Abstract
OBJECTIVE
Agents that augment GLP-1 effects enhance glucose-dependent β-cell insulin production and secretion and thus are hoped to prevent progressive impairment in insulin secretion characteristic of type 2 diabetes (T2D). The purpose of this study was to evaluate GLP-1 effects on β-cell secretory capacity, an in vivo measure of functional β-cell mass, early in the course of T2D.
RESEARCH DESIGN AND METHODS
We conducted a randomized controlled trial in 40 subjects with early T2D who received the GLP-1 analog exenatide (n = 14), the dipeptidyl peptidase IV inhibitor sitagliptin (n = 12), or the sulfonylurea glimepiride (n = 14) as an active comparator insulin secretagogue for 6 months. Acute insulin responses to arginine (AIRarg) were measured at baseline and after 6 months of treatment with 5 days of drug washout under fasting, 230 mg/dL (glucose potentiation of arginine-induced insulin release [AIRpot]), and 340 mg/dL (maximum arginine-induced insulin release [AIRmax]) hyperglycemic clamp conditions, in which AIRmax provides the β-cell secretory capacity.
RESULTS
The change in AIRpot was significantly greater with glimepiride versus exenatide treatment (P < 0.05), and a similar trend was notable for the change in AIRmax (P = 0.1). Within each group, the primary outcome measure, AIRmax, was unchanged after 6 months of treatment with exenatide or sitagliptin compared with baseline but was increased with glimepiride (P < 0.05). α-Cell glucagon secretion (AGRmin) was also increased with glimepiride treatment (P < 0.05), and the change in AGRmin trended higher with glimepiride than with exenatide (P = 0.06).
CONCLUSIONS
After 6 months of treatment, exenatide or sitagliptin had no significant effect on functional β-cell mass as measured by β-cell secretory capacity, whereas glimepiride appeared to enhance β- and α-cell secretion.
Introduction
The main pathophysiologic abnormalities in type 2 diabetes (T2D) are impaired tissue sensitivity to insulin action (i.e., insulin resistance) and impaired β-cell insulin secretion (1). Autopsy studies have observed a relative β-cell mass reduction of 40% from normal by the time impaired fasting glucose develops (≥110 mg/dL) and >60% reduction with overt T2D (2). This decline in β-cell mass has been associated with increased β-cell apoptosis (2), and an emerging role of defective autophagy-associated cell death is linked with the onset of β-cell dysfunction (3,4). Functional β-cell mass is best estimated in vivo as the β-cell secretory capacity derived from glucose potentiation of arginine-induced insulin secretion (5). Consistent with the autopsy data, metabolic studies have reported a relative β-cell secretory capacity reduction of >50% from normal as the fasting glucose increases over 110 mg/dL (1,5). The preservation of functional β-cell mass in T2D remains a major focus of research in hopes of stabilizing or reversing disease progression (6).
Agents that enhance GLP-1 action are purported to hold promise for the preservation of β-cell mass in T2D. GLP-1 is an incretin hormone secreted by L cells of the intestine in response to nutrient ingestion, enhances insulin production and secretion, and inhibits α-cell glucagon secretion in a glucose-dependent manner (7). The biologically active GLP-17–36 amide is rapidly inactivated by the ubiquitous protease dipeptidyl peptidase IV (DPP4). Raising GLP-1 to supraphysiologic levels improves β-cell sensitivity to glucose in T2D (8). Current strategies to enhance GLP-1 effects in T2D include the use of injectable GLP-1 analogues that resist inactivation by DPP4 and oral inhibitors of DPP4 that effectively increase endogenous GLP-1 levels. In rodent models, GLP-1 stimulates β-cell proliferation and exerts antiapoptotic effects, which, together with increased insulin production, is expected to augment functional β-cell mass in vivo (9,10). Whether GLP-1 can increase functional β-cell mass in human diabetes in vivo remains to be elucidated.
Current investigations suggest the acute improvement in β-cell sensitivity to glucose observed with enhancing GLP-1 effects in human T2D (8) may not extend to long-term effects on functional β-cell mass. Bunck et al. (11,12) demonstrated that 1-year treatment with exenatide in T2D significantly improved β-cell secretory capacity while on the drug; however, this benefit was not sustained 1 month after discontinuation. Similarly, there was a significant increase in β-cell secretory capacity in drug-naive T2D subjects treated with the DPP4 inhibitor vildagliptin for 1 year that again was not maintained after a 3-month washout period (13). These studies indicate an acute effect of GLP-1 analogues or DPP4 inhibitors to increase β-cell secretion but do not demonstrate a modifying effect of either drug on functional β-cell mass. These conflicting GLP-1 effects in rodents versus human T2D may be due to the lengthy duration of the washout period, during which glycemic control worsened in both clinical studies such that glucotoxicity may have obviated any previous improvement in β-cell secretory capacity (11–13).
The purpose of this investigation was to address if increasing GLP-1 effects early in the course of T2D would preserve or increase functional β-cell mass as measured by β-cell secretory capacity derived from the glucose-potentiated arginine (GPA) test. In this study, we present the results of a randomized controlled trial comparing the effects of exenatide or sitagliptin with glimepiride as an active comparator insulin secretogogue on β-cell secretory capacity before and after 6 months of treatment 5 days off drug in subjects with impaired fasting glucose or early T2D (fasting glucose ≥110 but <160 mg/dL). The 5-day washout period was designed to eliminate any acute effects of the study drugs on β-cell sensitivity to glucose while avoiding any deterioration in glycemic control to ensure that any trophic effects of the drugs would not be negated by the development of glucotoxicity. GLP-1 analogues and DPP4 inhibitors have been shown to increase both insulin production and secretion (14–16) and so were expected to have a positive effect on functional β-cell mass. A sulfonylurea was selected as an active comparator insulin secretogogue rather than a placebo to provide an agent that would control glycemia by increasing insulin secretion without affecting the functional β-cell mass. Since sulfonylureas induce insulin secretion without affecting production, they have been theorized to deplete insulin stores (17). We, therefore, hypothesized that exenatide and/or sitagliptin would increase β-cell secretory capacity compared with a decrease with glimepiride.
Research Design and Methods
Subjects
Subjects were males and females age 18–70 years with impaired fasting plasma glucose or early T2D as defined by a plasma glucose concentration between 110 and 159 mg/dL following a >12-h overnight fast performed off any antidiabetogenic agent for ≥2 weeks (6 weeks for thiazolinediones) and of stable body weight (±5%) for at least 2 weeks. Exclusion criteria included any prior exposure to GLP-1 analogues or DPP4 inhibitors and active cardiovascular, liver, or kidney disease and are provided in full detail under ClinicalTrials.gov identification number NCT00775684. The study protocol was approved by the University of Pennsylvania Institutional Review Board, and all subjects provided written informed consent. One hundred seventy subjects underwent the screening process, out of which 50 subjects were enrolled (Supplementary Fig. 1). Randomization was performed with stratification designed to balance sex and tiers of age (18–44 and 45–70 years), fasting glucose level (110–126 and 127–159 mg/dL), and BMI (<35 and 35–44 kg/m2) among the three groups.
Study Design
This study was a randomized controlled trial of open-label exenatide or sitagliptin versus an active comparator insulin secretagogue, glimepiride. The sulfonylurea glimepiride was chosen rather than a placebo to ensure adequate glycemic control was maintained in the comparator group while preventing confounding of β-cell effects by use of other agents that affect insulin sensitivity. Sulfonylureas bind to their receptor that inhibits the KATP on the β-cell membrane, leading to depolarization triggering insulin release (17–19). Glimepiride, in particular, was selected, as it has been shown to carry the least risk of hypoglycemia among the sulfonylureas (20). After completing a baseline oral glucose tolerance test (OGTT) and GPA test on separate days, subjects were randomized to receive exenatide 5 μg subcutaneous twice daily, sitagliptin 100 mg, or glimepiride 0.5 mg orally each morning. All subjects received a study glucometer and test strips (OneTouch Ultra; LifeScan, Milpitas, CA) to monitor glucose each morning and evening to detect and report any hypoglycemia (blood glucose <70 mg/dL). Exenatide was increased after 1 month per labeling to 10 μg twice daily. All subjects remained on this dose until completion of the study except for two subjects who experienced side effects but tolerated 5 or 10 μg once daily in each case. Sitagliptin remained at 100 mg for the duration of study and was well tolerated by all subjects. Glimepiride was increased by 0.5–1.0-mg increments in the morning or evening at weekly intervals (maximum total daily dose 4.0 mg, divided) to achieve an average fasting glucose level <110 mg/dL while avoiding any hypoglycemia. No clinically significant hypoglycemia was detected with any treatment arm. Augmentation of meal-related increases in levels of active GLP-17–36 amide in the sitagliptin group was documented by repeating the OGTT at the 5-month visit. Following 6 months of therapy, all drugs were held for 5–7 days (>10 half-lives; see Supplementary Data) prior to completing a final GPA test. The washout period was used to obviate any acute effect of exenatide, sitagliptin, or glimepiride on β-cell insulin or α-cell glucagon secretion during testing.
Subject Preparation for Metabolic Testing
All subjects arrived at the University of Pennsylvania Clinical and Translational Research Center on the morning of testing having fasted overnight after 2000 h for 12 h. By 0700 h, one catheter was placed in an antecubital vein for infusions, and one catheter was placed in a contralateral hand or forearm vein, retrograde when possible, for blood sampling, with the hand or arm warmed by a heating pad to promote arterialization of venous blood (21). Patency of the catheters was maintained with slow infusions of 0.9% saline. At least 20 min of acclimatization to catheter placement transpired prior to testing.
OGTT
After baseline blood sampling at −5 and −1 min, 75 g of anhydrous glucose in solution was ingested over a 5-min period. Blood samples were collected at 15, 30, 60, 90, and 120 min postingestion. At the 5-month visit, morning study medication was held until the completion of testing.
GPA Test
After baseline blood sampling at −5 and −1 min, 5 g arginine hydrochloride (10% solution) was injected over a 1-min period. Blood samples were collected at 2, 3, 4, and 5 min postinjection. After this baseline arginine stimulation test (AST), a hyperglycemic clamp technique (22) utilizing a variable rate of a 20% glucose solution was performed to achieve a plasma glucose level of 230 mg/dL. Blood samples were taken every 5 min and measured at the bedside by an automated glucose analyzer (YSI 2300; Yellow Springs Instruments, Yellow Springs, OH) and used to adjust the infusion rate to achieve the desired level. After 45-min of the glucose infusion, the AST was performed again. It has been demonstrated that the first administration of arginine has no effect on the subsequent response to arginine using this protocol (23). Then, after a 2-h period without glucose infusion, a hyperglycemic clamp was performed to achieve a plasma glucose level of 340 mg/dL. Forty-five minutes after initiation of the glucose infusion, another AST was performed.
Biochemical Analysis
Samples were collected on ice into tubes containing EDTA and protease inhibitor cocktail (and for OGTT samples, DPP4 inhibitor; Sigma-Aldrich, St. Louis, MO), centrifuged at 4°C, separated, and frozen at −80°C for subsequent analysis. Plasma glucose was determined in duplicate by an automated glucose analyzer (YSI 2300; Yellow Springs Instruments). Plasma insulin, proinsulin, and glucagon were measured in duplicate by double-antibody radioimmunoassays and GLP-17–36 amide by ELISA (Millipore, Billerica, MA). Each immunoassay for all time points in a given subject was conducted simultaneously, with representative subjects from each group included with each assay run.
Calculations
Plasma glucose, insulin, and GLP-1 responses during the OGTT were evaluated by the incremental area under the curve (AUC) calculated by the trapezoidal rule with the mean of the baseline values subtracted using the computer software Origin (Northampton, MA). The GPA test enables characterization of glucose-dependent insulin secretion from the glucose dose-response curve for acute insulin response to arginine (AIRarg) performed at fasting, 230 mg/dL, and 340 mg/dL glucose levels. The AST measures first-phase insulin release to a maximally stimulating dose of the nonglucose secretagogue arginine as the AIRarg. The AIRarg was determined as the mean of the 2-, 3-, 4-, and 5-min insulin levels minus the mean of the baseline values (8,24,25). The AIRarg performed during the 230 mg/dL glucose clamp enables determination of glucose potentiation of arginine-induced insulin release (AIRpot). The AIRarg performed during the 340 mg/dL glucose clamp allows for determination of the β-cell secretory capacity (AIRmax) since the AIRarg is maximal at plasma glucose concentrations >315 mg/dL (26,27). Between ∼60 and 250 mg/dL, the magnitude of AIRarg is a linear function of the plasma glucose level, so the difference in AIRarg at fasting and 230 mg/dL glucose levels divided by the difference in plasma glucose (ΔAIRarg/ΔPG) gives the glucose-potentiation slope (GPS) (8,24–26). Using the y-intercept (b) from the line created by these two points, the plasma glucose level at which half-maximal insulin secretion is achieved (PG50) is derived from solving the equation 1/2 (AIRmax) = (GPS · PG50) + b, and provides a measure of β-cell sensitivity to glucose (8,24–26). Insulin sensitivity (M/I) was determined by dividing the mean glucose infusion rate required during the 230 mg/dL glucose clamp (M) by the mean prestimulus insulin level (I) between 40 and 45 min of the glucose infusion (28). The proinsulin-to-insulin (PI/I) ratio was calculated as the molar concentration of proinsulin divided by the molar concentration of insulin × 100. Estimation of the PI/I ratio within the secretory granules of the β-cell is most reliable after acute stimulation of release (29); therefore, we examined the proinsulin secretory ratio in response to each injection of arginine from the respective acute PI/I responses to arginine by dividing the acute proinsulin responses by the acute insulin responses. The acute glucagon response to arginine (AGRarg), glucose inhibition of arginine-induced glucagon release (AGRinh), and minimum arginine-induced glucagon secretion (AGRmin) were calculated similarly as the mean of the postarginine values minus the mean of the prestimulus values under the fasting, 230 mg/dL, and 340 mg/dL glucose clamp conditions (26).
Statistical Analysis
The primary outcome was to determine if increasing GLP-1 effects by exenatide or sitagliptin preserved or increased functional β-cell mass measured as β-cell secretory capacity compared with glimepiride in subjects with early T2D. During year 4 of the study, a prespecified interim analysis was performed after 30 subjects had completed the study (10 subjects in each study group). This interim analysis demonstrated that the change in β-cell secretory capacity (AIRmax) was less in exenatide (P < 0.05) and not different in sitagliptin compared with the glimepiride group, an effect driven by an increase in β-cell secretory capacity in the glimepiride group (P < 0.05). These results effectively refuted our initial hypothesis such that no further subjects were enrolled, although those already active in the study completed the clinical trial to yield the final number of subjects reported. Subjects who did not complete the final GPA test were not included in the primary analysis.
All data are expressed as means ± SE. This was a two-factor experimental design with one repeated measure (baseline and final) and one nonrepeated measure (exenatide, sitagliptin, and glimepiride). To determine if exenatide or sitagliptin induced significant changes from baseline, the change for each measure (∆ = final − baseline) for each group was compared with the change in the glimepiride group using independent Student t tests or the Mann-Whitney U test for nonparametric data. One-way ANOVA was used to compare baseline measures across all three groups. Repeated-measures ANOVA was used to compare changes in weight, capillary blood glucose, and HbA1c over time between groups. When a statistical trend (P < 0.1) was evident with at least one of the between-group differences with glimepiride, within-group changes from baseline to final measures were compared with dependent Student t tests or the Wilcoxon matched-pairs test as appropriate. All comparisons were conducted using Statistica software (StatSoft, Inc., Tulsa, OK). Significance was considered at P < 0.05 (two-tailed).
Results
Subject Characteristics and Disposition
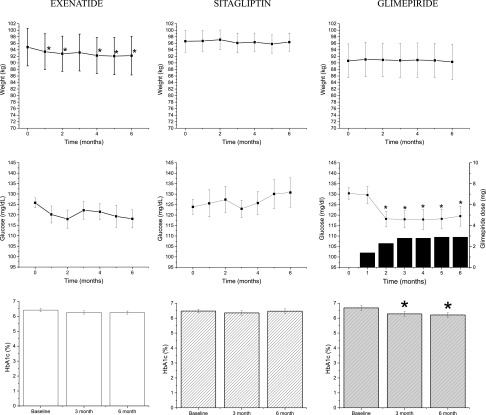
Of the 50 subjects enrolled, 47 subjects underwent randomization and completed baseline GPA testing (Supplementary Fig. 1) with 17 subjects randomized to exenatide, 13 to sitagliptin, and 17 to glimepiride. There were 7 dropouts, such that 14 subjects in the exenatide, 12 in the sitagliptin, and 14 in the glimepiride group completed the study for a total of 40 subjects used in the analysis. Reasons for discontinuation included noncompliance with study medication and/or procedures and adverse events (Supplementary Fig. 1). Baseline demographic measures were similar across all three groups (Table 1). At 6 months, change in weight or BMI were not significantly different across all three groups. Within the exenatide group, subjects had a decrease in weight over the 6-month course of study (Δ−2.6 ± 0.8; P < 0.05; Fig. 1) that was also reflected by a decrease in BMI (P < 0.05; Table 1). Fasting plasma glucose, insulin, and glucagon were similar at baseline and 6 months in all three groups (Table 1). The change in average capillary blood glucose over time was significantly different across all three groups (P < 0.01; Fig. 1). The glimepiride group had a greater reduction in capillary blood glucose than the sitagliptin group (P < 0.01) and the exenatide group by trend (P < 0.1). The lower glucose levels were achieved by 3 months when the average dose of glimepiride was 3 mg. Change in HbA1c over time was different by trend across all three groups (P = 0.07). The change in HbA1c in the exenatide group was not different from that in the glimepiride group, but the change in HbA1c in the sitagliptin group was less from that in the glimepiride group (P < 0.05; Fig. 1). Within group, HbA1c trended lower within the exenatide group (P = 0.09) at the end of 6 months, whereas it decreased significantly with glimepiride (P < 0.05). Lipid profiles were similar across all three groups at baseline, but at 6 months, there was an increase in HDL cholesterol with exenatide, but not sitagliptin treatment, that was significantly greater than that seen with glimepiride treatment (P < 0.05; Table 1).
Table 1.
Subject demographics at baseline and final (after 6 months of therapy) visits
| Exenatide (n = 14) |
Sitagliptin (n = 12) |
Glimepiride (n = 14) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 monthsa | Δ | Baseline | 6 monthsa | Δ | Baseline | 6 monthsa | Δ | |
| Age (years) | 57 ± 2 | — | — | 57 ± 3 | — | — | 52 ± 3 | — | — |
| Sex (% female) | 36 | — | — | 33 | — | — | 43 | — | — |
| Race (% African American) | 50 | — | — | 42 | — | — | 64 | — | — |
| Duration of T2D (years) | 3.3 ± 0.6 | — | — | 5.3 ± 1.7 | — | — | 3.4 ± 0.9 | — | — |
| BMI (kg/m2) | 33 ± 2 | 32 ± 2* | −1 ± 0 | 33 ± 1 | 33 ± 1 | 0 ± 0 | 31 ± 2 | 31 ± 2 | 0 ± 1 |
| HbA1c, %b (mmol/mol) | 6.4 ± 0.1 (46) | 6.2 ± 0.1 (44) | −0.2 ± 0.1 | 6.5 ± 0.1 (48) | 6.5 ± 0.2 (48) | −0.01 ± 0.1 | 6.7 ± 0.1 (50) | 6.2 ± 0.2 (44)* | −0.5 ± 0.2 |
| Fasting glucose (mg/dL) | 129 ± 3 | 128 ± 5 | −2 ± 5 | 131 ± 4 | 132 ± 8 | 1 ± 9 | 134 ± 4 | 126 ± 5 | −8 ± 6 |
| Fasting insulin (μU/mL) | 24 ± 6 | 21 ± 3 | −3 ± 4 | 17 ± 2 | 14 ± 2 | −2.6 ± 2 | 17 ± 2 | 15 ± 1.3 | −2 ± 2 |
| Fasting glucagon (pg/mL) | 40 ± 4 | 42 ± 8 | 2 ± 6 | 50 ± 6 | 47 ± 7 | −3 ± 6 | 51 ± 4 | 51 ± 5 | −0.2 ± 4 |
| Total cholesterol (mg/dL) | 168 ± 7 | 157 ± 6 | −10 ± 7 | 159 ± 12 | 162 ± 10 | 4 ± 6 | 172 ± 10 | 168 ± 11 | −4 ± 7 |
| Triglycerides (mg/dL) | 110 ± 15 | 85 ± 9 | −25 ± 14 | 104 ± 17 | 118 ± 20 | 11 ± 10 | 107 ± 18 | 93 ± 11 | −14 ± 16 |
| LDL cholesterol (mg/dL) | 104 ± 5 | 92 ± 6 | −12 ± 6 | 94 ± 10 | 101 ± 9 | 5 ± 6 | 110 ± 7 | 111 ± 11 | 2 ± 7 |
| HDL cholesterol (mg/dL) | 41 ± 2 | 48 ± 3* | 7 ± 2† | 44 ± 5 | 38 ± 2 | −3 ± 1 | 41 ± 3 | 38 ± 2 | −3 ± 2 |
Data are means ± SE.
Δ, change from baseline to 6 months with each value.
aFinal visits after 6 months of therapy were conducted following a 5- to 7-day drug washout.
bTo convert to mmol/mol, multiply by 10.93 and subtract 23.50.
*P < 0.05 when comparing values within each group.
†P < 0.05 when comparing Δ between the exenatide and glimepiride groups.
Figure 1.
Subject characteristics over the 6-month study period. Means ± SE of weight, fasting capillary glucose as determined by glucometer readings, and HbA1c in each group. Also shown for the glimepiride group is the average dose at each monthly visit. Changes in weight over time were not significantly different across the three groups [F(12, 222) = 1.1013; P = 0.4]. Average capillary glucose was significantly different [F(12, 204) = 2.53; P < 0.01] when comparing across all three groups. HbA1c was different by trend [F(4, 74) = 2.28; P < 0.1] when comparing across all three groups. *P < 0.05 when comparing Δ from baseline within each group at each time point.
Effects on Glucose, Insulin, and Endogenous GLP-1 During the OGTT
At 5 months, the changes in plasma glucose (Supplementary Fig. 2, top) and insulin (Supplementary Fig. 2, middle) responses were not significantly different with either exenatide or sitagliptin when compared with glimepiride treatment. The change in endogenous GLP-1 response was increased with sitagliptin compared with glimepiride treatment (ΔAUC 284 ± 70 vs. 31 ± 24 pmol · min/L; P < 0.01; Supplementary Fig. 2, bottom).
Effects on Insulin, Proinsulin, and Glucagon Responses During the GPA Test
There were no significant differences in the change in AIRarg after 6 months of exenatide or sitagliptin treatment compared with glimepiride (Supplementary Fig. 3 and Table 2), whereas the change in AIRpot was significantly lower in the exenatide group, but not in the sitagliptin group, when compared with the glimepiride group (P < 0.05 for exenatide vs. glimepiride; Table 2), and a similar trend was also evident for the change in AIRmax (P = 0.1 for exenatide vs. glimepiride; Table 2). In fact, within each group, β-cell secretory capacity (AIRmax) increased only in the glimepiride group at 6 months compared with baseline (P < 0.05; Table 2). β-Cell sensitivity to glucose (PG50) was not different at baseline and remained unchanged following any treatment (Table 2). The glucose infusion rates (M), second-phase insulin levels (I), and the resulting estimate of insulin sensitivity (M/I) were not different at baseline and after 6 months across all three groups (Table 2).
Table 2.
Measures of β-cell secretory capacity, β-cell sensitivity to glucose, insulin sensitivity, and glucagon secretion derived from the GPA test
| Exenatide (n = 14) |
Sitagliptin (n = 12) |
Glimepiride (n = 14) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 monthsa | Δ | Baseline | 6 monthsa | Δ | Baseline | 6 monthsa | Δ | |
| AIRarg (μU/mL) | 52 ± 14 | 52 ± 11 | −0.2 ± 9 | 35 ± 4 | 34 ± 6 | −2 ± 7 | 44 ± 6 | 42 ± 4 | −2 ± 7 |
| AIRpot (μU/mL) | 138 ± 31 | 108 ± 21 | −30 ± 20† | 83 ± 12 | 80 ± 15 | −2 ± 8 | 97 ± 16 | 119 ± 19 | 22 ± 12 |
| AIRmax (μU/mL) | 214 ± 60 | 188 ± 34 | −25 ± 50‡ | 149 ± 20 | 158 ± 30 | 9 ± 21 | 133 ± 19 | 202 ± 35* | 69 ± 33 |
| PG50 (mg/dL) | 175 ± 13 | 190 ± 14 | 25 ± 20 | 226 ± 12 | 209 ± 16 | −5 ± 24 | 168 ± 17 | 182 ± 10 | 10 ± 26 |
| M (mg · kg−1 · min−1) | 5.5 ± 0.3 | 5.8 ± 0.4 | 0.27 ± 0.4 | 5.4 ± 0.4 | 5.3 ± 0.4 | −0.1 ± 0.4 | 5.5 ± 0.4 | 5.8 ± 0.4 | 0.35 ± 0.4 |
| I (μU/mL) | 41 ± 13 | 39 ± 9 | −2 ± 9 | 22 ± 2 | 23 ± 4 | 1 ± 3 | 28 ± 7 | 26 ± 2 | −2 ± 5 |
| M/I (mg · kg−1 · min−1/μU/mL) | 0.3 ± 0.1 | 0.3 ± 0.1 | −0.01 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.04 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | −0.04 ± 0.0 |
| AGRarg (pg/mL) | 60 ± 12 | 63 ± 8 | 3 ± 17 | 77 ± 13 | 60 ± 11 | −17 ± 6 | 40 ± 9 | 62 ± 8 | 22 ± 17 |
| AGRinh (pg/mL) | 63 ± 12 | 58 ± 13 | −3 ± 6 | 64 ± 13 | 55 ± 16 | −10 ± 10 | 46 ± 7 | 59 ± 7 | 11 ± 7 |
| AGRmin (pg/mL) | 51 ± 12 | 52 ± 12 | 2 ± 5‡ | 55 ± 8 | 59 ± 19 | 4 ± 21 | 37 ± 6 | 59 ± 8* | 21 ± 8 |
Data are means ± SE.
Δ, change from baseline to 6 months with each value.
aFinal visits after 6 months of therapy were conducted following a 5- to 7-day drug washout.
*P < 0.05 when comparing values within each group.
†P < 0.05 when comparing Δ between the exenatide and glimepiride groups.
‡P ≤ 0.1 (statistical trend) when comparing Δ between exenatide and glimepiride groups.
Fasting proinsulin and APRs were not different across the groups at baseline or in response to treatment. PI/I ratios and proinsulin secretory ratios were unchanged from baseline to 6 months with no significant differences between the exenatide or sitagliptin and glimepiride groups (data not shown).
There were no significant differences in the change in AGRarg and AGRinh with exenatide or sitagliptin treatment after 6 months compared with glimepiride; however, there was a statistical trend when comparing the change in AGRmin with exenatide, but not sitagliptin, to glimepiride (P = 0.06 for exenatide vs. glimepiride; Table 2). Within each group, glucagon secretion was increased only in the glimepiride group at 6 months compared with baseline for AGRmin (P < 0.05; Table 2).
Conclusions
This study evaluated functional β-cell mass as determined by the β-cell secretory capacity in subjects with early T2D treated with exenatide, a GLP-1 analogue, or sitagliptin, a DPP4 inhibitor, compared against an active comparator sulfonylurea, glimepiride. Our results demonstrate that in early T2D, 6-month treatment with a GLP-1 analogue or DPP4 inhibitor does not increase functional β-cell mass relative to treatment with a sulfonylurea. While these data were contrary to our hypothesis, they are consistent with previous reports demonstrating no sustained effects of GLP-1 on β-cell secretory capacity (11–13). Unlike previous studies in which the drug washout period was ≥1 month, during which any potential beneficial effects could be reversed by documented worsening glycemic control, our results were obtained 5 days after discontinuation of the study medication to ensure effective drug washout while avoiding development of hyperglycemia. Indeed, fasting plasma glucose was controlled within each group at the 6-month visit compared with baseline. Thus, these are the first data to demonstrate a lack of improvement in β-cell secretory capacity with a GLP-1 analogue or a DPP4 inhibitor off drug and in the absence of overt hyperglycemia, while demonstrating a remarkable increase in β-cell secretory capacity with sulfonylurea treatment.
Exenatide-treated subjects experienced a decrease in weight and consequently BMI, although this was not statistically different from a neutral weight effect seen in the glimepiride group. In the exenatide-treated subjects, there was also an increase in plasma HDL cholesterol that has previously been demonstrated (30). Sitagliptin treatment was effective in increasing the endogenous GLP-1 response 2.3-fold to oral glucose in our study that is consistent with previous reports (31). In contrast to previous studies, there was no significant effect of exenatide or sitagliptin on HbA1c. This is likely attributable to the early T2D in our subjects who were at the threshold of overt diabetes (average HbA1c of 6.5% [48 mmol/mol]), while most outcomes studies have included subjects with more advanced T2D (average HbA1c ≥8.5% [69 mmol/mol]) (32,33). Our study is limited by its small sample size and short duration and thus does not allow us to determine whether prolonged treatment with exenatide or sitagliptin early in the course of T2D may prevent deterioration in glycemic control, perhaps through mechanisms other than affecting the β-cell secretory capacity.
Six months of treatment with glimepiride was effective in decreasing capillary blood glucose and lowering HbA1c without producing hypoglycemic episodes or weight gain with careful dose titration. Postmarketing reports and clinical trials have demonstrated significant increases in weight with glimepiride treatment but these observations were notably with higher concentrations of glimepiride (4–8 mg) titrated rapidly over a 1–4-week period (34). Similar to our finding of an increase in β-cell secretory capacity evident after 6 months, Karunakaran et al. (35) demonstrate a significant improvement in β-cell function with glicazide treatment for 1 year while demonstrating lower fasting plasma glucose and HbA1c without adversely effecting weight. Interpretation of our study’s results with glimepiride must be made cautiously, however, as glimepiride was included as an active comparator without a placebo group for comparison.
While the increase in β-cell secretory capacity after 6 months of treatment with glimepiride observed in this study requires confirmation and further assessment of durability, there are a few speculative mechanisms to explain such an effect. Compared with the exenatide and sitagliptin groups, the glimepiride-treated subjects experienced a reduction in capillary glucose. This improved glycemic control may have positively affected the β-cell secretory capacity. Another possibility may be a more specific effect of sulfonylureas against autophagy-associated cell death. Autophagy is a self-digestive mechanism that regulates protein turnover, and current evidence links impaired autophagy with accumulation of autophagic vacuoles in the β-cells of T2D when compared with nondiabetic islets (4). Altered autophagic mechanisms are evident when there is a mismatch between insulin production and secretion, as may occur in T2D, in which there is impaired β-cell sensitivity to glucose (36). As sulfonylureas such as glimepiride have no effect on insulin production but stimulate insulin secretion (36), these agents may correct a synthetic mismatch and protect against autophagy-associated cell death. Whether such effects may be associated with increases in functional β-cell mass as measured by β-cell secretory capacity warrants further study.
Curiously, α-cell glucagon secretion was increased after 6 months of exposure to glimepiride. The effect of sulfonylureas on α-cells remains unclear at present due to conflicting results that may be attributed to variation in model systems—i.e., with intact islets in which paracrine signaling remains intact versus diseased islets in which paracrine signaling is disrupted versus isolated α-cells (37,38). For example, Cheng-Xue et al. (37) demonstrated a glucagontrophic effect of tolbutamide when paracrine signaling by somatostatin was disrupted. These authors postulate that glucagon secretion is controlled by two mechanisms, one that is direct from the closure of KATP channels and one that is indirect via control from paracrine signaling (37). Thus, glimepiride may induce depolarization of the α-cell, and under normal, nondiseased conditions, glucose, insulin, and/or somatostatin via paracrine action can inhibit glucagon release. However, in T2D, there may be an uncoupling phenomenon in which glucagon secretion can become independent of these paracrine inhibitory signals, and the stimulatory effect of sulfonylureas at the α-cells predominates such that 6-month treatment may be glucagontrophic, as reported in this study. Whether long-term benefits of sulfonylureas that may enhance functional β-cell mass outweigh any adverse consequence of the increased glucagon secretion on glycemic control remains to be determined.
In conclusion, the implication of the current study is that a 6-month treatment with the GLP-1 analogue exenatide or DPP4 inhibitor sitagliptin does not increase β-cell secretory capacity in human T2D as purported in rodent models. Furthermore, our study indicates that the sulfonylurea glimepiride may be effective in at least short-term improvement in β-cell secretory capacity associated with improved glycemic control early in the course of T2D. Clinically, these findings support T2D treatment algorithms that place sulfonylureas ahead of incretin-based approaches (39), with special consideration for third-generation sulfonylureas such as glimepiride, in which careful dose titration as conducted in this study may avoid weight gain and hypoglycemia.
Supplementary Material
Article Information
Acknowledgments. The authors thank Dr. Anne Cappola, Perelman School of Medicine at the University of Pennsylvania, for serving as the medical monitor; Dr. John Angeloni, City Line Family Medicine, Bala Cynwyd, PA, for referring subjects to the study; Dr. Heather Collins, University of Pennsylvania Diabetes Research Center, for performance of the radioimmunoassays; and Huong-Lan Nguyen, Monell Chemical Senses Center at the University of Pennsylvania, for laboratory assistance.
Funding. This work was supported by the Pennsylvania Department of Health, Bureau of Health Statistics, and research grant 4100043362 (Commonwealth of Pennsylvania Center for Excellence in Regenerative Medicine), Public Health Service research grants UL1-TR-000003 (University of Pennsylvania Clinical and Translational Research Center) and P30-DK-19525 (University of Pennsylvania Diabetes Research Center), the Peterman-Arnold and Joanne and Raymond Welsh Research Fellowships (to L.G.), and the Human Metabolism Resource of the University of Pennsylvania Institute for Diabetes, Obesity and Metabolism. The study drugs used were purchased commercially and not provided by any company.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. L.G. participated in the conduct of the study, analyzed the data, and wrote the manuscript. N.K.R. coordinated the study and managed data acquisition and entry. C.S.F. participated in the conduct of the study. R.G. assisted with the study design, randomization procedures, and statistical analysis. M.H.S. assisted with the study design, in particular with drug selection and dosing. M.R.R. served as the principal investigator. All authors reviewed, edited, and approved the submitted manuscript. M.R.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented in poster form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013.
Footnotes
Clinical trial reg. no. NCT00775684, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-0398/-/DC1.
References
- 1.Kahn SE. Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 2001;86:4047–4058 [DOI] [PubMed] [Google Scholar]
- 2.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 10.2337/diabetes.52.1.102 [DOI] [PubMed] [Google Scholar]
- 3.Marchetti P, Masini M. Autophagy and the pancreatic beta-cell in human type 2 diabetes. Autophagy 2009;5:1055–1056 10.4161/auto.5.7.9511 [DOI] [PubMed] [Google Scholar]
- 4.Masini M, Bugliani M, Lupi R, et al. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia 2009;52:1083–1086 10.1007/s00125-009-1347-2 [DOI] [PubMed] [Google Scholar]
- 5.Kahn SE, Carr DB, Faulenbach MV, Utzschneider KM. An examination of beta-cell function measures and their potential use for estimating beta-cell mass. Diabetes Obes Metab 2008;10(Suppl. 4):63–76 10.1111/j.1463-1326.2008.00945.x [DOI] [PubMed] [Google Scholar]
- 6.U.K. Prospective Diabetes Study Group . U.K. Prospective Diabetes Study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995;44:1249–1258 10.2337/diab.44.11.1249 [DOI] [PubMed] [Google Scholar]
- 7.Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 1986;63:492–498 10.1210/jcem-63-2-492 [DOI] [PubMed] [Google Scholar]
- 8.Ahrén B, Larsson H, Holst JJ. Effects of glucagon-like peptide-1 on islet function and insulin sensitivity in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1997;82:473–478 [DOI] [PubMed] [Google Scholar]
- 9.Mu J, Petrov A, Eiermann GJ, et al. Inhibition of DPP-4 with sitagliptin improves glycemic control and restores islet cell mass and function in a rodent model of type 2 diabetes. Eur J Pharmacol 2009;623:148–154 10.1016/j.ejphar.2009.09.027 [DOI] [PubMed] [Google Scholar]
- 10.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999;48:2270–2276 10.2337/diabetes.48.12.2270 [DOI] [PubMed] [Google Scholar]
- 11.Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009;32:762–768 10.2337/dc08-1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunck MC, Cornér A, Eliasson B, et al. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 2011;34:2041–2047 10.2337/dc11-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley JE, Bunck MC, Möller-Goede DL, et al. Beta cell function following 1 year vildagliptin or placebo treatment and after 12 week washout in drug-naive patients with type 2 diabetes and mild hyperglycaemia: a randomised controlled trial. Diabetologia 2011;54:1985–1991 10.1007/s00125-011-2167-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A 1987;84:3434–3438 10.1073/pnas.84.10.3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drucker DJ. The biology of incretin hormones. Cell Metab 2006;3:153–165 10.1016/j.cmet.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 16.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 1987;2:1300–1304 10.1016/S0140-6736(87)91194-9 [DOI] [PubMed] [Google Scholar]
- 17.Almér LO, Johansson E, Melander A, Wåhlin-Boll E. Influence of sulfonylureas on the secretion, disposal and effect of insulin. Eur J Clin Pharmacol 1982;22:27–32 10.1007/BF00606421 [DOI] [PubMed] [Google Scholar]
- 18.Davies MJ, Metcalfe J, Day JL, Grenfell A, Hales CN, Gray IP. Effect of sulphonylurea therapy on plasma insulin, intact and 32/33 split proinsulin in subjects with type 2 diabetes mellitus. Diabet Med 1994;11:293–298 10.1111/j.1464-5491.1994.tb00274.x [DOI] [PubMed] [Google Scholar]
- 19.Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab 2005;90:501–506 10.1210/jc.2004-0699 [DOI] [PubMed] [Google Scholar]
- 20.Holstein A, Plaschke A, Egberts EH. Lower incidence of severe hypoglycaemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes Metab Res Rev 2001;17:467–473 10.1002/dmrr.235 [DOI] [PubMed] [Google Scholar]
- 21.McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol 1976;41:565–573 [DOI] [PubMed] [Google Scholar]
- 22.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 23.Larsson H, Ahrén B. Glucose-dependent arginine stimulation test for characterization of islet function: studies on reproducibility and priming effect of arginine. Diabetologia 1998;41:772–777 10.1007/s001250050986 [DOI] [PubMed] [Google Scholar]
- 24.Ward WK, Halter JB, Beard JC, Porte D, Jr. Adaptation of B and A cell function during prolonged glucose infusion in human subjects. Am J Physiol 1984;246:E405–E411 [DOI] [PubMed] [Google Scholar]
- 25.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D, Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984;74:1318–1328 10.1172/JCI111542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seaquist ER, Robertson RP. Effects of hemipancreatectomy on pancreatic alpha and beta cell function in healthy human donors. J Clin Invest 1992;89:1761–1766 10.1172/JCI115779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teuscher AU, Kendall DM, Smets YF, Leone JP, Sutherland DE, Robertson RP. Successful islet autotransplantation in humans: functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes 1998;47:324–330 10.2337/diabetes.47.3.324 [DOI] [PubMed] [Google Scholar]
- 28.Guldstrand M, Ahrén B, Adamson U. Improved beta-cell function after standardized weight reduction in severely obese subjects. Am J Physiol Endocrinol Metab 2003;284:E557–E565 [DOI] [PubMed] [Google Scholar]
- 29.Horwitz DL, Starr JI, Mako ME, Blackard WG, Rubenstein AH. Proinsulin, insulin, and C-peptide concentrations in human portal and peripheral blood. J Clin Invest 1975;55:1278–1283 10.1172/JCI108047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006;8:436–447 10.1111/j.1463-1326.2006.00602.x [DOI] [PubMed] [Google Scholar]
- 31.Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab 2006;91:4612–4619 10.1210/jc.2006-1009 [DOI] [PubMed] [Google Scholar]
- 32.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE, Sitagliptin Study 021 Group . Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006;29:2632–2637 10.2337/dc06-0703 [DOI] [PubMed] [Google Scholar]
- 33.Blevins T, Pullman J, Malloy J, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 2011;96:1301–1310 10.1210/jc.2010-2081 [DOI] [PubMed] [Google Scholar]
- 34.Goldberg RB, Holvey SM, Schneider J. A dose-response study of glimepiride in patients with NIDDM who have previously received sulfonylurea agents. The Glimepiride Protocol #201 Study Group. Diabetes Care 1996;19:849–856 10.2337/diacare.19.8.849 [DOI] [PubMed] [Google Scholar]
- 35.Karunakaran S, Hammersley MS, Morris RJ, Turner RC, Holman RR. The Fasting Hyperglycaemia Study: III. Randomized controlled trial of sulfonylurea therapy in subjects with increased but not diabetic fasting plasma glucose. Metabolism 1997;46(Suppl. 1):56–60 10.1016/S0026-0495(97)90319-X [DOI] [PubMed] [Google Scholar]
- 36.Uchizono Y, Alarcón C, Wicksteed BL, Marsh BJ, Rhodes CJ. The balance between proinsulin biosynthesis and insulin secretion: where can imbalance lead? Diabetes Obes Metab 2007;9(Suppl. 2):56–66 10.1111/j.1463-1326.2007.00774.x [DOI] [PubMed] [Google Scholar]
- 37.Cheng-Xue R, Gómez-Ruiz A, Antoine N, et al. Tolbutamide controls glucagon release from mouse islets differently than glucose: involvement of K(ATP) channels from both α-cells and δ-cells. Diabetes 2013;62:1612–1622 10.2337/db12-0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gregorio F, Ambrosi F, Cristallini S, Pedetti M, Filipponi P, Santeusanio F. Therapeutical concentrations of tolbutamide, glibenclamide, gliclazide and gliquidone at different glucose levels: in vitro effects on pancreatic A- and B-cell function. Diabetes Res Clin Pract 1992;18:197–206 10.1016/0168-8227(92)90146-I [DOI] [PubMed] [Google Scholar]
- 39.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–1596 10.1007/s00125-012-2534-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.