Abstract
Background & objectives:
The susceptibility of the mosquito to the invading pathogen is predominantly dictated by the complex interactions between the mosquito midgut and the surface proteins of the invading pathogen. It is well documented that the midgut microbiota plays an important role in determining the susceptibility of the mosquito to the pathogen. In the present study, we investigated the influence of Serratia odorifera, an endogenous cultivable midgut inhabitant of Aedes aegypti on the chikungunya virus (CHIKV) susceptibility to this mosquito.
Methods:
Ae. aegypti females free of gutflora were co-fed with CHIKV and either of the two midgut inhabitants namely, S. odorifeara and Microbacterium oxydans. CHIKV dissemination was checked on 10th day post feeding (DPF) using indirect immunoflurescence assay and plaque assay. CHIKV interacting proteins of the mosquito midgut were identified using virus overlay protein binding assay and MALDI TOF/TOF analysis.
Results:
The observations revealed that co-feeding of S. odorifera with CHIKV significantly enhanced the CHIKV susceptibility in adult Ae. aegypti, as compared to the mosquitoes fed with CHIKV alone and CHIKV co-fed with another midgut inhabitant, M. oxydans. Virus overlay protein binding assay (VOPBA) results revealed that porin and heat shock protein (HSP60) of Ae. aegypti midgut brush border membrane fraction interacted with CHIKV.
Interpretation & conclusions:
The results of this study indicated that the enhancement in the CHIKV susceptibility of Ae. aegypti females was due to the suppression of immune response of Ae. aegypti as a result of the interaction between S. odorifera P40 protein and porin on the gut membrane.
Keywords: CHIKV binding proteins, HSP60, porin, Serratia odorifera, transstadial transmission, vector susceptibility
The gut flora of vertebrates and invertebrates represents one of the most widespread and ancient symbiotic association. The symbiotic relationship between insect hosts and bacteria has been extensively studied in termites and cockroaches1. This symbiosis provides important functions to the host, including the synthesis of essential nutrients, resistance to the colonizing pathogens and stimulation of immune system1.
Chikungunya virus (CHIKV) produces a dengue-like illness in humans characterized by fever, rash, painful arthralgia, and sometimes arthritis. Chikungunya virus re-emerged after more than three decades years in Indian subcontinent and is causing frequent outbreaks since then2,3. Phylogenetic analysis based on the E1 gene and/or on the complete genome sequences of CHIKV strains revealed the existence of three distinct genotypes: West African; Asian; and Eastern, Central and Southern African (ECSA)2. CHIKV Asian strain was responsible for chikungunya epidemics in India since early sixties till 20052. The current CHIKV epidemic in India initiated in 2005 was associated with the shift of genotype from Asian to ECSA2. Chikungunya virus is endemic in Africa and Southeast Asia and is transmitted by Aedes mosquitoes through an urban or sylvatic transmission cycle. Ae. aegypti vector is responsible for frequent outbreaks of CHIKV in India2.
Several studies demonstrated that the midgut microbiota plays an important role in determining the vector competence of mosquitoes for the arboviruses4,5,6,7,8,9,10,11. These gut inhabitants have been shown to influence CHIKV, dengue virus (DENV) and yellow fever virus (YFV) infection in Aedes mosquitoes4,5,6,7,8,9 and the Japanese encephalitis virus (JEV) infection in Culex bitaeniorhyncus11. It has been demonstrated that midgut microbes can limit the replication of invading viruses in mosquitoes. Joyce et al12 showed that in vitro incubation of La Crosse virus (LACV) with the mixed mosquito midgut bacterial population decreased the infectivity of LACV to Vero cells. Native Wolbachia strains that harbour mosquito hosts have also been shown to limit the arboviral replication5,8,9. On the other hand, it was demonstrated that Serratia odorifera, a midgut inhabitant of Ae. aegypti, enhances its susceptibility to dengue-2 virus (DENV2)10.
In the previous study10 we employed culture dependent approach to understand the bacterial diversity of Ae. aegypti midgut and have demonstrated that the temperature of the larval habitat can influence the midgut microbiota they harbour10. We have also demonstrated that a midgut inhabitant, S. odorifera, enhances the DENV-2 susceptibility of Ae. aegypti by suppressing the immune response of the host through its interaction with prohibitin, a DENV 2 interacting molecule present on the surface of the midgut, with its polypeptide P4010. In India, CHIKV and DENV are transmitted by Ae. aegypti, hence, this study was undertaken to assess if this midgut inhabitant S. odorifera could also influence susceptibility of this vector to another virus, CHIKV (Togaviridae; Alphavirus).
Material & Methods
The study protocol was approved by the Institutional Animal Ethics Committee (IAEC) affiliated with National Institute of Virology (NIV), Pune, India. All animal and virology work was carried out in NIV and all molecular biology related work was performed in University, of Pune, India.
Virus stock preparation: CHIKV stock was prepared in Swiss albino mice (n=8; 1-2 days old). To prepare the virus stock, the suspension of Kolkata strain of CHIKV virus (634029) was reconstituted in 0.5 ml distilled water, and the dilutions were prepared in 1.25 per cent bovine serum albumin phosphate saline (BAPS), pH 7.4. One to two day old mice were inoculated intra-cerebrally with 0.02 ml of CHIKV suspension [8 × 107 (plaque forming unit)/ml). The mice were monitored for sickness. After about 8-10 days, when the mice showed clinical symptoms, they were sacrificed, and their brains were homogenized using 1.25 per cent BAPS (20% w/v). The homogenates were centrifuged at 16,000 g for 60 min. The supernatant of all brains were pooled and were distributed in vials and the titer of one of the vials was determined by plaque assay as described earlier10,13 (7 × 107 pfu/ml).
Plaque assay: The CHIKV copy number in the carcasses of CHIKV positive mosquitoes was determined using plaque assay. An individual mosquito was titurated in one ml of Mitsuhashi and Maramorosch medium (HiMedia, India) to release infectious virus. C6/36 cells were grown to confluent monolayers in 12-well plates, were infected with 10-fold serial dilutions of mosquito homogenates for one hour, and overlaid with carboxymethyl cellulose-nutrient mixture (Merck, Germany) After five days incubation at 37°C, the cells were stained with crystal violet solution. The viral titres were determined by counting plaques. The virus titre in the individual mosquito is reported as pfu per mosquito. The virus titres in the post blood meal fed mosquitoes were determined using the protocol mentioned earlier10.
Midgut inhabitant bacteria and bacterial lysates: S. odorifeara and Microbacterium oxydans cultures and bacterial lysates were prepared10.
Introduction of midgut bacterial isolates along with CHIKV in the blood meal of Ae. aegypti females: Four to six days old Ae. aegypti females, free of midgut flora were used for oral feeding experiments as mentioned earlier10. In the current study, we used 1.2 × 105 cfu (colony forming unit)/ml bacteria were used in the feeding experiments, which matched numerically to the natural inhabitant bacterial populations in the mosquito midgut. White leg horn fowls were bled through the heart, blood was defibrinated using glass beads and was then used for feeding experiments. The mosquitoes were allowed to feed for one hour through a goat intestine membrane covering the base of a glass feeder that carried the blood-virus or blood-virus-bacteria mixture maintained at 37°C. For an individual experiment, the infectious blood meal was given to three groups; Group 1: One ml of blood containing 250μl CHIKV (7×105 pfu/ml) (Total n= 169, about 60 females/experiment in three independent experiments), Group 2: One ml of blood mixture containing 250μl CHIKV (7×105 pfu/ml) along with 250μl of S. odorifera (1.2 × 105 cfu/ml) (Total n= 141, about 50 females / experiment in four independent experiments) and Group 3: One ml of blood mixture containing 250μl CHIKV (5 ×105 pfu/ml) along with 250μl of another Ae. aegypti midgut inhabitant bacterium M. oxydans (1.2 × 105 cfu/ml) (Total n= 167, about 60 females / experiment in three independent experiments). Fully engorged females were transferred to different containers and were maintained with 10 per cent glucose at 28±1°C for 10 days. To evaluate infection and dissemination rate and in turn vector competence on day 10 post feeding (DPF) surviving females were sacrificed by transferring them to -80°C.
Detection of CHIKV antigen in the head squashes by the indirect immunofluroscence assay (IFA): The presence of viral antigen was determined by IFA on 10th DPF by modifying the procedure described by Mourya and Mishra14. The head squashes were prepared on glass slides. The slides were immersed in the blocking buffer (0.1% Tween 20 and 2% BSA in PBS) for one hour at room temperature; the slides were incubated with mouse anti CHIKV antibodies, followed by FITC-conjugated goat anti-mouse IgG (Sigma Aldrich, USA) for 1 h each at 37° C. These slides were mounted with ProLong Gold anti-fade reagent (Promega, USA) with Evans Blue (Sigma Aldrich, USA), and were visualized under the fluorescent microscope. For each experiment, positive and negative controls were processed using the same protocol. These experiments were repeated three times.
Isolation of the midgut brush border membrane fraction (BBMF) from Ae. aegypti: BBMFs from the midgut epithelial cells of Ae. aegypti larvae and adults were prepared as described by Mourya et al15 and Paingankar et al16.
Virus overlay protein binding assay (VOPBA): VOPBA was performed to identify the Ae. aegypti BBMF polypeptides that were involved in the virus binding. The membrane proteins (50 μg) were separated on two 12.5 per cent SDS-polyacrylamide gel (SDS-PAGE). One part of gel was stained with Coomassie brilliant blue R-250 while the other gel was transferred to the nitrocellulose membranes (Hybond C) using a semi-dry blotting apparatus (BioRad Laboratories, USA) in 48 mmol Tris, 39 mmol glycine, and 20 per cent (vol/vol) methanol. The membranes were blocked with 2 per cent BSA (Sigma Aldrich, Germany) in PBST (phosphate-buffered saline pH 7.4, 0.5% Tween 20) at 4°C and washed three times for 30 min with PBST. The membranes were then incubated with native CHIKV in PBS at 37°C for 60 min and washed three times for 30 min with PBST. These were incubated for 60 min with the mouse antibody against CHIKV diluted 1:100 in PBS. After washing the membranes three times with PBST for 30 min, these were incubated for 60 min at room temperature with the rabbit anti-mouse IgG conjugated to peroxidase (Sigma Aldrich, Germany) (diluted 1:3,000 in BAPBS). Finally, the membranes were washed three times for 30 min with PBST. The membrane was developed with H2O2 and diaminobenzidine tetrahydrochloride (DAB, Sigma, Aldrich, Germany). Four independent experiments were carried out.
Protein identification using MALDI-TOF/TOF MS: The spots corresponding to the proteins of interest were excised from SDS-PAGE gels and subjected to alkylation followed by in-gel digestion with trypsin. The masses of the resultant peptides were analyzed by matrix-assisted laser desorption/ionization time of flight (MALDI-TOF / TOF)17 on Ultraflex TOF/TOF (Bruker Daltonics, Germany). MALDI TOF/TOF analysis was carried out at the Molecular Biophysics Unit, Indian Institute of Science, Bangalore, India. The mass spectrum generated by each sample was searched against protein databases (NCBInr, MSDB, and Swissprot) using the MASCOT search engine (www.matrixscience.com) and the proteins were identified based on the homology.
Statistical analysis: Analysis of variance (ANOVA) was used to investigate the effect of presence of different midgut bacteria on CHIKV dissemination rate. The groups were also compared by non parametric Mann-Whitney U test for the confirmation of the results. The statistical significance was determined by calculating ANOVA with Tukey's HSD and was further confirmed by analyzing the data by non-parametric Mann Whitney U test.
Results & Discussion
Earlier studies have demonstrated that the resident microbiota of the mosquito midgut can significantly influence vector susceptibility3,4,5,6,7,8,9,10,11. It has been documented that some bacteria negatively influence the vector competence5,8,9,11. However, the observed negative influence potentially could be due to an unnaturally high load of bacteria fed to the mosquitoes. On the other hand, some midgut inhabitants suppress the vector immune response for their survival in the host which is exploited by the invading pathogen to establish a successful infection in these vectors4,10. Mourya et al4 have demonstrated that Ae. aegypti fed with Aeromonas sp. and Escherichia coli showed enhanced susceptibility to DENV-2 although the underlying mechanism was not described. Similarly, a study by Apte-Deshpande et al10 showed that S. odorifeara, a midgut inhabitant of Ae. aegypti plays an important role in modulating its DENV-2 susceptibility. CHIKV and DENV-2 viruses prefer the same vector Ae. aegypti, for their transmission; therefore, we evaluated the ability of this midgut microbe to influence the susceptibility of Ae. aegypti for CHIKV infection.
The Ae. aegypti females free of midgut microflora were fed with CHIKV alone or with S. odorifeara or M. oxydans along with CHIKV via blood meal, and these three groups were monitored for dissemination of CHIKV. The group receiving S. odorifera along with CHIKV (group 2) showed significant increase in the susceptibility to CHIKV (69.7±10.1%) as compared to the groups that received only CHIKV (group 1) (41.5±10.6%; data of three independent experiments) or M. oxydans (group 3) (43.1±6.1%). The dissemination rates were significantly different among the three groups (P < 0.001) analyzed. The dissemination rates between only CHIKV fed and CHIKV + S. odorifera fed groups P<0.05) as well as CHIKV + M. oxydans and CHIKV + S. odorifera fed groups (P<0.05) were significantly different. Whereas, the dissemination rate was not significantly different between only CHIKV fed and CHIKV + M. oxydans fed groups (Fig. 1). The enhancement in dissemination rate was further confirmed by comparing CHIKV titres in all the groups immediately after blood meal and on 10th DPF. The titres were determined in individual mosquito carcasses. Immediately after blood meal, Ae. aegypti females had an average CHIKV titer of 3.23×103 (±630) pfu/mosquito for CHIKV fed group and 3.22×103 (±501) pfu/mosquito and 5.011×103 (±316) pfu/mosquito for the CHIKV + S. odorifera and CHIKV + M. oxydans groups, respectively, indicating almost equal number of viruses were ingested by all the groups. On 10th DPF, CHIKV fed group, CHIKV + M. oxydans and the CHIKV + S. odorifera groups showed titres of 4.93×107 (±2.87×104 pfu/mosquito, 4.15×107 (±1.53×104 pfu/mosquito, and 7.56×107 (±3.48×104 pfu/mosquito respectively indicating replication and dissemination of CHIKV in Ae. aegypti tissues. The group receiving S. odorifeara in the blood meal showed slightly higher titres of CHIKV as compared to only CHIKV and CHIKV+ M. oxydans groups indicative of enhanced entry/replication and dissemination of CHIKV in Ae. aegypti tissues. Virus titres in CHIKV negative mosquitoes were undetectable. These observations suggested that the presence of S. odorifera influenced the susceptibility of Ae. aegypti to CHIKV.
Fig. 1.
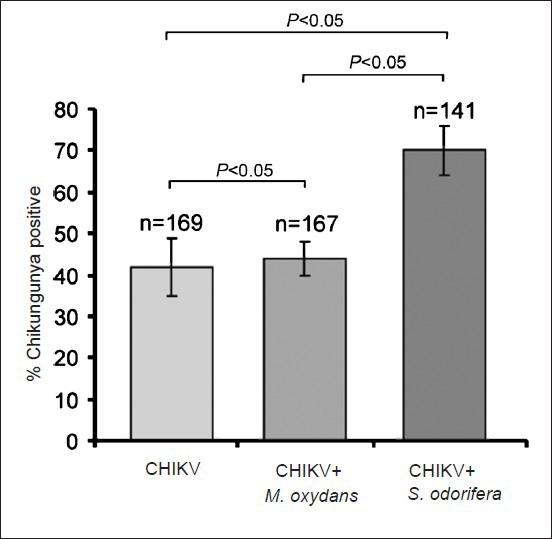
Significance of S. odorifera presence in the blood meal on CHIKV susceptibility of Ae. aegypti. The presence of S. odorifera in the blood meal significantly enhanced the CHIKV susceptibility (P<0.05) compared to M. oxydans.
These observations led to the speculation that S. odorifera modulated Ae. aegypti susceptibility either through its direct interaction with virus or virus interacting proteins or both. To understand the mechanism involved in the enhanced CHIK virus susceptibility, we investigated CHIKV interacting proteins present on the midgut brush border membrane fraction (BBMF) of unfed Ae. aegypti females. BBMF proteins were separated by SDS-PAGE and were immobilized on a nitrocellulose membrane. When this membrane was incubated with native CHIKV, two polypeptides (60 and 38 kDa) were recognized as CHIKV interacting proteins (Fig. 2). These proteins were excised from the gel and were analyzed by the mass spectrometry fingerprint analysis. They were identified as heat shock protein, 60 (HSP60) and porin respectively (Table). In our earlier study10, we have demonstrated that P40 protein of S. odorifera interact with proteins prohibitin and porin present on the BBMF of Ae. aegypti. The results obtained in this study and our earlier study10 indicate that porin interact with both CHIKV and S. odorifera protein P40.
Fig. 2.
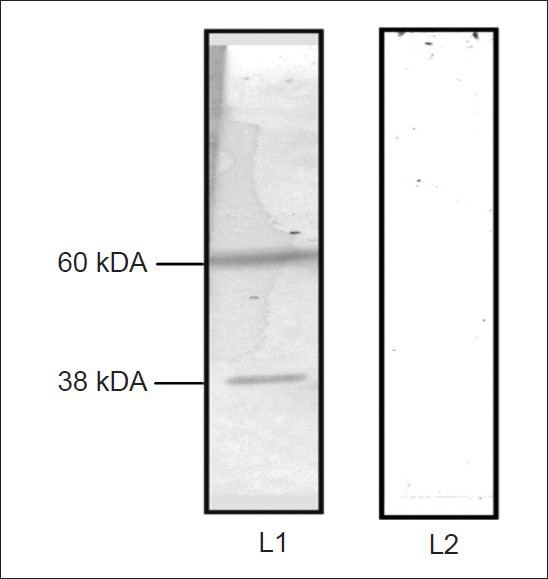
Virus overlay protein binding assay with CHIKV. The molecular weights of CHIKV binding proteins are shown on the left side. Lane L1-incubated with CHIKV, L2 – incubated with PBS, pH 7.4
Table.
Molecular identification of CHIKV binding proteins from female adult Ae. aegypti midgut membrane fraction
It is well documented that Alphaviruses can infect both insect and vertebrate cells and undergo a variety of biochemical and genetic modifications. They either use a ubiquitous receptor in different species, or are able to use multiple proteins as their receptors18. Molecules such as laminin, heparin sulphate, DC-SIGN and L-SIGN have been shown as parts of different alphavirus receptor complexes19,20,21. However, Alphavirus receptor/s have not been conclusively identified15. Arboviruses, including Japanese encephalitis22 chikungunya23 and DENV16,24 have been reported to use HSP family proteins as their cell receptors. In the VOPBA assay, we detected a 60 KDa heat shock protein as a CHIKV interacting protein and potential receptor for the entry into mosquito midgut cells. A recent report by Wintachai et al23 also demonstrated HSP60 as a CHIKV interacting protein and have claimed it as a potential candidate responsible for mediating the entry of CHIKV into the host cell. Earlier studies demonstrated prohibitin, Ae. aegypti midgut surface protein as DENV2 interacting protein16,23,25. In the current study, interaction of prohibitin with CHIKV was not observed. Porin, another mitochondrial protein present on the Ae. aegypti midgut interacted with CHIKV, probably due to the fact that these viruses belong to different strains/families and exploit different cellular proteins for their entry and replication.
Mitochondrial proteins play important role in immunity of insects and mammals. It has been shown that voltage dependent anion channels (VDAC) are part of the permeability transition pore complex in the mitochondrial membrane and play an important role in the mitochondria mediated immune response26. Porin, which was detected as a CHIKV binding protein in the current study, is a member of VDAC family of proteins. It has been documented that CHIKV mobilizes the apoptotic machinery to evade the host cell defense27. Recently, it has been documented that CHIKV-induced autophagy delays caspase-dependent cell death28. Li et al29 have suggested that VDAC2 functions to sense the presence of the virus and triggers the cell death to limit the viral proliferation. These observations suggested that CHIKV might inhibit cellular immune response and apoptosis by interacting with the mitochondrial proteins such as porin in the early infection stage to gain sufficient time for its replication.
In this study, mitochondrial protein porin was found to interact with CHIKV and P40. We speculate that like our earlier observation10, P40-porin interaction could lead to supression of immune response which could be exploited by CHIKV to establish its infection in this mosquito vector. Porin might also have a multifaceted role like the prohibitin molecule10. Further studies are essential to prove this hypothesis. Knocking-out the porin molecule might give better insight into its possible role in CHIK virus transmission and replication.
To conclude, our study showed that native S. odorifera was able to enhance susceptibility of CHIKV in Ae. aegypti potentially through its P40 protein. Interaction of P40 with mitochondrial protein porin perhaps downregulates the immune response of mosquito. CHIKV exploits this suppressed immune response in Ae. aegypti to establish its infection.
Acknowledgment
The authors thank Drs D. T. Mourya and Pradip Barde for suggestions on manuscript and for their help in carrying out experiments in NIV, Pune. We thank Dr A.C. Mishra, NIV, Pune for the facilities and encouragement and Dr Dipankar Chaterjee, Molecular Biophysics Unit, Indian Institute of Science, Bangalore for access to MALDI TOF/TOF. The authors acknowledge the University Grants Commission and the Indian Council of Medical Research, New Delhi for financial assistance. The second author (MP) acknowledges the Council of Scientific and Industrial Research, New Delhi for Senior Research Fellowship.
References
- 1.Dillon RJ, Vennard CT, Buckling A, Charnley AK. Diversity of locust gut bacteria protects against pathogen invasion. Ecol Lett. 2005;8:1291–8. [Google Scholar]
- 2.Arankalle VA, Shrivastava S, Cherian S, Gunjikar RS, Walimbe AM, Jadhav SM, et al. Genetic divergence of Chikungunya viruses in India (1963-2006) with special reference to the 2005-2006 explosive epidemic. J Gen Virol. 2007;88:1967–76. doi: 10.1099/vir.0.82714-0. [DOI] [PubMed] [Google Scholar]
- 3.Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–77. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 4.Mourya DT, Pidiyar V, Patole M, Gokhale MD, Shouche Y. Effect of midgut bacterial flora of Aedes aegypti on the susceptibility of mosquitoes to dengue viruses. Dengue Bull. 2002;26:190–4. [PubMed] [Google Scholar]
- 5.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell. 2009;139:1268–78. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez JL, Souza-Neto J, Torres Cosme R, Rovira J, Ortiz A, Pascale JM, et al. Reciprocal tripartite interactions between the Aedes aegypti midgut microbiota, innate immune system and dengue virus influences vector competence. PLoS Negl Trop Dis. 2012;6:e1561. doi: 10.1371/journal.pntd.0001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zouache K, Michelland RJ, Failloux AB, Grundmann GL, Mavingui P. Chikungunya virus impacts the diversity of symbiotic bacteria in mosquito vector. Mol Ecol. 2012;21:2297–309. doi: 10.1111/j.1365-294X.2012.05526.x. [DOI] [PubMed] [Google Scholar]
- 8.Mousson L, Zouache K, Arias-Goeta C, Raquin V, Mavingui P, Failloux AB, et al. The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus. PLoS Negl Trop Dis. 2012;6:e1989. doi: 10.1371/journal.pntd.0001989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Hurk AF, Hall-Mendelin S, Pyke AT, Frentiu FD, McElroy K, Day A, et al. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl Trop Dis. 2012;6:e1892. doi: 10.1371/journal.pntd.0001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apte-Deshpande A, Paingankar M, Gokhale MD, Deobagkar DN. Serratia odorifera a midgut inhabitant of Aedes aegypti mosquito enhances its susceptibility to dengue-2 Virus. PLoS One. 2012;7:e40401. doi: 10.1371/journal.pone.0040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mourya DT, Soman RS. Effect of gregarine parasite, Ascogregarina culicis & tetracycline on the susceptibility of Culex bitaeniorhyncus to JE virus. Indian J Med Res. 1985;81:247–50. [PubMed] [Google Scholar]
- 12.Joyce JD, Nogueira JR, Bales AA, Pittman KE, Anderson JR. Interactions between La Crosse virus and bacteria isolated from the digestive tract of Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2011;48:389–94. doi: 10.1603/me09268. [DOI] [PubMed] [Google Scholar]
- 13.Heller E. Enhancement of Chikungunya virus replication and inhibition of interferon production by actinomycin D. Virology. 1963;21:652–6. doi: 10.1016/0042-6822(63)90239-3. [DOI] [PubMed] [Google Scholar]
- 14.Mourya DT, Mishra AC. Antigen distribution pattern of Japanese encephalitis virus in Culex tritaeniorhynchus, C. vishnui & C. pseudovishnui. Indian J Med Res. 2000;111:157–61. [PubMed] [Google Scholar]
- 15.Mourya DT, Randive SN, Gokhale MD, Barde PV, Padbidri VS, Banerjee K. Putative chikungunya virus-specific receptor proteins on the midgut brush border membrane of Aedes aegypti mosquito. Indian J Med Res. 1998;107:10–4. [PubMed] [Google Scholar]
- 16.Paingankar MS, Gokhale MD, Deobagkar DN. Dengue-2-virus-interacting polypeptides involved in mosquito cell infection. Arch Virol. 2010;155:1453–61. doi: 10.1007/s00705-010-0728-7. [DOI] [PubMed] [Google Scholar]
- 17.Beavis RC, Chait BT. Matrix-assisted laser-desorption mass spectrometry using 355 nm radiation. Rapid Commun Mass Spectrom. 1989;3:436–9. doi: 10.1002/rcm.1290031208. [DOI] [PubMed] [Google Scholar]
- 18.Leung JY, Ng MM, Chu JJ. Replication of alphaviruses: a review on the entry process of alphaviruses into cells. Adv Virol. 2011;2011:249640. doi: 10.1155/2011/249640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang KS, Kuhn RJ, Strauss EG, Ou S, Strauss JH. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J Virol. 1992;66:4992–5001. doi: 10.1128/jvi.66.8.4992-5001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrnes AP, Griffin DE. Binding of Sindbis virus to cell surface heparan sulfate. J Virol. 1998;72:7349–56. doi: 10.1128/jvi.72.9.7349-7356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klimstra WB, Nangle EM, Smith MS, Yurochko AD, Ryman KD. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J Virol. 2003;77:12022–32. doi: 10.1128/JVI.77.22.12022-12032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu YZ, Cao MM, Wang WB, Wang W, Ren H, Zhao P, et al. Association of heat-shock protein 70 with lipid rafts is required for Japanese encephalitis virus infection in Huh7 cells. J Gen Virol. 2012;93:61–71. doi: 10.1099/vir.0.034637-0. [DOI] [PubMed] [Google Scholar]
- 23.Wintachai P, Wikan N, Kuadkitkan A, Jaimipuk T, Ubol S, Pulmanausahakul R, et al. Identification of prohibitin as a Chikungunya virus receptor protein. J Med Virol. 2012;84:1757–70. doi: 10.1002/jmv.23403. [DOI] [PubMed] [Google Scholar]
- 24.Reyes-Del Valle J, Chavez-Salinas S, Medina F, Del Angel RM. Heat shock protein 90 and heat shock protein 70 are components of dengue virus receptor complex in human cells. J Virol. 2005;79:4557–67. doi: 10.1128/JVI.79.8.4557-4567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuadkitkan A, Wikan N, Fongsaran C, Smith DR. Identification and characterization of prohibitin as a receptor protein mediating DENV-2 entry into insect cells. Virology. 2010;406:149–61. doi: 10.1016/j.virol.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Arnoult D, Carneiro L, Tattoli I, Girardin SE. The role of mitochondria in cellular defense against microbial infection. Semin Immunol. 2009;21:223–32. doi: 10.1016/j.smim.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Krejbich-Trotot P, Denizot M, Hoarau JJ, Jaffar-Bandjee MC, Das T, Gasque P. Chikungunya virus mobilizes the apoptotic machinery to invade host cell defenses. FASEB J. 2011;25:314–25. doi: 10.1096/fj.10-164178. [DOI] [PubMed] [Google Scholar]
- 28.Joubert PE, Werneke SW, de la Calle C, Guivel-Benhassine F, Giodini A, Peduto L, et al. Chikungunya virus-induced autophagy delays caspase–dependent cell death. J Exp Med. 2012;209:1029–47. doi: 10.1084/jem.20110996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Wang Y, Xue Y, Li X, Cao H, Zheng SJ. Critical role for voltage-dependent anion channel 2 in infectious bursal disease virus-induced apoptosis in host cells via interaction with VP5. J Virol. 2012;86:1328–38. doi: 10.1128/JVI.06104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


