Abstract
Purpose
Outcomes of patients with acute myeloid leukemia (AML) who are refractory to high-dose Cytarabine (HiDAC)-based induction are dismal. Allogeneic hematopoietic stem cell transplantation (AHSCT) as initial salvage may be effective and potentially superior to conventional salvage chemotherapy.
Methods
Eighteen percent (285 of 1597) of AML patients were primary refractory to HiDAC-based regimens at the MD Anderson Cancer Center between 1995 and 2009. AHSCT was the initial salvage in 28 cases. These patients were compared against 149 patients who received salvage chemotherapy, but never received AHSCT.
Results
Patients receiving salvage chemotherapy were older, had higher bone marrow blasts percentage, and higher incidence of unfavorable cytogenetics (P<0.001). Median time from induction to AHSCT was 76 days. Objective response was achieved in 23 of 28 patients (82%) undergoing AHSCT. The incidence of grade III/IV acute and chronic graft versus-host-disease was 11% and 29%, respectively. Median follow up for living patients is 80 months. Median overall survival (OS) was 15.7 months and 2.9 months for AHSCT and chemotherapy, respectively (P<0.001); the 3-year OS rates were 39% and 2%, respectively. ASHCT as initial salvage therapy was identified as an independent prognostic factor for survival in multivariate analysis (HR = 3.03; P < 0.001).
Conclusion
Initial salvage therapy with AHSCT in patients with primary HiDAC refractory AML is feasible and may yield superior outcomes to salvage chemotherapy.
Keywords: acute myeloid leukemia, primary refractory, allogeneic transplantation
Introduction
The outcome of patients with acute myeloid leukemia (AML) who are refractory to high-dose cytarabine (HiDAC) is dismal, with low response rates to salvage chemotherapy and poor long-term survival [1–3] Allogeneic hematopoietic stem cell transplantation (AHSCT) is the only salvage option with true curative potential in this scenario[4, 5]. The concept of performing AHSCT in patients who are not in complete remission (CR) is no totally novel. Studies have reported outcomes in patients with primary refractory AML who proceed to AHSCT while not in CR[5–9]. It must be noted that many of these studies used standard induction regimens consisting of cytarabine (100–200 mg/m2 for 7 days) in combination with an anthracycline, administered intravenously for 3 days[10]. However, induction therapy using HiDAC has been shown to improve not only long-term disease control but also overall survival (OS) in adult patients[11–13]. Many centers, including ours, have incorporated HiDAC into standard induction regimens for AML.
Approximately, 20–40% of adults with AML fail to achieve CR with 1 or 2 cycles of induction chemotherapy, and are deemed primary refractory. We have previously reported a dismal median OS of 3.8 months for patients with AML who are refractory to HiDAC-containing induction therapy (defined as 1gm/m2 cytarabine per dose)[2]. Salvage therapy in such patient populations yielded a response rate of 18% and median response duration of 9 months. AHSCT as initial salvage without exposure to further salvage chemotherapy in such primary HiDAC refractory patients may be effective and potentially superior to chemotherapy based salvage strategies. To better evaluate this hypothesis we reviewed the outcomes of patients with primary refractory AML who underwent AHSCT as initial salvage at our institution from 1995 and 2009 and herein report the results of this analysis.
Patients and Methods
This is a retrospective review of patients with AML treated at the University of Texas M D Anderson Cancer Center (UT/ MDACC) between November 1995 and December 2009. This study was approved by the Institutional Review Board. A departmental database was used to identify patients with AML who received induction chemotherapy.
A total of 1597 patients were treated with HiDAC-based (defined as ≥ 1 g/m2 cytarabine per dose) induction regimens at our institution. Among the 1597 patients treated with HiDAC-based induction, 285 (18%) were primary refractory. Twenty-eight (10%) of these patients underwent AHSCT as initial salvage. Among the remaining 257 primary refractory patients, follow up and outcome data was available in 149 (58%) patients who received salvage chemotherapy alone. Standard supportive care and antibiotic prophylaxis were implemented during induction and transplant. Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus and low dose methotrexate.
CR was defined by the presence of < 5% blasts in the bone marrow (BM) with > 1 × 109/L neutrophils and > 100 × 109/L platelets in the peripheral blood. The following covariates at diagnosis were studied: age, gender, performance status, white blood cell count (WBC), absolute neutrophil count (ANC), platelet count (PLT), hemoglobin (HB), peripheral blast percentage, bone marrow blast percentage, karyotype, presence of molecular mutations and history of a prior malignancy. Additional covariates were recorded for AHSCT patients: time to, and type of AHSCT (i.e., matched related, matched unrelated, mismatched, cord blood, or haploidentical), bone marrow blast percentage, peripheral blood blast percentage, WBC, ANC, PLT, HB at AHSCT, conditioning regimen, response post AHSCT, time to CR post AHSCT, presence and grade of graft-versus-host disease (GVHD). Outcomes of interest were CR duration, OS and disease-free survival (DFS). CR duration (CRD) was calculated from the time of CR until relapse. DFS was defined as time from CR to relapse or death from any cause. Overall survival (OS) was calculated from the time of CR until death or last follow-up. CR duration was calculated from the date of documented CR to the date of disease recurrence, death in CR or last documented follow-up.
Survival probabilities were estimated using the Kaplan-Meier method, and compared by the log-rank test. Differences among variables were evaluated by the chi- square test and Mann-Whitney U tests for categorical and continuous variables, respectively. Univariate and multivariate analyses were performed to identify potential prognostic factors associated with DFS and OS. These factors included age, gender, subtype of AML, AHSCT as first salvage, cytogenetics, history of an antecedent hematological disorder (AHD), prior chemotherapy or radiation (i.e., therapy-related disease), FLT3 mutation status, bone marrow blast percentage, WBC count, and platelet count. Multivariate analysis for DFS and OS used the Cox proportional hazard regression analysis. A p-value of <0.05 (two-tailed) was considered statistically significant.
Results
Patients Characteristics
Baseline characteristics for the two groups are presented in Table 1. Follow up and outcome data was available in 177 (62% of 285 refractory patients). Of these 177 patients, 149 (84%) received salvage chemotherapy alone and 28 (16%) received AHSCT. The patients were followed up for a median of 78 months (95% CI= 77 – 79). The median age for the patients who underwent AHSCT was 56 years, compared to 61 years for the group undergoing salvage chemotherapy (P = 0.01). The patients undergoing salvage chemotherapy alone had a significantly higher median bone marrow blast percentage at baseline (41% versus 19%; P = 0.003) and a higher frequency of unfavorable chromosomal abnormalities (−5, − 7, 36% vs. 21%; P = 0.01). Other baseline characteristics were similar between the two groups.
Table 1.
Patient characteristics and univariate analysis of factors associated with differences in the groups of study
| Parameter | AHSCT group N(%)/Median [range] | Salvage chemotherapy group N(%)/Median [range] | P-value | |
|---|---|---|---|---|
| N=28 | N=149 | |||
| Age (years) | 56 [20–77] | 61 [18–85] | 0.014 | |
| Bone marrow blasts percentage | 19 [0–82] | 41[0–94] | 0.003 | |
| WBC (× 109/L) | 2.0 [0.5–6.5] | 2.4 [0.1–97.0] | 0.113 | |
| Platelets (× 109/L) | 55 [4–317] | 27 [1–1105] | 0.119 | |
| Cytogenetics | −5/−7 | 6 (21) | 54 (36) | 0.014 |
| Diploid | 7 (25) | 43 (29) | ||
| Others | 15 (54) | 52 (35) | ||
| Gender | Female | 14 (50) | 58 (39) | 0.027 |
| Male | 14 (50) | 91 (61) | ||
| AHD | 14 (50) | 80 (54) | 0.719 | |
| Prior chemotherapy/radiation | 6 (21) | 29(19) | 0.810 | |
| FLT3 status | Mutated | 2 (7) | 18 (12) | 0.587 |
| Wild type | 10 (36) | 60 (40) | ||
| Not performed | 16 (57) | 71 (48) | ||
AHD= antecedent of hematologic disorder; WBC= white blood cell; AHSCT= Allogeneic hematopoietic stem cell transplantation
Transplantation Characteristics
For the transplant group, median time from induction to AHSCT was 76 days (range, 28 to 184 days). At time of transplant, median bone marrow blasts and median peripheral blood blasts were 19% (range, 0 to 82) and 4% (range, 0 to 41), respectively. Most patients underwent AHSCT from a matched sibling (64%). Seven patients (25%) had a matched unrelated donor, while 3 patients (11%) were transplanted using a related haploidentical donor. Conditioning regimens were fludarabine-busulfan in 9 patients (32%), fludarabine-melphalan in 7 (25%), and fludarabine-based in 5 patients (18%). Seven patients (25%) received other conditioning.
Effectiveness of therapeutic approaches
CR, CR with incomplete platelet recovery (CRp) or CR with incomplete peripheral blood count recovery (CRi) was achieved in 23 of 28 (82%) patients undergoing initial AHSCT, compared to 11% in the group undergoing salvage chemotherapy alone (P < 0.001). The incidence of Grade III/IV acute GVHD and chronic GVHD were 11% and 29%, respectively. Among the 23 responding patients, 12 patients (43%) relapsed within 5 months (range, 2 to 19 months). With a median follow-up of 80 months from the time of AHSCT (range, 28 to 118 months), eight patients (29%) remain alive in CR. Table 2 compares outcome with AHSCT versus chemotherapy alone.
Table 2.
Comparison of outcomes of allogeneic hematopoietic stem cell transplantation and salvage chemotherapy of patients with acute myeloid leukemia
| Parameter | AHSCT group, N=28 | Salvage chemotherapy group, N=149 | P-value |
|---|---|---|---|
| CR/CRp (%) | 23 (82) | 16 (11) | <0.001 |
| Median OS (months, range) | 15.7 (1.1–114) | 2.9 (0.1–128) | <0.001 |
| 3-year OS rate (%) | 39 | 2 | |
| Median DFS (months, range) | 14.8 (0.9–122) | 4.9 (0.6–126) | 0.076 |
| 3-year DFS (%) | 35 | 13 |
AHSCT= Allogeneic hematopoietic stem cell transplantation; CR= complete remission; CRp= complete remission with incomplete platelet recovery; OS= overall survival; DFS= disease-free survival
Outcome
The AHSCT group had superior median OS when compared to the salvage chemotherapy group (15.7 versus 2.9 months; P < 0.001) (Figure 1). There was a trend to improved median DFS for the group receiving AHSCT as compared to the group receiving initial salvage chemotherapy (14.8 versus 4.9 months; P = 0.08) Figure 2). The 3-year OS was 39% for patients undergoing AHSCT versus 2% for patients undergoing salvage chemotherapy (P<0.001). Similarly, DFS rates were 35%, and 13% for patients undergoing AHSCT and salvage chemotherapy, respectively (P=0.076).
Figure 1.
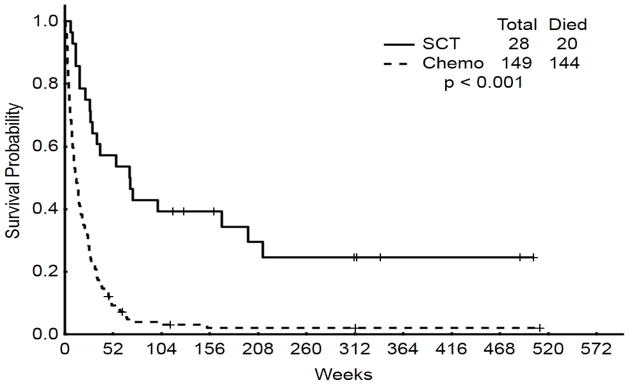
Overall survival: initial salvage AHSCT versus chemotherapy alone versus delayed AHSCT
Figure 2.
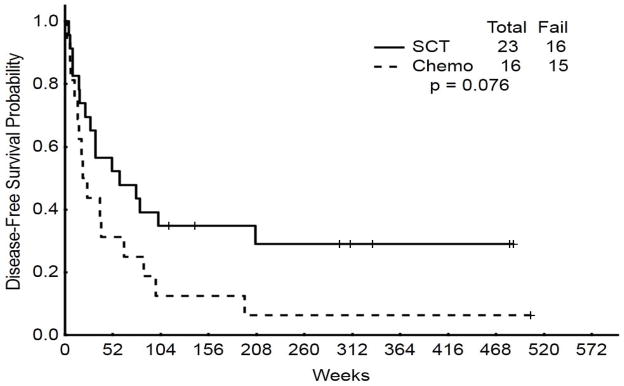
Disease-free survival: initial salvage AHSCT versus chemotherapy alone versus delayed AHSCT
We performed a multivariable analysis to determine predictors of DFS and OS. The only significant predictor of DFS was the WBC count (HR = 1.02, 95% CI = 1.000 – 1.044; P = 0.04). However for OS, patients not receiving AHSCT as initial salvage had a significant survival disadvantage on multivariable analysis (HR = 3.03, 95% CI = 1.766 – 5.200; P < 0.001). Other significant predictors of OS included cytogenetics (specifically presence of aberrations involving chromosome 5 or 7), percentage of BMBL and PLT count at the time of salvage strategy. A higher percentage of BMBL and a lower PLT count conferred a worse outcome. (Table 3).
Table 3.
Multivariate analysis for disease-free and overall survival
| Disease-free survival | Overall survival | |||
|---|---|---|---|---|
| Parameter | P-value | HR (95% CI) | P-value | HR (95% CI) |
| Salvage chemotherapy | 0.19 | 1.7 (0.76–3.90) | <0.001 | 3.03 (1.76–5.20) |
| Higher WBC at AHSCT | 0.04 | 1.02 (1.00–1.04) | 0.08 | 1.01 (0.99–1.02) |
| Lower PLT count at AHSCT | 0.22 | 0.99 (0.99–1.00) | 0.03 | 0.99 (0.99–1.00) |
| Higher BM blasts % at AHSCT | NS | NA | 0.003 | 1.01 (1.00–1.02) |
AHSCT= Allogeneic hematopoietic stem cell transplantation; WBC= white blood cell; PLT= platelet; BM= bone marrow; HR= hazard ratio; NS= not significant; NA= not applicable
Role of further salvage therapy prior to AHSCT versus upfront AHSCT
We did repeat the analysis including 15 patients with refractory AML who received further cytoreductive salvage therapy beyond their first salvage regimen and who received a subsequent AHSCT. The delayed AHSCT group had an inferior DFS (13.2 versus 4.9 months; P<0.01) and inferior median OS (39 months versus 14 months; P < 0.001). On multivariable analysis, initial AHSCT remained the most significant predictor of DFS and OS.
Discussion
We have shown that in patient’s refractory to HiDAC based induction therapy, initial salvage with AHSCT yields superior outcomes than salvage chemotherapy alone. In our experience, patients who fail initial induction with HiDAC based regimens have an extremely poor prognosis, with a median OS of less than 4 months[2]. Our historical data indicated that salvage approaches that did not include upfront AHSCT were independently associated with worse survival in this patients’ population. Herein, we have analyzed the role of upfront AHSCT in this patient population in greater detail.
We found a 39% 3-year OS rate for patients undergoing AHSCT as initial salvage, indicating the potential for cure in a significant proportion of primary refractory patients. Nevertheless, it is important to note that patients who receive initial salvage with AHSCT made up a small minority (16%) of the entire cohort of primary refractory patients. One must consider the factors that may have predisposed this group of patients to fare better than their counterparts who received salvage chemotherapy alone. Although patients were mostly well matched for baseline characteristics, the salvage chemotherapy group was older, and more likely to have abnormalities of chromosomes 5 or 7.
There is an inherent selection process for AHSCT that cannot be completely accounted for, intrinsically favoring younger patients, with less disease burden, and more favorable cytogenetic profiles[14]. Older age is a barrier to AHSCT, although the use of reduced intensity conditioning is making this a more attractive proposition for older patients. Another common reason for not undergoing transplant is the lack of a fully matched donor (i.e., MRD or MUD). Improvements in the use of alternative stem cell sources, such as umbilical cord blood or haploidentical donors, may enhance our ability to offer AHSCT to primary refractory patients. Alternative sources may also allow patients to proceed to transplant more rapidly, thereby reducing delays or drop outs secondary to infectious complications, comorbidities or leukemia relapse. Three patients in our study did indeed receive stem cells from a haploidentical donor, and such alternative donor transplants warrant further validation in larger studies.
AHSCT as initial salvage was the strongest independent predictor of survival in our analysis. This is consistent with a previous report supporting the notion that AHSCT immediately after induction failure was associated with a favorable prognosis in a large, heterogeneous group of relapsed AML patients[5]. We also found that a higher percentage of bone marrow blasts at the time of AHSCT was associated with inferior outcome. In the study conducted by Duval et al, presence of circulating blasts, but not bone marrow blasts, was associated with worse outcome[5]. Nevertheless, these conclusions suggest a benefit to cytoreduce prior to AHSCT. This entails administration of salvage chemotherapy with intent to achieve remission followed by AHSCT. Indeed, fifteen of our patients underwent salvage chemotherapies followed by AHSCT at later date. Unfortunately, their outcomes remained dismal with remission rates and overall survival rates inferior to patients who underwent up front AHSCT. Similarly, a large number of patients referred for AHSCT may not eventually receive AHSCT due to a variety of reasons as published by Estey et al.[15] Delays or occurrence of infections may further reduce the ability of patients to proceed to AHSCT. Thus, the best chance to receive AHSCT and benefit from it seems to be up front at failure to primary induction regimen.
Relapse was the major cause of treatment failure after AHSCT. This was despite the fact that more than 80% of patients who underwent AHSCT as initial salvage achieved CR, CR with incomplete platelet recovery or CR with incomplete blood count recovery. We have proposed that maintenance therapy may have a role in such patients who achieve morphological CR but have some degree of minimal residual disease, which may be identifiable by more sensitive modalities including multicolor flow- cytometry or polymerase-chain reaction testing. Low-dose azacitidine may serve not only as maintenance therapy but may also enhance the graft-versus-leukemia effect thus providing a dual benefit. This hypothesis is under prospective investigation[16, 17].
In conclusion, for patients with AML who are refractory to HiDAC-based induction chemotherapy, first salvage with AHSCT leads to superior OS and a trend toward superior DFS when compared to salvage chemotherapy. However, the majority of our patients were unable to proceed to AHSCT, and new approaches should be developed and tested to broaden the availability and shorten the time to AHSCT.
References
- 1.Estey EH. Treatment of relapsed and refractory acute myelogenous leukemia. Leukemia. 2000;14:476–479. doi: 10.1038/sj.leu.2401568. [DOI] [PubMed] [Google Scholar]
- 2.Ravandi F, Cortes J, Faderl S, et al. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood. 2010;116:5818–5823. doi: 10.1182/blood-2010-07-296392. quiz 6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanisic S, Kalaycio M. Treatment of refractory and relapsed acute myelogenous leukemia. Expert Rev Anticancer Ther. 2002;2:287–295. doi: 10.1586/14737140.2.3.287. [DOI] [PubMed] [Google Scholar]
- 4.Revesz D, Chelghoum Y, Le QH, et al. Salvage by timed sequential chemotherapy in primary resistant acute myeloid leukemia: analysis of prognostic factors. Ann Hematol. 2003;82:684–690. doi: 10.1007/s00277-003-0730-1. [DOI] [PubMed] [Google Scholar]
- 5.Duval M, Klein JP, He W, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zander AR, Dicke KA, Keating M, et al. Allogeneic bone marrow transplantation for acute leukemia refractory to induction chemotherapy. Cancer. 1985;56:1374–1379. doi: 10.1002/1097-0142(19850915)56:6<1374::aid-cncr2820560626>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Oyekunle AA, Kroger N, Zabelina T, et al. Allogeneic stem-cell transplantation in patients with refractory acute leukemia: a long-term follow-up. Bone Marrow Transplant. 2006;37:45–50. doi: 10.1038/sj.bmt.1705207. [DOI] [PubMed] [Google Scholar]
- 8.Forman SJ, Schmidt GM, Nademanee AP, et al. Allogeneic bone marrow transplantation as therapy for primary induction failure for patients with acute leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1991;9:1570–1574. doi: 10.1200/JCO.1991.9.9.1570. [DOI] [PubMed] [Google Scholar]
- 9.Mehta J, Powles R, Horton C, et al. Bone marrow transplantation for primary refractory acute leukaemia. Bone Marrow Transplant. 1994;14:415–418. [PubMed] [Google Scholar]
- 10.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–1163. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 11.Bishop JF, Matthews JP, Young GA, et al. A randomized study of high-dose cytarabine in induction in acute myeloid leukemia. Blood. 1996;87:1710–1717. [PubMed] [Google Scholar]
- 12.Kern W, Estey EH. High-dose cytosine arabinoside in the treatment of acute myeloid leukemia: Review of three randomized trials. Cancer. 2006;107:116–124. doi: 10.1002/cncr.21543. [DOI] [PubMed] [Google Scholar]
- 13.Buchner T, Hiddemann W, Wormann B, et al. Double induction strategy for acute myeloid leukemia: the effect of high-dose cytarabine with mitoxantrone instead of standard-dose cytarabine with daunorubicin and 6-thioguanine: a randomized trial by the German AML Cooperative Group. Blood. 1999;93:4116–4124. [PubMed] [Google Scholar]
- 14.Armistead PM, de Lima M, Pierce S, et al. Quantifying the survival benefit for allogeneic hematopoietic stem cell transplantation in relapsed acute myelogenous leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:1431–1438. doi: 10.1016/j.bbmt.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estey E, de Lima M, Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) Blood. 2007;109:1395–1400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]
- 16.Jabbour E, Giralt S, Kantarjian H, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer. 2009;115:1899–1905. doi: 10.1002/cncr.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116:5420–5431. doi: 10.1002/cncr.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]


