Abstract
Background
Purified microglia cultures are useful tools to study microglial behavior in vitro. Microglial cell lines serve as an attractive alternative to primary microglia culture, circumventing the costly and lengthy preparation of the latter. However, immortalization by genetic or pharmacologic manipulations may show altered physiology from primary microglia.
New Method
A novel microglial cell line was isolated from a primary glial culture of postnatal murine cerebral cortices. The culture contained a population of spontaneously transformed microglia that continued to divide without genetic or pharmacological manipulations. After several clones were isolated, one particular clone, SIM-A9, was analyzed for its microglial characteristics.
Results
SIM-A9 cells expressed macrophage/microglia-specific proteins, CD68 and Iba1. SIM-A9 cells were responsive to exogenous inflammatory stimulation with lipopolysaccharide and β-amyloid, triggering tyrosine kinase-based and NFκB signaling cascades as well as TNFα secretion. SIM-A9 cells also exhibited phagocytic uptake of fluorescent labeled β-amyloid and bacterial bioparticles. Furthermore, lipopolysaccharide increased the levels of inducible nitric oxide synthase and cyclooxygenase-2, whereas IL-4 stimulation increased arginase-1 levels demonstrating that SIM-A9 cells are capable of switching their profiles to pro- or anti-inflammatory phenotypes, respectively.
Comparison with Existing Methods
The use of SIM-A9 cells avoids expensive and lengthy procedures required for the preparation of primary microglia. Spontaneously immortalized SIM-A9 cells are expected to behave more comparably to primary microglia than virally transformed or pharmacologically induced microglial cell lines.
Conclusions
SIM-A9 cells exhibit key characteristics of cultured primary microglia and may serve as a valuable model system for the investigation of microglial behavior in vitro.
Keywords: Microglia, inflammation, lipopolysaccharide, beta-amyloid, phagocytosis, M1/M2 phenotype
1. INTRODUCTION
Microglia are the resident immune cells of the nervous system that continuously survey their local environment and become activated upon detection of abnormal and/or offensive signals to initiate inflammatory processes. Activated microglia have been described in a number of neurological conditions such as traumatic brain/spinal cord injury, ischemic strokes, infections, and neuroinflammatory diseases. Although microglial activation has been classically associated with inflammation and therefore implicated in pathogenesis of chronic neurodegenerative diseases such as Parkinson’s and Alzheimer’s diseases (McGeer and McGeer, 1995), newer evidence also supports a role of differentially activated microglia in regenerative processes with the expression of a distinct set of anti-inflammatory molecular markers (Chhor et al., 2013). Thus, understanding the mechanisms regulating specific phenotypes of microglia is of great interest to the field of neuroinflammatory diseases.
Purified microglia cultures are frequently used for the characterization of stimulus-triggered changes in their behavior. This in vitro model system provides a versatile tool, allowing direct application of exogenous stimulating or inhibiting agents to microglia, collection of secreted factors, and observation of microglial activities such as migration, proliferation and phagocytosis. In our laboratory, primary microglial cultures are routinely isolated from mixed cultures of neonatal mouse cerebral cortices and utilized to investigate responses to inflammatory stimuli as well as efficacy of anti-inflammatory agents (Dhawan et al., 2012; Floden et al., 2005; Nagamoto-Combs and Combs, 2010; Rojanathammanee et al., 2013; Sondag et al., 2009; Woster and Combs, 2007). However, isolation of primary microglia cultures requires a cumbersome procedure involving dissociation of brain tissue and 14 days of pre-culturing as a mixed cell population (Floden et al., 2005). Furthermore, the preparation method does not typically result in a large number of purified microglia thus requiring a substantial amount of brain tissue to obtain a sufficient number of microglia needed for an experiment.
In order to overcome the disadvantages of microglial culture, several research groups have created a few cell lines by transforming primary microglia with viral vectors (Blasi et al., 1990; Briers et al., 1994; Peudenier et al., 1991; Righi et al., 1989) or other genetic (Ohsawa et al., 1997) or pharmacological (Kanzawa et al., 2000) inductions. However, some issues with the long-term retention of primary microglia properties in these transformed cell lines have been reported (Ohsawa et al., 1997). Non-induced cell lines have also been isolated from spontaneously immortalized primary microglia from a mouse cerebellar organ culture (Alliot et al., 1996) and rat cerebral tissue culture (Cheepsunthorn et al., 2001). The novel microglial cell line described in this report is a cell line in this category, and has been isolated from a mixed glial culture of postnatal murine cerebral cortices that continued to proliferate for a number of passages without any genetic or pharmacologic manipulations. To our knowledge, this is the first spontaneously immortalized microglial cell line cloned from mouse cerebral tissue. In order to test whether our microglial cell line is a suitable alternative to the use of primary microglia culture, we have determined its phenotypic and functional properties that are characteristics of cultured primary microglia at rest as well as in response to exogenous proinflammatory stimuli.
2. MATERIALS AND METHODS
2.1. Materials
Dulbecco’s Modified Eagle Medium: Nutrient Mixture F12 (DMEM/F12) was purchased from Life Technologies, Corporation (Carlsbad, CA, USA). Mouse TNF-α ELISA kit was obtained from R&D Systems (Minneapolis, MN, USA). Lactate dehydrogenase assay (LDH) and Griess assay were purchased from Promega (Madison, WI, USA).
Primary antibodies against inducible nitric oxide synthase (iNOS; NOS2 [C-11])), cyclooxygenase-2 (COX-2, [N-20]), arginase I (Arg-I), α-tubulin, and horseradish peroxidase conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-β-amyloid antibody was obtained from Covance (Emeryville, CA, USA). Anti-TNFα antibody was from Abcam (Cambridge, MA, USA) and anti-CD68 antibody was purchased from Serotec (Raleigh, NC, USA). The antibodies for phospho-IκB, IκB, and glial fibrillary acidic protein (GFAP) were acquired from Cell Signaling Technology (Danvers, MA, USA). Anti-phospho-tyrosine (4G10) antibody was from EMD Millipore (Billerica, MA, USA), and anti-Iba1 antibody was from Wako Chemicals USA, Inc (Richmond, VA, USA). All the biotinylated secondary antibodies, Elite Vectastain ABC Kit, VIP Peroxidase Substrate Kit were obtained from Vector Laboratories, Inc. (Burlingame, CA, USA). Anti-microtubule associated protein 2 (MAP2), lipopolysaccharide (LPS) and other chemicals were obtained from Sigma (St. Louis, MO, USA).
2.2. Animals
The use of mice was approved by the University of North Dakota (UND) Institutional Animal Care and Use Committee (IACUC). The C57BL/6 strain of mice were housed in a room with 12-hr light/dark cycle and food and water were provided ad libitum in accordance with the National Research Council of the National Academies Guide for the Care and Use of Laboratory Animals (8th edition). Mice were bred in the UND animal facility, and newborn pups were housed in the same cage with their mother until sacrificed for tissue culture preparation.
2.3. Tissue culture
Mixed glial cultures were prepared as previously described (Floden et al., 2005). Briefly, cortical tissues were collected from mouse pups at postnatal day 1 (P1). The tissues were pooled and trypsinized after the removal of meninges, then the dissociated cells were plated in DMEM/F12 supplemented with L-glutamine (EMD Millipore; Billerica, MA, USA), 10% heat-inactivated fetal bovine serum and 5% heat-inactivated horse serum (Serum Source International., Charlotte, NC, USA) in a 75-mm culture flask. The cells were fed every 3 days for 14 days and microglia were harvested by vigorously shaking the flask at 120 rpm on a rotary shaker for 30 min. The detached microglia were resuspended in DMEM/F12 serum containing media, plated in a 6-well culture dish. Typically, purified microglia are used (i.e., treated, harvested or fixed) within 48 hours of plating. At one particular occasion, however, the cells were maintained for an additional 2 weeks. At this time, we unexpectedly observed extensive proliferation of the plated microglia. The cells were gently detached from the dish with phosphate buffered saline (PBS) containing 1 mM EDTA, 1 mM EGTA and 1 mg/ml glucose, and replated to test whether they had the ability to further propagate. The cells were passaged a total of 7 times over the course of an additional 4 weeks, each time aggressively proliferating to confluency. The cells were determined to have become spontaneously immortalized, and clonal populations of these cells were established. The cell line has been disclosed to the University of North Dakota Intellectual Property Commercialization and Economic Development Office, which can be contacted for material transfer agreements.
2.4. Limiting dilution
In a 96-well plate, the spontaneously immortalized microglia (SIM) cells were plated at approximately 5,000 cells/100 μL DMEM/F12 in each well of the first column. Ten μL of the cell suspension in the wells in this column was then plated in the wells of the next column containing 100 μL of the medium. This procedure was repeated across the plate (total of 12 columns) to establish a series of 1:10 dilutions. Upon completion of dilutions, the number of cells in each well was counted. Only a few of the wells contained a single cell at the time of plating, and this observation was confirmed by the presence of one colony formation in each of the wells 3–6 days later. These clonal cells were cultured individually and allowed to expand in DMEM/F12 serum containing medium. Aliquots of each clone were cryopreserved for future use. From the eleven resulting clones, a clone designated as “A9” was arbitrarily chosen in order to characterize its microglial properties.
2.5. Cell stimulation
SIM cells were detached from the culture dish, pelleted and resuspended in serum-free DMEM/F12 without or with 2.5 ng/mL LPS or 20 μM β-amyloid fibrils (Aβ1-42) (rPeptide, Athens, GA, USA). The cells were incubated at 37 °C for specified periods. For the enzyme linked-immuno-sorbent assay (ELISA), cells were stimulated in parallel with control, LPS, or Aβ for 24 hours at the density of 20,000 cells/well in 96-well plates, each containing 75 μL serum-free DMEM/F12 with or without the stimulus. To prepare cell lysates for western blot analyses, cells were plated in 35 mm-culture dishes, and treated in parallel with or without activating stimuli for 5 min to detect protein phosphorylation levels or for 24 hours to examine specific protein levels.
2.6. Nitrite assay
After a 24-hour incubation period, the culture medium from each well was collected to determine the amount of nitric oxide produced by the SIM-A9 cells. The collected media were incubated with Griess reagent according to the manufacturer’s instructions (Promega) in a 96-well plate at room temperature. The resulting nitrite, a stable derivative of nitric oxide, was colorimetrically quantified with a plate reader at 546 nm.
2.7. Bioparticle/Aβ1-42 uptake assays
Phagocytic activity of SIM-A9 cells was determined by quantifying uptake of Aβ1-42 or E. coli-derived (K-12 strain) bioparticles as described previously (Floden and Combs, 2006). In brief, SIM-A9 cells were incubated for 6 hours with 1 μM FITC-Aβ1-42 (rPeptide, Athens, GA, USA) or 0.125 mg/mL FITC-labeled E. coli bioparticles (Life Technologies, Carlsbad, CA, USA). After the incubation period, cells were rinsed with PBS containing 0.25 mg/mL trypan blue in order to quench extracellular fluorescent Aβ or bioparticles. Internalized FITC signals were quantified (480 nm excitation/520 nm emission) using a fluorescence plate reader (Bio-Tek, Winooski, VT, USA).
2.8. ELISA
All cells were stimulated in parallel with either Aβ peptides or LPS for stimulations. After 24 hours of stimulation, the medium was collected from each well and the levels of TNFα released into the media were quantified using ELISA analysis according to the manufacturer’s protocol (R&D Systems).
2.9. Western blot analysis
Following stimulation, cells were collected in RIPA buffer (20mM Tris, pH 7.4, 150mM NaCl, 1mM Na3VO4 10mM NaF, 1mM EDTA, 1mM EGTA, 0.2mM phenylmethylsulfonyl fluoride, 1% Triton X-100, 0.1% SDS, and 0.5% deoxycholate) and lysed by briefly sonicating the cell suspension. Cytosolic extracts were collected by centrifugation, and 5–10 μg of the lysates were used for SDS-PAGE. Resolved proteins were transferred to PVDF membranes and incubated in Tris-buffered saline with 0.1% TritonX-100 (TBS-T) containing 3% bovine serum albumin (BSA) for 1 hour to block background signals. The membranes were further incubated in a primary antibody diluted in the same buffer at 4°C for 12–18 hours with gentle rocking. The antibodies were used at the following dilutions: anti-phospho-tyrosine (1:1,000); anti-phospho-IκB (1:1,000); anti-IκB (1:1,000); anti-iNOS (1:1,000), anti-COX-2 (1:1,000); anti-Arg-1 (1:500); anti-α-tubulin (1:20,000). The membranes were rinsed in TBS-T and incubated in HRP-conjugated secondary antibodies (1:5,000 dilution in TBS-T containing 5% skim milk) for an hour prior to chemiluminescence detection using Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare, Pittsburgh, PA, USA). Resulting signals were detected and digitally captured using the UVP BioSpectrum® Imaging System (UVP, LLC. Upland, CA, USA). The documented western blot results were quantified using LI-COR Image Studio Lite Western Blot Software (LI-COR Biosciences, Lincoln, NE, USA). For qualitative presentation purposes, digital images were composed using Adobe Photoshop software (Adobe Systems, Inc., San Jose, CA, USA). When multiple protein detections were required from a set of samples, the PVDF membranes were stripped in 0.2 N NaOH for 5 min after the initial chemiluminescence detection, and rinsed briefly in water before being incubated in TBS-T with BSA for blocking.
2.10. Immunocytochemistry
SIM-A9 cells were plated onto poly-L-lysine coated glass coverslips then fixed with 4% paraformaldehyde for 15 min at room temperature and rinsed with PBS. The cells were blocked in PBS containing 5% normal goat serum, 0.5% BSA, and TritonX-100 for 1 hour at room temperature, and incubated in primary antibodies [anti-CD68 (1:1000); anti-Iba1 (1:250); anti-GFAP (1:1000); anti-MAP2 (1:1000)] diluted into the same buffer at 4°C for 12–18 hours, followed by a brief rinsing and a one-hour incubation with biotinylated secondary antibodies diluted into the same buffer. For enhanced signal visualization, the VECTASTAIN Elite ABC Kit was used with VIP substrate as a chromogen (Vector Labs, Burlingame, CA, USA). Immunostained cells were observed and representative images were photographed using the same light intensity across conditions for each antibody using an Olympus BX60 microscope equipped with a SPOT RT Slider camera and software (Diagnostics Instruments, Inc., Sterling Heights, MI, USA). The captured photomicrographs were labeled using Adobe Photoshop software (Adobe Systems, San Jose, CA, USA).
2.11. Statistical analysis
Sample values were averaged from three independent experimental conditions and are shown as mean ± standard error of the mean (SEM). Statistical significances between the experimental conditions were analyzed using the Student’s t-test or, when appropriate, One-way ANOVA with the associated p values determined using the Turkey-Kramer multiple comparisons post-test.
3. RESULTS
3.1. SIM-A9 cells expressed microglia/macrophage-specific proteins and exhibited microglia-like variations in morphology
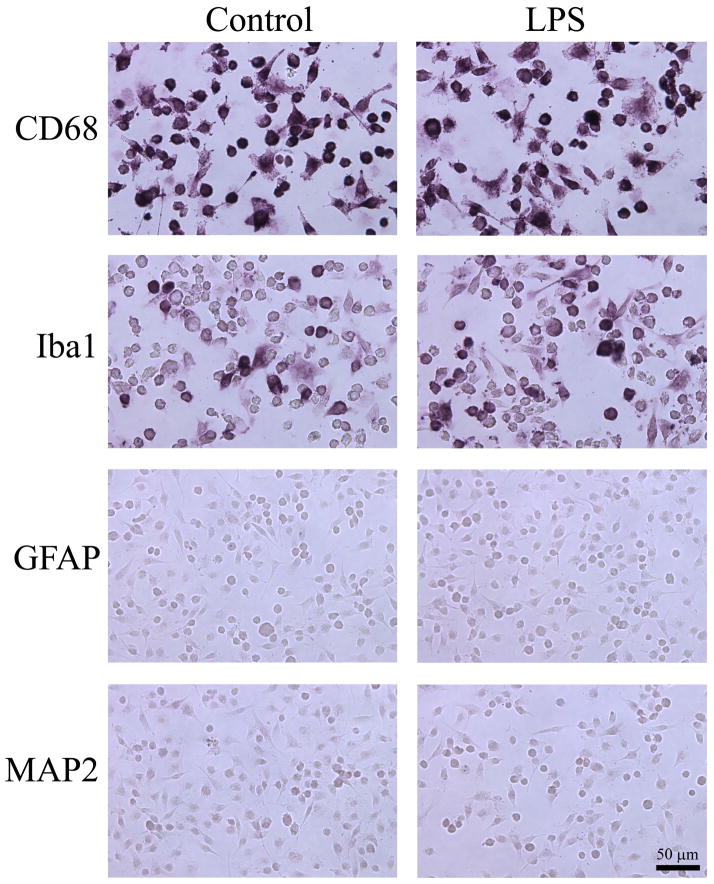
After the expansion of clonal SIM-A9 cells, the expression of microglia-specific proteins, CD68 and Iba1, were examined in order to determine whether the cells had retained microglial properties. Immunocytochemical staining showed that SIM-A9 cells were strongly immunoreactive to CD68 with essentially all cells stained regardless of LPS stimulation (Fig. 1). Similarly, SIM-A9 cells were immunoreactive to Iba1, although a subpopulation of the cells remained unstained without or with LPS (Fig. 1). On the contrary, SIM-A9 cells did not show immunoreactivity to either GFAP or MAP2, indicating that they did not express astrocyte- or neuron-specific proteins, respectively (Fig. 1). Taken together, SIM-A9 cells exhibited a microglia phenotype consistent with their expression of microglia/macrophage-specific proteins, CD68 and Iba1.
Fig. 1. Photomicrographs of SIM-A9 cells expressing microglia/macrophage-specific proteins.
SIM-A9 cells were treated without (Control; left panels) or with 2.5 ng/mL LPS (LPS; right panels) for 24 hours prior to immunostaining for microglial markers, CD68 or Iba1, as well as for astrocyte and neuronal marker proteins, GFAP and MAP2, respectively. Scale bar = 50 μm.
We also observed that SIM-A9 displayed different morphologies in culture, with or without multiple processes. Some of the cell shapes are highlighted with CD68-stained untreated SIM-A9 cells in Fig. 2. Round cells without obvious processes were often found to appear darker with CD68 staining, while bipolar or polygonal cells with multiple processes had flattened appearances and stained more faintly. The distal ends of the processes often fanned out to form lamellipodia (Fig. 2, arrowheads), suggesting that these cells are mobile. Since these variations in morphology are commonly observed in cultured microglia depending on their activation states and culture conditions (Giulian and Baker, 1986), SIM-A9 cells appeared to behave like their primary counterpart. After 2.5 ng/mL of LPS treatment for 24 hours in serum-free DMEM/F12, SIM-A9 cells did not display obvious changes in their morphology and remained relatively heterogeneous as described above (Fig. 1, right column).
Fig. 2. High magnification photomicrographs of SIM-A9 cells representing multiple microglia-like morphologies.
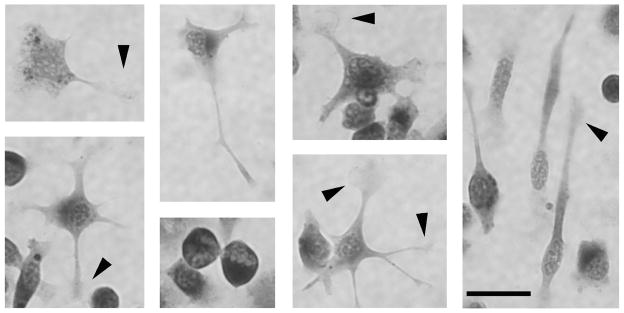
Untreated, CD68-immunostained SIM-A9 cells were photographed at a higher magnification to illustrate various morphologies resembling cultured primary microglia. Arrowheads point to lamellipodia at distal ends of microglial processes. Scale bar = 25 μm.
After examining their expression of characteristic microglial marker proteins, we next tested SIM-A9 cell function by examining their ability to secrete cytokines and nitric oxide as well as to phagocytose biological debris. Furthermore, stimulation of SIM-A9 cells into either a pro- or anti-inflammatory phenotype was also investigated by quantifying expression of phenotype-selective proteins.
3.2. SIM-A9 cells released TNFα upon stimulation with LPS or Aβ1-42
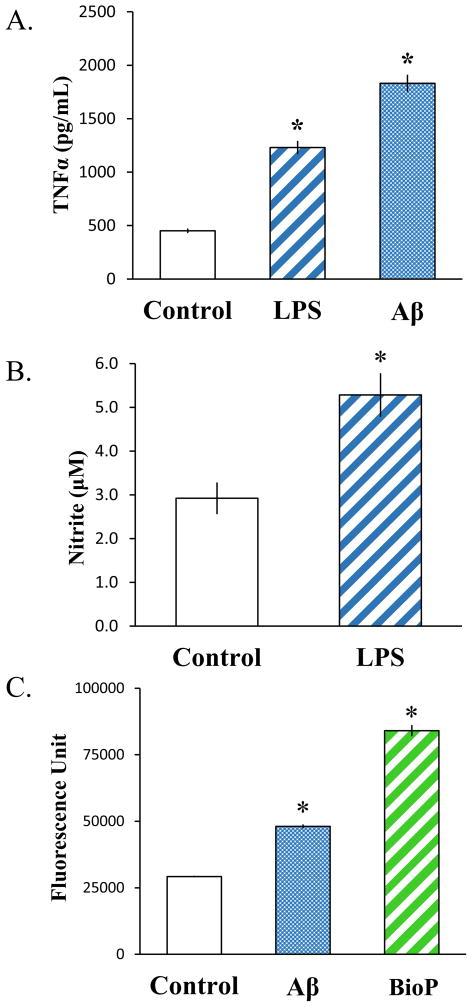
Microglia are known to produce and release cytokines when challenged with inflammatory stimuli. TNFα is one of the major cytokines secreted from microglia upon activation, and LPS and Aβ are well-characterized stimulators of TNFα production and release (Dhawan et al., 2012; Lee et al., 1993; Nagai et al., 2001). Thus, we tested whether SIM-A9 cells responded to these stimulatory agents by increasing TNFα secretion. SIM-A9 cells were incubated for 24 hours without or with 2.5 ng/mL LPS or 10 μM Aβ1-42, and their culture media were collected to quantify TNFα released using ELISA. After 24 hours in serum-free DMEM/F12, detectable amounts of TNFα were present in the culture media without any stimulation (Figure 3A, Control). This basal level of TNFα was significantly increased byapproximately 3- or 4- fold (p<0.001) following stimulation with LPS or Aβ1-42, respectively. This result indicated that SIM-A9 cells increase TNFα secretion in response to inflammatory stimuli similar to cultured primary microglia.
Fig. 3. Quantitative analyses of SIM-A9 immune responses.
The induced activities of SIM-A9 cells after a 24-hour stimulation without (Control) or with exogenous inflammatory stimuli (2.5 ng/mL LPS or 10 μM Aβ1-42) were examined by quantifying TNFα release (A) and nitricoxide production (B). The phagocytic activity of SIM-A9 cells were also measured by the uptake of FITC-labeled Aβ or E. coli bioparticles after a 6-hour incubation (C). Asterisks (*) indicate statistical significance with a p value <0.001 when compared to unstimulated SIM-A9 cells.
3.3. LPS or Aβ1-42 stimulated increased production of nitric oxide by SIM-A9 cells
Activation of microglia with inflammatory stimuli triggers increased expression of iNOS, resulting in elevated nitric oxide production and release (Chao et al., 1992; Zielasek et al., 1992). We therefore determined the ability of SIM-A9 cells to respond to stimuli by producing this signaling molecule. The amount of gaseous nitric oxide produced by SIM-A9 cells was correlated with the amount of its stable intermediate, nitrite that accumulated in the culture media. Stimulation of SIM-A9 cells with 2.5 ng/mL LPS for 24 hours modestly increased nitrite levels in the media (Fig. 3B). Although the magnitude of increase was relatively small, slightly less than two fold, the effect of LPS stimulation on nitrite accumulation was statistically significant (p<0.001). This result indicated that SIM-A9 cells, like primary microglia, increase nitric oxide production upon LPS challenge.
3.4. SIM-A9 cells displayed phagocytic activity for Aβ1-42 or E. coli bioparticles
Similar to macrophages and other peripheral leukocytes, microglia have the ability to phagocytose biological debris after injury or infections in order to remove damaged cells or pathogens. The ability of SIM-A9 cells to perform this important homeostatic function of microglia was examined using FITC-conjugated Aβ1-42 or E. coli-derived bioparticles. After a 6 hour incubation-period, the presence of intracellular fluorescent signal was detected in the SIM-A9 incubated with the labeled Aβ1-42 or E. coli bioparticles (Fig. 3C). The amount of internalized fluorescence was approximately 1.5 or 3 times greater than the background when the cells were incubated with FITC-Aβ1-42 or FITC-E. coli bioparticles, respectively (p<0.001). This result demonstrated that SIM-A9 cells possess phagocytic competency and are capable of engulfing biological debris as do microglia and macrophages.
3.5. SIM-A9 expressed M1/M2 phenotype-associated proteins following specific stimulations
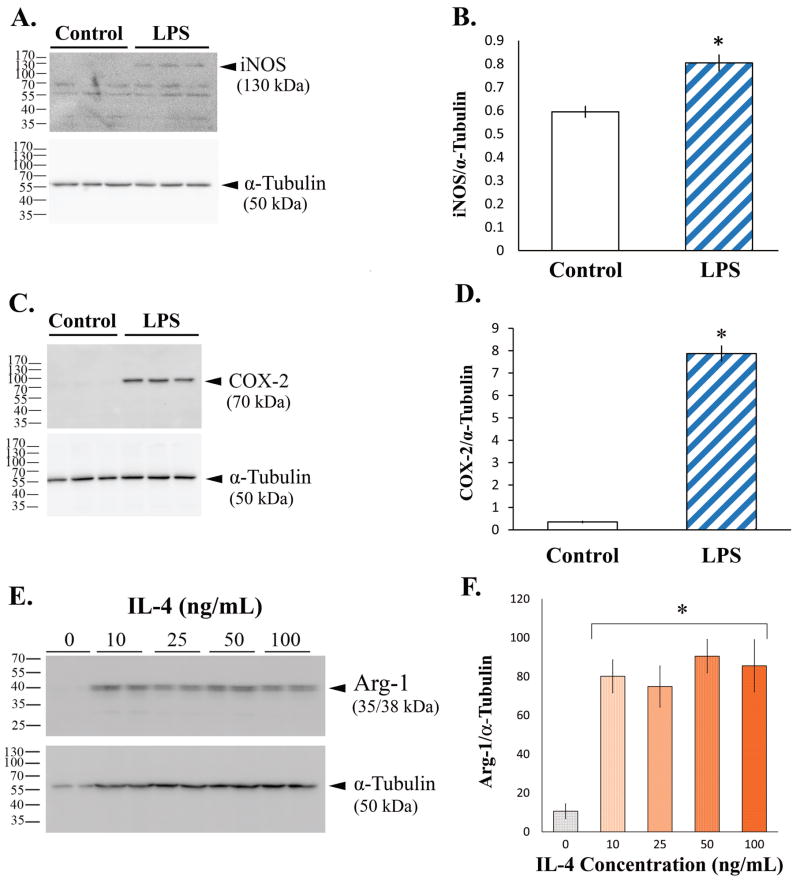
Diverse factors differentially activate microglia to elicit either proinflammatory/cytotoxic (M1) or anti-inflammatory/regenerative (M2) phenotypes. For example, LPS promotes the M1 phenotype in microglia, inducing the expression of iNOS and COX-2, while IL-4 promotes the M2 phenotype and triggers expression of Arg-1 (Chhor et al., 2013; Michelucci et al., 2009). Therefore, we examined whether LPS and IL-4 correspondingly induced M1 and M2 phenotypes in SIM-A9 cells. Changes in the levels of iNOS and COX-2 for the M1, and Arg-1 for the M2 phenotype, were determined using western blot analysis. In the absence of a stimulus, the levels of iNOS (Fig. 4, A & B) and COX-2 (Fig. 4, C & D) in SIM-A9 cells were virtually undetectable. However, the levels of both iNOS and COX-2 proteins were increased after incubation with LPS for 24 hours, as indicated by the presence of the immunoreactive bands at approximately 130 kDa and 70 kDa, respectively. Densitometric quantitation indicated that the LPS-mediated increase in iNOS was about 3-fold (Fig. 4B; n=3; p<0.01), while that of COX-2 was over 20-fold and more pronounced (Fig. 4D; n=3; p<0.001). Similar to iNOS and COX-2, very little Arg-1expression was observed in untreated SIM-A9 cells, but the level of Arg-1 was appreciably elevated by nearly 8-fold after a stimulation with 10 ng/mL IL-4 for 24 hours (Fig. 4, E & F; n=3; p<0.001). The amount of Arg-1 induced by this concentration of IL-4 was comparable to the induction by 100 ng/mL IL-4, indicating that the lower concentration of the cytokine already maximally increased Arg-1 levels (Fig. 4F). These data demonstrated that pro-/anti-inflammatory phenotypes can be induced in SIM-A9 by the same factors that shift microglial M1/M2 phenotypes.
Fig. 4. Western blot images and quantitation of M1/M2 phenotype-specific proteins in SIM-A9 cells.
The induction of M1 phenotype in SIM-A9 cells was examined by assessing the levels of iNOS (A & B) and COX-2 (C & D) after a 24-hour incubation without (Control) or with 2.5 ng/mL LPS, while the induction of M2 phenotype was determined by assessing the Arg-1 levels (E & F) after a 24-hour treatment without (Control) or with various concentrations of IL-4. Panels A, C and E show chemiluminescent detection of western blots, and graphs B, D and F depict quantitative values from the corresponding blots. Asterisks (*) indicate statistical significance with a p value <0.001 when compared to unstimulated SIM-A9 cells.
3.6. LPS or Aβ1-42 activated SIM-A9 via tyrosine kinase-based and NFκB signaling pathways
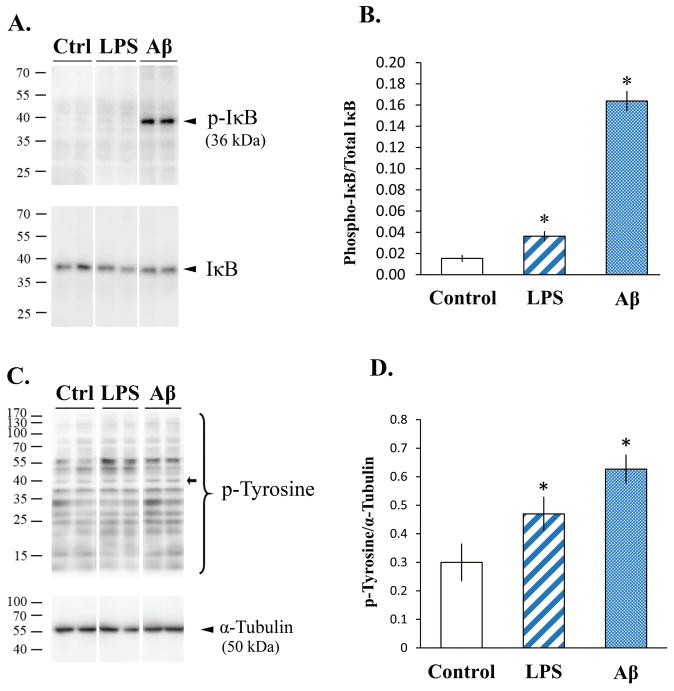
The microglial proinflammatory response to LPS is triggered by the association of LPS with Toll-like receptor 4 (TLR4), resulting in the activation of an NFκB signaling cascade (Fitzgerald et al., 2004). In order to validate that this LPS-mediated signaling pathway was also activated in SIM-A9 cells, IκB phosphorylation was examined in SIM-A9 cell lysates without or with LPS treatment for different time periods. Western blot analysis showed that minimal levels of phosphorylated IκB were present in untreated SIM-A9 cell lysates (Fig. 5, A & B). With LPS treatment (25 ng/mL), a 35-kDa band corresponding to phosphorylated IκB was visibly detectable after 5 minutes of stimulation, although it did not quite reach the level of statistical significance compared to unstimulated control. However, treatment of SIM-A9 cells with Aβ1-42 markedly increased IκB phosphorylation and resulted in a 10.6-fold induction (n=3; p<0.001) indicating that Aβ1-42 significantly activated the NFκB signaling pathway in SIM-A9 cells. Since activation of microglia by Aβ is also associated with increased tyrosine kinase activity (Combs et al., 1999; McDonald et al., 1997), we further examined tyrosine phosphorylation in SIM-A9 cells treated with Aβ1-42 and LPS (Fig. 5, C & D). Immunodetection of phosphorylated tyrosine residues showed a number of bands in the lysates of control and treated SIM-A9 cells (Fig. 5C). For quantitation purposes, we arbitrarily chose a band near the 40 kDa mark since proteins around this molecular weight have been reported to exhibit increased tyrosine phosphorylation with inflammatory stimulations (Fig. 5D, open arrow; Combs et al, 1999). Quantitation across the LPS- and Aβ-treated samples, respectively, showed 1.6- and 2.1-fold increases in the density of this band when compared to the control samples (n=3 per condition; p<0.05). This result suggested that increased tyrosine kinase-mediated signaling occurred after LPS or Aβ1-42 stimulation.
Fig. 5. Western blot images and quantitation of phospho-IκB (p-IκB) and phospho-tyrosine (p-Tyrosine) in SIM-A9 cells after LPS or Aβ stimulation.
SIM-A9 cells were incubated without (Ctrl or Control) or with 25 ng/mL LPS or 10 μM Aβ1-42 for 5 min and the levels of phosphorylated IκB (A & B) and tyrosine residues (C & D) were examined by western blot analysis. Panels A and C show chemiluminescent detection of western blots, and graphs B, and D depict quantitative values from the corresponding blots. Asterisks (*) indicate statistical significance with a p value <0.001 when compared to unstimulated SIM-A9 cells.
3.7 The microglial proinflammatory phenotype was preserved in SIM-A9 cells after a number of passages
In order to better determine the similarity of SIM-A9 cells to primary microglia, select phenotypic and behavioral features of the two cell types were directly compared. Furthermore, SIM-A9 cells that had undergone multiple passages were also analyzed in parallel since some cell lines may lose their original characteristics during extended culture passaging. The overall appearance of the SIM-A9 did not appreciably change after 40 passages (P40) from an earlier lot of cells (P6), with both exhibiting a variety of morphologies as described earlier (Fig. 6). Cultured primary microglia at 2 weeks in vitro mainly showed flattened morphologies. Elongated cells with two or more processes were observed in the cultures of all cell types. As shown in the SIM-A9 cells depicted in Fig. 1, cultured primary microglia demonstrated strong immunoreactivity to CD68 and, to a lesser extent, Iba1. The ability of SIM-A9 to express these microglia-specific proteins was retained at P40 although the staining intensity appeared somewhat diminished compared to P6 cells. Heterogeneity in immunostaining intensity was observed in all cell types (Fig. 6).
Fig. 6. Photomicrographs comparing the expression of microglial markers in primary microglia and SIM-A9 cells at different passages.
Primary microglia (left column), SIM-A9 after 6 passages (P6; middle column) or after 40 passages (P40; right column) were treated without (Control) or with 2.5 ng/mL LPS (LPS) for 24 hours prior to immunostaining for microglial markers, CD68 or Iba1. Scale bar = 100 μm.
Next, we evaluated TNFα secretion and phagocytosis to compare the abilities of microglia and SIM-A9 cells to respond to proinflammatory stimuli. In responses to LPS or Aβ, primary microglia elicited robust 40- and 13-fold increases, respectively, in TNFα secretion compared to controls (Fig. 7A). These stimuli also produced increases in cytokine secretion from the SIM-A9 cells at P6 but to a smaller degree of approximately 2–2.5 fold (Fig. 7B). At P40, the LPS-mediated TNFα secretion was slightly less than P6 (1.7-fold) while the Aβ-triggered cytokine secretion was 3.2-fold and remained comparable to P6 (Fig. 7C). Similarly, SIM-A9 cells at both P6 and P40 demonstrated phagocytic competency that paralleled primary microglia despite a small decrease in the amount of bioparticle uptake at P40 (Fig. 7D). These results indicated that fundamental immune responses of primary microglia to proinflammatory stimuli were preserved in SIM-A9 even after multiple passages, although the degree of responses may decline over time.
Fig. 7. Quantitative comparisons of TNFα release and phagocytic activities of primary microglia and SIM-A9 cells after different passages.
Primary microglia (A), SIM-A9 after 6 passages (B) or after 40 passages (C) were treated without (Control), with 2.5 ng/mL LPS (LPS), or 10 μM Aβ1-42 for 24 hours. The amounts of TNFα released in the culture media were quantified by ELISA. (D) The phagocytic activities of primary microglia and SIM-A9 at P6 and P40 were also measured by the uptake of FITC-labeled E. coli bioparticles after a 6-hour incubation. *p <0.01, ** p <0.001, *** p <0.0001 when compared to unstimulated SIM-A9 cells.
For additional comparison, the expression of inducible M1/M2 type differentiation molecules were also detected using western blot analysis in microglia and SIM-A9 cells at P6 and P40 after LPS or IL-4 treatment. The expression of COX-2, a proinflammatory mediator, was effectively increased by LPS in primary microglia as well as in SIM-A9 cells both at P6 and P40, indicating that this proinflammatory response was well maintained after multiple passages (Fig. 8). Interestingly, the IL-4-induced expression of Arg-1, typically used as a marker of an anti-inflammatory phenotype in microglia, was substantially reduced at P40. These results suggested that extended culturing of SIM-A9 cells might lead to a preferential selection of cells with a skewing towards primarily proinflammatory, M1-type activation.
Fig. 8. Western blot images of M1/M2 phenotype-specific proteins in primary microglia and SIM-A9 cells at different passages.
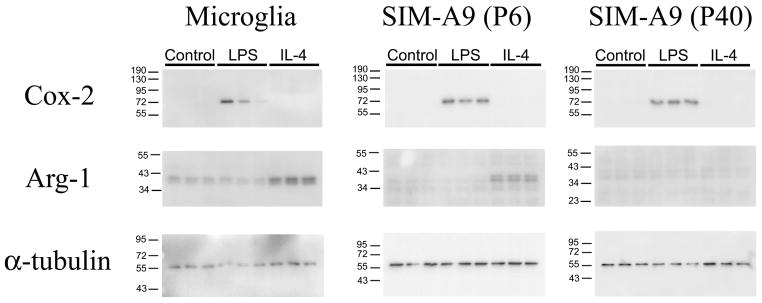
The induction of COX-2 and Arg-1 were evaluated in primary microglia and SIM-A9 cells at P6 and P40 after 24-hour incubation without (Control) or with 2.5 ng/mL LPS or with IL-4 (10 ng/mL) using western blot chemiluminescent detection.
4. DISCUSSION
We have isolated a clonal cell line, SIM-A9, from a population of cells that continuously proliferated in primary microglia culture. We report the phenotypic and functional property characterization of this novel, spontaneously immortalized cell line in order to assess its utility as an alternative tool for primary microglia cultures.
SIM-A9 cells aggressively propagated in serum-containing DMEM/F12. It has been reported that some commercially available animal sera were potentially contaminated with LPS, which influences the ability of microglia to proliferate (Ganter et al., 1992; Gebicke-Haerter et al., 1989). However, this is an unlikely reason for the spontaneous transformation of the SIM cells since numerous microglial cultures from many different animals were all grown using not only the same bottles of serum but also from additional bottles of serum from the same lot from the manufacturer. Thus, we concluded that our observation of the spontaneous microglia transformation which led to the SIM cells was an isolated event. We also continued to use the same culture media to propagate purified SIM cells without detecting any inhibition of proliferation.
We have not attempted to reproduce the immortalization phenomenon as we believe that it was a serendipitous event of uncertain causes arising either in vivo or in vitro and have no reason to expect, based upon our experience or any literature findings, that microglia are predisposed to undergo transformation in vitro in standard growth media with serum. We do feel that whatever the changes that occurred might offer some insight into overall cell transformation biology and perhaps to some understanding of forms of brain malignancy that may involve a microglial phenotype. Indeed, a recent review highlights a possible role of microglial transformation, in particular, in glioblastoma multiforme (Huysentruyt et al., 2011). Although our present report focused on the microglial phenotype characterization of the SIM-A9 cells, it would be of a great future interest to elucidate potential oncogenic factor(s) that prompted the transformation of the primary microglia. Genetic, epigenetic and proteomic analyses of SIM-A9 and other clones may provide insight to the development of central nervous system cellular transformation.
SIM-A9 cells displayed multiple microglia-like morphologies, such as polygonal, bipolar and spherical shapes with varying numbers of processes (Fig. 2). These morphological variations in SIM-A9 cells are corroborated by the observations that cultured primary microglia are also often found in diverse shapes, bearing spherical, ramified, amoeboid, and bipolar morphologies depending upon the culture conditions and/or presence of exogenous stimuli (Abd-El-Basset and Fedoroff, 1995; Han et al., 2002; Jordan and Thomas, 1987; Nakamura et al., 1999; Tanaka et al., 1998). Furthermore, the processes of some SIM-A9 cells contained lamellipodia, indicating that they are highly motile. Cellular motility is a functional feature of microglia and essential for local surveillance and migration to the site of insults (Horvath et al., 2008). As mentioned above, the presence of LPS alters the proliferation of primary microglia and the cells become more spherical (Ganter et al., 1992; Gebicke-Haerter et al., 1989), but our 24-hour LPS stimulation at 2.5 ng/mL did not have a noticeable effect on the morphology of SIM-A9 cells or primary microglia (Fig. 1 & 6).
The proteins CD68 and Iba1 are involved in phagocytosis and cellular motility and are specifically expressed in macrophages as well as microglia (Bhat et al., 1998; Giulian and Baker, 1986; Han et al., 2002). Therefore, these proteins are often used as molecular markers to identify microglia in tissue sections and mixed cultures. We demonstrated that SIM-A9 cells were immunoreactive for these microglial markers, with or without inflammatory stimulations (Fig. 1). Primary microglia also express these markers without stimulation, although their expressions have been reported to increase in inflammatory conditions (Boje and Arora, 1992; Giulian and Baker, 1986; Henn et al., 2009; Kakimura et al., 2002; Takata et al., 2010). We have confirmed the immunoreactivity of primary microglia to CD68 and Iba1 (Fig. 6). The CD68 staining was robust in primary microglia as well as the SIM-A9 cells, with or without LPS stimulation while the Iba1 staining was not as intense and also heterogeneous (Fig. 1 & 6). Although Iba1 immunoreactivity seemed to slightly increase with LPS stimulation, we did not detect obvious increases in the CD68-immunoreactivity after LPS treatment. It is possible that the regulatory mechanism of CD68 has been maximally activated perhaps due to the unknown trigger(s) that transformed these cells.
Typical behavioral responses of microglia to inflammatory stimuli such as LPS and Aβ include increased proinflammatory cytokine secretion and nitric oxide production as well as phagocytosis. Our results confirmed that SIM-A9 cells are capable of responding to LPS and Aβ1-42 by performing these microglial tasks (Fig. 3). This proinflammatory microglia phenotype, known as M1, is associated with elevated iNOS and COX-2 (Chhor et al., 2013) following the activation of the IκB- and tyrosine kinase pathways (Stansley et al., 2012). As expected, we observed increases in the amounts of iNOS and COX-2 (Fig. 4, A–D) as well as phosphorylated IκB (p-IκB) and tyrosine residues (p-Tyr) in SIM-A9 cells (Fig. 5). On the other hand, certain stimuli such as IL-4 differentiate microglia into a more regeneration-supportive M2 phenotype which can be identified by specific elevations in Arg-1 (Chhor et al., 2013). Stimulation with IL-4 effectively increased protein levels of Arg-1 in SIM-A9 cells, indicating that appropriate stimuli induce distinct M1/M2 phenotypes in this novel cell line.
Upon direct comparison of SIM-A9 cells and primary microglia, the latter exhibited greater responses to LPS and Aβ1-42 as determined by TNFα secretion (Fig. 7A). In addition, SIM-A9 cells showed a decreased ability to express M2 phenotype markers after a number of passages as was evident from the lack of IL-4 stimulated Arg-1 expression (Fig. 8). Reduced responses to external stimuli appear to be a common feature of immortalized cells and also have been observed with BV2 cells, a widely used virally transformed microglial cell line (Henn et al., 2009; Stansley et al., 2012). However, SIM-A9 cells showed competencies comparable to their primary counterpart with regard to bioparticle uptake (Fig. 7D) and COX-2 induction (Fig. 8) in response to proinflammatory stimuli. The microglial features of SIM-A9 cells and primary microglia examined in this study are summarized in Table 1. Related findings by other investigators using primary microglia and BV2 cells are also presented for comparison purposes. An extensive comparative assessment of various microglial cell lines has been reported by (Stansley et al., 2012).
TABLE 1. A summary of microglia-like features of SIM-A9 cells.
The results from SIM-A9 cells and primary microglia in the current study are summarized and compared to other published findings in primary microglia as well as a widely used microglia cell line, BV2. The items in parentheses indicate different materials and/or methods used in the referenced studies. For a more extensive summary of microglial cell line comparisons, see (Stansley et al., 2012).
| Features | SIM-A91 | Primary Microglia | BV2 |
|---|---|---|---|
| Species | Mouse | Mouse1,14/Rat2,3,5,6,7,8,9,12 | Mouse |
| Cause/method of immortalization | Spontaneous | N/A | Retrovirus |
| CD68-immunoreactivity | Positive | Positive1 | Data not available |
| Iba1-immunoreactivity | Positive | Positive1,8 | Positive8 |
| GFAP-immunoreactivity | Negative | Negative1,6 | Negative4 |
| MAP2- immunoreactivity | Negative | Negative1 | Data not available |
| LPS-induced TNFα production/secretion | Yes | Yes1,3,7,9,12 | No4/Yes7,13, |
| LPS-induced NO production | Yes | Yes3,5,7,12 | Yes7,8,13 |
| Phagocytosis | Yes (Aβ; BioP) | Yes (Aβ9; BioP1; latex beads6) | Yes (microorganisms4; Aβ10) |
| LPS-induced COX-2 expression | Yes | Yes1,7 | Yes7,13 |
| LPS-induced iNOS expression | Yes | Yes1,3,7 | Yes7,13 |
| IL-4 induced Arg-1 expression | Yes (may decrease with passage) | Yes1,14 | Yes14 |
| Activation of a MAPK signaling pathway(s) by LPS or Aβ stimulation | Phosphorylation of IκB and tyrosine residues by LPS, Aβ | Increased p38, ERK, and JNK substrate phosphorylation by LPS or Aβ2,12 | Phosphorylation of ERK1/2, p38, JNK, and Akt; NFκB nuclear translocation by LPS7,13,14 |
5. CONCLUSION
Our results validated that SIM-A9 cells have retained typical microglia characteristics after cloning, and they respond to inflammatory stimuli in a similar manner to primary microglia, especially with regard to phagocytic activity and proinflammatory signaling in response to LPS and Aβ stimulations. These cells propagate continuously in serum-containing media, and can be frozen stored at −80°C for at least up to 4 years. While some of the microglia-like functions may lessen with prolonged culturing and passaging, our data suggest that SIM-A9 cells may be used as a reliable, cost-effective, and time-saving research tool to investigate microglial functions and behaviors in vitro, likely to be particularly useful for screening immunomodulating compounds prior to in vivo assessment. Alternatively, the SIM-A9 cells may provide a model system for brain malignancy research as mentioned earlier. It is the authors’ intent to make the SIM-A9 cells available to interested researchers.
Highlights.
A novel spontaneously immortalized microglial cell line, SIM-A9, was characterized.
SIM-A9 cells express macrophage/microglia-specific proteins, CD68 and Iba1.
LPS and β-amyloid elicit microglia-like immunological behavior in SIM-A9 cells.
SIM-A9 cells can be induced to pro- or anti-inflammatory microglial phenotypes.
Acknowledgments
This study was supported by NIH grant 5R01AG042819-02 and 5P30GM103329-03.
Abbreviations
- SIM
spontaneously immortalized microglia
- LPS
lipopolysaccharide
- Aβ
beta-amyloid
- GFAP
Glial fibrillary acidic protein
- MAP2
microtubule-associated protein 2
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- IκB
inhibitor of κB
- TNFα
tumor necrosis factor alpha
- iNOS
inducible nitric oxide synthase
- COX-2
cyclooxygenase-2
- Arg-1
arginase-1
- IL-4
interleukin-4
- ELISA
enzyme-linked immunosorbent assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kumi Nagamoto-Combs, Email: kumi.combs@med.und.edu, Dept. of Basic Sciences, School of Medicine and Health Sciences, University of North Dakota, ADDRESS: 501 N. Columbia Road, Stop 9037, Grand Forks, North Dakota 58202-9037, USA, PHONE: 701-777-2559, FAX: 701-777-2477.
Joshua Kulas, Email: joshua.kulas@my.und.edu, Dept. of Basic Sciences, School of Medicine and Health Sciences, University of North Dakota, ADDRESS: 504 Hamline Street, Neuroscience Building, Grand Forks, North Dakota 58202, USA, PHONE: 701-777-0388, FAX: 701-777-4490.
Colin K. Combs, Email: colin.combs@med.und.edu, Dept. of Basic Sciences, School of Medicine and Health Sciences, University of North Dakota, ADDRESS: 504 Hamline Street, Neuroscience Building, Grand Forks, North Dakota 58202, USA, PHONE: 701-777-4025, FAX: 701-777-4490
References
- Abd-El-Basset E, Fedoroff S. Effect of bacterial wall lipopolysaccharide (LPS) on morphology, motility, and cytoskeletal organization of microglia in cultures. Journal of Neuroscience Research. 1995;41:222–37. doi: 10.1002/jnr.490410210. [DOI] [PubMed] [Google Scholar]
- Alliot F, Marty MC, Cambier D, Pessac B. A spontaneously immortalized mouse microglial cell line expressing CD4. Brain research Developmental brain research. 1996;95:140–3. doi: 10.1016/0165-3806(96)00101-0. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Xing B, de Almeida L, Dimayuga ER, Watterson DM, Van Eldik LJ. Microglial p38alpha MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Abeta) Journal of neuroinflammation. 2011;8:79. doi: 10.1186/1742-2094-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:1633–41. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. Journal of neuroimmunology. 1990;27:229–37. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Boje KM, Arora PK. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587:250–6. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- Briers TW, Desmaretz C, Vanmechelen E. Generation and characterization of mouse microglial cell lines. Journal of neuroimmunology. 1994;52:153–64. doi: 10.1016/0165-5728(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. Journal of immunology (Baltimore, Md : 1950) 1992;149:2736–41. [PubMed] [Google Scholar]
- Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia. 2001;35:53–62. doi: 10.1002/glia.1070. [DOI] [PubMed] [Google Scholar]
- Chhor V, Le Charpentier T, Lebon S, Ore MV, Celador IL, Josserand J, Degos V, Jacotot E, Hagberg H, Savman K, Mallard C, Gressens P, Fleiss B. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain, behavior, and immunity. 2013;32:70–85. doi: 10.1016/j.bbi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Cannady SB, Lehman TM, Landreth GE. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of beta-amyloid and prion proteins. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:928–39. doi: 10.1523/JNEUROSCI.19-03-00928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan G, Floden AM, Combs CK. Amyloid-beta oligomers stimulate microglia through a tyrosine kinase dependent mechanism. Neurobiology of aging. 2012;33:2247–61. doi: 10.1016/j.neurobiolaging.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, Rowe DC, Golenbock DT. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes and infection/Institut Pasteur. 2004;6:1361–7. doi: 10.1016/j.micinf.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Floden AM, Combs CK. Beta-amyloid stimulates murine postnatal and adult microglia cultures in a unique manner. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:4644–8. doi: 10.1523/JNEUROSCI.4822-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden AM, Li S, Combs CK. Beta-amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor alpha and NMDA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:2566–75. doi: 10.1523/JNEUROSCI.4998-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganter S, Northoff H, Mannel D, Gebicke-Harter PJ. Growth control of cultured microglia. J Neurosci Res. 1992;33:218–30. doi: 10.1002/jnr.490330205. [DOI] [PubMed] [Google Scholar]
- Gebicke-Haerter PJ, Bauer J, Schobert A, Northoff H. Lipopolysaccharide-free conditions in primary astrocyte cultures allow growth and isolation of microglial cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1989;9:183–94. doi: 10.1523/JNEUROSCI.09-01-00183.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1986;6:2163–78. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han IO, Kim KW, Ryu JH, Kim WK. p38 mitogen-activated protein kinase mediates lipopolysaccharide, not interferon-gamma, -induced inducible nitric oxide synthase expression in mouse BV2 microglial cells. Neurosci Lett. 2002;325:9–12. doi: 10.1016/s0304-3940(02)00218-5. [DOI] [PubMed] [Google Scholar]
- Han L, Yin K, Zhang S, Wu Z, Wang C, Zhang Q, Pan J, Chen B, Li J, Tan R, Xu Y. Dalesconols B inhibits lipopolysaccharide induced inflammation and suppresses NF-kappaB and p38/JNK activation in microglial cells. Neurochemistry international. 2013;62:913–21. doi: 10.1016/j.neuint.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Henn A, Lund S, Hedtjarn M, Schrattenholz A, Porzgen P, Leist M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. Altex. 2009;26:83–94. doi: 10.14573/altex.2009.2.83. [DOI] [PubMed] [Google Scholar]
- Horvath RJ, Nutile-McMenemy N, Alkaitis MS, Deleo JA. Differential migration, LPS-induced cytokine, chemokine, and NO expression in immortalized BV-2 and HAPI cell lines and primary microglial cultures. Journal of neurochemistry. 2008;107:557–69. doi: 10.1111/j.1471-4159.2008.05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysentruyt LC, Akgoc Z, Seyfried TN. Hypothesis: are neoplastic macrophages/microglia present in glioblastoma multiforme? ASN neuro. 2011;3 doi: 10.1042/AN20110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan FL, Thomas WE. Identification of microglia in primary cultures of mixed cerebral cortical cells. Brain research bulletin. 1987;19:153–9. doi: 10.1016/0361-9230(87)90180-8. [DOI] [PubMed] [Google Scholar]
- Kakimura J-i, Kitamura Y, Takata K, Umeki M, Suzuki S, Shibagaki K, Taniguchi T, Nomura Y, Gebicke-Haerter PJ, Smith MA, Perry G, Shimohama S. Microglial activation and amyloid-β clearance induced by exogenous heat-shock proteins. The FASEB Journal. 2002;16:601–3. doi: 10.1096/fj.01-0530fje. [DOI] [PubMed] [Google Scholar]
- Kanzawa T, Sawada M, Kato K, Yamamoto K, Mori H, Tanaka R. Differentiated regulation of allo-antigen presentation by different types of murine microglial cell lines. Journal of Neuroscience Research. 2000;62:383–8. doi: 10.1002/1097-4547(20001101)62:3<383::AID-JNR8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kopec KK, Carroll RT. Alzheimer’s beta-amyloid peptide 1-42 induces a phagocytic response in murine microglia. Journal of neurochemistry. 1998;71:2123–31. doi: 10.1046/j.1471-4159.1998.71052123.x. [DOI] [PubMed] [Google Scholar]
- Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. Journal of immunology (Baltimore, Md : 1950) 1993;150:2659–67. [PubMed] [Google Scholar]
- Liu D, Wang Z, Liu S, Wang F, Zhao S, Hao A. Anti-inflammatory effects of fluoxetine in lipopolysaccharide(LPS)-stimulated microglial cells. Neuropharmacology. 2011;61:592–9. doi: 10.1016/j.neuropharm.2011.04.033. [DOI] [PubMed] [Google Scholar]
- McDonald DR, Brunden KR, Landreth GE. Amyloid fibrils activate tyrosine kinase-dependent signaling and superoxide production in microglia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:2284–94. doi: 10.1523/JNEUROSCI.17-07-02284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain research Brain research reviews. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. Journal of neuroimmunology. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Nagai A, Nakagawa E, Hatori K, Choi HB, McLarnon JG, Lee MA, Kim SU. Generation and characterization of immortalized human microglial cell lines: expression of cytokines and chemokines. Neurobiology of disease. 2001;8:1057–68. doi: 10.1006/nbdi.2001.0437. [DOI] [PubMed] [Google Scholar]
- Nagamoto-Combs K, Combs CK. Microglial phenotype is regulated by activity of the transcription factor, NFAT (nuclear factor of activated T cells) The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:9641–6. doi: 10.1523/JNEUROSCI.0828-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Si QS, Kataoka K. Lipopolysaccharide-induced microglial activation in culture: temporal profiles of morphological change and release of cytokines and nitric oxide. Neuroscience Research. 1999;35:95–100. doi: 10.1016/s0168-0102(99)00071-1. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Imai Y, Nakajima K, Kohsaka S. Generation and characterization of a microglial cell line, MG5, derived from a p53-deficient mouse. Glia. 1997;21:285–98. [PubMed] [Google Scholar]
- Peudenier S, Héry C, Ng KH, Tardieu M. HIV receptors within the brain: A study of CD4 and MHC-II on human neurons, astrocytes and microglial cells. Research in Virology. 1991;142:145–9. doi: 10.1016/0923-2516(91)90051-4. [DOI] [PubMed] [Google Scholar]
- Pyo H, Jou I, Jung S, Hong S, Joe EH. Mitogen-activated protein kinases activated by lipopolysaccharide and beta-amyloid in cultured rat microglia. Neuroreport. 1998;9:871–4. doi: 10.1097/00001756-199803300-00020. [DOI] [PubMed] [Google Scholar]
- Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, Donini SD, Ricciardi-Castagnoli P. Monokine production by microglial cell clones. European journal of immunology. 1989;19:1443–8. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- Rojanathammanee L, Puig KL, Combs CK. Pomegranate polyphenols and extract inhibit nuclear factor of activated T-cell activity and microglial activation in vitro and in a transgenic mouse model of Alzheimer disease. The Journal of nutrition. 2013;143:597–605. doi: 10.3945/jn.112.169516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondag CM, Dhawan G, Combs CK. Beta amyloid oligomers and fibrils stimulate differential activation of primary microglia. Journal of neuroinflammation. 2009;6:1. doi: 10.1186/1742-2094-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansley B, Post J, Hensley K. A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. Journal of neuroinflammation. 2012;9:115. doi: 10.1186/1742-2094-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata K, Kitamura Y, Saeki M, Terada M, Kagitani S, Kitamura R, Fujikawa Y, Maelicke A, Tomimoto H, Taniguchi T, Shimohama S. Galantamine-induced amyloid-{beta} clearance mediated via stimulation of microglial nicotinic acetylcholine receptors. The Journal of biological chemistry. 2010;285:40180–91. doi: 10.1074/jbc.M110.142356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Toku K, Matsuda S, Sudo S, Fujita H, Sakanaka M, Maeda N. Induction of resting microglia in culture medium devoid of glycine and serine. Glia. 1998;24:198–215. [PubMed] [Google Scholar]
- Terazawa R, Akimoto N, Kato T, Itoh T, Fujita Y, Hamada N, Deguchi T, Iinuma M, Noda M, Nozawa Y, Ito M. A kavalactone derivative inhibits lipopolysaccharide-stimulated iNOS induction and NO production through activation of Nrf2 signaling in BV2 microglial cells. Pharmacological research : the official journal of the Italian Pharmacological Society. 2013;71:34–43. doi: 10.1016/j.phrs.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Woster AP, Combs CK. Differential ability of a thiazolidinedione PPARgamma agonist to attenuate cytokine secretion in primary microglia and macrophage-like cells. Journal of neurochemistry. 2007;103:67–76. doi: 10.1111/j.1471-4159.2007.04706.x. [DOI] [PubMed] [Google Scholar]
- Zhou X, Spittau B, Krieglstein K. TGFbeta signalling plays an important role in IL4-induced alternative activation of microglia. Journal of neuroinflammation. 2012;9:210. doi: 10.1186/1742-2094-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielasek J, Tausch M, Toyka KV, Hartung HP. Production of nitrite by neonatal rat microglial cells/brain macrophages. Cellular immunology. 1992;141:111–20. doi: 10.1016/0008-8749(92)90131-8. [DOI] [PubMed] [Google Scholar]