Abstract
OBJECTIVE
To assess the relative impact of an intensive lifestyle intervention (ILI) on use and costs of health care within the Look AHEAD trial.
RESEARCH DESIGN AND METHODS
A total of 5,121 overweight or obese adults with type 2 diabetes were randomly assigned to an ILI that promoted weight loss or to a comparison condition of diabetes support and education (DSE). Use and costs of health-care services were recorded across an average of 10 years.
RESULTS
ILI led to reductions in annual hospitalizations (11%, P = 0.004), hospital days (15%, P = 0.01), and number of medications (6%, P < 0.001), resulting in cost savings for hospitalization (10%, P = 0.04) and medication (7%, P < 0.001). ILI produced a mean relative per-person 10-year cost savings of $5,280 (95% CI 3,385–7,175); however, these were not evident among individuals with a history of cardiovascular disease.
CONCLUSIONS
Compared with DSE over 10 years, ILI participants had fewer hospitalizations, fewer medications, and lower health-care costs.
Introduction
The number of adults who both are overweight or obese and have type 2 diabetes is increasing rapidly (1). In the U.S., >35% of adults are obese (2), and the projected number of diabetes cases exceeds 30 million (3). Separately, obesity and diabetes markedly increase health-care costs (4–6), and their coexistence further increases costs (7). Strategies to reduce the economic impact of these trends are needed.
Lifestyle interventions aimed at promoting long-term weight loss and increased physical activity are recommended for overweight and obese individuals with type 2 diabetes. Although reduced health-care costs have been suggested to accompany these interventions (8–14), no studies have prospectively recorded their long-term effects on health-care costs among these individuals. The Action for Health in Diabetes (Look AHEAD) study is the first randomized clinical trial with sufficient size and duration to test whether behavioral intervention targeting weight loss and increased physical activity influences long-term health-care service use and costs.
Research Design and Methods
The design and methods of the Look AHEAD trial have been published previously (15), as have its CONSORT (Consolidated Standards of Reporting Trials) diagram and the results for its primary outcome (16). Look AHEAD was a single-blinded randomized controlled trial that recruited 5,145 individuals (from 2001 to 2004) who were overweight or obese and had type 2 diabetes. To be eligible, they had to meet the following criteria: 45–76 years of age, BMI >25 kg/m2 (>27 kg/m2 if treated with insulin), glycated hemoglobin (HbA1c) <11% (97 mmol/mol), blood pressure <160/<100 mmHg, triglyceride level <600 mg/dL, and successful completion of a maximum graded exercise test. Participants at the trial’s 16 sites were randomly assigned with equal probability to an intensive lifestyle intervention (ILI) or diabetes support and education (DSE) comparator. All participants provided informed consent. Local institutional review boards approved the protocols.
On 14 September 2012, the study was directed by its sponsor (National Institute of Diabetes and Digestive and Kidney Diseases) to terminate interventions based on recommendations from the trial’s data and safety monitoring board. This recommendation was based on an evaluation of statistical futility for the trial’s primary end point, a composite of death from cardiovascular disease, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for angina (16).
Interventions
ILI participants were assigned calorie, dietary fat, and physical activity goals (17). Trained interventionists provided instruction and encouragement in face-to-face group and individual meetings weekly for 6 months and three times per month for the next 6 months. Thereafter, ILI participants were offered an individual and group meeting each month and periodic refresher group meetings. DSE participants were invited to three group sessions on general education about diabetes self-care per year during the first 4 years and one per year thereafter (18). Look AHEAD investigators did not manage any medical care or medical service use (e.g., admit participants to the hospital, change dosages of or stop prescription medications, or prescribe rehabilitation). This management remained in the hands of the participants' health-care providers. The only exceptions were for temporary changes in glucose medications made by study staff to reduce the risk of hypoglycemia and the prescription of orlistat to 684 (27%) ILI participants, which was largely discontinued in 2008. The median duration of orlistat use among these 684 participants was 0.98 (interquartile range 0.43–1.66) years.
Assessments and Outcomes
At baseline, demographic data, medical history, and sources of medical care were collected by self-report. Weight and height were measured in duplicate using a digital scale and stadiometer. Hypertension was determined based on the use of antihypertensive medications or measured blood pressure. History of cardiovascular disease was defined by self-report of prior myocardial infarction, stroke, coronary or lower-extremity angioplasty, carotid endarterectomy, or coronary bypass surgery.
Hospitalizations, outpatient visits (office, hospital clinic, or other), outpatient tests and procedures, rehabilitation/long-term care, and home care were assessed annually through face-to-face interviews at clinic visits and at 6-month intervals by telephone. A validation study confirmed that information on hospitalizations for procedures unrelated to major study end points that had lengths of stay of ≤3 days could be collected by self-report. These accounted for 14% of hospitalizations. For the other 86% of hospitalizations, hospital records were reviewed for admission and discharge dates, reasons for hospitalization, and discharge status. Hospitalizations were categorized using the Clinical Classifications Software system from the Agency for Healthcare Research and Quality (www.hcup-us.ahrq.gov/toolssoftware/ccs/ccsfactsheet.jsp). Participants brought prescription medications to annual clinic visits for recording. Follow-up was censored at each participant’s last interview and, thus, excludes health-care use and costs associated with death. All data were collected by centrally trained staff who were masked to intervention assignment.
Regression models that included age, sex, discharge location, primary diagnosis, primary procedure, and length of stay as predictors were fitted to data from the Nationwide Inpatient Sample (19) to project hospitalization costs. Outpatient care costs were based on the Medicare Physician Fee Schedule (www.cms.gov/apps/physican-fee-schedule). Rehabilitation, long-term care, and home health services costs were based on Medicare Skilled Nursing Facility Prospective Payment System and National Home Health Utilization statistics for Medicare Parts A and B (www.cms.gov). Medication costs were based on adjusted average wholesale prices obtained from the Red Book (www.redbook.com/redbook) from January to May 2013. Medications were grouped by using this resource to disaggregate the broader U.S. Food and Drug Administration classifications (www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/investigationalnewdrugindapplication/ucm176533.htm). Costs were obtained for both brand-name and generic-brand drugs and assigned to medications proportional to the reported use (i.e., brand name vs. generic) by participants. Medication doses were not recorded; the most commonly prescribed doses were assumed. Adjustments accounted for the relationship between average wholesale prices and the cost of both the manufacturing and the purchasing of drugs (20,21). All costs were expressed in 2012 dollars, with adjustments made using the medical care component of the Consumer Price Index (www.bls.gov/data/inflation_calculator.htm).
Statistical Analysis
We report two types of outcomes: per-participant average annual rates and costs of medical service use and per-participant 10-year cumulative mean discounted costs. Data were collected from contacts with participants occurring before 14 September 2012. Hospitalizations reported later but that had admission dates before 14 September 2012 were also included.
Analyses followed intention-to-treat principles and used all available data, with participants included in their randomization group independent of adherence. For per-participant average annual estimates, frequencies and costs were tallied for each participant and divided by follow-up time to obtain observed counts and costs per year. For inference, weighted ANCOVA was used to compare intervention groups, with analytical weights proportional to participants’ lengths of follow-up. Clinic, the sole stratification factor in randomization, was a covariate. To accumulate costs over 10 years, annual estimates were discounted at 3% per year and summed. Bootstrapping was used for CIs of the 10-year differences in accumulated mean costs.
The Look AHEAD protocol prespecified subgroup comparisons for the primary outcome of major cardiovascular events were based on sex, history of cardiovascular disease, and race/ethnicity. Although this prespecification did not extend to other study outcomes, we report parallel comparisons for use rates and costs. We also report results for subgroups based on age and baseline BMI. Tests of interaction were used to assess the consistency of differences between intervention groups for each of these subgroups.
Results
Follow-up cost data were available from 5,121 of the 5,145 Look AHEAD participants (99.5%). Twenty-four participants withdrew from the trial or were lost to follow-up before the collection of any cost data. Collectively, these 5,121 individuals provided 50,498 person-years of follow-up (averaging 9.9 years per participant). Follow-up was terminated for refusal and lost contact in 6.2% of ILI and 6.5% of DSE participants (P = 0.62) and for death in 6.8% of ILI and 7.8% of DSE participants (P = 0.15).
Baseline Characteristics
At baseline (Table 1), 25% of participants were aged 45–54 years, 55% were aged 55–64 years, and 20% were aged 65–76 years. Fifteen percent were overweight (BMI 25.0–29.9 kg/m2), and 22% had class III obesity (BMI ≥40.0 kg/m2). Sixty percent were female; 54% had diabetes for at least 5 years; 14% had a history of cardiovascular disease; and 63% were non-Hispanic white. None of the characteristics in Table 1 differed significantly between intervention groups.
Table 1.
Characteristics at the time of enrollment into the Look AHEAD trial by intervention assignment
| Characteristic | DSE (n = 2,563) | ILI (n = 2,558) | P value‡ |
|---|---|---|---|
| Age | 0.15 | ||
| 45–54 years | 23 | 25 | |
| 55–64 years | 55 | 56 | |
| 65–76 years | 21 | 19 | |
| BMI | 0.16 | ||
| 25.0–29.9 kg/m2 | 14 | 16 | |
| 30.0–34.9 kg/m2 | 35 | 36 | |
| 35.0–39.9 kg/m2 | 29 | 26 | |
| >40 kg/m2 | 22 | 22 | |
| Sex | 0.75 | ||
| Female | 60 | 60 | |
| Male | 40 | 40 | |
| History of cardiovascular disease* | 0.45 | ||
| No | 86 | 86 | |
| Yes | 14 | 14 | |
| Race/ethnicity | 0.98 | ||
| African American | 16 | 16 | |
| American Indian | 5 | 5 | |
| Hispanic/Latino | 13 | 13 | |
| Non-Hispanic white | 63 | 63 | |
| Other/mixed | 3 | 3 | |
| Diabetes duration (n = 40 missing) | 0.20 | ||
| <5 years | 45 | 47 | |
| ≥5 years | 55 | 53 | |
| Education (n = 110 missing) | 0.54 | ||
| High school or less | 21 | 20 | |
| Post-high school | 38 | 37 | |
| College graduate | 41 | 42 | |
| Hypertension | 0.34 | ||
| No | 17 | 16 | |
| Yes | 83 | 84 | |
| Prescription medications, mean number | |||
| Antihypertensive | 1.20 | 1.24 | 0.29 |
| Diabetes | 1.40 | 1.38 | 0.51 |
| Lipid lowering | 0.53 | 0.53 | 0.86 |
| Annual household income (n = 503 missing) | 0.84 | ||
| <$40,000 | 34 | 34 | |
| $40,000 to <$80,000 | 37 | 37 | |
| ≥$80,000 | 30 | 29 | |
| Health insurance status† | |||
| Uninsured | 8 | 9 | 0.71 |
| Insured (self or work) | 77 | 77 | 0.66 |
| Medicare/Medicaid | 21 | 20 | 0.13 |
| Tricare/VA/military | 4 | 5 | 0.36 |
| Indian Health Service | 2 | 3 | 0.32 |
| Other/not reported | 4 | 4 | 0.56 |
| Usual source of health care (n = 13 missing) | 0.73 | ||
| Private physician’s office | 74 | 74 | |
| Hospital clinic/outpatient | 12 | 12 | |
| Community health center | 8 | 8 | |
| Other health-care facility | 5 | 6 | |
| No usual source of care | 1 | 1 |
Data are % unless otherwise indicated. VA, Veterans Affairs.
*History of cardiovascular disease included myocardial infarction, coronary artery bypass, angioplasty/stent procedure, peripheral vascular disease, stroke, stable angina, and class I/II heart failure.
†Participants were able to indicate more than one source of health insurance.
‡χ2 test.
Service Use
ILI participants averaged 0.177 hospitalizations per year compared with 0.199 hospitalizations per year for DSE participants, an 11% (P = 0.004) reduction (Table 2). Reductions in hospitalization rates reached nominal statistical significance for cardiovascular (11%, P = 0.04), pulmonary (27%, P = 0.05), and other (i.e., not among the named categories) (8%, P = 0.05) diseases. There was a significant 15% (P = 0.01) reduction in the average annual days in the hospital (0.69 vs. 0.81 days/year for ILI and DSE participants, respectively). ILI participants also had relative reductions of 14% (P = 0.05) and 16% (P = 0.001) in the annual rates of rehabilitation/long-term care and home care, respectively. There was no significant difference between the groups in the rates of outpatient visits, but the ILI group had lower rates for outpatient rehabilitation facilities, home care, and hospitalization.
Table 2.
Average annual rates of medical service use and average annual costs over follow-up for participants grouped by intervention assignment
| Category | DSE | ILI | Difference (SE) | Percent decrease (increase) in use | P value* |
|---|---|---|---|---|---|
| Per-participant average annual medical service use | |||||
| Hospitalizations | 0.199 (0.006) | 0.177 (0.006) | 0.023 (0.008) | 11 | 0.004 |
| Bone | 0.009 (0.001) | 0.008 (0.001) | 0.001 (0.001) | 11 | 0.20 |
| Cancer | 0.013 (0.002) | 0.012 (0.001) | 0.0005 (0.0024) | 4 | 0.84 |
| Cardiovascular | 0.066 (0.003) | 0.059 (0.002) | 0.008 (0.004) | 11 | 0.04 |
| Metabolism† | 0.010 (0.001) | 0.008 (0.001) | 0.002 (0.001) | 20 | 0.07 |
| Pulmonary | 0.011 (0.001) | 0.008 (0.001) | 0.003 (0.001) | 27 | 0.05 |
| Renal | 0.004 (0.001) | 0.003 (0.001) | 0.001 (0.001) | 25 | 0.22 |
| Other | 0.086 (0.003) | 0.079 (0.003) | 0.008 (0.004) | 8 | 0.05 |
| Days in hospital | 0.81 (0.03) | 0.69 (0.03) | 0.11 (0.04) | 15 | 0.01 |
| Outpatient services | |||||
| Visits | 11.75 (0.11) | 11.50 (0.11) | 0.25 (0.16) | 2 | 0.13 |
| Rehabilitation center/LTC (%) | 2.9 (0.17) | 2.5 (0.17) | 0.4 (0.20) | 14 | 0.05 |
| Home care (%) | 7.3 (0.26) | 6.1 (0.26) | 1.2 (0.37) | 16 | 0.001 |
| Medications | 4.96 (0.05) | 4.65 (0.05) | 0.31 (0.06) | 6 | <0.0001 |
| Diabetes | 1.45 (0.01) | 1.25 (0.01) | 0.19 (0.02) | 14 | <0.0001 |
| Lipid lowering | 0.65 (0.01) | 0.62 (0.01) | 0.04 (0.01) | 5 | 0.002 |
| Antihypertensive | 1.24 (0.02) | 1.19 (0.02) | 0.06 (0.02) | 4 | 0.02 |
| Other cardiovascular disease | 0.11 (0.005) | 0.10 (0.005) | 0.01 (0.01) | 9 | 0.14 |
| Psychiatric/neurologic | 0.29 (0.01) | 0.32 (0.01) | −0.03 (0.01) | (10) | 0.02 |
| Musculoskeletal | 0.20 (0.01) | 0.21 (0.01) | −0.003 (0.01) | (5) | 0.59 |
| Other | 1.02 (0.02) | 0.97 (0.02) | 0.05 (0.03) | 5 | 0.06 |
| Per-participant average annual medical service costs | |||||
| Hospitalization | 2,789 (96) | 2,506 (96) | 283 (136) | 10 | 0.04 |
| Bone | 136 (16) | 120 (16) | 16 (23) | 12 | 0.49 |
| Cancer | 219 (31) | 213 (31) | 6 (43) | 3 | 0.89 |
| Cardiovascular | 1,024 (54) | 948 (54) | 77 (77) | 3 | 0.32 |
| Metabolism | 123 (11) | 91 (11) | 32 (16) | 8 | 0.04 |
| Pulmonary | 137 (19) | 90 (19) | 47 (27) | 26 | 0.08 |
| Renal | 45 (7) | 32 (7) | 12 (10) | 27 | 0.22 |
| Other | 1,106 (47) | 1,012 (46) | 94 (66) | 8 | 0.16 |
| Outpatient services | 2,344 (41) | 2,313 (40) | 31 (57) | 1 | 0.59 |
| Visits | 1,513 (15) | 1,502 (15) | 11 (22) | 1 | 0.61 |
| Tests and procedures | 510 (7) | 502 (7) | 8 (10) | 2 | 0.48 |
| Rehabilitation center/LTC | 198 (29) | 201 (29) | −3 (41) | (2) | 0.93 |
| Home care | 123 (10) | 107 (10) | 16 (13) | 13 | 0.24 |
| Medications | 3,784 (44) | 3,503 (43) | 281 (61) | 7 | <0.0001 |
| Diabetes | 1,226 (18) | 1,012 (18) | 214 (25) | 17 | <0.0001 |
| Lipid lowering | 841 (12) | 793 (12) | 48 (17) | 6 | 0.005 |
| Antihypertensive | 436 (7) | 411 (7) | 25 (10) | 6 | 0.01 |
| Other cardiovascular disease | 111 (5) | 96 (5) | 15 (8) | 14 | 0.06 |
| Psychiatric/neurologic | 329 (13) | 367 (13) | −38 (19) | (12) | 0.04 |
| Musculoskeletal | 89 (4) | 96 (4) | −7 (6) | (8) | 0.23 |
| Other | 752 (19) | 727 (19) | 25 (27) | 3 | 0.35 |
| Total annual cost | 8,916 (133) | 8,321 (133) | 595 (188) | 7 | 0.002 |
Data are mean (SE) unless otherwise indicated. LTC, long-term care.
*Weighted ANCOVA.
†Metabolism includes hospitalizations for nondiabetic endocrine disorders (e.g., thyroid); fluid, electrolyte, and nutrition disorders; and other metabolic disorders (e.g., gout).
On average across follow-up, ILI participants attending clinic visits were taking 4.65 prescription medications compared with 4.96 medications in DSE, a reduction of 6% (P < 0.0001). The most significant reductions occurred for diabetes drugs (14%, P < 0.0001), lipid-lowering drugs (5%, P = 0.002), and antihypertensives (4%, P = 0.02). Use of psychiatric/neurologic drugs (the majority of which were agents that may be prescribed to treat depression, neuropathy, and insomnia) was 10% (P = 0.02) greater among ILI than among DSE participants.
Costs
The per-participant average annual cost of health-care services and medications was 7% less among ILI than among DSE participants ($8,321 vs. $8,916 per year; P = 0.002). This resulted from fewer hospitalizations and less medication use (Table 2). The annual 11% fewer hospitalizations translated to 10% ($283) lower per-participant average annual costs (P = 0.04). The 6% lower annual use of prescribed medications translated to a 7% ($281) lower annual medication cost (P < 0.0001). The most significant lower per-participant average annual medication costs were for diabetes drugs (17%, P < 0.0001), lipid-lowering drugs (6%, P = 0.005), and antihypertensive drugs (6%, P = 0.01). The 10% greater use of psychiatric/neurologic drugs for ILI participants translated to a 12% (P = 0.04) greater per-participant average annual psychiatric/neurologic drug cost.
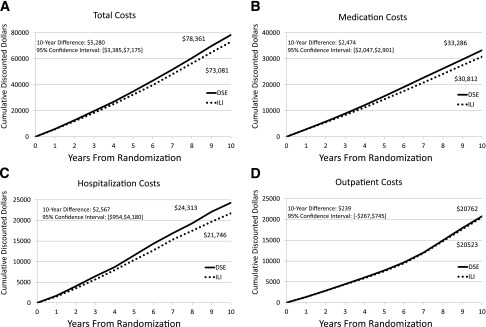
Ten-year discounted cumulative costs (Fig. 1A–D) were $78,361 (DSE) vs. $73,081 (ILI), a difference of $5,280 (95% CI $3,385–$7,175). Assignment to ILI yielded $2,567 ($954–$4,180) lower 10-year discounted hospitalization costs and $2,474 ($2,047–$2,901) lower discounted medication costs. Ten-year differences in discounted outpatient service costs were lower ($239 [−$267 to $745]).
Figure 1.
Per-participant average 10-year cumulative discounted costs. A: Ten-year cumulative total costs. B: Ten-year cumulative medication costs. C: Ten-year cumulative hospitalization costs. D: Ten-year cumulative outpatient care costs. Costs are reported in 2012 U.S. dollars and discounted at 3% per year.
From tests of interactions, the relative effects of ILI on average annual total costs were similar across subgroups based on age, baseline BMI, sex, and race/ethnicity (Table 3). Intervention effects varied according to the participant’s history of cardiovascular disease at baseline for outpatient (interaction P < 0.0001), medication (P = 0.02), and total costs (P = 0.02) but not for hospitalization costs (P = 0.71). For participants without a history of cardiovascular disease at baseline, ILI was associated with lower per-participant average annual costs of $133 (95% CI $13–$252) for outpatient care, $343 ($216–$470) for medications, and $801 ($417–$1,185) for total costs. For those with a history of cardiovascular disease, ILI was associated with greater per-participant average annual outpatient costs of $592 ($286–$889).
Table 3.
Mean annual costs (in 2012 dollars) over follow-up by intervention assignment for subgroups of participants based on baseline characteristics
| Subgroup | DSE | ILI | Difference (95% CI) | DSE vs. ILI P value† | Interaction P value† |
|---|---|---|---|---|---|
| Age | |||||
| 45–54 years | |||||
| Hospitalization | 1,674 (190) | 1,876 (184) | −203 (−721 to 316) | 0.44 | 0.14 |
| Outpatient | 1,902 (80) | 1,928 (78) | −26 (−246 to 193) | 0.81 | 0.85 |
| Medication | 3,439 (87) | 3,372 (84) | 67 (−170 to 305) | 0.58 | 0.13 |
| Total | 7,014 (263) | 7,176 (255) | −162 (−879 to 556) | 0.66 | 0.09 |
| 55–64 years | |||||
| Hospitalization | 2,713 (129) | 2,355 (128) | 358 (2 to 713) | 0.05 | — |
| Outpatient | 2,313 (54) | 2,272 (54) | 41 (−109 to 191) | 0.59 | — |
| Medication | 3,892 (59) | 3,560 (58) | 332 (170 to 495) | <0.0001 | — |
| Total | 8,918 (178) | 8,187 (177) | 731 (239 to 1,223) | 0.004 | — |
| 65–76 years | |||||
| Hospitalization | 4,321 (208) | 3,828 (218) | 493 (−97 to 1,084) | 0.10 | — |
| Outpatient | 2,954 (88) | 2,973 (92) | −19 (−269 to 230) | 0.88 | — |
| Medication | 3,911 (95) | 3,521 (100) | 390 (120 to 660) | 0.005 | — |
| Total | 11,186 (288) | 10,322 (302) | 864 (47 to 1,681) | 0.04 | — |
| BMI | |||||
| 25–29.9 kg/m2 | |||||
| Hospitalization | 2,125 (254) | 2,250 (240) | −125 (−810 to 561) | 0.72 | 0.36 |
| Outpatient | 2,085 (103) | 2,219 (97) | −134 (−424 to 156) | 0.36 | 0.43 |
| Medication | 3,476 (115) | 3,084 (109) | 392 (82 to 702) | 0.01 | 0.72 |
| Total | 7,686 (352) | 7,552 (333) | 133 (−818 to 1,084) | 0.78 | 0.58 |
| 30.0–39.9 kg/m2 | |||||
| Hospitalization | 2,864 (121) | 2,453 (122) | 411 (74 to 748) | 0.02 | — |
| Outpatient | 2,333 (51) | 2,296 (51) | 38 (−105 to 180) | 0.60 | — |
| Medication | 3,823 (55) | 3,574 (55) | 249 (97 to 401) | 0.001 | — |
| Total | 9,021 (168) | 8,322 (169) | 698 (231 to 1,165) | 0.003 | — |
| >40.0 kg/m2 | |||||
| Hospitalization | 3,001 (202) | 2,829 (201) | 172 (−387 to 731) | 0.55 | — |
| Outpatient | 2,537 (89) | 2,425 (88) | 112 (−134 to 358) | 0.37 | — |
| Medication | 3,867 (91) | 3,603 (91) | 264 (11 to 516) | 0.04 | — |
| Total | 9,405 (281) | 8,857 (279) | 548 (−228 to 1,323) | 0.17 | — |
| Sex | |||||
| Female | |||||
| Hospitalization | 2,311 (123) | 2,221 (123) | 90 (−252 to 432) | 0.60 | 0.08 |
| Outpatient | 2,293 (52) | 2,256 (52) | 37 (−108 to 182) | 0.62 | 0.90 |
| Medication | 3,651 (56) | 3,385 (56) | 266 (111 to 421) | 0.001 | 0.76 |
| Total | 8,255 (171) | 7,862 (171) | 393 (−82 to 867) | 0.10 | 0.18 |
| Male | |||||
| Hospitalization | 3,510 (151) | 2,931 (151) | 579 (160 to 998) | 0.007 | — |
| Outpatient | 2,420 (64) | 2,398 (64) | 23 (−155 to 201) | 0.80 | — |
| Medication | 3,984 (69) | 3,679 (68) | 305 (115 to 495) | 0.002 | — |
| Total | 9,914 (210) | 9,008 (209) | 906 (325 to 1,487) | 0.002 | — |
| History of cardiovascular disease | |||||
| No | |||||
| Hospitalization | 2,407 (101) | 2,082 (101) | 325 (46 to 604) | 0.02 | 0.71 |
| Outpatient | 2,295 (43) | 2,162 (43) | 133 (13 to 252) | 0.03 | <0.0001 |
| Medication | 3,667 (46) | 3,324 (46) | 343 (216 to 470) | <0.0001 | 0.02 |
| Total | 8,369 (139) | 7,568 (139) | 801 (417 to 1,185) | <0.0001 | 0.02 |
| Yes | |||||
| Hospitalization | 5,373 (261) | 5,191 (253) | 181 (−533 to 895) | 0.62 | — |
| Outpatient | 2,674 (112) | 3,266 (109) | −592 (−889 to −286) | 0.0002 | — |
| Medication | 4,569 (119) | 4,635 (116) | −67 (−392 to 259) | 0.69 | — |
| Total | 12,615 (360) | 13,093 (349) | −478 (−1,461 to 505) | 0.34 | — |
| Race/ethnicity | |||||
| African American | |||||
| Hospitalization | 2,118 (242) | 2,590 (241) | −473 (−1,142 to 197) | 0.17 | 0.08 |
| Outpatient | 2,090 (101) | 2,278 (101) | −189 (−469 to 91) | 0.19 | 0.32 |
| Medication | 3,437 (107) | 3,129 (107) | 308 (11 to 605) | 0.04 | 0.95 |
| Total | 7,644 (332) | 7,998 (331) | −353 (−1,271 to 565) | 0.45 | 0.13 |
| American Indian | |||||
| Hospitalization | 2,188 (431) | 1,956 (424) | 232 (−953 to 1,417) | 0.70 | — |
| Outpatient | 1,479 (180) | 1,607 (177) | −128 (−624 to 367) | 0.61 | — |
| Medication | 2,649 (191) | 2,503 (188) | 146 (−380 to 672) | 0.59 | — |
| Total | 6,316 (591) | 6,066 (581) | 250 (−1,375 to 1,874) | 0.76 | — |
| Hispanic/Latino | |||||
| Hospitalization | 2,276 (269) | 1,468 (267) | 808 (65 to 1,551) | 0.03 | — |
| Outpatient | 1,742 (113) | 1,545 (112) | 197 (−114 to 508) | 0.21 | — |
| Medication | 3,031 (119) | 2,654 (118) | 377 (47 to 707) | 0.02 | — |
| Total | 7,048 (369) | 5,666 (366) | 1,382 (363 to 2,401) | 0.008 | — |
| Non-Hispanic white | |||||
| Hospitalization | 3,072 (120) | 2,752 (120) | 320 (−13 to 652) | 0.06 | — |
| Outpatient | 2,583 (50) | 2,511 (50) | 72 (−67 to 211) | 0.31 | — |
| Medication | 4,104 (53) | 3,845 (53) | 260 (112 to 407) | 0.001 | — |
| Total | 9,760 (164) | 9,108 (164) | 652 (196 to 1,107) | 0.005 | — |
| Other/multiple races | |||||
| Hospitalization | 3,472 (569) | 2,225 (539) | 1,248 (−288 to 2,784) | 0.11 | — |
| Outpatient | 2,549 (238) | 2,749 (225) | −200 (−843 to 443) | 0.54 | — |
| Medication | 3,805 (252) | 3,551 (239) | 255 (−427 to 936) | 0.46 | — |
| Total | 9,827 (780) | 8,525 (738) | 1,303 (−803 to 3,408) | 0.22 | — |
Data are mean (SE) unless otherwise indicated. Included are results from ANCOVAs to assess the consistency of differences between intervention groups across subgroups. Negative differences occurred when costs among ILI participants exceeded those for DSE participants; positive differences occurred when costs among ILI participants were less than those for DSE participants. The DSE vs. ILI P values are for the mean difference between intervention groups within each strata. Interaction P values indicate whether the DSE vs. ILI differences were similar among subgroups.
†Weighted ANCOVA.
Conclusions
The Look AHEAD intervention produced sustained differences between intervention groups in mean weight loss (7.9 and 2.5 percentage points at 1 and 10 years in ILI and DSE, respectively) (16) and in physical fitness (22,23). Although not significantly reducing overall risk of major cardiovascular disease events (16), the intervention improved many measures of health, including markers of diabetes control, blood pressure, plasma lipid levels, sleep quality, physical function, and depression (24–27). Although these effects varied in magnitude and duration, and some were not sustained throughout follow-up, each could be associated with reduced health-care needs. The medical management of the participants was left to their own health-care providers, who were provided with clinically relevant information, such as annual lipid and blood pressure measurements.
Ten years of ILI broadly reduced the use of health-care services, including hospitalizations, selected outpatient services, and medications, and the total overall costs of health care, with significant savings for hospitalization and medication costs across many disease indications. The 11% overall relative decrease in 10-year hospitalization costs is less than the 17% relative reduction in 10-year inpatient care costs reported by the Diabetes Prevention Program among individuals initially free of diabetes (13). However, because the Look AHEAD cohort had more comorbidities and overall hospitalizations, the absolute savings associated with its intervention were greater ($2,567 vs. $1,309 [converted to 2012 dollars] for the Look AHEAD ILI vs. Diabetes Prevention Program behavioral intervention, respectively).
The significant reduction in hospitalizations for cardiovascular disease differs from the nonsignificant finding for the primary end point of Look AHEAD: a composite of the first occurrence of cardiovascular disease death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina (16). Unlike the primary end point, the cardiovascular hospitalization category presented in this article is more broadly defined and not limited to the first occurrence of events.
The rate of outpatient visits did not differ between intervention groups, supporting the decision of the study investigators not to provide ongoing medical management to participants but to leave this to their personal health-care providers. Thus, the reduction in other use and costs was not due to medical management by study investigators of ILI participants.
A clinical trial by Tsai et al. (14) found that behavioral weight loss intervention in an obese population can reduce medication costs, which agrees with self-controlled pre- and postobservational cohort studies of weight loss programs (28,29) and trials that have been analyzed as pre- and postobservational cohort studies (8,30). The Diabetes Prevention Program reported a 6% relative reduction in 10-year medication costs among participants assigned to its lifestyle intervention (13), resulting in a relative savings of $501 (converted to 2012 dollars). The 7% relative reduction produced by the Look AHEAD intervention yielded a savings of $2,474 (in 2012 dollars) because of the overall greater use of medications in its cohort. The finding of a greater use of psychiatric/neurologic medications over time among ILI compared with DSE participants follows a chance imbalance for greater antidepressant use at baseline among participants randomly assigned to ILI (17.4%) compared with DSE (15.1%; P = 0.02). Covariate adjustment for baseline antidepressant use eliminated differences between intervention groups in the use of these medications during follow-up (P = 0.63).
The savings in total medical care costs associated with ILI accrued gradually over time, resulting in a 10-year difference of $5,280. Similar patterns were seen for the accumulation of both hospital and medications costs, with 10-year differences of $2,567 and $2,474, respectively. There were only small overall differences during the first 4 years of follow-up when the interventions were most intense, although diabetes, hypertension, and hyperlipidemia medication costs were significantly lower among ILI participants even during the first year after randomization (31).
Health-care costs associated with obesity and diabetes accelerate in later life (32,33), and as expected, the rate at which costs accumulated in the Look AHEAD cohort increased with time. Future follow-up of the Look AHEAD cohort will assess whether the intervention has a legacy effect during the oldest years of life when health-care costs are highest (i.e., whether the differences in accumulated costs between the ILI and DSE cohorts continue to diverge).
Based on interaction tests, the savings on overall medical costs associated with ILI did not depend on participant age, BMI, sex, or race/ethnicity. In contrast, intervention effects on outpatient, medication, and total costs differed significantly according to baseline history of cardiovascular disease. For participants with no cardiovascular disease history, assignment to ILI significantly reduced the costs of outpatient care and medication; for those with a history, assignment to ILI significantly increased outpatient costs and had no effect on medication costs. The ILI effect on hospital costs did not differ between those who had or did not have a history of cardiovascular disease.
History of cardiovascular disease at baseline was prespecified as a subgroup for comparing intervention effects on the primary outcome. The hazard ratio for the intervention effect among participants with no history of cardiovascular disease for the primary outcome was 0.86 (95% CI 0.72–1.02) compared with 1.12 (0.90–1.42) for those with a history of cardiovascular disease. Although this trend did not reach nominal statistical significance (P = 0.06) (16), it resembles the difference we report for outpatient costs. Increased outpatient costs among ILI participants with prior cardiovascular disease were not triggered by an imbalance in cardiovascular disease events. Annual outpatient costs were 22% (P = 0.006) higher among ILI than among DSE participants before these events (including those individuals with no on-trial cardiovascular outcomes), which is similar to the overall difference in costs (Table 2). Why the intervention may affect costs differently among participants with and without a cardiovascular disease history is not clear; however, these results imply that the ILI is most beneficial to overweight and obese individuals with diabetes before cardiovascular disease is diagnosed.
The study had several limitations. The Look AHEAD cohort, although geographically and demographically diverse, comprised volunteers to a randomized clinical trial who were required to have a source of usual medical care; thus, the degree to which the findings may generalize to other populations is unclear. We relied on self-report to identify outpatient care and the occurrence of hospitalizations. We did not assess health-care costs related to death. As noted, there were slightly fewer deaths among the ILI than among the DSE participants, and the exclusion of these costs may have led to an underestimation of ILI benefits. Follow-up was not complete for all participants, and the results of the service use, cost, and subgroup analyses may be biased if follow-up lengths were different. However, we used a pattern-mixture approach (34) to assess the sensitivity of the findings, examining the consistency of results across strata based on length of follow-up, which confirmed the findings (data not shown). We did not report intervention costs or draw conclusions from a cost-benefit analysis; these are reserved for future study.
In conclusion, random assignment of overweight and obese individuals with type 2 diabetes to 10 years of an intensive behavioral intervention that focused on weight loss and increased physical activity resulted in relatively fewer hospitalizations, fewer days in the hospital, and less use of prescription medications. Cumulatively, these effects resulted in an average annual savings of almost $600 per participant relative to a comparison condition DSE.
Supplementary Material
Article Information
Duality of Interest. M.A.E. serves on monitoring boards for Terumo Medical Corporation and the Kowa Research Institute. He serves on a steering committee for Boehringer Ingelheim and has recently served on an advisory committee for Takeda Global Research. H.A.G. has twice been a sponsored lecturer by Merck. J.O.H. serves on advisory boards for Takeda and Novo Nordisk. E.S.H. has received consulting, advisory board, monitoring board, and speakers' board support from Amgen; Amylin Pharmaceuticals, LLC; Bristol-Myers Squibb/AstraZeneca; GI Dynamic, Inc.; Gilead Sciences, Inc.; International Medical Press Global Partnership for Effective Diabetes Management; Janssen Pharmaceuticals, Inc.; Merck Research Laboratories, Inc.; Sanofi, Inc.; Vivus, Inc.; Theracos Pharmaceuticals, Inc.; and Takeda Pharmaceuticals, Inc. E.N. has received research support from Eli Lilly and Company and GlaxoSmithKline. A.L.P. has consulted for Abbott Diabetes Care, Becton Dickinson, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, Lilly, Medtronic MiniMed, and Sanofi; has been on the speakers' bureau for Bristol-Myers Squibb/AstraZeneca and Novo Nordisk; has received research grant funding from Medtronic MiniMed; and has received editorial fees from Medscape. D.H.R. was a paid consultant/advisor to Novo Nordisk, Janssen, Takeda, Vivus, and Eisai and has an equity position in Scientific Intake. M.S. has received salary support from Amgen and diaDexus and has served as a consultant for diaDexus. T.A.W. serves on advisory boards for Novo Nordisk and Orexigen and is a consultant to Boehringer Ingelheim. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.A.E. and D.H. contributed to the data analysis and organization and drafting of the manuscript. H.A.G., A.B., M.E., T.K., W.C.K., M.G.M., and P.Z. organized the manuscript and wrote the initial drafts. F.L.B., G.A.B., J.M.Cl., J.M.Cu., C.E., J.P.F., S.G., E.W.G., H.P.H., J.O.H., E.S.H., V.S.H., J.M.J., R.W.J., K.C.J., S.E.K., A.E.K., A.K., C.E.L., M.M., A.M., D.M.N., E.N., J.P., A.L.P., X.P.-S., H.P., J.B.R., D.H.R., M.S., T.A.W., R.R.W., and S.Z.Y. designed and conducted the trial, collected data, and reviewed/edited the manuscript. J.R. contributed to the data analyses. A.G.T. contributed to the data analyses, designed and conducted the trial, collected data, and reviewed/edited the manuscript. M.A.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013.
Footnotes
Clinical trial reg. no. NCT00017953, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-0093/-/DC1.
Deceased.
References
- 1.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003;289:76–79 [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity in the United States, 2009-2010. NCHS data brief no. 82. Hyattsville, MD, National Center for Health Statistics, 2012 [PubMed] [Google Scholar]
- Huang ES, O’Grady M, Basu A, Capretta JC. Projecting the future diabetes population size and related costs for the U.S. Diabetes Care 2009;32:2225–2229 [DOI] [PMC free article] [PubMed]
- 4.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–w831 [DOI] [PubMed] [Google Scholar]
- 5.Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the USA: a quantitative systematic review. Obes Rev 2011;12:50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association Economic costs of diabetes in the US in 2007. Diabetes Care 2008;3:595–615 [DOI] [PubMed] [Google Scholar]
- 7.Liebl A, Breitscheidel L, Nicolay C, Happich M. Direct costs and health-related resource utilization in the 6 months after insulin initiation in German patients with type 2 diabetes mellitus in 2006: INSTIGATE study. Curr Med Res Opin 2008;24:2349–2358 [DOI] [PubMed] [Google Scholar]
- 8.Collins RW, Anderson JW. Medication cost savings associated with weight loss for obese non-insulin-dependent diabetic men and women. Prev Med 1995;24:369–374 [DOI] [PubMed] [Google Scholar]
- 9.Tsai AG, Glick HA, Shera D, Stern L, Samaha FF. Cost-effectiveness of a low-carbohydrate diet and a standard diet in severe obesity. Obes Res 2005;13:1834–1840 [DOI] [PubMed] [Google Scholar]
- 10.Herman WH, Hoerger TJ, Brandle M, et al. Diabetes Prevention Program Research Group . The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005;142:323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf AM, Siadaty M, Yaeger B, et al. Effects of lifestyle intervention on health care costs: Improving Control with Activity and Nutrition (ICAN). J Am Diet Assoc 2007;107:1365–1373 [DOI] [PubMed] [Google Scholar]
- 12.Krukowski RA, Tilford JM, Harvey-Berino J, West DS. Comparing behavioral weight loss modalities: incremental cost-effectiveness of an internet-based versus an in-person condition. Obesity (Silver Spring) 2011;19:1629–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diabetes Prevention Program Research Group . The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care 2012;35:723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai AG, Wadden TA, Volger S, et al. Cost-effectiveness of a primary care intervention to treat obesity. Int J Obes (Lond) 2013;37(Suppl. 1):S31–S37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD Research Group . Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–628 [DOI] [PubMed] [Google Scholar]
- 16.Wing RR, Bolin P, Brancati FL, et al. Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wadden TA, West DS, Delahanty L, et al. Look AHEAD Research Group . The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it [published correction appears in Obesity (Silver Spring) 2007;15:1339]. Obesity (Silver Spring) 2006;14:737–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesche-Thobaben JA. The development and description of the comparison group in the Look AHEAD trial. Clin Trials 2011;8:320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healthcare Cost and Utilization Project. Overview of the Nationwide Inpatient Sample (NIS) Available from http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed 15 September 2010
- 20.Levinson DR. Medicaid Drug Price Comparison: Average Sales Price to Average Wholesale Price. Publication OEI-03-05-00200. Washington, DC, Office of the Inspector General, Department of Health and Human Services, 2005
- 21.Levinson DR. Medicaid Drug Price Comparisons: Average Manufacturer Price to Published Prices. Publication OEI-05-05-00240. Washington, DC, Office of the Inspector General, Department of Health and Human Services, 2005
- 22.Wing RR, Look AHEAD Research Group . Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med 2010;170:1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakicic JM, Jaramillo SA, Balasubramanyam A, et al. Look AHEAD Study Group . Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD Study. Int J Obes (Lond) 2009;33:305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster GD, Borradaile KE, Sanders MH, et al. Sleep AHEAD Research Group of Look AHEAD Research Group . A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med 2009;169:1619–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin RR, Peyrot M, Gaussoin SA, et al. Look AHEAD Research Group . Four-year analysis of cardiovascular disease risk factors, depression symptoms, and antidepressant medicine use in the Look AHEAD (Action for Health in Diabetes) clinical trial of weight loss in diabetes. Diabetes Care 2013;36:1088–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregg E, Chen H, Wagenknecht L, et al.; Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012;308:2489–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rejeski WJ, Ip EH, Bertoni AG, et al. Look AHEAD Research Group . Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med 2012;366:1209–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenway FL, Ryan DH, Bray GA, Rood JC, Tucker EW, Smith SR. Pharmaceutical cost savings of treating obesity with weight loss medications. Obes Res 1999;7:523–531 [DOI] [PubMed] [Google Scholar]
- 29.Potteiger CE, Paragi PR, Inverso NA, et al. Bariatric surgery: shedding the monetary weight of prescription costs in the managed care arena. Obes Surg 2004;14:725–730 [DOI] [PubMed] [Google Scholar]
- 30.Davis WA, Bruce DG, Davis TME. Economic impact of moderate weight loss in patients with type 2 diabetes: the Fremantle Diabetes Study. Diabet Med 2011;28:1131–1135 [DOI] [PubMed] [Google Scholar]
- 31.Redmon JB, Bertoni AG, Connelly S, et al. Look AHEAD Research Group . Effect of the look AHEAD study intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care 2010;33:1153–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Köster I, von Ferber L, Ihle P, Schubert I, Hauner H. The cost burden of diabetes mellitus: the evidence from Germany—the CoDiM study. Diabetologia 2006;49:1498–1504 [DOI] [PubMed] [Google Scholar]
- 33.Wolfenstetter SB. Future direct and indirect costs of obesity and the influence of gaining weight: results from the MONICA/KORA cohort studies, 1995-2005. Econ Hum Biol 2012;10:127–138 [DOI] [PubMed] [Google Scholar]
- 34.Little RJA. Pattern-mixture models for multivariate incomplete data. J Am Stat Assoc 1993;87:1227–1237 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.