Abstract
We showed previously that zerumbone (ZER), a sesquiterpene isolated from subtropical ginger, inhibited in vitro (MCF-7 and MDA-MB-231 cells) and in vivo (MDA-MB-231 cells) growth of human breast cancer cells in association with apoptosis induction. Here, we investigated the role of Notch receptors in anticancer effects of ZER (cell migration inhibition and apoptosis induction) using breast cancer cells. Western blotting was performed to determine protein expression changes. Effect of ZER on transcriptional activity of Notch was assessed by luciferase reporter assays. Transfection with small hairpin RNA or small interfering RNA was performed for knockdown of Notch2 or Presenilin-1 protein. Cell migration and apoptosis were quantitated by Boyden chamber assay and flow cytometry, respectively. Exposure of MDA-MB-231, MCF-7, and SUM159 cells to ZER resulted in increased cleavage of Notch2 in each cell line. On the other hand, levels of cleaved Notch1 and Notch4 proteins were decreased following ZER treatment. Increased cleavage of Notch2 in ZER-treated cells was accompanied by induction of Presenilin-1 protein and transcriptional activation of Notch. Inhibition of cell migration as well as apoptosis induction resulting from ZER exposure was significantly augmented by knockdown of Notch2 protein. ZER-mediated cleavage of Notch2 protein in MDA-MB-231 cells was markedly attenuated upon RNA interference of Presenilin-1. Knockdown of Presenilin-1 protein also resulted in escalation of ZER-induced apoptosis. The present study indicates that Notch2 activation by ZER inhibits its proapoptotic and anti-migratory response at least in breast cancer cells.
Keywords: zerumbone, Notch2, presenilin-1, apoptosis
Introduction
The last two decades has witnessed remarkable progress toward early detection, risk-factor recognition, genomic landscape mapping, and targeted therapy of breast cancer [1–5]. Despite these advances, however, the breast cancer continues to be a prominent cause of mortality among women globally. In the United States alone >40,000 women succumb to breast cancer every year [6]. Therefore, novel non-toxic preventive interventions effective against major breast cancer subtypes are still desirable. Dietary phytochemicals owing to their favorable safety profile continue to draw attention for possible prevention and treatment of cancers [7]. Phytochemicals with anticancer activity against breast cancer have been isolated and characterized from edible (e.g., phenethyl isothiocyanate from cruciferous vegetables) as well as medicinal plants (e.g., withaferin A from Withania somnifera) [7–9].
Zerumbone (2,6,9,9-tetramethylcycloundeca-2,6,10-trien-1-one; hereafter abbreviated as ZER), a monocyclic sesquiterpene isolated from the rhizome of tropical ginger (Zingiber zerumbet), is one such promising phytochemical with in vivo activity against breast and other cancers in preclinical models [10–15]. An early published in vivo study with ZER showed a 46% reduction in frequency of azoxymethane-induced colonic aberrant crypt foci in rats coupled with suppression of cyclooxygenase-2 after 5-week of dietary administration at 0.05% [11]. A single topical 24 h pretreatment with ZER prior to carcinogen application (dimethylbenz[a]anthracene) resulted in inhibition of mouse skin tumor incidence and multiplicity by 60% and 80%, respectively, in ICR mice [12]. We have shown previously that the in vivo growth of MDA-MB-231 human breast cancer cells implanted in female athymic mice is significantly retarded by ZER administration in association with apoptosis induction and suppression of cell proliferation (Ki-67 expression) [10].
Cellular in vitro studies using a variety of cancer cell types have offered wealth of mechanistic insights into the anticancer effect of ZER. For example, ZER inhibited proliferation of human colon cancer cells by inducing mitochondrial dysfunction leading to apoptotic cell death [16]. Exposure of a normal rat liver cell line to ZER resulted in a significant induction of glutathione S-transferase, whereas this effect was not evident with its reduced analogues [17]. ZER was shown to abolish NF-κB and IκBα kinase activation in a panel of human cancer cells leading to suppression of anti-apoptotic and metastatic gene expression, induction of apoptotic cell death, and inhibition of cell invasion [18]. Cytotoxic effect of ZER in leukemia cells was found to be mediated through cell cycle arrest and Fas- and mitochondria-mediated apoptosis [19]. Modulation of Bax/Bcl-2 ratio favoring apoptosis, inhibition of Sonic hedgehog/GLI-mediated transcription, and downregulation of chemokine receptor CXCR4 concomitant with inhibition of CXCL12-induced breast and pancreatic cancer invasion were also shown after treatment of cancer cells with ZER [20–22].
Prior work from our laboratory has provided experimental evidence for apoptosis induction by ZER in vitro and in vivo in human breast cancer cells [10]. Immortalized embryonic fibroblasts from Bax and Bak double-knockout mice exhibited partial but statistically significant resistance toward ZER-mediated apoptosis when compared with wild-type fibroblasts [10]. The present study was undertaken to determine the role of Notch family receptors, which are known to be dysregulated in breast cancer [23], in anticancer effects (inhibition of cell migration and apoptosis induction) of ZER. For example, high Notch1 protein expression was suggested to be an early event in breast carcinogenesis and associated with the HER-2 molecular subtype [24]. Furthermore, a role for Notch2 in regulation of breast cancer cell migration as well as apoptosis was also suggested previously [25, 26].
Materials and methods
Chemicals, antibodies, and cell lines
ZER (purity >98%) was purchased from Sigma-Aldrich (St. Louis, MO). Reagents necessary for cell culture, including fetal bovine serum and antibiotics, and Oligofectamine were purchased from Invitrogen-Life Technologies (Carlsbad, CA). Antibodies for detection of cleaved Notch1, Jagged1, Jagged2, Notch2, cleaved poly-(ADP-ribose)-polymerase (PARP), cleaved caspase-3, Bcl-2, Presenilin-1, and Nicastrin were from Cell Signaling Technology (Beverly, MA); an antibody specific against cleaved Notch2 was from EMD-Millipore (Billerica, MA); anti- Notch4 (detects both full length and cleaved form) antibody was from Santa Cruz Biotechnology (Dallas, TX); an antibody specific for immunodetection of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was from GeneTex (Irvine, CA); and anti-actin antibody was from Sigma-Aldrich. The Notch2 and Presinilin1-targeted small interfering RNA (siRNA), Notch2-targeted small hairpin RNA (shRNA), and control shRNA were purchased from Santa Cruz Biotechnology.. A nonspecific control siRNA was purchased from Qiagen (Germantown, MD). MCF-7 and MDA-MB-231 cells were purchased from American Type Culture Collection (Manassas, VA) and maintained as described previously [27]. SUM159 cells (Asterand, Detroit, MI) were cultured as suggested by the supplier.
Western blotting
Stock solution of ZER was prepared in dimethyl sulfoxide (DMSO). An equal volume of DMSO (final concentration 0.1%) was added to controls. After treatment, cells were collected and processed for western blot analysis as previously described [28]. Actin or GAPDH band may be the same in some blots due to multiplexing. In some experiments, cell lysates after 6 h treatment were also used for western blotting. However, the results at 6 h are not shown because the data was inconsistent at this time point. Change in protein expression was determined by densitometric scanning and corrected for actin or GAPDH loading control.
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA from DMSO-treated control and ZER-treated cells was isolated using RNeasy kit (Qiagen). First-strand cDNA was synthesized using Superscript reverse transcriptase (Invitrogen-Life Technologies) with oligo (dT)20 primer. Primers were as follows: Notch2: forward 5′-AATCCCTGACTCCAGAACG-3′, reverse 5′-TGGTAGACCAAGTCTGTGATGAT-3′; GAPDH: forward 5′-GGACCTGACCTGCCGTCTAGAA-3′, reverse 5′-GGTGTCGCTGTTGAAGTCAGAG-3′. Quantitative real-time RT-PCR was done using 2× SYBR Green master mix (Applied Biosystems-Life Technologies) with 55°C annealing (45 seconds for 40 cycles.
Luciferase reporter assay
The Hairy and enhancer of split-1 (HES-1A/B) and Hairy/enhancer-of-split related with YRPW motif protein 1 (HEY-1) luciferase reporter constructs were generously provided by Dr. Kimberly E. Foreman (Department of Pathology, Loyola University Medical Center, Maywood, IL). Desired cells were transiently transfected with HES-1A/B or HEY-1 reporter constructs and pRL-CMV using Fugene6. Twenty-four hours after transfection, cells were treated with DMSO (control) or ZER (20 and 40 μM) for 12 or 24 hours. Luciferase activity was determined using Dual-Luciferase Reporter Assay kit from Promega (Madison, WI). Relative luciferase activity was normalized against protein concentration and renilla luciferase activity.
Stable knockdown of Notch2 protein
The MDA-MB-231 or SUM159 cells were transfected with 2 μg of control shRNA or Notch2-targeted shRNA using reagents from Santa Cruz Biotechnology. Cells with stable knockdown of Notch2 (hereafter abbreviated as Notch2 shRNA) and control shRNA transfected cells (control shRNA) were selected by culture in medium supplemented with 10 μg/mL of puromycin for 4 weeks.
Cell migration assay
Cell migration was determined using Transwell Boyden chamber containing 8 μm polycarbonate filter as described by us previously [29].
RNA interference of Notch2 or Presinilin-1 and apoptosis assay
Cells were transfected at ~50% confluency with control nonspecific siRNA or siRNA targeted against Notch2 or Presinilin-1 (100 nM) using Oligofectamine. Twenty-four hours after transfection, cells were treated with DMSO (control) or ZER (20 or 40 μM) for 24 h. Subsequently, cells were collected and processed for western blotting, cell migration and apoptosis assays. Apoptosis induction was assessed by flow cytometry using Annexin V/Propidium Iodide Apoptosis Detection kit according to the manufacturer’s instructions. In brief, after treatment with DMSO or ZER for 24 h, cells were harvested and washed with phosphate-buffered saline (PBS). Cells (1×105) were suspended in 100 μL of binding buffer, and stained with 4 μL of Annexin V-FITC and 2 μL of propidium iodide solution for 30 min at room temperature in the dark. Samples were then diluted with 200 μL of binding buffer and stained cells were analyzed using a flow cytometer.
Results
Effect of ZER on protein levels of cleaved Notch receptors in human breast cancer cells
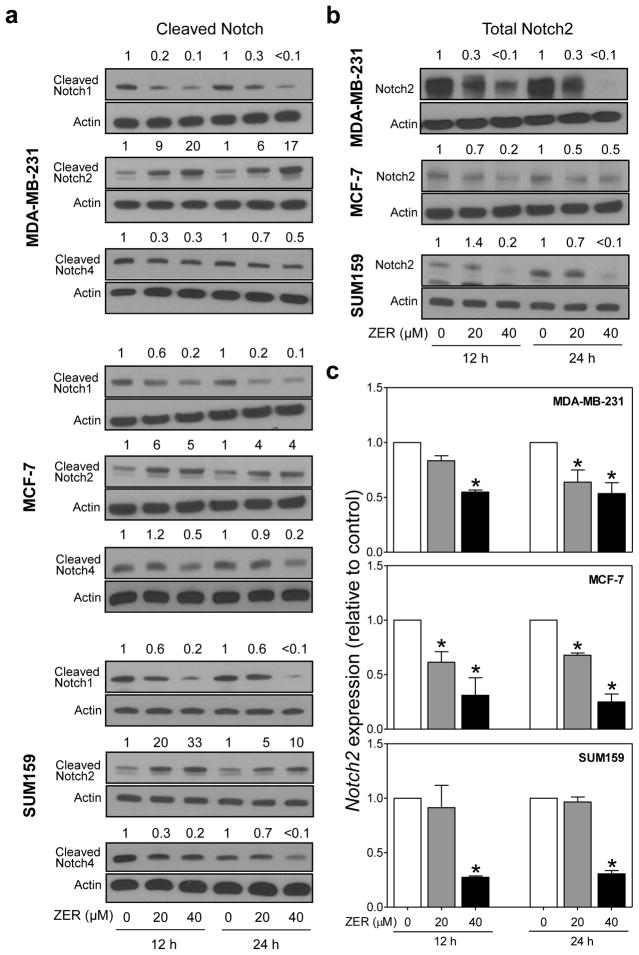
Western blotting was performed to determine the effect of ZER treatment on activation of Notch, which in mammalian cells comprises four receptors (Notch1–4) [30], using human breast cancer cells representing major subtypes (MDA-MB-231, MCF-7, and SUM159 cells). Ligand-dependent Notch activation involves proteolytic cleavage to intracellular domain and its subsequent translocation to the nucleus [30]. Levels of cleaved Notch1 and Notch4 proteins were generally decreased upon treatment with ZER in all three cell lines (Fig. 1a). In sharp contrast, ZER treatment resulted in an increase in levels of cleaved Notch2 protein (Fig. 1a). For example, the level of cleaved Notch2 protein was increased by up to 20-fold after ZER treatment in comparison with DMSO-treated control in MDA-MB-231 cells (40 μM, 12 h treatment). In general, ZER-mediated increase in Notch2 cleavage was more pronounced at 12 h time point in comparison with 24 h treatment in each cell line (Fig. 1a). On the other hand, level of total Notch2 protein was generally reduced after treatment with ZER in MDA-MB-231, MCF-7, and SUM159 cells (Fig. 1b). As can be seen in Figure 1c, ZER exposure also resulted in downregulation of Notch2 mRNA expression in each cell line as revealed by RT-PCR. Based on these results, we conclude that decrease in total Notch2 protein level after treatment of breast cancer cells with ZER is due to cleavage as well as transcriptional repression. These results also indicated differential effect of ZER on Notch2 versus Notch1 and Notch4 protein in each cell line.
Fig. 1.
ZER treatment increased levels of cleaved Notch2 protein in human breast cancer cells. a Western blot analysis for effect of ZER treatment (12 h or 24 h) on protein levels of cleaved Notch1, cleaved Notch2, and cleaved Notch4 in MDA-MB-231, MCF-7, and SUM159 cells. b Western blotting for effect of ZER on total Notch2 protein levels in MDA-MB-231, MCF-7, and SUM159 cells. Change in protein level relative to corresponding DMSO-treated control is shown above band. c Real-time quantitative RT-PCR for Notch2 mRNA in MDA-MB-231, MCF-7, and SUM159 cells after 12 h or 24 h of treatment with DMSO (control) or the indicated concentrations of ZER. Results shown are mean ± SD (n=3). *Significantly different (P<0.05) compared with corresponding DMSO-treated control by one-way ANOVA with Dunnett’s adjustment. Results were comparable in at least two independent experiments.
ZER exposure resulted in induction of Presenilin-1 protein
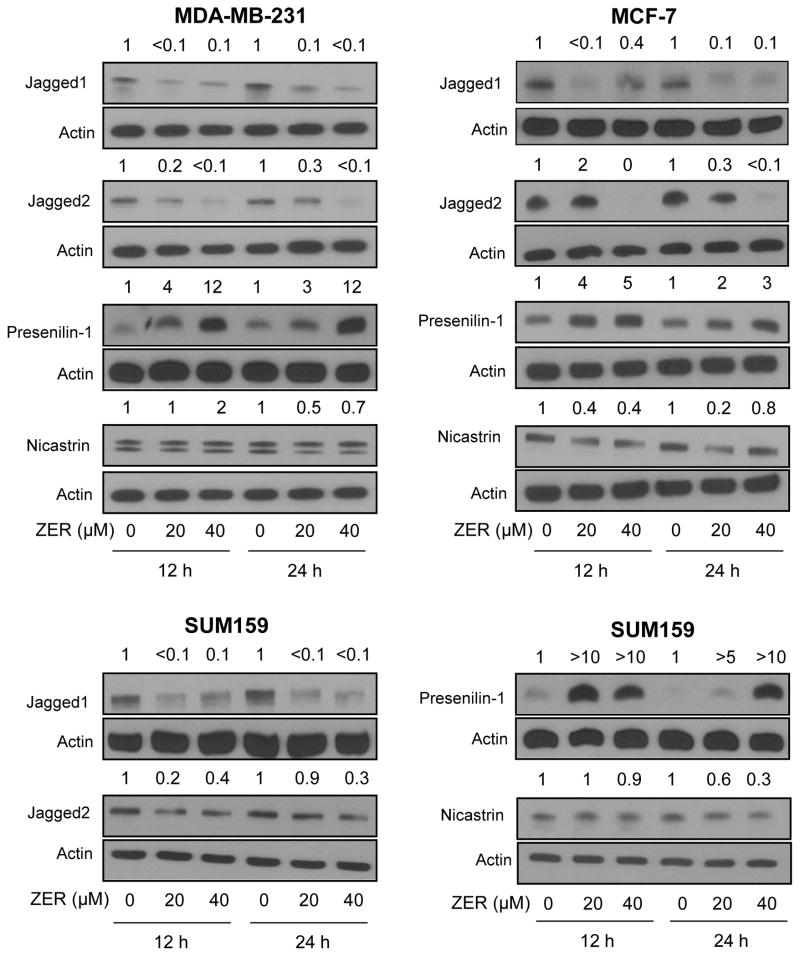
To gain insights into the increase in levels of cleaved Notch2 upon treatment with ZER, western blotting was performed for its ligands (Jagged1 and Jagged2) and γ-secretase complex components Presenilin-1 and Nicastrin (Fig. 2). Levels of both Jagged1 and Jagged2 were markedly suppressed after ZER treatment in MDA-MB-231, MCF-7, and SUM159 cells (Fig. 2). Likewise, ZER exposure resulted in downregulation of Nicastrin protein in each cell line at least at the 24 h time point. To the contrary, protein level of Presenilin-1 was clearly increased upon ZER treatment that varied between 3–12 fold in MDA-MB-231 cells, 2–5 fold in MCF-7 cells, and >5–>10 fold in SUM159 cells (Fig. 2). These results suggested that Notch2 cleavage after treatment with ZER might be related to induction of Presenilin-1 protein.
Fig. 2.
ZER treatment caused induction of Presenilin-1 protein in human breast cancer cells. Western blot analysis for effect of ZER on protein levels of Notch ligands (Jagged1 and Jagged2) and γ-secretase complex components (Presenilin-1 and Nicastrin) in MDA-MB-231, MCF-7, and SUM159 cells. Change in protein level relative to corresponding DMSO-treated control is shown above band. Results were comparable in at least two independent experiments.
Effect of ZER on transcriptional activity of Notch
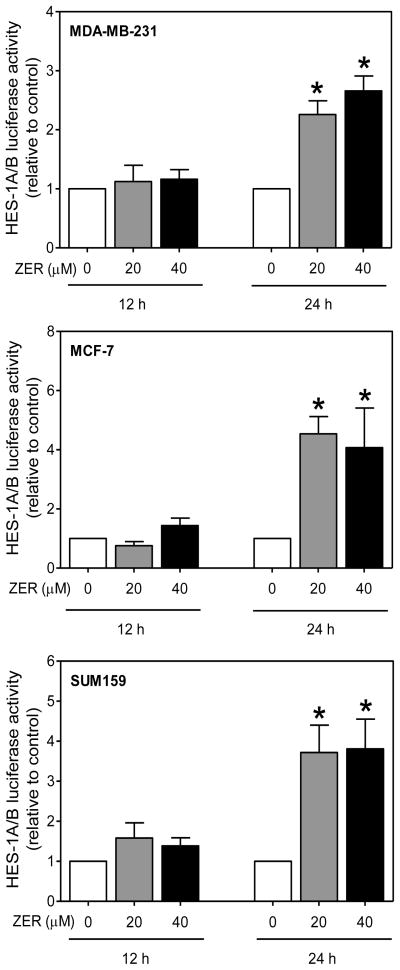
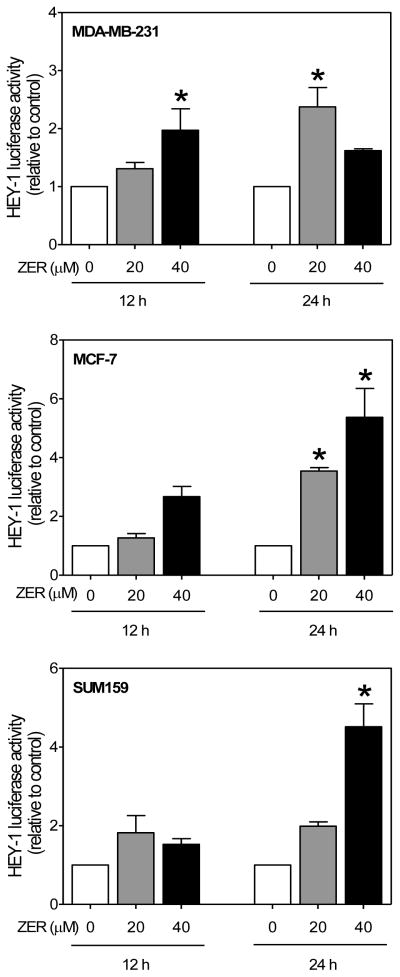
Because of opposite effects of ZER on levels of cleaved Notch2 versus cleaved Notch1 and Notch4 proteins, it was of interest to determine the impact of these changes on overall transcriptional activity of Notch. We approached this question by determining the effect of ZER on luciferase activities associated with downstream targets of Notch, including HES-1A/B and HEY1. The HES-1 and HEY-1, which are members of the basic helix-loop-helix family of transcriptional repressors are two independent primary targets of Notch [30, 31]. Exposure of MDA-MB-231 cells to ZER resulted in transcriptional activation of Notch as evidenced by an increase in HES-1A/B (Fig. 3) and HEY-1 (Fig. 4) luciferase reporter activities. ZER-mediated increase in HES-1A/B activity was obvious especially at the 24 h time point in each cell line (Fig. 3, 4). These results suggested that ZER might increase Notch transcriptional activity.
Fig. 3.
ZER treatment increased HES-1A/B-associated luciferase activity in breast cancer cells. HES-1A/B luciferase activity in MDA-MB-231, MCF-7, and SUM159 cells after 12 or 24 h treatment with ZER relative to corresponding DMSO-treated control. Results shown are mean ± SD (n=3). *Significantly different (P<0.05) compared with DMSO control by one-way ANOVA followed by Dunnett’s test. Results were comparable in replicate independent experiments.
Fig. 4.
ZER increased HEY-1-associated luciferase activity in breast cancer cells. HEY-1 luciferase activity in MDA-MB-231, MCF-7, and SUM159 cells after 12 or 24 h treatment with ZER relative to corresponding DMSO control. Data are mean ± SD (n=3). *Significantly different (P<0.05) compared with DMSO control by one-way ANOVA followed by Dunnett’s test. Results were comparable in replicate independent experiments.
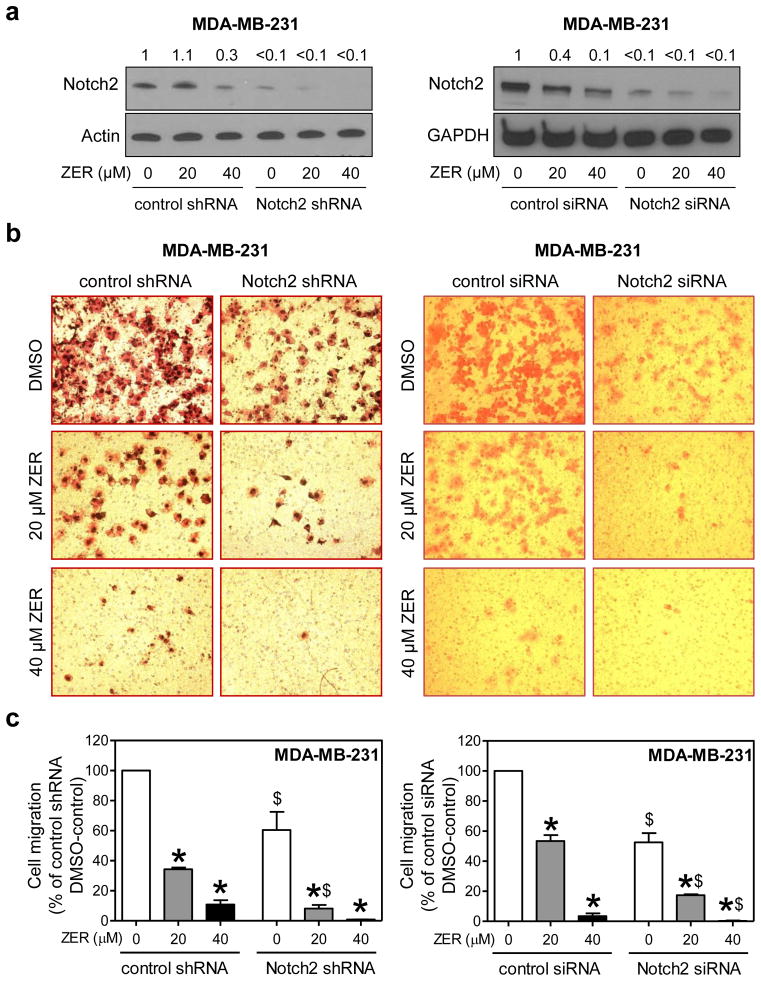
ZER-mediated inhibition of MDA-MB-231 cell migration was augmented by knockdown of Notch2
Because Notch2 activation was accompanied by an overall increase in Notch transcriptional activity (Fig. 3 and 4), we focused on this isoform to determine its role in cellular responses to ZER. Stable and transient knockdown of Notch2 protein in MDA-MB-231 cells was achieved by using shRNA and siRNA, respectively (Fig. 5a). Consistent with our previous observations [25, 32] knockdown of Notch2 itself inhibited MDA-MB-231 cell migration (Fig. 5b). In addition, ZER-mediated inhibition of cell migration was significantly augmented by Notch2 protein knockdown after transfection of MDA-MB-231 cells with shRNA (stable) as well as siRNA (transient) targeted against Notch2 (Fig. 5c). These results indicated that Notch2 activation by ZER impeded its inhibitory effect on MDA-MB-231 cell migration.
Fig. 5.
Knockdown of Notch2 augmented ZER-mediated inhibition of cell migration in MDA-MB-231 cells. a Western blotting for Notch2 in MDA-MB-231 cells stably or transiently transfected with control shRNA or siRNA or Notch2-targeted shRNA or siRNA and treated for 24 h with DMSO or ZER (20 or 40 μM). Change in protein level relative to DMSO-treated control shRNA or control siRNA transfected cells is shown above band. b Representative microscopic images depicting MDA-MB-231 cell migration after 24 h treatment with DMSO or ZER (20 or 40 μM) (100× magnification). c Quantitation of cell migration (mean ± S.D; n=3). Three-four fields on each filter were scored under an inverted microscope in each experiment. Significantly different (P<0.05) compared with *corresponding DMSO control, and $between control shRNA or siRNA and Notch2 shRNA or Notch2 siRNA transfected cells by one-way ANOVA followed by Bonferroni’s multiple comparison test. Cell migration relative to DMSO-treated control shRNA or control siRNA transfected cells is shown. Similar results were observed in two independent experiments. Representative data from one such experiment in triplicate are shown.
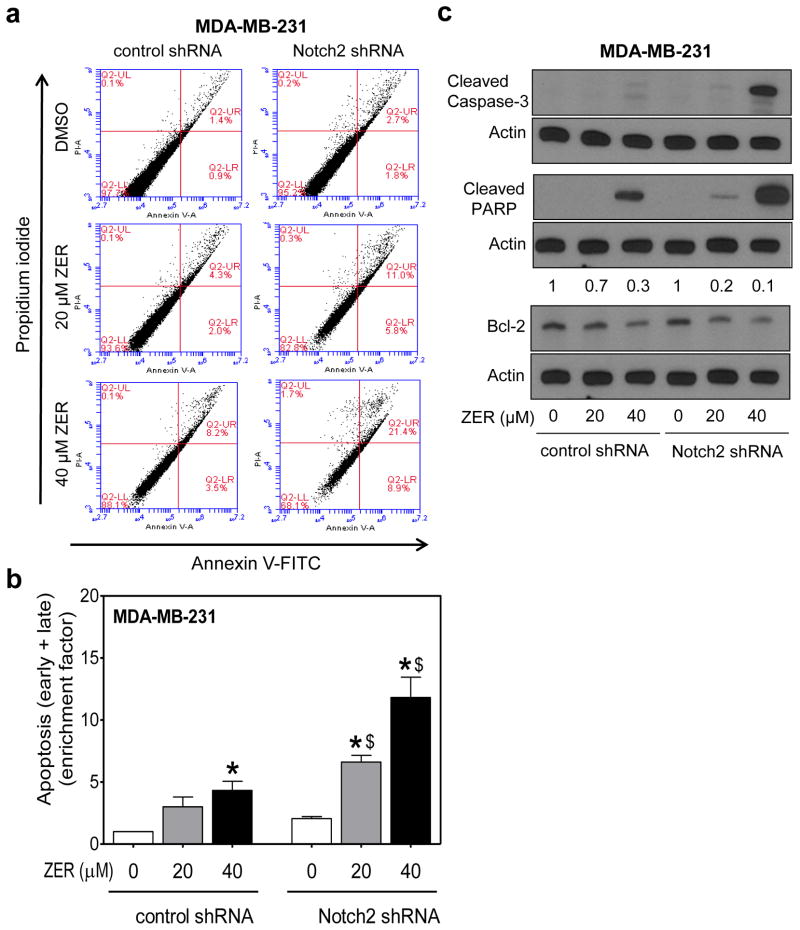
ZER-mediated apoptosis was augmented by Notch2 knockdown
Previous studies have shown that overexpression of intracellular domain of Notch2 triggers modest apoptosis in MDA-MB-231 cells even without any exogenous stimuli [26]. We therefore raised the question of whether proapoptotic effect of ZER was affected by Notch2 status. Fig. 6a shows flow histograms for apoptotic fraction (early and late apoptotic cells) in MDA-MB-231 cells stably transfected with the control shRNA or Notch2 shRNA and treated for 24 h treatment with DMSO (control) or ZER. Notch2 knockdown alone exhibited about 2-fold increase in early + late apoptotic fraction (Fig. 6a). Proapoptotic effect of ZER was intensified at both doses after stable knockdown of Notch2 protein (Fig. 6b). The enrichment of apoptosis was quantified relative to control shRNA cells treated with DMSO (Fig. 6b). Consistent with these results, ZER-mediated cleavage of PARP and procaspase-3 was markedly more pronounced in cells transfected with the Notch2 shRNA when compared with control shRNA-transfected MDA-MB-231 cells (Fig. 6c). Downregulation of anti-apoptotic Bcl-2 protein resulting from ZER exposure was also more pronounced in Notch2 silenced cells than in control shRNA transfected cells (Fig. 6c) providing explanation for increased sensitivity of Notch2 shRNA cells to ZER-induced apoptosis. These results indicated that similar to cell migration, apoptosis induction by ZER was attenuated by Notch2 activation. However, knockdown of Notch2 alone had only modest impact on apoptosis as the difference between control shRNA and Notch2 shRNA cells was statistically insignificant.
Fig. 6.
Stable knockdown of Notch2 augmented ZER-mediated apoptosis in MDA-MB-231 cells. a Representative flow histograms showing apoptotic fraction (Annexin V-FITC/propidium iodide method) in MDA-MB-231 cells stably transfected with control shRNA or Notch2 shRNA and treated for 24 h with DMSO or ZER (20 or 40 μM). b Quantitation of apoptotic fraction (early + late apoptotic cells). Apoptosis enrichment relative to DMSO-treated control shRNA transfected cells is shown as mean ± S.D (n=3). Significantly different (P<0.05) compared with *corresponding DMSO control, and $between control shRNA and Notch2 shRNA transfected cells by one-way ANOVA followed by Bonferroni’s multiple comparison test. c Immunoblotting for cleaved caspase-3, cleaved PARP, and Bcl-2 proteins after 24 h treatment of the indicated cells with DMSO or ZER. Change in protein level relative to DMSO-treated control shRNA transfected cells is shown above band. Quantitation of cleaved caspase-3 or cleaved PARP was not possible as the band was not detectable in DMSO-treated control shRNA transfected cells (first lane). Similar results were observed in two independent experiments.
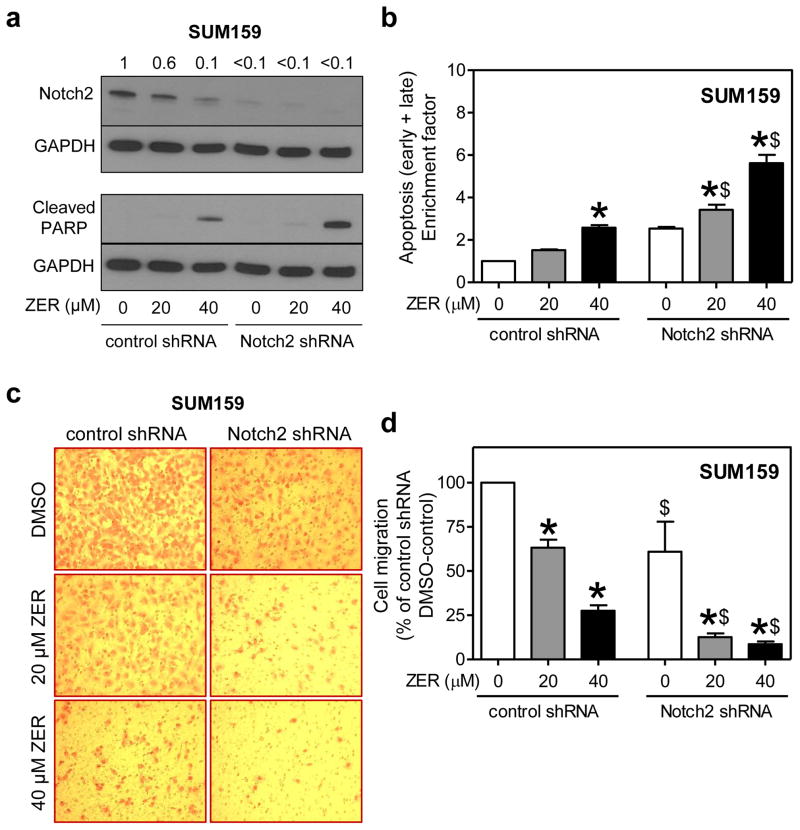
Effect of Notch2 knockdown on cell migration inhibition and apoptosis induction by ZER in SUM159 cells
Figure 7a shows knockdown of Notch2 protein in SUM159 cells after stable transfection with shRNA. Similar to MDA-MB-231 cells (Fig. 6c). Cleavage of PARP after treatment with ZER was relatively more pronounced in Notch2 silenced cells than in SUM159 cells stably transfected with control shRNA (Fig. 7a). In agreement with these results, ZER-induced apoptosis in SUM159 cells was significantly augmented by Notch2 knockdown (Fig. 7b). Effect of ZER treatment on SUM159 cell migration is shown in Figure 7c. Similar to MDA-MB-231 cells (Fig. 5c), inhibition of cell migration resulting from ZER treatment was significantly augmented by Notch2 knockdown in SUM159 cells (Fig. 7d). Collectively, these results indicated that modulation of ZER-mediated cell migration inhibition and apoptosis induction after Notch2 knockdown is not a cell line-specific phenomenon.
Fig. 7.
Stable knockdown of Notch2 augmented ZER-mediated apoptosis and cell migration inhibition in SUM159 cells. a Immunoblotting for Notch2 and cleaved PARP proteins in SUM159 cells stably transfected with control shRNA or Notch2-targeted shRNA and treated for 24 h with DMSO or ZER (20 or 40 μM). Change in protein level relative to DMSO-treated control shRNA transfected cells is shown above band. b Quantitation of apoptotic fraction (early + late apoptotic cells). Apoptosis enrichment relative to DMSO-treated control shRNA transfected cells is shown (mean ± S.D; n=3). c Representative microscopic images depicting SUM159 cell migration after 24 h treatment with DMSO or ZER (20 or 40 μM) (100× magnification). d Quantitation of cell migration (mean ± S.D; n=3). Significantly different (P<0.05) compared with *corresponding DMSO control, and $between control shRNA and Notch2 shRNA cells by one-way ANOVA followed by Bonferroni’s multiple comparison test. Similar results were observed in two independent experiments.
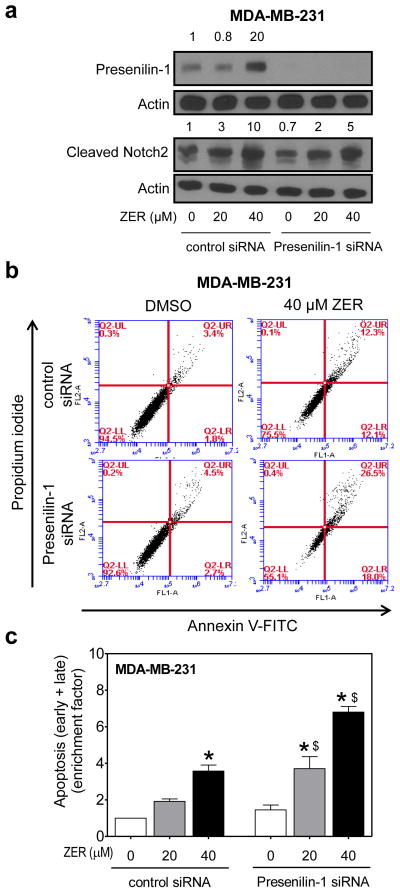
RNA interference of Presenilin-1 also augmented ZER-mediated apoptosis
Because ZER treatment resulted in induced expression of Presenilin-1 protein (Fig. 2), we further probed into the questions of whether Notch2 activation was related to this effect. The level of Presenilin-1 protein was decreased by >99% upon transient transfection of MDA-MB-231 cells with a siRNA specific for this protein when compared with control siRNA-transfected cells (Fig. 8a). RNA interference of Presenilin-1 also resulted in 50% decrease in cleaved Notch2 protein level especially at the 40 μM dose (Fig. 8a). Transient transfection with the Presenilin-1 siRNA alone had minimal effect on apoptosis as determined by Annexin V-FITC/propidium iodide method (Fig. 8b). On the other hand, ZER-induced apoptosis at both concentrations was significantly more pronounced in MDA-MB-231 cells transfected with the Presenilin-1 siRNA compared with those transfected with the control siRNA (Fig. 8c). Two conclusions can be drawn from these experiments: (a) Notch2 activation resulting from ZER treatment is partially due to Presenilin-1 induction; and (b) Presenilin-1 induction negatively regulates proapoptotic effect of ZER due to increased Notch2 activation.
Fig. 8.
RNA interference of Presenilin-1 augmented ZER-induced apoptosis in MDA-MB-231 cells. a Immunoblotting for Presenilin-1 and cleaved Notch2 in MDA-MB-231 cells transiently transfected with control siRNA or Presenilin1-specific siRNA and treated for 24 h with DMSO or ZER (20 or 40 μM). Change in protein level relative to DMSO-treated control siRNA-transfected cells is shown above band. b Flow histograms showing apoptosis in MDA-MB-231 cells after 24 h treatment with DMSO or ZER. c Quantitation of apoptotic fraction (early + late apoptotic cells). Enrichment of apoptotic fraction relative to DMSO-treated control siRNA-transfected cells is shown (mean ± S.D; n=3). Significantly different (P<0.05) compared with *corresponding DMSO control, and $between cells transfected with control siRNA and Presenilin-1 siRNA by one-way ANOVA followed by Bonferroni’s multiple comparison test.
Discussion
Data continues to accumulate to implicate Notch signaling in breast cancer development [33–38]. For example, high level of Jagged1 mRNA and protein expression is considered a predictor of poor outcome in breast cancer [33]. Overexpression of activated murine Notch1 and Notch3 in transgenic mice blocks mammary gland development but induces mammary tumors [34]. High co-expression of Jagged1 and Notch1 in human breast cancer is also associated with poor overall survival [36]. More recent studies have also implicated Notch1 and Notch4 in regulation of breast cancer stem cells [39, 40]. From these published reports, it is clear that Notch1, Notch3, and Notch4 have oncogenic role at least in breast cancer. The present study shows that ZER treatment not only decreases levels of cleaved Notch1 and Notch4 but also downregulates expression of their ligands Jagged1 and Jagged2. Noticeably, these effects of ZER are not influenced by the hormone receptor status of breast cancer cells. We have already shown previously that the in vivo growth of MDA-MB-231 xenografts is inhibited upon ZER administration [10]. Because of inhibitory effect of ZER on Notch1 and Notch4 activation, it is reasonable to hypothesize that ZER likely eliminates breast cancer stem cells. However, further work is necessary to explore this possibility.
Some studies suggest a tumor suppressor role for Notch2 in breast cancer [26, 41, 42]. The most convincing evidence for this association was provided by O’Neill and colleagues [26] who reported that overexpression of Notch2 intracellular domain in MDA-MB-231 cells inhibited tumor growth in vivo [26]. Analysis of Notch2 expression in normal mammary tissue and breast tumors in association with clinical outcome also suggested a tumor suppressor function for this protein [41]. On one hand, ZER-mediated activation of Nocth2 is a desirable outcome to increase its tumor suppressor function. On the other hand, proapoptotic effect of ZER is negatively regulated by Notch2 activation. We also show that Notch2 activation by ZER in breast cancer cells is partially dependent upon induction of Presenilin-1 protein. Consistent with this notion, apoptosis induction by ZER is also augmented by knockdown of Presenilin-1 protein. However, the precise mechanism by which ZER causes induction of Presenilin-1 protein remains to be determined.
It is interesting to note that a number of structurally-diverse naturally-occurring phytochemicals possess the ability to activate Notch2 in breast and prostate cancer cells [25, 32, 43, 44]. The examples include a steroidal lactone (withaferin A) isolated from the root and leaf of a medicinal plant (Withania somnifera) [25], aromatic isothiocyanates (benzyl isothiocyanate and phenethyl isothiocyanate) isolated from cruciferous vegetables (e.g., watercress and garden cress) [32, 43], and thioalkyl isothiocyanates from broccoli [44]. Interestingly, all these compounds are electrophilic in nature and capable of reacting with protein sulfhydryls [45, 46]. Thus, it is possible that a redox-sensitive mechanism is involved in Notch2 activation by these compounds, including ZER [16]. However, validation of this provocative hypothesis requires further experimentation. Nevertheless, the present study provides convincing evidence to indicate that the proapoptotic effect of ZER is negatively regulated by Notch2 activation.
In conclusion, the present study demonstrates that ZER treatment promotes cleavage of Notch2 leading to transcriptional activation of Notch in human breast cancer cell regardless of the hormone receptor status. We show further that the ZER-mediated apoptosis is significantly increased upon knockdown of Notch2 and Presenilin-1 proteins.
Acknowledgments
This investigation was supported by USPHS grant RO1 CA142604-05 and CA129347-07, awarded by the National Cancer Institute of the National Institutes of Health. This research project used the Flow Cytometry Facility supported in part by the Cancer Center Support Grant P30 CA047904 from the National Cancer Institute of the National Institutes of Health. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- DMSO
Dimethyl sulfoxide
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HES-1
Hairy and enhancer of split-1
- HEY-1
Hairy/enhancer-of-split related with YRPW motif protein 1
- PBS
Phosphate-buffered saline
- PARP
Poly-(ADP-ribose)-polymerase
- RT-PCR
Reverse transcription- polymerase chain reaction
- shRNA
Small hairpin RNA
- siRNA
Small interfering RNA
- ZER
Zerumbone
Footnotes
Conflict of interest: None of the authors has any conflict of interest.
References
- 1.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15(1):36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 2.Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007;7(9):659–672. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- 3.Higgins MJ, Baselga J. Breast cancer in 2010: novel targets and therapies for a personalized approach. Nature Rev Clin Oncol. 2011;8(2):65–66. doi: 10.1038/nrclinonc.2010.217. [DOI] [PubMed] [Google Scholar]
- 4.Van Zitteren M, van der Net JB, Kundu S, Freedman AN, van Duijn CM, Janssens AC. Genome-based prediction of breast cancer risk in the general population: a modeling study based on meta-analyses of genetic associations. Cancer Epidemiol Biomarkers Prev. 2011;20(1):9–22. doi: 10.1158/1055-9965.EPI-10-0329. [DOI] [PubMed] [Google Scholar]
- 5.Ellis MJ, Perou CM. The genomic landscape of breast cancer as a therapeutic roadmap. Cancer Discov. 2013;3(1):27–34. doi: 10.1158/2159-8290.CD-12-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 7.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 8.Singh SV, Kim SH, Sehrawat A, Arlotti JA, Hahm ER, Sakao K, Beumer JH, Jankowitz RC, Chandra-Kuntal K, Lee J, Powolny AA, Dhir R. Biomarkers of phenethyl isothiocyanate-mediated mammary cancer chemoprevention in a clinically relevant mouse model. J Natl Cancer Inst. 2012;104(16):1228–1239. doi: 10.1093/jnci/djs321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahm ER, Lee J, Kim SH, Sehrawat A, Arlotti JA, Shiva SS, Bhargava R, Singh SV. Metabolic alterations in mammary cancer prevention by withaferin A in a clinically-relevant mouse model. J Natl Cancer Inst. 2013;105(15):1111–1122. doi: 10.1093/jnci/djt153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sehrawat A, Arlotti JA, Murakami A, Singh SV. Zerumbone causes Bax- and Bak-mediated apoptosis in human breast cancer cells and inhibits orthotopic xenograft growth in vivo. Breast Cancer Res Treat. 2012;136(2):429–441. doi: 10.1007/s10549-012-2280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka T, Shimizu M, Kohno H, Yoshitani S, Tsukio Y, Murakami A, Safitri R, Takahashi D, Yamamoto K, Koshimizu K, Ohigashi H, Mori H. Chemoprevention of azoxymethane-induced rat aberrant crypt foci by dietary zerumbone isolated from Zingiber zerumbet. Life Sci. 2001;69(16):1935–1945. doi: 10.1016/s0024-3205(01)01277-2. [DOI] [PubMed] [Google Scholar]
- 12.Murakami A, Tanaka T, Lee JY, Surh YJ, Kim HW, Kawabata K, Nakamura Y, Jiwajinda S, Ohigashi H. Zerumbone, a sesquiterpene in subtropical ginger, suppresses skin tumor initiation and promotion stages in ICR mice. Int J Cancer. 2004;110(4):481–490. doi: 10.1002/ijc.20175. [DOI] [PubMed] [Google Scholar]
- 13.Kim M, Miyamoto S, Yasui Y, Oyama T, Murakami A, Tanaka T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int J Cancer. 2009;124(2):264–271. doi: 10.1002/ijc.23923. [DOI] [PubMed] [Google Scholar]
- 14.Abdelwahab SI, Abdul AB, Devi N, Taha MM, Al-zubairi AS, Mohan S, Mariod AA. Regression of cervical intraepithelial neoplasia by zerumbone in female Balb/c mice prenatally exposed to diethylstilboestrol: involvement of mitochondria-regulated apoptosis. Exp Toxicol Pathol. 2010;62(5):461–469. doi: 10.1016/j.etp.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Taha MM, Abdul AB, Abdullah R, Ibrahim TA, Abdelwahab SI, Mohan S. Potential chemoprevention of diethylnitrosamine-initiated and 2-acetylaminofluorene-promoted hepatocarcinogenesis by zerumbone from the rhizomes of the subtropical ginger (Zingiber zerumbet) Chem Biol Interact. 2010;186(3):295–305. doi: 10.1016/j.cbi.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Murakami A, Takahashi D, Kinoshita T, Koshimizu K, Kim HW, Yoshihiro A, Nakamura Y, Jiwajinda S, Terao J, Ohigashi H. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: the α,β-unsaturated carbonyl group is a prerequisite. Carcinogenesis. 2002;23(5):795–802. doi: 10.1093/carcin/23.5.795. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura Y, Yoshida C, Murakami A, Ohigashi H, Osawa T, Uchida K. Zerumbone, a tropical ginger sesquiterpene, activates phase II drug metabolizing enzymes. FEBS Lett. 2004;572(1–3):245–250. doi: 10.1016/j.febslet.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 18.Takada Y, Murakami A, Aggarwal BB. Zerumbone abolishes NF-κB and IκBα kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005;24(46):6957–6969. doi: 10.1038/sj.onc.1208845. [DOI] [PubMed] [Google Scholar]
- 19.Xian M, Ito K, Nakazato T, Shimizu T, Chen CK, Yamato K, Murakami A, Ohigashi H, Ikeda Y, Kizaki M. Zerumbone, a bioactive sesquiterpene, induces G2/M cell cycle arrest and apoptosis in leukemia cells via a Fas- and mitochondria-mediated pathway. Cancer Sci. 2007;98(1):118–126. doi: 10.1111/j.1349-7006.2006.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakinah SA, Handayani ST, Hawariah LP. Zerumbone induced apoptosis in liver cancer cells via modulation of Bax/Bcl-2 ratio. Cancer Cell Int. 2007;3(7):4. doi: 10.1186/1475-2867-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosoya T, Arai MA, Koyano T, Kowithayakorn T, Ishibashi M. Naturally occurring small-molecule inhibitors of hedgehog/GLI-mediated transcription. Chembiochem. 2008;9(7):1082–1092. doi: 10.1002/cbic.200700511. [DOI] [PubMed] [Google Scholar]
- 22.Sung B, Jhurani S, Ahn KS, Mastuo Y, Yi T, Guha S, Liu M, Aggarwal BB. Zerumbone down-regulates chemokine receptor CXCR4 expression leading to inhibition of CXCL12-induced invasion of breast and pancreatic tumor cells. Cancer Res. 2008;68(21):8938–8944. doi: 10.1158/0008-5472.CAN-08-2155. [DOI] [PubMed] [Google Scholar]
- 23.Zardawi SJ, O’Toole SA, Sutherland RL, Musgrove EA. Dysregulation of Hedgehog, Wnt and Notch signalling pathways in breast cancer. Histol Histopathol. 2009;24(3):385–398. doi: 10.14670/HH-24.385. [DOI] [PubMed] [Google Scholar]
- 24.Zardawi SJ, Zardawi I, McNeil CM, Millar EK, McLeod D, Morey AL, Crea P, Murphy NC, Pinese M, Lopez-Knowles E, Oakes SR, Ormandy CJ, Qiu MR, Hamilton A, Spillane A, Soon Lee C, Sutherland RL, Musgrove EA, O’Toole SA. High Notch1 protein expression is an early event in breast cancer development and is associated with the HER-2 molecular subtype. Histopathology. 2010;56(3):286–296. doi: 10.1111/j.1365-2559.2009.03475.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Sehrawat A, Singh SV. Withaferin A causes activation of Notch2 and Notch4 in human breast cancer cells. Breast Cancer Res Treat. 2012;136(1):45–56. doi: 10.1007/s10549-012-2239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Neill CF, Urs S, Cinelli C, Lincoln A, Nadeau RJ, León R, Toher J, Mouta-Bellum C, Friesel RE, Liaw L. Notch2 signaling induces apoptosis and inhibits human MDA-MB-231 xenograft growth. Am J Pathol. 2007;171(3):1023–1036. doi: 10.2353/ajpath.2007.061029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao D, Vogel V, Singh SV. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol Cancer Ther. 2006;5(11):2931–2945. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- 28.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24(5):891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 29.Sehrawat A, Singh SV. Benzyl isothiocyanate inhibits epithelial-mesenchymal transition in cultured and xenografted human breast cancer cells. Cancer Prev Res (Phila) 2011;4(7):1107–1117. doi: 10.1158/1940-6207.CAPR-10-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aithal MG, Rajeswari N. Role of Notch signalling pathway in cancer and its association with DNA methylation. J Genet. 2013;92(3):667–675. doi: 10.1007/s12041-013-0284-5. [DOI] [PubMed] [Google Scholar]
- 31.Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim Biophys Acta. 2011;1815(2):197–213. doi: 10.1016/j.bbcan.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SH, Sehrawat A, Singh SV. Notch2 activation by benzyl isothiocyanate impedes its inhibitory effect on breast cancer cell migration. Breast Cancer Res Treat. 2012;134(3):1067–1079. doi: 10.1007/s10549-012-2043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickson BC, Mulligan AM, Zhang H, Lockwood G, O’Malley FP, Egan SE, Reedijk M. High-level JAG1 mRNA and protein predict poor outcome in breast cancer. Mod Pathol. 2007;20(6):685–693. doi: 10.1038/modpathol.3800785. [DOI] [PubMed] [Google Scholar]
- 34.Hu C, Diévart A, Lupien M, Calvo E, Tremblay G, Jolicoeur P. Overexpression of activated murine Notch1 and Notch3 in transgenic mice blocks mammary gland development and induces mammary tumors. Am J Pathol. 2006;168(3):973–990. doi: 10.2353/ajpath.2006.050416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I, Karsan A. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med. 2007;204(12):2935–2948. doi: 10.1084/jem.20071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, Lockwood G, Egan SE. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65(18):8530–8537. doi: 10.1158/0008-5472.CAN-05-1069. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Fu L, Gu F, Ma Y. Notch1 is involved in migration and invasion of human breast cancer cells. Oncol Rep. 2011;26(5):1295–1303. doi: 10.3892/or.2011.1399. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi N, Oyama T, Ito E, Satoh H, Azuma S, Hayashi M, Shimizu K, Honma R, Yanagisawa Y, Nishikawa A, Kawamura M, Imai J, Ohwada S, Tatsuta K, Inoue J, Semba K, Watanabe S. NOTCH3 signaling pathway plays crucial roles in the proliferation of ErbB2-negative human breast cancer cells. Cancer Res. 2008;68(6):1881–1888. doi: 10.1158/0008-5472.CAN-07-1597. [DOI] [PubMed] [Google Scholar]
- 39.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, Bundred NJ, Clarke RB. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70(2):709–718. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez ME, Moore HM, Li X, Toy KA, Huang W, Sabel MS, Kidwell KM, Kleer CG. EZH2 expands breast stem cells through activation of Notch1 signaling. Proc Natl Acad Sci USA. 2014;111(8):3098–3103. doi: 10.1073/pnas.1308953111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parr C, Watkins G, Jiang WG. The possible correlation of Notch-1 and Notch-2 with clinical outcome and tumour clinicopathological parameters in human breast cancer. Int J Mol Med. 2004;14(5):779–786. doi: 10.3892/ijmm.14.5.779. [DOI] [PubMed] [Google Scholar]
- 42.Zarubin T, Jing Q, New L, Han J. Identification of eight genes that are potentially involved in tamoxifen sensitivity in breast cancer cells. Cell Res. 2005;15(6):439–446. doi: 10.1038/sj.cr.7290312. [DOI] [PubMed] [Google Scholar]
- 43.Kim SH, Sehrawat A, Sakao K, Hahm ER, Singh SV. Notch activation by phenethyl isothiocyanate attenuates its inhibitory effect on prostate cancer cell migration. PLoS One. 2011;6(10):e26615. doi: 10.1371/journal.pone.0026615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hahm ER, Chandra-Kuntal K, Desai D, Amin S, Singh SV. Notch activation is dispensable for D, L-sulforaphane-mediated inhibition of human prostate cancer cell migration. PLoS One. 2012;7(9):e44957. doi: 10.1371/journal.pone.0044957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mi L, Hood BL, Stewart NA, Xiao Z, Govind S, Wang X, Conrads TP, Veenstra TD, Chung FL. Identification of potential protein targets of isothiocyanates by proteomics. Chem Res Toxicol. 2011;24(10):1735–1743. doi: 10.1021/tx2002806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antony ML, Lee J, Hahm ER, Kim SH, Marcus AI, Kumari V, Ji X, Yang Z, Vowell CL, Wipf P, Uechi GT, Yates NA, Romero G, Sarkar SN, Singh SV. Growth arrest by the antitumor steroidal lactone withaferin A in human breast cancer cells is associated with down-regulation and covalent binding at cysteine 303 of β-tubulin. J Biol Chem. 2014;289(3):1852–1865. doi: 10.1074/jbc.M113.496844. [DOI] [PMC free article] [PubMed] [Google Scholar]