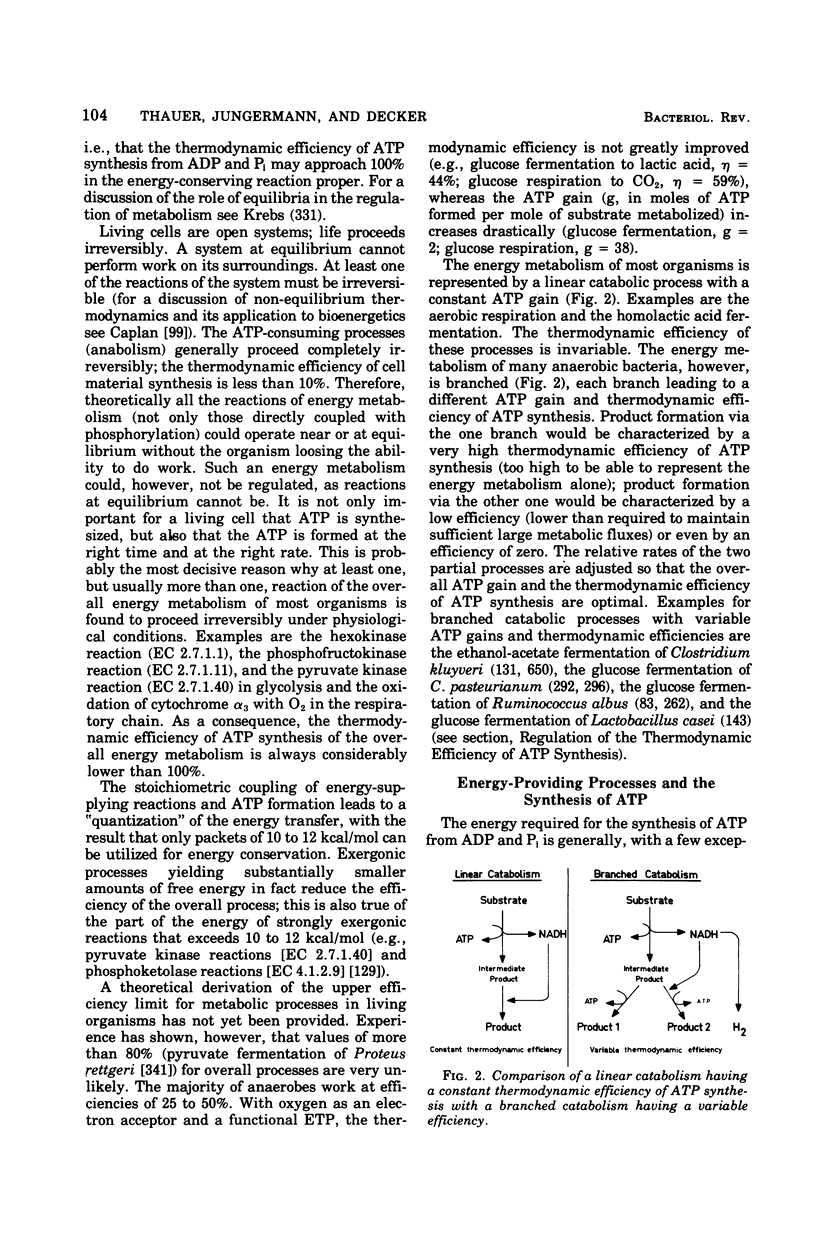
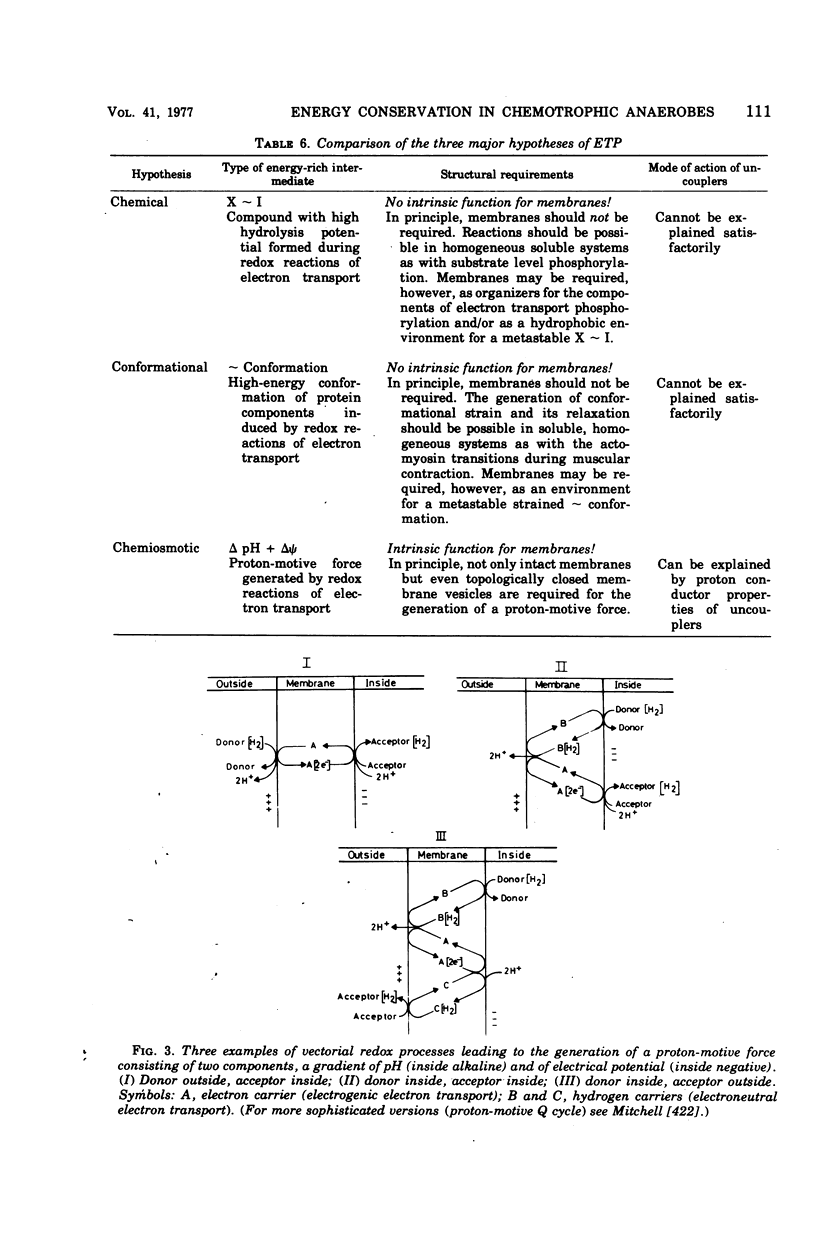
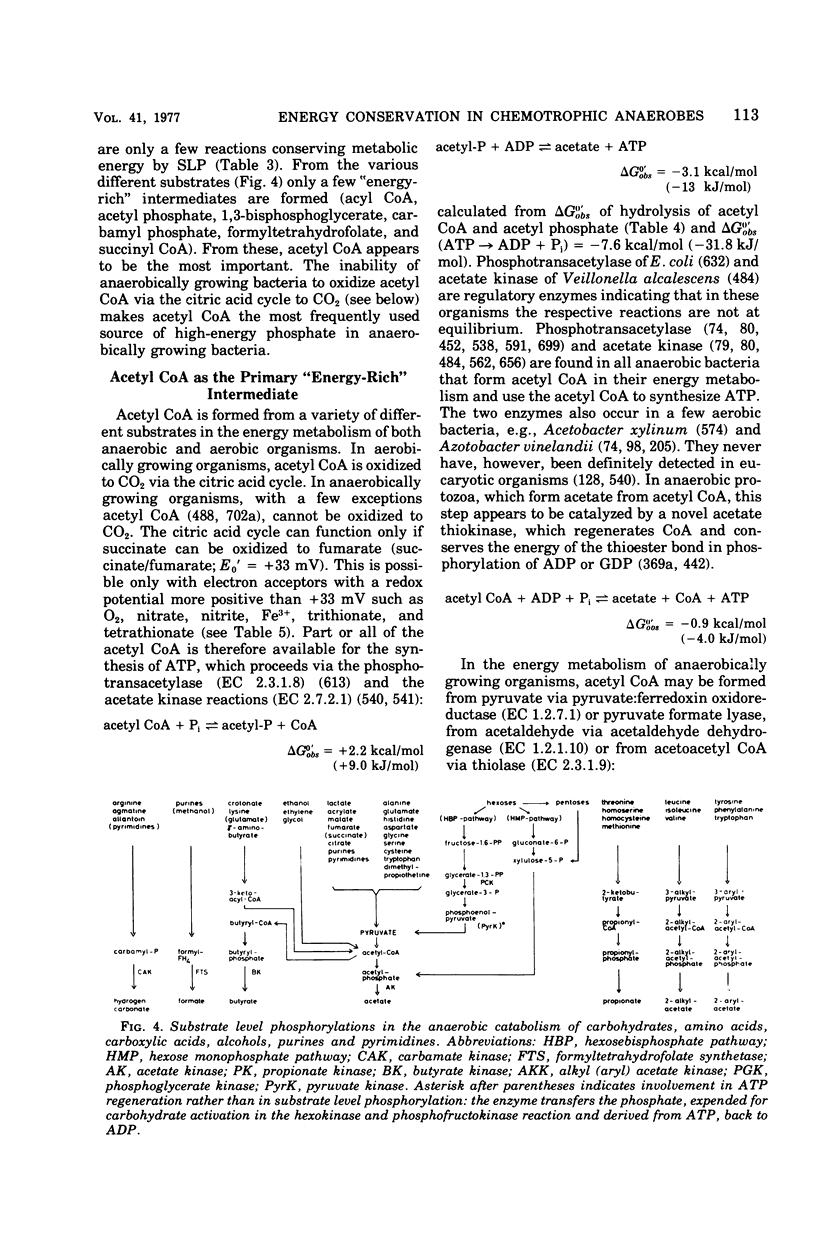
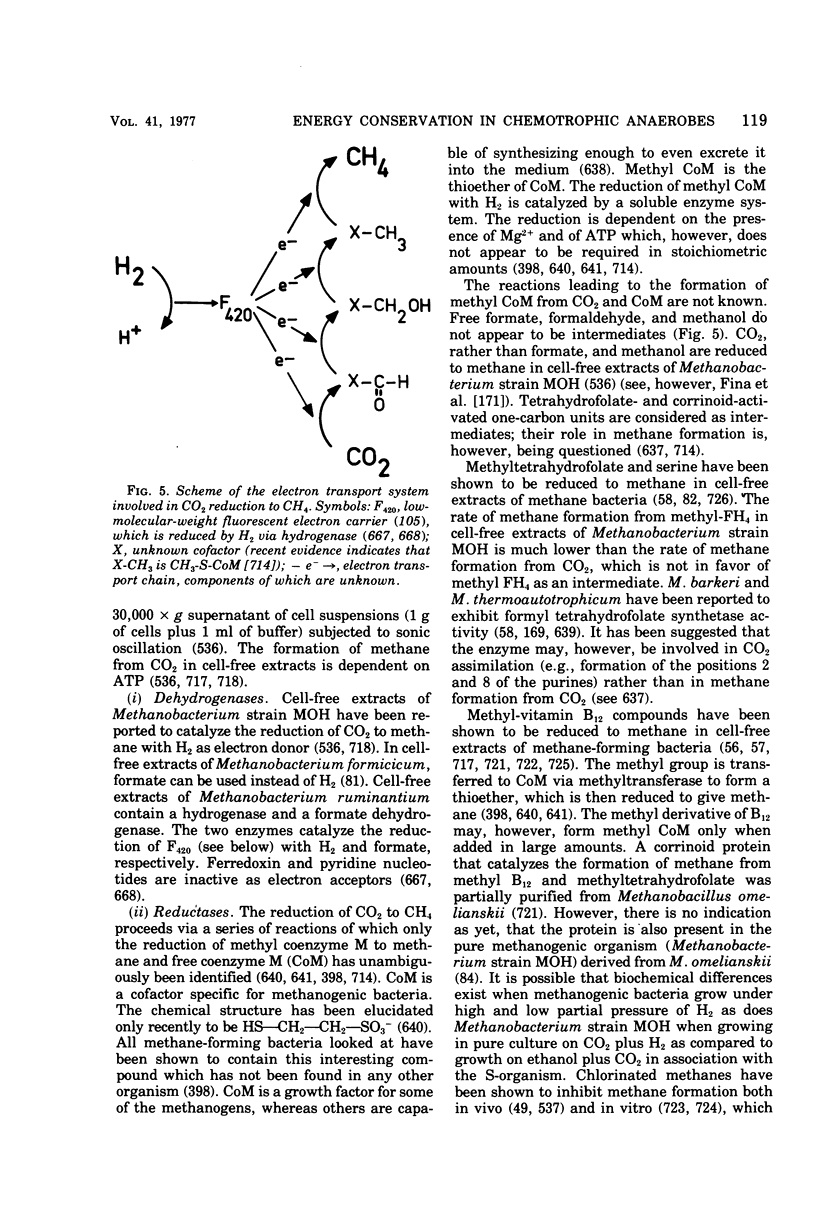
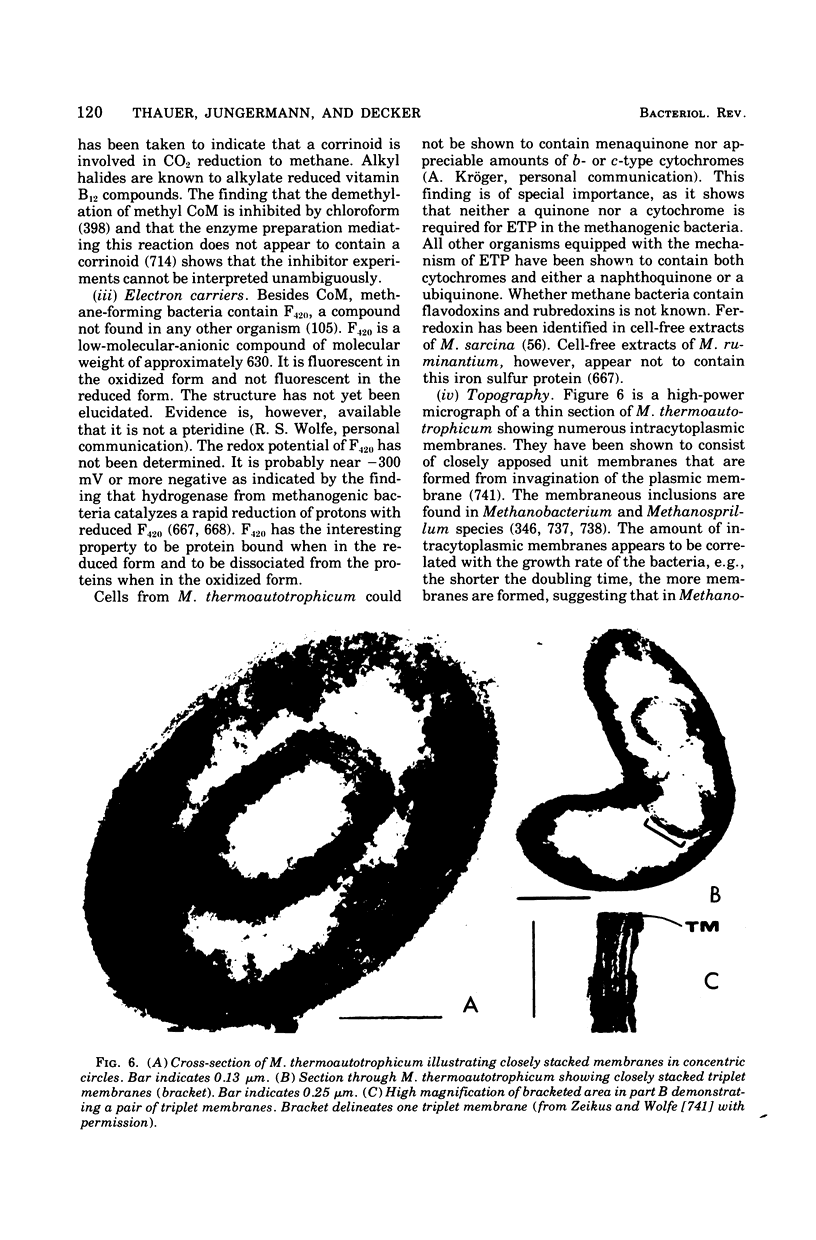
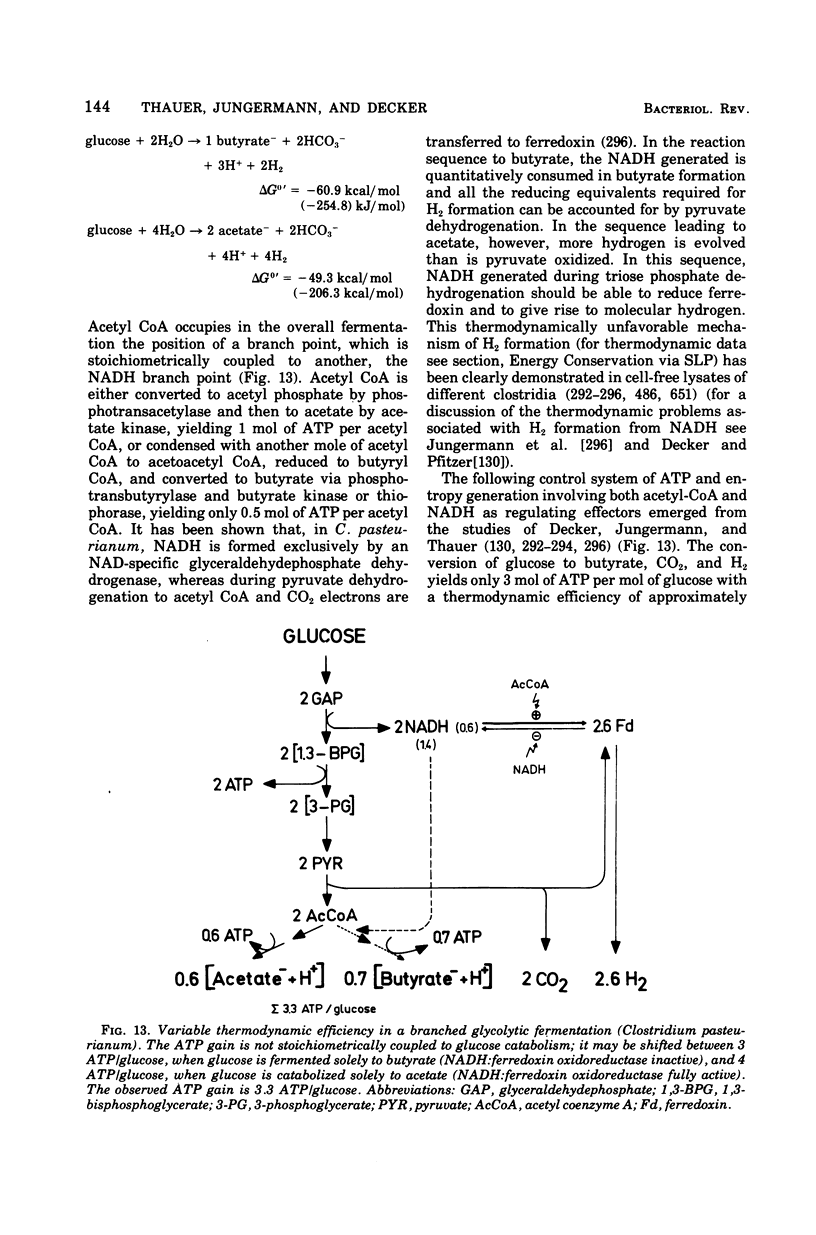
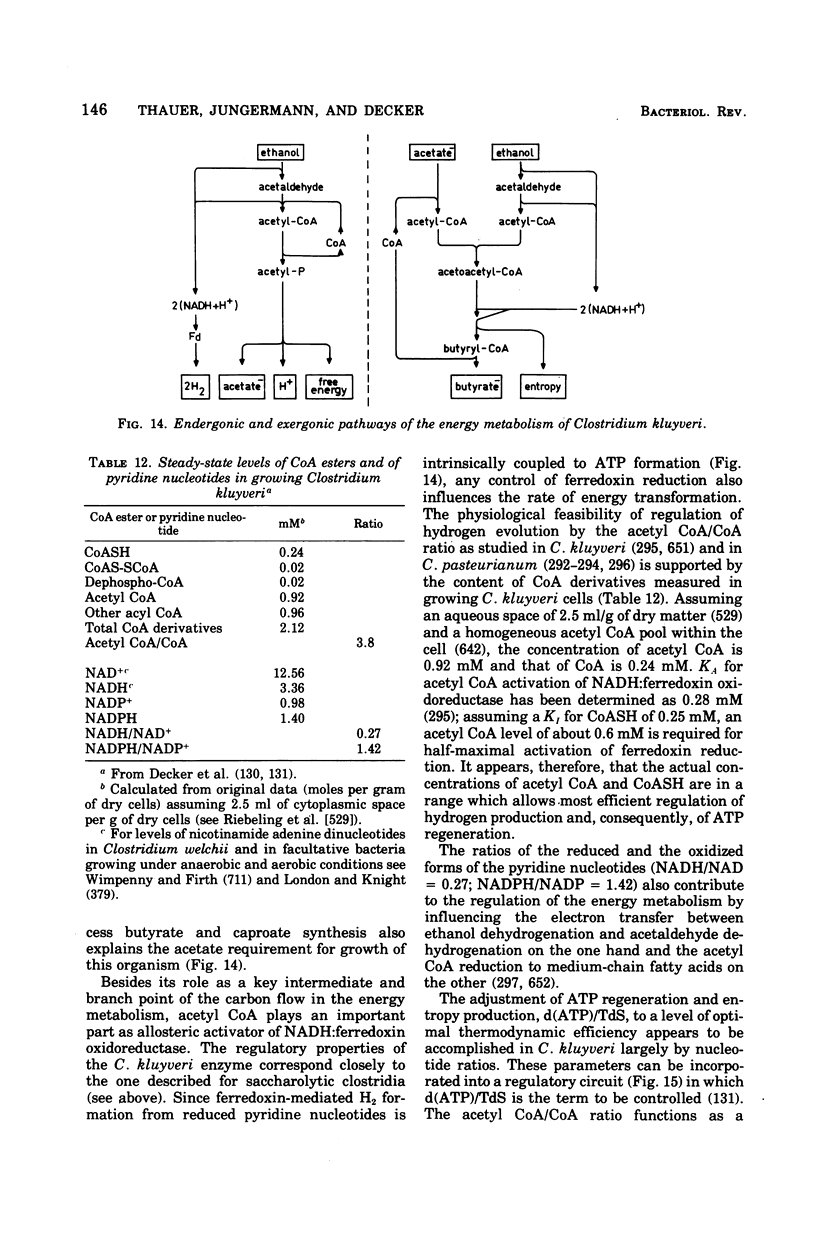
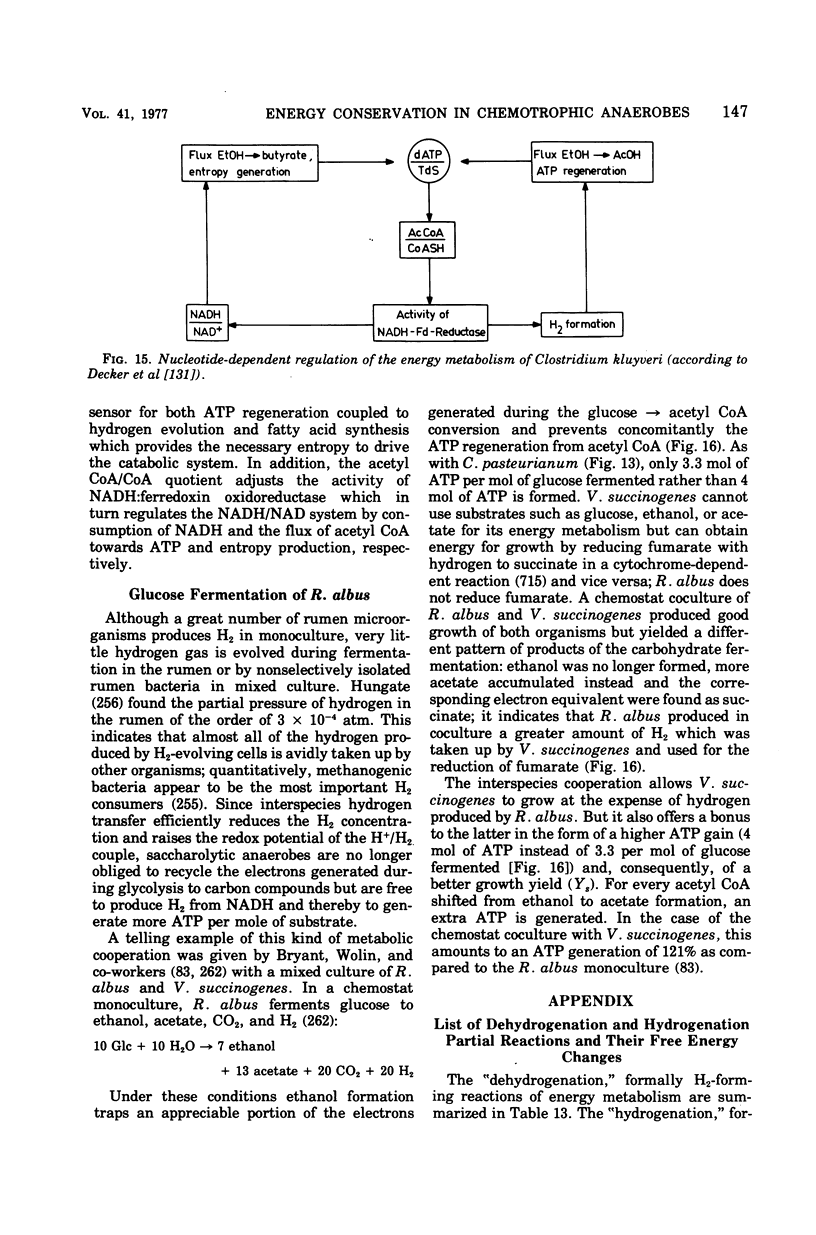
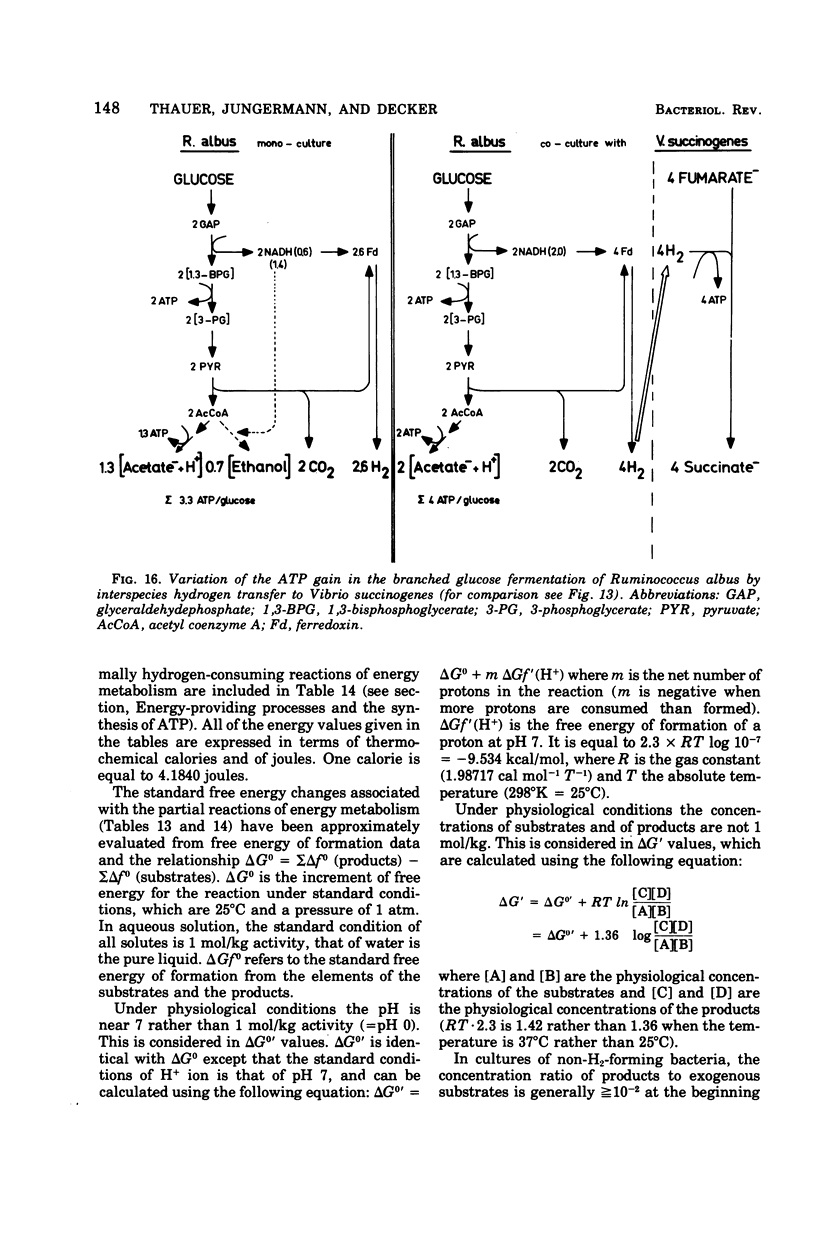
Full text
PDF















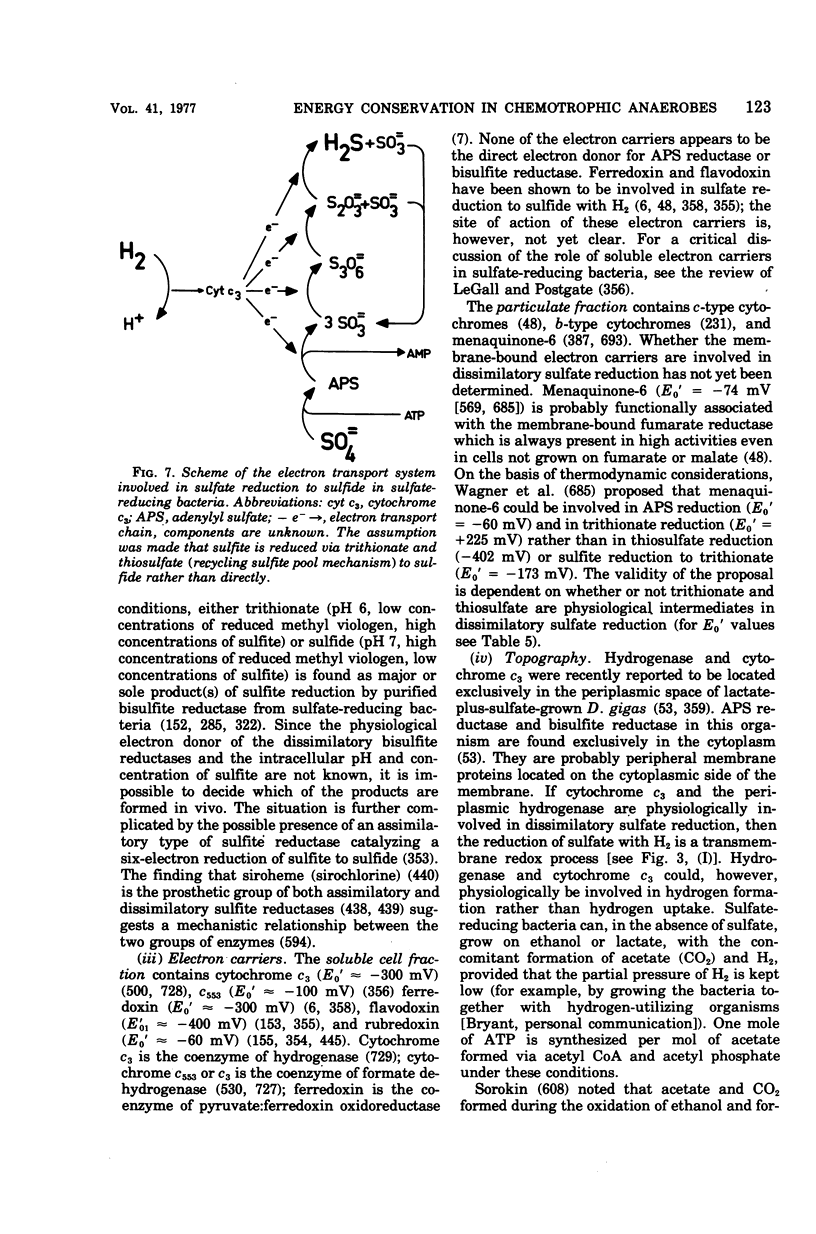


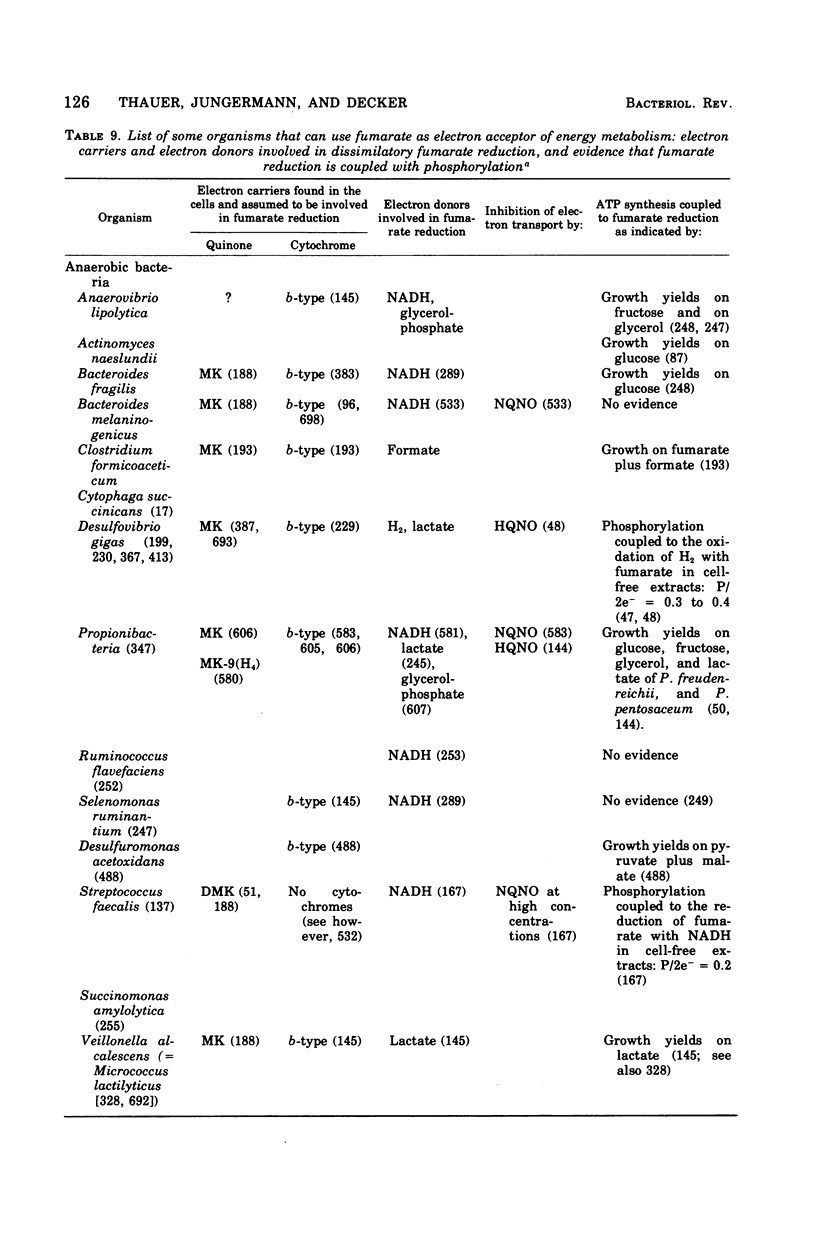
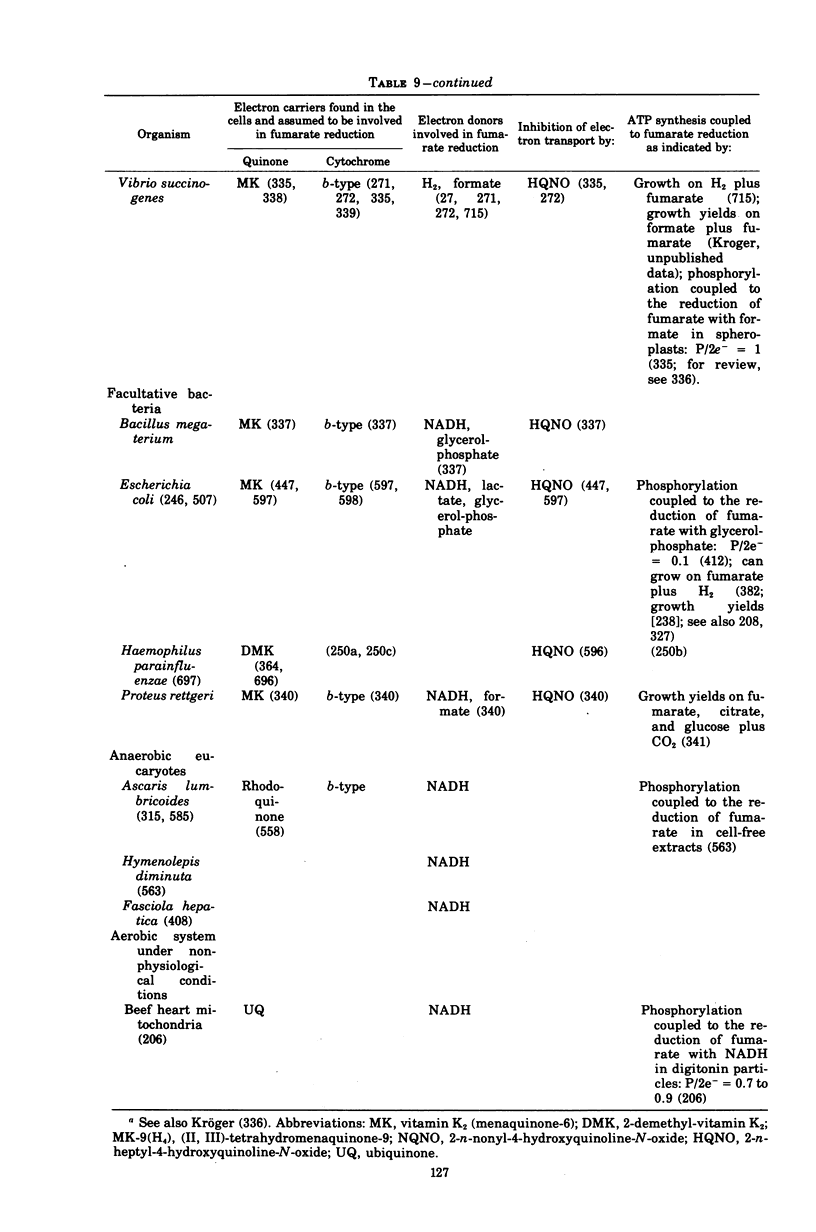



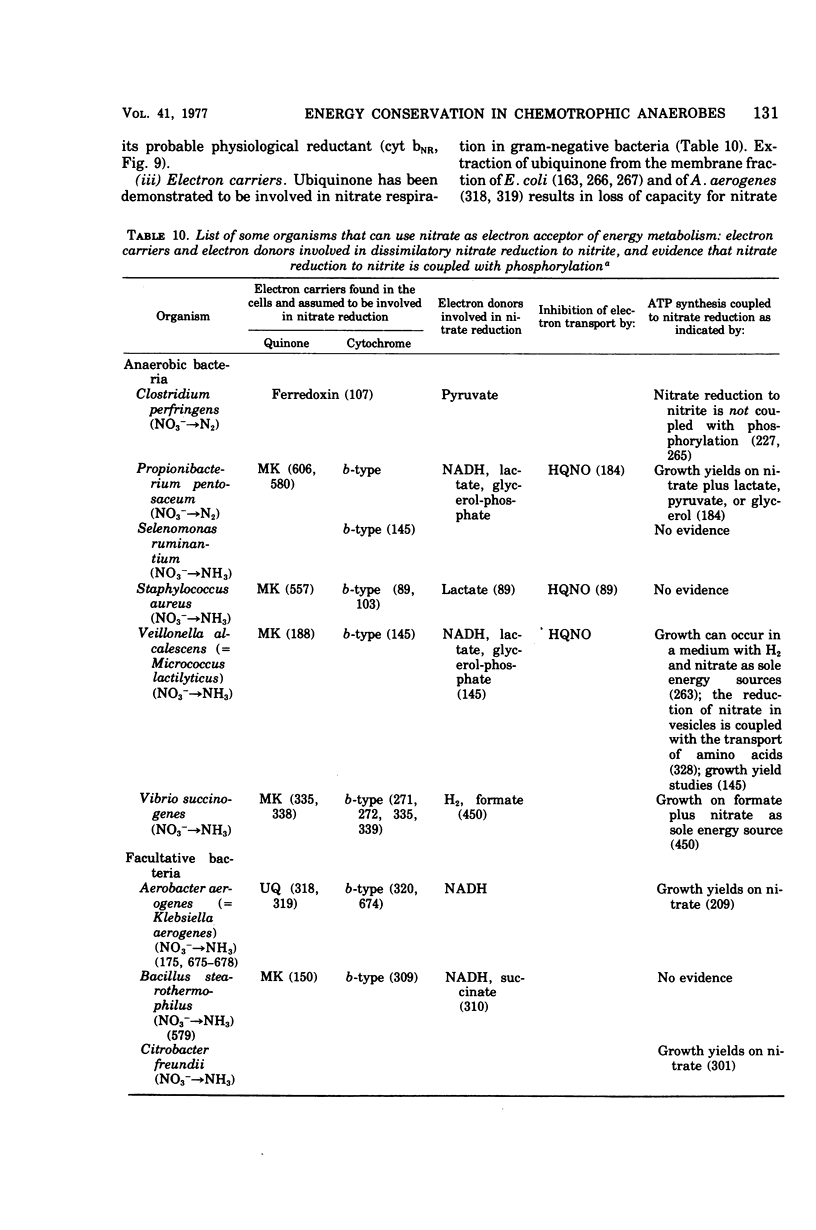









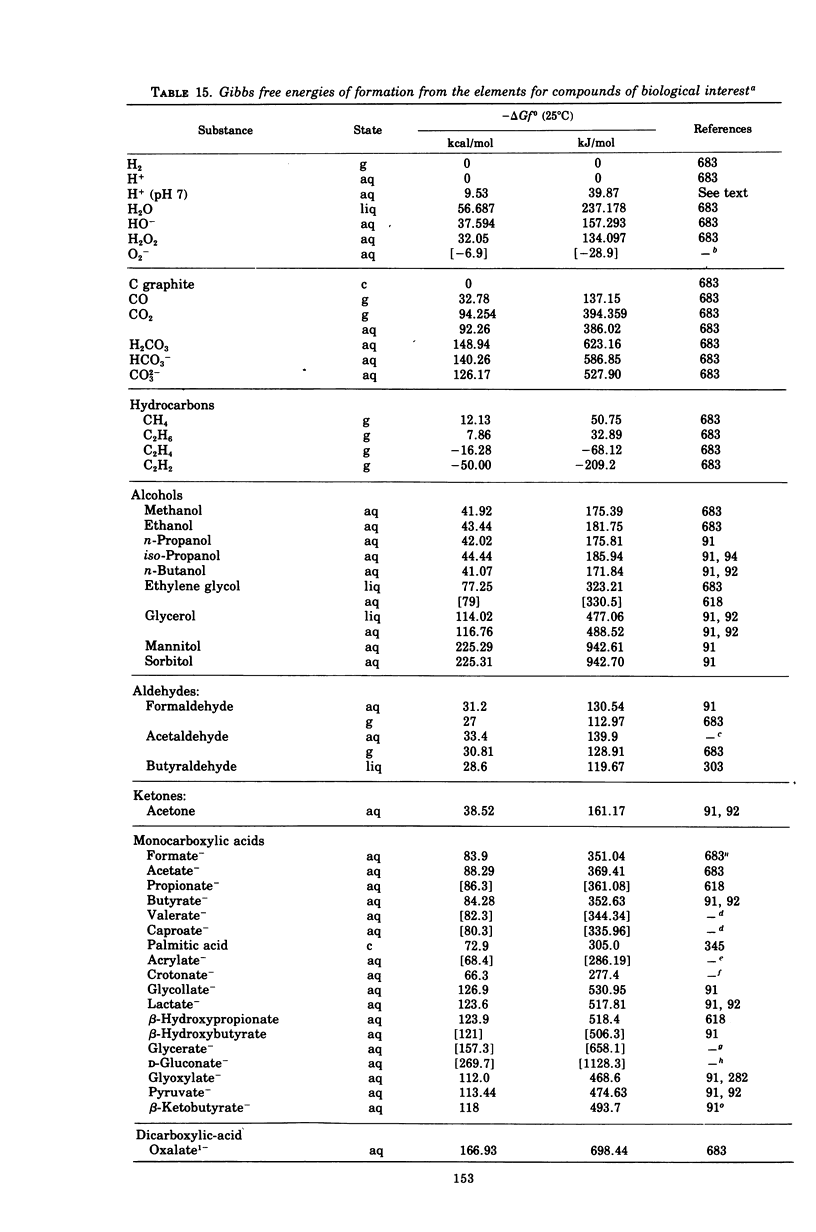
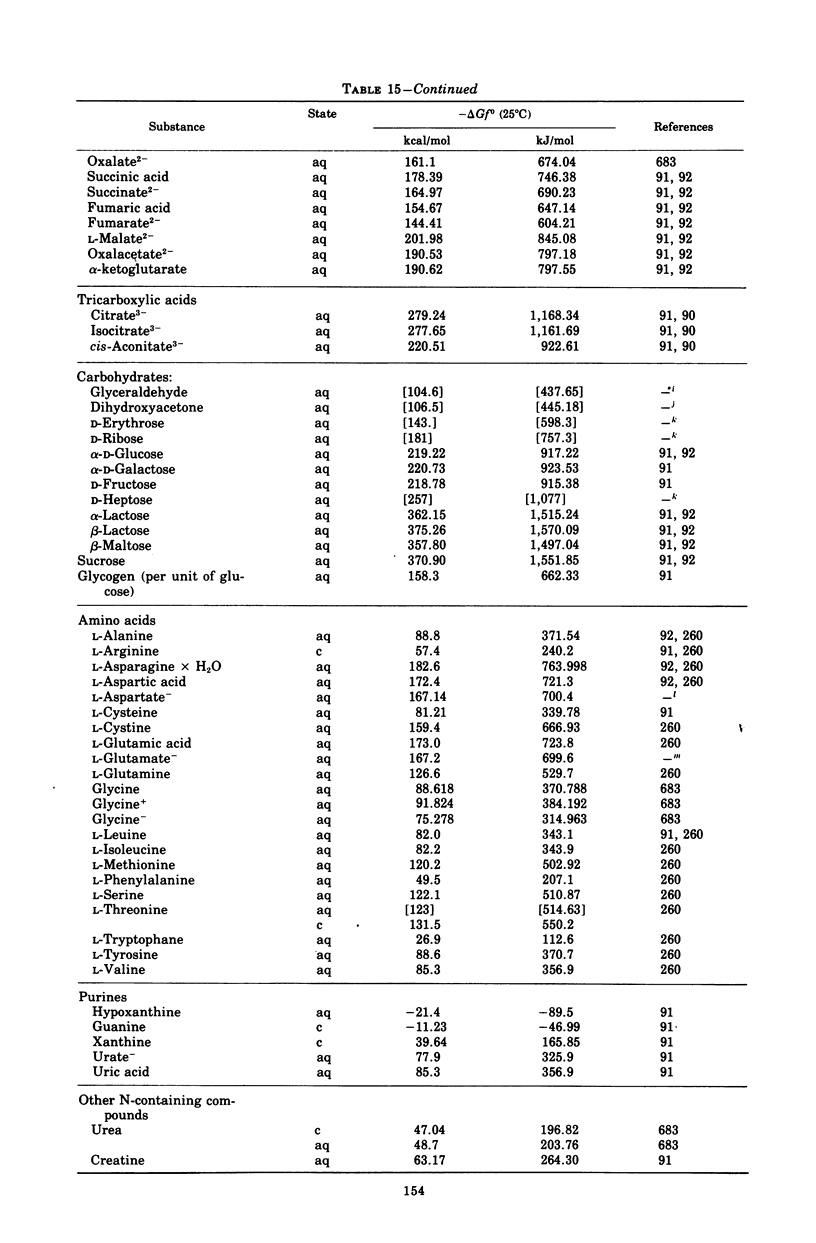
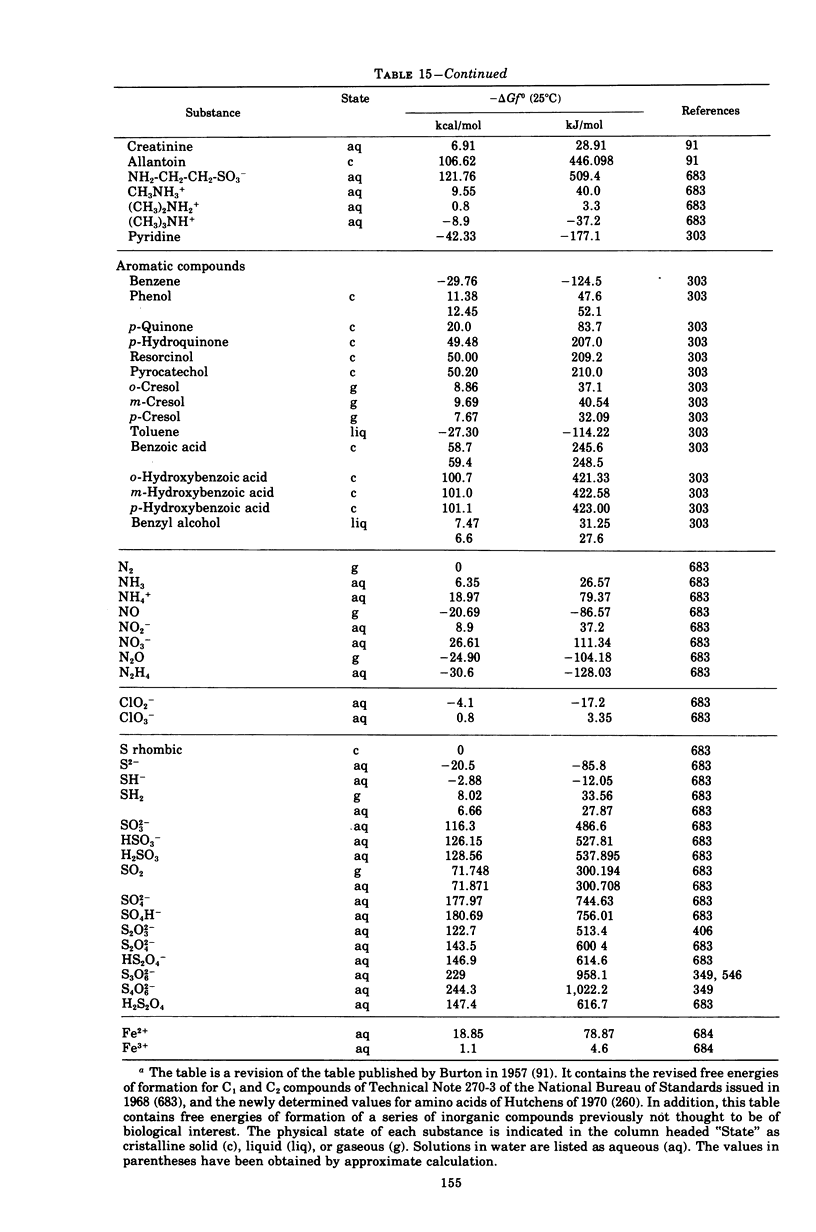

























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 't Riet J van, Stouthamer A. H., Planta R. J. Regulation of nitrate assimilation and nitrate respiration in Aerobacter aerogenes. J Bacteriol. 1968 Nov;96(5):1455–1464. doi: 10.1128/jb.96.5.1455-1464.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLEN M. B., VAN NIEL C. B. Experiments on bacterial denitrification. J Bacteriol. 1952 Sep;64(3):397–412. doi: 10.1128/jb.64.3.397-412.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLEN S. H., KELLERMEYER R. W., STJERNHOLM R. L., WOOD H. G. PURIFICATION AND PROPERTIES OF ENZYMES INVOLVED IN THE PROPIONIC ACID FERMENTATION. J Bacteriol. 1964 Jan;87:171–187. doi: 10.1128/jb.87.1.171-187.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON R. L., ORDAL E. J. CO2-dependent fermentation of glucose by Cytophaga succinicans. J Bacteriol. 1961 Jan;81:139–146. doi: 10.1128/jb.81.1.139-146.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDREW I. G., MORRIS J. G. THE BIOSYNTHESIS OF ALANINE BY CLOSTRIDIUM KLUYVERI. Biochim Biophys Acta. 1965 Jan 4;97:176–179. doi: 10.1016/0304-4165(65)90292-8. [DOI] [PubMed] [Google Scholar]
- Abrams A., Baron C. Reversible attachment of adenosine triphosphatase to streptococcal membranes and the effect of magnesium ions. Biochemistry. 1968 Feb;7(2):501–507. doi: 10.1021/bi00842a003. [DOI] [PubMed] [Google Scholar]
- Abrams A., Nolan E. A., Jensen C., Smith J. B. Tightly bound adenine nucleotide in bacterial membrane ATPase. Biochem Biophys Res Commun. 1973 Nov 1;55(1):22–29. doi: 10.1016/s0006-291x(73)80054-3. [DOI] [PubMed] [Google Scholar]
- Addanki A., Cahill F. D., Sotos J. F. Determination of intramitochondrial pH and intramitochondrial-extramitochondrial pH gradient of isolated heart mitochondria by the use of 5,5-dimethyl-2,4-oxazolidinedione. I. Changes during respiration and adenosine triphosphate-dependent transport of Ca++, Mg++, and Zn++. J Biol Chem. 1968 May 10;243(9):2337–2348. [PubMed] [Google Scholar]
- Akagi J. M., Adams V. Isolation of a bisulfite reductase activity from Desulfotomaculum nigrificans and its identification as the carbon monoxide-binding pigment P582. J Bacteriol. 1973 Oct;116(1):392–396. doi: 10.1128/jb.116.1.392-396.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi J. M., Campbell L. L. STUDIES ON THERMOPHILIC SULFATE-REDUCING BACTERIA III. : Adenosine Triphosphate-sulfurylase of Clostridium nigrificans and Desulfovibrio desulfuricans. J Bacteriol. 1962 Dec;84(6):1194–1201. doi: 10.1128/jb.84.6.1194-1201.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi J. M. Electron carries for the phosphoroclastic reaction of Desulfovibrio desulfuricans. J Biol Chem. 1967 May 25;242(10):2478–2483. [PubMed] [Google Scholar]
- Akagi J. M. The participation of a ferredoxin of Clostridium nigrificans in sulfite reduction. Biochem Biophys Res Commun. 1965 Oct 8;21(1):72–77. doi: 10.1016/0006-291x(65)90428-6. [DOI] [PubMed] [Google Scholar]
- Alberty R. A. Standard Gibbs free energy, enthalpy, and entropy changes as a function of pH and pMg for several reactions involving adenosine phosphates. J Biol Chem. 1969 Jun 25;244(12):3290–3302. [PubMed] [Google Scholar]
- Altendorf K., Harold F. M., Simoni R. D. Impairment and restoration of the energized state in membrane vesicles of a mutant of Escherichia coli lacking adenosine triphosphatase. J Biol Chem. 1974 Jul 25;249(14):4587–4593. [PubMed] [Google Scholar]
- Altendorf K., Hirata H., Harold F. M. Accumulation of lipid-soluble ions and of rubidium as indicators of the electrical potential in membrane vesicles of Escherichia coli. J Biol Chem. 1975 Feb 25;250(4):1405–1412. [PubMed] [Google Scholar]
- Ambler R. P. The amino acid sequence of cytochrome c-551.5 (Cytochrome c(7)) from the green photosynthetic bacterium Chloropseudomonas ethylica. FEBS Lett. 1971 Nov 1;18(2):351–353. doi: 10.1016/0014-5793(71)80484-2. [DOI] [PubMed] [Google Scholar]
- Anderson R. L., Wood W. A. Carbohydrate metabolism in microorganisms. Annu Rev Microbiol. 1969;23:539–578. doi: 10.1146/annurev.mi.23.100169.002543. [DOI] [PubMed] [Google Scholar]
- Andreesen J. R., El Ghazzawi E., Gottschalk G. The effect of ferrous ions, tungstate and selenite on the level of formate dehydrogenase in Clostridium formicoaceticum and formate synthesis from CO2 during pyruvate fermentation. Arch Mikrobiol. 1974 Mar 4;96(2):103–118. doi: 10.1007/BF00590167. [DOI] [PubMed] [Google Scholar]
- Andreesen J. R., Gottschalk G., Schlegel H. G. Clostridium formicoaceticum nov. spec. isolation, description and distinction from C. aceticum and C. thermoaceticum. Arch Mikrobiol. 1970;72(2):154–174. doi: 10.1007/BF00409521. [DOI] [PubMed] [Google Scholar]
- Andreesen J. R., Gottschalk G. The occurrence of a modified Entner-doudoroff pathway in Clostridium aceticum. Arch Mikrobiol. 1969;69(2):160–170. doi: 10.1007/BF00409760. [DOI] [PubMed] [Google Scholar]
- Andreesen J. R., Ljungdahl L. G. Formate dehydrogenase of Clostridium thermoaceticum: incorporation of selenium-75, and the effects of selenite, molybdate, and tungstate on the enzyme. J Bacteriol. 1973 Nov;116(2):867–873. doi: 10.1128/jb.116.2.867-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreesen J. R., Ljungdahl L. G. Nicotinamide adenine dinucleotide phosphate-dependent formate dehydrogenase from Clostridium thermoaceticum: purification and properties. J Bacteriol. 1974 Oct;120(1):6–14. doi: 10.1128/jb.120.1.6-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreesen J. R., Schaupp A., Neurauter C., Brown A., Ljungdahl L. G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO 2 . J Bacteriol. 1973 May;114(2):743–751. doi: 10.1128/jb.114.2.743-751.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar S. S., Levin E., Harold F. M. Accumulation of neutral amino acids by Streptococcus faecalis. Energy coupling by a proton-motive force. J Biol Chem. 1973 Aug 10;248(15):5225–5233. [PubMed] [Google Scholar]
- Aspen A. J., Wolin M. J. Solubilization and reconstitution of a particulate hydrogenase from Vibrio succinogenes. J Biol Chem. 1966 Sep 25;241(18):4152–4156. [PubMed] [Google Scholar]
- Atkinson D. E. Regulation of enzyme function. Annu Rev Microbiol. 1969;23:47–68. doi: 10.1146/annurev.mi.23.100169.000403. [DOI] [PubMed] [Google Scholar]
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Aue B. J., Deiel R. H. Fumarate reductase activity of Streptococcus faecalis. J Bacteriol. 1967 Jun;93(6):1770–1776. doi: 10.1128/jb.93.6.1770-1776.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALDWIN R. L., MILLIGAN L. P. ELECTRON TRANSPORT IN PEPTOSTREPTOCOCCUS ELSDENII. Biochim Biophys Acta. 1964 Dec 23;92:421–432. doi: 10.1016/0926-6569(64)90001-x. [DOI] [PubMed] [Google Scholar]
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- BAUM R. H., DOLIN M. I. ISOLATION OF 2-SOLANESYL-1,4-NAPHTHOQUINONE FROM STREPTOCOCCUS FAECALIS, 10 CL. J Biol Chem. 1965 Aug;240:3425–3433. [PubMed] [Google Scholar]
- BERGMEYER H. U., HOLZ G., KLOTZSCH H., LANG G. PHOSPHOTRANSACETYLASE AUS CLOSTRIDIUM KLUYVERI. ZUECHTUNG DES BACTERIUMS, ISOLIERUNG, KRISTALLISATION UND EIGENSCHAFTEN DES ENZYMS. Biochem Z. 1963;338:114–121. [PubMed] [Google Scholar]
- BLAYLOCK B. A., STADTMAN T. C. Biosynthesis of methane from the methyl moiety of methylcobalamin. Biochem Biophys Res Commun. 1963 Apr 2;11:34–38. doi: 10.1016/0006-291x(63)90023-8. [DOI] [PubMed] [Google Scholar]
- BOJANOWSKI R., GAUDY E., VALENTINE R. C., WOLFE R. S. OXAMIC TRANSCARBAMYLASE OF STREPTOCOCCUS ALLANTOICUS. J Bacteriol. 1964 Jan;87:75–80. doi: 10.1128/jb.87.1.75-80.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K., KREBS H. A. The free-energy changes associated with the individual steps of the tricarboxylic acid cycle, glycolysis and alcoholic fermentation and with the hydrolysis of the pyrophosphate groups of adenosinetriphosphate. Biochem J. 1953 Apr;54(1):94–107. doi: 10.1042/bj0540094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. The free energy change associated with the hydrolysis of the thiol ester bond of acetyl coenzyme A. Biochem J. 1955 Jan;59(1):44–46. doi: 10.1042/bj0590044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K., WILSON T. H. The free-energy changes for the reduction of diphosphopyridine nucleotide and the dehydrogenation of L-malate and L-glycerol 1-phosphate. Biochem J. 1953 Apr;54(1):86–94. doi: 10.1042/bj0540086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON R. M., STADTMAN E. R. The oxidation of acetaldehyde to acetyl coenzyme A. J Biol Chem. 1953 Jun;202(2):873–890. [PubMed] [Google Scholar]
- Baginsky M. L., Huennekens F. M. Electron transport function of a heat-stable protein and a flavoprotein in the oxidative decarboxylation of glycine by Peptococcus glycinophilus. Biochem Biophys Res Commun. 1966 Jun 13;23(5):600–605. doi: 10.1016/0006-291x(66)90441-4. [DOI] [PubMed] [Google Scholar]
- Baker J. J., Jeng I., Barker H. A. Purification and properties of L-erythro-3,5-diaminohexanoate dehydrogenase from a lysine-fermenting Clostridium. J Biol Chem. 1972 Dec 10;247(23):7724–7734. [PubMed] [Google Scholar]
- Baltscheffsky H., Baltscheffsky M. Electron transport phosphorylation. Annu Rev Biochem. 1974;43(0):871–897. doi: 10.1146/annurev.bi.43.070174.004255. [DOI] [PubMed] [Google Scholar]
- Banks B. E. Thermodynamics and biology. Chem Br. 1969 Nov;5(11):514–519. [PubMed] [Google Scholar]
- Banks B. E., Vernon C. A. Reassessment of the role of ATP in vivo. J Theor Biol. 1970 Nov;29(2):301–326. doi: 10.1016/0022-5193(70)90024-x. [DOI] [PubMed] [Google Scholar]
- Barker H. A. Citramalate lyase of Clostridium tetanomorphum. Arch Mikrobiol. 1967;59(1):4–12. doi: 10.1007/BF00406311. [DOI] [PubMed] [Google Scholar]
- Baron C., Abrams A. Isolation of a bacterial membrane protein, nectin, essential for the attachment of adenosine triphosphatase. J Biol Chem. 1971 Mar 10;246(5):1542–1544. [PubMed] [Google Scholar]
- Barton L. L., Le Gall J., Peck H. D., Jr Phosphorylation coupled to oxidation of hydrogen with fumarate in extracts of the sulfate reducing bacterium, Desulfovibrio gigas. Biochem Biophys Res Commun. 1970 Nov 25;41(4):1036–1042. doi: 10.1016/0006-291x(70)90189-0. [DOI] [PubMed] [Google Scholar]
- Bauchop T. Inhibition of rumen methanogenesis by methane analogues. J Bacteriol. 1967 Jul;94(1):171–175. doi: 10.1128/jb.94.1.171-175.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. R., LeGall L., Peck H. D. Evidence for the periplasmic location of hydrogenase in Desulfovibrio gigas. J Bacteriol. 1974 Nov;120(2):994–997. doi: 10.1128/jb.120.2.994-997.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt H., Schlegel H. G. Kinetics and properties of beta-ketothiolase from Clostridium pasteurianum. Arch Microbiol. 1975 Mar 12;103(1):21–30. doi: 10.1007/BF00436325. [DOI] [PubMed] [Google Scholar]
- Biebl H., Pfennig Growth of sulfate-reducing bacteria with sulfur as electron acceptor. Arch Microbiol. 1977 Feb 4;112(1):115–117. doi: 10.1007/BF00446664. [DOI] [PubMed] [Google Scholar]
- Blaylock B. A. Cobamide-dependent methanol-cyanocob(I)alamin methyltransferase of Methanosarcina barkeri. Arch Biochem Biophys. 1968 Mar 20;124(1):314–324. doi: 10.1016/0003-9861(68)90333-0. [DOI] [PubMed] [Google Scholar]
- Blaylock B. A., Stadtman T. C. Methane biosynthesis by Methanosarcina barkeri. Properties of the soluble enzyme system. Arch Biochem Biophys. 1966 Sep 26;116(1):138–152. doi: 10.1016/0003-9861(66)90022-1. [DOI] [PubMed] [Google Scholar]
- Bongers L. Yields of Hydrogenomonas eutropha from growth on succinate and fumarate. J Bacteriol. 1970 May;102(2):598–599. doi: 10.1128/jb.102.2.598-599.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra J., Huttunen M. T., Konings W. N. Anaerobic transport in Escherichia coli membrane vesicles. J Biol Chem. 1975 Sep 10;250(17):6792–6798. [PubMed] [Google Scholar]
- Boos W. Bacterial transport. Annu Rev Biochem. 1974;43(0):123–146. doi: 10.1146/annurev.bi.43.070174.001011. [DOI] [PubMed] [Google Scholar]
- Booth I. R., Morris J. G. Proton-motive force in the obligately anaerobic bacterium Clostridium pasteurianum: a role in galactose and gluconate uptake. FEBS Lett. 1975 Nov 15;59(2):153–157. doi: 10.1016/0014-5793(75)80364-4. [DOI] [PubMed] [Google Scholar]
- Bothe H., Falkenberg B., Nolteernsting U. Properties and function of the pyruvate: ferredoxin oxidoreductase from the blue-green alga Anabaena cylindrica. Arch Mikrobiol. 1974 Mar 28;96(4):291–304. doi: 10.1007/BF00590185. [DOI] [PubMed] [Google Scholar]
- Boxer D. H., Clegg R. A. A transmembrane location for the proton-translocating reduced ubiquinone leads to nitrate reductase segment of the respiration chain of Escherichia coli. FEBS Lett. 1975 Dec 1;60(1):54–57. doi: 10.1016/0014-5793(75)80417-0. [DOI] [PubMed] [Google Scholar]
- Boyer P. D., Cross R. L., Momsen W. A new concept for energy coupling in oxidative phosphorylation based on a molecular explanation of the oxygen exchange reactions. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2837–2839. doi: 10.1073/pnas.70.10.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P. D. Energy transduction and proton translocation by adenosine triphosphatases. FEBS Lett. 1975 Feb 1;50(2):91–94. doi: 10.1016/0014-5793(75)80464-9. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Chen C. H., Lehninger A. L. Stoichiometry of H+ ejection during respiration-dependent accumulation of Ca2+ by rat liver mitochondria. J Biol Chem. 1976 Feb 25;251(4):968–974. [PubMed] [Google Scholar]
- Bray R. C., Vincent S. P., Lowe D. J., Clegg R. A., Garland P. B. Electron-paramagnetic-resonance studies on the molybdenum of nitrate reductase from Escherichia coli K12. Biochem J. 1976 Apr 1;155(1):201–203. doi: 10.1042/bj1550201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresters T. W., Krul J., Scheepens P. C., Veeger C. Phosphotransacetylase associated with the pyruvate dehydrogenase complex from the nitrogen fixing Azotobacter vinelandii. FEBS Lett. 1972 May 15;22(3):305–309. doi: 10.1016/0014-5793(72)80257-6. [DOI] [PubMed] [Google Scholar]
- Brill W. J., Wolfe R. S. Acetaldehyde oxidation by methanobacillus--a new ferredoxin-dependent reaction. Nature. 1966 Oct 15;212(5059):253–255. doi: 10.1038/212253a0. [DOI] [PubMed] [Google Scholar]
- Brockman H. L., Wood W. A. D-Lactate dehydrogenase of Peptostreptococcus elsdenii. J Bacteriol. 1975 Dec;124(3):1454–1461. doi: 10.1128/jb.124.3.1454-1461.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman H. L., Wood W. A. Electron-transferring flavoprotein of Peptostreptococcus elsdenii that functions in the reduction of acrylyl-coenzyme A. J Bacteriol. 1975 Dec;124(3):1447–1453. doi: 10.1128/jb.124.3.1447-1453.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Akagi J. M. Purification of acetokinase from Desulfovibrio desulfuricans. J Bacteriol. 1966 Oct;92(4):1273–1274. doi: 10.1128/jb.92.4.1273-1274.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. D., Pereira C. R., Stormer F. C. Studies of the acetate kinase-phosphotransacetylase and the butanediol-forming systems in Aerobacter aerogenes. J Bacteriol. 1972 Dec;112(3):1106–1111. doi: 10.1128/jb.112.3.1106-1111.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., McBride B. C., Wolfe R. S. Hydrogen-oxidizing methane bacteria. I. Cultivation and methanogenesis. J Bacteriol. 1968 Mar;95(3):1118–1123. doi: 10.1128/jb.95.3.1118-1123.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Wolin E. A., Wolin M. J., Wolfe R. S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol. 1967;59(1):20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Pine L. Path of glucose breakdown and cell yields of a facultative anaerobe, Actinomyces naeslundii. J Gen Microbiol. 1967 Feb;46(2):225–236. doi: 10.1099/00221287-46-2-225. [DOI] [PubMed] [Google Scholar]
- Buckel W., Barker H. A. Two pathways of glutamate fermentation by anaerobic bacteria. J Bacteriol. 1974 Mar;117(3):1248–1260. doi: 10.1128/jb.117.3.1248-1260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke K. A., Lascelles J. Nitrate reductase system in Staphylococcus aureus wild type and mutants. J Bacteriol. 1975 Jul;123(1):308–316. doi: 10.1128/jb.123.1.308-316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG J. P., LASCELLES J. NITRATE REDUCTASE IN CELL-FREE EXTRACTS OF A HAEMIN-REQUIRING STRAIN OF STAPHYLOCOCCUS AUREUS. Biochem J. 1963 Dec;89:503–510. doi: 10.1042/bj0890503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN S. L. ENERGY REQUIREMENT FOR MICROBIAL GROWTH. Nature. 1964 Jun 13;202:1135–1136. doi: 10.1038/2021135b0. [DOI] [PubMed] [Google Scholar]
- CRANE R. K. Hypothesis for mechanism of intestinal active transport of sugars. Fed Proc. 1962 Nov-Dec;21:891–895. [PubMed] [Google Scholar]
- Caldwell D. R., White D. C., Bryant M. P., Doetsch R. N. Specificity of the heme requirement for growth of Bacteroides ruminicola. J Bacteriol. 1965 Dec;90(6):1645–1654. doi: 10.1128/jb.90.6.1645-1654.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F., Yates M. G. Pyruvate metabolism and nitrogen fixation in Azotobacter. FEBS Lett. 1973 Dec 1;37(2):203–206. doi: 10.1016/0014-5793(73)80459-4. [DOI] [PubMed] [Google Scholar]
- Campbell L. L., Postgate J. R. Classification of the spore-forming sulfate-reducing bacteria. Bacteriol Rev. 1965 Sep;29(3):359–363. doi: 10.1128/br.29.3.359-363.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeli C. Proton translocation induced by ATPase activity in chloroplasts. FEBS Lett. 1970 Apr 16;7(3):297–300. doi: 10.1016/0014-5793(70)80187-9. [DOI] [PubMed] [Google Scholar]
- Chambers L. A., Trudinger P. A. Are thiosulfate and trithionate intermediates in dissimilatory sulfate reduction? J Bacteriol. 1975 Jul;123(1):36–40. doi: 10.1128/jb.123.1.36-40.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelain R. Réduction des nitrites par Alcaligenes odorans var. viridans. Ann Inst Pasteur (Paris) 1969 Apr;116(4):498–500. [PubMed] [Google Scholar]
- Cheeseman P., Toms-Wood A., Wolfe R. S. Isolation and properties of a fluorescent compound, factor 420 , from Methanobacterium strain M.o.H. J Bacteriol. 1972 Oct;112(1):527–531. doi: 10.1128/jb.112.1.527-531.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Ishimoto M. Ferredoxin-linked nitrate reductase from Clostridium perfringens. J Biochem. 1973 Jun;73(6):1315–1318. doi: 10.1093/oxfordjournals.jbchem.a130208. [DOI] [PubMed] [Google Scholar]
- Chirpich T. P., Zappia V., Costilow R. N., Barker H. A. Lysine 2,3-aminomutase. Purification and properties of a pyridoxal phosphate and S-adenosylmethionine-activated enzyme. J Biol Chem. 1970 Apr 10;245(7):1778–1789. [PubMed] [Google Scholar]
- Clarke D. J., Morris J. G. Partial purification of a dicyclohexylcarbodi-imide-sensitive membrane adenosine triphosphatase complex from the obligately anaerobic bacterium Clostridium Pasteurianum. Biochem J. 1976 Mar 15;154(3):725–729. doi: 10.1042/bj1540725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg R. A. Purification and some properties of nitrate reductase (EC 1.7.99.4) from Escherichia coli K12. Biochem J. 1976 Mar 1;153(3):533–541. doi: 10.1042/bj1530533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell R. S., Harris E. J., Pressman B. C. Synthesis of ATP driven by a potassium gradient in mitochondria. Nature. 1967 Sep 30;215(5109):1487–1488. doi: 10.1038/2151487a0. [DOI] [PubMed] [Google Scholar]
- Cole J. A. Cytochrome c552 and nitrite reduction in Escherichia coli. Biochim Biophys Acta. 1968 Oct 1;162(3):356–368. doi: 10.1016/0005-2728(68)90122-9. [DOI] [PubMed] [Google Scholar]
- Cole J. A., Ward F. B. Nitrite reductase-deficient mutants of Escherichia coli K12. J Gen Microbiol. 1973 May;76(1):21–29. doi: 10.1099/00221287-76-1-21. [DOI] [PubMed] [Google Scholar]
- Cole J. S., 3rd, Aleem M. I. Electron transport-linked compared with proton-induced ATP generation in Thiobacillus novellus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3571–3575. doi: 10.1073/pnas.70.12.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Jr, Payne W. J. Separation of soluble denitrifying enzymes and cytochromes from Pseudomonas perfectomarinus. Can J Microbiol. 1973 Jul;19(7):861–872. doi: 10.1139/m73-137. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Gibson F. Studies on electron transport and energy-linked reactions using mutants of Escherichia coli. Biochim Biophys Acta. 1974 Apr 30;346(1):1–25. doi: 10.1016/0304-4173(74)90010-x. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane R. K. Na+ -dependent transport in the intestine and other animal tissues. Fed Proc. 1965 Sep-Oct;24(5):1000–1006. [PubMed] [Google Scholar]
- Cross R. L., de Sousa J. T., Packer L. Thiophosphate labelling of mitochondria-lack of evidence for an acyl-phosphate intermediate in oxidative phosphorylation. J Bioenerg. 1974;6(1):21–25. doi: 10.1007/BF01649013. [DOI] [PubMed] [Google Scholar]
- Czerkawski J. W., Harfoot C. G., Breckenridge G. The relationship between methane production and concentrations of hydrogen in the aqueous and gaseous phases during rumen fermentation in vitro. J Appl Bacteriol. 1972 Dec;35(4):537–551. doi: 10.1111/j.1365-2672.1972.tb03735.x. [DOI] [PubMed] [Google Scholar]
- DAWES E. A., FOSTER S. M. The formation of ethanol in Escherichia coli. Biochim Biophys Acta. 1956 Nov;22(2):253–265. doi: 10.1016/0006-3002(56)90148-2. [DOI] [PubMed] [Google Scholar]
- DEIBEL R. H., KVETKAS M. J. FUMARATE REDUCTION AND ITS ROLE IN THE DIVERSION OF GLUCOSE FERMENTATION BY STREPTOCOCCUS FAECALIS. J Bacteriol. 1964 Oct;88:858–864. doi: 10.1128/jb.88.4.858-864.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELWICHE C. C. Production and utilization of nitrous oxide by Pseudomonas denitrificans. J Bacteriol. 1959 Jan;77(1):55–59. doi: 10.1128/jb.77.1.55-59.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daesch G., Mortenson L. E. Sucrose catabolism in Clostridium pasteurianum and its relation to N2 fixation. J Bacteriol. 1968 Aug;96(2):346–351. doi: 10.1128/jb.96.2.346-351.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot G. N., Stouthamer A. H. Regulation of reductase formation in Proteus mirabilis. I. Formation of reductases and enzymes of the formic hydrogenlyase complex in the wild type and in chlorate-resistant mutants. Arch Mikrobiol. 1969;66(3):220–233. [PubMed] [Google Scholar]
- De Groot G. N., Stouthamer A. H. Regulation of reductase formation in Proteus mirabilis. II. Influence of growth with azide and of haem deficiency on nitrate reductase formation. Biochim Biophys Acta. 1970 Jun;208(3):414–427. doi: 10.1016/0304-4165(70)90214-x. [DOI] [PubMed] [Google Scholar]
- De Weer P., Lowe A. G. Myokinase equilibrium. An enzymatic method for the determination of stability constants of magnesium complexes with adenosine triphosphate, adenosine diphosphate, and adenosine monophosphate in media of high ionic strength. J Biol Chem. 1973 Apr 25;248(8):2829–2835. [PubMed] [Google Scholar]
- De Zoeten L. W., Posthuma D., Tipker J. Intermediary metabolism of the liver fluke Fasciola hepatica, I. Biosynthesis of propionic acid. Hoppe Seylers Z Physiol Chem. 1969 Jun;350(6):683–690. doi: 10.1515/bchm2.1969.350.1.683. [DOI] [PubMed] [Google Scholar]
- Decker K., Jungermann K., Thauer R. K. Energy production in anaerobic organisms. Angew Chem Int Ed Engl. 1970 Feb;9(2):138–158. doi: 10.1002/anie.197001381. [DOI] [PubMed] [Google Scholar]
- Decker K., Pfitzer S. Determination of steady-state concentrations of adenine nucleotides in growing C. kluyveri cells by biosynthetic labeling. Anal Biochem. 1972 Dec;50(2):529–539. doi: 10.1016/0003-2697(72)90063-2. [DOI] [PubMed] [Google Scholar]
- Deibel R. H. Utilization of arginine as an energy source for the growth of Streptococcus faecalis. J Bacteriol. 1964 May;87(5):988–992. doi: 10.1128/jb.87.5.988-992.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker E. E., Barker H. A. Identification and cobamide coenzyme-dependent formation of 3,5-diaminohexanoic acid, an intermediate in lysine fermentation. J Biol Chem. 1968 Jun 25;243(12):3232–3237. [PubMed] [Google Scholar]
- Delwiche E. A. Mechanism of Propionic Acid Formation by Propionibacterium pentosaceum. J Bacteriol. 1948 Dec;56(6):811–820. doi: 10.1128/jb.56.6.811-820.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzler D. N., Lais C. J., Magnani J. L., Leckie M. P. Maintenance of the energy charge in the presence of large decreases in the total adenylate pool of Escherichia coli and concurrent changes in glucose-6-p, fructose-p2 and glycogen synthesis. Biochem Biophys Res Commun. 1974 Oct 8;60(3):875–881. [PubMed] [Google Scholar]
- Douglas M. W., Ward F. B., Cole J. A. The formate hydrogenlyase activity of cytochrome c552-deficient mutants of Escherichia coli K12. J Gen Microbiol. 1974 Feb;80(2):557–560. doi: 10.1099/00221287-80-2-557. [DOI] [PubMed] [Google Scholar]
- Downey R. J. NAPHTHOQUINONE INTERMEDIATE IN THE RESPIRATION OF BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1962 Nov;84(5):953–960. doi: 10.1128/jb.84.5.953-960.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachev L. A., Jasaitis A. A., Kaulen A. D., Kondrashin A. A., Liberman E. A., Nemecek I. B., Ostroumov S. A., Semenov AYu, Skulachev V. P. Direct measurement of electric current generation by cytochrome oxidase, H+-ATPase and bacteriorhodopsin. Nature. 1974 May 24;249(455):321–324. doi: 10.1038/249321a0. [DOI] [PubMed] [Google Scholar]
- Drake H. L., Akagi J. M. Product analysis of bisulfite reductase activity isolated from Desulfovibrio vulgaris. J Bacteriol. 1976 May;126(2):733–738. doi: 10.1128/jb.126.2.733-738.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubourdieu M., Le Gall J. Chemical study of two flavodoxins extracted from sulfate reducing bacteria. Biochem Biophys Res Commun. 1970 Mar 12;38(5):965–972. doi: 10.1016/0006-291x(70)90816-8. [DOI] [PubMed] [Google Scholar]
- ELSDEN S. R., GILCHRIST F. M., LEWIS D., VOLCANI B. E. Properties of a fatty acid forming organism isolated from the rumen of sheep. J Bacteriol. 1956 Nov;72(5):681–689. doi: 10.1128/jb.72.5.681-689.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENTNER N., DOUDOROFF M. Glucose and gluconic acid oxidation of Pseudomonas saccharophila. J Biol Chem. 1952 May;196(2):853–862. [PubMed] [Google Scholar]
- Eagar R. G., Jr, Herbst M. M., Barker H. A., Richards J. H. Mechanism of action of coenzyme B 12 . Hydrogen transfer in the isomerization of -methylaspartate to glutamate. Biochemistry. 1972 Jan 18;11(2):253–264. doi: 10.1021/bi00752a017. [DOI] [PubMed] [Google Scholar]
- Eisenstein K. K., Wang J. H. Conversion of light to chemical free energy. I. Porphyrin-sensitized photoreduction of ferredoxin by glutathione. J Biol Chem. 1969 Apr 10;244(7):1720–1728. [PubMed] [Google Scholar]
- El Ghazzawi E. Neuisolierung von Clostridium aceticum Wieringa und stoffwechselphysiologische Untersuchungen. Arch Mikrobiol. 1967 May 17;57(1):1–19. [PubMed] [Google Scholar]
- Engel P. C., Massey V. Green butyryl-coenzyme A dehydrogenase. An enzyme-acyl-coenzyme A complex. Biochem J. 1971 Dec;125(3):889–902. doi: 10.1042/bj1250889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel P. C., Massey V. The purification and properties of butyryl-coenzyme A dehydrogenase from Peptostreptococcus elsdenii. Biochem J. 1971 Dec;125(3):879–887. doi: 10.1042/bj1250879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. Effects of molybdate, tungstate, and selenium compounds on formate dehydrogenase and other enzyme systems in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1032–1040. doi: 10.1128/jb.110.3.1032-1040.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. The purification and properties of formate dehydrogenase and nitrate reductase from Escherichia coli. J Biol Chem. 1975 Sep 10;250(17):6693–6705. [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. The role of a novel cytochrome b-containing nitrate reductase and quinone in the in vitro reconstruction of formate-nitrate reductase activity of E. coli. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1234–1241. doi: 10.1016/s0006-291x(74)80416-x. [DOI] [PubMed] [Google Scholar]
- Evans H. J., Wood H. G. The mechanism of the pyruvate, phosphate dikinase reaction. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1448–1453. doi: 10.1073/pnas.61.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEWSON C. A., NICHOLAS D. J. Nitrate reductase from Pseudomonas aeruginosa. Biochim Biophys Acta. 1961 May 13;49:335–349. doi: 10.1016/0006-3002(61)90133-0. [DOI] [PubMed] [Google Scholar]
- FINA L. R., SINCHER H. J., DECOU D. F. Evidence for production of methane from formic acid by direct reduction. Arch Biochem Biophys. 1960 Dec;91:159–162. doi: 10.1016/0003-9861(60)90482-3. [DOI] [PubMed] [Google Scholar]
- FORGET P., PICHINOTY F. INFLUENCE DE LA RESPIRATION ANA'EROBIE DU NITRATE ET DU FUMARATE SUR LE M'ETABOLISME FERMENTAIRE D'AEROBACTER AEROGENES. Biochim Biophys Acta. 1964 Feb 10;82:441–444. doi: 10.1016/0304-4165(64)90328-9. [DOI] [PubMed] [Google Scholar]
- Faust P. J., Vandemark P. J. Phosphorylation coupled to NADH oxidation with fumarate in Streptococcus faecalis 10Cl. Arch Biochem Biophys. 1970 Apr;137(2):392–398. doi: 10.1016/0003-9861(70)90454-6. [DOI] [PubMed] [Google Scholar]
- Ferry J. G., Wolfe R. S. Anaerobic degradation of benzoate to methane by a microbial consortium. Arch Microbiol. 1976 Feb;107(1):33–40. doi: 10.1007/BF00427864. [DOI] [PubMed] [Google Scholar]
- Flodgaard H., Fleron P. Thermodynamic parameters for the hydrolysis of inorganic pyrophosphate at pH 7.4 as a function of (Mg2+), (K+), and ionic strength determined from equilibrium studies of the reaction. J Biol Chem. 1974 Jun 10;249(11):3465–3474. [PubMed] [Google Scholar]
- Forget P. Les nitrate-réductases bactériennes. Solubilisation, purification et propriétés de l'enzyme A de Micrococcus denitrificans. Eur J Biochem. 1971 Feb 1;18(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01262.x. [DOI] [PubMed] [Google Scholar]
- Forget P. The bacterial nitrate reductases. Solubilization, purification and properties of the enzyme A of Escherichia coli K 12. Eur J Biochem. 1974 Mar 1;42(2):325–332. doi: 10.1111/j.1432-1033.1974.tb03343.x. [DOI] [PubMed] [Google Scholar]
- Forrest W. W. Adenosine triphosphate pool during the growth cycle in Streptococcus faecalis. J Bacteriol. 1965 Oct;90(4):1013–1018. doi: 10.1128/jb.90.4.1013-1018.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest W. W., Walker D. J. The generation and utilization of energy during growth. Adv Microb Physiol. 1971;5:213–274. doi: 10.1016/s0065-2911(08)60408-7. [DOI] [PubMed] [Google Scholar]
- Fujita T., Sato R. Studies on soluble cytochromes in Enterobacteriaceae. IV. Possible involvement of cytochrome c-552 in anaerobic nitrite metabolism. J Biochem. 1966 Dec;60(6):691–700. doi: 10.1093/oxfordjournals.jbchem.a128495. [DOI] [PubMed] [Google Scholar]
- GASTON L. W., STADTMAN E. R. Fermentation of ethylene glycol by Clostridium glycolicum, sp. n. J Bacteriol. 1963 Feb;85:356–362. doi: 10.1128/jb.85.2.356-362.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEST H. Oxidation and evolution of molecular hydrogen by microorganisms. Bacteriol Rev. 1954 Mar;18(1):43–73. doi: 10.1128/br.18.1.43-73.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., ENGLE L. P. VITAMIN K COMPOUNDS IN BACTERIA THAT ARE OBLIGATE ANAEROBES. Science. 1964 Dec 4;146(3649):1307–1309. doi: 10.1126/science.146.3649.1307. [DOI] [PubMed] [Google Scholar]
- GRAY C. T., GEST H. BIOLOGICAL FORMATION OF MOLECULAR HYDROGEN. Science. 1965 Apr 9;148(3667):186–192. doi: 10.1126/science.148.3667.186. [DOI] [PubMed] [Google Scholar]
- GRAY C. T., WIMPENNY J. W., HUGHES D. E., RANLETT M. A soluble c-type cytochrome from anaerobically grown Escherichia coli and various Enterobacteriaceae. Biochim Biophys Acta. 1963 Jan 8;67:157–160. doi: 10.1016/0006-3002(63)91809-2. [DOI] [PubMed] [Google Scholar]
- GROSSMAN J. P., POSTGATE J. R. The metabolism of malate and certain other compounds by Desulphovibrio desulphuricans. J Gen Microbiol. 1955 Jun;12(3):429–445. doi: 10.1099/00221287-12-3-429. [DOI] [PubMed] [Google Scholar]
- Galivan J. H., Allen S. H. Methylmalonyl coenzyme A decarboxylase. Its role in succinate decarboxylation by Micrococcus lactilyticus. J Biol Chem. 1968 Mar 25;243(6):1253–1261. [PubMed] [Google Scholar]
- Garland P. B., Downie J. A., Haddock B. A. Proton translocation and the respiratory nitrate reductase of Escherichia coli. Biochem J. 1975 Dec;152(3):547–559. doi: 10.1042/bj1520547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier D. K., Clark-Walker G. D., Garrard W. T., Jr, Lascelles J. Nitrate reductase and soluble cytochrome c in Spirillum itersonii. J Bacteriol. 1970 Jun;102(3):790–801. doi: 10.1128/jb.102.3.797-803.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghambeer R. K., Wood H. G., Schulman M., Ljungdahl L. Total synthesis of acetate from CO2. 3. Inhibition by alkylhalides of the synthesis from CO2, methyltetrahydrofolate, and methyl-B12 by Clostridium thermoaceticum. Arch Biochem Biophys. 1971 Apr;143(2):471–484. doi: 10.1016/0003-9861(71)90232-3. [DOI] [PubMed] [Google Scholar]
- Glynn I. M. Involvement of a membrane potential in the synthesis of ATP by mitochondria. Nature. 1967 Dec 30;216(5122):1318–1319. doi: 10.1038/2161318a0. [DOI] [PubMed] [Google Scholar]
- Goldner A. M., Schultz S. G., Curran P. F. Sodium and sugar fluxes across the mucosal border of rabbit ileum. J Gen Physiol. 1969 Mar;53(3):362–383. doi: 10.1085/jgp.53.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldner A. M. Sodium-dependent sugar transport in the intestine. Metabolism. 1973 May;22(5):649–656. doi: 10.1016/0026-0495(73)90236-9. [DOI] [PubMed] [Google Scholar]
- Gottwald M., Andreesen J. R., LeGall J., Ljungdahl L. G. Presence of cytochrome and menaquinone in Clostridium formicoaceticum and Clostridium thermoaceticum. J Bacteriol. 1975 Apr;122(1):325–328. doi: 10.1128/jb.122.1.325-328.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griniuviene B., Chmieliauskaite V., Grinius L. Energy-linked transport of permeant ions in Escherichia coli cells: evidence for membrane potential generation by proton-pump. Biochem Biophys Res Commun. 1974 Jan;56(1):206–213. doi: 10.1016/s0006-291x(74)80335-9. [DOI] [PubMed] [Google Scholar]
- Griniuviene B., Chmieliauskaite V., Melvydas V., Dzheja P., Grinius L. Conversion of Escherichia coli cell-produced metabolic energy into electric form. J Bioenerg. 1975 Mar;7(1):17–38. doi: 10.1007/BF01558460. [DOI] [PubMed] [Google Scholar]
- Guarraia L. J., Peck H. D., Jr Dinitrophenol-stimulated adenosine triphosphatase activity in extracts of Desulfovibrio gigas. J Bacteriol. 1971 Jun;106(3):890–895. doi: 10.1128/jb.106.3.890-895.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guynn R. W., Gelberg H. J., Veech R. L. Equilibrium constants of the malate dehydrogenase, citrate synthase, citrate lyase, and acetyl coenzyme A hydrolysis reactions under physiological conditions. J Biol Chem. 1973 Oct 25;248(20):6957–6965. [PubMed] [Google Scholar]
- Guynn R. W., Veech R. L. The equilibrium constants of the adenosine triphosphate hydrolysis and the adenosine triphosphate-citrate lyase reactions. J Biol Chem. 1973 Oct 25;248(20):6966–6972. [PubMed] [Google Scholar]
- Guynn R. W., Webster L. T., Jr, Veech R. L. Equilibrium constants of the reactions of acetyl coenzyme A synthetase and the hydrolysis of adenosine triphosphate to adenosine monophosphate and inorganic pyrophosphate. J Biol Chem. 1974 May 25;249(10):3248–3254. [PubMed] [Google Scholar]
- HAAS D. PHOSPHORYLATION COUPLED TO THE OXIDATION OF NADH BY FUMARATE IN DIGITONIN FRAGMENTS OF BEEF-HEART MITOCHONDRIA. Biochim Biophys Acta. 1964 Dec 23;92:433–439. doi: 10.1016/0926-6569(64)90002-1. [DOI] [PubMed] [Google Scholar]
- HADJIPETROU L. P., STOUTHAMER A. H. ENERGY PRODUCTION DURING NITRATE RESPIRATION BY AEROBACTER AEROGENES. J Gen Microbiol. 1965 Jan;38:29–34. doi: 10.1099/00221287-38-1-29. [DOI] [PubMed] [Google Scholar]
- HAGER L. P., LIPMANN F. Coupling between phosphorylation and flavin adenine dinucleotide reduction with the pyruvate oxidase of L. delbrueckii enzyme. Proc Natl Acad Sci U S A. 1961 Nov 15;47:1768–1772. doi: 10.1073/pnas.47.11.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDMAN J. K., STADTMAN T. C. METABOLISM OF OMEGA-AMINO ACIDS. V. ENERGETICS OF THE GAMMA-AMINOBUTYRATE FERMENTATION BY CLOSTRIDIUM AMINOBUTYRICUM. J Bacteriol. 1963 Jun;85:1326–1333. doi: 10.1128/jb.85.6.1326-1333.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDMAN J. K., STADTMAN T. C. Metabolism of amega-amino acids. III. Mechanism of conversion of gamma-aminobutyrate to gamma-hydroxybutryate by Clostridium aminobutyricum. J Biol Chem. 1963 Jun;238:2081–2087. [PubMed] [Google Scholar]
- HARDMAN J. K., STADTMAN T. C. Metabolism of omega-amino acids. I. Fermentation of gamma-aminobutyric acid by Clostridium aminobutyricum n. sp. J Bacteriol. 1960 Apr;79:544–548. doi: 10.1128/jb.79.4.544-548.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HART L. T., LARSON A. D., MCCLESKEY C. S. DENITRIFICATION BY CORYNEBACTERIUM NEPHRIDII. J Bacteriol. 1965 Apr;89:1104–1108. doi: 10.1128/jb.89.4.1104-1108.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEATH E. C., HURWITZ J., HORECKER B. L., GINSBURG A. Pentose fermentation by Lactobacillus plantarum. I. The cleavage of xylulose 5-phosphate by phosphoketolase. J Biol Chem. 1958 Apr;231(2):1009–1029. [PubMed] [Google Scholar]
- HENNING U. EIN REGULATIONSMECHANISMUS BEIM ABBAU DER BRENZTRAUBENSAEURE DURCH ESCHERICHIA COLI. Biochem Z. 1963 Jul 26;337:490–504. [PubMed] [Google Scholar]
- HIMES R. H., RABINOWITZ J. C. Formyltetrahydrofolate synthetase. II. Characteristics of the enzyme and the enzymic reaction. J Biol Chem. 1962 Sep;237:2903–2914. [PubMed] [Google Scholar]
- HIMES R. H., RABINOWITZ J. C. Formyltetrahydrofolate synthetase. III. Studies on the mechanism of the reaction. J Biol Chem. 1962 Sep;237:2915–2925. [PubMed] [Google Scholar]
- HIRSCH C. A., RASMINSKY M., DAVIS B. D., LIN E. C. A FUMARATE REDUCTASE IN ESCHERICHIA COLI DISTINCT FROM SUCCINATE DEHYDROGENASE. J Biol Chem. 1963 Nov;238:3770–3774. [PubMed] [Google Scholar]
- HOBSON P. N. CONTINUOUS CULTURE OF SOME ANEROBIC AND FACULTATIVELY ANAEROBIC RUMEN BACTERIA. J Gen Microbiol. 1965 Feb;38:167–180. doi: 10.1099/00221287-38-2-167. [DOI] [PubMed] [Google Scholar]
- HURWITZ J. Pentose phosphate cleavage by Leuconostoc mesenteroides. Biochim Biophys Acta. 1958 Jun;28(3):599–602. doi: 10.1016/0006-3002(58)90526-2. [DOI] [PubMed] [Google Scholar]
- Haaker H., Bresters T. W., Veeger C. Relation between anaerobic ATP synthesis from pyruvate and nitrogen fixation in Azotobacter vinelandii. FEBS Lett. 1972 Jun 15;23(2):160–162. doi: 10.1016/0014-5793(72)80330-2. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Kendall-Tobias M. W. Functional anaerobic electron transport linked to the reduction of nitrate and fumarate in membranes from Escherichia coli as demonstrated by quenching of atebrin fluorescence. Biochem J. 1975 Dec;152(3):655–659. doi: 10.1042/bj1520655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H. G., Henning U. Regulation of pyruvate dehydrogenase activity in Escherichia coli K12. Biochim Biophys Acta. 1966 Aug 10;122(2):355–358. doi: 10.1016/0926-6593(66)90076-2. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Levin E. Lactic acid translocation: terminal step in glycolysis by Streptococcus faecalis. J Bacteriol. 1974 Mar;117(3):1141–1148. doi: 10.1128/jb.117.3.1141-1148.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. I. The membrane potential. J Membr Biol. 1972;8(1):27–44. doi: 10.1007/BF01868093. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. II. Proton and sodium extrusion. J Membr Biol. 1972;8(1):45–62. doi: 10.1007/BF01868094. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Pavlasová E., Baarda J. R. A transmembrane pH gradient in Streptococcus faecalis: origin, and dissipation by proton conductors and N,N'-dicyclohexylcarbodimide. Biochim Biophys Acta. 1970;196(2):235–244. doi: 10.1016/0005-2736(70)90011-8. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Spitz E. Accumulation of arsenate, phosphate, and aspartate by Sreptococcus faecalis. J Bacteriol. 1975 Apr;122(1):266–277. doi: 10.1128/jb.122.1.266-277.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S. M., Hall J. B. The physiological function of nitrate reduction in Clostridium perfringens. J Gen Microbiol. 1975 Mar;87(1):120–128. doi: 10.1099/00221287-87-1-120. [DOI] [PubMed] [Google Scholar]
- Haschke R. H., Campbell L. L. Purification and properties of a hydrogenase from Desulfovibrio vulgaris. J Bacteriol. 1971 Jan;105(1):249–258. doi: 10.1128/jb.105.1.249-258.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchikian E. C., Le Gall J. Etude du métabolisme des acides dicarboxyliques et du pyruvate chez les bactéries sulfato-réductrices. I. Etude de l'oxydation enzymatique du fumarate en acétate. Ann Inst Pasteur (Paris) 1970 Feb;118(2):125–142. [PubMed] [Google Scholar]
- Hatchikian E. C., Le Gall J. Evidence for the presence of a b-type cytochrome in the sulfate-reducing bacterium Desulfovibrio gigas, and its role in the reduction of fumarate by molecular hydrogen. Biochim Biophys Acta. 1972 Jun 23;267(3):479–484. doi: 10.1016/0005-2728(72)90175-2. [DOI] [PubMed] [Google Scholar]
- Hatchikian E. C. On the role of menaquinone-6 in the electron transport of hydrogen: fumarate reductase system in the strict anaerobe Desulfovibrio gigas. J Gen Microbiol. 1974 Mar;81(1):261–266. doi: 10.1099/00221287-81-1-261. [DOI] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Hernandez E., Johnson M. J. Anaerobic growth yields of Aerobacter cloacae and Escherichia coli. J Bacteriol. 1967 Oct;94(4):991–995. doi: 10.1128/jb.94.4.991-995.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer P., Gottschalk G. Particulate nature of enzymes involved in the fermentation of ethanol and acetate by Clostridium kluyveri. FEBS Lett. 1972 Apr 1;21(3):351–354. doi: 10.1016/0014-5793(72)80200-x. [DOI] [PubMed] [Google Scholar]
- Hilton M. G. The metabolism of pyrimidines by proteolytic clostridia. Arch Microbiol. 1975;102(2):145–149. doi: 10.1007/BF00428359. [DOI] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Energy coupling in membrane vesicles of Escherichia coli. I. Accumulation of metabolites in response to an electrical potential. J Biol Chem. 1974 May 10;249(9):2939–2945. [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Role of an electrical potential in the coupling of metabolic energy to active transport by membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1804–1808. doi: 10.1073/pnas.70.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson P. N., Summers R. The continuous culture of anaerobic bacteria. J Gen Microbiol. 1967 Apr;47(1):53–65. doi: 10.1099/00221287-47-1-53. [DOI] [PubMed] [Google Scholar]
- Holländer R. Correlation of the function of demethylmenaquinone in bacterial electron transport with its redox potential. FEBS Lett. 1976 Dec 15;72(1):98–100. doi: 10.1016/0014-5793(76)80821-6. [DOI] [PubMed] [Google Scholar]
- Holländer R. Energy metabolism of some representatives of the Haemophilus group. Antonie Van Leeuwenhoek. 1976;42(4):429–444. doi: 10.1007/BF00410174. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Lehninger A. L., Thompson T. E. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc Natl Acad Sci U S A. 1968 Feb;59(2):484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopgood M. F., Walker D. J. Succinic acid production by rumen bacteria. I. Isolation and metabolism of Ruminococcus flavefaciens. Aust J Biol Sci. 1967 Feb;20(1):165–182. [PubMed] [Google Scholar]
- Hungate R. E. Hydrogen as an intermediate in the rumen fermentation. Arch Mikrobiol. 1967;59(1):158–164. doi: 10.1007/BF00406327. [DOI] [PubMed] [Google Scholar]
- Hungate R. E., Smith W., Bauchop T., Yu I., Rabinowitz J. C. Formate as an intermediate in the bovine rumen fermentation. J Bacteriol. 1970 May;102(2):389–397. doi: 10.1128/jb.102.2.389-397.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz C., Rosano C. L. The intracellular concentration of bound and unbound magnesium ions in Escherichia coli. J Biol Chem. 1967 Aug 25;242(16):3719–3722. [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- ITAGAKI E. THE ROLE OF LIPOPHILIC QUINONES IN THE ELECTRON TRANSPORT SYSTEM OF ESCHERICHIA COLI. J Biochem. 1964 Apr;55:432–445. doi: 10.1093/oxfordjournals.jbchem.a127905. [DOI] [PubMed] [Google Scholar]
- IWASAKI H., SHIDARA S., SUZUKI H., MOR T. Studies on denitrification. VII. Further purification and properties of denitrifying enzyme. J Biochem. 1963 Apr;53:299–303. [PubMed] [Google Scholar]
- Iannotti E. L., Kafkewitz D., Wolin M. J., Bryant M. P. Glucose fermentation products in Ruminococcus albus grown in continuous culture with Vibrio succinogenes: changes caused by interspecies transfer of H 2 . J Bacteriol. 1973 Jun;114(3):1231–1240. doi: 10.1128/jb.114.3.1231-1240.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inderlied C. B., Delwiche E. A. Nitrate reduction and the growth of Veillonella alcalescens. J Bacteriol. 1973 Jun;114(3):1206–1212. doi: 10.1128/jb.114.3.1206-1212.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto M., Umeyama M., Chiba S. Alteration of fermentation products from butyrate to acetate by nitrate reduction in Clostridium perfringens. Z Allg Mikrobiol. 1974;14(2):115–121. doi: 10.1002/jobm.3630140206. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Matsubara T. A nitrite reductase from Achromobacter cycloclastes. J Biochem. 1972 Apr;71(4):645–652. [PubMed] [Google Scholar]
- Iwasaki H., Matsubara T. Cytochrome c-557 (551) and cytochrome cd of Alcaligenes faecalis. J Biochem. 1971 May;69(5):847–857. doi: 10.1093/oxfordjournals.jbchem.a129536. [DOI] [PubMed] [Google Scholar]
- JACOBS N. J., WOLIN M. J. Electron-transport system of Vibrio succinogenes. I. Enzymes and cytochromes of electron-transport system. Biochim Biophys Acta. 1963 Jan 1;69:18–28. doi: 10.1016/0006-3002(63)91221-6. [DOI] [PubMed] [Google Scholar]
- JACOBS N. J., WOLIN M. J. Electron-transport system of Vibrio succinogenes. II. Inhibition of electron transport by 2-heptyl-4-hydroxyquinoline N-oxide. Biochim Biophys Acta. 1963 Jan 1;69:29–39. doi: 10.1016/0006-3002(63)91222-8. [DOI] [PubMed] [Google Scholar]
- JOHNS A. T. The mechanism of propionic acid formation by Clostridium propionicum. J Gen Microbiol. 1952 Feb;6(1-2):123–127. doi: 10.1099/00221287-6-1-2-123. [DOI] [PubMed] [Google Scholar]
- JOHNS A. T. The mechanism of propionic acid formation by Veillonella gazogenes. J Gen Microbiol. 1951 May;5(2):326–336. doi: 10.1099/00221287-5-2-326. [DOI] [PubMed] [Google Scholar]
- JOHNS A. T. The mechanism of propionic acid formation by propionibacteria. J Gen Microbiol. 1951 May;5(2):337–345. doi: 10.1099/00221287-5-2-337. [DOI] [PubMed] [Google Scholar]
- Jagendorf A. T., Uribe E. ATP formation caused by acid-base transition of spinach chloroplasts. Proc Natl Acad Sci U S A. 1966 Jan;55(1):170–177. doi: 10.1073/pnas.55.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng I. M., Somack R., Barker H. A. Ornithine degradation in Clostridium sticklandii; pyridoxal phosphate and coenzyme A dependent thiolytic cleavage of 2-amino-4-ketopentanoate to alanine and acetyl coenzyme A. Biochemistry. 1974 Jul 2;13(14):2898–2903. doi: 10.1021/bi00711a019. [DOI] [PubMed] [Google Scholar]
- Jeng I., Barker H. A. Purification and properties of l-3-aminobutyryl coenzyme A deaminase from a lysine-fermenting Clostridium. J Biol Chem. 1974 Oct 25;249(20):6578–6584. [PubMed] [Google Scholar]
- John P., Whatley F. R. Oxidative phosphorylation coupled to oxygen uptake and nitrate reduction in Micrococcus denitrificans. Biochim Biophys Acta. 1970 Sep 1;216(2):342–352. doi: 10.1016/0005-2728(70)90225-2. [DOI] [PubMed] [Google Scholar]
- Jones H. E., Skyring G. W. Effect of enzymic assay conditions on sulfite reduction catalysed by desulfoviridin from Desulfovibrio gigas. Biochim Biophys Acta. 1975 Jan 23;377(1):52–60. doi: 10.1016/0005-2744(75)90285-5. [DOI] [PubMed] [Google Scholar]
- Jones M. E., Lipmann F. CHEMICAL AND ENZYMATIC SYNTHESIS OF CARBAMYL PHOSPHATE. Proc Natl Acad Sci U S A. 1960 Sep;46(9):1194–1205. doi: 10.1073/pnas.46.9.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce B. K., Himes R. H. Formyltetrahydrofolate synthetase. A study of equilibrium reaction rates. J Biol Chem. 1966 Dec 10;241(23):5716–5724. [PubMed] [Google Scholar]
- Joyce B. K., Himes R. H. Formyltetrahydrofolate synthetase. Initial velocity and product inhibition studies. J Biol Chem. 1966 Dec 10;241(23):5725–5731. [PubMed] [Google Scholar]
- Joyner A. E., Jr, Baldwin R. L. Enzymatic studies of pure cultures of rumen microorganisms. J Bacteriol. 1966 Nov;92(5):1321–1330. doi: 10.1128/jb.92.5.1321-1330.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge W., Rumberg B., Schröder H. The necessity of an electric potential difference and its use for photophosphorylation in short flash groups. Eur J Biochem. 1970 Jul;14(3):575–581. doi: 10.1111/j.1432-1033.1970.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Kirchniawy H., Katz N., Thauer R. K. NADH, a physiological electron donor in clostridial nitrogen fixation. FEBS Lett. 1974 Jul 15;43(2):203–206. doi: 10.1016/0014-5793(74)81000-8. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Leimenstoll G., Rupprecht E., Thauer R. K. Demonstration of NADH-ferredoxin reductase in two caccharolytic Clostridia. Arch Mikrobiol. 1971;80(4):370–372. doi: 10.1007/BF00406223. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Rupprecht E., Ohrloff C., Thauer R., Decker K. Regulation of the reduced nicotinamide adenine dinucleotide-ferredoxin reductase system in Clostridium kluyveri. J Biol Chem. 1971 Feb 25;246(4):960–963. [PubMed] [Google Scholar]
- Jungermann K., Schön G. Pyruvate formate lyase in Rhodospirillum rubrum Ha adapted to anaerobic dark conditions. Arch Microbiol. 1974;99(2):109–116. doi: 10.1007/BF00696227. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Thauer R. K., Leimenstoll G., Decker K. Function of reduced pyridine nucleotide-ferredoxin oxidoreductases in saccharolytic Clostridia. Biochim Biophys Acta. 1973 May 30;305(2):268–280. doi: 10.1016/0005-2728(73)90175-8. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Thauer R. K., Rupprecht E., Ohrloff C., Decker K. Ferredoxin mediated hydrogen formation from NADPH in a cell-free system of Clostridium kluyveri. FEBS Lett. 1969 Apr;3(2):144–146. doi: 10.1016/0014-5793(69)80119-5. [DOI] [PubMed] [Google Scholar]
- KLEIN S. M., SAGERS R. D. Intermediary metabolism of Diplococcus glycinophilus. II. Enzymes of the acetategenerating system. J Bacteriol. 1962 Jan;83:121–126. doi: 10.1128/jb.83.1.121-126.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KMETEC E., BUEDING E. Succinic and reduced diphosphopyridine nucleotide oxidase systems of Ascaris muscle. J Biol Chem. 1961 Feb;236:584–591. [PubMed] [Google Scholar]
- KRASNA A. I., RIKLIS E., RITTENBERG D. The purification and properties of the hydrogenase of Desulfovibrio desulfuricans. J Biol Chem. 1960 Sep;235:2717–2720. [PubMed] [Google Scholar]
- KREBS H. A., KORNBERG H. L., BURTON K. A survey of the energy transformations in living matter. Ergeb Physiol. 1957;49:212–298. [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kamihara T. Ethanol utilization by Steptococcus faecalis. Arch Biochem Biophys. 1969 Aug;133(1):137–143. doi: 10.1016/0003-9861(69)90497-4. [DOI] [PubMed] [Google Scholar]
- Kandler O., Lauer E. Neuere Vorstellungen zur Taxonomie der Bifidobacterien. Zentralbl Bakteriol Orig A. 1974;228(1):29–45. [PubMed] [Google Scholar]
- Kaprálek F., Pichinoty F. The effect of oxygen on tetrathionate reductase activity and biosynthesis. J Gen Microbiol. 1970 Jul;62(1):95–105. doi: 10.1099/00221287-62-1-95. [DOI] [PubMed] [Google Scholar]
- Kaprálek F. The physiological role of tetrathionate respiration in growing citrobacter. J Gen Microbiol. 1972 Jun;71(1):133–139. doi: 10.1099/00221287-71-1-133. [DOI] [PubMed] [Google Scholar]
- Karlsson J. L., Volcani B. E., Barker H. A. The Nutritional Requirements of Clostridium aceticum. J Bacteriol. 1948 Dec;56(6):781–782. [PMC free article] [PubMed] [Google Scholar]
- Khosrovi B., Macpherson R., Miller J. D. Some observations on growth and hydrogen uptake by Desulfovibrio vulgaris. Arch Mikrobiol. 1971;80(4):324–337. doi: 10.1007/BF00406220. [DOI] [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Purification and properties of the flavine-stimulated anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli. J Bacteriol. 1972 Oct;112(1):539–547. doi: 10.1128/jb.112.1.539-547.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiszkiss D. F., Downey R. J. Localization and solubilization of the respiratory nitrate reductase of Bacillus stearothermophilus. J Bacteriol. 1972 Feb;109(2):803–810. doi: 10.1128/jb.109.2.803-810.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiszkiss D. F., Downey R. J. Physical aggregation and functional reconstitution of solubilized membranes of Bacillus stearothermophilus. J Bacteriol. 1972 Feb;109(2):811–819. doi: 10.1128/jb.109.2.811-819.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. 3. A flavin-linked dehydrogenase associated with the glycine cleavage system in Peptococcus glycinophilus. J Biol Chem. 1967 Jan 25;242(2):297–300. [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. I. Properties of the system catalyzing the exchange of bicarbonate with the carboxyl group of glycine in Peptococcus glycinophilus. J Biol Chem. 1966 Jan 10;241(1):197–205. [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. IV. Effect of borohydride reduction on the pyridoxal phosphate-containing glycine decarboxylase from Peptococcus glycinophilus. J Biol Chem. 1967 Jan 25;242(2):301–305. [PubMed] [Google Scholar]
- Knappe J., Blaschkowski H. P., Gröbner P., Schmitt T. Pyruvate formate-lyase of Escherichia coli: the acetyl-enzyme intermediate. Eur J Biochem. 1974 Dec 16;50(1):253–263. doi: 10.1111/j.1432-1033.1974.tb03894.x. [DOI] [PubMed] [Google Scholar]
- Knook D. L., Planta R. J. Function of ubiquinone in electron transport from reduced nicotinamide adenine dinucleotide to nitrate and oxygen in Aerobacter aerogenes. J Bacteriol. 1971 Feb;105(2):483–488. doi: 10.1128/jb.105.2.483-488.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knook D. L., Planta R. J. Restoration of electron transport in ultraviolet-irradiated membranes of Aerobacter aerogenes. FEBS Lett. 1971 Apr 12;14(1):54–56. doi: 10.1016/0014-5793(71)80273-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Seki Y., Ishimoto M. Biochemical studies on sulfate-ruducing bacteria. 8. Sulfite reductase from Desulfovibrio vulgaris--mechanism of trithionate, thiosulfate, and sulfide formation and enzymatic properties. J Biochem. 1974 Mar;75(3):519–529. doi: 10.1093/oxfordjournals.jbchem.a130420. [DOI] [PubMed] [Google Scholar]
- Kohn L. D., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. XV. Purification and properties of the membrane-bound D-lactate dehydrogenase from Escherichia coli. J Biol Chem. 1973 Oct 25;248(20):7012–7017. [PubMed] [Google Scholar]
- Koike I., Hattori A. Energy yield of denitrification: an estimate from growth yield in continuous cultures of Pseudomonas denitrificans under nitrate-, nitrite- and oxide-limited conditions. J Gen Microbiol. 1975 May;88(1):11–19. doi: 10.1099/00221287-88-1-11. [DOI] [PubMed] [Google Scholar]
- Koike I., Hattori A. Growth yield of a denitrifying bacterium, Pseudomonas denitrificans, under aerobic and denitrifying conditions. J Gen Microbiol. 1975 May;88(1):1–10. doi: 10.1099/00221287-88-1-1. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Boonstra J., De Vries W. Amino acid transport in membrane vesicles of obligately anaerobic Veillonella alcalescens. J Bacteriol. 1975 Apr;122(1):245–249. doi: 10.1128/jb.122.1.245-249.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Kaback H. R. Anaerobic transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3376–3381. doi: 10.1073/pnas.70.12.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman E. F., McLick J. ATP synthesis in oxidative phosphorylation: a direct-union stereochemical reaction mechanism. J Bioenerg. 1972 May;3(1):147–158. doi: 10.1007/BF01516005. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Roughton F. J. Carbonic anhydrase as a tool in studying the mechanism of reactions involving H(2)CO(3), CO(2) or HCO(3)'. Biochem J. 1948;43(4):550–555. doi: 10.1042/bj0430550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Veech R. L. Equilibrium relations between pyridine nucleotides and adenine nucleotides and their roles in the regulation of metabolic processes. Adv Enzyme Regul. 1969;7:397–413. doi: 10.1016/0065-2571(69)90030-2. [DOI] [PubMed] [Google Scholar]
- Krietsch W. K., Bücher T. 3-phosphoglycerate kinase from rabbit sceletal muscle and yeast. Eur J Biochem. 1970 Dec;17(3):568–580. doi: 10.1111/j.1432-1033.1970.tb01202.x. [DOI] [PubMed] [Google Scholar]
- Kröger A., Dadák V., Klingenberg M., Diemer F. On the role of quinones in bacterial electron transport. Differential roles of ubiquinone and menaquinone in Proteus rettgeri. Eur J Biochem. 1971 Aug 16;21(3):322–333. doi: 10.1111/j.1432-1033.1971.tb01472.x. [DOI] [PubMed] [Google Scholar]
- Kröger A., Dadák V. On the role of quinones in bacterial electron transport. The respiratory system of Bacillus megaterium. Eur J Biochem. 1969 Dec;11(2):328–340. doi: 10.1111/j.1432-1033.1969.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Kröger A. Electron-transport phosphorylation coupled to fumarate reduction in anaerobically grown Proteus rettgeri. Biochim Biophys Acta. 1974 May 22;347(2):273–289. doi: 10.1016/0005-2728(74)90051-6. [DOI] [PubMed] [Google Scholar]
- LADD J. N., WALKER D. J. The fermentation of lactate and acrylate by the rumen micro-organism LC. Biochem J. 1959 Feb;71(2):364–373. doi: 10.1042/bj0710364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARA F. J. The succinic dehydrogenase of Propionibacterium pentosaceum. Biochim Biophys Acta. 1959 Jun;33(2):565–567. doi: 10.1016/0006-3002(59)90153-2. [DOI] [PubMed] [Google Scholar]
- LEGALL J., MAZZA G., DRAGONI N. LE CYTOCHROME C3 DE DESULFOVIBRIO GIGAS. Biochim Biophys Acta. 1965 May 18;99:385–387. [PubMed] [Google Scholar]
- LENTZ K., WOOD H. G. Synthesis of acetate from formate and carbon dioxide by Clostridium thermoaceticum. J Biol Chem. 1955 Aug;215(2):645–654. [PubMed] [Google Scholar]
- LESTER R. L., WHITE D. C., SMITH S. L. THE 2-DESMETHYL VITAMIN K2'S. A NEW GROUP OF NAPHTHOQUINONES ISOLATED FROM HEMOPHILUS PARAINFLUENZAE. Biochemistry. 1964 Jul;3:949–954. doi: 10.1021/bi00895a018. [DOI] [PubMed] [Google Scholar]
- LEWIS D., ELSDEN S. R. The fermentation of L-threonine, L-serine, L-cysteine and acrylic acid by a gram-negative coccus. Biochem J. 1955 Aug;60(4):683–692. doi: 10.1042/bj0600683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIGHTBOWN J. W., JACKSON F. L. Inhibition of cytochrome systems of heart muscle and certain bacteria by the antagonists of dihydrostreptomycin: 2-alkyl-4-hydroxyquinoline N-oxides. Biochem J. 1956 May;63(1):130–137. doi: 10.1042/bj0630130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam Y., Nicholas D. J. A nitrite reductase with cytochrome oxidase activity from Micrococcus denitrificans. Biochim Biophys Acta. 1969 Aug 5;180(3):459–472. doi: 10.1016/0005-2728(69)90025-5. [DOI] [PubMed] [Google Scholar]
- Lam Y., Nicholas D. J. Aerobic and anaerobic respiration in Micrococcus denitrificans. Biochim Biophys Acta. 1969 Apr 8;172(3):450–461. doi: 10.1016/0005-2728(69)90141-8. [DOI] [PubMed] [Google Scholar]
- Langenberg K. F., Bryant M. P., Wolfe R. S. Hydrogen-oxidizing methane bacteria. II. Electron microscopy. J Bacteriol. 1968 Mar;95(3):1124–1129. doi: 10.1128/jb.95.3.1124-1129.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laris P. C., Pershadsingh H. A. Estimations of membrane potentials in Streptococcus faecalis by means of a fluorescent probe. Biochem Biophys Res Commun. 1974 Apr 8;57(3):620–626. doi: 10.1016/0006-291x(74)90591-9. [DOI] [PubMed] [Google Scholar]
- Le Gall J., Dragoni N. Dependance of sulfite reduction on a crystallized ferredoxin from Desulfovibrio gigas. Biochem Biophys Res Commun. 1966 Apr 19;23(2):145–149. doi: 10.1016/0006-291x(66)90519-5. [DOI] [PubMed] [Google Scholar]
- Le Gall J., Hatchikian E. C. Purification et propriétés d'une flavoprotéine intervenant dans la réduction du sulfite par Desulvovibrio gigas. C R Acad Sci Hebd Seances Acad Sci D. 1967 May 29;264(22):2580–2583. [PubMed] [Google Scholar]
- Le Gall J. Purification PARTIELLE ET 'ETUDE DE LA NAD: rubrédoxine oxydo-réductase de D. Gigas. Ann Inst Pasteur (Paris) 1968 Jan;114(1):109–115. [PubMed] [Google Scholar]
- Le Minor L., Piéchaud M., Pichinoty F., Coynault C. Etude par transduction sur les nitrate-, tétrathionate- et thiosulfate-réductases de Salmonella typhi-murium. Ann Inst Pasteur (Paris) 1969 Nov;117(5):637–644. [PubMed] [Google Scholar]
- Leach C. K., Carr N. G. Pyruvate: ferredoxin oxidoreductase and its activation by ATP in the blue-green alga Anabaena variabilis. Biochim Biophys Acta. 1971 Aug 6;245(1):165–174. doi: 10.1016/0005-2728(71)90019-3. [DOI] [PubMed] [Google Scholar]
- Lee J. P., LeGall J., Peck H. D., Jr Isolation of assimilatroy- and dissimilatory-type sulfite reductases from Desulfovibrio vulgaris. J Bacteriol. 1973 Aug;115(2):529–542. doi: 10.1128/jb.115.2.529-542.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. P., Peck H. D., Jr Purification of the enzyme reducing bisulfite to trithionate from Desulfovibrio gigas and its identification as desulfoviridin. Biochem Biophys Res Commun. 1971 Nov 5;45(3):583–589. doi: 10.1016/0006-291x(71)90457-8. [DOI] [PubMed] [Google Scholar]
- Lee J. P., Yi C. S., LeGall J., Peck H. D., Jr Isolation of a new pigment, desulforubidin, from Desulfovibrio desulfuricans (Norway strain) and its role in sulfite reduction. J Bacteriol. 1973 Jul;115(1):453–455. doi: 10.1128/jb.115.1.453-455.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legall J., DerVartanian D. V., Spilker E., Lee J. P., Peck H. D., Jr Evidence for the involvement of non-heme iron in the active site of hydrogenase from Desulfovibrio vulgaris. Biochim Biophys Acta. 1971 Jun 15;234(3):526–530. [PubMed] [Google Scholar]
- Lester R. L., DeMoss J. A. Effects of molybdate and selenite on formate and nitrate metabolism in Escherichia coli. J Bacteriol. 1971 Mar;105(3):1006–1014. doi: 10.1128/jb.105.3.1006-1014.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. H., Hinkle P. C. Reconstitution of ion transport and respiratory control in vesicles formed from reduced coenzyme Q-cytochrome c reductase and phospholipids. J Biol Chem. 1975 Nov 10;250(21):8467–8471. [PubMed] [Google Scholar]
- Lewis A. J., Miller J. D. Keto acid metabolism in Desulfovibrio. J Gen Microbiol. 1975 Oct;90(2):286–292. doi: 10.1099/00221287-90-2-286. [DOI] [PubMed] [Google Scholar]
- Li L. F., Ljungdahl L., Wood H. G. Properties of Nicotinamide Adenine Dinucleotide Phosphate-Dependent Formate Dehydrogenase from Clostridium thermoaceticum. J Bacteriol. 1966 Aug;92(2):405–412. doi: 10.1128/jb.92.2.405-412.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmark D. G., Müller M. Biochemical cytology of trichomonad flagellates. II. Subcellular distribution of oxidoreductases and hydrolases in Monocercomonas sp. J Protozool. 1974 May;21(2):374–378. doi: 10.1111/j.1550-7408.1974.tb03673.x. [DOI] [PubMed] [Google Scholar]
- Lindmark D. G., Müller M. Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate Tritrichomonas foetus, and its role in pyruvate metabolism. J Biol Chem. 1973 Nov 25;248(22):7724–7728. [PubMed] [Google Scholar]
- Lindmark D. G., Paolella P., Wood N. P. The pyruvate formate-lyase system of Streptococcus faecalis. I. Purification and properties of the formate-pyruvate exchange enzyme. J Biol Chem. 1969 Jul 10;244(13):3605–3612. [PubMed] [Google Scholar]
- Linke H. A. CO2-Fixierung durch Clostridium aceticum: 14CO2-Kurzzeiteinbau und Pyruvatstoffwechesel. Arch Mikrobiol. 1969;64(3):203–214. [PubMed] [Google Scholar]
- Ljungdahl L. G., Andreesen J. R. Tungsten, a component of active formate dehydrogenase from Clostridium thermoacetium. FEBS Lett. 1975 Jun 15;54(2):279–282. doi: 10.1016/0014-5793(75)80092-5. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G. Total synthesis of acetate from CO2 by heterotrophic bacteria. Annu Rev Microbiol. 1969;23:515–538. doi: 10.1146/annurev.mi.23.100169.002503. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L., Brewer J. M., Neece S. H., Fairwell T. Purification, stability, and composition of formyltetrahydrofolate synthetase from Clostridium thermoaceticum. J Biol Chem. 1970 Sep 25;245(18):4791–4797. [PubMed] [Google Scholar]
- London J., Knight M. Concentrations of nicotinamide nucleotide coenzymes in micro-organisms. J Gen Microbiol. 1966 Aug;44(2):241–254. doi: 10.1099/00221287-44-2-241. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- MOLINARI R., LARA F. J. The lactic dehydrogenase of Propionibacterium pentosaceum. Biochem J. 1960 Apr;75:57–65. doi: 10.1042/bj0750057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A., Normansell D. E. Purification and properties of nitrate reductase from Escherichia coli K12. J Biol Chem. 1974 Aug 25;249(16):5321–5327. [PubMed] [Google Scholar]
- MacGregor C. H. Solubilization of Escherichia coli nitrate reductase by a membrane-bound protease. J Bacteriol. 1975 Mar;121(3):1102–1110. doi: 10.1128/jb.121.3.1102-1110.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy J., Kulla H., Gottschalk G. H2-dependent anaerobic growth of Escherichia coli on L-malate: succinate formation. J Bacteriol. 1976 Feb;125(2):423–428. doi: 10.1128/jb.125.2.423-428.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy J., Probst I., Gottschalk G. Evidence for cytochrome involvement in fumarate reduction and adenosine 5'-triphosphate synthesis by Bacteroides fragilis grown in the presence of hemin. J Bacteriol. 1975 Aug;123(2):436–442. doi: 10.1128/jb.123.2.436-442.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P. C., Kashket E. R., Wilson T. H. A protonmotive force drives ATP synthesis in bacteria. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3896–3900. doi: 10.1073/pnas.71.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroc J., Azerad R., Kamen M. D., Le Gall J. Menaquinone (MK-6) in the sulfate-reducing obligate anaerobe, Desulfovibrio. Biochim Biophys Acta. 1970 Jan 13;197(1):87–89. doi: 10.1016/0005-2728(70)90012-5. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Iwasaki H. Enzymatic steps of dissimilatory nitrite reduction in Alcaligenes faecalis. J Biochem. 1971 May;69(5):859–868. doi: 10.1093/oxfordjournals.jbchem.a129537. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Iwasaki H. Nitric oxide-reducing activity of Alcaligenes faecalis cytochrome cd. J Biochem. 1972 Jul;72(1):57–64. doi: 10.1093/oxfordjournals.jbchem.a129897. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Mori T. Studies on denitrification. IX. Nitrous oxide, its production and reduction to nitrogen. J Biochem. 1968 Dec;64(6):863–871. doi: 10.1093/oxfordjournals.jbchem.a128968. [DOI] [PubMed] [Google Scholar]
- Matsubara T. Studies on denitrification. 8. Some properties of the N2O-anaerobically grown cell. J Biochem. 1971 Jun;69(6):991–1001. doi: 10.1093/oxfordjournals.jbchem.a129572. [DOI] [PubMed] [Google Scholar]
- Matsubara T. Studies on denitrification. XII. Gas production from amines and nitrite. J Biochem. 1970 Feb;67(2):229–235. doi: 10.1093/oxfordjournals.jbchem.a129246. [DOI] [PubMed] [Google Scholar]
- Matsubara T. The participation of cytochromes in the reduction of N20 to N2 by a denitryfying bacterium. J Biochem. 1975 Mar;77(3):627–632. doi: 10.1093/oxfordjournals.jbchem.a130764. [DOI] [PubMed] [Google Scholar]
- Mayhew S. G., Foust G. P., Massey V. Oxidation-reduction properties of flavodoxin from Peptostreptococcus elsdenii. J Biol Chem. 1969 Feb 10;244(3):803–810. [PubMed] [Google Scholar]
- Mayhew S. G., Massey V. Purification and characterization of flavodoxin from Peptostreptococcus elsdenii. J Biol Chem. 1969 Feb 10;244(3):794–802. [PubMed] [Google Scholar]
- Mayhew S. G. Properties of two clostridial flavodoxins. Biochim Biophys Acta. 1971 May 12;235(2):276–288. doi: 10.1016/0005-2744(71)90206-3. [DOI] [PubMed] [Google Scholar]
- McBride B. C., Wolfe R. S. A new coenzyme of methyl transfer, coenzyme M. Biochemistry. 1971 Jun 8;10(12):2317–2324. doi: 10.1021/bi00788a022. [DOI] [PubMed] [Google Scholar]
- McClare C. W. In defence of the high energy phosphate bond. J Theor Biol. 1972 May;35(2):233–246. doi: 10.1016/0022-5193(72)90036-7. [DOI] [PubMed] [Google Scholar]
- McCormick N. G., Ordal E. J., Whiteley H. R. DEGRADATION OF PYRUVATE BY MICROCOCCUS LACTILYTICUS I. : General Properties of the Formate-Exchange Reaction. J Bacteriol. 1962 Apr;83(4):887–898. doi: 10.1128/jb.83.4.887-898.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick N. G., Ordal E. J., Whiteley H. R. DEGRADATION OF PYRUVATE BY MICROCOCCUS LACTILYTICUS II. : Studies of Cofactors in the Formate-Exchange Reaction. J Bacteriol. 1962 Apr;83(4):899–906. doi: 10.1128/jb.83.4.899-906.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill D. J., Dawes E. A. Glucose and fructose metabolism in Zymomonas anaerobia. Biochem J. 1971 Dec;125(4):1059–1068. doi: 10.1042/bj1251059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger H. Biochemie einiger parasitische lebender Würmer und Protozoen und die Wirkungsweise chemotherapeutisch wichtiger Stoffe. Z Parasitenkd. 1970;34(4):271–295. doi: 10.1007/BF00260297. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Bartsch R. G., Kamen M. D. Cytochrome c 3 . A class of electron transfer heme proteins found in both photosynthetic and sulfate-reducing bacteria. Biochim Biophys Acta. 1971 Sep 7;245(2):453–464. doi: 10.1016/0005-2728(71)90162-9. [DOI] [PubMed] [Google Scholar]
- Miki K., Lin E. C. Anaerobic energy-yielding reaction associated with transhydrogenation from glycerol 3-phosphate to fumarate by an Escherichia coli system. J Bacteriol. 1975 Dec;124(3):1282–1287. doi: 10.1128/jb.124.3.1282-1287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Lin E. C. Electron transport chain from glycerol 3-phosphate to nitrate in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1288–1294. doi: 10.1128/jb.124.3.1288-1294.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Lin E. C. Enzyme complex which couples glycerol-3-phosphate dehydrogenation to fumarate reduction in Escherichia coli. J Bacteriol. 1973 May;114(2):767–771. doi: 10.1128/jb.114.2.767-771.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. D., Wakerley D. S. Growth of sulphate-reducing bacteria by fumarate dismutation. J Gen Microbiol. 1966 Apr;43(1):101–107. doi: 10.1099/00221287-43-1-101. [DOI] [PubMed] [Google Scholar]
- Mitchell P. A chemiosmotic molecular mechanism for proton-translocating adenosine triphosphatases. FEBS Lett. 1974 Jul 15;43(2):189–194. doi: 10.1016/0014-5793(74)80997-x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in energy transduction: a logical development of biochemical knowledge. J Bioenerg. 1972 May;3(1):5–24. doi: 10.1007/BF01515993. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Hypothesis: cation-translocating adenosine triphosphatase models: how direct is the participation of adenosine triphosphate and its hydrolysis products in cation translocation? FEBS Lett. 1973 Jul 15;33(3):267–274. doi: 10.1016/0014-5793(73)80209-1. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Proton translocation coupled to ATP hydrolysis in rat liver mitochondria. Eur J Biochem. 1968 May;4(4):530–539. doi: 10.1111/j.1432-1033.1968.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Performance and conservation of osmotic work by proton-coupled solute porter systems. J Bioenerg. 1973 Jan;4(1):63–91. doi: 10.1007/BF01516051. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Proton translocation mechanisms and energy transduction by adenosine triphosphatases: an answer to criticisms. FEBS Lett. 1975 Feb 1;50(2):95–97. doi: 10.1016/0014-5793(75)80465-0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett. 1975 Aug 1;56(1):1–6. doi: 10.1016/0014-5793(75)80098-6. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Translocations through natural membranes. Adv Enzymol Relat Areas Mol Biol. 1967;29:33–87. doi: 10.1002/9780470122747.ch2. [DOI] [PubMed] [Google Scholar]
- Miyata M., Matsubara T., Mori T. Studies on denitrification. XI. Some properties of nitric oxide reductase. J Biochem. 1969 Dec;66(6):759–765. doi: 10.1093/oxfordjournals.jbchem.a129205. [DOI] [PubMed] [Google Scholar]
- Miyata M., Mori T. Studies on denitrification. 8. Production of nitric oxide by denitrifying reaction in the presence of tetramethyl-p-phenylenediamine. J Biochem. 1968 Dec;64(6):849–861. doi: 10.1093/oxfordjournals.jbchem.a128967. [DOI] [PubMed] [Google Scholar]
- Miyata M., Mori T. Studies on denitrification. X. The "denitrifying enzyme" as a nitrite reductase and the electron donating system for denitrification. J Biochem. 1969 Oct;66(4):463–471. doi: 10.1093/oxfordjournals.jbchem.a129170. [DOI] [PubMed] [Google Scholar]
- Miyata M. Studies on denitrification. XIV. The electron donating system in the reduction of nitric oxide and nitrate. J Biochem. 1971 Aug;70(2):205–213. doi: 10.1093/oxfordjournals.jbchem.a129632. [DOI] [PubMed] [Google Scholar]
- Moore M. R., O'Brien W. E., Ljungdahl L. G. Purification and characterization of nicotinamide adenine dinucleotide-dependent methylenetetrahydrofolate dehydrogenase from Clostridium formicoaceticum. J Biol Chem. 1974 Aug 25;249(16):5250–5253. [PubMed] [Google Scholar]
- Moustafa H. H., Collins E. B. Molar growth yields of certain lactic acid bacteria as influenced by autolysis. J Bacteriol. 1968 Jul;96(1):117–125. doi: 10.1128/jb.96.1.117-125.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. Proton translocation quotient for the adenosine triphosphatase of rat liver mitochondria. FEBS Lett. 1973 Mar 15;30(3):317–320. doi: 10.1016/0014-5793(73)80678-7. [DOI] [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Kamin H., DerVartanian D. V., Lee J. P., LeGall J., Peck H. D., Jr An iron tetrahydroporphyrin prosthetic group common to both assimilatory and dissimilatory sulfite reductases. Biochem Biophys Res Commun. 1973 Sep 5;54(1):82–88. doi: 10.1016/0006-291x(73)90891-7. [DOI] [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M. Siroheme and sirohydrochlorin. The basis for a new type of porphyrin-related prosthetic group common to both assimilatory and dissimilatory sulfite reductases. J Biol Chem. 1973 Oct 10;248(19):6911–6919. [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Tove S. R., Kamin H. Siroheme: a new prosthetic group participating in six-electron reduction reactions catalyzed by both sulfite and nitrite reductases. Proc Natl Acad Sci U S A. 1974 Mar;71(3):612–616. doi: 10.1073/pnas.71.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. M., Weber A. The cooperative action of muscle proteins. Sci Am. 1974 Feb;230(2):58–71. [PubMed] [Google Scholar]
- MØLLER V. Simplified tests for some amino acid decarboxylases and for the arginine dihydrolase system. Acta Pathol Microbiol Scand. 1955;36(2):158–172. doi: 10.1111/j.1699-0463.1955.tb04583.x. [DOI] [PubMed] [Google Scholar]
- Müller M. Biochemistry of protozoan microbodies: peroxisomes, alpha-glycerophosphate oxidase bodies, hydrogenosomes. Annu Rev Microbiol. 1975;29:467–483. doi: 10.1146/annurev.mi.29.100175.002343. [DOI] [PubMed] [Google Scholar]
- NICHOLAS D. J., WILSON P. J. A DISSIMILATORY NITRATE REDUCTASE FROM NEUROSPORA CRASSA. Biochim Biophys Acta. 1964 Jun 8;86:466–476. doi: 10.1016/0304-4165(64)90086-8. [DOI] [PubMed] [Google Scholar]
- Naik M. S., Nicholas D. J. Phosphorylation associated with nitrate and nitrite reduction in Micrococcus denitrificans and Pseudomonas denitrificans. Biochim Biophys Acta. 1966 Mar 7;113(3):490–497. doi: 10.1016/s0926-6593(66)80007-3. [DOI] [PubMed] [Google Scholar]
- Newman D. J., Postgate J. R. Rubredoxin from a nitrogen-fixing variety of Desulfovibrio desulfuricans. Eur J Biochem. 1968 Dec;7(1):45–50. doi: 10.1111/j.1432-1033.1968.tb19571.x. [DOI] [PubMed] [Google Scholar]
- Newton N. A., Cox G. B., Gibson F. The function of menaquinone (vitamin K 2 ) in Escherichia coli K-12. Biochim Biophys Acta. 1971 Jul 20;244(1):155–166. doi: 10.1016/0304-4165(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Newton N. The two-haem nitrite reductase of Micrococcus denitrificans. Biochim Biophys Acta. 1969;185(2):316–331. doi: 10.1016/0005-2744(69)90425-2. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. Hamster brown-adipose-tissue mitochondria. The chloride permeability of the inner membrane under respiring conditions, the influence of purine nucleotides. Eur J Biochem. 1974 Dec 2;49(3):585–593. doi: 10.1111/j.1432-1033.1974.tb03862.x. [DOI] [PubMed] [Google Scholar]
- Niederman R. A., Wolin M. J. Requirement of succinate for the growth of Vibrio succinogenes. J Bacteriol. 1972 Feb;109(2):546–549. doi: 10.1128/jb.109.2.546-549.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojiri T., Tanaka F., Nakayama I. Purification and properties of phosphotransacetylase from Lactobacillus fermenti. J Biochem. 1971 Apr;69(4):789–801. doi: 10.1093/oxfordjournals.jbchem.a129527. [DOI] [PubMed] [Google Scholar]
- O'Brien W. E., Brewer J. M., Ljungdahl L. G. Purification and characterization of thermostable 5,10-methylenetetrahydrofolate dehydrogenase from Clostridium thermoaceticum. J Biol Chem. 1973 Jan 25;248(2):403–408. [PubMed] [Google Scholar]
- O'Brien W. E., Ljungdahl L. G. Fermentation of fructose and synthesis of acetate from carbon dioxide by Clostridium formicoaceticum. J Bacteriol. 1972 Feb;109(2):626–632. doi: 10.1128/jb.109.2.626-632.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHNISHI T. Oxidative phosphorylation coupled with nitrate respiration with cell free extract of Pseudomonas denitrificans. J Biochem. 1963 Jan;53:71–79. doi: 10.1093/oxfordjournals.jbchem.a127660. [DOI] [PubMed] [Google Scholar]
- OTA A., YAMANAKA T., OKUNUKI K. OXIDATIVE PHOSPHORYLATION COUPLED WITH NITRATE RESPIRATION. II. PHOSPHORYLATION COUPLED WITH ANAEROBIC NITRATE REDUCTION IN A CELL-FREE EXTRACT OF ESCHERICHIA COLI. J Biochem. 1964 Feb;55:131–135. [PubMed] [Google Scholar]
- Oesterhelt D. The purple membrane of Halobacterium halobium: a new system for light energy conversion. Ciba Found Symp. 1975;(31):147–167. doi: 10.1002/9780470720134.ch9. [DOI] [PubMed] [Google Scholar]
- Ottow J. C. Evaluation of iron-reducing bacteria in soil and the physiological mechanism of iron-reduction in Aerobacter aerogenes. Z Allg Mikrobiol. 1968;8(5):441–443. doi: 10.1002/jobm.3630080512. [DOI] [PubMed] [Google Scholar]
- Ottow J. C. Mechanism of iron-reduction by nitrate reductase inducible aerobic microorganisms. Naturwissenschaften. 1969 Jul;56(7):371–371. doi: 10.1007/BF00596937. [DOI] [PubMed] [Google Scholar]
- Ottow J. C. Selection, characterization and iron-reducing capacity of nitrate reductaseless (nit-) mutants of iron-reducing bacteria. Z Allg Mikrobiol. 1970;10(1):55–62. [PubMed] [Google Scholar]
- Ottow J. C. The distribution and differentiation of iron-reducing bacteria in gley soils. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1969;123(6):600–615. [PubMed] [Google Scholar]
- Owen C. S., Wilson D. F. Control of respiration by the mitochondrial phosphorylation state. Arch Biochem Biophys. 1974 Apr 2;161(2):581–591. doi: 10.1016/0003-9861(74)90341-5. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr Evidence for oxidative phosphorylation during the reduction of sulfate with hydrogen by Desulfovibrio desulfuricans. J Biol Chem. 1960 Sep;235:2734–2738. [PubMed] [Google Scholar]
- PECK H. D., Jr, SMITH O. H., GEST H. Comparative biochemistry of the biological reduction of fumaric acid. Biochim Biophys Acta. 1957 Jul;25(1):142–147. doi: 10.1016/0006-3002(57)90431-6. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr The role of adenosine-5'-phosphosulfate in the reduction of sulfate to sulfite by Desulfovibrio desulfuricans. J Biol Chem. 1962 Jan;237:198–203. [PubMed] [Google Scholar]
- PEEL J. L. The breakdown of pyruvate by cell-free extracts of the rumen micro-organism LC. Biochem J. 1960 Mar;74:525–541. doi: 10.1042/bj0740525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETRACK B., SULLIVAN L., RATNER S. Behavior of purified arginine desiminase from S. faecalis. Arch Biochem Biophys. 1957 Jul;69:186–197. doi: 10.1016/0003-9861(57)90485-x. [DOI] [PubMed] [Google Scholar]
- PICHINOTY F. A PROPOS DES NITRATE-R'EDUCTASES D'UNE BACT'ERIE D'ENITRIFIANTE. Biochim Biophys Acta. 1964 Aug 26;89:378–381. [PubMed] [Google Scholar]
- PICHINOTY F., BIGLIARDI-ROUVIER J. [Research on tetrathionate reductase of a facultative anaerobic bacterium]. Biochim Biophys Acta. 1963 Mar 12;67:366–378. doi: 10.1016/0006-3002(63)91843-2. [DOI] [PubMed] [Google Scholar]
- PICHINOTY F., D'ORNANO L. [Research on the reduction of nitrous oxide by Micrococcus denitrificans]. Ann Inst Pasteur (Paris) 1961 Sep;101:418–426. [PubMed] [Google Scholar]
- POSTGATE J. R. Cytochrome c3 and desulphoviridin; pigments of the anaerobe Desulphovibrio desulphuricans. J Gen Microbiol. 1956 Jul;14(3):545–572. doi: 10.1099/00221287-14-3-545. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R. The reduction of sulphur compounds by Desulphovibrio desulphuricans. J Gen Microbiol. 1951 Oct;5(4):725–738. doi: 10.1099/00221287-5-4-725. [DOI] [PubMed] [Google Scholar]
- Padan E., Rottenberg H. Respiratory control and the proton electrochemical gradient in mitochondria. Eur J Biochem. 1973 Dec 17;40(2):431–437. doi: 10.1111/j.1432-1033.1973.tb03212.x. [DOI] [PubMed] [Google Scholar]
- Papa S. Proton translocation reactions in the respiratory chains. Biochim Biophys Acta. 1976 Apr 30;456(1):39–84. doi: 10.1016/0304-4173(76)90008-2. [DOI] [PubMed] [Google Scholar]
- Papavassiliou J., Samaraki-Lyberopoulou V., Piperakis G. Production of tetrathionate reductase by Salmonella. Can J Microbiol. 1969 Feb;15(2):238–240. doi: 10.1139/m69-041. [DOI] [PubMed] [Google Scholar]
- Parker D. J., Wu T. F., Wood H. G. Total synthesis of acetate from CO 2 : methyltetrahydrofolate, an intermediate, and a procedure for separation of the folates. J Bacteriol. 1971 Nov;108(2):770–776. doi: 10.1128/jb.108.2.770-776.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R., Boos W. Energy coupling of the -methylgalactoside transport system of Escherichia coli. J Biol Chem. 1973 Jun 25;248(12):4429–4435. [PubMed] [Google Scholar]
- Pauling L. Structure of high-energy molecules. Chem Br. 1970 Nov;6(11):468–472. [PubMed] [Google Scholar]
- Payne W. J. Energy yields and growth of heterotrophs. Annu Rev Microbiol. 1970;24:17–52. doi: 10.1146/annurev.mi.24.100170.000313. [DOI] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J., Riley P. S., Cox C. D., Jr Separate nitrite, nitric oxide, and nitrous oxide reducing fractions from Pseudomonas perfectomarinus. J Bacteriol. 1971 May;106(2):356–361. doi: 10.1128/jb.106.2.356-361.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paynter M. J., Elsden S. R. Mechanism of propionate formation by Selenomonas ruminantium, a rumen micro-organism. J Gen Microbiol. 1970 Apr;61(1):1–7. doi: 10.1099/00221287-61-1-1. [DOI] [PubMed] [Google Scholar]
- Peck H. D., Jr Phosphorylation coupled with electron transfer in extracts of the sulfate reducing bacterium, Desulfovibrio gigas. Biochem Biophys Res Commun. 1966 Jan 4;22(1):112–118. doi: 10.1016/0006-291x(66)90611-5. [DOI] [PubMed] [Google Scholar]
- Peck H. D. THE ATP-DEPENDENT REDUCTION OF SULFATE WITH HYDROGEN IN EXTRACTS OF DESULFOVIBRIO DESULFURICANS. Proc Natl Acad Sci U S A. 1959 May;45(5):701–708. doi: 10.1073/pnas.45.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelroy R. A., Whiteley H. R. Regulatory properties of acetokinase from Veillonella alcalescens. J Bacteriol. 1971 Jan;105(1):259–267. doi: 10.1128/jb.105.1.259-267.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitdemange H., Bengone J. M., Cherrier C., Gay R. Influence de la source carboneé sur les activitiés NAD+ et NADP+-ferredoxine oxydoréductasiques de Clostridium tyrobutyricum. C R Acad Sci Hebd Seances Acad Sci D. 1974 May 20;278(21):2707–2710. [PubMed] [Google Scholar]
- Pfennig N., Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol. 1976 Oct 11;110(1):3–12. doi: 10.1007/BF00416962. [DOI] [PubMed] [Google Scholar]
- Phillips R. C., George P., Rutman R. J. Thermodynamic data for the hydrolysis of adenosine triphosphate as a function of pH, Mg2+ ion concentration, and ionic strength. J Biol Chem. 1969 Jun 25;244(12):3330–3342. [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- Pollock M. R., Knox R. Bacterial reduction of tetrathionate: A report to the medical research council. Biochem J. 1943 Oct;37(4):476–481. doi: 10.1042/bj0370476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Jr, McCarty R. E. Quantitative relationships between phosphorylation, electron flow, and internal hydrogen ion concentrations in spinach chloroplasts. J Biol Chem. 1976 Mar 25;251(6):1610–1617. [PubMed] [Google Scholar]
- Postgate J. R., Campbell L. L. Classification of Desulfovibrio species, the nonsporulating sulfate-reducing bacteria. Bacteriol Rev. 1966 Dec;30(4):732–738. doi: 10.1128/br.30.4.732-738.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postgate J. R. Recent advances in the study of the sulfate-reducing bacteria. Bacteriol Rev. 1965 Dec;29(4):425–441. doi: 10.1128/br.29.4.425-441.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash O. M., Sadana J. C. Purification, characterization and properties of nitrite reductase of Achromobacter fischeri. Arch Biochem Biophys. 1972 Feb;148(2):614–632. doi: 10.1016/0003-9861(72)90181-6. [DOI] [PubMed] [Google Scholar]
- Prakash O., Rao R. R., Sadana J. C. Purification and characterization of nitrite reductase from Achromobacter fischeri. Biochim Biophys Acta. 1966 May 5;118(2):426–429. doi: 10.1016/s0926-6593(66)80054-1. [DOI] [PubMed] [Google Scholar]
- Prins R. A., van Nevel C. J., Demeyer D. I. Pure culture studies of inhibitors for methanogenic bacteria. Antonie Van Leeuwenhoek. 1972;38(3):281–287. doi: 10.1007/BF02328099. [DOI] [PubMed] [Google Scholar]
- Quastel J. H., Stephenson M., Whetham M. D. Some Reactions of Resting Bacteria in Relation to Anaerobic Growth. Biochem J. 1925;19(2):304–317. doi: 10.1042/bj0190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABINOWITZ J. C., PRICER W. E., Jr Formyltetrahydrofolate synthetase. I. Isolation and crystallization of the enzyme. J Biol Chem. 1962 Sep;237:2898–2902. [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Enzymatic synthesis of adenosine-5'-phosphosulfate. J Biol Chem. 1958 Sep;233(3):686–690. [PubMed] [Google Scholar]
- ROSE I. A., GRUNBERG-MANAGO M., KOREY S. R., OCHOA S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954 Dec;211(2):737–756. [PubMed] [Google Scholar]
- Racker E., Kandrach A. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXXIX. Reconstitution of the third segment of oxidative phosphorylation. J Biol Chem. 1973 Aug 25;248(16):5841–5847. [PubMed] [Google Scholar]
- Racker E. The two faces of the inner mitochondrial membrane. Essays Biochem. 1970;6:1–22. [PubMed] [Google Scholar]
- Radcliffe B. C., Nicholas D. J. Some properties of a nitrate reductase from Pseudomonas denitrificans. Biochim Biophys Acta. 1970;205(2):273–287. doi: 10.1016/0005-2728(70)90257-4. [DOI] [PubMed] [Google Scholar]
- Radcliffe B. C., Nicholas D. J. Some properties of a nitrite reductase from Pseudomonas denitrificans. Biochim Biophys Acta. 1968 Apr 2;153(3):545–554. doi: 10.1016/0005-2728(68)90184-9. [DOI] [PubMed] [Google Scholar]
- Ragan C. I., Hinkle P. C. Ion transport and respiratory control in vesicles formed from reduced nicotinamide adenine dinucleotide coenzyme Q reductase and phospholipids. J Biol Chem. 1975 Nov 10;250(21):8472–8476. [PubMed] [Google Scholar]
- Raven J. A., Smith F. A. The evolution of chemiosmotic energy coupling. J Theor Biol. 1976 Apr;57(2):301–312. doi: 10.1016/0022-5193(76)90003-5. [DOI] [PubMed] [Google Scholar]
- Reddy C. A., Bryant M. P., Wolin M. J. Characteristics of S organism isolated from Methanobacillus omelianskii. J Bacteriol. 1972 Feb;109(2):539–545. doi: 10.1128/jb.109.2.539-545.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy C. A., Bryant M. P., Wolin M. J. Ferredoxin- and nicotinamide adenine dinucleotide-dependent H 2 production from ethanol and formate in extracts of S organism isolated from "Methanobacillus omelianskii". J Bacteriol. 1972 Apr;110(1):126–132. doi: 10.1128/jb.110.1.126-132.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy C. A., Bryant M. P., Wolin M. J. Ferredoxin-dependent conversion of acetaldehyde to acetate and H 2 in extracts of S organism. J Bacteriol. 1972 Apr;110(1):133–138. doi: 10.1128/jb.110.1.133-138.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves J. P. Transient pH changes during D-lactate oxidation by membrane vesicles. Biochem Biophys Res Commun. 1971 Nov;45(4):931–936. doi: 10.1016/0006-291x(71)90427-x. [DOI] [PubMed] [Google Scholar]
- Reeves R. E. A new enzyme with the glycolytic function of pyruvate kinase. J Biol Chem. 1968 Jun 10;243(11):3202–3204. [PubMed] [Google Scholar]
- Reeves R. E., Menzies R. A., Hsu D. S. The pyruvate-phosphate dikinase reaction. The fate of phosphate and the equilibrium. J Biol Chem. 1968 Oct 25;243(20):5486–5491. [PubMed] [Google Scholar]
- Reid R. A., Moyle J., Mitchell P. Synthesis of adenosine triphosphate by a protonmotive force in rat liver mitochondria. Nature. 1966 Oct 15;212(5059):257–258. doi: 10.1038/212257a0. [DOI] [PubMed] [Google Scholar]
- Renner E. D., Becker G. E. Production of nitric oxide and nitrous oxide during denitrification by Corynebacterium nephridii. J Bacteriol. 1970 Mar;101(3):821–826. doi: 10.1128/jb.101.3.821-826.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebeling V., Jungermann K. Properties and function of clostridial membrane ATPase. Biochim Biophys Acta. 1976 Jun 8;430(3):434–444. doi: 10.1016/0005-2728(76)90019-0. [DOI] [PubMed] [Google Scholar]
- Riebeling V., Thauer R. K., Jungermann K. The internal-alkaline pH gradient, sensitive to uncoupler and ATPase inhibitor, in growing Clostridium pasteurianum. Eur J Biochem. 1975 Jul 1;55(2):445–453. doi: 10.1111/j.1432-1033.1975.tb02181.x. [DOI] [PubMed] [Google Scholar]
- Riet J van't, Knook D. L., Planta R. J. The role of cytochrome b 1 in nitrate assimilation and nitrate respiration in Klebsiella aerogenes. FEBS Lett. 1972 Jun 1;23(1):44–46. doi: 10.1016/0014-5793(72)80280-1. [DOI] [PubMed] [Google Scholar]
- Riet J van't The participation of cytochromes in the process of nitrate respiration in klesbsiella (Aerobacter) aerogenes. Biochim Biophys Acta. 1973 Jan 18;292(1):237–245. doi: 10.1016/0005-2728(73)90268-5. [DOI] [PubMed] [Google Scholar]
- Rimerman E. A., Barker H. A. Formation and identification of 3-keto-5-aminohexanoic acid, a probable intermediate in lysine fermentation. J Biol Chem. 1968 Dec 10;243(23):6151–6160. [PubMed] [Google Scholar]
- Ritchey T. W., Seeley H. W. Cytochromes in Streptococcus faecalis var. zymogenes grown in a haematin-containing medium. J Gen Microbiol. 1974 Dec;85(2):220–228. doi: 10.1099/00221287-85-2-220. [DOI] [PubMed] [Google Scholar]
- Rizza V., Sinclair P. R., White D. C., Cuorant P. R. Electron transport system of the protoheme-requiring anaerobe Bacteroides melaninogenicus. J Bacteriol. 1968 Sep;96(3):665–671. doi: 10.1128/jb.96.3.665-671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton A. M., Wolfe R. S. Adenosine triphosphate pools in Methanobacterium. J Bacteriol. 1970 Apr;102(1):43–51. doi: 10.1128/jb.102.1.43-51.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A. M., Wolfe R. S. ATP requirement for methanogenesis in cell extracts of methanobacterium strain M.o.H. Biochim Biophys Acta. 1969 Dec 30;192(3):420–429. doi: 10.1016/0304-4165(69)90391-2. [DOI] [PubMed] [Google Scholar]
- Robinson J. R., Sagers R. D. Phosphotransacetylase from Clostridium acidiurici. J Bacteriol. 1972 Oct;112(1):465–473. doi: 10.1128/jb.112.1.465-473.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roon R. J., Barker H. A. Fermentation of agmatine in Streptococcus faecalis: occurrence of putrescine transcarbamoylase. J Bacteriol. 1972 Jan;109(1):44–50. doi: 10.1128/jb.109.1.44-50.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosing J., Slater E. C. The value of G degrees for the hydrolysis of ATP. Biochim Biophys Acta. 1972 May 25;267(2):275–290. doi: 10.1016/0005-2728(72)90116-8. [DOI] [PubMed] [Google Scholar]
- Ross R. A., Vernon C. A. A reply to Douglas Wilkie. Chem Br. 1970 Dec 12;6(12):539–540. [PubMed] [Google Scholar]
- Rossi E., Azzone G. F. The mechanism of ion translocation in mitochondria. 3. Coupling of K+ efflux with ATP synthesis. Eur J Biochem. 1970 Feb;12(2):319–327. doi: 10.1111/j.1432-1033.1970.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T., Avron M. Determination of pH in chloroplasts. I. Distribution of ( 14 C) methylamine. Eur J Biochem. 1972 Jan 31;25(1):54–63. doi: 10.1111/j.1432-1033.1972.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T., Avron M. Direct determination of DeltapH in chloroplasts, and its relation to the mechanisms of photoinduced reactions. FEBS Lett. 1971 Feb 12;13(1):41–44. doi: 10.1016/0014-5793(71)80659-2. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T. Determination of pH in chloroplasts. 3. Ammonium uptake as a measure of pH in chloroplasts and sub-chloroplast particles. Eur J Biochem. 1972 Jan 31;25(1):71–74. doi: 10.1111/j.1432-1033.1972.tb01668.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of transmembrane electrochemical proton gradients. J Bioenerg. 1975 May;7(2):61–74. doi: 10.1007/BF01558427. [DOI] [PubMed] [Google Scholar]
- Rudolph F. B., Purich D. L., Fromm H. J. Coenzyme A-linked aldehyde dehydrogenase from Escherichia coli. I. Partial purification, properties, and kinetic studies of the enzyme. J Biol Chem. 1968 Nov 10;243(21):5539–5545. [PubMed] [Google Scholar]
- Ruiz-Herrera J., DeMoss J. A. Nitrate reductase complex of Escherichia coli K-12: participation of specific formate dehydrogenase and cytochrome b1 components in nitrate reduction. J Bacteriol. 1969 Sep;99(3):720–729. doi: 10.1128/jb.99.3.720-729.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruíz-Herrera J., Alvarez A., Figueroa I. Solubilization and properties of formate dehydrogenases from the membrane of Escherichia coli. Biochim Biophys Acta. 1972 Dec 7;289(2):254–261. doi: 10.1016/0005-2744(72)90075-7. [DOI] [PubMed] [Google Scholar]
- SAGERS R. D., BENZIMAN M., GUNSALUS I. C. Acetate formation in Clostridium acidi-urici: acetokinase. J Bacteriol. 1961 Aug;82:233–238. doi: 10.1002/path.1700820136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRAMM M., KLYBAS V., RACKER E. Phosphorolytic cleavage of fructose-6-phosphate by fructose-6-phosphate phosphoketolase from Acetobacter xylinum. J Biol Chem. 1958 Dec;233(6):1283–1288. [PubMed] [Google Scholar]
- SEIDMAN I., ENTNER N. Oxidative enzymes and their role in phosphorylation in sarcosomes of adult Ascaris lumbricoides. J Biol Chem. 1961 Mar;236:915–919. [PubMed] [Google Scholar]
- SENEZ J. C. Some considerations on the energetics of bacterial growth. Bacteriol Rev. 1962 Jun;26:95–107. [PMC free article] [PubMed] [Google Scholar]
- STADTMAN T. C., ELLIOTT P., TIEMANN L. Studies on the enzymic reduction of amino acids. III. Phosphate esterification coupled with glycine reduction. J Biol Chem. 1958 Apr;231(2):961–973. [PubMed] [Google Scholar]
- STADTMAN T. C. The participation of a quinone in the enzymic reduction of glycine by Clostridium sticklandii. Biochem Z. 1958;331(1):46–48. [PubMed] [Google Scholar]
- SZULMAJSTER J. [Carbamyl phosphate, intermediate in the degradation of creatinine by enzymatic extracts of Eubacterium sarcosinogenum]. Biochim Biophys Acta. 1960 Oct 21;44:173–175. doi: 10.1016/0006-3002(60)91539-0. [DOI] [PubMed] [Google Scholar]
- Sapshead L. M., Wimpenny J. W. The influence of oxygen and nitrate on the formation of the cytochrome pigments of the aerobic and anaerobic respiratory chain of Micrococcus denitrificans. Biochim Biophys Acta. 1972 May 25;267(2):388–397. doi: 10.1016/0005-2728(72)90126-0. [DOI] [PubMed] [Google Scholar]
- Sasarman A., Purvis P., Portelance V. Role of menaquinone in nitrate respiration in Staphylococcus aureus. J Bacteriol. 1974 Feb;117(2):911–913. doi: 10.1128/jb.117.2.911-913.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Yamada K., Ozawa H. Rhodoquinone specificity in the reactivation of succinoxidase activity of acetone-extracted Ascaris mitochondria. Biochem Biophys Res Commun. 1972 Jan 31;46(2):578–582. doi: 10.1016/s0006-291x(72)80178-5. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Iyanagi T., Yamazaki I. Relation between redox potentials and rate constants in reactions coupled with the system oxygen-superoxide. Biochemistry. 1975 Aug 26;14(17):3761–3764. doi: 10.1021/bi00688a007. [DOI] [PubMed] [Google Scholar]
- Scardovi V., Sgorbati B., Zani G. Starch gel electrophoresis of fructose-6-phosphate phophoketolase in the genus Bifidobacterium. J Bacteriol. 1971 Jun;106(3):1036–1039. doi: 10.1128/jb.106.3.1036-1039.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaupp A., Ljungdahl L. G. Purification and properties of acetate kinase from Clostridium thermoaceticum. Arch Microbiol. 1974;100(2):121–129. doi: 10.1007/BF00446312. [DOI] [PubMed] [Google Scholar]
- Scheibel L. W., Saz H. J., Bueding E. The anaerobic incorporation of 32P into adenosine triphosphate by Hymenolepis diminuta. J Biol Chem. 1968 May 10;243(9):2229–2235. [PubMed] [Google Scholar]
- Schimke R. T., Berlin C. M., Sweeney E. W., Carroll W. R. The generation of energy by the arginine dihydrolase pathway in Mycoplasma hominis 07. J Biol Chem. 1966 May 25;241(10):2228–2236. [PubMed] [Google Scholar]
- Schmidt G. B., Rosano C. L., Hurwitz C. Evidence for a magnesium pump in Bacillus cereus T. J Bacteriol. 1971 Jan;105(1):150–155. doi: 10.1128/jb.105.1.150-155.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnebli H. P., Abrams A. Membrane adenosine triphosphatase from Streptococcus faecalis. Preparation and homogeneity. J Biol Chem. 1970 Mar 10;245(5):1115–1121. [PubMed] [Google Scholar]
- Schnebli H. P., Vatter A. E., Abrams A. Membrane adenosine triphosphatase from Streptococcus faecalis. Molecular weight, subunit structure, and amino acid composition. J Biol Chem. 1970 Mar 10;245(5):1122–1127. [PubMed] [Google Scholar]
- Schoberth S., Gottschalk G. Considerations on the energy metabolism of Clostridium kluyveri. Arch Mikrobiol. 1969;65(4):318–328. doi: 10.1007/BF00412211. [DOI] [PubMed] [Google Scholar]
- Scholes P. B., McLain G., Smith L. Purification and properties of a c-type cytochrome from Micrococcus denitrificans. Biochemistry. 1971 May 25;10(11):2072–2076. doi: 10.1021/bi00787a017. [DOI] [PubMed] [Google Scholar]
- Scholes P. B., Smith L. Composition and properties of the membrane-bound respiratory chain system of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):363–375. doi: 10.1016/0005-2728(68)90081-9. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Acid-base titration across the plasma membrane of Micrococcus denitrificans: factors affecting the effective proton conductance and the respiratory rate. J Bioenerg. 1970 Jun;1(1):61–72. doi: 10.1007/BF01516089. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Respiration-driven proton translocation in Micrococcus denitrificans. J Bioenerg. 1971 Sep;1(3):309–323. doi: 10.1007/BF01516290. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Rottenberg H., Avron M. Determination of pH in chloroplasts. 2. Fluorescent amines as a probe for the determination of pH in chloroplasts. Eur J Biochem. 1972 Jan 31;25(1):64–70. doi: 10.1111/j.1432-1033.1972.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Rottenberg H., Avron M. Membrane potential as a driving force for ATP synthesis in chloroplasts. FEBS Lett. 1972 Dec 1;28(2):173–176. doi: 10.1016/0014-5793(72)80704-x. [DOI] [PubMed] [Google Scholar]
- Schulman M., Ghambeer R. K., Ljungdahl L. G., Wood H. G. Total synthesis of acetate from CO2. VII. Evidence with Clostridium thermoaceticum that the carboxyl of acetate is derived from the carboxyl of pyruvate by transcarboxylation and not by fixation of CO2. J Biol Chem. 1973 Sep 25;248(18):6255–6261. [PubMed] [Google Scholar]
- Schulman M., Parker D., Ljungdahl L. G., Wood H. G. Total synthesis of acetate from CO 2 . V. Determination by mass analysis of the different types of acetate formed from 13 CO 2 by heterotrophic bacteria. J Bacteriol. 1972 Feb;109(2):633–644. doi: 10.1128/jb.109.2.633-644.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulp J. A., Stouthamer A. H. The influence of oxygen, glucose and nitrate upon the formation of nitrate reductase and the respiratory system in Bacillus licheniformis. J Gen Microbiol. 1970 Dec;64(2):195–203. doi: 10.1099/00221287-64-2-195. [DOI] [PubMed] [Google Scholar]
- Schwartz A. C., Schäfer R. New amino acids, and heterocyclic compounds participating in the Stickland reaction of Clostridium sticklandii. Arch Mikrobiol. 1973 Nov 2;93(3):267–276. doi: 10.1007/BF00412026. [DOI] [PubMed] [Google Scholar]
- Schwartz A. C., Sporkenbach J. The electron transport system of the anaerobic Propionibacterium shermanii: cytochrome and inhibitor studies. Arch Microbiol. 1975 Mar 10;102(3):261–273. doi: 10.1007/BF00428377. [DOI] [PubMed] [Google Scholar]
- Schwartz A. C. Terpenoid quinones of the anaerobic Propionibacterium shermanii. I. (II, 3)-Tetrahydromenaquinone-9. Arch Mikrobiol. 1973 Jun 6;91(3):273–279. doi: 10.1007/BF00408913. [DOI] [PubMed] [Google Scholar]
- Senior A. E. The structure of mitochondrial ATPase. Biochim Biophys Acta. 1973 Dec 31;301(3):249–277. doi: 10.1016/0304-4173(73)90006-2. [DOI] [PubMed] [Google Scholar]
- Shahak Y., Hardt H., Avron M. Acid-base driven reverse electron flow in isolated chloroplasts. FEBS Lett. 1975 Jun 15;54(2):151–154. doi: 10.1016/0014-5793(75)80063-9. [DOI] [PubMed] [Google Scholar]
- Shikama K. Standard free energy maps for the hydrolysis of ATP as a function of pH, pMg and pCa. Arch Biochem Biophys. 1971 Nov;147(1):311–317. doi: 10.1016/0003-9861(71)90338-9. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Suzuki T., Kameda K. Y., Abiko Y. Phosphotransacetylase of Escherichia coli B, purification and properties. Biochim Biophys Acta. 1969;191(3):550–558. doi: 10.1016/0005-2744(69)90348-9. [DOI] [PubMed] [Google Scholar]
- Showe M. K., DeMoss J. A. Localization and regulation of synthesis of nitrate reductase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1305–1313. doi: 10.1128/jb.95.4.1305-1313.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum A. C., Murphy J. C. Effects of selenium compounds on formate metabolism and coincidence of selenium-75 incorporation and formic dehydrogenase activity in cell-free preparations of Escherichia coli. J Bacteriol. 1972 Apr;110(1):447–449. doi: 10.1128/jb.110.1.447-449.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Postma P. W. The energetics of bacterial active transport. Annu Rev Biochem. 1975;44:523–554. doi: 10.1146/annurev.bi.44.070175.002515. [DOI] [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. Anaerobic transport of amino acids coupled to the glycerol-3-phosphate-fumarate oxidoreductase system in a cytochrome-deficient mutant of Escherichia coli. Biochim Biophys Acta. 1976 Mar 12;423(3):450–461. doi: 10.1016/0005-2728(76)90200-0. [DOI] [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. Reduced nicotinamide adenine dinucleotide dependent reduction of fumarate coupled to membrane energization in a cytochrome deficient mutant of Escherichia coli K12. Biochim Biophys Acta. 1975 Aug 11;396(2):229–241. doi: 10.1016/0005-2728(75)90037-7. [DOI] [PubMed] [Google Scholar]
- Skulachev V. P. Enzymic generators of membrane potential in mitochondria. Ann N Y Acad Sci. 1974 Feb 18;227:188–202. doi: 10.1111/j.1749-6632.1974.tb14384.x. [DOI] [PubMed] [Google Scholar]
- Slater E. C., Rosing J., Mol A. The phosphorylation potential generated by respiring mitochondria. Biochim Biophys Acta. 1973 Apr 5;292(3):534–553. doi: 10.1016/0005-2728(73)90003-0. [DOI] [PubMed] [Google Scholar]
- Slater E. C. The coupling between energy-yielding and energy-utilizing reactions in mitochondria. Q Rev Biophys. 1971 Feb;4(1):35–71. doi: 10.1017/s0033583500000391. [DOI] [PubMed] [Google Scholar]
- Slayman C. L. Adenine nucleotide levels in Neurospora, as influenced by conditions of growth and by metabolic inhibitors. J Bacteriol. 1973 May;114(2):752–766. doi: 10.1128/jb.114.2.752-766.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone N., Kitsutani S. The redox reactions in propionic acid fermentation. II. Purification of NAD-independent glycerolphosphate dehydrogenase bound to minute particles from supernatant fraction of Propionibacterium arabinosumm. J Biochem. 1972 Aug;72(2):291–297. doi: 10.1093/oxfordjournals.jbchem.a129908. [DOI] [PubMed] [Google Scholar]
- Sone N. The redox reactions in propionic acid fermantation. I. Occurrence and nature of an electron transfer system in Propionibacterium arabinosum. J Biochem. 1972 Jun;71(6):931–940. doi: 10.1093/oxfordjournals.jbchem.a129864. [DOI] [PubMed] [Google Scholar]
- Sone N. The redox reactions in propionic acid fermentation. IV. Participation of menaquinone in the electron transfer system in Propionibacterium arabinosum. J Biochem. 1974 Jul;76(1):137–145. doi: 10.1093/oxfordjournals.jbchem.a130538. [DOI] [PubMed] [Google Scholar]
- Sorokin Y. I. Role of carbon dioxide and acetate in biosynthesis by sulphate-reducing bacteria. Nature. 1966 Apr 30;210(5035):551–552. doi: 10.1038/210551a0. [DOI] [PubMed] [Google Scholar]
- Spangler W. J., Gilmour C. M. Biochemistry of nitrate respiration in Pseudomonas stutzeri. I. Aerobic and nitrate respiration routes of carbohydrate catabolism. J Bacteriol. 1966 Jan;91(1):245–250. doi: 10.1128/jb.91.1.245-250.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Isolation and properties of fumarate reductase mutants of Escherichia coli. J Bacteriol. 1973 May;114(2):563–570. doi: 10.1128/jb.114.2.563-570.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperl G. T., Hoare D. S. Denitrification with methanol: a selective enrichment for Hyphomicrobium species. J Bacteriol. 1971 Nov;108(2):733–736. doi: 10.1128/jb.108.2.733-736.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher M., Switzer R. L., Sprinson D. B. Stereochemistry of the glutamate mutase reaction. J Biol Chem. 1966 Feb 25;241(4):864–867. [PubMed] [Google Scholar]
- Stadtman T. C. Glycine reduction to acetate and ammonia: identification of ferredoxin and another low molecular weight acidic protein as components of the reductase system. Arch Biochem Biophys. 1966 Jan;113(1):9–19. doi: 10.1016/0003-9861(66)90151-2. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Methane fermentation. Annu Rev Microbiol. 1967;21:121–142. doi: 10.1146/annurev.mi.21.100167.001005. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C., Renz P. Anaerobic degradation of lysine. V. Some properties of the cobamide coenzyme-dependent beta-lysine mutase of Clostridium sticklandii. Arch Biochem Biophys. 1968 Apr;125(1):226–239. doi: 10.1016/0003-9861(68)90657-7. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium biochemistry. Science. 1974 Mar 8;183(4128):915–922. doi: 10.1126/science.183.4128.915. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H. A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Van Leeuwenhoek. 1973;39(3):545–565. doi: 10.1007/BF02578899. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Influence of hydrogen acceptors on growth and energy production of Proteus mirabilis. Antonie Van Leeuwenhoek. 1972;38(1):81–90. doi: 10.1007/BF02328079. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. A reevaluation of the method for the determination of ATP production by measuring molar growth yields. Biochim Biophys Acta. 1973 Feb 12;301(1):53–70. doi: 10.1016/0304-4173(73)90012-8. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H. Biochemistry and genetics of nitrate reductase in bacteria. Adv Microb Physiol. 1976;14(11):315–375. doi: 10.1016/s0065-2911(08)60230-1. [DOI] [PubMed] [Google Scholar]
- Sun A. Y., Ljungdahl L., Wood H. G. Total synthesis of acetate from CO2. II. Purification and properties of formyltetrahydrofolate synthetase from Clostridium thermoaceticum. J Bacteriol. 1969 May;98(2):842–844. doi: 10.1128/jb.98.2.842-844.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. Phosphotransacetylase of Escherichia coli B, activation by pyruvate and inhibition by NADH and certain nucleotides. Biochim Biophys Acta. 1969;191(3):559–569. doi: 10.1016/0005-2744(69)90349-0. [DOI] [PubMed] [Google Scholar]
- Switzer R. L., Baltimore B. G., Barker H. A. Hydrogen transfer between substrates and deoxyadenosylcobalamin in the glutamate mutase reaction. J Biol Chem. 1969 Oct 10;244(19):5263–5268. [PubMed] [Google Scholar]
- Switzer R. L., Barker H. A. Purification and characterization of component S of glutamate mutase. J Biol Chem. 1967 Jun 10;242(11):2658–2674. [PubMed] [Google Scholar]
- Synthesis and sideedness of membrane-bound respiratory nitrate reductase (EC1.7.99.4) in Escherichia coli lacking cytochromes. Biochem J. 1975 May;148(2):329–333. [PMC free article] [PubMed] [Google Scholar]
- TANIGUCHI S., ITAGAKI E. Nitrate reductase of nitrate respiration type from E. coli. I. Solubilization and purification from the particulate system with molecular characterization as a metalloprotein. Biochim Biophys Acta. 1960 Nov 4;44:263–279. doi: 10.1016/0006-3002(60)91562-6. [DOI] [PubMed] [Google Scholar]
- THORNE K. J., JONES M. E. CARBAMYL AND ACETYL PHOSPHOKINASE ACTIVITIES OF STREPTOCOCCUS FAECALIS AND ESCHERICHIA COLI. J Biol Chem. 1963 Sep;238:2992–2998. [PubMed] [Google Scholar]
- TWAROG R., WOLFE R. S. Enzymatic phosphorylation of butyrate. J Biol Chem. 1962 Aug;237:2474–2477. [PubMed] [Google Scholar]
- TWAROG R., WOLFE R. S. ROLE OF BUTYRYL PHOSPHATE IN THE ENERGY METABOLISM OF CLOSTRIDIUM TETANOMORPHUM. J Bacteriol. 1963 Jul;86:112–117. doi: 10.1128/jb.86.1.112-117.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. D., McBride B. C., Wolfe R. S., Bryant M. P. Coenzyme M, essential for growth of a rumen strain of Methanobacterium ruminantium. J Bacteriol. 1974 Nov;120(2):974–975. doi: 10.1128/jb.120.2.974-975.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. A simplified assay for coenzyme M (HSCH2CH2SO3). Resolution of methylcobalamin-coenzyme M methyltransferase and use of sodium borohydride. J Biol Chem. 1974 Aug 10;249(15):4886–4890. [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. Structure and methylation of coenzyme M(HSCH2CH2SO3). J Biol Chem. 1974 Aug 10;249(15):4879–4885. [PubMed] [Google Scholar]
- Thauer R. K. CO 2 reduction to formate in Clostridium acidi-urici. J Bacteriol. 1973 Apr;114(1):443–444. doi: 10.1128/jb.114.1.443-444.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K. CO(2)-reduction to formate by NADPH. The initial step in the total synthesis of acetate from CO(2) in Clostridium thermoaceticum. FEBS Lett. 1972 Oct 15;27(1):111–115. doi: 10.1016/0014-5793(72)80421-6. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Henninger H., Wenning J., Decker K. The energy metabolism of Clostridium kluyveri. Eur J Biochem. 1968 Apr 3;4(2):173–180. doi: 10.1111/j.1432-1033.1968.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Rupprecht E., Decker K. Hydrogen formation from NADH in cell-free extracts of Clostridium kluyveri. Acetyl coenzyme A requirement and ferredoxin dependence. FEBS Lett. 1969 Jul;4(2):108–112. doi: 10.1016/0014-5793(69)80208-5. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Kirchniawy F. H., Jungermann K. A. Properties and function of the pyruvate-formate-lyase reaction in clostridiae. Eur J Biochem. 1972 May 23;27(2):282–290. doi: 10.1111/j.1432-1033.1972.tb01837.x. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Käufer B., Fuchs G. The active species of 'CO2' utilized by reduced ferredoxin:CO2 oxidoreductase from Clostridium pasteurianum. Eur J Biochem. 1975 Jun 16;55(1):111–117. doi: 10.1111/j.1432-1033.1975.tb02143.x. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Käufer B., Scherer P. The active species of "CO2" utilized in ferredoxin-linked carboxylation reactions. Arch Microbiol. 1975 Aug 28;104(3):237–240. doi: 10.1007/BF00447330. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Rupprecht E., Jungermann K. Glyoxylate inhibition of clostridial pyruvate synthase. FEBS Lett. 1970 Aug 31;9(5):271–273. doi: 10.1016/0014-5793(70)80374-x. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Rupprecht E., Jungermann K. The synthesis of one-carbon units from CO(2) via a new ferredoxin dependent monocarboxylic acid cycle. FEBS Lett. 1970 Jun 27;8(5):304–307. doi: 10.1016/0014-5793(70)80293-9. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Rupprecht E., Ohrloff C., Jungermann K., Decker K. Regulation of the reduced nicotinamide adenine dinucleotide phosphate-ferredoxin reductase system in Clostridium kluyveri. J Biol Chem. 1971 Feb 25;246(4):954–959. [PubMed] [Google Scholar]
- Thayer W. S., Hinkle P. C. Stoichiometry of adenosine triphosphate-driven proton translocation in bovine heart submitochondrial particles. J Biol Chem. 1973 Aug 10;248(15):5395–5402. [PubMed] [Google Scholar]
- Thayer W. S., Hinkle P. C. Synthesis of adenosine triphosphate by an artificially imposed electrochemical proton gradient in bovine heart submitochondrial particles. J Biol Chem. 1975 Jul 25;250(14):5330–5335. [PubMed] [Google Scholar]
- Tisdale H., Hauber J., Prager G., Turini P., Singer T. P. Studies on succinate dehydrogenase. 15. Isolation, molecular properties, and isoenzymes of fumarate reductase. Eur J Biochem. 1968 May;4(4):472–477. doi: 10.1111/j.1432-1033.1968.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Trebst A., Hauska G. Energiekonservierung in der photosynthetischen Membran der Chloroplasten. Naturwissenschaften. 1974 Jul;61(7):308–316. doi: 10.1007/BF00599561. [DOI] [PubMed] [Google Scholar]
- Trudinger P. A. Metabolism of thiosulfate and tetrathionate by heterotrophic bacteria from soil. J Bacteriol. 1967 Feb;93(2):550–559. doi: 10.1128/jb.93.2.550-559.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L., Stadtman T. C. Anaerobic degradation of lysine. IV. Cobamide coenzyme-dependent migration of an amino group from carbon 6 of beta-lysine (3,6-diaminohexanoate) to carbon 5 forming a new naturally occurring amino acid, 3,5-diaminohexanoate. Arch Biochem Biophys. 1968 Apr;125(1):210–225. doi: 10.1016/0003-9861(68)90656-5. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Stadtman T. C. Purification of protein components of the clostridial glycine reductase system and characterization of protein A as a selenoprotein. Arch Biochem Biophys. 1973 Jan;154(1):366–381. doi: 10.1016/0003-9861(73)90069-6. [DOI] [PubMed] [Google Scholar]
- Tuttle J. H., Jannasch H. W. Dissimilatory reduction of inorganic sulfur by facultatively anaerobic marine bacteria. J Bacteriol. 1973 Sep;115(3):732–737. doi: 10.1128/jb.115.3.732-737.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng S. F., Wolfe R. S., Bryant M. P. Factor 420-dependent pyridine nucleotide-linked hydrogenase system of Methanobacterium ruminantium. J Bacteriol. 1975 Jan;121(1):184–191. doi: 10.1128/jb.121.1.184-191.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzing S. F., Bryant M. P., Wolfe R. S. Factor 420-dependent pyridine nucleotide-linked formate metabolism of Methanobacterium ruminantium. J Bacteriol. 1975 Jan;121(1):192–196. doi: 10.1128/jb.121.1.192-196.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe E. G., Jagendorf A. T. Membrane permeability and internal volume as factors in ATP synthesis by spinach chloroplasts. Arch Biochem Biophys. 1968 Nov;128(2):351–359. doi: 10.1016/0003-9861(68)90041-6. [DOI] [PubMed] [Google Scholar]
- Uyeda K., Rabinowitz J. C. Pyruvate-ferredoxin oxidoreductase. IV. Studies on the reaction mechanism. J Biol Chem. 1971 May 25;246(10):3120–3125. [PubMed] [Google Scholar]
- VALENTINE R. C., BOJANOWSKI R., GAUDY E., WOLFE R. S. Mechanism of the allantoin fermentation. J Biol Chem. 1962 Jul;237:2271–2277. [PubMed] [Google Scholar]
- VALENTINE R. C., WOLFE R. S. Phosphorolysis of carbamyl oxamic acid. Biochim Biophys Acta. 1960 Dec 4;45:389–391. doi: 10.1016/0006-3002(60)91467-0. [DOI] [PubMed] [Google Scholar]
- VALENTINE R. C., WOLFE R. S. Purification and role of phosphotransbutyrylase. J Biol Chem. 1960 Jul;235:1948–1952. [PubMed] [Google Scholar]
- Van 't Riet J., Planta R. J. Purification, structure and properties of the respiratory nitrate reductase of Klebsiella aerogenes. Biochim Biophys Acta. 1975 Jan 30;379(1):81–94. doi: 10.1016/0005-2795(75)90010-0. [DOI] [PubMed] [Google Scholar]
- Van Gent-Ruijters M. L., DeVries W., Southamer A. H. Influence of nitrate on fermentation pattern, molar growth yields and synthesis of cytochrome b in Propionibacterium pentosaceum. J Gen Microbiol. 1975 May;88(1):36–48. doi: 10.1099/00221287-88-1-36. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Raijman L., Krebs H. A. Equilibrium relations between the cytoplasmic adenine nucleotide system and nicotinamide-adenine nucleotide system in rat liver. Biochem J. 1970 Apr;117(3):499–503. doi: 10.1042/bj1170499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega J. M., Garrett R. H. Siroheme: a prosthetic group of the Neurospora crassa assimilatory nitrite reductase. J Biol Chem. 1975 Oct 25;250(20):7980–7989. [PubMed] [Google Scholar]
- Vetter H., Jr, Knappe J. Flavodoxin and ferredoxin of Escherichia coli. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):433–446. doi: 10.1515/bchm2.1971.352.1.433. [DOI] [PubMed] [Google Scholar]
- Von Tigerstrom R. G., Razzell W. E. Aldehyde dehydrogenase. I. Purification and properties of the enzyme from Pseudomonas aeruginosa. J Biol Chem. 1968 May 25;243(10):2691–2702. [PubMed] [Google Scholar]
- Vosjan J. H. ATP generation by electron transport in Desulfovibrio desulfuricans. Antonie Van Leeuwenhoek. 1970;36(4):584–586. [PubMed] [Google Scholar]
- WALKER G. C., NICHOLAS D. J. Nitrite reductase from Pseudomonas aeruginosa. Biochim Biophys Acta. 1961 May 13;49:350–360. doi: 10.1016/0006-3002(61)90134-2. [DOI] [PubMed] [Google Scholar]
- WARRINGA M. G., SMITH O. H., GIUDITTA A., SINGER T. P. Studies on succinic dehydrogenase. VIII. Isolation of a succinic dehydrogenase-fumaric reductase from an obligate anaerobe. J Biol Chem. 1958 Jan;230(1):97–109. [PubMed] [Google Scholar]
- WHITE D. C., BRYANT M. P., CALDWELL D. R. Cytochromelinked fermentation in Bacteroides ruminicola. J Bacteriol. 1962 Oct;84:822–828. doi: 10.1128/jb.84.4.822-828.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. THE FUNCTION OF 2-DEMETHYL VITAMIN K2 IN THE ELECTRON TRANSPORT SYSTEM OF HEMOPHILUS PARAINFLUENZAE. J Biol Chem. 1965 Mar;240:1387–1394. [PubMed] [Google Scholar]
- WHITTENBURY R. HYDROGEN PEROXIDE FORMATION AND CATALASE ACTIVITY IN THE LACTIC ACID BACTERIA. J Gen Microbiol. 1964 Apr;35:13–26. doi: 10.1099/00221287-35-1-13. [DOI] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- WOLIN M. J., WOLIN E. A., JACOBS N. J. Cytochrome-producing anaerobic Vibrio succinogenes, sp. n. J Bacteriol. 1961 Jun;81:911–917. doi: 10.1128/jb.81.6.911-917.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN M. J., WOLIN E. A., WOLFE R. S. ATP-DEPENDENT FORMATION OF METHANE FROMMETHYLCOBALAMIN BY EXTRACTS OF METHANOBACILLUS OMELIANSKII. Biochem Biophys Res Commun. 1963 Aug 20;12:464–468. doi: 10.1016/0006-291x(63)90316-4. [DOI] [PubMed] [Google Scholar]
- Wagner G. C., Kassner R. J., Kamen M. D. Redox potentials of certain vitamins K: implications for a role in sulfite reduction by obligately anaerobic bacteria. Proc Natl Acad Sci U S A. 1974 Feb;71(2):253–256. doi: 10.1073/pnas.71.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallnöfer P., Baldwin R. L. Pathway of propionate formation in Bacteroides ruminicola. J Bacteriol. 1967 Jan;93(1):504–505. doi: 10.1128/jb.93.1.504-505.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Barker H. A. Activation of L-citramalate hydrolyase from clostridium tetanomorphum. J Biol Chem. 1969 May 25;244(10):2527–2538. [PubMed] [Google Scholar]
- Wang C. C., Barker H. A. Purification and properties of L-citramalate hydrolyase. J Biol Chem. 1969 May 25;244(10):2516–2526. [PubMed] [Google Scholar]
- Ware D. A., Postgate J. R. Physiological and chemical properties of a reductant-activated inorganic pyrophosphatase from Desulfovibrio desulfuricans. J Gen Microbiol. 1971 Aug;67(2):145–160. doi: 10.1099/00221287-67-2-145. [DOI] [PubMed] [Google Scholar]
- Weber M. M., Matschiner J. T., Peck H. D. Menaquinone-6 in the strict anaerobes Desulfovibrio vulgaris and Desulfovibrio gigas. Biochem Biophys Res Commun. 1970 Jan 23;38(2):197–204. doi: 10.1016/0006-291x(70)90696-0. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W. Accumulation of bicarbonate in intact chloroplasts following a pH gradient. Biochim Biophys Acta. 1972 Dec 14;283(3):430–441. doi: 10.1016/0005-2728(72)90260-5. [DOI] [PubMed] [Google Scholar]
- West I. C., Mitchell P. The proton-translocating ATPase of Escherichia coli. FEBS Lett. 1974 Mar 15;40(1):1–4. doi: 10.1016/0014-5793(74)80880-x. [DOI] [PubMed] [Google Scholar]
- White D. C., Sinclair P. R. Branched electron-transport systems in bacteria. Adv Microb Physiol. 1971;5:173–211. doi: 10.1016/s0065-2911(08)60407-5. [DOI] [PubMed] [Google Scholar]
- Whiteley H. R., Pelroy R. A. Purification and properties of phosphotransacetylase from Veillonella alcalescens. J Biol Chem. 1972 Mar 25;247(6):1911–1917. [PubMed] [Google Scholar]
- Whitfield C. D., Mayhew S. G. Evidence that apo-reduced nicotinamide adenine dinucleotide dehydrogenase and apo-electron-transferring flavoprotein from Peptostreptococcus elsdenii are identical. J Biol Chem. 1974 May 10;249(9):2811–2815. [PubMed] [Google Scholar]
- Whitfield C. D., Mayhew S. G. Purification and properties of electron-transferring flavoprotein from Peptostreptococcus elsdenii. J Biol Chem. 1974 May 10;249(9):2801–2810. [PubMed] [Google Scholar]
- Widdel F., Pfennig N. A new anaerobic, sporing, acetate-oxidizing, sulfate-reducing bacterium, Desulfotomaculum (emend.) acetoxidans. Arch Microbiol. 1977 Feb 4;112(1):119–122. doi: 10.1007/BF00446665. [DOI] [PubMed] [Google Scholar]
- Williams R. J. Proton-driven phosphorylation reactions in mitochondrial and chloroplast membranes. FEBS Lett. 1975 May 1;53(2):123–125. doi: 10.1016/0014-5793(75)80001-9. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Stubbs M., Oshino N., Erecińska M. Thermodynamic relationships between the mitochondrial oxidation-reduction reactions and cellular ATP levels in ascites tumor cells and perfused rat liver. Biochemistry. 1974 Dec 17;13(26):5305–5311. doi: 10.1021/bi00723a008. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Stubbs M., Veech R. L., Erecińska M., Krebs H. A. Equilibrium relations between the oxidation-reduction reactions and the adenosine triphosphate synthesis in suspensions of isolated liver cells. Biochem J. 1974 Apr;140(1):57–64. doi: 10.1042/bj1400057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpenny J. W., Firth A. Levels of nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide in facultative bacteria and the effect of oxygen. J Bacteriol. 1972 Jul;111(1):24–32. doi: 10.1128/jb.111.1.24-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt H. T. Coupling of quanta, electrons, fields, ions and phosphrylation in the functional membrane of photosynthesis. Results by pulse spectroscopic methods. Q Rev Biophys. 1971 Nov;4(4):365–477. doi: 10.1017/s0033583500000834. [DOI] [PubMed] [Google Scholar]
- Wolfe R. S. Microbial formation of methane. Adv Microb Physiol. 1971;6:107–146. doi: 10.1016/s0065-2911(08)60068-5. [DOI] [PubMed] [Google Scholar]
- Wolin E. A., Wolfe R. S., Wolin M. J. Viologen dye inhibition of methane formation by Methanobacillus omelianskii. J Bacteriol. 1964 May;87(5):993–998. doi: 10.1128/jb.87.5.993-998.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M., Allam A. M., Brill W. J., Wolfe R. S. Formation of methane from serine by cell-free extracts of Methanobacillus omelianskii. J Biol Chem. 1965 Dec;240(12):4564–4569. [PubMed] [Google Scholar]
- Wood J. M., Kennedy F. S., Rosen C. G. Synthesis of methyl-mercury compounds by extracts of a methanogenic bacterium. Nature. 1968 Oct 12;220(5163):173–174. doi: 10.1038/220173a0. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Kennedy F. S., Wolfe R. S. The reaction of multihalogenated hydrocarbons with free and bound reduced vitamin B 12. Biochemistry. 1968 May;7(5):1707–1713. doi: 10.1021/bi00845a013. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Wolfe R. S. Components required for the formation of CH-4 from methylcobalamin by extracts of Methanobacillus omelianskii. J Bacteriol. 1966 Sep;92(3):696–700. doi: 10.1128/jb.92.3.696-700.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M., Wolfe R. S. Propylation and purification of a B12 enzyme involved in methane formation. Biochemistry. 1966 Nov;5(11):3598–3603. doi: 10.1021/bi00875a031. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Wolin M. J., Wolfe R. S. Formation of methane from methyl factor B and methyl factor 3 by cell-free extracts of Methanobacillus omelianskii. Biochemistry. 1966 Jul;5(7):2381–2384. doi: 10.1021/bi00871a030. [DOI] [PubMed] [Google Scholar]
- Wood N. P., Jungermann K. Inactivation of the pyruvate formate lyase of Clostridium butyricum. FEBS Lett. 1972 Oct 15;27(1):49–52. doi: 10.1016/0014-5793(72)80407-1. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T. IDENTITY OF PSEUDOMONAS CYTOCHROME OXIDASE WITH PSEUDOMONAS NITRITE REDUCTASE. Nature. 1964 Oct 17;204:253–255. doi: 10.1038/204253a0. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., OKUNUKI K. Crystalline Pseudomonas cytochrome oxidase. I. Enzymic properties with special reference to the biological specificity. Biochim Biophys Acta. 1963 Mar 12;67:379–393. doi: 10.1016/0006-3002(63)91844-4. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., OTA A., OKUNUKI K. Oxidative phosphorylation coupled with nitrate respiration. I. Evidence for phosphorylation coupled with nitrate reduciton in a cell-free extract of Pseudomonas aeruginosa. J Biochem. 1962 Apr;51:253–258. doi: 10.1093/oxfordjournals.jbchem.a127529. [DOI] [PubMed] [Google Scholar]
- Yagi T. Formate: cytochrome oxidoreductase of Desulfovibrio vulgaris. J Biochem. 1969 Oct;66(4):473–478. doi: 10.1093/oxfordjournals.jbchem.a129171. [DOI] [PubMed] [Google Scholar]
- Yagi T., Honya M., Tamiya N. Purification and properties of hydrogenases of different origins. Biochim Biophys Acta. 1968 Apr 2;153(3):699–705. doi: 10.1016/0005-2728(68)90197-7. [DOI] [PubMed] [Google Scholar]
- Yagi T., Maruyama K. Purification and properties of cytochrome c 3 of Desulfovibrio vulgaris, Miyazaki. Biochim Biophys Acta. 1971 Aug 27;243(2):214–224. doi: 10.1016/0005-2795(71)90078-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Tonomura Y. pH jump-induced phosphorylation of adenosine diphosphate in thylakoidal membranes. J Biochem. 1975 Jan 1;77(1?):137–146. [PubMed] [Google Scholar]
- Yong F. C., King T. E. Respiratory control and oxidative phosphorylation of the cytochrome c-cytochrome oxidase complex. Biochem Biophys Res Commun. 1972 Apr 28;47(2):380–386. doi: 10.1016/0006-291x(72)90724-3. [DOI] [PubMed] [Google Scholar]
- Zappia V., Barker H. A. Studies on lysine-2,3-aminomutase. Subunit structure and sulfhydryl groups. Biochim Biophys Acta. 1970 Jun 23;207(3):505–513. doi: 10.1016/s0005-2795(70)80013-7. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Bowen V. G. Comparative ultrastructure of methanogenic bacteria. Can J Microbiol. 1975 Feb;21(2):121–129. doi: 10.1139/m75-019. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Bowen V. G. Fine structure of Methanospirillum hungatii. J Bacteriol. 1975 Jan;121(1):373–380. doi: 10.1128/jb.121.1.373-380.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Fine structure of Methanobacterium thermoautotrophicum: effect of growth temperature on morphology and ultrastructure. J Bacteriol. 1973 Jan;113(1):461–467. doi: 10.1128/jb.113.1.461-467.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhilina T. N. Tonkoe stroenie metanosartsiny. Mikrobiologiia. 1971 Jul-Aug;40(4):674–680. [PubMed] [Google Scholar]
- de Vries W., Gerbrandy S. J., Stouthamer A. H. Carbohydrate metabolism in Bifidobacterium bifidum. Biochim Biophys Acta. 1967 Apr 25;136(3):415–425. doi: 10.1016/0304-4165(67)90001-3. [DOI] [PubMed] [Google Scholar]
- de Vries W., Kapteijn W. M., van der Beek E. G., Stouthamer A. H. Molar growth yields and fermentation balances of Lactobacillus casei L3 in batch cultures and in continuous cultures. J Gen Microbiol. 1970 Nov;63(3):333–345. doi: 10.1099/00221287-63-3-333. [DOI] [PubMed] [Google Scholar]
- de Vries W., van Wijck-Kapteyn W. M., Oosterhuis S. K. The presence and function of cytochromes in Selenomonas ruminantium, Anaerovibrio lipolytica and Veillonella alcalescens. J Gen Microbiol. 1974 Mar;81(1):69–78. doi: 10.1099/00221287-81-1-69. [DOI] [PubMed] [Google Scholar]
- de Vries W., van Wyck-Kapteyn W. M., Stouthamer A. H. Generation of ATP during cytochrome-linked anaerobic electron transport in propionic acid bacteria. J Gen Microbiol. 1973 May;76(1):31–41. doi: 10.1099/00221287-76-1-31. [DOI] [PubMed] [Google Scholar]
- van Riet J., van Ed J. H., Wever R., van Gelder B. F., Planta R. J. Characterization of the respiratory nitrate reductase of Klebsiella aerogenes as a molybdenum-containing iron-sulfur enzyme. Biochim Biophys Acta. 1975 Oct 20;405(2):306–317. doi: 10.1016/0005-2795(75)90096-3. [DOI] [PubMed] [Google Scholar]
- von Hugo H., Gottschalk G. Distribution of 1-phosphofructokinase and PEP:fructose phosphotransferase activity in Clostridia. FEBS Lett. 1974 Sep 15;46(1):106–108. doi: 10.1016/0014-5793(74)80345-5. [DOI] [PubMed] [Google Scholar]