Abstract
Background and Aim:
Autoimmune hemolytic anemia (AIHA) is characterized by the production of autoantibodies directed against red cell antigens. Most patients of AIHA arrive in the emergency or out-patient department (OPD) with severe anemia requiring urgent blood transfusion. Here we share our experience of managing these patients with incompatible blood transfusions and suggest the minimal test required to assure patient safety.
Materials and Methods:
A total of 14 patients admitted with severe anemia, diagnosed with AIHA and requiring blood transfusion urgently were included in the study. A series of immunohematological investigations were performed to confirm the diagnosis and issue best match packed red blood cells (PRBC) to these patients.
Results:
A total of 167 PRBC units were crossmatched for 14 patients of which 46 units (28%) were found to be best match ones and 26 (56.5%) of these units were transfused. A mean turn around time of 222 min was observed in issuing the “best match” blood. Severe hemolysis was observed in all patients with a median hemoglobin increment of 0.88 g/dl after each unit PRBC transfusion.
Conclusion:
Decision to transfuse in AIHA should be based on the clinical condition of the patient. No critical patient should be denied blood transfusion due to serological incompatibility. Minimum investigations such as direct antiglobulin test (DAT), antibody screening and autocontrol should be performed to ensure transfusion safety in patients. All transfusion services should be capable of issuing “best match” PRBCs in AIHA.
Keywords: Alloantibody, autoantibody, autoimmune hemolytic anemia, best match blood, gel technology
Introduction
Autoimmune hemolytic anemia (AIHA) is characterized by the production of autoantibodies directed against red cell antigens.[1] The disease is uncommon and the overall incidence is 1 in 80,000-100,000 of a given population per year in the Caucasians.[2] The basic cause of autoantibody production in AIHA is the individual's immune system not able to recognize the host or self-antigens and this has been attributed to the failure of T cell regulation of B cells and less likely the subtle alteration in structure of the antigens on the patient's red cells.[3] These autoantibodies cause accelerated clearance of red cells thus reducing the in vivo life span of patient's own red cells. When the rate of in vivo red cell destruction is greater than the rate of marrow compensation, anemia develops.[3] AIHA is classified as warm, cold and mixed according to the characteristic temperature reactivity of the autoantibody or by etiology as primary (idiopathic) or secondary.[1,2] Serologic evidence of an autoantibody is provided by a positive direct antiglobulin test (DAT) and auto control and subsequent identification of an autoantibody in the red cell eluate and possibly the serum.[1,4] However, in patients with history of transfusion, pregnancy or transplantation underlying alloantibodies can produce a mixed-field positive auto control.[5,6] Advanced immunohematological tests such as adsorption study and antigenic phenotyping on eluted red cells can differentiate these alloantibodies from autoantibodies.[1,7] To our observation most patients of AIHA develop weakness, dizziness, fatigue and dyspnea and arrive in the emergency or out-patient department (OPD) with severe anemia where urgent blood transfusion is the only live saving management. Here we share our experience of managing these patients with incompatible blood transfusion and suggest the minimal test required to assure patient safety.
Materials and Methods
The prospective study was conducted over a period of 10 months. A total of 14 patients admitted with severe anemia, diagnosed with AIHA and requiring blood transfusion urgently were included in the study. For all these patients, a request for packed red blood cells (PRBC) and blood samples in ethylenediamine tetra acetic acid (EDTA) and plain vials for blood grouping and crossmatching were received from the respective wards. For all samples, blood grouping was confirmed and crossmatching of PRBC units was performed by the technologist on duty. Detailed immunohematological investigations were done once PRBC units subjected to crossmatch were found incompatible using the gel technology (DiaMed, Cressier s/Morat, Switzerland). These investigations which included DAT, antibody class detection, cold acid elution, indirect antiglobulin test (IAT), autocontrol and adsorption using polyethylene glycol (PEG) were done as described previously by Das et al.[4,7,8] In all tests, the finding of agglutination reactions were graded as 4+, 3+, 2+, 1+ and negative and documented accordingly. Blood group discrepancy was resolved using eluted red cells and adsorbed serum and incubation at different thermal amplitudes. For patient who has developed underlying alloantibody, corresponding antigen negative blood was obtained from the inventory using special antisera for minor blood grouping (DiaMed, Cressier s/Morat, Switzerland). Hematological and biochemical indicators of in vivo hemolysis such as hemoglobin (Hb), percentage of reticulocyte (retic %), total serum bilirubin (S.Bil) and serum lactate dehydrogenase (LDH) were obtained from the patient file.[9,10] PRBC units whose reaction strength was found less than that of the autocontrol strength were designated as “best match” or “least incompatible” units and selected for transfusion as discussed previously.[11,12] The turnaround time (TAT) between the receipt of blood sample and requisition in the blood bank to the issue of blood to the patient was documented in minutes. All transfusions were done slowly under close supervision and regular monitoring of vital signs.
Statistical analysis
SPSS statistical software (version 13, USA) was applied for all statistical analysis. Hematological and biochemical values of patients were analyzed using the simple box plot separate variable analysis.
Results
Table 1 describes that a total of 14 patients aged between 18 and 64 years (M:F = 1:2.5) received 26 units of “best match” PRBCs at the dose of 1.86 units/patient. While secondary AIHA was observed in 7 patients, single type autoantibody (only IgG) was demonstrated in 11 patients (78.6%). A total of 167 PRBC units were crossmatched for these 14 patients of which 46 units (28%) were found to be “best match” ones. The crossmatch transfusion ratio (C:T) was calculated to be 6.4. Underlying alloantibody (anti-C) was detected in one patient who was transfused two units of ‘C’ antigen negative “best match” PRBCs. A mean TAT of 222 min (range: 160-430 min) was observed in issuing “best match” blood to the patients. The process flow of detailed immunohematological work-up for issuing “best match” blood to the AIHA patients has been depicted in Figure 1. Figure 2, a box plot analysis describes the indicator values of in vivo hemolysis in our patients. Severe hemolysis was observed in all patients with pre-transfusion median Hb of 5.4 g/dl. A median Hb increment of 0.88 g/dl was observed after each unit of PRBC transfusion.
Table 1.
Details of the AIHA patients (N = 14)
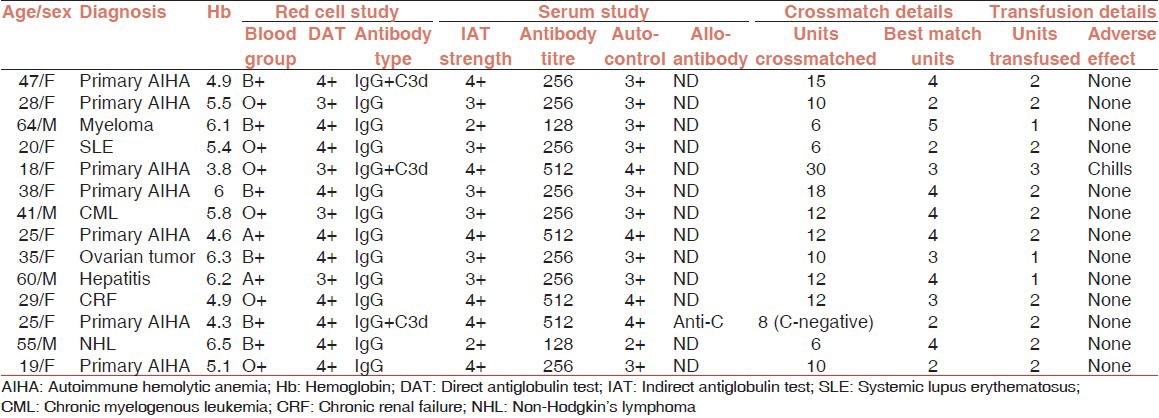
Figure 1.
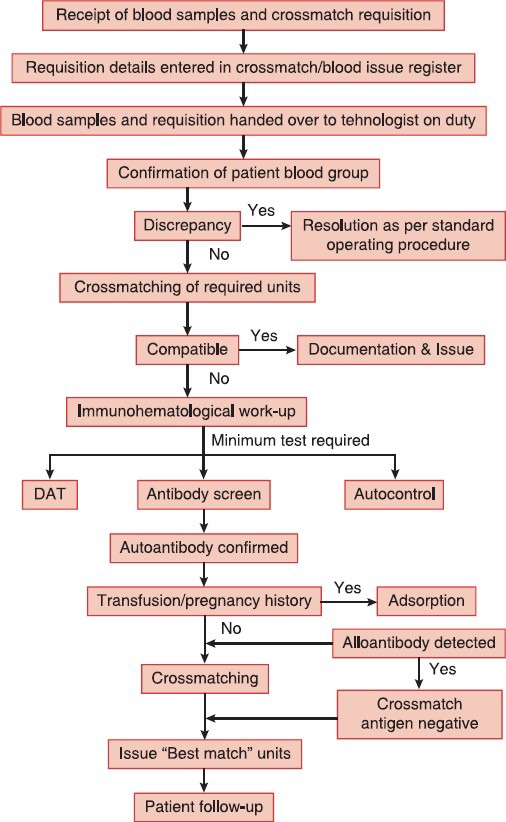
Process flow of immunohematological work-up for issuing ‘Best match’ blood in AIHA
Figure 2.
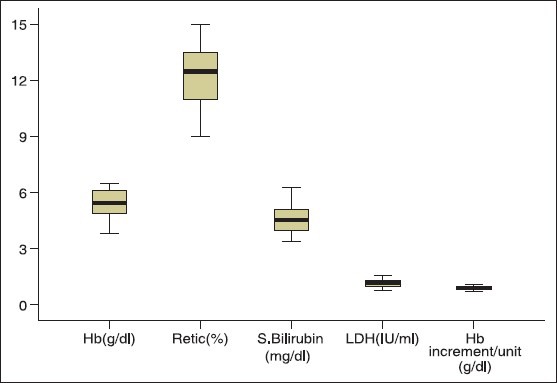
Box plot analysis of the indicators of in vivo hemolysis and hemoglobin increment per unit packed red blood cells in autoimmune hemolytic anemia patients (N = 14)
Discussion
Decision to transfuse in AIHA should be based on the clinical condition of the patient rather than correcting the laboratory values.[11] On one hand, delay in blood transfusion due to incompatible cross-match, lack of adequate blood bank infrastructure, series of immunohematological tests, lack of skilled person and on the other hand critical patient condition make the transfusion management more challenging. More delay has been observed in patients with blood group discrepancy or patients suspected to carry underlying alloantibody due to previous blood transfusion or pregnancy. Blood group discrepancy in one patient was resolved using eluted red cells and adsorbed serum. Grouping discrepancy in AIHA is not uncommon and has been documented previously.[13,14] Due to simplicity and rapidity, we selected cold acid elution and PEG adsorption for resolution of blood group discrepancy, characterization of autoantibody and detection of underlying alloantibody. Figure 1 describes our process flow of immunohematological work-up for issuing “best match” blood in AIHA. We suggested that minimum investigations such as DAT, antibody screen and autocontrol should be performed to ensure transfusion safety in AIHA. Although the blood issuing TAT in this category of patients has not been documented elsewhere but mean TAT of 222 min in the present study can be considered very high particularly when patients are in anemic crisis. The delay in issuing blood was observed more when special techniques like elution and adsorption has to be done or when more blood has to be crossmatched due to high autocontrol strength (≥3+). Approximately 12-40% of transfused patients develop clinically significant alloantibodies inducing rapid hemolysis and causing hemolytic transfusion reactions.[15,16] History of blood transfusions in all our patients necessitated adsorption study. Extended red cell phenotyping was not possible in any patient due to recent blood transfusion. In India, baseline pre-transfusion red cell phenotyping is not a routine practice hence transfusion of phenotype matched PRBC is almost impossible. We selected cold acid elution because it is an effective and rapid method of obtaining concentrated antibody and is probably the widely applied elution technique in AIHA.[4] As discussed earlier mere presence of red cell bound immunoglobulins does not indicate overt disease and a combination of clinical and laboratory evidences of hemolysis are required to establish the diagnosis of autoimmune hemolysis.[1] Conventional criteria such as Hb concentration, percentage of reticulocyte, unconjugated bilirubin concentration, LDH activity and haptoglobin concentration may collectively be of value in assessing the severity of the disease process in AIHA.[17] As depicted in Figure 2, severe in vivo hemolysis was observed in all patients that has necessitated the blood transfusion. The average Hb increment after each unit transfusion was 0.88 g/dl (range: 0.7-1.1 g/dl). The poor increment may be attributed to the destruction of transfused allogeneic red cells with the patient's own red cells. However, large sample size studies are required to comment on the possible Hb increment in AIHA. In vivo hemolysis in AIHA is multifactorial depending on thermal amplitude, quantity, class, subclass and titer of autoantibodies.[9,18] All patients in the present study presented with a high DAT strength (2+ to 4+) with IgG alone or combined with C3d. We observed that a high titer of free autoantibody in the serum (>128) or a high IAT strength (>2+) necessitated more blood to be tested to obtain the required “best match” units. High C:T of 6.4 could not be denied because most patient's physician were reluctant to transfuse the ordered number of PRBCs once they were aware of the incompatibility. We observed that crossmatch to probability of obtaining a “best match” unit ratio was high (4.3 vs. 1.35) when the free autoantibody concentration in serum was high (IAT >2+ or titer >128). In one young lady with primary AIHA and severe anemia with development of underlying alloantibody – anti ‘C’, it took 430 min to issue 2 units of “best match” PRBCs. A number of factors such as allogeneic PEG adsorption, obtaining PRBCs devoid of “C” antigen in a population where 85% are positive for “C” antigen and high titer of free antibodies in serum may be responsible for the long TAT. However, all transfusions were uneventful except one critically ill female patient who complained of chills.
Conclusion
We conclude that decision to transfuse in AIHA should be based on the clinical condition of the patient. No critical patient should be denied blood transfusion due to serological incompatibility. All transfusion services should be capable of performing the minimum test required to issue “best match” PRBCs in AIHA. Specialized techniques such as elution and adsorption which at times are helpful in enhancing blood safety in AIHA should be established in all blood banks.
Footnotes
Source of Support: Nil
Conflicting Interest: None declared
References
- 1.Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69:258–71. doi: 10.1002/ajh.10062. [DOI] [PubMed] [Google Scholar]
- 2.Duffy TP. Autoimmune hemolytic anemia and paroxysmal nocturnal hemoglobinuria. In: Simon TL, Dzik WH, Synder EL, Stowell CP, Strauss RG, editors. Rossi's Principles of Transfusion Medicine. 3rd ed. Philadelphia, USA: Lippincott Williams and Wilkins Publication; 2002. pp. 345–66. [Google Scholar]
- 3.Issit P. Serological diagnosis and characterization of causative antibody-Methods in Hematology. In: Chaplin H Jr, editor. Immune Hemolytic Anemia. 1st ed. USA: Churchill Livingston; 1985. pp. 47–94. [Google Scholar]
- 4.Bercher ME. Technical Manual. 15th ed. Bethesda, Maryland: AABB; 2005. The positive direct antiglobulin test and immune mediated red cell destruction. In: American Association of Blood Banks ed; pp. 453–82. [Google Scholar]
- 5.Toy PT, Chin CA, Reid ME, Burns MA. Factors associated with positive direct antiglobulin tests in pretransfusion patients: A case-control study. Vox Sang. 1985;49:215–20. doi: 10.1111/j.1423-0410.1985.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 6.Clark JA, Tanley PC, Wallace CH. Evaluation of patients with positive direct antiglobulin tests and nonreactive eluates discovered during pretransfusion testing. Immunohematology. 1992;8:9–12. [PubMed] [Google Scholar]
- 7.Das SS, Chaudhary R. Utility of adsorption techniques in serological evaluation of warm autoimmune haemolytic anaemia. Blood Transfus. 2009;7:300–4. doi: 10.2450/2009.0079-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das SS, Nityanand S, Chaudhary R. Clinical and serological characterization of autoimmune hemolytic anemia in a tertiary care hospital in North India. Ann Hematol. 2009;88:727–32. doi: 10.1007/s00277-008-0674-6. [DOI] [PubMed] [Google Scholar]
- 9.Wikman A, Axdorph U, Gryfelt G, Gustafsson L, Björkholm M, Lundahl J. Characterization of red cell autoantibodies in consecutive DAT-positive patients with relation to in vivo haemolysis. Ann Hematol. 2005;84:150–8. doi: 10.1007/s00277-004-0959-3. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler CA, Calhoun L, Blackall DP. Warm reactive autoantibodies: Clinical and serologic correlations. Am J Clin Pathol. 2004;122:680–5. doi: 10.1309/CJAW-6N8J-6H0H-R2WM. [DOI] [PubMed] [Google Scholar]
- 11.Petz LD. A physician's guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol. 2004;124:712–6. doi: 10.1111/j.1365-2141.2004.04841.x. [DOI] [PubMed] [Google Scholar]
- 12.Das SS, Chaudhary R. Transfusion support in autoimmune hemolytic anemia. Indian J Hematol Blood Transfus. 2006;1:9–13. [Google Scholar]
- 13.Zhu JY, Lan JC, Hu LY, Meng QB, Luo HQ. Study on blood ABO typing in patients with autoimmune hemolytic anemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2004;12:525–7. [PubMed] [Google Scholar]
- 14.Garratty G. Problems associated with compatibility testing for patients with autoimmune hemolytic anemia. Southeast Asian J Trop Med Public Health. 1993;24(Suppl 1):76–9. [PubMed] [Google Scholar]
- 15.Laine EP, Leger RM, Arndt PA, Calhoun L, Garratty G, Petz LD. In vitro studies of the impact of transfusion on the detection of alloantibodies after autoadsorption. Transfusion. 2000;40:1384–7. doi: 10.1046/j.1537-2995.2000.40111384.x. [DOI] [PubMed] [Google Scholar]
- 16.Leger RM, Garratty G. Evaluation of methods for detecting alloantibodies underlying warm autoantibodies. Transfusion. 1999;39:11–6. doi: 10.1046/j.1537-2995.1999.39199116889.x. [DOI] [PubMed] [Google Scholar]
- 17.Chaplin H., Jr Red cell-bound immunoglobulin as a predictor of severity of hemolysis in patients with autoimmune hemolytic anemia. Transfusion. 1990;30:576–8. doi: 10.1046/j.1537-2995.1990.30790385512.x. [DOI] [PubMed] [Google Scholar]
- 18.Sokol RJ, Hewitt S, Booker DJ, Bailey A. Red cell autoantibodies, multiple immunoglobulin classes, and autoimmune hemolysis. Transfusion. 1990;30:714–7. doi: 10.1046/j.1537-2995.1990.30891020331.x. [DOI] [PubMed] [Google Scholar]


