnight with Alexa Fluor 488 or Alexa Fluor 568 (Invitrogen) anti-rabbit secondary antibody diluted 1:1000 in blocking solution. Immunostained embryos were imaged on a LSM7 MP microscope (Zeiss; Objective: 20×/1.0 DIC(UV) VIS-IR 421452-9800) equipped with a Chameleon Ti:Sapphire pulsed laser (Coherent, Inc.). Alexa Fluor 488 and Alexa Flour 568 were alternatively excited at 904 nm and 1057 nm, respectively, on each section during image acquisition.
miRNA Duplexes
RNA oligonucleotides (Integrated DNA technologies) corresponding to the mature and star sequences of zebrafish let-7a, let-7c, let-7f, and, let-7g (seeTable S1) were diluted to 250 µM in nuclease-free water. Equal volumes of mature and star oligonucleotides were combined, heated to 95°C and annealed at 37°C for 30 minutes. miRNA duplexes were aliquotted and stored at −80°C. 2 nl of 50 µM miRNA duplexes were injected into embryos. A mis-match duplex in which 4 out of 8 bases in the seed sequenced were changed (Table S1) was used as a negative control (referred to as “control duplex”).
Quantification of endothelial gene expression
Transcript levels were quantified using the NanoString nCounter gene expression system (Nanostring Technologies, Seattle, WA; (Geiss et al., 2008). Total RNA was isolated from embryos using a Qiagen RNAeasy kit. For embryos injected with let-7a or control duplex, RNA was isolated at 15 ss. To assess over-expression of Etv2 and let-7a, embryos were co-injected with let-7a duplex as above along with 50 pg of mRNA encoding Etv2 or Etv2 minus its DNA binding domain [Etv2(-DBD)] and RNA was isolated at shield stage. For each experiment, 100 ng of total RNA was hybridized for 12 to 20 hrs with the Nanostring probeset (Table S2) at 650C in a thermocycler. Samples were then loaded into the nCounter prep station and fluorescence signal was quantified using the nCounter Digital Analyzer. Gene normalization and fold change calculations were done using Nsolver Analysis Software (Nanostring Technologies). In all cases, biological triplicates were performed and gene counts were normalized to eukaryotic translation elongation factor 1 alpha 1 like 1 (eef1a1l1) and actin, beta 2 (actb2). Either the average normalized gene count or the average fold change was plotted and error bars represent the Standard Error of the Mean (SEM).
In situ hybridization
An antisense DIG-labeled etv2 riboprobe was synthesized by linearizing pCS2-etv2 with EcoRI followed by in vitro transcription using T7 polymerase. A gata1a riboprobe was synthesized as described elsewhere (Hart et al., 2009). Whole mount in situ hybridization was performed according to standard protocols (Hauptmann, 1999).
Mosaic analysis
Tg(fli1a:egfp)y1 embryos were used as donors in all cases and 0.35% miniRuby (dextran, tetramethylrhodamine and biotin 10,000MW; Invitrogen) was co-injected as a lineage tracer. To assess the effect of Etv2 overexpression, we injected 100pg of myc-etv2 or mCherry mRNA into 1-cell stage donor embryo. For let-7a overexpression we injected 2 nl of either 50µm control or let-7a duplex. At sphere stage, approximately 20 cells were transplanted from the ventral blastoderm margin of donors into wild type hosts, which were subsequently screened at 30 hpf for the appearance of red and green fluorescence. Embryos were imaged using an MZFLIII fluorescent dissection microscope or using a using a Leica DMIRE2 confocal microscope (Objective: HC PL APO 20×/0.70CS). The proportion of successfully transplanted embryos (i.e. exhibiting miniRuby-positive cells in any trunk tissue) with contribution to blood vessels was determined in three separate experiments and significance was calculated by Fisher’s Exact test. p < 0.05 was deemed significant.
Northern
Northern blot analysis for microRNA expression was performed as previously described (Kim et al., 2010). Zebrafish RNA was isolated using a miRNeasy Micro kit (Qiagen) and 5µg of total RNA was loaded per lane. Blots were hybridized with a DIG labeled let-7a locked-nucleic acid probe (Exiqon), stripped using boiling water, and hybridized with a DIG-labeled 5s rRNA DNA probe (see Table S1). Chemilumenscence detection was performed following incubation with a horseradish peroxidase-conjugated antibody against DIG. Northern blots were performed using RNAs from three separate experiments and quantified by measuring the optical density of bands using ImageJ to compare levels in uninjected versus let-7a injected embryos. Average fold difference from three independent experiments was plotted and error bars represent SEM. Significance was measured using a student t-test.
3’ RACE and Etv2 3’ UTR cloning
3’ RACE was performed using the SMART RACE kit (Clontech). etv2-specific primers for primary and nested PCR are listed in Table S1. Amplified fragments were gel purified, cloned into pGEM-t (Promega) and verified by sequencing.
Quantitative PCR of miRNAs
RNA was purified from uninjected zebrafish embryos injected at 24 hpf or those injected with 1 ng lin28a mRNA using a miRNeasy micro kit (Qiagen). qRT-PCR to detect mature miRNAs was performed using the miScript System (Qiagen). Two µg of whole RNA was used to synthesize cDNA. qPCR was performed from 100 ng of cDNA template with commercially available primers for indicated miRNA (Qiagen) and the miScript universal primer using the miScript SYBR green PCR Kit (Qiagen). snord61.2 expression was assessed in parallel and used to normalize microRNA expression levels. PCR quantification was performed on a StepOnePlus Real-time PCR system (Applied Biosystems). Each reaction was run in triplicate and performed on at least two experimental replicates and 2-log fold change calculated by comparing uninjected to Lin28a injected.
Supplementary Material
Highlights.
Etv2 expression is highly dynamic during early development.
Etv2 function is restricted to early endothelial commitment.
Etv2 is subjected to post-transcriptional regulation.
microRNAs can contribute to Etv2 repression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John C. Moore, Email: john2.moore@umassmed.edu.
Sarah Sheppard, Email: sarah.sheppard@umassmed.edu.
Ilya A. Shestopalov, Email: ishestopalov@enders.tch.harvard.edu.
James K. Chen, Email: jameschen@stanford.edu.
References
- Anderson KL, et al. Myeloid development is selectively disrupted in PU.l null mice. Blood. 1998a;91:3702–3710. [PubMed] [Google Scholar]
- Anderson KL, et al. Neutrophils deficient in PU.1 do not terminally differentiate or become functionally competent. Blood. 1998b;92:1576–1585. [PubMed] [Google Scholar]
- Barton K, et al. The Ets-1 transcription factor is required for the development of natural killer cells in mice. Immunity. 1998;9:555–563. doi: 10.1016/s1074-7613(00)80638-x. [DOI] [PubMed] [Google Scholar]
- Bertrand JY, et al. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development. 2007;134:4147–4156. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TC, et al. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3384–3389. doi: 10.1073/pnas.0808300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B, et al. Production of maternal-zygotic mutant zebrafish by germ-line replacement. Proc Natl Acad Sci U S A. 2002;99:14919–14924. doi: 10.1073/pnas.222459999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver O, Krieg PA. Molecular mechanisms of vascular development. In: Harvey RP, Rosenthal N, editors. Heart Development. San Diego: Academic Press; 1999. pp. 221–252. [Google Scholar]
- Covassin LD, et al. A genetic screen for vascular mutants in zebrafish reveals dynamic roles for Vegf/Plcgl signaling during artery development. Dev Biol. 2009;329:212–226. doi: 10.1016/j.ydbio.2009.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell. 2009;16:180–195. doi: 10.1016/j.devcel.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Val S, et al. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell. 2008;135:1053–1064. doi: 10.1016/j.cell.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E, et al. Foxs and Ets in the transcriptional regulation of endothelial cell differentiation and angiogenesis. Biochim Biophys Acta. 2007;1775:298–312. doi: 10.1016/j.bbcan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Djebali S, et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development. 2005;132:4645–4652. doi: 10.1242/dev.02070. [DOI] [PubMed] [Google Scholar]
- Ferdous A, et al. Nkx2-5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc Natl Acad Sci USA. 2009;106:814–819. doi: 10.1073/pnas.0807583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss GK, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- Ginsberg M, et al. Efficient Direct Reprogramming of Mature Amniotic Cells into Endothelial Cells by ETS Factors and TGFbeta Suppression. Cell. 2012;151:559–575. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez AJ, et al. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308:833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- Gomez G, et al. Identification of vascular and hematopoietic genes downstream of etsrp by deep sequencing in zebrafish. PLoS One. 2012;7:e31658. doi: 10.1371/journal.pone.0031658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez GA, et al. Discovery and characterization of novel vascular and hematopoietic genes downstream of etsrp in zebrafish. PLoS One. 2009;4:e4994. doi: 10.1371/journal.pone.0004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart DO, et al. Selective interaction between Trf3 and Taf3 required for early development and hematopoiesis. Dev Dyn. 2009;238:2540–2549. doi: 10.1002/dvdy.22083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann G. Two-color detection of mRNA transcript localizations in fish and fly embryos using alkaline phosphatase and beta-galactosidase conjugated antibodies. Development genes and evolution. 1999;209:317–321. doi: 10.1007/s004270050258. [DOI] [PubMed] [Google Scholar]
- Hayashi M, et al. Endothelialization and altered hematopoiesis by persistent Etv2 expression in mice. Exp Hematol. 2012;40:738–750. doi: 10.1016/j.exphem.2012.05.012. e11. [DOI] [PubMed] [Google Scholar]
- Heo I, et al. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell. 2008;32:276–284. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Hollenhorst PC, et al. Expression profiles frame the promoter specificity dilemma of the ETS family of transcription factors. Nucleic Acids Res. 2004;32:5693–5702. doi: 10.1093/nar/gkh906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenhorst PC, et al. Genomic and biochemical insights into the specificity of ETS transcription factors. Annu Rev Biochem. 2011;80:437–471. doi: 10.1146/annurev.biochem.79.081507.103945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iljin K, et al. Role of ets factors in the activity and endothelial cell specificity of the mouse Tie gene promoter. FASEB J. 1999;13:377–386. doi: 10.1096/fasebj.13.2.377. [DOI] [PubMed] [Google Scholar]
- Johnson SM, et al. RAS is regulated by tlAt-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Kappel A, et al. Role of SCL/Tal-1, GATA, and ets transcription factor binding sites for the regulation of flk-1 expression during murine vascular development. Blood. 2000;96:3078–3085. [PubMed] [Google Scholar]
- Kim SW, et al. A sensitive non-radioactive northern blot method to detect small RNAs. Nucleic Acids Res. 2010;38:e98. doi: 10.1093/nar/gkp1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano-Nakagawa N, et al. Etv2 is expressed in the yolk sac hematopoietic and endothelial progenitors and regulates Lmo2 gene expression. Stem Cells. 2012;30:1611–1623. doi: 10.1002/stem.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehbacher A, et al. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Lee D, et al. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelievre E, et al. The Ets family contains transcriptional activators and repressors involved in angiogenesis. Int J Biochem Cell Biol. 2001;33:391–407. doi: 10.1016/s1357-2725(01)00025-5. [DOI] [PubMed] [Google Scholar]
- Liu F, Patient R. Genome-wide analysis of the zebrafish ETS family identifies three genes required for hemangioblast differentiation or angiogenesis. Circ Res. 2008;103:1147–1154. doi: 10.1161/CIRCRESAHA.108.179713. [DOI] [PubMed] [Google Scholar]
- Maroulakou IG, et al. Differential expression of ets-1 and ets-2 proto-oncogenes during murine embryogenesis. Oncogene. 1994;9:1551–1565. [PubMed] [Google Scholar]
- Mayr C, Bartel DP. Widespread shortening of 3’UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell. 2009;138:673–684. doi: 10.1016/j.cell.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKercher SR, et al. Targeted disruption of the PU.l gene results in multiple hematopoietic abnormalities. The EMBO journal. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- Nam Y, et al. Molecular basis for interaction of let-7 microRNAs with Lin28. Cell. 2011;147:1080–1091. doi: 10.1016/j.cell.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoli S, et al. miR-221 is required for endothelial tip cell behaviors during vascular development. Dev Cell. 2012;22:418–429. doi: 10.1016/j.devcel.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoli S, et al. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X, et al. Versatile synthesis and rational design of caged morpholinos. I Am ChemSoc. 2009;131:13255–13269. doi: 10.1021/ja809933h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham VN, et al. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol. 2007;303:772–783. doi: 10.1016/j.ydbio.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, et al. Asclla regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nature cell biology. 2010;12:1101–1107. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W, Flamme I. Vasculogenesis. Annual review of cell and developmental biology. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Roman BL, et al. Disruption of acvrll increases endothelial cell number in zebrafish cranial vessels. Development. 2002;129:3009–3019. doi: 10.1242/dev.129.12.3009. [DOI] [PubMed] [Google Scholar]
- Sandberg R, et al. Proliferating cells express mRNAs with shortened 3’. untranslated regions and fewer microRNA target sites. Science. 2008;320:1643–1647. doi: 10.1126/science.1155390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EW, et al. Requirement of transcription factor PU.l in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- Sharrocks AD. The ETS-domain transcription factor family. Nature reviews. Molecular cell biology. 2001;2:827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- Shestopalov IA, et al. Light-controlled gene silencing in zebrafish embryos. Nat Chem Biol. 2007;3:650–651. doi: 10.1038/nchembio.2007.30. [DOI] [PubMed] [Google Scholar]
- Spyropoulos DD, et al. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Flil transcription factor. Molecular and cellular biology. 2000;20:5643–5652. doi: 10.1128/mcb.20.15.5643-5652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler P, et al. The c-ets-1 proto-oncogenes in Xenopus laevis: expression during oogenesis and embryogenesis. Mech Dev. 1993;41:163–174. doi: 10.1016/0925-4773(93)90046-z. [DOI] [PubMed] [Google Scholar]
- Sumanas S, Lin C. Etsl-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4:elO. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teruyama K, et al. Neurophilin-1 is a downstream target of transcription factor Ets-1 in human umbilical vein endothelial cells. FEBS Lett. 2001;504:1–4. doi: 10.1016/s0014-5793(01)02724-7. [DOI] [PubMed] [Google Scholar]
- Thornton JE, Gregory RI. How does Lin28 let-7 control development and disease? Trends in cell biology. 2012;22:474–482. doi: 10.1016/j.tcb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitsky I, et al. Extensive alternative polyadenylation during zebrafish development. Genome research. 2012;22:2054–2066. doi: 10.1101/gr.139733.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman MB, Lin S. Etsrp/Etv2 is directly regulated by Foxcla/b in the zebrafish angioblast. Circulation research. 2012;110:220–229. doi: 10.1161/CIRCRESAHA.111.251298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villefranc IA, et al. Gateway compatible vectors for analysis of gene function in the zebrafish. Dev Dyn. 2007;236:3077–3087. doi: 10.1002/dvdy.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan SR, et al. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakiya K, et al. A cAMP response element and an Ets motif are involved in the transcriptional regulation of fit-1 tyrosine kinase (vascular endothelial growth factor receptor 1) gene. J Biol Chem. 1996;271:30823–30828. doi: 10.1074/jbc.271.48.30823. [DOI] [PubMed] [Google Scholar]
- Wareing S, et al. The Flkl-Cre-Mediated Deletion of ETV2 Defines Its Narrow Temporal Requirement During Embryonic Hematopoietic Development. Stem Cells. 2012;30:1521–1531. doi: 10.1002/stem.1115. [DOI] [PubMed] [Google Scholar]
- Wei G, et al. Etsl and Ets2 are required for endothelial cell survival during embryonic angiogenesis. Blood. 2009;114:1123–1130. doi: 10.1182/blood-2009-03-211391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei GH, et al. Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. The EMBO journal. 2010;29:2147–2160. doi: 10.1038/emboj.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. Eugene, Oregon: University of Oregon Press; 1993. [Google Scholar]
- Wienholds E, et al. The microRNA-producing enzyme Dicerl is essential for zebrafish development. Nat Genet. 2003;35:217–218. doi: 10.1038/ng1251. [DOI] [PubMed] [Google Scholar]
- Wong KS, et al. Identification of vasculature-specific genes by microarray analysis of Etsrp/Etv2 overexpressing zebrafish embryos. Dev Dyn. 2009;238:1836–1850. doi: 10.1002/dvdy.21990. [DOI] [PubMed] [Google Scholar]
- Yu F, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.