Abstract
Introduction:
Guḍūci Sattva is a highly valued formulation among ayurvedic physicians, commonly recommended in conditions such as Jvara (fever), Dāha (burning sensation) and other conditions of Pitta predominance. In spite of its numerous medicinal attributes, no published work is available until date on manufacturing guidelines along with its quality control parameters.
Aims and Objectives:
The aim of this study is to develop the standard manufacturing procedure for preparation of Guḍūci Sattva and its tablets.
Materials and Methods:
A total of 15 batches of Guḍūci Sattva were prepared in the laboratory. During its preparation, pharmaceutical findings and observations were systematically recorded. To maintain quality control, Guḍūci Sattva tablets were further subjected to analysis such as shape, diameter, width, hardness, weight variation, disintegration time (DT) and friability. Qualitative analysis to detect the presence of various functional groups and high performance thin layer chromatography (HPTLC) profile were also carried out.
Results and Conclusion:
The average percentage of dried Sattva obtained was 3.8%. The tablets were prepared by direct compression method as per pharmacopoeal specifications. Optimum hardness, weight of tablets, DT and friability of Guḍūci Sattva tablets were found complying with official standards. Alkaloids, carbohydrates and starch were found present in Sattva tablets. Number of peaks obtained in HPTLC also corresponds to this finding. Data obtained by present study may be considered as standard for future studies.
Conclusion:
The average percentage of dried Sattva obtained was 3.8%. The tablets were prepared by direct compression method as per pharmacopoeal specifications. Optimum hardness, weight of tablets, DT and friability of Guḍūci Sattva tablets were found complying with official standards. Alkaloids, carbohydrates and starch were found present in Sattva tablets. Number of peaks obtained in HPTLC also corresponds to this finding. Data obtained by present study may be considered as standard for future studies.
KEY WORDS: Guḍūci, Sattva, standard manufacturing procedure, tablets
INTRODUCTION
India is sitting on a gold mine of well-recorded and well-practiced knowledge of traditional herbal medicine. Although, during the past 20 years, herbal products have enjoyed a renaissance among consumers throughout both developing as well as developed nations,[1] one of the impediments in their acceptance is the lack of uniform standard manufacturing protocols (SMPs). Standardization of process is an important step in standardization. This is the stage where physical, chemical and biological properties of a drug undergo significant changes. Change in chemical constituent level even creates perplexity in pharmacological activity of its organic constituents. Thus, products with high quality standards in manufacturing procedures are needed to maintain quality assurance, uniformity in different batches so as to help patients make safe use of these phyto-therapeutic products.
Sattva or Sāra of a herb is the essence or active part and here it refers to the water extractable solid substance collected from herbal drug.[2] It can be considered as an secondary derivative of Hima Kalpanā (cold infusion) because a part of pharmaceutical process involved in it is analogous to Hima Kalpanā. Among all herbal Sattvas, Guḍūci Sattva (aqueous extract of Tinospora cordifolia) is a widely used formulation in Indian system of medicine as febrifuge and a general tonic. Foremost citation of Guḍūci Sattva is available in Rasendra Mangalam.[3] Its pharmaceutical processing has been mentioned elaborately in Yoga Ratnākara,[4] Rasa Yoga Sāgara[5] and Siddha Yoga Saṅgraha.[6] All these texts have mentioned different methods of Sattva preparation. However, exact quantity of water to be used in its preparation is not mentioned in any classical text. Earlier studies in the pharmaceutical aspects of Guḍūci Sattva have reported quantitative variation in the final product.[7,8] The reason behind this may be due to collection of raw material from different seasonal and climatic conditions. Even though Guḍūci Sattva is widely used in practice, published information on its SMP is lacking among manufacturers, hence posing a challenge to establish an acceptable processing method.
The data compiled after several (at least three) repetitions of the same procedure is to be compared to generate a standard protocol for any formulation. Vaṭi (tablet) is a widely accepted dosage form, as it retains potency for long time, helps in dose fixation and is convenient to the patients. Guḍūci Sattva is available in market in fine powder form and its dose is lower,[9] thus leading to inconvenience to patients. Hence, we decided to convert it into tablets. The present study may prove to be a guide to large scale manufacturers to achieve standardization.
MATERIALS AND METHODS
The whole pharmaceutical procedure is arranged in the following two unit processes, i.e., preparation of Guḍūci Sattva and its tablets.
Pharmaceutical Performa was prepared for maintaining SMPs during pharmaceutical study. A total of 15 batches of Guḍūci Sattva were prepared by adopting classical method.[6] Before dealing with main pharmaceutical study of the formulation, three pilot studies were carried out to investigate the possible common problems which may impact the process. Tablets were prepared as per pharmacopeal specifications by following the principle of direct compression.[10] The main pharmaceutical steps involved are mentioned as follows.
Procurement and identification of Guḍūci stem
Fresh Guḍūci (Tinospora cordifolia (Willd.) Miers) stem spreading over Nimba (Azadirachta indica) tree [Figure 1] was procured from the campus of Gujarat Ayurved University, Jamnagar in the month of December-January 2012 and authenticated at Pharmacognosy laboratory. The physical impurities and outer loose skin were removed and the stem was washed thoroughly with water. Necessary equipment such as stainless steel (SS) vessel, SS ladle, cotton cloth, measuring jar, SS spoon etc., were arranged prior to beginning of the pharmaceutical procedure.
Figure 1.

Natural habitat of collected Nimba Guḍūci
Preparation of Guḍūci Sattva
5 kg of fresh Guḍūci stem of 1.6-2.0 cm thick was taken and chopped into pieces of 1.5-2.0 inches length. These pieces then were pounded thoroughly and converted into a slimy paste. The mass so obtained was kept for soaking overnight (12 h) in 4 times of potable water (w/v) in a SS vessel. The next morning, the mass was macerated in water thoroughly with hands for about 1 h and filtered slowly through a clean cotton cloth folded four times. The liquid was kept aside undisturbed for 4 h; thereafter the supernatant liquid was carefully siphoned off. White and smooth starchy sediment settled at the bottom was collected into a SS tray, air-dried under a running fan and stored in dry airtight glass jars under sterile conditions. 14 more batches were prepared to ensure SMP by following similar process. Whole unit operating process is depicted in Figure 2.
Figure 2.

Unit operating procedure of Guḍūci Sattva preparation
Preparation of tablets of Guḍūci Sattva
Prepared Sattva was taken in SS vessel and converted into granules (size 20) with the help of a 20 mesh sieve, taken into a SS tray and kept in the oven at 50°C until it completely dried. Next day they were mixed with 1% binding agent (Gum acacia). Granules were passed through a tablet punching machine to prepare tablets of 500 mg. A 16 station single rotary tableting machine was used for tableting. The tablets were collected, weighed and stored in air-tight sterile glass containers along with small pieces of cotton in them. Equipments and their respective specifications used in preparation of Guḍūci Sattva and its tablets preparation are listed in Appendix 1 and 2 respectively.
General observations
As the maceration was completed after 1 h, the sliminess of pulp was almost reduced which indicates complete extraction and separation of starch contents or Sattva from Guḍūci. The color of liquid after straining with the four folded cloth was greenish brown. The color of final collected air dried Sattva was Shaṅkhābha (clear white).
Analytical study
Organoleptic characters of fresh Guḍūci stem, liquid after maceration and dried Sattva were noted. The tablets obtained were subjected for testing with pharmacopoeial standard parameters such as hardness,[11] weight variation,[12] disintegration time (DT),[13] friability[13] etc., Qualitative analysis for various functional groups was also done.[14,15] Powdered sample of Guḍūci Sattva tablets was further subjected to high performance thin layer chromatography (HPTLC) study.
HPTLC profile
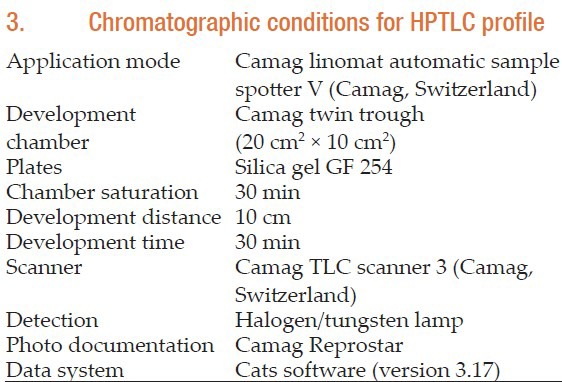
Initially sample solutions were prepared. Accurately weighed and powdered 500 mg Guḍūci Sattva tablets were taken in methanol and was filtered through Whatman's I filter paper (Nair Industries, Mumbai). The filtrate was further subjected to chromatographic separation. The Solvent system used was Chloroform: Methanol (9:1% v/v). A volume of 5 μl of sample solution was applied on pre-coated silica gel 60 F254 TLC plate (E. Merck) of uniform thickness of 0.2 mm and the plate was developed in the solvent system up to a distance of 8 cm. The plate was visualized under short and long ultraviolet (UV) radiation and density of the separated spots was recorded using scanner III. The plate was sprayed with Vaniline- sulfuric acid reagent and observed in visible light. The Rf values were recorded [Figure 3]. Peak display densitogram of Guḍūci Sattva tablets at 254 nm and 366 nm is shown in Figure 4. Chromatographic conditions for HPTLC profile are listed in Appendix 3.
Figure 3.
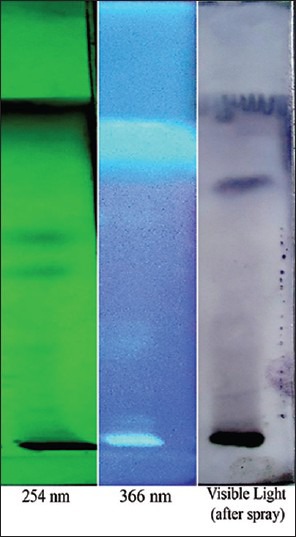
Visualization of Guḍūci Sattva tablets at 254 nm, 366 nm and in visible light
Figure 4.
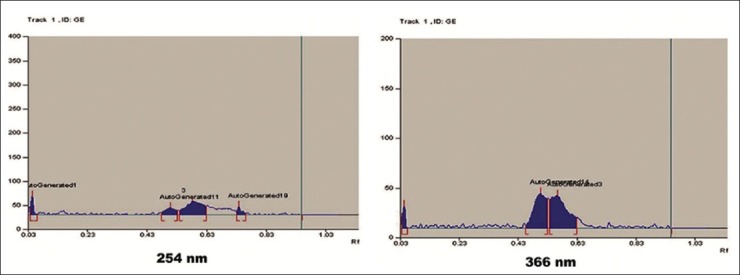
Peak display densitogram of Guḍūci Sattva tablets at 254 nm and 366 nm
RESULTS AND DISCUSSION
Raw drug was collected in December and January months, as more extraction of Sattva is observed in this season.[16] Fresh Guḍūci was taken in present study as per classical guideline-Sadaiva ardrā prayojyeta (always use in fresh state).[17] Guḍūci grown on Neem trees was selected for pharmaceutical processing, because it is said to be the best as the synergy between these plants enhance its efficacy.[18] Pilot batches of Guḍūci Sattva were prepared for fixing the suitable method of preparation and to avoid the variation from batch to batch. Thus, to develop SMP, 15 batches of each of the trial drugs were prepared [Table 1]. Initially, separation of physical impurities and outer skin was done. First post-harvest processing includes primary cutting or comminuting. In classics, “Aṅguṣṭha pramāṇa” (thumb size) of Guḍūci stem has been mentioned to use for preparing Guḍūci based formulations.[19] Accordingly, thumb sized or medium size stems having average diameter 1.6-2.0 cm were selected for study.[8] The diameter of the stem, size of the pieces and soaking time were same in all batches. Properly washed Guḍūci stem was made into pieces up to 1-2 inches, because in the pilot study we found it to be the suitable size for extraction of Sattva. It was then crushed into coarse slimy mass in Ūlūkhala Yantra (pounding apparatus). Particle size reduction provides a larger surface area for drug to interact with water for adequate transfer of constituents and hence better extraction. As there are no references about the ratio of material and water for preparation of Guḍūci Sattva, the reference of recent texts was followed in the present study.[20] Further, overnight soaking was done for proper penetration of water into the cells of the drug. Here, it is suggested to decide the duration of soaking according to the weather, as it was observed in pilot batches that soaking for a long time leads to unwanted microbial growth and foul smell. On the other hand, if soaking done for a short period, amount of extraction may be less and it may hamper the final yield of Sattva.
Table 1.
Details of Guḍūci Sattva in different batches
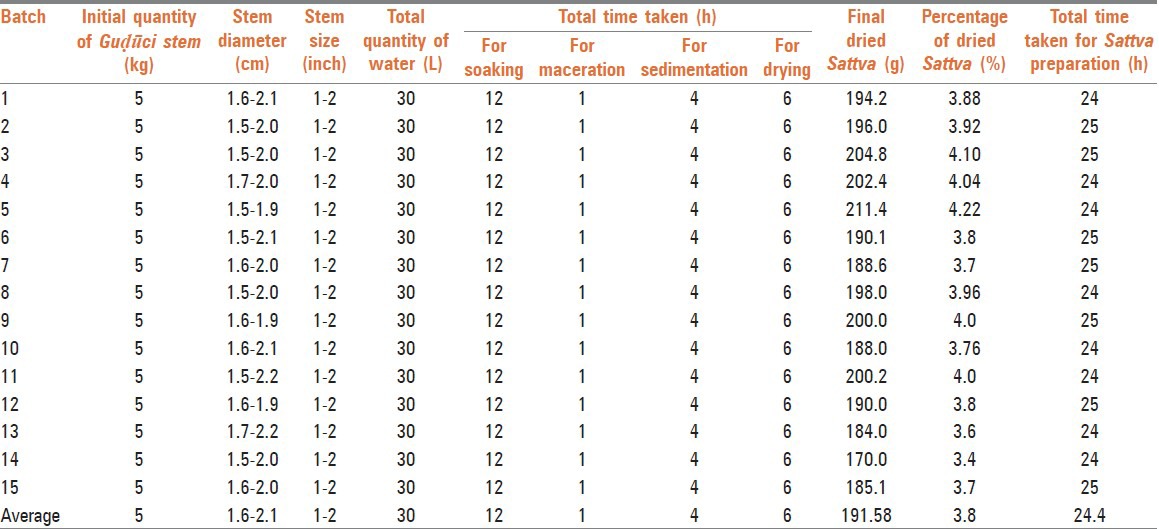
Next morning, maceration of the fine mass was done thoroughly up to reduction in sliminess of pulp, to ensure complete extraction of Sattva. Maceration was continued until the coarse mass lost stickiness. It took 1 h for complete extraction and separation of starch contents or Sattva from Guḍūci. The color of liquid after straining with a four folded cloth was greenish brown. This may be due to the chlorophyll content present in the wall of green Guḍūci stem. Total time taken for preparation of Sattva was between 23 h and 25 h (up to complete drying) and it was almost uniform in all the batches. The average percentage of dried Sattva obtained was 3.8%.
Organoleptic characters which correspond to the pancajñānendriya parīkṣhā (perception by five sense organs) of Ayurveda, were documented at three stages of preparation (for fresh Guḍūci stem, liquid after maceration and powdered Sattva), [Table 2] because these parameters can change at different stages. As per classics, the taste of Guḍūci Sattva is svādu (palatable/pleasant),[21] but recent experts describe the Sattva to be slight bitter.[22,23] However, in present study, it was found tasteless in all batches, thus, corroborating observations in the classics. It can be assumed that freshly prepared Sattva is palatable and will not have bitter taste.
Table 2.
Organoleptic characters of raw (fresh Guḍūci stem), liquid after maceration and finished product
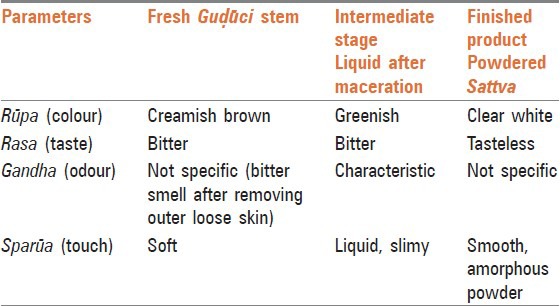
In Rasa Yoga Sāgara, the color of Sattva is mentioned as “Śubhrakhaṇḍanibha” (clear white like sugar cubes)[5] and Yoga Ratnakara explains it as “Śaṅkhanibha” (clear white like conch shell).[4] Current texts mention it as greenish white[22] or grayish white[23] in colour. In present work it was found clear white in color which resembles with classical description. The organoleptic characters of extracted and dried Sattva resemble the chief desired characters mentioned in classics. This indicates that if prompt care taken during pharmaceutical process, genuineness of Sattva can be maintained.
Similar observations were found in each batch, thus establishing the SMP followed in the preparation of Guḍūci Sattva. The operative process of the formulation can be validated on the basis of data generated by repeated pharmaceutical procedures.
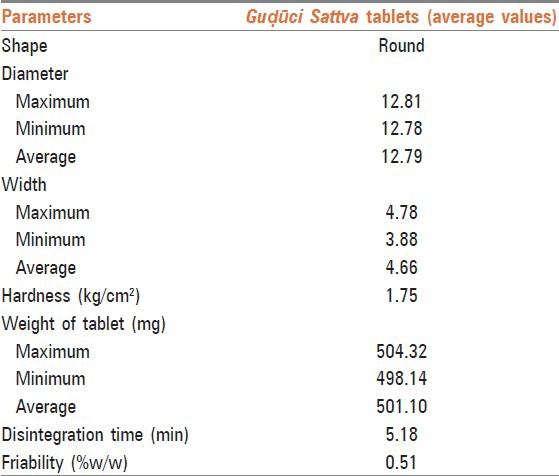
Tablets of Guḍūci Sattva were made and evaluated for various parameters such as organoleptic, shape, hardness, uniformity in weight, Disintegration time (DT) and friability, which were found to be acceptable as per the pharmacopoeial specifications. From a drug stability point of view, to make tablets is more acceptable than its powder state, as in classics shelf life of tablets is more than their powders.[24]
Shape of Guḍūci Sattva tablet was circular with flat facets. The hardness of the tablets was found to be 1.75 kg/cm2. It is desirable that every tablet in a batch should be uniform in weight, but in case of variation, allowable permissible limit is ± 5% for tablets weighing 250 mg or more. The average weight of tablets was found to be 501.10 mg. DT is the time required to break the tablet into smaller particles after swallowing. Thus, it determines the durability and absorption of tablets. In general, Indian Pharmacopoeia prescribes an upper limit of 15 min of DT for plain/uncoated tablets. The DT for Sattva tablets was found to be 5.18 min. The friability of the tablets was 0.51% w/w (i.e. below prescribed limit of 1%); indicating a good mechanical resistance. Thus, the objectives of Kalpanā viz.: increasing shelf life, making it palatable, making it easy to dispense, dose fixation, etc., were achieved in present work.
Qualitative tests were done to detect the presence of functional groups. They revealed the presence of alkaloids, carbohydrates and starch in Guḍūci Sattva tablets, whereas glycosides, tannins, saponins, flavanoids, phenols, proteins and sterols were absent. Detailed analysis of Guḍūci Sattva tablets are shown in Tables 3-6. Chromatographic study (HPTLC) was also carried out under 254 nm and 366 nm UV to establish fingerprinting [Table 7].
Table 3.
Process validation for tableting of Guḍūci Sattva
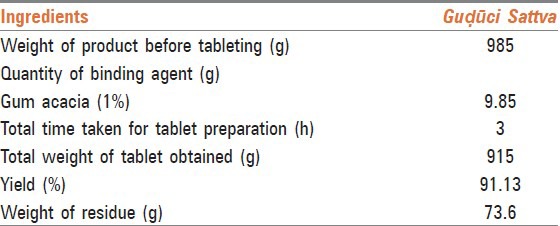
Table 6.
Qualitative analysis for various functional groups in Guḍūci Sattva tablets
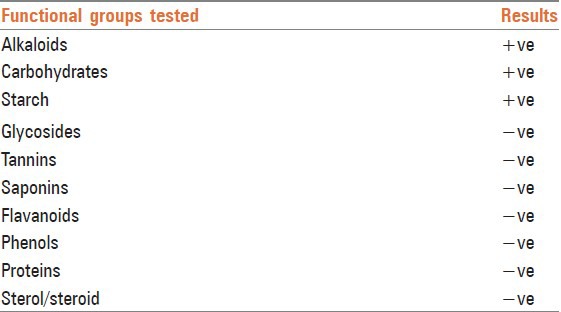
Table 7.
HPTLC profile of Guḍūci Sattva

Table 4.
Organoleptic parameters of Guḍūci Sattva tablets

Table 5.
Physical analysis of Guble Sattva tablets
As Guḍūci Sattva was observed to have hygroscopic nature, it readily gains moisture if kept exposed to environment, which in turn may promote microbial growth in it. Thus, it is recommended to store it in dry, sterile and airtight containers. A small piece of cotton was kept with tablets in container to avoid regaining of moisture and lumping of tablets. Thus it could prevent any change in disintegration characteristics and hardness of tablets. It also prevents breakage of tablets due to friction during handling.
CONCLUSION
Among various dosage forms evolved in Ayurvedic pharmaceutics, Guḍūci Sattva is highly recommended, palatable and acceptable to patients. The average percentage of Guḍūci Sattva obtained from 15 batches is 3.8%. December and January are considered to be best for maximum extraction of Sattva. The organoleptic characters were found in accordance with classical statements and we have shown them to be almost similar in nature for all batches. The tablets exhibited optimum DT, weight uniformity and hardness. The findings of present study ensure the uniformity in the operative procedures. Thus the SMP of Guḍūci Sattva has been established. As no published reports are available on the standardization of preparation of Guḍūci Sattva tablets, the observations of our present study can be considered as standards for further studies.
APPENDIX
1. Equipments and their specifications in Guḍūci Sattva preparation

2. Equipments and their specifications used in Vati (Tablets) preparation

3. Chromatographic conditions for HPTLC profile
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1. [Last accessed on 2012 Feb 13]. Available from: http://www.freepatentsonline.com/6806090.html .
- 2.2nd ed. Part-1. New Delhi: Controller of Publications, Ministry of Health and Family Welfare, Govt. of India; 2003. The Ayurvedic Formulary of India; p. 560. [Google Scholar]
- 3.Nagarjuna . Rasendra Mangalam of Nagarjuna. 3\112. In: Sharma HS, editor. Varanasi: Choukhamba Orientalia; 2008. p. 84. [Google Scholar]
- 4.Shastri B, editor. 7th ed. Varanasi: Choukhamba Sanskrit Sansthan; 2002. Commentary Vidhyotani of Shastri L on Yogaratnakara of Anonymous; p. 118. [Google Scholar]
- 5.Sharma H, Rasa Yoga Sagara. Reprint ed. Varanasi: Chaukhamba Krishnadas Academy; 2004. p. 378. [Google Scholar]
- 6.Acharya YT. 13th ed. Ch. 14. Nagpur: Shri Baidhnath Ayurved Bhavan Ltd; 2008. Siddha Yoga Sangraha. Rajyakshma Urahkshatadhikar; pp. 83–4. [Google Scholar]
- 7.Mehra PN, Puri HS. Studies on Guduchi satwa. Indian J Pharmacol. 1969;31:180–82. [Google Scholar]
- 8.Sharma R, Harisha CR, Galib R, Patgiri BJ, Prajapati PK. Quantitative estimation of Satva extracted from different stem sizes of Guduchi (Tinospora cordifolia (Willd.) Miers. J Pharm Sci Innov. 2012;1:38–40. [Google Scholar]
- 9.Hiremath SG, Satva Kalpana. 2nd revised ed. Part 1. Ch. 19. Varanasi: Chaukhamba Orientalia; 2005. A Text Book Bhaishajya Kalpana; p. 221. [Google Scholar]
- 10.3rd ed. I. Delhi: The Controller of Publication; 1985. The Indian Pharmacopoeia; p. 501. [Google Scholar]
- 11.Vol. 2. New Delhi: Controller of Publications, Government of India, Ministry of Health and Family Welfare; 1996. Indian Pharmacopoeia; p. 734. [Google Scholar]
- 12.Vol. 2. New Delhi: Controller of Publications, Government of India, Ministry of Health and Family Welfare; 1996. Indian Pharmacopoeia; p. 736. [Google Scholar]
- 13.Appendix-7.1: A-80. New Delhi: Controller of Publications, Government of India, Ministry of Health and Family Welfare; 1996. Indian Pharmacopoeia; p. 2. [Google Scholar]
- 14.Baxi AJ, Shukla VJ, Bhatt UB. 1st ed. Jamnagar: Gujarat Ayurved University; 2001. Methods of Qualitative Testing of some Ayurvedic Formulations; pp. 5–12. [Google Scholar]
- 15.Khandelwal KR. 16th ed. Pune: Nirali Prakashan; 2006. Practical Pharmacognosy Techniques and Experimental; pp. 149–56. [Google Scholar]
- 16.Sharma R, Shukla VJ, Ravishankar B, Prajapati PK. MD Thesis. Jamnagar: Gujarat Ayurveda University, IPGT and RA; 2012. The effect of two different dosage forms of Guduchi, i.e. Satva and Ghana WSR Antihyperglycemic effect on Madhumeha (NIDDM) pp. 132–3. [Google Scholar]
- 17.Shastri P, editor. 6th ed. 1/45 Varanasi: Chaukhamba Orientalia; 2005. Sharangadhara Samhita of Sharangadhara, Prathama Khanda, with the commentary of Adhamalla's Dipika and Kasiram's Gudhartha Dipika; p. 11. [Google Scholar]
- 18.Vol. 1. New Delhi: Indian Council of Medical Research; 2003. Quality Standards of Indian Medicinal Plants; p. 212. [Google Scholar]
- 19.Acharya YT. 13th ed. Ch. 1. Nagpur: Shri Baidhnath Ayurved Bhavan Ltd; 2008. Siddha Yoga Sangraha. Jwaradhikar; p. 4. [Google Scholar]
- 20.Hiremath SG. Part-1, Ch. 19. Varanasi: Chaukhamba Orientalia; 2005. A Text Book of Bhaishajya Kalpana (Indian Pharmaceutics) p. 220. [Google Scholar]
- 21.Navare K, editor. 1st ed. Part 1. Delhi: Choukhambha Sanskrit Sansthan; 2011. Nighantu Ratnakar of Panshikar VL and Soman KV; p. 75. [Google Scholar]
- 22.Reddy KR. 2nd ed. Ch. 4. Varanasi: Chaukhamba Samskrita Bhawan; 2005. Ausadha Kalpana. Bhaishajya Kalpana Vijnanam; p. 234. [Google Scholar]
- 23.Hiremath SG. A Text Book Bhaishajya Kalpana. 2nd revised ed. Part 1 Ch 19. Varanasi: Chaukhamba Orientalia; 2005. Satva Kalpana; p. 220. [Google Scholar]
- 24.Shastri P. 1st ed. Varanasi: Chaukhamba Surbharati Prakashan; 2006. Sharangadhara Samhita of Sharangadhara, Prathama Khanda, with the commentary of Adhamalla's Dipika and Kasiram's Gudhartha Dipika; p. 13. 1/51-52. [Google Scholar]


