Abstract
Although the incidence of gastric cancer in the Dominican Republic is not high, the disease remains a significant health problem. We first conducted a detailed analysis of Helicobacter pylori status in the Dominican Republic. In total, 158 patients (103 females and 55 males; mean age 47.1±16.2 years) were recruited. The status of H. pylori infection was determined based on four tests: rapid urease test, culture test, histological test and immunohistochemistry. The status of cagA and vacA genotypes in H. pylori was examined using PCR and gene sequencing. The overall prevalence of H. pylori infection was 58.9 %. No relationship was found between the H. pylori infection rate and the age range of 17–91 years. Even in the youngest group (patients aged <29 years), the H. pylori infection rate was 62.5 %. Peptic ulcer was found in 23 patients and gastric cancer was found in one patient. The H. pylori infection rate in patients with peptic ulcer was significantly higher than that in patients with gastritis (82.6 versus 54.5 %, P<0.01). The cagA-positive/vacA s1m1 genotype was the most prevalent (43/64, 67.2 %). Compared with H. pylori-negative patients, H. pylori-positive patients showed more severe gastritis. Furthermore, the presence of cagA was related to the presence of more severe gastritis. All CagA-positive strains had Western-type CagA. In conclusion, we found that H. pylori infection is a risk factor for peptic ulcer in the Dominican Republic. Patients with cagA-positive H. pylori could be at higher risk for severe inflammation and atrophy.
Introduction
Helicobacter pylori is a spiral, Gram-negative bacterium that chronically colonizes the human stomach and is currently recognized as playing a causative role in the pathogenesis of various gastroduodenal diseases, including gastritis, peptic ulcer, gastric cancer and mucosa-associated lymphoid tissue lymphoma (Peek & Blaser, 2002; Suerbaum & Michetti, 2002). Gastric cancer remains a significant health problem, although its incidence greatly varies geographically. Countries can be categorized as high risk (e.g. Japan), intermediate risk (e.g. Vietnam) or low risk (e.g. the United States) for gastric cancer based on the age-standardized incidence rate (ASR) of the malignancy (Ferlay et al., 2010) (this information is also available from the International Agency for Research on Cancer; GLOBOCAN2012, http://globocan.iarc.fr/). Although the association between H. pylori infection and gastric cancer is well established (Parsonnet et al., 1991; Uemura et al., 2001), a high prevalence of H. pylori infection is not always related to a high incidence of gastric cancer. Interestingly, despite the high prevalence of H. pylori infection in Africa and South Asia, the incidence of gastric cancer in these areas is much lower than that in other countries; this phenomenon is the so-called African and Asian enigma (Malaty, 2007).
The Dominican Republic is a nation that occupies the western part of an island shared with the nation of Haiti in the Caribbean Sea. The ASRs of gastric cancer in Caribbean countries are reportedly <10 : 100 000 year–1 (http://globocan.iarc.fr/). The ASR of gastric cancer in the Dominican Republic is reportedly 7.3 : 100 000 year–1 (http://globocan.iarc.fr/). Although the prevalence of H. pylori infection has been determined in several countries with different socioeconomic, cultural and racial makeups (Azevedo et al., 2009; Goh et al., 2011; Tonkic et al., 2012), the prevalence of H. pylori infection in the Dominican Republic has not yet been investigated thoroughly. A previous study reported that the age-adjusted seroprevalence of H. pylori infection in the Dominican Republic was 62.1 % (Aoki et al., 2004). This finding suggested that the low virulence of H. pylori in the Dominican Republic contributed to the low incidence of gastric cancer. However, serological H. pylori antibody titres varied greatly depending on the test kit used (Burucoa et al., 2013; Miki, 2011). Furthermore, previous studies of the prevalence of chronic atrophic gastritis in the Dominican Republic were undertaken based on pepsinogen measurements, not histology (Aoki et al., 2004, 2005). Variable cut-off values for pepsinogen I and the pepsinogen I/II ratio were applied in previous studies, and might have affected the sensitivity and specificity of the results (Brenner et al., 2007; Leung et al., 2008). In this study, we determined the infection rate of H. pylori in the Dominican Republic using multiple tests: rapid urease test, culture test, histological test and immunohistochemistry (IHC). We also identified the virulence genes of H. pylori in the Dominican Republic.
Methods
Study population.
We recruited 158 patients with dyspeptic symptoms (103 females and 55 males; mean age 47.1±16.2 years, range 17–91 years) at the Digestive Disease Center, Dr Luis E. Aybar Health and Hygiene City, Santo Domingo, Dominican Republic, in February 2011. Patients with a history of partial gastric resection or previous treatment for H. pylori infection were excluded. Written informed consent was obtained from all participants, and the protocol was approved by the ethics committees of Dr Luis E. Aybar Health and Hygiene City and Oita University Faculty of Medicine, Japan.
During each endoscopy session, four gastric biopsy specimens were obtained (three from the antrum and one from the corpus). The antrum specimens were used for H. pylori culture, rapid urease test and histological examination. The corpus specimen was used for histological examination. Peptic ulcer and gastric cancer were identified using endoscopy. Gastritis was diagnosed in the absence of peptic ulcer or gastric malignancy.
Status of H. pylori infection.
To maximize the diagnostic accuracy, we used four methods for the diagnosis of H. pylori infection: rapid urease test, culture test, histological test and IHC. The CLOtest (Kimberly Clark Ballard Medical Products) was used as a rapid urease test to detect the presence of H. pylori urease. H. pylori culture was performed as described previously (Yamaoka et al., 1998b). For histology, all biopsy specimens were fixed in 10 % buffered formalin for 24 h and then embedded in paraffin. Serial sections were stained with haematoxylin–eosin and May–Giemsa. The degree of bacterial load was classified into four grades according to the updated Sydney System (Dixon et al., 1996). Grade ≥1 bacterial load was defined as H. pylori-positive. Patients were considered H. pylori-negative when all four tests were negative, whereas the H. pylori-positive status required at least one positive result.
IHC.
IHC was performed as described previously (Uchida et al., 2007). Briefly, after antigen retrieval and inactivation of endogenous peroxidase activity, tissue sections were incubated with anti-H. pylori antibody (Dako) overnight at 4 °C. After washing, the sections were incubated with biotinylated goat anti-rabbit IgG (Nichirei), followed by incubation with a solution of avidin-conjugated horseradish peroxidase (Vector). In all cases, we performed Giemsa staining using a serial section to identify the presence of H. pylori. If the H. pylori identified by Giemsa staining was positively immunostained, we considered the patient to be H. pylori-positive.
Staging for gastritis.
The degree of gastritis was classified using four grades according to the updated Sydney System (Dixon et al., 1996); samples of grade ≥1 were considered atrophy-positive, according to a previous report (Bornschein et al., 2012). In addition, the gastritis stage was assessed based on the severity and topographic locations (antrum and corpus) according to the Operative Link on Gastritis Assessment (OLGA) system (Rugge et al., 2005, 2007).
Isolation and genotyping of H. pylori.
H. pylori DNA was extracted from H. pylori cultured to confluence on plates using a commercially available kit (Qiagen). The status of cagA was determined with PCR for a conserved region of cagA and for direct sequencing as described previously (Yamaoka et al., 2000). The cagA genotype (East-Asian type and Western type) was confirmed by sequencing the PCR products as described previously (Xia et al., 2009). We performed vacA genotyping (s1, s2, m1 and m2) as described previously (Atherton et al., 1995; Yamaoka et al., 1999a). The PCR products were analysed by gel electrophoresis using 1.5 % agarose gel containing ethidium bromide.
Statistical analysis.
Data were analysed using SPSS version 19 (SPSS). Statistical evaluation was performed with the χ2 test to compare discrete variables, and with the Mann–Whitney U test and the t-test to compare continuous variables. To match age and sex, we used multiple backward stepwise logistic regression analyses to examine the associations of peptic ulcer with the main predictor variables. Predictor variables for peptic ulcer consisted of age, sex and H. pylori status. For each variable, the odds ratio and 95 % confidence interval were calculated. A two-tailed P<0.05 was considered significant.
Results
Prevalence of H. pylori infection in the Dominican Republic
Table 1 shows the H. pylori-positive rate for each test. The histology and IHC results matched completely, and are shown as ‘histological examination’. Compared with the positive rate of the culture, that of histological examination was significantly higher (P = 0.01). Although not statistically significant, the rapid urease test showed a positive rate lower than that of the histological examination. Overall, the prevalence of H. pylori infection in the Dominican Republic was 58.9 % (93/158). Fig. 1 shows the prevalence of H. pylori infection in various age groups. No relationship was found between H. pylori infection rate and age ranging from 17 to 91 years (P = 0.81).
Table 1. Prevalence [n (%)] of H. pylori infection determined by diagnostic tests.
| Age (years) | Total | |||||
| 17–29 | 30–39 | 40–49 | 50–59 | 60-91 | ||
| Total no. subjects | 24 | 28 | 40 | 30 | 36 | 158 |
| Rapid urease test | 13 (54.2) | 14 (50.0) | 22 (55.0) | 16 (53.3) | 13 (36.1) | 78 (49.4) |
| Culture | 10 (41.7) | 14 (50.0) | 21 (52.5) | 12 (40.0) | 11 (30.6) | 68 (43.0) |
| Histological examination | 14 (58.3) | 17 (60.7) | 24 (60.0) | 16 (53.3) | 18 (50.0) | 89 (56.3) |
| Final | 15 (62.5) | 17 (60.7) | 25 (62.5) | 18 (60.0) | 18 (50.0) | 93 (58.9) |
Fig. 1.
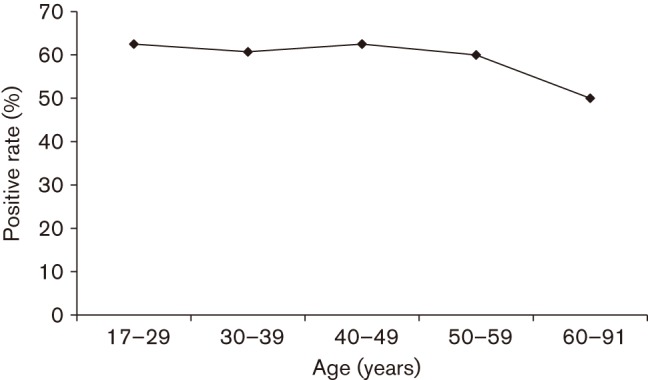
Prevalence of H. pylori infection in various age groups in the Dominican Republic. H. pylori infection was examined using four methods: rapid urease test, culture test, histological test and IHC. Patients were considered H. pylori-negative when all four tests were negative, whereas the H. pylori-positive status required at least one positive test result.
Endoscopic findings and H. pylori infection rate
Gastritis was the most common finding (134/158, 84.8 %). Among 134 patients with gastritis, four had gastric polyps and one had a submucosal tumour. Gastric and duodenal ulcers were found in 13 (8.2 %) and 10 (6.3 %) patients, respectively. Gastric cancer was found in one patient (0.6 %). Fig. 2 shows the prevalence of H. pylori infection in patients with gastritis and peptic ulcer. A high infection rate was detected among patients with gastric ulcer (84.6 %) and duodenal ulcer (80.0 %), whereas 52.8 % of the patients with gastritis were H. pylori-positive. When gastric and duodenal ulcers were defined as peptic ulcers, the prevalence of H. pylori infection in peptic ulcer was significantly higher than that in gastritis (82.6 versus 54.5 %, P = 0.008). Multiple logistic analysis after adjustment for age and gender showed that H. pylori positivity was significantly associated with peptic ulcer (odds ratio 3.96; 95 % confidence interval 1.28–12.29). The patient with gastric cancer was infected with H. pylori.
Fig. 2.
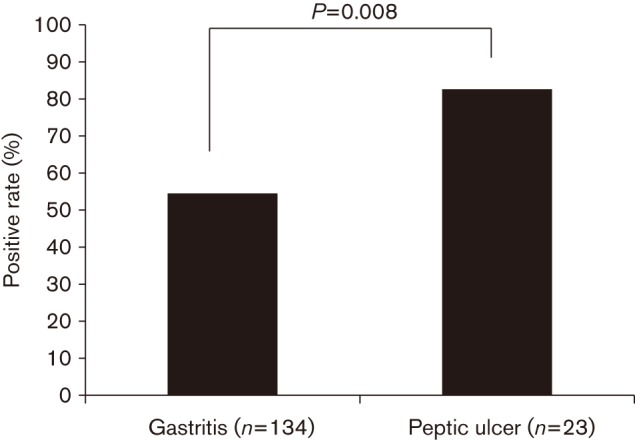
Prevalence of H. pylori infection in patients with gastritis and peptic ulcer.
Virulence genes of H. pylori
DNA was successfully extracted from 64 of 68 cultured strains and virulence genes were examined in these 64 strains (47 from patients with gastritis, 16 from patients with peptic ulcer and one from the patient with gastric cancer). The distributions of the cagA and vacA genotypes in the Dominican Republic are shown in Table 2. The prevalence of cagA was 75.0 % (48/64). The vacA s1 genotype was the most common (48/64, 75.0 %). The prevalence of the vacA m1 genotype was 71.9 % (46/64). Among the genotypes combining the vacA s and m regions, 44 (68.8 %) were s1m1, four (6.3 %) were s1m2, two (3.1 %) were s2m1 and 14 (21.9 %) were s2m2. Among the genotypes combining cagA and vacA, the cagA-positive/vacA s1m1 genotype was the most prevalent (43/64, 67.2 %). The types of cagA and vacA were compared between patients with gastritis and peptic ulcer (Table 2). No differences in the cagA and vacA genotypes were found between the gastritis and peptic ulcer groups (all P>0.05).
Table 2. Virulence genes [n (%)] of H. pylori in the Dominican Republic.
| Total | Gastritis | Peptic ulcer | Gastric cancer | |
| N | 64 | 47 | 16 | 1 |
| cagA (+) | 48 (75.0 %) | 36 (76.6) | 12 (75.0) | 0 (0.0) |
| cagA (–) | 16 (25.0) | 11 (23.4) | 4 (25.0) | 1 (100.0) |
| vacA s1 | 48 (75.0) | 36 (76.6) | 12 (75.0) | 0 (0.0) |
| vacA s2 | 16 (25.0) | 11 (23.4) | 4 (25.0) | 1 (100.0) |
| vacA m1 | 46 (71.9) | 34 (72.3) | 12 (75.0) | 0 (0.0) |
| vacA m2 | 18 (28.1) | 13 (27.7) | 4 (25.0) | 1 (100.0) |
| vacA s1m1 | 44 (68.8) | 33 (70.2) | 11 (68.8) | 0 (0.0) |
| vacA s1m2 | 4 (6.3) | 3 (6.4) | 1 (6.3) | 0 (0.0) |
| vacA s2m1 | 2 (3.1) | 1 (2.1) | 1 (6.3) | 0 (0.0) |
| vacA s2m2 | 14 (21.9) | 10 (21.3) | 3 (18.8) | 1 (100.0) |
| cagA (+) vacA s1m1 | 43 (67.2) | 32 (68.1) | 11 (68.8) | 0 (0.0) |
| cagA (+) vacA s1m2 | 3 (4.7) | 2 (4.3) | 1 (6.3) | 0 (0.0) |
| cagA (+) vacA s2m1 | 1 (1.6) | 1 (2.1) | 0 (0.0) | 0 (0.0) |
| cagA (+) vacA s2m2 | 1 (1.6) | 1 (2.1) | 0 (0.0) | 0 (0.0) |
| cagA (–) vacA s1m1 | 1 (1.6) | 1 (2.1) | 0 (0.0) | 0 (0.0) |
| cagA (–) vacA s1m2 | 1 (1.6) | 1 (2.1) | 0 (0.0) | 0 (0.0) |
| cagA (–) vacA s2m1 | 1 (1.6) | 0 (0.0) | 1 (6.3) | 0 (0.0) |
| cagA (–) vacA s2m2 | 13 (20.3) | 9 (19.1) | 3 (18.8) | 1 (100.0) |
The CagA types derived from the cagA genotypes by gene sequencing were also examined. Thirty-nine strains were sequenced successfully for cagA. All 39 showed Western-type CagA. Among the 39 Western-type CagA strains, 33 (84.6 %) were ABC type. Strains with multiple C segments (i.e. ABCC) were found in three patients (two with gastritis and one with gastric ulcer). Other types of CagA (AB, AABC and ABBC) were found in one patient each.
Gastric mucosa status
Histological findings classified 35 patients as grade 0 for atrophy in the antrum; 106 patients had grade 1, 17 had grade 2 and none had grade 3. Atrophy in the corpus was classified as grades 0, 1 and 2 for 117, 37 and four patients, respectively. When samples of grade ≥1 were considered atrophy-positive, 123 patients (77.8 %) were found to have mucosal atrophy in the antrum, and 41 (25.9 %) patients also had mucosal atrophy in both the antrum and the corpus.
The staging of gastritis was also assessed according to the OLGA system. Stage 0 was found in 22.1 %, stage I in 64.5 % (102/158) and stage II in 13.2 % (21/158). Stages III and IV were not found. The differences in histological scores according to the status of H. pylori infection are shown in Table 3. Compared with H. pylori-negative subjects, H. pylori-positive subjects showed significantly greater activity, inflammation and atrophy in both the antrum and the corpus (all P<0.0001). The OLGA score in H. pylori-positive subjects was also significantly higher than that in negative patients (1.08±0.55 versus 0.68±0.56, P<0.0001). Eleven and seven subjects had intestinal metaplasia in the antrum and corpus, respectively. The prevalence of intestinal metaplasia in the antrum was not significantly different between H. pylori-positive and H. pylori-negative subjects (8.6 versus 6.1 %, P = 0.56). Likewise, no differences in the presence of intestinal metaplasia in the corpus were found between H. pylori-positive and H. pylori-negative subjects (5.3 versus 3.0 %, P = 0.48).
Table 3. Differences in histological scores according to the status of H. pylori infection.
| H. pylori (+) | H. pylori (–) | P | |
| N | 93 | 65 | |
| Antrum | |||
| Activity | 1.33±0.64 | 0.12±0.37 | <0.0001 |
| Inflammation | 1.81±0.63 | 0.51±0.71 | <0.0001 |
| Atrophy | 1.04±0.53 | 0.66±0.53 | <0.0001 |
| Intestinal metaplasia | 0.14±0.52 | 0.08±0.40 | 0.33 |
| Bacterial density | 1.58±0.82 | 0.00±0.00 | <0.0001 |
| Corpus | |||
| Activity | 1.00±0.67 | 0.11±0.40 | <0.0001 |
| Inflammation | 1.26±0.56 | 0.32±0.58 | <0.0001 |
| Atrophy | 0.40±0.55 | 0.12±0.37 | <0.0001 |
| Intestinal metaplasia | 0.10±0.44 | 0.05±0.27 | 0.48 |
| Bacterial density | 1.60±0.75 | 0.00±0.00 | <0.0001 |
| OLGA score | 1.08±0.55 | 0.68±0.56 | <0.0001 |
Histological scores according to cagA status are shown in Table 4. Compared with cagA-negative patients, cagA-positive patients had significantly higher scores for atrophy in the antrum and corpus (P = 0.03 and 0.01, respectively). Inflammation scores in the corpus of cagA-positive patients were also significantly higher than in those of cagA-negative patients (P = 0.03). Interestingly, none of the 16 cagA-negative patients had intestinal metaplasia in the antrum or corpus. The OLGA score in cagA-positive patients was also significantly higher than that in cagA-negative patients (1.15±0.50 versus 0.81±0.54, P = 0.03).
Table 4. Histological scores according to the status of cagA.
| cagA (+) | cagA (–) | P | |
| N | 48 | 16 | |
| Antrum | |||
| Activity | 1.48±0.61 | 1.25±0.44 | 0.20 |
| Inflammation | 1.94±0.56 | 1.75±0.57 | 0.24 |
| Atrophy | 1.15±0.50 | 0.81±0.54 | 0.03 |
| Intestinal metaplasia | 0.13±0.53 | 0.00±0.00 | 0.31 |
| Bacterial density | 1.79±0.68 | 1.63±0.80 | 0.48 |
| Corpus | |||
| Activity | 1.06±0.63 | 0.81±0.65 | 0.17 |
| Inflammation | 1.31±0.55 | 0.94±0.57 | 0.03 |
| Atrophy | 0.42±0.49 | 0.06±0.25 | 0.01 |
| Intestinal metaplasia | 0.06±0.43 | 0.00±0.00 | 0.56 |
| Bacterial density | 1.81±0.67 | 1.38±0.71 | 0.03 |
| OLGA score | 1.15±0.50 | 0.81±0.54 | 0.03 |
Discussion
Using a combination of four tests, we determined the prevalence of H. pylori in the Dominican Republic to be 58.9 %. Similar results have been reported in neighbouring countries, such as Haiti (60.0 %) and Jamaica (50.8 %) (Hisada et al., 2001; Shak et al., 2011). In contrast with those in developed countries, H. pylori infections in the developing world occur earlier in life and with a higher frequency (Goh et al., 2011). The prevalence of H. pylori infection was reported to be >70 % in developing countries (Calvet et al., 2013; Leja et al., 2012; Porras et al., 2013). The present study showed that the prevalence was high even in young age groups (58.3 % in patients aged ≤29 years) in the Dominican Republic. The high infection rate in the younger age group in our study was consistent with that reported in a study conducted in 2001–2002 (Aoki et al., 2005). Sanitary conditions (e.g. availability of necessary equipment for water and sewage management) are considered important factors in H. pylori infection (Goh et al., 2011). Poor sanitary conditions are positively associated with a high rate of H. pylori positivity (Dattoli et al., 2010; Strebel et al., 2010). Furthermore, the prevalence of H. pylori infection decreased along with improvements in clean public water systems after World War II in Japan (Asaka et al., 1992). The World Health Organization and UNICEF have reported that the percentage of improved sanitation facilities in 2011 was 82 % in the Dominican Republic. By contrast, that figure was 100 % in Japan and the United States, where H. pylori infection rates are decreasing (http://www.unicef.org/). Unimproved sanitary conditions in the Dominican Republic may be associated with high H. pylori infection rates. Nevertheless, the prevalence of H. pylori infection was lowest in the older age group and we reported previously that the prevalence of H. pylori infection decreased with age in Bhutan, which is also a developing country (Vilaichone et al., 2013). The decrease in H. pylori infection rate with age might be due to frequent antibiotic use in infectious diseases in general, but further studies are necessary to clarify the factors influencing the H. pylori infection rate.
Our finding that the prevalence of H. pylori in patients with peptic ulcer was significantly higher than that in patients with gastritis was consistent with the findings of previous studies (Huang et al., 2002; Malfertheiner et al., 2012; Papatheodoridis et al., 2006). This relationship suggests that H. pylori infection is a risk factor for the development of peptic ulcer and gastric cancer in the Dominican Republic. Furthermore, histological scores were higher in H. pylori-positive patients than in H. pylori-negative patients, consistent with the results of other studies (Kodama et al., 2012b). Successful eradication is effective for the prevention of not only peptic ulcer, but also gastric cancer (Asaka et al., 2010). Additionally, 10-year prospective follow-up studies have shown that successful eradication therapy reduces significantly mucosal inflammation, activity and atrophy (Kodama et al., 2012a, b). Therefore, eradication therapy for H. pylori infection can be useful in reducing the occurrence of peptic ulcer and gastric cancer in the Dominican Republic.
We found that 77.8 % of subjects had mucosal atrophy in the antrum, and 25.9 % subjects also had mucosal atrophy in both the antrum and the corpus in the Dominican Republic. We reported previously that mucosal atrophy was found in the antrum in 91.9 % of subjects and in the corpus in 37.7 % of subjects in Bhutan, where the incidence of gastric cancer is high (17.2 : 100 000 year–1) (Shiota et al., 2013). Another staging system for gastritis (OLGA) showed that 22.1 % of subjects were classified as stage 0, 64.5 % as stage I and 13.2 % as stage II in the Dominican Republic. Stages III and IV were not observed. On the contrary, ~40 % of subjects were classified as stages III and IV in a study of Japanese patients (Satoh et al., 2008). Milder gastritis might be related to the low incidence of gastric cancer in the Dominican Republic despite the high H. pylori infection rate.
To our knowledge, this report is the first to reveal the virulence factors of H. pylori in the Dominican Republic. The cagA gene, which encodes a highly immunogenic protein (CagA), is the most extensively studied H. pylori virulence factor (Covacci et al., 1993; Tummuru et al., 1993). Almost all H. pylori isolates from East-Asian countries and ~60–80 % of isolates from Western countries are cagA positive (Matsuda et al., 2011; Suzuki et al., 2012; Yamaoka et al., 1999b). In Western countries, individuals infected with cagA-positive H. pylori strains reportedly have a higher risk of peptic ulcer or gastric cancer than those infected with cagA-negative strains (van Doorn et al., 1998; Yamaoka et al., 2002). Furthermore, CagA can be divided into two types (East-Asian and Western) according to the repeat sequences of the 3′ region of cagA (Yamaoka, 2010). The repeat regions contain the Glu-Pro-Ile-Tyr-Ala (EPIYA) motifs. Sequences have been annotated according to segments (20–50 aa) flanking the EPIYA motifs (i.e. segments EPIYA-A, -B, -C and -D) (Yamaoka, 2010). Compared with individuals with Western-type CagA strains containing EPIYA-C segments, those infected with East-Asian-type CagA strains containing EPIYA-D segments reportedly have an increased risk of peptic ulcer or gastric cancer (Matsunari et al., 2012; Vilaichone et al., 2004). We determined the prevalence of cagA to be 75.0 % in the Dominican Republic, which is similar to that of neighbouring countries. The cagA-positive rate is reportedly 73.2 % in Cuban strains (Torres et al., 2009). Furthermore, all of the 39 strains sequenced successfully were Western-type CagA; in particular, 84.6 % of CagA strains showed the ABC pattern. The prevalence of strains with multiple C segments was only 7.7 % and all of them were ABCC, but not ABCCC. The percentage of strains with ABCC was lower than that in Cuban strains (20 %) (Torres et al., 2012). In Western countries, the incidence of gastric cancer in patients infected with strains carrying multiple EPIYA-C repeats is higher than the incidence in those infected with strains with a single repeat (Argent et al., 2004; Azuma et al., 2002; Xia et al., 2009; Yamaoka et al., 1998a, 1999a). For example, 31 % of H. pylori strains had multiple EPIYA-C repeats in Colombia, where the incidence of gastric cancer is relatively high (17.4 : 100 000 year–1) (Yamaoka et al., 1999a). Western-type CagA and the lower percentage of multiple EPIYA-C repeats in the Dominican Republic may account for the lower incidence of gastric cancer in the Dominican Republic than in East-Asian countries. However, we found that cagA-positive patients had significantly greater atrophy in the antrum and corpus than cagA-negative patients. Inflammation score in the corpus was also significantly higher in these patients. Interestingly, none of 16 cagA-negative patients had intestinal metaplasia in the antrum or corpus. These results suggest that patients infected with cagA-positive H. pylori strains can be a high-risk population, even in the Dominican Republic.
Many studies in Western countries have shown that individuals infected with vacA s1 or m1 H. pylori strains have a higher risk of peptic ulcer or gastric cancer than those infected with s2 or m2 strains (Atherton et al., 1995; Cover & Blaser, 1992; Sugimoto & Yamaoka, 2009; Sugimoto et al., 2009). The vacA s1m1 genotype was predominant in the Dominican Republic, as in Jamaica and Cuba (Hisada et al., 2001; Torres et al., 2009). On the contrary, 21.9 % of subjects had the vacA s2m2 genotype – a prevalence higher than that in Japan, where most cases are vacA s1m1 (Yamaoka et al., 1998b). Furthermore, vacA s2m1 or vacA s2m2 genotypes were found even in cagA-positive strains and this is consistent with findings in Cuban strains (Torres et al., 2009). Less virulent types of vacA may also be related to the lower incidence of gastric cancer in Caribbean countries including the Dominican Republic.
However, our study had several limitations. The prevalence of H. pylori infection in this study was determined based on a combination of four analyses. Although several clinical tests have been developed to diagnose H. pylori infection, no ‘gold standard’ has been established. In this study, histological examination showed the highest positive rate. The histological diagnosis of H. pylori infection is very much dependent on the expertise of the pathologists. Rapid urease tests, such as CLOtest, can be useful for rapid diagnosis. However, the accuracy of this test can be affected by the bacterial load (Megraud & Lehours, 2007) and a reportedly low sensitivity (Megraud, 1997). The results of cultures from biopsy specimens are dependent on transport conditions and careful handling in the laboratory (Megraud & Lehours, 2007). Other global tests, such as the urea breath test or the stool H. pylori antigen test, should be used in future studies.
In conclusion, we found that the prevalence of H. pylori infection in the Dominican Republic was high despite the low incidence of gastric cancer. Lower virulence of H. pylori and mild gastritis in the Dominican Republic might contribute to the low incidence of gastric cancer. However, the presence of H. pylori was related to severe clinical outcomes and more severe gastritis. In addition, the presence of cagA was related to more severe gastritis. Therefore, eradication therapy for H. pylori would likely contribute to decreasing H. pylori-related diseases such as peptic ulcer and gastric cancer in the Dominicaln Republic.
Acknowledgements
This report is based on work supported in part by grants from the National Institutes of Health [DK62813 (Y. Y.) and DK56338 (D. Y. G.)], Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan [22390085, 22659087, 24406015 and 24659200 (Y. Y.), and 23790798 (S. S.)], the Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation of the Japan Society for the Promotion of Science, the Strategic Funds for the Promotion of Science and Technology from the Japan Science and Technology Agency, and the National Fund for Innovation and Scientific and Technological Development from the Ministry of Higher Education Science and Technology of the Dominican Republic (M. C.).
Abbreviations:
- ASR
age-standardized incidence rate
- IHC
immunohistochemistry
- OLGA
Operative Link for Gastritis Assessment
References
- Aoki K., Kihaile P. E., Castro M., Disla M., Nyambo T. B., Misumi J. (2004). Seroprevalences of Helicobacter pylori infection and chronic zatrophic gastritis in the United Republic of Tanzania and the Dominican Republic. Environ Health Prev Med 9, 170–175 10.1007/BF02898097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K., Kihaile P. E., Wenyuan Z., Xianghang Z., Castro M., Disla M., Nyambo T. B., Misumi J. (2005). Comparison of prevalence of chronic atrophic gastritis in Japan, China, Tanzania, and the Dominican Republic. Ann Epidemiol 15, 598–606 10.1016/j.annepidem.2004.11.002 [DOI] [PubMed] [Google Scholar]
- Argent R. H., Kidd M., Owen R. J., Thomas R. J., Limb M. C., Atherton J. C. (2004). Determinants and consequences of different levels of CagA phosphorylation for clinical isolates of Helicobacter pylori. Gastroenterology 127, 514–523 10.1053/j.gastro.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Asaka M., Kimura T., Kudo M., Takeda H., Mitani S., Miyazaki T., Miki K., Graham D. Y. (1992). Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology 102, 760–766 [DOI] [PubMed] [Google Scholar]
- Asaka M., Kato M., Takahashi S., Fukuda Y., Sugiyama T., Ota H., Uemura N., Murakami K., Satoh K., Sugano K., Japanese Society for Helicobacter Research (2010). Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter 15, 1–20 10.1111/j.1523-5378.2009.00738.x [DOI] [PubMed] [Google Scholar]
- Atherton J. C., Cao P., Peek R. M., Jr, Tummuru M. K., Blaser M. J., Cover T. L. (1995). Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem 270, 17771–17777 10.1074/jbc.270.30.17771 [DOI] [PubMed] [Google Scholar]
- Azevedo N. F., Huntington J., Goodman K. J. (2009). The epidemiology of Helicobacter pylori and public health implications. Helicobacter 14 (Suppl 1), 1–7 10.1111/j.1523-5378.2009.00703.x [DOI] [PubMed] [Google Scholar]
- Azuma T., Yamakawa A., Yamazaki S., Fukuta K., Ohtani M., Ito Y., Dojo M., Yamazaki Y., Kuriyama M. (2002). Correlation between variation of the 3′ region of the cagA gene in Helicobacter pylori and disease outcome in Japan. J Infect Dis 186, 1621–1630 10.1086/345374 [DOI] [PubMed] [Google Scholar]
- Bornschein J., Selgrad M., Wex T., Kuester D., Malfertheiner P. (2012). Serological assessment of gastric mucosal atrophy in gastric cancer. BMC Gastroenterol 12, 10. 10.1186/1471-230X-12-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H., Rothenbacher D., Weck M. N. (2007). Epidemiologic findings on serologically defined chronic atrophic gastritis strongly depend on the choice of the cutoff-value. Int J Cancer 121, 2782–2786 10.1002/ijc.22992 [DOI] [PubMed] [Google Scholar]
- Burucoa C., Delchier J. C., Courillon-Mallet A., de Korwin J. D., Mégraud F., Zerbib F., Raymond J., Fauchère J. L. (2013). Comparative evaluation of 29 commercial Helicobacter pylori serological kits. Helicobacter 18, 169–179 10.1111/hel.12030 [DOI] [PubMed] [Google Scholar]
- Calvet X., Ramírez Lázaro M. J., Lehours P., Mégraud F. (2013). Diagnosis and epidemiology of Helicobacter pylori infection. Helicobacter 18 (Suppl 1), 5–11 10.1111/hel.12071 [DOI] [PubMed] [Google Scholar]
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z. & other authors (1993). Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A 90, 5791–5795 10.1073/pnas.90.12.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Blaser M. J. (1992). Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem 267, 10570–10575 [PubMed] [Google Scholar]
- Dattoli V. C., Veiga R. V., da Cunha S. S., Pontes-de-Carvalho L. C., Barreto M. L., Alcântara-Neves N. M. (2010). Seroprevalence and potential risk factors for Helicobacter pylori infection in Brazilian children. Helicobacter 15, 273–278 10.1111/j.1523-5378.2010.00766.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M. F., Genta R. M., Yardley J. H., Correa P. (1996). Classification and grading of gastritis: the updated Sydney System. Am J Surg Pathol 20, 1161–1181 10.1097/00000478-199610000-00001 [DOI] [PubMed] [Google Scholar]
- Ferlay J., Shin H. R., Bray F., Forman D., Mathers C., Parkin D. M. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127, 2893–2917 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- Goh K. L., Chan W. K., Shiota S., Yamaoka Y. (2011). Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter 16 (Suppl 1), 1–9 10.1111/j.1523-5378.2011.00874.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada M., Lee M. G., Hanchard B., Owens M., Song Q., van Doorn L. J., Cutler A. F., Gold B. D. (2001). Characteristics of Helicobacter pylori infection in Jamaican adults with gastrointestinal symptoms. J Clin Microbiol 39, 212–216 10.1128/JCM.39.1.212-216.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. Q., Sridhar S., Hunt R. H. (2002). Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet 359, 14–22 10.1016/S0140-6736(02)07273-2 [DOI] [PubMed] [Google Scholar]
- Kodama M., Murakami K., Okimoto T., Abe T., Nakagawa Y., Mizukami K., Uchida M., Inoue K., Fujioka T. (2012a). Helicobacter pylori eradication improves gastric atrophy and intestinal metaplasia in long-term observation. Digestion 85, 126–130 10.1159/000334684 [DOI] [PubMed] [Google Scholar]
- Kodama M., Murakami K., Okimoto T., Sato R., Uchida M., Abe T., Shiota S., Nakagawa Y., Mizukami K., Fujioka T. (2012b). Ten-year prospective follow-up of histological changes at five points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J Gastroenterol 47, 394–403 10.1007/s00535-011-0504-9 [DOI] [PubMed] [Google Scholar]
- Leja M., Cine E., Rudzite D., Vilkoite I., Huttunen T., Daugule I., Rumba-Rozenfelde I., Pimanov S., Liepniece-Karele I. & other authors (2012). Prevalence of Helicobacter pylori infection and atrophic gastritis in Latvia. Eur J Gastroenterol Hepatol 24, 1410–1417 10.1097/MEG.0b013e3283583ca5 [DOI] [PubMed] [Google Scholar]
- Leung W. K., Wu M. S., Kakugawa Y., Kim J. J., Yeoh K. G., Goh K. L., Wu K. C., Wu D. C., Sollano J. & other authors (2008). Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol 9, 279–287 10.1016/S1470-2045(08)70072-X [DOI] [PubMed] [Google Scholar]
- Malaty H. M. (2007). Epidemiology of Helicobacter pylori infection. Best Pract Res Clin Gastroenterol 21, 205–214 10.1016/j.bpg.2006.10.005 [DOI] [PubMed] [Google Scholar]
- Malfertheiner P., Megraud F., O’Morain C. A., Atherton J., Axon A. T., Bazzoli F., Gensini G. F., Gisbert J. P., Graham D. Y. & other authors (2012). Management of Helicobacter pylori infection – the Maastricht IV/Florence Consensus Report. Gut 61, 646–664 10.1136/gutjnl-2012-302084 [DOI] [PubMed] [Google Scholar]
- Matsuda M., Shiota S., Matsunari O., Watada M., Murakami K., Fujioka T., Yamaoka Y. (2011). Prevalence of two homologous genes encoding glycosyltransferases of Helicobacter pylori in the United States and Japan. J Gastroenterol Hepatol 26, 1451–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunari O., Shiota S., Suzuki R., Watada M., Kinjo N., Murakami K., Fujioka T., Kinjo F., Yamaoka Y. (2012). Association between Helicobacter pylori virulence factors and gastroduodenal diseases in Okinawa, Japan. J Clin Microbiol 50, 876–883 10.1128/JCM.05562-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégraud F. (1997). A growing demand for Helicobacter pylori culture in the near future? Ital J Gastroenterol Hepatol 29, 574–576 [PubMed] [Google Scholar]
- Mégraud F., Lehours P. (2007). Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev 20, 280–322 10.1128/CMR.00033-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K. (2011). Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels – “ABC method”. Proc Jpn Acad, Ser B, Phys Biol Sci 87, 405–414 10.2183/pjab.87.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatheodoridis G. V., Sougioultzis S., Archimandritis A. J. (2006). Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: a systematic review. Clin Gastroenterol Hepatol 4, 130–142 10.1016/j.cgh.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. (1991). Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 325, 1127–1131 10.1056/NEJM199110173251603 [DOI] [PubMed] [Google Scholar]
- Peek R. M., Jr, Blaser M. J. (2002). Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2, 28–37 10.1038/nrc703 [DOI] [PubMed] [Google Scholar]
- Porras C., Nodora J., Sexton R., Ferreccio C., Jimenez S., Dominguez R. L., Cook P., Anderson G., Morgan D. R. & other authors (2013). Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701). Cancer Causes Control 24, 209–215 10.1007/s10552-012-0117-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugge M., Genta R. M., OLGA Group (2005). Staging gastritis: an international proposal. Gastroenterology 129, 1807–1808 10.1053/j.gastro.2005.09.056 [DOI] [PubMed] [Google Scholar]
- Rugge M., Meggio A., Pennelli G., Piscioli F., Giacomelli L., De Pretis G., Graham D. Y. (2007). Gastritis staging in clinical practice: the OLGA staging system. Gut 56, 631–636 10.1136/gut.2006.106666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K., Osawa H., Yoshizawa M., Nakano H., Hirasawa T., Kihira K., Sugano K. (2008). Assessment of atrophic gastritis using the OLGA system. Helicobacter 13, 225–229 10.1111/j.1523-5378.2008.00599.x [DOI] [PubMed] [Google Scholar]
- Shak J. R., Sodikoff J. B., Speckman R. A., Rollin F. G., Chery M. P., Cole C. R., Suchdev P. S. (2011). Anemia and Helicobacter pylori seroreactivity in a rural Haitian population. Am J Trop Med Hyg 85, 913–918 10.4269/ajtmh.2011.11-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota S., Mahachai V., Vilaichone R. K., Ratanachu-ek T., Tshering L., Uchida T., Matsunari O., Yamaoka Y. (2013). Seroprevalence of Helicobacter pylori infection and gastric mucosal atrophy in Bhutan, a country with a high prevalence of gastric cancer. J Med Microbiol 62, 1571–1578 10.1099/jmm.0.060905-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K., Rolle-Kampczyk U., Richter M., Kindler A., Richter T., Schlink U. (2010). A rigorous small area modelling-study for the Helicobacter pylori epidemiology. Sci Total Environ 408, 3931–3942 10.1016/j.scitotenv.2010.03.045 [DOI] [PubMed] [Google Scholar]
- Suerbaum S., Michetti P. (2002). Helicobacter pylori infection. N Engl J Med 347, 1175–1186 10.1056/NEJMra020542 [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Yamaoka Y. (2009). The association of vacA genotype and Helicobacter pylori-related disease in Latin American and African populations. Clin Microbiol Infect 15, 835–842 10.1111/j.1469-0691.2009.02769.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto M., Zali M. R., Yamaoka Y. (2009). The association of vacA genotypes and Helicobacter pylori-related gastroduodenal diseases in the Middle East. Eur J Clin Microbiol Infect Dis 28, 1227–1236 10.1007/s10096-009-0772-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R., Shiota S., Yamaoka Y. (2012). Molecular epidemiology, population genetics, and pathogenic role of Helicobacter pylori. Infect Genet Evol 12, 203–213 10.1016/j.meegid.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkic A., Tonkic M., Lehours P., Mégraud F. (2012). Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter 17 (Suppl 1), 1–8 10.1111/j.1523-5378.2012.00975.x [DOI] [PubMed] [Google Scholar]
- Torres L. E., Melián K., Moreno A., Alonso J., Sabatier C. A., Hernández M., Bermúdez L., Rodríguez B. L. (2009). Prevalence of vacA, cagA and babA2 genes in Cuban Helicobacter pylori isolates. World J Gastroenterol 15, 204–210 10.3748/wjg.15.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres L. E., González L., Melián K., Alonso J., Moreno A., Hernández M., Reyes O., Bermúdez L., Campos J. & other authors (2012). EPIYA motif patterns among Cuban Helicobacter pylori CagA positive strains. Biomedica 32, 23–31 [DOI] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. (1993). Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun 61, 1799–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Kanada R., Tsukamoto Y., Hijiya N., Matsuura K., Yano S., Yokoyama S., Kishida T., Kodama M. & other authors (2007). Immunohistochemical diagnosis of the cagA-gene genotype of Helicobacter pylori with anti-East Asian CagA-specific antibody. Cancer Sci 98, 521–528 10.1111/j.1349-7006.2007.00415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura N., Okamoto S., Yamamoto S., Matsumura N., Yamaguchi S., Yamakido M., Taniyama K., Sasaki N., Schlemper R. J. (2001). Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345, 784–789 10.1056/NEJMoa001999 [DOI] [PubMed] [Google Scholar]
- van Doorn L. J., Figueiredo C., Sanna R., Plaisier A., Schneeberger P., de Boer W., Quint W. (1998). Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology 115, 58–66 10.1016/S0016-5085(98)70365-8 [DOI] [PubMed] [Google Scholar]
- Vilaichone R. K., Mahachai V., Tumwasorn S., Wu J. Y., Graham D. Y., Yamaoka Y. (2004). Molecular epidemiology and outcome of Helicobacter pylori infection in Thailand: a cultural cross roads. Helicobacter 9, 453–459 10.1111/j.1083-4389.2004.00260.x [DOI] [PubMed] [Google Scholar]
- Vilaichone R. K., Mahachai V., Shiota S., Uchida T., Ratanachu-ek T., Tshering L., Tung N. L., Fujioka T., Moriyama M., Yamaoka Y. (2013). Extremely high prevalence of Helicobacter pylori infection in Bhutan. World J Gastroenterol 19, 2806–2810 10.3748/wjg.v19.i18.2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Yamaoka Y., Zhu Q., Matha I., Gao X. (2009). A comprehensive sequence and disease correlation analyses for the C-terminal region of CagA protein of Helicobacter pylori. PLoS ONE 4, e7736. 10.1371/journal.pone.0007736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y. (2010). Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol 7, 629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y., Kodama T., Kashima K., Graham D. Y., Sepulveda A. R. (1998a). Variants of the 3′ region of the cagA gene in Helicobacter pylori isolates from patients with different H. pylori-associated diseases. J Clin Microbiol 36, 2258–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y., Kodama T., Kita M., Imanishi J., Kashima K., Graham D. Y. (1998b). Relationship of vacA genotypes of Helicobacter pylori to cagA status, cytotoxin production, and clinical outcome. Helicobacter 3, 241–253 10.1046/j.1523-5378.1998.08056.x [DOI] [PubMed] [Google Scholar]
- Yamaoka Y., El-Zimaity H. M., Gutierrez O., Figura N., Kim J. K., Kodama T., Kashima K., Graham D. Y. (1999a). Relationship between the cagA 3′ repeat region of Helicobacter pylori, gastric histology, and susceptibility to low pH. Gastroenterology 117, 342–349 10.1053/gast.1999.0029900342 [DOI] [PubMed] [Google Scholar]
- Yamaoka Y., Kodama T., Gutierrez O., Kim J. G., Kashima K., Graham D. Y. (1999b). Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol 37, 2274–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y., Osato M. S., Sepulveda A. R., Gutierrez O., Figura N., Kim J. G., Kodama T., Kashima K., Graham D. Y. (2000). Molecular epidemiology of Helicobacter pylori: separation of H. pylori from East Asian and non-Asian countries. Epidemiol Infect 124, 91–96 10.1017/S0950268899003209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y., Kikuchi S., el-Zimaity H. M., Gutierrez O., Osato M. S., Graham D. Y. (2002). Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology 123, 414–424 10.1053/gast.2002.34781 [DOI] [PubMed] [Google Scholar]


