Abstract
Objective
To evaluate whether counts of circulating colony forming unit-endothelial cells (CFU-ECs), cells co-expressing CD34, CD133, and CD31 (CD34+CD133+CD31+), and CD34+CD45− cells are altered in adolescents with type 1 diabetes and if the changes in counts correlate with endothelial dysfunction.
Study design
Adolescents with diabetes (ages 18 to 22 years) and race- and sex-matched control subjects were studied. We assessed circulating CFU-ECs, using colony assays, and CD34+CD133+CD31+ and CD34+CD45− cells, using poly-chromatic flow cytometry. CFU-ECs and CD34+CD133+CD31+ are hematopoietic-derived progenitors that inversely correlate with cardiovascular risk in adults. CD34+CD45− cells are enriched for endothelial cells with robust vasculogenic potential. Vascular reactivity was tested by laser Doppler iontophoresis.
Results
Subjects with diabetes had lower CD34+CD133+CD31+ cells, a trend toward reduced CFU-ECs, and increased CD34+CD45− cells compared with control subjects. Endothelium-dependent vasodilation was impaired in subjects with diabetes, which correlated with reductions in circulating CD34+CD133+CD31+ cells.
Conclusions
Long-term sequelae of type 1 diabetes include vasculopathies. Endothelial progenitor cells promote vascular health by facilitating endothelial integrity and function. Lower CD34+CD133+CD31+ cells may be a harbinger of future macrovascular disease risk. Higher circulating CD34+CD45− cells may reflect ongoing endothelial damage. These cells are potential biomarkers to guide therapeutic interventions to enhance endothelial function and to prevent progression to overt vascular disease.
The primary sources of morbidity and mortality in individuals with diabetes are microvascular and macrovascular atherosclerotic complications. Despite advances in diabetes care, early cardiovascular disease remains common in persons with type 1 diabetes.1,2 Progression of vascular disease is related to the degree of glycemic control over time, and intensive management of glycemia in persons with type 1 diabetes can affect the rate of cardiovascular events.3 Currently, hemoglobin A1C (A1C) is the primary variable used to predict risk of subsequent vasculopathy. Yet, the rate and timing of complication development varies from individual to individual and is not solely determined by glycemic measurements. No specific biomarkers are available to identify which children with type 1 diabetes are at highest risk of later vascular diseases.
Endothelial dysfunction is a predictor of cardiovascular disease.4-6 Given that diabetes is characterized by inflammation7 and oxidative excess,8 the endothelium in individuals with type 1 diabetes is continually exposed to multiple stressors that promote the development of endothelial dysfunction.
Homeostatic regulation of the endothelium is complex, requiring dynamic interactions between endothelial cells resident in vessel walls and cells circulating in the peripheral blood to sustain endothelial integrity and function. Circulating endothelial progenitor cells (EPCs) are purported to participate in both of these processes by promoting neovascularization and reendothelialization. Vascular reactivity studies have documented endothelial dysfunction in adolescents and young adults with type 1 diabetes. These include studies demonstrating reductions in brachial flow-mediated dilation,9-11 increases in carotid intima-media thickness,10-12 and higher radial artery stiffness by tonometry.13
Studies in adults, including subjects with type 1 and type 2 diabetes, demonstrate an inverse correlation between vascular disease risk and circulating EPC numbers.14-17 In these studies, a variety of methods were used to quantify circulating EPCs including colony formation assays and polychromatic flow cytometry to detect cells that co-express CD34, CD133, CD31, and/or vascular endothelial growth factor receptor 2. However, because these important observations were made, the definition of an EPC has been questioned due to data demonstrating that these methods do not identify endothelial precursors, but rather subpopulations of hematopoietic progenitor cells that facilitate vascular repair and angiogenesis via secretion of paracrine growth factors (reviewed in Reference 18).18 Specifically, circulating progenitor cells (CPCs) are primitive hematopoietic progenitor cells, and colony forming unit-endothelial cells (CFU-ECs) are not endothelial cells but angiogenic macrophages.18-21 In addition, a distinct subpopulation of peripheral blood cells expresses CD34 but not CD45 (CD34+CD45−), which is enriched for endothelial colony forming cells (ECFCs).21 ECFCs are true endothelial cells with robust proliferative, clonogenic, and vasculogenic potential. Interestingly, ECFCs are elevated in patients with severe coronary artery disease22 and an increase in circulating CD34+CD45− cells positively correlates with vascular disease progression.19
Despite ongoing study of the identity of these cells, multiple studies in adults demonstrate that a reduction in the circulating numbers of CFU-ECs and CPCs correlate with increased vascular disease risk,14,16,23,24 suggesting that rare populations of circulating cells have an important role in protecting from vascular morbidities. However, limited studies have been conducted in children or young adults to examine whether a reduction in CPCs is present early in disease and whether alterations in circulating progenitor subpopulations correlate with a measure of vascular dysfunction. Using colony formation assays and novel poly-chromatic flow cytometry methods, we questioned whether adolescents with type 1 diabetes for >5 years have reduced CFU-ECs and CPCs that correlate with evidence of endothelial dysfunction assessed by laser Doppler iontophoresis.
Methods
The study was approved by the Indiana University Institutional Review Board. Patients with type 1 diabetes for at least 5 years and healthy sex- and race-matched control subjects ages 18 to 22 years were recruited. Control subjects were recruited both through advertisements and by asking patients to ask a friend without diabetes to participate with them. Subjects were excluded if they had evidence of insulin resistance (acanthosis, extreme obesity), were smokers, or were on medications other than insulin or oral contraceptive pills. Consent was obtained from all subjects.
All assessments were done in the morning after subjects had been fasting for at least 3 hours. Subjects with diabetes took their morning insulin as usual (adjusting for the fasting conditions). Height, weight, and blood pressure were obtained. Subjects completed the International Physical Activity Questionnaire, Short Format, to assess physical activity over 7 days before the visit.25 Peripheral blood was collected for A1C, fructosamine, lipid profiles, high-sensitivity c-reactive protein (hsCRP), colony formation assays, and flow cytometry. Vascular reactivity was assessed by laser Doppler iontophoresis.
Preparation of Mononuclear Cells
Peripheral blood was collected in citrate cell preparation tubes (BD Biosciences, Franklin Lakes, New Jersey) and processed at the Angiogenesis and Endothelial Progenitor Cell Core at Indiana University. Tubes were centrifuged at 1600g for 30 minutes, and low-density mononuclear cells (MNCs) were collected similar to previous studies20 for colony formation assays and flow cytometry studies.
Colony Formation Assay
CFU-ECs were cultured from MNCs using the EndoCult Liquid Medium Kit (StemCell Technologies, Vancouver, British Columbia, Canada) per the manufacturer’s protocol and as previously described.20 Briefly, MNCs were resuspended in complete EndoCult medium (Cambrex, Walkersville, Maryland) and seeded on fibronectin-coated tissue culture plates (BD Biosciences). After 48 hours, nonadherent cells were collected and replated in fibronectin-coated tissue culture plates for 3 days. Colonies were identified as elongated sprouting cells radiating from a central core of round cells. Colony numbers were scored in a blinded fashion.
Poly-Chromatic Flow Cytometry
MNCs were incubated with Fc block for 10 minutes at 4 °C (Miltenyi Biotec, Auburn, California) before staining with directly conjugated monoclonal antibodies against human antigens, CD31 fluoroscein isothyocyanate (FITC, BD Pharmingen, San Diego, California, cat. No. 555445), CD34 phycoerythrin (PE, BD Pharmingen, cat. No. 550761), CD133 allophycocyanin (APC, Miltenyi Biotec, Auburn, California, cat. No. 130-090-826), CD45 APC-AlexaFluor (AF) 750 (Invitrogen, Carlsbad, California, cat. No. MHCD4527), as well as the viability marker ViViD (Invitrogen) and CD41a (BD Pharmingen, cat. No. 555465), and CD235a (glyA, R&D Systems, Minneapolis, Minnesota, cat. No. MAB1228), both conjugated to Pacific Blue (PacB, Invitrogen) for the exclusion of platelets and red blood cells, respectively. Cells were incubated with antibodies for 30 minutes at 4 °C, washed twice in PBS with 2% FBS, and fixed in 300μL 1% paraformaldehyde (Sigma Aldrich, St. Louis, Missouri). Fluorescence minus 1 controls were prepared as negative gating controls and anti-mouse Ig BD Comp-Beads (BD Biosciences, Bedford, Massachusetts) were stained with each of the individual test antibodies to serve as single-color compensation controls. The frequency of phenotypically defined cell populations was analyzed using a Becton Dickinson LSR II flow cytometer and FlowJo software, version 8.7.3 (Tree Star, Inc., Ashland, Oregon). At least 300 000 events were collected and analyzed for each sample.
Vascular Reactivity Studies
Participants’ forearms were measured using a tape measure and a probe connected to a laser Doppler instrument (Periflux 5001, Perimed, Stockholm, Sweden) was attached at the midpoint of the subject’s forearm. The probe temperature was at 32 °C during all tests. Basal skin perfusion was measured for 2 minutes; then, either acetylcholine (Miochol-E, Novartis, Stein, Switzerland), to assess endothelium-dependent vasodilation, or sodium nitroprusside (Hospira Inc., Lake Forest Illinois), to assess endothelium-independent vasodilation, was introduced into the skin using iontophoresis via the PeriIont Micropharmacology System, PF382b (Perimed). Acetylcholine stimulations were done first and then subsequently sodium nitroprusside on the opposite arm. Iontophoretic stimulation took place for 20 seconds at 60-second intervals and was repeated 6 times to achieve maximum stimulated perfusion. The electrical current used for iontophoresis was minimal at 0.1 mA and was delivered through a device that uses a 9-V battery as the power source. Vasodilation was calculated from changes in the laser Doppler signal measured during basal and acetylcholine or nitroprusside stimulated periods. Laser Doppler output was expressed in perfusion units (PU) of output voltage (1 PU = 10 mV). Percent change in perfusion was calculated by dividing PUs measured at each stimulation by basal PUs × 100. In our lab, in young adults, we found that the coefficient of variation of the percent change in perfusion for 9 assessments on 3 subjects was 22%, similar to other reports using this technique.26-28
Statistical Analyses
The primary goal of this study was to obtain comparative data of EPC counts and function for adolescents with and without type 1 diabetes. A power analysis was not conducted prospectively because no EPC data were available to estimate sample size a priori. Descriptive statistics were calculated for all variables including EPC counts. Means ± standard deviations are reported unless otherwise noted. Two-sample t tests and Wilcoxon rank sum tests were used to compare the type 1 diabetes group with the control group. Vascular reactivity data were analyzed using repeated-measures ANOVA with unstructured covariance. Covariates included baseline outcome, time, and group. Appropriate transformation was applied to meet the normality assumption. For the acetylcholine induced vasodilation data, the model-based mean plot over time clearly showed that the difference between the groups increased over time, although there was no significant interaction effect between time and group. Therefore, the interaction term was included in the model and group difference was tested at each time point. SAS version 9.1 (SAS Institute Inc., Cary, North Carolina) and SPSS version 16.0 (SPSS Inc., Chicago, Illinois) were used for all statistical analyses.
Results
Seventeen adolescents with type 1 diabetes (8 males; 2 black) and 18 control subjects (9 males; 1 black) were enrolled with a mean age of 20.3 ± 1.4 years. Individuals with type 1 diabetes had been diagnosed for a mean of 10.7 ± 3.5 years (range, 4.9 to 17.6) and had no clinical evidence of vascular disease (no microalbuminuria or history of retinopathy). No significant differences were observed between the groups except in A1C and fructosamine (Table). Physical activity scores were not significantly different between groups (44% of control subjects and 53% of type 1 diabetes reported high physical activity).
Table. Clinical and laboratory data for subjects.
| Diabetic patients (n=17) |
Control subjects (n=18) |
P value | |
|---|---|---|---|
| Height (cm) | 169.7 ± 9.0 | 173.1 ± 11.0 | .335 |
| Weight (kg) | 73.8 ± 13.2 | 72.1 ± 15.8 | .741 |
| Body mass index (kg/m2) | 25.7 ± 4.4 | 23.9 ± 3.3 | .179 |
| Blood pressure (mm Hg) | |||
| Systolic blood pressure | 116.1 ±13.1 | 120.9 ±15.5 | .351 |
| Diastolic blood pressure | 67.3 ± 8.1 | 65.4 ± 11.9 | .606 |
| Hemoglobin A1C (%) | 9.4 ± 1.6 | 5.1 ± 0.3 | <.001 |
| Fructosamine (μM) | 409.3 ± 81.8 | 236.2 ± 16.3 | <.001 |
| Total cholesterol (mg/dL) | 165 (138-323) | 159 (133-333) | .647 |
| Median (range) | |||
| HDL cholesterol (mg/dL) | 59.2 ± 11.9 | 60.1 ± 9.8 | .795 |
| Triglycerides (mg/dL) | 88(40-381) | 107.5 (29-242) | .807 |
| Median (range) | |||
| hsCRP (ng/mL) | 3159 ± 4126 | 3729 ± 8294 | .802 |
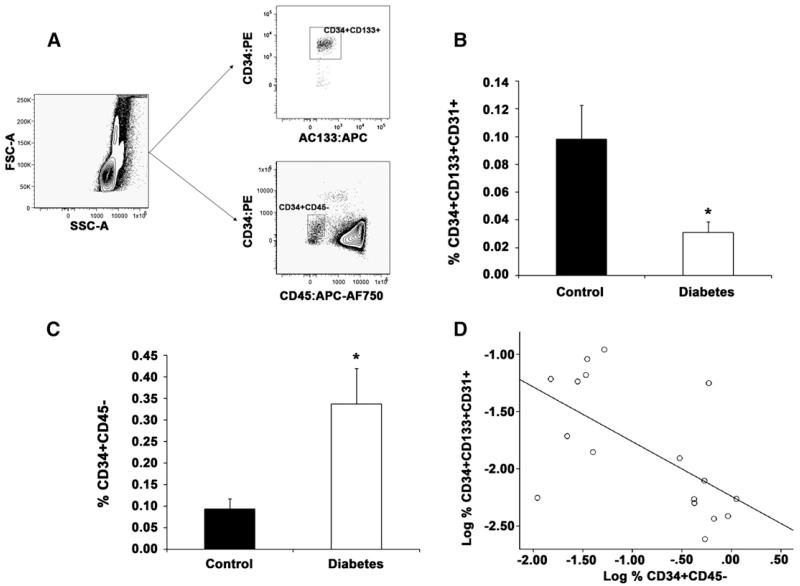
To examine whether adolescents with type 1 diabetes have a reduction in circulating progenitor cell populations that facilitate angiogenesis and inversely correlate with vascular disease risk, well-established flow cytometric and CFU-EC assays were conducted. Samples were analyzed from all 17 patients with diabetes and 16 control subjects (insufficient cells were recovered from 2 control specimens). Figure 1, A illustrates the gating strategy used for flow cytometric analyses. Interestingly, fewer CD34+CD133+CD31+ cells or CPCs were detected in subjects with diabetes compared with control subjects (Figure 1, B). In addition, CFU-EC frequency trended lower in subjects with diabetes compared with control subjects (13.2 ± 18.2 versus 26.5 ± 33.1 colonies/10−6 MNCs, respectively, P = .119), which is consistent with studies in older adults with long-standing diabetes.15,17,23
Figure 1.
Young adults with type 1 diabetes have reduced circulating CD34+CD133+CD31+ cells and increased CD34+CD45− cells. Poly-chromatic flow cytometry demonstrates alterations in circulating subpopulations. Peripheral blood MNCs were evaluated for the proportion of CD34+CD133+CD31+ and CD34+CD45− cells using flow cytometry. A, Representative dot plots demonstrating the gating strategy for CD34, CD133, and CD45 expression is shown. All CD34+CD133+ cells co-expressed CD31. The mean proportion of circulating B, CD34+CD133+CD31+ and C, CD34+CD45− cells are shown. Error bars represent SEM, n = 17 for type 1 diabetes samples and n = 16 for control samples. D, Correlation between CD34+CD133+CD31+ and CD34+CD45− cell populations was examined for the diabetic patients, *P < .05.
Previously, we reported that CD34+CD45− cells are enriched for ECFCs, endothelial cells with functional properties characteristic of progenitor cells.21 Given recent studies demonstrating that an increase in circulating CD34+CD45− cells positively correlates with vascular disease progression,19,22 we examined whether circulating CD34+CD45− cells were elevated in subjects with diabetes. Adolescents with type 1 diabetes had a significant increase in CD34+CD45− cells compared with control subjects (Figure 1, C). We speculated that this observation was indicative of endothelial injury and subsequent mobilization into the circulation. Therefore, an inverse correlation should exist between circulating levels of CD34+CD45− and CD34+CD133+CD31+ cells in subjects with diabetes. Linear regression analysis supported this hypothesis (Figure 1, D, r = −0.604, P = .01). Together these data demonstrate that adolescents with type 1 diabetes without clinically evident indications of vascular impairment already exhibit altered levels of circulating CD34+CD133+CD31+ and CD34+ CD45− subpopulations.
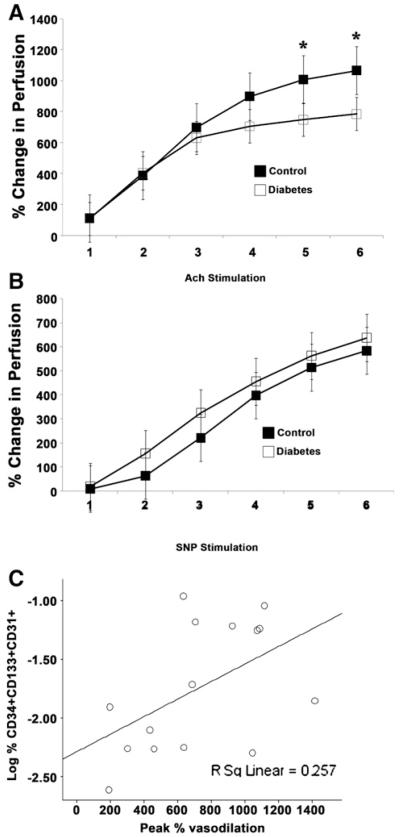
To examine whether altered circulating CD34+CD133+ CD31+ and CD34+CD45− cells in subjects with diabetes correlated with impaired endothelial function, vascular reactivity studies were conducted using laser Doppler iontophoresis.29,30 Iontophoretic stimulation with acetylcholine was used to examine endothelial-dependent vasodilation, and iontophoretic delivery of nitroprusside evaluated endothelial-independent vasodilation. Interestingly, adolescents with type 1 diabetes exhibited a significant reduction in acetylcholine induced vasodilation, both assessed as peak percent change (653% ± 329%; range, 164% to 1371% in diabetics vs 1002% ± 499%; range, 388% to 1969% in control subjects, P < .03) and as mean over time (Figure 2, A) compared with control subjects, though had intact vasodilation in response to nitroprusside (Figure 2, B). Importantly, these data demonstrate that adolescents with type 1 diabetes exhibit endothelial dysfunction. To determine whether enumeration of circulating CD133+ CD34+ and/or CD34+CD45− cell numbers may predict the degree of endothelial dysfunction, we next examined whether circulating frequencies of these defined subpopulations correlated with endothelial dependent vasodilation. As expected, peak acetylcholine induced vasodilation directly correlated with circulating CD133+CD34+ cell numbers (Figure 2, C, r = 0.507, P = .05), indicating greater endothelial dysfunction in individuals with lower numbers of circulating CD133+CD34+ cells. Interestingly, a trend towards a negative correlation between CD34+CD45− cell numbers and peak acetylcholine induced vasodilation was detected, though not statistically significant (r = −0.461, P = .08).
Figure 2.
The degree of endothelial dysfunction in young adults with type 1 diabetes positively correlates with CD34+CD133+CD31+ cells in the peripheral blood. Vascular reactivity was evaluated using a laser Doppler iontophoresis methodology. A, Endothelium-dependent function was assessed by measuring vasodilation in response to 6 repeated stimulations with acetylcholine (Ach), n = 17 per group,* P < .05. B, Endothelium-independent function was assessed by measuring vasodilation in response to 6 repeated stimulations with nitroprusside (SNP), n = 17 control subjects, n = 16 subjects with diabetes. C, Correlation between CD34+CD133+CD31+ cells and maximal vasodilation after iontophoretic stimulation with acetylcholine was examined for the subjects with diabetes, * P < .05.
No correlations existed between fructosamine or A1C with circulating CD34+CD133+CD31+, CD34+CD45−, or vascular reactivity in subjects with diabetes, indicating that mean glycemic control in individuals with established diabetes over the preceding 1 to 3 months was not related to these outcomes. There were also no significant correlations between hsCRP and any of these measures, probably reflecting that this was overall a young and healthy population. Collectively, these data demonstrate adverse effects of type 1 diabetes on circulating progenitor cell numbers and endothelial function at a young age, suggesting that an early intervention may be of benefit to impact the high risk for these individuals to develop vascular morbidities.
Discussion
Multiple studies in adults demonstrate that circulating CFU-ECs and CPCs inversely correlate with risk of subsequent cardiovascular disease.14-17 However, no studies have been conducted in children, and rarely have studies included adolescents with a predisposition to develop vascular morbidities. Interventions to enhance vascular health are likely to be most successful early in disease prior to sustaining irreversible vascular damage, emphasizing the importance of studies in high-risk children and adolescents. Persons with type 1 diabetes have a greater than 10-fold increase in cardiovascular disease compared with nondiabetic age-matched peers.3 Given that the majority of individuals with type 1 diabetes are diagnosed in childhood, the availability of a noninvasive marker to predict those children at highest risk of developing vascular disease may result in modification of current treatments as well as enhance the potential for discovery of novel therapies. Though optimizing A1C affects vascular disease risk in patients with type 1 diabetes,3 glycemic indices are not sensitive predictors for subsequent vasculopathy. Collectively, these observations provided the rationale for the current study measuring circulating progenitor subpopulations and endothelial function in adolescents with type 1 diabetes.
Our data demonstrate reduced CPCs (CD34+CD133+ CD31+ cells) and a trend toward decreased CFU-ECs in subjects with type 1 diabetes. Previous studies in adults with type 1 diabetes that had no evidence of vascular disease found a significant decrease in the number of circulating progenitors, evaluated by culturing techniques and not flow cytometry, compared with age- and sex-matched healthy control subjects.17 However, the subjects in these studies were middle-aged (mean age, 40.7 years), with a mean diabetes duration of 21.1 years. Recently, Sibal et al11 reported decreases in circulating CD34+VE-cadherin+, CD133+VE-cadherin+, and CD133+VEGFR-2+ cell populations in persons with diabetes ages 16 to 35 years. In addition, higher CD34+VE-cadherin+, CD133+VEGFR-2+, and CD34+ counts were associated with better vascular reactivity assessed using flow-mediated dilation of the brachial artery. However, our study focuses on alterations in circulating progenitor populations in adolescents with type 1 diabetes. Our observation that poly-chromatic flow cytometry detected larger differences than the CFU-EC assay is not surprising because it is a highly sensitive method and requires minimal cell manipulation before analysis. Furthermore, toward the future of identifying biomarkers for enhanced vascular disease risk, a poly-chromatic flow cytometric method is more amenable to the standardization required for clinical testing compared with an in vitro culture method.19
Interestingly, we also detected increased circulating CD34+CD45− cell concentrations in subjects with type 1 diabetes. These novel findings are the first to report alterations in circulating CD34+CD45− cells in any diabetic population. Given that CD34+CD45− cells are elevated in patients with severe coronary artery disease,22 these data suggest that adolescents with type 1 diabetes may have ongoing vascular injury. Furthermore, because CD34+CD45− cells contain ECFCs, which are enriched in the endothelium,18 these data suggest that the diabetic environment may mobilize ECFCs from the endothelium into the circulation. Future studies evaluating the concentration and function of this circulating subpopulation in children and older subjects with type 1 diabetes will be important to understand how enumeration of these cells may be used as a biomarker to assess vascular damage and to enhance our understanding of the pathogenesis of vascular disease in type 1 diabetes. Promising studies in patients with cancer illustrate the potential clinical utility of measuring both CD34+CD45− and CD34+CD133+ CD31+ cell concentrations.19 Collectively, poly-chromatic flow cytometry data support the idea that lower CD34+ CD133+CD31+ counts reflect reduced vascular repair capability, and higher CD34+CD45− counts may indicate ongoing vascular injury.
Remarkably, in adolescents with diabetes, we observed a positive correlation between circulating CD34+CD133+ CD31+ cells and acetylcholine-induced vasodilation as measured by laser Doppler iontophoresis; a non-invasive method for assessing endothelial dysfunction. Given that endothelial dysfunction precedes progression to overt vascular disease, this finding lends further support to the concept that enumeration of circulating progenitor subpopulations may be a harbinger of future vascular disease risk.
There were some limitations of our study protocol and methods. Although we matched control subjects to subjects with diabetes by race and sex, we did not minimize individual variations that can affect both EPC and vascular reactivity (such as immediately antecedent physical activity) and had a relatively small number of subjects. We also chose to study only subjects with relatively long-standing diabetes. Future study protocols could be designed to study further the effects of inter-individual variables in a larger population of subjects.
Interestingly, there were no correlations between A1C or hsCRP with progenitor subpopulations or endothelial function, which is sobering because these measures are currently used clinically to assess risk of future microvascular and macrovascular disease. Although this finding may be, in part, due to small sample size, it may also reflect that these assessments evaluate different components of the diabetic milieu that increase the risk of vascular events. Several other cross-sectional studies of adolescents and young adults with type 1 diabetes found no correlations between A1C and vascular reactivity measures.11,31-33 Our subjects were otherwise healthy, so enhanced inflammation, as measured by hsCRP, may not be a major contributing factor to vascular dysfunction in young individuals with type 1 diabetes. Furthermore, other studies also failed to demonstrate correlations of A1C with measures of vascular reactivity in individuals with diabetes.34 Postprandial glucose values may be a stronger predictor of macrovascular disease than fasting or preprandial numbers.35 Additionally, in a recent study by our group, maternal pregestational diabetes resulted in dramatic impairments in neonatal ECFC numbers and function; however, the mothers in our study had A1C values in the normal range.36 Together, these observations suggest that other factors besides an “average” of glycemic values (such as blood glucose variability) may affect circulating progenitor cell numbers and vascular reactivity at a given moment in time. Currently, it is unknown whether reducing glucose variability can achieve a greater risk reduction in the rate of diabetes complications than simply changing A1C.
In summary, either as stand-alone measures or as part of algorithms assessing multiple risk factors, enumeration of circulating progenitor subpopulations by poly-chromatic flow cytometry may serve as a biomarker to assess risk of future vascular disease. In addition, quantitative measurement of circulating progenitor cells could be exploited to evaluate responsiveness to novel treatments that may prevent and/or delay vascular disease progression, similar to ongoing studies assessing antiangiogenic drugs in cancer patients. Finally, studies in younger subjects with type 1 diabetes and long-term longitudinal studies are warranted to understand better the complex pathophysiologic basis for vascular dysfunction and disease progression in children with type 1 diabetes.
Acknowledgments
Support was provided by Indiana University Department of Pediatrics Protocol Development Team, in particular, research nurses Lucy Miller and Tammy Garrett. We thank Janice Walls for her expert administrative assistance in preparation of the manuscript. Finally, we acknowledge the state-of-the-art facilities and expertise in both the Angiogenesis and Endothelial Progenitor Cell Core and Flow Cytometry Core within the Indiana University Simon Cancer Center.
Supported by UL1RR025761 Indiana Clinical and Translational Sciences Institute (L.D. and D.I), P30 CA08709 (D.I. and L.H.), and the Riley Children’s Foundation (D.I. and L.H.).
Glossary
- A1C
Hemoglobin A1C
- CFU-ECs
Colony forming unit-endothelial cells
- CPCs
Circulating progenitor cells
- ECFCs
Endothelial colony-forming cells
- EPCs
Endothelial progenitor cells
- hsCRP
High-sensitivity c-reactive protein
- MNCs
Mononuclear cells
- PU
Perfusion units
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Linda A. DiMeglio, Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN.
Aneesh Tosh, Divisions of Adolescent Medicine and Pediatric Endocrinology, University of Missouri School of Medicine, Columbia, MO.
Chandan Saha, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN.
Myka Estes, Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN.
Julie Mund, Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN.
Laura E. Mead, Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN.
David A. Ingram, Herman B. Wells Center for Pediatric Research, Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, IN.
Laura S. Haneline, Herman B. Wells Center for Pediatric Research, Department of Microbiology and Immunology, Indiana University School of Medicine, Indianapolis, IN.
References
- 1.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55:1463–9. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 2.Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. High risk of cardiovascular disease in patients with type 1 diabetes in the UK: a cohort study using the general practice research database. Diabetes Care. 2006;29:798–804. doi: 10.2337/diacare.29.04.06.dc05-1433. [DOI] [PubMed] [Google Scholar]
- 3.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105:32S–9. doi: 10.1016/s0002-9343(98)00209-5. [DOI] [PubMed] [Google Scholar]
- 5.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 6.Kuvin JT, Karas RH. Clinical utility of endothelial function testing: ready for prime time? Circulation. 2003;107:3243–7. doi: 10.1161/01.CIR.0000075928.54461.33. [DOI] [PubMed] [Google Scholar]
- 7.Basu S, Larsson A, Vessby J, Vessby B, Berne C. Type 1 diabetes is associated with increased cyclooxygenase- and cytokine-mediated inflammation. Diabetes Care. 2005;28:1371–5. doi: 10.2337/diacare.28.6.1371. [DOI] [PubMed] [Google Scholar]
- 8.Jain SK, McVie R, Bocchini JA., Jr. Hyperketonemia (ketosis), oxidative stress and type 1 diabetes. Pathophysiology. 2006;13:163–70. doi: 10.1016/j.pathophys.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Jin SM, Noh CI, Yang SW, Bae EJ, Shin CH, Chung HR, et al. Endothelial dysfunction and microvascular complications in type 1 diabetes mellitus. J Korean Med Sci. 2008;23:77–82. doi: 10.3346/jkms.2008.23.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarvisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, et al. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004;109:1750–5. doi: 10.1161/01.CIR.0000124725.46165.2C. [DOI] [PubMed] [Google Scholar]
- 11.Sibal L, Aldibbiat A, Agarwal SC, Mitchell G, Oates C, Razvi S, et al. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia. 2009;52:1464–73. doi: 10.1007/s00125-009-1401-0. [DOI] [PubMed] [Google Scholar]
- 12.Chin MH, Cook S, Jin L, Drum ML, Harrison JF, Koppert J, et al. Barriers to providing diabetes care in community health centers. Diabetes Care. 2001;24:268–74. doi: 10.2337/diacare.24.2.268. [DOI] [PubMed] [Google Scholar]
- 13.Haller MJ, Samyn M, Nichols WW, Brusko T, Wasserfall C, Schwartz RF, et al. Radial artery tonometry demonstrates arterial stiffness in children with type 1 diabetes. Diabetes Care. 2004;27:2911–7. doi: 10.2337/diacare.27.12.2911. [DOI] [PubMed] [Google Scholar]
- 14.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 15.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–6. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 16.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 17.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–9. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 18.Case J, Ingram DA, Haneline LS. Oxidative stress impairs endothelial progenitor cell function. Antioxid Redox Signal. 2008;10:1895–907. doi: 10.1089/ars.2008.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc. 2007;2:805–10. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–18. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Guven H, Shepherd RM, Bach RG, Capoccia BJ, Link DC. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J Am Coll Cardiol. 2006;48:1579–87. doi: 10.1016/j.jacc.2006.04.101. [DOI] [PubMed] [Google Scholar]
- 23.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–57. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 24.Sugawara J, Mitsui-Saito M, Hayashi C, Hoshiai T, Senoo M, Chisaka H, et al. Decrease and senescence of endothelial progenitor cells in patients with preeclampsia. J Clin Endocrinol Metab. 2005;90:5329–32. doi: 10.1210/jc.2005-0532. [DOI] [PubMed] [Google Scholar]
- 25.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 26.Farkas K, Kolossvary E, Jarai Z, Nemcsik J, Farsang C. Non-invasive assessment of microvascular endothelial function by laser Doppler flow-metry in patients with essential hypertension. Atherosclerosis. 2004;173:97–102. doi: 10.1016/j.atherosclerosis.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Kubli S, Waeber B, le-Ave A, Feihl F. Reproducibility of laser Doppler imaging of skin blood flow as a tool to assess endothelial function. J Cardiovasc Pharmacol. 2000;36:640–8. doi: 10.1097/00005344-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Morris SJ, Shore AC, Tooke JE. Responses of the skin microcirculation to acetylcholine and sodium nitroprusside in patients with NIDDM. Diabetologia. 1995;38:1337–44. doi: 10.1007/BF00401767. [DOI] [PubMed] [Google Scholar]
- 29.Goodfellow J, Bellamy MF, Gorman ST, Brownlee M, Ramsey MW, Lewis MJ, et al. Endothelial function is impaired in fit young adults of low birth weight. Cardiovasc Res. 1998;40:600–6. doi: 10.1016/s0008-6363(98)00197-7. [DOI] [PubMed] [Google Scholar]
- 30.Martin H, Gazelius B, Norman M. Impaired acetylcholine-induced vascular relaxation in low birth weight infants: implications for adult hypertension? Pediatr Res. 2000;47:457–62. doi: 10.1203/00006450-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Haller MJ, Stein JM, Shuster JJ, Theriaque D, Samyn MM, Pepine C, et al. Pediatric Atorvastatin in Diabetes Trial (PADIT): a pilot study to determine the effect of atorvastatin on arterial stiffness and endothelial function in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2009;22:65–8. doi: 10.1515/jpem.2009.22.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullen MJ, Clarkson P, Donald AE, Thomson H, Thorne SA, Powe AJ, et al. Effect of enalapril on endothelial function in young insulin-dependent diabetic patients: a randomized, double-blind study. J Am Coll Cardiol. 1998;31:1330–5. doi: 10.1016/s0735-1097(98)00099-0. [DOI] [PubMed] [Google Scholar]
- 33.Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–6. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 34.Haller MJ, Stein J, Shuster J, Theriaque D, Silverstein J, Schatz DA, et al. Peripheral artery tonometry demonstrates altered endothelial function in children with type 1 diabetes. Pediatr Diabetes. 2007;8:193–8. doi: 10.1111/j.1399-5448.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 35.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Ingram DA, Lien IZ, Mead LE, Estes M, Prater DN, Derr-Yellin E, et al. In vitro hyperglycemia or a diabetic intrauterine environment reduces neonatal endothelial colony forming cell numbers and function. Diabetes. 2008;57:724–31. doi: 10.2337/db07-1507. [DOI] [PubMed] [Google Scholar]