Abstract
Background & objectives:
With the advent of serum chemistry autoanalyzer and routine estimation of serum calcium as a part of annual physical examination, there has been a dramatic change in the presentation of primary hyperparathyroidism (PHPT) from symptomatic to asymptomatic disease in the United States. However, such trend has not been documented from India. We carried out this retrospective study to analyse the changes in clinical presentations of PHPT patients over a period of two decades in a tertiary care centre in north India.
Methods:
This retrospective study included patients with PHPT treated at a single centre of north India between March1990 and October 2010. Two decades were divided into four different time periods, i.e. 1990 to 1994, 1995 to 1999, 2000 to 2004 and 2005 to 2010. Clinical presentations, biochemical parameters and surgical outcomes were compared between different time periods using appropriate statistical methods.
Results:
Data of 202 patients with PHPT with male: female ratio of 3:7 were analyzed. There was a rise in the number of cases of PHPT diagnosed in the last decade compared to the previous decade (28 cases vs 174 cases, P<0.001). Change in the mean age, male: female ratio, lag time for the diagnosis of PHPT and clinical presentations of PHPT (predominance of bone and stone symptoms) did not differ across different time periods. Non-significant decrease in serum calcium levels at the time of diagnosis of PHPT and a significant, decline in the serum alkaline phosphatase levels (P<0.01) were found in the last decade, however, iPTH levels were higher in the last decade (P<0.05). There was no change in the site and size of parathyroid adenoma in the two decades, however, postoperative symptomatic hypocalcemia was less frequent in the last decade.
Interpretation & conclusions:
The findings of this retrospective analysis show that the PHPT still remains symptomatic disease with increasing awareness over the last two decades in our center. There was not much change in the clinical presentation, in the past two decades.
Keywords: Biochemical, changing presentation, primary hyperparathyroidism
Primary hyperparathyroidism (PHPT) is characterized by inappropriately elevated serum parathyroid hormone (PTH) level despite elevated serum calcium. PHPT is the most common cause of hypercalcemia1. In early 1940s, PHPT was described classically as “a disease of bones, stones, abdominal groans, psychic moan and fatigue overtones”2. In 1970s, two changes namely, introduction of serum chemistry autoanalyzer in the clinical chemistry departments and routine estimation of serum calcium as a part of annual physical examination in the United States had significant impact on the clinical presentation of PHPT3. Following this, two major events have occurred; first, in the United States and other developed countries there was a steady rise in the apparent incidence of the disease and second, there was a dramatic shift in the clinical pattern of presentation4. Presently, the majority of PHPT patients in the developed countries are asymptomatic. From the time of Felix Mandel who performed first curative parathyroidectomy in 19255, surgery remains the main treatment for symptomatic PHPT.
Due to the changed demographics of hyperparathyroidism with the advent of serum chemistry autoanalyzer, questions arose as to when should a patient with asymptomatic PHPT be treated? This has led to two National Institutes of Health (NIH) Consensus Conferences on this topic6. However, such changes in presentations of PHPT have not been reported from India. Recent studies on presentations of PHPT still report the disease as symptomatic only7,8,9. The awareness relating to the diagnosis of PHPT among physicians has increased and many recent studies have reported increasing incidence of symptomatic PHPT form India10.
However, no study from India has reported changes in presentation of PHPT over a period of time. Considering this question in mind, this study was undertaken in a tertiary care teaching institution of north India to observe the changes if any, in the clinical presentation of PHPT patients in a tertiary care centre in north India.
Material & Methods
Patient selection: In this retrospective study, data were retrieved from the PHPT registry of the department of Endocrinology, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. Record of patients with histopathologically proved PHPT between March 1990 and October 2010 were analyzed. Diagnosis of PHPT was based on inappropriately elevated PTH despite elevated albumin adjusted calcium. Patients with secondary or tertiary hyperparathyroidism and multilple endocrine neoplasia (MEN) syndrome were excluded. Lag time was described as time interval between first symptom of PHPT to diagnosis of PHPT. Clinical presentations, biochemical parameters and parathyroid gland weight were recorded and compared in different time period. The time period was arbitrarily divided into four different time periods i.e. 1990-1994, 1995-1999, 2000-2004 and 2005-2010. Cure of PHPT was defined as > 50 per cent decrease in PTH with normalization of serum calcium levels which were estimated between 3rd and 7th postoperative day. This study protocol was approved by the Institutional Ethics committee.
Laboratory methods: From 1990 to 2005, serum intact parathyroid hormone (iPTH) and 25- hydroxy vitamin D [25 (OH) D] were measured with radioimmunoassay (RIA) (Immunotech kit, Hitachi, Germany). Serum calcium (8.5-11.4 mg/dl) was measured by Clark and Collip method11 and albumin adjusted calcium was calculated. After 2005, serum iPTH (15-65 pg/ml) and 25(OH)D (total) (detection range 11.1-42.9 ng/ml, normal 20-100 ng/ml) were measured by chemiluminescence assay using commercially available kits (Elecsys 2010 system, Roche diagnostic, Germany). Serum calcium (reference range 8.6-10.2 mg/dl), inorganic phosphate (reference range 2.7-4.5 mg/dl), albumin (reference range 3.4-4.8 mg/dl), alkaline phosphatase (reference range 40-129 IU/l), creatinine (reference range 0.4-1.2 mg/dl) were measured by autoanalyzer (Roche diagnostics, Modular P 800 Germany). Serum haemoglobin (8.5-11.4 mg/dl) was measured by Schiff's method12 before and after 2005 by autoanalyzer (Automated blood cell counter coulter LH 750 (Beckman Coulter, Miami FL, USA).
Localization method: Ultrasonography of the parathyroid glands was performed with a high-resolution linear array transducer of 7.5–10 MHz (ATLHDI 5000, Philips, Bothell, WA, USA). Dual phase sestamibi imaging (MIBI) was used as other modality to localize the parathyroid glands. Single photon emission computed tomography (SPECT) imaging was performed in patients where planner imaging failed to localize the abnormal gland.
Treatment outcome: Postoperatively, parathyroid adenoma was weighted and subjected to histopatholgogical examination. All patients were monitored for sign and symptoms of hyporcalcemia for at least 3 to 7 days postoperatively. Hypocalcemia was defined as serum calcium level less than 8.2 mg/dl. Serum iPTH level was estimated between 3rd to 7th days after surgery.
Statistical methods: SPSS 17 (Statistical Package for Social Sciences 17, 2008, USA) was used for analysis of data. Chi- square test was used to compare categorical variables. Normality of data was checked using Kolmogorov- test. ANOVA test was used for normally distributed data while Kruskal-Wallis test was applied for skewed data for comparisons of variables among different time period.
Results
Baseline characteristic of patients with PHPT: A total of 202 PHPT patient records were retrieved and analyzed for changes in clinical and biochemical presentations over a period of two decade. Of these, 60 were men (29.7%) and 142 were women (70.3%) with a male: female ratio of 3:7. Number of PHPT cases diagnosed during the last two decades and gender distribution is shown in the Fig. There was significant increase in the diagnosis of PHPT in the last decade compared to the previous decade (28 cases vs 174 cases, P<0.001). Mean ages of presentation in the years 1990-1994, 1995-1999, 2000-2004 and 2005-2010 were 34.7± 15.3, 35.8±13.5, 37.5±11.9 and 39.4±14.6 years, respectively. Thirty two patients (15.8%) were < 25 years of age. There was no change in the mean age of the PHPT presentation over the past two decades. Similarly, male to female ratio in PHPT cases remained unchanged. Time from symptoms suggestive of PHPT to diagnosis of PHPT was 82.3± 78.7 months during the period 1990-1994 and reduced to 44.5± 36.5 months during 1995-1999 (P=0.01), but later remained the same (43.8±42.1, 41.9±37.2 months during 2000-2004 and 2005-2010 respectively.
Fig.
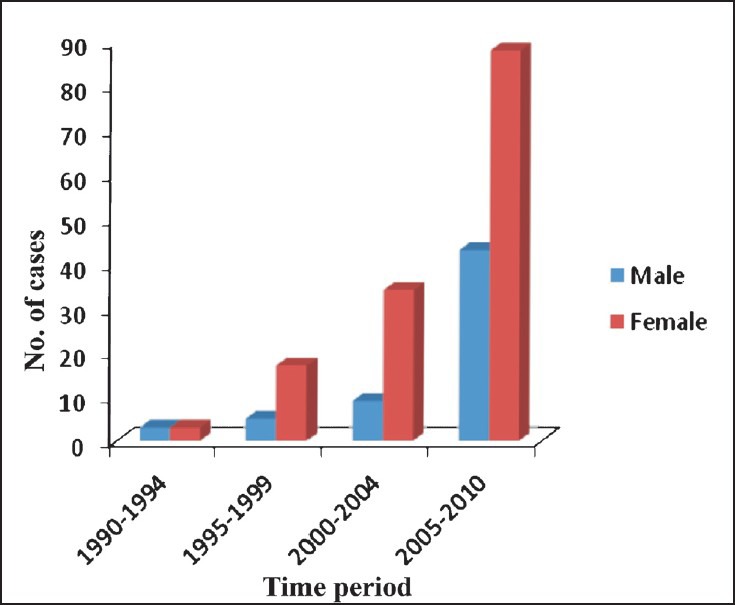
Number of cases and gender-wise distribution of PHPT patients accoridng to different time period (n=202).
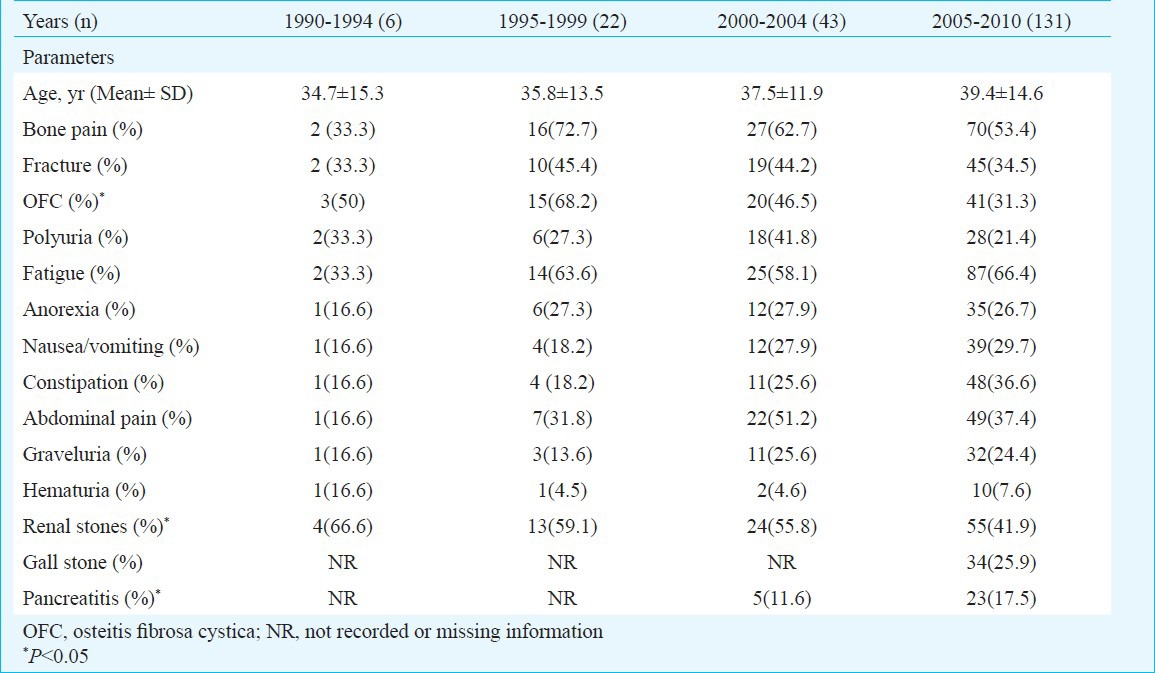
Difference in clinical presentation over two decades: Of the 202 patients with PHPT, only two patients were asymptomatic and the remaining (99.1%) were symptomatic (n=200). The common clinical presentations of PHPT during 1990-1994 (n=6) were renal stones (n=4), osteitis fibrosa cystica (n=3), bone pain (n=2), fractures (n=2), polyuria (n=2) and fatigue (n=2) while gastrointestinal symptoms were less common (n=1). The clinical presentations of PHPT in the period of 1994-1999 (n=22) were bone pain (n=16), osteitis fibrosa cystica (n=15), fatigue (n=14) and renal stones (n=13). The presentations in the last decade (2000-2004 and 2005-2010) were characterized by bone pain (62.7, 53.4%, respectively), fatigue (58.1, 66.4%, respectively) and renal stones (n=27, n=70 respectively. Gastrointestinal presentations (anorexia, nausea/vomiting/abdominal pain and constipation) though more commonly found in the last decade compared to previous decade remained non-significant. The main change in the clinical presentations of PHPT over these 20 years was recognition of PHPT among patients with gallstones (n=34) and pancreatitis (n=28) in last decade (2000-2010) while bone and stone symptoms were still prevalent in similar magnitude. The details of clinical presentations during different time periods are shown in Table I.
Table I.
Difference in clinical presentations according to different time period (n=202)
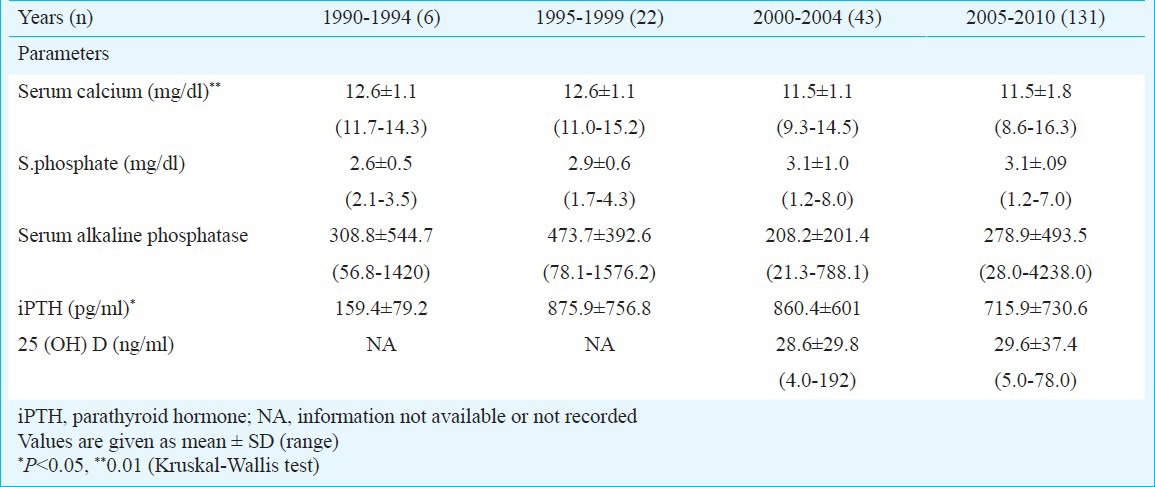
Difference in biochemical presentation over two decades: Serum calcium at the time of diagnosis of PHPT was lower in last decade compared to the previous decade (P=0.01). Similarly, there was a decline in serum alkaline phosphatase level (P=0.01) but iPTH levels were higher in the last decade (P=0.05). Before 2005, seven patients with PHPT (10%) were normocalcemic while after 2005 two patients (1.5%) were normocalcemic. There was no significant change in the haemoglobin, 25 (OH) D levels and renal parameters over a period of past 10 years (There were no data before 2000). The difference in biochemical parameters among different time periods is shown in Table II.
Table II.
Difference in biochemical presentation according to different time period (n=202)
Differences in outcomes over two decades: There was no change in percentage of postoperative cure of PHPT among different time periods. The most common site of adenoma was left inferior parathyroid gland n=94 (46.4%) followed by right inferior n=61 (30.1%), left superior n=21 (10.4%) and least in right superior parathyroid gland n=18 (9.1%), the remaining patients had parathyroid carcinoma and ectopic parathyroid glands. There was no difference in the site of parathyroid adenoma and size of adenoma (weight of gland) over different periods. Majority of them were parathyroid adenoma except in three patients who had parathyroid carcinoma. Mean weight of parathyroid adenoma was 4.7±1.6gm. Post-operative symptomatic hypocalcaemia was noted in (1990-1994) n=4 (66.6%), (1995-1999) n=15 (68.1%), (2000-2004) n=24 (55.8%) and (2005-2010) n=38 (29.2%) (P<0.01). Total nine deaths occurred of the 186 PHPT patients (mortality information was missing in 16 cases). Causes of death were severe hypocalcemia in two, sepsis in two, chronic renal failure in two, respiratory failure due to multiple rib fracture in one and pancreatitis in two.
Discussion
This study revealed that in our center the number of PHPT patients increased over the past two decades, but PHPT still remained a symptomatic disease, and major presenting features were bone pain, fractures, renal stones. Trend of diagnosing PHPT changed from the classical symptoms to underrecognized symptoms like gallstones and pancreatitis in the last five years. Post-operative complications like hypocalcemia decreased significantly. Further, in our center patients with PHPT were young with female predominance and there was no change in the mean age of diagnosis of PHPT over the past two decades. Studies from India have also reported that PHPT patients are young with female predominance8,9,10,13. The reason for the younger age of presentation is not known. Contrary to India, PHPT in West, usually occurs in post-menopausal period and often picked up on routine screening (asymptomatic).
In a study from in Rochester, Minnesota, USA, the increasing incidence of PHPT due to routine screening, was largely due to increasing estimation of serum calcium14. In India availability of autoanalyzer is limited to the secondary and tertiary health centers and routine annual physical examination is not in practice.
Despite, increase in number of cases of PHPT diagnosed in our center, the disease still remains symptomatic and characterized by stones and bone symptoms. This has been reported by many other studies from India8,10,15. This is in contrast to many developed countries where asymptomatic disease predominates. The reasons for symptomatic disease in India could be the) lack of awareness of the disease at primary care physician level, limited availability of autoanalyzer at primary care facility, and absence of routine annual health check up practiced in India. The severity of the disease may be due to prevalent vitamin D deficiency13. In the last decade, there was an increased occurrence of gallstone and pancreatitis among PHPT patients. Though there are several reports of high incidence of gallstones and pancreatitis in patients with PHPT form India, but these remain controversial as there is no cause and effect relationship established between these symptoms and PHPT and lack of population based studies to prove these associations16,17. Large population based study from USA failed to demonstrate higher incidence of pancreatitis in patients with PHPT (mainly asymptomatic) compared to general population18, however, such studies have not been documented from other parts of the world.
In the present study, mean serum calcium level at the time of diagnosis of PHPT was lower in the last decade compared to the previous decade. This was possibly due to recognition of PHPT at earlier stage than before19. Paradoxically, we noticed higher level of iPTH in last decade compared to the previous decade despite modest hypercalcemia at the time of diagnosis; this might be due to the recent availability of more sensitive PTH assay.
The retrospective design is one of the limitations of the study. Furthermore, this study reflects the changes or trend of presentation of PHPT from a single center and hence these findings may not be applicable to the other parts of India,
In conclusion, PHPT still remains symptomatic disease in India with increasing incidence over last two decades. Though, there was not much change in the clinical presentation, progressive decline in serum calcium at the time of diagnosis of PHPT was noted reflecting trend towards picking up asymptomatic PHPT in near future. Though there was no change in site and size of parathyroid adenoma, but postoperative complications were reduced in the last decade. Prospective studies need to be done from different parts of the country with large sample sizes to understand the trend.
References
- 1.Bilezikian JP, Silverberg SJ. Clinical practice. Asymptomatic primary hyperparathyroidism. N Engl J Med. 2004;350:1746–51. doi: 10.1056/NEJMcp032200. [DOI] [PubMed] [Google Scholar]
- 2.Mundy GR, Cove DH, Fisken R. Primary hyperparathyroidism changes in the pattern of clinical presentation. Lancet. 1980;1:1317–20. doi: 10.1016/s0140-6736(80)91783-3. [DOI] [PubMed] [Google Scholar]
- 3.Wermers RA, Khosla S, Atkinson EJ, Achenbach SJ, Oberg AL, Grant CS, et al. Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993-2001: an update on the changing epidemiology of the disease. J Bone Miner Res. 2006;21:171–7. doi: 10.1359/JBMR.050910. [DOI] [PubMed] [Google Scholar]
- 4.Almquist M, Bergenfelz A, Mårtensson H, Thier M, Nordenström E. Changing biochemical presentation of primary hyperparathyroidism. Langenbecks Arch Surg. 2010;395:925–8. doi: 10.1007/s00423-010-0675-5. [DOI] [PubMed] [Google Scholar]
- 5.Mandl F. Therapeutisher versuch bein falls von ostitis fibrosa generalisata mittles. Extirpation eines epithelkorperchen tumors. Wien Klin Woecheshr Zentral. 1926;143:245–84. [Google Scholar]
- 6.Bilezikian JP, Khan AA, Potts JT., Jr Third International Workshop on the Management of Asymptomatic Primary Hyperthyroidism. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94:335–9. doi: 10.1210/jc.2008-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah VN, Bhadada SK, Bhansali A, Behera A, Mittal BR, Vadera B. Influence of age and gender on presentation of symptomatic primary hyperparathyroidism. J Postgrad Med. 2012;58:107–11. doi: 10.4103/0022-3859.97171. [DOI] [PubMed] [Google Scholar]
- 8.Bhansali A, Masoodi SR, Reddy KS, Behra A, Das Radotra B, Mittal BR, et al. Primary hyperparathyroidism in north India: a description of 52 cases. Ann Saudi Med. 2005;25:29–35. doi: 10.5144/0256-4947.2005.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopal RA, Acharya SV, Bandgar T, Menon PS, Dalvi AN, Shah NS. Clinical profile of primary hyperparathyroidism from western India: a single center experience. J Postgrad Med. 2010;56:79–84. doi: 10.4103/0022-3859.65279. [DOI] [PubMed] [Google Scholar]
- 10.Pradeep PV, Jayashree B, Mishra A, Mishra SK. Systematic review of primary hyperparathyroidism in India: the past, present, and the future trends. Int J Endocrinol. 2011;2011:921814. doi: 10.1155/2011/921814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark EP, Collip JB. A study of the Tisdall method for the determination of blood serum calcium with a suggested modification. J Biol Chem. 1925;63:461–4. [Google Scholar]
- 12.Balasubramaniam P, Malathi A. Comparative study of haemoglobin estimated by Drabkin's and Sahil's method. J Postgrad Med. 1992;38:8–9. [PubMed] [Google Scholar]
- 13.Harinarayan CV, Gupta N, Kochupillai N. Vitamin D status in primary hyperparathyroidism in India. Clin Endocrinol (Oxf) 1995;43:351–8. doi: 10.1111/j.1365-2265.1995.tb02043.x. [DOI] [PubMed] [Google Scholar]
- 14.Wermers RA, Khosla S, Atkinson EJ, Hodgson SF, O’Fallon WM, Melton LJ., 3rd The rise and fall of primary hyperparathyroidism: a population-based study in Rochester, Minnesota, 1965-1992. Ann Intern Med. 1997;126:433–40. doi: 10.7326/0003-4819-126-6-199703150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Bhadada SK, Bhansali A, Dutta P, Behera A, Chanukya GV, Mittal BR. Characteristics of primary hyperparathyroidism in adolescents. J Pediatr Endocrinol Metab. 2008;21:1147–53. doi: 10.1515/jpem.2008.21.12.1147. [DOI] [PubMed] [Google Scholar]
- 16.Bhadada SK, Udawat HP, Bhansali A, Rana SS, Sinha SK, Bhasin DK. Chronic pancreatitis in primary hyperparathyroidism: comparison with alcoholic and idiopathic chronic pancreatitis. J Gastroenterol Hepatol. 2008;23:959–64. doi: 10.1111/j.1440-1746.2007.05050.x. [DOI] [PubMed] [Google Scholar]
- 17.Bhadada SK, Bhansali A, Shah VN, Behera A, Ravikiran M, Santosh R. High prevalence of cholelithiasis in primary hyperparathyroidism: a retrospective analysis of 120 cases. Indian J Gastroenterol. 2011;30:100–1. doi: 10.1007/s12664-011-0101-0. [DOI] [PubMed] [Google Scholar]
- 18.Khoo TK, Vege SS, Abu-Lebdeh HS, Ryu E, Nadeem S, Wermers RA. Acute pancreatitis in primary hyperparathyroidism: a population-based Study. J Clin Endocrinol Metab. 2009;94:2115–8. doi: 10.1210/jc.2008-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzaglia PJ, Berber E, Kovach A, Milas M, Esselstyn C, Siperstein AE. The changing presentation of hyperparathyroidism over 3 decades. Arch Surg. 2008;143:260–6. doi: 10.1001/archsurg.143.3.260. [DOI] [PubMed] [Google Scholar]


