Abstract
Background
The regulation of intestinal barrier permeability is important in the maintenance of normal intestinal physiology. Sphingosine-1-phosphate (S1P) has been shown to play a pivotal role in enhancing barrier function in several non-intestinal tissues. The current study determined whether S1P regulated function of the intestinal epithelial barrier by altering expression of E-cadherin, an important protein in adherens junctions.
Methods
Studies were performed upon cultured differentiated IECs (IEC-Cdx2L1 line) using standard techniques.
Results
S1P treatment significantly increased levels of E-cadherin protein and mRNA in intestinal epithelial cells (IECs) and also led to E-cadherin localizing strongly to the cell–cell border. S1P also improved the barrier function as indicated by a decrease in 14C-mannitol paracellular permeability and an increase in transepithelial electrical resistance (TEER) in vitro.
Conclusions
These results indicate that S1P increases levels of E-cadherin, both in cellular amounts and at the cell–cell junctions, and leads to improved barrier integrity in cultured intestinal epithelial cells.
Keywords: Sphingosine, Intestinal barrier, Ca2+ signaling, Intestinal epithelium
Introduction
The gastrointestinal (GI) mucosa is lined by an epithelial cell layer which forms an important barrier protecting subepithelial tissue from injury by noxious chemicals, allergens, and infectious pathogens. Intestinal barrier dysfunction has been implicated in a wide variety of pathologic conditions, such as infection, trauma, inflammation, and malignancy [1, 2], and the integrity of this layer strongly depends on specialized structures involved in cell–cell contacts, including tight junctions, adherens junctions, and desmosomes [3–8]. These junctions maintain normal epithelial structure and surround the subapical region of epithelial cells [5, 9, 10], and they work in concert both to seal [3, 9, 11, 12] and to mediate strong cell–cell adhesion [5, 10, 13, 14].
The cadherins are a group of Ca2+-dependent adhesion molecules essential for the induction and maintenance of cell–cell contacts [5, 7]. E-cadherin is primarily found at the adherens junctions and is the major cadherin expressed in epithelial cells, including those of the GI tract [5, 7, 15, 16]. It is thought that the strong E-cadherin-mediated cell–cell adhesions necessary for barrier integrity are brought about by E-cadherin homodimerization to neighboring cells, and its conserved cytoplasmic domain interacting with the cytoskeleton through actin-binding proteins, leading to strengthened cell–cell adhesion [7, 17–20].
Sphingosine-1-phosphate (S1P) is a bioactive lysophospholipid with important biological functions, which include cell adhesion, barrier regulation, proliferation, differentiation, migration, and survival [21–27]. S1P is ubiquitous in cells and is produced intracellularly, though its broad spectrum of effects on distinct cellular functions occur via specific cell surface receptors [25, 28–31]. Through its interaction with the endothelial-differentiation-gene (EDG) family of receptors, S1P is shown to have protective effects in a growing number of tissues, including the pancreas, thyroid, heart, kidney, and liver [32–39], as well as in the lung, where S1P increases transepithelial electrical resistance (TEER), thus, enhancing barrier function [21, 24, 26, 31, 40–43]. Although the exact mechanism underlying S1P in mediating its protective effects on endothelial barrier integrity remain unclear, there is a growing pool of data suggesting that the protective effect of S1P is mediated in part through associations with cortactin (an F-actin cross-linking polypeptide) and myosin light chain kinase [21], as well as the enhancement of cadherin-based adherens junctions [44, 45]. Interestingly, although S1P is most abundant in intestinal tissue [46, 47] and its receptors and S1P synthetic enzymes are highly expressed in intestinal epithelial cells [48], there is little data available regarding the role of S1P on normal intestinal epithelial cell (IEC) integrity.
The aim of the current study was to test the hypothesis that S1P is involved in the regulation of intestinal barrier function by altering the expression of adherens junctions through a process involving Ca2+. First, we examined the effects of S1P treatment on the expression and distribution of E-cadherin and β-catenin in differentiated intestinal epithelial cells. Second, we ascertained whether S1P would affect permeability in these cells.
Materials and Methods
Chemicals and Supplies
Disposable cultureware was purchased from Corning Glass Works (Corning, NY). Tissue culture media and dialyzed fetal bovine serum (dFBS) were obtained from GIBCO-BRL (Gaithersburg, MD), and biochemicals, including S1P, were purchased from Sigma (St. Louis, MO). The monoclonal antibodies against E-cadherin, and β-catenin, were purchased from BD Transduction Laboratories (Lexington, KY). The antibody against JAM-1 was obtained from R&D Systems (Minneapolis, MN). The antibody against S1P1 was purchased from Abcam Incorporated (Cambridge, MA). The 12-mm Transwell filters (0.4-μm pore size, clear polyester) were obtained from Costar (Cambridge, MA). Fluorescein-conjugated goat antimouse and goat antirabbit antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). 14C-labeled mannitol was obtained from Amersham Pharmacia Biotech (Piscataway, NJ).
Cell Culture and General Experimental Protocols
The stable Cdx2-transfected-IEC-6 cell line was developed and characterized by Suh and Traber [49], and was a kind gift from Dr. Peter G. Traber (University of Pennsylvania, Philadelphia, PA). The expression vector LacSwitch System (Stratagene, La Jolla, CA) was used for directing the conditional expression of Cdx2, and isopropyl–D-thioga-lactopyranoside (IPTG) served as the inducer for gene expression. IEC-6 cells were transfected with pOP-RSVCdx2 by an electroporation technique and clones resistant to selection medium containing 0.6 mg/ml G418 and 0.3 mg/ml hygromycin B were isolated and screened for Cdx2 expression by Northern blot, RNase protection assays, and electrophoretic mobility shift assay. Stock stable Cdx2-transfected IEC-6 cells were grown in the DMEM used in parental nontransfected IEC-6 cells. Before the experiments, cells were grown in DMEM containing 4 mM IPTG for 16 days to induce cell differentiation. In the first series of studies, we examined the effects of S1P treatment on the expression and cellular distribution of E-cadherin, β-catenin, and JAM-1 in IEC-Cdx2L1 cells. Cells were grown in control media until confluent, at which point the cells were treated with solutions according to the assays to be conducted. The monolayers were washed three times with ice-cold Dulbecco’s PBS (DPBS). In the second series of studies, we investigated whether the depletion of [Ca2+]cyt followed by S1P treatment altered E-cadherin expression. In the Ca2+-free medium, 1.8 mM CaCl2 was replaced by 1.8 mM MgCl2 and an additional 0.1 mM EGTA was added to chelate the residual Ca2+. Free Ca2+ in the Ca2+-free medium was <0.002 mM. Levels of E-cadherin mRNA and protein were measured at various times after sequential treatment with Ca2+-free medium followed by S1P in control medium. In the third series of studies, we investigated whether observed changes in E-cadherin expression after S1P treatment alter paracellular permeability in the differentiated IEC-Cdx2L1 cells. Permeability was measured by paracellular tracer flux assays and the membrane-impermeable molecule [14C] mannitol served as the molecular tracer, as well as changes in TEER.
Reverse Transcription and Real-Time PCR
Total RNA was isolated by using an RNeasy Mini Kit (Qiagen, Valencia, CA). Equal amounts of total RNA (2 μg) were transcribed to synthesize single-strand cDNA with a reverse transcription (RT)-PCR kit (Invitrogen Life Technologies, Carlsbad, CA), as previously described [50]. Primers for E-cadherin were purchased from R&D Systems Inc. (Minneapolis, MN) and yielded a 397-bp fragment. The levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) PCR product were assessed to monitor the even RNA input in RT–PCR samples. Real-time quantitative PCR (Q-PCR) was performed using an Applied Biosystems instrument (Foster City, CA) using specific primers, probes, and software (Applied Biosystems). The levels of E-cadherin mRNA were quantified by Q-PCR analysis and normalized to a control.
Western Blot Analysis
Cell samples dissolved in ice-cold RIPA buffer (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1 mM DTT, 0.5 mM EDTA, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin, 2 μg/ml leupeptin, and 2 mM sodium orthovanadate) were sonicated and centrifuged at 14,000 rpm for 15 min at 4°C. The protein concentration of the supernatant was measured by the methods described by Bradford [51] and each lane was loaded with 2–5 μg of protein equivalent. The supernatant was boiled for 5 min and then subjected to electrophoresis on 7.5% acrylamide gels according to Laemmli [52]. Briefly, after the transfer of protein onto nitrocellulose filters, the filters were incubated for 1 h in 5% nonfat dry milk in 1× TBS-T buffer (Tris-buffered saline, pH 7.4, with 0.1% Tween 20). Immunologic evaluation was then performed overnight at 4°C in 5% nonfat dry milk-TBST buffer containing specific antibodies against E-cadherin, β-catenin, ZO-1, ZO-2, occluding, EDG-1, or JAM-1 proteins. The filters were subsequently washed with 1× TBST and incubated with the secondary antibodies conjugated with horseradish per-oxidase (HRP) for 1 h at room temperature. The immunocomplexes on the filters were reacted for 1 min with chemiluminescence reagent (NEL-100, DuPont NEN).
Immunofluorescence Staining
The immunofluorescence procedure was carried out according to the method of Vielkind and Swierenga [53] with minor changes [54, 55]. Cells were incubated with the primary antibody against E-cadherin, occludin, EDG-1, and JAM-1 at 4°C overnight and then incubated with secondary antibody conjugated with FITC for 2 h at room temperature. After the slides were rinsed three times, they were mounted and viewed through a Zeiss confocal microscope (model LSM410). Images were processed with Photoshop software (Adobe, San Jose, CA). For the actin staining, the cells were treated similarly, except that incubation occurred with 1:40 dilution of rhodamine-labeled phalloidin in PBS/BSA for 45 min at room temperature and then the cells were visualized under confocal microscopy.
RNA Interference
The siRNA (small interfering RNA) were designed to cleave specifically the EDG-1 (S1P1) mRNA (siEDG-1); these, as well as the control (C-siRNA—which had no specific homology to any one gene) were synthesized and purchased from Dharmacon Inc. (Lafayette, CO). For each 60-mm cell culture dish, 25 μl of the 20 μM stock siRNA EDG-1 or C-siRNA was mixed with 500 μl of Opti-MEM medium (Invitrogen). This mixture was gently added to a solution containing 10 μl Lipofectamine™ 2000 (Invitrogen) in 500 μl Opti-MEM medium. The solution was incubated for 20 min at room temperature and gently overlaid onto the monolayer of cells in 4 ml of medium, and the cells were harvested for various assays after 48 h of incubation.
Measurement of [Ca2+]cyt
Details of the digital imaging methods used for measuring [Ca2+]cyt are described in our previous publications [56, 57]. Briefly, cells were plated on 25-mm coverslips and were incubated in culture medium containing 3.3 μM fura 2-AM for 30–40 min at room temperature (22–24°C) under an atmosphere of 10% CO2 in air. Fluorescent images were obtained with a microchannel plate image intensifier (Amperex XX1381; Opelco, Washington, DC) coupled by fiber optics to a PULNiX charge-coupled device (CCD) video camera (Stanford Photonics, Stanford, CA). Image acquisition and analysis were performed with a MetaMorph Imaging System (Universal Imaging, Ypsilanti, MI). [Ca2+]cyt was calculated from fura-2 fluorescence emission excited at 380 and 360 nm by the ratio method [58].
Paracellular Tracer Flux Assay
Flux assays were performed with the 12-mm Transwell filters as described by Wong and Gumbiner [59] with minor modification. Briefly, IEC-Cdx2L1 cells were grown in control cultures and then trypsinized, plated at a confluent density of 4 × 105 cells/cm2 on the insert, and maintained under the same culture conditions for an additional 48 h to establish tight monolayers. [14C] mannitol (mol wgt 184) served as the paracellular tracer in this experiment. At the beginning of the flux assay, both sides of the bathing wells of the Transwell filters were replaced with fresh medium containing 5 mM unlabeled mannitol. [14C] mannitol was added to a final concentration of 3.6 nM to the apical bathing wells that contained 0.5 ml of medium. The basal bathing well had no added tracer and contained 1.5 ml of the same flux assay medium as in the apical compartment. All flux assays were performed at 37°C, and the basal medium was collected 2 h after the addition of [14C] mannitol for counting in a Beckman liquid scintillation counter. The results were expressed as the percentage of total count values of each tracer. As an adjunct, TEER was measured by a method described previously [60, 61]. Transwell inserts containing the cell monolayers were placed inside the EndOhm-12 chamber (World Precision Instruments, Sarasota, FL) and TEER was measured across the monolayers using an Epithelial Tissue Voltohmmeter (World Precision Instruments, Sarasota, FL) and was expressed as ohms per centimeter square.
Statistics
All data are expressed as means ± standard errors (SE) from six or eight samples. Autoradiographic and immunofluorescence labeling experiments were repeated three times. The significance of the difference between means was determined by two-way analysis of variance (ANOVA). The level of significance was determined with the Duncan’s multiple-range test.
Results
Presence of EDG-1 (S1P1) Receptors in Intestinal Epithelial Cells
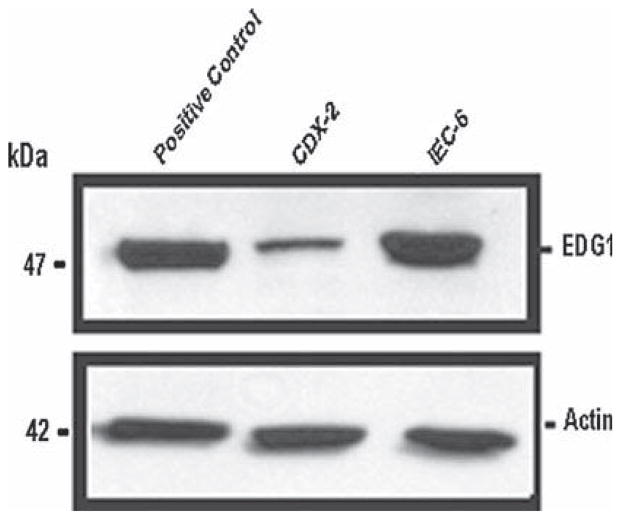
To determine whether intestinal epithelial cells possess EDG (S1P) receptors, we harvested protein from intestinal epithelial cells and used Western blotting techniques to determine the expression of EDG-1 receptor proteins. Figure 1 shows the presence of the EDG-1 (S1P1) protein in both parental IEC-6 cells and differentiated IEC-Cdx2L1 cells. We also verified their presence in Caco-2 cells (data not shown). Further studies also determined the absence of the EDG-3 (S1P3) receptor (data not shown). We also assessed these cells for sphingosine kinase 1 (SphK1) and found that it was present in both undifferentiated and differentiated IECs, but appeared to be greater in the undifferentiated cells (data not shown). These results indicate that both differentiated and undifferentiated intestinal epithelial cells elaborate EDG-1 receptors but not the EDG-3 receptors, and that they each demonstrate SphK1.
Fig. 1.
Presence of effectors of S1P in IECs. Demonstration of EDG-1 (S1P1) receptor in IEC-6 and IEC-Cdx2L1 cells grown to confluence in control conditions, demonstrating the presence of the EDG-1 protein at 47 kDa in Western blots probing with specific antibody. The second panel demonstrates the absence of EDG-3 (S1P3) receptor in Cdx2 cells and IEC-6 cells. Actin (42 kDa) immunoblotting was performed as an internal control for equal loading. Two experiments were performed that showed similar results
S1P Enhances Epithelial Cell Barrier Properties In Vitro
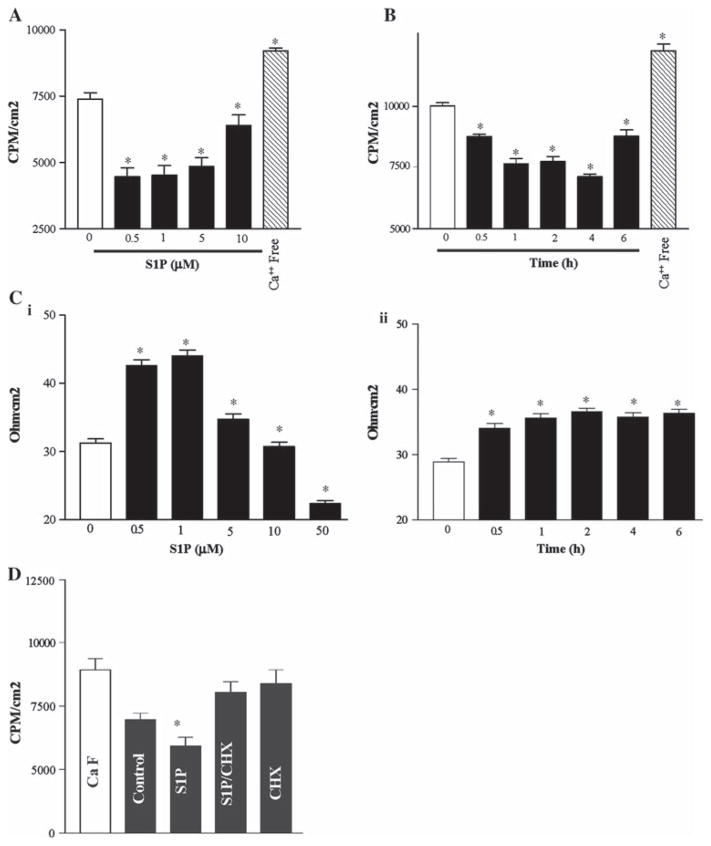
We have previously examined influences on intestinal barrier function in differentiated IECs [50, 62], as measured by the assessment of TEER and 14C-mannitol permeability, and we sought to see if S1P exposure resulted in enhanced intestinal epithelial barrier function. Cells were grown to confluence to establish a tight monolayer. As shown in Fig. 2, S1P enhanced the epithelial barrier, as indicated by an increase in TEER and a decrease in paracellular permeability. This effect was maximal at an S1P dose of 0.5 μM (14C-mannitol permeability improved by ~40% and TEER improved from 31.3 ± 1.5 to 44 ± 2 Ω/cm2, n = 12, P <0.05) and was significant up to 10 μM (higher doses demonstrated no effect). The duration of S1P exposure (at 0.5 μM) also affected the barrier function, with significant effects noted as early as 30 min and lasting up to 24 h, and with the maximal effect noted at 4 h (14C-mannitol permeability improved by ~31%). Exposure of the cells to Ca2+-free medium served as a positive control, and as expected (Fig. 2), showed dramatically increased paracellular permeability to 14C-mannitol at 2 h. These results indicate that S1P decreases the intestinal epithelial paracellular permeability in a dose- and time-dependent fashion.
Fig. 2.
Epithelial cell barrier function after treatment with S1P. A Paracellular permeability. Dose response of cells exposed to S1P for 4 h. B Time course response to S1P (0.5 μM). (C) TEER data for conditions replicating A (i) and B (ii). Values are means ± SE of data from six samples. * P <0.05 compared with control IECs unexposed to S1P. D Paracellular permeability comparing S1P (0.5 μM) with co-incubation of cycloheximide (CHX—25 μg/ml) alone and CHX + S1P. * P <0.05 compared with control IECs unexposed to S1P. The results shown are means from six experiments. All cells were viable (early passage) at the time of experimentation
We next performed co-administration of S1P with cycloheximide (CHX) in order to demonstrate that the observed enhancement in barrier properties was (at least in part) due to protein expression. Figure 2d demonstrates that the observed S1P-induced improvement in barrier function is ablated with CHX treatment.
Effect of S1P Treatment on the Expression of E-Cadherin, β-Catenin, and JAM-1
Previously, we have demonstrated that, in differentiated Cdx2L1 cells, decreased E-cadherin levels result in impaired barrier function [62]. As such, since S1P induced decreases in paracellular permeability, we sought to determine whether S1P regulated cell–cell junctional interactions. As shown in Fig. 3A, treatment with S1P for 4 h dose-dependently increased the E-cadherin levels, with significant induction in the levels of E-cadherin noted at 100 nM and a maximal response at S1P exposure of 0.5 μM. In contrast, S1P did not have an effect on β-catenin or JAM-1 levels (JAM-1 data not shown). Additionally, Fig. 3B shows that S1P initiated a significant effect on E-cadherin levels in 1 h, and also that this selective effect of S1P (0.5 μM) on E-cadherin was maximal at 3 h, at which point the effect is seen to plateau. Finally, real-time PCR analysis of E-cadherin mRNA expression after exposure to S1P at (0.5 μM) increased E-cadherin levels by 2.6× within 1 h of exposure (Fig. 3C); this effect, however, did not exactly parallel the increased protein levels, as the increase returned to control levels after 4 h. These results show that, in intestinal epithelial cells, S1P rapidly increases E-cadherin, both protein levels, and mRNA levels. The results due to S1P were specific, as related peptides such as ceramide did not demonstrate similar effects on the E-cadherin level (Fig. 3D).
Fig. 3.
Protein and mRNA levels of E-cadherin in IECs after treatment with S1P. A Dose response to S1P for 4 h. Representative autoradiograms (a) and quantitative densitometric analysis (b) derived from Western blots. E-cadherin (120 kDa) was identified by probing nitrocellulose with specific antibody. Actin (42 kDa) immunoblotting was performed as an internal control for equal loading. B Time course response of cells exposed to S1P (0.5 μM). Striped bars, control; solid bars, S1P treatment. The results for A and B are representative of results from three experiments. C RT–PCR and real-time PCR Analysis: total RNA was isolated and 1.5 μg of each sample was then used as a template for reverse transcription synthesis of the first-strand cDNA. Real-time Q-PCR was performed using an Applied Biosystems instrument (ABI PRISM 6700) using specific primers, probes, and software (Applied Biosystems). Values are means ± SEM of data from three separate experiments; relative levels of E-cadherin were corrected for loading as measured by densitometry of actin or to GAPDH levels. * P <0.05 compared with control IECs unexposed to S1P, five experiments were performed that showed similar results. D Western blots as above, here for E-cadherin comparing control media to S1P (2.5 mM, 4 h) and ceramide (2.5 mM, 4 h). Two experiments were performed that showed similar results for D
Effects of S1P Treatment on the Cellular Distribution of E-Cadherin and β-Catenin
To determine whether S1P altered the subcellular distribution of E-cadherin, immunofluorescence staining was performed in this study. In comparing cells treated with S1P (0.5 μM) for 4 h to control cells (Fig. 4), immunoreactivities for E-cadherin were markedly increased along the cell–cell contact region. On the other hand, the distribution of β-catenin was not seen to be altered under the same conditions.
Fig. 4.
Effect of S1P on the cellular distribution of E-cadherin and β-catenin. Cells were plated in a four-well chamber slide and grown in control medium to confluence. Cells were treated and then fixed for immunostaining. Cells were incubated with the specific antibody against E-cadherin and β-catenin and then with anti-IgG conjugated with FITC. Slides were viewed through a Zeiss confocal microscope. The results are representative data from five experiments
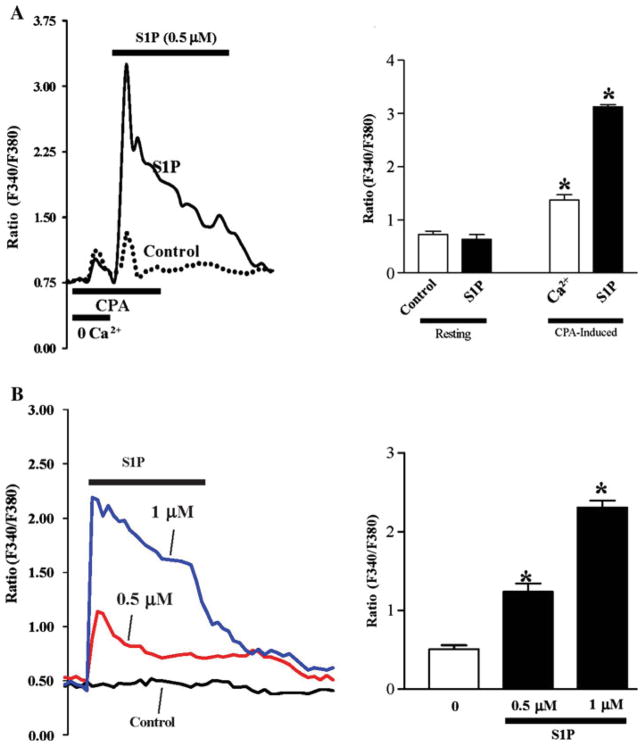
S1P Increase [Ca2+]cyt
To determine the possibility that exposure of intestinal epithelial cells to S1P resulted in increased cytoplasmic Ca2+ concentration and to see whether these changes were attributable to receptor-operated calcium (ROC) influx via S1P receptor binding, we measured individual IEC [Ca2+]cyt upon exposure to S1P under control conditions and after cyclopiazonic acid (CPA)-induced Ca2+ store depletion. Exposure to CPA in the absence of extracellular Ca2+ (0 Ca2+) resulted in an initial transient increase in [Ca2+]cyt, representing the mobilization of Ca2+ from the intracellular stores, that then returned to baseline; subsequent return of extracellular Ca2+ to the perfusate caused a more pronounced increase in [Ca2+]cyt, representing Ca2+ re-entry. The return of extracellular Ca2+ to the perfusate in the presence of S1P (0.5 μM) resulted in a ~4× increase in [Ca2+]cyt versus Ca2+ alone (Fig. 5a). As shown in Fig. 5b, S1P treatment at 0.5 and 1 μM significantly increased [Ca2+]cyt influx in a dose-dependent fashion. Cells were of normal viability and were plated 2D prior to experimentation.
Fig. 5.
Effect of S1P on calcium-mediated changes in resting free cytosolic Ca2+ concentration ([Ca2+]cyt) in differentiated IEC-Cdx2L1 cells. a Representative record showing cyclopiazonic acid (CPA)-induced Ca2+ store depletion performed followed by S1P (0.5 μM) treatment. CPA-induced store depletion allows for singling out extracellular calcium entry from intracellular sources. The results are depicted graphically in the right panel. Experiments were performed three times with over 50 cells each day. b S1P induced changes at 0.5 μM and 1 μM versus control. The results are depicted graphically in the right panel
[Ca2+]cyt on E-Cadherin Expression and Permeability
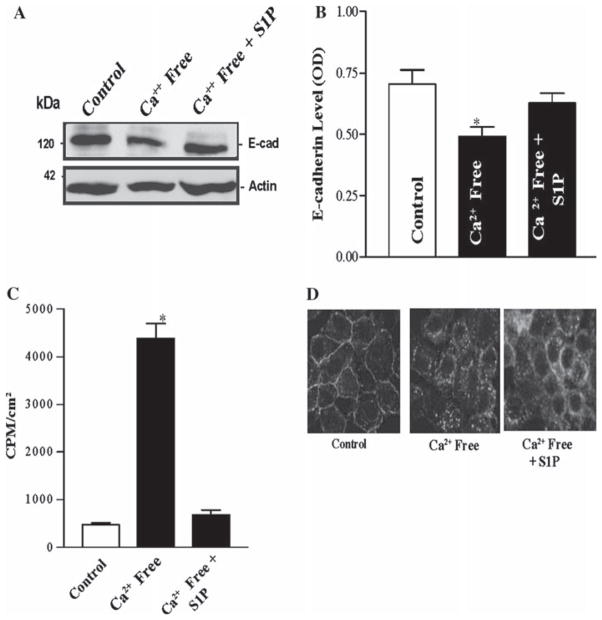
We have previously demonstrated that altering [Ca2+]cyt regulates E-cadherin expression [56, 62]. In this study, we tested the possibility that S1P preserved levels of E-cadherin during Ca2+-free (CaF) conditions. In the first study, we examined whether the decreased [Ca2+]cyt seen upon the removal of extracellular Ca2+ alters the expression of E-cadherin mRNA and protein in intestinal epithelial cells. The removal of extracellular Ca2+ decreased E-cadherin mRNA (data not shown) and also inhibited E-cadherin protein expression at 4 h after exposure to the CaF media (Fig. 6); however, pretreatment with S1P (0.5 μM, 1 h) prevented this decrease in E-cadherin. No effect was seen on β-catenin in parallel experiments under the same conditions (data not shown). We next determined whether S1P would improve paracellular permeability during CaF conditions, which we have shown leads to dramatic increases in intestinal epithelial paracellular permeability. CaF media at 2 h led to dramatic increased permeability to 14C-mannitol (Fig. 6c), and co-incubation with S1P (pretreatment of 0.5 μM, 1 h) was able to preserve permeability at a level unchanged versus control conditions. This effect was time-sensitive, however, as S1P was unable to reverse the permeability changes from a 6-h exposure to CaF media (data not shown). Immunofluorescence staining was repeated for E-cadherin in CaF conditions; pretreatment with S1P (0.5 μM, 2 h) demonstrated partial maintenance of the cortical staining (Fig. 6d). These results indicate that S1P is able to at least partially prevent CaF-associated decreases in E-cadherin levels and increases in paracellular permeability.
Fig. 6.
Effect of S1P on E-cadherin levels and permeability in IEC-Cdx2L1 cells pretreated by Ca2+-free media. a Representative Western blots depicting E-cadherin levels in response to Ca2+-free media with and without 1 h pretreatment of S1P (0.5 μM). The results of densitometry are depicted in b. Values are means ± SEM of data from three separate experiments. c 14C-mannitol permeability of cells described in a, plus additionally 6-h exposure to Ca2+-free media. d Immunostaining of cells for E-cadherin. CaF cells were exposed to control media and S1P containing (0.5 μM) media for 2 h prior to CaF conditions. Pictures were obtained after 1 h of CaF media exposure. Values are means ± SEM of data from six samples. * P <0.05 compared with control IEC-Cdx2L1 cells unexposed to S1P
Discussion
An increasing body of evidence indicates that S1P is a critical regulator of cellular barrier function in mammalian cells [21, 23, 24, 40] and that this, in part, involves the manipulation of the proteins involved in cell–cell adhesion [21, 42]. Though S1P is abundant in the mammalian intestine [46–48], a specific role of S1P in the regulation of intestinal barrier integrity remains to be elucidated. The present studies demonstrate that S1P modulated the expression and cortical location of E-cadherin, as it did similarly in other mammalian systems [5, 21, 24]. The most significant of the new findings was that S1P augments intestinal barrier function in differentiated rat intestinal epithelial cells. Also, the markedly increased permeability at the intestinal barrier seen with calcium-free conditions could be reversed partly through S1P exposure. These findings indicate that S1P plays a critical role in modulating the integrity of the intestinal barrier, and it may indicate a role of S1P in pathological conditions where this barrier is injured.
The role of S1P in enhancing the integrity of the endothelial barrier has been extensively studied in both in vivo and in vitro systems. S1P primarily induces its downstream effects via specific receptor-mediated interactions, such as EDG-1 (S1P1) and EDG-3 (S1P-3) in cell surface receptors. This alteration in the barrier is not due to transcellular transport, but, rather, changes in paracellular transport [63]. Garcia et al. have reported that S1P plays a critical role in endothelial junction integrity via the cytoskeletal rearrangement of actin-binding proteins, such as myosin light-chain kinase and cortactin [21, 24]. S1P-regulated barrier function is dependent on its interacting with the EDG-1 receptor, and is also related to activation of the downstream target PI3K and to actin rearrangements, as silencing at any of these steps abolished the protective effect of S1P [26, 41]. The relevance of EDG-1 is further underscored by the fact that EDG-1 knockouts were lethal in utero [64]. In the current study, we demonstrate the presence of EDG-1, but not EDG-3, in IECs (Fig. 1), and we have recently [65] demonstrated that S1P’s effect on the differentiated IECs is blocked by the silencing of either the EDG-1 receptor or of PI3K inhibition.
S1P is also shown to induce the peripheral enhancement of VE-cadherin, a member of the cadherin family of proteins, with localization of VE-cadherin to areas of cell–cell contact [41]. It has been shown that the adherens junction acts to integrate data from varied signaling transduction pathways involved in cell–cell adhesion and cellular cytoskeletal arrangement to regulate intestinal epithelial barrier function. E-cadherin is a calcium-dependent cell-surface component of the adherens junction and its cytoplasmic domain interacts with the catenin family of proteins [41]. This cadherin–catenin complex, in turn, associates with the actin cytoskeleton to bolster cell–cell adhesion, thus, functionally regulating the intestinal epithelial barrier [62]. E-cadherin forms these cadherin–catenin complexes during the assembly of the adherens intercellular junctions and they continue to strengthen and develop into mature adherens junctions. The silencing of E-cadherin with specific antibodies against it lead to disruption of the adherens junctions and increased permeability [66, 67], and dominant negative mutants for E-cadherin have demonstrated assembly failure of the tight and adherens junctions, and, consequently, increased paracellular permeability. Thus, S1P has been demonstrated to elaborate permeability changes through mechanisms involving E-cadherin, and E-cadherin has been demonstrated [62] to be integral to intestinal permeability, but S1P’s relevance in intestinal barrier function has not previously been demonstrated. In the present study, we show that S1P exposure led to dramatic increases in cytosolic calcium concentrations (Fig. 5) and in E-cadherin mRNA and protein expression versus the control (Fig. 3). We have reported previously [62] that increased E-cadherin expression alone can explain improved barrier function, and we have shown that S1P specifically is lessening paracellular permeability in intestinal epithelial cells that is, at least partly, due to the increased expression of E-cadherin. The exact mechanistic details of S1P-regulated E-cadherin expression is still under investigation; recent reports [68] have implicated zinc-finger transcriptional repressors snail and slug for this epithelial–mesenchymal transition (EMT); however, we have not seen evidence that S1P alters snail or slug in intestinal epithelial cells (data not shown).
The current study further demonstrates that S1P modulates the subcellular localization of E-cadherin in differentiated Cdx2L1 cells (Fig. 4). In endothelium, S1P modulates barrier function via the S1P1/Akt/Rac small GTPase pathway. Additionally, several proteins, including VE-cadherin and JAM-1, were observed to co-localize in cellular junctional areas when treated with S1P, suggesting a functional involvement in S1P-induced cell–cell interactions and barrier integrity. While we did notice the change in E-cadherin expression and co-localization with S1P exposure, it is of note that we did not find similar changes in JAM-1 or β-catenin in the intestinal cells (Figs. 3 and 4, and some data not shown). Clearly, further studies are needed to define the exact mechanism for this specific protein response.
The results presented in Fig. 3 show that S1P significantly decreased the permeability of confluent Cdx2L1 cells to [14C] mannitol and concomitantly increased the TEER of these cells as well. Significant S1P-mediated enhancement of barrier integrity has been demonstrated by Garcia et al. in pulmonary endothelial cells [21, 41], and was thought to be due, in part, to the strengthening of the cytoskeleton by S1P-mediated distribution/assembly of adherens proteins. Our data (Fig. 3) demonstrate that intestinal epithelial cells show significantly increased resistance at 30 min of exposure, similar to previously published reports [21]. The rapidity of this response would imply that this is due to both calcium-mediated events and to S1P assembling and stabilizing of the adherens junction (and, therefore, the entire paracellular junctional complex), and the co-localization of these proteins to the cell–cell contact area has been shown to occur within 1 h of S1P stimulation [21, 44]. Despite this rapid response, and especially coupled with the relatively short half life in blood of S1P (under 1 h), it would be surprising that the effects of S1P last as long as they did in our experiments; however, our time course is entirely consistent with the observed effects seen on the pulmonary barrier [39], where a single injection of S1P results in persistent effects for up to 24 h. This suggests that S1P attenuates a long-term response via the activation or repression of transcriptional and translational pathways, and our data from Fig. 3 show a protective effect at the barrier that extends for several hours after initial exposure.
The results presented in Fig. 6 show that S1P attenuates the response of CaF media on paracellular permeability in intestinal epithelial cells. S1P has been shown to protect canine lungs from acute lung injury from various inflammatory insults [39], and further studies are underway to investigate the extent of the protective effect of S1P on the intestinal mucosa.
In summary, these results indicate that S1P has a barrier-protective effect on differentiated intestinal epithelial cells. S1P increases the levels and distribution of E-cadherin, which has been shown previously to play a major role in cellular adhesion and the cytoskeletal arrangement. This specific increase in E-cadherin leads to improved barrier integrity in both physiological conditions and, potentially, under pathological conditions.
Acknowledgments
This work was supported by a Research Career Development Award and a VA Merit Award (to D.J.T.) from the Department of Veterans Affairs.
Contributor Information
Jose Greenspon, Cell Biology Group, Department of Surgery, University of Maryland School of Medicine, Baltimore, MD, USA.
Ruiyun Li, Department of Surgery, Baltimore Veterans Affairs Medical Center, 10 N. Greene Street, Baltimore, MD 21201, USA. Cell Biology Group, Department of Surgery, University of Maryland School of Medicine, Baltimore, MD, USA.
Lan Xiao, Department of Surgery, Baltimore Veterans Affairs Medical Center, 10 N. Greene Street, Baltimore, MD 21201, USA. Cell Biology Group, Department of Surgery, University of Maryland School of Medicine, Baltimore, MD, USA.
Jaladanki N. Rao, Department of Surgery, Baltimore Veterans Affairs Medical Center, 10 N. Greene Street, Baltimore, MD 21201, USA. Cell Biology Group, Department of Surgery, University of Maryland School of Medicine, Baltimore, MD, USA
Rex Sun, Department of Gastroenterology, University of Maryland School of Medicine, Baltimore, MD, USA.
Eric D. Strauch, Cell Biology Group, Department of Surgery, University of Maryland School of Medicine, Baltimore, MD, USA
Terez Shea-Donohue, Department of Gastroenterology, University of Maryland School of Medicine, Baltimore, MD, USA.
Jian-Ying Wang, Department of Surgery, Baltimore Veterans Affairs Medical Center, 10 N. Greene Street, Baltimore, MD 21201, USA. Cell Biology Group, Department of Surgery, University of Maryland School of Medicine, Baltimore, MD, USA. Department of Pathology, University of Maryland School of Medicine, Baltimore, MD, USA.
Douglas J. Turner, Email: dturner@smail.umaryland.edu, Department of Surgery, Baltimore Veterans Affairs Medical Center, 10 N. Greene Street, Baltimore, MD 21201, USA. Cell Biology Group, Department of Surgery, University of Maryland School of Medicine, Baltimore, MD, USA
References
- 1.Carneiro-Filho BA, Lima IP, Araujo DH, et al. Intestinal barrier function and secretion in methotrexate-induced rat intestinal mucositis. Dig Dis Sci. 2004;49:65–72. doi: 10.1023/b:ddas.0000011604.45531.2c. [DOI] [PubMed] [Google Scholar]
- 2.Lima AA, Silva TM, Gifoni AM, et al. Mucosal injury and disruption of intestinal barrier function in HIV-infected individuals with and without diarrhea and cryptosporidiosis in northeast Brazil. Am J Gastroenterol. 1997;92:1861–1866. [PubMed] [Google Scholar]
- 3.Cereijido M, Shoshani L, Contreras RG. Molecular physiology and pathophysiology of tight junctions. I. Biogenesis of tight junctions and epithelial polarity. Am J Physiol Gastrointest Liver Physiol. 2000;279:G477–G482. doi: 10.1152/ajpgi.2000.279.3.G477. [DOI] [PubMed] [Google Scholar]
- 4.Collares-Buzato CB, McEwan GT, Jepson MA, et al. Paracellular barrier and junctional protein distribution depend on basolateral extracellular Ca2+ in cultured epithelia. Biochim Biophys Acta. 1994;1222:147–158. doi: 10.1016/0167-4889(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 5.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 6.Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- 7.Takeichi M. Cadherins: a molecular family important in selective cell–cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- 8.Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 9.Cereijido M, Valdés J, Shoshani L, et al. Role of tight junctions in establishing and maintaining cell polarity. Annu Rev Physiol. 1998;60:161–177. doi: 10.1146/annurev.physiol.60.1.161. [DOI] [PubMed] [Google Scholar]
- 10.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madara JL. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol. 1998;60:143–159. doi: 10.1146/annurev.physiol.60.1.143. [DOI] [PubMed] [Google Scholar]
- 12.Nusrat A, Parkos CA, Verkade P, et al. Tight junctions are membrane microdomains. J Cell Sci. 2000;113(Pt 10):1771–1781. doi: 10.1242/jcs.113.10.1771. [DOI] [PubMed] [Google Scholar]
- 13.Adams CL, Nelson WJ, Smith SJ. Quantitative analysis of cadherin–catenin–actin reorganization during development of cell–cell adhesion. J Cell Biol. 1996;135:1899–1911. doi: 10.1083/jcb.135.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angres B, Barth A, Nelson WJ. Mechanism for transition from initial to stable cell–cell adhesion: kinetic analysis of E-cadherin-mediated adhesion using a quantitative adhesion assay. J Cell Biol. 1996;134:549–557. doi: 10.1083/jcb.134.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanby AM, Chinery R, Poulsom R, et al. Downregulation of E-cadherin in the reparative epithelium of the human gastrointestinal tract. Am J Pathol. 1996;148:723–729. [PMC free article] [PubMed] [Google Scholar]
- 16.Shore EM, Nelson WJ. Biosynthesis of the cell adhesion molecule uvomorulin (E-cadherin) in Madin–Darby canine kidney epithelial cells. J Biol Chem. 1991;266:19672–19680. [PubMed] [Google Scholar]
- 17.Angst BD, Marcozzi C, Magee AI. The cadherin superfamily. J Cell Sci. 2001;114:625–626. doi: 10.1242/jcs.114.4.625. [DOI] [PubMed] [Google Scholar]
- 18.Rimm DL, Koslov ER, Kebriaei P, et al. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Troxell ML, Chen YT, Cobb N, et al. Cadherin function in junctional complex rearrangement and posttranslational control of cadherin expression. Am J Physiol. 1999;276:C404–C418. doi: 10.1152/ajpcell.1999.276.2.C404. [DOI] [PubMed] [Google Scholar]
- 20.Weiss EE, Kroemker M, Rüdiger AH, et al. Vinculin is part of the cadherin–catenin junctional complex: complex formation between alpha-catenin and vinculin. J Cell Biol. 1998;141:755–764. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dudek SM, Jacobson JR, Chiang ET, et al. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279:24692–24700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 22.English D, Kovala AT, Welch Z, et al. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. J Hematother Stem Cell Res. 1999;8:627–634. doi: 10.1089/152581699319795. [DOI] [PubMed] [Google Scholar]
- 23.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 24.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem. 2004;92:1075–1085. doi: 10.1002/jcb.20088. [DOI] [PubMed] [Google Scholar]
- 25.Siess W. Athero- and thrombogenic actions of lysophosphatidic acid and sphingosine-1-phosphate. Biochim Biophys Acta. 2002;1582:204–215. doi: 10.1016/s1388-1981(02)00173-7. [DOI] [PubMed] [Google Scholar]
- 26.Singleton PA, Dudek SM, Chiang ET, et al. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J. 2005;19:1646–1656. doi: 10.1096/fj.05-3928com. [DOI] [PubMed] [Google Scholar]
- 27.Singleton PA, Dudek SM, Ma SF, et al. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation: novel role for hyaluronan and cd44 receptor family. J Biol Chem. 2006;281:34381–34393. doi: 10.1074/jbc.M603680200. [DOI] [PubMed] [Google Scholar]
- 28.English D, Welch Z, Kovala AT, et al. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J. 2000;14:2255–2265. doi: 10.1096/fj.00-0134com. [DOI] [PubMed] [Google Scholar]
- 29.Nugent D, Xu Y. Sphingosine-1-phosphate: characterization of its inhibition of platelet aggregation. Platelets. 2000;11:226–232. doi: 10.1080/09537100050057675. [DOI] [PubMed] [Google Scholar]
- 30.Ryu Y, Takuwa N, Sugimoto N, et al. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ Res. 2002;90:325–332. doi: 10.1161/hh0302.104455. [DOI] [PubMed] [Google Scholar]
- 31.Schaphorst KL, Chiang E, Jacobs KN, et al. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am J Physiol Lung Cell Mol Physiol. 2003;285:L258–L267. doi: 10.1152/ajplung.00311.2002. [DOI] [PubMed] [Google Scholar]
- 32.Björklund S, Palmberg S, Rask S, et al. Effects of sphingosine 1-phosphate on calcium signaling, proliferation and S1P2 receptor expression in PC Cl3 rat thyroid cells. Mol Cell Endocrinol. 2005;231:65–74. doi: 10.1016/j.mce.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Chen PF, Chin TY, Chueh SH. Ca2+ signaling induced by sphingosylphosphorylcholine and sphingosine 1-phosphate via distinct mechanisms in rat glomerular mesangial cells. Kidney Int. 1998;54:1470–1483. doi: 10.1046/j.1523-1755.1998.00162.x. [DOI] [PubMed] [Google Scholar]
- 34.Dahm F, Nocito A, Bielawska A, et al. Distribution and dynamic changes of sphingolipids in blood in response to platelet activation. J Thromb Haemost. 2006;4:2704–2709. doi: 10.1111/j.1538-7836.2006.02241.x. [DOI] [PubMed] [Google Scholar]
- 35.Davaille J, Li L, Mallat A, et al. Sphingosine 1-phosphate triggers both apoptotic and survival signals for human hepatic myofibroblasts. J Biol Chem. 2002;277:37323–37330. doi: 10.1074/jbc.M202798200. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko T, Murakami T, Kawana H, et al. Sphingosine-1-phosphate receptor agonists suppress concanavalin A-induced hepatic injury in mice. Biochem Biophys Res Commun. 2006;345:85–92. doi: 10.1016/j.bbrc.2006.04.067. [DOI] [PubMed] [Google Scholar]
- 37.Karliner JS, Honbo N, Summers K, et al. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33:1713–1717. doi: 10.1006/jmcc.2001.1429. [DOI] [PubMed] [Google Scholar]
- 38.Laychock SG, Sessanna SM, Lin MH, et al. Sphingosine 1-phosphate affects cytokine-induced apoptosis in rat pancreatic islet beta-cells. Endocrinology. 2006;147:4705–4712. doi: 10.1210/en.2006-0456. [DOI] [PubMed] [Google Scholar]
- 39.Peng X, Hassoun PM, Sammani S, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 40.Finigan JH, Dudek SM, Singleton PA, et al. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 41.McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 2005;17:131–139. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Mehta D, Konstantoulaki M, Ahmmed GU, et al. Sphingosine 1-phosphate-induced mobilization of intracellular Ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J Biol Chem. 2005;280:17320–17328. doi: 10.1074/jbc.M411674200. [DOI] [PubMed] [Google Scholar]
- 43.Shikata Y, Birukov KG, Garcia JG. S1P induces FA remodeling in human pulmonary endothelial cells: role of Rac, GIT1, FAK, and paxillin. J Appl Physiol. 2003;94:1193–1203. doi: 10.1152/japplphysiol.00690.2002. [DOI] [PubMed] [Google Scholar]
- 44.Lee M-J, Thangada S, Claffey KP, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 45.Lee M-J, Van Brocklyn JR, Thangada S, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 46.Thamilselvan V, Li W, Sumpio BE, et al. Sphingosine-1-phosphate stimulates human Caco-2 intestinal epithelial proliferation via p38 activation and activates ERK by an independent mechanism. In Vitro Cell Dev Biol Anim. 2002;38:246–253. doi: 10.1290/1071-2690(2002)038<0246:SPSHCI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 47.Yatomi Y, Welch RJ, Igarashi Y. Distribution of sphingosine 1-phosphate, a bioactive sphingolipid, in rat tissues. FEBS Lett. 1997;404:173–174. doi: 10.1016/s0014-5793(97)00121-x. [DOI] [PubMed] [Google Scholar]
- 48.Kohno M, Momoi M, Oo ML, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol. 1996;16:619–625. doi: 10.1128/mcb.16.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo X, Rao JN, Liu L, et al. Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1159–G1169. doi: 10.1152/ajpgi.00407.2004. [DOI] [PubMed] [Google Scholar]
- 51.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 52.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 53.Vielkind U, Swierenga SH. A simple fixation procedure for immunofluorescent detection of different cytoskeletal components within the same cell. Histochemistry. 1989;91:81–88. doi: 10.1007/BF00501916. [DOI] [PubMed] [Google Scholar]
- 54.Guo X, Rao JN, Liu L, et al. Polyamines regulate beta-catenin tyrosine phosphorylation via Ca(2+) during intestinal epithelial cell migration. Am J Physiol Cell Physiol. 2002;283:C722–C734. doi: 10.1152/ajpcell.00054.2002. [DOI] [PubMed] [Google Scholar]
- 55.Wang JY, McCormack SA, Johnson LR. Role of nonmuscle myosin II in polyamine-dependent intestinal epithelial cell migration. Am J Physiol. 1996;270:G355–G362. doi: 10.1152/ajpgi.1996.270.2.G355. [DOI] [PubMed] [Google Scholar]
- 56.Rao JN, Li L, Golovina VA, et al. Ca2+-RhoA signaling pathway required for polyamine-dependent intestinal epithelial cell migration. Am J Physiol Cell Physiol. 2001;280:C993–C1007. doi: 10.1152/ajpcell.2001.280.4.C993. [DOI] [PubMed] [Google Scholar]
- 57.Wang JY, Wang J, Golovina VA, et al. Role of K(+) channel expression in polyamine-dependent intestinal epithelial cell migration. Am J Physiol Cell Physiol. 2000;278:C303–C314. doi: 10.1152/ajpcell.2000.278.2.C303. [DOI] [PubMed] [Google Scholar]
- 58.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 59.Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balda MS, Whitney JA, Flores C, et al. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical–basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu TS, Musch MW, Sugi K, et al. Protective role of HSP72 against Clostridium difficile toxin A-induced intestinal epithelial cell dysfunction. Am J Physiol Cell Physiol. 2003;284:C1073–C1082. doi: 10.1152/ajpcell.00134.2002. [DOI] [PubMed] [Google Scholar]
- 62.Guo X, Rao JN, Liu L, et al. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. Am J Physiol Cell Physiol. 2003;285:C1174–C1187. doi: 10.1152/ajpcell.00015.2003. [DOI] [PubMed] [Google Scholar]
- 63.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 64.Liu Y, Wada R, Yamashita T, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Investig. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greenspon J, Li R, Xiao L, et al. Sphingosine-1-phosphate protects intestinal epithelial cells from apoptosis through the Akt signaling pathway. Dig Dis Sci. 2008;54:499–510. doi: 10.1007/s10620-008-0393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corada M, Liao F, Lindgren M, et al. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–1684. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- 67.Corada M, Mariotti M, Thurston G, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Z, Zhang H, Kumar R. Regulation of E-cadherin. Breast Cancer Online. 2005;8:e15. [Google Scholar]