This is the thirteenth installment of the comprehensive survey series in high throughput chemistry.1 Biologically active libraries reported in 2009 are captured in Tables 1–5 under the headings of proteases, nonproteolytic enzymes, GPCRs, nonGPCRs, and oncolytics/antiinfectives. Table 6 lists molecular probes. Compound collections without disclosed biological activity are delineated in Tables 7–10 under the headings of scaffold derivatization/acyclic synthesis, monocyclic-, bicyclic/spirocyclic-, and polycyclic/macrocyclic synthesis. Polymer-supported reagents/scavengers/linkers are presented in Tables 11 (nonfluorous) and Table 12 (fluorous). There are 370 libraries and 24 molecular probes extracted from 355 literature citations.2–491 Approximately 90% of the citations originated from academic laboratories, with the majority of these from European and Asian laboratories. Solution-phase methodology accounted for ca. 85% of chemical library synthesis.
Table 1.
Chemical Libraries Targeting Proteases.
|
Asterisk is the point of attachment to resin.
Table 5.
Chemical Libraries Yielding Cytotoxic and Antiinfective Agents.
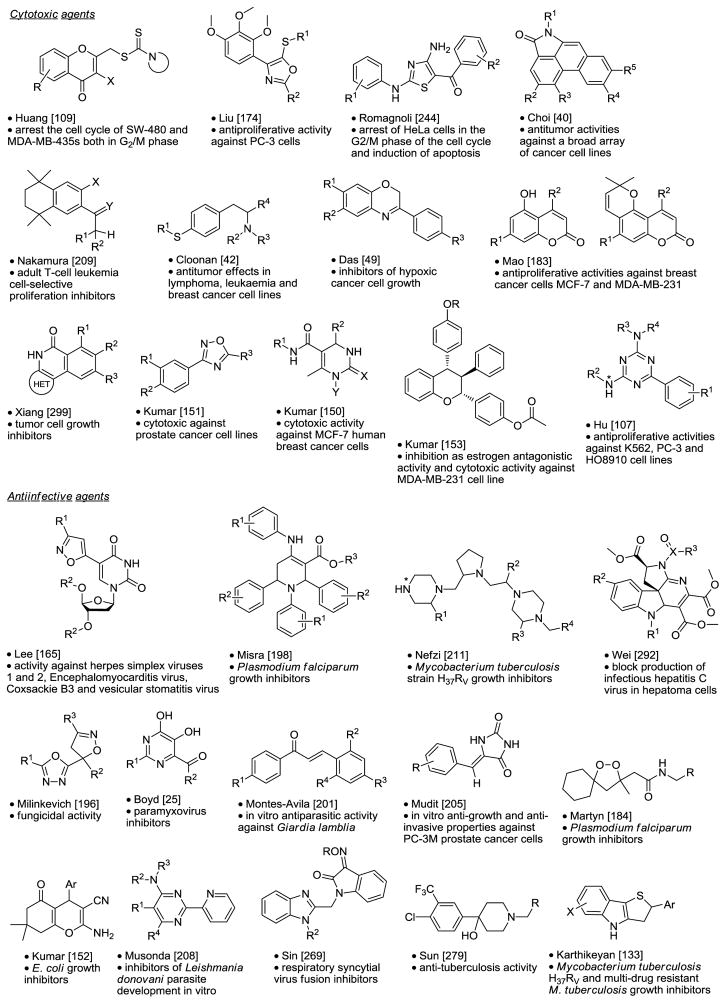
|
Asterisk is the point of attachment to resin.
Table 6.
Selected Molecular Probes.
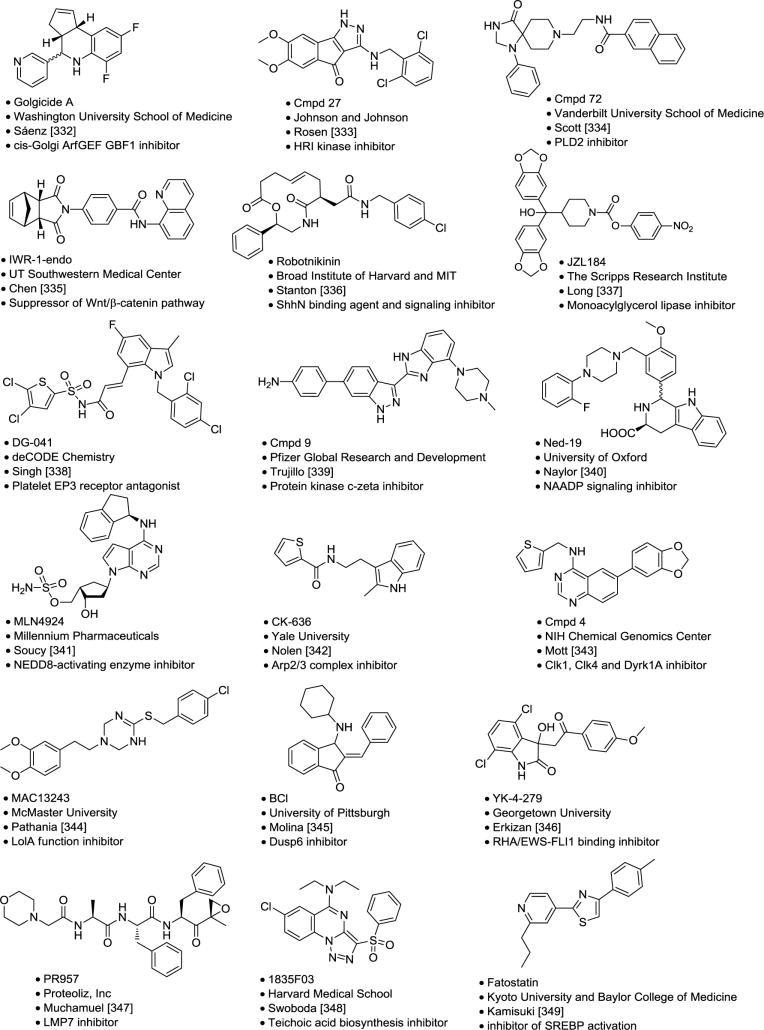
|
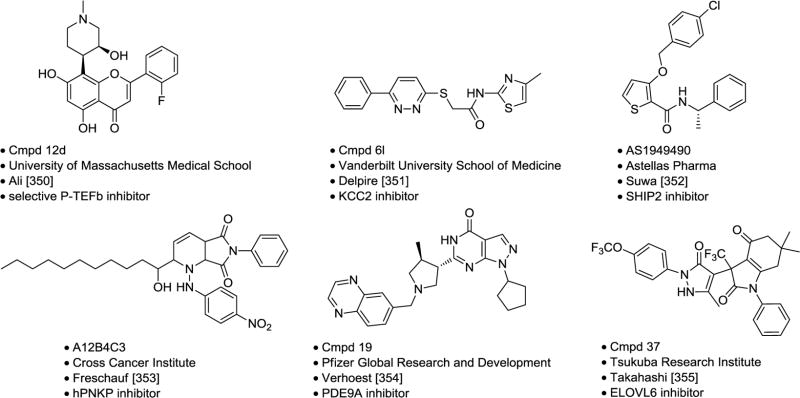
|
Table 7.
Scaffold Derivatization and Acyclic Synthesis.
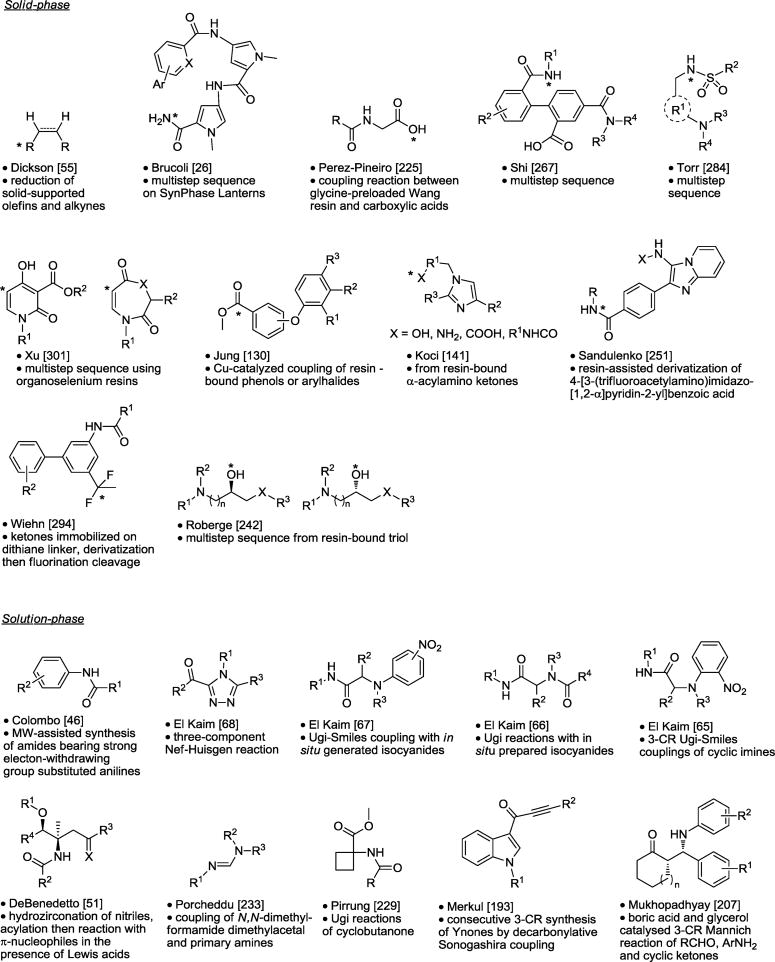
|
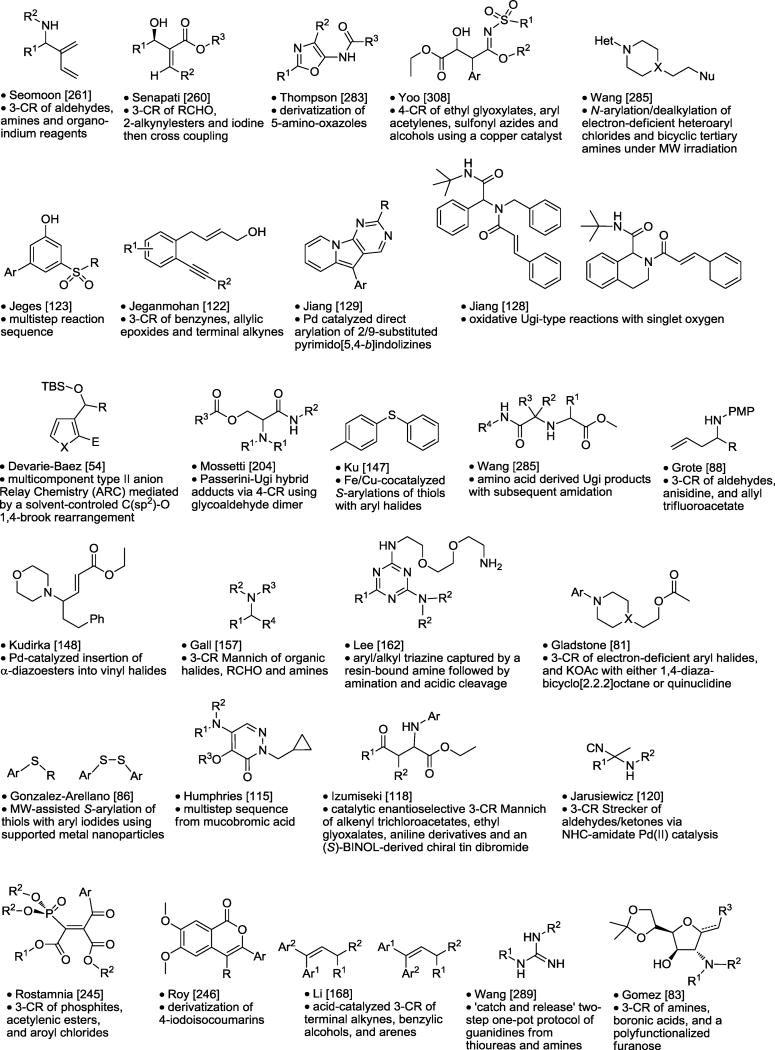
|
Asterisk is the point of attachment to resin.
Table 10.
Polycyclic and Macrocyclic Synthesis.
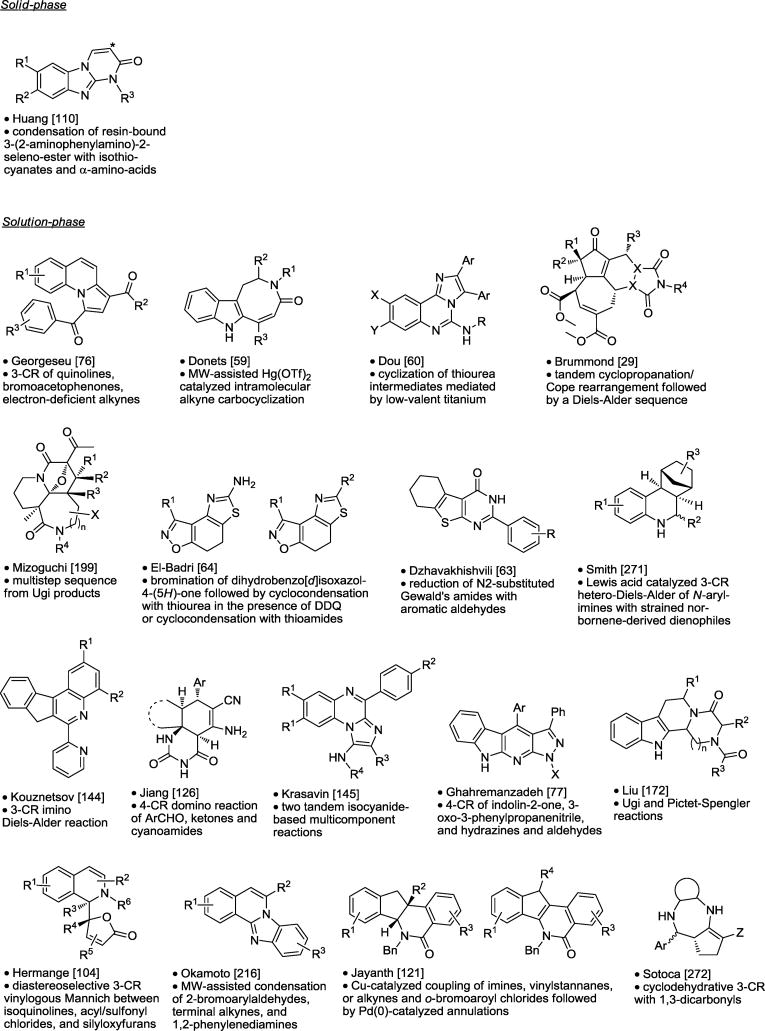
|
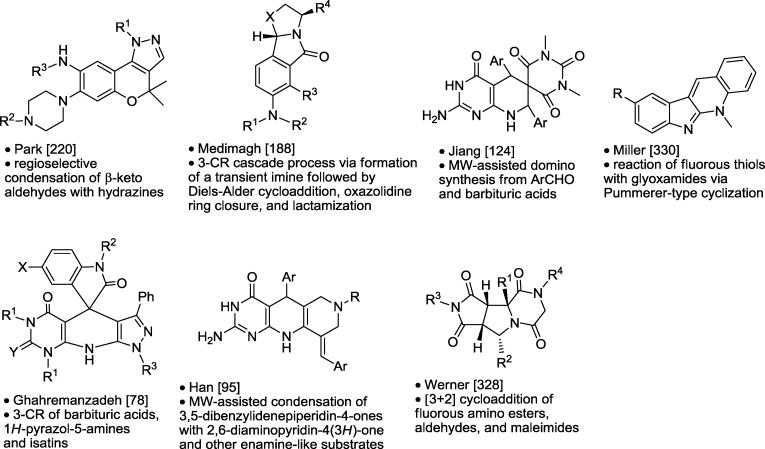
|
Asterisk is the point of attachment to resin.
Table 11.
Polymer-Supported Reagents, Scavengers, and Linkers.
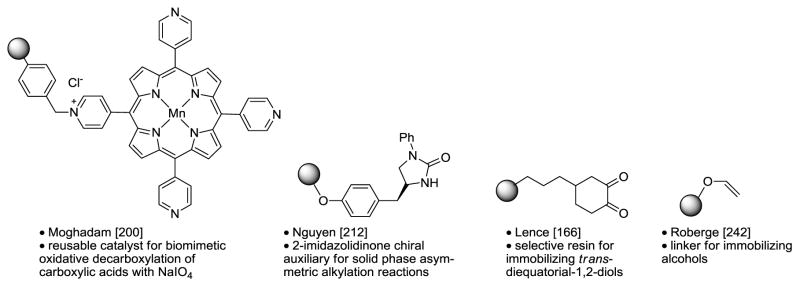
|
Table 12.
Fluorous Catalysts, Reagents, Scavengers, Linkers and Library Synthesis.
|
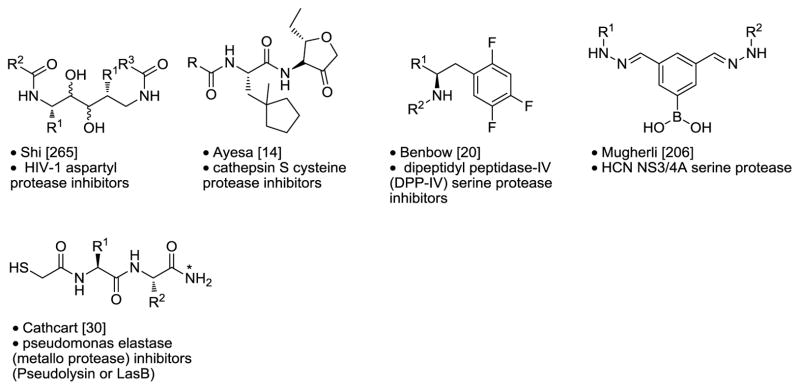
This year’s 18 vignettes include (a) biologically active libraries: HDAC1/HDAC2 inhibitors,134 H3 antagonists,136 glucagon receptor antagonists,179 purinergic P2Y12 receptor antagonists,221 heat shock protein 90 (Hsp90) inhibitors,41 selective norepinephrine reuptake inhibitors,73 allosteric modulators of mGluR4287 and mGluR571 Bcl-2 inhibitors via DOS library;182 (b) molecular probes: Ned-19340 and DG-041;338 (c) high throughput chemical methodology: catch and release synthesis of substituted guanidines.289 and substituted pyrimidines via a 3-component reaction;158 and (d) fluorous technology: displaceable fluorous dihydropyran322 and isonnitrile linkers,327 fluorous synthesis of 1,4-benzodiazepine-2,5-dione, 327 piperazinedion-fulsed tricyclic328 compound libraries and a fluorous mixture synthesis of natural product resorcyclic acid lactone library.329
Related publications and reviews appeared in 2009 on microwave-assisted of N-heterocyclic synthesis356 and convertible isonitriles,365 click chemistry,358 dynamic combinatorial chemistry,363 large scale preparation of silicon-functionalized SynPhase lanterns,370 kinase inhibitors,357 heat shock protein 90 inhibitors,376 biologically active benzoannelated nitrogen heterocycles,361 library automation and analysis,360,368 library design,375 NMR-ased screening,377,378 fragement libraries and fragement hopping,366,367,369 review of Ellman’s libraries,374 solid-phase resin specifications,359 microelectrode arrays for monitoring ligand-receptor binding events,364 yactoliter-scale DNA reactor for small molecule evolution,362 DNA encoded libraries,379 and fluorous chemistry and separations.380–383
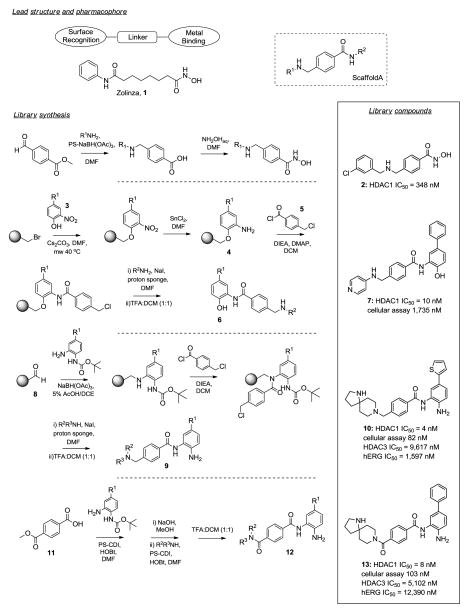
Selective HDAC1/HDAC2 Inhibitors.134
Histone deacetylases (HDACs) are enzymes involved in the remodeling of chromatin. Several classes of HDAC inhibitors have been found to have potent and specific anticancer activities and the common pharmacophore identified contains a surface recognition domain, a linker region and a metal binding region. Kattar and co-workers set out to improve upon the selectivity and tolerability of Zolinza 1, Merck’s first-in-class mixed HDAC 1, 2, 3 and 6 inhibitor used for the treatment of cutaneous manifestation of T-cell lymphoma (Figure 1).134 In their initial library, keeping the metal binding domain constant and varying both linker and surface recognition domains, scaffold A had already been identified. Reacting 4-formylmethyl-benzoate and polystyrene bound sodium triacetoxyborohydride with 48 R1-amines, followed by hydroxamate formation with NH2OH for their first follow-up library, 3-chlorobenzylamine was found to be preferred in the R1-position over phenethyl or aniline analogs, yielding compound 2 (HDAC IC50 = 348 nM). Modifying the metal binding domain next, 2-aminophenols were chosen to replace the hydroxamate moiety. R1-substituted 2-nitrophenols 3 were reacted with bromo-Wang resin, cesium carbonate and DMF under microwave irradiation at 40 °C. The nitro group was reduced with SnCl2 in DMF and the resulting resin-bound anilines 4 coupled with the linker unit 4-chloromethyl benzoyl chloride 5, DMAP and DIEA in DCM. Attachment of R2-amines was accomplished using sodium iodide and a proton sponge in DMF. Final compounds 6 were released from resin with TFA/DCM. Aromatic substituents in R1 and anilines in R2 demonstrated 10–30-fold increased potency for HDAC1 (e.g. 7, IC50 = 10 nM, cellular assay = 1,735 nM). To improve cellular potency the aminophenol metal binding site was replaced with a primary amine. Mono protected R1-substituted di-anilines were bound to aldehyde resin 8 via reductive amination utilizing sodium triacetoxyborohydride in AcOH/DCE. 4-Chloromethyl benzoyl chloride was attached next, followed by reaction with surface binding domain R2-amines, sodium iodide and a proton sponge. Exposure to TFA/DCM yielded final products 9. Amino spirocyclic building blocks in the R2-position retained affinity towards HDAC1 and improved selectivity over HDAC3 as well as improved potency in the cellular assay. Unfortunately this series exhibited activity against hERG (10: HDAC1 IC50 = 4 nM, cellular assay = 82 nM, HDAC3 IC50 = 9,617 nM, hERG IC50 = 1,597 nM). Eliminating one amino-functionality by switching to a terephthalamide linkage successfully mitigated hERG liabilities. This last set of compounds was synthesized via solution-phase, starting from 11 and mono protected R1-substituted di-anilines which were combined with PS-CDI and HOBt in DMF. After ester saponification, the spirocyclic amine was coupled using PS-CDI again. The final 26 compounds 12 were obtained after deprotection with TFA/DCM. Compound 13 showed the best overall profile (HDAC1: IC50 = 8 nM, cellular assay = 103 nM, HDAC3: IC50 = 5,102 nM, hERG IC50 = 12,390 nM) and demonstrated in vivo efficacy in an acute PD HCT-116 xenograph model study. This successful combination of solid and solution phase library synthesis led to the efficient optimization of a novel HDAC inhibitor series, continuously improving additional properties within each library iteration.
Figure 1.
HDAC1/HDAC2 inhibitors.134
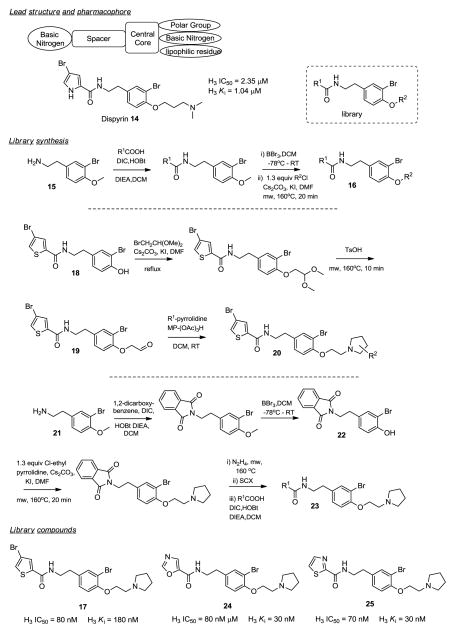
H3 Antagonists.136
The histamine H3 receptor is an attractive G protein–coupled receptor drug target that regulates neurotransmission in the central nervous system. There has been considerable effort by both academic and industrial laboratories to develop potent and selective H3 receptor antagonists for the potential treatment of attention-deficit hyperactivity disorder, dementias, schizophrenia, as well as obesity and sleep disorders resulting in a refined H3 antagonist pharmacophore model containing two basic nitrogens separated by a spacer and a central core that also carries a polar group and a lipophilic residue (Figure 2). After having completed its total synthesis, Kennedy and co-workers recognized that the marine alkaloid dispyrin 14 perfectly maps onto this pharmacophore model.136 Dispyrin 14 indeed displayed some H3 antagonist activity (IC50 = 2.35 μM) and was used as the starting point for a natural product guided iterative parallel synthesis campaign. Starting from 3-bromo-4-methoxyphenylethylamine 15 five heterocyclic carboxylic acids R1 were coupled using DIC, HOBt and DIEA in DCM. The methyl ether was removed with BBr3 and the resulting phenols alkylated with five R2-aminoalkyl chlorides under microwave irradiation conditions generating 25 different compounds 16. All the compounds showed activity against H3 with more potent compounds containing an ethyl pyrrolidinyl residue in the R2 position (Ki’s < 200 nM), with the best compound 17 (H3 Ki = 80 nM, H3 IC50 = 180 nM). Retaining the 4-bromo-thiophene residue from 17, functionalized pyrrolidines were explored next. Phenol 18 was reacted first with 2-bromo-1,1-dimethoxy ethane and then with tosylic acid to yield aldehyde 19. Final compounds 20 were obtained via reductive amination utilizing resin-bound triacetoxyborohydride together with the functionalized pyrrolidines. No potency improvement was observed in final compounds 20.
Figure 2.
Histamine H3 antagonists.136
The last library reexamined the R1 residue, including additional heterocycles and aromatic moieties. Protecting bromomethoxy phenethyl amine 21 as the phthalimide by reacting it with 1,2-dicarboxybenzene, DIC, HOBt and DIEA, followed by BBr3 demethylation yielded phenol 22. Alkylation with chloroethyl pyrrolidine and deprotection with hydrazine, both under microwave conditions, followed by coupling with a diverse set of R1-acids with DIC, HOBt yielded final compounds 23. Five membered heterocycles in the R1-position showed superior activities compared to pyridine and substituted benzenes, with 5-oxazole (24) and 2-thiazole (25) displaying the best potencies, Kis = 32 nM and IC50 = 72–83 nM, respectively. This iterative parallel library campaign successfully optimized H3 antagonism properties of a natural product lead structure over 30-fold.
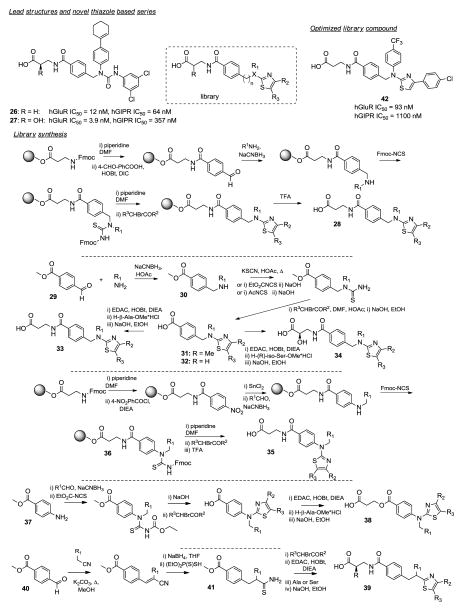
Human Glucagon Receptor Antagonists.179
The glucagon receptor is a member of the class B G-protein coupled family of receptors. Glucagon maintains glucose homeostasis during the fasting state by promoting hepatic gluconeogenesis and glycogenolysis. Antagonizing the glucagon receptor is expected to result in reduced hepatic glucose overproduction, leading to overall glycemic control and a possible treatment for type 2 diabetes. Only a few classes of non-peptidic glucagon receptor antagonists are known (Figure 3). Madsen and co-workers had previously described β-alanine and isoserine urea based human glucagon receptor (hGluR) antagonists 26 and 27, whereby 27 showed improved selectivity over the related human glucose-dependent insulinotropic receptor (hGIPR). Here the authors explored the replacement of the urea linkage with a heterocyclic scaffold, thus rigidifying the molecule.179 A library assisted optimization strategy was pursued, combining solid and solution phase synthesis approaches, with the goal of finding a novel potent, orally available, hGluR selective antagonists. For the first library on solid support, 4-formylbenzoic acid was coupled to deprotected Fmoc-β-Ala-Wang resin with HOBt/DIC, followed by reductive amination with R1-amines and treated with Fmoc-NCS to afford resin-bound Fmoc protected thioureas. Thiazole formation was accomplished through reaction with α-bromoketones after Fmoc-deprotection and final compounds 28 were obtained after cleavage from resin with TFA. Over 800 analogs were prepared via this procedure. Compounds were also prepared via solution-phase starting from 4-formylbenzoic acid methyl ester 29, which was reductively aminated with R1-amines utilizing sodium cyanoborohydride. The benzylic amines 30 were converted to their corresponding thioureas employing different methods depending on the reactivity of the respective secondary amine. The aminothiazole 31 was obtained via reaction with α-bromoketones in acetic acid. The methyl ester was cleaved with NaOH and the resulting carboxylic acid 32 was coupled with either β-alanine or (R)-isoserine methyl ester, followed by hydrolysis to yield the final compounds 33 and 34, respectively. Compounds 35 with the aminothiazole directly attached to the aromatic ring were prepared in similar fashion both on solid support and solution-phase. 4-Nitrobenzoyl chloride was reacted with resin-bound β-alanine, the nitro group was reduced with SnCl2, followed by reductive alkylation with R1-aldehydes. Thiourea 36 was obtained after reaction with Fmoc-isothiocyanate. After deprotection, cyclization with α-bromoketones and cleavage from resin final compounds 35 were obtained in high yields and purities. Using a solution-phase approach, compounds from this type were obtained by reductive amination of 4-aminomethyl benzoate 37 with R1-aldehydes, followed by reactions with EtO2C-NCS, NaOH, α-bromoketones and subsequent EDAC, HOBt coupling with β-alanine methyl ester and hydrolysis to give 38. Finally, compounds 39 containing a 5-alkylthiazole core were prepared starting with a Knoevenagel condensation of 4-methyl benzoate 40 with phenyl acetonitriles, followed by reduction with NaBH4 in THF and treatment with dithiophosphoric acid O,O-diethylester. The resulting saturated thioamide 41 was reacted with α-bromoketones and coupled to β-alanine methyl ester followed by hydrolysis. Aliphatic R1 groups were not well tolerated. Compounds with 4-CF3-, 4-OCF3- and 4-SCF3-phenyl in the R1 position displayed good binding affinities and superior rat PK properties. Compound 42 (hGluR IC50 = 93 nM, hGIPR IC50 = 1,100 nM) showed high oral bioavailability (58%), low clearance (1mL/min)/kg, long plasma T1/2 (228 min after i.v. administration), and extremely high plasma exposure (Cmax = 2100 ng/mL) in the rat (3 mg/kg, p.o.). Changing the β-alanine to isoserine in this series did lead to a loss of mGluR activity. Compounds with modified core structures retained binding affinities but displayed less favorable PK profiles. The SAR for R2 and R3 residues is of minor importance as long as the substituents on R2 are lipophilic and in meta or para position of the phenyl ring. Compound 42 was tested in a non-human primate model of hyperglucagonaemia and hyperglycaemia. It dose-dependently decreased glucagon stimulated glycaemia and abolished the hyperglycemic effect of exogenously administered glucagon completely at i.v. doses of 1 and 3 mg/kg. Compound 42 also showed high plasma exposure and a long plasma half-life in monkeys. This library approach demonstrated that the urea linkage in previously reported hGluR antagonists can be successfully replaced by a number of different thiazole cores and established SARs for binding, selectivity and PK properties for this novel chemical class.
Figure 3.
Glucogon receptor antagonists.179
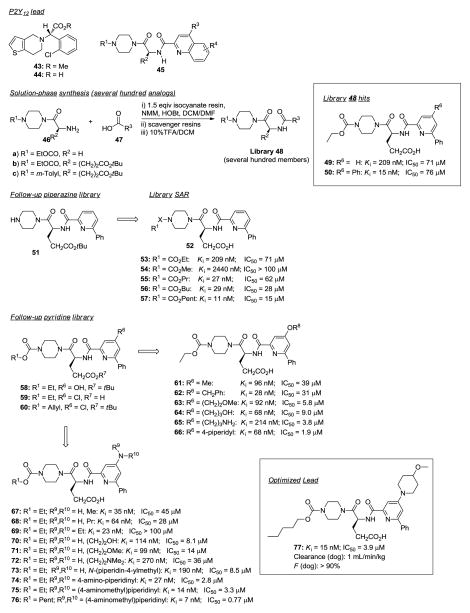
Purinergic P2Y12 receptor antagonists.221
Plavix® (clopidogrel, 43) is an antiplatelet agent approved for stroke and myocardial infarction in patients with atherosclerosis. Its mechanism of action is thought to proceed via metabolism to acid 44 followed by irreversible inactivation of P2Y12, a platelet specific GPCR (Figure 4). This in turn leads to a reduction in adenosine diphosphate (ADP)-stimulated platelet aggregation and the formation of platelet aggregates thereby providing therapeutic benefit. Because 43 is a prodrug requiring metabolic activation, it cannot be used effectively in patients that require emergency treatment (delayed time of action) or in patients who cannot metabolize 43. Recognizing the shortcomings of 43, Parlow and colleagues sought to find a new class of P2Y12 receptor antagonists having direct action on the receptor.221 P2Y12-based quinoline antagonists were previously reported by Berlex and this class was used as a starting point for library design and SAR exploration. Initial efforts focused on replacing the quinoline moiety employing polymer-assisted solution-phase chemistry. Piperazine derivatives 46 were coupled with heteroaromatic carboxylic acids 47 in the presence of resin-bound carbodiimide and HOBt. Excess reactants were sequestered with resin-bound isocyanate and a resin-bound secondary amine providing clean intermediates that, post TFA treatment, afforded desired carboxylic-acid-containing analogs 48. Hundreds of compounds were prepared using this approach. The library was evaluated in a human-derived P2Y12 receptor binding assay, screening for % inhibition at 10 μM. Ki values and inhibitory effects in a human platelet rich plasma (PRP) assay were obtained for the more potent compounds. The results from the first round of library evaluation indicated that replacing the original quinoline group with pyridine generally produced compounds with nanomolar Ki values, although with poor PRP inhibitory effects. Since the most potent compounds had one or two phenyl groups on the pyridine ring (49 and 50, respectively), the authors selected 49 as the lead for further SAR exploration and optimization. The next step consisted of SAR exploration around the piperazine moiety 51. A broad range of alkylating, acylating and sulfonating agents were used in conjunction with a scavenger resin to remove residual electrophile reactants. Biological assays showed that activity was driven by the carbamate group where the longer the aliphatic carbamate chain, the greater receptor affinity binding and, importantly, inhibitory PRP activity (53-57: Kis = 2440 to 11 nM; IC50s = >100 to 15 μM).
Figure 4.
P2Y12 receptor antagonists.221
The next SAR campaign focused on the 4-position of the pyridine ring. The authors opted to make chloro- and phenol-containing intermediates (58-60) to use these functional groups as handles to introduce chemical diversity. Phenol 58 was O-alkylated with alcohols under Mitsunobu reaction conditions (DEAD, PPh3, THF) or with halides (Cs2CO3, KI, DMF) ultimately yielding carboxylic acid ethers 61-66. Although the SAR was rather flat, a trend emerged for increased PRP activity with the phenolic ethers (65, 66: IC50 = 3.8 and 1.9 μM, respectively).
In addition to 4-ether substitution, 4-amino substitution was explored. This was carried out by treating carbamate 55 with 20 equiv of amine to displace the 4-chloro group yielding 4-aminopyridine ethyl carbamates 67-76. Simultaneous variation of the 4-position and the carbamate moiety was achieved using the same 4-chloro displacement strategy with 60 followed by alloc deprotection, N-piperidine carbamoylation and TFA deprotection. Small secondary alkyl amines were preferred over tertiary amines (IC50s for 67 and 68 versus 69) while amino ethers exhibited higher PRP inhibitory activity (70, 71); particularly effective were the amino piperidyl and amino piperazine groups (72-75). Lastly, increasing the length of the alkyl carbamate chain led to further increases in Ki and IC50 (76: Ki = 7 nM; IC50 = 0.77 μM). Evaluation of the most potent candidates in this series led to the discovery of 77 with a satisfactory in vivo profile, oral bioavailability, and some 340-fold more selective for the P2Y12 receptor versus its closest homolog, the P2Y13 receptor.
Heat shock protein 90 (Hsp90) Inhibitors.41
Hsp90 has gained attention in the pharmaceutical industry due to its participation in multiple cell signaling pathways in cancer cells (e.g., PI3K/Akt). Hsp90 is an ATPase protein that acts as a chaperone, binding to multiple oncology relevant client proteins (e.g., Her2, cKit, MET), stabilizing these proteins so to permit their cell signaling function. Inhibition of Hsp90 prevents the necessary folding required to bind and stabilize client proteins. This results in the degradation of the client proteins via the ubiquitin proteasome pathway making Hsp90 an attractive oncology target. Geldanamycin and close synthetic analog 17-allylamino-17-demethoxygeldanamycin (17-AAG) bind to the ATP active site in the N-terminal domain inhibiting Hsp90 function validating this to be a viable mode of action to disrupt Hsp90’s biological function. Poor water solubility and hepatotoxicity limit the clinical utility of these agents and thus, new small molecules are sought targeting the ATP binding site to inhibit Hsp90 function. Radicicol is an inhibitor of Hsp90 via ATP competition binding. Promising results have been noted by several research programs with resorcinol-based Hsp90 inhibitors. However, a common limitation to this compound class is that the phenol groups can be substrate sites of metabolic degradation processes (e.g., glucuronidation) resulting in fast in vivo clearance.
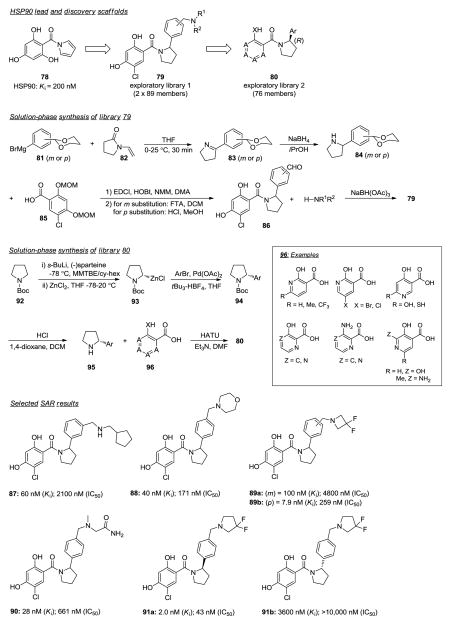
Cho-Schultz and colleagues focused their attention on compound 78, an amide-containing polyphenol and ATP binding site-directed inhibitor of Hsp90 (Figure 5).41 A two-stage solution-phase strategy was devised to explore the chemical space and develop an SAR around 78 to produce new potent Hsp90 inhibitors without phenol groups. The first library of compounds focused on the solution-phase synthesis of 79. The objective was to improve binding affinity and reduce or eliminate glucuronidation by increasing hydrophilicity via the introduction of basic amino fragments. Library synthesis commenced by the addition of Grignard intermediate 81, derived from m- and p-1,3-dioxane-subsituted bromobenzene to N-vinyl pyrrolidinone 82 affording 2-pyrrolines 83 (23–29% yields). These cyclic imines were reduced to 84 with NaBH4 and then coupled to phenol-protected benzoic acid 85. Deprotection of the phenol groups with either TFA or HCl resulted in aldehydes 86 that were subjected to reductive amination with ca. 90 amines to afford the 178-compound library 79. Compounds were tested in a tritium-labeled-ligand competitive binding assay (Ki) followed by the measurement of Akt-degradation in H1299 lung cancer cells (IC50). The SAR indicated that para substituted compounds had higher binding affinity against Hsp90 and demonstrated superior activity in the cell-based assay (Kis = 5.4–40 nM; IC50s = 112–668 nM) versus the meta substituted compounds (Kis = 60–100 nM; IC50s = 2100–4800 nM). A cocrystal structure of Hsp90α with 91a (PDB code: 3HEK, resolution: 1.95 Å) established the (R) stereochemistry of the pyrrolidine group as crucial for the molecule to adopt the most favorable, lower-energy conformation to bind at the ATP site. In this configuration, the resorcinol group exhibits a salient H-bond interaction with Asp93, the amide group retains its expected planarity, the 3,3-difluroropyrrolidine group is placed into a hydrophobic region (Tyr137, Val136, Gly135), and the pendant phenyl group is also accommodated in a hydrophobic pocket. Chiral separation of other racemates confirmed the (R)-enantiomers bound far more potently against Hsp90 than their corresponding (S)-enantiomers. Unfortunately, all of the (R)-enantiomers exhibited high clearance in human hepatocytes. Armed with this knowledge, a second library was conceived focusing on the synthesis of (R)-enantiomers and the replacement of the phenol groups to improve the ADME profile. The chemistry relied on the enantioselective α-arylation of N-Boc-pyrrolidine 92 with (−)-sparteine to prepare chiral 93. Chiral organometallic 93 was coupled with aromatic bromides to afford key intermediate 94. Following Boc deprotection, amines 95 were coupled to a diverse group of aromatic carboxylic acids 96 using HATU to afford library 80. With this methodology enantiomeric ratios of >96:4 were achieved and allowed for the synthesis of a ca.112-membered library. Unfortunately, none of the compounds of library 80 exhibited inhibitory activity against Hsp90 when tested at 10 μM in the binding assay. Lastly, selected synthesis of mono-phenol analogs revealed that both phenol groups are essential for binding since the removal of either phenol group led to a >190-fold loss in potency.
Figure 5.
Hsp90 inhibitors.41
Selective Norepinephrine Reuptake Inhibitors.73
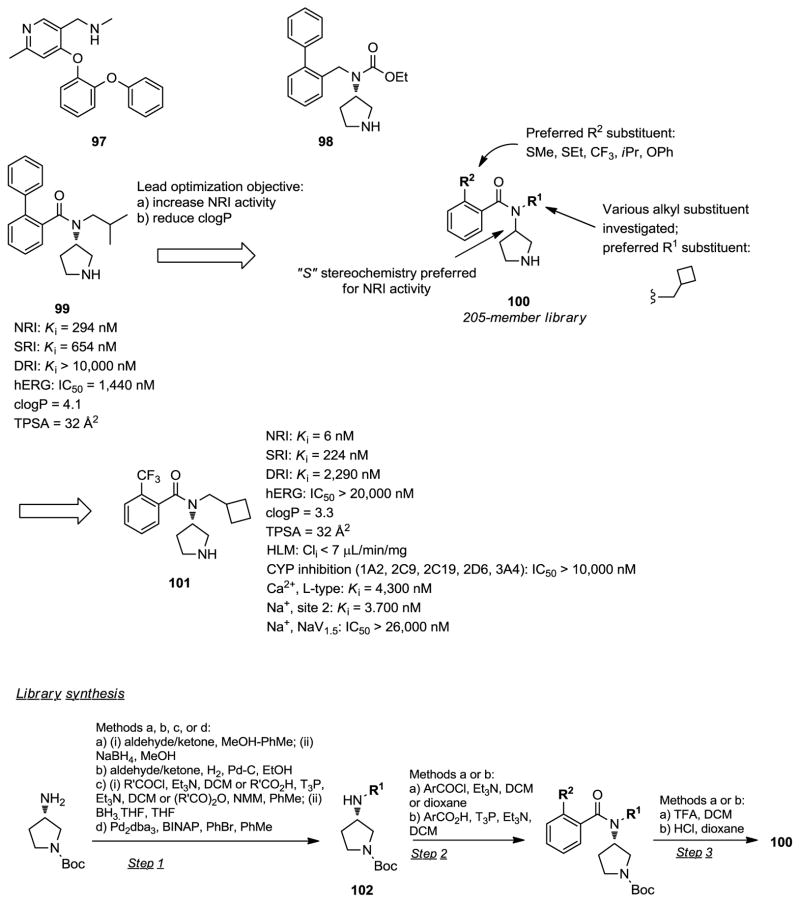
The norepinephrine transporter is a membrane bound protein which regulates the uptake of the neurotransmitter norepinephrine (NE) from the presynaptic cleft of noradrenergic neurons during synaptic transmission. It therefore plays an important role in regulating the physiological functions of NE, the deficiency of which has been implicated in a number of neurological disorders. Norepinephrine reuptake inhibitors (NRIs) such as reboxetine and atomoxetine have been used clinically for the treatment of major depressive disorder and attention deficit hyperactivity disorder (ADHD), respectively. Researchers at Pfizer disclosed recently several new chemical series of selective norepinephrine reuptake inhibitors (sNRI).73 The two lead compounds 97 and 98, investigated clinically, were discontinued due to hepatotoxicity (Figure 6). This safety issue identified with 97 and 98 was thought to be related to the high lipophilicity of these compounds (clogP = 4.2–4.4). A search for new NRI templates, providing ligands with reduced lipophilicity when compared to 97 or 98, was then undertaken. The benzamide derivative 99 was identified, from previous lead identification work, as a weak norepinephrine reuptake inhibitor with low selectivity over the serotonin transporter (Ki (NET) = 294 nM; Ki (SERT) = 654 nM). The lead optimization objective was to improve the affinity and the selectivity for the norepinephrine transporter, while simultaneously reducing the lipophilicity in this series. Analogs of 99 (205-member library; general structure 100) were readily available via solution phase parallel synthesis methodology. The N-alkyl or N-aryl substituted 3-amino-1-Boc-(S)-pyrrolidines 102, obtained from 3-amino-1-Boc-(S)-pyrrolidine using common methodologies, were converted to the target compounds 100 via amide formation followed by N-Boc deprotection. The binding affinity of the library compounds at the human norepinephrine, serotonin, and dopamine transporters was determined using scintillation proximity assay (SPA) technology. SAR analysis in this series revealed that potent norepinephrine reuptake inhibition could be achieved over a range of lipophilicity (clogP: 3.0–4.4). The lead optimization campaign led to the discovery of compound 101, a potent norepinephrine reuptake inhibitor (Ki = 6 nM) displaying 37-fold and 380-fold selectivity over the serotonin and dopamine transporters, respectively. The lipophilicity of 101 (clogP = 3.3) was also significantly reduced when compared to previous lead compounds 97 and 98 investigated clinically (clogP = 4.4 and 4.2, respectively). Further studies demonstrated that 101 has excellent metabolic stability in human liver microsomes and human hepatocytes, weak CYP (1A2, 2C9, 2C19, 2D6, 3A4) inhibition, and good membrane permeability. In light of its weak inhibitory activities at the hERG (IC50 >20,000 nM) and NaV1.5 (IC50 > 26,000 nM) ion channels, 101 is expected to have a satisfactory cardiovascular safety profile. Off-target profiling of 101 at a panel of 110 receptors, enzymes and ion channels revealed only weak affinity at the M4 (Ki = 4,300 nM) and M5 (Ki = 1,800 nM) muscarinic receptors. In vivo rat microdialysis experiments demonstrated that 101 produces a rapid increase in NE levels in the prefrontal cortex after subcutaneous administration demonstrating good CNS penetration. Based on its favorable profile, 101 (PF-3409409) was selected for preclinical evaluation.
Figure 6.
Selective noradrenaline reuptake inhibitors.73
Positive Allosteric Modulators of mGluR4.287
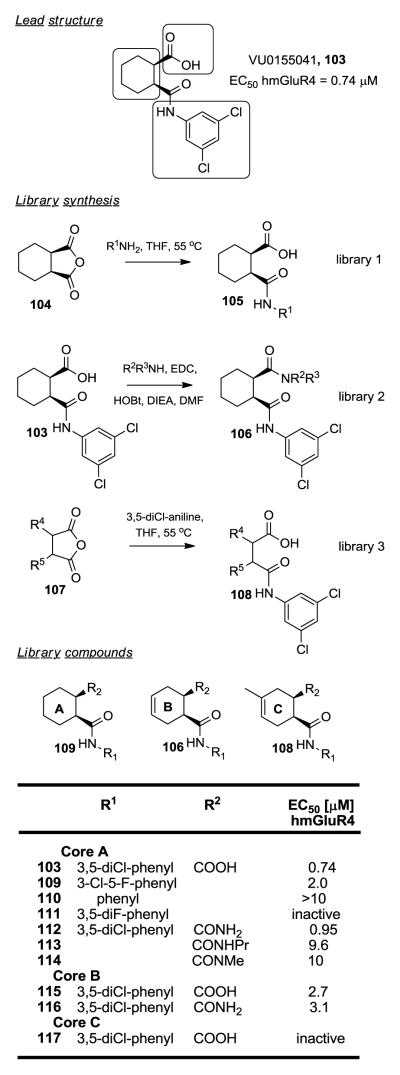
Metabotropic glutamate receptors (mGluRs) play important roles in a broad range of central nervous system functions and have therapeutic potential in a variety of neurological and psychiatric disorders. Activation of metabotropic glutamate receptor 4 (mGluR4) has been shown to modulate neurotransmission in the basal ganglia and results in antiparkinsonian effects in rodent models of Parkinson’s Disease (PD). Allosteric binding sites, as opposed to traditional orthosteric binding sites, offer unparalleled opportunities for drug discovery by providing high levels of selectivity, mimicking physiological conditions and affording fewer side effects. VU0155041 103, a novel mGluR4 positive allosteric modulator (PAM) discovered in a high throughput screen was the starting point of a detailed SAR analysis of this compound class (Figure 7). Three parts of the lead molecule were explored separately through an iterative parallel synthesis approach, the aromatic amide moiety, the carboxylic acid residue and the cyclohexyl core. The first library centered around commercially available cis-1,2-cyclohexanedicarboxylic anhydride 104, which was reacted with respective R1-amines in THF at 55 °C to give library compounds 105. Several 3,5- and 3,4-substituted phenyl, unsubstituted phenyl, benzyl and pyridyl, morpholino and cyclo alkyl analogs were evaluated. Besides the original 3,5-dichlorophenyl lead 103 (EC50 hmGluR4 = 0.74 μM), only the 3-Cl-5-F-phenyl amide 109 retained some activity (EC50 hmGluR4 = 2 μM), all other substitutions led to a loss of activity. Carboxylic acid replacements were investigated next, starting from VU0155041 103, coupling respective R2,R3-amines with EDC, HOBt and DIEA in DMF a library of diamides 106 was synthesized. All compounds showed at least a tenfold loss of activity, with the exception of the primary carboxamide 112, which retained similar submicromolar activity as the lead (EC50 hmGluR4 = 0.95 μM). Variations of the core structure were then examined. Commercially available cyclic anhydrides 107 were reacted with 3,5-dichloroaniline in THF at 55 °C. Only the cyclohexene analog 115 retained some activity (EC50 hmGluR4 = 2.7 μM), substituted cyclohexene cores (e.g. 117) resulted in inactive compounds. Changing the substitution pattern to a 1,3-orientation led to a total loss of activity. Combining the cyclohexene core with a primary carboxamide in compound 116 showed some activity (EC50 hmGluR4 = 3.1 μM).
Figure 7.
Positive allosteric modulators of mGluR4.297
This SAR evaluation around VU0155041 103, utilizing a parallel synthesis approach, illustrates another example of the rather flat SAR common to positive allosteric modulators.
mGluR5 allosteric modulators.71
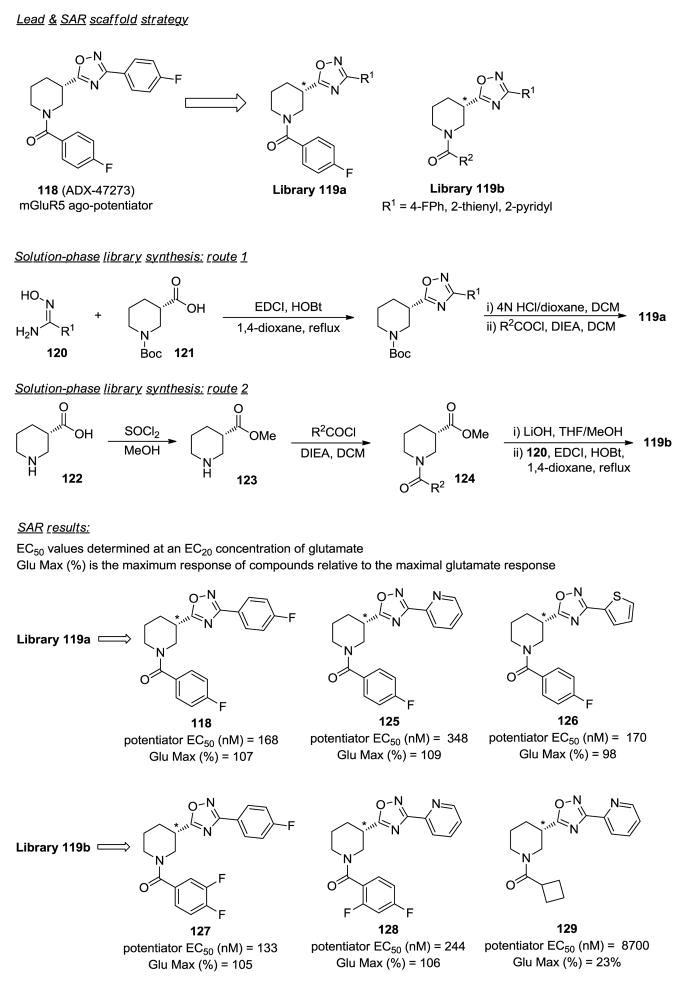
Excess dopamine transmission in the brain is believed to be one cause of schizophrenia and antipsychotic agents that antagonize the dopamine D2 receptor are routinely prescribed. Many of these agents display off target pharmacology against a range of neurotransmitters leading to side effects. It was observed clinically that administering glycine, an N-methyl-D-aspartate (NMDA) receptor co-agonist, elicited a modest improvement in schizophrenic patients suggesting that activation of the NMDA receptor could be an option for therapeutic treatment. Potentiation of NMDA receptor transmission can occur directly by modulating NMDA receptor sites or indirectly by activating NMDA-receptor-function dependent GPCRs. Metabotropic glutamate receptor 5, mGluR5, is a GPCR that upon activation can potentiate indirectly the NMDA receptor. Using ADX-47273 (118), a previously reported positive allosteric modulator of mGluR5 demonstrating in vivo activity in cognition and schizophrenia animal models, Engers and colleagues generated solution-phase libraries around scaffold 119 to flesh out the SAR of 118 and improve its overall physicochemical properties (Figure 8).71 Two routes were developed for library synthesis. The first route involved simultaneous coupling-cyclization of (Z)-N′-hydroxyimidamides 120 and (S)-1-(tert-butoxycarbonyl)piperidine-3-carboxylic acid 121 using EDCI and HOBt under reflux to give oxadiazoles. Removal of the Boc group and acylation of the piperidine intermediates afforded library 119a. The second route consisted of the esterification of (S)-piperidine-3-carboxylic acid 122, acylating ester 123, saponifying N-acyl esters 124 with LiOH and then simultaneous coupling-cyclization with (Z)-N′-hydroxyimidamides 120 to afford desired library 119b.
Figure 8.
mGluR5 allosteric modulators.71
In the first exploratory library, the 4-fluorobenzylpiperamide was kept constant and the 4-fluorophenyl ring on the oxadiazole unit was replaced. It was found that 2-pyridyl and 2-thienyl groups were reasonable substitutes for the 4-fluorophenyl group while the 3- and 4-pyridyl and 2-pyrazinyl groups were not. In the follow-up library 119b, which retained the original 4-fluorophenyl and now included the 2-thienyl and 2-pyridyl (R1) groups, the acyl group (R2) was varied. Biological evaluation of this library revealed the following trends. For R2, most mono- and di-fluorophenyl groups exhibited submicromolar ago-potentiation (potentiation of the agonist activity of glutamate at its EC90) while the 2,6-difluorophenyl group did not. The 2-pyridyl analogs e.g. 125, were 2-times less potent than their 4-fluorophenyl and 2-thienyl counterparts. Unexpectedly pyridyl compounds, e.g. 128, displayed pure mGluR5 positive allosteric modulation as these compounds had no inherent agonist activity. The corresponding HCl salts of the pyridyl analogs exhibited expected improved water solubility. Interestingly, the cyclobutyl analog 129 was a negative allosteric potentiator, an example of an unusual functional switch. Finally, in order to establish the importance of the chiral center, enantiomeric separations were carried out on 118, 125 and 126. The (R)-enantiomers were in general 9- to 10-fold less potent than the (S)-enantiomers, yet display similar in vitro efficacy. With this study the SAR features of 118 were quickly identified, particularly the relevance of the (S)-chiral center.
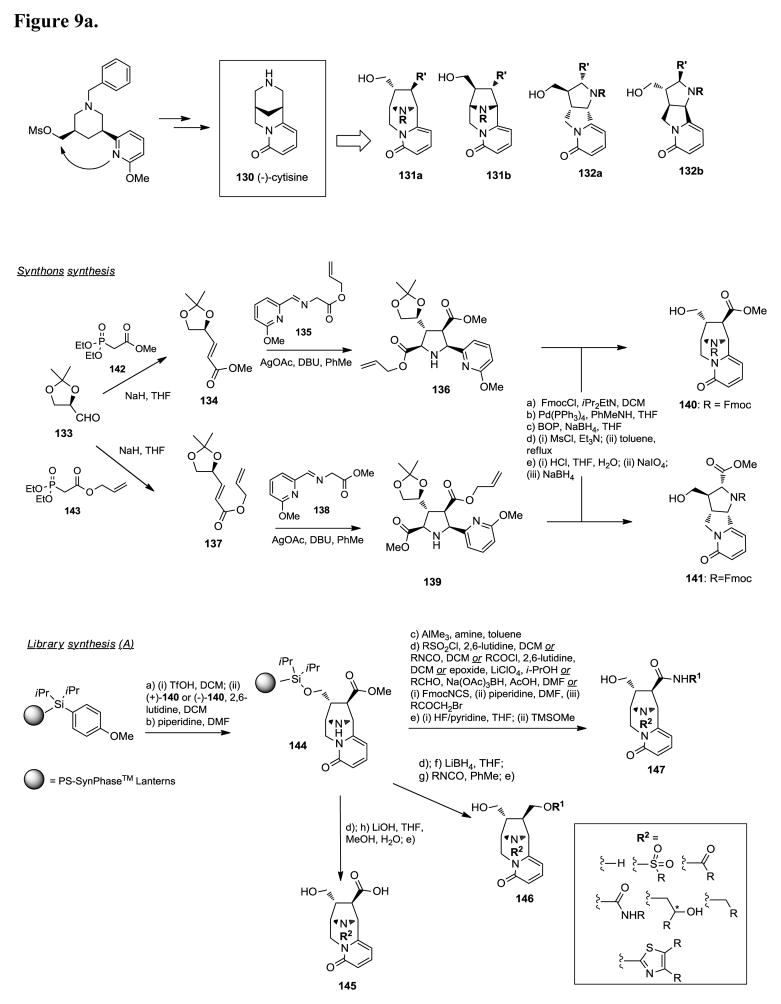
Bcl-2 inhibitors: Diversity-Oriented Synthesis Library.182
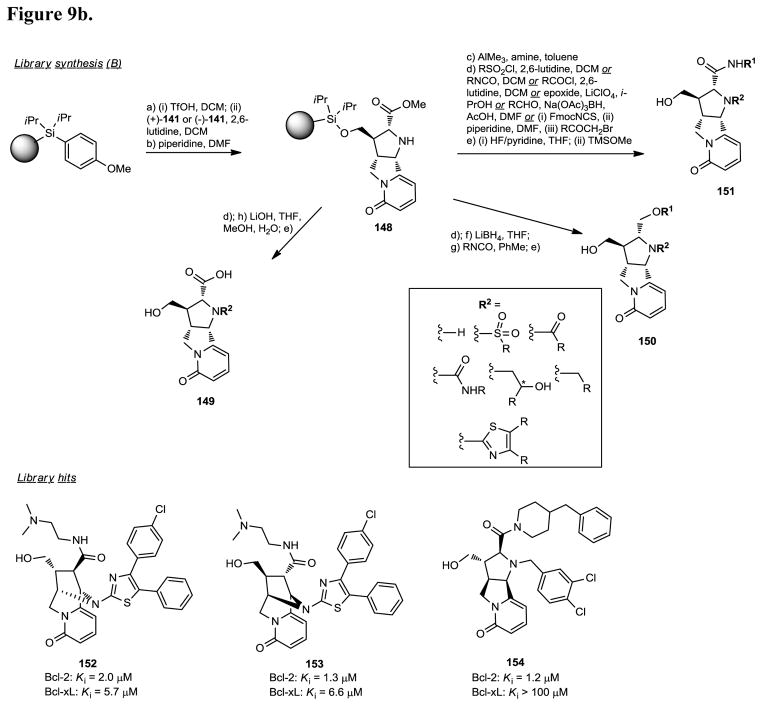
Apoptosis, or programmed cell death, is important for normal development, host defense, and suppression of oncogenesis, and disregulation of apoptosis has been implicated in cancer and many other human diseases. The Bcl-2 family proteins are central regulators of apoptosis and comprise anti-apoptotic proteins such as Bcl-2, Bcl-xL, and Mcl-1 and pro-apoptotic proteins such as Bak, Bax, Bim, Bid, and Bad. Overexpression of the Bcl-2 membrane protein has been observed in 70% of breast cancer, 30–60% of prostate cancer, 80% of B-cell lymphomas, 90% of colorectal adenocarcinomas, and many other forms of cancer. The expression levels of Bcl-2 proteins also correlate with resistance to a wide spectrum of chemotherapeutic drugs and γ-radiation therapy. Bcl-2 is therefore a promising molecular target for the design of an entirely new class of anticancer drugs aimed at overcoming resistance of cancer cells to apoptosis. Consequently, design of non-peptide small molecule inhibitors of Bcl-2 and Bcl-xL is currently an exciting research area for the development of new anticancer agents. In an effort to identify novel inhibitors of Bcl-2, scientists at Infinity Pharmaceuticals designed and prepared a 15,000-member pyridone library (Figure 9a/b), via discovery oriented synthesis (DOS).182 The design of this library was based on the structure of the nicotinic agonist (−)-cytisine (130) and previous work describing the total synthesis of this natural product. In particular, the pyridone cyclization step, key transformation for the total synthesis of (−)-cytisine, was exploited for the diversity oriented synthesis of heterocycles 131a and 132a as well as their corresponding enantiomers 131b and 132b, respectively. The synthons 140 and 141 used for the diversity oriented library synthesis were prepared according to Figure 9a. Condensation of D-glyceraldehyde acetonide 133 with phosphonate 142, under Horner-Emmons conditions, provided the α,β-unsaturated ester 134, which reacted with the imine 135 in toluene in the presence of AgOAc and DBU to provide the corresponding [3+2] azomethine ylide-alkene cycloaddition product 136 in high yield and excellent diastereoselectivity (>95:5). The pyrrolidine derivative 136 was then converted to the bridge bicyclic scaffold 140 in 5 steps, i.e. a) protection of the NH functionality of 136 as the N-Fmoc derivative, b) deprotection of the allyl ester functionality by treatment with Pd(PPh3)4, c) conversion of the resulting carboxylic acid to the corresponding primary alcohol, d) activation of the resulting alcohol with mesyl chloride followed by formation of the pyridone ring via intramolecular cyclization, and e) conversion of the 1,3-dioxolane, 2,2-dimethyl functionality to the corresponding primary alcohol according to a 3-step sequence. The tricyclic synthon 141 was prepared from phosphonate 143 via a synthetic sequence similar to the one described for the preparation of 140. The pyridone scaffolds 140, 141, prepared in large scale (>75g), were then loaded onto silicon-functionalized Lanterns via activation with TfOH (average loading level of 15 mmol of compound per Lantern). Deprotection of the N-Fmoc functionality provided the resins 144 and 148 which were converted to the library compounds 145-147 and 149-151 via classical solid-phase derivatization methodologies (Figure 9b). The 15,000 library compounds, prepared with purities greater than 75% for over 75% of the library, were screened for binding affinity for Bcl-2 and Bcl-xL. The most potent compounds derived from the bridged bicyclic pyridone scaffolds 131a and 131b, exemplified by compound 152 [Ki (Bcl-2) = 2.0 μM; Ki (Bcl-xL) = 5.7 μM)] and its enantiomeric analog 153 [Ki (Bcl-2) = 1.3 μM; Ki (Bcl-xL) = 6.6 μM)], contained a chloro-substituted diphenyl 2-aminothiazole and a diamine at the R and R′ position, respectively. Compound 154 was the best ligand identified from the tricyclic pyridone cores 132a and 132b. This compound selectivitly binds with micromolar affinity at Bcl-2 (Ki = 1.2 μM) and displays, in contrast to 152,153, high (> 100-fold) selectivity for Bcl-2 over Bcl-xL (Figure 9b).
Figure 9.
Figure 9a. Bcl-2 inhibitors via DOS library.182
Figure 9b. Bcl-2 inhibitors via DOS library (continued).
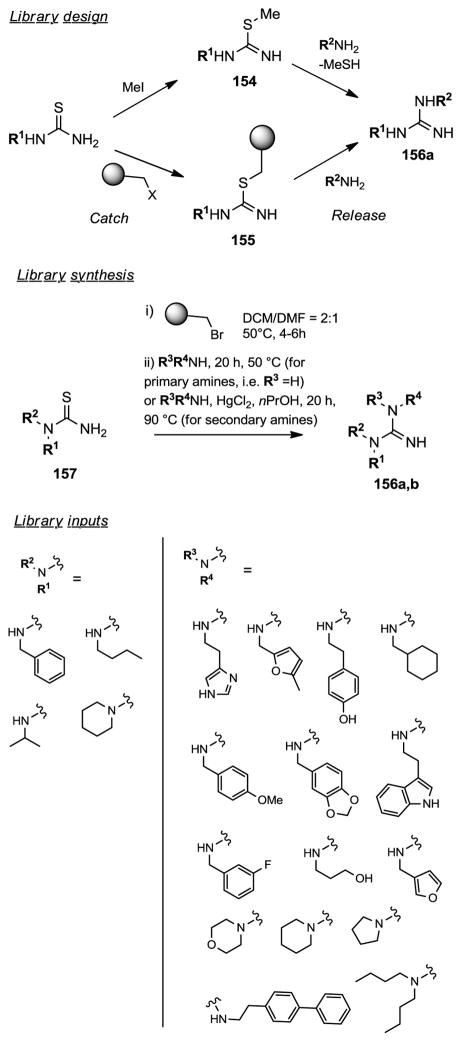
Catch and Release Synthesis of Substituted Guanidines.289
One of the most common methods to prepare substituted guanidines 156 involves the condensation of amines with S-methylated thioureas 156 (Figure 10). This method suffers from the formation of noxious and toxic methyl mercaptan side product, which limits application for high-throughput parallel synthesis. To overcome this problem, scientists at Abbott designed a solid phase strategy for the synthesis of N,N′,N″-substituted guanidines using a catch and release methodology inspired from the solution phase synthesis described above.289 In this method, the thioureas 155 immobilized on solid support react with various amines to provide the desired guanidine derivatives, free of mercaptan side products. Using this new methodology, and after several optimization of the reaction conditions, various substituted guanidines were prepared from thioamides in a one-pot process. Hence, loading of N-substituted thioamides 157 to brominated polystyrene resin in a mixture DCM/DMF (2:1) at 50°C for 4–6 h provided the thiourea resins 155, which could be washed, dried, and stored or used without isolation for the next step. Condensation of the resin-bound thioureas with primary amines at 50 °C for 20 h provided, in high yield, the desired substituted guanidines 156a,b purified by HPLC and isolated as their TFA salts. The reaction conditions were modified to allow the formation of N,N′,N″-trisubstituted guanidines from the corresponding secondary amines in good yields. In the modified method, the condensation of secondary amines to the resin-bound thioureas was conducted in methoxyethanol in the presence of 1.5 equiv. of HgCl2. Using this new solid phase methodology, a total of 17 N,N′-disustituted or N,N′,N″-trisubstituted guanidines 156a,b were prepared, from the corresponding thioamides, in isolated yields ranging from 42% to 99%.
Figure 10.
Catch and release synthesis of substituted guanidines.289
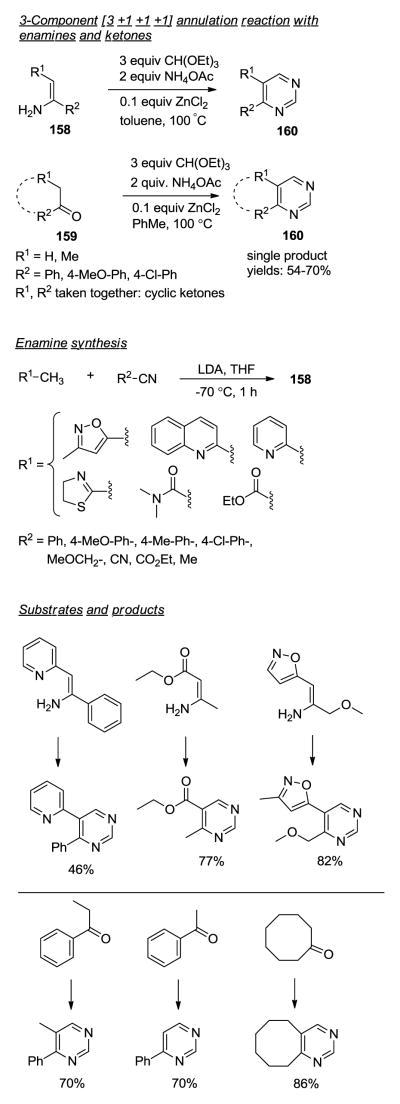
Substituted Pyrimidines via a 3-component reaction.158
Konakahara and coworkers developed a novel three-component coupling reaction of enamines 158, triethyl orthoformate and ammonium acetate providing a facile route to 4,5-disubstituted pyrimidine derivatives 160 (Figure 11). Pyrimidine derivatives exhibit biological activity against all major classes of molecular targets including kinases, proteases, nuclear hormone receptors and GPCRs. Bredereck- and Pinner-type chemistries are established routes for the preparation of pyrimidines. However, challenging multistep synthesis of reactive intermediates and harsh reaction conditions limit their general utility, particularly for the preparation of pyrimidine-based arrays. The initial 3-CR was conducted by simply heating enamines 158 with 3 equiv of orthoester and 2 equiv of NH4OAc in toluene at 100 °C for 20 h. Optimized reaction conditions included the addition of 0.1 equiv of ZnCl2 which improved the overall yield (>70%), accelerated reaction time and enhanced the range of substrates. Non-commercially available enamines were prepared in high yield (>90%) by the addition of electron-withdrawing stabilized carbon nucleophiles to arylnitriles. The ZnCl2-catalyzed three-component reaction was expanded by using ketones 159 in place of enamines. The 3-CR with ketone substrates required 72 h for completion affording the desired products in good yields (54–70%).
Figure 11.
MCR to pyrimidine derivatives.158
Discovery of Ned-19.340
Second messengers play enormously important roles in transduction of cellular signals and govern cellular responses to stimuli. Molecular and cellular biology has benefited greatly from molecular tools like Forskolin which alters cellular levels of the critical second messenger cAMP. Nicotinic acid adenine dinucleotide phosphate (NAADP, 161) is recognized as a critical regulator of Ca2+ release in numerous human tissues in response to several reported stimuli (Figure 12). Controversies surrounding the exact nature of NAADP as a secondary messenger and its mechanism of action permeate the literature. Furthermore, the lack of chemical probes of this important biomolecule hamper studies into the cause and consequences associated with Ca2+ release. To rectify this inadequacy, Churchill and coworkers reported the discovery of Ned-19 (162), a potent inhibitor of NAADP signaling.340 Utilizing the chemical structure (including electrostatic surface assessments) of NAADP, a ligand-based virtual screen was performed in which novel structures were sought with sufficient overlap with NAADP while maintaining drug-like properties in the ‘hit’ compounds. One agent that was discovered was an (S)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate designated as Ned-19. This commercially available agent inhibited NAADP signaling in a sea urchin egg assay at nanomolar concentrations. The stereochemistry at the 1 position of the tetrahydro-1H-pyrido ring was explored via an independent synthesis. The synthesis was initiated by installment of a chloromethyl group at the 3-position of commercially available 4-methoxybenzaldehyde. The resulting aldehyde was subjected into a Pictet-Spengler reaction with (S)-methyl 2-amino-3-(1H-indol-3-yl)propanoate to afford the core (S)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate scaffold. Displacement of the benzyl chloride with 1-(2-fluorophenyl)piperazine and saponification of the methyl ester provided Ned-19. The diastereomeric mixture was separated following the Pictet-Spengler reaction. The specific activity of both the cis and trans isomers of Ned-19 were evaluated and the trans isomer was significantly more active than the cis isomer. Biochemically, Ned-19 was able to eliminate all NAADP-mediated Ca2+ signaling at 100 μM without altering inositol 1,4,5-trisphosphate-mediated or cADP-ribose-mediated Ca2+ release. These experiments suggest that Ned-19 is selective as a NAADP signaling probe rather than a nonspecific modulator of Ca2+ release. Ned-19 blocks NAADP signaling in intact cells and it can serve as a fluorescent label for NAADP receptors. Finally, Ned-19 was used to demonstrate a role for NAADP signaling in glucose signaling and Ca2+ release in pancreatic beta cells. While further studies with Ned-19 are required to fully validate its use as a probe of NAADP signaling, this work offers a novel agent to begin to fully dissect the various roles of this important secondary messenger.
Figure 12.
Discovery of Ned-19.340
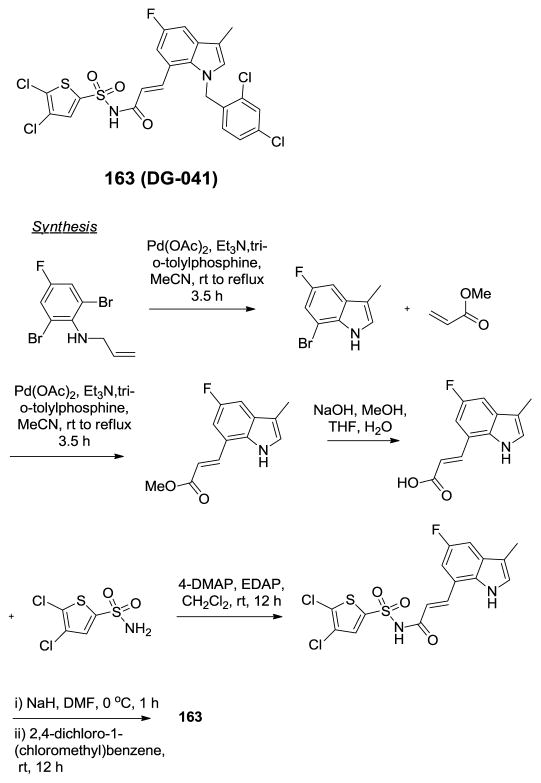
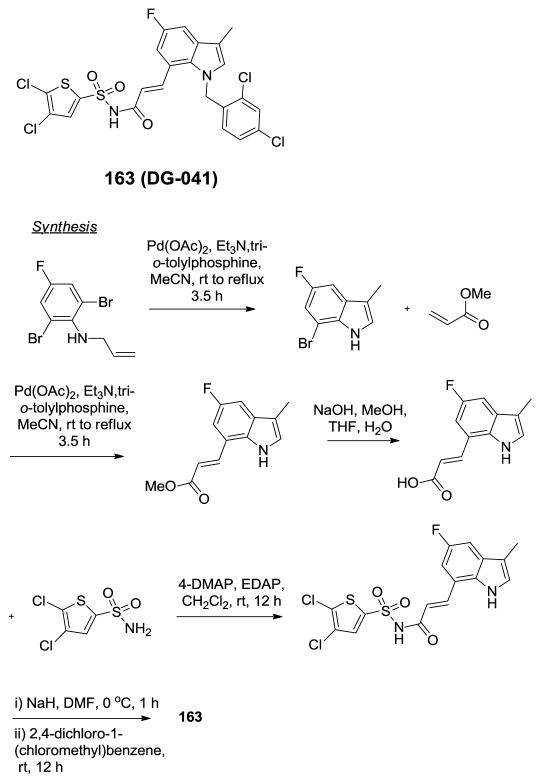
Discovery of DG-041.338
There exists a complex network of signaling events that govern platelet activation and aggregation and great strides have been made in modulating these relevant signaling events to avoid catastrophic acute thrombosis during vascular events like myocardial infarction and stroke. The recognition that aspirin (acetylsalicylic acid) acts as an antagonist of platelet aggregation can be considered a starting point for research into defining the critical targets associated with platelet function. More recently, the purinergic P2Y12 receptor has been shown to play an essential role in platelet signaling following platelet collagen and von Willebrand factor release and Ca2+ stimulation of TxA2 and release of ADP. P2Y12 receptor antagonists including prasugrel have proven more effective than aspirin in preventing recurrent myocardial infarction. Both aspirin and P2Y12 receptor antagonists, however, have the unwanted side-effect of severe, prolonged bleeding events due to their global impairment of platelet aggregation. Research has continued to shed light on the complex signaling cascade associated with platelet aggregation and today it is known that multiple GPCRs are responsible for both Ca2+ release and activation/inactivation of adenylcyclase. One target that is gaining attention is the EP3 receptor. The EP3 receptor responds to prostaglandin E2 (PGE2) which is produced in low concentrations within atherosclerotic plaque. Importantly, signaling through the EP3 receptor is not sufficient to produce an aggregation response without co-signaling events at one or more additional receptors that are associated with platelet activation. Studies have showed that platelets lacking the EP3 receptor were protected from thrombotic events in several models and it has been hypothesized that, since healthy tissues do not produce PGE2, blocking of the EP3 receptor should have a diminished effect on bleeding events. To examine this theory, researchers at deCODE chemistry sought out new EP3 receptor antagonists through a ligand-based design strategy (Figure 13).338 The basic strategy was to seek out scaffolds that mimic the three-dimensional shape and electrostatic profile of the native ligand PGE2. Ultimately efforts focused on a collection of substituted indoles with appendages at the 1 and 7 positions. Their initial leads possessed low-nanomolar activity at the EP3 receptor and lead optimization included blocking metabolically labile positions at C3 and C5 of the indole ring. The optimization campaign led to the discovery of DG-041 (163). DG-041 (163) displayed IC50 values of 8.1 nM and 4.6 nM in a Ca2+ response assay and a radioligand displacement assay, respectively. DG-041 had no relevant activity versus the functionally related, platelet-associated GPCR targets nor was this agent active across a panel of 50 random GPCRs. DG-041 was also found to be acceptable for both oral and i.v. dosing with appropriate PK properties for use as a tool compound in models of platelet aggregation. The synthesis of this agent was accomplished via sequential intramolecular and intermolecular Heck couplings to arrive at an appropriately 7-substituted indole intermediate. Saponification followed by coupling to 4,5-dichlorothiophene-2-sulfonamide and subsequent N-alkylation with 2,4-dichloro-1-(chloromethyl)benzene provided DG-041. DG-041 was found to inhibit human and rat platelet aggregation in vitro in a collagen induction experiment with several co-agonists and, importantly, in the presence of high serum concentrations. Within in vivo systems, DG-041 inhibited platelet aggregation at a dose of 10 mg/kg while there was no prolongation of bleeding up to a concentration of 100 mg/kg. The authors present several other findings that further validate DG-041 as an important new tool for studying the EP3 receptor and reference, as yet, unpublished data suggesting that DG-041 is efficacious in a human patient population.
Figure 13.
Discovery of DG-041.338
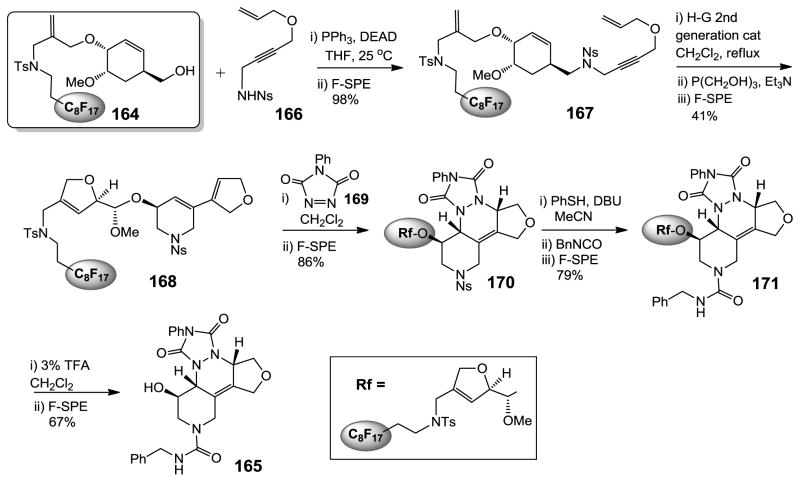
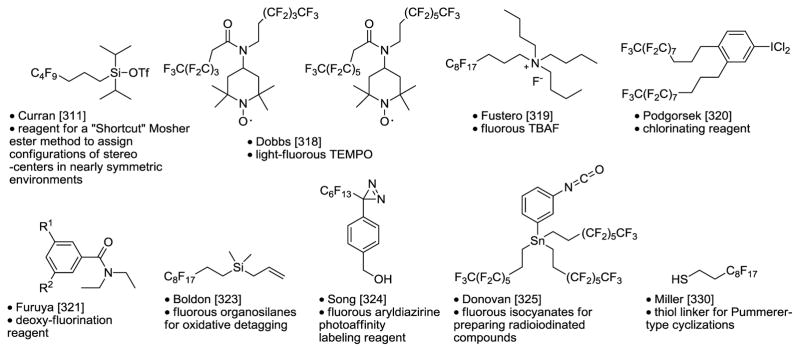
Displaceable fluorous dihydropyran linker.322
Fluorous linker combined with fluorous solid-phase extraction (F-SPE) is a powerful reaction and purification technique for solution-phase synthesis.380 The Nelson group introduced fluorous dihydropyran linkers for the protection of amino and hydroxyl groups. Figure 14 highlights the utility of displaceable linker 164 in the synthesis of highly condensed heterocyclic compound 165.322 The fluorous linker was attached to sulfonamide 166 through Fukuyama-Mitsunobu reaction to form compound 167. Cascade metathesis using Hoveyda-Grubbs second generation catalyst cleaved the central dihydropyran ring and produced compound 168. This compound was then used for Diels-Alder reaction with 4-phenyl-[1,2,4]-triazole-3,5-dione 169 to afford 170 as a single diastereomer. The removal of the o-nitrophenylsulfonyl (Ns) group from 170 followed by the reaction with an isocyanate gave urea 171. Finally, the fluorous linker was removed by the treatment of 3% TFA to afford product 165. In this multistep synthesis, all the intermediates and the final product were isolated from the reaction mixture by F-SPE.
Figure 14.
Fluorous dihydropyran linker.322
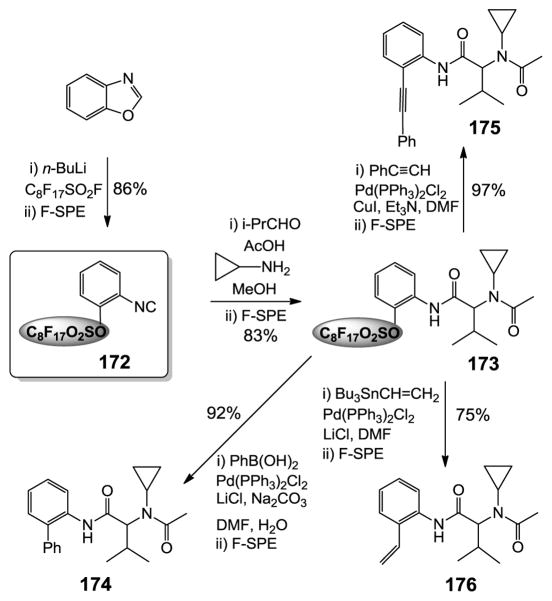
Displaceable fluorous isonitrile linker.327
Isonitriles are versatile reagents for organic reactions. However, their synthetic advantages are offset by the notorious odor associated with conventional isonitriles. The Pirrung group developed a new process using base-promoted ring-opening of oxazoles to produce aromatic isonitriles which no longer have the unpleasant odors.228 During the reaction process a fluorous sulfonate group could be introduced to form a fluorous linker. The utility of the displaceable fluorous isonitrile has been demonstrated in the multistep synthesis involving Ugi four-component reaction (U-4CR) and metal catalyst-promoted linker cleavage reactions. Freshly prepared isonitrile 172 was used as the limiting agent for the Ugi reaction to form 173 (Figure 15). This intermediate was then subject to different coupling reactions to remove the fluorous linker and also to introduce a new functional group to the Ugi condensation product. The developed post-Ugi reactions included Suzuki coupling to form product 174, Sonogashira reaction to form compound 175, and Stille coupling to form compound 176. The protocol is suitable for diversity-oriented synthesis of compound libraries since four substitution groups can be introduced during the Ugi reactions and different linker cleavage reactions could lead to an array of scaffolds.
Figure 15.
Fluorous isonitrile linker.327
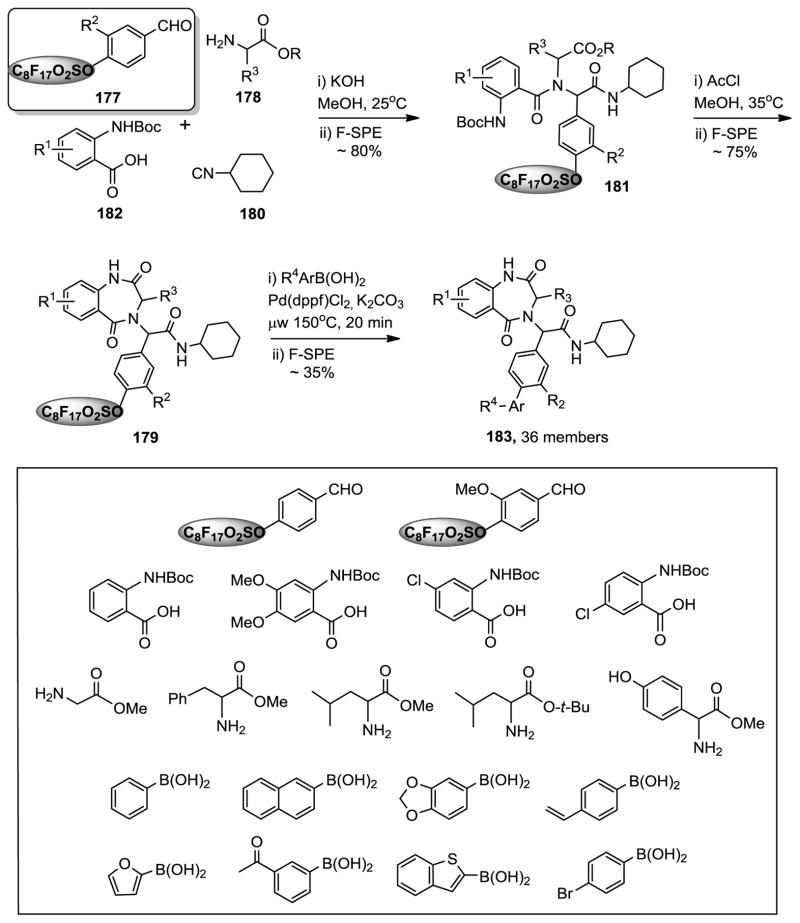
Fluorous synthesis of 1,4-benzodiazepine-2,5-dione library.327
In the solution-phase parallel synthesis of a 1,4-benzodiazepine-2,5-dione library, the Yang group developed a new U-4CR using fluorous benzaldehydes instead of fluorous isonitriles (Figure 16) as the displaceable linker.327 The library synthesis was accomplished in three reaction steps. At the first step of the Ugi reactions, fluorous benzaldehydes 177 were used as the limiting agent to react with Boc-anthranilic acids 178, amino esters 179, and cyclohexyl isonitrile 180. After F-SPE purification, Ugi products 181 were used for the second step of acetyl chloride-promoted de-Boc/cyclizations to form 1,4-benzodiazepine-2,5-diones 182. The cyclization reactions were selective and only attacked the ester group to form the seven-membered ring. The last step Suzuki reactions were carried out under microwave heating to cleave the fluorous sulfonyl group and introduce the biaryl functional group to form products 183. The Yang group also used 2-nitrobenzoic acids as anthranilic acid alternatives for the U-4CRs. A demonstration library of 36 analogs was produced with building blocks R1 to R4 variants.
Figure 16.
Fluorous synthesis of 1,4-benzodiazepinedione library.327
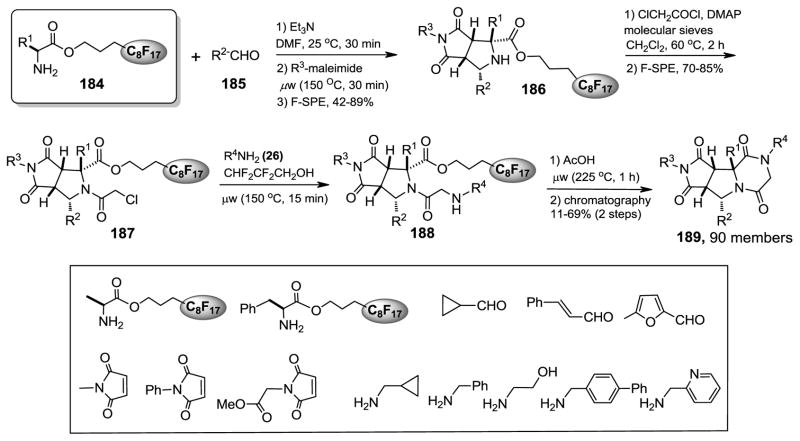
Fluorous synthesis of piperazinedione-fused tricyclic compound library.328
Fluorous amino esters are useful linkers for library synthesis. In a joint work conducted by the Werner and the Zhang groups, fluorous amino esters 184 were used for solution-phase parallel synthesis of novel piperazinedione-fused tricyclic library compounds 189 (Figure 17).328 The compounds are structurally similar to the tricyclic thrombin inhibitors and diketopiperazine-based inhibitors of human hormone-sensitive lipase. At the first library synthesis step, azomethine ylides generated from 184 and aldehydes 185 underwent microwave-assisted 1,3-dipolar cycloadditions with maleimides to form 186 in stereoselective fashion. The adducts were treated with chloroacetyl chloride to afford N-acylated products 187. The chloro group of 187 was displaced with amines to afford 188. The last step was the microwave-promoted cyclizations to cleave the fluorous linker and generate the piperazinedione ring. The final products were purified by HPLC to ensure >95% purities. Library 189 containing 90 compounds was synthesized with assorted building blocks for R1 to R4.
Figure 17.
Fluorous synthesis of tricyclic library.328
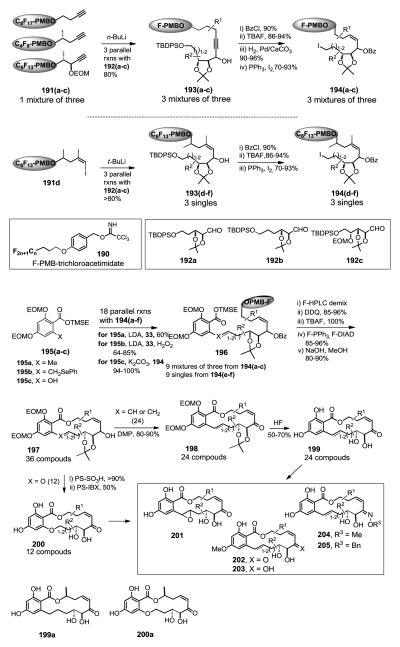
Fluorous mixture synthesis of natural product resorcylic acid lactone (RAL) library.329
Natural resorcylic acid lactones (RAL) containing a cis-enone moiety such as radicicol A, L-783277, and LL-Z1640-2 are known to be potent and irreversible kinase inhibitors. For QSAR studies, the Winssinger group developed a fluorous mixture synthesis (FMS) method based on the previously reported fluorous total synthesis of radicicol A and synthesized a compound library containing 51 analogs (Figure 18).329 In the FMS, three propargyl alcohols were used each attached to a different p-methoxybenzyl (PMB) linker by reacting with F-PMB-trichloroacetimidates 190 to form 191(a–c) (Scheme 5). The equimolar mixture of 191(a–c) was split to three portions and each reacted with one of three aldehydes 192(a–c) to afford 3 mixtures of 193(a–c). After sequential hydroxyl group protection, TBDPS deprotection, and iodination, compounds 193(a–c) were converted to 3 mixtures of 194(a–c) each containing three components. Through a similar pathway, compound 191d reacted with aldehydes 192(a–c) to afford compounds 194(d–f). The second stage of FMS involved the reaction of three compounds of 195(a–c) with each of 6 compounds of 194(a–f) to afford compounds 196. Among the 18 pools of 196, 9 are three-component mixtures generated from 194(a–c) and 9 are single compounds generated from 194(d–f). At this point, those 9 three-component mixtures were demixed on HPLC with a fluorous column to afford 27 individual compounds of 196. These 27 compound and another 9 compounds of 196 generated from 194(e–f) were treated with DDQ and TBAF to remove both the fluorous linker and the TMSE group. The macrocyclization reactions were promoted by Mitsunobu reaction conditions using fluorous PPh3 and DIAD. The fluorous derivatives were easily removed from the reaction mixture by F-SPE. The treatment of NaOH removed the Bz group to afford total of 36 macrocyclic compounds 197. Among them, 24 (X = CH or CH2) were treated with DMP followed by HF to afford products 199. Another 12 of 197 were treated with PS-SO3H and PS-IBX to afford products 200. Compounds 199 and 200 can be converted to 201-205 through simple transformations to afford a total of 51 macrocyclic compounds. A subset of 28 representative library compounds was assayed against a panel of 19 kinases representing those bearing the adequately positioned cysteine residue, bearing a cysteine residue at a different position within the ATP binding pocket, and those that do not bear a cysteine residue. The screening results indicated that there is very little difference in relative selectivity of kinase inhibition throughout the library compounds. VEGF-R2 is the most highly inhibited kinase followed by PDGFR-α, VEGR-R3, Flt3, VEGF-R1, MEK1 SESE and KIT. Two representative compounds 199a and 200a were evaluated in a larger panel of 402 kinases and also evaluated against a series of mutations of Fli3 and KIT. The screening results provided valuable QSAR information and also led to the identification of several potent inhibitors of multiple oncogenic kinases.
Figure 18.
Fluorous mixture synthesis of resorcylic acid lactone library.329
Table 2.
Chemical Libraries Targeting Nonproteolytic Enzymes.
|
|
Asterisk is the point of attachment to resin.
Table 3.
Chemical Libraries Targeting G-Protein Coupled Receptors.
|
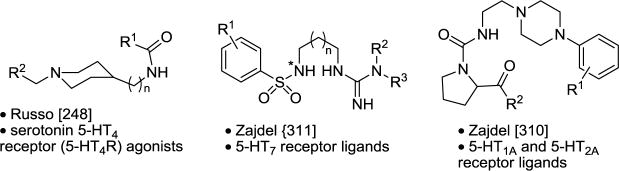
|
Asterisk is the point of attachment to resin.
Table 4.
Chemical Libraries Targeting Non-G-Protein-Coupled Receptors.
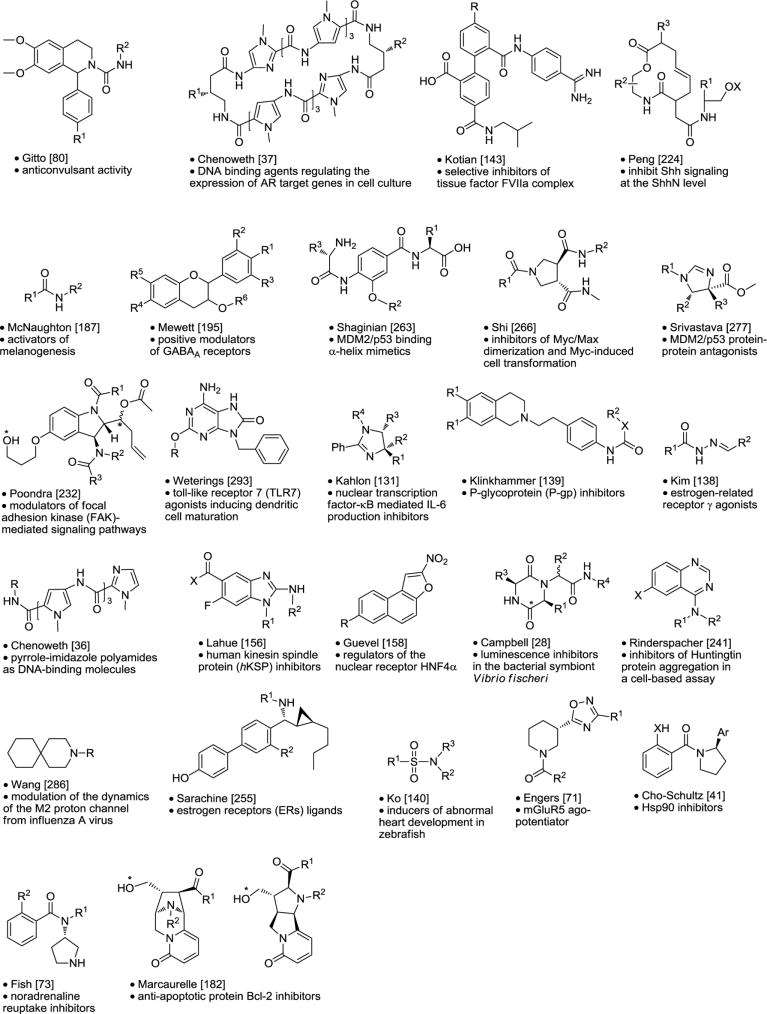
|
Asterisk is the point of attachment to resin.
Table 8.
Monocyclic Synthesis.
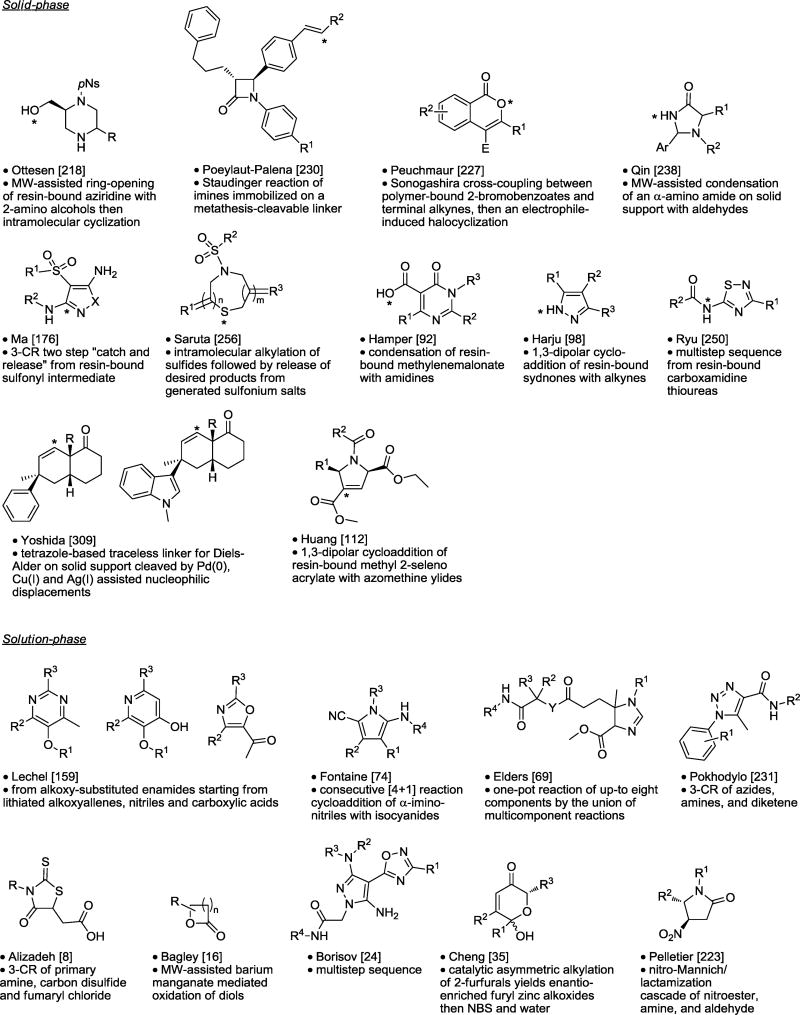
|
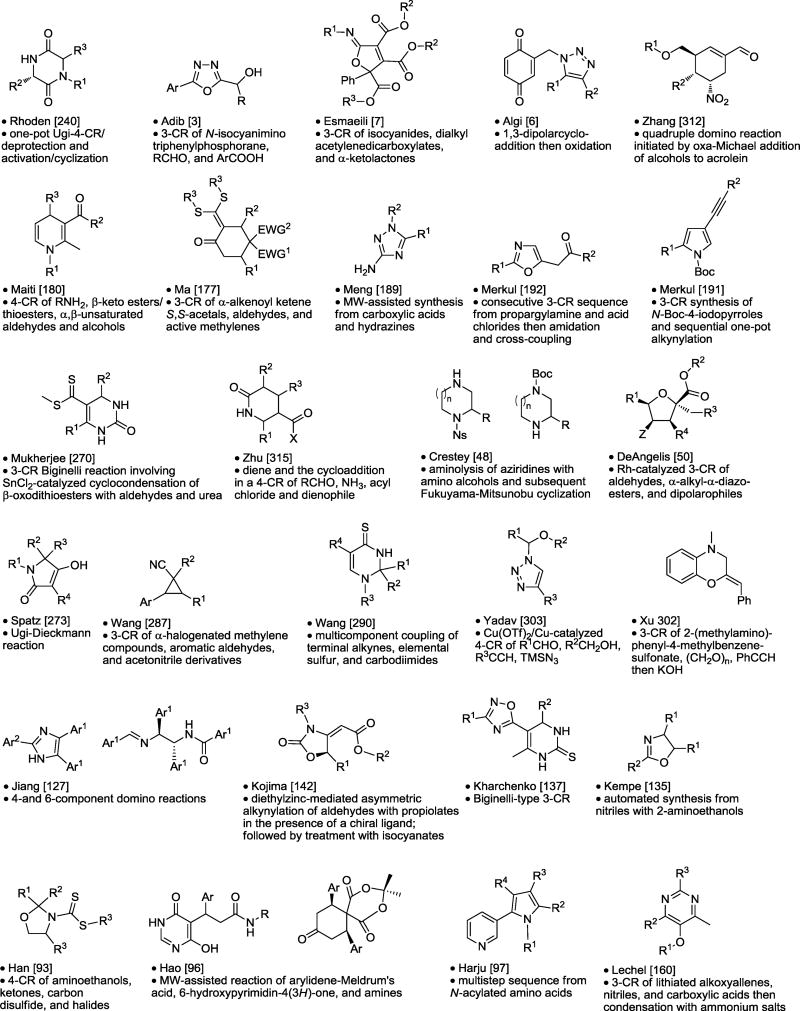
|
|
Asterisk is the point of attachment to resin.
Table 9.
Bicyclic and Spirocyclic Synthesis.
|
|
|
Asterisk is the point of attachment to resin.
References
- 1.Dolle RE, Le Bourdonnec B, Goodman AJ, Morales GA, Thomas CJ, Zhang W. J Comb Chem. 2009;11:739–790. doi: 10.1021/cc9000828. [DOI] [PubMed] [Google Scholar]
- 2.Abadi AH, Ibrahim TM, Abouzid KM, Lehmann J, Tinsley HN, Gary BD, Piazza GA. Bioorg Med Chem. 2009;17:5974–5982. doi: 10.1016/j.bmc.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adib M, Kesheh MR, Ansari S, Bijanzadeh HR. Synlett. 2009:1575–1578. [Google Scholar]
- 4.Aguado L, Camarasa M-J, Perez-Perez M-J. J Comb Chem. 2009;11:210–212. doi: 10.1021/cc800169d. [DOI] [PubMed] [Google Scholar]
- 5.Airiau E, Girard N, Mann A, Salvadori J, Taddei M. Org Lett. 2009;11:5314–5317. doi: 10.1021/ol902279m. [DOI] [PubMed] [Google Scholar]
- 6.Algi F, Balci M. Synthesis. 2009:1341–1347. [Google Scholar]
- 7.Ali Esmaeili A, Vesalipoor H. Synthesis. 2009:1635–1638. [Google Scholar]
- 8.Alizadeh A, Zohreh N. Synlett. 2009:2146–2148. [Google Scholar]
- 9.Allan M, Manku S, Therrien E, Nguyen N, Styhler S, Robert M-F, Goulet A-C, Petschner AJ, Rahil G, Robert MacLeod A, Deziel R, Besterman JM, Nguyen H, Wahhab A. Bioorg Med Chem Lett. 2009;19:1218–1223. doi: 10.1016/j.bmcl.2008.12.075. [DOI] [PubMed] [Google Scholar]
- 10.Ankati H, Biehl E. Tetrahedron Lett. 2009;50:4677–4682. doi: 10.1016/j.tetlet.2011.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annis DA, Cheng CC, Chuang C-C, McCarter JD, Nash HM, Nazef N, Rowe T, Kurzeja RJM, Shipps GW., Jr Comb Chem High Throughput Screening. 2009;12:760–771. doi: 10.2174/138620709789104870. [DOI] [PubMed] [Google Scholar]
- 12.Antonysamy S, Hirst G, Park F, Sprengeler P, Stappenbeck F, Steensma R, Wilson M, Wong M. Bioorg Med Chem Lett. 2009;19:279–282. doi: 10.1016/j.bmcl.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 13.Awuah E, Capretta A. Org Lett. 2009;11:3210–3213. doi: 10.1021/ol901043q. [DOI] [PubMed] [Google Scholar]
- 14.Ayesa S, Lindquist C, Agback T, Benkestock K, Classon B, Henderson I, Hewitt E, Jansson K, Kallin A, Sheppard D, Samuelsson B. Bioorg Med Chem. 2009;17:1307–1324. doi: 10.1016/j.bmc.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Baghbanzadeh M, Molnar M, Damm M, Reidlinger C, Dabiri M, Kappe CO. J Comb Chem. 2009;11:676–684. doi: 10.1021/cc900036a. [DOI] [PubMed] [Google Scholar]
- 16.Bagley MC, Lin Z, Phillips DJ, Graham AE. Tetrahedron Lett. 2009;50:6823–6825. [Google Scholar]
- 17.Barluenga J, Mendoza A, Rodriguez F, Fananas FJ. Angew Chem, Int Ed. 2009;48:1644–1647. doi: 10.1002/anie.200805519. [DOI] [PubMed] [Google Scholar]
- 18.Barton NP, Bellenie BR, Doran AT, Emmons AJ, Heer JP, Salvagno CM. Bioorg Med Chem Lett. 2009;19:528–532. doi: 10.1016/j.bmcl.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Baskovc J, Bevk D, Stanovnik B, Svete J. J Comb Chem. 2009;11:500–507. doi: 10.1021/cc900032c. [DOI] [PubMed] [Google Scholar]
- 20.Benbow JW, Andrews KA, Aubrecht J, Beebe D, Boyer D, Doran S, Homiski M, Hui Y, McPherson K, Parker JC, Treadway J, VanVolkenberg M, Zembrowski WJ. Bioorg Med Chem Lett. 2009;19:2220–2223. doi: 10.1016/j.bmcl.2009.02.099. [DOI] [PubMed] [Google Scholar]
- 21.Beswick PJ, Blackaby AP, Bountra C, Brown T, Browning K, Campbell IB, Corfield J, Gleave RJ, Guntrip SB, Hall RM, Hindley S, Lambeth PF, Lucas F, Mathews N, Naylor A, Player H, Price HS, Sidebottom PJ, Taylor NL, Webb G, Wiseman J. Bioorg Med Chem Lett. 2009;19:4509–4514. doi: 10.1016/j.bmcl.2009.02.089. [DOI] [PubMed] [Google Scholar]
- 22.Blanco-Ania D, Hermkens PHH, Sliedregt LAJM, Scheeren HW, Rutjes FPJT. J Comb Chem. 2009;11:527–538. doi: 10.1021/cc800190z. [DOI] [PubMed] [Google Scholar]
- 23.Blanco-Ania D, Hermkens PHH, Sliedregt LAJM, Scheeren HW, Rutjes FPJT. J Comb Chem. 2009;11:539–546. doi: 10.1021/cc800191w. [DOI] [PubMed] [Google Scholar]
- 24.Borisov AV, Detistov OS, Pukhovaya VI, Zhuravel IO, Kovalenko SM. J Comb Chem. 2009;11:1023–1029. doi: 10.1021/cc900070m. [DOI] [PubMed] [Google Scholar]
- 25.Boyd VA, Mason J, Hanumesh P, Price J, Russell CJ, Webb TR. J Comb Chem. 2009;11:1100–1104. doi: 10.1021/cc900111u. [DOI] [PubMed] [Google Scholar]
- 26.Brucoli F, Howard PW, Thurston DE. J Comb Chem. 2009;11:576–586. doi: 10.1021/cc900009r. [DOI] [PubMed] [Google Scholar]
- 27.Brummond KM, Mao S, Shinde SN, Johnston PJ, Day BW. J Comb Chem. 2009;11:486–494. doi: 10.1021/cc900024p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell J, Blackwell HE. J Comb Chem. 2009;11:1094–1099. doi: 10.1021/cc900115x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao J, Gao H, Bemis G, Salituro F, Ledeboer M, Harrington E, Wilke S, Taslimi P, Pazhanisamy S, Xie X, Jacobs M, Green J. Bioorg Med Chem Lett. 2009;19:2891–2895. doi: 10.1016/j.bmcl.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 30.Cathcart GR, Gilmore BF, Greer B, Harriott P, Walker B. Bioorg Med Chem Lett. 2009;19:6230–6232. doi: 10.1016/j.bmcl.2009.08.099. [DOI] [PubMed] [Google Scholar]
- 31.Che J, Raghavendra MS, Lam Y. J Comb Chem. 2009;11:378–384. doi: 10.1021/cc800193r. [DOI] [PubMed] [Google Scholar]
- 32.Chen C-H, Chien M-H, Kuo M-L, Chou C-T, Lai J-J, Lin S-F, Thummanagoti S, Sun C-M. J Comb Chem. 2009;11:1038–1046. doi: 10.1021/cc900084s. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Bai J, Jiao L, Guo Z, Yin Q, Li X. Bioorg Med Chem. 2009;17:3980–3986. doi: 10.1016/j.bmc.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 34.Cheng CC, Shipps GW, Yang Z, Sun B, Kawahata N, Soucy KA, Soriano A, Orth P, Xiao L, Mann P, Black T. Bioorg Med Chem Lett. 2009;19:6507–6514. doi: 10.1016/j.bmcl.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 35.Cheng K, Rowley Kelly A, Kohn RA, Dweck JF, Walsh PJ. Org Lett. 2009;11:2703–2706. doi: 10.1021/ol900905r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chenoweth DM, Harki DA, Dervan PB. J Am Chem Soc. 2009;131:7175–7181. doi: 10.1021/ja901307m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chenoweth DM, Harki DA, Phillips JW, Dose C, Dervan PB. J Am Chem Soc. 2009;131:7182–7188. doi: 10.1021/ja901309z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cherian PT, Koikov LN, Wortman MD, Knittel JJ. Bioorg Med Chem Lett. 2009;19:2215–2219. doi: 10.1016/j.bmcl.2009.02.115. [DOI] [PubMed] [Google Scholar]
- 39.Cho C-H, Neuenswander B, Lushington GH, Larock RC. J Comb Chem. 2009;11:900–906. doi: 10.1021/cc9000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi YL, Kim JK, Choi S-U, Min Y-K, Bae M-A, Kim BT, Heo J-N. Bioorg Med Chem Lett. 2009;19:3036–3040. doi: 10.1016/j.bmcl.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 41.Cho-Schultz S, Patten MJ, Huang B, Elleraas J, Gajiwala KS, Hickey MJ, Wang J, Mehta PP, Kang P, Gehring MR, Kung P-P, Sutton SC. J Comb Chem. 2009;11:860–874. doi: 10.1021/cc900056d. [DOI] [PubMed] [Google Scholar]
- 42.Cloonan SM, Keating JJ, Butler SG, Knox AJS, Jorgensen AM, Peters GH, Rai D, Corrigan D, Lloyd DG, Williams DC, Meegan MJ. Eur J Med Chem. 2009;44:4862–4888. doi: 10.1016/j.ejmech.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 43.Coffinier D, El Kaim L, Grimaud L. Org Lett. 2009;11:995–997. doi: 10.1021/ol8029438. [DOI] [PubMed] [Google Scholar]
- 44.Cole AG, Metzger A, Brescia M-R, Qin L-Y, Appell KC, Brain CT, Hallett A, Ganju P, Denholm AA, Wareing JR, Ritchie TJ, Drake GM, Bevan SJ, MacGloinn A, McBryde A, Patel V, Oakley PJ, Nunez X, Gstach H, Schneider P, Baldwin JJ, Dolle RE, McDonald E, Henderson I. Bioorg Med Chem Lett. 2009;19:119–122. doi: 10.1016/j.bmcl.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Cole AG, Stauffer TM, Rokosz LL, Metzger A, Dillard LW, Zeng W, Henderson I. Bioorg Med Chem Lett. 2009;19:378–381. doi: 10.1016/j.bmcl.2008.11.066. [DOI] [PubMed] [Google Scholar]
- 46.Colombo M, Bossolo S, Aramini A. J Comb Chem. 2009;11:335–337. doi: 10.1021/cc900011z. [DOI] [PubMed] [Google Scholar]
- 47.Covel JA, Santora VJ, Smith JM, Hayashi R, Gallardo C, Weinhouse MI, Ibarra JB, Schultz JA, Park DM, Estrada SA, Hofilena BJ, Pulley MD, Smith BM, Ren A, Suarez M, Frazer J, Edwards J, Hauser EK, Lorea J, Semple G, Grottick AJ. J Med Chem. 2009;52:5603–5611. doi: 10.1021/jm900857n. [DOI] [PubMed] [Google Scholar]
- 48.Crestey F, Witt M, Jaroszewski JW, Franzyk H. J Org Chem. 2009;74:5652–5655. doi: 10.1021/jo900441s. [DOI] [PubMed] [Google Scholar]
- 49.Das BC, Madhukumar AV, Anguiano J, Mani S. Bioorg Med Chem Lett. 2009;19:4204–4206. doi: 10.1016/j.bmcl.2009.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeAngelis A, Taylor MT, Fox JM. J Am Chem Soc. 2009;131:1101–1105. doi: 10.1021/ja807184r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeBenedetto MV, Green ME, Wan S, Park J-H, Floreancig PE. Org Lett. 2009;11:835–838. doi: 10.1021/ol802764j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeNinno MP, Andrews M, Bell AS, Chen Y, Eller-Zarbo C, Eshelby N, Etienne JB, Moore DE, Palmer MJ, Visser MS, Yu LJ, Zavadoski WJ, Michael Gibbs E. Bioorg Med Chem Lett. 2009;19:2537–2541. doi: 10.1016/j.bmcl.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 53.Deschrijver T, Verwilst P, Broos K, Deckmyn H, Dehaen W, De Borggraeve WM. Tetrahedron. 2009;65:4521–4529. [Google Scholar]
- 54.Devarie-Baez NO, Kim W-S, Smith AB, III, Xian M. Org Lett. 2009;11:1861–1864. doi: 10.1021/ol900434k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dickson DP, Toh C, Lunda M, Yermolina MV, Wardrop DJ, Landrie CL. J Org Chem. 2009;74:9535–9538. doi: 10.1021/jo901764u. [DOI] [PubMed] [Google Scholar]
- 56.Ding Q, Huang X-G, Wu J. J Comb Chem. 2009;11:1047–1049. doi: 10.1021/cc900085p. [DOI] [PubMed] [Google Scholar]
- 57.Ding Q, He X, Wu J. J Comb Chem. 2009;11:587–591. doi: 10.1021/cc900027c. [DOI] [PubMed] [Google Scholar]
- 58.Dockendorff C, Jin S, Olsen M, Lautens M, Coupal M, Hodzic L, Spear N, Payza K, Walpole C, Tomaszewski MJ. Bioorg Med Chem Lett. 2009;19:1228–1232. doi: 10.1016/j.bmcl.2008.12.095. [DOI] [PubMed] [Google Scholar]
- 59.Donets PA, Van Hecke K, Van Meervelt L, Van der Eycken EV. Org Lett. 2009;11:3618–3621. doi: 10.1021/ol901356h. [DOI] [PubMed] [Google Scholar]
- 60.Dou G, Wang M, Shi D. J Comb Chem. 2008;11:151–154. doi: 10.1021/cc8001469. [DOI] [PubMed] [Google Scholar]
- 61.Dou G, Shi D. J Comb Chem. 2009;11:1073–1077. doi: 10.1021/cc9001058. [DOI] [PubMed] [Google Scholar]
- 62.Duval R, Kolb S, Braud E, Genest D, Garbay C. J Comb Chem. 2009;11:947–950. doi: 10.1021/cc900140f. [DOI] [PubMed] [Google Scholar]
- 63.Dzhavakhishvili SG, Gorobets NY, Shishkina SV, Shishkin OV, Desenko SM, Groth UM. J Comb Chem. 2009;11:508–514. doi: 10.1021/cc9000373. [DOI] [PubMed] [Google Scholar]
- 64.El-Badri MH, Kurth MJ. J Comb Chem. 2009;11:228–238. doi: 10.1021/cc800141s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El Kaim L, Grimaud L, Oble J, Wagschal S. Tetrahedron Lett. 2009;50:1741–1743. [Google Scholar]
- 66.El Kaim L, Grimaud L, Schiltz A. Org Biomol Chem. 2009;7:3024–3026. [Google Scholar]
- 67.El Kaim L, Grimaud L, Schiltz A. Synlett. 2009:1401–1404. [Google Scholar]
- 68.El Kaim L, Grimaud L, Wagschal S. Synlett. 2009:1315–1317. [Google Scholar]
- 69.Elders N, van der Born D, Hendrickx LJD, Timmer BJJ, Krause A, Janssen E, de Kanter FJJ, Ruijter E, Orru RVA. Angew Chem, Int Ed. 2009;48:5856–5859. S5856/1–S5856/58. doi: 10.1002/anie.200902683. [DOI] [PubMed] [Google Scholar]
- 70.Elford TG, Ulaczyk-Lesanko A, De Pascale G, Wright GD, Hall DG. J Comb Chem. 2009;11:155–168. doi: 10.1021/cc8001487. [DOI] [PubMed] [Google Scholar]
- 71.Engers DW, Rodriguez AL, Williams R, Hammond AS, Venable D, Oluwatola O, Sulikowski GA, Conn PJ, Lindsley CW. ChemMedChem. 2009;4:505–511. doi: 10.1002/cmdc.200800357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Erb W, Neuville L, Zhu J. J Org Chem. 2009;74:3109–3115. doi: 10.1021/jo900210x. [DOI] [PubMed] [Google Scholar]
- 73.Fish PV, Wakenhut F, Ryckmans T, Stobie A. Bioorg Med Chem Lett. 2009;19:4579–4583. doi: 10.1016/j.bmcl.2009.06.096. [DOI] [PubMed] [Google Scholar]
- 74.Fontaine P, Masson G, Zhu J. Org Lett. 2009;11:1555–1558. doi: 10.1021/ol9001619. [DOI] [PubMed] [Google Scholar]
- 75.Fu J, Shuttleworth SJ, Connors RV, Chai A, Coward P. Bioorg Med Chem Lett. 2009;19:4264–4267. doi: 10.1016/j.bmcl.2009.05.124. [DOI] [PubMed] [Google Scholar]
- 76.Georgescu E, Caira MR, Georgescu F, Draghici B, Popa MM, Dumitrascu F. Synlett. 2009:1795–1799. [Google Scholar]
- 77.Ghahremanzadeh R, Ahadi S, Bazgir A. Tetrahedron Lett. 2009;50:7379–7381. [Google Scholar]
- 78.Ghahremanzadeh R, Sayyafi M, Ahadi S, Bazgir A. J Comb Chem. 2009;11:393–396. doi: 10.1021/cc8001958. [DOI] [PubMed] [Google Scholar]
- 79.Giannini G, Marzi M, Pezzi R, Brunetti T, Battistuzzi G, Marzo MD, Cabri W, Vesci L, Pisano C. Bioorg Med Chem Lett. 2009;19:2346–2349. doi: 10.1016/j.bmcl.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 80.Gitto R, Pagano B, Citraro R, Scicchitano F, De Sarro G, Chimirri A. Eur J Med Chem. 2009;44:1349–1354. doi: 10.1016/j.ejmech.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 81.Gladstone SG, Earley WG, Acker JK, Martin GS. Tetrahedron Lett. 2009;50:3813–3816. [Google Scholar]
- 82.Gleave RJ, Beswick PJ, Brown AJ, Giblin GMP, Haslam CP, Livermore D, Moses A, Nicholson NH, Page LW, Slingsby B, Swarbrick ME. Bioorg Med Chem Lett. 2009;19:6578–6581. doi: 10.1016/j.bmcl.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 83.Gomez AM, Barrio A, Pedregosa A, Valverde S, Lopez JC. J Org Chem. 2009;74:6323–6326. doi: 10.1021/jo9012817. [DOI] [PubMed] [Google Scholar]
- 84.Goncalves S, Wagner A, Mioskowski C, Baati R. Tetrahedron Lett. 2009;50:274–276. [Google Scholar]
- 85.Gong Y-D, Ryu IA. J Comb Chem. 2009;11:626–630. doi: 10.1021/cc900020j. [DOI] [PubMed] [Google Scholar]
- 86.Gonzalez-Arellano C, Luque R, Macquarrie DJ. Chem Commun. 2009:1410–1412. doi: 10.1039/b818767c. [DOI] [PubMed] [Google Scholar]
- 87.Green DM, Goljer I, Andraka DS, Chengalvala M, Shanno L, Hurlburt W, Pelletier JC. J Comb Chem. 2009;11:117–125. doi: 10.1021/cc800106h. [DOI] [PubMed] [Google Scholar]
- 88.Grote RE, Jarvo ER. Org Lett. 2009;11:485–488. doi: 10.1021/ol8026297. [DOI] [PubMed] [Google Scholar]
- 89.Guchhait SK, Madaan C. Synlett. 2009:628–632. [Google Scholar]
- 90.Guo C, Hou X, Dong L, Dagostino E, Greasley S, Ferre R, Marakovits J, Johnson MC, Matthews D, Mroczkowski B, Parge H, VanArsdale T, Popoff I, Piraino J, Margosiak S, Thomson J, Los G, Murray BW. Bioorg Med Chem Lett. 2009;19:5613–5616. doi: 10.1016/j.bmcl.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 91.Kyle Hadden M, Hill SA, Davenport J, Matts RL, Blagg BSJ. Bioorg Med Chem. 2009;17:634–640. doi: 10.1016/j.bmc.2008.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamper BC, Kesselring AS, Chott RC, Yang S. J Comb Chem. 2009;11:469–480. doi: 10.1021/cc900010v. [DOI] [PubMed] [Google Scholar]
- 93.Han F-B, Ge Z-M, Cheng T-M, Li R-T. Synlett. 2009:648–650. [Google Scholar]
- 94.Han Z-G, Tu S-J, Jiang B, Yan S, Zhang X-H, Wu S-S, Hao W-J, Cao X-D, Shi F, Zhang G, Ma N. Synthesis. 2009:1639–1646. [Google Scholar]
- 95.Han Z-G, Zhang G, Jiang B, Ma N, Shi F, Tu S-J. J Comb Chem. 2009;11:809–812. doi: 10.1021/cc800196u. [DOI] [PubMed] [Google Scholar]
- 96.Hao W-J, Jiang B, Tu S-J, Wu S-S, Han Z-G, Cao X-D, Zhang X-H, Yan S, Shi F. J Comb Chem. 2009;11:310–314. doi: 10.1021/cc800175n. [DOI] [PubMed] [Google Scholar]
- 97.Harju K, Manevski N, Yli-Kauhaluoma J. Tetrahedron. 2009;65:9702–9706. [Google Scholar]
- 98.Harju K, Vesterinen J, Yli-Kauhaluoma J. Org Lett. 2009;11:2219–2221. doi: 10.1021/ol900704b. [DOI] [PubMed] [Google Scholar]
- 99.Heng S, Gryncel KR, Kantrowitz ER. Bioorg Med Chem. 2009;17:3916–3922. doi: 10.1016/j.bmc.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Henry C, Haupt A, Turner SC. J Org Chem. 2009;74:1932–1938. doi: 10.1021/jo802439d. [DOI] [PubMed] [Google Scholar]
- 101.Heravi MM, Baghernejad B, Oskooie HA. Tetrahedron Lett. 2009;50:767–769. [Google Scholar]
- 102.Heravi MM, Baghernejad B, Oskooie HA. Synlett. 2009:1123–1125. [Google Scholar]
- 103.Heravi MM, Sadjadi S, Mokhtari Haj N, Oskooie HA, Shoar RH, Bamoharram FF. Tetrahedron Lett. 2009;50:943–945. [Google Scholar]
- 104.Hermange P, Dau METH, Retailleau P, Dodd RH. Org Lett. 2009;11:4044–4047. doi: 10.1021/ol901452d. [DOI] [PubMed] [Google Scholar]
- 105.Honda T, Terao T, Aono H, Ban M. Bioorg Med Chem. 2009;17:699–708. doi: 10.1016/j.bmc.2008.11.060. [DOI] [PubMed] [Google Scholar]
- 106.Hu F, Chou CJ, Gottesfeld JM. Bioorg Med Chem Lett. 2009;19:3928–3931. doi: 10.1016/j.bmcl.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu Z, Ma T, Chen Z, Ye Z, Zhang G, Lou Y, Yu Y. J Comb Chem. 2009;11:267–273. doi: 10.1021/cc800157k. [DOI] [PubMed] [Google Scholar]
- 108.Huang H, Jiang H, Chen K, Liu H. J Org Chem. 2009;74:5476–5480. doi: 10.1021/jo901101v. [DOI] [PubMed] [Google Scholar]
- 109.Huang W, Ding Y, Miao Y, Liu M-Z, Li Y, Yang G-F. Eur J Med Chem. 2009;44:3687–3696. doi: 10.1016/j.ejmech.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 110.Huang X, Huang J, Cao J. J Comb Chem. 2009;11:515–518. doi: 10.1021/cc900006z. [DOI] [PubMed] [Google Scholar]
- 111.Huang X, Xu J. J Org Chem. 2009;74:8859–8861. doi: 10.1021/jo901628a. [DOI] [PubMed] [Google Scholar]
- 112.Huang X, Xu J-F. J Comb Chem. 2009;11:350–354. doi: 10.1021/cc800161g. [DOI] [PubMed] [Google Scholar]
- 113.Humphries PS, Balan G, Bechle BM, Conn EL, Dirico KJ, Hui Y, Oliver RM, Southers JA, Yang X. Tetrahedron Lett. 2009;50:2140–2143. [Google Scholar]
- 114.Humphries PS, Do Q-QT, Wilhite DM. Tetrahedron Lett. 2009;50:2552–2554. [Google Scholar]
- 115.Humphries PS, Oliver RM. Tetrahedron Lett. 2009;50:2682–2684. [Google Scholar]
- 116.Ibrahim N, Legraverend M. J Comb Chem. 2009;11:658–666. doi: 10.1021/cc900066v. [DOI] [PubMed] [Google Scholar]
- 117.Ito S, Hirata Y, Nagatomi Y, Satoh A, Suzuki G, Kimura T, Satow A, Maehara S, Hikichi H, Hata M, Ohta H, Kawamoto H. Bioorg Med Chem Lett. 2009;19:5310–5313. doi: 10.1016/j.bmcl.2009.07.145. [DOI] [PubMed] [Google Scholar]
- 118.Izumiseki A, Yoshida K, Yanagisawa A. Org Lett. 2009;11:5310–5313. doi: 10.1021/ol9022613. [DOI] [PubMed] [Google Scholar]
- 119.Jadidi K, Ghahremanzadeh R, Bazgir A. J Comb Chem. 2009;11:341–344. doi: 10.1021/cc800167h. [DOI] [PubMed] [Google Scholar]
- 120.Jarusiewicz J, Choe Y, Yoo KS, Park CP, Jung KW. J Org Chem. 2009;74:2873–2876. doi: 10.1021/jo900163w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jayanth TT, Zhang L, Johnson TS, Malinakova HC. Org Lett. 2009;11:815–818. doi: 10.1021/ol802755z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jeganmohan M, Bhuvaneswari S, Cheng C-H. Angew Chem, Int Ed. 2009;48:391–394. doi: 10.1002/anie.200804873. [DOI] [PubMed] [Google Scholar]
- 123.Jeges G, Nagy T, Meszaros T, Kovacs J, Dorman G, Kowalczyk A, Goodnow RA. J Comb Chem. 2009;11:327–334. doi: 10.1021/cc800172h. [DOI] [PubMed] [Google Scholar]
- 124.Jiang B, Cao L-J, Tu S-J, Zheng W-R, Yu H-Z. J Comb Chem. 2009;11:612–616. doi: 10.1021/cc900038g. [DOI] [PubMed] [Google Scholar]
- 125.Jiang B, Hao W-J, Wang X, Shi F, Tu S-J. J Comb Chem. 2009;11:846–850. doi: 10.1021/cc900052b. [DOI] [PubMed] [Google Scholar]
- 126.Jiang B, Tu S-J, Kaur P, Wever W, Li G. J Am Chem Soc. 2009;131:11660–11661. doi: 10.1021/ja904011s. [DOI] [PubMed] [Google Scholar]
- 127.Jiang B, Wang X, Shi F, Tu S-J, Ai T, Ballew A, Li G. J Org Chem. 2009;74:9486–9489. doi: 10.1021/jo902204s. [DOI] [PubMed] [Google Scholar]
- 128.Jiang G, Chen J, Huang J-S, Che C-M. Org Lett. 2009;11:4568–4571. doi: 10.1021/ol9018166. [DOI] [PubMed] [Google Scholar]
- 129.Jiang M, Li T, Meng L, Yang C, Xie Y, Ding J. J Comb Chem. 2009;11:806–808. doi: 10.1021/cc800162k. [DOI] [PubMed] [Google Scholar]
- 130.Jung N, Braese S. J Comb Chem. 2009;11:47–71. doi: 10.1021/cc800032q. [DOI] [PubMed] [Google Scholar]
- 131.Kahlon DK, Lansdell TA, Fisk JS, Tepe JJ. Bioorg Med Chem. 2009;17:3093–3103. doi: 10.1016/j.bmc.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 132.Kang SS, Cuendet M, Endringer DC, Croy VL, Pezzuto JM, Lipton MA. Bioorg Med Chem. 2009;17:1044–1054. doi: 10.1016/j.bmc.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 133.Karthikeyan SV, Perumal S, Shetty KA, Yogeeswari P, Sriram D. Bioorg Med Chem Lett. 2009;19:3006–3009. doi: 10.1016/j.bmcl.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 134.Kattar SD, Surdi LM, Zabierek A, Methot JL, Middleton RE, Hughes B, Szewczak AA, Dahlberg WK, Kral AM, Ozerova N, Fleming JC, Wang H, Secrist P, Harsch A, Hamill JE, Cruz JC, Kenific CM, Chenard M, Miller TA, Berk SC, Tempest P. Bioorg Med Chem Lett. 2009;19:1168–1172. doi: 10.1016/j.bmcl.2008.12.083. [DOI] [PubMed] [Google Scholar]
- 135.Kempe K, Lobert M, Hoogenboom R, Schubert US. J Comb Chem. 2009;11:274–280. doi: 10.1021/cc800174d. [DOI] [PubMed] [Google Scholar]
- 136.Phillip Kennedy J, Jeffrey Conn P, Lindsley CW. Bioorg Med Chem Lett. 2009;19:3204–3208. doi: 10.1016/j.bmcl.2009.04.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kharchenko JV, Detistov OS, Orlov VD. J Comb Chem. 2009;11:216–219. doi: 10.1021/cc800111h. [DOI] [PubMed] [Google Scholar]
- 138.Kim Y, Koh M, Kim D-K, Choi H-S, Park SB. J Comb Chem. 2009;11:928–937. doi: 10.1021/cc900081j. [DOI] [PubMed] [Google Scholar]
- 139.Klinkhammer W, Mueller H, Globisch C, Pajeva IK, Wiese M. Bioorg Med Chem. 2009;17:2524–2535. doi: 10.1016/j.bmc.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 140.Ko S-K, Jin HJ, Jung D-W, Tian X, Shin I. Angew Chem, Int Ed. 2009;48:7809–7812. S7809/1–S7809/13. doi: 10.1002/anie.200902370. [DOI] [PubMed] [Google Scholar]
- 141.Koci J, Pudelova N, Krchnak V. J Comb Chem. 2009;11:397–402. doi: 10.1021/cc800210q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kojima N, Nishijima S, Tanaka T. Synlett. 2009:3171–3174. [Google Scholar]
- 143.Kotian PL, Krishnan R, Rowland S, El-Kattan Y, Saini SK, Upshaw R, Bantia S, Arnold S, Sudhakar Babu Y, Chand P. Bioorg Med Chem. 2009;17:3934–3958. doi: 10.1016/j.bmc.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 144.Kouznetsov VV, Bohorquez ARR, Saavedra LA. Synthesis. 2009:4219–4225. [Google Scholar]
- 145.Krasavin M, Shkavrov S, Parchinsky V, Bukhryakov K. J Org Chem. 2009;74:2627–2629. doi: 10.1021/jo900050k. [DOI] [PubMed] [Google Scholar]
- 146.Krupkova S, Soural M, Hlavac J, Hradil P. J Comb Chem. 2009;11:951–955. doi: 10.1021/cc900068w. [DOI] [PubMed] [Google Scholar]
- 147.Ku X, Huang H, Jiang H, Liu H. J Comb Chem. 2009;11:338–340. doi: 10.1021/cc800182q. [DOI] [PubMed] [Google Scholar]
- 148.Kudirka R, Devine SKJ, Adams CS, Van Vranken DL. Angew Chem, Int Ed. 2009;48:3677–3680. S3677/1–S3677/29. doi: 10.1002/anie.200805483. [DOI] [PubMed] [Google Scholar]
- 149.Kumar A, Maurya RA, Ahmad P. J Comb Chem. 2009;11:198–201. doi: 10.1021/cc8001876. [DOI] [PubMed] [Google Scholar]
- 150.Prashantha Kumar BR, Sankar G, Nasir Baig RB, Chandrashekaran S. Eur J Med Chem. 2009;44:4192–4198. doi: 10.1016/j.ejmech.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 151.Kumar D, Patel G, Johnson EO, Shah K. Bioorg Med Chem Lett. 2009;19:2739–2741. doi: 10.1016/j.bmcl.2009.03.158. [DOI] [PubMed] [Google Scholar]
- 152.Kumar D, Reddy VB, Sharad S, Dube U, Kapur S. Eur J Med Chem. 2009;44:3805–3809. doi: 10.1016/j.ejmech.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 153.Kumar S, Deshpande S, Chandra V, Kitchlu S, Dwivedi A, Nayak VL, Konwar R, Prabhakar YS, Sahu DP. Bioorg Med Chem. 2009;17:6832–6840. doi: 10.1016/j.bmc.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 154.Kysil V, Khvat A, Tsirulnikov S, Tkachenko S, Ivachtchenko A. Tetrahedron Lett. 2009;50:2854–2856. [Google Scholar]
- 155.Lafleur K, Huang D, Zhou T, Caflisch A, Nevado C. J Med Chem. 2009;52:6433–6446. doi: 10.1021/jm9009444. [DOI] [PubMed] [Google Scholar]
- 156.Lahue BR, Ma Y, Shipps GW, Seghezzi W, Herbst R. Bioorg Med Chem Lett. 2009;19:3405–3409. doi: 10.1016/j.bmcl.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 157.Le Gall E, Haurena C, Sengmany S, Martens T, Troupel M. J Org Chem. 2009;74:7970–7973. doi: 10.1021/jo901704s. [DOI] [PubMed] [Google Scholar]
- 158.Le Guevel R, Oger F, Lecorgne A, Dudasova Z, Chevance S, Bondon A, Barath P, Simonneaux G, Salbert G. Bioorg Med Chem. 2009;17:7021–7030. doi: 10.1016/j.bmc.2009.07.079. [DOI] [PubMed] [Google Scholar]
- 159.Lechel T, Lentz D, Reissig H-U. Chem--Eur J. 2009;15:5432–5435. doi: 10.1002/chem.200900386. [DOI] [PubMed] [Google Scholar]
- 160.Lechel T, Moehl S, Reissig H-U. Synlett. 2009:1059–1062. [Google Scholar]
- 161.Lee JM, Yu E-A, Park JY, Ryu IA, Shin DS, Gong Y-D. Bull Korean Chem Soc. 2009;30:1325–1330. [Google Scholar]
- 162.Lee JW, Bork JT, Ha H-H, Samanta A, Chang Y-T. Aust J Chem. 2009;62:1000–1006. [Google Scholar]
- 163.Lee T, Park J-H, Jeon M-K, Gong Y-D. J Comb Chem. 2009;11:288–293. doi: 10.1021/cc8001684. [DOI] [PubMed] [Google Scholar]
- 164.Lee T, Park J-H, Lee D-H, Gong Y-D. J Comb Chem. 2009;11:495–499. doi: 10.1021/cc900023s. [DOI] [PubMed] [Google Scholar]
- 165.Lee Y-S, Park SM, Kim BH. Bioorg Med Chem Lett. 2009;19:1126–1128. doi: 10.1016/j.bmcl.2008.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lence E, Castedo L, Gonzalez-Bello C. Tetrahedron Lett. 2009;50:1795–1798. [Google Scholar]
- 167.Litvinov YM, Shestopalov AA, Rodinovskaya LA, Shestopalov AM. J Comb Chem. 2009;11:914–919. doi: 10.1021/cc900076j. [DOI] [PubMed] [Google Scholar]
- 168.Li H-H, Jin Y-H, Wang J-Q, Tian S-K. Org Biomol Chem. 2009;7:3219–3221. doi: 10.1039/b911954j. [DOI] [PubMed] [Google Scholar]
- 169.Liu G, Wurst JM, Tan DS. Org Lett. 2009;11:3670–3673. doi: 10.1021/ol901437f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li Y, Yu Y, Giulianotti M, Houghten RA. J Org Chem. 2009;74:2183–2185. doi: 10.1021/jo802583t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu H, Dagousset G, Masson G, Retailleau P, Zhu J. J Am Chem Soc. 2009;131:4598–4599. doi: 10.1021/ja900806q. [DOI] [PubMed] [Google Scholar]
- 172.Liu H, Domling A. J Org Chem. 2009;74:6895–6898. doi: 10.1021/jo900986z. [DOI] [PubMed] [Google Scholar]
- 173.Liu R, Zhang J. Chem--Eur J. 2009;15:9303–9306. doi: 10.1002/chem.200901386. [DOI] [PubMed] [Google Scholar]
- 174.Liu X-H, Lv P-C, Xue J-Y, Song B-A, Zhu H-L. Eur J Med Chem. 2009;44:3930–3935. doi: 10.1016/j.ejmech.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 175.Loaiza PR, Loeber S, Huebner H, Gmeiner P. Bioorg Med Chem. 2009;17:5482–5487. doi: 10.1016/j.bmc.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 176.Ma W, Peterson B, Kelson A, Laborde E. J Comb Chem. 2009;11:697–703. doi: 10.1021/cc900045t. [DOI] [PubMed] [Google Scholar]
- 177.Ma Y, Wang M, Li D, Bekturhun B, Liu J, Liu Q. J Org Chem. 2009;74:3116–3121. doi: 10.1021/jo900217g. [DOI] [PubMed] [Google Scholar]
- 178.MacLeod C, Tuthill PA, Dolle RE. Synlett. 2009:2857–2861. [Google Scholar]
- 179.Madsen P, Kodra JT, Behrens C, Nishimura E, Jeppesen CB, Pridal L, Andersen B, Knudsen LB, Valcarce-Aspegren C, Guldbrandt M, Christensen IT, Jorgensen AS, Ynddal L, Brand CL, Bagger MA, Lau J. J Med Chem. 2009;52:2989–3000. doi: 10.1021/jm8016249. [DOI] [PubMed] [Google Scholar]
- 180.Maiti S, Menendez JC. Synlett. 2009:2249–2252. [Google Scholar]
- 181.Malik L, Kelly NM, Ma J-N, Currier EA, Burstein ES, Olsson R. Bioorg Med Chem Lett. 2009;19:1729–1732. doi: 10.1016/j.bmcl.2009.01.085. [DOI] [PubMed] [Google Scholar]
- 182.Marcaurelle LA, Johannes C, Yohannes D, Tillotson BP, Mann D. Bioorg Med Chem Lett. 2009;19:2500–2503. doi: 10.1016/j.bmcl.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 183.Mao W-W, Wang T-T, Zeng H-P, Wang Z-Y, Chen J-P, Shen J-G. Bioorg Med Chem Lett. 2009;19:4570–4573. doi: 10.1016/j.bmcl.2009.06.098. [DOI] [PubMed] [Google Scholar]
- 184.Martyn DC, Beletsky G, Cortese JF, Tyndall E, Liu H, Fitzgerald MM, O’Shea TJ, Liang B, Clardy J. Bioorg Med Chem Lett. 2009;19:5657–5660. doi: 10.1016/j.bmcl.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 185.McGowan D, Nyanguile O, Cummings MD, Vendeville S, Vandyck K, Van den Broeck W, Boutton CW, De Bondt H, Quirynen L, Amssoms K, Bonfanti J-F, Last S, Rombauts K, Tahri A, Hu L, Delouvroy F, Vermeiren K, Vandercruyssen G, Van der Helm L, Cleiren E, Mostmans W, Lory P, Pille G, Van Emelen K, Fanning G, Pauwels F, Lin T-I, Simmen K, Raboisson P. Bioorg Med Chem Lett. 2009;19:2492–2496. doi: 10.1016/j.bmcl.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 186.McGuinness BF, Carroll CD, Zawacki LG, Dong G, Yang C, Hobbs DW, Jacob-Samuel B, Hall JW, Jenh C-H, Kozlowski JA, Anilkumar GN, Rosenblum SB. Bioorg Med Chem Lett. 2009;19:5205–5208. doi: 10.1016/j.bmcl.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 187.McNaughton BR, Gareiss PC, Jacobs SE, Fricke AF, Scott GA, Miller BL. ChemMedChem. 2009;4:1583–1589. doi: 10.1002/cmdc.200900194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Medimagh R, Marque S, Prim D, Marrot J, Chatti S. Org Lett. 2009;11:1817–1820. doi: 10.1021/ol9003965. [DOI] [PubMed] [Google Scholar]
- 189.Meng J, Kung P-P. Tetrahedron Lett. 2009;50:1667–1670. [Google Scholar]
- 190.Mentel M, Schmidt AM, Gorray M, Eilbracht P, Breinbauer R. Angew Chem, Int Ed. 2009;48:5841–5844. doi: 10.1002/anie.200901643. [DOI] [PubMed] [Google Scholar]
- 191.Merkul E, Boersch C, Frank W, Muller TJJ. Org Lett. 2009;11:2269–2272. doi: 10.1021/ol900581a. [DOI] [PubMed] [Google Scholar]
- 192.Merkul E, Grotkopp O, Mueller TJJ. Synthesis. 2009:502–507. [Google Scholar]
- 193.Merkul E, Oeser T, Mueller TJJ. Chem--Eur J. 2009;15:5006–5011. doi: 10.1002/chem.200900119. [DOI] [PubMed] [Google Scholar]
- 194.Metzger A, Qin L-Y, Cole AG, Saionz KW, Brescia M-R, Gstach H, Wareing JR, Zimmermann J, Brill WKD, Baldwin JJ, Dolle RE, Henderson I. Tetrahedron Lett. 2009;50:7082–7085. [Google Scholar]
- 195.Mewett KN, Fernandez SP, Pasricha AK, Pong A, Devenish SO, Hibbs DE, Chebib M, Johnston GAR, Hanrahan JR. Bioorg Med Chem. 2009;17:7156–7173. doi: 10.1016/j.bmc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 196.Milinkevich KA, Yoo CL, Sparks TC, Lorsbach BA, Kurth MJ. Bioorg Med Chem Lett. 2009;19:5796–5798. doi: 10.1016/j.bmcl.2009.07.139. [DOI] [PubMed] [Google Scholar]
- 197.Minond D, Saldanha SA, Subramaniam P, Spaargaren M, Spicer T, Fotsing JR, Weide T, Fokin VV, Sharpless KB, Galleni M, Bebrone C, Lassaux P, Hodder P. Bioorg Med Chem. 2009;17:5027–5037. doi: 10.1016/j.bmc.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Misra M, Pandey SK, Pandey VP, Pandey J, Tripathi R, Tripathi RP. Bioorg Med Chem. 2009;17:625–633. doi: 10.1016/j.bmc.2008.11.062. [DOI] [PubMed] [Google Scholar]
- 199.Mizoguchi H, Oguri H, Tsuge K, Oikawa H. Org Lett. 2009;11:3016–3019. doi: 10.1021/ol901020a. [DOI] [PubMed] [Google Scholar]
- 200.Moghadam M, Tangestaninejad S, Mirkhani V, Mohammadpoor-baltork I, Sirjanian N, Parand S. Bioorg Med Chem. 2009;17:3394–3398. doi: 10.1016/j.bmc.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 201.Montes-Avila J, Diaz-Camacho SP, Sicairos-Felix J, Delgado-Vargas F, Rivero IA. Bioorg Med Chem. 2009;17:6780–6785. doi: 10.1016/j.bmc.2009.02.052. [DOI] [PubMed] [Google Scholar]
- 202.Moro WB, Yang Z, Kane TA, Zhou Q, Harville S, Brouillette CG, Brouillette WJ. J Comb Chem. 2009;11:617–625. doi: 10.1021/cc9000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Morton D, Leach S, Cordier C, Warriner S, Nelson A. Angew Chem, Int Ed. 2009;48:104–109. doi: 10.1002/anie.200804486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mossetti R, Pirali T, Tron GC. J Org Chem. 2009;74:4890–4892. doi: 10.1021/jo9005969. [DOI] [PubMed] [Google Scholar]
- 205.Mudit M, Khanfar M, Muralidharan A, Thomas S, Shah GV, van Soest RWM, El Sayed KA. Bioorg Med Chem. 2009;17:1731–1738. doi: 10.1016/j.bmc.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 206.Mugherli L, Burchak ON, Balakireva LA, Thomas A, Chatelain F, Balakirev MY. Angew Chem, Int Ed. 2009;48:7639–7644. doi: 10.1002/anie.200901139. [DOI] [PubMed] [Google Scholar]
- 207.Mukhopadhyay C, Datta A, Butcher RJ. Tetrahedron Lett. 2009;50:4246–4250. [Google Scholar]
- 208.Musonda CC, Whitlock GA, Witty MJ, Brun R, Kaiser M. Bioorg Med Chem Lett. 2009;19:401–405. doi: 10.1016/j.bmcl.2008.11.098. [DOI] [PubMed] [Google Scholar]
- 209.Nakamura M, Hamasaki T, Tokitou M, Baba M, Hashimoto Y, Aoyama H. Bioorg Med Chem. 2009;17:4740–4746. doi: 10.1016/j.bmc.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 210.Nasr G, Petit E, Supuran CT, Winum J-Y, Barboiu M. Bioorg Med Chem Lett. 2009;19:6014–6017. doi: 10.1016/j.bmcl.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 211.Nefzi A, Appel J, Arutyunyan S, Houghten RA. Bioorg Med Chem Lett. 2009;19:5169–5175. doi: 10.1016/j.bmcl.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 212.Nguyen QPB, Kim JN, Kim TH. Tetrahedron Lett. 2009;50:4015–4018. [Google Scholar]
- 213.Nikulnikov M, Tsirulnikov S, Kysil V, Ivachtchenko A, Krasavin M. Synlett. 2009:260–262. [Google Scholar]
- 214.Ohta Y, Chiba H, Oishi S, Fujii N, Ohno H. J Org Chem. 2009;74:7052–7058. doi: 10.1021/jo901328q. [DOI] [PubMed] [Google Scholar]
- 215.Ohta Y, Kubota Y, Watabe T, Chiba H, Oishi S, Fujii N, Ohno H. J Org Chem. 2009;74:6299–6302. doi: 10.1021/jo901090u. [DOI] [PubMed] [Google Scholar]
- 216.Okamoto N, Sakurai K, Ishikura M, Takeda K, Yanada R. Tetrahedron Lett. 2009;50:4167–4169. [Google Scholar]
- 217.Olsen CA, Ghadiri MR. J Med Chem. 2009;52:7836–7846. doi: 10.1021/jm900850t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ottesen LK, Olsen CA, Witt M, Jaroszewski JW, Franzyk H. Chem- -Eur J. 2009;15:2966–2978. doi: 10.1002/chem.200802044. [DOI] [PubMed] [Google Scholar]
- 219.Owen DR, Rodriguez-Lens M, Corless MD, Gaulier SM, Horne VA, Kinloch RA, Maw GN, Pearce DW, Rees H, Ringer TJ, Ryckmans T, Stammen BLC. Bioorg Med Chem Lett. 2009;19:1702–1706. doi: 10.1016/j.bmcl.2009.01.106. [DOI] [PubMed] [Google Scholar]
- 220.Park SO, Kim J, Koh M, Park SB. J Comb Chem. 2009;11:315–326. doi: 10.1021/cc800197s. [DOI] [PubMed] [Google Scholar]
- 221.Parlow JJ, Burney MW, Case BL, Girard TJ, Hall KA, Hiebsch RR, Huff RM, Lachance RM, Mischke DA, Rapp SR, Woerndle RS, Ennis MD. Bioorg Med Chem Lett. 2009;19:4657–4663. doi: 10.1016/j.bmcl.2009.06.075. [DOI] [PubMed] [Google Scholar]
- 222.Pasquini S, Mugnaini C, Brizzi A, Ligresti A, Di Marzo V, Ghiron C, Corelli F. J Comb Chem. 2009;11:795–798. doi: 10.1021/cc9000955. [DOI] [PubMed] [Google Scholar]
- 223.Pelletier SMC, Ray PC, Dixon DJ. Org Lett. 2009;11:4512–4515. doi: 10.1021/ol901640v. [DOI] [PubMed] [Google Scholar]
- 224.Peng LF, Stanton BZ, Maloof N, Wang X, Schreiber SL. Bioorg Med Chem Lett. 2009;19:6319–6325. doi: 10.1016/j.bmcl.2009.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Perez-Fernandez R, Pittelkow M, Belenguer AM, Lane LA, Robinson CV, Sanders JKM. Chem Commun. 2009:3708–3710. doi: 10.1039/b902842k. [DOI] [PubMed] [Google Scholar]
- 226.Pettersson H, Bulow A, Ek F, Jensen J, Ottesen LK, Fejzic A, Ma J-N, Del Tredici AL, Currier EA, Gardell LR, Tabatabaei A, Craig D, McFarland K, Ott TR, Piu F, Burstein ES, Olsson R. J Med Chem. 2009;52:1975–1982. doi: 10.1021/jm801534c. [DOI] [PubMed] [Google Scholar]
- 227.Peuchmaur M, Lisowski V, Gandreuil C, Maillard LT, Martinez J, Hernandez J-F. J Org Chem. 2009;74:4158–4165. doi: 10.1021/jo900281a. [DOI] [PubMed] [Google Scholar]
- 228.Pirrung MC, Ghorai S, Ibarra-Rivera TR. J Org Chem. 2009;74:4110–4117. doi: 10.1021/jo900414n. [DOI] [PubMed] [Google Scholar]
- 229.Pirrung MC, Wang J. J Org Chem. 2009;74:2958–2963. doi: 10.1021/jo802170k. [DOI] [PubMed] [Google Scholar]
- 230.Poeylaut-Palena AA, Mata EG. J Comb Chem. 2009;11:791–794. doi: 10.1021/cc900072z. [DOI] [PubMed] [Google Scholar]
- 231.Pokhodylo NT, Matiychuk VS, Obushak MD. J Comb Chem. 2009;11:481–485. doi: 10.1021/cc900012w. [DOI] [PubMed] [Google Scholar]
- 232.Poondra RR, Kumar NN, Bijian K, Prakesch M, Campagna-Slater V, Reayi A, Reddy PT, Choudhry A, Barnes ML, Leek DM, Daroszewska M, Lougheed C, Xu B, Schapira M, Alaoui-Jamali MA, Arya P. J Comb Chem. 2009;11:303–309. doi: 10.1021/cc8001525. [DOI] [PubMed] [Google Scholar]
- 233.Porcheddu A, Giacomelli G, Piredda I. J Comb Chem. 2009;11:126–130. doi: 10.1021/cc8001124. [DOI] [PubMed] [Google Scholar]
- 234.Portela-Cubillo F, Scott JS, Walton JC. J Org Chem. 2009;74:4934–4942. doi: 10.1021/jo900629g. [DOI] [PubMed] [Google Scholar]
- 235.Presset M, Coquerel Y, Rodriguez J. Org Lett. 2009;11:5706–5709. doi: 10.1021/ol9024056. [DOI] [PubMed] [Google Scholar]
- 236.Pudelova N, Krchnak V. J Comb Chem. 2009;11:851–859. doi: 10.1021/cc9000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pudelova N, Krchnak V. J Comb Chem. 2009;11:370–374. doi: 10.1021/cc800181y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Qin L-Y, Cole AG, Metzger A, O’Brien L, Sun X, Wu J, Xu Y, Xu K, Zhang Y, Henderson I. Tetrahedron Lett. 2009;50:419–422. [Google Scholar]
- 239.Rawls KA, Therese Lang P, Takeuchi J, Imamura S, Baguley TD, Grundner C, Alber T, Ellman JA. Bioorg Med Chem Lett. 2009;19:6851–6854. doi: 10.1016/j.bmcl.2009.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rhoden CRB, Rivera DG, Kreye O, Bauer AK, Westermann B, Wessjohann LA. J Comb Chem. 2009;11:1078–1082. doi: 10.1021/cc900106u. [DOI] [PubMed] [Google Scholar]
- 241.Rinderspacher A, Cremona ML, Liu Y, Deng S-X, Xie Y, Gong G, Aulner N, Tobben U, Myers K, Chung C, Andersen M, Vidovic D, Schurer S, Branden L, Yamamoto A, Landry DW. Bioorg Med Chem Lett. 2009;19:1715–1717. doi: 10.1016/j.bmcl.2009.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Roberge JY, Harikrishnan LS, Kamau MG, Ruan Z, Van Kirk K, Liu Y, Cooper CB, Poss MA, Dickson JK, Gavai AV, Chao ST, Leith LW, Bednarz MS, Mathur A, Kakarla R, Schnur DM, Vaz R, Lawrence RM. J Comb Chem. 2008;11:72–82. doi: 10.1021/cc800051u. [DOI] [PubMed] [Google Scholar]
- 243.Rolfe A, Young K, Volp K, Schoenen F, Neuenswander B, Lushington GH, Hanson PR. J Comb Chem. 2009;11:732–738. doi: 10.1021/cc900025e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Romagnoli R, Baraldi PG, Carrion MD, Cruz-Lopez O, Lopez Cara C, Basso G, Viola G, Khedr M, Balzarini J, Mahboobi S, Sellmer A, Brancale A, Hamel E. J Med Chem. 2009;52:5551–5555. doi: 10.1021/jm9001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rostamnia S, Alizadeh A, Zhu L-G. J Comb Chem. 2009;11:143–145. doi: 10.1021/cc800128j. [DOI] [PubMed] [Google Scholar]
- 246.Roy S, Roy S, Neuenswander B, Hill D, Larock RC. J Comb Chem. 2009;11:1128–1135. doi: 10.1021/cc9001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Roy S, Roy S, Neuenswander B, Hill D, Larock RC. J Comb Chem. 2009;11:1061–1065. doi: 10.1021/cc9000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Russo O, Cachard-Chastel M, Riviere C, Giner M, Soulier J-L, Berthouze M, Richard T, Monti J-P, Sicsic S, Lezoualc’h F, Berque-Bestel I. J Med Chem. 2009;52:2214–2225. doi: 10.1021/jm801327q. [DOI] [PubMed] [Google Scholar]
- 249.Ryckmans T, Edwards MP, Horne VA, Correia AM, Owen DR, Thompson LR, Tran I, Tutt MF, Young T. Bioorg Med Chem Lett. 2009;19:4406–4409. doi: 10.1016/j.bmcl.2009.05.062. [DOI] [PubMed] [Google Scholar]
- 250.Ryu IA, Park JY, Han HC, Gong Y-D. Synlett. 2009:999–1003. [Google Scholar]
- 251.Sandulenko Y, Komarov A, Krasavin M. Lett Org Chem. 2009;6:491–495. [Google Scholar]
- 252.Sanganee HJ, Baxter A, Barber S, Brown AJH, Grice D, Hunt F, King S, Laughton D, Pairaudeau G, Thong B, Weaver R, Unitt J. Bioorg Med Chem Lett. 2009;19:1143–1147. doi: 10.1016/j.bmcl.2008.12.104. [DOI] [PubMed] [Google Scholar]
- 253.Sanudo M, Garcia-Valverde M, Marcaccini S, Delgado JJ, Rojo J, Torroba T. J Org Chem. 2009;74:2189–2192. doi: 10.1021/jo8025862. [DOI] [PubMed] [Google Scholar]
- 254.Sanz-Cervera JF, Blasco R, Piera J, Cynamon M, Ibanez I, Murguia M, Fustero S. J Org Chem. 2009;74:8988–8996. doi: 10.1021/jo9016265. [DOI] [PubMed] [Google Scholar]
- 255.Sarachine MJ, Janjic JM, Wipf P, Day BW. Bioorg Med Chem Lett. 2009;19:2404–2408. doi: 10.1016/j.bmcl.2009.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Saruta K, Ogiku T, Fukase K. Tetrahedron Lett. 2009;50:4364–4367. [Google Scholar]
- 257.Sasada T, Kobayashi F, Sakai N, Konakahara T. Org Lett. 2009;11:2161–2164. doi: 10.1021/ol900382j. [DOI] [PubMed] [Google Scholar]
- 258.Scott DE, Dawes GJ, Ando M, Abell C, Ciulli A. ChemBioChem. 2009;10:2772–2779. doi: 10.1002/cbic.200900537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Scott SA, Selvy PE, Buck JR, Cho HP, Criswell TL, Thomas AL, Armstrong MD, Arteaga CL, Lindsley CW, Brown HA. Nat Chem Biol. 2009;5:108–117. doi: 10.1038/nchembio.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Senapati BK, Hwang G-S, Lee S, Ryu DH. Angew Chem, Int Ed. 2009;48:4398–4401. doi: 10.1002/anie.200900351. [DOI] [PubMed] [Google Scholar]
- 261.Seomoon D, AJ, Lee PH. Org Lett. 2009;11:2401–2404. doi: 10.1021/ol9005213. [DOI] [PubMed] [Google Scholar]
- 262.Shaabani A, Rezayan AH, Keshipour S, Sarvary A, Ng SW. Org Lett. 2009;11:3342–3345. doi: 10.1021/ol901196z. [DOI] [PubMed] [Google Scholar]
- 263.Shaginian A, Whitby LR, Hong S, Hwang I, Farooqi B, Searcey M, Chen J, Vogt PK, Boger DL. J Am Chem Soc. 2009;131:5564–5572. doi: 10.1021/ja810025g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sharma S, Kundu B. J Comb Chem. 2009;11:720–731. doi: 10.1021/cc9000345. [DOI] [PubMed] [Google Scholar]
- 265.Shi H, Liu K, Leong WWY, Yao SQ. Bioorg Med Chem Lett. 2009;19:3945–3948. doi: 10.1016/j.bmcl.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 266.Shi J, Stover JS, Whitby LR, Vogt PK, Boger DL. Bioorg Med Chem Lett. 2009;19:6038–6041. doi: 10.1016/j.bmcl.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Shi S, Zhu S, Gerritz SW, Rachwal B, Ruan Z, Hutchins R, Kakarla R, Sofia MJ, Sutton J, Cheney D. Bioorg Med Chem Lett. 2009;19:6477–6480. doi: 10.1016/j.bmcl.2009.08.100. [DOI] [PubMed] [Google Scholar]
- 268.Shih H-W, Guo C-W, Lo K-H, Huang M-Y, Cheng W-C. J Comb Chem. 2009;11:281–287. doi: 10.1021/cc800158t. [DOI] [PubMed] [Google Scholar]
- 269.Sin N, Venables BL, Combrink KD, Gulgeze HB, Yu K-L, Civiello RL, Thuring J, Wang XA, Yang Z, Zadjura L, Marino A, Kadow KF, Cianci CW, Clarke J, Genovesi EV, Medina I, Lamb L, Krystal M, Meanwell NA. Bioorg Med Chem Lett. 2009;19:4857–4862. doi: 10.1016/j.bmcl.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 270.Singh OM, Devi NS. J Org Chem. 2009;74:3141–3144. doi: 10.1021/jo802585b. [DOI] [PubMed] [Google Scholar]
- 271.Smith CD, Gavrilyuk JI, Lough AJ, Batey RA. J Org Chem. 2009;75:702–715. doi: 10.1021/jo9021106. [DOI] [PubMed] [Google Scholar]
- 272.Sotoca E, Allais C, Constantieux T, Rodriguez J. Org Biomol Chem. 2009;7:1911–1920. doi: 10.1039/b821343g. [DOI] [PubMed] [Google Scholar]
- 273.Spatz JH, Welsch SJ, Duhaut D-E, Jaeger N, Boursier T, Fredrich M, Allmendinger L, Ross G, Kolb J, Burdack C, Umkehrer M. Tetrahedron Lett. 2009;50:1705–1707. [Google Scholar]
- 274.Spivey AC, Tseng C-C, Jones TC, Kohler AD, Ellames GJ. Org Lett. 2009;11:4760–4763. doi: 10.1021/ol902038y. [DOI] [PubMed] [Google Scholar]
- 275.Sridharan V, Maiti S, Menendez JC. J Org Chem. 2009;74:9365–9371. doi: 10.1021/jo9021309. [DOI] [PubMed] [Google Scholar]
- 276.Srinivasan R, Tan LP, Wu H, Yang P-Y, Kalesh KA, Yao SQ. Org Biomol Chem. 2009;7:1821–1828. doi: 10.1039/b902338k. [DOI] [PubMed] [Google Scholar]
- 277.Srivastava S, Beck B, Wang W, Czarna A, Holak TA, Dömling A. J Comb Chem. 2009;11:631–639. doi: 10.1021/cc9000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Stokes BJ, Jovanovic B, Dong H, Richert KJ, Riell RD, Driver TG. J Org Chem. 2009;74:3225–3228. doi: 10.1021/jo9002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sun D, Scherman MS, Jones V, Hurdle JG, Woolhiser LK, Knudson SE, Lenaerts AJ, Slayden RA, McNeil MR, Lee RE. Bioorg Med Chem. 2009;17:3588–3594. doi: 10.1016/j.bmc.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sun Z-Y, Zhu Z, Ye Y, McKittrick B, Czarniecki M, Greenlee W, Mullins D, Guzzi M. Bioorg Med Chem Lett. 2009;19:6801–6805. doi: 10.1016/j.bmcl.2009.09.048. [DOI] [PubMed] [Google Scholar]
- 281.Tan LP, Wu H, Yang P-Y, Kalesh KA, Zhang X, Hu M, Srinivasan R, Yao SQ. Org Lett. 2009;11:5102–5105. doi: 10.1021/ol9023419. [DOI] [PubMed] [Google Scholar]
- 282.Thansandote P, Gouliaras C, Turcotte-Savard M-O, Lautens M. J Org Chem. 2009;74:1791–1793. doi: 10.1021/jo8025725. [DOI] [PubMed] [Google Scholar]
- 283.Thompson MJ, Adams H, Chen B. J Org Chem. 2009;74:3856–3865. doi: 10.1021/jo900425w. [DOI] [PubMed] [Google Scholar]
- 284.Torr JE, Large JM, McDonald E. Comb Chem High Throughput Screening. 2009;12:275–284. doi: 10.2174/138620709787581747. [DOI] [PubMed] [Google Scholar]
- 285.Wang H-J, Wang Y, Csakai AJ, Earley WG, Herr RJ. J Comb Chem. 2009;11:355–363. doi: 10.1021/cc8001662. [DOI] [PubMed] [Google Scholar]
- 286.Wang J, Cady SD, Balannik V, Pinto LH, DeGrado WF, Hong M. J Am Chem Soc. 2009;131:8066–8076. doi: 10.1021/ja900063s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wang Q-F, Song X-K, Chen J, Yan C-G. J Comb Chem. 2009;11:1007–1010. doi: 10.1021/cc900005v. [DOI] [PubMed] [Google Scholar]
- 288.Wang W, Dömling A. J Comb Chem. 2009;11:403–409. doi: 10.1021/cc9000136. [DOI] [PubMed] [Google Scholar]
- 289.Wang Y, Sauer DR, Djuric SW. Tetrahedron Lett. 2009;50:5145–5148. [Google Scholar]
- 290.Wang Z, Wang Y, Zhang W-X, Hou Z, Xi Z. J Am Chem Soc. 2009;131:15108–15109. doi: 10.1021/ja9069419. [DOI] [PubMed] [Google Scholar]
- 291.Ward TR, Turunen BJ, Haack T, Neuenswander B, Shadrick W, Georg GI. Tetrahedron Lett. 2009;50:6494–6497. doi: 10.1016/j.tetlet.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wei W, Cai C, Kota S, Takahashi V, Ni F, Strosberg AD, Snyder JK. Bioorg Med Chem Lett. 2009;19:6926–6930. doi: 10.1016/j.bmcl.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Weterings JJ, Khan S, van der Heden van Noort GJ, Melief CJM, Overkleeft HS, van der Burg SH, Ossendorp F, van der Marel GA, Filippov DV. Bioorg Med Chem Lett. 2009;19:2249–2251. doi: 10.1016/j.bmcl.2009.02.095. [DOI] [PubMed] [Google Scholar]
- 294.Wiehn MS, Furniss D, Brase S. J Comb Chem. 2009;11:982–1006. doi: 10.1021/cc9000899. [DOI] [PubMed] [Google Scholar]
- 295.Wiehn MS, Lindell SD, Brase S. J Comb Chem. 2009;11:960–981. doi: 10.1021/cc900090p. [DOI] [PubMed] [Google Scholar]
- 296.Wilk W, Noeren-Mueller A, Kaiser M, Waldmann H. Chem--Eur J. 2009;15:11976–11984. doi: 10.1002/chem.200901797. [DOI] [PubMed] [Google Scholar]
- 297.Williams R, Johnson KA, Gentry PR, Niswender CM, Weaver CD, Conn PJ, Lindsley CW, Hopkins CR. Bioorg Med Chem Lett. 2009;19:4967–4970. doi: 10.1016/j.bmcl.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Worlikar SA, Neuenswander B, Lushington GH, Larock RC. J Comb Chem. 2009;11:875–879. doi: 10.1021/cc900057n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Xiang J, Yang H, Che C, Zou H, Yang H, Wei Y, Quan J, Zhang H, Yang Z, Lin S. PLoS ONE. 2009;4:e4361. doi: 10.1371/journal.pone.0004361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Xie Y, Liu Y, Gong G, Smith DH, Yan F, Rinderspacher A, Feng Y, Zhu Z, Li X, Deng S-X, Branden L, Vidovic D, Chung C, Schuerer S, Morisseau C, Hammock BD, Landry DW. Bioorg Med Chem Lett. 2009;19:2354–2359. doi: 10.1016/j.bmcl.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Xu J-F, Huang X. J Comb Chem. 2009;11:938–942. doi: 10.1021/cc900086e. [DOI] [PubMed] [Google Scholar]
- 302.Xu X, Liang L, Liu J, Yang J, Mai L, Li Y. Tetrahedron Lett. 2009;50:57–59. [Google Scholar]
- 303.Yadav JS, Subba Reddy BV, Madhusudhan Reddy G, Rehana Anjum S. Tetrahedron Lett. 2009;50:6029–6031. [Google Scholar]
- 304.Yaziji V, Coelho A, El Maatougui A, Brea J, Loza MI, Garcia-Mera X, Sotelo E. J Comb Chem. 2009;11:519–522. doi: 10.1021/cc900044k. [DOI] [PubMed] [Google Scholar]
- 305.Yermolayev SA, Gorobets NY, Desenko SM. J Comb Chem. 2008;11:44–46. doi: 10.1021/cc800118v. [DOI] [PubMed] [Google Scholar]
- 306.Yu X, Wu J. J Comb Chem. 2009:895–899. doi: 10.1021/cc900079s. [DOI] [PubMed] [Google Scholar]
- 307.Yongye AB, Appel JR, Giulianotti MA, Dooley CT, Medina-Franco JL, Nefzi A, Houghten RA, Martinez-Mayorga K. Bioorg Med Chem. 2009;17:5583–5597. doi: 10.1016/j.bmc.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Yoo EJ, Park SH, Lee SH, Chang S. Org Lett. 2009;11:1155–1158. doi: 10.1021/ol900023t. [DOI] [PubMed] [Google Scholar]
- 309.Yoshida M, Hedberg C, Kaiser M, Waldmann H. Chem Commun. 2009:2926–2928. doi: 10.1039/b901041f. [DOI] [PubMed] [Google Scholar]
- 310.Zajdel P, Subra G, Verdie P, Bojarski AJ, Duszynska B, Basista K, Obniska J, Martinez J, Pawlowski M. Eur J Med Chem. 2009;44:800–808. doi: 10.1016/j.ejmech.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 311.Zajdel P, Subra G, Verdie P, Gabzdyl E, Bojarski AJ, Duszynska B, Martinez J, Pawlowski M. Bioorg Med Chem Lett. 2009;19:4827–4831. doi: 10.1016/j.bmcl.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 312.Zhang F-L, Xu A-W, Gong Y-F, Wei M-H, Yang X-L. Chem--Eur J. 2009;15:6815–6818. doi: 10.1002/chem.200900613. [DOI] [PubMed] [Google Scholar]
- 313.Zhang L, Xiao Q, Ma C, Xie X-Q, Floreancig PE. J Comb Chem. 2009;11:640–644. doi: 10.1021/cc800200h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zhao C, Tovar C, Yin X, Xu Q, Todorov IT, Vassilev LT, Chen L. Bioorg Med Chem Lett. 2009;19:319–323. doi: 10.1016/j.bmcl.2008.11.093. [DOI] [PubMed] [Google Scholar]
- 315.Zhu W, Mena M, Jnoff E, Sun N, Pasau P, Ghosez L. Angew Chem, Int Ed. 2009;48:5880–5883. doi: 10.1002/anie.200806065. [DOI] [PubMed] [Google Scholar]
- 316.Zhu Z, Sun Z-Y, Ye Y, McKittrick B, Greenlee W, Czarniecki M, Fawzi A, Zhang H, Lachowicz JE. Bioorg Med Chem Lett. 2009;19:5218–5221. doi: 10.1016/j.bmcl.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 317.Wang DM, Sun MN, Liu G. J Comb Chem. 2009;11:556–575. doi: 10.1021/cc800198p. [DOI] [PubMed] [Google Scholar]
- 318.Dobbs AP, Jones P, Penny MJ, Rigby SE. Tetrahedron. 2009;65:5271–5277. [Google Scholar]
- 319.Fustero S, Sancho AG, Acena JL, Sanz-Cervera JF. J Org Chem. 2009;74:6398–6401. doi: 10.1021/jo901245m. [DOI] [PubMed] [Google Scholar]
- 320.Podgorsek A, Jurisch M, Stavber S, Zupan M, Iskra J, Gladysz JA. J Org Chem. 2009;74:3133–3140. doi: 10.1021/jo900233h. [DOI] [PubMed] [Google Scholar]
- 321.Furuya T, Nomoto T, Fukuhara T, Hara S. J Fluorine Chem. 2009;130:348–353. [Google Scholar]
- 322.O’Leary-Steele C, Cordier C, Hayes J, Warriner S, Nelson A. Fluorous-tagged “safety catch” linker for preparing heterocycles by ring-closing metathesis. Org Lett. 2009;11:915–918. doi: 10.1021/ol802848j. (Example I) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Boldon S, Moore JE, Gouverneur V. J Fluorine Chem. 2009;130:1151–1156. [Google Scholar]
- 324.Song S, Zhang Q. Org Lett. 2009;11:4882–4885. doi: 10.1021/ol901955y. [DOI] [PubMed] [Google Scholar]
- 325.Donovan AC, Valliant JF. J Org Chem. 2009;74:8133–8138. doi: 10.1021/jo901475d. [DOI] [PubMed] [Google Scholar]
- 326.Sancho AG, Wang X, Sui B, Curran DP. Adv Synth Catal. 2009;351:1035–1040. doi: 10.1002/adsc.200900061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Liu A, Zhou H, Su G, Zhang W, Yan B. J Comb Chem. 2009;11:1083–1093. doi: 10.1021/cc900109e. (Example III) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Werner S, Nielsen SD, Wipf P, Turner DM, Chambers PG, Geib SJ, Curran DP, Zhang W. J Comb Chem. 2009;11:452–459. doi: 10.1021/cc900003q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Jogireddy R, Dakas P-Y, Valot G, Barluenga S, Winssinger N. Chem Eur J. 2009;15:11498–11506. doi: 10.1002/chem.200901375. [DOI] [PubMed] [Google Scholar]
- 330.Miller M, Vogel JC, Tsang W, Merrit A, Procter DJ. Org Biomol Chem. 2009;7:589–597. doi: 10.1039/b816608k. [DOI] [PubMed] [Google Scholar]
- 331.Curran DP, Sui B. J Am Chem Soc. 2009;131:5411–5413. doi: 10.1021/ja900849f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Sáenz JB, Sun WJ, Chang JW, Li J, Bursulaya B, Gray NS, Haslam DB. Nature Chem Biol. 2009;5:157–165. doi: 10.1038/nchembio.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Rosen MD, Woods CR, Goldberg SD, Hack MD, Bounds AD, Yang Y, Wagaman PC, Phuong VK, Ameriks AP, Barrett TD, Kanelakis KC, Chang J, Shankley NP, Rabinowitz MH. Bioorg Med Chem Lett. 2009;19:6548–6551. doi: 10.1016/j.bmcl.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 334.Scott SA, Selvy PE, Buck JR, Cho HP, Criswell TL, Thomas AL, Armstrong MD, Arteaga CL, Lindsley CW, Brown HA. Nature Chem Biol. 2009;5:108–117. doi: 10.1038/nchembio.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan C-W, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Nature Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Stanton BZ, Peng LF, Maloof N, Nakai K, Wang X, Duffner JL, Taveras KM, Hyman JM, Lee SW, Koehler AN, Chen JK, Fox JL, Mandinova A, Schreiber SL. Nature Chem Biol. 2009;5:154–156. doi: 10.1038/nchembio.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Nature Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Singh J, Zeller W, Zhou N, Hategen G, Mishra R, Polozov A, Yu P, Onua E, Zhang J, Zembower D, Kiselyov A, Ramírez JL, Sigthorsson G, Bjornsson JM, Thorsteinsdottir M, Andrésson T, Bjarnadottir M, Magnusson O, Fabre J-E, Stefansson K, Gurney ME. ACS Chem Biol. 2009;4:115–126. doi: 10.1021/cb8002094. [DOI] [PubMed] [Google Scholar]
- 339.Trujillo JI, Kiefer JR, Huang W, Thorarensen A, Xing L, Caspers NL, Day JE, Mathis KJ, Kretzmer KK, Reitz BA, Weinberg RA, Stegeman RA, Wrightstone A, Christine L, Compton R, Li X. Bioorg Med Chem Lett. 2009;19:908–911. doi: 10.1016/j.bmcl.2008.11.105. [DOI] [PubMed] [Google Scholar]
- 340.Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, Rosen D, Thomas JM, Izumi M, Ganesan A, Galione A, Churchill GC. Nature Chem Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KW, Cardin DP, Critchley S, Cullis CA, Doucette A, Garnsey JJ, Gaulin JL, Gershman RE, Lublinsky McDonald A, Mizutani H, Narayanan U, Olhava EJ, Peluso S, Rezaei M, Sintchak MD, Talreja T, Thomas MP, Traore T, Vyskocil S, Weatherhead GS, Yu J, Zhang J, Dick LR, Claiborne CF, Rolfe M, Bolen JB, Langston SP. Nature. 2009;458:732–737. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 342.Nolen BJ, Tomasevic N, Russell A, Pierce DW, Jia Z, McCormick CD, Hartman J, Sakowicz R, Pollard TD. Nature. 2009;460:1031–1035. doi: 10.1038/nature08231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Mott BT, Tanega C, Shen M, Maloney DJ, Shinn P, Leister W, Marugan JJ, Inglese J, Austin CP, Misteli T, Auld DS, Thomas CJ. Bioorg Med Chem Lett. 2009;19:6700–6705. doi: 10.1016/j.bmcl.2009.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Pathania R, Zlitne S, Barker C, Das R, Gerritsma DA, Lebert J, Awuah E, Melacini G, Capretta FA, Brown ED. Nature Chem Biol. 2009;5:849–856. doi: 10.1038/nchembio.221. [DOI] [PubMed] [Google Scholar]
- 345.Molina G, Vogt A, Bakan A, Dai W, de Oliveira PQ, Znosko W, Smithgall TE, Bahar I, Lazo JS, Day BW, Tsang M. Nature Chem Biol. 2009;5:680–687. doi: 10.1038/nchembio.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Erkizan HV, Kong Y, Mechant M, Schlottmann S, Barger-Rotenberg JS, Yuan L, Abaan OD, Chou T-h, Dakshanamurthy S, Brown ML, Űren A, Toretsky JA. Nature Med. 2009;15:750–757. doi: 10.1038/nm.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Muchamuel T, Basler M, Aujay MA, Suzuki E, Kalim KW, Lauer C, Sylvain C, Ring ER, Shields J, Jiang J, Shwonek P, Parlati F, Demo SD, Bennett MK, Kirk CJ, Groettrup M. Nature Med. 2009;15:781–788. doi: 10.1038/nm.1978. [DOI] [PubMed] [Google Scholar]
- 348.Swoboda JG, Meredith TC, Campbell J, Brown S, Suzuki T, Bollenbach T, Malhowski AJ, Kishony R, Gilmore MS, Walker S. ACS Chem Biol. 2009;4:875–883. doi: 10.1021/cb900151k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kamisuki S, Mao Q, Abu-Elheiga L, Gu Z, Kugimiya A, Kwon Y, Shinohara T, Kawazoe Y, Sato S-I, Asakura K, Choo H-YP, Sakai J, Wakil SJ, Uesugi M. Chem Biol. 2009;16:882–892. doi: 10.1016/j.chembiol.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 350.Ali A, Ghosh A, Nathans RS, Sharova N, O’Brien S, Cao H, Stevenson M, Rana TM. ChemBioChem. 2009;10:2072–2080. doi: 10.1002/cbic.200900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Delpire E, Days E, Lewis LM, Mi D, Kim K, Lindsley CW, Weaver CD. Proc Natl Acad Sci USA. 2009;106:5383–5388. doi: 10.1073/pnas.0812756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Suwa A, Yamamoto T, Sawada A, Minoura K, Hosogai N, Tahara A, Kurama T, Shimokawa T, Aramori I. Br J Pharmacol. 2009;158:879–887. doi: 10.1111/j.1476-5381.2009.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Freschauf GK, Karimi-Busheri F, Ulaczyk-Lesanko A, Mereniuk TR, Ahrens A, Koshy JM, Rasouli-Nia A, Pasarj P, Holmes CFB, Rininsland F, Hall DG, Weinfeld M. Cancer Res. 2009;69:7739–7746. doi: 10.1158/0008-5472.CAN-09-1805. [DOI] [PubMed] [Google Scholar]
- 354.Verhoest PR, Proulx-Lafrance C, Corman M, Chenard L, Helal CJ, Hou X, Kleiman R, Liu S, Marr E, Menniti FS, Schmidt CJ, Vanase-Frawley M, Schmidt AW, Willimas RD, Nelson FR, Fonseca KR, Liras S. J Med Chem. 2009;52:7946–7949. doi: 10.1021/jm9015334. [DOI] [PubMed] [Google Scholar]
- 355.Takahashi T, Nagase T, Sasaki T, Nagumo A, Shimamura K, Miyamoto Y, Kitazawa H, Kanesaka M, Yoshimoto R, Aragane K, Tokita S, Sato N. J Med Chem. 2009;52:3142–3145. doi: 10.1021/jm900391x. [DOI] [PubMed] [Google Scholar]
- 356.Abid M, Torok B, Huang X. Aust J Chem. 2009;62:208–222. [Google Scholar]
- 357.Akritopoulou-Zanze I, Hajduk PJ. Drug Discovery Today. 2009;14:291–297. doi: 10.1016/j.drudis.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 358.Bartels JL, Lu P, Walker A, Maurer K, Moeller KD. Chem Commun. 2009:5573–5575. doi: 10.1039/b910577h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Bouillon I, Soural M, Miller MJ, Krchnak V. J Comb Chem. 2009;11:213–215. doi: 10.1021/cc800143e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.FitzGibbons J, Op S, Hobson A, Schaffter L. J Comb Chem. 2009;11:592–597. doi: 10.1021/cc800209w. [DOI] [PubMed] [Google Scholar]
- 361.Gil C, Braese S. J Comb Chem. 2009;11:175–197. doi: 10.1021/cc800102t. [DOI] [PubMed] [Google Scholar]
- 362.Hansen MH, Blakskjaer P, Petersen LK, Hansen TH, Hoejfeldt JW, Gothelf KV, Hansen NJVJ. J Am Chem Soc. 2009;131:1322–1327. doi: 10.1021/ja808558a. [DOI] [PubMed] [Google Scholar]
- 363.Herrmann A. Org Biomol Chem. 2009;7:3195–3204. doi: 10.1039/b908098h. [DOI] [PubMed] [Google Scholar]
- 364.Hu L, Bartels JL, Bartels JW, Maurer K, Moeller KD. J Am Chem Soc. 2009;131:16638–16639. doi: 10.1021/ja907000m. [DOI] [PubMed] [Google Scholar]
- 365.Hulme C, Chappeta S, Dietrich J. Tetrahedron Lett. 2009;50:4054–4057. doi: 10.1016/j.tetlet.2010.06.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Hung AW, Silvestre HL, Wen S, Ciulli A, Blundell TL, Abell C. Angew Chem, Int Ed. 2009;48:8452–8456. doi: 10.1002/anie.200903821. [DOI] [PubMed] [Google Scholar]
- 367.Ji H, Li H, Martasek P, Roman LJ, Poulos TL, Silverman RB. J Med Chem. 2009;52:779–797. doi: 10.1021/jm801220a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Kagan M, Chlenov M, Melnikov S, McConnell O, Bach AC, Carter G, Failli A, Caggiano TJ, Shumsky JS, Lubda D. J Comb Chem. 2009;11:704–719. doi: 10.1021/cc9000407. [DOI] [PubMed] [Google Scholar]
- 369.Oyarzabal J, Howe T, Alcazar J, Andres JI, Alvarez RM, Dautzenberg F, Iturrino L, Martinez S, Van der Linden I. J Med Chem. 2009;52:2076–2089. doi: 10.1021/jm8016199. [DOI] [PubMed] [Google Scholar]
- 370.Ryba TD, Depew KM, Marcaurelle LA. J Comb Chem. 2009;11:110–116. doi: 10.1021/cc8000986. [DOI] [PubMed] [Google Scholar]
- 371.Scott WL, O’Donnell MJ. J Comb Chem. 2009;11:3–13. doi: 10.1021/cc800183m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Scott WL, Alsina J, Audu CO, Babaev E, Cook L, Dage JL, Goodwin LA, Martynow JG, Matosiuk D, Royo M, Smith JG, Strong AT, Wickizer K, Woerly EM, Zhou Z, O’Donnell MJ. J Comb Chem. 2008;11:14–33. doi: 10.1021/cc800184v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Scott WL, Audu CO, Dage JL, Goodwin LA, Martynow JG, Platt LK, Smith JG, Strong AT, Wickizer K, Woerly EM, O’Donnell MJ. J Comb Chem. 2009;11:34–43. doi: 10.1021/cc800185z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Shelat AA, Guy RK. Bioorg Med Chem. 2009;17:1088–1093. doi: 10.1016/j.bmc.2008.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Stocks MJ, Wilden GRH, Pairaudeau G, Perry MWD, Steele J, Stonehouse JP. ChemMedChem. 2009;4:800–808. doi: 10.1002/cmdc.200900050. [DOI] [PubMed] [Google Scholar]
- 376.Taldone T, Sun W, Chiosis G. Bioorg Med Chem. 2009;17:2225–2235. doi: 10.1016/j.bmc.2008.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Vulpetti A, Hommel U, Landrum G, Lewis R, Dalvit C. J Am Chem Soc. 2009;131:12949–12959. doi: 10.1021/ja905207t. [DOI] [PubMed] [Google Scholar]
- 378.Xie J, Thapa R, Reverdatto S, Burz DS, Shekhtman A. J Med Chem. 2009;52:3516–3522. doi: 10.1021/jm9000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Clark Matthew A, Acharya Raksha A, Arico-Muendel Christopher C, Belyanskaya Svetlana L, Benjamin Dennis R, Carlson Neil R, Centrella Paolo A, Chiu Cynthia H, Creaser Steffen P, Cuozzo John W, Davie Christopher P, Ding Yun, Franklin G Joseph, Franzen Kurt D, Gefter Malcolm L, Hale Steven P, Hansen Nils JV, Israel David I, Jiang Jinwei, Kavarana Malcolm J, Kelley Michael S, Kollmann Christopher S, Li Fan, Lind Kenneth, Mataruse Sibongile, Medeiros Patricia F, Messer Jeffrey A, Myers Paul, O’Keefe Heather, Oliff Matthew C, Rise Cecil E, Satz Alexander L, Skinner Steven R, Svendsen Jennifer L, Tang Lujia, van Vloten Kurt, Wagner Richard W, Yao Gang, Zhao Baoguang, Morgan Barry A. Nature Chem Biol. 2009;5:647–654. doi: 10.1038/nchembio.211. [DOI] [PubMed] [Google Scholar]
- 380.Zhang W. In: Linker Strategies in Solid-Phase Organic Synthesis. Scott P, editor. Wiley; 2009. pp. 553–576. [Google Scholar]
- 381.Zhang W. Chem Rev. 2009;109:749–795. doi: 10.1021/cr800412s. [DOI] [PubMed] [Google Scholar]
- 382.Zhang W. Green Chem. 2009;11:911–920. [Google Scholar]
- 383.Zhang W. In: Fluorine in Medicinal Chemistry and Chemical Biology. Ojima I, editor. Wiley-Blackwell; 2009. pp. 335–359. [Google Scholar]