Abstract
Background & objectives:
Recurrent spontaneous abortion (RSA) is a reproductive problem that occurs in women in reproductive age with a frequency of 1-3 per cent. Previous studies have reported high levels of serum androgens to be associated with RSAs. At the molecular level, the effect of androgens is mediated through the activation of the androgen receptor (AR). The CAG and GGN repeat polymorphisms of the AR gene are associated with the AR activity. We hypothesize that the AR CAG/GGN repeat polymorphism may be associated with levels of serum androgens. Thus, this study as undertaken to evaluate the relationship between CAG/GGN repeats in exon 1 of the AR gene in women with RSAs.
Methods:
This case-control study was performed in Ningxia, PR China, including 149 women with RSAs and 210 controls. The CAG and GGN repeats of the AR gene were genotyped using a PCR-based assay and were analyzed using Peak Scanner Software v1.0 to determine the CAG/GGN repeat length.
Results:
CAG repeats ranged from 15 to 29 in the RSA patients, compared to 14 to 35 in the control group. The median value of CAG repeats was 22 for the RSA group and 24 for control group. The total AR CAG alleles (≤22 repeats), shorter AR CAG alleles (≤22 repeats), and biallelic means (≤22.5 repeats) were significantly different in the RSA group in comparison to the control group (P<0.001, P<0.01). The median value of the GGN repeats was 23 for the cases and 22 for controls. The total number of AR GGN alleles (≤23 repeats) was significantly different in the RSA group compared to the control group (P<0.5). There was no difference between the RSA group and the control groups in regards to shorter alleles, longer alleles, and biallelic means.
Interpretation & conclusions:
Our observation suggests that the CAG and GGN repeat length is shorter in women with RSAs as compared with controls and that shorter CAG and GGN repeats may be pathogenic for RSAs in Chinese women. Further studies need to be done in different ethnic populations.
Keywords: Androgen, androgen receptor gene, CAG repeats, GGN repeats, recurrent spontaneous abortion
Recurrent spontaneous abortions (RSA) is defined as repeated occurrence of three or more miscarriages before 24th week of gestation1. Epidemiological studies have suggested that RSAs comprise a multifactorial condition enhanced by a variety of genetic and environmental factors2. Some known aetiologic factors of RSAs include parental chromosome abnormalities, uterine abnormalities, endocrinologic diseases, infections, thrombophilia, immunologic factors, nutritional, and environmental factors. However, the true cause of RSAs cannot be determined in approximately 50 per cent of cases3.
Hormonal factors have been proposed to contribute to RSAs4,5. Hormonal disorders may result from problems with certain endocrine glands, such as the pituitary, thyroid, adrenal, as well as the ovaries6. An increased miscarriage rate has been observed in women with polycystic ovarian syndrome (PCOS), a disorder which is characterized by amenorrhoea, hirsutism, and an increase in androgen concentrations4,7. The prevalence of PCOS in women with RSAs has been reported to be between 44 and 63 per cent8,9. The presence of PCOS has not been shown to predict miscarriage, however, those women who miscarried have been shown to demonstrate higher levels of total testosterone, may be associated with RSAs4.
Androgens are steroid hormones with several physiologic effects in both sexes, and their production is associated with androgen insensitivity syndromes of different degrees10. These affect the metabolism of lipoprotein, the vascular endothelium and smooth muscle, and blood coagulation. At the molecular level, the effect of androgens is mediated through the activation of the androgen receptor (AR). The androgen receptor (AR) gene is a transcription factor mediating the physiologic effects of androgens11. AR is essential for male sexual differentiation and maintenance of normal reproductive functions12. In females, the AR is expressed in the ovary, mainly on the granulosa cells, suggesting some involvement in folliculogenesis13. It is located on chromosome Xq11-12 and has a variable NH2-terminal domain that contains two functionally polymorphic microsatellites, a polyglutamine tract encoded by CAG repeats, and a polyglycine tract (GGN) encoded by (GGT)3 GGG (GGT)2 (GGC)n repeat14.
Studies have shown that longer CAG repeats are associated with reduced AR transactivation activity and weaker transcriptional potential14, while androgen responsiveness is increased in cell cultures with shorter GGN repeat15. Increased androgen bioactivity may result from elevated circulating androgen concentrations11. Sugawa and co-workers16 found that shorter CAG repeat lengths would increase serum levels of androgens.
In a study that has examined the AR exon 1 CAG repeat variation in RSAs, Aruna and associates6 have reported that longer CAG repeat lengths are associated with increased odds for RSAs in Indian women with statistical power estimated to be 90 per cent. To our knowledge, there is no study that has examined the GGN repeat variation in the setting of RSAs. For comprehensive assessment of the functional competence of the AR as a transcriptional regulator, both the CAG repeat and the GGN repeat need to be considered17,18. Therefore, we undertook this study to investigate the possible role of the CAG and GGN repeat polymorphisms in the manifestation of RSAs in Chinese women.
Material & Methods
Venous blood samples (5 ml) were collected from 149 randomly selected women (27.97±4.49 yr, 19-42 yr) who had been diagnosed with RSAs during 2006-2011 for which no aetiology was known at the General Hospital of Ningxia Medical University, Ningxia, PR China. For the control group, blood samples from 210 women (27.67±3.67 yr, 20-36 yr), representing similar age, ethnicity, and socio-economic status as compared to the RSA group, were recruited from Yincuan Maternal Child Health Care Hospital in Ningxia. Women who had the spontaneous loss of three or more consecutive pregnancies before 20 weeks of gestation were included in the RSA group. The patients were investigated to exclude established causes of RSA, such as chromosomal abnormalities, uterine abnormalities, hereditary thrombophilias, endocrinologic disorders, immunologic factors and infections. Women in the control group were included if they had no history of abortions or fertility treatments and had normal menstrual cycles. This study was approved by the Institutional Ethical Committee and informed written consent was obtained from all women included in the study.
Genomic DNA was extracted by the TIANamp Blood DNA Kit (TianGen Biotech, Bejing, China) and quantified spectrophotometrically. The CAG and GGN repeats were genotyped using a PCR-based assay. Primer sequences were F:5’-FAM-TCCAGAATCTGTTCCAGAGCGTGC-3’ and R:5’ -GCTGTGAAGGTTGCTGTTCCTCAT-3’ for the CAG18 repeat, and primer sequences were F:5’-FAM-TCCTGGCACACTCTCTTCAC-3’ and R:5’-GCCAGGGTACCACACA- TCAGGT-3’ for the GGN17 repeat. PCR conditions for CAG amplification were: initial denaturation of 94°C for 5 min, followed by 30 cycles of 30 sec at 94°C, 30 sec at 65°C and 45 sec at 72°C and finally 7 min at 72°C. PCR conditions for GGN amplification were: initial denaturation at 94°C for 5 min, followed by 30 cycles of 30 sec at 94°C, 30 sec at 60°C and 45 sec at 72°C and finally 7 min at 72°C. All PCR reactions were performed in a 12.5 μl volume of PCR MIX 6.25 μl, Taq DNA polymerase 0.125 μl (5U/μl), 0.25 μl of each primer, DNA 0.5 μl, double distilled water 5.125 μl.
For genotyping, 0.5 μl of PCR product was mixed with 0.1 μl of LIZ500™ (Applied Biosystems, Foster City, CA) and 7.4 μl of Hi-Di formamide (Applied Biosystems, Foster City, CA) and analyzed on ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA), using Peak Scanner software V1.0 to ascertain the size of AR alleles. PCR and Gene Scan analysis were repeated for all samples to confirm the number of repeats.
As women have two AR alleles (for the two X chromosomes), similar to the earlier method19 analyses were done in three different modes of allele representation; (i) the mean value of the two alleles (biallelic mean), (ii) the shorter allele alone (shorter), and (iii) the longer allele alone (longer). The use of the terms shorter and longer is relative for each individual and does not represent an absolute CAG repeat number or range of numbers.
Statistical analysis: An independent t-test (α=0.05) was performed to test the significance in difference in mean CAG repeats between RSA and control populations. Chi-squared analysis was done to test for the homogeneity of allele frequencies between the case and control groups. Logistic regression analysis was performed and odds ratios were obtained for perceived risk alleles to determine if a significant association to RSAs could be identified. Data were analyzed using SPSS 11.5 statistical software (SPSS, Chicago, IL).
Results
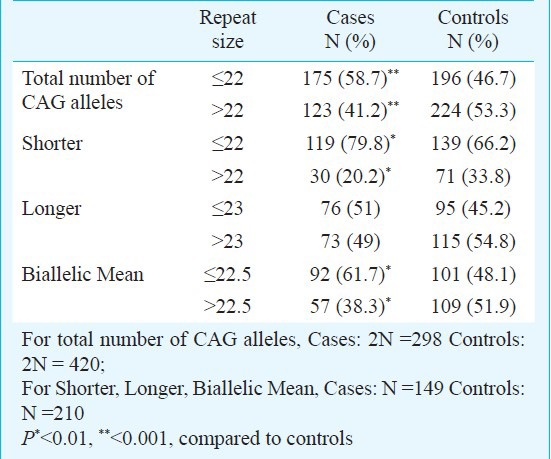
CAG repeats ranged from 15 to 29 in the RSA patients, compared to 14 to 35 in the control group (Fig. 1). The average CAG repeat length was significantly greater in the control group as compared with RSA group (P=0.041, P=0.038; Table I). The mean number of longer CAG repeats for the RSA and control groups was similar (Table I). The median value of CAG repeats was 22 for the RSA group and 24 for control group. For qualitative comparisons, the median value of 22 repeats was considered, which bisects the entire polymorphic range providing three categories viz. <22, 22, and >22 repeats. The total AR CAG alleles (≤22 repeats) and shorter AR CAG alleles (≤22 repeats), were significantly (P<0.001, P<0.01) different in the RSA group in comparison to the control group. There was no significant difference between the two groups for longer AR CAG alleles (Table II). When the biallelic mean was categorized into >22.5 and ≤22.5 repeats, a significant difference was seen in the biallelic mean distribution pattern (Fig. 1) between the RSA and control groups (P<0.01, Table II).
Fig. 1.
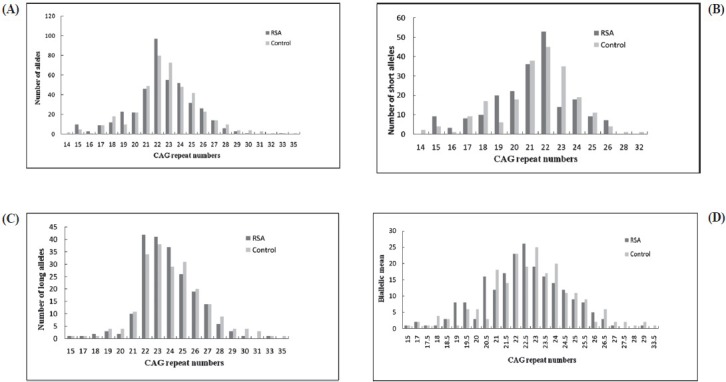
Distribution of CAG repeat numbers in exon 1 of the AR gene in RSA cases and controls. (A) Total alleles, (B) short CAG allele, (C) long CAG alleles, (D) biallelic mean.
Table I.
Comparison of CAG repeat length for case and controls
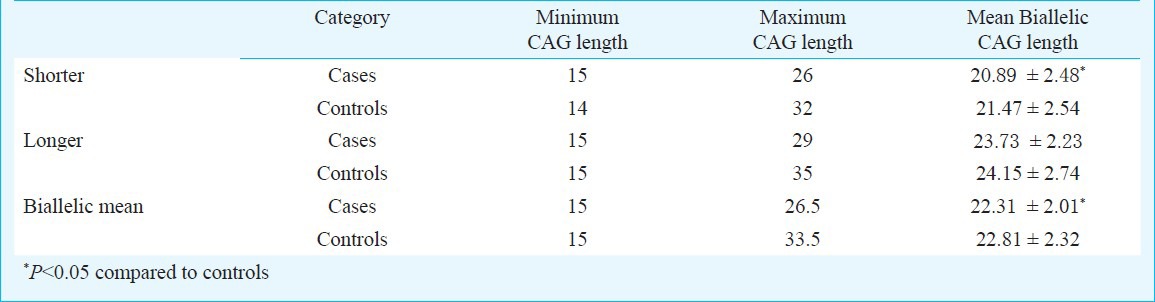
Table II.
Distribution of RSA cases and controls according to the qualitative categories of CAG repeats
The androgen receptor GGN repeats ranged from 18-28 and 16-28, respectively (Fig. 2). The median value of the GGN repeats was 23 for the cases and 22 for controls. The mean number of shorter GGN repeat length was significantly greater in control women as compared with RSA women (Table III). For qualitative comparisons, the median value of 23 repeats was considered, which bisects the entire polymorphic range providing three categories viz. <23, 23 and >23 repeats. The total AR GGN alleles were significantly different in the RSA group in comparison to the control group (P<0.05, Table IV). No significant difference was found in the shorter and longer alleles distribution pattern (Fig. 2) between the RSA cases and controls (Table IV). When the biallelic mean was categorized into >23 and ≤23 repeats, then also we did not find a significant difference in the shorter alleles and biallelic mean distribution pattern (Fig. 2) between the RSA cases and controls.
Fig. 2.
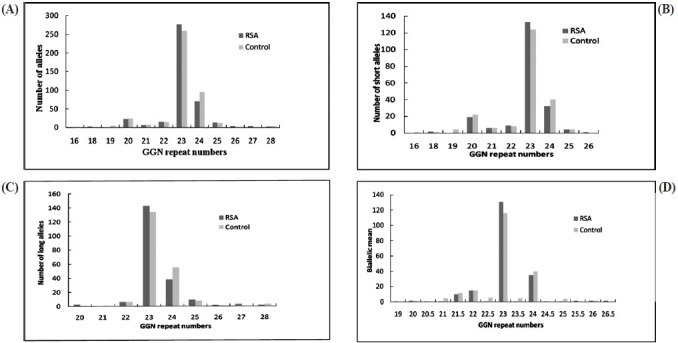
Distribution of GGN repeat numbers in exon 1 of the AR gene in RSA cases and controls. (A) Total alleles, (B) short GGN alleles, (C) long GGN alleles, (D) biallelic mean.
Table III.
Comparison of GGN repeat length for case and controls
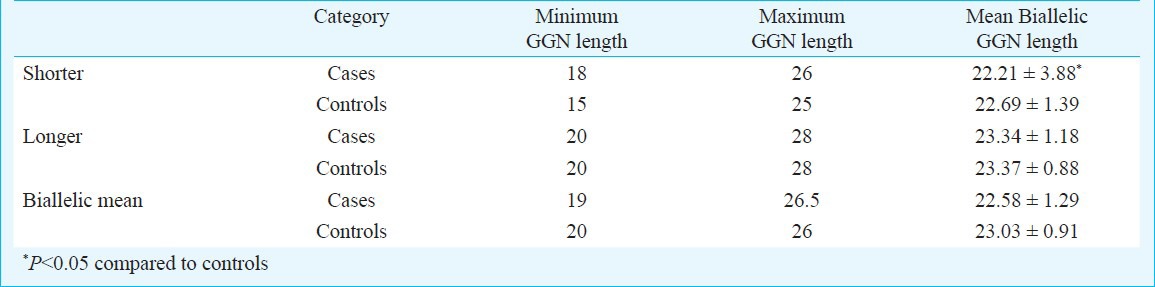
Table IV.
Distribution of RSA cases and controls according to the qualitative categories of GGN repeats
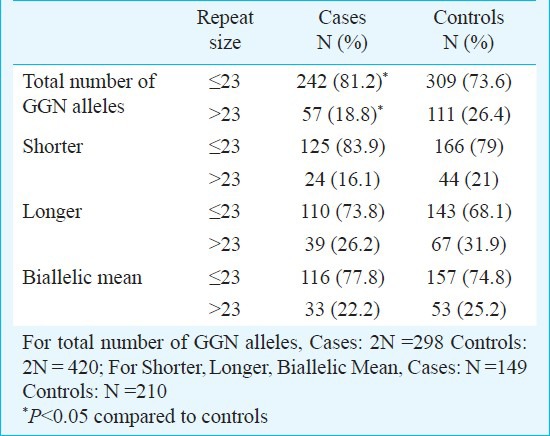
Table V.
Results of logistic regression analysis for the extreme CAG repeat in total allele (CAG RN), shorter and biallelic means (BAM) and GGN repeat in total allele (GGN RN)
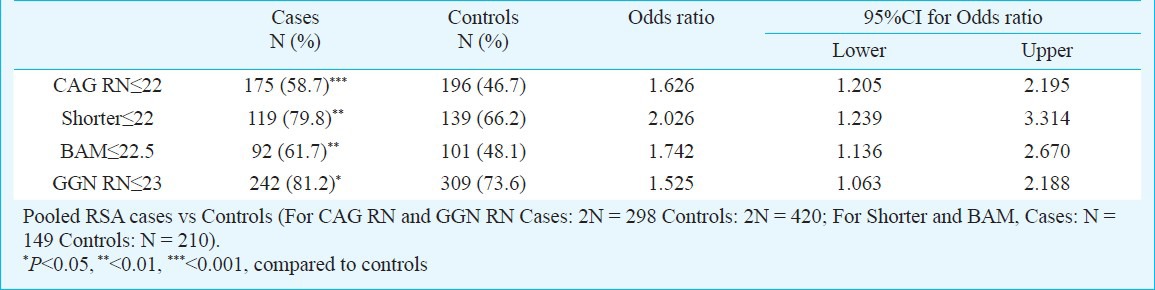
Owing to the significant heterogeneity in the biallelic mean frequency between the RSA cases and the controls, the odds ratio was also computed to examine if there was any effect of repeat numbers in the upper polymorphic range of the allele spectrum in the manifestation of RSAs. Odds were computed between the RSA cases and the controls for the CAG repeats ≤ 22 vs. > 22 both for the total and shorter alleles and ≤ 22.5 vs. > 22.5 for the biallelic mean, and for the GGN repeats ≤23 vs. >23 for the total alleles (Table V). Highly significant odds were obtained for each of the categories suggesting strong association of extreme CAG repeat lengths with RSA. The result of logistic regression analysis illustrated that shorter CAG and shorter GGN repeat lengths might be associated with increased odds of RSA.
Discussion
The outcome of pregnancy relies on the success rate of various early events, such as implantation, establishment of foeto-maternal circulation and maintenance of increased blood flow to the implantation site11,20. The development of RSA is complex and is regulated by multiple genetic pathways. Different genes encoding for proteins involved in various biologic pathways have been reported to be associated with recurrent spontaneous abortion21,22. Several studies were performed to investigate whether polymorphisms of different candidate genes could be risk factors for RSA. However, the results are often controversial, leading to uncertain conclusions about the role of these polymorphisms in RSA pathogenesis.
There is evidence that androgens and their receptor are involved in uterine cell proliferation in rats23. Several groups6,11,24 have investigated the relationship between AR gene polymorphisms and RSA. So, we hypothesized that polymorphisms in this gene might affect normal gene function and could be associated with RSA.
The effect of androgens is mediated through the activation of the androgen receptor. Ackerman et al25 have recently reported that there may be potential ethnic differences in androgenic pathway activity and androgen sensitivity. In this study, the relative AR (CAG)(n) repeat length in the different groups was: Afro-Caribbean (shortest repeat lengths and greatest predicted AR activity) < Caucasian < Hispanic < Thai (longest repeat length and lowest predicted AR activity). Lundin et al26 have shown that AR alleles with 23 GGN repeats have the strongest trans-activational activity and each change in the GGN tract length causes a slight impairment of receptor function. The length of the GGN repeat (number of glycine residues) was linearly and inversely associated with AR protein content in cell cultures, and longer GGN tracts resulted in a linearly reduced AR activity per cell11.
Our findings demonstrated that women in the RSA group had a significantly greater frequency of total AR CAG alleles (≤ 22 repeat), shorter AR CAG alleles (≤ 22 repeat), and biallelic means (≤ 22.5 repeat) than women in the control group. The shorter CAG repeat lengths were associated with an increased risk of RSAs. In contrast to our study, Aruna et al6 reported that women with RSAs had a significantly greater frequency of longer AR CAG alleles than the control women, and that these longer CAG repeat lengths were associated with an increased risk of RSAs. Our study provides evidence of the association of shorter CAG alleles with RSAs, which is in contrast to the findings by Aruna et al6. We found a significant difference in the CAG allele distribution in the RSA group compared with the control group. We also found a significant difference in the GGN total allele distribution in the RSA group as compared to the control group, thus it could perhaps be used as a genetic marker for the assessment of risk for RSA in women. However, there may be potential ethnic differences in the androgenic pathway activity and androgen sensitivity. So it is necessary to do more studies to discover the relationship between AR CAG / GGN repeat polymorphisms and RSA in other populations of the region.
In conclusion, there is a paucity of information in the literature regarding the relationship AR CAG and GGN polymorphisms in relation to RSAs. Thus, our study provides the necessary foundation so that other investigators may contribute to the development of relevant endocrine therapeutic strategies for the prevention of recurrent miscarriages.
Acknowledgment
The study was funded by the National Natural Science Foundation of China (Grant No. 31060147), PR China.
References
- 1.Reiss HE, editor. Oxford: Oxford University Press; 1998. Reproductive medicine: from A to Z 1998. [Google Scholar]
- 2.Cramer DW, Wise LA. The epidemiology of recurrent pregnancy loss. Semin Reprod Med. 2000;18:331–9. doi: 10.1055/s-2000-13722. [DOI] [PubMed] [Google Scholar]
- 3.Litridis I, Kapnoulas N, Natisvili T, Agiannitopoulos K, Peraki O, Ntostis P, et al. Genetic variation in the CYP17 gene and recurrent spontaneous abortions. Arch Gynecol Obstet. 2011;283:289–93. doi: 10.1007/s00404-009-1348-x. [DOI] [PubMed] [Google Scholar]
- 4.Tulppala M, Stenman UH, Cacciatore B, Ylikorkala O. Polycystic ovaries and levels of gonadotropins and androgens in recurrent miscarriage: prospective study in 50 women. Br J Obstet Gynaecol. 1993;100:348–52. doi: 10.1111/j.1471-0528.1993.tb12978.x. [DOI] [PubMed] [Google Scholar]
- 5.Clifford K, Rai R, Watson H, Regan L. An informative protocol for the investigation of recurrent miscarriage: preliminary experience of 500 consecutive cases. Hum Reprod. 1994;9:1328–32. doi: 10.1093/oxfordjournals.humrep.a138703. [DOI] [PubMed] [Google Scholar]
- 6.Aruna M, Dasgupta S, Sirisha PV, Andal Bhaskar S, Tarakeswari S, Singh L, et al. Role of androgen receptor CAG repeat polymorphism and X-inactivation in the manifestation of recurrent spontaneous abortions in Indian women. PLoS One. 2011:6. doi: 10.1371/journal.pone.0017718. e17718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bussen S, Sütterlin M, Steck T. Endocrine abnormalities during the follicular phase in women with recurrent spontaneous abortion. Hum Reprod. 1999;14:18–20. doi: 10.1093/humrep/14.1.18. [DOI] [PubMed] [Google Scholar]
- 8.Maryam K, Bouzari Z, Basirat Z, Kashifard M, Zadeh MZ. The comparison of insulin resistance frequency in patients with recurrent early pregnancy loss to normal individuals. BMC Res Notes. 2012;5:133. doi: 10.1186/1756-0500-5-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty P1, Goswami SK, Rajani S, Sharma S, Kabir SN, Chakravarty B, et al. Recurrent pregnancy loss in polycystic ovary syndrome: role of hyperhomocysteinemia and insulin resistance. PLoS One. 2013;8:e64446. doi: 10.1371/journal.pone.0064446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev. 1995;16:271–21. doi: 10.1210/edrv-16-3-271. [DOI] [PubMed] [Google Scholar]
- 11.Karvela M, Stefanakis N, Papadopoulou S, Tsitilou SG, Tsilivakos V, Lamnissou K. Evidence for association of the G1733A polymorphism of the androgen receptor gene with recurrent spontaneous abortions. Fertil Steril 2008. 2010;90:e9–12. doi: 10.1016/j.fertnstert.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 12.Hiort O. Neonatal endocrinology of abnormal male sexual differentiation: molecular aspects. Horm Res. 2000;53(Supp1):38–41. doi: 10.1159/000053203. [DOI] [PubMed] [Google Scholar]
- 13.Kimura S, Matsumoto T, Matsuyama R, Shiina H, Sato T, Takeyama K, et al. Androgen receptor function in folliculogenesis and its clinical implication in premature ovarian failure. Trends Endocrinol Metab. 2007;18:183–9. doi: 10.1016/j.tem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Lee DK, Chang C. Endocrine mechanisms of disease: expression and degradation of androgen receptor: mechanism and clinical implication. J Clin Endocrinol Metab. 2003;88:4043–54. doi: 10.1210/jc.2003-030261. [DOI] [PubMed] [Google Scholar]
- 15.Ding D, Xu L, Menon M, Reddy GP, Barrack ER. Effect of GGC (glycine) repeat length polymorphism in the human androgen receptor on androgen action. Prostate. 2005;62:133–9. doi: 10.1002/pros.20128. [DOI] [PubMed] [Google Scholar]
- 16.Sugawa F, Wada Y, Maruyama T, Uchida H, Ishizuka B, Ogata T. Premature ovarian failure and androgen receptor gene CAG repeat lengths weighted by X chromosome inactivation patterns. Fertil Steril. 2009;91:649–52. doi: 10.1016/j.fertnstert.2007.11.085. [DOI] [PubMed] [Google Scholar]
- 17.Welzel M, Schwarz HP, Hedderich J, Dörr HG, Binder G, Brämswig JH, et al. No correlation between androgen receptor CAG and GGN repeat length and the degree of genital virilization in females with 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2010;95:2443–50. doi: 10.1210/jc.2009-1338. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig AH, Murawska M, Panek G, Timorek A, Kupryjanczyk J. Androgen, progesterone, and FSH receptor polymorphisms in ovarian cancer risk and outcome. Endocr Relat Cancer. 2009;16:1005–16. doi: 10.1677/ERC-08-0135. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee S, Singh R, Kadam S, Maitra A, Thangaraj K, Meherji P, et al. Longer CAG repeat length in the androgen receptor gene is associated with premature ovarian failure. Hum Reprod. 2009;24:3230–5. doi: 10.1093/humrep/dep296. [DOI] [PubMed] [Google Scholar]
- 20.Karvela M, Papadopoulou S, Tsaliki E, Konstantakou E, Hatzaki A, Florentin-Arar L, et al. Endothelial nitric oxide synthase gene polymorphisms in recurrent spontaneous abortions. Arch Gynecol Obstet. 2008;278:349–52. doi: 10.1007/s00404-008-0577-8. [DOI] [PubMed] [Google Scholar]
- 21.Buchholz T, Lohse P, Kosian E, Thaler CJ. Vasoconstrictively acting AT1R A1166C and NOS3 4/5 polymorphisms in recurrent spontaneous abortions (RSA) Am J Reprod Immunol. 2004;51:323–8. doi: 10.1111/j.1600-0897.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- 22.Tempfer C, Unfried G, Zeillinger R, Hefler L, Nagele F, Huber JC. Endothelial nitric oxide synthase gene polymorphism in women with idiopathic recurrent miscarriage. Hum Reprod. 2001;16:1644–7. doi: 10.1093/humrep/16.8.1644. [DOI] [PubMed] [Google Scholar]
- 23.Weihua Z, Ekman J, Almkvist A, Saji S, Wang L, Warner M, et al. Involvement of androgen receptor in 17beta-estradiol-induced cell proliferation in rat uterus. Biol Reprod. 2002;67:616–23. doi: 10.1095/biolreprod67.2.616. [DOI] [PubMed] [Google Scholar]
- 24.Jahaninejad T, Ghasemi N, Kalantar SM, Sheikhha MH, Pashaiefar H. StuI polymorphism on the androgen receptor gene is associated with recurrent spontaneous abortion. J Assist Reprod Genet. 2013;30:437–40. doi: 10.1007/s10815-013-9949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ackerman CM, Lowe LP, Lee H, Hayes MG, Dyer AR, Metzger BE, et al. Hapo Study Cooperative Research Group. Ethnic variation in allele distribution of the androgen receptor (AR) (CAG)n repeat. J Androl. 2012;33:210–5. doi: 10.2164/jandrol.111.013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundin KB, Giwercman A, Dizeyi N, Giwercman YL. Functional in vitro characterisation of the androgen receptor GGN polymorphism. Mol Cell Endocrinol. 2007;264:184–7. doi: 10.1016/j.mce.2006.11.008. [DOI] [PubMed] [Google Scholar]


