Full text
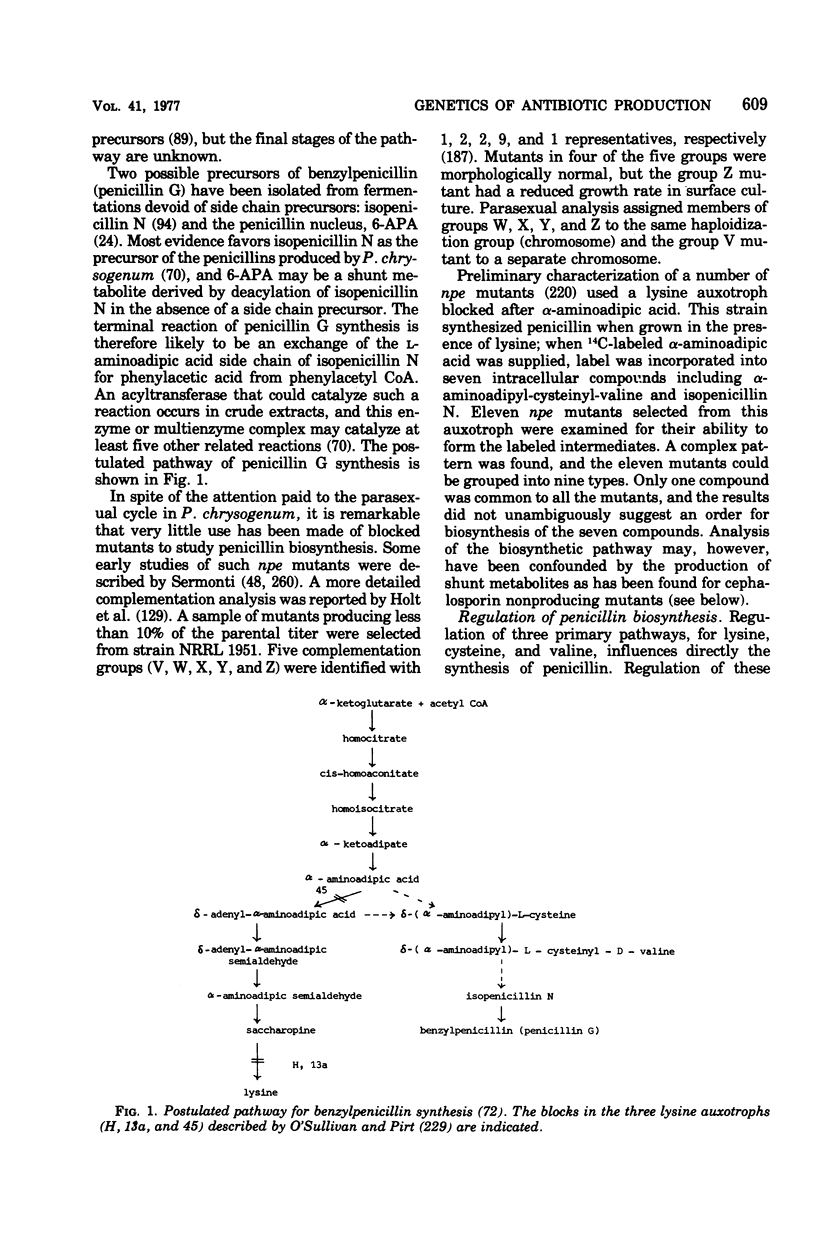
PDF















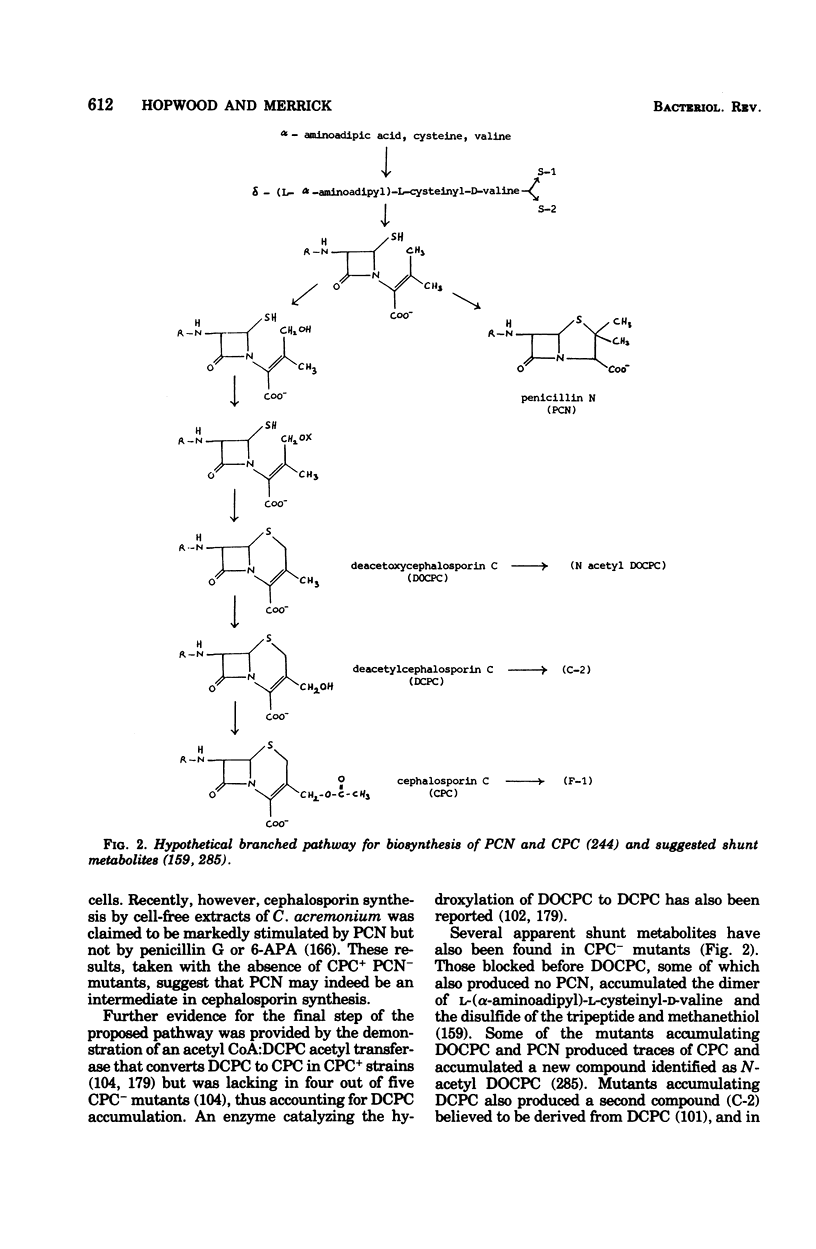






















Selected References
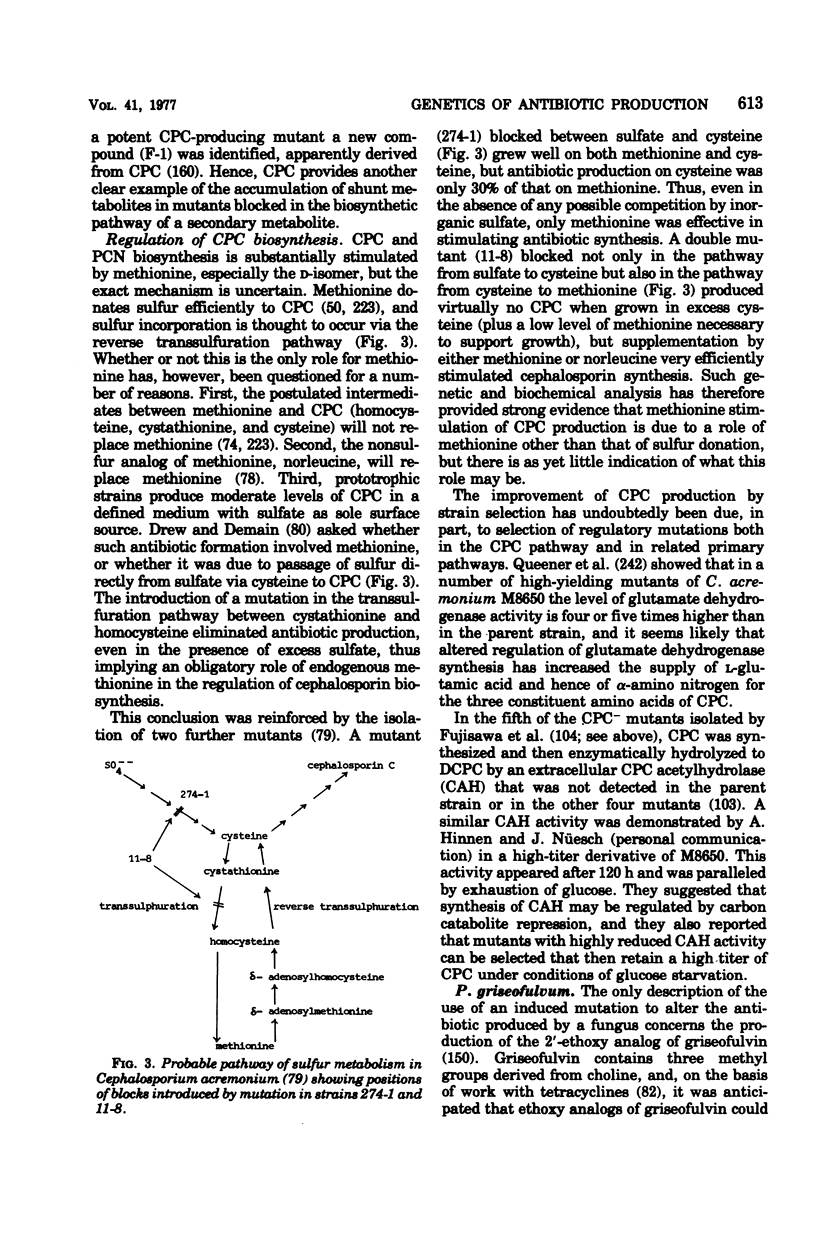
These references are in PubMed. This may not be the complete list of references from this article.
- ALACEVIC M. Interspecific recombination in Streptomyces. Nature. 1963 Mar 30;197:1323–1323. doi: 10.1038/1971323a0. [DOI] [PubMed] [Google Scholar]
- ALIKHANIAN S. I., BORISOVA L. N. Recombination in Actinomyces aureofaciens. J Gen Microbiol. 1961 Sep;26:19–28. doi: 10.1099/00221287-26-1-19. [DOI] [PubMed] [Google Scholar]
- ALIKHANIAN S. I., ILJINA T. S., LOMOVSKAYA N. D. Transduction in Actionmycetes. Nature. 1960 Oct 15;188:245–246. doi: 10.1038/188245a0. [DOI] [PubMed] [Google Scholar]
- ARNSTEIN H. R., MORRIS D., TOMS E. J. Isolation of a tripeptide containing alpha-aminoadipic acid from the mycelium of Penicillium chrysogenum and its possible significance in penicillin biosynthesis. Biochim Biophys Acta. 1959 Oct;35:561–562. doi: 10.1016/0006-3002(59)90417-2. [DOI] [PubMed] [Google Scholar]
- Akagawa H., Okanishi M., Umezawa H. A plasmid involved in chloramphenicol production in Streptomyces venezuelae: evidence from genetic mapping. J Gen Microbiol. 1975 Oct;90(2):336–346. doi: 10.1099/00221287-90-2-336. [DOI] [PubMed] [Google Scholar]
- Alikhanian S. Applied aspects of microbial genetics. Curr Top Microbiol Immunol. 1970;53:91–148. doi: 10.1007/978-3-642-95180-0_2. [DOI] [PubMed] [Google Scholar]
- Anne J., Eyssen H., De Somer P. Somatic hybridisation of Penicillium roquefortii with P. chrysogenum after protoplast fusion. Nature. 1976 Aug 19;262(5570):719–721. doi: 10.1038/262719a0. [DOI] [PubMed] [Google Scholar]
- Anné J., Peberdy J. F. Induced fusion of fungal protoplasts following treatment with polyethylene glycol. J Gen Microbiol. 1976 Feb;92(2):413–417. doi: 10.1099/00221287-92-2-413. [DOI] [PubMed] [Google Scholar]
- Audit C., Anagnostopoulos C. Genetic studies relating to the production of transformed clones diploid in the tryptophan region of the Bacillus subtilis genome. J Bacteriol. 1973 Apr;114(1):18–27. doi: 10.1128/jb.114.1.18-27.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATCHELOR F. R., DOYLE F. P., NAYLER J. H., ROLINSON G. N. Synthesis of penicillin: 6-aminopenicillanic acid in penicillin fermentations. Nature. 1959 Jan 24;183(4656):257–258. doi: 10.1038/183257b0. [DOI] [PubMed] [Google Scholar]
- BRADLEY S. G., LEDERBERG J. Heterokaryosis in Streptomyces. J Bacteriol. 1956 Aug;72(2):219–225. doi: 10.1128/jb.72.2.219-225.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAENDLE D. H., SZYBALSKI W. Heterokaryotic compatibility, metabolic cooperation, and genic recombination in Streptomyces. Ann N Y Acad Sci. 1959 Sep 30;81:824–853. doi: 10.1111/j.1749-6632.1959.tb49369.x. [DOI] [PubMed] [Google Scholar]
- BU'LOCK J. D. Intermediary metabolism and antibiotic synthesis. Adv Appl Microbiol. 1961;3:293–342. doi: 10.1016/s0065-2164(08)70514-8. [DOI] [PubMed] [Google Scholar]
- Bainbridge B. W., Roper J. A. Observations on the effects of a chromosome duplication in Aspergillus nidulans. J Gen Microbiol. 1966 Mar;42(3):417–424. doi: 10.1099/00221287-42-3-417. [DOI] [PubMed] [Google Scholar]
- Bal J., Bartnik W., Goryluk B., Pienízek N. J. An easy way of obtaining Aspergillus nidulans haploids in the parasexual cycle using N-glycosyl polifungin. Genet Res. 1975 Jun;25(3):249–252. doi: 10.1017/s0016672300015676. [DOI] [PubMed] [Google Scholar]
- Balassa G. Biochemical genetics of bacterial sporulation. I. Unidirectional pleiotropic interactions among genes controlling sporulation in Bacillus subtilis. Mol Gen Genet. 1969;104(1):73–103. [PubMed] [Google Scholar]
- Ball C. Haploidization analysis in Penicillium chrysogenum. J Gen Microbiol. 1971 Apr;66(1):63–69. doi: 10.1099/00221287-66-1-63. [DOI] [PubMed] [Google Scholar]
- Baumann R., Hütter R., Hopwood D. A. Genetic analysis in a melanin-producing streptomycete, Streptomyces glaucescens. J Gen Microbiol. 1974 Apr;81(2):463–474. doi: 10.1099/00221287-81-2-463. [DOI] [PubMed] [Google Scholar]
- Birch A. J. Biosynthesis of polyketides and related compounds. Science. 1967 Apr 14;156(3772):202–206. doi: 10.1126/science.156.3772.202. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu'Lock J. D., Hamilton D., Hulme M. A., Powell A. J., Smalley H. M., Shepherd D., Smith G. N. Metabolic development and secondary biosynthesis in Penicillium urticae. Can J Microbiol. 1965 Oct;11(5):765–778. doi: 10.1139/m65-104. [DOI] [PubMed] [Google Scholar]
- Butcher A. C. Non-allelic interactions and genetic isolation in wild populations of Aspergillus nidulans. Heredity (Edinb) 1969 Nov;24(4):621–631. doi: 10.1038/hdy.1969.82. [DOI] [PubMed] [Google Scholar]
- Butcher A. C. The relationship between sexual outcrossing and heterokaryon incompatibility in Aspergillus nidulans. Heredity (Edinb) 1968 Aug;23(3):443–452. doi: 10.1038/hdy.1968.55. [DOI] [PubMed] [Google Scholar]
- Bérdy J. Recent developments of antibiotic research and classification of antibiotics according to chemical structure. Adv Appl Microbiol. 1974;18(0):309–406. [PubMed] [Google Scholar]
- CAGLIOTI M. T., SERMONTI G. A study of the genetics of penicillin-producing capacity in Penicillium chrysogenum. J Gen Microbiol. 1956 Feb;14(1):38–46. doi: 10.1099/00221287-14-1-38. [DOI] [PubMed] [Google Scholar]
- CROWDY S. H., GROVE J. F., McCLOSKEY P. The translocation of antibiotics in higher plants. 4. Systemic fungicidal activity and chemical structure in griseofulvin relatives. Biochem J. 1959 Jun;72(2):241–249. doi: 10.1042/bj0720241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caltrider P. G., Niss H. F. Role of methionine in cephalosporin synthesis. Appl Microbiol. 1966 Sep;14(5):746–753. doi: 10.1128/am.14.5.746-753.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats J. H., Roeser J. Genetic recombination in Streptomyces bikiniensis var. zorbonensis. J Bacteriol. 1971 Mar;105(3):880–885. doi: 10.1128/jb.105.3.880-885.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronelli C., Tamoni G., Lancini G. C. Gardimycin, a new antibiotic from Actinoplanes. II. Isolation and preliminary characterization. J Antibiot (Tokyo) 1976 May;29(5):507–510. doi: 10.7164/antibiotics.29.507. [DOI] [PubMed] [Google Scholar]
- Coronelli C., White R. J., Lancini G. C., Parenti F. Lipiarmycin, a new antibiotic from Actinoplanes. II. Isolation, chemical, biological and biochemical characterization. J Antibiot (Tokyo) 1975 Apr;28(4):253–259. doi: 10.7164/antibiotics.28.253. [DOI] [PubMed] [Google Scholar]
- Cross T., Goodfellow M. Taxonomy and classification of the actinomycetes. Soc Appl Bacteriol Symp Ser. 1973 Jan;2:11–112. [PubMed] [Google Scholar]
- DEMAIN A. L. Inhibition of penicillin formation by lysine. Arch Biochem Biophys. 1957 Mar;67(1):244–246. doi: 10.1016/0003-9861(57)90265-5. [DOI] [PubMed] [Google Scholar]
- Demain A. L. Biochemistry of penicillin and cephalosporin fermentations. Lloydia. 1974 Jun;37(2):147–167. [PubMed] [Google Scholar]
- Demain A. L. How do antibiotic-producing microorganisms avoid suicide? Ann N Y Acad Sci. 1974 May 10;235(0):601–612. doi: 10.1111/j.1749-6632.1974.tb43294.x. [DOI] [PubMed] [Google Scholar]
- Demain A. L., Inamine E. Biochemistry and regulation of streptomycin and mannosidostreptomycinase (alpha-D-mannosidase) formation. Bacteriol Rev. 1970 Mar;34(1):1–19. doi: 10.1128/br.34.1.1-19.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain A. L. Industrial fermentations and their relation to regulatory mechanisms. Adv Appl Microbiol. 1966;8:1–27. doi: 10.1016/s0065-2164(08)70490-8. [DOI] [PubMed] [Google Scholar]
- Demain A. L., Masurekar P. S. Lysine inhibition of in vivo homocitrate synthesis in Penicillium chrysogenum. J Gen Microbiol. 1974 May;82(1):143–151. doi: 10.1099/00221287-82-1-143. [DOI] [PubMed] [Google Scholar]
- Dennen D. W., Carver D. D. Sulfatase regulation and antibiotic synthesis in Cephalosporium acremonium. Can J Microbiol. 1969 Feb;15(2):175–181. doi: 10.1139/m69-029. [DOI] [PubMed] [Google Scholar]
- Dimroth P., Ringelmann E., Lynen F. 6-Methylsalicylic acid synthetase from Penicillium patulum. Some catalytic properties of the enzyme and its relation to fatty acid synthetase. Eur J Biochem. 1976 Sep 15;68(2):591–596. doi: 10.1111/j.1432-1033.1976.tb10847.x. [DOI] [PubMed] [Google Scholar]
- Dobrzanski W. T., Osowiecki H. Isolation and some properties of the competence factor from group H Streptococcus strain Challis. J Gen Microbiol. 1967 Aug;48(2):299–304. doi: 10.1099/00221287-48-2-299. [DOI] [PubMed] [Google Scholar]
- Drew S. W., Demain A. L. Methionine control of cephalosporin C formation. Biotechnol Bioeng. 1973 Jul;15(4):743–754. doi: 10.1002/bit.260150408. [DOI] [PubMed] [Google Scholar]
- Drew S. W., Demain A. L. Production of cephalosporin C by single and double sulfur auxotrophic mutants of Cephalosporium acremonium. Antimicrob Agents Chemother. 1975 Jul;8(1):5–10. doi: 10.1128/aac.8.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANTINI A. A. Genetics and antibiotic production of Emericellopsis species. Genetics. 1962 Feb;47:161–177. doi: 10.1093/genetics/47.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANTINI A. A., OLIVE L. S. Sexual recombination in a homothallic, antibiotic producing fungus. Science. 1960 Dec 2;132(3440):1670–1670. doi: 10.1126/science.132.3440.1670. [DOI] [PubMed] [Google Scholar]
- Forrester P. I., Gaucher G. M. Conversion of 6-methylsalicylic acid into patulin by Penicillium urticae. Biochemistry. 1972 Mar 14;11(6):1102–1107. doi: 10.1021/bi00756a025. [DOI] [PubMed] [Google Scholar]
- Francis M. M., Cella R., Vining L. C. Genetic recombination in a chloramphenicol-producing strain of Streptomyces species 3022a. Can J Microbiol. 1975 Aug;21(8):1151–1159. doi: 10.1139/m75-172. [DOI] [PubMed] [Google Scholar]
- Freeman R. F., Bibb M. J., Hopwood D. A. Chloramphenicol acetylransferase-independent chloramphenicol resistance in Streptomyces coelicolor A3(2). J Gen Microbiol. 1977 Feb;98(2):453–465. doi: 10.1099/00221287-98-2-453. [DOI] [PubMed] [Google Scholar]
- Friend E. J., Hopwood D. A. The linkage map of Streptomyces rimosus. J Gen Microbiol. 1971 Oct;68(2):187–197. doi: 10.1099/00221287-68-2-187. [DOI] [PubMed] [Google Scholar]
- Fripp Y. J., Caten C. E. Genotype-environmental interactions in Schizophyllum commune. I. Analysis and character. Heredity (Edinb) 1971 Dec;27(3):393–407. doi: 10.1038/hdy.1971.103. [DOI] [PubMed] [Google Scholar]
- Froyshov O. Enzyme-bound intermediates in the biosynthesis of bacitracin. Eur J Biochem. 1975 Nov 1;59(1):201–206. doi: 10.1111/j.1432-1033.1975.tb02442.x. [DOI] [PubMed] [Google Scholar]
- Fuchs P. G., Zajdel J., Dobrzański W. T. Possible plasmid nature of the determinant for production of the antibiotic nisin in some strains of Streptococcus lactis. J Gen Microbiol. 1975 May;88(1):189–192. doi: 10.1099/00221287-88-1-189. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y., Kanzaki T. Occurrence of a new cephalosporoate in a culture broth of a Cephalosporium acremonium mutant. J Antibiot (Tokyo) 1975 May;28(5):372–378. doi: 10.7164/antibiotics.28.372. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y., Shirafugi H., Kida M., Nara K., Yoneda M., Kanzaki T. New findings on cephalosporin C biosynthesis. Nat New Biol. 1973 Dec 5;246(153):154–155. doi: 10.1038/newbio246154a0. [DOI] [PubMed] [Google Scholar]
- Furumai T., Suzuki M. Studies on the biosynthesis of basic 16-membered macrolide antibiotic, platenomycins. I. Selection of and cosynthesis by non-platenomycin-producing mutants. J Antibiot (Tokyo) 1975 Oct;28(10):770–774. doi: 10.7164/antibiotics.28.770. [DOI] [PubMed] [Google Scholar]
- Furumai T., Takeda K., Suzuki M. Studies on the biosynthesis of basic 16-membered macrolide antibiotics, platenomycins. IV. Biosynthesis of platenomycins. J Antibiot (Tokyo) 1975 Oct;28(10):789–797. doi: 10.7164/antibiotics.28.789. [DOI] [PubMed] [Google Scholar]
- GODTFREDSEN W. O., JAHNSEN S., LORCK H., ROHOLT K., TYBRING L. Fusidic acid: a new antibiotic. Nature. 1962 Mar 10;193:987–987. doi: 10.1038/193987a0. [DOI] [PubMed] [Google Scholar]
- GREGORY K. F., HUANG J. C. TYROSINASE INHERITANCE IN STREPTOMYCES SCABIES. I. GENETIC RECOMBINATION. J Bacteriol. 1964 Jun;87:1281–1286. doi: 10.1128/jb.87.6.1281-1286.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGORY K. F., HUANG J. C. TYROSINASE INHERITANCE IN STREPTOMYCES SCABIES. II. INDUCTION OF TYROSINASE DEFICIENCY BY ACRIDINE DYES. J Bacteriol. 1964 Jun;87:1287–1294. doi: 10.1128/jb.87.6.1287-1294.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRINDLE M. Heterokaryon compatibility of unrelated strains in the Aspergillus nidulans group. Heredity (Edinb) 1963 May;18:191–204. doi: 10.1038/hdy.1963.21. [DOI] [PubMed] [Google Scholar]
- Goulden S. A., Chattaway F. W. End-product control of acetohydroxyacid synthetase by valine in Penicillium chrysogenum Q 176 and a high penicillin-yielding mutant. J Gen Microbiol. 1969 Nov;59(1):111–118. doi: 10.1099/00221287-59-1-111. [DOI] [PubMed] [Google Scholar]
- Goulden S. A., Chattaway F. W. Lysine control of alpha-aminoadipate and penicillin synthesis in Penicillium chrysogenum. Biochem J. 1968 Dec;110(4):55P–56P. doi: 10.1042/bj1100055p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E., Morell J. L. The structure of nisin. J Am Chem Soc. 1971 Sep 8;93(18):4634–4635. doi: 10.1021/ja00747a073. [DOI] [PubMed] [Google Scholar]
- HOPWOOD D. A. Linkage and the mechanism of recombination in Streptomyces coelicolor. Ann N Y Acad Sci. 1959 Sep 30;81:887–898. doi: 10.1111/j.1749-6632.1959.tb49374.x. [DOI] [PubMed] [Google Scholar]
- HOPWOOD D. A. NEW DATA ON THE LINKAGE MAP OF STREPTOMYCES COELICOLOR. Genet Res. 1965 Jul;6:248–262. doi: 10.1017/s0016672300004134. [DOI] [PubMed] [Google Scholar]
- Haavik H. I., Froyshov O. Function of peptide antibiotics in producer organisms. Nature. 1975 Mar 6;254(5495):79–82. doi: 10.1038/254079a0. [DOI] [PubMed] [Google Scholar]
- Haneishi T., Terahara A., Arai M., Hata T., Tamura C. New antibiotics, methylenomycins A and B. II. Structures of methylenomycins A and B. J Antibiot (Tokyo) 1974 Jun;27(6):393–399. doi: 10.7164/antibiotics.27.393. [DOI] [PubMed] [Google Scholar]
- Harold R. J., Hopwood D. A. A rapid method for complemenntation testing of ultraviolet-sensitive (UVS) mutants of Streptomyces coelicolor. Mutat Res. 1972 Sep;16(7):27–34. doi: 10.1016/0027-5107(72)90060-7. [DOI] [PubMed] [Google Scholar]
- Harold R. J., Hopwood D. A. Ultraviolet-sensitive mutants of Streptomyces coelicolor. II. Genetics. Mutat Res. 1970 Nov;10(5):439–448. doi: 10.1016/0027-5107(70)90004-7. [DOI] [PubMed] [Google Scholar]
- Hastie A. C. Benlate-induced instability of Aspergillus diploids. Nature. 1970 May 23;226(5247):771–771. doi: 10.1038/226771a0. [DOI] [PubMed] [Google Scholar]
- Higgens C. E., Hamill R. L., Sands T. H., Hoehn M. M., Davis N. E. Letter: The occurrence of deacetoxycephalosporin C in fungi and streptomycetes. J Antibiot (Tokyo) 1974 Apr;27(4):298–300. doi: 10.7164/antibiotics.27.298. [DOI] [PubMed] [Google Scholar]
- Hirai K., Nozoe S., Tsuda K., Iitaka Y., Ishibashi K., Shirasaka M. The structure of siccainin. Tetrahedron Lett. 1967 Jun;23:2177–2179. doi: 10.1016/s0040-4039(00)90792-5. [DOI] [PubMed] [Google Scholar]
- Hogg R. W., Broquist H. P. Homocitrate formation in Neurospora crassa. Relation to lysine biosynthesis. J Biol Chem. 1968 Apr 25;243(8):1839–1845. [PubMed] [Google Scholar]
- Holt G., Macdonald K. D. Isolation of strains with increased penicillin yield after hybridization in Aspergillus nidulans. Nature. 1968 Aug 10;219(5154):636–637. doi: 10.1038/219636a0. [DOI] [PubMed] [Google Scholar]
- Holt G., Macdonald K. D. Penicillin production and its mode of inheritance in Aspergillus nidulans. Antonie Van Leeuwenhoek. 1968;34(4):409–416. doi: 10.1007/BF02046463. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Chater K. F., Dowding J. E., Vivian A. Advances in Streptomyces coelicolor genetics. Bacteriol Rev. 1973 Sep;37(3):371–405. doi: 10.1128/br.37.3.371-405.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A. Genetic analysis and genome structure in Streptomyces coelicolor. Bacteriol Rev. 1967 Dec;31(4):373–403. doi: 10.1128/br.31.4.373-403.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A. Genetics of the Actinomycetales. Soc Appl Bacteriol Symp Ser. 1973 Jan;2:131–153. [PubMed] [Google Scholar]
- Hopwood D. A. Lack of Constant Genome Ends in STREPTOMYCES COELICOLOR. Genetics. 1966 Nov;54(5):1177–1184. doi: 10.1093/genetics/54.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M. A plasmid of Streptomyces coelicolor carrying a chromosomal locus and its inter-specific transfer. J Gen Microbiol. 1973 Dec;79(2):331–342. doi: 10.1099/00221287-79-2-331. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M., Bibb M. J., Cohen S. N. Genetic recombination through protoplast fusion in Streptomyces. Nature. 1977 Jul 14;268(5616):171–174. doi: 10.1038/268171a0. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M. Genetic studies on SCP1-prime strains of Streptomyces coelicolor A3(2). J Gen Microbiol. 1976 Jul;95(1):107–120. doi: 10.1099/00221287-95-1-107. [DOI] [PubMed] [Google Scholar]
- Houghton J. A. A new class of slow-growing non-perithecial mutants of Aspergillus nidulans. Genet Res. 1970 Dec;16(3):285–292. doi: 10.1017/s0016672300002548. [DOI] [PubMed] [Google Scholar]
- Iwaki M., Shimura K., Kanda M., Kaji E., Saito Y. Some mutants of Bacillus brevis deficient in gramicidin S formation. Biochem Biophys Res Commun. 1972 Jul 11;48(1):113–118. doi: 10.1016/0006-291x(72)90351-8. [DOI] [PubMed] [Google Scholar]
- JACKSON M., DULANEY E. L., PUTTER I., SHAFER H. M., WOLF F. J., WOODRUFF H. B. Transethylation in antibiotic biosynthesis. II. Production of the 2'-ethoxy analogue of griseofulvin by biosynthesis. Biochim Biophys Acta. 1962 Aug 27;62:616–619. doi: 10.1016/0006-3002(62)90258-5. [DOI] [PubMed] [Google Scholar]
- JINKS J. L. Somatic selection in fungi. Nature. 1954 Aug 28;174(4426):409–410. doi: 10.1038/174409b0. [DOI] [PubMed] [Google Scholar]
- Jinks J. L., Caten C. E., Simchen G., Croft J. H. Heterokaryon incompatibility and variation in wild populations of Aspergillus nidulans. Heredity (Edinb) 1966 May;21(2):227–239. doi: 10.1038/hdy.1966.20. [DOI] [PubMed] [Google Scholar]
- Jinks L. J., Connolly V. Determination of the environmental sensitivity of selection lines by the selection environment. Heredity (Edinb) 1975 Jun;34(3):401–406. doi: 10.1038/hdy.1975.49. [DOI] [PubMed] [Google Scholar]
- Jones A., Westlake D. W. Regulation of chloramphenicol synthesis in Streptomyces sp. 3022a. Properties of arylamine synthetase, an enzyme involved in antibiotic biosynthesis. Can J Microbiol. 1974 Nov;20(11):1599–1611. doi: 10.1139/m74-247. [DOI] [PubMed] [Google Scholar]
- KAFER E. An 8-chromosome map of Aspergillus nidulans. Adv Genet. 1958;9:105–145. [PubMed] [Google Scholar]
- KUTZNER H. J., WAKSMAN S. A. Streptomyces coelicolor Mueller and Streptomyces violaceoruber Waksman and Curtis, two distinctly different organisms. J Bacteriol. 1959 Oct;78:528–538. doi: 10.1128/jb.78.4.528-538.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambe M., Imae Y., Kurahashi K. Biochemical studies on gramicidin S non-producing mutants of Bacillus brevis ATCC 9999. J Biochem. 1974 Mar;75(3):481–493. doi: 10.1093/oxfordjournals.jbchem.a130417. [DOI] [PubMed] [Google Scholar]
- Kanzaki T., Fukita T., Shirafuji H., Fujisawa Y., Kitano K. Letter: Occurrence of a 3-methylthiomethylcephem derivative in a culture broth of Cephalosporium mutant. J Antibiot (Tokyo) 1974 May;27(5):361–362. doi: 10.7164/antibiotics.27.361. [DOI] [PubMed] [Google Scholar]
- Kawamoto I., Takasawa S., Okachi R., Koakura M., Takahashi I. A new antibiotic victomycin (XK 49-1-B-2). I. Taxonomy and production of the producing organism. J Antibiot (Tokyo) 1975 May;28(5):358–365. doi: 10.7164/antibiotics.28.358. [DOI] [PubMed] [Google Scholar]
- Kirby R., Hopwood D. A. Genetic determination of methylenomycin synthesis by the SCP1 plasmid of Streptomyces coelicolor A3(2). J Gen Microbiol. 1977 Jan;98(1):239–252. doi: 10.1099/00221287-98-1-239. [DOI] [PubMed] [Google Scholar]
- Kirby R., Wright L. F., Hopwood D. A. Plasmid-determined antibiotic synthesis and resistance in Streptomyces coelicolor. Nature. 1975 Mar 20;254(5497):265–267. doi: 10.1038/254265a0. [DOI] [PubMed] [Google Scholar]
- Kohsaka M., Demain A. L. Conversion of penicillin N to cephalosporin(s) by cell-free extracts of Cephalosporium acremonium. Biochem Biophys Res Commun. 1976 May 17;70(2):465–473. doi: 10.1016/0006-291x(76)91069-x. [DOI] [PubMed] [Google Scholar]
- Kojima M., Sato A. Letter: Microbial semi-synthesis of aminoglycosidic antibiotics by mutants of S. ribosidificus and S. kanamyceticus. J Antibiot (Tokyo) 1973 Dec;26(12):784–786. doi: 10.7164/antibiotics.26.784. [DOI] [PubMed] [Google Scholar]
- Kozar W., Rajchert-Trzpil M., Dobrzański W. T. The effect of proflavin, ethidium bromide and an elevated temperature on the appearance of nisin-negative clones in nisin-producing strains of Streptococcus lactis. J Gen Microbiol. 1974 Aug;83(2):295–302. doi: 10.1099/00221287-83-2-295. [DOI] [PubMed] [Google Scholar]
- Käfer E. Origins of translocations in Aspergillus nidulans. Genetics. 1965 Jul;52(1):217–232. doi: 10.1093/genetics/52.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähler R., Noack D. Action of acridine orange and ethidium bromide on growth and antibiotic activity of Streptomyces hygroscopicus JA 6599. Z Allg Mikrobiol. 1974;14(6):529–533. doi: 10.1002/jobm.3630140610. [DOI] [PubMed] [Google Scholar]
- LHOAS P. Mitotic haploidization by treatment of Aspergillus niger diploids with para-fluorophenylalanine. Nature. 1961 May 20;190:744–744. doi: 10.1038/190744a0. [DOI] [PubMed] [Google Scholar]
- Laland S. G., Zimmer T. L. The protein thiotemplate mechanism of synthesis for the peptide antibiotics produced by Bacillus brevis. Essays Biochem. 1973;9:31–57. [PubMed] [Google Scholar]
- Lemke P. A., Nash C. H. Mutations that affect antibiotic synthesis by Cephalosporium acremonium. Can J Microbiol. 1972 Feb;18(2):255–259. doi: 10.1139/m72-038. [DOI] [PubMed] [Google Scholar]
- Lepesant-Kejzlarová J., Lepesant J. A., Walle J., Billault A., Dedonder R. Revision of the linkage map of Bacillus subtilis 168: indications for circularity of the chromosome. J Bacteriol. 1975 Mar;121(3):823–834. doi: 10.1128/jb.121.3.823-834.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhoas P. Genetic analysis by means of the parasexual cycle in Aspergillus niger. Genet Res. 1967 Aug;10(1):45–61. doi: 10.1017/s0016672300010752. [DOI] [PubMed] [Google Scholar]
- Loder P. B., Abraham E. P. Isolation and nature of intracellular peptides from a cephalosporin C-producing Cephalosporium sp. Biochem J. 1971 Jul;123(3):471–476. doi: 10.1042/bj1230471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomovskaya N. D., Voeykova T. A., Mkrtumian N. M. Construction and properties of hybrids obtained in interspecific crosses between Streptomyces coelicolor A3(2) and Streptomyces griseus Kr-15. J Gen Microbiol. 1977 Jan;98(1):187–198. doi: 10.1099/00221287-98-1-187. [DOI] [PubMed] [Google Scholar]
- MACDONALD K. D., HUTCHINSON J. M., GILLETT W. A. FORMATION AND SEGREGATION OF HETEROZYGOUS DIPLOIDS BETWEEN A WILD-TYPE STRAIN AND DERIVATIVES OF HIGH PENICILLIN YIELD IN PENICILLIUM CHRYSOGENUM. J Gen Microbiol. 1963 Dec;33:385–394. doi: 10.1099/00221287-33-3-385. [DOI] [PubMed] [Google Scholar]
- MACDONALD K. D., HUTCHINSON J. M., GILLETT W. A. HETEROKARYON STUDIES AND THE GENETIC CONTROL OF PENICILLIN AND CHRYSOGENIN PRODUCTION IN PENICILLIUM CHRYSOGENUM. J Gen Microbiol. 1963 Dec;33:375–383. doi: 10.1099/00221287-33-3-375. [DOI] [PubMed] [Google Scholar]
- MACDONALD K. D., HUTCHINSON J. M., GILLETT W. A. ISOLATION OF AUXOTROPHS OF PENICILLIUM CHRYSOGENUM AND THEIR PENICILLIN YIELDS. J Gen Microbiol. 1963 Dec;33:365–374. doi: 10.1099/00221287-33-3-365. [DOI] [PubMed] [Google Scholar]
- MACDONALD K. D., HUTCHINSON J. M., GILLETT W. A. PROPERTIES OF HETEROZYGOUS DIPLOIDS BETWEEN STAINS OF PENICILLIUM CHRYSOGENUM SELECTED FOR HIGH PENICILLIN YIELD. Antonie Van Leeuwenhoek. 1964;30:209–224. doi: 10.1007/BF02046727. [DOI] [PubMed] [Google Scholar]
- MacDonald K. D. Segregants from a heterozygous diploid of Penicillium chrysogenum following different physical and chemical treatments. J Gen Microbiol. 1971 Aug;67(2):247–250. doi: 10.1099/00221287-67-2-247. [DOI] [PubMed] [Google Scholar]
- Macdonald K. D. Differences in diploids synthesized between the same parental strains of Penicillium chrysogenum. Antonie Van Leeuwenhoek. 1966;32(4):431–441. doi: 10.1007/BF02097495. [DOI] [PubMed] [Google Scholar]
- Macdonald K. D., Holt G. Genetics of biosynthesis and overproduction of penicillin. Sci Prog. 1976 Winter;63(252):547–573. [PubMed] [Google Scholar]
- Macdonald K. D., Hutchinson J. M., Gillett W. A. Heterozygous diploids of Penicillium chrysogenum and their segregation patterns. Genetica. 1965;36(4):378–397. doi: 10.1007/BF01557170. [DOI] [PubMed] [Google Scholar]
- Macdonald K. D. The persistence of parental genome segregation in Penicillium chrysogenum after nitrogen mustard treatment. Mutat Res. 1968 Mar-Apr;5(2):302–305. doi: 10.1016/0027-5107(68)90029-8. [DOI] [PubMed] [Google Scholar]
- Majer J., Martin J. R., Egan R. S., Corcoran J. W. Antibiotic glycosides. 8. Erythromycin D, a new macrolide antibiotic. J Am Chem Soc. 1977 Mar 2;99(5):1620–1622. doi: 10.1021/ja00447a055. [DOI] [PubMed] [Google Scholar]
- Maragoudakis M. E., Holmes H., Strassman M. Control of lysine biosynthesis in yeast by a feedback mechanism. J Bacteriol. 1967 May;93(5):1677–1680. doi: 10.1128/jb.93.5.1677-1680.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. R., Perun T. J., Girolami R. L. Studies on the biosynthesis of the erythromycins. I. Isolation and structure of an intermediate glycoside, 3-alpha-L-mycarosylerythronolide B. Biochemistry. 1966 Sep;5(9):2852–2856. doi: 10.1021/bi00873a011. [DOI] [PubMed] [Google Scholar]
- Martin J. R., Perun T. J. Studies on the biosynthesis of the erythromycins. 3. Isolation and structure of 5-deoxy-5-oxoerythronolide B, a shunt metabolite of erythromycin biosynthesis. Biochemistry. 1968 May;7(5):1728–1733. doi: 10.1021/bi00845a016. [DOI] [PubMed] [Google Scholar]
- Martin J. R., Rosenbrook W. Studies on the biosynthesis of the erythromycins. II. Isolation and structure of a biosynthetic intermediate, 6-deoxyerythronolide B. Biochemistry. 1967 Feb;6(2):435–440. doi: 10.1021/bi00854a010. [DOI] [PubMed] [Google Scholar]
- Masurekar P. S., Demain A. L. Impaired penicillin production in lysine regulatory mutants of Penicillium chrysogenum. Antimicrob Agents Chemother. 1974 Sep;6(3):366–368. doi: 10.1128/aac.6.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masurekar P. S., Demain A. L. Insensitivity of homocitrate synthase in extracts of Penicillium chyrosogenum to feedback inhibition by lysine. Appl Microbiol. 1974 Aug;28(2):265–270. doi: 10.1128/am.28.2.265-270.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully K. S., Forbes E. The use of p-fluorophenylalanine with 'master strains' of Aspergillus nidulans for assigning genes to linkage groups. Genet Res. 1965 Nov;6(3):352–359. doi: 10.1017/s0016672300004249. [DOI] [PubMed] [Google Scholar]
- Merrick M. J. A morphological and genetic mapping study of bald colony mutants of Streptomyces coelicolor. J Gen Microbiol. 1976 Oct;96(2):299–315. doi: 10.1099/00221287-96-2-299. [DOI] [PubMed] [Google Scholar]
- Merrick M. J., Caten C. E. The inheritance of penicillin titre in wild-type isolates of Aspergillus nidulans. J Gen Microbiol. 1975 Feb;86(2):283–293. doi: 10.1099/00221287-86-2-283. [DOI] [PubMed] [Google Scholar]
- Merrick M. J. Hybridization and selection for increased penicillin titre in wild-type isolates of Aspergillus nidulans. J Gen Microbiol. 1975 Dec;91(2):278–286. doi: 10.1099/00221287-91-2-278. [DOI] [PubMed] [Google Scholar]
- Merrick M. J. The inheritance of penicillin titre in crosses between lines of Aspergillus nidulans selected for increased productivity. J Gen Microbiol. 1975 Dec;91(2):287–294. doi: 10.1099/00221287-91-2-287. [DOI] [PubMed] [Google Scholar]
- Murphy G., Lynen F. Patulin biosynthesis: the metabolism of m-hydroxybenzyl alcohol and m-hydroxybenzaldehyde by particulate preparations from Penicillium patulum. Eur J Biochem. 1975 Oct 15;58(2):467–475. doi: 10.1111/j.1432-1033.1975.tb02394.x. [DOI] [PubMed] [Google Scholar]
- Musílek V., Cerná J., Sasek V., Semerdzieva M., Vondrácek M. Antifungal antibiotic of the basidiomycete Oudemansiella mucida. I. Isolation and cultivation of a producing strain. Folia Microbiol (Praha) 1969;14(4):377–387. doi: 10.1007/BF02872707. [DOI] [PubMed] [Google Scholar]
- Nagaoka K., Demain A. L. Mutational biosynthesis of a new antibiotic, streptomutin A, by an idiotroph of Streptomyces griseus. J Antibiot (Tokyo) 1975 Sep;28(9):627–635. doi: 10.7164/antibiotics.28.627. [DOI] [PubMed] [Google Scholar]
- Nagarajan R., Boeck L. D., Gorman M., Hamill R. L., Higgens C. E., Hoehn M. M., Stark W. M., Whitney J. G. Beta-lactam antibiotics from Streptomyces. J Am Chem Soc. 1971 May 5;93(9):2308–2310. doi: 10.1021/ja00738a035. [DOI] [PubMed] [Google Scholar]
- Nash C. H., Huber F. M. Antibiotic synthesis and morphological differentiation of Cephalosporium acremonium. Appl Microbiol. 1971 Jul;22(1):6–10. doi: 10.1128/am.22.1.6-10.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan C. Y., Pirt S. J. Penicillin production by lysine auxotrophs of Penicillium chrysogenum. J Gen Microbiol. 1973 May;76(1):65–75. doi: 10.1099/00221287-76-1-65. [DOI] [PubMed] [Google Scholar]
- Okanishi M., Hamana K., Umezawa H. Factors affecting infection of protoplasts with deoxyribonucleic acid of actinophage PK-66. J Virol. 1968 Jul;2(7):686–691. doi: 10.1128/jvi.2.7.686-691.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanishi M., Ita T., Umezawa H. Possible control of formation of aerial mycelium and antibiotic production in Streptomyces by episomic factors. J Antibiot (Tokyo) 1970 Jan;23(1):45–47. doi: 10.7164/antibiotics.23.45. [DOI] [PubMed] [Google Scholar]
- Okanishi M., Suzuki K., Umezawa H. Formation and reversion of Streptomycete protoplasts: cultural condition and morphological study. J Gen Microbiol. 1974 Feb;80(2):389–400. doi: 10.1099/00221287-80-2-389. [DOI] [PubMed] [Google Scholar]
- Okanishi M., Utahara R., Okami Y. Infection of the protoplasts of Streptomyces kanamyceticus with deoxyribonucleic acid preparation from actinophage PK-66. J Bacteriol. 1966 Dec;92(6):1850–1852. doi: 10.1128/jb.92.6.1850-1852.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976 Sep;40(3):681–697. doi: 10.1128/br.40.3.681-697.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONTECORVO G., KAFER E. Genetic analysis based on mitotic recombination. Adv Genet. 1958;9:71–104. [PubMed] [Google Scholar]
- PONTECORVO G., ROPER J. A., HEMMONS L. M., MACDONALD K. D., BUFTON A. W. J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- PONTECORVO G., SERMONTI G. Para-sexual recombination in Penicillium chrysogenum. J Gen Microbiol. 1954 Aug;11(1):94–104. doi: 10.1099/00221287-11-1-94. [DOI] [PubMed] [Google Scholar]
- Peterson E. A., Gillespie D. C., Cook F. D. A wide-spectrum antibiotic produced by a species of Sorangium. Can J Microbiol. 1966 Apr;12(2):221–230. doi: 10.1139/m66-031. [DOI] [PubMed] [Google Scholar]
- Pirali G., Somma S., Lancini G. C., Sala F. Inhibition of peptide chain initiation in Escherichia coli by thermorubin. Biochim Biophys Acta. 1974 Oct 28;366(3):310–318. doi: 10.1016/0005-2787(74)90291-3. [DOI] [PubMed] [Google Scholar]
- Polsinelli M., Beretta M. Genetic Recombination in Crosses Between Streptomyces aureofaciens and Streptomyces rimosus. J Bacteriol. 1966 Jan;91(1):63–68. doi: 10.1128/jb.91.1.63-68.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia A. M., Spada-Sermonti I., Basile S., Misuraca F., Sermonti G. Infectious transfer of a fertility factor in Streptomyces coelicolor. Genet Res. 1973 Apr;21(2):107–113. doi: 10.1017/s0016672300013288. [DOI] [PubMed] [Google Scholar]
- Queener S. W., Capone J. J., Radue A. B., Nagarajan R. Synthesis of deactoxycephalosporin C by a mutant of Cephalosporium acremonium. Antimicrob Agents Chemother. 1974 Sep;6(3):334–337. doi: 10.1128/aac.6.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queener S. W., McDermott J., Radue A. B. Glutamate dehydrogenase specific activity and cephalosporin C synthesis in the M8650 series of Cephalosporium acremonium mutants. Antimicrob Agents Chemother. 1975 May;7(5):646–651. doi: 10.1128/aac.7.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROPER J. A. Production of heterozygous diploids in filamentous fungi. Experientia. 1952 Jan 15;8(1):14–15. doi: 10.1007/BF02168881. [DOI] [PubMed] [Google Scholar]
- Redshaw P. A., McCann P. A., Sankaran L., Pogell B. M. Control of differentiation in streptomycetes: involvement of extrachromosomal deoxyribonucleic acid and glucose repression in aerial mycelia development. J Bacteriol. 1976 Feb;125(2):698–705. doi: 10.1128/jb.125.2.698-705.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenberg H. V. Cyclic AMP in prokaryotes. Annu Rev Microbiol. 1974;28(0):353–369. doi: 10.1146/annurev.mi.28.100174.002033. [DOI] [PubMed] [Google Scholar]
- Rinehart K. L., Jr, Stroshane R. M. Biosynthesis of aminocyclitol antibiotics. J Antibiot (Tokyo) 1976 Apr;29(4):319–353. doi: 10.7164/antibiotics.29.319. [DOI] [PubMed] [Google Scholar]
- Roland I., Froyshov O. On the presence of pantothenic acid in the three complementary enzymes of bacitracin synthetase. FEBS Lett. 1975 Dec 15;60(2):305–308. doi: 10.1016/0014-5793(75)80736-8. [DOI] [PubMed] [Google Scholar]
- Russi S., Carere A., Fratello B., Khoudokormoff V. Caratterizzazione biochimica di alcuni mutanti di Streptomyces coelicolor richiedenti istidina. Ann Ist Super Sanita. 1966;2(4):506–522. [PubMed] [Google Scholar]
- SAITO H., IKEDA Y. Cytogenetic studies on Streptomyces griseoflavus. Ann N Y Acad Sci. 1959 Sep 30;81:862–878. doi: 10.1111/j.1749-6632.1959.tb49372.x. [DOI] [PubMed] [Google Scholar]
- SERMONTI G. Complementary genes which affect penicillin yields. J Gen Microbiol. 1956 Dec;15(3):599–608. doi: 10.1099/00221287-15-3-599. [DOI] [PubMed] [Google Scholar]
- SERMONTI G. Genetics of penicillin production. Ann N Y Acad Sci. 1959 Sep 30;81:950–973. doi: 10.1111/j.1749-6632.1959.tb49380.x. [DOI] [PubMed] [Google Scholar]
- Sankaran L., Pogell B. M. Biosynthesis of puromycin in Streptomyces alboniger: regulation and properties of O-demethylpuromycin O-methyltransferase. Antimicrob Agents Chemother. 1975 Dec;8(6):721–732. doi: 10.1128/aac.8.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempf H., Bujard H., Hopwood D. A., Goebel W. Isolation of covalently closed circular deoxyribonucleic acid from Streptomyces coelicolor A3(2). J Bacteriol. 1975 Feb;121(2):416–421. doi: 10.1128/jb.121.2.416-421.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp T., Hutter R., Hopwood D. A. Genetic recombination in Nocardia mediterranei. J Bacteriol. 1975 Jan;121(1):128–136. doi: 10.1128/jb.121.1.128-136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermonti G., Bandiera M., Spadasermonti I. New approach to the genetics of Streptomyces coelicolor. J Bacteriol. 1966 Jan;91(1):384–392. doi: 10.1128/jb.91.1.384-392.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermonti G., Puglia A. M., Ficarra G. The time course of recombinant production in Streptomyces coelicolor. Genet Res. 1971 Oct;18(2):133–145. doi: 10.1017/s0016672300012532. [DOI] [PubMed] [Google Scholar]
- Sermonti G, Mancinelli A, Spada-Sermonti I. Heterogeneous Clones ("Heteroclones") in Streptomyces Coelicolor a 3(2). Genetics. 1960 Jun;45(6):669–672. doi: 10.1093/genetics/45.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shier W. T., Ogawa S., Hichens M., Rinehart K. L., Jr Chemistry and biochemistry of the neomycins. XVII. Bioconversion of aminocyclitols to aminocyclitol antibiotics. J Antibiot (Tokyo) 1973 Oct;26(10):551–561. doi: 10.7164/antibiotics.26.551. [DOI] [PubMed] [Google Scholar]
- Shier W. T., Rinehart K. L., Jr, Gottlieb D. Preparation of four new antibiotics from a mutant of Streptomyces fradiae. Proc Natl Acad Sci U S A. 1969 May;63(1):198–204. doi: 10.1073/pnas.63.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shier W. T., Schaefer P. C., Gottlieb D., Rinehart K. L., Jr Use of mutants in the study of aminocyclitol antibiotic biosynthesis and the preparation of the hybrimycin C complex. Biochemistry. 1974 Dec 3;13(25):5073–5078. doi: 10.1021/bi00722a002. [DOI] [PubMed] [Google Scholar]
- Smith B., Warren S. C., Newton G. G., Abraham E. P. Biosynthesis of penicillin N and cephalosporin C. Antibiotic production and other features of the metabolism of Cephalosporium sp. Biochem J. 1967 Jun;103(3):877–890. doi: 10.1042/bj1030877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapley E. O., Jackson M., Hernandez S., Zimmerman S. B., Currie S. A., Mochales S., Mata J. M., Woodruff H. B., Hendlin D. Cephamycins, a new family of beta-lactam antibiotics. I. Production by actinomycetes, including Streptomyces lactamdurans sp. n. Antimicrob Agents Chemother. 1972 Sep;2(3):122–131. doi: 10.1128/aac.2.3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova N. L. Geneticheskii analiz izmenchivosti kol'tsevykh khromosom drozofily. Soobshchenie II. Vliianie vozrasta roditelei. Genetika. 1973 Dec;9(12):69–75. [PubMed] [Google Scholar]
- TARDREW P. L., JOHNSON M. J. Sulfate utilization by penicillin-producing mutants of Penicillium chrysogenum. J Bacteriol. 1958 Oct;76(4):400–405. doi: 10.1128/jb.76.4.400-405.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976 Sep;40(3):722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasawa S., Kawamoto I., Takahashi I., Koakura M., Okachi R. Platomycins A and B I Taxonomy of the producing strain and production, isolation and biological properties of platomycins. J Antibiot (Tokyo) 1975 Sep;28(9):656–661. doi: 10.7164/antibiotics.28.656. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Koyama Y., Nagai T., Marumo H., Omura S. Nanomycins, new antibiotics produced by a strain of Streptomyces. II. Structure and biosynthesis. J Antibiot (Tokyo) 1975 Nov;28(11):868–875. doi: 10.7164/antibiotics.28.868. [DOI] [PubMed] [Google Scholar]
- Taylor H. D., Schmitz H. Antibiotics derived from a mutant of Bacillus circulans. J Antibiot (Tokyo) 1976 May;29(5):532–535. doi: 10.7164/antibiotics.29.532. [DOI] [PubMed] [Google Scholar]
- Testa R. T., Wagman G. H., Daniels P. J., Weinstein M. J. Mutamicins; biosynthetically created new sisomicin analogues. J Antibiot (Tokyo) 1974 Dec;27(12):917–921. doi: 10.7164/antibiotics.27.917. [DOI] [PubMed] [Google Scholar]
- Tokunaga T., Mizuguchi Y., Suga K. Genetic recombination in mycobacteria. J Bacteriol. 1973 Mar;113(3):1104–1111. doi: 10.1128/jb.113.3.1104-1111.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxler P., Treichler H. J., Nüesch J. Synthesis of N-acetyldeacetoxy-cephalosporin C by a mutant of Cephalosporium acremonium. J Antibiot (Tokyo) 1975 Aug;28(8):605–606. doi: 10.7164/antibiotics.28.605. [DOI] [PubMed] [Google Scholar]
- Upshall A., Käfer E. Detection and identification of translocations by increased specific nondisjunction in Aspergillus nidulans. Genetics. 1974 Jan;76(1):19–31. doi: 10.1093/genetics/76.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDERHAEGHE H., VANDIJCK P., DESOMER P. IDENTITY OF RAMYCIN WITH FUSIDIC ACID. Nature. 1965 Feb 13;205:710–711. doi: 10.1038/205710a0. [DOI] [PubMed] [Google Scholar]
- Vaks B., Zuckerberg A., Rosenberg E. Purification and partial characterization of an antibiotic produced by Myxococcus xanthus. Can J Microbiol. 1974 Feb;20(2):155–161. doi: 10.1139/m74-025. [DOI] [PubMed] [Google Scholar]
- Walker J. B. Enzymatic reactions involved in streptomycin biosynthesis and metabolism. Lloydia. 1971 Dec;34(4):363–371. [PubMed] [Google Scholar]
- Wheelis L. The genetics of dissimilarity pathways in Pseudomonas. Annu Rev Microbiol. 1975;29:505–524. doi: 10.1146/annurev.mi.29.100175.002445. [DOI] [PubMed] [Google Scholar]
- White R. J., Martinelli E., Gallo G. G., Lancini G., Beynon P. Rifamycin biosynthesis studied with 13C enriched precursors and carbon magnetic resonance. Nature. 1973 Jun 1;243(5405):273–277. doi: 10.1038/243273a0. [DOI] [PubMed] [Google Scholar]
- White R. J., Martinelli E., Lancini G. Ansamycin biogenesis: studies on a novel rifamycin isolated from a mutant strain of Nocardia mediterranei. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3260–3264. doi: 10.1073/pnas.71.8.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth H. Surface structure of streptomycete spores as revealed by negative staining and freeze-etching. J Bacteriol. 1970 Jan;101(1):318–322. doi: 10.1128/jb.101.1.318-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. T., Khan M. R. Antibiotics--a soil microbiologist's viewpoint. Postepy Hig Med Dosw. 1974 Jul-Aug;28(4):395–408. [PubMed] [Google Scholar]
- Williams S. T., Sharples G. P., Bradshaw R. M. The fine structure of the Actinomycetales. Soc Appl Bacteriol Symp Ser. 1973 Jan;2:113–130. [PubMed] [Google Scholar]
- Wright L. F., Hopwood D. A. Actinorhodin is a chromosomally-determined antibiotic in Streptomyces coelicolar A3(2). J Gen Microbiol. 1976 Oct;96(2):289–297. doi: 10.1099/00221287-96-2-289. [DOI] [PubMed] [Google Scholar]
- Wright L. F., Hopwood D. A. Identification of the antibiotic determined by the SCP1 plasmid of Streptomyces coelicolor A3(2). J Gen Microbiol. 1976 Jul;95(1):96–106. doi: 10.1099/00221287-95-1-96. [DOI] [PubMed] [Google Scholar]



