Abstract
Background:
Evidence has shown beneficial effects of probiotics in the treatment of irritable bowel syndrome (IBS); however, there is still a lack of data in this regard. We evaluated the efficacy of a multi-strain probiotic compound on IBS symptoms and quality-of-life (QOL).
Materials and Methods:
Adult IBS patients (n = 132) were randomized to receive a probiotic compound containing seven bacteria species including Lactobacillus strains, Bifidobacterium strains and Streptococcus thermophiles or similar placebo, twice daily after a meal for 14 consecutive days. Improvement of IBS symptoms was assessed in categories of abdominal pain and distension and improvement of bowel habit. Improvement in patients QOL was assessed by the IBS-QOL instrument. Patients were evaluated for symptoms and QOL at baseline and then 1 month after completion of the treatment.
Results:
After treatment, there was a decrease in abdominal pain and distension severity in both probiotic and the placebo groups (P<0.001), but there was no difference between the two groups in this regard (P>0.05). Improvement in bowel habit was observed in 33.3% of the probiotic and 36.5% of the placebo group (P = 0.910). There was no significant difference between the two groups in QOL after the treatment (P >0.05).
Conclusions:
We found no beneficial effects over placebo for a 2-week treatment with the above mentioned multi-strain probiotic compound in the treatment of IBS. Further, trials are yet required before a clear conclusion in this regards.
Keywords: Irritable bowel syndrome, probiotics, therapy
INTRODUCTION
Irritable bowel syndrome (IBS) is the most common functional bowel disorder characterized by abdominal pain/discomfort accompanying with disturbed bowel habits. It is estimated that 3-15% of the general population have IBS world-wide, modestly more prevalent in women than in men.[1] IBS possesses a chronic nature and affects patients physically, psychologically and economically and therefore, it is associated with impaired quality-of-life (QOL) and causes a major economic burden.[2]
The pathophysiology of IBS is still not completely understood. Studies have shown that the etiology is most likely multifactorial and abnormal brain-gut interaction, food intolerance; altered microflora, post-infectious or inflammatory changes and genetic and psychological factors contribute to the pathogenesis of IBS.[3,4] Heterogeneous pathophysiology and nature of IBS has a substantial impact on the efficacy of therapies for IBS. Only few therapies have been found to be effective in IBS treatment and treatments are not satisfactory in about half of the patients.[5]
The role of altered gut microbiota in the pathogenesis of IBS is highlighted by evidences showing changes in fecal and mucosa associated microflora, post-infectious IBS phenomenon and the link with intestinal bacterial overgrowth and dysregulation of mucosal immune system. Therefore, investigators have tried to see the effects of alterations in the intestinal microflora on IBS symptoms.[6] In this regard, the role of probiotics for intestinal functions and altered bowel microbiota, found in patients with IBS, has drawn attention toward these agents. Previous studies have shown beneficial effects of probiotics in the treatment of IBS and their safety has also contributed in to their popularity.[7,8,9] However, the benefits are likely to be strain-specific[10] and Bifidobacterium infantis has resulted in significant improvement in almost all IBS symptoms.[9]
Based on some evidence, a mixture of probiotics that contains several species of bacteria could be more effective than a single species of bacteria in the treatment of some gastrointestinal diseases. It is assumed that multi-strain probiotics have synergistic effects that increase their effectiveness.[11,12] However, whether a probiotic mixture is more effective than a single agent in treatment of IBS is remained un-answered yet. Another remained concern is the world-wide generalizability of current studies results. Almost all clinical studies with microbial therapies are carried out with people from developed countries.[7,8,9] Since, there is a variety in gut flora between different world's regions[13,14] the efficacy of probiotics may be affected by different ethnic groups of patients from different countries. There is no published report about probiotics efficacy on IBS treatment in Iran yet. Moreover, most of the previous studies are limited by small sample size.[15] Considering the above mentioned questions and lack of qualified studies, the purpose of our study was to evaluate the efficacy of a multi-strain probiotic compound, Balance® (Protexin Co., Somerset, UK), in the treatment of Iranian IBS patients. Balance® contains seven species of bacteria including Lactobacillus and Bifidobacterium species that separately have been shown helpful for treatment of IBS.[9,16] We hypothesized that this probiotic compound would decrease symptoms of IBS and increase the QOL.
MATERIALS AND METHODS
Patients and setting
This randomized placebo-controlled triple-blinded study was conducted on adult patients with IBS referred to gastroenterology clinics of Alzahra University Hospital in Isfahan City (central Iran). IBS was diagnosed by a single gastroenterologist according to the Rome II criteria (reference is needed). Patients with symptoms presented at least for 2 days/week in the preceding 2 weeks were included. Those with any infectious diseases before or during the study that need antibiotic therapy, immune-deficient disease, history of surgery on the gastrointestinal tract and history of using antibiotics or probiotics within 4 weeks before the study were not included. Considering type I error = 0.05, study power = 0.8 and expecting 10 point increase in the QOL score,[17] the study sample size was calculated as 66 cases per group. The study was approved by the Ethics Committee of Isfahan University of Medical Sciences and registered in the U.S. National Institutes of Health Protocol Registration System (available at clinicaltrials.gov NCT01837472). Informed consent was obtained from all patients after full explanation of the study aim and protocol.
Intervention
Patients were randomized into probiotic and placebo groups based on random table list. Patients in the probiotic group received the probiotic compound Balance® (Protexin Co., Somerset, UK), twice daily after a meal for 14 consecutive days. Balance® capsules contain seven bacteria species including Lactobacillus strains (Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus acidophilus and Lactobacillus bulgaricus), Bifidobacterium strains (Bifidobacterium breve and Bifidobacterium longum) and Streptococcus thermophiles. Total viable count is 1 × 108 CFU/per capsule. Other Ingredients are Fructo-oligosaccharide as prebiotic, magnesium stearate and hydroxypropyl methyl cellulose. Those in the placebo group received placebo capsules with the same order as probiotic group. No other treatment was prescribed for patients during the study period.
Assessments
Primary outcome of this study was improvement of IBS symptoms that was assessed in categories of abdominal pain and distension (from 0: No symptom to 3: Severe symptom) and improvement of bowel habit (get worse, no change, get better). Secondary outcome was improvement in patients QOL that was assessed by applying the IBS-QOL instrument. The IBS-QOL is a reliable and valid instrument for the assessment of IBS patients’ QOL. It contains 34 items with 5-point response scale (1: No problem to 5: Extreme problem). The total score is converted into 0-100 points for better interpretation.[17] The Persian version of the IBS-QOL with sufficient psychometric properties was used in this study.[18] Patients were evaluated for symptoms and QOL at baseline and then 1 month after completion of the treatment.
Statistical analyses
This study was designed as a triple-blind study and attending physicians, patients, principal investigators as well as data analyzer were all unaware about the treatment arms and drug codes. A third colleague who coded the probiotic and placebo capsules clarified the codes after data analyses. Data were analyzed using the SPSS software for windows version 16.0. Quantitative data and qualitative data were compared between the two groups with independent sample t-test and Chi-square/Mann-Whitney U tests, respectively. Paired t-test and Wilcoxon test were applied to assess changes in QOL score and symptoms severity within each group, respectively. A P value of less than 0.05 was considered as indicating a statistical significant difference in all analyses.
RESULTS
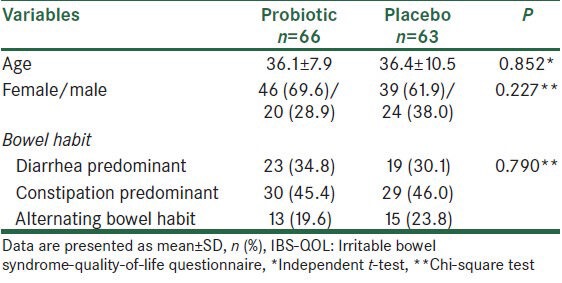
A total of 160 patients were evaluated during the study period. Twenty eight patients were not included owing to unwillingness to participate, receiving antibiotics or probiotics at the time of the study. Thus, 132 patients were randomized into the probiotic and placebo groups. Three patients from the placebo group did not come for follow-up evaluations, not due to drug side effects. Thus, 129 patients completed the trial; 66 patients in the probiotic and 63 ones in the placebo group. Mean age was 36.2 ± 9.3 years and 85 (65.9%) cases were female. The two groups were similar regarding demographic and baseline clinical characteristics [Table 1].
Table 1.
Comparison of the two groups with regards to demographic and baseline clinical characteristics
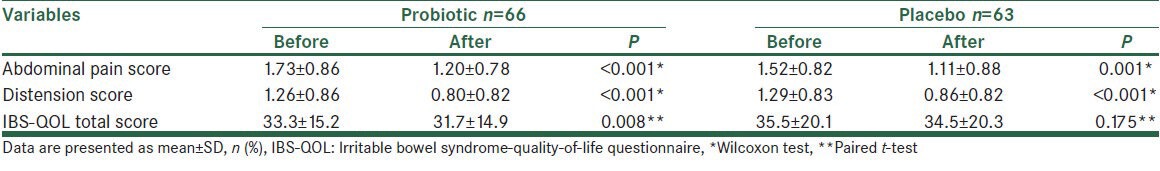
After the treatment period, there was a significant decrease in abdominal pain severity in both the probiotic (P < 0.001) and the placebo (P = 0.001) groups. There was no difference between the two groups in this regard (P = 0.558). Furthermore, distension severity decreased significantly in both probiotic (P < 0.001) and the placebo (P < 0.001) groups, but no difference was observed between the two groups in this regard (P = 0.673). Improvement in bowel habit was observed in 33.3% of the probiotic and 36.5% of the placebo group (P = 0.910). With regard to QOL, an improvement was observed in the probiotic (P = 0.008), but not in the placebo group (P = 0.175); though, there was no significant difference between the two groups in this regard (P = 0.372), Table 2. Further analysis in each subgroup of bowel habit did not change these results.
Table 2.
Comparison of the two groups with regards to symptoms and quality-of-life after treatment
In the probiotic group, three patients experienced mild abdominal pain and nausea at the beginning of therapy, which disappeared by continuing the drug. No specific side-effect was reported in the placebo group.
DISCUSSION
The aim of the present study was to evaluate the efficacy of a multi-strain probiotic compound, which contains seven species of bacteria including Lactobacillus and Bifidobacterium species in the treatment of Iranian IBS patients. We found no beneficial effects for this probiotic compound over placebo in reliving IBS symptoms. Although there was a statistically significant improvement in QOL score in the probiotic group, this change was not clinically important, which might be due to short duration of the study.
Previous studies on the efficacy of multi-strain probiotic compounds in the treatment of IBS have provided different results. Two small studies by Kim et al. on a probiotic compound containing Bifidobacterium, Lactobacillus and Streptococcus salivarius species for 4-8 weeks found beneficial effects of these probiotics over placebo only for bloating and flatulence symptoms.[19,20] Ki Cha et al. have evaluated a probiotic mixture containing Lactobacillus and Bifidobacterium and S. thermophilus for 8 weeks and found an overall response rate of 48% versus 12% with placebo; though, it had no significant effect on individual symptoms.[21] The study by Williams et al. on a combination of Lactobacillus and Bifidobacterium species also found greater improvement in IBS symptom severity and also in QOL compared with placebo over the 8-week intervention period.[22] Two other studies by Kajander et al. showed that a probiotic mixture of Lactobacillus and Bifidobacterium species for 5 months can stabilize the intestinal microbiota and alleviate IBS symptoms.[23,24] In contrast to these reports, the study by Søndergaard on a probiotic fermented milk containing Lactobacillus and Bifidobacterium species has found no special effect over acidified milk for 8 weeks.[25] A similar study by Simrén et al. also found no positive effect of such treatment.[26] Our study also found no beneficial effects of multi-strain probiotic over placebo in the treatment of IBS. According to systematic reviews and meta-analyses on this subject, there is a considerable heterogeneity among previous clinical trials regarding study design, which prevent a clear conclusion. Studies are different considering the duration of treatment (2 weeks to 5 months), outcome assessments and the type and amount (drug dose) of intervention. A publication bias toward positive results also should be considered.[9] Future studies should follow Rome Committee recommendations for appropriate design of clinical trials in this field.[27]
There are some limitations for this study. We assessed abdominal pain, the most important IBS symptom for patients as well as other symptoms with a Likert scale. This type of assessment is based on clinical importance of change; however, it might not detect small changes. Therefore, it is better for future studies to apply more comprehensive evaluation of symptoms.[28] Furthermore, the IBS-QOL evaluates QOL of the patient in the preceding 30 days; thus, 1 month was not an appropriate interval for expecting change in QOL and the study needed longer follow-up for evaluation of changes in QOL.
CONCLUSIONS
We found no beneficial effects for a 2-weeks treatment with a multi-strain probiotic compound containing Lactobacillus and Bifidobacterium species over placebo in the treatment of Iranian IBS patients. Further trials with longer duration of treatment and follow-ups are yet required before a clear conclusion in this regards. Such research should also focus on the type, optimal dose of probiotics and the subgroups of patients who are likely to benefit the most.
ACKNOWLEDGMENT
This study was supported by the Isfahan University of Medical Sciences. Authors are thankful to Dr. Ali Gholamrezaei for helping us in data analysis and editing this report.
Footnotes
Source of Support: Isfahan University of Medical Sciences.
Conflict of Interest: None declared.
REFERENCES
- 1.Cremonini F, Talley NJ. Irritable bowel syndrome: Epidemiology, natural history, health care seeking and emerging risk factors. Gastroenterol Clin North Am. 2005;34:189–204. doi: 10.1016/j.gtc.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Chang L. Review article: Epidemiology and quality of life in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20(Suppl 7):31–9. doi: 10.1111/j.1365-2036.2004.02183.x. [DOI] [PubMed] [Google Scholar]
- 3.Chang JY, Talley NJ. Current and emerging therapies in irritable bowel syndrome: From pathophysiology to treatment. Trends Pharmacol Sci. 2010;31:326–34. doi: 10.1016/j.tips.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775–85. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 5.American College of Gastroenterology Task Force on Irritable Bowel Syndrome. Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(Suppl 1):S1–35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 6.Parkes GC, Brostoff J, Whelan K, Sanderson JD. Gastrointestinal microbiota in irritable bowel syndrome: Their role in its pathogenesis and treatment. Am J Gastroenterol. 2008;103:1557–67. doi: 10.1111/j.1572-0241.2008.01869.x. [DOI] [PubMed] [Google Scholar]
- 7.Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: A systematic review. Gut. 2010;59:325–32. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 8.Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: Probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9:15. doi: 10.1186/1471-230X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: A systematic review. Am J Gastroenterol. 2009;104:1033–49. doi: 10.1038/ajg.2009.25. [DOI] [PubMed] [Google Scholar]
- 10.Whelan K. Probiotics and prebiotics in the management of irritable bowel syndrome: A review of recent clinical trials and systematic reviews. Curr Opin Clin Nutr Metab Care. 2011;14:581–7. doi: 10.1097/MCO.0b013e32834b8082. [DOI] [PubMed] [Google Scholar]
- 11.Chapman CM, Gibson GR, Rowland I. Health benefits of probiotics: Are mixtures more effective than single strains? Eur J Nutr. 2011;50:1–17. doi: 10.1007/s00394-010-0166-z. [DOI] [PubMed] [Google Scholar]
- 12.Chapman CM, Gibson GR, Rowland I. In vitro evaluation of single-and multi-strain probiotics: Inter-species inhibition between probiotic strains, and inhibition of pathogens. Anaerobe. 2012;18:405–13. doi: 10.1016/j.anaerobe.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Ghoshal UC, Park H, Gwee KA. Bugs and irritable bowel syndrome: The good, the bad and the ugly. J Gastroenterol Hepatol. 2010;25:244–51. doi: 10.1111/j.1440-1746.2009.06133.x. [DOI] [PubMed] [Google Scholar]
- 14.Ursell LK, Clemente JC, Rideout JR, Gevers D, Caporaso JG, Knight R. The interpersonal and intrapersonal diversity of human-associated microbiota in key body sites. J Allergy Clin Immunol. 2012;129:1204–8. doi: 10.1016/j.jaci.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, et al. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut. 2013;62:159–76. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke G, Cryan JF, Dinan TG, Quigley EM. Review article: Probiotics for the treatment of irritable bowel syndrome: Focus on lactic acid bacteria. Aliment Pharmacol Ther. 2012;35:403–13. doi: 10.1111/j.1365-2036.2011.04965.x. [DOI] [PubMed] [Google Scholar]
- 17.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: Development and validation of a new measure. Dig Dis Sci. 1998;43:400–11. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 18.Gholamrezaei A, Zolfaghari B, Farajzadegan Z, Nemati K, Daghaghzadeh H, Tavakkoli H, et al. Linguistic validation of the Irritable Bowel Syndrome-Quality of Life Questionnaire for Iranian patients. Acta Med Iran. 2011;49:390–5. [PubMed] [Google Scholar]
- 19.Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687–96. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 21.Ki Cha B, Mun Jung S, Hwan Choi C, Song ID, Woong Lee H, Joon Kim H, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46:220–7. doi: 10.1097/MCG.0b013e31823712b1. [DOI] [PubMed] [Google Scholar]
- 22.Williams EA, Stimpson J, Wang D, Plummer S, Garaiova I, Barker ME, et al. Clinical trial: A multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2009;29:97–103. doi: 10.1111/j.1365-2036.2008.03848.x. [DOI] [PubMed] [Google Scholar]
- 23.Kajander K, Hatakka K, Poussa T, Färkkilä M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: A controlled 6-month intervention. Aliment Pharmacol Ther. 2005;22:387–94. doi: 10.1111/j.1365-2036.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- 24.Kajander K, Myllyluoma E, Rajilić-Stojanović M, Kyrönpalo S, Rasmussen M, Järvenpää S, et al. Clinical trial: Multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27:48–57. doi: 10.1111/j.1365-2036.2007.03542.x. [DOI] [PubMed] [Google Scholar]
- 25.Søndergaard B, Olsson J, Ohlson K, Svensson U, Bytzer P, Ekesbo R. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: A randomized, placebo-controlled trial. Scand J Gastroenterol. 2011;46:663–72. doi: 10.3109/00365521.2011.565066. [DOI] [PubMed] [Google Scholar]
- 26.Simrén M, Ohman L, Olsson J, Svensson U, Ohlson K, Posserud I, et al. Clinical trial: The effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome-a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31:218–27. doi: 10.1111/j.1365-2036.2009.04183.x. [DOI] [PubMed] [Google Scholar]
- 27.Design of Treatment Trials Committee. Irvine EJ, Whitehead WE, Chey WD, Matsueda K, Shaw M, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2006;130:1538–51. doi: 10.1053/j.gastro.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 28.Gholamrezaei A, Nemati K, Emami MH. Which end point is more comprehensive in reflecting changes in irritable bowel syndrome treatment trials? Am J Gastroenterol. 2009;104:2859. doi: 10.1038/ajg.2009.486. [DOI] [PubMed] [Google Scholar]


