Abstract
Mass spectrometry (MS) is known for highly specific and sensitive analysis. The general applicability of this technique makes it a good candidate for biological applications over a much broader range than is now the case. The limiting factors preventing MS from being applied at the biologist's bench or in a physician's office are identified as the large size of the systems, as well as the complicated analytical procedures required. An approach for developing miniature MS analysis systems with simplified operational procedures is described and the associated technical developments are discussed.
Mass spectrometry (MS) plays an irreplaceable role in biochemical analysis and clinical diagnosis. It has high specificity by providing the possible molecular formulas of chemical and biological compounds based on molecular weights measured by MS analysis, as well as chemical structures confirmed by the MS/MS process. MS can also provide high sensitivity (low detection and quantitation limits) in analysis, even with soft ionization methods where relatively low energies are involved in the ionization process for biological compounds; however, it is vulnerable to matrix effects, which suppress the sensitivity of some compounds due to a competition by others in a same mixture, which have much higher ionization efficiency. This problem has been effectively taken care of by careful analyte extraction from the raw samples and subsequent chromatographic separation of the extracted analytes prior to MS analysis. The corresponding instrumentation development has led to modern LC–MS systems [1] for chemical and biological analysis. With their high specificity and sensitivity, these systems are particularly powerful in analysis of unknown analytes in complex mixtures and have also been used for routine quantitation of target analytes.
In this article, we discuss the emerging miniature MS analysis systems as a divergent process from the continuous development that has led to the current LC–MS systems. We expect miniature MS systems to play a role in bioanalysis distinct from the laboratory systems; for instance, they will be used on the biologist's bench or in the physician's office. To achieve this goal, the analytical systems need to be adequately small to fit on the desktop and the operational procedures and maintenance need to be simple enough for users without analytical trainings. These small systems will need to take raw samples, such as blood, urine or tissue, and provide the analytical reports understandable to the end users, instead of MS spectra or chromatograms. The range of the applicability in terms of types of samples might well be very narrow for each individual system, in comparison with the current highly versatile LC–MS systems in analytical laboratories, but they will allow the users to get the needed information readily to assist the decision-making in disease diagnosis or therapy.
A distinctive feature of MS has been its versatility in general purpose chemical analysis. However, to develop miniature MS systems with highly autonomous functions we might have to select subsets of capabilities for MS-based analysis and put them into different specialized packages. It is hoped that the consequent ease of use for these systems would lead to a significantly widened range of applications for MS outside those possible in current analytical laboratories. For the actual design of systems for different target sample analyses, a general MS analysis instrument platform could possibly be used with only the scan function specified differently. However, the procedures for sample pretreatment and ionization for these new applications might have to change significantly. Efficient extraction of the biomarker compound from the complex sample matrix is still necessary to retain adequate sensitivity during the ionization. There is an ongoing incremental effort on shrinking the equipment and developing automated procedures based on current LC–MS systems. While this approach might eventually provide some solutions, an alternative approach based on the ambient ionization methods has certainly already been shown to be promising for direct MS analysis of biological samples.
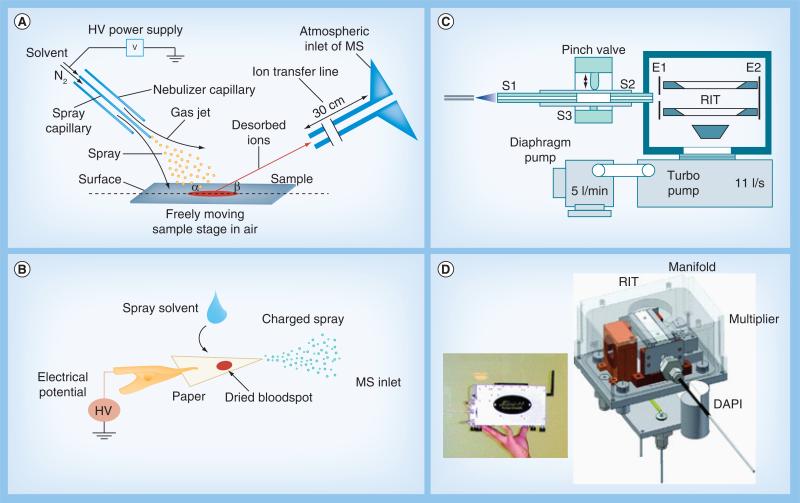
Ambient ionization methods are used for directly sampling and ionizing analytes from untreated samples. There have been more than 40 ambient ionization methods developed since 2004 [2–6]. Among several developed at Purdue University (IN, USA), desorption electrospray ionization (DESI; Figure 1A) [7] and paper spray (Figure 1B) [8] have been intensively applied for analysis of biological samples. In DESI, a primary beam of charged droplets is generated using conditions that resemble those in electrospray ionization (ESI); however, instead of purified analytes in the primary droplets being ionized as in ESI, the analytes are desorbed directly from the raw sample by the impinging solvent droplets and at the same time they are ionized for MS analysis. Biological samples such as tissue sections can be analyzed using DESI without any pretreatment or chromatographic separation. In paper spray, samples such as biofluids can be deposited on a triangle paper substrate to form a dried spot, which is easy for storage and transfer; when it is analyzed, a small amount of solvent, such as 10–20 μl methanol, is deposited on the paper and a high voltage of approximately 4 kV is applied to cause spray ionization at the tip of the wetted substrate. Quantitation with high precision has been achieved for analysis of the drug compounds in blood samples [9].
Figure 1. Examples of ambient ionization methods and miniature mass spectrometers.
(A) Desorption electrospray ionization (DESI) and (B) paper spray ionization, two of many ambient ionization methods. (C) Configuration of a miniature MS with a discontinuous atmospheric pressure interface and a miniature pumping system. (D) Mini 11 handheld MS that can be used for analysis of biological compounds from condensed phase samples.
DAPI: Discontinuous atmospheric pressure interface; HV: High voltage; RIT: Rectilinear ion trap.
(A, B, C & D) are reproduced with permission from [7,8,12,13], respectively.
The development of the ambient ionization methods has largely been performed with commercial MS instruments on the laboratory scale. Significant performance improvement has been demonstrated for direct analysis of complex mixtures. It becomes increasingly convincing that these methods can be successfully transferred for the use with miniature mass spectrometers as an approach for developing complete analysis systems with simple operations [10,11]. In the practical implementation of this approach, analyte ions generated by ambient ionization of biological samples need to be transferred to the mass analyzer operating in vacuum for mass analysis. This requires an atmospheric pressure interface for the miniaturized MS in the analysis system, which is quite difficult to implement since large pumping systems are typically required for supporting the atmospheric pressure interface with a continuous gas flow. This challenge was addressed in our previous research by using a discontinuous atmospheric pressure interface (DAPI; Figure 1C) [12], which was demonstrated with the handheld Mini 10 (10 kg) [12] and Mini 11 (4 kg; Figure 1D) [13] mass spectrometers. The DAPI opens periodically and the ions are introduced in a pulsed fashion to avoid a large continuous gas flow that cannot be handled by the small pumping system. The effectiveness of DAPI was demonstrated with traditional ESI and atmospheric pressure chemical ionization, as well as ambient ionization methods including DESI and low temperature plasma probes. The same instrument architecture has been used in the development of the MS portion for the Mini 12 system [14], which was developed as a first complete miniature MS analysis system for bioanalysis based on an approach that combines ambient ionization with a miniature MS.
Many achievements have been made by researchers in the areas of ambient ionization [2–6] and MS miniaturization [10]. In this article, we use some of the work we have carried out in the recent past as examples to support the arguments we make for the future development of self-sustained miniature MS systems.
Highly specific analysis with extremely simplified operating procedures
Many applications have been demonstrated using ambient ionization with their potential being realized by the specific molecular information now readily available through direct MS analysis. For instance, a panel of acylcarnitines can be quantitatively profiled by paper spray mass analysis of a single dried blood spot [15]. Tissue analysis, that used to require labor-intense procedures for sample extraction and purification, can now be performed with a single step MS analysis using one of several ambient ionization methods [16–19]. Tissue imaging actually requires sample treatment to be minimized such that the original distribution of the chemical and biological compounds in the tissue samples can be preserved [20]. Using DESI, a tissue section can be directly analyzed by scanning a primary beam of charged droplets across its surfaces. Mass spectra can be recorded for each pixel at a resolution of 200 μm or better [21]. Highly specific molecular information becomes readily available to assist biological studies and clinical diagnosis. DESI imaging has been used to characterize drug delivery throughout the body of a mouse [22]. The distributions of the phospholipids and fatty acids can also be readily obtained and used as potential biomarkers to distinguish normal and diseased tissue sections [16].
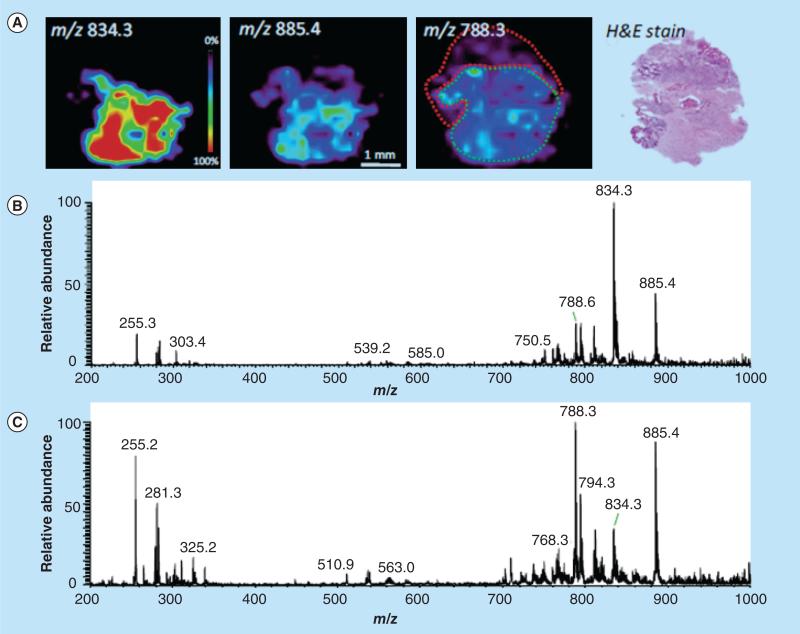
A series of preliminary studies on small sample sets of human liver [23], bladder [24], kidney [25], prostate [26], testicular [27] and brain [28,29] cancers performed using DESI-MS imaging shows correlations of lipid distributions with pathology (Figure 2) [30]. In these examples, diagnostic conclusions were not based on unique bio-markers; instead, they are based upon the up- or down-regulation of these lipid species observed when comparing normal- and disease-state samples, which can only be provided by MS analysis. Many of the same lipid species are present in multiple types of normal tissues, while the aberrant metabolism [31] that occurs during malignancy is unique to each type of cancer, resulting in distinctive lipid profiles that can be easily recognized by DESI-MS and definitively associated with a specific disease [32]. Multivariate statistical analysis was used to visualize the disease state of the tissue and create a diagnostic rule for characterizing the tissue sections. DESI-MS ion images correlated well with the statistically generated images, which in turn correlated with the pathological diagnosis. This approach was successful for human bladder cancer [24]. Particular glycerophospholipids, fatty acids and their dimers were observed at increased intensities in cancerous tissue in the negative ion mode, and they function as putative molecular markers of disease. Cancerous and adjacent normal tissues from two types of cancer papillary renal cell carcinoma and clear cell renal cell carcinoma were distinguished from adjacent normal tissue [25]. Human seminoma tissue and adjacent normal tissues were also analyzed using DESI-MS imaging [27]. Seminolipids were found exclusively in normal tubule tissue, allowing for the identification of this tissue type. DESI-MS imaging has also been performed on human glioma samples, including the subtypes oligodendroglioma, astrocytoma and oligoastrocytoma, of different histologic grades and of varied tumor cell concentration [29]. The lipidomic data obtained with DESI imaging potentially could be used for classification for subtype, grade and other features of the cancers, including features that provide prognostic information.
Figure 2. Desorption electrospray ionization-MS imaging of surgical sample, astrocytoma grade IV, showing regions of heterogeneity as recognized by histopathological evaluation.
(A) Desorption ESI (DESI)-MS ion images showing the distribution of m/z 834.3, m/z 885.4 and m/z 788.3, and optical image of H&E-stained tissue section. DESI-MS spectra of (B) low tumor cell concentration region infiltrating into gray matter (delineated in green in the same ion image) and (C) dense tumor region (delineated in red in the m/z 788.3 ion image), with high tumor cell concentration.
H&E: Hematoxylin and eosin.
Reproduced with permission from [30].
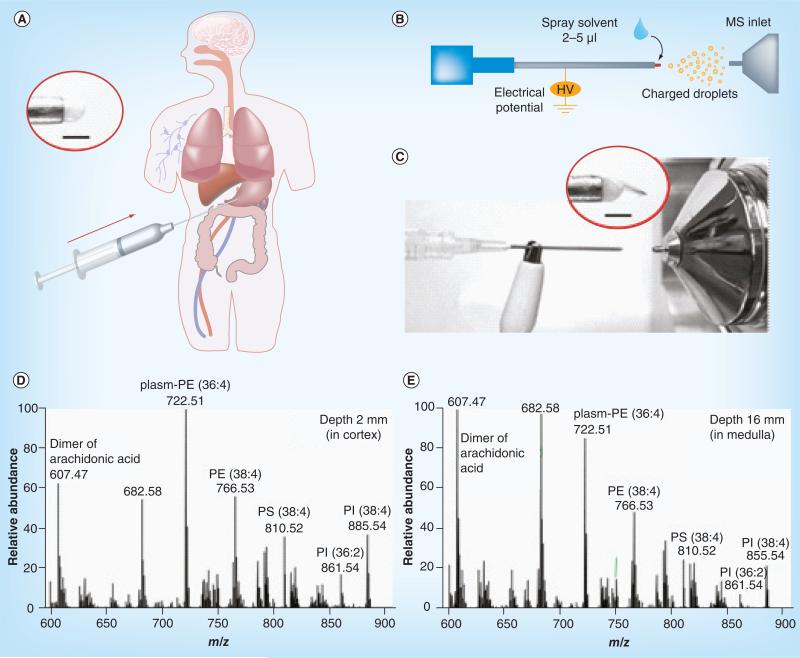
With the otherwise complex sampling and ionization process simplified into a simple, one-step ambient ionization process, the direct analysis of the raw samples can potentially be integrated into existing medical procedures with great flexibility. For example, this has been demonstrated in a previous study by direct analysis of the tissue samples taken by needle biopsy [18]. A small amount of tissue sample was taken from a block of tissue using an aspiration needle (Figure 3A); 2–5 μl methanol was then applied to the tissue sample at the needle tip and a high voltage was applied to produce spray ionization (Figure 3B & C). This method was applied for analysis of mouse spinal cord tissue, depth profiling of porcine kidney, and differentiation of normal and cancerous human kidney tissue. In another study, the conditions for DESI were modified for biosafety appropriate to in vivo sampling ionization (i.e., no high voltage or organic solvent was involved in the desorption ionization process); the analytes sampled from the tissue by the primary droplets were transferred in a 1/16” inner diameter tubing of 4 m length and mass analyzed in real time to produce lipidomic profiles [33]. This method potentially can be used to develop an in vivo MS analysis probe that can be used with laparoscopic or endoscopic procedures to examine the tissue. The local microorganism environment in the human body, which can be enterotoxigentic and subsequently cancerogenic [34], can also be characterized in vivo.
Figure 3. Direct analysis of tissue samples using tissue spray.
(A) Direct spray ionization from a biopsy needle for biological tissue analysis. The inset shows biological tissue held by the needle without added spray solvent. (B) Schematic and (C) photo of direct spray ionization with needle biopsy on tissue. Inset shows Taylor cone formed at the tissue after applying high voltage and spray solvent. Scale bar is 1 mm. MS spectra of tissue samples at (D) 2 mm and (E) 16 mm from a porcine kidney surface, recorded using an Exactive Orbitrap.
HV: High voltage.
Adapted with permission from [18].
Adequate sensitivity without extensive purification
What ambient ionization methods provide for mixture analysis is real time, fast extraction of the analytes from complex mixtures and their ionization simultaneously or immediately afterwards. The conditions of the extraction or desorption do need to be optimized for the target analytes [35,36], which allows excellent LOD to be achieved for targeted analysis. Some examples include the detection of chemical warfare agents at low ppb levels using direct analysis in real time [37], 0.62 pg/ml nicotine in gas phase samples using extractive ESI [38], 100 fmol of peptides using electrospray-assisted laser desorption ionization [39], and 0.2–40 ng drug molecules in plasma using DESI [40]. Using the extraction nano-ESI method developed recently, LODs as low as 0.1 ng/ml were obtained for therapeutic or illicit drug compounds in blood, with an amount of sample consumed as low as 0.2 μl [41]. If these kinds of sensitivities along with the low amounts of sample required can be transferred into analysis for clinical diagnosis, it means mandatory sensitivities might be achieved using minimally invasive methods for sampling, such as a finger prick instead of a venous blood draw. Preclinical studies could also benefit from these methods by greatly reducing the number of small animals being sacrificed. Complete profiling of drug metabolites for individual animal also becomes possible.
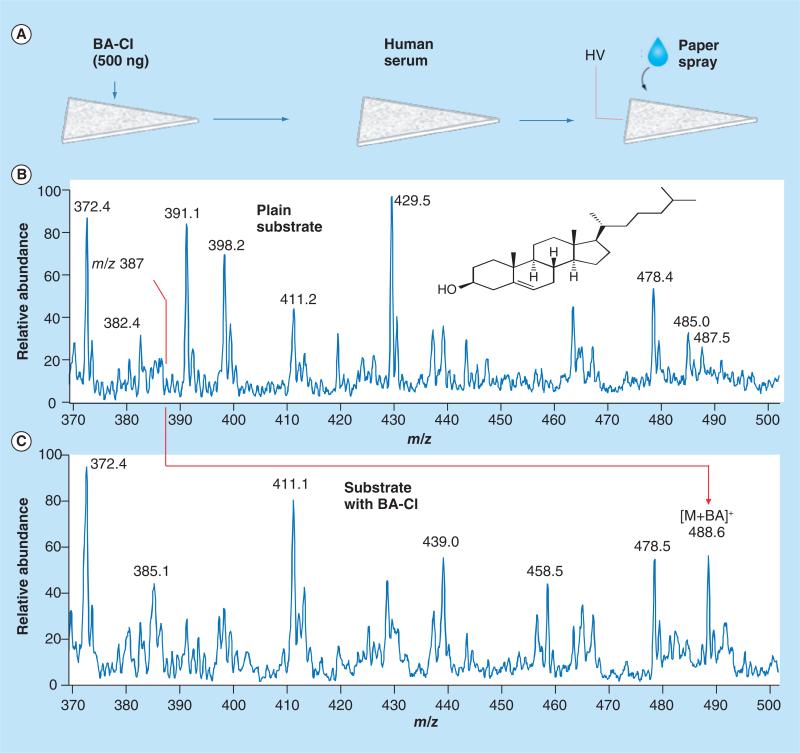
In addition to optimization of analyte extraction, real-time chemical reactions can also be incorporated in ambient ionization to significantly improve the sensitiv ity, as well as the selectivity of the direct analysis. In the analysis of anabolic steroids in urine samples [42], reactive DESI was implemented by adding hydroxylamine to the spray solvent. The protonated hydroxylamine reacted with the carbonyl group on neutral steroids in the dried urine sample, which produced ionized, derivatized steroids. A 10–100-times improvement was obtained in LODs for analysis of the steroids [42]. An on-paper reaction was also implemented for direct analysis of cholesterol in serum using paper spray. Owing to the relative low efficiency of spray ionization, it is difficult to observe cholesterol directly when analyzing the dried serum spots on paper using paper spray MS. To enable an on-paper reaction, betaine aldehyde chloride was coated on the paper substrate before the serum sample was added to form the dried spot (Figure 4). The consequent derivatization of cholesterol with the betaine aldehyde produces a product compound with a much higher ionization efficiency during paper spray process and hereby a much improved sensitivity in direct analysis [43].
Figure 4. Improvement of the sensitivity using on-paper chemical derivatization.
(A) Paper spray MS analysis of cholesterol in human serum with (B) blank paper and (C) paper preloaded with betaine aldehyde chloride (5 μl, 100 μg/ml). A total of 2 μl of human serum spotted onto the paper and 10 μl of ACN/CHCl3 (1:1, v:v) utilized as solvent for paper spray.
BA: Betaine aldehyde; HV: High voltage; M: Molecular.
Adapted with permission from [43].
Quantitation: can it be done with simple procedures?
A significant advantage of using MS for biological analysis is that highly precise quantitation can be achieved at relatively low concentrations. For regulatory applications in clinical diagnosis, the same mandatory quantitative criteria, which are met by the current in-laboratory instrumental systems and analytical procedures, will apply to miniature systems using ambient ionization for direct analysis. Semi-quantitative analysis might be achieved using ambient ionization without internal standards (IS) and could be further improved; however, high precision quantitation might be performed much more easily if IS were used. This has been demonstrated extensively with paper spray [9,44]. Table 1 lists some examples, where adequate LOQs, dynamic concentration ranges and quantitation precisions (RSDs) have been achieved for the corresponding targeted applications. This kind of performance was obtained with direct analysis by paper spray MS with IS mixed into the samples prior to MS analysis.
Table 1.
Quantitation by paper spray MS.
| Analyte/sample | LOQ (ng/ml) | Linear range (ng/ml) | RSD (%) | Ref. |
|---|---|---|---|---|
| Imatinib/blood | 10 | 10-4000 | <5 | [45] |
| Sitamaquine/blood | 5 | 5-1000 | <10 | [9] |
| Amitriptyline/blood | 0.9 | 0.9-443 | <10 | [9] |
| Hydralazine/tissue | 16 | 16-2000 | <20 | [17] |
| Cocaine/blood | 0.5 | 0.5-1000 | <12 | [44] |
| Benzoylecgonine/blood | 1 | 1-800 | <12 | [44] |
| Methamphetamine/blood | 5 | 5-500 | <12 | [44] |
| Nicotine/blood | 1 | 1-100 | <15 | [46] |
| Cotinine/blood | 3 | 3-200 | <15 | [46] |
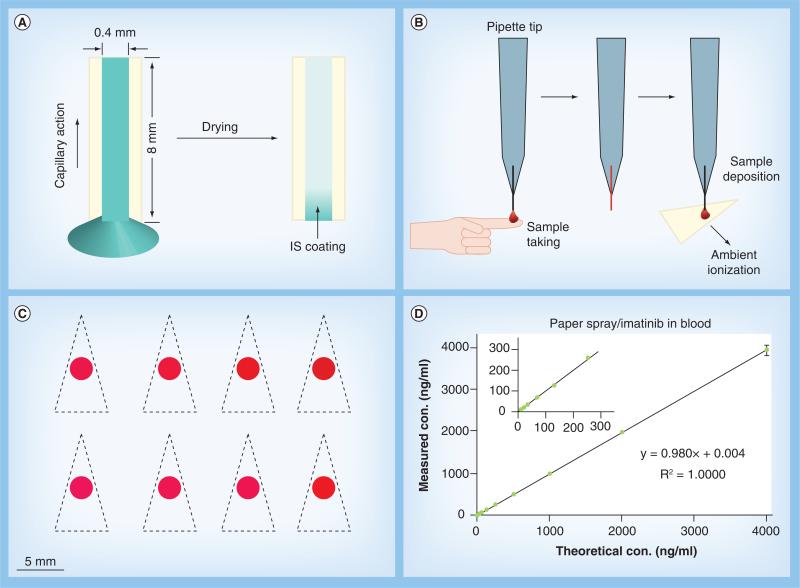
A critical issue for incorporating the IS into direct sampling ionization is whether this could be done without requiring laboratory techniques such as pipetting, since the miniature MS systems need to be used by personnel without experience in analytical chemistry. Paper spray ionization only requires a tiny amount of sample (~1 μl) to conduct quantitative analysis. It is also practically impossible to spike IS into such a small amount of blood using laboratory methods. Ordinary pipetting would introduce a volume error as high as 10% when collecting microliter blood from a finger prick, a method that would not be allowed for clinical testing anyway. Adoption of traditional laboratory methods for sample collection and IS spiking for miniature MS systems might adversely affect the accuracy and precision in the results. As one attempt in a series of efforts to develop simple procedures, a capillary sampler with IS coating was used to take samples for direct analysis [45]. The capillary sampler was fabricated by cutting a glass tube of 0.4 mm diameter into small sections of approxiamtely 8 mm long (1 μl volume). The inner surface of the capillary was precoated with IS by filling an IS solution through capillary action and letting it dry (Figure 5A). When collecting a sample, such as blood by finger prick and capillary action, the IS is dissolved and mixed into the sample automatically (Figure 5B). The collected sample is then transferred onto a paper substrate (Figure 5C) and subsequently analyzed using multireaction monitoring for quantitative analysis. The mixing of the IS into the sample was found to be efficient and reproducible. Highly precise quantitation with RSD better than 5% has been achieved using the IS-coated capillary sampler with three ambient ionization methods (Figure 5D), namely paper spray, DESI and low temperature plasma probe [45].
Figure 5. Incorporation of internal standards in direct analysis of blood samples.
(A) Coating internal standard inside a capillary sampler (0.4 mm inner diameter, ~8 mm long). The internal standard solution is sucked into the capillary via capillary action and allowed to dry. (B) Collecting finger prick blood sample using a capillary sampler with a pipette tip holder. (C) Photograph of eight dried blood spots prepared on chromatography paper for paper spray ionization measurement. Paper triangles are cut out along the dashed lines manually using scissors. (D) Quantitative analysis of imatinib in blood using capillary sampler for dried blood spot preparation and paper spray ionization for detection.
con.: Concentration.
Adapted with permission from [45].
Miniature MS analytical systems: beyond small mass analyzers & miniature mass spectrometers
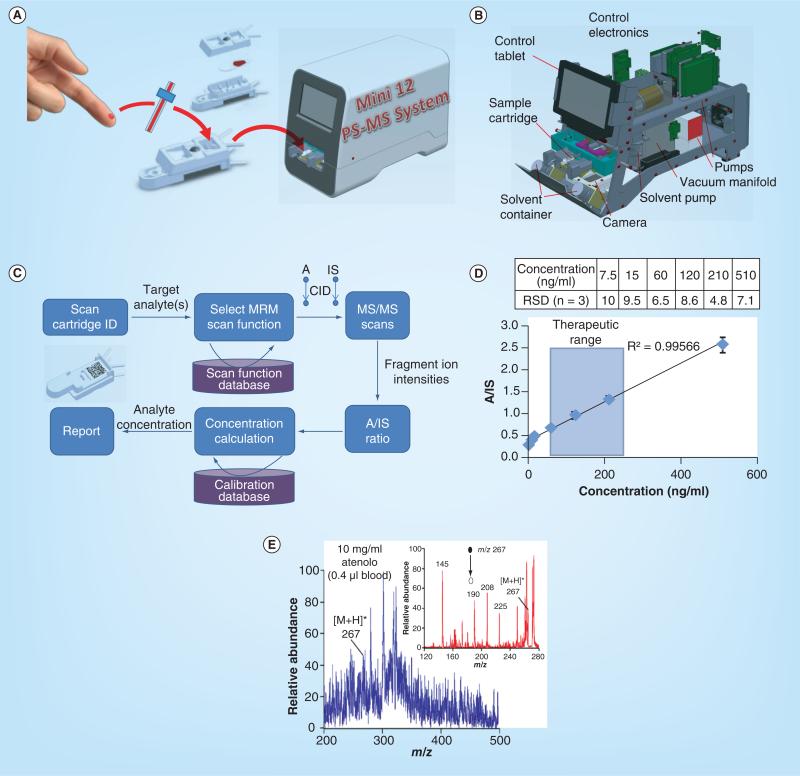
The effort on miniaturization of MS systems dates back two decades. Much of the previous effort was focused on the miniaturization of the mass analyzers and later, the packaging of small mass spectrometers [10]. As discussed above, miniaturization of the mass spectrometer itself is not adequate for enabling chemical analysis outside the analytical laboratory. A complete system needs to be self-sustained and have the capability of processing the raw samples and using the spectral data to produce a report on site. As an exploration with the approach of combining ambient ionization with miniature mass spectrometers, we recently developed two systems, a Mini 12 MS system for biological and point-of-care (POC) analysis [14] and a backpack MS system for in-field chemical detection [47]. Both systems use a rectilinear ion trap [48] as a mass analyzer and a DAPI for coupling with ambient ionization sources, but they have different means for sampling ionization, with paper spray used for the Mini 12 (Figure 6A & B) and a low temperature plasma probe for the backpack MS. In comparison with in-field systems, the miniature MS systems for POC analysis might be more tolerant in terms of size and power consumption, similar to requirements for a desktop computer, but subject to much more restrictive requirements for quantitation and standardized simple procedures.
Figure 6. Direct analysis of biological samples using miniature MS systems.
(A) Blood analysis using the Mini 12 MS system with paper spray sample cartridges. (B) Configuration of Mini 12 system. (C) Procedure for autonomous analysis. (D) Quantitation of amitriptyline in bovine blood using amitriptyline-d6 as internal standard. (E) MS and MS/MS analysis of 10 μg/ml atenolol in 0.4 μl bovine blood using paper spray and Mini 10 handheld MS.
A: Analyte; IS: Internal standard.
(A–D) are adapted with permission from [14]; (E) reproduced with permission from [8].
The Mini 12 of approximately 25 kg was designed for nurses or physicians to perform a simple procedure (Figure 6C) to obtain a report based on a quantitative MS analysis. The finger prick blood sample can be taken using an IS-coated capillary and transferred to a sample cartridge containing a paper substrate. Once the cartridge is pushed into the Mini 12 system, an analytical procedure is triggered and executed automatically. A bar code on the cartridge is read through a camera (Figure 6B), the required target analysis procedure is identified and the corresponding scan function is automatically loaded (Figure 6C). The solvent pump deposits the spray solvent onto the cartridge and a high voltage is added to generate the paper spray. MS/MS scans are performed for the target analyte and its IS, respectively, and the measured intensities are used to calculate the analyte concentration based on a calibration curve presaved in the system. The entire process requires no intervention by the operator, or any spectral interpretation or data analysis. With the improved stability in ion current using extraction nano-ESI, quantitation of amitriptyline in blood samples with an LOQ of 7.5 ng/ml and RSD better than 10% was achieved, which is very promising in terms of meeting US FDA requirements for therapeutic drug monitoring [49].
The Mini 12 was developed as a POC prototype in the exploration of the concept of combining ambient ionization with miniature mass spectrometer for development of a self-sustained analysis system. Its potential for POC applications has been demonstrated, while it is also being tested for some critical technical challenges. The ion transfer from ambient ionization source in air into the mass analyzer located in a vacuum is essential for the approach but high efficiency in transfer is difficult to achieve with the vacuum sustained by a small pumping system with a low capacity. The DAPI (Figure 1D) has been shown to be effective, but some potential negative impacts on quantitation due to the pressure fluctuations remain to be tested. The transfer of ambient ionization methods from commercial laboratory scale MS to miniature MS systems is not straightforward. Further optimization or even major revision might be required. The performance is highly dependent on the atmospheric pressure interface [41]. Further study on the electro–hydrodynamic effects [50] on the transfer of charged droplets and formation of dried ions would significantly help the development of the interface and the overall analysis efficiency.
Finally, MS/MS capabilities are essential for implementing the strategy discussed here for developing miniature MS systems. As for laboratory scale systems, the MS/MS capability is necessary for the differentiation of the target analyte from its isomers and isobars. However, MS/MS is also particularly important for achieving adequate sensitivity for the MS analysis with ambient ionization, which is the key to the procedural simplification and instrument miniaturization, but does lead to significantly more chemical noise in the MS spectra. As shown by the comparison in Figure 6E for analysis of the atenolol in blood with paper spray, MS/MS analysis can significantly improve the S/N and thereby the sensitivity and the quantitation accuracy. The justified importance of the MS/MS also leads to an implication for candidates for mass analyzers in small analysis systems. Linear ion traps are certainly one type of good candidates due to their MS/MS capability, large trapping capacities, as well as tolerance to relatively high operation pressures [10].
Future perspective
As a versatile analytical technique, MS has advanced in instrument development with increasingly comprehensive capability, which adversely prevents its use outside analytical laboratories and by users without training in proper operation of the complicated analytical systems. Miniaturization of the MS analytical system, more importantly with the simplification of the operation procedures, is a path forward for exploring the opportunity of applying MS by novices at the locations convenient for the tests. The development of self-sustained, integrated package, beyond just the mass spectrometer itself, is the critical step for delivering valid solutions for bioanalysis.
Ambient ionization has been shown to be a valid approach to bypass the conventional procedures for sample preparation and purification, which has been essential for applying MS for analysis of complex biological samples. The future development in this field can take advantage of the techniques previously developed in sample extraction and chromatographic separation. Fast real-time extraction and rough separation of the target compounds from the sample matrices in time scale of seconds will be a primary goal for the development. High precision quantitation will be necessary and the use of IS has been demonstrated to be of a significant advantage. The challenge will be the implementation with consumable cartridges and without requiring training with analytical laboratory techniques.
The Mini 12 system using an ion trap is presented here as an example, of small size and weight, but also with the necessary interface for ambient ionization source and MS/MS capability important for analysis of complex mixtures. However, in contrast to the miniature instruments used for in-field applications, MS systems of a wide range of miniaturized sizes and reduced power are foreseen to be feasible for bioanalysis in biologists’ laboratories or physicians’ offices. The development of pumping technologies will also facilitate the delivery of very quiet systems in operation.
Simplified user interface with autonomous testing can be enabled with specialized bioanalysis applications. A variety of different MS system packages with the same mass spectrometer core but different sample cartridges and specialized user interface might well be the model for developing the commercial products. In this way, a universal technique can be used in a simple fashion by different users for their specialized needs.
Key terms.
Ambient ionization: Integrated process for sampling and ionizing the analytes directly from samples in their ambient states.
Discontinuous atmospheric pressure interface: An atmospheric pressure interface allows the ions to be transferred from air into the vacuum manifold for mass analysis. It is typically constantly open and supported by differential pumping system. A discontinuous atmospheric pressure interface is closed for most of the time except for a short opening of approximately 10–20 ms to introduce ions in a pulsed fashion. It is suitable for miniature MS with compact pumping system of low power.
Key term.
Rectilinear ion trap: 2D or linear ion trap with simplified geometry. It is composed with four flat radio frequency electrodes, in comparison with hyperbolic or round electrodes used in commercial MS.
Executive summary.
General strategy for developing miniature MS systems
Simple operation of the small MS systems is important for performing bioanalysis outside the traditional analytical laboratories.
Combination of ambient ionization and miniature mass spectrometers is a valid approach for the future development of small analytical systems with simple operation procedures.
Analytical performance
High sensitivity and high specificity can be obtained with ambient ionization without sample preparation.
High precision quantitation can also be achieved without requiring laboratory techniques.
Challenges & solutions for technical development
Interface between the ambient ionization source and miniature mass spectrometer is the key for the described approach and discontinuous atmospheric pressure interface has been demonstrated to be effective.
Tandem MS is important for improvement of S/N ratio and retain the high specificity of the analysis.
Acknowledgments
The work reported was supported by National Science Foundation (Grant CHE 0847205 and CHE1307264) and the NIH (1R01GM106016–01, 8R21GM103454, 1R21EB015722 and 1R21EB009459).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Ackermann BL, Berna MJ, Eckstein JA, Ott LW, Chaudhary AK. Current applications of liquid chromatography/mass spectrometry in pharmaceutical discovery after a decade of innovation. Annu. Rev. Anal. Chem. 2008;1(1):357–396. doi: 10.1146/annurev.anchem.1.031207.112855. [DOI] [PubMed] [Google Scholar]
- 2•.Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Ambient mass spectrometry. Science. 2006;311(5767):1566–1570. doi: 10.1126/science.1119426. [A comprehensive introduction of the concept for ambient ionization.] [DOI] [PubMed] [Google Scholar]
- 3.Ouyang Z, Zhang XR. Ambient mass spectrometry. Analyst. 2010;135(4):659–660. doi: 10.1039/c003812c. [DOI] [PubMed] [Google Scholar]
- 4.Huang M-Z, Yuan C-H, Cheng S-C, Cho Y-T, Shiea J. Ambient ionization mass spectrometry. Annu. Rev. Anal. Chem. 2010;3(1):43–65. doi: 10.1146/annurev.anchem.111808.073702. [DOI] [PubMed] [Google Scholar]
- 5.Nemes P, Vertes A. Ambient mass spectrometry for in vivo local analysis and in situ molecular tissue imaging. Trends Anal. Chem. 2012;34:22–34. [Google Scholar]
- 6•.Monge ME, Harris GA, Dwivedi P, Fernandez FM. Mass spectrometry. Recent advances in direct open air surface sampling/ionization. Chem. Rev. 2013;113(4):2269–2308. doi: 10.1021/cr300309q. [The most recent review on ambient ionization.] [DOI] [PubMed] [Google Scholar]
- 7.Takats Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306(5695):471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 8•.Wang H, Liu J, Cooks RG, Ouyang Z. Paper spray for direct analysis of complex mixtures using mass spectrometry. Angew. Chem. Int. Ed. 2010;49(5):877–880. doi: 10.1002/anie.200906314. [Introduction of the paper spray, which has been used for design of consumable sample cartridges.] [DOI] [PubMed] [Google Scholar]
- 9.Manicke NE, Abu-Rabie P, Spooner N, Ouyang Z, Cooks RG. Quantitative analysis of therapeutic drugs in dried blood spot samples by paper spray mass spectrometry: an avenue to therapeutic drug monitoring. J. Am. Soc. Mass Spectrom. 2011;22(9):1501–1507. doi: 10.1007/s13361-011-0177-x. [DOI] [PubMed] [Google Scholar]
- 10•.Ouyang Z, Cooks RG. Miniature mass spectrometers. Annu. Rev. Anal. Chem. 2009;2:187–214. doi: 10.1146/annurev-anchem-060908-155229. [A comprehensive review on miniature MS and the introduction of the concept for small analytical systems with simple operation procedures.] [DOI] [PubMed] [Google Scholar]
- 11.Ouyang Z, Noll RJ, Cooks RG. Handheld miniature ion trap mass spectrometers. Anal. Chem. 2009;81(7):2421–2425. doi: 10.1021/ac900292w. [DOI] [PubMed] [Google Scholar]
- 12.Gao L, Cooks RG, Ouyang Z. Breaking the pumping speed barrier in mass spectrometry: discontinuous atmospheric pressure interface. Anal. Chem. 2008;80(11):4026–4032. doi: 10.1021/ac800014v. [DOI] [PubMed] [Google Scholar]
- 13.Gao L, Sugiarto A, Harper JD, Cooks RG, Ouyang Z. Design and characterization of a multisource hand-held tandem mass spectrometer. Anal. Chem. 2008;80(19):7198–7205. doi: 10.1021/ac801275x. [DOI] [PubMed] [Google Scholar]
- 14•.Li L, Chen T-C, Ren Y, Hendricks PI, Cooks RG, Ouyang Z. Mini 12, miniature mass spectrometer for clinical and other applications – introduction and characterization. Anal. Chem. 2014;86(6):2909–2916. doi: 10.1021/ac403766c. [Introduction of Mini 12, the first complete miniature MS system designed for point-of-care applications.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Q, Manicke NE, Wang H, Petucci C, Cooks RG, Ouyang Z. Direct and quantitative analysis of underivatized acylcarnitines in serum and whole blood using paper spray mass spectrometry. Anal. Bioanal. Chem. 2012;404(5):1389–1397. doi: 10.1007/s00216-012-6211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiseman JM, Ifa DR, Song QY, Cooks RG. Tissue imaging at atmospheric pressure using desorption electrospray ionization (DESI) mass spectrometry. Angew. Chem. Int. Ed. 2006;45(43):7188–7192. doi: 10.1002/anie.200602449. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Manicke NE, Yang Q, et al. Direct analysis of biological tissue by paper spray mass spectrometry. Anal. Chem. 2011;83(4):1197–1201. doi: 10.1021/ac103150a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Cooks RG, Ouyang Z. Biological tissue diagnostics using needle biopsy and spray ionization mass spectrometry. Anal. Chem. 2011;83(24):9221–9225. doi: 10.1021/ac202626f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemes P, Woods AS, Vertes A. Simultaneous imaging of small metabolites and lipids in rat brain tissues at atmospheric pressure by laser ablation electrospray ionization mass spectrometry. Anal. Chem. 2010;82(3):982–988. doi: 10.1021/ac902245p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu JJ, Ouyang Z. Mass spectrometry imaging for biomedical applications. Anal. Bioanal. Chem. 2013;405(17):5645–5653. doi: 10.1007/s00216-013-6916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ifa DR, Wiseman JM, Song Q, Cooks RG. Development of capabilities for imaging mass spectrometry under ambient conditions with desorption electrospray ionization (DESI). Int. J. Mass Spectrom. 2007;259(1–3):8–15. [Google Scholar]
- 22.Kertesz V, Van Berkel GJ, Vavrek M, Koeplinger KA, Schneider BB, Covey TR. Comparison of drug distribution images from whole-body thin tissue sections obtained using desorption electrospray ionization tandem mass spectrometry and autoradiography. Anal. Chem. 2008;80(13):5168–5177. doi: 10.1021/ac800546a. [DOI] [PubMed] [Google Scholar]
- 23.Wiseman JM, Puolitaival SM, Takats Z, Cooks RG, Caprioli RM. Mass spectrometric profiling of intact biological tissue by using desorption electrospray ionization. Angew. Chem. Int. Ed. 2005;44(43):7094–7097. doi: 10.1002/anie.200502362. [DOI] [PubMed] [Google Scholar]
- 24.Dill AL, Eberlin LS, Costa AB, et al. Multivariate statistical identification of human bladder carcinomas using ambient ionization imaging mass spectrometry. Chem. Eur. J. 2011;17(10):2897–2902. doi: 10.1002/chem.201001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dill AL, Eberlin LS, Zheng C, et al. Multivariate statistical differentiation of renal cell carcinomas based on lipidomic analysis by ambient ionization imaging mass spectrometry. Anal. Bioanal. Chem. 2010;398(7–8):2969–2978. doi: 10.1007/s00216-010-4259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberlin LS, Dill AL, Costa AB, et al. Cholesterol sulfate imaging in human prostate cancer tissue by desorption electrospray ionization mass spectrometry. Anal. Chem. 2010;82(9):3430–3434. doi: 10.1021/ac9029482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masterson TA, Dill AL, Eberlin LS, et al. Distinctive glycerophospholipid profiles of human seminoma and adjacent normal tissues by desorption electrospray ionization imaging mass spectrometry. J. Am. Soc. Mass Spectrom. 2011;22(8):1326–1333. doi: 10.1007/s13361-011-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eberlin LS, Dill AL, Golby AJ, et al. Discrimination of human astrocytoma subtypes by lipid analysis using desorption electrospray ionization imaging mass spectrometry. Angew. Chem. Int. Ed. 2010;49(34):5953–5956. doi: 10.1002/anie.201001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eberlin LS, Norton I, Dill AL, et al. Classifying human brain tumors by lipid imaging with mass spectrometry. Cancer Res. 2012;72(3):645–654. doi: 10.1158/0008-5472.CAN-11-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eberlin LS, Norton I, Orringer D, et al. Ambient mass spectrometry for the intraoperative molecular diagnosis of human brain tumors. Proc. Natl. Acad. Sci. USA. 2013;110(5):1611–1616. doi: 10.1073/pnas.1215687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prien JM, Huysentruyt LC, Ashline DJ, Lapadula AJ, Seyfried TN, Reinhold VN. Differentiating N-linked glycan structural isomers in metastatic and nonmetastatic tumor cells using sequential mass spectrometry. Glycobiology. 2008;18(5):353–366. doi: 10.1093/glycob/cwn010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooks RG, Manicke NE, Dill AL, et al. New ionization methods and miniature mass spectrometers for biomedicine: DESI imaging for cancer diagnostics and paper spray ionization for therapeutic drug monitoring. Faraday Discuss. 2011;149:247–267. doi: 10.1039/c005327a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C-H, Lin Z, Garimella S, et al. Development of a mass spectrometry sampling probe for chemical analysis in surgical and endoscopic procedures. Anal. Chem. 2013;85(24):11843–11850. doi: 10.1021/ac4025279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dejea C, Wick E, Sears CL. Bacterial oncogenesis in the colon. Future Microbiol. 2013;8(4):445–460. doi: 10.2217/fmb.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Xu W, Manicke NE, Cooks RG, Ouyang Z. Silica coated paper substrate for paper-spray analysis of therapeutic drugs in dried blood spots. Anal. Chem. 2012;84(2):931–938. doi: 10.1021/ac202058w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren Y, Wang H, Liu J, Zhang Z, McLuckey M, Ouyang Z. Analysis of biological samples using paper spray mass spectrometry: an investigation of impacts by the substrates, solvents and elution methods. Chromatographia. 2013;76(19–20):1339–1346. doi: 10.1007/s10337-013-2458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nilles JM, Connell TR, Durst HD. Quantitation of chemical warfare agents using the direct analysis in real time (DART) technique. Anal. Chem. 2009;81(16):6744–6749. doi: 10.1021/ac900682f. [DOI] [PubMed] [Google Scholar]
- 38.Berchtold C, Meier L, Zenobi R. Evaluation of extractive electrospray ionization and atmospheric pressure chemical ionization for the detection of narcotics in breath. Int. J. Mass Spectrom. 2011;299(2–3):145–150. [Google Scholar]
- 39.Peng IX, Loo RRO, Margalith E, Little MW, Loo JA. Electrospray-assisted laser desorption ionization mass spectrometry (ELDI-MS) with an infrared laser for characterizing peptides and proteins. Analyst. 2010;135(4):767–772. doi: 10.1039/b923303b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy JH, Wiseman JM. Evaluation and performance of desorption electrospray ionization using a triple quadrupole mass spectrometer for quantitation of pharmaceuticals in plasma. Rapid Commun. Mass Spectrom. 2010;24(3):309–314. doi: 10.1002/rcm.4390. [DOI] [PubMed] [Google Scholar]
- 41.Ren Y, Liu J, Li L, McLuckey MN, Ouyang Z. Direct mass spectrometry analysis of untreated samples of ultralow amounts using extraction nano-electrospray. Analytical Methods. 2013;5(23):6686–6692. doi: 10.1039/C3AY41149D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang G, Chen H, Zhang X, Cooks RG, Ouyang Z. Rapid screening of anabolic steroids in urine by reactive desorption electrospray ionization. Anal. Chem. 2007;79(21):8327–8332. doi: 10.1021/ac0711079. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Wang H, Manicke NE, Lin J-M, Cooks RG, Ouyang Z. Development, characterization, and application of paper spray ionization. Anal. Chem. 2010;82(6):2463–2471. doi: 10.1021/ac902854g. [DOI] [PubMed] [Google Scholar]
- 44.Su Y, Wang H, Liu J, Wei P, Cooks RG, Ouyang Z. Quantitative paper spray mass spectrometry analysis of drugs of abuse. Analyst. 2013;138(16):4443–4447. doi: 10.1039/c3an00934c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Liu J, Cooks RG, Ouyang Z. Enabling quantitative analysis in ambient ionization mass spectrometry: internal standard coated capillary samplers. Anal. Chem. 2013;85(12):5632–5636. doi: 10.1021/ac401056q. [Demonstration of achieving high precision quantitation with small sample amounts and simple analytical procedures.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Ren Y, McLuckey MN, et al. Direct quantitative analysis of nicotine alkaloids from biofluid samples using paper spray mass spectrometry. Anal. Chem. 2013;85(23):11540–11544. doi: 10.1021/ac402798m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hendricks PI, Dalgleish JK, Shelley JT, et al. Autonomous in situ analysis and real-time chemical detection using a backpack miniature mass spectrometer: concept, instrumentation development, and performance. Anal. Chem. 2014;86(6):2900–2908. doi: 10.1021/ac403765x. [DOI] [PubMed] [Google Scholar]
- 48.Ouyang Z, Wu GX, Song YS, Li HY, Plass WR, Cooks RG. Rectilinear ion trap: concepts, calculations, and analytical performance of a new mass analyzer. Anal. Chem. 2004;76(16):4595–4605. doi: 10.1021/ac049420n. [DOI] [PubMed] [Google Scholar]
- 49.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine . Guidance for Industry–Bioanalytical Method Validation. Center for Drug Evaluation and Research (CDER); MD, USA: 2001. pp. 1–22. [Google Scholar]
- 50.Garimella S, Zhou X, Ouyang Z. Simulation of rarefied gas flows in atmospheric pressure interfaces for mass spectrometry systems. J. Am. Soc. Mass Spectrom. 2013;24(12):1890–1899. doi: 10.1007/s13361-013-0736-4. [DOI] [PubMed] [Google Scholar]